Abstract
Background
physical activity in older age has been associated with better cognitive function, but the role of earlier life physical activity is less well understood.
Objective
determine associations between physical activity throughout the lifespan and cognitive function in older age.
Design
cross-sectional study.
Setting
the Rancho Bernardo Study of Healthy Aging in southern California.
Subjects
A total of 1,826 community-dwelling men and women (60–99 years) who attended a research visit in 1988–92.
Methods
participants underwent cognitive testing at older age, and reported physical activity as a teenager, at age 30 years, 50 years and currently. For each time-point, participants were classified as regularly active (3+ times/week) or inactive.
Results
regular physical activity was associated with better cognitive function, with physical activity at older ages showing the strongest associations. Physical activity in older age was associated with better global cognitive function, executive function and episodic memory, regardless of intensity. Intense physical activity in teenage years was associated with better late-life global cognitive function in women. Teenage physical activity interacted with older age physical activity on executive function; those active at both periods performed better than those active at only one period. Similar patterns of associations were observed after excluding individuals with poor health.
Conclusions
regular physical activity in older age, regardless of intensity, is associated with better cognitive function. Physical activity in teenage years may enhance cognitive reserve to protect against age-related decline in executive function. Further research is needed to assess the effect of physical activity across the lifespan on healthy brain ageing.
Keywords: executive function, cognitive ageing, cognitive function, cognitive reserve, physical activity, older people
Key points
This study evaluated the association of PA throughout the lifespan on later-life cognitive function.
Regular PA in older age, regardless of intensity, was most strongly associated with better cognitive function and reduced odds of cognitive impairment.
Individuals physically active as a teenager and in older age showed highest level of executive function.
Associations between PA and cognitive function were similar for men and women.
Introduction
With the ageing of the population, identifying approaches to preserve cognitive function is of critical importance to maintain quality of life and independence in later years. Regular physical activity (PA) in older age has been associated with slower cognitive decline and lower rates of dementia [1, 2]. However, the role of PA earlier in life on later cognitive function remains unclear. If PA elicits enduring brain changes that confer resilience against cognitive ageing, early-life PA may provide additional protection against age-related cognitive decline.
Studies of older adults have shown associations of PA with better cognitive performance [3–7]. However, few examined associations of early-life PA with cognitive ageing, or directly compared PA at different points across the lifespan with older age cognitive function. PA in young adulthood has been associated with faster processing speed in later life, but this effect was limited to men and not observed for global cognitive function [8]. In contrast, women who were active at several ages throughout life had lower rates of cognitive impairment in older age, and this association was strongest for teenage PA [9]. One study reported associations of lifelong PA with cognitive performance in older women, but did not compare effects of PA at different ages [10]. Others found that lifelong [11] or early life [12] PA and cardiorespiratory fitness [13] were associated with better executive function, memory and processing speed in midlife, but did not report late-life cognitive function. Further research is needed to clarify the relative influence of PA at various time-points throughout life on cognitive ageing in men and women, to identify which cognitive domains are most responsive to PA, and to determine if effects are modified by activity intensity.
We examined associations of PA at different points throughout the lifespan with performance on multiple cognitive tests in a well-characterized cohort of community-dwelling older men and women. We hypothesized that PA in older age would be associated with better cognitive performance, and that PA in youth would show an additional positive association.
Methods
Participants
Participants were members of the Rancho Bernardo Study (RBS) of Healthy Ageing, a longitudinal study of community-dwelling older adults in southern California established in 1972–74.
Eligible study participants included 2,030 individuals who completed cognitive testing at the initial cognitive assessment (1988–92). After excluding 167 participants under age 60 because of our focus on late-life cognitive function, and 37 with incomplete PA information, the final sample included 1,826 participants (Appendix 1).
Study procedures were approved by the University of California, San Diego Human Research Protections Program Board and participants provided informed written consent prior to participation.
Cognitive assessment
Cognitive function was assessed with four standard tests administered by a trained examiner. Tests included the Mini-Mental State Exam (MMSE) [14], a measure of global cognitive function; the Trails Making Test, Part B (Trails B) of the Halstead–Reitan Battery [15], a test of psychomotor tracking and executive function; the category fluency (animal naming) test [16], a test of semantic memory and executive function; and the Buschke-Fuld Selective Reminding test [17], a measure of verbal episodic memory (total recall analyzed in this study).
Health and lifestyle assessment
Health and lifestyle information was collected using standardized self-administered questionnaires. At the 1988–92 assessment, participants were asked retrospectively how many times per week they engaged in light, moderate and strenuous exercise, as a teenager, at age 30, at age 50, and currently (henceforth referred to as older age). Individuals were classified as active if they engaged in any level of PA three or more times per week. At each time-point the Godin Leisure-Time Exercise score [18], a composite of the frequency of light, moderate and strenuous exercise, was computed to assess PA intensity.
Participants reported how many alcoholic beverages they consumed during an average week, and whether they never, previously or currently smoked cigarettes. Education level was acquired at enrollment. Height and weight were measured and body mass index (BMI; kg/m2) was calculated. Self-assessed health was reported on a five-point scale (excellent, very good, good, fair, poor). Blood pressure was calculated as the mean of two resting, seated measurements (Hypertension Detection and Follow-Up Program Cooperative Group, 1976). Participants were considered hypertensive if systolic blood pressure was ≥140, diastolic blood pressure was ≥90, or if they reported taking antihypertensive medication.
Statistical analysis
Demographic and behavioural characteristics were compared between groups using independent sample t-tests or univariate analysis of variance (ANOVA) for continuous measures, and chi-squared tests for categorical variables.
To test effects of PA on cognitive function, separate ANOVAs were run for each cognitive test, at each time-point. Fixed factors included PA (active/inactive) and sex, and models included a sex by PA interaction. To assess whether intensity of PA was associated with cognitive function independent of frequency of activity, separate linear regressions were run for each test, at each time-point, with Godin score as the predictor and controlling for activity status (active/inactive). Regressions were repeated stratified by sex. ANOVAs and linear regressions were adjusted for age and education, and additionally adjusted for health-related factors (alcohol consumption, smoking, hypertension, BMI and self-rated health).
Binary logistic regressions, adjusted for age, sex, and education, were performed to test whether PA at each time point was associated with likelihood of cognitive impairment, defined as an MMSE score >1.5 standard deviations below sex-, age- and education-adjusted means based on the National Alzheimer’s Coordinating Center Uniform Data Set [19].
To assess whether teenage and older age PA demonstrate interactive effects on late-life cognitive function, ANOVAs were performed with factors for teenage PA, older age PA, and sex, with all two-way interactions and the three-way interaction of sex by teenage and older age PA. Models were repeated to evaluate interactions of older age PA with age 30 PA and with age 50 PA. Analyses underwent covariate adjustment as described above.
Sensitivity analyses excluded individuals with fair or poor self-reported health.
P-values for two-sided tests are shown. For analysis of demographics and binary logistic regressions, P < 0.05 was considered statistically significant. After Bonferroni correction for multiple comparisons across four tests, P < 0.0125 was considered significant for analyses of cognitive function.
Results
Participant characteristics
The mean age of participants at cognitive assessment was 75.1 ± 7.7 years (range 60–99); 60% were women. Overall, 1,501 (82%) participants reported regular PA as a teenager, 1,130 (62%) at age 30, 1,249 (68%) at age 50 and 1,294 (71%) in older age. Physically active participants at time of cognitive assessment were younger, more educated, had lower rates of current smoking and hypertension, lower BMI and reported better health (Ps < 0.05, Table 1). At all time-points, a greater proportion of men than women reported being physically active and Godin scores were higher for men than women (all Ps < 0.001).
Table 1.
Characteristics for participants at older age cognitive assessment by older age physical activity status.
| Inactive | Active | P-value | |
|---|---|---|---|
| N = 532 | N = 1294 | ||
| Age (years) | 76.8 ± 8.0 | 74.4 ± 7.4 | <0.001 |
| Sex (% women) | 68 | 57 | <0.001 |
| Education (years) | 14.2 ± 2.2 | 14.5 ± 2.3 | 0.009 |
| Alcohol (drinks/week) | 5.1 ± 7.2 | 5.7 ± 7.2 | 0.16 |
| Smoking (% former/current) | 45/12 | 51/6 | <0.001 |
| Hypertension (%) | 66 | 59 | 0.005 |
| BMI (kg/m2)a | 25.3 ± 4.1 | 25.0 ± 3.5 | <0.05 |
| Health (% fair/poor) | 7 | 3 | <0.001 |
| Active in teenage (%) | 78 | 84 | 0.004 |
| Active age 30 (%) | 54 | 65 | <0.001 |
| Active age 50 (%) | 52 | 75 | <0.001 |
| Godin teenage | 43 ± 35 | 54 ± 49 | <0.001 |
| Godin age 30 | 21 ± 25 | 29 ± 34 | <0.001 |
| Godin age 50 | 14 ± 18 | 27 ± 24 | <0.001 |
| Godin older age | 2 ± 3 | 26 ± 18 | <0.001 |
Values are mean ± SD or percent (%).
aAdjusted for sex.
PA across adulthood and later-life cognitive function
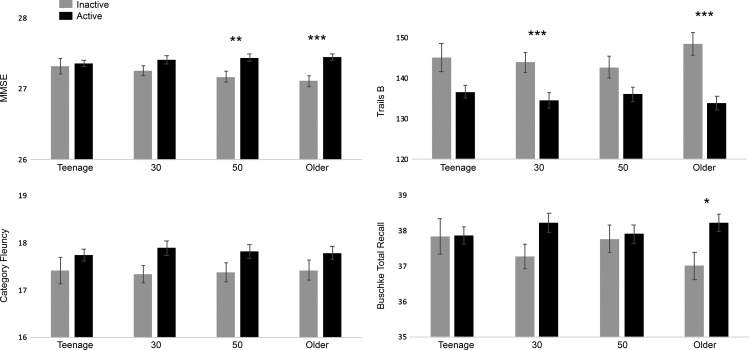
Figure 1 shows cognitive performance as a function of activity status at each age period. For the MMSE, PA at age 50 (base model: F(1,1798) = 9.35, P = 0.002; fully adjusted model: F(1,1786) = 6.40, P = 0.01) and in older age (base: F(1,1798) = 13.58, P < 0.001; fully adjusted: F(1,1786) = 8.09, P = 0.005) was associated with better MMSE scores. For Trails B, PA at age 30 (base: F(1,1475) = 11.15, P < 0.001; fully adjusted: F(1,1464) = 12.14, P < 0.001) and older age (base: F(1,1475) = 18.51, P < 0.001; fully adjusted: F(1,1464) = 14.26, P < 0.001) was associated with better Trails B performance. Total recall scores were higher in those physically active in older age (base: F(1,1466) = 6.42, P = 0.01; fully adjusted: F(1,1455) = 5.79, P = 0.02). Category fluency performance was not affected by PA at any age (Ps > 0.01). Sex did not interact with PA (Ps > 0.05). PA in older age, but not at any other age, was associated with reduced odds of cognitive impairment (OR: 0.77; 95% CI: 0.60–0.99).
Figure 1.
Cognitive function by physical activity at different age periods. Cognitive function scores (adjusted for age, sex and education) for participants classified as physically active (black bars) or inactive (grey bars) at teenage, age 30, age 50 and older age (mean = 75 years). Note that for Trails B higher scores reflect poorer performance. *P < 0.05, **P < 0.01, ***P < 0.001 for active versus inactive.
PA intensity and later-life cognitive function
Higher intensity teenage PA was associated with better MMSE scores (base: β (per SD Godin score) = 0.13, P = 0.01; fully adjusted: β = 0.14, P = 0.007). Sex-stratified regressions showed that this association was present for women (base: β = 0.17, p = 0.02; fully adjusted: β = 0.18, P = 0.01), but not for men (P = 0.20). Intensity of PA at other ages was not associated with cognitive function (Ps > 0.10).
Earlier life and later-life PA and late-life cognitive function
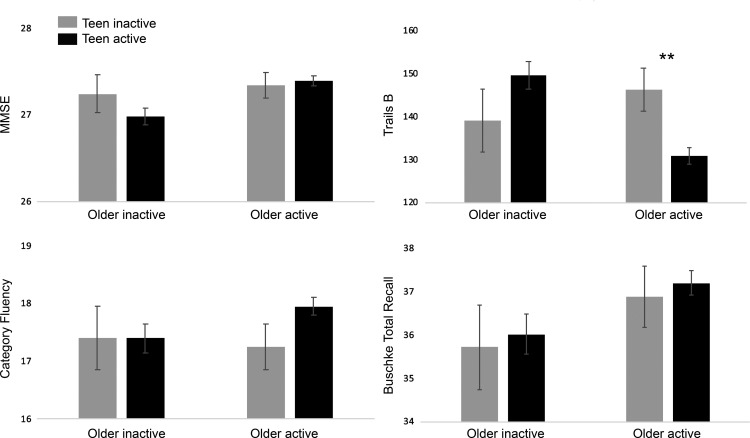
To assess whether early-life PA modified the association between older age PA and cognitive function, we examined interactions between teenage and older age PA on cognitive performance (Figure 2). There was an interaction between teenage and older age PA for Trails B (base: F(1,1471) = 7.42, P = 0.007; fully adjusted: F(1,1460) = 7.63, P = 0.006); active older individuals performed better if they were also active as teenagers (P < 0.001), but not if they were inactive as teenagers (P > 0.50). This effect did not differ by sex (P = 0.22). Older age PA did not interact with age 30 or age 50 PA for men or women (Ps > 0.10).
Figure 2.
Cognitive function by teenage and older age physical activity. Cognitive function scores (adjusted for age, sex and education) for participants classified according to physical activity in teenage and older age. Note that for Trails B higher scores reflect poorer performance. **P < 0.01.
PA and cognitive function in healthy individuals
Fair or poor health was reported by 76 participants. These individuals were older (P < 0.001), more likely to have hypertension (P = 0.003), and consumed less alcohol (P = 0.004). Similar associations between PA and cognitive performance were observed when excluding these individuals (MMSE with age 50 PA: F(1,1723) = 4.08, P = 0.04; MMSE with older age PA: F(1,1723) = 4.42, P = 0.04; Trails B with age 30 PA: F(1,1414) = 9.67, P = 0.002; Trails B with older age PA: F(1,1414) = 11.89, P < 0.001; total recall with older age PA: F(1,1393) = 4.93, P = 0.03). There was a similar pattern of interaction between teenage and older age PA on Trails B scores (F(1,1410) = 5.75, P = 0.02).
Discussion
In this study, regular PA at different points across the lifespan was associated with better late-life function in multiple cognitive domains. The strongest associations were observed for current PA, although PA in youth and midlife were also important. Individuals who were physically active during both teenage years and older age showed the highest level of cognitive function. Greater intensity of PA in teen years, but not at later ages, was associated with better global cognitive function, particularly for women.
Our finding that PA in older age was most strongly associated with better global cognitive function, executive function and episodic memory supports growing evidence for a role of PA in promoting healthy cognitive ageing [5–7]. Our observation that PA in older age, but not at younger ages, was associated with reduced odds of cognitive impairment contrasts with the Study of Osteoporotic Factors (SOF), in which teenage PA more strongly predicted cognitive impairment than older age PA [9]. Several differences between our studies may contribute to our discrepant findings. In the SOF, women were characterized as active based upon any reported PA, whereas our active groups were based on reported activity at least three times per week. Regular PA in older age may be a more important predictor of cognitive function than occasional PA. In addition, the SOF used a set cutoff on the modified MMSE to determine cognitive impairment, whereas our definition was based on age-, sex-, and education-specific norms for the full MMSE, and thus was likely more sensitive [19]. The greater proportion of cognitively impaired participants in our sample is consistent with this (23% vs. 10%).
Teenage PA also predicted preserved executive function in older age, in accord with the reduced risk of cognitive impairment for active teenagers reported in the SOF [9]. Older age PA was positively associated with executive function only for individuals who were also active as teenagers. In contrast, there was no interaction of activity at ages 30 or 50 with older age PA. These findings are consistent with previously reported associations of an active youth with healthy cognitive ageing [8, 9], which may result from increased resilience to effects of ageing or disease, a concept referred to as ‘cognitive reserve’ [20]. Enduring protective effects of early-life activity may arise from lasting brain changes, such as stimulation of neurogenesis and neuronal growth, production of neurotrophic factors, or brain vascularization, which may optimize brain development [21–24]. Because brain white matter is still developing through early adulthood [25], regular PA in teenage years may promote white matter connectivity [26], providing a substrate for cognitive reserve. This may buffer against ageing-related white matter decline that contributes to decline in executive function [27, 28].
Several studies have reported benefits of moderate PA on cognitive decline [29], but there is conflicting evidence over whether strenuous activity is beneficial [11] or detrimental [8, 10]. Here, more intense teenage PA was associated with better late-life global cognitive function in women, suggesting that higher intensity exercise in youth may further contribute to cognitive reserve. Notably, activity intensity in later years was not associated with cognitive function. Thus, even light regular PA, such as gardening or housework, may be sufficient to promote healthy cognitive ageing.
Men and women may engage in distinct patterns of PA for cultural or biological reasons [30], yet it remains unresolved whether PA elicits similar effects on the ageing male and female brain. There is limited evidence that exercise may be more cognitively protective for women than men [31], and particularly so for executive function [32]. We observed no sex difference in the association between PA and executive function as assessed with the Trails B test, although more intense PA as a teenager was associated with better global cognitive function for women only. While our weak evidence for a stronger association between PA and cognitive ageing in women aligns with prior reports, our findings generally suggest that an active lifestyle imparts cognitive benefits for both men and women.
Limitations of this study include self-reported PA, which may be more accurate for current activity and less reliable in cognitively impaired individuals. We cannot exclude the contribution of reverse causation, i.e. that declining health or cognition leads to reduced PA. However, PA at ages 30 and 50, prior to the age at which cognitive decline typically manifests, was also associated with better cognitive function in later life. Furthermore, similar patterns of associations were observed after adjusting for various health measures and after excluding individuals who reported fair to poor health. Strengths include a large, relatively homogeneous sample of mostly white, middle-class participants, which minimizes confounding due to socioeconomic status, race and access to healthcare.
In conclusion, this study identified positive associations between PA throughout the lifespan and later-life cognitive function among community-dwelling adults. Cognitive associations were strongest for older age PA and were not modified by activity intensity, suggesting that even light or moderate PA in later years may help to preserve brain health. Interactive positive effects of teenage PA suggest that regular PA during youth can contribute to cognitive reserve, in turn promoting healthy cognitive ageing.
Supplementary Material
Declaration of Conflict of Interest: None.
Declaration of Sources of Funding: This work was supported by the National Institute on Alcohol Abuse and Alcoholism (R01 AA021187); the National Institute of Aging (AG028507, AG007181); the National Institute of Diabetes and Digestive and Kidney Diseases (DK031801). Some study data were collected and managed using REDCap electronic data capture tools hosted at University of California San Diego and supported by a grant from the National Institutes of Health (UL1TR001442). The funding agencies had no role in design, execution, analysis and interpretation of data, or writing of the study.
References
- 1. Blondell SJ, Hammersley-Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia? A systematic review and meta-analysis of longitudinal studies. BMC Public Health 2014; 14: 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gallaway PJ, Miyake H, Buchowski MS et al. . Physical activity: a viable way to reduce the risks of mild cognitive impairment, Alzheimer’s disease, and vascular dementia in older adults. Brain Sci 2017; 7: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh-Manoux A, Hillsdon M, Brunner E, Marmot M. Effects of physical activity on cognitive functioning in middle age: evidence from the Whitehall II prospective cohort study. Am J Public Health 2005; 95: 2252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flicker L, Almeida OP, Acres J et al. . Predictors of impaired cognitive function in men over the age of 80 years: results from the Health in Men Study. Age Ageing 2005; 34: 77–80. [DOI] [PubMed] [Google Scholar]
- 5. Christensen H, Korten A, Jorm AF, Henderson AS, Scott R, Mackinnon AJ. Activity levels and cognitive functioning in an elderly community sample. Age Ageing 1996; 25: 72–80. [DOI] [PubMed] [Google Scholar]
- 6. Clarkson-Smith L, Hartley AA. Relationships between physical exercise and cognitive abilities in older adults. Psychol Aging 1989; 4: 183–9. [DOI] [PubMed] [Google Scholar]
- 7. Barnes DE, Blackwell T, Stone KL et al. . Cognition in older women: the importance of daytime movement. J Am Geriatr Soc 2008; 56: 1658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dik M, Deeg DJ, Visser M, Jonker C. Early life physical activity and cognition at old age. J Clin Exp Neuropsychol 2003; 25: 643–53. [DOI] [PubMed] [Google Scholar]
- 9. Middleton LE, Barnes DE, Lui LY, Yaffe K. Physical activity over the life course and its association with cognitive performance and impairment in old age. J Am Geriatr Soc 2010; 58: 1322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tierney MC, Moineddin R, Morra A, Manson J, Blake J. Intensity of recreational physical activity throughout life and later life cognitive functioning in women. J Alzheimers Dis 2010; 22: 1331–8. [DOI] [PubMed] [Google Scholar]
- 11. Dregan A, Gulliford MC. Leisure-time physical activity over the life course and cognitive functioning in late mid-adult years: a cohort-based investigation. Psychol Med 2013; 43: 2447–58. [DOI] [PubMed] [Google Scholar]
- 12. Hoang TD, Reis J, Zhu N et al. . Effect of early adult patterns of physical activity and television viewing on midlife cognitive function. JAMA Psychiatr 2016; 73: 73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu N, Jacobs DR Jr., Schreiner PJ et al. . Cardiorespiratory fitness and cognitive function in middle age: the CARDIA study. Neurology 2014; 82: 1339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tombaugh TN, McDowell I, Kristjansson B, Hubley AM. Mini-Mental State Examination (MMSE) and the Modified MMSE (3MS): a psychometric comparison and normative data. Psychol Assess 1996; 8: 48–59. [Google Scholar]
- 15. Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills 1958; 8: 271–6. [Google Scholar]
- 16. Borkowsk JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia 1967; 5: 135–40. [Google Scholar]
- 17. Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 1974; 24: 1019–25. [DOI] [PubMed] [Google Scholar]
- 18. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci 1985; 10: 141–6. [PubMed] [Google Scholar]
- 19. Shirk SD, Mitchell MB, Shaughnessy LW et al. . A web-based normative calculator for the uniform data set (UDS) neuropsychological test battery. Alzheimer’s Res Ther 2011; 3: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scarmeas N, Stern Y. Cognitive reserve: implications for diagnosis and prevention of Alzheimer’s disease. Curr Neurol Neurosci Rep 2004; 4: 374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uda M, Ishido M, Kami K, Masuhara M. Effects of chronic treadmill running on neurogenesis in the dentate gyrus of the hippocampus of adult rat. Brain Res 2006; 1104: 64–72. [DOI] [PubMed] [Google Scholar]
- 22. van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 1999; 2: 266–70. [DOI] [PubMed] [Google Scholar]
- 23. Marais L, Stein DJ, Daniels WM. Exercise increases BDNF levels in the striatum and decreases depressive-like behavior in chronically stressed rats. Metab Brain Dis 2009; 24: 587–97. [DOI] [PubMed] [Google Scholar]
- 24. Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab 1992; 12: 110–9. [DOI] [PubMed] [Google Scholar]
- 25. Paus T, Zijdenbos A, Worsley K et al. . Structural maturation of neural pathways in children and adolescents: in vivo study. Science 1999; 283: 1908–11. [DOI] [PubMed] [Google Scholar]
- 26. Herting MM, Colby JB, Sowell ER, Nagel BJ. White matter connectivity and aerobic fitness in male adolescents. Dev Cogn Neurosci 2014; 7: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perry ME, McDonald CR, Hagler DJ Jr. et al. . White matter tracts associated with set-shifting in healthy aging. Neuropsychologia 2009; 47: 2835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kennedy KM, Raz N. Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia 2009; 47: 916–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sofi F, Valecchi D, Bacci D et al. . Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med 2011; 269: 107–17. [DOI] [PubMed] [Google Scholar]
- 30. Rosenfeld CS. Sex-dependent differences in voluntary physical activity. J Neurosci Res 2017; 95: 279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci 2003; 14: 125–30. [DOI] [PubMed] [Google Scholar]
- 32. Barha CK, Davis JC, Falck RS, Nagamatsu LS, Liu-Ambrose T. Sex differences in exercise efficacy to improve cognition: a systematic review and meta-analysis of randomized controlled trials in older humans. Front Neuroendocrinol 2017; 46: 71–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.