Abstract
Hepatic hydrothorax, a rare and debilitating complication of cirrhosis, carries high morbidity and mortality. First-line treatment consists of dietary sodium restriction and diuretic therapy. Some patients, mainly those who are refractory to medical management, will require invasive pleural drainage. The authors report the case of a 76-year-old man in a late cirrhotic stage of alcoholic chronic liver disease, presenting with recurrent right-sided hepatic hydrothorax, portal hypertension, hepatosplenomegaly and thrombocytopaenia. After recurrent admissions and complications, the potential for adjusting diuretic therapy was limited. After unsuccessful talc pleurodesis, an indwelling tunnelled pleural catheter was placed with effective symptomatic control. One month later, the patient was readmitted with empyema due to Acinetobacter radioresistens. Despite optimised medical and surgical treatment, the patient died 4 weeks later.
Keywords: alcoholic liver disease, cirrhosis, pleural infection
Background
Hepatic hydrothorax, a debilitating complication of cirrhosis, is a pleural effusion associated with portal hypertension that cannot be attributed to any cardiac or pulmonary cause.1 2 Its prevalence in patients with cirrhosis varies from 0.4% to 15%3 4 and it is associated with high morbidity and mortality rates (25% 1-year mortality after diagnosis).5 Although other presentations could occur, hepatic hydrothorax is commonly seen in conjunction with ascites (up to 97% of patients) and is more often right-sided (65%–85% of cases).3 6 The most accepted mechanism is the direct passage of ascitic fluid into the pleural space through minute (and exceptionally congenital) diaphragmatic defects enhanced by negative intrapleural pressure, even in patients without clinically apparent ascites.1 6 7
Patients may be asymptomatic or they may present with signs and symptoms related to pleural effusion, depending on the amount and time of fluid accumulation and current cardiopulmonary status.7 For diagnostic purposes, thoracentesis and pleural fluid analysis are warranted. Most frequently, a transudative effusion is elicited and an underlying exudative process such as infection, inflammation or malignancy may be ruled out.6
First-line treatment consists of dietary sodium restriction and diuretic therapy.8 Occasionally, the use of diuretics is limited by concomitant renal dysfunction, electrolyte abnormalities, worsening of encephalopathy or ineffective symptomatic control. For patients resistant to medical management, estimated to be 26%,1 invasive pleural drainage is required.
Approaches to pleural drainage such as thoracentesis, chest tube placement, pleurodesis, indwelling tunnelled pleural catheter (ITPC) and transjugular intrahepatic portosystemic shunt (TIPS) have varying success and complication rates, and average 30-day mortality between 18% and 22%.1 2 9
Thoracentesis is generally well tolerated and effective in relieving symptoms, though repeat procedures are usually required, carrying a cumulative high risk of complications (such as pneumothorax, bleeding and infection).10 Serial thoracentesis should be reserved for patients who are not candidates for alternative therapies, because of short life expectancy, or those requiring drainage less often than once every 2 weeks.7
Chest tubes with continued pleural drainage should be discouraged as these can result in sudden volume depletion, electrolyte abnormalities and renal dysfunction, which in turn lead to increased morbidity and mortality.11–13 Whenever possible, and when ascites is present, paracentesis should be performed prior to thoracentesis to improve respiratory mechanics and to minimise rapid recurrence of pleural fluid.2
Liver transplantation is the treatment of choice for suitable patients when available and feasible. It is the only option that offers a cure, although not all patients are able to benefit from it in a timely manner.14
Recently developed ITPCs have been used to successfully relieve dyspnoea in patients with malignant pleural effusions, with good outcomes.15–18 These catheters allow patients to control pleural fluid output and global hydro and electrolyte states outside the hospital. Curiously, few studies have explored the safety and efficacy of ITPCs for refractory benign pleural effusions, and more specifically, transudative hepatic hydrothorax in patients with advanced cirrhotic liver disease.7 19–23
Case presentation
The authors report a case of a 76-year-old man with arterial hypertension, dyslipidaemia, severe centrilobular pulmonary emphysema, stage 2 chronic kidney disease and late cirrhotic stage alcoholic chronic liver disease (Child-Pugh score B, MELD score 22 points) which lead to recurrent right-sided hepatic hydrothorax, portal hypertension, hepatosplenomegaly and thrombocytopaenia. Throughout the previous year, he underwent five long inpatient admissions due to decompensated chronic liver disease and recurrent right-sided hepatic hydrothorax without clinically significant ascites (figure 1) requiring nearly weekly pleural drainage for effective symptom relief. In addition to clinical and symptomatic worsening related to the hydrothorax, the patient had concomitant worsening of renal dysfunction and relapsing portosystemic encephalopathy that limited the optimisation of diuretic therapy. A few of these admissions were also complicated with nosocomial infections (mainly urinary and respiratory) and multiple broad-spectrum antibiotics such as piperacillin/tazobactam were prescribed.
Figure 1.
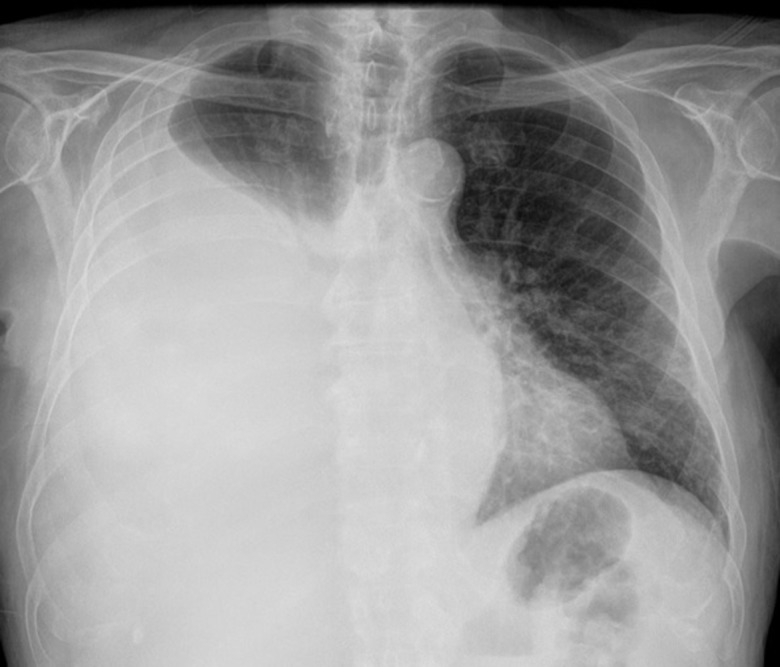
Chest radiograph demonstrating a large right-sided pleural effusion, with mild mediastinal shift, previously managed through insertion of a conventional chest drain and talc pleurodesis.
Pleural fluid analysis consistently demonstrated transudates; other underlying exudative processes such as infection (including spontaneous bacterial pleuritis), inflammation or malignancy were excluded. After multidisciplinary discussion, talc pleurodesis through a conventional chest tube was attempted without efficacy. Three weeks later, an ITPC was placed, resulting in effective symptomatic control and diuretic management (figure 2). The patient was successfully discharged for day-hospital management with weekly pleural drainages (about 750 mL each) through ITPC.
Figure 2.
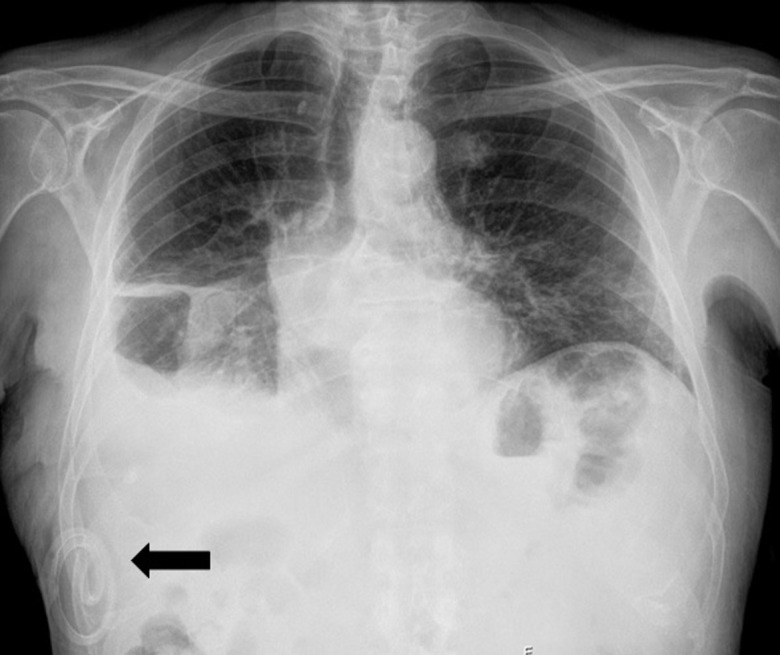
Chest radiograph immediately after ITPC placement (black arrow), demonstrating a residual small right-sided pleural effusion and pleural hypotransparencies related to previous talc pleurodesis. ITPC, indwelling tunnelled pleural catheter.
A month later, the patient presented at the emergency room with a week-long history of mild mucous productive cough, polypnoea and asthenia. Mild hypoxaemia was noted, other vital signs being unremarkable. Blood tests showed usual anaemia (haemoglobin 90 g/L) and thrombocytopaenia (74×109/L), new onset renal dysfunction (serum creatinine 154.72 µmol/L and glomerular filtration rate estimated as 0.62 mL/s) and moderate elevation of inflammatory markers (C-reactive protein 125 mg/L and leucocyte count unaltered). Chest X-ray showed increased right-sided pleural effusion and no clear signs of parenchymal infiltrate (figure 3).
Figure 3.
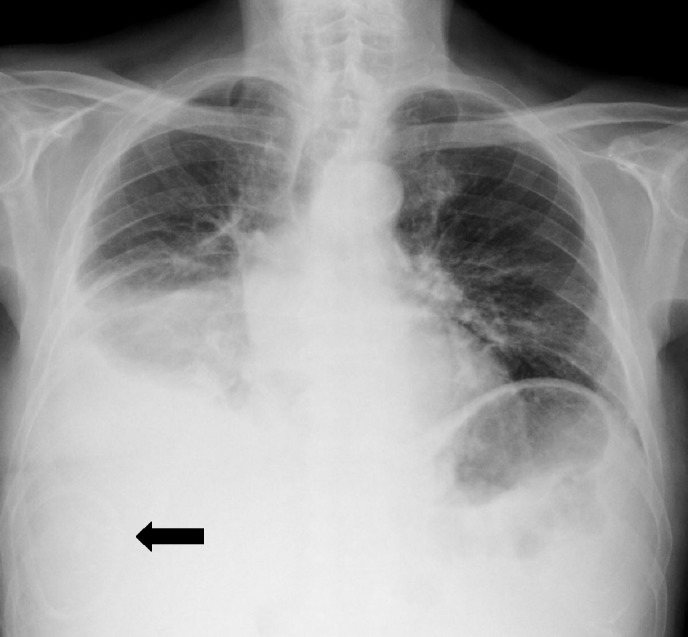
Chest radiograph at emergency room admission where the diagnosis of empyema was suspected; it shows ITPC previously placed (black arrow), increased right-sided pleural effusion and no clear signs of parenchymal infiltrate. ITPC, indwelling tunnelled pleural catheter.
On admission, pleural drainage was performed, and presuming a respiratory infection, ceftriaxone and azithromycin were started empirically. Soon after, mild erythema without significant exudates was noticed near the ITPC insertion site. The appearance of pleural fluid was mildly purulent with an uncharacteristic odour. Pleural effusion analysis was compatible with exudate/empyema (pH 7.06, leucocyte count 10.53×109/L, glucose <0.28 mmol/L and lactate dehydrogenase 370 U/L) and an Acinetobacter radioresistens (with susceptibility mainly to meropenem, gentamicin and trimethoprim/sulfamethoxazole) was isolated in two different pleural fluid samples. Considering this, his comorbidities and recent in-hospital admission, the ITPC was replaced for a conventional chest tube and directed antibiotic treatment with meropenem was started.
For the first time, the patient also presented mild to moderate ascites, with diagnostic ascitic fluid analysis (namely microbiological) unremarkable.
Outcome and follow-up
One week after directed antibiotic treatment with meropenem, thoracic CT revealed persistent moderate loculated hydropneumothorax with passive atelectasis of adjacent lung segments and pleural hyperdensities related to previous talc pleurodesis (figure 4).
Figure 4.
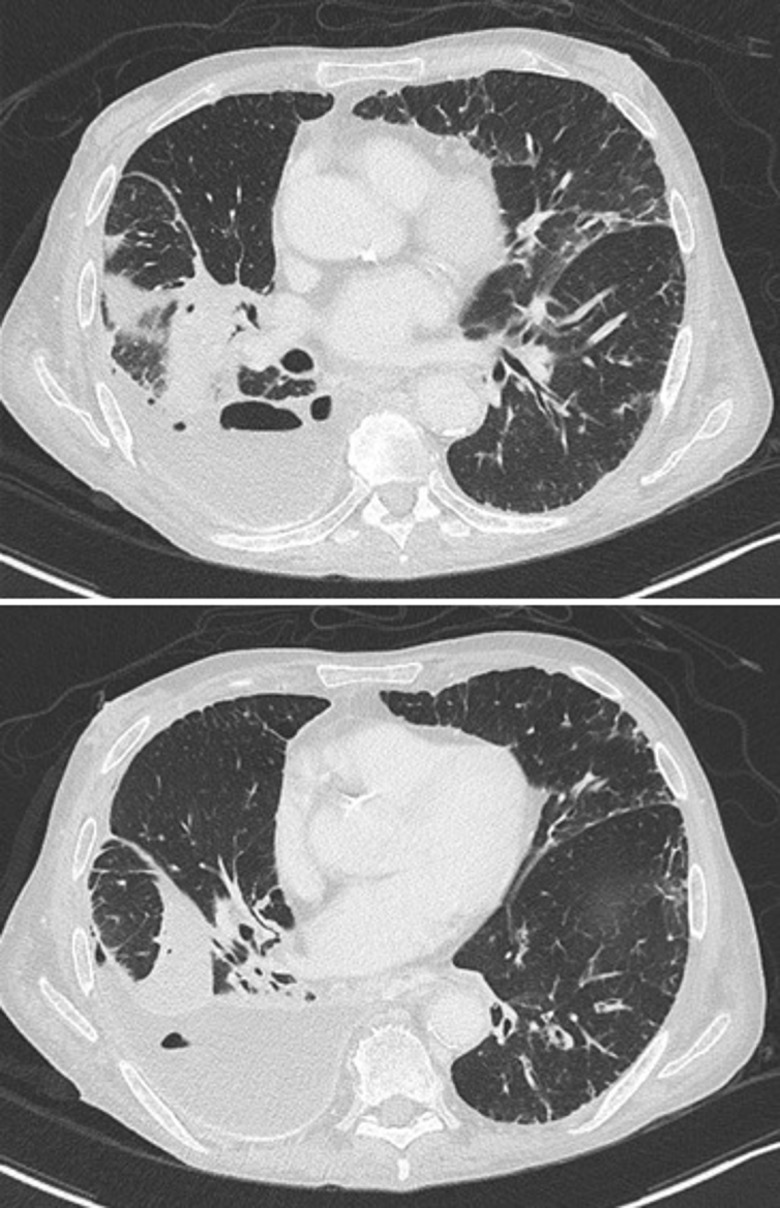
Thoracic CT after 1 week of adequate antibiotherapy (meropenem) for empyema; it reveals persistent moderate loculated right-sided hydropneumothorax with passive atelectasis of adjacent lung segments and pleural hyperdensities related to previous talc pleurodesis.
Pending case discussion with thoracic surgery, surgical (by video-assisted thoracoscopic surgery) empyemectomy and pleural lavage were performed, avoiding effective lung decortication due to high risk of haemorrhage. No effective lung expansion was achieved.
Despite all medical and surgical management in the best of our efforts, the patient died 4 weeks later due to progression to multiorgan dysfunction.
Discussion
Management of hepatic hydrothorax, particularly the refractory types, remains a clinical challenge. For those who are not eligible for TIPS or liver transplantation and in whom palliation and symptom control are the primary goals of care, repeated thoracentesis might be the best option if the need for pleural drainage is sporadic and life expectancy is limited. In patients who require more frequent drainage and have a longer life expectancy, ITPC placement has been considered as a therapeutic option.7
The rate of spontaneous pleurodesis after ITPC placement seems to be similar in malignant and non-malignant pleural effusions (about 30%–50%),19–21 24 although the average time to pleurodesis is longer for the last group of patients (about two to four times longer).18 25 The rate of pleural infection in patients with ITPCs placed for hepatic hydrothorax seems to be higher (8%–16%) than for ITPCs placed for malignant pleural effusion, where rates of 3% have been reported.11 20 22 26 A recent multicentre study examining outcomes related to ITPC use in hepatic hydrothorax found a 10% overall infection rate (the majority with cellulitis at the site of catheter insertion) and a 2.5% mortality rate (due to ITPC-related empyema and sepsis/septic shock), with a median time from ITPC removal to death of 106 days (range, 17–347 days).23
In our patient’s case, although considering palliation and symptom control as the primary goal, a multidisciplinary discussion (internal medicine, pneumology and thoracic surgery) first adopted conventional approaches, delaying ITPC placement. Possible explanations could lie in the lack of experience and low accessibility of appropriate equipment needed at our centre. Despite all efforts and proper management, our patient’s ITPC placement was complicated by an infection (empyema) due to A. radioresistens, which directly affected the outcome.
A. radioresistens is a non-spore forming and aerobic gram-negative coccobacillus, which is ubiquitous in soil and water, and a skin commensal.27 Its ability to withstand extreme desiccation and radiation exposure (hence the name radioresistens) helps it to survive in extreme weather conditions and cause nosocomial infections.28 However, the detection of A. radioresistens in clinical samples is rare, and its role as a causative agent of infection is controversial.28–32 So far, there is only scarce anecdotal evidence reporting this agent as a cause of infection: a middle ear/sinus infection complicated by septicaemia in an immunocompromised patient30; a pneumonia in an elderly and multimorbid patient suffering from chronic obstructive pulmonary disease33; and finally, a few reports of nosocomial outbreaks of A. radioresistens from hospital pneumatic tube systems.
Treatment of A. radioresistens infection needs antibiotic guidance by local antibiotic susceptibility reports. Duration of treatment may be longer than 2 weeks and dependent on the patient’s clinical response. Although the bacteria in this case had a favourable pattern, antibiotic susceptibilities are usually variable, and there is evidence of carbapenem, fluoroquinolone and tetracycline resistance in the medical literature.27 34
Contrasting with a study about the use of indwelling catheters for hepatic hydrothorax, in which isolated bacteria were Staphylococcus spp and Stenotrophomonas maltophilia,22 this case adds to evidence that A. radioresistens could be a rare but important clinical pathogen of infection on this kind of vulnerable patients.
Based on reported evidence, the authors believe that immunological abnormalities occurring in cirrhosis, such as a depressed reticuloendothelial system, neutrophil dysfunction, reduced serum complement and low bactericidal function, account for the increased susceptibility of this patient to bacterial seeding and spreading of uncommon pathogens like A. radioresistens.35 It is supposed that the severity, rather than the aetiology of liver disease, is predominantly responsible for that immune deficiency.36 Our patient’s outcome could also be related to his comorbidities, mainly pulmonary, as it was found in an observational study that patients with pleural effusion combined with cardiopulmonary diseases had a significantly higher risk of death in patients with cirrhosis than those without those comorbidities.37 Apart from ITPC insertion site complications or permanent use of invasive foreign material, no other specific risk factors for bacterial infection in patients with cirrhosis with ITPC were found in the literature.
Learning points.
Indwelling tunnelled pleural catheter may be an option for alleviating symptoms associated with recurrent hepatic hydrothorax that is refractory to conventional medical therapy.
Proper discussion and evaluation of patients with hepatic hydrothorax on centres with relevant experience on this pathology and procedures are crucial.
This case adds to evidence that Acinetobacter radioresistens could be a rare but important clinical pathogen for infection in vulnerable patients.
Footnotes
Contributors: All authors were attending physicians involved on the management of the patient our ward. CSC, VT and PON: drafting of the manuscript. RBM: revised critically the manuscript for its design and important intellectual content. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Next of kin consent obtained.
References
- 1. Singh A, Bajwa A, Shujaat A. Evidence-based review of the management of hepatic hydrothorax. Respiration 2013;86:155–73. 10.1159/000346996 [DOI] [PubMed] [Google Scholar]
- 2. Porcel JM. Management of refractory hepatic hydrothorax. Curr Opin Pulm Med 2014;20:352–7. 10.1097/MCP.0000000000000058 [DOI] [PubMed] [Google Scholar]
- 3. Mercky P, Sakr L, Heyries L, et al. Use of a tunnelled pleural catheter for the management of refractory hepatic hydrothorax: a new therapeutic option. Respiration 2010;80:348–52. 10.1159/000282493 [DOI] [PubMed] [Google Scholar]
- 4. Hou F, Qi X, Guo X. Effectiveness and safety of pleurodesis for hepatic hydrothorax: A systematic review and meta-analysis. Dig Dis Sci 2016;61:3321–34. 10.1007/s10620-016-4260-9 [DOI] [PubMed] [Google Scholar]
- 5. Walker SP, Morley AJ, Stadon L, et al. Nonmalignant pleural effusions: A prospective study of 356 consecutive unselected patients. Chest 2017;151:1099–105. 10.1016/j.chest.2016.12.014 [DOI] [PubMed] [Google Scholar]
- 6. Gurung P, Goldblatt M, Huggins JT, et al. Pleural fluid analysis and radiographic, sonographic, and echocardiographic characteristics of hepatic hydrothorax. Chest 2011;140:448–53. 10.1378/chest.10-2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haas KP, Chen AC. Indwelling tunneled pleural catheters for the management of hepatic hydrothorax. Curr Opin Pulm Med 2017;23:351–6. 10.1097/MCP.0000000000000386 [DOI] [PubMed] [Google Scholar]
- 8. Lazaridis KN, Frank JW, Krowka MJ, et al. Hepatic hydrothorax: pathogenesis, diagnosis, and management. Am J Med 1999;107:262–7. 10.1016/S0002-9343(99)00217-X [DOI] [PubMed] [Google Scholar]
- 9. Cerfolio RJ, Bryant AS. Efficacy of video-assisted thoracoscopic surgery with talc pleurodesis for porous diaphragm syndrome in patients with refractory hepatic hydrothorax. Ann Thorac Surg 2006;82:457–9. 10.1016/j.athoracsur.2006.03.057 [DOI] [PubMed] [Google Scholar]
- 10. Xiol X, Castellote J, Cortes-Beut R, et al. Usefulness and complications of thoracentesis in cirrhotic patients. Am J Med 2001;111:67–9. 10.1016/S0002-9343(01)00744-6 [DOI] [PubMed] [Google Scholar]
- 11. Chen CH, Shih CM, Chou JW, et al. Outcome predictors of cirrhotic patients with spontaneous bacterial empyema. Liver Int 2011;31:417–24. 10.1111/j.1478-3231.2010.02447.x [DOI] [PubMed] [Google Scholar]
- 12. Orman ES, Lok AS. Outcomes of patients with chest tube insertion for hepatic hydrothorax. Hepatol Int 2009;3:582–6. 10.1007/s12072-009-9136-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu LU, Haddadin HA, Bodian CA, et al. Outcome analysis of cirrhotic patients undergoing chest tube placement. Chest 2004;126:142–8. 10.1378/chest.126.1.142 [DOI] [PubMed] [Google Scholar]
- 14. European Association for the Study of the Liver. EASL clinical practice guidelines: liver transplantation. J Hepatol 2016;64:433–85. [DOI] [PubMed] [Google Scholar]
- 15. Chalhoub M, Ali Z, Sasso L, et al. Experience with indwelling pleural catheters in the treatment of recurrent pleural effusions. Ther Adv Respir Dis 2016;10:566–72. 10.1177/1753465816667649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Putnam JB, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer 1999;86:1992–9. [PubMed] [Google Scholar]
- 17. Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006;129:362–8. 10.1378/chest.129.2.362 [DOI] [PubMed] [Google Scholar]
- 18. Suzuki K, Servais EL, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011;6:762–7. 10.1097/JTO.0b013e31820d614f [DOI] [PubMed] [Google Scholar]
- 19. Shah R, Succony L, Gareeboo S. Use of tunneled pleural catheters for the management of refractory hepatic hydrothorax. BMJ Case Rep 2011;2011:doi: bcr0520114213 10.1136/bcr.05.2011.4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chalhoub M, Harris K, Castellano M, et al. The use of the PleurX catheter in the management of non-malignant pleural effusions. Chron Respir Dis 2011;8:185–91. 10.1177/1479972311407216 [DOI] [PubMed] [Google Scholar]
- 21. Potechin R, Amjadi K, Srour N. Indwelling pleural catheters for pleural effusions associated with end-stage renal disease: a case series. Ther Adv Respir Dis 2015;9:22–7. 10.1177/1753465814565353 [DOI] [PubMed] [Google Scholar]
- 22. Chen A, Massoni J, Jung D, et al. Indwelling tunneled pleural catheters for the management of hepatic hydrothorax. A pilot study. Ann Am Thorac Soc 2016;13:862–6. 10.1513/AnnalsATS.201510-688BC [DOI] [PubMed] [Google Scholar]
- 23. Shojaee S, Rahman N, Haas K, et al. Indwelling tunneled pleural catheters for refractory hepatic hydrothorax in patients with cirrhosis: A multicenter study. Chest 2018(18):32281–5. 10.1016/j.chest.2018.08.1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patil M, Dhillon SS, Attwood K, et al. Management of benign pleural effusions using indwelling pleural catheters: A systematic review and meta-analysis. Chest 2017;151:626–35. 10.1016/j.chest.2016.10.052 [DOI] [PubMed] [Google Scholar]
- 25. Warren WH, Kalimi R, Khodadadian LM, et al. Management of malignant pleural effusions using the Pleur(x) catheter. Ann Thorac Surg 2008;85:1049–55. 10.1016/j.athoracsur.2007.11.039 [DOI] [PubMed] [Google Scholar]
- 26. Fortin M, Tremblay A. Pleural controversies: indwelling pleural catheter vs. pleurodesis for malignant pleural effusions. J Thorac Dis 2015;7:1052–7. 10.3978/j.issn.2072-1439.2015.01.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brady AC, Lewis JS, Pfeiffer CD. Rapid detection of blaOXA in carbapenem-susceptible Acinetobacter radioresistens bacteremia leading to unnecessary antimicrobial administration. Diagn Microbiol Infect Dis 2016;85:488–9. 10.1016/j.diagmicrobio.2016.04.025 [DOI] [PubMed] [Google Scholar]
- 28. Jawad A, Snelling AM, Heritage J, et al. Exceptional desiccation tolerance of acinetobacter radioresistens. J Hosp Infect 1998;39:235–40. 10.1016/S0195-6701(98)90263-8 [DOI] [PubMed] [Google Scholar]
- 29. Leone S, Sturiale L, Pessione E, et al. Detailed characterization of the lipid A fraction from the nonpathogen Acinetobacter radioresistens strain S13. J Lipid Res 2007;48:1045–51. 10.1194/jlr.M600323-JLR200 [DOI] [PubMed] [Google Scholar]
- 30. Visca P, Petrucca A, De Mori P, et al. Community-acquired Acinetobacter radioresistens bacteremia in an HIV-positive patient. Emerg Infect Dis 2001;7:1032–5. 10.3201/eid0706.010621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verma R, Baroco AL. Acinetobacter radioresistens septicemia associated with pneumonia in a patient with chronic obstructive pulmonary disease and hepatitis C. Infectious Diseases in Clinical Practice 2017;25:e12–e13. 10.1097/IPC.0000000000000521 [DOI] [Google Scholar]
- 32. Poirel L, Figueiredo S, Cattoir V, et al. Acinetobacter radioresistens as a silent source of carbapenem resistance for Acinetobacter spp. Antimicrob Agents Chemother 2008;52:1252–6. 10.1128/AAC.01304-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Savov E, Pfeifer Y, Wilharm G, et al. Isolation of Acinetobacter radioresistens from a clinical sample in Bulgaria. J Glob Antimicrob Resist 2016;4:57–9. 10.1016/j.jgar.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 34. Higgins PG, Zander E, Seifert H. Identification of a novel insertion sequence element associated with carbapenem resistance and the development of fluoroquinolone resistance in Acinetobacter radioresistens. J Antimicrob Chemother 2013;68:720–2. 10.1093/jac/dks446 [DOI] [PubMed] [Google Scholar]
- 35. Arvaniti V, D’Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010;139:1246–56. 10.1053/j.gastro.2010.06.019 [DOI] [PubMed] [Google Scholar]
- 36. Runyon BA. Bacterial infections in patients with cirrhosis. J Hepatol 1993;18:271–2. 10.1016/S0168-8278(05)80267-3 [DOI] [PubMed] [Google Scholar]
- 37. Hou F, Qi X, Ning Z, et al. Prevalence, risk factors, and in-hospital outcome of pleural effusion in liver cirrhosis: A retrospective observational study. International Journal of Clinical and Experimental Medicine 2016;9:3265–79. [Google Scholar]


