Abstract
Following central nervous system (CNS) injury, activated astrocytes form a glial scar that inhibits the migration of axons ultimately leading to regeneration failure. Biomaterials developed for CNS repair can provide local delivery of therapeutics and/or guidance mechanisms to encourage cell migration into damaged regions of the brain or spinal cord. Electrospun fibers are a promising type of biomaterial for CNS injury since these fibers can direct cellular and axonal migration while slowly delivering therapeutic to the injury site. In this study, it was hypothesized that inclusion of an anti-metabolite, 6-aminonicotinamide (6AN), within poly-L-lactic acid (PLLA) electrospun fibers could attenuate astrocyte metabolic activity while still directing axonal outgrowth. Electrospinning parameters were varied to produce highly aligned electrospun fibers that contained 10% or 20% (w/w) 6AN. 6AN release from the fiber substrates occurred continuously over two weeks. Astrocytes placed onto drug-releasing fibers were less active than those cultured on scaffolds without 6AN. Dorsal root ganglia (DRG) placed onto control and drug-releasing scaffolds were able to direct neurites along the aligned fibers. However, neurite outgrowth was stunted by fibers that contained 20% 6AN. These results show that 6AN release from aligned, electrospun fibers can decrease astrocyte activity while still directing axonal outgrowth.
Keywords: astrocyte, electrospun fibers, controlled drug-release, central nervous system injury, neural tissue engineering
1. Introduction
Aligned, electrospun fibers direct neurite outgrowth (1–8) especially when a high degree of fiber alignment is attained (7,8). In addition to fiber alignment, other physical characteristics such as fiber diameter also impact neurite extension (9), and when these fibers possess the proper diameter and degree of alignment, longer, more directed neurite extension occurs (8). Typically these studies utilize an in vitro dorsal root ganglia (DRG) explant model, but neuritogenesis is also seen in models using primary motor neurons (10).
Significant progress is being made to optimize the physical characteristics (fiber alignment, diameter, and selection of materials) of electrospun fibers for neural repair (8,9). The focus on these physical characteristics predominates in comparison to studies that attempt to release therapies that alter glial or neuronal response. While studies have incorporated chemotherapies (11–13) or protein (14–15) within or on the surface of fibers to improve cellular adhesion or enhance axonal regeneration, few studies attempt to vigorously control electrospinning parameters so that aligned, electrospun substrates are fabricated that also release therapy.
Examination of harmful astrocyte behavior on electrospun fibers does not often occur. Additionally, when therapies are selected for release from electrospun fibers, they are not often selected for the purpose of directly affecting astrocyte activity. Of recent significance, it was discovered that astrocytes cultured on polydioxanone electropun fibers helped facilitate axonal outgrowth from DRG neurons (1). A more recent in vitro model using a DRG/non-reactive astrocyte coculture system in the presence of polyamide nanofibers coupled to fibroblast growth factor-2 (FGF-2) enhanced axonal extension (15). These studies suggest that astrocytes may play a beneficial role in the regeneration process when used in combination with aligned, electrospun fibers. However, in vivo astrocytes create an impassable barrier in response to injury (16), and this is believed to be a protective response that separates damaged tissue from healthy tissue (17). If this scar tissue remains after injury, even if the extent of scar formation is diminished (18), then regeneration cannot occur. It has been suggested that if astrocytes are prevented from properly forming this scar tissue, then regeneration may occur (19).
The mechanisms that lead to astrocyte reactivity are still unclear due to the inherent complexity of astrocytes, but significant efforts to better understand activation pathways and methods to prevent reactivity are underway (16). The anti-metabolite 6-aminonicotinamide (6AN) is known to inhibit the pentose phosphate shunt (PPP) and effectively deplete astrocytes of metabolites (20–21) and nucleotides (21). 6AN affects the metabolic activity of astrocytes more so than neurons (22), making it an attractive agent for spinal cord injury applications. This has been demonstrated in a nerve crush injury where increased axon recovery was seen when 6AN was applied (23).
The goal of this study was to design highly aligned electrospun fibers that release different amounts of 6AN for a sustained period of time. Once fabricated, it was hypothesized that these 6AN-containing fibers would attenuate astrocyte metabolism but still allow for directed extension of regenerating axons from DRG explants. Electrospinning parameters were varied to produce highly aligned fiber scaffolds that contained 6AN. Release of 6AN from the aligned fiber substrates occurred over several days. Neurite outgrowth from DRG explants cultured on some 6AN releasing fibers was not significantly altered when compared to fibers that did not release 6AN. Dissociated astrocytes were less metabolically active than their counterparts cultured on fibers not releasing 6AN. This preliminary study suggests low concentrations of 6AN loaded into aligned, electrospun fibers may be capable of directing axonal extension while reducing astrocyte activity without significantly reducing neurite growth.
2. Methods
2.1 Electrospinning
Poly-L-lactic acid (PLLA, NatureWorks™; grade 6201D, Lot #9051-89-2, density: 1.25, MW being 78 kDa, and number average MW 48 kDa) was purchased from Cargill Dow LLC (Minnetonka, MN). 1,1,1,6,6,6-hexafluoro-2-propanol (HFP), chloroform, dichloromethane, and 6-aminonicotinamide (6AN) were purchased from Sigma (St. Louis, MO).
240mg of PLLA was dissolved in 3g of HFP to create a control solution of 8% wt/wt solution of PLLA. 10% 6AN and 20% 6AN (wt 6AN/wt PLLA) fibers had 24mg and 48mg respectively of 6AN added to a 10% PLLA solution (240mg PLLA/2.4g HFP) (Table 1).
Table 1.
Variable electrospinning parameters for each experimental group.
| Weight PLLA (mg) | Weight HFP (g) | Weight 6AN (mg) | Collection Time (min) | Voltage (kV) | |
|---|---|---|---|---|---|
| Control | 240 | 3 | 0 | 10 | 15 |
| 10% 6AN | 240 | 2.4 | 24 | 5 | 14 |
| 20% 6AN | 240 | 2.4 | 48 | 5 | 14 |
Basic electrospinning design and setup has been previously described (7). Briefly, each group was spun using a 5 mL syringe with a 22 gauge needle of length 38 mm onto an aluminum disk 220 mm in diameter and 10 mm thick, spinning at 1800 rpm with a collection distance of 60 mm. All groups were electrospun at a relative humidity level of 33% + 3%. All fibers were electrospun onto 15x15 mm glass coverslips with a thin film of PLLA cast onto the coverslips using a solution containing 4% wt/wt PLLA in a 1:1 mixture of chloroform and dichloromethane. The control group was electrospun for 10 minutes at 15 kV and all other groups were electrospun at 14 kV for 5 minutes. All control and experimental groups were electrospun in triplicate.
2.2 Scanning electron microscopy (SEM)
SEM was conducted for fiber characterization using a Hitachi S-4700 field emission scanning electron microscope (FESEM) at an accelerating voltage of 1 kV. Prior to imaging, each sample was coated with a 5 nm thick layer of gold/palladium by a Hummer 6.2 sputter coater (Anatech Ltd. Denver, NC).
2.3 Fiber characterization
Each fiber group had three samples imaged, one from each independent fabrication. In order to ensure unbiased sampling, all information associating each image to a sample group was removed and analyzed by a person unaffiliated with the project.
2.3.1 Fiber alignment
To quantify the alignment and diameter of fibers, 99 fibers for each fiber group (33 fibers from each independently fabricated sample) were analyzed using ImageJ (NIH, Bethesda, MD). The median angle of each sample set was subtracted from each angle to give the degree of deviation. Each angle was placed into a data bin of 2°, so that all angles between 0° and 2° were placed in one bin, and all angles between 2° and 4° were placed in another bin, and so on. Angles ranged from −90° to 90° with 0° being the median of the fiber sample.
2.3.2 Fiber diameter
33 fibers from independently fabricated samples were analyzed to determine fiber diameter (n=99 per test group). Cross sectional diameter was measured using ImageJ (25).
2.3.3 Fiber Surface Coverage
In order to determine fiber density, a reference line perpendicular to the general fiber orientation was drawn and the number of fibers along the reference line was counted (n=3 per test group). The density was converted to the number of fibers per mm for each experimental group.
Previous studies have calculated fiber mesh size to determine the area between the fibers (8,9). However, in an ideal situation of perfectly aligned fibers the gap area or mesh size is infinite rendering a mesh size calculation irrelevant. It is however necessary to know the surface area coverage of a fiber sample to ensure uniformity between groups. In order to show similarities between test groups for fiber coverage the mean diameter of a sample is multiplied by the density of the sample to get the diameter-density product (DDP).
The diameter density product gives a number representing the general fiber surface area coverage for a sample.
2.4 Drug release characterization
In order to determine the quantity of drug released from the fibers, a standard curve was created by dissolving 6-AN in HFP and diluted to various concentrations with PBS to a volume of 200 μl. These calibrated solutions were then incubated at 37°C and 5% CO2 for 24 hours to imitate culture conditions. In order to remain above the detection limit, PBS (200 μl) was dropped into silicon molds (4mm x 13mm) placed on all sample groups. After 24 hours, PBS solutions in contact with fiber sample or solutions created for the calibration curve were then reacted with 20 μl of a 25 mg/ml solution of fluorescamine (Sigma, St. Louis, MO) for 55 minutes. Fluorescamine is a reactant that produces fluorescent products when in the presence of a primary amine group. Samples were then analyzed for fluorescent products (ex: 390nm, em: 465nm) using a Biotek Synergy HT plate reader (Winooski, VT). 6AN release from the fiber samples was monitored for 14 days.
2.5 Cell culture
2.5.1 DRG culture
E9 chick DRG and E10 chick spinal cord were isolated in accordance with procedures approved by the Institutional Animal Care and Use Committee (IACUC) at Michigan Technological University. Full DRG were dropped in 150 μl of Neurobasal medium supplemented with L-glutamine, penicillin/streptomycin, and B-27 serum free supplement (Invitrogen, Green Island, NY) on top of a fiber sample. DRG were allowed to attach onto fiber samples (n=6) that were not coated with any neuronal adherent proteins or serum solutions for approximately 14 hours within a tissue culture incubator (37°C, 5% CO2). Then Neurobasal medium was added to a total volume of 2mL with a final concentration of 50 ng/ml of nerve growth factor (NGF; Calbiochem, La Jolla, CA). The DRG were then incubated for 60 hours before fixing and labeling. Cultures were performed in triplicate to ensure consistency in results.
2.5.2 Astrocyte culture
Astrocytes were isolated from E10 chick spinal cords as described previously (24). Briefly, astrocytes were dissociated from three chick spinal cords and incubated in DMEM:F12 supplemented with penicillin/streptomycin, GlutaMax, fungizone, and fetal bovine serum (FBS) (all purchased from Invitrogen, Green Island, NY). Cells were cultured for seven days and then split and incubated for another five days in medium containing 1:100 G5 supplement (Invitrogen, Green Island, NY) prior to culturing on fibers. G5 contains growth factors such as epidermal growth factor (EGF) that are known to differentiate astrocytes to maturity. 75,000 differentiated cells were plated on fibers in 150μl of Neurobasal medium for 14 hours. Neurobasal medium was added to a total volume of 2mL with 50ng/ml concentration of NGF and incubated for 60 hours.
2.6 Immunocytochemistry
After 60 hours in culture, DRG or astrocyte cultures were fixed by adding 2 ml of PBS (10 mM phosphate, 150 mM sodium chloride) solution containing 8% (wt/volume) paraformaldehyde (Sigma, St. Louis, MO) to the 2 ml of culture solution for one hour. Cells were washed three times with PBS, and then blocked with a PBS solution containing 10% normal goat serum and 0.4% triton X-100 (Sigma, St. Louis, MO) for 1 hour. Blocking solution was then removed and incubated (37°C, 5% CO2) with rabbit anti-neurofilament primary antibody for DRG (1:800 dilution, Chemicon, Temecula, CA) for 14 hours. Specimens were then washed and incubated with Alexa Fluor 488 (1:1000, Invitrogen, Green Island, NY) for an hour. Cells were then incubated for 15 minutes in a 10μg/ml solution of DAPI in PBS. The DAPI solution was removed and cells were washed 3 times prior to imaging. DRG and astrocytes were imaged using a Zeiss Axiovert 200 M microscope equipped with an AxioCam fluorescence camera.
2.7 DRG Size and Length of neurite outgrowth
In order to ensure unbiased sampling, each DRG image was stripped of all information associating it with an experimental group and analyzed by a person unaffiliated with the project. All DRG that visibly attached, seen by the naked eye, to the fiber sample before antibody labeling were analyzed. If a DRG did not attach to a fiber sample an additional trial was performed until a total of 6 DRG attached. This approach was used as opposed to a previous method of DRG selection that only analyzed DRG having a certain neurite length (9) in order to examine the effects of 6AN on neurite extension.
The perimeter of each selected DRG was marked and measured to determine the area. Then the 10 longest neurites from one side of the DRG were measured and averaged to generate the average neurite length for that particular side of the DRG. The same analysis was then conducted on the other side of the DRG. Six DRG were analyzed for each experimental group. Since each DRG has two sides of neurite outgrowth, 12 length measurements (n = 12) were gathered for each condition. All measurements were taken using Image J software (25).
2.8 Degree of perpendicular neurite outgrowth
To determine how 6AN release affected neurite extension perpendicular to the axis of the aligned fibers, the degree of perpendicular neurite outgrowth was quantified. The perimeter of each DRG characterized in Section 2.7 was marked using NIH ImageJ software (25). The distance from the top and the bottom of the explants to the maximum neurite extension that occurred perpendicular to the aligned fibers was measured as neurite perpendicular outgrowth (n = 12 for each fiber condition).
2.9 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay for cell activity
Before fixing astrocytes, fiber slides were transferred to a 12-well plate and each fiber sample was incubated for four hours in 500 μl of fresh Neurobasal culture medium with 100 μl of MTS assay solution prepared according to the manufacturers protocol (Promega, Madison, WI). After 4 hours, 100 μl of culture medium was removed and placed in a 96-well plate to be analyzed for transmittance in a plate reader (Biotek). The MTS assay forms a water soluble formazan product when metabolized by dehydrogenase enzymes, thus forming formazan product with respect to the degree of metabolic activity. The formazan product is then read for absorbance at 490nm.
2.10 Statistical analysis
Statistical analyses were performed using JMP IN software (Release 8.0.1; SAS; Cary, NC). A one-way ANOVA was run first to determine statistical differences between groups in fiber density (N = 99), neurite length (N = 12), DRG surface area (n=6), and MTS transmittance (n=3). For those that showed differences in ANOVA, post-hoc Tukey–Kramer HSD tests were used to compare all pairs individually. The Brown–Forsythe test was run to determine statistical differences in fiber alignment (N = 99). Linear Regression was performed on 6AN release from the fibers to determine release rates.
3 Results
3.1 Physical Fiber Characteristics
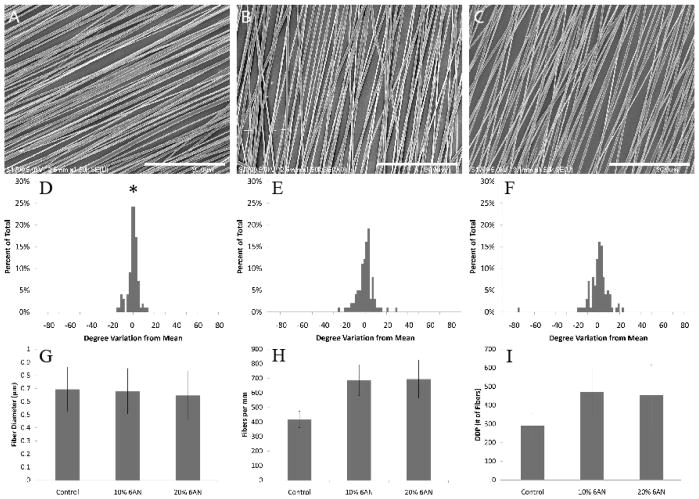
To evaluate the effect of 6AN on electrospun fiber characteristics, the alignment, diameter, and density was determined (Figure 1). Alignment of the control group was statistically different from both groups containing 6AN (p<0.01) but 6AN groups did not show statistical difference from each other. None of the groups showed statistical differences when examining fiber density, diameter, or DDP. Although not significantly different, there was a trend to a smaller number of fibers in the Control sample set.
Figure 1.
Analysis of physical fiber properties. SEM images of (A) Control, (B) 10% 6AN loaded fibers, and (C) 20% 6AN fibers and corresponding alignment analysis of each (D–F). Fiber diameter (G), density (H) and the product of diameter and density (I). Scale bars are 30μm (A–C) and *p<0.01.
3.2 Drug Release Characteristics
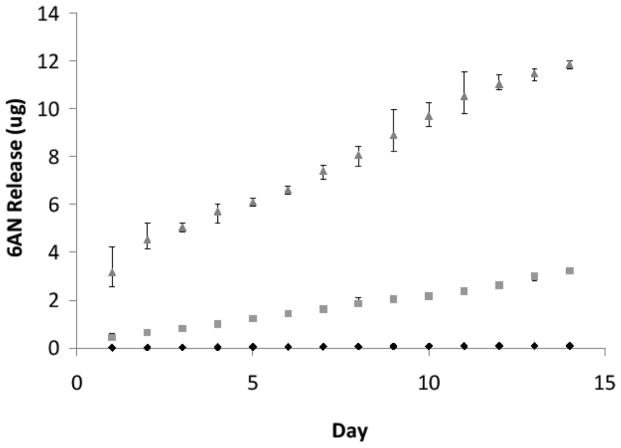
200μl of PBS was placed on 3 independently fabricated samples for each test group then incubated for 24 hours. PBS was then removed and reacted with fluorescamine before analyzing for fluorescence. Fluorescent readings were compared to a standard curve to determine amount of 6AN release (Figure 2). The 20% 6AN fibers showed a large burst release for the first day followed by a relatively constant release for the next 14 days. Unlike the 20% 6AN fibers, the 10% 6AN fibers showed a smaller burst release and maintained a nearly linear release for 14 days. The control showed no release.
Figure 2.
Daily 6AN release over a two-week period. Control (⋄), 10% 6AN (□, R2 = 0.86), and 20% 6AN (△, R2 = 0.64).
A major difference between the 6AN groups besides the presence of burst release and release rates was the variation within the groups of the release rates. There was far less variation in 10% 6AN fiber release rate (R2 = 0.87) than the 20% 6AN fibers (R2 = 0.64) when a linear regression was performed. Only one sample in the 20% 6AN fibers showed a decrease in 6AN release that compared to the 10% 6AN release rate.
3.3 DRG Culture
Full DRG were cultured on three independently fabricated samples for each group (n=6 per group). The DRG did not always attach to the fiber samples, and this was seen to be a more frequent occurrence in the 20% 6AN group. 6 of 7 DRG trials attached to the control fibers, 6 of 8 DRG attached to the 10% 6AN fibers, and 6 of 12 DRG attached to 20% 6AN fibers. In the event of a DRG failing to attach to the fiber sample an additional trial was performed until a DRG successfully attached. The data represents all DRG that attached (Figure 3).
Figure 3.
DRG culture after 60 hours. (A) Control, (B) 10% 6AN, and (C) 20% 6AN stained with neurofilament (green) and DAPI nuclear stain (blue). DRG area was measured (D) and growth was analyzed by measuring neurite extension along the length of the fibers (E) and perpendicular to the fibers (F). Scale bars are 500μm and *p<0.05.
While neurite extension between the 20% 6AN fibers (778 μm ± 160 μm) and the other groups was statistically different (p<0.01), the Control (980 μm ± 259 μm) and 10% 6AN (934 μm ± 254 μm) groups did not show a large difference. Analysis of DRG size showed that DRG cultured on 20% 6AN fibers (0.204mm2 ± 0.051mm2) were larger (p<0.05) than the both the Control (0.131mm2 ± 0.023mm2) and the 10% 6AN (0.141mm2 ± 0.028mm2). Perpendicular neurite extension analysis showed no differences between the groups.
One noticeable difference between the groups was the morphology of extending neurites. Nearly all control and 10% 6AN groups DRG showed well defined neurites (Figure 3A) while the 20% 6AN DRG had few (Figure 3C).
3.4 Astrocyte Culture
75,000 astrocytes were seeded on fiber scaffolds in 150μl of Neurobasal and incubated for 14 hours. Neurobasal culture medium was then added to a total volume of 2 mL. After 56 hours, fresh culture media was placed in the culture dishes and MTS assay was added. After 4 hours, the culture media with MTS assay was analyzed for absorbance (Figure 4). Absorbance values for each test group for the MTS assay showed statistically different results (p<0.0001) with the control showing the greatest absorbance and the 20% 6AN showing the least. The formazan product from MTS catalysis is what is measured for absorbance. Thus the more formazan product formed the higher the absorbance values.
Figure 4.

Absorbance values of culture media incubated for 4 hours with MTS assay. Higher absorbance indicates higher metabolic activity, *p<0.0001.
4 Discussion
4.1 Physical Characteristics of 6AN-PLLA Fibers
While many groups have included a drug or protein into electrospun fibers for controlled release (12–15) few have attempted to do so in aligned fibers, and to the best of our knowledge no one has attempted to do so with a highly controlled result. One of the goals of this study was to determine the effects of adding a drug or protein to an electrospinning process known to produce highly aligned fibers and determine if a high degree of control over physical characteristics could be maintained. Inclusion of 6AN, a small molecular weight analog of nicotinic acid, drastically changed the physical properties of the electrospun fibers. The fibers became less aligned than fibers electrospun from solutions containing the same amount of PLLA but with no 6AN. It was necessary to account for the decrease in fiber diameter due to addition of 6AN by increasing the PLLA concentration from the Control (8% PLLA) for the experimental groups containing 6AN (10% PLLA). The decrease in size is consistent with previous work that shows the addition of any substance to a particular electrospinning method reduces the maximum fiber size (13).
Despite our ability to achieve fibers of similar diameter, creating fibers of similar orientation was problematic. This could be due to a number of reasons. First the added charge to the solution by including 6AN may have increased the drawing force for a similar voltage (26). This would cause the fibers to be drawn to the collector faster preventing the rotating wheel from elongating the Taylor cone as efficiently as before. Another possibility is that the addition of the 6AN stabilized the HFP solution decreasing its evaporation rate. The decrease in evaporation rate would likely increase the Taylor cone radius (27) thus making it more difficult to align on the collecting disk. Both situations account for the decrease in diameter size, although the decrease in diameter may have also come from the 6AN disrupting the HFP solution by decreasing its surface tension thus requiring less force to draw solution from the tip. In any case it is clear that the inclusion of 6AN significantly increases the collection rate at a lower voltage since in a 5 minute span a similar number of fibers could be collected compared to solution not containing 6AN taking 10 minutes.
4.2 Controlled Release of 6AN
For the purposes of this study we chose to monitor release of 6AN for 14 days because this is the approximate length of time the blood brain barrier (BBB) remains porous after injury (17). When the BBB becomes porous, astrocytes are exposed to various factors that induce reactivity. However, our current data does not give any indication as to the total amount of 6AN released. To determine an approximate amount of 6AN contained in each set of fibers, we dissolved 25 samples of each sample set in dichloromethane. Dichloromethane dissolves PLLA but not 6AN. After centrifuging the supernatant was removed and additional dichloromethane was added to remove any remaining PLLA. After centrifuging a second time and removing the supernatant, the dichloromethane was allowed to evaporate off and the 6AN remaining was weighed. Using this method we were able to determine that the average sample weight of 6AN was 330μg for the 20% 6AN fibers and 160 μg for the 10% 6AN fibers. Taking into account the area analyzed using the molds compared to the total surface area of the fiber samples, approximately 15% of the 6AN was released from the 20% 6AN fibers and 8.5% was released from the 10% 6AN fibers over a 14 day period.
Previous studies analyzing drug release kinetics have determined that release rate depends on a number of factors including polymer-drug affinity (11, 12). The drug solubility in the polymer determines whether the drug forms amorphously within the polymer as opposed to forming as a crystalline solid. If the drug forms as a crystalline solid, and is thus insoluble in the polymer, then a rapid release rate is seen (12). A similar rapid release rate is seen when hydrophilic drugs are incorporated into electrospun fibers (11). The release rate of 6AN in the 10% 6AN group is nearly linear with very little to no burst release. This indicates a concentration of 6AN below its solubility limit in PLLA. The 20% 6AN group on the other hand shows a large burst release during the first day and shows sporadic bursts throughout the 14 day period. This indicates a concentration of 6AN above the solubility limit in PLLA.
Another contributing factor to release rate is the hydrophobicity of the drug. If the drug is hydrophobic, then a longer and more linear release rate is seen (12, 28, 29) versus a rapid release for a hydrophilic drug (11). Because 6AN is highly hydrophobic and appears to be soluble in PLLA, it is expected that the release rate be linear. While the variation between 10% 6AN samples showed a reasonable correlation to a linear regression (R2 = 0.86) each individual sample showed a strong correlation (R2 range = 0.97-0.99). Thus, we conclude the release rate of 6AN shows a strong linear release rate and the variation likely comes from small differences in fiber density or diameter in each sample. The 20% 6AN shows a lower correlation with individual samples (R2 range = 0.94-0.97) and the combined show even greater variation (R2 = 0.64).
4.3 The Effect of 6AN Release on DRG Growth
While 6AN has been used in CNS injury models to impart injury or reduce regeneration, it is known that low levels of 6AN is permissive to nerve regeneration while causing necrosis in reactive astrocytes (23). One of the goals of this study is to determine if PLLA fibers are capable of releasing sufficient levels of 6AN that allow nerve growth while simultaneously inhibiting astrocyte metabolic activity. Despite the difference in PLLA control and 10% 6AN electrospun fiber alignment, there was no statistical difference in axonal extension. Both of these groups were statistically different from the 20% 6AN group, and while the alignment was different from the Control it was not different from the 10% 6AN fibers. This suggests that it is the 6AN, and not the physical parameters, that are ultimately limiting the growth of the extending axons. It should be noted also that a large number of attempts were necessary to successfully attach DRG to fiber samples in the 20% 6AN group compared to the other groups. If this material is capable of releasing enough 6AN to reduce axonal extension then the quantities released should be sufficient to reduce astrocyte activity.
It should be recognized that this data is ultimately biased due to the DRG selection process. Because cultures were repeated until a DRG successfully attached, a number of additional factors may have played a role in DRG attachment and neurite extension. One issue could be that there is a variation in the number of cells that survive the DRG isolation process. If fewer cells survive, but are still capable of attaching, then the DRG may have attached to the Control and the 10% 6AN fibers while having too many cells killed by the 20% 6AN to attach. Thus the 20% 6AN trials that attached may have been DRG that had a larger number of cells surviving the isolation process. This may account for the larger DRG size. Another issue with the DRG selection process is that due to the variation in drug release between the samples, it is possible that DRG only attached to samples that released less 6AN. This does not appear to diminish the significance of the data, but rather has the potential to increase the validity of these conclusions.
One interesting aspect of the DRG data is the significant increase in DRG size in the 20% 6AN group. While this may be attributed to the DRG selection process, as described above, ultimately we find this to be in agreement with previous literature. Previously 6AN was used to cause demyelination of axons in both the CNS (30) and peripheral nervous system (30, 31) due to degenerating Schwann cells and Oligodendrocytes. These studies suggest that 6AN has a more dramatic effect on Schwann cells, Oligodendrocytes, and astrocytes than neurons. If this is true then the increase in DRG size might be explained by the significant effect it is having on the Schwann cells to migrate out of the DRG body. While we have previously shown that the S100 antibody, labeling for Schwann cells, covers the majority of all cells stained with DAPI, we cannot use this data to accurately measure Schwann cell migration out of the DRG body. If the DRG selection process is biasing the data then it is necessary to account for Schwann cells remaining in the DRG body by using the S100 antibody. Based on inspection it appears that there is a significant decrease in Schwann cell migration, however we would not be able to determine that the Schwann cells are remaining in the DRG body since both the Schwann cells and neuron nuclei are stained by the DAPI. The S100 label would be necessary to determine that Schwann cells are remaining in the DRG body.
4.4 The Effect of 6AN Release on Astrocyte Activity
In spite of the beneficial roles that have been demonstrated in vitro, astrocytes remain a difficult issue in vivo. It is known that astrocytes become ‘reactive’ and migrate to the injury site to form dense, impassable networks while proliferating and releasing factors inhibiting axonal extension. Astrocyte reactivity, which has been defined as a range of phenotypic changes in astrocytes responding to CNS insult (16), is thought to be a neuroprotective response to foreign molecules and factors released by invading macrophages (17). When TGF-β1 and TGF-β2, two factors that induce astrocyte reactivity, were inhibited by use of antibodies to block membrane receptors, a reduction in glial scar formation was seen despite little to no axon regeneration (18). However, almost completely preventing scar formation in a knockout model for GFAP and Vimentin, two essential intermediate filaments for the formation of astrocyte networks in the glial scar, showed scar formation was significantly decreased and nerve regeneration occurred (19). Thus, while it is necessary to consider the macrophage and neuroprotective components to the glial scar formation as well as the growth promoting factors previously demonstrated (1, 15), ultimately the glial scar must be overcome in order for regeneration to occur.
Because the mechanism to induce astrocyte reactivity is complex and depends on a number of factors, we chose to use the G5 supplement which contains EGF and FGF. EGF is known to induce a strong response in astrocytes causing a drastic increase in chondroitin sulfate proteoglycans (CSPGs; 32) and when combined with FGF is known to induce GFAP expression (33). Since GFAP and CSPG expression is seen in reactive astrocytes we determined that the G5 supplement should adequately induce a limited degree of astrocyte reactivity.
This study attempts to demonstrate how electrospun fibers might be used to address this issue while using the physical parameters of the fibers to direct neurite growth. While the effect of 6AN release from some fiber samples (10% 6AN) appears to have minimal effects on neurite extension, astrocyte metabolism was significantly decreased in both groups of 6AN fibers compared to the control. The mechanism of 6AN is competitive inhibition of certain dehydrogenase enzymes in the pentose phosphate pathway PPP, which deplete reducing agents and ribose supplies (20, 21). Although 6AN acts primarily on the PPP, it also appears to inhibit the glycolytic pathway (20). It is clear why both pathways would be upregulated in reactive astrocytes due to the genetic precursors and energy needed for these proliferative cells to release growth factors, intermediate filaments, and chondroitin sulfate proteoglycans (CSPGs).
An interesting part of the astrocyte data is that unlike the DRG experiments that required a number of additional trials in order to get DRG attached only three astrocyte trials per test group were conducted. Due to the lack of clarity of our cell staining or the limitations of our microscope we could not get accurate cells counts. However, based on inspection of the images we did not see a decrease in the astrocyte coverage as we might have expected. Because we could not get an accurate cell count, it is possible that the decrease in the MTS assay may be due to a decrease in astrocyte attachment or cell death rather than a decrease in metabolic activity. Since there isn’t the same degree of variability for astrocyte seeding as there is for DRG isolation, we believe that these results still indicate a significant decrease in astrocyte activity either through cell death, attachment, metabolic activity or some combination of these.
Despite these promising preliminary results, there are additional factors to take into consideration when using 6AN not addressed here. While our data indicates it is possible to release appropriate amounts of 6AN to inhibit astrocyte activity while permitting little reduction in neurite extension, previous studies indicate other cells must be taken into consideration. 6AN is known to affect the ability of Schwann cells and Oligodendrocytes to myelinate axons (30, 31). It is possible that the amount of drug needed to be released to inhibit astrocytes will cause these cells to demyelinate axons outside of the injured region. Also, we do not know if 6AN affects the ability of these cells to migrate, but if it does as this data may suggest then these cells may not be able to assist in nerve growth as previously indicated (34, 35).
Another potential issue with the use of these fibers is the macrophage response. While 6AN may induce macrophage activation (36), 6AN is also shown to decrease macrophage sensitivity to lipopolysaccharide (LPS) and interferon-lambda (37). If 6AN does not decrease macrophage activation then by preventing the glial scar to form, macrophages would be permitted to migrate into uninjured tissue and cause additional damage. If 6AN does decrease macrophage activation, the next question is whether 6AN is released at high enough levels to quickly prevent macrophage activation thus preventing macrophage migration into undamaged tissue.
5 Conclusions
This study investigated the possibility of using aligned, electrospun fibers loaded with a metabolic inhibitor (6AN) to selectively target astrocytes while allowing nerve growth. While it was difficult to design aligned fibers that release 6AN with characteristics shown to be conducive to nerve growth, proper parameters were determined to electrospin fibers that allowed similar growth to that of highly aligned scaffolds. These novel fiber scaffolds showed a high degree of control in releasing 6AN and at levels sufficient to significantly reduce astrocyte activity while in some cases, did not affect axonal extension from DRG neurons. The reduction in metabolism warrants further study to determine the specific effects 6AN has on astrocytes once they become reactive.
Acknowledgments
This work was supported by NIH NINDS R21NS62392 and NICHD R15HD61096 to Ryan Gilbert. The authors would also like to acknowledge the SURF program at Michigan Technological University for partial funding of Nicholas Schaub. The authors acknowledge the contributions Hanbing Wang’s electrospinning expertise, Connor McCarthy’s maintenance of the electrospinning environment, Will Paces for analyzing DRG and electrospun fiber images, and Nils Bergman’s assistance with FESEM. Finally, the authors acknowledge Dr. Jerry Silver (Case Western Reserve University, Department of Neuroscience) for suggesting 6-aminonicotinimde as a therapy for release from our electrospun fiber scaffolds.
References
- 1.Chow WN, Simpson DG, Bigbee JW, Colello RJ. Evaluating neuronal and glial growth on electrospun polarized matrices: bridging the gap in percussive spinal cord injuries. Neuron Glia Bio. 2007;3(2):119–26. doi: 10.1017/S1740925X07000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie J, MacEwan MR, Xiaoran L, Sakyama-Elbert SE, Younan X. Neurite Outgrowth on Nanofiber Scaffolds with Different Orders, Structures, and Surface Properties. ACS Nano. 2009;3(5):1151–159. doi: 10.1021/nn900070z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corey JM, Lin DY, Mycek KB, Chen Q, Samuel S, Feldman EL. Aligned electrospun nanofibers specify the direction of dorsal root ganglia neurite growth. J Biomed Mater Res A. 2007;83(3):636–645. doi: 10.1002/jbm.a.31285. [DOI] [PubMed] [Google Scholar]
- 4.Kim YT, Haftel VK, Kumar S, Bellamkonda RV. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials. 2008;29(21):3117–3127. doi: 10.1016/j.biomaterials.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wen X, Tresco PA. Effect of filament diameter and extracellular matrix molecule precoating on neurite outgrowth and Schwann cell behavior on multifilament entubulation bridging device in vitro. J Biomed Mater Res A. 2006;76(3):626–637. doi: 10.1002/jbm.a.30520. [DOI] [PubMed] [Google Scholar]
- 6.Schnell E, Klinkhammer K, Balzer S, Brook G, Klee D, Dalton P. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials. 2007;28(19):3012–3025. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Wang HB, Mullins ME, Cregg JM, Hurtado A, Oudega M, Trombley MT, Gilbert RJ. Creation of highly aligned electrospun poly-L-lactic acid fibers for nerve regeneration applications. J Neural Eng. 2009;6(1):016001. doi: 10.1088/1741-2560/6/1/016001. [DOI] [PubMed] [Google Scholar]
- 8.Wang HB, Mullins ME, Craig JM, McCarthy CW, Gilbert RJ. Varying the Diameter of Aligned Electrospun Fibers Alters Neurite Outgrowth and Schwann Cell Migration. Acta Biomater. 2010;6(8):2970–2978. doi: 10.1016/j.actbio.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 9.He L, Liao S, Quan D, Ma K, Chan C, Ramakrishna S, Lu J. Synergistic Effects of Electrospun PLLA Fiber Dimension and Pattern on Neonatal Mouse Cerbellum C17.2 Stem Cells. Acta Biomater. 2010;6(8):2960–2969. doi: 10.1016/j.actbio.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 10.Gertz CC, Leach MK, Birrel LK, Martin DC, Feldman EL, Corey JM. Accelerated Neuritogenesis and Maturation of Primary Spinal Motor Neurons in Response to Nanofibers. Dev Neurobiol. 2010;70(8):589–603. doi: 10.1002/dneu.20792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, Jadjiargyrou M. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J Control Release. 2004;98(1):47–56. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Zeng J, Yang L, Liang Q, Zhang X, Guan H, Xu X, Chen X, Jing X. Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation. J Control Release. 2005;105(1–2):43–51. doi: 10.1016/j.jconrel.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 13.Zeng J, Xu X, Chen X, Liang Q, Bian X, Yang L, Jing X. Biodegradable electrospun fibers for drug delivery. J Control Release. 2003;92(3):227–31. doi: 10.1016/s0168-3659(03)00372-9. [DOI] [PubMed] [Google Scholar]
- 14.Chew SY, Mi R, Hoke A, Leong KW. Aligned Protein-Polymer Composite Fibers Enhance Nerve Regeneration: A Potential Tissue-Engineering Platform. Adv Funct Mater. 2007;17(8):1288–1296. doi: 10.1002/adfm.200600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delgago-Rivera R, Harris SL, Ahmed I, Babu AN, Patel RP, Ayres V, Flowers F, Meiners S. Increased FGF-2 Secretion and Ability to Support Neurite Outgrowth by Astrocytes Cultured on Polyamide Nanofibrillar Matrices. Matrix Biol. 2009;28(3):137–147. doi: 10.1016/j.matbio.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Sofroniew MV. Molecular Dissection of Reactive Astrogliosis and Glial Scar Formation. Trends Neurosci. 2009;32(12):638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silver J, Miller JH. Regeneration beyond the Glial Scar. Nat Rev Neuro. 2004;5(2):146. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 18.Moon LDF, Fawcett JW. Reduction in CNS Scar Formation without Concomitant Increase in Axon Regeneration following Treatment of Adult Rat Brain with a Combination of Antibodies to TGFbeta1 and Beta2. Eur J Neuro. 2001;14(10):1667. doi: 10.1046/j.0953-816x.2001.01795.x. [DOI] [PubMed] [Google Scholar]
- 19.Wilhelmsson U, Li L, Pekna M, Berthold C, Blom S, Eliasson C, Renner O, Bushong E, Ellisman M, Morgan TE, Pekny M. Absence of Glial Fibrillary Acidic Protein and Vimentin Prevents Hypertrophy of Astrocytic Processes and Improves Post-Traumatic Regeneration. J Neuro. 2004;24(21):5016. doi: 10.1523/JNEUROSCI.0820-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haghighat N, McCandless DW. Effect of 6-Aminonicotinamide on Metabolism of Astrocytes and C6-Glioma Cells. Metab Brain Dis. 1997;12(1):29–45. doi: 10.1007/BF02676352. [DOI] [PubMed] [Google Scholar]
- 21.Hunting D, Gowans B, Henderson JF. Effects of 6-Aminonicotinamide on Cell Growth, Poly(ADP-Ribose) Synthesis and Nucleotide Metabolism. Biochem Pharmacol. 1985;34(22):3999–4003. doi: 10.1016/0006-2952(85)90379-x. [DOI] [PubMed] [Google Scholar]
- 22.Sotelo C. Cerebellar Neuroglia: Morphological and Histochemical Aspects. Prog Brain Res. 1967;25:226–250. doi: 10.1016/S0079-6123(08)60966-8. [DOI] [PubMed] [Google Scholar]
- 23.Politis MJ. 6-Aminonicotinamide selectively causes necrosis in reactive astroglia cells in vivo. J Neurol Sci. 1989;92(1):71–79. doi: 10.1016/0022-510x(89)90176-7. [DOI] [PubMed] [Google Scholar]
- 24.Taylor AR, Robinson MB, Milligan CE. In Vitro Methods to Prepare Astrocyte and Motor neuron Cultures for the Investigation of Potential in Vivo Interactions. Nature Protocols. 2007;2(6):1499–1507. doi: 10.1038/nprot.2007.208. [DOI] [PubMed] [Google Scholar]
- 25.Rasband WS. ImageJ. U. S. National Institutes of Health; Bethesda, Maryland, USA: 1997–2009. http://rsb.info.nih.gov/ij/ [Google Scholar]
- 26.Reneker DH, Yarin AL, Fong H, Koombhongse S. Bending instability of electrically charged liquid jets of polymer solution in electrospinning. J App Phys. 2000;87:4531–4547. [Google Scholar]
- 27.Yarin AL, Reneker DH, Koombhongse S. Bending instability in electrospinning of nanofibers. J App Phys. 2000;87:4531–4547. [Google Scholar]
- 28.Natu MV, de Sousa HC, Gil MH. Effects of drug solubility, state and loading on controlled release in bicomponent electrospun fibers. Int J Pharm. 2010;397(1–2):50–58. doi: 10.1016/j.ijpharm.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 29.Verreck G, Chun I, Rosenblatt J, Peeters J, Dijck AV, Mensch J, Noppe M, Brewster ME. Incorporation of drugs in an amorphous state into electrospun nanofibers composed of a water-insoluble, nonbiodegradable polymer. J Control Release. 2003;92(3):349–60. doi: 10.1016/s0168-3659(03)00342-0. [DOI] [PubMed] [Google Scholar]
- 30.Blakemore WF. Remyelination by Schwann Cells of Axons Demyelinated by Intraspinal Injection of 6-aminonicotinamide in the Rat. J Neurocytology. 1975;4(6):745–57. doi: 10.1007/BF01181634. [DOI] [PubMed] [Google Scholar]
- 31.Friede R, Bischhausen R. How Do Axons Control Myelin Formation? The Model of 6-aminonicotinamide Neuropathy. J Neuro Sci. 1978;35(2–3):341–53. doi: 10.1016/0022-510x(78)90014-x. [DOI] [PubMed] [Google Scholar]
- 32.Avola Roberto, Di Tullio Maria Antonietta, Fisichella Alfredo, Tayebati Seyed Khosrow, Tomassoni Daniele. Glial Fibrillary Acidic Protein and Vimentin Expression Is Regulated by Glucocorticoids and Neurotrophic Factors in Primary Rat Astroglial Cultures. Clinical and Experimental Hypertension. 2004;26(4):323–33. doi: 10.1081/ceh-120034137. [DOI] [PubMed] [Google Scholar]
- 33.Smith George M, Strunz Celia. Growth Factor and Cytokine Regulation of Chondroitin Sulfate Proteoglycans by Astrocytes. Glia. 2005;52(3):209–18. doi: 10.1002/glia.20236. [DOI] [PubMed] [Google Scholar]
- 34.Bunge MB. Novel Combination Strategies to Repair the Injured Mammalian Spinal Cord. J Spinal Cord Med. 2008;31(3):262–69. doi: 10.1080/10790268.2008.11760720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seggio AM, Narayanaswamy A, Roysam B, Thompson DM. Self-aligned Schwann Cell Monolayers Demonstrate an Inherent Ability to Direct Neurite Outgrowth. J Neural Eng. 2010;7(4) doi: 10.1088/1741-2560/7/4/046001. [DOI] [PubMed] [Google Scholar]
- 36.Hothersall JS, Gordge M, Noronha-Dutra AA. Inhibition of NADPH Supply by 6-aminonicotinamide: Effect on Glutathione, Nitric Oxide and Superoxide in J774 Cells. FEBS Letters. 1998;434(1–2):97–100. doi: 10.1016/s0014-5793(98)00959-4. [DOI] [PubMed] [Google Scholar]
- 37.Kaur C, Yong ES, Ling EA. Studies of Activated Microglia and Macrophages in Lumbosacral Spinal Cord following an Intraperitoneal Injection of 6-aminonicotinamide into Adult Rats. Hist Histopathol. 1993;8(4):699–707. [PubMed] [Google Scholar]