Abstract
Aims
Reconstruction of the acetabulum after resection of a periacetabular malignancy is technically challenging and many different techniques have been used with varying success. Our aim was to prepare a systematic review of the literature dealing with these techniques in order to clarify the management, the rate of complications and the outcomes.
Patients and Methods
A search of PubMed and MEDLINE was conducted for English language articles published between January 1990 and February 2017 with combinations of key search terms to identify studies dealing with periacetabular resection with reconstruction in patients with a malignancy. Studies in English that reported radiographic or clinical outcomes were included. Data collected from each study included: the number and type of reconstructions, the pathological diagnosis of the lesions, the mean age and follow-up, gender distribution, implant survivorship, complications, functional outcome, and mortality. The results from individual studies were combined for the general analysis, and then grouped according to the type of reconstruction.
Results
A total of 57 studies met the inclusion criteria and included 1700 patients. Most lesions were metastatic (41%), followed by chondrosarcoma (29%), osteosarcoma (10%), Ewing’s sarcoma (7%), and multiple myeloma (2%). The techniques of reconstruction were divided into seven types for analysis: those involving a Harrington reconstruction, a saddle prosthesis, an allograft and allograft prosthesis composite, a pasteurised autograft, a porous tantalum implant, a custom-made prosthesis and a modular hemipelvic reconstruction. The rate of complications was 50%, with infection (14%) and instability (8%) being the most common. Mortality data were available for 1427 patients (84%); 50% had died of disease progression, 23% were alive with disease, and 27% had no evidence of disease at a mean follow-up of 3.4 years (0 to 34).
Conclusion
Both the rate of complications and mortality are high following resection of oncological periacetabular lesions and reconstruction. Many types of reconstruction have been used with unique challenges and complications for each technique. Newer prostheses, including custom-made prostheses and porous tantalum implants and augments, have shown promising early functional and radiographic outcomes.
Cite this article: Bone Joint J 2018;100-B(1 Supple A):22–30.
Keywords: Arthroplasty, Hip
Periacetabular bony tumours present a difficult clinical challenge. Although most are metastatic,1 they can also be primary malignancies.2,3 Limb salvage surgery, with internal hemipelvectomy and subsequent reconstruction of the hip, has become routine management for most of these lesions given the acceptable rate of local recurrence and the improvement in function.4 While iliofemoral arthrodesis and resection are options,5 most surgeons currently prefer to use reconstructive techniques, of which there are many different types, including Harrington-type reconstruction,6 allograft reconstruction with or without a prosthesis,7 and reconstruction using a pasteurised autograft,8, a saddle-type prosthesis9 a modular endoprosthesis10 a custom-made hemipelvic prosthesis11 or porous tantalum acetabular components.4,12 These cases are challenging due to the large bony defects and soft-tissue loss following resection of the tumour, peri-operative complications and the post-operative treatment of the tumour or its progression. Previous authors have reported high complication rates and marginal functional results with early reconstructive techniques.13
In an effort to understand the burden of the condition and the surgical complications, mid-term component, patient survivorship and functional outcomes of different reconstruction techniques, we aimed to perform a systematic review of the literature on reconstruction of the hip after resection of oncological periacetabular lesions.
Patients and Methods
In March 2017, a search of PubMed and MEDLINE was conducted for English language articles published between January 1990 and February 2017. Combinations of the terms “pathologic”, “metastatic”, “oncologic”, “tumor”, “neoplasm”, “limb salvage”, “hip arthroplasty”, “hip reconstruction”, “acetabular reconstruction”, and “pelvic reconstruction” were used. The reference lists of the resulting articles were reviewed for inclusion. Inclusion criteria were: studies published in English; gender of patients of all ages; human studies; studies that reported radiographic or clinical outcomes; all levels of evidence; and studies reporting clinical outcomes for total hip arthroplasty (THA) in patients with a pathological lesion involving the hip. Exclusion criteria were: studies with patients who had outcomes included in an earlier study; non-clinical studies and studies that involved pathological lesions that were diagnosed following THA. All studies were reviewed by two authors (TSB, CGS).
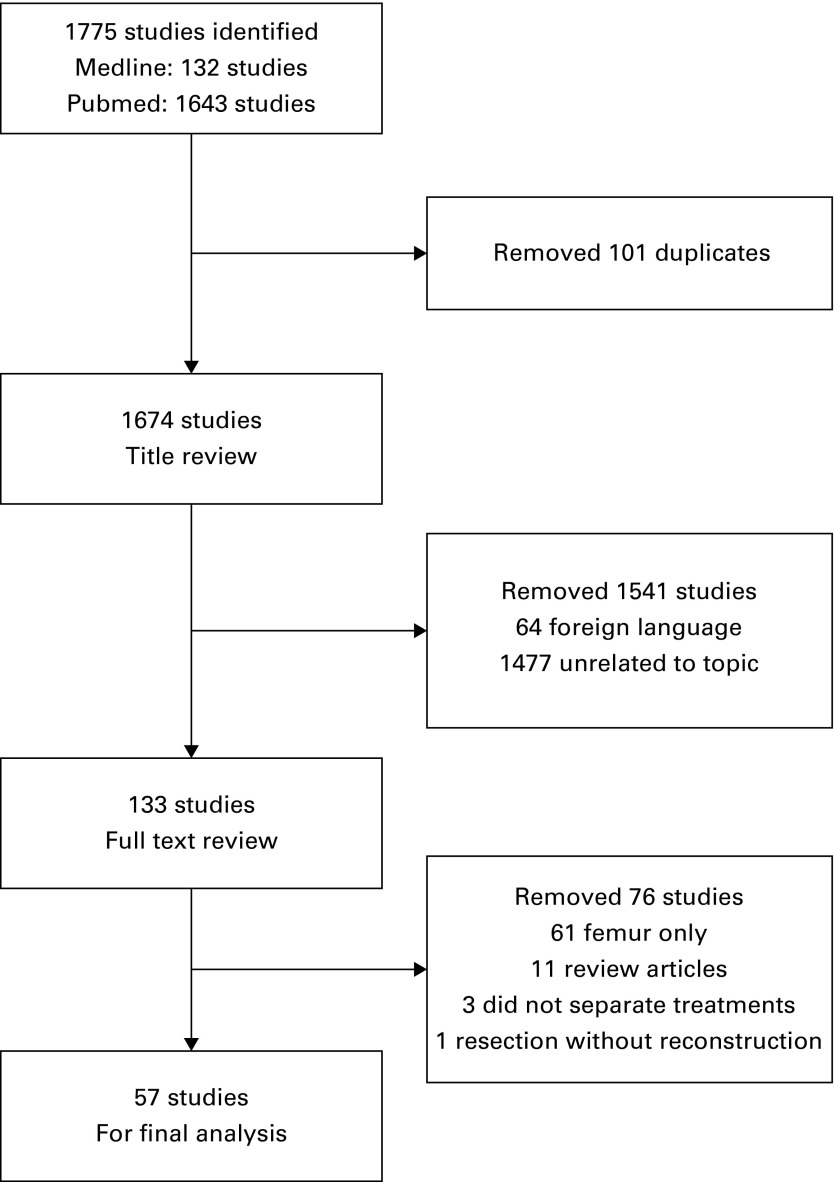
The initial search yielded 1674 studies (Fig. 1). Following review of their titles, the full text of 133 were studied. After removing review articles, those dealing with femoral reconstruction for malignancy, those that involved resection alone as treatment and articles that did not separate outcomes based on type of reconstruction, 57 studies remained for the final analyses (Fig. 1).1,4-12,14-60
Fig. 1.
Flow chart showing search strategy for study identification.
The data were collected after a review of the design, the number of patients and risk of bias within each study. All were retrospective case series. The following data were collected from each study: the number and type of reconstruction, the pathology of the lesions, the mean age of the patients and range, the gender of patients, the mean follow-up and range, implant survivorship, the incidence and types of complication, the functional outcome scores and mortality. The results from the individual studies were combined as a whole for the analysis. Then, after grouping the studies according to the type of reconstruction, the demographic, clinical and functional data were combined for each type. Although mortality data can be a reflection of the oncological diagnosis and initial resection rather than the type of reconstruction, these data were included in the discussion of each technique. Musculoskeletal Tumor Society (MSTS) functional scores61were used in most studies and were therefore used in this review to combine and compare functional outcomes.
Results
In the 57 studies reviewed, a total of 1700 reconstructions were performed. The mean age at the time of reconstruction was 49 years (5 to 92). There were 829 male patients (49%). The pathology was osseous metastatic disease in 700 (41%), chondrosarcoma in 487 (29%), osteosarcoma in 169 (10%), Ewing’s sarcoma in 111 (7%), multiple myeloma in 26 (2%), and other or not specified in 207 (11%) (Table I).
Table I.
Clinical characteristics of the patients
| Characteristic | |
|---|---|
| Papers, n | 57 |
| Reconstructions, n | 1700 |
| Mean age in yrs, n (range) | 49 (5 to 92) |
| Male patients, n (%) | 829 (49) |
| Diagnosis, n (%) | |
| Metastatic disease | 700 (41) |
| Chondrosarcoma | 487 (29) |
| Osteosarcoma | 169 (10) |
| Ewing’s sarcoma | 111 (7) |
| Multiple myeloma | 26 (2) |
| Other, not specified | 207 (11) |
Data about mortality were available for 1427 reconstructions (84%). At a mean follow-up of 3.4 years (0 to 34), 710 (50%) had died of progression of disease (DOD), 326 (23%) were alive with evidence of disease (AWD), and 391 (27%) had no evidence of disease (NED) (Table II). When isolating mortality data for reconstruction following resection of a metastatic lesion, 347 (76.3%) were DOD, 107 (23.5%) were AWD, and only one (0.2%) had NED (Table II).
Table II.
Patient mortality and complications following reconstruction
| Patients, n | 1427 |
| All patients, n (%) | |
| DOD | 710 (50) |
| AWD | 326 (23) |
| NED | 391 (27) |
| Metastatic disease, n (%) | |
| DOD | 347 (76.3) |
| AWD | 107 (23.5) |
| NED | 1 (0.2) |
| Reconstructions, n | 1700 |
| Overall complications (n, %) | 857 (50) |
| Deep infection | 228 (14) |
| Dislocation | 144 (8) |
| Wound necrosis, delayed healing | 105 (6) |
| Local recurrence | 101 (6) |
| Nerve palsy | 52 (3) |
| Thrombotic complication | 35 (2) |
DOD, dead of disease; AWD, alive with disease; NED, no evidence of disease
There were 857 complications (50%) in the 1700 reconstructions. Deep prosthetic joint infection was the most common, occurring in 228 patients (14%). Dislocation occurred in 144 patients (8%). Problems with wound healing occurred in 105 patients (6%), nerve palsy in 52 (3%) and thrombosis in 32 (2%) (Table II).
A Harrington-type reconstruction62 was used in 14 studies, involving 415 patients.6,15,18,21,32,36,39,41,46,53,55-58 All were performed for metastatic disease. The mean age was 62 years (15 to 92). There were 189 male patients (46%). The mean follow-up was 1.4 years (0 to 9.3). The mean MSTS functional score was 61% in the surviving patients at the final follow-up, at which time 288 patients (69%) were DOD and 89 (21%) were AWD. Dislocation was the most common complication, occurring in 35 patients (8%), followed by deep infection occurring in 22 (5%), aseptic loosening in nine (2%), periprosthetic fracture in six (1%), thrombosis in five (1%), and nerve palsy in five (1%) (Table III).
Table III.
Harrington reconstruction
| Studies, n | 14 |
| Reconstructions, n | 415 |
| Metastatic disease, n (%) | 415 (100) |
| Mean age in yrs, n (range) | 61.5 (27 to 92) |
| Male patients, n (%) | 189 (45.5) |
| Mean follow-up in yrs, n (range) | 1.4 (0 to 9.3) |
| MSTS score at final follow-up, % | 61 |
| Patient mortality, n (%) | |
| AWD | 89 (21) |
| DOD | 288 (69) |
| Complications, n (%) | |
| Dislocation | 35 (8) |
| Infection | 22 (5) |
| Aseptic loosening | 9 (2) |
| Fracture | 6 (1) |
| Thrombotic complication | 5 (1) |
| Nerve palsy | 5 (1) |
MSTS; Muscoskeletal Tumor Society; AWD, alive with disease; DOD, dead of disease
A saddle prosthesis was used in eight studies, involving 135 patients.9,23,25,29-31,43,48 Most, 103 (76%), were for a primary malignancy. The mean age was 53 years (17 to 79). There were 78 male patients (58%). The mean follow-up was 4.6 years (0.1 to 16.8). The mean MSTS functional score was 51% in the surviving patients at the final follow-up, at which time 64 patients (47%) were DOD, 43 (32%) had NED, and 11 (8%) were AWD. Deep infection was the most common complication, occurring in 32 patients (24%). Dislocation occurred in 22 patients (16%), nerve palsy in ten (7%), prosthetic dissociation or failure in eight (6%), periprosthetic fracture in eight (6%), thrombosis in seven (5%), and problems with wound healing in four (3%) (Table IV).
Table IV.
Results of the saddle prosthesis
| Studies, n | 8 |
| Reconstructions, n | 135 |
| Metastatic disease, n (%) | 32 (24) |
| Primary malignancy, n (%) | 103 (76) |
| Mean age in yrs, n (range) | 53 (17 to 79) |
| Male patients, n (%) | 78 (58) |
| Mean follow-up in yrs, n (range) | 4.6 (0.1 to 16.8) |
| MSTS score at final follow-up, % | 51 |
| Patient mortality, n (%) | |
| AWD | 11 (8) |
| NED | 43 (32) |
| DOD | 64 (47) |
| Complications, n (%) | |
| Infection | 32 (24) |
| Dislocation | 22 (16) |
| Nerve palsy | 10 (7) |
| Prosthetic dissociation/failure | 8 (6) |
| Fracture | 8 (6) |
| Thrombotic complication | 7 (5) |
| Wound necrosis | 4 (3) |
MSTS, Muscoskeletal Tumor Society; AWD, alive with disease; NED, no evidence of disease; DOD, dead of disease
Allograft and allograft prosthesis composite reconstructions were used in nine studies, involving 133 patients.7,14,19,22,24,26,43,50,54 Most, 123 (93%), were for a primary malignancy. The mean age was 42 years (5 to 71). There were 84 male patients (63%). The mean follow-up was 5.8 years (0.8 to 19). The mean MSTS functional score in the surviving patients at final follow-up was 72%. At this time, 37 patients (28%) were DOD, 55 (41%) had NED and eight (6%) were AWD. Deep infection was the most common complication, occurring in 20 patients (15%). Local recurrence occurred in 15 (11%), dislocation in ten (8%), problems with wound healing in seven (5%), prosthetic migration or failure in six (5%), and late allograft fracture in five (4%) (Table V).
Table V.
Results of allograft reconstruction
| Studies, n | 9 |
| Reconstructions, n | 133 |
| Metastatic disease, n (%) | 10 (7) |
| Primary malignancy, n (%) | 123 (93) |
| Mean age in yrs, n (range) | 42 (5 to 71) |
| Male patients, n (%) | 63 (63) |
| Mean follow-up in yrs, n (range) | 5.8 (0.8 to 19) |
| MSTS score at final follow-up, % | 72 |
| Patient mortality, n (%) | |
| AWD | 8 (6) |
| NED | 55 (41.4) |
| DOD | 37 (27.8) |
| Complications, n (%) | |
| Infection | 20 (15) |
| Local recurrence | 15 (11) |
| Dislocation | 10 (8) |
| Wound necrosis | 7 (5) |
| Prosthesis migration/failure | 6 (5) |
| Fracture | 5 (4) |
MSTS, Muskoskeletal Tumor Society; AWD, alive with disease; NED, no evidence of disease; DOD, dead of disease
Autograft reconstructions were used in five studies, involving 54 patients.8,14,27,33,60 In this technique, the resected hemipelvis is pasteurised with heat to sterilise the malignancy and re-implanted with plates, screws, and prostheses. Most, 47 (87%), were for a primary malignancy. The mean age was 41 years (13 to 65). There were 27 male patients (50%). The mean follow-up was 5.2 years (0.7 to 11.8). The mean MSTS functional score in the surviving patients at final follow-up was 72%, at which time 18 patients (33%) were DOD, 30 (56%) had NED and six (11%) were AWD. Dislocation was the most common complication, occurring in eight patients (15%). Deep infection occurred in five (13%), aseptic loosening in four (10%), late graft fracture in four (10%), local recurrence in three (6%) and problems with wound healing in two (5%) (Table VI).
Table VI.
Results of autograft reconstruction
| Studies, n | 5 |
| Reconstructions, n | 54 |
| Metastatic disease, n (%) | 7 (13) |
| Primary malignancy, n (%) | 47 (87) |
| Mean age in yrs, n (range) | 41 (13 to 65) |
| Male patients, n (%) | 27 (50) |
| Mean follow-up in yrs, n (range) | 5.2 (0.7 to 11.8) |
| MSTS score at final follow-up, % | 72 |
| Patient mortality, n (%) | |
| AWD | 6 (11) |
| NED | 30 (56) |
| DOD | 18 (33) |
| Complications, n (%) | |
| Dislocation | 8 (15) |
| Infection | 5 (13) |
| Aseptic loosening | 4 (10) |
| Late graft fracture | 4 (10) |
| Local recurrence | 3 (6) |
| Wound healing | 2 (5) |
MTSTS; Muscoskeletal Tumor Scoety; AWD, alive with disease; NED, no evidence of disease; DOD, dead of disease
Porous tantalum implants were used in two recent studies, involving 30 patients.4,12 Most, 22 (73%), were for a primary malignancy. The mean age was 58 years (22 to 80). There were six male patients of the ten reported by gender (60%). The mean follow-up was 4.7 years (0.7 to 9.4), at which time the mean Harris Hip Score63 in the surviving patients was 74. At final follow-up, one patient (3%) had DOD and 21 (70%) were AWD. Implant survivorship free from all-cause revision was 100%. Dislocation was the most common complication occurring in four patients (13%). Superficial infection occurred in two (7%), thrombosis in two (7%), bleeding complications in two (7%), and problems with wound healing in one (3%) (Table VII).
Table VII.
Results of porous tantalum reconstruction
| Studies, n | 2 |
| Reconstructions, n | 30 |
| Metastatic disease, n (%) | 8 (27) |
| Primary malignancy, n (%) | 22 (73) |
| Mean age in yrs, n (range) | 58 (22 to 80) |
| Male patients, n (%) | 6 (60) |
| Mean follow-up in yrs, n (range) | 4.7 (0.7 to 9.4) |
| Harris Hip Score at final follow-up | 74 |
| Patient mortality | |
| AWD, n (%) | 21 (70) |
| DOD, n (%) | 1 (3) |
| Implant survivorship from all-cause revision at final follow-up, % | 100 |
| Complications, n (%) | |
| Dislocation | 4 (13) |
| Superficial Infection | 2 (7) |
| Thrombotic complication | 2 (7) |
| Bleeding complication | 2 (7) |
| Wound healing | 1 (3) |
AWD, alive with disease; DOD, dead of disease
Custom endoprostheses were used in five studies, involving 182 patients.11,17,34,35,40 Most, 156 (86%), were for a primary malignancy. The mean age was 42 years (10 to 81). There were 87 male patients (48%). The mean follow-up was 4.4 years (0.2 to 33.5). The mean MSTS functional score in the surviving patients at final follow up was 63%. Implant survivorship from all-cause revision was 61% at final follow-up. Deep infection was the most common complication, occurring in 42 patients (23%). Local recurrence occurred in 40 (22%) and dislocation in 31 patients (17%) (Table VIII).
Table VIII.
Results of custom prostheses reconstruction and modular endoprosthesis reconstruction
| Custom prostheses reconstruction | |
| Studies, n | 5 |
| Reconstructions, n | 182 |
| Metastatic disease, n (%) | 26 (14) |
| Primary malignancy, n (%) | 156 (86) |
| Mean age in yrs, n (range) | 42 (10 to 81) |
| Male patients, n (%) | 88 (48) |
| Mean follow-up in yrs, n (range) | 4.4 (0.2 to 33.5) |
| MSTS score, % | 63 |
| Patient mortality, n (%) | |
| AWD | 75 (41) |
| DOD | 63 (35) |
| NED | 31 (19) |
| Implant survivorship from all-cause revision at final follow-up, % | 61 |
| Complications, n (%) | |
| Infection | 41 (23) |
| Local recurrence | 40 (22) |
| Dislocation | 31 (17) |
| Thrombotic complication | 11 (6) |
| Wound healing | 10 (5) |
| Nerve palsy | 6 (3) |
| Aseptic loosening | 2 (1) |
| Modular endoprosthesis reconstruction | |
| Studies, n | 5 |
| Reconstructions, n | 143 |
| Metastatic disease, n (%) | 31 (22) |
| Primary malignancy, n (%) | 112 (78) |
| Mean age in yrs, n (range) | 47 (12 to 81) |
| Male patients, n (%) | 84 (59) |
| Mean follow-up in yrs, n (range) | 5 (0.8 to 23.8) |
| MSTS score, % | 64 |
| Patient mortality, n (%) | |
| AWD | 50 (35) |
| DOD | 28 (20) |
| NED | 31 (22) |
| Implant survivorship from all-cause revision at final follow-up, % | 69 |
| Complications, n (%) | |
| Infection | 34 (24) |
| Dislocation | 18 (13) |
| Recurrence | 16 (11) |
| Aseptic loosening | 6 (4) |
| Wound healing | 3 (2) |
MSTS, Musculoskeletal Tumor Society; AWD, alive with disease; DOD, dead of disease; NED, no evidence of disease
Modular hemipelvis reconstructions were used in five studies, involving 143 reconstructions.1,10,38,49,59 Most, 112 (78%), were for a primary malignancy. The mean age was 47 years (12 to 81). There were 84 male patients (59%). The mean follow-up was five years (0.8 to 23.8). The mean MSTS functional score in the surviving patients at final follow-up was 69%, at which time, 28 patients (20%) were DOD, 50 (35%) were AWD and 31 (22%) had NED. Once again, deep infection was the most common complication, occurring in 34 patients (24%). Dislocation occurred in 18 (13%), local recurrence in 16 (11%), aseptic loosening in six (4%) and problems with wound healing in three (2%) (Table VIII).
Discussion
Periacetabular oncological lesions may present in various areas of the acetabulum and be of varying size. Many types of reconstruction are available for their treatment. Our aim in this systematic review was to present the largest and most comprehensive review of acetabular reconstruction after resection of these lesions, in order to aid their management and to clarify the clinical outcomes.
Foremost, we found that most periacetabular lesions were from metastatic disease (41%), followed by chondrosarcoma (29%), osteosarcoma (10%), and Ewing’s sarcoma (7%). The mean age of the patients, of 49 years, was younger than that of most patients who undergo THA, median age 69 years.64
Importantly, this review provides a historical perspective on reconstructive techniques that may no longer be frequently encountered. One technique, the Harrington-type reconstruction,62 has been used exclusively in metastatic disease for palliative pain relief, whereas all other techniques are predominately used for primary malignancies. While the overall rate of complications in all the studies was 50%, deep infection (14%) and dislocation (8%) were the most common complications. Further developments should focus on decreasing these complications and this might involve the use of vancomycin powder65 and dual-mobility constructs,42 respectively.
Henderson et al66 described the mechanisms of failure of endoprostheses as being either mechanical, due to soft-tissue failure, aseptic loosening or structural failure, or non-mechanical due to infection or progression of disease. While it was difficult to separate complications from revisions in this review, future studies should strive to describe implant survivorship using these guidelines.
There are limitations to this study. Foremost, the data presented are retrospective. Moreover, the demographic data and pre-operative diagnoses were heterogenous which had an impact on the rates of complication and long-term outcomes.
Reconstruction after resection of an oncological periacetabular lesion is technically challenging. The evidence presented in this review can guide the discussion with patients during pre-operative planning. Newer techniques, including custom-made prostheses and reconstruction with porous tantalum acetabular implants and augments offer promising early results.
Take home message:
- Hip reconstruction should be considered for most periacetabular lesions that present to the orthopaedic surgeon in 2017.
- Metastatic lesions predominate, followed by chondrosarcoma, osteosarcoma, Ewing’s sarcoma, and other primary bone tumours.
- Multiple methods for reconstruction are available, each with its own risks and benefits.
- Overall complication rate is high, but implant survivorship is improved with newer technology and improved methods of reconstruction.
References
- 1.Guo W, Li D, Tang X, Yang Y, Ji T. Reconstruction with modular hemipelvic prostheses for periacetabular tumor. Clin Orthop Relat Res 2007;461:180–188. [DOI] [PubMed] [Google Scholar]
- 2.Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg [Am] 1978;60-A:731–746. [PubMed] [Google Scholar]
- 3.Hipfl C, Stihsen C, Puchner SE, Kaider A, Dominkus M, et al. Pelvic reconstruction following resection of malignant bone tumours using a stemmed acetabular pedestal cup. Bone Joint J 2017;99-B:841–848. [DOI] [PubMed] [Google Scholar]
- 4.Abdel MP, von Roth P, Perry KI, et al. Early results of acetabular reconstruction after wide periacetabular oncologic resection. J Bone Joint Surg [Am] 2017;99-A:9. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs B, O'Connor MI, Kaufman KR, Padgett DJ, Sim FH. Iliofemoral arthrodesis and pseudarthrosis: a long-term functional outcome evaluation. Clin Orthop Relat Res 2002;397:29–35. [DOI] [PubMed] [Google Scholar]
- 6.Walker RH. Pelvic reconstruction/total hip arthroplasty for metastatic acetabular insufficiency. Clin Orthop Relat Res 1993;294:170–175. [PubMed] [Google Scholar]
- 7.Bell RS, Davis AM, Wunder JS, et al. Allograft reconstruction of the acetabulum after resection of stage-IIB sarcoma. Intermediate-term results. J Bone Joint Surg [Am] 1997;79-A:1663–1674. [DOI] [PubMed] [Google Scholar]
- 8.Jeon DG, Kim MS, Cho WH, Song WS, Lee SY. Reconstruction with pasteurized autograft-total hip prosthesis composite for periacetabular tumors. J Surg Oncol 2007;96:493–502. [DOI] [PubMed] [Google Scholar]
- 9.Aboulafia AJ, Buch R, Mathews J, Li W, Malawer MM. Reconstruction using the saddle prosthesis following excision of primary and metastatic periacetabular tumors. Clin Orthop Relat Res 1995;314:203–213. [PubMed] [Google Scholar]
- 10.Uchida A, Myoui A, Araki N, et al. Prosthetic reconstruction for periacetabular malignant tumors. Clin Orthop Relat Res 1996;326:238–245. [DOI] [PubMed] [Google Scholar]
- 11.Dai KR, Yan MN, Zhu ZA, Sun YH. Computer-aided custom-made hemipelvic prosthesis used in extensive pelvic lesions. J Arthroplasty 2007;22:981–986. [DOI] [PubMed] [Google Scholar]
- 12.Khan FA, Rose PS, Yanagisawa M, Lewallen DG, Sim FH. Surgical technique: porous tantalum reconstruction for destructive nonprimary periacetabular tumors. Clin Orthop Relat Res 2012;470:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wunder JS, Ferguson PC, Griffin AM, Pressman A, Bell RS. Acetabular metastases: planning for reconstruction and review of results. Clin Orthop Relat Res 2003;(415 Suppl):S187–S197. [DOI] [PubMed] [Google Scholar]
- 14.Harrington KD. The use of hemipelvic allografts or autoclaved grafts for reconstruction after wide resections of malignant tumors of the pelvis. J Bone Joint Surg [Am] 1992;74-A:331–341. [PubMed] [Google Scholar]
- 15.Allan DG, Bell RS, Davis A, Langer F. Complex acetabular reconstruction for metastatic tumor. J Arthroplasty 1995;10:301–306. [DOI] [PubMed] [Google Scholar]
- 16.Windhager R, Karner J, Kutschera HP, et al. Limb salvage in periacetabular sarcomas: review of 21 consecutive cases. Clin Orthop Relat Res 1996;331:265–276. [PubMed] [Google Scholar]
- 17.Abudu A, Grimer RJ, Cannon SR, Carter SR, Sneath RS. Reconstruction of the hemipelvis after the excision of malignant tumours. Complications and functional outcome of prostheses. J Bone Joint Surg [Br] 1997;79-B:773–779. [DOI] [PubMed] [Google Scholar]
- 18.Vena VE, Hsu J, Rosier RN, O'Keefe RJ. Pelvic reconstruction for severe periacetabular metastatic disease. Clin Orthop Relat Res 1999;362:171–180. [PubMed] [Google Scholar]
- 19.Boyle MJ, Hornicek FJ, Robinson DS, Mnaymneh W. Internal hemipelvectomy for solitary pelvic thyroid cancer metastases. J Surg Oncol 2000;75:3–10. [DOI] [PubMed] [Google Scholar]
- 20.Marco RA, Sheth DS, Boland PJ, et al. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg [Am] 2000;82-A:642–651. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson J, Gustafson P, Fornander P, Ornstein E. The Harrington reconstruction for advanced periacetabular metastatic destruction: good outcome in 32 patients. Acta Orthop Scand 2000;71:591–596. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida Y, Osaka S, Mankin HJ. Hemipelvic allograft reconstruction after periacetabular bone tumor resection. J Orthop Sci 2000;5:198–204. [DOI] [PubMed] [Google Scholar]
- 23.Cottias P, Jeanrot C, Vinh TS, Tomeno B, Anract P. Complications and functional evaluation of 17 saddle prostheses for resection of periacetabular tumors. J Surg Oncol 2001;78:90–100. [DOI] [PubMed] [Google Scholar]
- 24.Langlais F, Lambotte JC, Thomazeau H. Long-term results of hemipelvis reconstruction with allografts. Clin Orthop Relat Res 2001;388:178–186. [DOI] [PubMed] [Google Scholar]
- 25.Natarajan MV, Bose JC, Mazhavan V, Rajagopal TS, Selvam K. The Saddle prosthesis in periacetabular tumours. Int Orthop 2001;25:107–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pant R, Moreau P, Ilyas I, Paramasivan ON, Younge D. Pelvic limb-salvage surgery for malignant tumors. Int Orthop 2001;24:311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwameis E, Dominkus M, Krepler P, et al. Reconstruction of the pelvis after tumor resection in children and adolescents. Clin Orthop Relat Res 2002;402:220–235. [DOI] [PubMed] [Google Scholar]
- 28.Satcher Jr RL, O'Donnell RJ, Johnston JO. Reconstruction of the pelvis after resection of tumors about the acetabulum. Clin Orthop Relat Res 2003;409:209–217. [DOI] [PubMed] [Google Scholar]
- 29.Benevenia J, Cyran FP, Biermann JS, Patterson FR, Leeson MC. Treatment of advanced metastatic lesions of the acetabulum using the saddle prosthesis. Clin Orthop Relat Res 2004;426:23–31. [DOI] [PubMed] [Google Scholar]
- 30.Aljassir F, Beadel GP, Turcotte RE, et al. Outcome after pelvic sarcoma resection reconstructed with saddle prosthesis. Clin Orthop Relat Res 2005;438:36–41. [DOI] [PubMed] [Google Scholar]
- 31.Kitagawa Y, Ek ET, Choong PF. Pelvic reconstruction using saddle prosthesis following limb salvage operation for periacetabular tumour. J Orthop Surg (Hong Kong) 2006;14:155–162. [DOI] [PubMed] [Google Scholar]
- 32.Ghert M, Alsaleh K, Farrokhyar F, Colterjohn N. Outcomes of an anatomically based approach to metastatic disease of the acetabulum. Clin Orthop Relat Res 2007;459:122–127. [DOI] [PubMed] [Google Scholar]
- 33.Kim HS, Kim KJ, Han I, Oh JH, Lee SH. The use of pasteurized autologous grafts for periacetabular reconstruction. Clin Orthop Relat Res 2007;464:217–223. [DOI] [PubMed] [Google Scholar]
- 34.Falkinstein Y, Ahlmann ER, Menendez LR. Reconstruction of type II pelvic resection with a new periacetabular reconstruction endoprosthesis. J Bone Joint Surg [Br] 2008;90-B:371–376. [DOI] [PubMed] [Google Scholar]
- 35.Jaiswal PK, Aston WJ, Grimer RJ, et al. Periacetabular resection and endoprosthetic reconstruction for tumours of the acetabulum. J Bone Joint Surg [Br] 2008;90-B:1222–1227. [DOI] [PubMed] [Google Scholar]
- 36.Tillman RM, Myers GJ, Abudu AT, Carter SR, Grimer RJ. The three-pin modified ‘Harrington’ procedure for advanced metastatic destruction of the acetabulum. J Bone Joint Surg [Br] 2008;90-B:84–87. [DOI] [PubMed] [Google Scholar]
- 37.Deloin X, Dumaine V, Biau D, et al. Pelvic chondrosarcomas: surgical treatment options. Orthop Traumatol Surg Res 2009;95:393–401. [DOI] [PubMed] [Google Scholar]
- 38.Menendez LR, Ahlmann ER, Falkinstein Y, Allison DC. Periacetabular reconstruction with a new endoprosthesis. Clin Orthop Relat Res 2009;467:2831–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clayer M. The survivorship of protrusio cages for metastatic disease involving the acetabulum. Clin Orthop Relat Res 2010;468:2980–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo Z, Li J, Pei GX, Li XD, Wang Z. Pelvic reconstruction with a combined hemipelvic prostheses after resection of primary malignant tumor. Surg Oncol 2010;19:95–105. [DOI] [PubMed] [Google Scholar]
- 41.Ho L, Ahlmann ER, Menendez LR. Modified Harrington reconstruction for advanced periacetabular metastatic disease. J Surg Oncol 2010;101:170–174. [DOI] [PubMed] [Google Scholar]
- 42.Philippeau JM, Durand JM, Carret JP, et al. Dual mobility design use in preventing total hip replacement dislocation following tumor resection. Orthop Traumatol Surg Res 2010;96:2–8. [DOI] [PubMed] [Google Scholar]
- 43.Donati D, Di Bella C, Frisoni T, Cevolani L, DeGroot H. Alloprosthetic composite is a suitable reconstruction after periacetabular tumor resection. Clin Orthop Relat Res 2011;469:1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gebert C, Wessling M, Hoffmann C, et al. Hip transposition as a limb salvage procedure following the resection of periacetabular tumors. J Surg Oncol 2011;103:269–275. [DOI] [PubMed] [Google Scholar]
- 45.Donati D, D'Apote G, Boschi M, Cevolani L, Benedetti MG. Clinical and functional outcomes of the saddle prosthesis. J Orthop Traumatol 2012;13:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoell S, Dedy N, Gosheger G, et al. The Burch-Schneider cage for reconstruction after metastatic destruction of the acetabulum: outcome and complications. Arch Orthop Trauma Surg 2012;132:405–410. [DOI] [PubMed] [Google Scholar]
- 47.Sherman CE, O’Connor MI, Sim FH. Survival, local recurrence, and function after pelvic limb salvage at 23 to 38 years of followup. Clin Orthop Relat Res 2012;470:712–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jansen JA, van de Sande MA, Dijkstra PD. Poor long-term clinical results of saddle prosthesis after resection of periacetabular tumors. Clin Orthop Relat Res 2013;471:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ueda T, Kakunaga S, Takenaka S, Araki N, Yoshikawa H. Constrained total hip megaprosthesis for primary periacetabular tumors. Clin Orthop Relat Res 2013;471:741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu S, Shi X, Zhou G, Lu M, Li C. Composite reconstruction of the hip following resection of periacetabular tumors: middle-term outcome. J Arthroplasty 2013;28:537–542. [DOI] [PubMed] [Google Scholar]
- 51.Shahid M, Saunders T, Jeys L, Grimer R. The outcome of surgical treatment for periacetabular metastases. Bone Joint J 2014;96-B:132–136. [DOI] [PubMed] [Google Scholar]
- 52.Angelini A, Calabro T, Pala E, et al. Resection and reconstruction of pelvic bone tumors. Orthopedics 2015;38:87–93. [DOI] [PubMed] [Google Scholar]
- 53.Bernthal NM, Price SL, Monument MJ, et al. Outcomes of modified Harrington reconstructions for nonprimary periacetabular tumors: an effective and inexpensive technique. Ann Surg Oncol 2015;22:3921–3928. [DOI] [PubMed] [Google Scholar]
- 54.Karim SM, Colman MW, Lozano-Calderón SA, et al. What are the functional results and complications from allograft reconstruction after partial hemipelvectomy of the pubis? Clin Orthop Relat Res 2015;473:1442–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kiatisevi P, Sukunthanak B, Pakpianpairoj C, Liupolvanish P. Functional outcome and complications following reconstruction for Harrington class II and III periacetabular metastasis. World J Surg Oncol 2015;13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsagozis P, Wedin R, Brosjo O, Bauer H. Reconstruction of metastatic acetabular defects using a modified Harrington procedure. Acta Orthop 2015;86:690–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Erol B, Aydemir AN, Onay T, Topkar MO. Reconstruction of advanced periacetabular metastatic lesions with modified Harrington procedure. Acta Orthop Traumatol Turc 2016;50:178–185. [DOI] [PubMed] [Google Scholar]
- 58.Lozano-Calderon SA, Kaiser CL, Osler PM, Raskin KA. Cemented total hip arthroplasty with retrograde ischioacetabular Steinmann pin reconstruction for periacetabular metastatic carcinoma. J Arthroplasty 2016;31:1555–1560. [DOI] [PubMed] [Google Scholar]
- 59.Bus MP, Szafranski A, Sellevold S, et al. LUMiC® endoprosthetic reconstruction after periacetabular tumor resection: Short-term Results. Clin Orthop Relat Res 2017;475:686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo X, Li X, Liu T, Shuai C, Zhang Q. Pasteurized autograft reconstruction after resection of periacetabular malignant bone tumours. World J Surg Oncol 2017;15:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res 1993;286:241–246. [PubMed] [Google Scholar]
- 62.Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg [Am] 1981;63-A:653. [PubMed] [Google Scholar]
- 63.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg [Am] 1969;51:737–755. [PubMed] [Google Scholar]
- 64.No authors listed. NJR annual report 2015. http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/12th%20annual%20report/NJR%20Online%20Annual%20Report%202015.pdf?ver=2015-09-14-170656-847 (date last accessed 11 August 2017).
- 65.Otte JE, Politi JR, Chambers B, Smith CA. Intrawound vancomycin powder reduces early prosthetic joint infections in revision hip and knee arthroplasty. Surg Technol Int 2017;30:30. [PubMed] [Google Scholar]
- 66.Henderson ER, Groundland JS, Pala E, et al. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg [Am] 2011;93-A:418–429. [DOI] [PubMed] [Google Scholar]