Abstract
Control of arbovirus transmission remains focused on vector control through application of insecticides directly to the environment. However, these insecticide applications are often reactive interventions that can be poorly-targeted, inadequate for localized control during outbreaks, and opposed due to environmental and toxicity concerns. In this study, we developed endectocide-treated feed as a systemic endectocide for birds to target blood feeding Culex tarsalis, the primary West Nile virus (WNV) bridge vector in the western United States, and conducted preliminary tests on the effects of deploying this feed in the field. In lab tests, ivermectin (IVM) was the most effective endectocide tested against Cx. tarsalis and WNV-infection did not influence mosquito mortality from IVM. Chickens and wild Eurasian collared doves exhibited no signs of toxicity when fed solely on bird feed treated with concentrations up to 200 mg IVM/kg of diet, and significantly more Cx. tarsalis that blood fed on these birds died (greater than 80% mortality) compared to controls (less than 25% mortality). Mosquito mortality following blood feeding correlated with IVM serum concentrations at the time of blood feeding, which dropped rapidly after the withdrawal of treated feed. Preliminary field testing over one WNV season in Fort Collins, Colorado demonstrated that nearly all birds captured around treated bird feeders had detectable levels of IVM in their blood. However, entomological data showed that WNV transmission was non-significantly reduced around treated bird feeders. With further development, deployment of ivermectin-treated bird feed might be an effective, localized WNV transmission control tool.
Author summary
West Nile virus (WNV) is a mosquito-borne virus that causes significant disease and death every year in humans, domesticated animals, and wildlife. Control of WNV transmission is focused on controlling the mosquito vector through applications of insecticides directly to the environment. In this study, we evaluate a novel control strategy for WNV transmission by targeting the main mosquito bridge vector in the Great Plains region, Culex tarsalis, through its blood feeding behavior. Because Culex tarsalis favor taking blood meals from particular bird species, our strategy aims to target these bird species with endectocide-treated bird feed that will result in lethal blood meals for Cx. tarsalis. In this study, we developed a safe and effective formulation of ivermectin-treated diet that resulted in increased mortality for Cx. tarsalis blood fed on birds consuming this treated diet as compared to mosquitoes feeding on control birds. We also conducted a pilot field trial in Fort Collins, Colorado to test this strategy in a natural transmission cycle, which demonstrated promising results.
Introduction
West Nile virus (WNV) is an arthropod-borne flavivirus, and the leading cause of domestically acquired arboviral disease in the United States [1,2], resulting in significant disease and death every year in humans, domesticated animals, and wildlife. From 1999–2017, >48,000 cases of human WNV disease and >2000 deaths were reported to the CDC [3], but the total number of individuals in the U.S. who have been made ill from WNV is estimated to be greater than 1 million, or approximately 1 of every 5 persons infected (>5 million infected individuals) [4]. Control of WNV transmission remains focused on vector control through larvicide and adulticide applications [5]. Larvicide applications are generally preferred to adulticide applications as they are more cost-effective and less environmentally-damaging due to more direct and efficient targeting of mosquitoes [6,7]. While previous studies have demonstrated the effectiveness of larvicide applications to catch basins, a common Culex larval habitat, in reducing the number of mosquitoes [8,9], the efficacy may vary significantly with suboptimal catch basin design or environmental conditions [10,11]. Aerial spraying can be costly [12], but is effective in reducing target mosquito populations [13–16], and has been linked to reductions in human WNV cases in a treated area relative to an untreated area [15] and in entomological measures of WNV risk [16]. Similar ground ultra-low volume application of adulticides may reduce target mosquito populations under ideal conditions, but studies have provided inconclusive data on their effect on WNV infection rates in mosquitoes or subsequent virus transmission [17–20]. Additionally, off-target effects can occur despite optimal calibration of adulticide applications to host-seeking and active times for target vector species [21–23]. Insecticide applications also often face community opposition due to environmental and toxicity/allergenicity concerns [24–28] and are often restricted to urban and semi-urban communities that can afford to fund them [29,30].
WNV is maintained in an enzootic cycle between Culex mosquitoes and avian hosts. The highest WNV disease incidence occurs along the Great Plains region of the United States [31], as the irrigated agriculture provides a supportive habitat for the main WNV bridge vector of the region, Culex tarsalis [32].Therefore, blood meals by Cx. tarsalis from often-bitten avian species may be utilized to selectively target adult females through their blood feeding behavior. Given that the majority of Cx. tarsalis blood meals on the northern Colorado plains may come from select species during the WNV transmission season [33], effective targeting of these preferred hosts with endectocide-treated bird feed could result in control of WNV transmission.
Previous studies have assessed the use of systemic endectocides provided to wild animals to control tick vector populations. Pound et al. evaluated ivermectin (IVM)-treated corn that was fed to white-tailed deer (Odocoileus virginianus) in a treatment pasture to control tick populations [34]. Amblyomma americanum collections from treatment pastures showed a 83.4% reduction in adults, 92.4% in nymphs, and 100.0% in larvae compared to control pastures [34]. IVM-treated feed provided to O. virginianus, which is the definitive host for the reproductive stage of Ixodes scapularis, has also been explored as a method for controlling this vector of Lyme disease. Rand et al. provided an island community of white-tailed deer with IVM-treated corn for 5 consecutive spring and fall seasons [35]. A treatment effect was observed in island deer that reached target IVM sera concentrations resulting in reductions in adult tick density, engorgement, and oviposition rates as well as reduced rates of larval eclosion from any laid eggs compared to collections from untreated deer on a control island [35]. Dolan et al. also conducted a field study that targeted the rodent reservoirs of Lyme disease to reduce the infection prevalence of Borrelia burgdorferi and Anaplasma phagocytophilum with antibiotic-treated bait. Between treated and control areas, they found that B. burgdorferi prevalence was reduced by 87% and A. phagocytophilum by 74% in small mammals, and in questing nymphal ticks, B. burgdorferi prevalence was reduced by 94% and A. phagocytophilum by 92% [36]. A field study testing the passive application of topical acaricide during bait consumption showed reductions of 68% and 84% of nymphal and larval I. scapularis found on white-footed mice, accompanied by a 53% reduction in the B. burgdorferi infection rate of white-footed mice and a 77% decrease in the questing adult I. scapularis abundance between control and treated properties [37].
Rodent baits with feed-through and systemic insecticide activity have also been evaluated to control the phlebotomine sand fly vectors of zoonotic cutaneous leishmaniasis and visceral leishmaniasis. A wide variety of insecticides have been tested for efficacy against multiple phlebotomine sand fly species using larval and adult blood feeding bioassays in multiple rodents. Methoprene, pyriproxyfen, novularon, eprinomectin, ivermectin, and diflubenzuron have been tested for efficacy within the lab [38–41], while fipronil has been additionally tested in field studies [40,42,43]. Systemic insecticides have also been used to target plague transmission, where field trials have assessed imidalcloprid-treated bait for controlling flea populations in California ground squirrels (Spermphilus beechyi), black-tailed prairie dogs (Cynomys ludovicianus), and other rodents [44–46]. To our knowledge, this strategy of endectocide-treated baits has not been evaluated in birds for arbovirus control.
IVM use in birds is primarily off-label; however, IVM has been administered to treat multiple species of parasites that infest birds, including falcons, cockerels, and chickens [47–50]. Moreno et al. characterized the pharmacokinetics, metabolism, and tissue profiles of IVM in laying hens (Gallus gallus) with IVM delivered using intravenous (IV) and oral routes [51]. For both IV and oral routes, expected pharmacokinetic profiles and tissue distributions consistent for a highly lipophilic drug were observed [51]. Bennett et al. demonstrated transfer of IVM through crop milk when adult pigeon pairs were given 3.3 μg/mL IVM dosed in drinking water and housed with brooding squab, and IVM was subsequently detected in squabs following 3 days of daily adult pigeon IVM dosing [52].
In this present study, we evaluated endectocide-treated bird feed as a systemic endectocide to target Cx. tarsalis. 50% lethal concentrations for selamectin, eprinomectin, and ivermectin were determined in artificial blood meals. IVM-treated bird feed was evaluated for safety and consumption rates in chickens. Mosquitocidal effects in Cx. tarsalis fed on IVM-treated birds were also characterized. Lastly, we present the results of a pilot field trial conducted in Fort Collins, CO in 2017 that examined the safety of IVM-treated bird feed in the field and efficacy on entomological indices of WNV transmission.
Methods
Ethics statement
Animal research was done under CSU IACUC study protocol 16-6552A. Animal euthanasia was applied using sodium pentobarbital as approved in the IACUC study protocol. Field research was done under Colorado Parks and Wildlife Scientific Collection License #17TRb2104 and Fort Collins Natural Areas Permit #914–2017.
Mosquito membrane feeding assays
Cx. tarsalis (Bakersfield colony) were reared in standard insectary conditions (28 ˚C, 16:8 light cycle). Approximately 150 larvae were reared in roughly 3 gallons of water and fed 2.5 grams of powdered Tetramin fish food daily until pupation. Adults were housed at approximately 300 per cage and fed ad libitum sugar and water until separated for bioassays. Mosquito bioassays were performed to determine the lethal concentrations resulting in 50% mortality (LC50) by adding drug (eprinomectin, selamectin, and IVM) into defibrinated calf blood (Colorado Serum Company) at serial dilutions for artificial membrane feeding. Following blood feeding, Cx. tarsalis were knocked down with CO2, and fully-engorged females were collected and held for 5 days in the same insectary conditions. For all bioassays, mosquito mortality was recorded every 24 hours and analyzed using Kaplan-Meier survival curves and compared using Mantel-Cox (log-rank) test. LC50 values were calculated using a nonlinear mixed model with probit analysis [53].
Artificial membrane blood feeds were also used to test the effects of IVM and WNV on Cx. tarsalis mortality. The WNV strain used was a 2012 Colorado isolate propagated in Vero cells. Negative controls were DMEM (Dulbecco’s Modified Eagle Media) and DMSO (dimethyl sulfoxide) at the same volumes as WNV and IVM, respectively. For the concurrent blood feed of WNV and IVM, IVM at 73.66 ng/mL (LC75) and WNV at low titer (5x105 PFU/mL) or high titer (107 PFU/mL) were fed in a membrane blood meal to Cx. tarsalis and mortality was observed as described above. For the WNV-exposure followed by an IVM blood feed, mosquitoes were fed a first blood meal containing 107 PFU/mL of WNV or DMEM for a mock-exposure. Fully engorged females were sorted and held for 10 days, then fed a second blood meal containing 73.66 ng/mL IVM, after which fully blood fed females were sorted and mortality observed.
Birds
4–6 weeks old white leghorn chickens were divided into groups (n = 4) that were housed separately, and which were provided clean water daily and control (untreated) diet consisting of a cracked corn mix (Chick Start and Grow, Northern Colorado Feeders Supply) mixed with any additives that were also added to IVM-treated diet for 3 or 7 consecutive days. IVM-treated diet consisted of two formulations: an Ivomec formulation where liquid Ivomec (Merial) was mixed directly into the cracked corn mix and a powder IVM formulation where powder IVM (Sigma-Aldrich) was mixed into all-purpose flour at 5% and then added to the cracked corn mixture to aid in even powder distribution. Chickens were fed ad libitum and feed consumed by each group was measured daily. Chickens were weighed daily and observed for clinical signs of toxicity, including diarrhea, mydriasis, ptosis, stupor and ataxia. The amount of chicken feed consumed was compared between groups using the students t-test and chicken growth rates were compared using linear regression. Blood was collected from these chickens through venipuncture at the end of their IVM diet regimen and for two days following IVM diet withdrawal. Serum was then isolated from the blood samples and stored at -80°C until further analysis.
Eurasian collared doves (Streptopelia decaocto) were captured by mist net in Wellington, CO and brought back to CSU. They were housed in groups of three and provided ad libitum clean water and either control diet or powder IVM formulation diet of 200 mg IVM/kg of diet for 10 days. Three doves were fed each control and powder IVM formulation diet and then used for mosquito bioassays.
Mosquito bioassays following blood feeding on birds were conducted on the last day of the IVM diet regimen for each group and for two days following IVM diet removal. For direct blood feeding on birds, the downy breast feathers were trimmed, and the exposed bird breast was placed on top of the mosquito cage. The birds were gently restrained for 30 minutes while the mosquitoes blood fed through the mosquito cage organdy. Given the difficulties of direct mosquito blood feeding on live chickens, supplemental serum-replacement membrane blood feeds were also performed, where frozen chicken serum was used in reconstituted blood meals using red blood cells from defibrinated calf blood [54,55].
All research with animals was reviewed and conducted under authorization by the Colorado State University Institutional Animal Care and Use Committee, protocol 16-6552A. Colorado State University Animal Care and Use is Public Health Service (PHS) and Office for Laboratory Animal Welfare (OLAW) assured (#A3572-01), United States Department of Agriculture (USDA) registered (#84-R-0003), and Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited (#000834).
IVM extraction and derivatization
All chemicals used in derivatization were HPLC grade and purchased from Sigma-Aldrich. IVM was extracted from serum following methanol precipitation [56]. 400 μL of methanol was added to 100 μL serum and vortexed for 1.5 min. Methanol precipitation was carried out at -80˚C overnight. Samples were centrifuged for 30 min at 16,000 x g. Supernatants were transferred and evaporated to dryness using a Speedvac concentrator (Savant). The dry residue was dissolved in 20 μL acetonitrile. Samples were derivatized according to previously published literature [57].
HPLC quantification
A Waters 700 autosampler system was used to quantify IVM by high-performance liquid chromatography (HPLC)-fluorescence. A mobile phase of acetonitrile/water (3:1, v/v) was pumped through a C8 column (Waters, XBridge BEH C8 XP, 130 Å, 2.5 μm, 2.1x100 mm) at a rate of 0.45 mL/min. Excitation and emission spectra were 365 and 470 nm, respectively. 10 μL of derivatized sample was injected by the autosampler.
Precision was quantified as coefficient of variation (%CV). This was calculated interday and intraday, evaluating drug-free chicken serum samples (n = 5) spiked with IVM at 25, 50, and 100 ng/mL. Instrument CV was 6.11%. Intraday CV ranged between 4.36 and 9.77%. Interday reproducibility was 15.39%. Retention time CV was 1.77%.
The method was linear across the range of concentrations tested in the standard curve (3.125–100 ng/mL). Linear regression curves containing fortified IVM serum samples with concentrations of 3.125, 6.25, 12.5, 25, 50, and 100 ng/mL had a R-square value of 0.9974. Limits of detection and quantification were 1.56 ng/mL and 3.125 ng/mL, respectively.
Pilot field trial
For the 2017 pilot trial, field sites were located in urban and suburban areas in the City of Fort Collins (mainly in city open space areas and near water sources) that were weekly mosquito trapping sites used by the city for WNV surveillance efforts and have been maintained since 2006 (S1 Fig). Six field sites were chosen based on historical WNV surveillance data from all city trapping sites as those having the highest number of WNV-positive Cx. tarsalis pools since 2006, while excluding trap sites in neighborhoods that are regularly treated with adulticides or used as sentinel sites for the Colorado WNV surveillance system by the state department of health. The 6 chosen sites were all in east Fort Collins and were randomly placed into the treatment group (3 sites; mosquito traps surrounded by IVM-treated bird feed stations) or the control group (3 sites; mosquito traps surrounded by control un-treated bird feed stations). At each field site, an array of three bird feed stations was placed in an approximate triangular perimeter around the mosquito trap at a distance of 50 m (S1 Fig). IVM-treated bird feed was used at a concentration of 200 mg/kg of diet, and the diet was a mixture of white proso millet, cracked corn, and flour (47.5:47.5:5, v/v/v). IVM-treated bird feed was changed daily to account for any effects of IVM degradation due to exposure, which also allowed for daily monitoring of any obvious adverse effects of IVM in local fauna.
Motion-activated trail cameras were used to document bird visits to feeders, with each field site having a motion-activated trail camera placed at one of the three feeders. Photos were screened using the Sibley Guide to Birds [58]. Due to an overabundance of pictures, only a random sampling of 6 days from the 2017 season were counted. Bird trapping and sampling of their blood was performed at two IVM sites. Birds were caught using mist nets placed approximately 10 m from an IVM-treated bird feeder. Blood was collected from netted birds using jugular venipuncture and placed into serum separator tubes. Bird sera were analyzed using HPLC-fluorescence and a subset of samples was analyzed using LC-MS. Because 200 μL of blood could not be drawn from the sparrows as needed for HPLC-fluorescence quantification, IVM analysis for sparrows was only documented as presence or absence. Control sera from house sparrows caught in spring 2014 were used as negative controls. Serum from one IVM-positive grackle was also used in a serum-replacement blood feed with colony Cx. tarsalis for a mosquito survival bioassay.
Mosquitoes were processed as part of the Fort Collins WNV surveillance program according to established protocols [59]. Briefly, mosquitoes were collected weekly by Vector Disease Control International using miniature CDC light traps baited with CO2. Mosquitoes were sorted to species and pooled into groups of typically no more than 50. Mosquito pools were screened at CSU using qRT-PCR using the following primer sequences: forward 5’ 1160-TCAGCGATCTCTCCACCAAAG 3’, reverse 5’ 1209-GGGTCAGCACGTTTGTCATTG 3’, probe 5’ FAM-1186-TGCCCGACCATGGGAGAAGCTC 3’ [59].
Bird sampling was done under Colorado Parks and Wildlife Scientific Collection License #17TRb2104 and Fort Collins Natural Areas Permit #914–2017.
Statistical analysis
Chicken feed consumption was compared between groups using a t-test. Linear regression was done on chicken weights and the rate of weight gain was compared using Analysis of Covariance.
For mosquito bioassays, survival was analyzed using Kaplan-Meier survival curves and compared using Mantel-Cox (log-rank) test. LC50 values were calculated using a nonlinear mixed model with probit analysis [53]. IVM sera concentrations from chickens were compared using ANOVA. IVM sera concentrations from individual chickens were correlated to cumulative mosquito morality from bioassays conducted on the respective chickens using Spearman correlation.
The field trial utilized control and treatment sites located in the City of Fort Collins; however, it was an exploratory trial to test a new trial design and sites, and so was not powered for detecting differences in Cx. tarsalis abundance and WNV infection. Cx. tarsalis abundance from control and treatment sites were compared against each other using a generalized linear mixed model with negative binomial distribution that included site, week of trapping, and treatment. Cx. tarsalis abundance was also shown in comparison to historical data from 2006–2016 (which lacked any bird feed stations surrounding the traps). WNV infection rate was calculated as maximum likelihood estimate (MLE) using the Excel PooledInfRate Add In [60], but Fisher’s exact test was again used to compare the total number of WNV-positive and WNV-negative pools between control and treatment sites.
Statistical analyses were done in GraphPad Prism (Version 7) and R (Version 3.3.1).
Results
IVM has mosquitocidal effects in Cx. tarsalis
Mosquitocidal concentrations of IVM, selamectin, and eprinomectin were determined with mosquito bioassays following blood feeds with serially diluted drug (S2 Fig). IVM had the lowest LC50 concentration at 49.94 ng/mL (Table 1) as compared to eprinomectin with a LC50 of 101.59 ng/mL and selamectin with a LC50 of 151.46 ng/mL. With the lowest effective concentrations, ivermectin was chosen for further characterization in birds.
Table 1. Lethal concentrations (LCx) of ivermectin (IVM) for Cx. tarsalis when added to membrane blood meals.
| LC(x) | [IVM] (ng/mL) |
|---|---|
| LC5 | 19.35 [10.52–26.80] |
| LC10 | 23.86 [14.29–31.62] |
| LC25 | 33.85 [23.58–42.10] |
| LC50 | 49.94 [39.71–59.93] |
| LC75 | 73.66 [61.37–92.96] |
| LC100 | 104.52 [84.38–149.54] |
Brackets indicate 95% Confidence Intervals
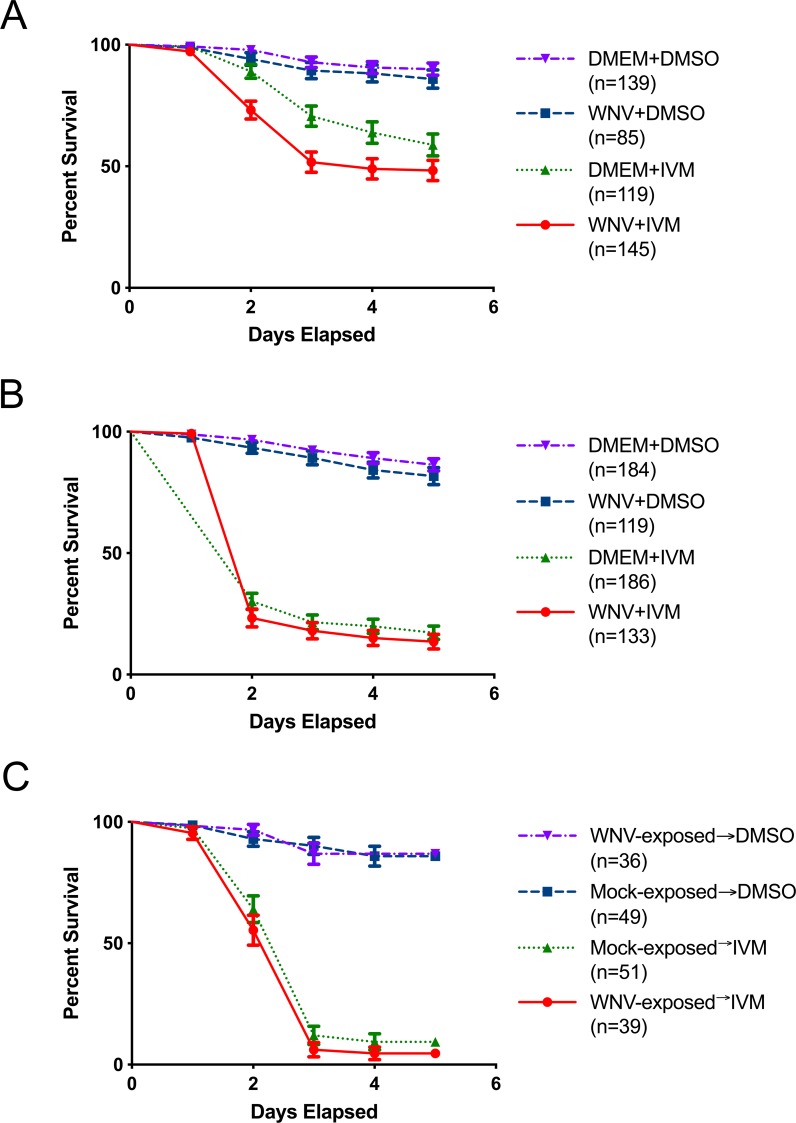
Potential interactions of IVM and WNV on Cx. tarsalis mortality were assessed in a simultaneous blood meal containing IVM (LC75) and WNV. Feeding with IVM only resulted in significantly increased mortality compared to DMSO controls; however, the observed 41% and 83% mortality for IVM control groups (Fig 1A and 1B) reflect the variability of mosquito bioassays, especially for intermediate ranges of lethal concentrations. WNV (both low and high titer) exposure in the absence of IVM did not affect Cx. tarsalis mortality over 5 days immediately after the blood meal (Fig 1A and Fig 1B), or following a second untreated blood meal 10 days later (Fig 1C). On the other hand, Cx. tarsalis given a concurrent blood meal containing low-titer WNV and IVM exhibited significantly increased mortality at 51% compared to the control IVM group not fed WNV with 41% morality (p = 0.0268, χ2 = 4.904) (Fig 1A). However, there was no significant difference (p = 0.2529, χ2 = 1.307) in mortality between Cx. tarsalis fed a concurrent blood meal containing high titer WNV and IVM compared to the control (Fig 1B). Similarly, Cx. tarsalis given a first blood meal of either DMEM control or high titer WNV, and then a second blood meal containing IVM 10 days later, showed no significant differences in mortality (p = 0.1637, χ2 = 1.940) (Fig 1C).
Fig 1. Cx. tarsalis survival in bioassays with IVM and WNV.
(A) Cx. tarsalis survival following a membrane blood meal containing IVM at 73.66 ng/mL (LC75) and WNV at a titer of (A) 5x105 PFU/mL or (B) 107 PFU/mL. (C) Cx. tarsalis survival following a second membrane blood meal of IVM at 73.66 ng/mL given 10 days after a first blood meal of 107 PFU/mL WNV. Error bars indicate standard error.
Birds safely consume IVM-treated diet
Over 7 days of observation, there were no observable clinical signs of IVM neurotoxicity—diarrhea, mydriasis, ptosis, stupor, and ataxia–in groups that consumed either liquid Ivomec or powder formulations of IVM of 200 mg IVM/kg of diet.
For the Ivomec formulation diet, the chickens consumed an average 59.3 g of feed per chicken daily. This was significantly less than the corresponding control group which averaged 121.6 g of feed per chicken per day (p = 0.0045, t = 3.490). Consequently, there was also a significant difference (p <0.0001, F = 19.45) in the rate of weight gain between Ivomec and control groups (S3A Fig). For the powder IVM formulation diet, the IVM group consumed 60.97 g of feed per chicken each day, which was not significantly different from daily control group consumption of 55.2 g of feed per chicken (p = 0.2928, t = 1.100). This was also reflected in similar rates of weight gain between powder IVM and control groups (p = 0.0680, F = 4.022) (S3B Fig).
Blood feeding on IVM-treated birds resulted in significantly increased Cx. tarsalis mortality
Cx. tarsalis mortality following blood feeding on IVM-treated chickens increased as IVM concentration within the diet increased (S4 Fig). There were significant differences in mosquito mortality following blood feeding on chickens given 50 mg IVM/kg of diet (p = 0.0132, χ2 = 6.146) and 100 mg IVM/kg of diet (<0.0001, χ2 = 86.48). However, the largest increase in mortality (p<0.0001, χ2 = 461.1) following blood feeding was at 200 mg IVM/kg of diet with 95.2% mortality in mosquitoes fed on IVM-treated chickens and 2.7% mortality in mosquitoes fed on control chickens. All subsequent experiments used IVM-treated feed at 200 mg IVM/kg of diet.
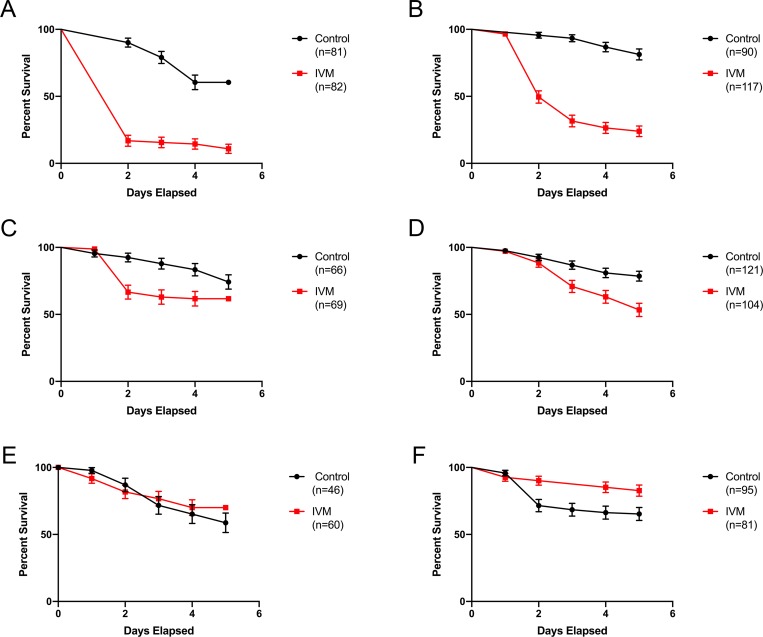
For the Ivomec formulation at 200 mg IVM/kg of diet, there was a significantly increased mortality in mosquitoes blood fed on chickens consuming Ivomec-diet for either 3 or 7 days as compared to mosquitoes blood fed on control chickens (Fig 2; left and right panels, respectively). On the last day of Ivomec feed administered, for both 3 or 7 days, there was a significant increase (p<0.0001, χ2 = 80.22 and χ2 = 76.41, respectively) in mortality between mosquitoes blood fed on chickens consuming an Ivomec diet with upwards of 80% mortality as compared to mosquitoes blood fed on control chickens with less than 40% mortality (Fig 2A and 2B). This difference in mosquito mortality between treatment and controls decreased when the blood feed occurred 1 day following the withdrawal of the Ivomec diet in the treatment group (Fig 2C and 2D). After 2 days following Ivomec diet withdrawal, there was no significant difference in mosquito mortality between those blood fed on Ivomec-consuming chickens as compared to mosquitoes blood fed on control chickens in the 3 day group, but there was a significant difference in the 7 day IVM group (p = 0.0117, χ2 = 6.354) which is likely due to the variability in mosquito bioassays (Fig 2E and 2F). In addition, the time administered Ivomec-treated diets (3 vs. 7 days) did not affect mosquito survival curves following direct blood feeding on chickens, regardless if the mosquitoes were blood fed on the last day of chicken time on the diets, or if the chickens were 1 or 2 days post withdrawal of the diets (Fig 2, left vs. right panels).
Fig 2. Blood feeding on Ivomec-treated chickens increased Cx. tarsalis mortality.
Cx. tarsalis survivorship following direct blood feeding on chickens given Ivomec-formulation diet at a concentration of 200 mg IVM/kg of diet for 3 (left panels: A, C, E) and 7 (right panels: B, D, F) days. (Top panels: A, B) Blood feeding occurred on the last day treated diet was given to the IVM groups. (Middle panels: C, D) Blood feeding occurred one day after treated diet was withdrawn from the IVM groups. (Bottom panels: E, F) Blood feeding occurred on the second day after treated diet was withdrawn from the IVM groups. Error bars indicate standard error.
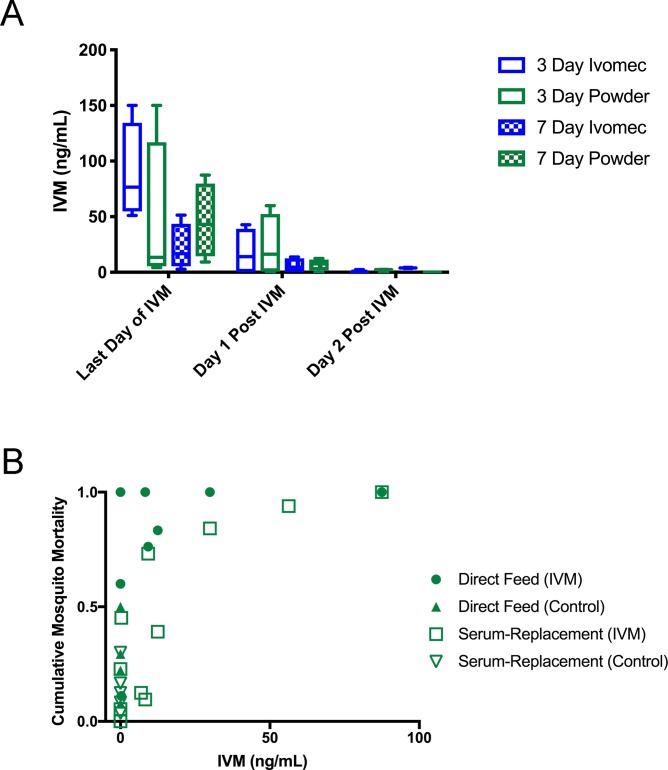
There was also significantly increased mosquito mortality in mosquitoes blood fed on chickens consuming the powder formulation of IVM (200 mg IVM/kg of diet) compared to mosquitoes fed on control chickens (S5 Fig). Because bioassays from the Ivomec formulation and a preliminary powder formulation indicated no differences between mosquitocidal effects for groups given IVM for 3 or 7 days, these and subsequent experiments focused on the 7 day time point. A direct blood feed of mosquitoes on chickens given a powder IVM diet for 7 days resulted in 92.3% mosquito mortality as compared to 25.7% mosquito mortality from those blood fed on control chickens (p<0.0001, χ2 = 41.23) (S5A Fig), while an indirect, serum-replacement blood feed using sera from chickens given a powder IVM diet for 7 days resulted in 79.0% mosquito mortality as compared to 16.7% mortality from those blood fed on control chicken serum (p<0.0001, χ2 = 42.83) (S5B Fig). Furthermore, the mosquito survival curves between those blood fed directly on IVM-treated chickens as compared to sera from IVM-treated chickens were significantly different (red lines in S5A Fig vs. S5B; p<0.0001; hazard ratio 2.007). At 1 day post-powder IVM diet withdrawal, there was still a significant difference (p = 0.001, χ2 = 10.86) in mosquito mortality between those directly blood fed on IVM-diet vs control-diet chickens (S5C Fig; 90.9% vs. 0% mortality). However, this mosquitocidal effect was not apparent in a serum-replacement blood feed derived from chicken blood taken 1 day after IVM diet withdrawal (p = 0.7445, χ2 = 0.1062) (S5D Fig). As above, the mosquito survival curves between those blood fed directly vs. indirectly on treated chickens 1 day post-diet withdrawal were also significantly different (red lines in S5C Fig vs. S5D; p<0.0001; hazard ratio 6.742). At 2 days post-IVM diet withdrawal, blood/serum from treated chickens was no longer mosquitocidal in either direct blood feeding (p = 0.8402, χ2 = 0.04065) or serum-replacement (p = 0.1792, χ2 = 1.804) assays (S5E and S5F Fig).
Direct blood feeds of Cx. tarsalis were also conducted on six wild caught Eurasian Collared Doves fed either a powder IVM formulation diet of 200 mg IVM/kg or control diet in the laboratory (Fig 3). There was a significant difference in mosquito mortality (p<0.0001, χ2 = 60.34) with 88.5% mortality in Cx. tarsalis fed on IVM-treated doves as compared to 14.3% mortality from mosquitoes blood fed on control doves. Additionally, there were no clinical signs of IVM toxicity observed in this treated bird species.
Fig 3. Blood feeding on powder IVM-treated doves increased Cx. tarsalis mortality.
Cx. tarsalis survival following direct blood feeding on captured wild Eurasian collared doves fed powder IVM-diet at a concentration of 200 mg IVM/kg diet for 7 to 10 days. Error bars indicate standard error.
Chicken IVM serum concentrations correlated with Cx. tarsalis mortality in bioassays
Neither the IVM formulation nor the time for which the chickens consumed IVM-treated diet resulted in significant differences in average IVM serum concentrations (p = 0.2715, F = 1.472) (Fig 4A, blue vs. green bars). On the last day of IVM diet, the average IVM serum concentrations (with SD) were 88.575 (±43.613) ng/mL for 3-day Ivomec, 45.255 (±70.051) ng/mL for 3-day powder IVM, 21.910 (±20.914) ng/mL for 7-day Ivomec, 45.745 (±33.852) ng/mL for 7-day powder IVM. Chicken IVM serum concentrations decreased following withdrawal of the IVM diet and were nearly undetectable at 2 days post-withdrawal, which corresponded with mosquito bioassay results showing decreases in mosquitocidal activity following IVM-diet removal. Additionally, IVM serum concentrations were correlated to resulting mosquito mortality from blood feeding on these corresponding IVM-powder fed chickens (Fig 4B). There was a higher correlation between IVM serum concentrations and mortality from serum-replacement feeds with a Spearman r of 0.8629 (P = 0.0007), while the correlation between IVM serum concentrations and mortality from direct blood feeds was 0.4153 (p = 0.3062).
Fig 4. Characterization of chicken IVM sera concentrations.
(A) Chicken sera IVM concentrations measured in IVM-treated groups and taken on the last day treated diet was given, or one or two days after treated diet was withdrawn. Lines indicate median values, boxes indicate 25–75 percentiles, whiskers indicate minimum and maximum values. (B) Individual chicken IVM serum concentrations versus corresponding cumulative Cx. tarsalis mortality on day 5 post blood feeding for both direct and serum-replacement blood feeds on chickens given powder IVM-formulation feed at a concentration of 200 mg IVM/kg of feed.
Pilot field trial
For a pilot trial testing IVM feed in a natural transmission cycle, feeder stations were placed in urban and suburban areas within the City of Fort Collins (S1 Fig) and randomized to treatment or control sites. Bird visits to IVM feeders at all sites were dominated by grackles with infrequent visits by house (Passer domesticus) and sagebrush sparrows (Artemisiospiza nevadensis) and black-capped chickadees (Poecile atricapillus) (Table 2). There were also two visits by blue jays (Cyanocitta cristata), and a few other birds which could not be identified from the photographs. A more homogenous mix of grackles, house and brewers (Spizella breweri) sparrows, blue jays, black-capped chickadees, bushtits, and squirrels visited control feeders (Table 2).
Table 2. 2017 bird visitation data as documented by field cameras on bird feeders.
| Animal | Control Sites | IVM Sites |
|---|---|---|
| Grackle | 92.5% (n = 1219) | 22.8% (n = 31) |
| Blue Jay | 0.15% (n = 2) | 22.8% (n = 31) |
| Sagebrush sparrow | 0.075% (n = 2) | 1.5% (n = 2) |
| Squirrel | 1.3% (n = 15) | 19.9% (n = 27) |
| Raccoons | 0.5% (n = 7) | |
| House sparrow | 0.075% (n = 1) | 0.74% (n = 1) |
| Black-capped chickadee | 2.5% (n = 33) | 6.6% (n = 9) |
| Unidentified | 2.8% (n = 37) | 3.7% (n = 5) |
| Bushtit | 2.9% (n = 4) | |
| Brewer’s sparrow | 19.1% (n = 26) |
Percentages indicate proportion of visits composed of indicated species and n indicates number of documented visits made by the indicated species.
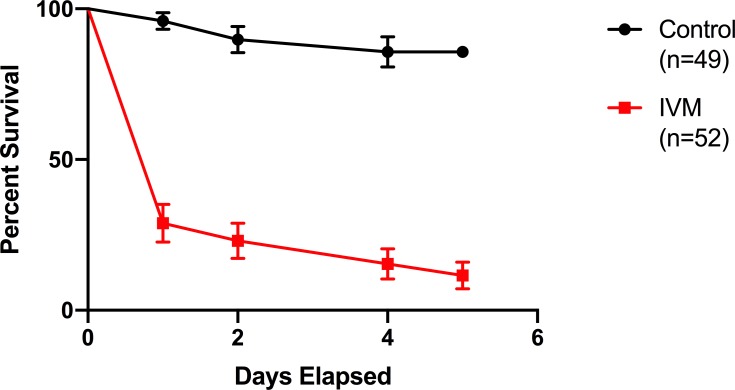
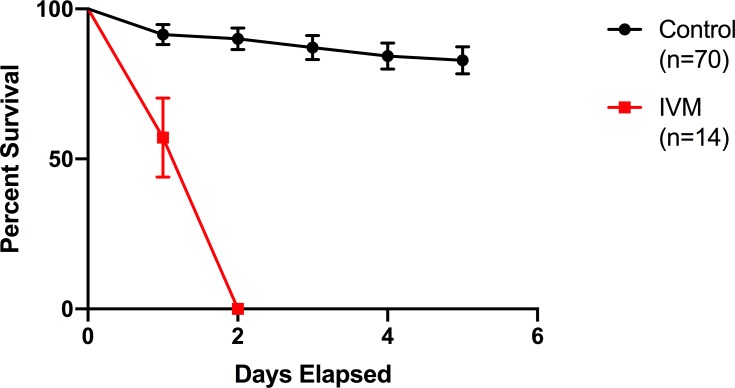
Birds were also caught by mist net and their sera assayed for IVM at the end of the field season. Ten grackles and 5 sparrows were caught over 4 mornings of sampling on August 30th and September 2nd, 3rd, and 7th. Most birds had been observed feeding from the IVM-treated feeder immediately preceding mist net capture. Nine grackles and 4 sparrows (87% of tested sera) had detectable levels of IVM within their serum, and the negative control sparrow serum from 2014 had no detectable IVM (Table 3). Serum from grackle #5 (Table 3) was plentiful and thus further used in a LC-MS assay to confirm the presence of IVM, and also tested in a serum-replacement bioassay. Interestingly, even though the IVM serum concentration in grackle #5 was measured as 5.7 ng/mL, there was strong mosquitocidal effect from this serum (100% mortality within 2 days; p<0.0001, χ2 = 54.15) compared to control mosquitoes fed on control calf serum (Fig 5).
Table 3. Detection and quantification of IVM in field-caught birds in 2017.
| Bird | IVM Detection | IVM Quantification |
|---|---|---|
| Sparrow 1 | Positive | NA |
| Sparrow 2 | Positive | NA |
| Sparrow 3 | Positive | NA |
| Sparrow 4 | Positive | NA |
| Sparrow 5 | Positive | NA |
| Sparrow 6 | Positive | NA |
| Sparrow 7 | Positive | NA |
| Sparrow 8 | Positive | NA |
| Sparrow 9 | Positive | NA |
| Sparrow 10 | Negative | NA |
| Grackle 1 | Negative | NA |
| Grackle 2 | Positive | Below LOQ |
| Grackle 3 | Positive | Below LOQ |
| Grackle 4 | Positive | Below LOQ |
| Grackle 5 | Positive | 5.7 ng/mL |
Sera from field-caught birds was assayed for IVM. Sparrow sera were only used for detection due to low volumes available. Limit of quantification (3.125 ng/mL) is indicated as LOQ.
Fig 5. Blood feeding on wild-caught grackle serum resulted in increased Cx. tarsalis mortality.
Cx. tarsalis survival following a serum-replacement blood feed using serum from a wild-caught grackle in which IVM was detected compared to control calf blood.
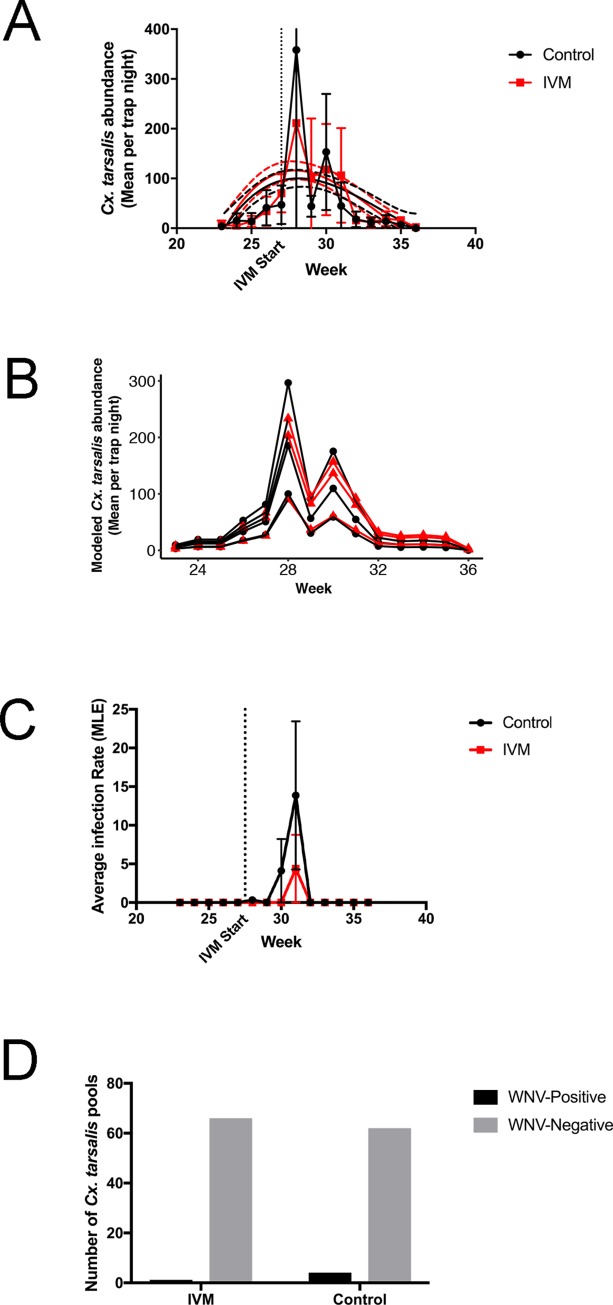
Cx. tarsalis abundance over time in 2017 at the urban and suburban field sites was similar to historical data collected from the same traps for 10 years prior (Fig 6A). A generalized linear mixed model with negative binomial distribution did not find a significant difference between Cx. tarsalis abundance at IVM sites compared to control sites (p = 0.161, z = 1.401) (Fig 6B). The low number of WNV infections did not allow for robust statistical analysis, although MLE was calculated (Fig 6C). A combined Fisher’s Exact Test of all 6 field sites showed a non-significant decrease in the proportion of WNV-positive pools to WNV-negative pools among control and treatment traps (p = 0.2081) (Fig 6D).
Fig 6. Cx. tarsalis abundance and infection for 2017 field sites.
Dotted lines indicate the start of IVM-treated feed at IVM sites. (A) Historical data from the same sites (2006–2016) were fit to non-linear regression (smoothed solid lines) with 95% CIs (smoothed dashed lines), and 2017 data were plotted as connected points. (B) Modeled Cx. tarsalis abundance from each IVM (red) and control (black) site over the season. Red lines indicate IVM treatment. (C) Average infection rate (MLE) from IVM and control sites. (D) Number of WNV-positive and WNV-negative pools at IVM and control sites is shown.
Discussion
This study presents a novel characterization of IVM-treated bird feed as a systemic endectocide to control WNV transmission. Lab studies characterized the effects of IVM-treated bird feed in both domestic and wild birds, especially mosquitocidal effects in Cx. tarsalis blood fed on birds consuming this IVM-containing diet. In addition, a pilot field trial was performed over a WNV season to gather preliminary efficacy data on the effects of IVM-treated bird feed within a natural WNV transmission cycle between wild birds and mosquitoes.
IVM was determined to be the most effective endectocide tested with the lowest lethal concentrations for Cx. tarsalis. In addition, there did not appear to be a synergistic effect of IVM and WNV on Cx. tarsalis mortality in either a simultaneous blood feed of IVM and high titer WNV or sequential blood feeds, the first containing WNV and the second containing IVM. There was a statistical difference between survival curves of Cx. tarsalis fed a concurrent blood meal of a low WNV titer IVM compared to Cx. tarsalis fed only IVM. However, this increased mortality was likely due to the variable survival response of mosquitoes to IVM particularly at intermediate lethal concentrations, rather than a biologically significant interaction between WNV and IVM as there was no mortality difference between mosquitoes fed a concurrent higher titer WNV+IVM blood meal compared to mosquitoes fed DMEM+IVM. There was also no difference between mosquitoes previously exposed to WNV and then fed IVM as compared to mosquitoes unexposed to WNV and then fed IVM. While there is a study suggesting that IVM can inhibit WNV replication by targeting NS3 helicase activity, this was an in vitro cell-culture study using mammalian cells, and the concentration of IVM needed to inhibit 50% of the RNA synthesis in the Vero cells infected with WNV was considerably higher than what was achieved in our chickens following IVM feed consumption [61].
No clinical signs of toxicity were observed in any of the birds consuming either formulation of IVM feed. This was not surprising as IVM is given therapeutically in bird species in a wide range of doses (0.2 mg/kg to 2 mg/kg), depending on route of administration. However, more detailed studies of IVM toxicity should be conducted in multiple bird species in future controlled experiments. Previous studies have identified neurotoxic effects in pigeons following long-term consumption of a diet containing avermectin [62,63], of which IVM is a safer derivative [64]. Specifically, Chen et al. observed clinical signs of neurotoxicity, ranging from reduced activity and food intake following avermectin consumption for 60 days on a 20 mg/kg diet, to ataxia and spasms following avermectin consumption for 30 days on a 60 mg/kg diet [63]. On the other hand, a characterization of IVM pharmacokinetics, metabolism, and tissue distribution in laying hens treated intravenously (400 μg/kg) or consuming IVM-treated water (400 μg/kg/day) for 5 days did not report any ill effects in the birds [51]. Following the intravenous injection of the hens, the highest IVM plasma concentrations (739.6 ± 50.2 ng/mL) were 30 minutes after administration and plasma concentrations remained below 10 ng/mL after 24 hours [51]. Mean IVM concentrations in our chickens fed exclusively on an IVM-containing diet for 3 and 7 days were approximately 45 ng/mL, and similarly we did not observe any neurotoxicity. It remains to be determined if these results vary among different bird species or longer times on the diet. However, in the field studies, it is unlikely that the IVM-treated bird feed was the sole or even primary source of food for the wild birds visiting the feeders given the abundance of alternative food sources during summer.
While chickens on the powder IVM and control diets consumed equivalent quantities of food, there was a significant difference in feed consumption among chicken fed the Ivomec diet and their controls. This may be a result of the glycerol formal and propylene glycol carriers in Ivomec that could give an unpleasant taste, as propylene glycol has been identified as a unpleasant and unpalatable feed additive in cattle [65]. Consequently, the decreased Ivomec feed consumption relative to control feed consumption is likely responsible for the significantly reduced rate of weight gain in the Ivomec group as compared to controls.
Chickens that consumed either a powder IVM or Ivomec diet reached mosquitocidal levels of IVM in their blood within 3 days, as demonstrated by both the IVM serum concentrations in the chickens as well as the significant difference in survival curves of mosquitoes blood fed on IVM-treated chickens compared to controls. There were no notable differences between either IVM diet formulations in mosquitocidal efficacy when considering either time to achieve a mosquitocidal effect and IVM persistence in chicken serum following IVM withdrawal. Furthermore, the time the chickens were placed on the two IVM diets (3 and 7 days) did not significantly affect mosquito mortality, serum concentrations, or the elimination time of IVM from serum following feed withdrawal. This is corroborated by the similar IVM serum concentrations at all time points among the different IVM administration times and formulations. A mosquitocidal effect, but no observable bird toxicity, was demonstrated for wild-caught Eurasian collared doves following consumption of the 200 mg IVM/kg diet, indicating similar mosquitocidal efficacy of the approach in one other bird species and thus potential application to other wild bird species in field settings.
The mosquito mortality in control groups had a greater variation for direct blood feeds (17.75% CV) relative to control groups for serum-replacement blood feeds (3.57% CV), indicating that direct blood feeds results in more inherent variability in mosquito mortality. This increased variability could be a result of increased mosquito handling and rougher conditions during direct blood feeding on birds. It is also possible this higher variability is partly due to smaller sample sizes from the direct blood feeds due to the low success of our colony mosquitoes imbibing full blood meals from live chickens. Regardless, the higher variability among direct blood feed data led to a weaker correlation between IVM serum concentrations and mosquito mortality compared to that from serum-replacement blood feed data. However, despite this higher variability, cumulative mosquito mortality from these direct blood feeds was higher (consistently above 75%) compared to that from the serum-replacement feeds, and mostly independent of measured IVM concentration in the chickens’ sera. One likely possibility for this discrepancy is that the IVM concentration within serum extracted from venous blood may not always be an accurate representation of the IVM concentration in subdermal capillary blood on which mosquitoes blood feed. It has been previously proposed that because IVM is extremely lipophilic and sequestered in fatty tissues, there may exist a concentration gradient of higher IVM or IVM metabolite concentrations in adipose tissue and blood of the surrounding capillaries compared with venous blood [66]. This is also one explanation for the observation that the IVM serum concentrations in chickens correlated with higher cumulative mosquito mortality than would be predicted from the LCx values calculated using artificial membrane feeds. A useful future analysis would be to compare mosquito mortality results from direct skin blood feeding on chickens, membrane blood feeds using venous blood drawn from the chickens, and serum replacement blood feeds using unfrozen serum from the same chickens.
The mosquitocidal effect from chickens on an IVM-containing diet did not extend past one day after IVM-feed withdrawal, and this corresponded with the IVM serum concentrations that were generally below detectable limits by two days post-IVM feed withdrawal. This could potentially be a concern for applying this strategy in the field as it would suggest that frequent bird visits would be necessary to maintain their mosquitocidal blood concentrations of IVM. However, our field data indicated that wild birds were visiting the bird feeders and did have detectable levels of IVM within their sera during multiple days throughout the trial. In addition, one grackle from our 2017 field trial had strongly mosquitocidal serum as assessed in a bioassay, even though the IVM concentration in that serum was surprisingly low. It is promising that a majority of the birds tested had detectable levels of IVM within their sera, indicating that there was an unexpectedly high coverage of IVM in captured birds. However, the placement of mist nets at roughly a 10 m distance from an IVM feeder may have biased the sampling towards birds that visited the feeder, so future studies should more intensively sample birds at wider radii from the feeders. Understanding IVM coverage and persistence within wild birds is an important component of determining the efficacy of this strategy and should be supplemented with detection of IVM in wild-caught blood fed Cx. tarsalis in future field seasons. This could also be coupled with mosquito survival bioassays using wild bird sera to assess mosquitocidal activity as we performed here.
This use of IVM-treated feed as a systemic endectocide to control WNV transmission is based on targeting Cx. tarsalis by medicating its preferred host species. Previous studies in California implicate Cx. tarsalis as a regionally adaptive, opportunistic blood feeder with a preference for avian hosts, and the diversity of available blood meal sources is reflected in the composition of its blood meals [67–71]. Important avian hosts for Cx. tarsalis in small rural towns within Weld County, which is adjacent to our Fort Collins field site area, include American Robins, doves, and other Passeriformes [33]. American Robins are an important Cx. tarsalis blood meal source and WNV amplification host that does not frequent bird feeders and would not be targeted by this current strategy [33,72,73]. However, doves and passerines are preferred blood meal sources of Cx. tarsalis and contribute to the cumulative number of WNV-positive Cx. tarsalis at estimated rates of approximately 30% in June, 60% in July, and 85% in August [33]. This represents a large proportion of Cx. tarsalis blood meal sources and WNV-positive contributions from birds that consume grain and seed that could be targeted throughout the summer season. However, our trail camera data did not show a large proportion of visits from these species identified as regionally important. For example, grackles were predominantly visiting our IVM-treated feeders, while control feeders were visited mostly by grackles, blue jays, brewer’s sparrows, and squirrels. However, the single trail camera we employed per site may not have fully documented bird visits to other feeders at the field site. Camera placement was limited to tree-filled areas where a feeder could be placed with a camera locked to a tree across from the feeder, and this may have biased the camera data against bird species that feed in open space or brush rather than among trees. This limitation of the field camera data is illustrated by our detection of IVM in house sparrows caught by mist net, but we had no documentation of sparrow visits on the trail camera for this specific field site. An important future direction will also be to gather a more updated understanding of the Cx. tarsalis blood meal sources within urban and suburban area of the City of Fort Collins, which might allow for specific targeting of these bird species with attractive bird feed compositions and an optimized bird feeder design.
In addition to a better characterization of avian blood meal sources for Cx. tarsalis, a more complete understanding of bird and Cx. tarsalis spatial dynamics is also important for determining the best placement for the IVM-treated feeders. Because our field sites were chosen based on historical mosquito and WNV surveillance, we did not account for crucial bird parameters that may have influenced mosquito sampling. For example, birds may have fed at the IVM-treated feeders and returned to their communal roosts where they would have been blood fed on by Cx. tarsalis [33,70,74], representing a treatment effect in a different population of Cx. tarsalis than sampled at our traps. Accounting for these bird-mosquito spatial dynamics by placing IVM-treated feeders near communal roosts of granivorous birds and sampling mosquitoes within close range may show the greatest entomological treatment effect, especially as Kent et al. gives an example of a house sparrow roost serving as both a major blood meal and amplification source of WNV-positive Cx. tarsalis [33]. While communal bird roosts could present a critical target, this strategy should continue to be tested in areas of increased human use such as parks and backyards. This highlights that future studies should also consider the best placement of bird feeders in the context of both human land use, and bird and mosquito interactions.
Our pilot field trial was ultimately inconclusive and did not find a significant difference in Cx. tarsalis abundance or WNV infection due to IVM treatment. This is likely due to three field sites for each trial arm being underpowered to observe a significant effect. However, these preliminary field data will serve as important effect size variables with which to properly power future field trials. In addition, this strategy of controlling vector pathogen transmission with an endectocide like IVM is based on shifting the mosquito population age structure in a treatment area from older, infectious mosquitoes to younger, non-infectious mosquitoes, and is less dependent on reducing total mosquito abundance. This has been modeled, as well as observed with empirical data, in trials testing IVM for malaria transmission control [75,76]. We would also expect to see a shift in the age structure of the population to fewer older, infectious Cx. tarsalis and more uninfected, younger mosquitoes. However, our preliminary results from ovary dissections and parity scoring according to Detinova [77] showed consistently high parous rates within the field-caught Cx. tarsalis. This suggested that autogeny, or the ability to develop a batch of eggs without imbibing a blood meal, could be present among the Cx. tarsalis in our study area and confounded our data, and we chose to not conduct further parity scoring during our pilot field trial. As determining age structure of the wild Cx. tarsalis population would be additional way to evaluate this control strategy, future studies should integrate other age-grading techniques such as near infrared spectroscopy (NIRS) [78,79].
Our characterization of IVM as a systemic endectocide in birds demonstrates its feasibility to be developed into a novel WNV transmission control tool. We have demonstrated that birds readily consume IVM-treated feed in the lab and field with our formulation and concentration, while not displaying any observable clinical signs of toxicity following consumption. Furthermore, Cx. tarsalis mosquitoes blood feed on these IVM-treated birds and often die as a result. Our pilot field trial testing IVM-treated feed in natural transmission cycles within wild birds and mosquitoes was ultimately inconclusive, but did provide critical effect size variables to inform future trial design. Important future directions will be to optimize treated bird feed formulations for the field and better characterize the pharmacokinetics and pharmacodynamics of this diet within multiple bird species, especially in relation to mosquitocidal activity and physiological/clinical signs of toxicity. In addition, a more-updated, regionally-specific understanding of the blood meal host preferences of Cx. tarsalis across urban, suburban and rural habitats would allow for better targeting of these preferred host species through the design of an attractive bird feed composition, discriminating bird feeders, and optimized bird feeder location for application to different geographic areas. Finally, our field study provides an important template for future field studies across multiple WNV seasons that will be adequately-powered for measuring effect sizes in entomological and other outcomes.
Supporting information
Panel A depicts the WNV surveillance trap sites within the city of Fort Collins. The 3 control (black circles) and 3 IVM sites (red circles) are shown. Panel B shows a representative field site with an array of 3 bird feeders (red squares) surrounding one mosquito trap (yellow circle). The figure was created using LandsatLookViewer (http://landsatlook.usgs.gov/).
(TIF)
Cx. tarsalis mortality following blood feeding on IVM (A), selamectin (B), and eprinomectin (B). Cx. tarsalis were blood fed on increasing concentrations of endectocides and their mortality was observed to calculate lethal concentrations. Error bars indicate standard error.
(TIF)
Linear relationship between chicken weight and days elapsed is shown where black lines indicate control groups and red lines indicate ivermectin-treated groups of chickens fed (A) Ivomec-formulation diet or (B) powder IVM-formulation diet. Error bars indicate standard deviation.
(TIF)
Cx. tarsalis survival following direct blood feeding on chickens that were fed Ivomec-formulation diet for 7 consecutive days at concentrations of 50 mg IVM/kg of diet, 100 mg IVM/kg of diet, and 200 mg IVM/kg of diet. Error bars indicate standard error.
(TIF)
Cx. tarsalis survivorship following direct (left panels: A, C, E) or serum-replacement (right panels: B, D, F) blood feeding on chickens given powder-IVM diet at a concentration of 200 mg IVM/kg of diet for 7 days. (Top panels: A, B) Blood feeding occurred on, or using serum taken on, the last day treated diet was given to the IVM groups. (Middle panels: C, D) Blood feeding occurred on, or using serum taken on, the day after treated diet was withdrawn from the IVM groups. (Bottom panels: E, F) Blood feeding occurred on, or using serum taken on, the second day after treated diet was withdrawn from the IVM groups. Error bars indicate standard error.
(TIF)
Acknowledgments
The authors would like to thank Jasmine Donkoh, Wojtek Kuklinski, Taylor Clarkson, Cole Mills, Erin Markle, Megan Miller, and Jon Seaman for their help with experiments and rearing Cx. tarsalis. We appreciate Barb Graham’s assistance with statistical analysis. We are also grateful to Dr. Dean Crick for the use of HPLC-fluorescence instrumentation and Dr. Aaron Brault for negative control sparrow serum. We would also like to thank CSU and the City of Fort Collins for allowing us to conduct our pilot field trial. Additionally, we appreciate the discussion and support of Dr. Adrienne LeBailly, Dr. Ann Powers, Matt Parker, and additional members of the Fort Collins West Nile virus technical advisory committee.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was funded by National Institutes of Health award 1R41AI122543-01A1 received by authors BC and BDF. This study was also funded by Colorado State University Infectious Diseases Fund award 1491360 received by author BDF. This work was also supported by the annual City of Fort Collins West Nile virus Surveillance Grant, received by author GDE. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Reimann CA, Hayes EB, Diguiseppi C, Hoffman R, Lehman JA, Lindsey NP, et al. Epidemiology of Neuroinvasive Arboviral Disease in the United States. Am J Trop Med Hyg. 2008;76(6):974–9. [PubMed] [Google Scholar]
- 2.Lindsey NP, Staples JE, Lehman JA, Fischer M, Centers for Disease Control and Prevention (CDC). Surveillance for human West Nile virus disease—United States, 1999–2008. MMWR Surveill Summ. 2010. April 2;59(2):1–17. [PubMed] [Google Scholar]
- 3.CDC. West Nile virus disease cases and deaths reported to CDC by year and clinical presentation, 1999–2015. 2016. [Google Scholar]
- 4.Fox JL. Uncertainties in Tracking West Nile Virus Undermine Vaccine Development. Microbe Mag. 2015. March 1;10(3):98–9. [Google Scholar]
- 5.Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310(3):308–15. 10.1001/jama.2013.8042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thier A. Balancing the Risks: Vector Control and Pesticide Use in Response to Emerging Illness. J Urban Heal Bull New York Acad Med. 2001;78(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gubler DJ, Campbell GL, Nasci R, Komar N, Petersen L, Roehrig JT. West Nile Virus in the United States: Guidelines for Detection, Prevention, and Control. Viral Immunol. 2000;13(4):469–75. 10.1089/vim.2000.13.469 [DOI] [PubMed] [Google Scholar]
- 8.Stockwell PJ, Wessell N, Reed DR, Kronenwetter-Koepel TA, Reed KD, Turchi TR, et al. A Field Evaluation of Four Larval Mosquito Control Methods in Urban Catch Basins. J Am Mosq Control Assoc. 2006. January 20;22(4):666–71. 10.2987/8756-971X(2006)22[666:AFEOFL]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 9.Anderson JF, Ferrandino FJ, Dingman DW, Main AJ, Andreadis TG, Becnel JJ. Control of Mosquitoes in Catch Basins in Connecticut With Bacillus thuringiensis israelensis, Bacillus sphaericus, and Spinosad. J Am Mosq Control Assoc. 2011. March 29;27(1):45–55. 10.2987/10-6079.1 [DOI] [PubMed] [Google Scholar]
- 10.Harbison, Sinacore J, Henry M, Xamplas C, Dugas L, O’Hara Ruiz M. Identification of Larvicide-Resistant Catch Basins from Three Years of Larvicide Trials in a Suburb of Chicago, IL. Environ Health Insights. 2014;8(S2):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harbison, Layden J, Xamplas C, Zazra D, Henry M, O’Hara Ruiz M. Observed Loss and Ineffectiveness of Mosquito Larvicides Applied to Catch Basins in the Northern Suburbs of Chicago IL, 2014. Environ Health Insights. 2015;9:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barber LM, Schleier JJ, Peterson RKD, Peterson RKD. Economic cost analysis of West Nile virus outbreak, Sacramento County, California, USA, 2005. Emerg Infect Dis. 2010. March;16(3):480–6. 10.3201/eid1603.090667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmisano CT, Taylor V, Caillouet K, Byrd B, Wesson DM. Impact of West Nile Virus Outbreak Upon St. Tammany Parish Mosquito Abatement District. J Am Mosq Control Assoc. 2005;21(1):33–8. 10.2987/8756-971X(2005)21[33:IOWNVO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 14.Simpson JE. Emergency Mosquito Aerial Spray Response to the 2004 Florida Hurricanes Charley, Frances, Ivan, and Jeanne: An Overview of Control Results. J Am Mosq Control Assoc. 2006;22(3):457–63. 10.2987/8756-971X(2006)22[457:EMASRT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 15.Carney RM, Husted S, Jean C, Glaser C, Kramer V. Efficacy of aerial spraying of mosquito adulticide in reducing incidence of West Nile Virus, California, 2005. Emerg Infect Dis. 2008. May;14(5):747–54. 10.3201/eid1405.071347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macedo PA, Schleier III JJ, Reed M, Kelley K, Goodman GW, Brown DA, et al. Evaluation of Efficacy and Human Health Risk of Aerial Ultra-Low Volume Applications of Pyrethrins and Piperonyl Butoxide for Adult Mosquito Management in Response to West Nile Virus Activity in Sacramento County, California. J Am Mosq Control Assoc. 2010;26(1):57–66. 10.2987/09-5961.1 [DOI] [PubMed] [Google Scholar]
- 17.Lothrop HD, Lothrop BB, Gomsi DE, Reisen WK. Intensive Early Season Adulticide Applications Decrease Arbovirus Transmission Throughout the Coachella Valley, Riverside County, California. Vector-Borne Zoonotic Dis. 2008;8(4):475–89. 10.1089/vbz.2007.0238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lothrop H, Lothrop B, Palmer M, Wheeler S, Gutierrez A, Gomsi D, et al. Evaluation of pyrethrin and permethrin ground ultra-low volume applications for adult Culex control in rural and urban environments of the Coachella Valley of California. Source J Am Mosq Control Assoc. 2007;23(2):190–207. [DOI] [PubMed] [Google Scholar]
- 19.Reddy MR, Spielman A, Lepore TJ, Henley D, Kiszewski AE, Reiter P. Efficacy of Resmethrin Aerosols Applied from the Road for Suppressing Culex Vectors of West Nile Virus. Vector-borne Zoonotic Dis. 2006;6(2):117–27. 10.1089/vbz.2006.6.117 [DOI] [PubMed] [Google Scholar]
- 20.Mutebi J-P, Delorey MJ, Jones RC, Plate DK, Gerber SI, Gibbs KP, et al. The Impact of Adulticide Applications on Mosquito Density in Chicago, 2005. J Am Mosq Control Assoc. 2011. March 29;27(1):69–76. 10.2987/10-6045.1 [DOI] [PubMed] [Google Scholar]
- 21.Ultra-low-volume space sprays in mosquito control: a critical review. Med Vet Entomol. 2012;26:121–30. 10.1111/j.1365-2915.2011.00992.x [DOI] [PubMed] [Google Scholar]
- 22.Urquhart C, Paulsen D, Fryxell RTT. La Crosse Virus Vectors Are Host-Seeking and Ovipositing After 1700 H in Eastern Tennessee. J Am Mosq Control Assoc. 2017. September 30;33(3):233–6. 10.2987/16-6620R.1 [DOI] [PubMed] [Google Scholar]
- 23.Farajollahi A, Healy SP, Unlu I, Gaugler R, Fonseca DM. Effectiveness of Ultra-Low Volume Nighttime Applications of an Adulticide against Diurnal Aedes albopictus, a Critical Vector of Dengue and Chikungunya Viruses. Shiff C, editor. PLoS One. 2012. November 8;7(11):e49181 10.1371/journal.pone.0049181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oberhauser KS, Manweiler SA, Lelich R, Blank M, Batalden R V, De Anda A. Impacts of Ultra-Low Volume Resmethrin Applications on Non-Target Insects. Source J Am Mosq Control Assoc. 25(1):83–93. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen JJ, Wiberg-Larsen P, Kristensen EA, Cedergreen N, Friberg N. Pyrethroid effects on freshwater invertebrates: A meta-analysis of pulse exposures. Environ Pollut. 2013. November 1;182:479–85. 10.1016/j.envpol.2013.08.012 [DOI] [PubMed] [Google Scholar]
- 26.Godfray HCJ, Blacquière T, Field LM, Hails RS, Petrokofsky G, Potts SG, et al. A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc R Soc B Biol Sci. 2014;281(1786):20140558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner-Schuman M, Richardson JR, Auinger P, Braun JM, Lanphear BP, Epstein JN, et al. Association of pyrethroid pesticide exposure with attention-deficit/hyperactivity disorder in a nationally representative sample of U.S. children. Environ Heal. 2015;14(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross SM, McManus IC, Harrison V, Mason O. Neurobehavioral problems following low-level exposure to organophosphate pesticides: a systematic and meta-analytic review. Crit Rev Toxicol. 2013. January 19;43(1):21–44. 10.3109/10408444.2012.738645 [DOI] [PubMed] [Google Scholar]
- 29.Winters AM, Eisen RJ, Lozano-Fuentes S, Moore CG, Pape WJ, Eisen L. Predictive spatial models for risk of West Nile virus exposure in eastern and western Colorado. Am J Trop Med Hyg. 2008;79(4):581–90. [PMC free article] [PubMed] [Google Scholar]
- 30.Tedesco C, Ruiz M, McLafferty S. Mosquito politics: Local vector control policies and the spread of West Nile Virus in the Chicago region. Heal Place. 2010;16(6):1188–95. [DOI] [PubMed] [Google Scholar]
- 31.CDC. Average annual incidence of West Nile virus neuroinvasive disease reported to CDC by county, 1999‐2012. 2013;2013. [Google Scholar]
- 32.Eisen L, Barker CM, Moore CG, Pape WJ, Winters AM, Cheronis N. Irrigated agriculture is an important risk factor for West Nile virus disease in the hyperendemic Larimer-Boulder-Weld area of north central Colorado. J Med Entomol. 2010;47(5):939–51. [DOI] [PubMed] [Google Scholar]
- 33.Kent R, Juliusson L, Weissmann M, Evans S, Komar N. Seasonal Blood-Feeding Behavior of Culex tarsalis (Diptera: Culicidae) in Weld County, Colorado, 2007. J Med Entomol. 2009;46(2):380–90. [DOI] [PubMed] [Google Scholar]
- 34.Pound JM, Miller JA, George JE, Oehler DD, Harmel DE. Systemic treatment of white-tailed deer with ivermectin-medicated bait to control free-living populations of lone star ticks (Acari:Ixodidae). J Med Entomol. 1996;33(3):385–94. [DOI] [PubMed] [Google Scholar]
- 35.Rand PW, Lacombe EH, Holman MS, Lubelczyk C, Smith RP. Attempt to Control Ticks (Acari: Ixodidae) on Deer on an Isolated Island Using Ivermectin-Treated Corn. J Med Entomol. 2000;37(1):126–33. [DOI] [PubMed] [Google Scholar]
- 36.Dolan MC, Schulze TL, Jordan RA, Dietrich G, Schulze CJ, Hojgaard A, et al. Elimination of Borrelia burgdorferi and Anaplasma phagocytophilum in Rodent Reservoirs and Ixodes scapularis Ticks Using a Doxycycline Hyclate-Laden Bait. Am J Trop Med Hyg. 2011;85(6):1114–20. 10.4269/ajtmh.2011.11-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dolan MC, Maupin GO, Schneider BS, Hamon N, Cole C, Zeidner NS, et al. Control of Immature Ixodes scapularis (Acari: Ixodidae) on Rodent Reservoirs of Borrelia burgdorferi in a Residential Community of Southeastern Connecticut. J Med Entomol. 2004;41(6):1043–54. [DOI] [PubMed] [Google Scholar]
- 38.Mascari TM, Mitchell MA, Rowton ED, Foil LD. Evaluation of juvenile hormone analogues as rodent feed-through insecticides for control of immature phlebotomine sandflies. Med Vet Entomol. 2011;25(2):227–31. 10.1111/j.1365-2915.2010.00919.x [DOI] [PubMed] [Google Scholar]
- 39.Mascari TM, Stout RW, Foil LD. Evaluation of three feed-through insecticides using two rodent and two sand fly species as models. J Am Mosq Control Assoc. 2012;28(3):260–2. 10.2987/11-6215R.1 [DOI] [PubMed] [Google Scholar]
- 40.Ingelhoff K, Garlapati R, Poche D, Singh MI, Remmers JL, Poche RM. Feed-through insecticides for the control of the sand fly Phlebotomus argentipes. Vet Entomol. 2013;27:10–8. [DOI] [PubMed] [Google Scholar]
- 41.Mascari TM, Mitchell MA, Rowton ED, Foil LD. Laboratory Evaluation of Diflubenzuron as a Feed-Through for Control of Immature Sand Flies (Diptera: Psychodidae). J Med Entomol. 2007. March 1;44(2):171–4. [DOI] [PubMed] [Google Scholar]
- 42.Derbali M, Polyakova L, Boujaâma A, Burruss D, Cherni S, Barhoumi W, et al. Laboratory and field evaluation of rodent bait treated with fipronil for feed through and systemic control of Phlebotomus papatasi. Acta Trop. 2014;135:27–32. 10.1016/j.actatropica.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 43.Poché RM, Garlapati R, Singh MI, Poché DM. Evaluation of fipronil oral dosing to cattle for control of adult and larval sand flies under controlled conditions. J Med Entomol. 2013;50(4):833–7. [PubMed] [Google Scholar]
- 44.Borchert JN, Davis RM, Poché RM. Field efficacy of rodent bait containing the systemic insecticide imidacloprid against the fleas of California ground squirrels. J Vector Ecol. 2009;34(1):92–8. 10.1111/j.1948-7134.2009.00011.x [DOI] [PubMed] [Google Scholar]
- 45.Jachowski DS, Skipper S, Gompper ME. Field evaluation of imidacloprid as a systemic approach to flea control in black-tailed prairie dogs, Cynomys ludovicianus. J Vector Ecol. 2011. June 1;36(1):100–7. 10.1111/j.1948-7134.2011.00146.x [DOI] [PubMed] [Google Scholar]
- 46.Borchert JN, Enscore RE, Eisen RJ, Atiku LA, Owor N, Acayo S, et al. Evaluation of Rodent Bait Containing Imidacloprid for the Control of Fleas on Commensal Rodents in a Plague-Endemic Region of Northwest Uganda. J Med Entomol. 2010;47(5):842–50. [DOI] [PubMed] [Google Scholar]
- 47.Lierz M. Evaluation of the dosage of ivermectin in falcons. Vet Rec. 2001;148:596–600. [DOI] [PubMed] [Google Scholar]
- 48.Clyde VL, Patton S. Diagnosis, Treatment, and Control of Common Parasites in Companion and Aviary Birds. [Google Scholar]
- 49.Zeman P. Systemic efficacy of ivermectin against Dermanyssus gallinae (De Geer, 1778) in fowls. Vet Parasitol. 1987;23:141–6. [DOI] [PubMed] [Google Scholar]
- 50.Sharma R, Bhat T. Anthelmintic activity of ivermectin against experimental Ascaridia galli infection in chickens. Vet Parasitol. 1990;37:307–14. [DOI] [PubMed] [Google Scholar]
- 51.Moreno L, Domínguez P, Farias C, Cantón L, Virkel G, Maté L, et al. Ivermectin Pharmacokinetics, Metabolism, and Tissue/Egg Residue Profiles in Laying Hens. J Agric Food Chem. 2015;63:10327–32. 10.1021/acs.jafc.5b04632 [DOI] [PubMed] [Google Scholar]
- 52.Bennett DC, Cheng KM. Ivermectin residues in squab. Poult Sci. 2012;91:2808–11. 10.3382/ps.2012-02529 [DOI] [PubMed] [Google Scholar]
- 53.Kobylinski KC, Deus KM, Butters MP, Hongyu T, Gray M, da Silva IM, et al. The effect of oral anthelmintics on the survivorship and re-feeding frequency of anthropophilic mosquito disease vectors. Acta Trop. 2010;116(2):119–26. 10.1016/j.actatropica.2010.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bousema T, Dinglasan RR, Morlais I, Gouagna LC, Van Warmerdam T, Awono-Ambene PH, et al. Mosquito Feeding Assays to Determine the Infectiousness of Naturally Infected Plasmodium falciparum Gametocyte Carriers. PLoS One. 2012;7(8):e42821 10.1371/journal.pone.0042821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chaccour CJ, Hammann F, Alustiza M, Castejon S, Tarimo BB, Abizanda G, et al. Cytochrome P450/ABC transporter inhibition simultaneously enhances ivermectin pharmacokinetics in the mammal host and pharmacodynamics in Anopheles gambiae. Sci Rep. 2017. December 17;7(1):8535 10.1038/s41598-017-08906-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prieto JG, Merino G, Pulido MM, Estevez E, Molina AJ, Vila L, et al. Improved LC method to determine ivermectin in plasma. J Pharm Biomed Anal. 2003;31(4):639–45. [DOI] [PubMed] [Google Scholar]
- 57.Berendsen BJA, Mulder PPJ, van Rhijn H (J.)A. The derivatisation of avermectins and milbemycins in milk: New insights and improvement of the procedure. 2007. p. 126–33. [DOI] [PubMed] [Google Scholar]
- 58.Sibley DA. The Sibley Guide to Birds. Second. New York: Alfred A. Knopf; 2014. [Google Scholar]
- 59.Fauver JR, Pecher L, Schurich JA, Bolling BG, Calhoon M, Grubaugh ND, et al. Temporal and Spatial Variability of Entomological Risk Indices for West Nile Virus Infection in Northern. J Med. 2016;53(2):425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biggerstaff BJ. PooledInfRate, Version 4.0: a Microsoft Office Excel Add-In to compute prevalence estimates from pooled samples. 2009. [Google Scholar]
- 61.Mastrangelo E, Pezzullo M, De Burghgraeve T, Kaptein S, Pastorino B, Dallmeier K, et al. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J Antimicrob Chemother. 2012. August;67(8):1884–94. 10.1093/jac/dks147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li M, You T-Z, Zhu W-J, Qu J-P, Liu C, Zhao B, et al. Antioxidant response and histopathological changes in brain tissue of pigeon exposed to avermectin. Ecotoxicology. 2013. October 14;22(8):1241–54. 10.1007/s10646-013-1112-7 [DOI] [PubMed] [Google Scholar]
- 63.Chen L-J, Sun B-H, Cao Y, Yao H-D, Qu J-P, Liu C, et al. The effects of avermectin on amino acid neurotransmitters and their receptors in the pigeon brain. Pestic Biochem Physiol. 2014. March 1;110:13–9. 10.1016/j.pestbp.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 64.Shoop WL, Mrozik H, Fisher MH. Structure and activity of avermectins and milbemycins in animal health. Vet Parasitol. 1995;59(59):139–56. [DOI] [PubMed] [Google Scholar]
- 65.Nielsen NI, Ingvartsen KL. Propylene glycol for dairy cows A review of the metabolism of propylene glycol and its effects on physiological parameters, feed intake, milk production and risk of ketosis. Anim Feed Sci Technol. 2004;115(115):191–213. [Google Scholar]
- 66.Chaccour C, Hammann F, Rabinovich NR. Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety. Malar J. 2017. December 24;16(1):161 10.1186/s12936-017-1801-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tempelis CH, Reeves WC, Bellamy RE, Lofy MF. A three-year study of the feeding habits of Culex tarsalis in Kern County, California. Am J Trop Med Hyg. 1965;14(1):170–7. [DOI] [PubMed] [Google Scholar]
- 68.Thiemann TC, Lemenager DA, Kluh S, Carroll BD, Lothrop HD, Reisen WK. Spatial Variation in Host Feeding Patterns of Culex tarsalis and the Culex pipiens complex (Diptera: Culicidae) in California. J Med Entomol. 2012. July 1;49(4):903–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campbell R, Thiemann TC, Lemenager D, Reisen WK. Host-Selection Patterns of Culex tarsalis (Diptera: Culicidae) Determine the Spatial Heterogeneity of West Nile Virus Enzootic Activity in Northern California. J Med Entomol. 2013. November 1;50(6):1303–9. [DOI] [PubMed] [Google Scholar]
- 70.Komar N, Panella NA, Young GR, Brault AC, Levy CE. Avian Hosts of West Nile Virus in Arizona. Am J Trop Med Hyg. 2013;89(3):474–81. 10.4269/ajtmh.13-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lothrop HD, Reisen WK. Landscape affects the host-seeking patterns of Culex tarsalis (Diptera: Culicidae) in the Coachella Valley of California. J Med Entomol. 2001;38(2):325–32. [DOI] [PubMed] [Google Scholar]
- 72.McKenzie VJ, Goulet NE. Bird community composition linked to human West Nile virus cases along the Colorado front range. Ecohealth. 2010;7(4):439–47. 10.1007/s10393-010-0360-8 [DOI] [PubMed] [Google Scholar]
- 73.Komar N, Panella NA, Burkhalter KL. Focal amplification and suppression of West Nile virus transmission associated with communal bird roosts in northern Colorado. J Vector Ecol. 2018. December 1;43(2):220–34. 10.1111/jvec.12306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Komar N, Colborn JM, Horiuchi K, Delorey M, Biggerstaff B, Damian D, et al. Reduced West Nile Virus Transmission Around Communal Roosts of Great-Tailed Grackle (Quiscalus mexicanus). Ecohealth. 2014. December 6; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alout H, Krajacich BJ, Meyers JI, Grubaugh ND, Brackney DE, Kobylinski KC, et al. Evaluation of ivermectin mass drug administration for malaria transmission control across different West African environments. Malar J. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sylla M, Kobylinski KC, Gray M, Chapman PL, Sarr MD, Rasgon JL, et al. Mass drug administration of ivermectin in south-eastern Senegal reduces the survivorship of wild-caught, blood fed malaria vectors. Malar J. 2010;9(1):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Detinova TS. Age-grouping methods in Diptera of medical importance with special reference to some vectors of malaria. World Heal Organ Monogr Ser. 1962;47:13–191. [PubMed] [Google Scholar]
- 78.Krajacich BJ, Meyers JI, Alout H, Dabiré RK, Dowell FE, Foy BD. Analysis of near infrared spectra for age-grading of wild populations of Anopheles gambiae. Parasit Vectors. 2017. December 7;10(1):552 10.1186/s13071-017-2501-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Milali MP, Sikulu-Lord MT, Kiware SS, Dowell FE, Povinelli RJ, Corliss GF. Do NIR spectra collected from laboratory-reared mosquitoes differ from those collected from wild mosquitoes? Lazzari CR, editor. PLoS One. 2018. May 31;13(5):e0198245 10.1371/journal.pone.0198245 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Panel A depicts the WNV surveillance trap sites within the city of Fort Collins. The 3 control (black circles) and 3 IVM sites (red circles) are shown. Panel B shows a representative field site with an array of 3 bird feeders (red squares) surrounding one mosquito trap (yellow circle). The figure was created using LandsatLookViewer (http://landsatlook.usgs.gov/).
(TIF)
Cx. tarsalis mortality following blood feeding on IVM (A), selamectin (B), and eprinomectin (B). Cx. tarsalis were blood fed on increasing concentrations of endectocides and their mortality was observed to calculate lethal concentrations. Error bars indicate standard error.
(TIF)
Linear relationship between chicken weight and days elapsed is shown where black lines indicate control groups and red lines indicate ivermectin-treated groups of chickens fed (A) Ivomec-formulation diet or (B) powder IVM-formulation diet. Error bars indicate standard deviation.
(TIF)
Cx. tarsalis survival following direct blood feeding on chickens that were fed Ivomec-formulation diet for 7 consecutive days at concentrations of 50 mg IVM/kg of diet, 100 mg IVM/kg of diet, and 200 mg IVM/kg of diet. Error bars indicate standard error.
(TIF)
Cx. tarsalis survivorship following direct (left panels: A, C, E) or serum-replacement (right panels: B, D, F) blood feeding on chickens given powder-IVM diet at a concentration of 200 mg IVM/kg of diet for 7 days. (Top panels: A, B) Blood feeding occurred on, or using serum taken on, the last day treated diet was given to the IVM groups. (Middle panels: C, D) Blood feeding occurred on, or using serum taken on, the day after treated diet was withdrawn from the IVM groups. (Bottom panels: E, F) Blood feeding occurred on, or using serum taken on, the second day after treated diet was withdrawn from the IVM groups. Error bars indicate standard error.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.