Abstract
The development and pre-clinical evaluation of nano-texturised, biomimetic, surfaces of titanium (Ti) implants treated with titanium dioxide (TiO2) nanotube arrays is reviewed. In vitro and in vivo evaluations show that TiO2 nanotubes on Ti surfaces positively affect the osseointegration, cell differentiation, mineralisation, and anti-microbial properties. This surface treatment can be superimposed onto existing macro and micro porous Ti implants creating a surface texture that also interacts with cells at the nano level. Histology and mechanical pull-out testing of specimens in rabbits indicate that TiO2 nanotubes improves bone bonding nine-fold (p = 0.008). The rate of mineralisation associated with TiO2 nanotube surfaces is about three times that of non-treated Ti surfaces. In addition to improved osseointegration properties, TiO2 nanotubes reduce the initial adhesion and colonisation of Staphylococcus epidermidis. Collectively, the properties of Ti implant surfaces enhanced with TiO2 nanotubes show great promise.
Cite this article: Bone Joint J 2018;100-B(1 Supple A):9–16.
Keywords: Nanotubes, Titanium dioxide, Osseointegration, Antimicrobial, Mineralisation, Nano-texturing
Titanium (Ti) and its alloys have been widely used in orthopaedic implants for decades, due to their biocompatibility, low toxicity, and excellent mechanical properties.1 When designed with macro porous and micro-textured features for enhanced osseointegration, implants made from Ti alloys bond with adjacent bone surfaces relatively well compared with those made from other materials with similar macro and micro features. However, complications such as aseptic loosening and infection persist.2-4 Inadequate osseointegration remains a complication associated with implants that rely on osseointegration for proper function, particularly those with relatively flat and small surface areas that have high shear loading, such as non-cemented uni and total condylar knee tibial trays.5,6 Faster osseointegration can enhance recovery as a result of improved load distribution and a more stable bone-implant interface.
Over the past six decades, the surface technologies of implants have progressed from bioinert surfaces such as porous Ti and tantalum, to bioactive surfaces such as plasma-sprayed hydroxyapatite and other ceramics, to the most recent and probable future generation of biomimetic engineered, nano-texturised surfaces such as Ti treated with Ti oxide (TiO2) nanotube arrays. These surfaces mimic the nano-morphology of the external cellular membranes of the osteoblasts that surround the implant. A substantially increased surface area has unique chemical characteristics provided by the nanotubes, allowing increased interaction between the surface of the implant and adjacent cells. Nano-surface mechanisms increase the rate of initial osseointegration between Ti alloys and the surrounding tissue, greatly increasing the strength of the bond between implant and bone.7
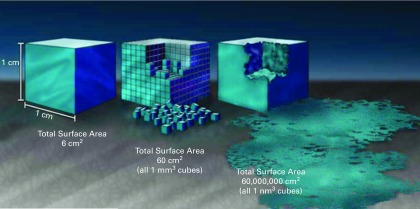
Nano-technology is the control of matter at a scale of approximately 1 to 100 nanometres (nm), where novel properties and function occur because of size.8 Thus, for the surface of an implant truly to possess nano-technology, aspects of shape and structure must be formed at the nano-scale which specifically enhance the properties of the implant. The impact that nano-texturising has on the interactions of the surface of the implant can be illustrated by imagining the macro, micro, and nano-surface area of a 1 cm × 1 cm × 1 cm cubic implant, which has 6 cm2 of macro surface area (about the size of a sugar cube), as shown in Figure 1.8 If such an implant is divided into 1 mm cubes, the total surface area increases to 60 cm2. If it is further divided into 1 nm cubes, the surface area increases to 60 000 000 cm2, equivalent to approximately 1.5 acres of surface area. Likewise, nano-texturing an implant with features such as tubes that have both internal and external surface areas greatly increases the area available for osseointegration between the nano-scale features of the tubes and those of the cells.
Fig. 1.
Graphic illustration of the increased surface area provided by nano-texturising. Adapted with permission from the National Nanotechnology Coordination Office ITRI, Inc.8
TiO2 nanotube arrays are formed on Ti surfaces through a specialised efficient anodisation process that is followed by heat treatment.9-14 The arrays consist of rows of vertically aligned nanotubes with engineered and reproducible internal diameters, outer diameters, and lengths, resulting in a nano-textured surface of nanotubes that has defined, non-random physical and chemical properties. In order to treat a Ti implant, it is first immersed in a fluoride-rich electrolyte. As a voltage is applied, a thicker compact layer of TiO2 develops on the outer surface. Then, during an extended anodisation process of approximately ten minutes to 45 minutes, depending on the volume and surface area of the implant, billions of vertically aligned nanotubes are integrated into the outer TiO2 layer, creating a surface of nano-texturised TiO2 nanotubes. After anodisation, the surface is heat treated to 500°C for two hours to convert the TiO2 nanotubes from an amorphous phase to an anatase phase. This post-anodisation heat treatment improves both the toughness of the nanotubes and their osseointegration potential.15 The resultant surface is not a coating but a transformed TiO2 surface, which not only greatly increases the available surface area for processes such as cell adhesion, but also provides a nano-texture that interacts with outer cell membrane surfaces. Such TiO2 nano-texturised surfaces exhibit tensile and shear adhesion strengths that exceed the typical loads experienced during the introduction of implants, their use and their removal.16
The anodisation process can be adjusted to create nano-texturised surfaces of vertically aligned nanotubes with accurately controlled pore diameters. For example, specimens can be made with a mean outside diameter of the nanotube of 30 nm (sd 10 nm), 70 nm (sd 10 nm), or 100 nm (sd 10 nm), and with specific thicknesses and heights of the walls. A nano-texturised surface with a mean diameter of the nanotube of 100 nm has a high hydrophilicity when compared with a non-nano-texturised Ti surface or a nano-texturised surface with a mean diameter of 30 nm.17,18 The hydrophilicity of the surface of a nanotube array is inversely related to the diameter of the tube. The higher the hydrophilicity, the more absorbent the surface and the smaller the contact angle surrounding liquid on the surface.17,18 The formation of vertically aligned TiO2 nanotubes on the surface of a Ti implant can positively affect the osseointegration, cell differentiation, mineralisation, and antimicrobial properties. Figure 2 shows an example of a tibial tray that incorporates macro features, micro porosity, and nano-texturing to enhance fixation.
Fig. 2.
Titanium alloy tibial tray incorporating macro fixation with pegs and keels; micro fixation with additive manufactured micrometer scale porosity, and nano-scale texturing throughout the porosity with anodised titanium dioxide nanotubes. Image courtesy of Optimotion Implants, LLC, Orlando, Florida. Macro (mm scale); Micro (μm scale); Nano (nm scale).
We present a review of recent research to summarise the outcomes that can be achieved by the creation of vertically aligned TiO2 nanotubes on a Ti implant surface.
Osseointegration and cell differentiation
Oh et al15,19 studied the in vitro behaviour of osteoblasts cultured on vertically aligned TiO2 nanotubes and investigated the effect of such a nano-structure on the morphology of osteoblasts and the kinetics of cell proliferation. A layer of vertically aligned TiO2 nanotubes on a Ti surface was created by anodisation, and MC3T3-E1 mouse osteoblast cells were seeded on the experimental substrate and on a control substrate of pure non-texturised Ti. The presence of nanotubes positively affected the adhesion and propagation of the osteoblasts, with the filopodia of the growing cells spreading across the pores of the nanotube arrays, producing an interlocked cell structure. With the passage of time, the number of adhered cells on the TiO2 nanotubes increased significantly by approximately 300% to 400% compared with the number of cells adhering to the Ti metal surface. This effect is most likely to be due to the increased surface area, the increased hydrophilicity, and the unique topography of nanotubes that increases the negative charge on the outer rim of the tubes.19
Gongadze et al20 have suggested that the attraction between the negatively charged Ti surface and a negatively charged osteoblast is mediated by charged proteins with a distinctive distribution of quadrupolar internal charge. Similarly, cation-mediated attraction between fibronectin molecules and the Ti surface is expected to be more efficient for a high surface charge density, resulting in integrin mediated osteoblast adhesion. The osteoblasts are most strongly bound along the sharp convex edges of the surfaces of the TiO2 nanotube where the magnitude of the density of the negative surface charge is the highest. A vertically aligned nanotube configuration may be a useful route for accelerating the proliferation of various other types of cells in addition to osteoblasts.17
As described above, the pore diameter of TiO2 nanotubes can be controlled by adjusting the potential during anodisation. In an in vitro examination of human mesenchymal stem cells (hMSC), Oh et al15 created TiO2 nanotubes with pore diameters ranging from 30 nm to 100 nm. Varying the pore diameters of nano-tubular-shaped TiO2 surface structures independently allowed either augmented hMSC adhesion or a specific differentiation of hMSCs into osteoblasts by using only the geometric cues, without the introduction of osteogenic-inducing media. The behaviour of hMSC in response to the varied sizes of nanotubes revealed a significant change in a relatively narrow range of sizes. As shown in Figure 3, small (~30 nm diameter) nanotubes promoted adhesion without noticeable differentiation, whereas larger (~70 nm to 100 nm diameter) nanotubes elicited a dramatic elongation of stem cells (~10-fold increase), which induced cytoskeletal stress and selective differentiation into osteoblast-like cells.
Fig. 3.
Mesenchymal stem cells at 24 hours. Upper row (left to right): scanning electron microscope micrographs of human mesenchymal stem cells (hMSCs) on flat Ti and 30 nm, 50 nm, 70 nm and 100 nm diameter Ti dioxide (TiO2) nanotube surfaces after two hours of culture. Lower row (left to right): fluorescent images of hMSCs on flat Ti and 30 nm, 50 nm, 70 nm, and 100 nm diameter TiO2 nanotube surfaces showing increase in cell elongation with increasing nanotube diameter.
Similarly, osteoblasts can exhibit increased elongation when cultured on TiO2 nanotubes of increasing diameter. Brammer18prepared TiO2 nanotubes of 30 nm, 50 nm, 70 nm and 100 nm pore diameter on Ti substrates by anodisation, and seeded the substrates with MC3T3-E1 mouse osteoblasts to investigate cellular behaviour in response to the different sizes of nanotube. They observed that a change in osteoblast behaviour was obtained in a relatively narrow range of nanotube dimensions; those with a small diameter (~30 nm) stimulated the highest degree of osteoblast adhesion, while those with a larger diameter (70 nm to 100 nm) stimulated a lower population of cells with extremely elongated cellular morphology and much higher levels of alkaline phosphatase, as shown in Figure 4. Increased elongation of nuclei is also seen with larger diameter nanotubes.18
Fig. 4.
Scanning electron microscope micrographs of osteoblasts (which appear dark) on (left to right) flat titanium (Ti) and 30 nm, 50 nm, 70 nm, 100 nm diameter Ti dioxide (TiO2)nanotube surfaces after 24 hours of incubation. The arrows indicate strikingly long cellular extensions across the substrate on the 100 nm nanotubes. Red brackets show increased cellular elongation on the larger ~70 nm to 100 nm diameter nanotubes. Flat and more rounded cells are shown on Ti and 30 nm to 50 nm TiO2 nanotube surfaces.
The rate of cell adhesion may be significantly increased on nano-textured surfaces. Peng et al21found, in an in vitro study comparing the cellular response with different textured surfaces, that the adhesion of C3H10T1/2 mouse cells on the surface of Ti specimens treated with TiO2 nanotube arrays, 30 nm or 80 nm in diameter, was significantly enhanced when compared with control samples cultured on polished Ti and acid-etched Ti. A surface analysis of the four groups (group one, polished Ti; group two, acid etched Ti; group three, nanotubes with a mean diameter of 80 nm; and group four, nanotubes with a mean diameter of 30 nm) identified increased surface roughness, decreased water contact angles and an enhanced concentration of oxygen and fluorine atoms on the surfaces of TiO2 nanotubes. The density on the 30 nm and 80 nm TiO2 nanotube arrays was found to be higher than that on the mechanically polished and acid-etched Ti sheets (p < 0.01), and the cell density on the 80 nm TiO2 nanotube arrays was markedly higher than that of the other groups at two, eight, 12, and 24 hours. The authors concluded that the surface of a TiO2 nanotube can reduce bacterial colonisation (as described below) and enhance C3H10T1/2 cell adhesion. Many physical and chemical properties of the surface of a TiO2 nanotube may contribute to these effects.
Adhesion strength, or implant-bone bonding, was measured in an in vivo study comparing the surface of a TiO2 nanotube implant with the surface of a Tigrit-blasted implant. Bjursten et al7implanted discs with TiO2 nanotube surfaces and control discs with Ti grit-blasted surfaces in compression on the flat cortical bone non-load-bearing surface of the proximal anterior tibias of rabbits. Weight-bearing was allowed immediately following surgery. The grit blasted surfaces had a micro-surface roughness with features approximately 6 μm long by approximately 2 μm deep and an outer layer of approximately 5 nm of amorphous TiO2. The TiO2 nanotube treated implants had an approximately 250 nm to 300 nm thick layer of anatase TiO2 nanotubes that were 100 nm outer diameter by 80 nm inner diameter formed by anodisation. After removing the tibial periosteum in the area of attachment of the implant, and reaming the exposed bone surface flat with a circular reamer, the implants were held in compression by Teflon (DuPont Co., Wilmington, Delaware) coverings that were installed over the disc-shaped implants to hold them securely.
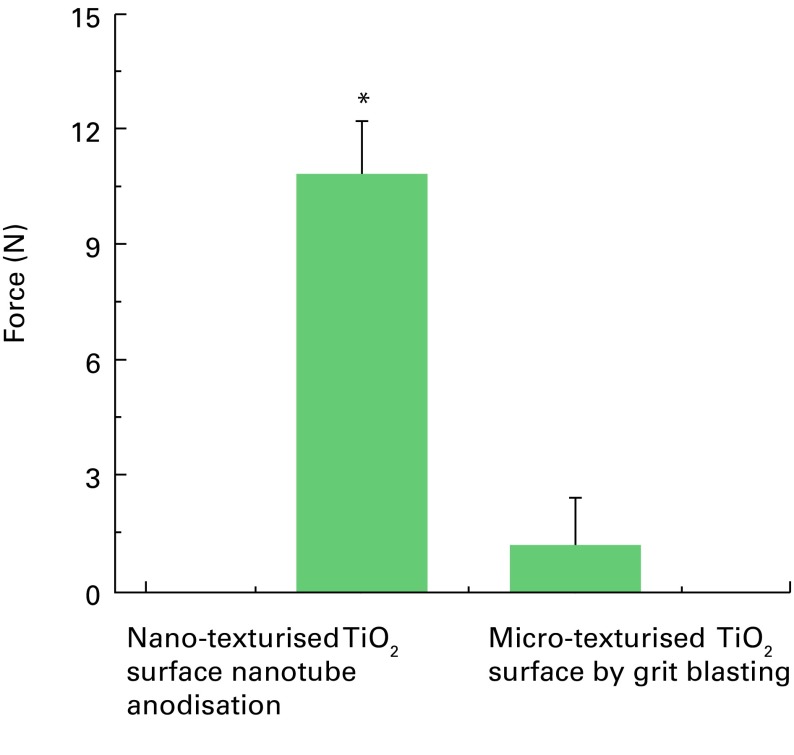
After four weeks, the coverings were removed, and screws were attached to the discs in harvested necropsy samples and pulled slowly at 0.1 mm/sec to measure adhesion strength in pure tension. Pull-out testing indicated that the strength of bonding of the TiO2 nanotubes improved by nine-fold (p = 0.008) compared with the blasted micro-textured surface (mean 10.8 N (sd 3.1 N) vs mean 1.2 N (sd 2.7 N)), as shown in Figure 5. Furthermore, the TiO2 nanotube implants showed significantly higher bone-implant contact area (mean 78.3% (sd 33.3)) compared with the micro-textured implants (mean 21.7% (sd 24.7)). Histological analysis, illustrated in Figure 6, confirmed greater bone-implant contact area, new bone formation and increased levels of calcium and phosphorus on the surfaces of the nanotubes compared with the micro-textured implants. Energy dispersive radiograph mapping of the interface after tensile testing indicated a higher surface area and increased calcium and phosphorus on the TiO2 nanotube surfaces compared with the blasted implant surfaces. The percentage of surface area covered by calcium and phosphorous, which is indicative of strong osseointegration, was approximately 41.7% on TiO2 nanotube surfaces versus only 8.3% for the micro-textured surfaces. The bond between newly formed bone and the surfaces of the TiO2 nanotubes was so strong that fracture occurred within the growing bone rather than at the implant-bone interface.
Fig. 5.
Results of tensile pull-out tests for titanium dioxide (TiO2) nanotube versus TiO2 micro-texturised, grit blasted disk implants (*p-value = 0.008).
Fig. 6.
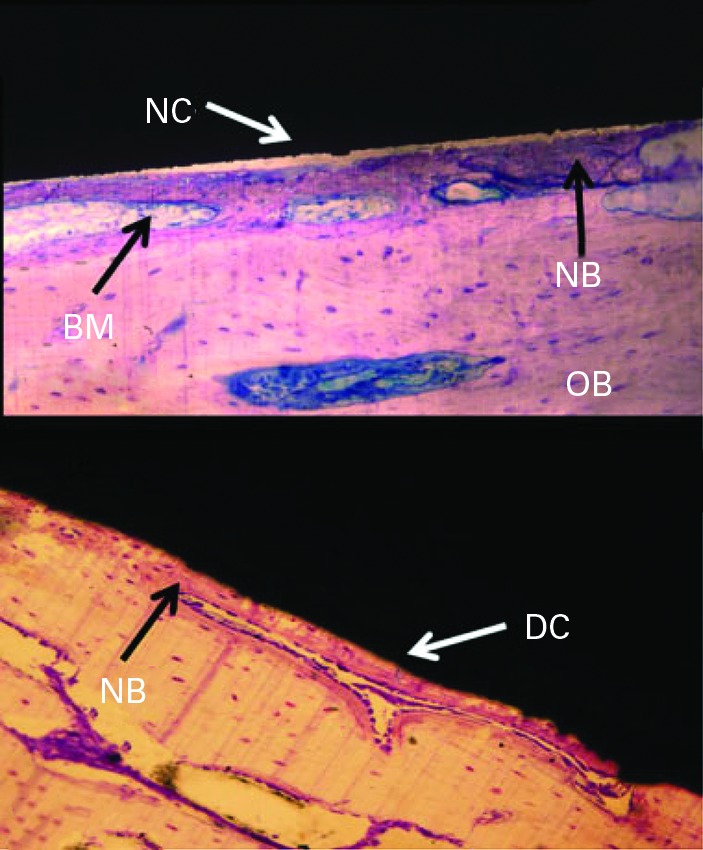
Haematoxylin and eosin stained ground sections with thickness of 25 μm to 50 μm showing direct contact (DC) or non-direct contact (NC) with bone on (top) titanium (Ti) micro-texturised grit blasted implant and (bottom) Ti dioxide nano-texturised nanotube implant. Bone marrow (BM), old bone (OB), and new bone (NB) are indicated.
A nano-surfaced material in the range below 100 nm more closely mimics the natural hydroxyapatite and collagen constituents of bone than a micro-scale textured surface alone. The nano-topography of the TiO2 nanotubes more closely resembles the porous structure of native bone, allowing for more optimal interactions for osteogenesis.7 As protein adsorption on the surface occurs first on implantation, the nanotubes provide a more favourable structure to attract proteins, such as vitronectin and fibronectin, which promote the adhesion of osteoblasts onto the surface of the implant. Nanotubes improve adhesion and proliferation for osteoblasts through their improved focal adhesion.15Thus, one of the major benefits of nano-surfacing seems to be the initial enhancement of osteoblast attachment and accelerated osseointegration. When sufficient time is allowed without micro-movement between the implant and bone, an implant surface without nano-structure eventually seems to catch up, although at a slower rate, and produces a comparable degree of bone formation with a nano-textured surface. However, the quality of osseointegration and bone bonding may still not match that of a nano-textured surface.
Ti nanotubes may also be formed on Ti coatings applied to cobalt chrome (CoCr) implants. Figure 7 shows in vitro osteoblast formation on a CoCr implant with a Ti coating and TiO2 nanotubes after 24 hours.22 Similar to TiO2 nanotubes on solid Ti implants, the osteoblasts are elongated, with prominent filopodia. These results are like the results seen in Figure 4 (osteoblast cells on TiO2 nanotubes on a Ti implant). In addition, the cells may show large focal adhesions, which are vital for proper cell function.
Fig. 7.
Scanning electron microscope micrographs of osteoblast formation on cobalt/chrome (CoCr) implant (a), and on CoCr implant with titanium coating and titanium dioxide nanotubes after 24 hours (b). Both images are 10 μm. The yellow arrows show the location of cellular extension.
Mineralisation
Bone mineralisation occurs when an inorganic substance, such as calcium or phosphorus, precipitates in an organic matrix such as an osteoblast. As reported by Frandsen et al,22 TiO2 surfaces with Ti nanotubes show an increased rate of mineralisation compared with a pure Ti surface. In this in vitro study, the behaviour of human osteoblast cells on TiO2 nanotubes and tantalum (Ta) coated TiO2 nanotube surfaces of nearly identical nano-topography were compared to assess the effect of changes in surface characteristics due to a Ta coating alone. Although the rate of mineralisation of the surface of a TiO2 nanotube array is about three times faster than that of Ti micro-textured by blasting, and about twice as fast as micro-textured Ta, the rate can be further enhanced by adding nano-particles of Ta to the nanotubes. The ‘osteofunctionality’ was enhanced on the Ta surface as measured by alkaline phosphatase activity, bone nodule formation, and the deposition of matrix minerals. The Ta surface promoted an approximately 30% faster rate of mineralisation and bone-nodule formation compared with the results on bare TiO2 nanotubes. Table I and Figure 8 compare the rates of mineralisation for phosphorus and calcium on Ti and Ta substrates, on TiO2 surfaces with Ti nanotubes and on Ti nanotubes further enhanced with a Ta nano-particle coating. The Ta enhanced nanotubes provide an almost four-fold increase in the rate compared with the micro-texturised Ti surfaces used in most implants today. These findings enhance our understanding of cell behaviour in response to subtle alterations in nanostructure and surface chemistry and offer further insights into the potential for compelling manipulation of biomaterial surfaces.
Fig. 8.
Atomic percentage of phosphorus and calcium as a function of time, on titanium (Ti), tantalum (Ta), titanium dioxide (TiO2) nanotubes (NT), and TiO2 NT coated with Ta.
Table I.
Rates of calcium and phosphorus deposition by substrate
| Substrate | Rate of phosphorous deposition | Rate of calcium deposition |
|---|---|---|
| Titanium (Ti) | 0.498 | 0.565 |
| Tantalum (Ta) | 0.695 | 0.830 |
| Ti dioxide (TiO2) nanotubes | 1.247 | 1.594 |
| Ta-coated TiO2 nanotubes | 1.616 | 2.081 |
Antimicrobial properties
TiO2 nanotube arrays on Ti implants have shown the additional benefit of antimicrobial capabilities. Peng et al,21 in combination with the increased cell adhesion reported above, found in an in vitro evaluation that the inclusion of TiO2 nanotubes on Ti surfaces reduced the initial adhesion and colonisation of Staphylococcus epidermidis compared with polished or acid-etched Ti surfaces. Bacterial colonisation on nanotubes decreased significantly from the colonisation present on polished Ti or acid-etched Ti. The nanotubes with the larger mean diameter (80 nm) had the highest antimicrobial effect. This effect, along with the increased cell adhesion reported above, may be due to many factors, including both physical characteristics and chemical composition. It is hypothesised that the negative charge of the hydrophilic nanotube surface attaches to positively charged osteoblasts while the same surface repels the negatively charged microbes, reducing the build-up of biofilm and resisting infection. These findings show promise for clinical and commercial application, since Staphylococcus epidermidis is the most common pathogen associated with orthopaedic infections.23
Ta and silver have been shown to reduce bacterial adherence to implants.24,25 However, the entire implant or its whole surface need not be coated with these materials to have a measureable effect. Nano-particles of silver on the surface of an implant have been shown to decrease the formation of biofilms on implants.26-28 Apart from being bacterial resistant, nano-particles of silver are non-cytotoxic in appropriate doses and can provide long-term antimicrobial effects.28 The fundamental idea behind this dual treatment is to enhance the osseointegration of Ti implants at the same time as improving their resistance to infection in vivo.
Yavari et al26prepared scaffolds of porous Ti with TiO2 nanotubes by anodisation and then soaked the nanotubes in silver nitrate having low (0.02 M), medium (0.1 M), and high (0.5 M) concentrations. The antimicrobial behaviour and viability of the cells of the treated nanotube scaffolds were assessed. At up to 24 hours after treatment, the biomaterials were found to be extremely effective in preventing the formation of biofilms on the scaffolds and decreasing the number of planktonic bacteria, especially for the medium and high concentrations of silver ions. The antimicrobial effects of the biomaterials, particularly the ones with greater concentrations of antimicrobial agents, continued until two weeks however, for the groups with the highest concentrations of silver, the viability of the cells was adversely affected. The potency of the biomaterials in decreasing the number of planktonic bacteria and deterring the formation of biofilms make them promising candidates for fighting implant-associated infections.
The specific geography of a nano-textured surface can affect bacterial adhesion to the surface. Stolzoff et al28 investigated bacterial growth on nano-textured surfaces having varied topography. Ion beam assisted deposition was used to coat Ti coupons with 0.5 μm to 1 μm of textured Ti. In order to generate varied levels of nano-texture, the energy of the argon ion beam and the density of the current were modified relative to the rate of deposition. Five samples were created with varied surface topography, although some samples had similar surface roughness. The samples were subjected to different ion energy densities, ranging from 0 mJ/cm3 to 90.3 mJ/cm3, to vary the surface topography. In the analysis of bacterial adhesion, samples one and two, which had negligible differences in roughness compared with samples three and five, had the greatest decrease in bacterial adhesion, while sample four, with the largest roughness was no different. The main differences in samples one and two were that their topography included evenly spaced small peaks compared with larger, more randomly positioned peaks found in samples three and five. It was concluded that the value of roughness generally did not predict the levels of bacterial colonisation. Instead, surface topography was confirmed as a key factor in the prevention of bacterial adhesion and proliferation.
In conclusion, the formation of TiO2 nanotube arrays on Ti surfaces has been shown to increase osseointegration based on measurements of improved adhesion and propagation of osteoblasts,19 stem cell differentiation,15 increased elongation of cells,17,29 higher cell density,18 and strength of adhesion 7 when compared with non-nano-texturised Ti surfaces. The formation of TiO2 nanotube arrays on Ti surfaces also offers improved bone mineralisation29 and anti-microbial properties22,26-28 compared with non-nano-texturised Ti surfaces. Additionally, TiO2 nanotube arrays formed on Ti-coated CoCr surfaces show enhanced cell attachment.22 When applied to orthopaedic implants, this biomimetic engineered layer may allow for earlier weight-bearing and a decreased risk of infection, thus speeding up recovery times and enhancing the health and mobility of patients. These observations suggest great promise for the development of Ti nano-texturised orthopaedic implants. In demanding applications, even the most technologically advanced macro and micro porous Ti structures can fail to integrate clinically. The potential for osseointegration of any surface is not guaranteed due to complex biological and mechanical factors. However, the surfaces of an implant that have been nano-textured with TiO2 nanotubes show significant improvement in osseointegration.
Take home message:
- The texture of an implant surface at the nano level impacts osseointegration and anti-microbial properties.
- TiO2 nanotube arrays are superimposed nano-texturised features on macro and micro porous tissue ingrowth surfaces.
- Macro, micro and nano texturisation is being considered for the next generation of orthopaedic joint implants.
References
- 1.Long M, Rack HJ. Titanium alloys in total joint replacement – a materials science perspective. Biomaterials 1998;19:1621–1639. [DOI] [PubMed] [Google Scholar]
- 2.Puckett SD, Taylor E, Raimondo T, Webster TJ. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 2010;31:706–713. [DOI] [PubMed] [Google Scholar]
- 3.Carr AJ, Robertsson O, Graves S, et al. Knee replacement. Lancet 2012;379:1331–1340. [DOI] [PubMed] [Google Scholar]
- 4.Dalury DF. Cementless total knee arthroplasty. Bone Joint J 2016; 98-B:867–873. [DOI] [PubMed] [Google Scholar]
- 5.Drees P, Eckardt A, Gay RE, Gay S, Huber LC. Mechanisms of disease: molecular insights into aseptic loosening of orthopedic implants. Nat Clin Pract Rheumatol 2007;3:165–171. [DOI] [PubMed] [Google Scholar]
- 6.Bordini B, Stea S, De Clerico M, et al. Factors affecting aseptic loosening of 4750 total hip arthroplasties: multivariate survival analysis. BMC Musculoskelet Disord 2007;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjursten LM, Rasmusson L, Oh S, et al. Titanium dioxide nanotubes enhance bone bonding in vivo. J Biomed Mater Res A 2010;92:1218–1224. [DOI] [PubMed] [Google Scholar]
- 8.No authors listed. National Nanotechnology Initiative, What’s So Special about the Nanoscale? https://www.nano.gov/nanotech-101/special (date last accessed 21 August 2017).
- 9.Jin S, Oh SCompositions comprising nanostructures for cell, tissue and artificial organ growth, and methods for making and using same. United States Patent 8414908. 9 April 2013. https://www.google.com/patents/US8414908 (date last accessed 30 August 2017).
- 10.Zwilling V, Darque-Ceretti E, Boutry-Forveille A, et al. Structure and physicochemistry of anodic oxide films on titanium and TA6V alloy. Surf Interface Anal 1999;27:629–637. [Google Scholar]
- 11.Gong D, Grimes CA, Varghese OK, et al. Titanium oxide nanotube arrays prepared by anodic oxidation. J Mater Res 2001;16:3331–3334. [Google Scholar]
- 12.Varghese OK, Gong D, Paulose M, Grimes CA, Dickey EC. Crystallization and high-temperature structural stability of titanium oxide nanotube arrays. J Mater Res 2003;18:157–165. [Google Scholar]
- 13.Mor GK, Varghese OK, Paulose M, Mukherjee N, Grimes CA. Fabrication of tapered, conical-shaped titania nanotubes. J Mater Res 2003;18:2588–2593. [Google Scholar]
- 14.Cai Q, Paulose M, Varghese OK, Grimes CA. The effect of electrolyte composition on the fabrication of self-organized titanium oxide nanotube arrays by anodic oxidation. J Mater Res 2005;20:230–236. [Google Scholar]
- 15.Oh S, Brammer KS, Li YSJ, et al. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci USA 2009;106:2130–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Descamps S, Awitor KO, Raspal V, et al. Mechanical properties of nanotextured titanium orthopedic screws for clinical applications. J Med Device 2013;7:210051–210055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frandsen CJAn extensive analysis of modified nanotube surfaces for next-generation orthopedic implants. http://escholarship.org/uc/item/4v60v29j?query=frandsen (date last accessed 21 August 2017).
- 18.Brammer KSControlled nanostructures for enhanced biological responses and release of incorporated biomolecules. http://escholarship.org/uc/item/9612567k#page-1 (date last accessed 21 August 2017).
- 19.Oh S, Daraio C, Chen LH, et al. Significantly accelerated osteoblast cell growth on aligned TiO 2 nanotubes. J Biomed Mater Res A 2006;78:97–103. [DOI] [PubMed] [Google Scholar]
- 20.Gongadze E, Kabaso D, Bauer S, et al. Adhesion of osteoblasts to a nanorough titanium implant surface. Int J Nanomedicine 2011;11:1801–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng Z, Ni J, Zheng K, et al. Dual effects and mechanism of TiO2 nanotube arrays in reducing bacterial colonization and enhancing C3H10T1/2 cell adhesion. Int J Nanomedicine 2013;8:3093–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frandsen CJ, Brammer KS, Noh K, Johnston GW, Jin S. Tantalum coating on TiO2 nanotubes induces superior rate of matrix mineralization and osteofunctionality in human osteoblasts. Mater Sci Eng C Mater Biol Appl 2014;37:332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valour F, Trouillet-Assant S, Rasigade J- P. Staphylococcus epidermidis in orthopedic device infections: the role of bacterial internalization in human osteoblasts and biofilm formation. PLoS One 2013;8:67240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schildauer TA, Robie B, Muhr G, Köller M. Bacterial adherence to tantalum versus commonly used orthopaedic implant materials. J Orthop Trauma 2006;20:476–484. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Cui Q, Feng B, et al. Antibacterial activity of TiO2 nanotubes: influence of crystal phase, morphology and Ag deposition. Appl Surf Sci 2013;284:179–183. [Google Scholar]
- 26.Amin Yavari S, Loozen L, Paganelli FL, et al. Antibacterial behavior of additively manufactured porous titanium with nanotubular surfaces releasing silver ions. ACS Appl Mater Interfaces 2016;8:17080–17089. [DOI] [PubMed] [Google Scholar]
- 27.Shivaram A, Bose S, Bandyopadhyay A. Mechanical degradation of TiO2 nanotubes with and without nanoparticulate silver coating. J Mech Behav Biomed Mater 2016;59:508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stolzoff M, Burns JE, Aslani A, et al. Decreased bacterial growth on titanium nanoscale topographies created by ion beam assisted evaporation. Intl J Nanomedicine 2017;12:1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ni J, Frandsen CJ, Noh K, et al. Fabrication of thin film TiO2 nanotube arrays on Co-28Cr-6Mo alloy by anodization. Mater Sci Eng C Mater Biol Appl 2013;33:1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]