Abstract
The Pseudomonas putida flhA-flhF-fleN-fliA cluster encodes a component of the flagellar export gate and three regulatory elements potentially involved in flagellar biogenesis and other functions. Here we show that these four genes form an operon, whose transcription is driven from the upstream PflhA promoter. A second promoter, PflhF, provides additional transcription of the three distal genes. PflhA and PflhF are σN-dependent, activated by the flagellar regulator FleQ, and negatively regulated by FleN. Motility, surface adhesion and colonization defects of a transposon insertion mutant in flhF revealed transcriptional polarity on fleN and fliA, as the former was required for strong surface adhesion and biofilm formation, and the latter was required for flagellar synthesis. On the other hand, FlhF and FleN were necessary to attain proper flagellar location and number for a fully functional flagellar complement. FleN, along with FleQ and the second messenger c-di-GMP differentially regulated transcription of lapA and the bcs operon, encoding a large adhesion protein and cellulose synthase. FleQ positively regulated the PlapA promoter and activation was antagonized by FleN and c-di-GMP. PbcsD was negatively regulated by FleQ and FleN, and repression was antagonized by c-di-GMP. FleN promoted FleQ binding to both PlapA and PbcsD in vitro, while c-di-GMP antagonized interaction with PbcsD and stimulated interaction with PlapA. A single FleQ binding site in PlapA was critical to activation in vivo. Our results suggest that FleQ, FleN and c-di-GMP cooperate to coordinate the regulation of flagellar motility and biofilm development.
Introduction
Bacterial life cycles in the environment are commonly characterized by the alternation of a single cell-based free-living planktonic stage and a sessile stage during which they develop structured highly cooperative surface-associated communities, also known as biofilms [1]. While the planktonic lifestyle allows bacterial cells to colonize new niches and gain access to fresh resources (and also escape from unfavourable habitats), biofilm growth has been shown to promote positive interactions between organisms, such as syntrophism and horizontal genetic transfer, while providing a nurturing, protective environment [2,3]. For many bacteria, motility during the planktonic stage is directed by flagella, molecular engines that enable swimming in liquid and semi-solid media by engaging in propeller-like rotation driven by the protonmotive force [4]. Flagella are complex organelles requiring the hierarchical synthesis and assembly of multiple structural and functional elements [5]. On the other hand, biofilm formation is generally considered a form of coordinated collective behaviour reminiscent of some developmental processes in higher organisms. Biofilm development proceeds through stages of adhesion, proliferation and microcolony formation and maturation, and is terminated by programmed biofilm dispersal [6,7]. Transition between the planktonic and biofilm lifestyles and the ordered succession of such stages arguably requires a variety of signal transduction and regulatory pathways to connect environmental and physiological signals to the adequate physiological responses [7].
Pseudomonas putida is a well-characterized Gram-negative soil and rhizosphere bacterium and a highly versatile model organism for biodegradation of organic toxicants, and bioremediation of polluted sites [8]. During planktonic growth, P. putida displays unipolar lophotrichous flagellation (i.e., carries a tuft of flagella at a single pole) [9]. In the reference strain KT2440 genome [10], genes encoding the structural components of the flagella and the chemotaxis signal transduction system, as well as a number of dedicated regulatory proteins, are encoded in a near-contiguous 70.7 kbp cluster containing 70 ORFs, of which at least 63 are apparently related to flagellar motility and chemotaxis. In addition, 27 genes encoding MCP chemoreceptors are scattered elsewhere in the genome [11]. The ability to form biofilms on both biotic and abiotic surfaces is a key to P. putida survival in its natural environment, and several factors relevant to biofilm development in P. putida have been identified [12]. The high molecular weight adhesin proteins LapA and LapF are important determinants for cell-surface and cell-cell interactions [13], flagella have been shown to contribute to initial surface attachment and to the maturation stage [12]. The major component of the extracellular matrix in P. putida biofilms is a mixture of exopolysaccharides (EPS), whose synthesis and export functions are encoded in four separate gene clusters in the P. putida chromosome, and the contribution of different types of EPS to the extracellular biofilm matrix and biofilm stability has been explored [14–17].
The nucleotide c-di-GMP is ubiquitously used in bacteria for intracellular signalling of the transition between the planktonic and sessile lifestyles. c-di-GMP is synthesized from GTP by diguanylate cyclase (DGC) activities, and degraded by specific phosphodiesterase (PDE) activities. Bacterial genomes often encode multiple proteins displaying one or both of these activities, implying that the c-di-GMP levels are likely regulated in a complex fashion. Changes in c-di-GMP concentration are sensed by effectors, which in turn regulate a variety of processes, generally related to motility, biofilm development or virulence, acting at the transcriptional, translational or posttranslational levels. The biology of c-di-GMP signalling has been extensively reviewed (see for example [18,19]).
FleQ has long been known as an enhancer-binding protein of the NtrC/NifA family of σN-dependent promoter activators and as the master regulator of flagellar biogenesis in Pseudomonas aeruginosa and other bacteria [20–23]. In P. aeruginosa FleQ is a c-di-GMP-responsive transcription factor that reversely regulates genes involved in flagellar motility and surface adhesion in response to changes in the intracellular levels of this second messenger [24]. Recent work by our lab and others has also highlighted the importance of FleQ and c-di-GMP in the switch from the planktonic to the sessile lifestyle and vice versa in P. putida [23,25]. Transcription of the flagellar cluster is subjected to a regulatory cascade in which the σN-dependent activator FleQ is the master regulator, c-di-GMP antagonizes FleQ activation, and the flagellar σ factor FliA directs transcription of late flagellar and chemotaxis genes. On the other hand, synthesis of key components of the biofilm matrix, such as the high molecular weight adhesin LapA or the cellulose synthase complex is subjected to FleQ- and c-di-GMP-dependent regulation to ensure maximum expression under high c-di-GMP regimes [23]. Finally, biofilm dispersal is triggered by a decrease in c-di-GMP concentration provoked by the PDE BifA [26], which is indirectly regulated by FleQ via the flagellar cascade, thus providing a regulatory link between the termination of the biofilm lifestyle and the synthesis of new flagella [23].
FlhF and FleN (also known as FlhG, YlxH or MinD2), two members of the SIMIBI family of nucleotide-binding proteins, have been characterized as responsible of the spatial and numerical regulation of flagellar biogenesis in a variety of bacteria showing polar flagellation [27–30], as well as the peritrichously-flagellated Bacillus subtilis [31]. FlhF is a Signal Recognition Particle (SRP)-type GTPase responsible of the polar localization of flagella in Vibrio, Shewanella, Campylobacter, Helicobacter, and Pseudomonas aeruginosa [32–37]. FleN is a MinD/ParA-type ATPase involved in restricting the number of flagella to that characteristic for each organism [31,32,38–41]. Based on biochemical, genetic and structural evidence, it has been proposed that GTP-bound FlhF initiates flagellar biogenesis by recruiting early components of the flagellar basal body to the pole, and FleN antagonizes FlhF function by stimulating its GTPase activity once the correct number of flagella (or flagellar basal bodies) is attained, but many mechanistic details of this model are as of yet unexplored [4,28,42].
In addition to their roles in determination of flagellar location and number, FlhF and FleN have been involved in transcriptional regulation of the flagellar genes in different organisms. FlhF and the flagellar type-III secretion system protein FlhA negatively regulate flagellar gene transcription in Helicobacter pylori [37]. FleN antagonizes FleQ-dependent activation of Class II flagellar promoters in a c-di-GMP-dependent manner in P. aeruginosa, etc [43]. In addition, FleN modulates FleQ-dependent regulation of the pel, pea and cdrA operons, encoding EPS components and a large adhesin, in P. aeruginosa [44], and has recently been shown to play a similar role in the lapA and bcs operons in P. putida [25].
Recently, we described the isolation of a transposon insertion mutant in flhF in P. putida KT2442, a rifampicin-resistant derivative of the P. putida reference strain KT2440, displaying major defects in biofilm formation and flagellar motility [45]. Here we address the origin of the phenotypic defects observed in this mutant and explore the roles of FlhF and FleN in the regulation of flagellar gene expression and flagellar biogenesis and in the transcriptional regulation of biofilm matrix components.
Results
The flhAF-fleN-fliA gene cluster is co-transcribed as an operon
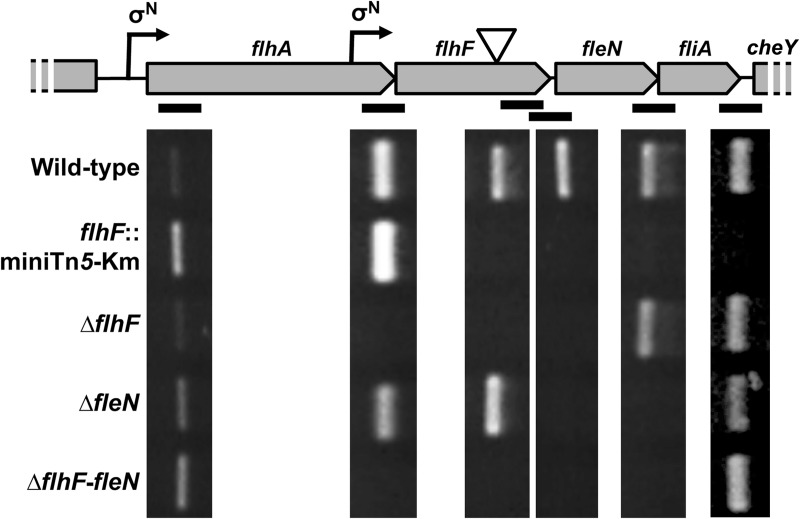
In a genetic screen for transposon mutant derivatives of P. putida KT2442 (a rifampicin-resistant derivative of the model strain KT2440) with defects in biofilm formation we recently isolated MRB49, bearing a miniTn5-Km insertion in the flagellar gene flhF. MRB49 displayed severe defects in both biofilm formation and flagellar motility that were comparable to those of a fleQ mutant [45], suggesting that the mutation impairs a key step in the coordinated regulation of flagellar biogenesis and surface adhesion. The gene flhF is the second of a string of fourteen genes (flhAF-fleN-fliA-cheYZAB-motCD-pp4334-pp4333-cheW-pp4331) transcribed in the same orientation. We have identified a FleQ-activated, σN-dependent (i.e., Class II) promoter activity upstream from flhA [23]. On the other hand, Rodríguez-Herva et al. (2010) [46] identified cheY as a target for FliA regulation, suggesting that cheY and the genes downstream are part of a different, FliA-dependent (i.e., Class IV) transcriptional unit. However, the transcriptional organization of the flhA-flhF-fleN-fliA region is currently unresolved, as Pandza et al. (2000) [47] proposed the transcriptional units flhA and flhF-fleN-fliA, and Rodríguez-Herva et al. (2010) [46] proposed the transcriptional units flhAF and fleN-fliA. To solve this discrepancy, semiquantitative RT-PCR was performed using RNA from the wild-type strain KT2442 and the flhF::miniTn5-Km mutant MRB49 as templates and specific primers amplifying a proximal segment of flhA, the flhA-flhF, flhF-fleN, fleN-fliA and fliA-cheY intergenic regions, and a distal segment of flhF (downstream from the transposon insertion) (Fig 1).
Fig 1. Transcriptional organization of the flhA-flhF-fleN-fliA cluster.
(Top) Cartoon of the of the flhA-flhF-fleN-fliA-cheY genes showing the location of the putative σN-dependent promoters (bent arrows) and the location of the transposon insertion in the flhF::miniTn5-Km mutant (Bottom) Ethidium bromide-stained agarose gel showing the results of semiquantitative RT-PCR assays using total RNA from the wild-type, flhF::miniTn5-Km. ΔflhF, ΔfleN and ΔflhF-fleN strains, and primers annealing to the flhA coding sequence, the flhF coding sequence (distal region), and flanking each intergenic region (black bars).
RT-PCR from the wild-type strain yielded amplified products from all six primer pairs. In contrast, RNA from MRB49 (flhF::miniTn5-Km) yielded bands from the flhA and flhA-flhF segments, but no product was obtained with any of the oligonucleotide pairs annealing downstream from the transposon insertion point, indicating that the transposon insertion is polar on the downstream fleN and fliA. In disagreement with previous reports [46,47], these results rule out the presence of transcriptional terminators at the flhA-flhF, flhF-fleN, fleN-fliA and fliA-cheY junctions, indicating that flhA, flhF, fleN, fliA and cheY are co-transcribed as part of the same operon.
As the miniTn5-Km insertion in flhF present in MRB49 causes transcriptional polarity on fleN and fliA, we questioned whether the phenotypes displayed by the flhF::miniTn5-Km mutant may be due to the defect in expression of one of these genes, rather than the inactivation of flhF. To test this hypothesis, we used allelic replacement to construct KT2442-derived mutant strains MRB69, MRB71 and MRB78, bearing unmarked complete deletions of flhF, fleN and both genes simultaneously (see Materials and Methods below). RNA from MRB69, MRB71 and MRB78 was also used as template for RT-PCR, confirming that each mutant failed to transcribe the deleted gene(s), while expression of the downstream genes was unaffected (Fig 1).
Examination of the RT-PCR results provided two additional pieces of information regarding the expression of the flhAF-fleN-fliA operon. Firstly, the signal corresponding to the proximal segment of flhA was increased in all three mutants not expressing FleN relative to the wild-type and ΔflhF mutant. Notwithstanding the low sensitivity of this technique for quantitative analysis, this result hints to a possible negative effect of FleN on transcription from the PflhA promoter. Secondly, all amplified products downstream from flhA were more abundant than the corresponding products obtained with the proximal flhA primers in every genetic background, suggesting that a second promoter activity may contribute to increase the expression of flhF, fleN and fliA.
Two FleQ- and FleN-regulated promoters control flhAF-fleN-fliA transcription
Inspection of the sequences upstream from flhF and flhA revealed two regions displaying high similarity to the σN binding motif consensus (TGGCACG-N4-TTGCW) [48]. The first of these regions, bearing the sequence TGGAAAGcttcTTGCA (matches to the consensus underlined), is located between positions -71 and -57 relative to the flhA start codon, and likely corresponds to the promoter activity we previously designated PflhA [23]. The second region, bearing the sequence TGGAACAgattTTGCT (matches to the consensus underlined), is located within the flhA coding region, between positions -298 and -284 relative to the flhF start codon, and corresponds to the P1 promoter previously identified in this region [49]. We have designated this putative σN-dependent promoter PflhF (Fig 1).
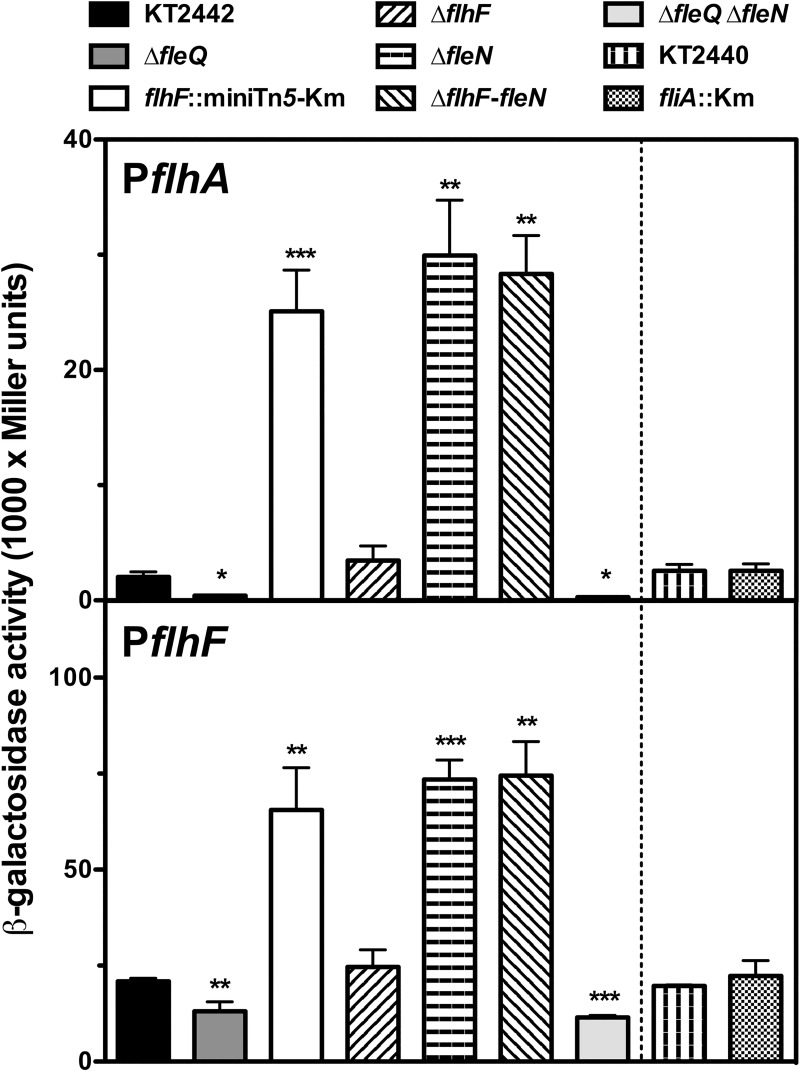
To evaluate the transcription from these promoters we used plasmids pMRB115 and pMRB158, bearing transcriptional fusions of PflhA and PflhF in the broad host-range dual gfp-lacZ reporter plasmid pMRB3 [23]. The resulting PflhA-gfp-lacZ and PflhF-gfp-lacZ fusion plasmids were transferred to P. putida KT2442 and its ΔfleQ, flhF::miniTn5-Km, ΔflhF, ΔfleN and ΔflhF-fleN mutant derivatives by mating, and expression was assessed by means of β-galactosidase assays (Fig 2).
Fig 2. Expression of the PflhA and PflhF promoters in P. putida.
β-galactosidase assays of the PflhA and PflhF promoter fusions in wild-type (KT2442), ΔfleQ (MRB52), flhF:miniTn5-km (MRB49), ΔflhF (MRB69), ΔfleN (MRB71), ΔflhF-fleN (MRB78), and ΔfleQΔfleN (MRB101) backgrounds (left panels) and wild type (KT2440) and fliA- backgrounds (right panels). Bars represent the averages and standard deviations of at least three independent assays. Stars designate p-values for the Student's t-test for unpaired samples not assuming equal variance. *: p<0.05; **: p<0.01; ***:p<0.005.
Expression from the PflhA promoter was all but abolished in the absence of FleQ relative to that in the wild-type strain, as expected from the fact that PflhA is a Class II flagellar promoter [23]. Consistently with the RT-PCR results above, PflhA transcription was increased 12- to 15-fold in the three backgrounds not expressing FleN (flhF::miniTn5-Km, ΔfleN and ΔflhF-fleN). In contrast, expression in the ΔflhF mutant was not significantly different from that in KT2442. The β-galactosidase activity levels from the PflhA-gfp-lacZ fusion were also determined in the fliA mutant KT2440fliA::aph-3 and its parent strain, KT2440. Expression in both strains was indistinguishable from that in KT2442. The putative PflhF promoter showed similarly high expression levels in the wild-type KT2442 and KT2440 strains, indicating that a promoter activity is indeed present in the distal region of flhA. The regulatory pattern of PflhF was very similar to that observed with PflhA, but the extent of the regulation was smaller in PflhF. Accordingly, PflhF expression was decreased 2-fold in the ΔfleQ mutant and increased 3- to 4-fold in the flhF::miniTn5-Km, ΔfleN and ΔflhF-fleN strains, relative to the wild-type. No significant differences in β-galactosidase levels were observed with the ΔflhF and fliA mutants. Taken together, these results indicate that both PflhA and PflhF are positively regulated by FleQ and negatively regulated by FleN. PflhA and PflhF expression was also assessed in the double ΔfleQΔfleN mutant derivative of KT2442 MRB101 (Fig 2). Expression was in both cases low and similar to that obtained in a ΔfleQ mutant, suggesting that FleN-dependent regulation operates via FleQ in both promoters. The β-galactosidase activity values and the intensities of the amplified RT-PCR fragments suggest that PflhA is a weaker, more stringently regulated promoter, while PflhF is a stronger, moderately regulated promoter. We propose that PflhF is responsible for most of the transcription of flhF, fleN and fliA, while PflhA drives flhA transcription and has a minor contribution to the expression of the three distal genes. Moreover, it is worth noting that, unlike its P. aeruginosa counterpart [21], P. putida fliA transcription is positively regulated by FleQ. Kim et al. (1995) [49] described the regulation of PflhF, and the presence of additional, σ70-dependent promoters that may be accountable for the high basal levels.
FlhF and FleN are required for fully functional lophotrichous flagellation
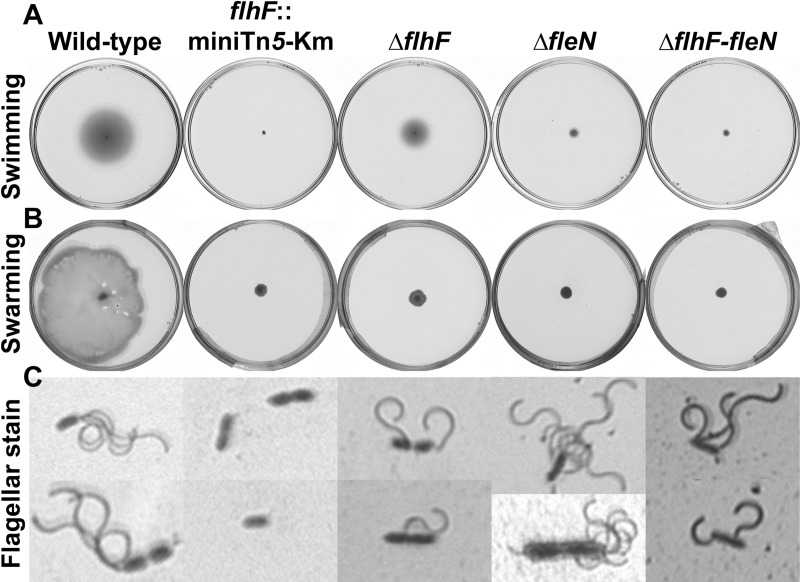
The flhF::miniTn5-Km, Δflh, ΔfleN and ΔflhF-fleN mutants were tested, along with the wild-type strain KT2442, for a variety of phenotypes related to flagellar motility. The flhF::miniTn5-Km mutant was non-flagellated and non-motile, as judged by light microscopy observation of flagellar stains and swimming and swarming motility assays (Fig 2A, 2B and 2C). In contrast, the ΔflhF, ΔfleN and ΔflhF-fleN mutants were flagellated and motile, thus indicating that neither of these two genes is absolutely required for the biogenesis of rotating flagella. However, the deletion mutants showed alterations in flagellar number and location (Fig 3C and S1 Fig). Thus, while the wild-type strain displayed a tuft of 3–4 polar flagella, the ΔflhF mutant cells displayed one or rarely two flagella, often located at a subpolar or lateral position, consistent with a role of FlhF in determining the point of flagellar insertion in one of the cell poles. The ΔfleN mutant displayed a bundle of flagella in a polar position, but the number of flagella (4–7) was on average greater than that in the wild-type. Finally, the ΔflhF-fleN mutant MRB78 showed a combination of the phenotypes of the single deletion mutants, as it displayed several flagella (2–4) inserted at polar, subpolar or lateral positions on the cell surface. All three deletion mutants were defective in swimming motility (Fig 3A): the ΔflhF mutant displayed a 57±1% decrease in the diameter of the swimming halo, and the corresponding values for the ΔfleN and the double ΔflhF-fleN mutant were 82±1% and 89±1%, respectively. Finally, swarming motility was undetectable in all three deletion mutants. These results indicate that even though all three deletion mutants are flagellated and motile, the observed changes in flagellar number and location point likely result in a diminished ability to coordinate flagellar rotation to achieve efficient swimming and swarming.
Fig 3. Flagellar motility of the flhF and fleN mutants.
(A) Swimming motility assays, (B) swarming motility assays and (C) flagellar stain of the wild-type KT2442, the flhF:miniTn5-km mutant MRB49, the ΔflhF mutant MRB69, the ΔfleN mutant MRB71 and the ΔflhF-fleN mutant MRB78 strains. Each picture is a representative one out of at least three biological replicates.
To investigate further the observed motility phenotypes, light microscopy-coupled video imaging was used to assess the near-surface motility patterns of the wild-type, ΔflhF, ΔfleN and ΔflhF-fleN strains (S2 Fig and S1–S4 Movies). Motile cells of the wild-type strain displayed a characteristic swimming pattern in which smooth and fast directional runs alternated with abrupt stops often followed by periods of slower, erratic swimming with frequent changes in direction (S2 Fig and S1 Movie). Long runs were rarely observed in motile cells of the ΔflhF mutant, erratic motility was highly prevalent in this organism, and cells were often propelled or tumble sideways or diagonally (S2 Fig and S2 Movie). Motile cells of the ΔfleN mutant showed faster near-surface motility than wild-type cells. Stops and direction changes were less frequent than in the wild-type, and uninterrupted runs often showed a tendency to bend in the clockwise direction to draw near-circular trajectories (S2 Fig and S3 Movie). The behaviour of the ΔflhF-fleN mutant combined fast long runs with an exacerbated tendency to clockwise-bent trajectories, along with cells displaying a "tumbling" sideways or diagonal motility pattern (S2 Fig and S4 Movie). Taken together, these results indicate that the swimming and swarming defects observed in the ΔflhF, ΔfleN and ΔflhF-fleN mutants are not due an inability of the flagellar apparatus to propel the cells, but rather to poor coordination of the flagellar apparatus that results in unusual motility patterns, such as "tumbling", infrequent stops and direction changes, or curved swimming trajectories.
The phenotypes of the single ΔflhF, ΔfleN or the double ΔflhF-fleN mutants do not suffice to explain the complete lack of flagella and swimming motility observed with the flhF::miniTn5-Km mutant. However, we have shown above that the transposon insertion is polar on fliA transcription, while the deletion mutants display wild-type levels of fliA mRNA (Fig 1). A null fliA mutant derivative of P. putida KT2440 was previously characterized and found to be non-flagellated and non-motile [46]. Accordingly, we propose that the lack of FliA due to transcriptional polarity is the cause of the non-flagellated, non-motile phenotypes of the flhF::miniTn5-Km mutant.
FleN, but not FlhF, is required for normal biofilm formation
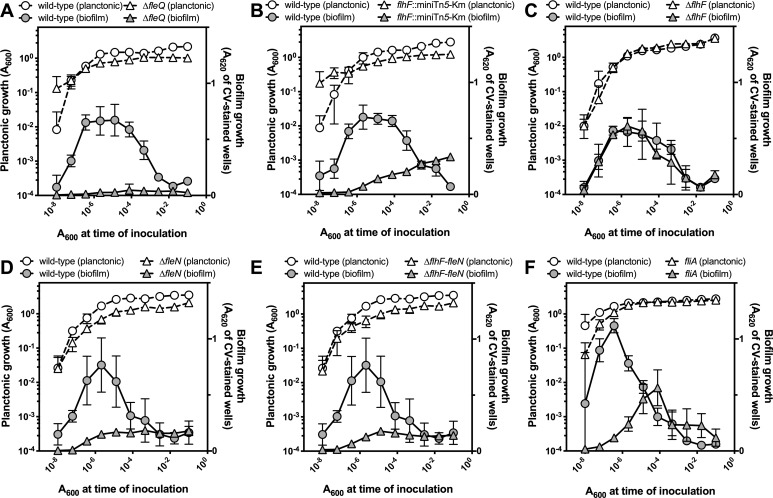
Biofilm formation by the flhF::miniTn5-Km, ΔflhF, ΔfleN and ΔflhF-fleN mutants was assessed by means of serial dilution-based growth curves. This method uses a dilution set and a single incubation time to recapitulate the time-course of planktonic and biofilm growth in microtiter plate wells [50]. The wild-type strain KT2442 and the ΔfleQ mutant MRB52 were used as positive and negative controls, respectively. The ΔfleQ mutant did not display any biofilm formation (Fig 4A), consistent with the phenotype documented for a fleQ::miniTn5-Km insertion mutant [45]. As previously shown [45], the flhF::miniTn5-Km mutant was impaired in biofilm formation (Fig 4B), albeit the phenotype was not so severe as in the ΔfleQ mutant. A similar phenotype was observed with the ΔfleN and ΔflhF-fleN mutants (Fig 4D and 4E), while the ΔflhF mutant displayed a biofilm growth and dispersal pattern similar to that of the wild-type strain (Fig 4B). Since the flhF::miniTn5-Km insertion is also polar on fliA and inactivation of fliA was previously proposed to impair surface attachment and biofilm formation [46], we also assessed planktonic and biofilm growth of KT2440 fliA::aphA-3, a fliA mutant derivative P. putida KT2442 parent strain, KT2440. As expected, the wild-type control, KT2440, displayed a planktonic and biofilm growth behavior similar to that of KT2442. The fliA mutant showed delayed biofilm formation and dispersal, and somewhat decreased levels of biofilm biomass (Fig 4E), a phenotype resembling that observed in a plethora of flagella-defective mutants [45], but clearly distinct and less severe than those displayed by the flhF::miniTn5-Km, ΔfleN and ΔflhFfleN mutants. Interestingly, the single common trait of these three mutants is that they do not express fleN. Therefore, these results strongly suggest that FleN is required for biofilm formation, while FlhF and FliA are not major players in the regulation of biofilm development.
Fig 4. Serial dilution-based growth curves of flhF, fleN and fliA mutants.
Planktonic (left axes, open symbols) or biofilm growth (right axes, closed symbols) is plotted against the initial A600 of each dilution. Circles represent the wild-type strain and squares represent the ΔfleQ mutant MRB52 (A), the flhF:miniTn5-km mutant MRB49 (B), the ΔflhF mutant MRB69 (C) the ΔfleN mutant MRB71 (D), the ΔflhF-fleN mutant MRB78 (E) or the fliA- mutant KT2440 fliA::aphA-3 (F). Plots display one representative experiment of at least three biological replicates. Error bars represent the standard deviation of the six technical replicates.
We also assessed the impact of these mutations on surface adhesion by means of a simple attachment assay. Cells of the wild-type strain and the flhF::miniTn5-Km, ΔflhF, ΔfleN and ΔflhF-fleN mutants were incubated for three hours on the surface of polystyrene microtiter plate wells, the plates were placed under phase-contrast microscopy and attachment was determined by means of pairs of micrographs taken with one minute time interval. Attachment was evaluated from the brownian motility of the cells and their ability to form microcolonies (Fig 5).
Fig 5. Microscopy adhesion assay of flhF and fleN mutants.
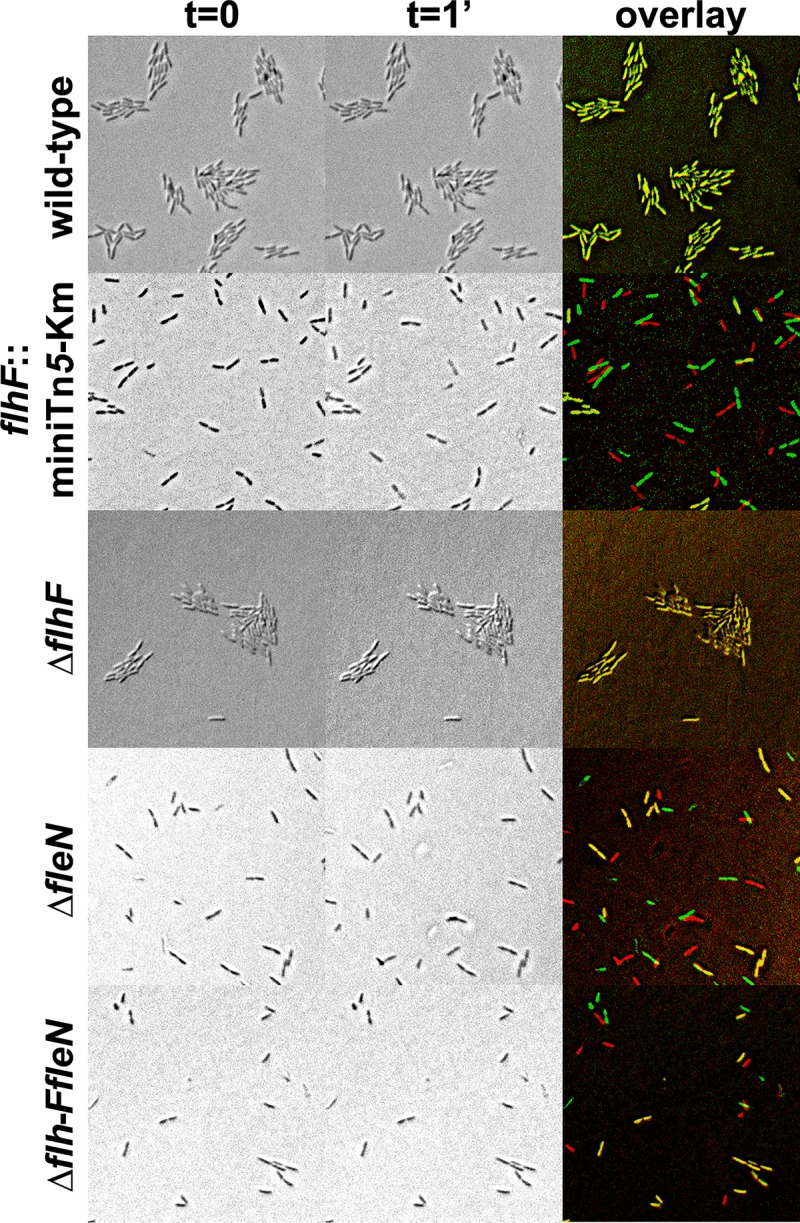
Phase-contrast micrographs of (from top to bottom) the wild-type strain KT2442, the flhF:miniTn5-km mutant MRB49, the ΔflhF mutant MRB69, the ΔfleN mutant MRB71 and the ΔflhF-fleN mutant MRB78. Two frames of the same field were taken in a 1-minute interval (left and center). The right panel shows an overlay of the two images of each strain digitally colored red (t = 0) or green (t = 1 min).
Analysis of the microscopic images confirmed that the wild-type strain attaches strongly to the substrate. Attached cells were immobilized on the surface and formed microcolonies, likely due to successive divisions of substrate-attached cells. Similarly, the ΔflhF mutant also displayed immobilized cells in microcolonies, suggesting that the lack of FlhF does not impair surface attachment. In contrast, the strains not producing FleN (i.e., the flhF::miniTn5-Km, ΔfleN and ΔflhF-fleN mutants), incubated under the same conditions displayed a mixture of moving and immobilized cells that were seldom grouped in microcolonies. These results suggest that the lack of FleN impairs permanent attachment required for stable surface colonization.
FleN regulates the synthesis of biofilm matrix components LapA and cellulose
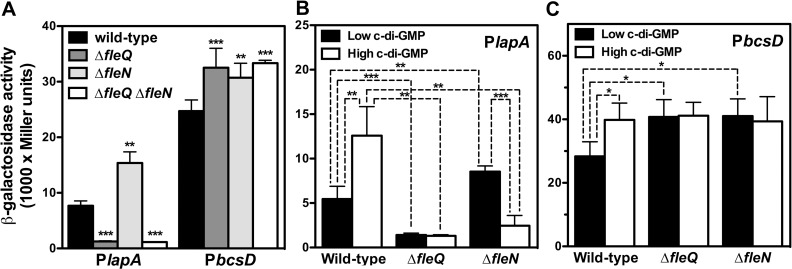
Expression of lapA, encoding the high molecular weight adhesin LapA is positively regulated by FleQ [13,23,51]. Similarly, the bcs operon, encoding the cellulose synthesis and export cellulose synthase complex, was recently shown to be negatively regulated by FleQ [23,51]. Since mutants devoid of FleN are defective in biofilm formation, and FleN acts as an auxiliary factor to FleQ regulation in P. aeruginosa [43,44], we questioned whether FleN may also influence the expression of the PlapA and PbcsD promoters. To test this hypothesis, we transferred the PlapA-gfp-lacZ and PbcsD-gfp-lacZ fusion plasmids pMRB67 and pMRB112 to the wild-type strain KT2442 and its ΔfleQ, ΔfleN and ΔfleQ ΔfleN derivatives MRB52, MRB71 and MRB101 by mating, and expression was assessed by means of β-galactosidase assays (Fig 6A).
Fig 6. Expression of the PlapA and PbcsD promoters in P. putida.
β-galactosidase assays of the PlapA and PbcsD promoter fusions in the wild-type KT2442, ΔfleQ (MRB52), ΔfleN (MRB71) and ΔfleQΔfleN (MRB101) backgrounds (A). PlapA (B) and PbcsD (C) promoter fusions in the wild-type KT2442, ΔfleQ (MRB52), ΔfleN (MRB71) strains bearing the YhjH-producing plasmid pMRB89 (low c-di-GMP) or the YedQ- producing plasmid pYedQ (high c-di-GMP). Bars represent the averages and standard deviations of three independent assays. Stars designate p-values for the Student's t-test for unpaired samples not assuming equal variance. *: p<0.05, **: p<0.01; ***: p<0.001.
As previously described [23], PlapA expression was decreased (6-fold) in the absence of FleQ. In contrast, the β-galactosidase levels were increased 2-fold in the ΔfleN background. The ΔfleQΔfleN mutant displayed low expression levels, similar to those in the ΔfleQ background. These results are consistent with the notion that, similarly to the observations with the PflhA and PflhF promoters, FleN antagonizes FleQ activation of PlapA. PbcsD expression was modestly but significantly increased (1.3- to 1.4-fold) in the ΔfleQ, ΔfleN and ΔfleQ ΔfleN backgrounds. These results indicate that FleQ and FleN are both negative regulators of the bcs operon.
The second messenger c-di-GMP was recently shown to modulate expression of both lapA and the bcs cluster [23,51]. In P. aeruginosa, FleQ activity is regulated by c-di-GMP levels and FleN is proposed to modulate the response of FleQ to c-di-GMP [43]. To test the possible interaction of the two regulatory factors with this second messenger, the wild-type, ΔfleQ and ΔfleN strains bearing the PlapA-gfp-lacZ and PbcsD-gfp-lacZ fusion plasmids were electroporated with plasmids pMRB89, overexpressing the E. coli PDE YhjH, and pYedQ, overexpressing the E. coli DGC YedQ, which provoke c-di-GMP depletion and overproduction, respectively, and the resulting strains were assayed for β-galactosidase activity as above.
The expression patterns of the PlapA and PbcsD promoters under the c-di-GMP depletion regime were similar to those observed in the strains with physiological c-di-GMP levels: PlapA was 4-fold downregulated in the ΔfleQ strain and slightly (1.6-fold) upregulated in the ΔfleN strain (Fig 6B), while PbcsD was 1.4-fold upregulated in both mutant backgrounds (Fig 6C). These results suggest that relatively low intracellular c-di-GMP levels are naturally present in our strains under our experimental conditions. On the other hand, c-di-GMP overproduction resulted in increased expression of PlapA and PbcsA, indicating that c-di-GMP acts as an inducer on both promoters. In these conditions, PlapA was downregulated 10- and 5-fold, respectively, in the ΔfleQ and ΔfleN backgrounds, indicating that, while FleQ is an activator of the PlapA promoter under all c-di-GMP regimes, the presence of high c-di-GMP levels causes FleN to switch from antagonizing to promoting FleQ-dependent activation. In these conditions, downregulation of the PbcsD promoter in ΔfleQ and ΔfleN backgrounds was not observed, thus indicating that c-di-GMP acts as an inducer of PbcsD by releasing FleQ- and FleN-dependent repression.
FleN and c-di-GMP modulate the interaction of FleQ with PlapA and PbcsD
To advance in the understanding of how FleN influences the expression of the PlapA and PbcsD promoters, P. putida KT2440 FleN was overproduced heterologously in E. coli NCM631 as a N-terminal CBD-intein-FleN fusion, purified by chitin affinity chromatography, and the tag was subsequently self-cleaved to release the native FleN protein. Purity of the FleN preparation was estimated to be ~60% (S3 Fig), and ATPase activity was 2,74 ± 0,54 nmol phosphate-1 / h-1 nmol FleN-1. This value is similar to that measured for Vibrio alginolyticus FlhG and ~10-fold lower than that found in P. aeruginosa FleN [39,43]
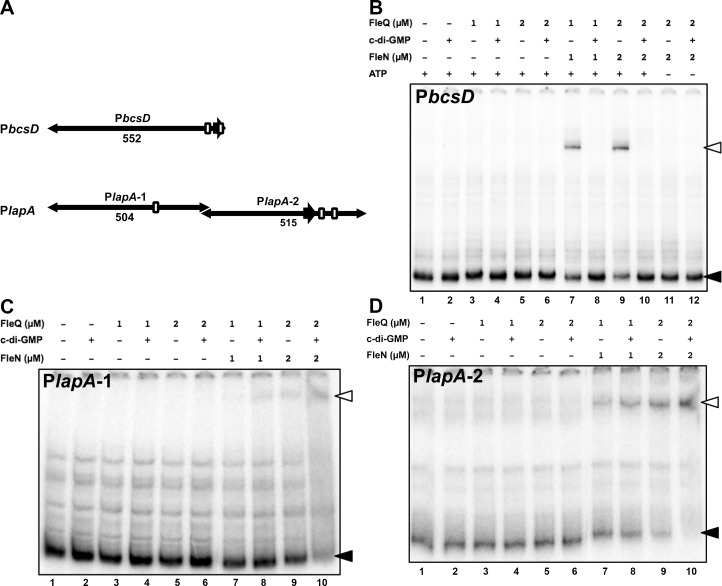
Interaction of FleQ and FleN with the PbcsD and PlapA promoter regions was also assessed by means of gel mobility shift assays. To this end, three PCR products were amplified, radiolabeled and used as probes (Fig 7A): a fragment containing PbcsD sequences from -571 to -21 (relative to the start codon)(PbcsD probe), a fragment containing PlapA sequences from -997 to -494 (PlapA-1 probe), and a fragment containing PlapA sequences from -513 to +2 (PlapA-2 probe). The PbcsD probe is predicted to contain two FleQ binding motifs, while the PlapA-1 and PlapA-2 probes are predicted to contain one and two FleQ binding motifs, respectively [23,52] (S4 Fig). Binding reactions were performed in which each protein was added separately at concentrations of 1 or 2 μM, or together at equimolar concentrations in a binding buffer containing 50 μM ATP. In these conditions FleQ alone did not detectably retard any of the three probes (Fig 7B–7D and S5 Fig), and neither did FleN alone (S5 Fig). In contrast, the equimolar mixture of FleQ and FleN resulted in a retarded band in all three probes that was intensified as protein concentration was increased from 1 to 2 μM (Fig 7B–7D). The significantly slower relative migration rates compared with those observed previously with FleQ alone [23,51] suggests that these complexes indeed contain both proteins. We have shown above (Fig 6) that c-di-GMP acts an antagonist for FleQ repression of PbcsD, but as an inducer of FleQ activation at PlapA. Addition of c-di-GMP alone or to mixtures containing only FleQ did not alter the mobility of the probes (lanes 2, 4 and 6 in Fig 7B–7D). In contrast, a clear effect was observed upon addition of c-di-GMP to reactions containing both FleQ and FleN (lanes 8 and 10 in Fig 7B–7D), as the mobility shift of the PbcsD probe was completely abolished, while the retarded bands corresponding to the PlapA-1 and PlapA-2 probes were intensified. Finally, the effect of ATP on FleQ-FleN binding was assessed on the PbcsD promoter. In the absence of ATP, the retarded band caused by the simultaneous presence of FleQ and FleN was not observed, regardless of the presence of c-di-GMP (lanes 11 and 12 in Fig 7B). Taken together, our in vitro results strongly suggest that (i) FleQ directly regulates lapA and the bcs operon transcription by interacting with their promoter regions; (ii) FleQ interacts with at least one binding site at the PbcsD promoter region and at least two sites at the PlapA promoter region; (iii) FleQ binding to PbcsD and PlapA is stimulated by FleN, in a process that requires ATP (at least for PbcsD); and (iv) c-di-GMP acts differentially by antagonizing FleQ-FleN binding to PbcsD and stimulating FleQ-FleN binding to PlapA.
Fig 7. Gel mobility shift assays on the PlapA and PbcsD promoters.
Panel A: Cartoon of the probes used for each of the promoter regions, indicating the sizes (in bp) of the resulting fragments and the location of the predicted FleQ binding motifs (open boxes). Drawn to scale. Panels B, C and D: Autoradiograph of a representative PAGE gel containing the indicated probe, 0, 1 or 2 μM FleQ and 0, 1 or 2 μM FleN. Assays were performed in the absence (-) or in the presence (+) of c-di-GMP and in the absence (-) or in the presence (+) of ATP. Closed arrowheads denote the free DNA probes and open arrowheads denote the retarded complexes.
A single FleQ binding site is critical to FleQ activation of lapA transcription
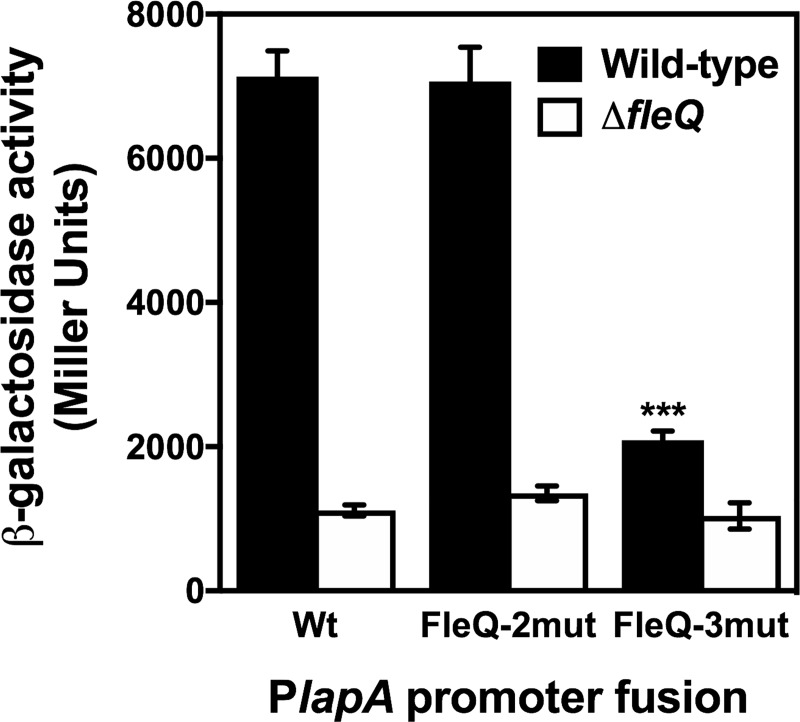
To assess the relevance of the putative FleQ binding sites at the PlapA promoter region, we focused on the FleQ-2 and FleQ-3 sites, located in the vicinity of PlapA3, the main promoter of the six directing lapA transcription [52] (S2 Fig). To this end, we performed site-directed mutagenesis on the conserved residues of the FleQ-2 and FleQ-3 motifs, according to the current FleQ binding site consensus [53] (S3 Fig). Fragments containing the resulting mutant FleQ-2mut and FleQ-3mut sites were transferred to pMRB2 to yield the mutant PlapA-gfp-lacZ reporter plasmids pMRB80 and pMRB81, respectively. These constructs, along with the wild-type fusion plasmid pMRB67, were transferred to the wild-type and ΔfleQ strains, and PlapA expression was assessed from β-galactosidase assays (Fig 8).
Fig 8. Effect of FleQ binding sites mutation on PlapA expression.
β-galactosidase assays of the PlapA promoter fusion and FleQ-2 and FleQ-3 mutated promoters fusions in the wild-type strain KT2442 and ΔfleQ mutant (MRB52). Bars represent the averages and standard deviations of three independent assays. Stars designate p-values for the Student's t-test for unpaired samples not assuming equal variance. *: p<0.05, **: p<0.01; ***: p<0.001.
Expression of the wild-type PlapA promoter region was high in the wild-type background, and was decreased 6-fold in the absence of FleQ, as shown above. A similar behavior was also observed with the fusion bearing the FleQ-2mut mutant motif, suggesting that, despite its proximity to the PlapA3 promoter, this site is not highly relevant to lapA transcriptional regulation. In contrast, introduction of the FleQ-3mut mutation provoked a >3-fold decrease in expression in the wild-type background, while the β-galactosidase levels were essentially unaltered in the ΔfleQ mutant. Still, a residual 2-fold FleQ-dependent activation was observed. These results strongly suggest that FleQ-2 is a bona fide FleQ binding site whose integrity is critical to FleQ activation of LapA transcription, although low level residual FleQ-dependent regulation may be supported by other sites.
Discussion
The transcriptional organization of the flhA-flhF-fleN-fliA region was previously unresolved, as two separate studies proposed the transcriptional units flhA and flhFfleNfliA [47] or flhAF and fleN-fliA [46]. However, our RT-PCR results showing evidence of transcription across all three intergenic regions indicate that all four genes are co-transcribed as part of an operon (Fig 1). Furthermore, we have provided evidence that in addition to the main promoter located upstream from flhA, an internal promoter located within the flhA coding region and designated PflhF directs transcription of the three distal genes (Fig 2). While FleQ-independent transcription of fliA has been documented in P. aeruginosa [21], our RT-PCR results (Fig 1), along with the fact that a miniTn5-Km insertion in flhF results in a nonmotile, non-flagellated phenotype, while deletion of flhF, fleN or both does not prevent motility (Fig 3) strongly suggest that fliA transcription in P. putida is strictly dependent on the upstream PflhA and PflhF promoters and the phenotype of the insertion mutant is due to transcriptional polarity on fliA. The fact that readthrough transcription was detected at the fliA-cheY intergenic region in the wild-type, ΔflhF, ΔfleN and ΔflhF-fleN strains but not in the flhF::miniTn5-Km mutant indicates that transcription from PflhA and PflhF extends beyond fliA into cheY. This is in sharp contrast with the previous suggestion that cheY transcription is FliA-dependent [46]. We note however that such evidence was obtained from transcriptomics analysis performed with a fliA::Km insertion mutant, and therefore the lack of cheY transcription may be explained by transcriptional polarity of the insertion, as shown above for the flhF::miniTn5-Km mutant. In this regard, bioinformatics analysis failed to reveal significant matches to the FliA-RNA polymerase binding consensus at the fliA-cheY intergenic region, and a transcriptional fusion encompassing 263 bp of proximal cheY sequence, the 106 bp intergenic region and 289 bp of distal fliA sequence failed to reveal any significant FliA-regulated transcription (Antonio Leal-Morales, unpublished results). Given the short length of the subsequent cheY-cheZ (11 bp), cheZ-cheA (21 bp), cheA-cheB (47 bp), cheB-motC (0 bp) and motC-motD (2 bp) intergenic regions and the lack of predicted terminator or promoter sequences in them, we consider it likely that the PflhA- and PflhF-initiated transcriptional units extend across this region to encompass these six additional chemotaxis and flagellar motor genes.
Expression analysis revealed that the PflhA and PflhF are positively regulated by FleQ and negatively regulated by FleN, while FliA has no effect on either of the promoters (Fig 2). Epistasis analysis strongly suggests that FleN regulation is dependent on FleQ function, consistent with the proposed role of FleN as an antagonist for FleQ-dependent activation [43,44]. PflhA was previously shown to be a σN-dependent Class II promoter [23]. A σN-dependent promoter was previously described upstream from flhF in P. putida ATCC 12633 [47]. Although we cannot formally exclude that FleQ- and FleN-dependent regulation of the PflhF promoter is indirect, we consider it likely that PflhF is also a Class II promoter. Consistently with our observations, FleQ binding to genomic regions consistent with the location of the PflhA and PflhF promoters described here [54]. It is worth noting that PflhF displays considerable FleQ-independent basal expression (Fig 2). This is consistent with the presence of additional weak σ70-dependent promoters in this region, as previously shown [47]. As noted above, fliA lacks a promoter of its own, and should therefore be considered a Class II flagellar gene, unlike the Class I classification in P. aeruginosa [41]. FleQ-dependent regulation of FliA transcription has been documented in Legionella pneumophila and Pseudomonas syringae pv tomato [55,56].
While regulated flagellar location involving 'landmark' proteins has been documented for multiple bacterial species showing all types of flagellation patterns, a stochastic nucleation pattern leading to random flagellar localization has been proposed for E. coli and Salmonella [42,57]. In a P. putida ΔflhF mutant, the polar tuft of 3–4 flagella found in the wild-type was replaced by 1–2 single, randomly located flagella (Fig 3C). These results confirm a role for FlhF in determining the polar location of flagella, as shown previously in P. putida [47] and other organisms [32–37]. We propose that, in the absence of the 'landmark' protein FlhF, P. putida is still able to assemble flagella, albeit less efficiently (hence the small number of flagella) by means of stochastic nucleation, leading to randomly located flagella that still retain partial functionality. As a likely consequence of this aberrant flagellation pattern, smooth swimming was sometimes substituted by rolling diagonally or perpendicularly to the cell's long axis, a pattern that was exacerbated when flhF and fleN deletions were combined (S1 Fig, S1–S4 Movies). This motility behavior is reminiscent of tumbling motility typical of Listeria, a genus of Gram-positive bacteria bearing 1 to 5 peritrichous flagella [58]. Interestingly, the ΔflhF mutant displayed a relatively modest defect in swimming but was unable to swarm (Fig 3B and 3C), suggesting that polar flagellation is not a requisite for flagellar motility, but it is required for coordination of cell populations during swarming.
Similarly, we have confirmed a role of FleN in determining the number of flagella in P. putida. Strains lacking FleN displayed more flagella (Fig 3C) and faster swimming than their FleN+ counterparts, with smooth, rarely interrupted trajectories that often bent clockwise. Interestingly, changes in the number of flagella did not significantly influence the swimming speed and frequency of tumbles in E. coli [57,59], while longer run times with high directional persistence correlated with smaller numbers of flagella in Bacillus subtilis [60]. The contrast between these results and our observation suggest that the increase of swimming speed with the flagellar numbers may be a trait specific of polarly flagellated bacteria. On the other hand, bent swimming trajectories in flagellar motility are the result of a hydrodynamic effect due to wall-induced torque occurring in the presence of a nearby solid surface [61]. P. putida flagella can propel the cells in the forward (pushing) or in the backwards direction (pulling). These two forms of motility are distinguished by their tendency to clockwise- or counterclockwise-bent trajectories, respectively [62]. Accordingly, our results suggest that fleN inactivation results in a bias towards forward (pushing) motility, that may result in the observed defects in the swimming and swarming assays.
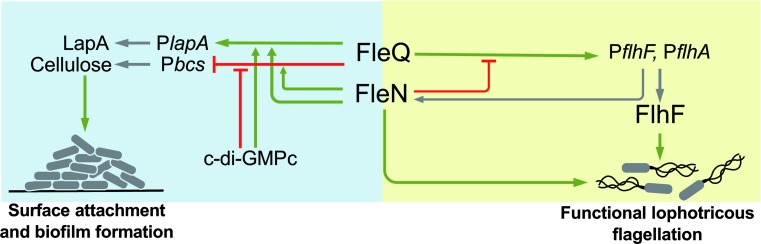
Our results position FleN as a major player in the transition between the planktonic and sessile lifestyles (Fig 9). Firstly, FleN antagonizes FleQ-dependent activation of the PflhA and PflhF promoters of the flhAFfleNfliA operon, encoding the regulatory elements relevant to flagellar biogenesis FlhF, FleN and FliA, as well as the flagellar secretion protein FlhA (Fig 2); secondly, FleN is required for normal surface attachment and biofilm formation (Fig 5); and finally, FleN is directly involved, along with FleQ and c-di-GMP, in the transcriptional regulation of the PlapA promoter, driving the synthesis of a high molecular weight adhesin, and the PbcsD promoter, responsible for transcription of the bcs cluster, encoding the cellulose synthase complex (Fig 6A).
Fig 9. Model of the role of FleN in the planktonic-to-biofilm lifestyle switch.
Green arrows denote positive effects. Capped red lines denote negative effects. Grey arrows connect promoters with end-products.
We have found that FleN acts synergistically with FleQ to regulate transcription of lapA and the bcs cluster. In the case of the PbcsD promoter, FleN promotes FleQ repression in vivo at low c-di-GMP concentrations (Fig 6C). Such repression is released by an increase of intracellular c-di-GMP levels. Accordingly, FleN promotes FleQ interaction with the PbcsD promoter region in vitro and c-di-GMP antagonizes such effect (Fig 7B). Similar binding assays performed with a different PbcsD promoter fragment were recently reported [25], with equivalent results. Remarkably, the fragments used in both assays have an overlap region of only 41 bp (-61 to -21) relative to the annotated start codon. This region contains a putative FleQ binding site centered at position -34.5, matching 12 out of the 14 positions of the proposed consensus [53]. A putative σ70-type promoter overlaps this motif, thus providing a sound rationale for PbcsD promoter repression by blocking access of RNA polymerase to the promoter region [63,64]. A second, less conserved putative FleQ binding site is centered at position -74.5 and may contribute to the observed FleQ binding and repression observed here and previously [25,53], but further evidence is lacking.
Our results revealed that FleN acts synergistically with FleQ in the activation of lapA transcription in vivo at high c-di-GMP levels, but limits FleQ-dependent activation at a low c-di-GMP regime. Interestingly, c-di-GMP stimulated lapA transcription in the presence of FleN, but antagonized FleQ activation in its absence (Fig 6B). FleQ binds in vitro to two different PlapA fragments. In both cases, FleN stimulated FleQ-DNA interaction, which was increased further by the addition of c-di-GMP (Fig 7C and 7D). Similar results were recently obtained with PlapA probes largely overlapping the PlapA-2 fragment shown here [25]. Analysis of the interaction of FleQ with FleN and c-di-GMP at the PlapA promoter region and its functional consequences is complicated by the presence of six functional lapA promoters, up to four putative FleQ binding sites and six binding sites for an additional transcription factor, Fis, which is also a positive regulator of lapA transcription [52]. Our PlapA-2 probe contains two putative FleQ binding sites, as well as four promoters, including the strong PlapA3 promoter [52,53], suggesting that this region is likely relevant to the regulation. While our site-directed mutagenesis results revealed that a single site (FleQ-2) is critical for most of the FleQ-dependent regulation observed (Fig 8), the presence of multiple, closely clustered FleQ binding sites may be relevant to the ability of FleN to modulate the effect of c-di-GMP on FleQ-dependent activation, as previously observed in the regulation of the P. aeruginosa pel promoter [65]. On the other hand, our PlapA-2 probe contains two weak lapA promoters and a putative FleQ binding motif. This fragment is located upstream from the fragments recently assessed by Nie et al. (2017) [25], and therefore FleQ-DNA interaction in this region was not previously documented. The similar behavior in the interaction of FleQ with FleN and c-di-GMP in the two promoter fragments suggests a complex coordinated regulatory mechanism involving several FleQ binding sites and multiple promoters may be involved in the regulation of lapA transcription.
Materials and methods
Bacterial strains and growth conditions
Bacterial strains used in this work are summarized in Table 1. Planktonic cultures of E. coli and P. putida strains were routinely grown in Luria-Bertani (LB) broth [66] at 37°C and 30°C, respectively, with 180 rpm shaking. For solid media, Bacto-Agar (Difco) was added to a final concentration of 18 g l-1. Antibiotics and other additions were used, when required, at the following concentrations: ampicillin (100 mg l-1), carbenicillin (0.5 g l-1), kanamycin (25 mg l-1), rifampicin (10 mg l-1), chloramphenicol (15 mg l-1), gentamycin (10 mg l-1), tetracycline (5 mg l-1), 5-bromo-4-chloro-3-indoyl-β-D-galactopyranoside (X-gal) (25 mg l-1) and sodium salicylate (2 mM). All reagents were purchased from Sigma-Aldrich, except for X- Gal, which was purchased from Fermentas.
Table 1. Bacterial strains, plasmids and oligonucleotides used in this work.
Underlined bases indicate oligonucleotide positions that differ from the corresponding templates.
| Bacterial strain | Genotype/phenotype | Reference/source |
| E. coli | ||
| DH5α | Ψ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17 (rk- mk+) supE44 thi-1 gyrA relA1 | [71] |
| P. putida | ||
| KT2440 | mt-2 hsdR1 (r- m+) | [72] |
| KT2442 | mt-2 hsdR1 (r- m+) Rifr | [72] |
| KT2440 fliA::aphA-3 | KT2440 fliA::Km. Kmr | [46] |
| MRB49 | KT2442 flhF::miniTn5-Km. Rifr Kmr | [45] |
| MRB52 | KT2442 ΔfleQ | This work |
| MRB69 | KT2442 ΔflhF | This work |
| MRB71 | KT2442 ΔfleN | This work |
| MRB78 | KT2442 ΔflhF-fleN | This work |
| MRB101 | KT2442 ΔfleN ΔfleQ | This work |
| Plasmid | Genotype/phenotype | Reference/source |
| pENTR/D-TOPO | Vector for directional TOPO cloning. Kmr | Thermo Fisher Scientific |
| pEX18-Tc | Gene replacement vector with MCS from pUC18. Tcr Sacs | [73] |
| pMPO284 | pPS854-derived vector containing pUTminiTn5Km Kmr gene flanked by the FRT sites, Apr Kmr | [26] |
| pMRB1 | pBBR1-MCS4-derived broad host-range gfpmut3::lacZ transcriptional fusion vector. Apr | [26] |
| pMRB2 | pBBR1-MCS4-derived vector containing the Gateway conversion cassette attR1-Cmr-ccdB-attR2. Apr Cmr | [26] |
| pMRB3 | pBBR1-MCS4-derived vector containing the Gateway conversion cassette attR1-Cmr-ccdB-attR2. Apr Cmr | [23] |
| pMRB67 | pMRB2-derived vector containing a PlapA-gfpmut3-lacZ transcriptional fusion. Apr | [23] |
| pMRB89 | Expression vector for E. coli YhjH containing nahR-Psal-TnasF-yhjH. Strr | [26] |
| pMRB95 | pTYB12-based vector for FleQ overproduction. Apr | This work |
| pMRB96 | pEX18-Tc with Kmr gene flanked by FRT sites cloned between fleQ flanking regions. Tcr Kmr Sacs | This work |
| pMRB104 | pEX18-Tc with Kmr gene flanked by FRT sites cloned between flhF flanking regions. Tcr Kmr Sacs | This work |
| pMRB105 | pEX18-Tc with Kmr gene flanked by FRT sites cloned between fleN flanking regions. Tcr Kmr Sacs | This work |
| pMRB112 | pMRB2-derived vector containing a PbcsD-gfpmut3-lacZ transcriptional fusion. Apr | [23] |
| pMRB115 | pMRB3-derived vector containing a PflhA-gfpmut3-lacZ transcriptional fusion. Apr | [23] |
| pMRB132 | pTYB12-based vector for FleN overproduction. Apr | This work |
| pMRB144 | pEX18-Tc with Kmr gene flanked by FRT sites cloned between flhF-fleN flanking regions. Tcr Kmr Sacs | This work |
| pMRB158 | pMRB3-derived vector containing a PflhF-gfpmut3-lacZ transcriptional fusion. Apr | This work |
| pRK2013 | Helper plasmid for triparental mating. ColE1 replicon. Kmr | [74] |
| pTYB12 | Expression vector for N-terminal Intein–chitin-binding domain protein fusions. Apr | New England Biolabs |
| pYedQ | pRK404A-derived plasmid expressing E. coli YedQ from the Plac promoter. Tcr | [75]. |
| Oligonucleotide | Sequence (5’ to 3’) | |
| flhF-up-Fwd | ACCCGAATTCCGAACTGGCGATCAACCC | |
| flhF-up-Rev | CGCAGGATCCGCATTATCCCCTACCTCA | |
| flhF-down-Fwd | CGGCGGATCCGTTGACCATGAAGCGTGT | |
| flhF-down-Rev | TGCAAAGCTTGCTCGACAAAGAACTCCA | |
| fleN-up-Fwd | CCTGGAATTCCTGCTGGAAGTGCAACTC | |
| fleN-up-Rev | TGCAGGATCCCCATGTCTGTTCTTTACC | |
| fleN-down-Fwd | GGACGGATCCTATGAACGCCAGCGGCTT | |
| fleN-down-Rev: | TTTTAAGCTTCTTGTCCAATTAGACCTC | |
| flhA-RT-fwd | TCTTCACGTTCAACATCGCC | |
| flhA-RT-rev | TCCATCGAGCCGTAGAACTC | |
| Pint_FlhA_Fwd | CACCCTCTGAAGACATGGGCAAG | |
| Pint_FlhA_Rev2 | CACGGACCAGTTTCATGGCC | |
| flhA-flhF-RT-fwd | CTTCTGGAACCTAGCATGGC | |
| flhA-flhF-RT-rev | AATGCGCGTGTGGGTCTT | |
| flhF-miniTn5DST2-fwd | CATGCAACTGGAAACCTTGG | |
| flhF-miniTn5DST2-rev | GCCGTGGGTTGTGATAGAGA | |
| flhF-fleN-RT-fwd | GAGCCTTGCCATCAGTCATG | |
| flhF-fleN-RT-rev | CAGCAACACGTCGACATTGG | |
| fleN-fliA-RT-fwd | GACCGCTTCCTTGACGTTG | |
| fleN-fliA-RT-rev | CGTCGTATTTGTTGGCCACT | |
| fliA-cheY-RT-fwd | CAAGGAAATCGGTGAGGTGC | |
| fliA-cheY-RT-rev | CCGCTTCAATGATCTGGTCG | |
| PflhF-fwd | CACCCTCTGAAGACATGGGCAAG | |
| PflhF-rev | CACGGACCAGTTTCATGGCC | |
| fleN-fwd-NdeI | AACACATATGGGTAGCATGCATCCC | |
| fleN-rev-EcoRI | CCGCGAATTCTCATAGTACGGGTCCCGC | |
| PlapA1-fwd-HindIII | TGACAAGCTTCGACTGACTTCGGATTCC | |
| PlapA1-rev | AGCTATGTGTCGCAAATCAA | |
| PlapA2-fwd | TTGATTTGCGACACATAGCT | |
| PlapA2-rev-BamHI | GCCCGGATCCATTGGACTCTCCGTGTGACC | |
| PbcsD-fwd-HindIII | CCCAAGCTTGTTGATCGCCAGCACCTGG | |
| PbcsD-rev | GACTCATGTCAAAAAAACGACAAAAATGA | |
| lapA-qRT-fwd | AGCATTGTCGGCCAGGTTATT | |
| lapA-qRT-rev | TCGATAAGTACACGCCGGATG | |
| bcsD-qRT-fwd | CATTCCGCCTTCAAACCGTTT | |
| bcsD-qRT-rev | GGAAATCGCCAACAACTGCAT |
Plasmid and strain construction
Plasmids and oligonucleotides used in this work are summarized in Table 1. All DNA manipulations were performed following standard protocols [66]. Restriction and modification enzymes were used according to the manufacturers' instructions (Fermentas, Roche and NEB). When required, blunt ends were generated using the Klenow fragment or T4 DNA polymerase. E. coli DH5α was used as a host in cloning procedures. All cloning steps involving PCR were verified by commercial sequencing (Secugen). Plasmid DNA was transferred to E. coli and P. putida strains by transformation [67], triparental mating [68] or electroporation [69]. Site-specific integration of miniTn7 derivatives in P. putida strains was performed essentially as described [70].
To construct a P. putida KT2442 derivative with an in-frame deletion of the flhF gene (MRB69), 811 bp and 894 bp from the upstream and downstream chromosomal regions flanking flhF were PCR-amplified with oligonucleotide pairs FlhF-up-Fw/FlhF-up-Rev (upstream region) and FleF-dw-Fwd/FlhF-dw-Rev (downstream region). The PCR products were cleaved with EcoRI and BamHI or BamHI and HindIII, respectively, and three-way ligated into EcoRI- and HindIII- digested pEX18Tc. A BamHI-excised FRT-flanked kanamycin resistance cassette from pMPO284 was then cloned into the BamHI site, yielding pMRB104. This plasmid was transferred to P. putida KT2442 by electroporation. Selection of integration, allelic replacement and FLP-mediated excision of the kanamycin resistance marker was performed essentially as described [73,76]. This strategy led to the complete deletion of flhF minus its start and stop codons and its replacement with a short ORF spanning the start codon, the single FRT scar and the stop codon. ΔfleQ, ΔfleN and ΔflhF-fleN mutants were generated using an equivalent approach. Plasmids containing 800–900 pb upstream and downstream regions flanking the target genes and the FRT-flanked kanamycin resistance cassette were constructed. These plasmids, named pMRB96, pMPRB105 and pMRB144, were transferred by electroporation to KT2442 to generate MRB52 (ΔfleQ), MRB71 (ΔfleN) and MRB78 (ΔflhF-fleN). A ΔfleQΔfleN mutant (MRB101) was also constructed by using pMRB105 to electroporate MRB53 (ΔfleQ). The structure of the deleted loci was verified by PCR and Southern blot.
For the construction of pMRB132, a plasmid overproducing a chitin-binding domain (CBD)- intein-FleN fusion for protein purification, the fleN open reading frame was PCR amplified with FleN_Fwd_NdeI/FleN_Rev_EcoRI primers and cloned into NdeI- and EcoRI-cleaved pTYB12 (New England Biolabs).
A 1381 pb fragment containing the putative PflhF promoter region (positions -1323 pb to +57 relative to the flhF start codon) was amplified using oligonucleotides Pint_FlhA_Fwd and Pint_FlhA_Rev as primers, and inserted in the directional TOPO cloning vector pENTRTM/D-TOPO. This region was subsequently transferred using the Gateway recombination technology (Thermo Fisher Scientific) into the Gateway gfpmut3-lacZ fusion vector pMRB2, to produce pMRB158.
Biofilm growth and quantification
For most procedures involving biofilm growth, overnight cultures grown in LB or K10T-1 broth [77] were diluted in the same medium to an A600 of 0.1 and 150 μl were dispensed into wells of Costar 96 microtiter polystyrene plates (Corning). The plates were incubated at 25°C with moderate shaking (150 rpm) for the desired period of time and processed for planktonic and biofilm growth quantification, essentially as described [78]. Serial dilution-based growth curves were performed as described [45]. For each experiment, at least 3 biological replicates were assayed in octuplicate.
For pellicle and aggregate detection, fresh colonies were inoculated in glass tubes containing 5 ml of K10T-1 broth. Cultures were incubated overnight at 30°C with shaking, after which the tubes were placed on a rack for 10 minutes and documented by digital photography.
Flagellar motility (swimming and swarming) assays
Swimming assays were adapted from [79]. Tryptone motility plates containing 0.3% Bacto-agar (Difco) were toothpick-inoculated with fresh colonies and incubated for 12 h at 30°C. Digital photographs were taken, and the swimming zone diameter was measured and normalized that of the wild-type. At least 3 biological replicates were assayed for each strain.
Swarming assays were performed essentially as described by [80], with some modifications. Two and a half microliters of overnight LB cultures were spotted onto the center of plates containing 0.5% PG-agar [proteose peptone No. 3 (Difco 212693), 0.5%, and glucose, 0.2%, with Difco Bacto-Agar] and plates were incubated at 25ºC for 40 hours. At least two biological replicates were assayed in three separate experiments.
Microscopy techniques
Phase contrast microscopy of surface adhesion was performed on a Leica DMI4000B inverted microscope using 20x objective and 1.6x ocular magnification. Cells from mid-exponential (A600 = 0.2–0.3) LB cultures of the selected P. putida strains were serially diluted (102−104) in LB and samples were transferred to wells of Costar 96 microtiter polystyrene plates (Corning). Attachment was allowed to proceed for 30 minutes, planktonic cells were removed by washing twice with 150 μl LB, and then 50 μl LB was added to the wells. For short-term assessment of surface attachment, plates were incubated at room temperature for 3 hours, and then cells deposited on the plane of the well surface were video recorded for 1 minute.
The Leifson flagellum staining procedure for light microscopy was carried out according to Clark [81], with some modifications. A 1.2% (w/v) solution of basic fuchsine prepared in 95% (v/v) ethanol and left overnight at 25°C was mixed with an equal volume of a solution of 0.75% NaCl (w/v) and 1.5% (w/v) tannic acid prepared in double-deionized water. The final pH of the dye was adjusted with 1 N NaOH to pH 5.0. Swarmer cells were resuspended in 10 mM MgCl2, adjusted to an OD600 of 1.0, and fixed by adding 1 ml of 4% (v/v) formaldehyde solution per ml of culture for 20 min. The suspension then was centrifuged, washed with double-deionized water, and resus- pended in 1 ml of double-deionized water. The slides were cleaned by soaking them for 24 h at room temperature in acid dichromate solution, rinsed with double-deionized water, and then air dried. A large drop of culture suspension was deposited on the centre of the slide and was allowed to run down its length and then air dried. Subsequently, 1 ml of dye solution was added and kept for 40 min, and the slide was washed with tap water, air dried, mounted with Merko-glass, and examined under a Zeiss Axioskop microscope.
β-galactosidase assays
For β-galactosidase assays of lacZ fusions, overnight cultures bearing the corresponding fusion plasmids were diluted in 5 ml LB to an A600 of 0.01 and incubated for 24 hours at 30°C with shaking. Growth was then stopped, and β-galactosidase activity was determined from sodium dodecyl sulfate- and chloroform-permeabilized cells as described [82].
RNA preparation and RT-PCR
Total RNA was prepared from stationary phase cultures essentially as described [83]. Reverse transcription (RT) of total RNA (5 μg) was carried out using the high-capacity cDNA Archive kit (Applied Biosystems), with random hexamers as primers. The primer pairs flhA-RT-fwd/ flhA-RT-rev, flhA-flhF-RT-fwd/ fleN-fliA-RT-fwd, flhF-miniTn5DST2-fwd/flhF-miniTn5DST2-rev, flhF-fleN-RT-fwd/flhF-fleN-RT-rev, fleN-fliA-RT-fwd /fleN-fliA-RT-rev and fliA-cheY-RT-fwd/fliA-cheY-RT-rev was used to amplify fragments of 457 pb, 452 pb, 359 pb, 454 pb, 432 pb and 496 pb, respectively. Reactions were performed with 50 ng cDNA as template in a 25-cycle PCR program. Negative and positive controls were performed with total RNA or genomic DNA as templates, respectively. The RT-PCR products obtained were resolved by 1% agarose gel electrophoresis and visualized by ethidium bromide staining.
Protein purification and gel mobility shift assays
FleQ and FleN were overproduced from the T7 promoter as a N-terminal intein-CBD fusion from the overproducing strain NCM631 harbouring pMRB95 or pMRB132 and pIZ227 by using the IMPACT kit from NEB (#E6901S Beverly, MA) as previously described for FleQ [23]. A summary of FleN overproduction an purification procedure is shown in S2 Fig. Protein concentration was calculated using the Protein Assay kit from Bio-Rad, according to the manufacturer’s protocol, and process efficiency and purity was estimated by SDS-PAGE.
Gel mobility shift assays were performed essentially as described [44]. The PlapA-1, PlapA-2 and PbcsD fragments were PCR amplified from genomic DNA and the products were cleaved with HindIII and gel purified. DNA fragments were labelled by filling in 5′ over-hanging ends using the Klenow fragment in a reaction mixture containing [α-32P]-dCTP. Unincorporated nucleotides were removed using the MSB Spin PCRapace kit (Invitek). FleQ-DNA complexes were formed at room temperature for 30 min in 10 μl reaction containing 10 ng/μl labelled-probe, 0.2 μg/μl poly-dI-dC and increasing amounts of purified FleQ in binding buffer (10 mM Tris-HCl [pH 7.8], 50 mM KCl, 8 mM magnesium acetate, 50 ng/l BSA, 5% glycerol). When required, 50 μM ATP, 300 μM c-di-GMP or different concentrations of FleN were added to the binding reactions. Reactions were stopped with 3 μl of loading buffer (0.125% w/v bromophenol blue, 0.125% w/v xylene cyanol, 10 mM Tris HCl (pH 8), 1 mM EDTA, 30% glycerol) and samples were separated on a 5% polyacrylamide native gel in TGE buffer (10 mM Tris [pH 8.8], 380 mM glycine, 1 mM EDTA) at 4°C. Dried gels were exposed on a phosphoscreen and were visualized with a Typhoon 9410 scanner and analysed using the ImageQuant software (Amersham).
ATPase activity
FleN ATPase activity was assayed by measuring the inorganic phosphate production using the Enzchek Phosphate Assay Kit (Molecular Probes). Reactions were set up essentially as previously described [43]. FleN was added to the reaction mixtures at 1.3 μM and incubated for 30 min at room temperature before 2 mM ATP was added. A360 was measured after 3 h and used to calculate the specific activity.
Supporting information
Flagellar stain of the wild-type KT2442, the ΔflhF mutant MRB69, the ΔfleN mutant MRB71 and the ΔflhF-fleN mutant MRB78 strains.
(PDF)
Five cells from each video sequence of the wild-type, ΔflhF, ΔfleN and ΔflhF-fleN strains were monitored over time. Tracks were generated using the MtrackJ plugin of ImageJ.
(PDF)
Lane 1: uninduced overproducing strain, whole cells; lane 2: induced overproducing strain, whole cells; lane 3: induced overproducing strain, clarified soluble extract; lane 4: chitin affinity resin flow-through; lane 5: wash buffer eluate; lane 6: chitin-bound protein prior to cleavage; lane 7: DTT wash solution prior to incubation; lane 8: chitin-bound protein after cleavage; lane 9: eluted protein after DTT incubation. M: molecular weight marker (sizes in kDa)
(PDF)
(PDF)
Panels A, B and C: Autoradiograph of a representative PAGE gel containing the indicated probe, FleQ and/or FleN at the indicated concentrations. Assays were performed in the absence (-) or in the presence (+) of c-di-GMP and in the absence (-) or in the presence (+) of ATP. Closed arrowheads denote the free DNA probes and open arrowheads denote the retarded complexes.
(PDF)
(MOV)
(MOV)
(MOV)
(MOV)
Acknowledgments
We wish to thank María Trinidad Gallego for valuable help in the microscopy procedures. Tim Tolker-Nielsen (University of Copenhagen, Denmark) and Victoria Shingler (University of Umeå, Sweden) for providing plasmids and strains, Maribel Ramírez de Verger, María G. Velasco and Ana Hernández for technical assistance and all members of the Govantes and Santero laboratories at CABD for providing materials and critical discussion.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by Ministerio de Ciencia e Innovación, Spain (http://www.idi.mineco. gob.es/) and European Regional Development fund (http://ec.europa.eu/regional_policy/en/funding/erdf/), Grant BIO2010-17853, awarded to FG; Ministerio de Economía y Competitividad, Spain (http://www.idi.mineco.gob.es/) and European Regional Development fund (http://ec.europa.eu/regional_policy/en/funding/erdf/), Grant BIO2013-420173-P, awarded to FG; and Consejo Superior de Investigaciones Científicas, Spain (http://www.csic.es/), JAE-Predoc 2010 scholarship, awarded to AJ-F. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial Biofilms. Annu Rev Microbiol. 1995;49: 711–745. 10.1146/annurev.mi.49.100195.003431 [DOI] [PubMed] [Google Scholar]
- 2.Davey ME, O’Toole GA. Microbial Biofilms: from Ecology to Molecular Genetics. Microbiol Mol Biol Rev. 2000;64: 847–867. 10.1128/MMBR.64.4.847-867.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDougald D, Rice SA, Barraud N, Steinberg PD, Kjelleberg S. Should we stay or should we go: Mechanisms and ecological consequences for biofilm dispersal. Nat Rev Microbiol. 2012;10: 39–50. 10.1038/nrmicro2695 [DOI] [PubMed] [Google Scholar]
- 4.Altegoer F, Schuhmacher J, Pausch P, Bange G. From molecular evolution to biobricks and synthetic modules: A lesson by the bacterial flagellum. Biotechnol Genet Eng Rev. 2014;30: 49–64. 10.1080/02648725.2014.921500 [DOI] [PubMed] [Google Scholar]
- 5.Chevance FF V, Hughes KT. Coordinating assembly of a bacterial macromolecular machine. Nat Rev Microbiol. 2008;6: 455–465. 10.1038/nrmicro1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Toole G, Kaplan HB, Kolter R. Biofilm Formation as Microbial Development. Annu Rev Microbiol. 2000;54: 49–79. 10.1146/annurev.micro.54.1.49 [DOI] [PubMed] [Google Scholar]
- 7.Monds RD, O’Toole GA. The developmental model of microbial biofilms: ten years of a paradigm up for review. Trends in Microbiology. 2009. pp. 73–87. 10.1016/j.tim.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 8.Martins Dos Santos VAP, Heim S, Moore ERB, Strätz M, Timmis KN. Insights into the genomic basis of niche specificity of Pseudomonas putida KT2440. Environ Microbiol. 2004;6: 1264–1286. 10.1111/j.1462-2920.2004.00734.x [DOI] [PubMed] [Google Scholar]
- 9.Harwood CS, Fosnaugh K, Dispensa M. Flagellation of Pseudomonas putida and analysis of its motile behavior. J Bacteriol. 1989;171: 4063–4066. 10.1128/jb.171.7.4063-4066.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson KE, Weinel C, Paulsen IT, Dodson RJ, Hilbert H, Martins dos Santos VAP, et al. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ Microbiol. 2002;4: 799–808. 10.1046/j.1462-2920.2002.00366.x [DOI] [PubMed] [Google Scholar]
- 11.Corral-Lugo A, De la Torre J, Matilla MA, Fernández M, Morel B, Espinosa-Urgel M, et al. Assessment of the contribution of chemoreceptor-based signalling to biofilm formation. Environ Microbiol. 2016;18: 3355–3372. 10.1111/1462-2920.13170 [DOI] [PubMed] [Google Scholar]
- 12.Klausen M, Gjermansen M, Kreft JU, Tolker-Nielsen T. Dynamics of development and dispersal in sessile microbial communities: Examples from Pseudomonas aeruginosa and Pseudomonas putida model biofilms. FEMS Microbiology Letters. 2006. pp. 1–11. 10.1111/j.1574-6968.2006.00280.x [DOI] [PubMed] [Google Scholar]
- 13.Martínez-Gil M, Ramos-González MI, Espinosa-Urgel M. Roles of cyclic Di-GMP and the Gac system in transcriptional control of the genes coding for the Pseudomonas putida adhesins LapA and LapF. J Bacteriol. 2014;196: 1484–1495. 10.1128/JB.01287-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camesano TA, Abu-Lail NI. Heterogeneity in bacterial surface polysaccharides, probed on a single-molecule basis. Biomacromolecules. 2002;3: 661–667. 10.1021/bm015648y [DOI] [PubMed] [Google Scholar]
- 15.Chang WS, Halverson LJ. Reduced water availability influences the dynamics, development, and ultrastructural properties of Pseudomonas putida biofilms. J Bacteriol. 2003;185: 6199–6204. 10.1128/JB.185.20.6199-6204.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang WS, Van De Mortel M, Nielsen L, De Guzman GN, Li X, Halverson LJ. Alginate production by Pseudomonas putida creates a hydrated microenvironment and contributes to biofilm architecture and stress tolerance under water-limiting conditions. J Bacteriol. 2007. pp. 8290–8299. 10.1128/JB.00727-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson M, Chiang WC, Fazli M, Gjermansen M, Givskov M, Tolker-Nielsen T. Influence of putative exopolysaccharide genes on Pseudomonas putida KT2440 biofilm stability. Environ Microbiol. 2011;13: 1357–1369. 10.1111/j.1462-2920.2011.02447.x [DOI] [PubMed] [Google Scholar]
- 18.Boyd CD, O’Toole GA. Second Messenger Regulation of Biofilm Formation: Breakthroughs in Understanding c-di-GMP Effector Systems. Annu Rev Cell Dev Biol. 2012;28: 439–462. 10.1146/annurev-cellbio-101011-155705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Römling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 2013;77: 1–52. 10.1128/MMBR.00043-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arora SK, Ritchings BW, Almira EC, Lory S, Ramphal R. A transcriptional activator, FleQ, regulates mucin adhesion and flagellar gene expression in Pseudomonas aeruginosa in a cascade manner. J Bacteriol. 1997;179: 5574–5581. 10.1128/jb.179.17.5574-5581.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dasgupta N, Wolfgang MC, Goodman AL, Arora SK, Jyot J, Lory S, et al. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol Microbiol. 2003;50: 809–824. 10.1046/j.1365-2958.2003.03740.x [DOI] [PubMed] [Google Scholar]
- 22.Smith TG, Hoover TR. Chapter 8 Deciphering bacterial flagellar gene regulatory networks in the genomic Era. Adv Appl Microbiol. 2009. pp. 257–295. 10.1016/S0065-2164(08)01008-3 [DOI] [PubMed] [Google Scholar]
- 23.Jiménez-Fernández A, López-Sánchez A, Jiménez-Díaz L, Navarrete B, Calero P, Platero AI, et al. Complex Interplay between FleQ, cyclic diguanylate and multiple σ factors coordinately regulates flagellar motility and biofilm development in Pseudomonas putida. PLoS One. 2016;11: 1–26. 10.1371/journal.pone.0163142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baraquet C, Harwood CS. FleQ DNA binding consensus sequence revealed by studies of FleQ-dependent regulation of biofilm gene expression in Pseudomonas aeruginosa. J Bacteriol. 2016;198: 178–186. 10.1128/JB.00539-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nie H, Xiao Y, Liu H, He J, Chen W, Huang Q. FleN and FleQ play a synergistic role in regulating lapA and bcs operons in Pseudomonas putida KT2440. Environ Microbiol Rep. 2017;9: 571–580. 10.1111/1758-2229.12547 [DOI] [PubMed] [Google Scholar]
- 26.Jimenez-Fernandez A, Lopez-Sanchez A, Calero P, Govantes F. The c-di-GMP phosphodiesterase BifA regulates biofilm development in Pseudomonas putida. Environ Microbiol Rep. 2015;7: 78–84. 10.1111/1758-2229.12153 [DOI] [PubMed] [Google Scholar]
- 27.Leipe DD, Wolf YI, Koonin E V, Aravind L. Classification and evolution of P-loop GTPases and related ATPases. J Mol Biol. 2002;317: 41–72. 10.1006/jmbi.2001.5378 [DOI] [PubMed] [Google Scholar]
- 28.Kazmierczak BI, Hendrixson DR. Spatial and numerical regulation of flagellar biosynthesis in polarly flagellated bacteria. Mol Microbiol. 2013. pp. 655–663. 10.1111/mmi.12221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bange G, Kümmerer N, Grudnik P, Lindner R, Petzold G, Kressler D, et al. Structural basis for the molecular evolution of SRP-GTPase activation by protein. Nat Struct Mol Biol. 2011;18: 1376–1380. 10.1038/nsmb.2141 [DOI] [PubMed] [Google Scholar]
- 30.Kusumoto A, Kamisaka K, Yakushi T, Terashima H, Shinohara A, Homma M. Regulation of polar flagellar number by the flhF and flhG genes in Vibrio alginolyticus. J Biochem. 2006;139: 113–121. 10.1093/jb/mvj010 [DOI] [PubMed] [Google Scholar]
- 31.Guttenplan SB, Shaw S, Kearns DB. The cell biology of peritrichous flagella in Bacillus subtilis. Mol Microbiol. 2013;87: 211–229. 10.1111/mmi.12103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Correa NE, Peng F, Klose KE. Roles of the regulatory proteins FlhF and FlhG in the Vibrio cholerae flagellar transcription hierarchy. J Bacteriol. 2005;187: 6324–6332. 10.1128/JB.187.18.6324-6332.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray TS, Kazmierczak BI. FlhF Is required for swimming and swarming in Pseudomonas aeruginosa. J Bacteriol. 2006;188: 6995–7004. 10.1128/JB.00790-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green JCD, Kahramanoglou C, Rahman A, Pender AMC, Charbonnel N, Fraser GM. Recruitment of the earliest component of the bacterial flagellum to the old cell division pole by a membrane-associated Signal Recognition Particle family GTP-binding protein. J Mol Biol. 2009;391: 679–690. 10.1016/j.jmb.2009.05.075 [DOI] [PubMed] [Google Scholar]
- 35.Gao T, Shi M, Ju L, Gao H. Investigation into FlhFG reveals distinct features of FlhF in regulating flagellum polarity in Shewanella oneidensis. Mol Microbiol. 2015;98: 571–585. 10.1111/mmi.13141 [DOI] [PubMed] [Google Scholar]
- 36.Balaban M, Joslin SN, Hendrixson DR. FlhF and its GTPase activity are required for distinct processes in flagellar gene regulation and biosynthesis in Campylobacter jejuni. J Bacteriol. 2009;191: 6602–6611. 10.1128/JB.00884-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niehus E, Gressmann H, Ye F, Schlapbach R, Dehio M, Dehio C, et al. Genome-wide analysis of transcriptional hierarchy and feedback regulation in the flagellar system of Helicobacter pylori. Mol Microbiol. 2004;52: 947–961. 10.1111/j.1365-2958.2004.04006.x [DOI] [PubMed] [Google Scholar]
- 38.Ono H, Takashima A, Hirata H, Homma M, Kojima S. The MinD homolog FlhG regulates the synthesis of the single polar flagellum of Vibrio alginolyticus. Mol Microbiol. 2015;98: 130–141. 10.1111/mmi.13109 [DOI] [PubMed] [Google Scholar]
- 39.Gulbronson CJ, Ribardo DA, Balaban M, Knauer C, Bange G, Hendrixson DR. FlhG employs diverse intrinsic domains and influences FlhF GTPase activity to numerically regulate polar flagellar biogenesis in Campylobacter jejuni. Mol Microbiol. 2016;99: 291–306. 10.1111/mmi.13231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dasgupta N, Arora SK, Ramphal R. fleN, a gene that regulates flagellar number in Pseudomonas aeruginosa. J Bacteriol. 2000;182: 357–364. 10.1128/JB.182.2.357-364.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dasgupta N, Ramphal R. Interaction of the antiactivator FleN with the transcriptional activator FleQ regulates flagellar number in Pseudomonas aeruginosa. J Bacteriol. 2001;183: 6636–6644. 10.1128/JB.183.22.6636-6644.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuhmacher JS, Rossmann F, Dempwolff F, Knauer C, Altegoer F, Steinchen W, et al. MinD-like ATPase FlhG effects location and number of bacterial flagella during C-ring assembly. Proc Natl Acad Sci. 2015;112: 3092–7. 10.1073/pnas.1419388112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baraquet C, Harwood CS. Cyclic diguanosine monophosphate represses bacterial flagella synthesis by interacting with the Walker A motif of the enhancer-binding protein FleQ. Proc Natl Acad Sci. 2013;110: 18478–83. 10.1073/pnas.1318972110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hickman JW, Harwood CS. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol Microbiol. 2008;69: 376–389. 10.1111/j.1365-2958.2008.06281.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.López-Sánchez A, Leal-Morales A, Jiménez-Díaz L, Platero AI, Bardallo-Pérez J, Díaz-Romero A, et al. Biofilm formation-defective mutants in Pseudomonas putida. Rumbaugh K, editor. FEMS Microbiol Lett. 2016;363: fnw127 10.1093/femsle/fnw127 [DOI] [PubMed] [Google Scholar]
- 46.Rodríguez-Herva JJ, Duque E, Molina-Henares MA, Navarro-Avilés G, van Dillewijn P, de la Torre J, et al. Physiological and transcriptomic characterization of a fliA mutant of Pseudomonas putida KT2440. Environ Microbiol Rep. 2010;2: 373–380. 10.1111/j.1758-2229.2009.00084.x [DOI] [PubMed] [Google Scholar]
- 47.Pandza S, Baetens M, Park CH, Au T, Keyhan M, Matin A. The G-protein FIhF has a role in polar flagellar placement and general stress response induction in Pseudomonas putida. Mol Microbiol. 2000;36: 414–423. 10.1046/j.1365-2958.2000.01859.x [DOI] [PubMed] [Google Scholar]
- 48.Barrios H, Valderrama B, Morett E. Compilation and analysis of σ54-dependent promoter sequences. Nucleic Acids Res. 1999;27: 4305–4313. 10.1093/nar/27.22.4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim Y, Watrud LS, Matin A. A carbon starvation survival gene of Pseudomonas putida is regulated by σ54. J Bacteriol. 1995;177: 1850–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.López-Sánchez A, Jiménez-Fernández A, Calero P, Gallego LD, Govantes F. New methods for the isolation and characterization of biofilm-persistent mutants in Pseudomonas putida. Environ Microbiol Rep. 2013;5: 679–685. 10.1111/1758-2229.12067 [DOI] [PubMed] [Google Scholar]
- 51.Xiao Y, Nie H, Liu H, Luo X, Chen W, Huang Q. C-di-GMP regulates the expression of LapA and BCS operons via FLeQ in Pseudomonas putida KT2440. Environ Microbiol Rep. 2016;8: 659–666. 10.1111/1758-2229.12419 [DOI] [PubMed] [Google Scholar]
- 52.Ainelo H, Lahesaare A, Teppo A, Kivisaar M, Teras R. The promoter region of lapA and its transcriptional regulation by Fis in Pseudomonas putida. PLoS One. 2017;12: e0185482 10.1371/journal.pone.0185482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molina-Henares MA, Ramos-González MI, Daddaoua A, Fernández-Escamilla AM, Espinosa-Urgel M. FleQ of Pseudomonas putida KT2440 is a multimeric cyclic diguanylate binding protein that differentially regulates expression of biofilm matrix components. Res Microbiol. 2017;168: 36–45. 10.1016/j.resmic.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 54.Blanco-Romero E, Redondo-Nieto M, Martínez-Granero F, Garrido-Sanz D, Ramos-González MI, Martín M, et al. Genome-wide analysis of the FleQ direct regulon in Pseudomonas fluorescens F113 and Pseudomonas putida KT2440. Sci Rep. 2018;8: 13145 10.1038/s41598-018-31371-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schulz T, Rydzewski K, Schunder E, Holland G, Bannert N, Heuner K. FliA expression analysis and influence of the regulatory proteins RpoN, FleQ and FliA on virulence and in vivo fitness in Legionella pneumophila. Arch Microbiol. 2012;194: 977–989. 10.1007/s00203-012-0833-y [DOI] [PubMed] [Google Scholar]
- 56.Nogales J, Vargas P, Farias GA, Olmedilla A, Sanjuán J, Gallegos MT. FleQ coordinates flagellum-dependent and -independent motilities in Pseudomonas syringae pv. tomato DC3000. Appl Environ Microbiol. 2015;81: 7533–7545. 10.1128/AEM.01798-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mears PJ, Koirala S, Rao C V, Golding I, Chemla YR. Escherichia coli swimming is robust against variations in flagellar number. Elife. 2014;2014: e01916 10.7554/eLife.01916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allerberger F. Listeria: Growth, phenotypic differentiation and molecular microbiology. FEMS Immunol Med Microbiol. 2003;35: 183–189. 10.1016/S0928-8244(02)00447-9 [DOI] [PubMed] [Google Scholar]
- 59.Darnton NC, Turner L, Rojevsky S, Berg HC. On torque and tumbling in swimming Escherichia coli. J Bacteriol. 2007;189: 1756–1764. 10.1128/JB.01501-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Najafi J, Shaebani MR, John T, Altegoer F, Bange G, Wagner C. Flagellar number governs bacterial spreading and transport efficiency. Sci Adv. 2018;4: eaar6425 10.1126/sciadv.aar6425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lauga E, Powers TR. The hydrodynamics of swimming microorganisms. Reports Prog Phys. 2009;72: 096601 10.1088/0034-4885/72/9/096601 [DOI] [Google Scholar]
- 62.Hintsche M, Waljor V, Großmann R, Kühn MJ, Thormann KM, Peruani F, et al. A polar bundle of flagella can drive bacterial swimming by pushing, pulling, or coiling around the cell body. Sci Rep. 2017;7: 16771 10.1038/s41598-017-16428-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rojo F. Repression of transcription initiation in bacteria. J Bacteriol. 1999. pp. 2987–2991. Available: http://www.ncbi.nlm.nih.gov/pubmed/10321997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rojo F. Mechanisms of transcriptional repression. Curr Opin Microbiol. 2001;4: 145–151. 10.1016/S1369-5274(00)00180-6 [DOI] [PubMed] [Google Scholar]
- 65.Baraquet C, Murakami K, Parsek MR, Harwood CS. The FleQ protein from Pseudomonas aeruginosa functions as both a repressor and an activator to control gene expression from the Pel operon promoter in response to c-di-GMP. Nucleic Acids Res. Oxford University Press; 2012;40: 7207–7218. 10.1093/nar/gks384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sambrook J, Russell D, Russell D. Molecular Cloning, a Laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- 67.Inoue H, Nojima H, Okayama H. High efficiency transformation of Escherichia coli with plasmids. Gene. 1990;96: 23–28. 10.1016/0378-1119(90)90336-P [DOI] [PubMed] [Google Scholar]
- 68.Espinosa-Urgel M, Salido A, Ramos J-L. Genetic analysis of functions involved in adhesion of Pseudomonas putida to seeds. J Bacteriol. 2000;182: 2363–2369. 10.1128/JB.182.9.2363-2369.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi KH, Kumar A, Schweizer HP. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: Application for DNA fragment transfer between chromosomes and plasmid transformation. J Microbiol Methods. 2006;64: 391–397. 10.1016/j.mimet.2005.06.001 [DOI] [PubMed] [Google Scholar]
- 70.Choi KH, Gaynor JB, White KG, Lopez C, Bosio CM, Karkhoff-Schweizer RAR, et al. A Tn7-based broad-range bacterial cloning and expression system. Nat Methods. 2005;2: 443–448. 10.1038/nmeth765 [DOI] [PubMed] [Google Scholar]
- 71.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166: 557–580. 10.1016/S0022-2836(83)80284-8 [DOI] [PubMed] [Google Scholar]
- 72.Franklin FC, Bagdasarian M, Bagdasarian MM, Timmis KN. Molecular and functional analysis of the TOL plasmid pWWO from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta cleavage pathway. Proc Natl Acad Sci. 1981;78: 7458–7462. 10.1073/pnas.78.12.7458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212: 77–86. 10.1016/S0378-1119(98)00130-9 [DOI] [PubMed] [Google Scholar]
- 74.Figurski DH, Helinski DR. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci. 1979;76: 1648–1652. 10.1073/pnas.76.4.1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ausmees N, Mayer R, Weinhouse H, Volman G, Amikam D, Benziman M, et al. Genetic data indicate that proteins containing the GGDEF domain possess diguanylate cyclase activity. FEMS Microbiol Lett. 2001;204: 163–167. 10.1111/j.1574-6968.2001.tb10880.x [DOI] [PubMed] [Google Scholar]
- 76.Llamas MA, Ramos JL, Rodríguez-Herva JJ. Mutations in each of the tol genes of Pseudomonas putida reveal that they are critical for maintenance of outer membrane stability. J Bacteriol. 2000;182: 4764–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Monds RD, Newell PD, Schwartzman JA, O’Toole GA. Conservation of the Pho regulon in Pseudomonas fluorescens Pf0-1. Appl Environ Microbiol. 2006;72: 1910–1924. 10.1128/AEM.72.3.1910-1924.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O’Toole GA, Pratt LA, Watnick PI, Newman DK, Weaver VB, Kolter R. Genetic approaches to study of biofilms. Methods Enzymol. 1999;310: 91–109. 10.1016/S0076-6879(99)10008-9 [DOI] [PubMed] [Google Scholar]
- 79.Parkinson JS. cheA, cheB, and cheC genes of Escherichia coli and their role in chemotaxis. J Bacteriol. 1976;126: 758–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matilla MA, Ramos JL, Duque E, De Dios Alché J, Espinosa-Urgel M, Ramos-González MI. Temperature and pyoverdine-mediated iron acquisition control surface motility of Pseudomonas putida. Environ Microbiol. 2007;9: 1842–1850. 10.1111/j.1462-2920.2007.01286.x [DOI] [PubMed] [Google Scholar]
- 81.Clark WA. A simplified Leifson flagella stain. J Clin Microbiol. 1976;3: 632–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller J. A Short Course in Bacterial Genetics: a Laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 83.García-González V, Govantes F, Porrúa O, Santero E. Regulation of the Pseudomonas sp. strain ADP cyanuric acid degradation operon. J Bacteriol. 2005;187: 155–167. 10.1128/JB.187.1.155-167.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flagellar stain of the wild-type KT2442, the ΔflhF mutant MRB69, the ΔfleN mutant MRB71 and the ΔflhF-fleN mutant MRB78 strains.
(PDF)
Five cells from each video sequence of the wild-type, ΔflhF, ΔfleN and ΔflhF-fleN strains were monitored over time. Tracks were generated using the MtrackJ plugin of ImageJ.
(PDF)
Lane 1: uninduced overproducing strain, whole cells; lane 2: induced overproducing strain, whole cells; lane 3: induced overproducing strain, clarified soluble extract; lane 4: chitin affinity resin flow-through; lane 5: wash buffer eluate; lane 6: chitin-bound protein prior to cleavage; lane 7: DTT wash solution prior to incubation; lane 8: chitin-bound protein after cleavage; lane 9: eluted protein after DTT incubation. M: molecular weight marker (sizes in kDa)
(PDF)
(PDF)
Panels A, B and C: Autoradiograph of a representative PAGE gel containing the indicated probe, FleQ and/or FleN at the indicated concentrations. Assays were performed in the absence (-) or in the presence (+) of c-di-GMP and in the absence (-) or in the presence (+) of ATP. Closed arrowheads denote the free DNA probes and open arrowheads denote the retarded complexes.
(PDF)
(MOV)
(MOV)
(MOV)
(MOV)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.