Abstract
General surgeons, orthopedists, and pulmonologists individually treat patients with thoracic insufficiency syndrome (TIS). The benefits of growth-sparing procedures such as Vertical Expandable Prosthetic Titanium Rib (VEPTR)insertionfor treating patients with TIS have been demonstrated. However, at present there is no objective assessment metricto examine different thoracic structural components individually as to their roles in the syndrome, in contributing to dynamics and function, and in influencing treatment outcome. Using thoracic dynamic MRI (dMRI), we have been developing a methodology to overcome this problem. In this paper, we extend this methodology from our previous structural analysis approaches to examining lung tissue properties. We process the T2-weighted dMRI images through a series of steps involving 4D image construction of the acquired dMRI images, intensity non-uniformity correction and standardization of the 4D image, lung segmentation, and estimation of the parameters describing lung tissue intensity distributions in the 4D image. Based on pre- and post-operative dMRI data sets from 25 TIS patients (predominantly neuromuscular and congenital conditions), we demonstrate how lung tissue can be characterized by the estimated distribution parameters. Our results show that standardized T2-weighted image intensity values decrease from the pre- to post-operative condition, likely reflecting improved lung aeration post-operatively. In both pre- and post-operative conditions, the intensity values decrease also from end-expiration to end-inspiration, supporting the basic premise of our results.
Keywords: Thoracic insufficiency syndrome (TIS), dynamic magnetic resonance imaging (dMRI), lung function, vertical expandable prosthetic titanium rib (VEPTR)
1. INRODUCTION
Spine deformity surgery is common in the US, with an estimated 20,000 spine fusions performed annually for adolescent idiopathic scoliosis (AIS) and a smaller number of chest wall and spinal deformity growth-sparing surgeries performed for younger patients. Failure to arrest progressive scoliosis can lead to severe deformities of the spine and chest with increased mortality from restrictive lung disease. The real issues of long-term survival and quality of life depend on intact pulmonary function provided by the thorax.Thorax is a dynamic complex biomechanical chamber of respiration formed by the spine, rib cage, and sternum, powered by diaphragmatic excursion. The latter provides for ~80% of the forced vital capacity andthe remainder comes from rib cage expansion.
The inability of the thorax to support normal respiration or lung growth is known as thoracic insufficiency syndrome (TIS) [1]. TIS is associated with at least 28 pediatric disease conditions, often due to a thoracic volume depletion deformity. The incidence of TIS is estimated as 12.4 births per 10,000, with approximately 3,000 births annually in the US, leading to millions of dollars in health care expenditures incurred for individual patients.There are several growth-sparing procedures that have been used, including Vertical Expandable Prosthetic Titanium Rib (VEPTR), to expand and support the thorax and allow for further lung growth[2–6].Unfortunately, thereareno published data which quantitatively evaluate the pre-operative to post-operative changes in the lung dynamicsor tissue characteristics in TIS patients following these procedures.In this paper, we attempt to assess for presence of significant changes in lung tissue via image intensity and texture characteristics of the lungs based on dynamic magnetic resonance imaging (dMRI) following VEPTR surgery.
In Section 2, we briefly describe the various steps involved in our analytics method and present results in Section 3. We summarize our conclusions in Section 4.
2. METHODS
Image data
This study was conducted following approval from the Institutional Review Board at the Children’s Hospital of Philadelphia and University of Pennsylvania along with a Health Insurance Portability and Accountability Act waiver. Image data sets utilized in this study all pertain to pediatric thoracic dMRI of TIS patients. In our image acquisition protocol, for each sagittal slice position, 2D slice images are acquired continuously at a rate of about 200 ms/slice over several breathing cycles without the help of any devices to gate or synchronize data acquisition with the respiratory phenomenon. The data sets consist of 25 dMRI scans from TIS patients both pre-operatively and approximately 1 year after VEPTR surgery. Imaging protocol is as follow: True fast imaging with steady-state precession (True FISP) with TR/TE ~ 4.3/2.2 msec, magnetic field strength of 1.5 Tesla, with voxel size of 1.17×1.17×5.0 mm3and 3D scene size of 224×256×34, and the number of respiratory time points varying from 4 to 8.
Image processing
The acquired images are processed through several key steps in order to isolate the dynamic lungs from the acquired dMRI data sets: 1) 4D image construction. 2) Image non-uniformity correction and intensity standardization. 3) Lung segmentation.
1). 4D image construction:
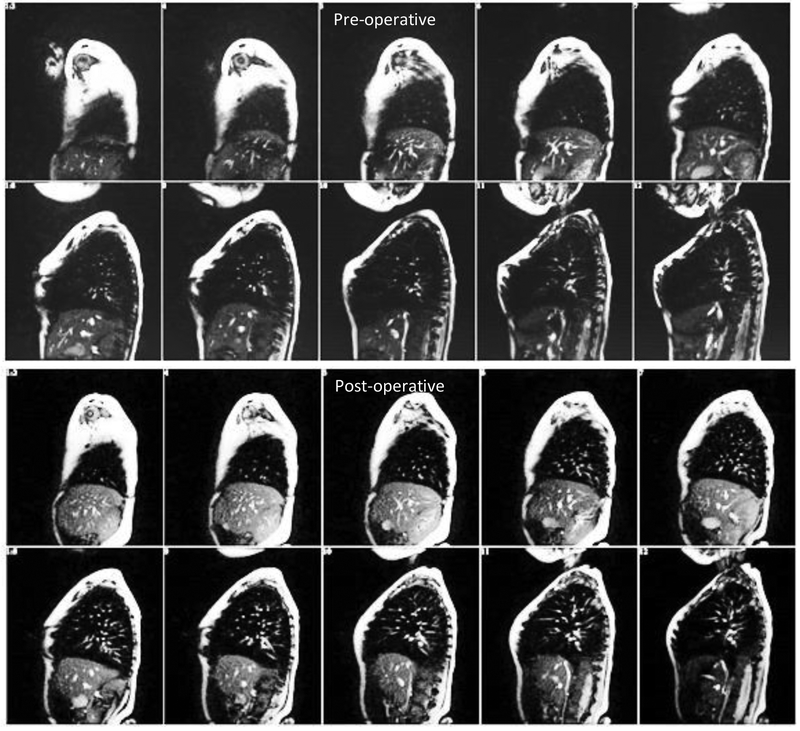
Our dMRI acquisition results in typically over 2500 sagittal slices covering the entire chest spatially and at 4 to 8 respiratory phases over several cycles. Our 4D construction method [7] builds an optimal 4D volume consisting of about 4 to 8 respiratory phases, where for each phase, the thoracic volume is represented by about 35 sagittal slices through the chest. Thus, a typical 4D volume consists of about 200 slices.This small set of slices constituting a 4D image is selected from the acquired slices using a graph-based optimization technique. For all subsequent analysis, this constructed 4D image is used. Figure 1 below shows some selected slices from a 3D volume corresponding to one respiratory phase of one of our patient cohort pre-operatively and postoperatively.
Figure 1.
Some selected sagittal slices from one 3D volume of the 4D image of a TIS patient pre-operatively and post-operatively.
2). Image non-uniformity correction and intensity standardization:
The main challenges in MR image analysis are non-uniformity of image intensity in each given image and non-standardness of the intensities across different subjects. Non-uniformity refers to the presence of slow-varying background components of intensity which may make the same tissue appear with widely different intensities in different locations of the same acquired image data set. Non-standardness refers to significant variation of intensities of the same tissue region in images of the same subject acquired on the same scanner with same imaging sequence pre-operatively and post-operatively and images of different subjects acquired on the same scanner with same imaging sequence [8]. We perform non-uniformity correction as explained in [9], followed by a process to correct for the non-standardness of MR image intensities by mapping all image intensities to a standard scale as described in [10].
3). Lung segmentation:
The dMRI images have poor definition of the lung boundary due to low MRI signal from bone and other connective tissues in the vicinity of the lung boundary (see Figure 1). We developed an interactive version of the Iterative Relative Fuzzy Connectedness method wherein seeds are specified on slices in the lung tissue and in neighboring background tissues and segmentation is carried out in a slice-by-slice manner. Typically, the seeds can be propagated in adjoining slices without having to change them. The segmentation process proceeds under user supervision and when it goes wrong, the user intervenes and re-specifies seeds.
Image analysis
The isolated lung regions within the 4D images are further analyzed to study the properties of the lung tissues preoperatively and post-operatively through two image analysis steps: 1) Estimation of the parameters of standardized intensity distribution within lung tissue. 5) Comparison of intensity arameters between different patient states.
1). Estimation of lung tissue intensity distribution parameters:
Within the segmented lung space, standardized intensity distributions are computed via histograms for several states including end-inspiration, end-expiration, pre- operative condition, and post-operative condition. Several parameters of the histogram are computed including: mean, median, mode, quartiles, peak height, skewness, and kurtosis.
2). Comparison of intensity parameters between different patient states:
Comparisons of the histogram parameters were made via paired t-test for each of the parameters between several pairs of states: (end-inspiration, end-expiration) pre-operatively; (end-inspiration, end-expiration) post-operatively; Comparisons were made for the whole lung and separately for the left and right lungs.
3. RESULTS
An exemplary result is shown in Table 1. Mean values of some of the standardized intensity parameters which showed statistically significant difference in end-inspiration or end-expiration for the whole lung are listed in the table along with the P value of the t-test. None among quartiles, skewness, kurtosis, and peak height showed any significant difference. Simple averages/ mid values (mean, median, mode) for both end-inspiration and end-expiration states showed statistically significant differences. What are shown in the 3rd and 4th columns are the mean values of the parameters under consideration shown in the second column.
Table 1.
Parameters for intensity properties of lungs in Pre-operative and Post-operative conditions.
| Object | Parameter | Pre-operative | Post-operative | P-value |
|---|---|---|---|---|
| Whole lung (end expiration) | Mode | 47.68 | 26.48 | 0.037 |
| Median | 116.64 | 67.6 | 0.044 | |
| Mean | 164.20 | 102.87 | 0.028 |
We observe that these means (of the mid value parameters) of the whole lung statistically significantly decrease following surgery. This trend consistently holds for left lung in both end-inspiration and end-expiration, but only in end-expiration for the right lung. The decrease for end-inspiration for the right lung from pre- to post-operative condition is not statistically significant. Since our images are T2-weighted, these results are consistent with improved aeration of the lungs following surgery. We observed also a decrease in average intensity of the lungs from end-inspiration to end-expiration, again consistent with decreased air content in the lungs at end-expiration relative to end-inspiration. From Figures 2a and 2b, it can be seen that there is increase in lung volume and a corresponding decrease in lung intensity following surgical intervention.
Figure2a.
Pre-operative segmented right lung with its standardized intensity in end-inspiration.
Figure 2b.
The same lung as in Figure 2a but post-operatively.
4. CONCLUSIONS
In the study of TIS, currently used clinical measures are grossly inadequate to understand the progression of the disease and its treatment outcomes. Dynamic MR imaging and analysis offer numerous avenues to devise assessment measures that are truly functional and capture dynamics as well as tissue characteristics. As such, we preliminarily demonstrate in this paper the feasibility of assessing changes in lung tissue properties following surgery in TIS patients via standardized MRI intensity properties.
General surgeons, orthopedists, and pulmonologists individually treat patients with TIS, but the dynamic metrics to measure the severity of TIS and effectiveness of treatment are non-existent. Current procedures are generally based on surgical intuition and limited past experiences. In this paper, we assess for presence of significant changes in the intensity of the lungs, potentially depicting changes in parenchymal tissue properties, particularly ventilation, based on dynamic MRI following VEPTR insertion. Image texture analysis may shed further light on the changes in lung tissue characteristics in relation to ventilation and surgical intervention.
ACKNOWLEDGMENT
This work is supported by a grant from the National Institutes of Health 1R21HL124462–01A1 and a “Frontier Grant” from the Children’s Hospital of Philadelphia.
5. REFERENCES:
- [1].Campbell RM, smith MD, “Thoracic insufficiency syndrome and exotic,” J Bone Surg Am. 89 Suppl 1:108–22 (2007). [DOI] [PubMed] [Google Scholar]
- [2].Skaggs DL, Akbarnia BA, Flynn JM, and et al. , “A classification of growth friendly spine implants,” J Pediatr Orthop; 34: 260–274 (2014). [DOI] [PubMed] [Google Scholar]
- [3].Redding Gregory J., [Thoracic Insufficiency Syndrome], Springer-Verlag; Berlin, Heidelberg, (2016). [Google Scholar]
- [4].Mai Vu M., Chen Qun, Li Wei, Hatabu Hiroto, and Edelman Robert R., “Effect of Respiratory Phases on MR Lung Signal Intensity and Lung Conspicuity Using Segmented Multiple Inversion Recovery Turbo Spin Echo (MIR-TSE),” Magnetic Resonance in Medicine 43:760–763 (2000). [DOI] [PubMed] [Google Scholar]
- [5].Failo R, Wielopolski PA, Tiddens HAWM, Hop WCJ, Pozzi Mucelli R and Lequin MH, “Lung morphology assessment using MRI: A robust ultra-short TR/TE 2D steady state free precession sequence used in cystic fibrosis patients,”. Magn. Reson. Med, 61: 299–306 (2009). [DOI] [PubMed] [Google Scholar]
- [6].Balioglu Mehmet Bulent, Albayrak Akif, Akman Yunus Emre, Atici Yunus, Kargin Deniz, and Kaygusuz Mehmet Akif “The effect of vertical expandable prosthetic titanium rib on growth in congenital scoliosis,” J Craniovertebr Junction Spine. 6(4): 200–205 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tong Y, Udupa JK, Ciesielski KC, Wu C, McDonough JM, Mong DA, Campbell RM Jr., “Retrospective 4D MR image construction from free breathing slice acquisitions: A novel graph-based approach,”Medical Image Analysis, 35:345–359 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Madabhushi A and Udupa JK, “Interplay between intensity standardization and inhomogeneity correction in MR image processing,” IEEE Trans. Med. Imaging 24(5), 561–576 (2005). [DOI] [PubMed] [Google Scholar]
- [9].Zhuge J Udupa K, Liu J, and Saha PK, “Image background inhomogeneity correction in MRI via intensity standardization,” Comput. Med. Imaging Graphics 33(1), 7–16 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nyúl LG and Udupa JK, “On standardizing the MR image intensity scale,” Magn. Reson. Med 42(6), 1072–1081 (1999). [DOI] [PubMed] [Google Scholar]
- [11].Tong Y, Udupa JK, Odhner D, Wu C, Zhao Y, McDonough JM, Capraro A, Torigian DA, Campbell RM, “Interactive iterative relative fuzzy connectedness lung segmentation on thoracic 4D dynamic MR images,” Proc. SPIE, 10137: 1013723–1–1013723–6, doi: 10.1117/12.2254968 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]