Abstract
Studies form our laboratory and others show that the oncogenic tyrosine kinase and serine threonine kinase signaling pathways are essential for cone photoreceptor survival. These pathways are downregulated in mouse models of retinal degenerative diseases. In the present study, we found that activation mutants of mTOR delayed the death of cones in a mouse model of retinal degeneration. These studies suggest that oncogenic protein kinases may be useful as therapeutic agents to treat retinal degenerations that affect cones.
XX.1. Introduction
Studies from our laboratory clearly suggest that insulin receptor (IR) is functionally important for cone photoreceptor survival. Deletion of IR from cones resulted in age-related cone degeneration. We found a ligand-independent IR activation in both rod and cone photoreceptor cells (Rajala et al., 2016; Rajala et al., 2002). This activation occurs through inhibition of a protein tyrosine phosphatase by phosphorylated Grb14. Cone-opsin bleaching promotes phosphorylation of a non-receptor tyrosine kinase, Src, which in turn phosphorylates Grb14 (Rajala et al., 2007; Rajala et al., 2016). Thus, activated IR further activates the downstream signaling cascade, which includes mammalian target of rapamycin (mTOR) signaling. Our studies also suggest that mice deficient in photobleaching of opsins and with mutations in the rhodopsin gene fail to promote Src activation and subsequent Grb14 phosphorylation (Rajala et al., 2007; Rajala et al., 2016). This failure results in increased PTP1B activity, and thus dephosphorylates IR and inactivates its signaling. It has also been shown that stimulation of insulin/mTOR pathway promotes cone survival in animal models of retinitis pigmentosa (RP; (Punzo et al., 2009). Insulin receptor, Src, and mTOR are protein tyrosine or serine/threonine kinases; their overexpression in cancer cells indicates a poor prognosis. Interestingly, these oncogenic proteins promote cone survival. Photoreceptors are highly metabolic, and their energy demands are equivalent to those of cancer cells (Rajala and Gardner, 2016; Warburg, 1956).
mTOR is the downstream effector of IR/PI3K/Akt signaling (Schmelzle and Hall, 2000), which was shown to be reduced in cones of rd1 mice after the rods had died, but could be rescued by insulin injections (Punzo et al., 2009). mTOR integrates the input, including insulin, growth factors, and amino acids, from upstream pathways and senses cellular nutrient, oxygen, and energy levels (Kim et al., 2013; Reiling and Sabatini, 2006; Sabatini, 2006; Sarbassov et al., 2005). mTOR exists in two distinct protein complexes: 1) mTORC1, which is rapamycin-sensitive and consists of mTOR, Raptor, and mLST8 (Sato et al., 2010; Urano et al., 2007), and 2) mTORC2, which is rapamycin-insensitive and consists of mTOR, Rictor, mLST8, and mSin (Urano et al., 2007). Complex 1 phosphorylates S6K and 4E-BP1, and thereby regulates protein synthesis (Urano et al., 2007). Complex 2 phosphorylates Akt and regulates the actin cytoskeleton (Sabatini, 2006).
Cepko’s lab proposed that cone cell death in RP mouse models is due to starvation that occurs through a downregulation of the insulin/mTOR signaling pathway in cones (Punzo et al., 2009). If so, nutrition-independent constitutive activation mutants of mTOR should rescue cone cell death due to starvation in rd1 mice (Punzo et al., 2009). Two different point mutations in mTOR, S2215Y and R2505P, each of which confers constitutive activation of mTOR signaling even under nutrient starvation conditions, have been identified in the human cancer genome database (Sato et al., 2010). In the mammalian system, two additional point mutations in mTOR, L1460P and E2419K, have also been identified and are constitutively activated in nutrient-starved cells (Urano et al., 2007). These point mutants have been shown to form functional complexes with mTORC1 and mTORC2 in the activation (phosphorylation) of their respective substrates, Akt, S6K, and 4E-BP1 (Sato et al., 2010). In the present study, we examined whether the mutant mTOR-E2419K is able to rescue the cone degeneration in rd1 mice.
XX.2. Materials and Methods
XX.2.1. Plasmids and vectors
The pcDNA3-Au1-mTOR-wild-type (Addgene plasmid # 26036) and pcDNA3-AU1-mTOR-E2419K (Addgene plasmid # 19994) were gifts from Dr. Fuyuhiko Tamanoi (Urano et al., 2007). A breeding colony of Balb/C, rd1, Nrl−/− and LacZ mice under the control of human red/green cone opsin promoter (provided by Dr. Jeremy Nathans, Johns Hopkins School of Medicine) was maintained in our vivarium on cyclic light (5 lux; 12 h on/12 h off). Experiments were carried out with postnatal day 5 (P5) male and female pups.
XX.2.2. Subretinal injection
The subretinal injections were performed via the transscleral route. Mice were anesthetized by intramuscular injection of a ketamine (80–100 mg/kg) and xylazine (5 mg/kg) mixture of approximately 0.1 ml, until mice did not display a blink reflex to a touch on the corneal surface. Eyes were dilated with 1% cyclopentolate hydrochloride ophthalmic solution applied to the cornea (Akron, Lake Forest, IL). The mice were kept on a 37°C regulated heating pad under a surgical microscope (Carl Zeiss Surgical, NY). An insulin syringe with a beveled 30-gauge needle was used to puncture a hole in the cornea. Next, a 33-gauge blunt-end needle attached to a 10-μl Nanofil® syringe controlled by a UMP3 pump controller (World Precision Instruments, Sarasota, FL) was positioned toward the superior nasal portion of the retina. Then, 1 μl of LPD nanoparticles (~85 ng of DNA) were injected into the subretinal space. The needle was retracted 10–15 s after injection, when a bleb of retinal detachment was visible. Following complete removal of the injection needle, the eye was carefully observed for any indication of post-surgical complications, such as iris and sub-retinal bleeding, pronounced retinal detachment or damage, or excessive vitreous loss. Saline and GelTeal lubricant eye gel (Alcon, Fort Worth, TX) were applied topically to the eye 3–4 times daily for 3–4 days after injection, to keep the eye continually moist. The severity of acute post-surgical complications and subsequent long-term complications, including eye infection, loss of visual function, and atrophy, were carefully evaluated. In the absence of any severe complications, the procedure was deemed successful and the animal remained in the study.
XX.2.3. X-gal staining
Two months later, flat mounts were prepared and stained with X-gal to examine cone cell density, according to the method described earlier (Punzo and Cepko, 2007). Retinal whole-mounts were imaged using a Nikon Eclipse E800 (Tokyo, Japan) microscope.
XX.3. Results
XX.3.1. Expression of mTOR signaling proteins in wild-type and cone-dominant Nrl knockout mouse retinas
We examined the expression of mTOR signaling proteins on immunoblots in both wild-type Balb/c and cone-dominant Nrl knockout mouse retinas (Mears et al., 2001). Our results revealed the expression of mTOR, p70S6K, and 4E-BP. It is interesting to note that the levels of these proteins are much higher in cone-dominant retina than in rod-dominant retina (Fig. 1). We also found that these proteins undergo phosphorylation on specific residues. The rod and cone cell-specific markers show that rhodopsin is absent from Nrl knockout mice, whereas M-opsin was higher in Nrl knockout mice than in wild-type mice.
Figure 1. Expression of mTOR signaling proteins in wild-type and cone-dominant Nrl knockout mice.
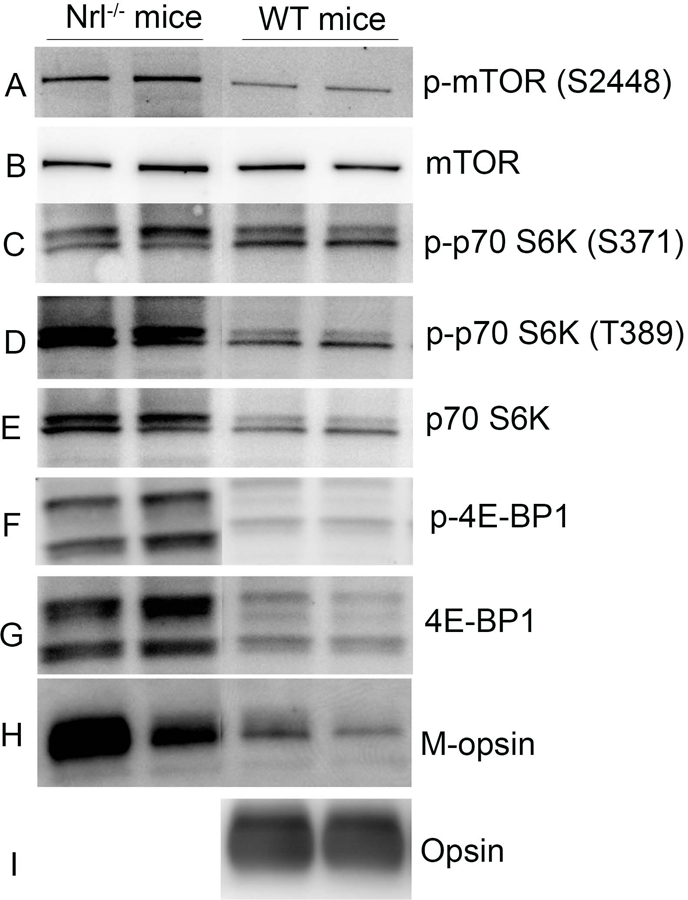
Retinal proteins from rod-dominant wild-type and cone-dominant Nrl knockout mouse retinas (in duplicate) were subjected to immunoblot analysis with mTOR, phospho-mTOR (S2448), phospho-70S6K (S371), phospho-70S6K (T389), p70S6K, phospho-4E-BP, 4E-BP1, M-opsin, and opsin antibodies.
XX.3.2. Mutant mTOR promotes cone survival in rd1 mice
We have a mouse model that expresses β-galactosidase (LacZ) under the control of human red and green cone pigment promoter (Fig. 1A; (Wang et al., 1992) and have expressed an rd1 mutation in LacZ mice (Fig. 1A). We used lipid nanoparticles to deliver wild-type and mutant mTOR to P5 rd1/rd1-LacZ mice and examined cone density after two months. Flat mounts stained with X-gal (LacZ converts colorless X-gal substrate to a blue dye) showed that cone density is well preserved in wild-type LacZ mice (Fig. 1A), but not in rd1/rd1-LacZ mice (Fig. 1B). Cone cell loss could be delayed by subretinal injection of mutant mTOR-E2419K (Fig. 1D), but not wild-type mTOR (Fig. 1C).
XX.4. Discussion
The insulin/mTOR pathway is active and promotes cone survival. In degenerative mouse models, this pathway is downregulated. It has been recently reported that conditional ablation of negative regulators of mTOR pathway promotes cone survival in mouse models of retinal degeneration (Venkatesh et al., 2015). Very recently, we reported that activation of Src, which signals in the activation of insulin, promotes cone survival in a mouse model of cone degeneration (Rajala et al., 2016). In the present study, we found that activated mutant of mTOR promotes cone survival in rd1 mice. We used pcDNA3 vector carrying wild-type mTOR and mutant mTOR under the control of cytomegalovirus promoter (CMV), a strong promoter used to obtain expression of exogenous genes in mammalian and other higher eukaryotic cells. Our results with mutant mTOR construct showed that cone cell density was much weaker in rd1/rd1 mice than in wild-type mice (Fig. 1D vs 1A). This finding could be due to the nature of the general promoter without cell specificity. As a proof of principle, we could delay the death of cones with activated mutants of mTOR. In the future, we will target the expression specifically to cone photoreceptor cells, and will also study cone function. These findings set the stage for future studies examining the idea that oncogenic proteins may be useful as therapeutic agents to treat retinal degenerations that affect cones.
Figure 2. Effect of mutant mTOR on cone cell death in an RP mouse model.
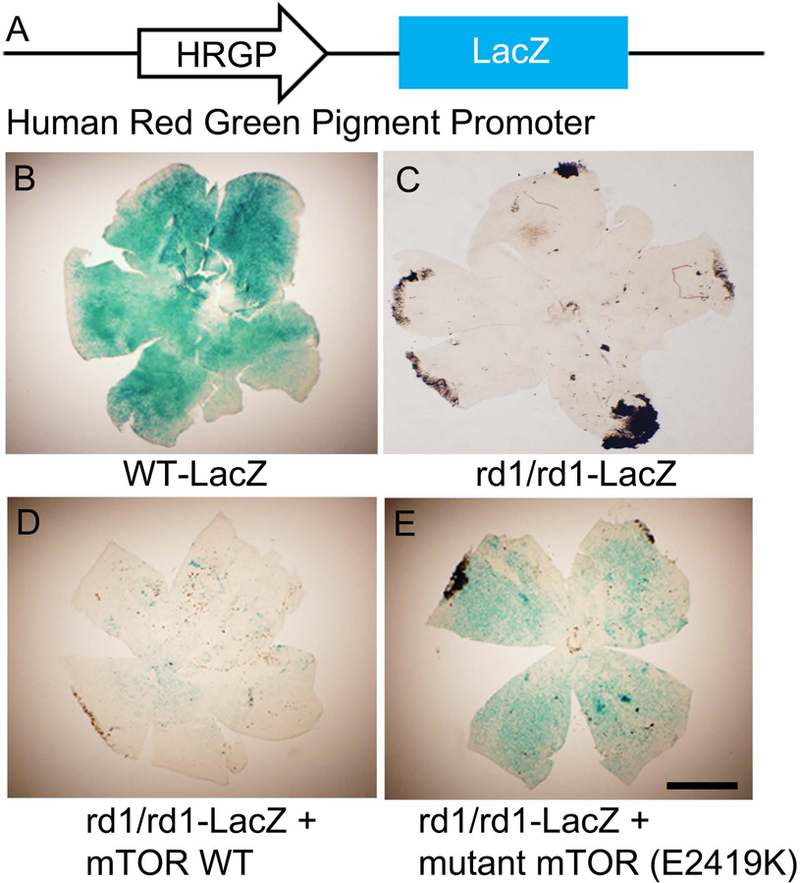
Schematic diagram of HRGP (COP)-LacZ mice (A). Wild-type mTOR (D) and mutant mTOR E2419K (E) were subretinally injected into P5 rd1/rd1-LacZ mice under the control of CMV promoter. Two months later, flat mounts were prepared and stained with X-gal to examine cone cell density (B-E). Controls for this experiment include wild-type-LacZ mice (B) and untreated rd1/rd1-LacZ mice (C). Scale bar: 1 mm.
Acknowledgments:
This study was supported by grants from the National Institutes of Health (EY00871, and NEI Core grant EY021725) and an unrestricted grant from Research to Prevent Blindness, Inc. to the Department of Ophthalmology. The authors thank Dr. Claudio Punzo for proving us with the LacZ mice under the control of human red/green cone opsin promoter, which were generated in Dr. Jeremy Nathan’s laboratory at Johns Hopkins School of Medicine. The authors acknowledge Ms. Kathy J. Kyler, Staff Editor, University of Oklahoma Health Sciences Center, for editing this manuscript.
References
- Kim SG, Buel GR, Blenis J (2013) Nutrient regulation of the mTOR Complex 1 signaling pathway. Mol Cells 73:4429–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears AJ, Kondo M, Swain PK et al. (2001) Nrl is required for rod photoreceptor development. Nat Genet 29:447–452. [DOI] [PubMed] [Google Scholar]
- Punzo C, Cepko C (2007) Cellular responses to photoreceptor death in the rd1 mouse model of retinal degeneration. Invest Ophthalmol Vis Sci 48:849–857. [DOI] [PubMed] [Google Scholar]
- Punzo C, Kornacker K, Cepko CL (2009) Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci 12:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala A, Anderson RE, Ma JX et al. (2007) G-protein-coupled Receptor Rhodopsin Regulates the Phosphorylation of Retinal Insulin Receptor. J Biol Chem 282:9865–9873. [DOI] [PubMed] [Google Scholar]
- Rajala A, Wang Y, Rajala RV (2016) Activation of oncogenic tyrosine kinase signaling promotes insulin receptor-mediated cone photoreceptor survival. Oncotarget 7:46924–46942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala RV, Gardner TW (2016) Burning fat fuels photoreceptors. Nat Med 22:342–343. [DOI] [PubMed] [Google Scholar]
- Rajala RV, McClellan ME, Ash JD et al. (2002) In vivo regulation of phosphoinositide 3-kinase in retina through light-induced tyrosine phosphorylation of the insulin receptor beta-subunit. J Biol Chem 277:43319–43326. [DOI] [PubMed] [Google Scholar]
- Reiling JH, Sabatini DM (2006) Stress and mTORture signaling. Oncogene 25:6373–6383. [DOI] [PubMed] [Google Scholar]
- Sabatini DM (2006) mTOR and cancer: insights into a complex relationship. Nat Rev Cancer 6:729–734. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM (2005) Growing roles for the mTOR pathway. Curr Opin Cell Biol 17:596–603. [DOI] [PubMed] [Google Scholar]
- Sato T, Nakashima A, Guo L et al. (2010) Single amino-acid changes that confer constitutive activation of mTOR are discovered in human cancer. Oncogene 29:2746–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzle T, Hall MN (2000) TOR, a central controller of cell growth. Cell 103:253–262. [DOI] [PubMed] [Google Scholar]
- Urano J, Sato T, Matsuo T et al. (2007) Point mutations in TOR confer Rheb-independent growth in fission yeast and nutrient-independent mammalian TOR signaling in mammalian cells. Proc Natl Acad Sci U S A 104:3514–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh A, Ma S, Le YZ et al. (2015) Activated mTORC1 promotes long-term cone survival in retinitis pigmentosa mice. J Clin Invest 125:1446–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Macke JP, Merbs SL et al. (1992) A locus control region adjacent to the human red and green visual pigment genes. Neuron 9:429–440. [DOI] [PubMed] [Google Scholar]
- Warburg O (1956) On the origin of cancer cells. Science 123:309–314. [DOI] [PubMed] [Google Scholar]


