Summary
Hyper-phosphorylation of RB controls its interaction with E2F and inhibits its tumor suppressor properties. However, during G1 active RB can be mono-phosphorylated on any one of 14 CDK phosphorylation sites. Here we used quantitative proteomics to profile protein complexes formed by each mono-phosphorylated RB isoform (mP-RB) and identified the associated transcriptional outputs. The results show that the 14 sites of mono-phosphorylation co-ordinate RB’s interactions and confer functional specificity. All 14 mP-RBs interact with E2F/DP proteins but they provide different shades of E2F regulation. RB mono-phosphorylation at S811, for example, alters RB transcriptional activity by promoting its association with NuRD complexes. The greatest functional differences between mP-RBs are evident beyond the cell cycle machinery. RB mono-phosphorylation at S811 or T826 stimulates the expression of oxidative phosphorylation genes, increasing cellular oxygen consumption. These results indicate that RB activation signals are integrated in a phosphorylation code that determines the diversity of RB activity.
eTOC:
Sanidas et al show how RB activity is tailored by mono-phosphorylation in G1-phase cells. Fourteen different forms of RB interact with specific sets of proteins and provide distinct transcriptional outputs. In this way, RB mono-phosphorylation generates a functional diversity that extends beyond the regulation of the E2F program.
Introduction
RB, the protein product of the retinoblastoma tumor susceptibility gene (RB1), is essential for normal development and suppresses several types of cancer. Although RB has been studied for many years, its precise molecular mechanism of action remains an enigma (Dyson, 2016). It is generally agreed that RB suppresses the E2F transcription factor, an important regulator of genes needed for cell proliferation. However, hundreds of proteins have been reported to physically associate with RB and have been proposed to be involved in RB’s tumor suppressor activity (Morris and Dyson, 2001; Dick and Rubin, 2013; Dick et al., 2018). RB/E2F complexes can associate with several types of chromatin-associated protein complexes (Dick and Rubin, 2013) and RB has been implicated in both the activation and repression of transcription (Markey et al., 2007; Ianari et al., 2009). RB has been reported to bind to several transcription factors in addition to E2F, to target both euchromatic and heterochromatic regions, and to suppress transcription of repetitive sequences (Benevolenskaya et al., 2005; Siddiqui et al., 2007; Longworth and Dyson, 2010; Ishak et al., 2016; Dick et al., 2018). RB also has transcription-independent activities that impact cell cycle control and cell survival. For example, RB associates with Skp2 and APC and interferes with Skp2-mediated degradation of p27 (Ji et al., 2004; Binne et al., 2007). A pool of RB localizes to mitochondria and interacts with Bax (Ferecatu et al., 2009; Hilgendorf et al., 2013). RB can also be recruited to sites of DNA damage (Cook et al., 2015; Velez-Cruz et al., 2016) and it interacts with replication proteins (Sterner et al., 1998; Mendoza-Maldonado et al., 2010). According to current interaction databases at least 322 human proteins interact with RB (Table S1).
The large number of putative RB-associated proteins may explain the diverse functions ascribed to RB, with the caveat that only a few interactions have been studied in detail. It is unclear, however, how one protein can have so many different partners or how such large number of interactions are orchestrated. Since RB associates at sub-stoichiometric levels with each of its proposed targets, different pools of RB may have distinct functions. Alternatively, specific conditions may allow RB to differentiate between its partners and target it for specific roles. To date, there has not been a comprehensive analysis of RB-associated proteins and RB’s action has been investigated by studying specific properties of RB or a few cherry-picked interactors at a time.
Independent studies from the Dowdy and Rubin laboratories offered a new way to think about the RB “interactome”. It is known that cyclin dependent kinases (CDKs) inactivate RB each time a normal cell divides. Experiments with synchronized cells indicated that Cyclin D-dependent kinases provide the initial phosphorylation of RB in G1, while Cyclin E/CDK2 drives the hyper-phosphorylation of RB at the G1/S transition that inactivates RB’s nuclear functions (Hinds et al., 1992; Dowdy et al., 1993; Hinds et al., 1994; Sherr, 1996). Seth Rubin’s laboratory examined the impact of CDK phosphorylation on RB and discovered that modification of individual residues causes major changes to the RB protein structure. Phosphorylation of RB carboxyl terminal domain (RBC) at S788 and S795 directly inhibits RB association with E2F-DP heterodimers, while RBC phosphorylation at T821 and T826 indirectly reduces RB binding to E2F-DP by stimulating an intramolecular interaction between RBC and the RB pocket domain (Rubin et al., 2005). Phosphorylation of T373 promotes a conformational change that allows the RB amino terminal domain (RBN) to dock against the pocket domain. Phosphorylation of S608 also triggers another conformational change in which a loop containing the phosphorylation site interacts with part of the pocket domain (Burke et al., 2010; Burke et al., 2012). Remarkably, the structural changes driven by single phosphorylation events alter specific binding domains without compromising the overall integrity of the protein, leaving other binding surfaces intact. These findings suggest that individual phosphorylation events might limit the spectrum of proteins that RB interacts with.
Complementary experiments from the Dowdy laboratory demonstrated that RB isolated from asynchronously dividing tissue culture cells exists in two general states (Narasimha et al., 2014). A population of hyper-phosphorylated RB generated by cell cycle progression appears to be phosphorylated on all 14 in vivo CDK phosphorylation sites. A second population, that predominates in G1 (and previously called “hypo-phosphorylated” RB) is comprised primarily of mono-phosphorylated RB (mP-RB). Strikingly, the single phosphorylation event on mP-RB can occur at many sites – at perhaps all 14 known CDK phosphorylation sites. Isoelectric focusing gels, that separate phosphorylated RB by charge, revealed that mP-RB is the exclusive form of RB in contact inhibited cells and in cells arrested by DNA damage; situations where RB is active and blocks cell cycle progression (Narasimha et al., 2014). These results suggest that “active” RB is actually the integrated effect of many mP-RB isoforms, perhaps as many as 14 different mP-RBs. This may explain why the 32P tryptic peptide maps of RB isolated from G1 and G2 arrested cells were identical (Lees et al., 1991).
Together these observations suggested a new model for RB function (Dick and Rubin, 2013; Rubin, 2013; Narasimha et al., 2014; Dyson, 2016). The central concept is that cells contain multiple isoforms of active RB in which mono-phosphorylation tailors the properties of RB by inhibiting its interactions with specific proteins and, potentially, by promoting interactions with others. Part of the appeal of this model is that it immediately suggests how different aspects of RB function might be controlled. At present, however, there are major gaps in the evidence for this model: it is not yet known which proteins interact with each mP-RB; it is unclear whether mP-RBs regulate the same genes or different subsets of genes; it is uncertain whether the various mP-RBs co-exist or are generated at different times; perhaps most fundamentally, it is unclear whether the mP-RBs are functionally distinct, and if they are different, it is unknown what roles individual isoforms play.
Here, we test the hypothesis that RB action is controlled by a code of mono-phosphorylation. We generated a panel of isogenic cell lines that can be induced to replace the endogenous RB protein with mutant RB proteins containing just a single CDK phosphorylation site. We developed techniques to profile RB-associated proteins by mass spectrometry and used this experimental system to identify the proteins that interact with each form of RB carrying a single CDK phosphorylation site. We report significant differences in the composition of the protein complexes formed by individual mP-RBs and show that these provide functional specificity that extends beyond the regulation of the cell cycle. Illustrating these functional differences, we demonstrate that RB phosphorylation at S811 promotes association with the NuRD chromatin-remodeling complex and alters the spectrum of genes repressed by RB. Proteomic and functional studies have indicated that mitochondrial changes are a major consequence of RB inactivation. We find that RB phosphorylation at S811 or T826 strongly enhances the expression of oxidative phosphorylation genes, elevating cellular oxygen consumption. Collectively these results reveal a regulatory code for RB, demonstrating that mP-RBs interact with different sets of proteins, regulate different sets of targets, and control different aspects of RB function.
Results
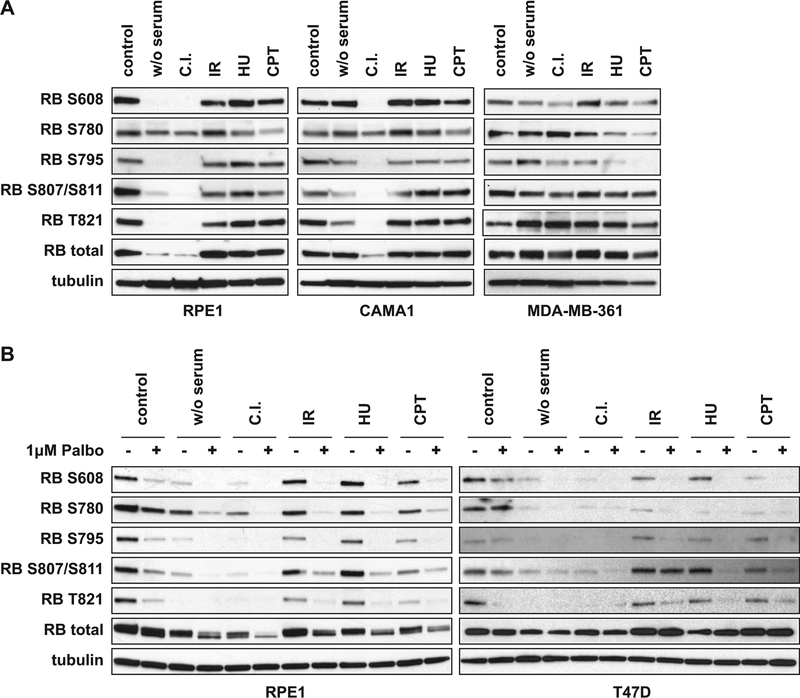
To understand the relationship between mP-RBs we first asked whether the levels of different mP-RBs fluctuate in a fixed ratio, or whether cell cycle arrest conditions favor specific mP-RBs. A small panel of cell lines that express wild-type RB and are sensitive to CDK4/6 inhibitors were exposed to conditions reported to activate RB (serum starvation, contact inhibition, treatment with DNA damaging agents). Modification of specific sites was followed by western blot analysis using five commercially-available phospho-specific RB antibodies (Fig. S1A). Results obtained with hTERT-immortalized human retina epithelial cells (RPE1) and three estrogen receptor positive breast cancer cell lines (CAMA1, MDA-MB-361 and T47D) are shown in Fig. 1 (quantitation shown in Fig. S1B and S1C). Even with a small panel, it is clear that different cell lines give different profiles. While some conditions had similar effects on all sites, other conditions showed specific changes. In RPE1 and CAMA1 cells, contact inhibition caused de-phosphorylation of S608, S795, S807/S811 and T821, and the preferential RB phosphorylation on S780. In contrast, in T47D cells, gamma-irradiation or treatment with hydroxyurea generated RB selectively phosphorylated on S807/S811. In MDA-MB-361 cells, camptothecin treatment preferentially reduced phosphorylation of S780 and S795. These changes could not simply be attributed to differences in cell cycle position (Fig. S1D). While treatment of RPE1 and T47D cells with the CDK4/6 kinase inhibitor palbociclib generally reduced RB phosphorylation (Fig. 1B and S1C), not all RB phosphorylation isoforms were suppressed (e.g. S807/S811 phosphorylation in irradiated T47D cells) suggesting that some context-specific phosphorylation events may have additional regulation.
Figure 1: Cellular stress triggers differential RB phosphorylation.
(A) Western blots of cell lysates from RPE1, CAMA1 and MDA-MB-361 cells treated under stress conditions or untreated (control) were probed with the indicated antibodies. Serum starvation for 24h (w/o serum), contact inhibition for 5 days (C.I.), 10 Gy gamma-irradiation (IR), 5mM Hydroxyurea for 6h (HU) and 1 μM Camptothecin for 6h (CPT). (B) RPE1 and T47D cells were treated with 1 μM palbociclib (CDK4/6 inhibitor) or DMSO for 6h, concurrently with the induction of cellular stress as in Figure 1A.
These results add to published studies showing that cell differentiation causes RB phosphorylation at S612 (Hattori et al., 2014) and that cellular stress signals can induce p38-mediated phosphorylation of RB at S249 and T252 (Gubern et al., 2016). We conclude that the picture of RB phosphorylation is far more complex than typically described: the patterns differ between cell lines with some conditions causing similar fluctuations in RB phosphorylation at multiple sites, while others favor site-specific RB phosphorylation. To understand what these changes might mean, we sought to identify the shared and unique properties of the mP-RBs.
An experimental strategy to generate isogenic cell lines expressing different phosphorylation isoforms of RB.
Studying mP-RBs required an experimental approach that allows the expression of specific forms of RB. We avoided using RB null cells. Genetic studies show that RB-family members (p107 and p130) partially compensate for RB when RB null cell are put in long-term culture (Sage et al., 2003) and the constitutive RB1 inactivation causes genomic instability (Hernando et al., 2004). Conversely, long-term overexpression of RB reduces viability of non-transformed human cells (Calo et al., 2010; Hilgendorf et al., 2013). To minimize the opportunity for compensation and the caveats associated with adaptation of cells in long-term culture, we created a transient doxycycline (DOX) inducible system that replaces the endogenous RB protein with exogenous RB mutants. Human RPE1 cells were chosen for this analysis because they are non-transformed cells that lack perturbations in the RB/E2F pathway and have been widely used for RB research.
RPE1 cells were infected with a lentiviral vector expressing a DOX-inducible shRNA that targets the 3’-UTR of RB1 mRNA. This gave efficient depletion of the endogenous wild type RB (Fig. 2A). We then generated lentiviral vectors with DOX-inducible constructs that express FLAG tagged forms of RB that are not targeted by the shRNA and introduced these into the same cells. Addition of DOX now triggered the efficient replacement of endogenous RB by exogenous tagged wild type protein (Fig. 2B) or RBΔcdk, in which all of the known in vivo CDK phosphorylation sites were mutated to alanine (Fig. 2C) (Narasimha et al., 2014). The exogenous proteins are expressed at levels comparable to endogenous RB (Fig. 2B and 2C). Replacement of endogenous RB with exogenous wild type RB had no effect on cell cycle distribution or proliferation, but replacement of endogenous RB with RBΔcdk triggered a robust G1-arrest (Fig. 2D and 2E). A shift in the mobility of the overall pool of RB within 8–16hrs of DOX treatment illustrates the replacement of wild type endogenous protein with unphosphorylated RBΔcdk (Fig. 2C).
Figure 2: An inducible RB replacement system generates isogenic cell lines expressing different FLAG-RB isoforms.
(A) Timecourse of DOX-inducible knock down of the endogenous RB in RPE1 cells. (B) Timecourse of DOX-inducible substitution of the endogenous RB by exogenous wild type RB or (C) RBΔcdk in RPE1 cells. Note that a-Rb1 detects all RB protein but a-FLAG detects only exogenous RB (D-E) Cell cycle analysis of the same cell cultures, before and after 48h DOX treatment. (F) Western blots probed with a-FLAG show the expression of RB wild-type, RBΔcdk or the 14 mP-RB isoforms in RPE1 cells depleted of endogenous RB, after 48h DOX treatment.
This method was used to prepare a panel of 16 isogenic cell lines that, when treated with DOX, replaced endogenous RB with exogenous FLAG-tagged wild-type RB, or RBΔcdk, or any one of the 14 mutant RB proteins that contain just one CDK phosphorylation site (Fig. 2F). The 14 mP-RB mutant alleles were generated by restoring a single phosphorylation site into RBΔcdk (Narasimha et al., 2014). Using phospho-RB antibodies, we verified that the mutant proteins were efficiently phosphorylated on the appropriate sites (Fig. S2A). Biochemical fractionation showed that all 14 mP-RBs had a similar subcellular distribution with predominantly nuclear localization (Fig. S2B). Interestingly, only wild-type RB showed a significant fraction in the cytoplasm, a finding consistent with evidence that hyperphosphorylated RB has cytoplasmic functions (Zhang et al., 2016). Although there is some variation in levels of mP-RB expression (Fig. 2F), all 14 mP-RBs arrested cells in G1-phase (Fig. S2C), confirming that all are active. The efficiency of G1-arrest varied (with T356 and S788 giving the greatest G1 increase) but a lack of correlation between the degree of arrest and the level of RB expression suggests that the mP-RBs likely differ in activity.
Proteomic analysis of the complexes formed by distinct RB phosphorylation isoforms identifies interactions regulated by individual modifications.
One reason why RB’s mechanism of action has remained mysterious is that no one has succeeded in purifying endogenous RB-complexes in quantities sufficient for comprehensive analysis. Using the inducible RB replacement system and taking advantage of tools developed for FLAG-tagged protein purification, we optimized previous protocols to isolate endogenous RB complexes. Western blot analysis of immunoprecipitated wild-type RB or RBΔcdk complexes confirmed the enrichment of known binding partners (E2F1, E2F3, TFDP1, Cyclin A, Cyclin D1 and CDK2) (Fig. S3A). Complexes formed by FLAG-tagged wild-type RB, RBΔcdk and the 14 mP-RBs were purified from the isogenic cell lines and analyzed by multiplexed quantitative mass spectrometry-based proteomics. For negative controls we profiled anti-FLAG immunoprecipitates from cells expressing DOX-induced un-tagged wild-type RB or RBΔcdk (Fig. 2F, S2A). Three independent experiments were performed per isogenic cell line (Fig. S3D). The Median Coefficient of Variation among all triplicates was 13%.
The mass spectrometry profiles of proteins bound to wild-type RB or RBΔcdk included all the proteins that we had detected by western blot, and the specific binding properties of wild type RB or RBΔcdk were clear using either method. Volcano plots (Fig. 3A and 3B) display the fold enrichment of proteins in RB immunoprecipitates compared to control precipitates (x-axis) and the P-value generated from three independent replicates (y-axis). Significant interactions with fold change greater than 1.5 relative to control are boxed. As expected, wild-type RB and RBΔcdk interact with multiple E2F/DP proteins (Fig. 3A, 3B and S3A). The quantitative proteomic analysis revealed that E2F3 and TFDP1 are more abundant in RBΔcdk complexes, while wild-type RB complexes have more E2F1. Cyclin A and CDK2 have a striking binding preference for wild-type RB, while Cyclin D1 was detected only in RBΔcdk complexes. Overall, we identified 282 proteins with a statistical significant enrichment (1.5-fold change and FDR<25%) in complexes with wild-type RB. Surprisingly, this number was much higher than the 42 proteins detected in complexes with RBΔcdk (Fig. 3C).
Figure 3: RB mono-phosphorylation alters the composition of the endogenous RB complexes.
(A) Quantitative proteomics analysis of RB complex from RPE1 cells expressing the FLAG-RB wild-type or (B) FLAG-RBΔcdk. The volcano plots show the log2 fold change in the abundance of a protein in the RB complex relative to control. Positions of several known and novel interactors are indicated. (C) Venn diagram presentation of the number of common proteins between RB wild-type and RBΔcdk complexes or (D) between RB wild-type, RBΔcdk and the combination of the 14 mP-RB isoforms complexes. Proteins with statistical significant enrichment (1.5-fold change and FDR<25%) are shown. (E) Heatmap presentation of the log2 fold changes in the abundance of associated proteins in RB complexes relative to control immunoprecipitates. Data are presented for complexes formed by RB wild-type, RBΔcdk, and 14 mP-RB mutants. Yellow, orange and green colors denote mP-RB complexes that are grouped together by hierarchical clustering. (F) Distribution of the 14 CDK phosphorylation sites in RB. Positions of N-terminal (RBN), pocket, and C-terminal (RBC) domains are shown.
The differences between wild-type RB and RBΔcdk complexes highlighted the question of whether RB phosphorylation might promote interactions. We examined this hypothesis and tested the idea that mP-RBs have specific binding properties by examining the proteins associated with each of the 14 mP-RBs. Since RBΔcdk has cell cycle arrest properties that are similar to mP-RBs (Fig. S2C), anti-FLAG immunoprecipitates from arrested cells expressing untagged RBΔcdk were used as a negative control for complexes formed by the Flag-tagged mP-RBs.
Collectively, this mass spectrometric analysis identified 438 proteins with a statistically significant enrichment in complexes with at least one of the 16 forms of RB examined (Table S2). The 22 proteins significantly enriched with all forms of RB included multiple E2F and DP proteins. Importantly, 85 of the 260 proteins that were found in wild-type RB complexes but not in RBΔcdk complexes were also found in complexes with one or more of the 14 mP-RBs (Fig. 3D). This number underlines the major impact of mono-phosphorylation on the composition of RB complexes, but is likely to be an underestimate of phosphorylation-dependent interactions since each form of mP-RB represents only a small percentage of the pool of wild-type RB, and wild-type RB complexes were isolated from asynchronous cell populations in which only 47% cells were in G1 (Fig. 2E), the stage of the cell cycle where RB is mono-phosphorylated. Indeed, the profiles of mP-RB complexes revealed 136 interactors that did not reach the same cut-offs for enrichment and statistical significance with either wild-type RB or RBΔcdk. Strikingly, individual mP-RBs associate with different numbers of proteins and have different numbers of common interactors with wild-type RB or RBΔcdk (Fig. S3B and S3C). We also note that 175 RB-associated proteins were only detected with wild-type RB; these likely include interactions that depend on the hyper-phosphorylation of RB that occurs outside of G1 (Table S3).
Unbiased hierarchical clustering analysis of the statistical significant interactions with fold change greater than 1.5 relative to control in at least one of the 16 RB isoforms’ complexes (Fig. 3E) showed that the profiles of all mP-RBs more closely resemble RBΔcdk than wild-type RB. Although the mP-RBs induce G1-arrest to differing degrees, none of the mP-RB-interactors significantly correlated with these differences. Indeed, mP-RB-T356 and mP-RB-S788 that gave the largest G1-population (Fig. S2C) were separated in the clustering analysis (Fig. 3E).
Each mP-RB had a unique set of associated proteins. Interestingly, mP-RBs containing phosphorylation sites in related protein domains grouped together (Fig. 3E, 3F). mP-RBs with phosphorylation sites in RBC (S807, S811, T821 and T826) or sites close to the pocket domain (S612, S780, S788 and S795) were jointly classified, while mP-RBs that contain a phosphorylation site in RBN (S230, S249 and T252) grouped together with RBΔcdk. T373 phosphorylation has been reported to trigger a major conformational change in RB by settling RBN next to the pocket domain (Burke et al., 2012), and it is notable that complexes formed by mP-RB-T373 show the greatest contrast to the other mP-RBs. The hierarchical clustering revealed interactions that are dependent or independent on RB mono-phosphorylation (Table S4). E2F3, E2F4 and E2F5 clustered with TFDP1 and TFPD2, forming a group of proteins that associate equally well with each of the 14 mP-RBs (Fig. S4A). The enrichment of this group of endogenous proteins in RB complexes was unaffected by mono-phosphorylation at T373, or S608, or at RBC residues that have been reported to interfere with E2F binding in vitro (Burke et al., 2010; Burke et al., 2012; Burke et al., 2014). Of all of the E2F- and DP-family members, E2F1 was the one protein that showed the clearest preference for specific mP-RBs (Fig. S4B). RB mono-phosphorylation at 10 of the 14 CDK sites enhanced association with E2F1, with mP-RBs S780 and T373 showing the strongest E2F1 enrichment.
The clustering analysis revealed several groups of proteins whose interactions with RB depend greatly on sites of mono-phosphorylation. To illustrate the variety of effects, we describe RB’s interactions with two important sets of proteins: one that is inhibited, and another that is stimulated by site-specific RB phosphorylation.
Site-specific mono-phosphorylation disrupts RB’s interaction with CyclinD1 and CDK6.
CyclinD1 and CDK6 did not associate significantly with wild-type RB but bound very strongly to RBΔcdk (Fig. 3A and 3B). Interestingly, the re-introduction of single phosphorylation sites reduced these interactions (Fig. 4A). Multiple sites impacted binding, in particular the inclusion of T373, T821, or T826 strongly reduced the co-precipitation of CyclinD1 with RB. These observations were confirmed by western blot analysis (Fig. S4C). To verify that RB association with CyclinD1 is disrupted by CDK4/6 kinase activity, we compared complexes formed by wild type RB in cells treated with the CDK4/6 inhibitor palbociclib or DMSO. As expected, CDK4/6 inhibition stimulated CyclinD1 binding to wild type RB but this effect was lost when phospho-mimicking amino acids substituted T373 or T826 (Fig. 4B). An unexplained feature of the RB mono-phosphorylation model is the idea that G1 CDK complexes can phosphorylate RB on many sites, but that each molecule is phosphorylated only once. Our results suggest that, for at least half of the sites, once CyclinD1:CDK6 kinase has phosphorylated RB on a single residue, it now has greatly reduced affinity for the mono-phosphorylated protein. This presumably limits the potential for multisite phosphorylation of RB by Cyclin D1 during G1. We note that, since several mP-RBs do bind to CyclinD1 and CDK6, additional mechanisms must exist to explain the absence of multisite RB phosphorylation during G1 (Narasimha et al., 2014).
Figure 4: Mono-phosphorylation reduces or enhances RB affinity to specific proteins.
(A) Heatmap presentation of the log2 fold enrichment of CDK6 or Cyclin D1 in RB complexes relative to control. (B) Treatment with 450 nM palbociclb for 16h increases the co-immunoprecipitation of Cyclin D1 with RB in RPE1 cells expressing wild type RB but this effect is lost in cells expressing phospho-mimicking RB mutants. (C) Heatmap presentation of the log2 fold enrichment of the NuRD complex components in RB complexes relative to control. (D) Western blot detection of NuRD complex components in RB immunoprecipitates.
Site-specific mono-phosphorylation enhances interaction between RB and NuRD complexes.
Nucleosome Remodeling and Deacetylase (NuRD) complex was the most abundant chromatin-remodeling complex detected in RB complexes. NuRD failed to bind to wild-type RB but multiple components of NuRD (including MTA1, MTA2 and HDACs) were significantly enriched in one or more type of RB complexes (Fig. 4C). Interestingly, NuRD components grouped together in the hierarchical clustering analysis and formed a group that associate more strongly with mP-RB S811 than with constitutive active RBΔcdk (Fig. 4C and Table S4). Conversely RB mono-phosphorylation at other residues (T373, S780 or T826) inhibited these interactions. The RB isoform-specific interaction with NuRD components was confirmed using western blots (Fig. 4D). A central element of the RB phosphorylation code is the notion that individual phosphorylation events regulate specific activities. The RB/NuRD interaction illustrates the fact that phosphorylation events do not simply disrupt RB interactions but that certain sites promote specific associations.
Different mono-phosphorylation RB alleles have distinct transcriptional outputs.
Evidence that site-specific mono-phosphorylation changes the composition of RB complexes prompted us to ask whether mP-RB alleles have different functional properties. To investigate this, RNA sequencing was used to profile the transcriptional changes caused by replacing endogenous RB with exogenous wild-type RB, RBΔcdk or the 14 mP-RB isoforms. The log2 fold change of the normalized read count for each annotated feature was calculated between cells expressing RB mutant alleles and cells expressing wild-type RB. Scores were analyzed by hierarchical clustering analysis for all mapped transcripts (Fig. 5A), or for transcripts in the previously identified RB-loss signature (Markey et al., 2007) (Fig. 5B). A large category of genes upregulated following RB loss contain elements that are controlled by E2F (Markey et al., 2007). Like RBΔcdk, all 14 mP-RB isoforms repressed these genes (Fig. 5B, bottom of the heatmap), consistent with the fact that all of these isoforms associate with E2F proteins and generate a robust G1 arrest (Fig. S2C). Interestingly, most mP-RBs have a weaker repressor activity than RBΔcdk, while mP-RB isoforms S230, S612, and S788 repressed E2F targets similarly to RBΔcdk. Remarkably, mono-phosphorylation of T356 suppressed E2F program even more effectively than RBΔcdk, indicating that this specific site enhances the repressive activity of unphosphorylated RB.
Figure 5: Mono-phosphorylation controls RB transcriptional activity.
(A) Heatmap presentation of the log2 fold change of the normalized read count for each annotated feature or (B) transcripts that are regulated by acute RB loss (Markey et al., 2007) in cells expressing RB mutant alleles relative to cells expressing RB wild-type. (C) IPA analysis of genes showing a significant Spearman correlation with the association of at least three components of the NuRD complex with RB. Plot shows the -log(p-value) for the five most significant canonical pathways. Z-score indicates upregulation (orange), downregulation (blue) or non-specific expression pattern (grey) for the genes involved in each pathway. (D) Heatmap presentation of the expression levels of 16 genes that are involved in the role of BRCA1 in DNA damage response and show correlation with NuRD complex association with RB. (E) RPE1 cells expressing mP-RB S811 were transfected with siControl, siMTA1, siMTA2 or siMTA1+siMTA2. 48h after transfection, TOPBP1, MRE11, BRCA1, BRCA2 and MSH2 mRNA levels were measured by real time RT-PCR. Bars show the fold change in gene expression (mean ± SD) in siMTA1-siMTA2-transfected cells relative to siControl-transfected cells. (F) RPE1 cells (upper) and T47D cells (bottom) expressing RB wild-type, RB S811A or RB S811D were treated with 10 Gy gamma-irradiation (IR). 6h after IR, BRCA1 and BRCA2 mRNA levels were measured by real time RT-PCR. Bars show the fold change in gene expression (mean ± SD) in irradiated cells relative to control-untreated cells. (E, F; two-way ANOVA, ****p<0.0001; ***p<0.001; **p<0.01; *p<0.05). (G) Gamma-irradiation-induced mP-RB S811 supresses the genes involved in the role of BRCA1 in DNA damage response in a NuRD-depended manner.
While the transcription profiles of cells expressing the mP-RBs were related they were also different, indicating that the mP-RBs have different functional outputs. mP-RBs containing phosphorylation sites in RBC (S811, T821 and T826) showed the most distinctive transcriptional signatures (Fig. 5A). Within the RB loss signature, the major differences between mP-RBs was evident at targets that are not typical E2F targets (Fig. 5B, upper part of the heatmap) and the most diverse transcriptional outputs were generated by mP-RBs containing phosphorylation sites at S811 or T821 (Fig. 5B). The clusters of RB-regulated genes that were specifically regulated by a single mP-RB were prominent in cells expressing RB mono-phosphorylated at S811. Strikingly, these groups of genes were transcribed at uniform levels in cells expressing any other RB phosphorylation isoform, including wildtype and RBΔcdk. Here, we focus on the isoform-specific programs controlled by S811.
RB mono-phosphorylation at S811 causes NuRD–dependent transcriptional regulation.
NuRD components were the most abundant chromatin regulators in the RB complexes. Since NuRD showed the strongest association with mP-RB S811 (Fig. 4C) it seemed likely to be involved in mP-RB S811–mediated repression. To identify targets of the RB/NuRD interaction we mined the proteome and transcription data for transcripts whose levels showed the tightest correlation to the abundance of NuRD components in the 15 different versions of repressive RB (RBΔcdk and 14 mP-RBs). Genes showing a significant Spearman correlation with the association of at least three components of the NuRD complex with RB were analyzed by Ingenuity Pathway Analysis (IPA) software (Table S5A). This highlighted genes linked to the role of BRCA1 in DNA damage response (Fig. 5C). A Pearson correlation analysis gave similar results (Table S5B and Fig. S5A). Indeed, 16 of the 78 genes in this pathway had expression profiles that significantly correlated with the association of RB with NuRD. Importantly, while all 14 mP-RB isoforms inhibited the expression of these genes relative to wild-type RB, mP-RB S811 showed the strongest degree of suppression (Fig. 5D).
The genes involved in the role of BRCA1 in DNA damage are also involved in homologous recombination (HR) DNA repair, a mechanism that is strongly suppressed in G1-cells (Orthwein et al., 2015). We noted that, in addition to cells expressing mP-RB S811, these genes were also repressed in cells expressing mP-RBs T356, S612 or S788 (Fig. 5D). These mP-RB isoforms caused the strongest G1arrest (Fig. S2C), suggesting that G1-arrest may also contribute to gene regulation. The promoters of many of these genes (BRCA1, BRCA2, BARD1, CHEK1, MSH2 and RFC3) contain E2F-response elements that are anticipated to be regulated by all forms of active RB. We hypothesized therefore that RB’s interaction with NuRD is unlikely to be the sole mechanism of regulation at these targets, but that interaction between RB and NuRD may provide an additional suppressor mechanism that effectively silences the expression of these genes in G1. To test this, we depleted MTA1 and MTA2, scaffold proteins for the assembly of NuRD complexes, in cells expressing wild-type or active RB mutants (Fig. S5B). We examined the expression of TOPBP1, MRE11, BRCA1, BRCA2 and MSH2 because the promoters of these genes are known to be bound by RB in G1-arrested cells (Chicas et al., 2010). Strikingly, the depletion of NuRD complex activity increased the expression of these genes in cells expressing mP-RB S811 (Fig. 5E), but not in cells expressing other forms of RB (Fig. S5C). Importantly, the depletion of MTA1 and MTA2 did not alter the repression of other E2F target-genes in cells expressing mP-RB S811 (RBL1 and CDKN2C; Fig. S5D). These results suggest that NuRD is important for transcriptional regulation by mP-RB S811, and that it enhances repression by this RB isoform at a specific subset of E2F-regulated genes.
RB phosphorylation at S807/S811 is preserved, or stimulated, in RPE1 and T47D cells by gamma-irradiation induced DNA damage (Fig. 1B). To examine the significance of RB phosphorylation at S811 in transcriptional regulation in this context, we generated RPE1 and T47D cell lines that, when treated with DOX, replace endogenous RB with exogenous single alanine mutant RB in position 811 (RB S811A). In both cell lines, gamma-irradiation increased the expression of BRCA1 and BRCA2, but only in cells expressing RB that cannot be phosphorylated at position 811 (Fig. 5F). Interestingly, substitution of serine 811 by the phospho-mimicking aspartate (RB S811D) had the exact opposite effect and generated RB with increased repressor activity (Fig. 5F, right upper panel). To test whether the suppressor activity of the phosphorylated RB at S811 after irradiation is NuRD-dependent, we monitored the expression of TOPBP1, MRE11, BRCA1, BRCA2 and MSH2 genes in RPE1 cells expressing RB wild-type, RB S811A or S811D mutants, before and after knock down of MTA1 and MTA2. As expected, NuRD complexes did not regulate the expression of these genes in cells expressing RB S811A (Fig. S5E, right upper panel). However, when cells were irradiated, depletion of MTA1 and MTA2 increased the expression of TOPBP1, BRCA2 and MSH2 in cells expressing wild-type RB and increased the expression of BRCA2 and MSH2 in cells expressing the strong repressor RB S811D (Fig. S5E). Collectively, these data support the idea that under specific stress conditions, the mono-phosphorylation of RB at S811 enhances the repression of specific targets in a NuRD-dependent manner (Fig. 5G).
Mono-phosphorylation directs RB to the control of specific cellular functions.
To understand the types of genes controlled by each mP-RB we performed Gene Set Enrichment Analysis (GSEA), comparing the gene expression profiles of cells expressing individual isoforms to either wild-type RB (Fig. 6A) or un-phosphorylated RBΔcdk (Fig. 6B). For an initial analysis we used the hallmark gene set collection that represents well-defined biological states or processes (Liberzon et al., 2015).
Figure 6: The transcriptional signatures of the 14 mP-RB isoforms disclose functional specificity.
(A) GSEA analysis of the transcriptome of the cells expressing mutant RB alleles relative to RB wildtype or (B) RBΔcdk for the hallmark gene-set collection. Heatmaps presenting the Normalized Enrichment Score for gene sets with false discovery rate FDR<15%. Red, blue and grey colors indicate up-, down- or non-significant regulation respectively.
Like RBΔcdk, all 14 mP-RB alleles repressed the expression of E2F targets and genes involved in G2/M checkpoint relative to wild-type RB (Fig. 6A). However, when compared with RBΔcdk (Fig. 6B), 10 of the 14 mP-RBs showed an impaired repression of E2F targets. This may explain their reduced ability to promote G1 (Fig. S2C). Significantly, the most extensive differences between mP-RBs were seen outside the traditional paradigm of RB as a repressor of E2F-targets. For example, genes encoding proteins that are involved in purine and pyrimidine metabolism and nucleotide excision during DNA repair were upregulated by mono-phosphorylation at S811 while they are inhibited by other mP-RB isoforms (Fig. 6A). In addition, inhibition of WNT signaling correlates with specific RB mono-phosphorylation at T356, T373, S788 and T821 (Fig. 6A). Remarkably, mP-RB T356 showed enhanced repressor activity relative to RBΔcdk that was evident on many gene-sets in addition to classic E2F targets. Genes repressed by mP-RB T356 are involved in several cellular functions, including cell proliferation, differentiation, and proinflammatory responses (Fig. 6B). mP-RBs containing phosphorylation sites in RBC activated the expression of a broad set of genes regulating metabolic pathways, adipocyte and myocyte differentiation and immune response (Fig. 6A). Of these, genes encoding proteins involved in oxidative phosphorylation (OXPHOS) show the most robust activation. RB mono-phosphorylation at S811 is particularly important for this transcriptional program (Fig. S6A). Importantly, the increased expression of the OXPHOS genes was evident when mP-RB S811 and mP-RB T826 were compared to either wild-type RB or RBΔcdk (Fig. S6B), suggesting that this transcriptional switch is a gain of function mediated via RB mono-phosphorylation.
RB mono-phosphorylation at S811 and T826 stimulates mitochondrial oxidative phosphorylation.
Decreased mitochondrial activity and defective oxidative phosphorylation are known consequences of RB loss in mouse tissues and human cell lines (Nicolay et al., 2015; Varaljai et al., 2015) but the idea that specific forms of RB might control mitochondrial function has not been examined. When we compared the promoters of the genes in the GSEA OXPHOS gene-set with RB ChIP-seq data (Chicas et al., 2010) we found that almost 60% of these promoters are bound by RB and likely to be direct targets (Fig. S7A). Strikingly, 83% of the OXPHOS genes bound by RB were upregulated in cells expressing mP-RB S811 and 58% were upregulated in cells expressing mP-RB T826 (Fig. S7A). The coordinated induction of these targets suggested that specific phosphorylation sites likely control this aspect of RB function.
To assess the functional significance of RB phosphorylation at S811 or T826 on mitochondrial activity we monitored the oxygen consumption rate (OCR) in cells expressing wild-type or mutant RB alleles. Strikingly, cells expressing mP-RB S811 or mP-RB T826 had significantly increased basal OCR and protonophore-induced OCR (maximal respiration capacity) relative to both wild-type RB or RBΔcdk (Fig. 7A, 7B and S7B). Additionally, OCR measurements in cells expressing RB with a single alanine mutation at position 811 showed that this site is needed to maintain the maximal respiration capacity (Fig. S7D). We note that the effects of S811 on OCR were NuRD-independent (Fig. S7E). Although RB is important to maintain the basal OCR in RPE1 cells (Nicolay et al., 2015), cells expressing wild-type RB or the constitutive active RBΔcdk had similar rates of cellular respiration (Fig. 7A and S7B). RBΔcdk represses E2F and arrests cells in G1 but wild-type RB does not, yet neither of these RB proteins gave the increased OCR seen with mP-RB S811 and mP-RB T826 suggesting that RB control of mitochondrial activity is independent of E2F regulation or cell cycle arrest.
Figure 7: RB mono-phosphorylation at S811 and T826 leads to increased mitochondria number and cell respiration.
(A) OCR measurements in RPE1 cells expressing RB wild-type, RBΔcdk and mP-RB S811 or (B) mP-RB T826. After obtaining the basal respiration levels, oligomycin, FCCP and Antimycin A were added to calculate the ATP-linked respiration, the maximal respiration capacity and the nonmitochondrial respiration respectively. (A, B; Mean ± SD, n=5 replicates, repeated twice; two-way ANOVA comparisons between mP-RB and RB wild-type (blue) or RBΔcdk (orange) expressing cells). (C) Ratio of mitochondrial DNA (mtDNA) to nuclear DNA (NucDNA) in RPE1 cells expressing RB wild-type, RBΔcdk or the 14 mP-RB isoforms (n=3 per cell line, repeated twice) (D) Integrated density per cell or (E) ratio of integrated density to cell surface of the MitoTracker-DeepRedFM in RPE1 cells expressing RB wild-type, RBΔcdk, mP-RB S811 or mP-RB T826. (C, D, E; Mean ± SD, n=50 per cell line; one-way ANOVA relative to RBΔcdk expressing cells). (****p<0.0001; ***p<0.001; **p<0.01; *p<0.05)
To ask whether the differences in OCR reflect changes in mitochondrial functionality, we calculated the respiratory control ratio (RCR) in cells expressing different RB isoforms. RCR, the ratio of the maximal respiration to oligomycin-insensitive respiration, reflects the coupling of oxygen consumption with ATP synthesis and shows the functional integrity of mitochondria independent of mitochondrial mass (Brand and Nicholls, 2011). Interestingly, all cell lines tested had similar RCR values suggesting that the differences in OCR were likely due to changes in mitochondria number (Fig. S7C). When we calculated the ratio of the mitochondrial DNA (MtDNA) to nuclear DNA (NucDNA) to obtain a relative index of mitochondria number (Rooney et al., 2015) we found that cells expressing mP-RB S811 or mP-RB T826 contained significantly more mitochondria than cells expressing any other RB mutant allele (Fig. 7C). Cells expressing wild-type RB contained fewer mitochondria than cells expressing RBΔcdk and this likely explains the slight difference in the maximal respiratory capacity seen between these two conditions (Fig. 7A). MitoTracker-DeepRedFM staining also showed a significant increase in mitochondrial staining in cells expressing mP-RB S811 or mP-RB T826 (Fig. 7D). To ensure that these differences were not an indirect consequence of changes in cell size, we calculated the ratio of the integrated MitoTracker density to cell surface. Indeed, cells expressing mP-RB S811 or mP-RB T826 had increased mitochondrial staining relative to cells expressing wild-type RB or RBΔcdk, independent of cell size (Fig. 7E). Collectively these results show that the impact of RB on oxidative phosphorylation is not a generic property of all forms of RB but is a property of specific mP-RBs.
Discussion
The results described here show that there are many different forms of active RB. Different RB isoforms interact with different sets of proteins, they mediate different patterns of transcriptional regulation, and they have distinct functional properties. Our findings provide strong support the idea that RB is controlled, at least in part, by a code of mono-phosphorylation, and these results provide a framework for thinking about the properties of the various mP-RBs. It is now clear that, when interpreting studies of RB function, it will be necessary to know which specific isoforms of RB are expressed, which types of proteins are engaged, and what types of functional output are being regulated. New insights inevitably raise new questions and four key issues are discussed below.
First, how many functionally different forms of RB are there? Because of the knowledge that RB is regulated by CDKs and the evidence that cells with active RB can contain exclusively monophosphorylated RB, we focused first on the properties of RB isoforms with single sites of CDK phosphorylation. However, we appreciate that RB is also controlled by additional post-translational modifications and these likely tailor RB in different ways. The p300/CPB transcriptional co-activator complex and its associated factor P/CAF acetylate RB at K873/K874, a modification that activates RB during cell differentiation and DNA-damage response (Chan et al., 2001; Nguyen et al., 2004; Markham et al., 2006; Pickard et al., 2010), whereas Tip60-dependent acetylation triggers proteasomal degradation of RB (Leduc et al., 2006). Additionally, several methyl-transferases methylate RB at K810 (Carr et al., 2011; Cho et al., 2012), K873 (Munro et al., 2010) and K860 (Saddic et al., 2010) regulating interactions that control RB’s transcriptional activity and function in G1/S-checkpoint and DNA-damage response. Recently, arginine RB methylation at R775, R787 and R798 reported to reduce RB ability to retain E2F1 activity (Kim et al., 2015). Adding to this picture are potential roles for RB ubiquitination (Miwa et al., 2006) and SUMOylation (Ledl et al., 2005; Meng et al., 2016). Given this variety of post-translational modifications, we anticipate that the mechanism of RB action will ultimately prove to be far more complex than the “simple” analysis of the 14 different mP-RBs described here.
A second set of questions center on the puzzle of how the abundance of the different forms of RB is controlled. In other examples where proteins have a modification code that generates functional diversity, such as histones (Kouzarides, 2007) and p53 (Dai and Gu, 2010), specificity is provided by enzymes that make different modifications or that act on specific sites. At first glance RB regulation seems different because all of the well-studied sites of RB phosphorylation are modified by CDKs. We note that individual mP-RBs have different affinities for CyclinD1 and CDK6 (Fig. 4A) as well as protein phosphatase PP1 (Table S4). It is possible that CDKs provide more site-selective regulation of RB than is currently appreciated (Hattori et al., 2014) or that regulation occurs via selective dephosphorylation (Lentine et al., 2012). In addition to CDKs, other kinases have also been reported to contribute to differential RB phosphorylation. For example, cellular stress conditions promote RB phosphorylation by p38 stress-activated protein kinase, generating RB isoforms resistant to hyper-phosphorylation by CDKs (Gubern et al., 2016). Chk1/2 phosphorylates RB at S612 during DNA damage response (Inoue et al., 2007) while Aurora B phosphorylates RB at S780 to prevent post-mitotic endoreplication (Nair et al., 2009). Although some cellular conditions modulate levels of phosphorylation at all CDK sites on RB, these observations show that there is a variable mosaic of RB phosphorylation.
The complexity of the different forms of RB raises a third question: will it ever be possible to make a complete list of the proteins that associate with RB? Here we identified 438 proteins with a statistically significant enrichment in RB immunoprecipitates, however there are several reasons for thinking that these lists remain incomplete. We only examined a single cell type, yet RB has tissue-specific functions and it seems inevitable that cell type specific interactors must exist. RB is activated in response to several different stress signals and it is known that some of these conditions promote different types of interactions. Although our mass spectrometry identified many new interactors, we did not recover all of the proteins that have been previously linked to RB. In particular our RB complexes contained relatively few chromatin regulators. This likely reflects the difficulty of preserving weak interactions through the harsh conditions needed to remove RB complexes from chromatin, and we anticipate that the use of chemical cross-linkers and further refinement of extraction procedures will further expand the lists of RB-associated proteins.
The results described here raise a fourth and very important set of questions regarding the functional diversity of RB and our understanding of “active” RB protein. Some properties of RB are shared between isoforms. For example, RBΔcdk the all mP-RBs associate with E2F/DP proteins and repress E2F targets. Despite this, their repression properties are not identical: mP-RB T356 gave stronger repression than RBΔcdk and affected a more diverse set of targets, mP-RB S811 also has differential effects on RB/E2F targets and acts in a NuRD-dependent manner. Strikingly, 10 of the 14 mP-RBs gave less robust repression of E2F and proliferation genes than RBΔcdk. These differences may be significant in contexts that favor the accumulation of specific mP-RBs. We note that the reduced E2F-repression by the majority of mP-RBs may help explain why p16 mutant, cdk4 mutant or Cyclin D1 overexpressing cells have impaired cell cycle control. To some degree, these differences in E2F regulation might be described as different “shades” of activity, but the ability of the three C-terminal phosphorylation sites to regulate OXPHOS genes and to control mitochondrial activity more closely resemble molecular switches. Curiously mP-RB S811, mP-RB T821 and mP-RB T826 induce the expression of overlapping but distinct sets of mitochondrial genes, raising the possibility that these three mP-RBs may act co-operatively to promote mitochondrial activity. Hierarchical clustering identified several clusters of phosphorylation sites that interact with similar sets of proteins (Table S4), suggesting that groups of mP-RBs likely control RB activities that extend beyond E2F regulation; we speculate that specific phosphorylation sites may determine the “non-canonical” RB functions recently described (Ishak et al., 2016; Dick et al., 2018). The properties of mP-RB S811 show that a single phosphorylation site can increase the transcription of one group of targets, while repressing transcription of another group. NURD was needed for the specific effects of mP-RB S811 on HR genes, but not for the concurrent activation of OXPHOS genes. This suggests that the divergent effects are mediated by different interactions and illustrates the need for further studies of the mP-RBs.
The evidence that specific RB functions are mediated by particular mP-RBs, the fact that mP-RBs have more associated proteins than RBΔcdk, and the observation that each mP-RB associates with a different set of proteins, raise serious doubts about the long-held belief that unphosphorylated RB is the fully active form of the protein. Dowdy and co-workers arrived at a similar conclusion (Narasimha et al., 2014; Dowdy, 2018), albeit for different reasons. When assayed for effects of RB on mitochondrial function, mP-RB S811 and mP-RB T826 were far more active than RBΔcdk or wild-type RB. Whether RB isoforms should be described as “active” or “inactive” clearly depends on the phenotype being examined. Further work is needed to determine which proteins, from the large number that associated with wild-type RB but were not detected with RBΔcdk or the mP-RBs, preferentially associate with hyperphosphorylated RB. These may explain the increased stabilization of the RB protein in cellular thermal shift assay (CETSA) when cells bypass the G1/S checkpoint (Dai et al., 2018), a signal that reflects the modulations in protein interactions. Other studies have shown that phosphorylated RB has effects in the cytoplasm (Zhang et al., 2016) and it is possible that hyperphosphorylated RB is not truly inactive, as traditionally described, but that it simply associates with groups of proteins that are different from RBΔcdk or the mP-RBs.
The biological role of RB has been inferred from experiments that examined cells that had lost RB, or express inactive RB. These types of experiments cannot explain how RB performs different functions or how the different roles of RB are co-ordinated. For this, it is necessary to study cells that contain active RB. The experimental system described here, allowing the transient replacement of endogenous RB with mutant isoforms, reveals that the mechanism of RB action is more complex than previously imagined. The fact that there are so many functionally distinct forms of RB shows that RB’s mechanism of action will be difficult to dissect by simply overexpressing an RB cDNA or by knocking it out. Further studies are clearly needed to fully understand the “readers” and “writers” of this mono-phosphorylation code.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Nicholas J. Dyson (dyson@helix.harvard.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell culture:
The telomerase-expressing non-transformed human retina epithelial cells RPE1 were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 5% Fetal Bovine Serum (FBS) and antibiotics (100 units/ml Penicillin and 100 μg/ml Streptomycin; P/S). The estrogen receptor positive (ER+) human breast cancer cell lines CAMA1 and MDA-MB-361 were cultured in DMEM/Nutricient mixture F12 medium supplemented with 10% FBS and antibiotics (P/S). The ER+ human breast cancer cell line T47D were cultured in RPMI 1640 medium supplemented with 10% FBS and antibiotics (P/S). The human embryonic kidney 293T cells were cultured in DMEM supplemented with 10% FBS and antibiotics (P/S). RB1 knock down or replacement of the endogenous by exogenous RB protein in RPE1 and T47D cells transduced with pINDUCER11 and/or pINDUCER20 constructs were induced by addition of 0.5 μg/ml DOX (Sigma, Cat. No. D9891) for the indicating time. Cellular stress was induced in RPE1, CAMA1, MDA-MB-361 and T47D cells by culturing cells in media without FBS for 24h, maintaining cells in contact inhibition for 5 days, or treatment by DNA damaging conditions; 10 Gy gamma-irradiation, treatment with 5mM Hydroxyurea (Sigma, Cat. No. H8627) or 1 μM Camptothecin (Sigma, Cat. No. C9911) for 6h. To inhibit CDK4/6 kinase activity, cells were treated with 1 μM palbociclb (ChemieTek; Cat.No. CT-PD2991) for the indicating time. The concentration of palbociclib that is used is at least 800 folds greater than the IC50 of this drug for the inhibition of T47D cells proliferation (T47D palbociclib IC50 = 1.21 nM, https://www.cancerrxgene.org) and at least 15 folds greater than the IC50 of palbociclib for the inhibition of RB phosphorylation at S780 and S795 in MDA-MB-435 cells (Pfizer’s brochure).
METHODS DETAILS
Cloning and site-directed mutagenesis:
FLAG-tagged or untagged RB wild-type, RBΔcdk and the 14 FLAG-tagged mP-RB mutant alleles were amplified by PCR from the corresponding pcDNA3 constructs (Narasimha et al., 2014) that were kindly provided by Dr. Steve Dowdy, using the oligos described in Table S6, and were transferred to the pENTR/D-TOPO cloning vector, in accordance with manufacturer’s instructions (Invitrogen, Cat. No. 45–0218). The pcDNA3-HA-RBΔcdk+ S780 construct that we received from Dr. Dowdy had a missense mutation Lys524Gln. To recover the mP-RB S780 mutant allele, we replaced the Alanine 780 with Serine by site-directed mutagenesis in the pENTR-FLAG-RBΔcdk plasmid. pENTR-FLAG-RBΔcdk-15A mutant allele, in which 15 CDK sites are mutated to Alanine, including Threonine 5, was generated by replacing Threonine 5 with Alanine by site-directed mutagenesis in the pENTR-FLAG-RBΔcdk plasmid. RB1 single alanine mutation at position 811 and RB1 phospho-mimicking mutant alleles were generated by site-directed mutagenesis in pENTR-FLAG-RB wild type plasmid. Site-directed mutagenesis performed using the QuickChange II XL Site-Directed Mutagenesis Kit (Agilent, Cat. No. 200521) and the mutagenesis primers described in Table S6.RB wild-type and mutants cDNA were subsequently transferred into pINDUCER20 (Addgene; 44012) with LR clonase reaction (Invitrogen, cat. No. 11791–020). shRNA that targets the 3’-UTR of RB1 mRNA at the sequence 5’- CAGAGATCGTGTATTGAGATT-3’ was subcloned in XhoI – MluI sites in pINDUCER11 (Addgene; 44363), as it was described before (Meerbrey et al., 2011).
Transfections and infections:
pINDUCER11 and pINDUCER20 lentiviral constructs were packaged in 293T cells by transient transfection, in combination with the envelope plasmid pCMV-VSV-G (Addgene; 8454) and the packaging plasmid pCMV-dR8.2 dvpr (Addgene; 8455). Transfections were carried out using X-tremeGENE™ 9 DNA Transfection Reagent (Sigama, Cat. No. 6365787001). RPE1 and T47D cells were infected with the lentiviral particles in the presence of 5 μg/ml polybrene (Sigma, Cat. No. 107689). Depending on the selection marker, stable RPE1 and T47D cell lines were selected for expression of the green fluorescence protein by fluorescence-activated cell sorting or resistance to 400μg/ml G-418 (Gibco, Cat. No. 10131035). siRNAs (20 nM final concentration) were transfected into RPE1 cells by using the Lipofectamine RNAiMAX Transfection Reagent (Invitrogen, Cat. No. 13778100).
Western Blot analysis:
Cells were washed with phosphate-buffered saline (PBS) and total cell lysates were collected in lysis buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 1mM PMSF) supplement with protease (Roche, Cat. No. 04693159001) and phosphatase (Roche, Cat. No. 04906837001) inhibitor-cocktail. Lysates were passed 10 times through a 27-gauge needle, clarified by centrifugation at 16,000 × g for 10 min at 4°C, analyzed on Criterion TGX gels (BioRad, Cat. No. 5671084) and transferred on PVDF membranes (BioRad, Cat. No. 1704273). Primary antibodies were used in 5% bovine serum albumin (BSA; Boston BioProducts, Cat. No. P-753) in Tris-buffered saline (TBS) containing 0.1% Tween 20 (Santa Cruz, Cat. No. sc-362311). Secondary antibodies were obtained from GE and were used at 1:4,000 dilutions. The relative intensity of the protein bands was quantified using the Fiji software.
Cell fractionation:
Approximately 5×106 RPE1 cells were trypsinized, washed with PBS and resuspended in 200 μl of ice-cold Buffer A (hypotonic buffer; 10 mM Hepes-KOH pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 0.5 mM DTT, 1 mM PMSF, Roche protease inhibitors). Cells were allowed to swell on ice for 10 min. Then, 200 μl of ice-cold Buffer A + 0.4% NP-40 was added and cells were incubated on ice for additional 10 min. 300 × g centrifugation for 5 min at 4°C separated crude nuclear pellet from the cytoplasmic supernatant fraction. Pellet was resuspended in 100 μl buffer A, laid onto a 1.2 ml cushion of 0.8 M sucrose in buffer A and centrifuged at 2,800 × g for 10 min at 4°C. The nuclear pellet was washed with 1 ml PBS and resuspended in 100 μl EB buffer (10 mM PIPES pH6.8, 250 mM ammonium sulphate, 300 mM sucrose, 1 mM EGTA, 1 mM PMSF), for 5 min on ice. Supernatant nucleoplasmic fraction was collected by 2,800 × g centrifugation for 5 min at 4°C. Pelet was digested with DNAse I (5 units/100 μl) (NEB, Cat. No. M0303) in 100 μl digestion buffer (10 mM PIPES pH6.8, 300 mM sucrose, 50 mM NaCl, 2.5 mM MgCl2, 0.5 mM CaCl2, 0.5% Triton X-100, 1 mM PMSF), at 37°C for 30 min. Supernatant DNAse fraction and pellet nuclear matrix were collected by 5,000 × g centrifugation for 5 min at 4°C. Different cell fractions were analyzed by western blot. Gel loading was adjusted to give equivalent cell numbers in each lane.
Immunoprecipitation of RB complexes:
RB complex extraction and immunoprecipitation (IP) was modified from previous protocol (Dick et al., 2000) that was optimized to maximize the extraction of active RB, without losing RB’s association with known interacting proteins. The optimization process involved protein extraction in high salt concentration (250 mM NaCl), sonication that induces chromatin fractionation and collection of cell lysates in 10% glycerol to prevent protein denaturation. RPE1 cells transduced with pINDUCER11-shRB1 and pINDUCER20- FLAG-tagged or untagged RB wild-type, RBΔcdk or the 14 FLAG-tagged mP-RB mutant alleles were treated with 0.5 μg/ml DOX for 48h to induce the replacement of the endogenous by exogenous RB protein. Threonine 5 was not included in this analysis because it was reported not to be phosphorylated in vivo (Narasimha et al., 2014) and it is not evolutionary conserved below primates. In addition, we found that the mutation of Threonine 5 to Alanine in RBΔcdk, in effect introducing 15 alanine mutations into the wild-type RB sequences (RBΔcdk-15), generates a mutant protein that is unable to arrest RPE1 cells in G1-phase (Fig. S2D). Approximately 2×107 cells per culture were washed twice with ice-cold PBS and cell lysates were collected in 1 ml E1Agl250 buffer (50 mM Hepes-KOH pH 7.4, 250 mM NaCl, 0.1% NP-40, 10% glycerol, 1 mM PMSF) supplement with Roche protease and phosphatase inhibitors. Lysates were incubated on ice for 10 min and then were sonicated using the Fisher Scientific Sonic Dismembrator 550 with 2 × 15 sec pulses (45 sec interval) and amplitude 50%. Lysates were centrifuged at 16,000 × g for 10 min at 4°C and supernatants were incubated with anti-FLAG M2 magnetic beads under rotation for 2h at 4°C (40 μl of magnetic beads per IP). Beads were washed three times with 1 ml E1Agl150 buffer (50 mM Hepes-KOH pH 7.4, 150 mM NaCl, 0.1% NP-40, 10% glycerol, 1 mM PMSF) and immunoprecipitates were eluted twice with 100 μl elution buffer (100 μg/ml FLAG peptide; Sigma, Cat. No. F3290, in TBS; 10 mM Tris-HCl pH 7.5, 150 mM NaCl). Elutions were combined and analyzed by western blot or quantitative proteomics analysis. For western blot analysis, 5% of input cell lysate had been kept before IP. Anti-FLAG immunoprecipitates from cells expressing DOX-induced un-tagged wild-type RB or RBΔcdk were used as negative controls. The group of proteins that show statistically significant enrichment in the untagged-control pull-downs (Fig. 3A and 3B) are strongly enriched for proteins that were reported to associate non-specifically with anti-FLAG M2 magnetic beads (Mellacheruvu et al., 2013).
Cell Cycle analysis:
Before or 48h after DOX-induction RPE1 cells were incubated with 20 μM 5-ethynyl-2’-deoxyuridine (EdU; Life technologies, Cat. No. A10044) for 2h, fixed with 3.7% formaldehyde in PBS for 15 min, blocked with 3% BSA in PBS for 1 min, permeabilized with 0.5% Triton X-100 in PBS for 30 min and stained with ClickIT reaction (100mM Tris-HCl pH 7.5, 3mM CuSO4, 50 mM Ascorbic Acid, 2.5 μM Alexa Fluor-647 azide; Life Technologies, Cat. No. A10277) for 30 minutes and 3μM 4’,6-Diamidino-2-Phenylindole Dihydrochloride (DAPI; Life Technologies, Cat. No. D1306) in staining buffer (100mM Tris-HCl pH 7.5, 150mM NaCl, 1mM CaCl2, 0.5mM MgCl2, 0.1% NP-40), for 15 minutes. FACS analysis was performed with LSR II flow cytometer (BD Bioscences).
Real-time RT-PCR:
Total cell RNA was extracted using the Direct-zol RNA MiniPrep Plus kit (Zymo Research, Cat. No. R2073). cDNA was synthesized from 1 μg total RNA, using oligo-dT priming and the TaqMan Reverse Transcription kit (Applied biosystems, Cat. No. N8080234). Relative expression of transcripts was quantified by real-time PCR, using the FastStart Universal SYBR Green Master mix (Roche, Cat. No. 4913914001) and the LightCycler 480 System (Roche). mRNA levels were normalized to ACTB and GAPDH. Primer sets are listed on the Table S6.
Cell respiration analysis:
Oxygen consumption rates (OCR) were measured with XF24–3 Extracellular Flux Analyzer (Seahorse Bioscience), using the Seahorse XF24 FluxPak (Agilent, Cat. No. 100850–001). 24h after DOX-induction or 48h after DOX-induction and 24h after siRNA transfection, RPE1 cells were seeded at 50×103 cells per well in XF24 24-well cell culture microplates in 200 μl DMEM supplemented with 5% FBS, antibiotics and 0.5 μg/ml DOX and were incubated at 37°C in 5% CO2 incubator. 24h later, medium was replaced with 1 ml of the assay medium DMEM without NaHCO3 pH7.4 (Sigma, Cat. No. D1152) supplemented with 5% FBS, antibiotics and 0.5 μg/ml DOX and plates were placed in the XF24 Extracellular Flux Analyzer for OCR measurements. Each measurement was performed over 4 min after 2 min mix and 2 min wait period. Basal OCR was collected 5 times. 3 measurements were collected after injection of the ATP synthase inhibitor oligomycin (Sigma, Cat. No. 75351) in 0.5 μM final concentration, 3 measurements were collected after injection of the proton translocator carbonyl cyanide4 (trifluoromethoxy) phenylhydrazone (FCCP; Sigma, Cat. No. C2920) in 10 μM final concentration and 3 measurements were collected after injection of the electron transport chain complex III inhibitor antimycin A (Sigma, Cat. No. A8674) in 2 μM final concentration.
Mitochondria DNA copy number:
48h after DOX-induction, DNA was isolated from RPE1 cells expressing RB wild-type, RBΔcdkor the 14 mP-RB mutant alleles. DNA extraction performed using the DNeasy Blood and Tissue kit (Qiegen, Cat. No. 69506). Quantitative-PCR was used to calculate the ratio of the mitochondria DNA to nuclear DNA, as it was described before (Rooney et al., 2015).
MitoTracker staining:
48h after DOX-induction, RPE1 cells expressing RB wild-type, RBΔcdk or mP-RB mutant alleles and growing on coverslips were stained with 200 nM MitoTracker-DeepRedFM (Invitrogen, Cat. No. M22426) for 30 min at 37°C in 5% CO2 incubator, in accordance with the manufacturer’s protocol. Mitochondria mass was determined by the MitoTracker fluorescence levels. Images were acquired in a 710 Zeiss confocal microscope and the integrated density and cell surface area measurements were done by Fiji software.
Ingenuity Pathway Analysis:
Correlation analysis between the abundance of NuRD complex components in the 15 different active RB complexes (RBΔcdk and the 14 mP-RB complexes) and the transcriptional profiles of cells expressing these RB mutant alleles was performed using R. Genes showing significant Spearman or Pearson correlation with the association of at least three of the NuRD complex components with RB were analyzed by Ingenuity Pathway Analysis (IPA) software (Ingenuity® Systems, http://www.ingenuity.com).
RNA sequencing Analysis:
Data can be found under GEO accession: GSE116346
RNA extraction, library construction and sequencing:
48h after DOX-induction, total RNA was extracted from RPE1 cells expressing RB wild-type, RBΔcdk or the 14 mP-RB mutant alleles, using the Direct-zol RNA MiniPrep Plus kit (Zymo Research, Cat. No. R2073). Multiplexed RNA sequencing libraries were prepared using the TruSeq library prep kit (Illumina, Cat. No. RS-122–2301/2) and paired-end 75bp reads were collected in a NextSeq 500 sequencing system (Illumina), using the NextSeq™ 500 High Output Kit (Illumina, Cat. No. FC-404–1002) across 4 sequencing lanes.
Read Alignment and Functional Analysis:
Paired-end read quality was assessed using the FASTQC tool (FastQC A Quality Control tool for High Throughput Sequence Data http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ by S. Andrews). Reads were trimmed to remove poor quality regions and adaptor sequences using the Trimmomatic v0.3 tool (default parameters) (Bolger et al., 2014). The remaining high-quality reads were aligned to the GRCh38 (v85) annotation of the human genome using TopHat (default parameters; v2.1.0) (Kim et al., 2013). Sample BAM files were merged across sequencing lanes using the MergeSAMFiles function from PICARD tools (v2.0.1; http://broadinstitute.github.io/picard). HTseq-count v0.6.1 (Anders et al., 2015) was used to calculate the raw read counts associated with each annotated feature (coding and non-coding transcripts). DESeq2 (Love et al., 2014) was used to normalize the counts across samples. Normalized read count fold change ratios were calculated between each of the 14 mono-phosphorylation-site samples and the two control samples (RB wild-type and RBΔcdk). Based on the fold change ratios a ranked list of genes was generated for each RB-mutant allele expressing sample. A pre-ranked geneset enrichment analysis (GSEA) (weighted scoring scheme, 1000 permutations) was performed on each ranked list using the Hallmark genesets collection from MSigDB (Liberzon et al., 2015). Enriched genesets were identified using an FDR q-value threshold of 0.25. Read alignment and GSEA analyses were run using R.
Quantitative proteomics:
FLAG immunoprecipitates were purified from RPE1 cells expressing FLAG-tagged RB wild-type, RBΔcdk or the 14 mP-RB mutant alleles, or from RPE1 cells expressing untagged RB wild-type or RBΔcdk (negative controls), after 48h DOX-induction. Three independent experiments were performed per isogenic cell line. Eluted proteins were reduced with DTT, alkylated with iodoacetamide, purified by MeOH/CHCl3 precipitation, and digested with LysC and trypsin essentially as described before (Lapek et al., 2017). Peptide concentration differences across all samples were determined using multiplexed quantitative proteomics applying tandem-mass-tag (TMT) technology. TMT10plex reagents were used to label the peptides and samples were pooled into groups of either nine (twelve sets) or ten (3 sets) samples. Two bridge samples from pooling all IPs into one sample were used to compare protein concentrations across different TMT sets (Lapek et al., 2017). The TMT sample sets were analyzed in duplicate on an Orbitrap Fusion and an Orbitrap Lumos mass spectrometer as described previously (Lapek et al., 2017) but omitting the off-line fractionation by basic pH reversed phase chromatography. Synchronous precursor selection (SPS) supported MS3 was used to obtain accurate TMT-based quantification. MS2 data were annotated using SEQUEST (Huttlin et al., 2010) searching data against the Uniprot database of human protein sequences. A smaller than 1 % false discovery filter for both peptide and protein assignments was applied through the target-decoy database search strategy (Elias and Gygi, 2007) and using linear discriminant analysis and posterior error histogram sorting (Huttlin et al., 2010). Peptides with unambiguous protein annotations were assigned to the protein with most matching peptides (Huttlin et al., 2010). For quantification, we extracted TMT reporter ion intensities as those of the most intense ions within a 0.03 Th window around the predicted MS3 reporter ion intensities. Only MS3 with an average signal-to-noise value of larger than 20 per reporter ion as well as with an isolation specificity (Ting et al., 2011) of larger than 0.75 were considered for quantification. TMT-intensities were first normalized for each protein based on the median average protein intensity calculated for all proteins. Then, a median of the normalized intensities was calculated from all protein intensities in each TMT channel and the protein intensities were normalized to the median value of these median intensities. Proteomics data can be found at https://massive.ucsd.edu/; accession number MSV000082562.
QUANTIFICATION AND STATISTICAL ANALYSIS
Hierarchical clustering and statistical analysis:
Hierarchical clustering analysis of the proteomic data was performed with Ward’s method using the JMP pro 12 software. Hierarchical clustering analysis of the transcriptomic data and the GSEA normalized enrichment scores were performed with Ward’s method using R. Statistical analysis was performed using the GraphPad Prism 6 software. Statistical comparison among groups was carried out with one-way ANOVA, Dunnett’s multiple comparisons test or two-way ANOVA, Sidak’s multiple comparisons test. The calculated statistical significance is indicated as ****p<0.0001; ***p<0.001; **p<0.01; *p<0.05.
Supplementary Material
Highlights:
Mono-phosphorylation controls RB’s association with other proteins.
Distinct mono-phosphorylated forms of RB have different transcriptional outputs.
RB mono-phosphorylation at S811 promotes NuRD-dependent transcriptional repression.
Mono-phosphorylation of RB’s C-terminal domain enhances expression of OXPHOS genes.
Acknowledgments:
We thank B.J. Drapkin and W.O. Miles for critical reading of the manuscript; L. Zou, D.V. Titov, M. Stanzione, B. Krishnan and A. Guarner for helpful discussions and technical help; S.F. Dowdy for sharing reagents and for helpful discussion. Seahorse experiments were carried out at V.K. Mootha laboratory. This work was supported by NIH grants CA236538 and GM117413 to N.J.D.
Footnotes
Declaration of Interests: The authors declare no competing interests.
DATA AND SOFTWARE AVAILABILITY
Proteomics data: https://massive.ucsd.edu/; accession number MSV000082562.
RNA sequencing data: GEO accession: GSE116346.
Mendeley Data: http://dx.doi.org/10.17632/4nhtvzz5rv.1.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Anders S, Pyl PT, and Huber W (2015). HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevolenskaya EV, Murray HL, Branton P, Young RA, and Kaelin WG Jr. (2005). Binding of pRB to the PHD protein RBP2 promotes cellular differentiation. Mol Cell 18, 623–635. [DOI] [PubMed] [Google Scholar]
- Binne UK, Classon MK, Dick FA, Wei W, Rape M, Kaelin WG Jr., Naar AM, and Dyson NJ (2007). Retinoblastoma protein and anaphase-promoting complex physically interact and functionally cooperate during cell-cycle exit. Nat Cell Biol 9, 225–232. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, and Usadel B (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, and Nicholls DG (2011). Assessing mitochondrial dysfunction in cells. Biochem J 435, 297312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JR, Deshong AJ, Pelton JG, and Rubin SM (2010). Phosphorylation-induced conformational changes in the retinoblastoma protein inhibit E2F transactivation domain binding. J Biol Chem 285, 16286–16293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JR, Hura GL, and Rubin SM (2012). Structures of inactive retinoblastoma protein reveal multiple mechanisms for cell cycle control. Genes Dev 26, 1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JR, Liban TJ, Restrepo T, Lee HW, and Rubin SM (2014). Multiple mechanisms for E2F binding inhibition by phosphorylation of the retinoblastoma protein C-terminal domain. J Mol Biol 426, 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo E, Quintero-Estades JA, Danielian PS, Nedelcu S, Berman SD, and Lees JA (2010). Rb regulates fate choice and lineage commitment in vivo. Nature 466, 1110–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr SM, Munro S, Kessler B, Oppermann U, and La Thangue NB (2011). Interplay between lysine methylation and Cdk phosphorylation in growth control by the retinoblastoma protein. EMBO J 30, 317327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan HM, Krstic-Demonacos M, Smith L, Demonacos C, and La Thangue NB (2001). Acetylation control of the retinoblastoma tumour-suppressor protein. Nat Cell Biol 3, 667–674. [DOI] [PubMed] [Google Scholar]
- Chicas A, Wang X, Zhang C, McCurrach M, Zhao Z, Mert O, Dickins RA, Narita M, Zhang M, and Lowe SW (2010). Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer Cell 17, 376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HS, Hayami S, Toyokawa G, Maejima K, Yamane Y, Suzuki T, Dohmae N, Kogure M, Kang D, Neal DE, et al. (2012). RB1 methylation by SMYD2 enhances cell cycle progression through an increase of RB1 phosphorylation. Neoplasia 14, 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R, Zoumpoulidou G, Luczynski MT, Rieger S, Moquet J, Spanswick VJ, Hartley JA, Rothkamm K, Huang PH, and Mittnacht S (2015). Direct involvement of retinoblastoma family proteins in DNA repair by non-homologous end-joining. Cell Rep 10, 2006–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, and Gu W (2010). p53 post-translational modification: deregulated in tumorigenesis. Trends Mol Med 16, 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Zhao T, Bisteau X, Sun W, Prabhu N, Lim YT, Sobota RM, Kaldis P, and Nordlund P (2018). Modulation of Protein-Interaction States through the Cell Cycle. Cell. [DOI] [PubMed] [Google Scholar]
- Dick FA, Goodrich DW, Sage J, and Dyson NJ (2018). Non-canonical functions of the RB protein in cancer. Nat Rev Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick FA, and Rubin SM (2013). Molecular mechanisms underlying RB protein function. Nat Rev Mol Cell Biol 14, 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick FA, Sailhamer E, and Dyson NJ (2000). Mutagenesis of the pRB pocket reveals that cell cycle arrest functions are separable from binding to viral oncoproteins. Mol Cell Biol 20, 3715–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdy SF (2018). Death of a Dogma: Cyclin D Activates Rb by Mono-phosphorylation In Cyclins D-type and Cancer Current Cancer Research, Hinds PW, and Brown NE, eds. (Springer, Cham; ), pp. 133–147. [Google Scholar]
- Dowdy SF, Hinds PW, Louie K, Reed SI, Arnold A, and Weinberg RA (1993). Physical interaction of the retinoblastoma protein with human D cyclins. Cell 73, 499–511. [DOI] [PubMed] [Google Scholar]
- Dyson NJ (2016). RB1: a prototype tumor suppressor and an enigma. Genes Dev 30, 1492–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias JE, and Gygi SP (2007). Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods 4, 207–214. [DOI] [PubMed] [Google Scholar]
- Ferecatu I, Le Floch N, Bergeaud M, Rodriguez-Enfedaque A, Rincheval V, Oliver L, Vallette FM, Mignotte B, and Vayssiere JL (2009). Evidence for a mitochondrial localization of the retinoblastoma protein. BMC Cell Biol 10, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubern A, Joaquin M, Marques M, Maseres P, Garcia-Garcia J, Amat R, Gonzalez-Nunez D, Oliva B, Real FX, de Nadal E, et al. (2016). The N-Terminal Phosphorylation of RB by p38 Bypasses Its Inactivation by CDKs and Prevents Proliferation in Cancer Cells. Mol Cell 64, 25–36. [DOI] [PubMed] [Google Scholar]
- Hattori T, Uchida C, Takahashi H, Yamamoto N, Naito M, and Taya Y (2014). Distinct and sitespecific phosphorylation of the retinoblastoma protein at serine 612 in differentiated cells. PLoS One 9, e86709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernando E, Nahle Z, Juan G, Diaz-Rodriguez E, Alaminos M, Hemann M, Michel L, Mittal V, Gerald W, Benezra R, et al. (2004). Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature 430, 797–802. [DOI] [PubMed] [Google Scholar]
- Hilgendorf KI, Leshchiner ES, Nedelcu S, Maynard MA, Calo E, Ianari A, Walensky LD, and Lees JA (2013). The retinoblastoma protein induces apoptosis directly at the mitochondria. Genes Dev 27, 1003–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds PW, Dowdy SF, Eaton EN, Arnold A, and Weinberg RA (1994). Function of a human cyclin gene as an oncogene. Proc Natl Acad Sci U S A 91, 709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds PW, Mittnacht S, Dulic V, Arnold A, Reed SI, and Weinberg RA (1992). Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell 70, 993–1006. [DOI] [PubMed] [Google Scholar]
- Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villen J, Haas W, Sowa ME, and Gygi SP (2010). A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianari A, Natale T, Calo E, Ferretti E, Alesse E, Screpanti I, Haigis K, Gulino A, and Lees JA (2009). Proapoptotic function of the retinoblastoma tumor suppressor protein. Cancer Cell 15, 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Kitagawa M, and Taya Y (2007). Phosphorylation of pRB at Ser612 by Chk1/2 leads to a complex between pRB and E2F-1 after DNA damage. EMBO J 26, 2083–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishak CA, Marshall AE, Passos DT, White CR, Kim SJ, Cecchini MJ, Ferwati S, MacDonald WA, Howlett CJ, Welch ID, et al. (2016). An RB-EZH2 Complex Mediates Silencing of Repetitive DNA Sequences. Mol Cell 64, 1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji P, Jiang H, Rekhtman K, Bloom J, Ichetovkin M, Pagano M, and Zhu L (2004). An Rb-Skp2-p27 pathway mediates acute cell cycle inhibition by Rb and is retained in a partial-penetrance Rb mutant. Mol Cell 16, 47–58. [DOI] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, and Salzberg SL (2013). TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14, R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Wang DH, Campbell M, Huerta SB, Shevchenko B, Izumiya C, and Izumiya Y (2015). PRMT4-mediated arginine methylation negatively regulates retinoblastoma tumor suppressor protein and promotes E2F-1 dissociation. Mol Cell Biol 35, 238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T (2007). Chromatin modifications and their function. Cell 128, 693–705. [DOI] [PubMed] [Google Scholar]
- Lapek JD Jr., Greninger P, Morris R, Amzallag A, Pruteanu-Malinici I, Benes CH, and Haas W (2017). Detection of dysregulated protein-association networks by high-throughput proteomics predicts cancer vulnerabilities. Nat Biotechnol 35, 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledl A, Schmidt D, and Muller S (2005). Viral oncoproteins E1A and E7 and cellular LxCxE proteins repress SUMO modification of the retinoblastoma tumor suppressor. Oncogene 24, 3810–3818. [DOI] [PubMed] [Google Scholar]
- Leduc C, Claverie P, Eymin B, Col E, Khochbin S, Brambilla E, and Gazzeri S (2006). p14ARF promotes RB accumulation through inhibition of its Tip60-dependent acetylation. Oncogene 25, 4147–4154. [DOI] [PubMed] [Google Scholar]
- Lees JA, Buchkovich KJ, Marshak DR, Anderson CW, and Harlow E (1991). The retinoblastoma protein is phosphorylated on multiple sites by human cdc2. EMBO J 10, 4279–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentine B, Antonucci L, Hunce R, Edwards J, Marallano V, and Krucher NA (2012). Dephosphorylation of threonine-821 of the retinoblastoma tumor suppressor protein (Rb) is required for apoptosis induced by UV and Cdk inhibition. Cell Cycle 11, 3324–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, and Tamayo P (2015). The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 1, 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longworth MS, and Dyson NJ (2010). pRb, a local chromatin organizer with global possibilities. Chromosoma 119, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey MP, Bergseid J, Bosco EE, Stengel K, Xu H, Mayhew CN, Schwemberger SJ, Braden WA, Jiang Y, Babcock GF, et al. (2007). Loss of the retinoblastoma tumor suppressor: differential action on transcriptional programs related to cell cycle control and immune function. Oncogene 26, 6307–6318. [DOI] [PubMed] [Google Scholar]
- Markham D, Munro S, Soloway J, O’Connor DP, and La Thangue NB (2006). DNA-damageresponsive acetylation of pRb regulates binding to E2F-1. EMBO Rep 7, 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerbrey KL, Hu G, Kessler JD, Roarty K, Li MZ, Fang JE, Herschkowitz JI, Burrows AE, Ciccia A, Sun T, et al. (2011). The pINDUCER lentiviral toolkit for inducible RNA interference in vitro and in vivo. Proc Natl Acad Sci U S A 108, 3665–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellacheruvu D, Wright Z, Couzens AL, Lambert JP, St-Denis NA, Li T, Miteva YV, Hauri S, Sardiu ME, Low TY, et al. (2013). The CRAPome: a contaminant repository for affinity purificationmass spectrometry data. Nat Methods 10, 730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Maldonado R, Paolinelli R, Galbiati L, Giadrossi S, and Giacca M (2010). Interaction of the retinoblastoma protein with Orc1 and its recruitment to human origins of DNA replication. PLoS One 5, e13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Qian J, Yue H, Li X, and Xue K (2016). SUMOylation of Rb enhances its binding with CDK2 and phosphorylation at early G1 phase. Cell Cycle 15, 1724–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa S, Uchida C, Kitagawa K, Hattori T, Oda T, Sugimura H, Yasuda H, Nakamura H, Chida K, and Kitagawa M (2006). Mdm2-mediated pRB downregulation is involved in carcinogenesis in a p53independent manner. Biochem Biophys Res Commun 340, 54–61. [DOI] [PubMed] [Google Scholar]
- Morris EJ, and Dyson NJ (2001). Retinoblastoma protein partners. Adv Cancer Res 82, 1–54. [DOI] [PubMed] [Google Scholar]
- Munro S, Khaire N, Inche A, Carr S, and La Thangue NB (2010). Lysine methylation regulates the pRb tumour suppressor protein. Oncogene 29, 2357–2367. [DOI] [PubMed] [Google Scholar]
- Nair JS, Ho AL, Tse AN, Coward J, Cheema H, Ambrosini G, Keen N, and Schwartz GK (2009). Aurora B kinase regulates the postmitotic endoreduplication checkpoint via phosphorylation of the retinoblastoma protein at serine 780. Mol Biol Cell 20, 2218–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimha AM, Kaulich M, Shapiro GS, Choi YJ, Sicinski P, and Dowdy SF (2014). Cyclin D activates the Rb tumor suppressor by mono-phosphorylation. Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DX, Baglia LA, Huang SM, Baker CM, and McCance DJ (2004). Acetylation regulates the differentiation-specific functions of the retinoblastoma protein. EMBO J 23, 1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolay BN, Danielian PS, Kottakis F, Lapek JD Jr., Sanidas I, Miles WO, Dehnad M, Tschop K, Gierut JJ, Manning AL, et al. (2015). Proteomic analysis of pRb loss highlights a signature of decreased mitochondrial oxidative phosphorylation. Genes Dev 29, 1875–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orthwein A, Noordermeer SM, Wilson MD, Landry S, Enchev RI, Sherker A, Munro M, Pinder J, Salsman J, Dellaire G, et al. (2015). A mechanism for the suppression of homologous recombination in G1 cells. Nature 528, 422–426. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pickard A, Wong PP, and McCance DJ (2010). Acetylation of Rb by PCAF is required for nuclear localization and keratinocyte differentiation. J Cell Sci 123, 3718–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney JP, Ryde IT, Sanders LH, Howlett EH, Colton MD, Germ KE, Mayer GD, Greenamyre JT, and Meyer JN (2015). PCR based determination of mitochondrial DNA copy number in multiple species. Methods Mol Biol 1241, 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin SM (2013). Deciphering the retinoblastoma protein phosphorylation code. Trends Biochem Sci 38, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin SM, Gall AL, Zheng N, and Pavletich NP (2005). Structure of the Rb C-terminal domain bound to E2F1-DP1: a mechanism for phosphorylation-induced E2F release. Cell 123, 1093–1106. [DOI] [PubMed] [Google Scholar]
- Saddic LA, West LE, Aslanian A, Yates JR 3rd, Rubin SM, Gozani O, and Sage J (2010). Methylation of the retinoblastoma tumor suppressor by SMYD2. J Biol Chem 285, 37733–37740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage J, Miller AL, Perez-Mancera PA, Wysocki JM, and Jacks T (2003). Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature 424, 223–228. [DOI] [PubMed] [Google Scholar]
- Sherr CJ (1996). Cancer cell cycles. Science 274, 1672–1677. [DOI] [PubMed] [Google Scholar]
- Siddiqui H, Fox SR, Gunawardena RW, and Knudsen ES (2007). Loss of RB compromises specific heterochromatin modifications and modulates HP1alpha dynamics. J Cell Physiol 211, 131–137. [DOI] [PubMed] [Google Scholar]
- Sterner JM, Dew-Knight S, Musahl C, Kornbluth S, and Horowitz JM (1998). Negative regulation of DNA replication by the retinoblastoma protein is mediated by its association with MCM7. Mol Cell Biol 18, 2748–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting L, Rad R, Gygi SP, and Haas W (2011). MS3 eliminates ratio distortion in isobaric multiplexed quantitative proteomics. Nat Methods 8, 937–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varaljai R, Islam AB, Beshiri ML, Rehman J, Lopez-Bigas N, and Benevolenskaya EV (2015). Increased mitochondrial function downstream from KDM5A histone demethylase rescues differentiation in pRB-deficient cells. Genes Dev 29, 1817–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez-Cruz R, Manickavinayaham S, Biswas AK, Clary RW, Premkumar T, Cole F, and Johnson DG (2016). RB localizes to DNA double-strand breaks and promotes DNA end resection and homologous recombination through the recruitment of BRG1. Genes Dev 30, 2500–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xu K, Liu P, Geng Y, Wang B, Gan W, Guo J, Wu F, Chin YR, Berrios C, et al. (2016). Inhibition of Rb Phosphorylation Leads to mTORC2-Mediated Activation of Akt. Mol Cell 62, 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.