Abstract
The nitric oxide – guanylyl cyclase-1 – cyclic guanylate monophosphate (NO-GC-1–cGMP) pathway has emerged as a potential pathogenic mechanism for glaucoma, a common intraocular pressure (IOP)-related optic neuropathy characterized by the degeneration of retinal ganglion cells (RGCs) and their axons in the optic nerve. NO activates GC-1 to increase cGMP levels, which are lowered by cGMP-specific phosphodiesterase (PDE) activity. This pathway appears to play a role in both the regulation of IOP, where reduced cGMP levels in mice leads to elevated IOP and subsequent RGC degeneration. Here, we investigated whether potentiation of cGMP signaling could protect RGCs from glaucomatous degeneration. We administered the PDE5 inhibitor tadalafil orally (10 mg/kg/day) in murine models of two forms of glaucoma – primary open angle glaucoma (POAG; GC-1−/− mice) and primary angle-closure glaucoma (PACG; Microbead Occlusion Model) - and measured RGC viability at both the soma and axon level. To determine the direct effect of increased cGMP on RGCs in vitro, we treated axotomized whole retina and primary RGC cultures with the cGMP analogue 8-Br-cGMP. Tadalafil treatment increased plasma cGMP levels in both models, but did not alter IOP or mean arterial pressure. Nonetheless, tadalafil treatment prevented degeneration of RGC soma and axons in both disease models. Treatment of whole, axotomized retina and primary RGC cultures with 8-Br-cGMP markedly attenuated both necrotic and apoptotic cell death pathways in RGCs. Our findings suggest that enhancement of the NO-GC-1-cGMP pathway protects the RGC body and axon in murine models of POAG and PACG, and that enhanced signaling through this pathway may serve as a novel glaucoma treatment, acting independently of IOP.
Keywords: cGMP, retinal ganglion cell, glaucoma, nitric oxide, PDE5
Introduction
Glaucoma, a common optic neuropathy, is the leading cause of irreversible blindness worldwide. By the year 2040, an estimated 111 million people will have glaucoma, many of which will be bilaterally blind 1, 2. Glaucoma is characterized by degeneration of retinal ganglion cells (RGCs), whose axons form the optic nerve 3. There are two major forms of the disease - primary angle closure glaucoma (PACG) and primary open angle glaucoma (POAG), with the latter being most prevalent 4. Several risk factors for glaucoma have been identified, however the only risk factor currently amenable to treatment is the level of intraocular pressure (IOP). Animal models of glaucoma illustrate a strong correlation between cumulative IOP exposure and optic neuropathy 5, 6. Large clinical trials indicate that significant and sustained IOP lowering by pharmacological, laser or surgical intervention slows vision loss in glaucoma patients 7, 8. However, human studies demonstrate that substantial IOP lowering does not completely halt disease progression 7, 9. Furthermore lowering IOP in patients with ocular hypertension slows, but does not completely prevent the onset of glaucomatous disease 10. These findings underscore the need to find strategies that directly protect the RGCs in clinical glaucoma management.
Multiple genetic epidemiology studies implicate impaired nitric oxide – guanylate cyclase-1 – cyclic guanylate monophosphate (NO-GC1-cGMP) signaling as a possible pathogenic mechanism in POAG 11-14 and PACG 15-17. NO activates GC-1 to increase levels of cGMP and cGMP levels are lowered by degradation via cGMP-specific phosphodiesterases (PDE). Among women the relation between NOS3 polymorphisms and POAG is significantly modified by age at menarche, parity 18, and postmenopausal hormone use 11. Similarly, variants in the promoter region of NOS3 were identified in 20% of familial POAG patients 19 and the NOS3 variant rs2070744 is significantly associated with a subset of normal tension glaucoma patients with optic nerve head hemorrhage 20. Furthermore, variants in the genomic region that encompasses GUCY1A3 and GUCY1B3, the genes encoding the α1 and β1 subunit of GC-1, are associated with female POAG cases that exhibit early paracentral vision loss in the Glaucoma Genes and Environment (GLAUGEN) study 12. Likewise, genetic variants in upstream components of the NO-GC-1-cGMP pathway are also associated with open-angle glaucoma21-25; specifically loci between CAV1 and CAV2, the genes encoding for Caveolin 1/2 which control NO production by NOS3 26 are associated with POAG. Physiological studies suggest that NO and cGMP regulate aqueous humor (AqH) outflow, and optic nerve head hemodynamics in pre-clinical models 15-18, 12, 27, 28 and have clinical relevance for glaucoma treatment 29-35. Collectively these studies highlight a role for the NO-GC-1-cGMP pathway in POAG and PACG pathogenesis, representing important steps toward understanding the contribution of the NO-GC-1-cGMP pathway in the etiology of glaucoma.
We recently implicated a role for GC-1 in the regulation of IOP in glaucoma 12, 36. Our findings indicated that female mice lacking the alpha-1 subunit of GC-1 (formerly soluble guanlyate cyclase or sGC), develop age-related glaucoma mimicking human POAG 12. GC-1 disruption leads to age-related elevated IOP and RGC degeneration. Here, we investigated whether preventing breakdown of cGMP by phosphodiesterase type 5 (PDE5) could, conversely, protect RGCs from glaucomatous degeneration. We found that the PDE5 inhibitor, tadalafil, increases serum cGMP levels and impedes RGC loss in murine models of both POAG (GC-1−/− mice) and PACG (Microbead Occlusion Model; MOM) without altering IOP. Additionally, we determined that cGMP directly impedes axotomy-induced necrosis and apoptosis in whole retina as well as in primary RGCs. Our findings suggest that increased bioavailability of cGMP prevents IOP-related RGC degeneration in an IOP-independent manner. We provide evidence for IOP-independent neuroprotection in both POAG and PACG pre-clinical models, indicating that NO-GC-1–cGMP pathway is a strong candidate for therapeutic targeting.
Methods
Animals.
All animal studies were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the IACUC committee at the Massachusetts General Hospital and Vanderbilt Medical Center. For RGC cultures, we obtained timed-pregnant Sprague-Dawley rats from (Charles River Laboratories, Wilmington, MA). Two – four days after birth, pups were collected, sacrificed by decapitation and retinas were processed for primary cultures of RGCs. For GC-1−/− studies, age-matched female wild type Sv/129S6 (WT) and GC-1−/− mice on an Sv/129S6 background were bred and housed at the Massachusetts General Hospital animal facility 37. For microbead studies, age-matched (WT Sv/129S6) 8-week old female mice were bred in the animal facility at Massachusetts General Hospital. All mice were euthanized with 10 mg intraperitoneal (IP) pentobarbital injection. Observers masked to animal genotype, experimental group and diet performed all data acquisition and analyses described.
Microbead Model.
Microbead-induced IOP elevation was performed, as previously described 38. WT mice were anesthetized with ketamine (100 mg/kg IP; Ketaset; Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (9 mg/kg; TranquiVed; Vedco, Inc., St. Joseph, MO). Additional analgesia was provided by topical application of tetracaine (1%; Bausch & Lomb, Tampa, FL). IOP elevation was induced unilaterally by 2μl injection of polystyrene microbeads (FluoSpheres; Invitrogen, Carlsbad, CA; 15-μm diameter) into the anterior chamber of the right eye under a surgical microscope. Microbeads were formulated at 5.0 × 106 beads/ml in sterile saline solution. The central cornea was gently punctured using a sharp 30-gauge needle (World Precision Instruments Inc., Sarasota, FL). Just prior to microbead injection, an air bubble was injected into the anterior chamber via a micropipette connected with a Hamilton syringe, coupled to a syringe pump to prevent excessive AqH egress from the eye. A small volume (2 μl) of microbeads was then injected through the pre-formed anterior chamber entry site using the micropipette - Hamilton syringe-syringe pump equipment. Mice were placed on a heating pad for recovery after the injection, and a triple-antibiotic ointment (neomycin/polymyxin B/bacitracin) (Dechra Veterinary Products, Overland Park, KS) was applied topically onto the injected eye to prevent infection. In this study, microbeads were injected at Day 0 and Day 10 to maintain IOP elevation In control groups, mice received 2μl injections of vehicle (saline) at Day 0 and Day 10.
IOP measurements.
IOPs were measured at baseline, and post-injection at week 1, 2, 3 and 4 in the PACG model and at 2, 4, 6, 8 and 10 months after tadalafil treatment in the POAG model. IOP measurements were taken at the same time of day (between 1 and 3 PM). Mice were anesthetized by sequential isoflurane inhalation of 5% for 30 seconds, 3% for 1 min and then 2% for a further 1 min before measurements were taken. Isoflurane was delivered in 95% oxygen with a precision vaporizer and IOP measurements were taken after 2 min when animals lost toe pinch and blink reflex. IOPs were acquired with a TonoLab rebound tonometer (iCare, Franconia, NH, USA) after topical application of anesthesia (0.5% proparacaine hydrochloride). Five TonoLab final readings each were averaged to obtain a single IOP value per eye.
Retinal explants.
For ex vivo retinal preparations, eyes were enucleated from WT mice and retinas rapidly removed and prepared as previously described 39. Briefly, explants were placed on organotypic culture inserts (0.4 mm pore; Millipore, Temecula, CA) held within culturing plates containing modified Neurobasal A media (2% B27 and 1% N2 supplements, 2 mM l-glutamine, 100 μM inosine, 0.1% gentamic in, 50 ng/mL BDNF, 20 ng/mL CNTF, and 10 ng/mL bFGF), and allowed to equilibrate overnight in an incubator at 37°C with 5% CO2. Explants were maintained at ambient pressure in a standard incubator for 3 days and all experiments using explants were performed minimally in triplicate.
RGC cell separation and culture.
Isolation of RGCs was carried out as previously described 40. Eyes (n ≥ 16/preparation) from postnatal day 2 to 4 Sprague-Dawley rats were enucleated, retinas were dissected from each and stored on ice in Dulbecco modified Eagle medium plus 5% glucose (DMEM/glu; Gibco, Carlsbad, CA). Tissue was dissociated first by lightly vortexing for 10 seconds, and then by centrifugation (70xg for 6 min at 4°C). Retinas were triturated by pipetting and incubated for 15 min at 37°C in 1 mg/mL papain (Worthington, Lakewood, NJ) and 0.01% DNase I in Earle’s Balanced Salt Solution. RGCs were purified by immunomagnetic separation, as previously described 41-43. Briefly, to first remove Muller glia, the cell suspension was centrifuged (4°C at 250xg) and re-suspended in DMEM/Glu with a polyclonal mouse anti-CD44 IgG (4μg/mL, Catalog no: ab157107; Abcam). The suspension was incubated on ice for 10 min while shaking, before centrifugation (4°C at 250xg) and incubation on ice for 15 min, shaking, with anti-mouse immunoglobulin G (IgG) secondary antibody conjugated to magnetic microbeads. The suspension was then loaded into a pre-equilibrated column in the presence of a magnetic field (Miltenyi Biotech, Auburn CA). To isolate RGCs, the remaining cell suspension was incubated with monoclonal mouse anti-Thy1.1/CD90 IgG on ice, while shaking for 10 min (5μg/mL; Catalog no. 554895; BD Pharmingen, San Diego CA), and then for 15 min on ice, shaking, with antimouse IgG-labelled secondary and passed through a column in a magnetic field. The resulting Thy1.1/CD90-positive cells were plated into eight-chamber glass slides coated with laminin (0.01 mg/mL; Sigma) and grown in serum-free, B27-supplemented medium (NeuroBasal; Gibco), as previously described 40. The growth medium also contained 2 mM glutamine, 0.1% genomycin, 1% N2 supplement (insulin 500 μg/mL; transferrin 10 mg/mL; progesterone 630 ng/mL; putrescine 1.6 mg/mL and selenite 520 ng/mL; Gibco), 50 ng/mL brain-derived neurotrophic factor (Invitrogen, Carlsbad, CA), 20 ng/mL ciliary neurotrophic factor (Invitrogen), 10 ng/mL bFGF (Invitrogen), and 100 μM inos ine (Sigma). Before experiments, RGCs were maintained with the medium described above in a standard incubator containing 5% CO2 until homeostasis was reached, as determined by neurite outgrowth and a stable level of viability (5–7 days).
Pharmacological Interventions.
For in vivo studies, food pellets containing cGMP-enhancing-compound (40mg/kg tadalafil) were ordered from Research Diets, Inc. (New Brunswick, NJ, USA). All mice were fed with an ad libitum diet. The duration of tadalafil treatment was determined by the timeline of pathology in each model, such that tadalafil treatment was provided throughout pathogenesis and progression. For hemodynamic (WT) and cGMP (WT and GC-1−/−) studies described below, age-matched mice were fed tadalafil-containing chow (10mg/kg/day) for 4 weeks prior to measurements. For microbead studies, WT mice were fed tadalafil-containing chow three days before the time of microbead injection, and then for 4 weeks until sacrifice and endpoints for the study were carried out. For chronic GC1−/− studies, tadalafil treatment began at maturity (3-month-old WT and GC-1−/− mice) and continued for 10 months until they were sacrificed. For ex vivo and in vitro studies, the cGMP analogue 8-Br-cGMP was used. For retinal explant experiments, explants were kept in culture for 24h to equilibrate before incubation in the presence or absence of 8-Br-cGMP for another 48h. Cell culture medium was then removed for LDH analysis (see below). Remaining retinal tissue was then sonicated and protein extracted for western blot analysis (see below). For primary RGC cultures, cells were kept in culture for 5-7 days before addition of 8-Br-cGMP for an extra 48h with or without 8-Br-cGMP.
cGMP Quantification.
Whole blood was collected in K2EDTA tubes (Becton Dickinson) from 3-month-old WT and GC-1−/− mice maintained on tadalafil chow for 4 weeks. Following centrifugation (4 °C for 15 min at 1000 xg), serum was collected and snap-frozen in liquid nitrogen until analysis. cGMP concentrations were measured using the acetylation protocol of a cGMP ELISA Kit (Cayman Chemical) after sample dilution with supplied ELISA buffer as per manufacturer’s specifications. Samples were standardized to protein concentration measured by BCA Protein Assay Kit (Pierce).
Hemodynamic measurements.
Mean arterial pressure (MAP) was measured in 3-month-old WT mice maintained on tadalafil chow for 4 weeks. Mice were placed on a temperature-controlled surgical table with a rectal temperature probe for the maintenance of body temperature at 37 °C. The age-matched animals were anesthetized with 1.5 volume% isoflurane and 0.8 L/min O2 flow. The right carotid artery was cannulated with a custom-made PE-10 catheter connected to a pressure transducer for monitoring of systemic blood pressure. After reaching a steady state, the MAP was recorded.
Retinal wholemount immunohistochemistry and RGC quantification.
Eyes were enucleated, a small incision made in the cornea and post-fixed for 2 hours in 4% paraformaldehyde (Boston Bioproducts, Ashland, MA) at 4°C. Retinas were isolated from the eyecup and cuts made to create quadrants. Dissected retinas were blocked with 0.1 % Triton-X100 and 2 % BSA in PBS for 1 hour, followed by incubation with the primary antibody β-III-tubulin, (1:400, 1 ug/ml, Millipore) overnight at 4°C. The following day, retinas were washed 3x in PBS and incubated for 2 hours at room temperature with the secondary antibody anti-mouse Alexa Fluoro 594 (1:500, 4 ug/ml, Life Technologies). Finally, the 4’,6-diamidino-2-phenylindole counterstain was added in PBS (1:1000) for 15 min at room temperature. Flat-mount stained retinas were placed on SuperFrost Plus slides (VWR, Batavia, IL) and coverslipped with mounting medium (VectaShield, Vector Lab, Burlingame, CA). Flat-mounted retina each with 4 unidirectional segments were imaged with a confocal microscope (Leica TSC SP5). For each retina, we employed a consistent sampling pattern that accounted for eccentricity whereby16-20 non-overlapping images were taken from mid and peripheral regions of the retina (around 4-5 per unidirectional segment; at least two mid-central images and two peripheral per segment). The identity of each image was masked and anti-βIII-tubulin positive cells, denoting RGCs, were counted by two investigators, first by hand and then using CellProfiler and CellProfilerAnalyst software, as described previously 44. For each animal RGC counts were averaged and expressed as mean RGCs per mm2 of retina.
Optic nerve axon quantification.
Optic nerves were harvested and fixed in 2.5 % Karnovsky fixative (2.5 % formaldehyde/2.5 % glutaraldehyde) at 4°C. Semi-thin optic nerve cross sections (1 μm) were stained with 1 % paraphenylenediamine (PPD, Fisher Scientific Co., Fair Lawn, NJ) and imaged on an upright microscope (Nikon bright-field microscope). Each section of optic nerve was imaged with 10-12 non-overlapping images taken per section at 100x; at least 5-6 central regions and 5-6 peripheral regions equidistant from the center of and circumference of the nerve were taken. Quantification of myelinated axons on 100x images were carried out both manually and in ImageJ using the AxonJ plug-in by at least two masked investigators. AxonJ is an automated quantification tool used to count rodent axons on sections stained with PPD 45. The software uses an algorithm to detect axons and evaluates axon number in each section. To calculate axon density, axon counts were divided by the area counted (mm2). Axon density is expressed as mean axons/mm2.
LDH cell death assay.
After seeding, test compounds were prepared and added to primary RGC or retinal explants in culture medium. Culture medium was retained and LDH leakage measured using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega). Absorbances were measured at 490 nm using the SpectraMax M Series Multi-Mode Microplate Reader (Molecular Devices) and the SoftMax Pro Software (Molecular Devices). For data analysis, background absorbance was subtracted from sample absorbance. For every experiment 8 technical repeats of each sample were run, and the experiment repeated 3 times using a new retinal explant or isolation of primary cells.
Western blot analysis.
Primary RGCs were collected from wells and centrifuged at (1200 relative centrifugal force for 5 minutes at 4°C), washed twice in ice-cold PBS and the remaining pellet re-suspended in 60μl radioimmunoprecipitation buffer (RIPA) buffer containing 50mM Tris-HCl, 150mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, halt Protease inhibitor cocktail (ThermoFisher), and halt Phosphatase inhibitor cocktail (ThermoFisher). Cells were homogenized using an insulin syringe and vortexed for 3 seconds. Lysates were incubated for 20 minutes on ice and then centrifuged (15 min at >10,000xg). The supernatant was transferred into fresh Eppendorf tubes, snap frozen with liquid nitrogen, and stored at −80°C if not immediately used. For retinal samples, each retina was placed into 50μl RIPA buffer and homogenized by sonication (Fischer Scientific, FB50). Protein was measured in samples using the Pierce 660nn Protein Assay kit (ThermoFisher). Samples (20μg protein) were separated by SDS-PAGE on a 4-12% Bolt Bis-Tris gel (Invitrogen), electroblotted onto PVDF membranes and probed with the following primary antibodies: Cleaved Caspase-3 (9661S; 1:1000) Cell Signaling, Caspase-3 (9665S; 1:1000) cell signaling, and GAPDH (2118S; 1:1000) Cell Signaling. Proteins were detected using IRDye 680LT or IRDye 800CW secondary antibodies, Odyssey Blocking Buffer and a Li-Cor Odyssey Infrared Imaging System (Li-Cor Inc., Lincoln, NE) following manufacturer’s protocol.
Statistical Analysis.
All data are presented as means ± standard deviation (SD). GraphPad Prism 6 (La Jolla, CA) was used to perform statistical analyses of the data. For multiple comparisons in GC-1−/− and microbead studies, one-way ANOVA and the Welch’s-corrected t-test were used. Mann-Whitney Rank Sum Tests were used for data sets that did not meet normality and/or equal variance requirements. A p value of 0.05 was considered significant.
Results
Tadalafil increases plasma cGMP in mice without elevating systemic blood pressure.
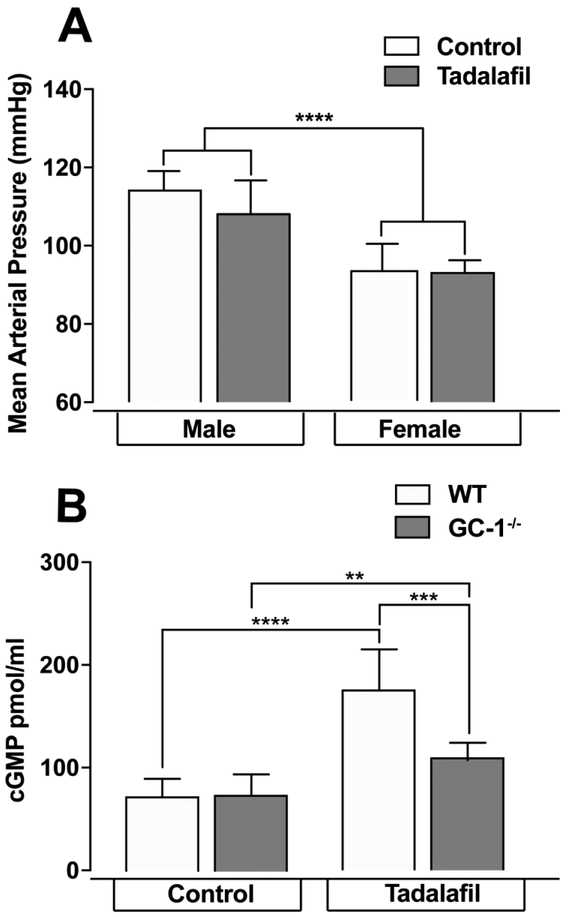
Current indications for tadalafil treatment relate to its ability to increase smooth muscle relaxation in blood vessels via prolonged enhancement of cGMP levels. Since systemic blood pressure can affect AqH outflow and IOP 46, 47, we first confirmed that the dose of tadalafil in this study was beneath that required to induce systemic blood pressure effects. We maintained two-month old WT mice on control or tadalafil chow, dosed at 10mg/kg/day for 4 weeks. Tadalafil treatment did not alter MAP in either male or female mice (p>0.05; n=7 for all groups, Figure 1A), though MAP was 19% higher in male mice than in female mice, regardless of diet (p<0.005 for both control and tadalafil diets; Figure 1A). This is a strain-dependent blood pressure phenomenon and has been reported previously 48, and therefore only female mice were used throughout the remainder of the study.
Figure 1. Tadalafil does not alter MAP in male or female mice and increases plasma cGMP after 4 weeks.
Naïve WT or GC-1−/− mice were maintained on 40mg/Kg tadalafil chow for 4 weeks before MAP or cGMP measurements were taken, n=7-9 for all groups A). MAP does not change after tadalafil treatment, B) cGMP levels increase in both naïve WT and GC-1−/− mice. Data expressed as mean ± S.D. **p=0.004, ***p=0.0009 and ****p<0.00001.
To validate the ability of tadalafil to enhance cGMP levels, we measured cGMP in plasma of WT and GC-1−/− female mice fed a control or tadalafil diet. In mice maintained on control chow for 4 weeks, baseline cGMP levels were similar in GC-1−/− mice and WT mice (Figure 1B). In mice treated with tadalafil, (4 weeks) plasma cGMP levels increased 2.4-fold and 1.5-fold in WT mice and GC-1−/− mice, respectively (p < 0.05 for both; n=9 and n=7 respectively, Figure 1B). cGMP levels in GC-1−/− maintained on tadalafil were 37% lower than tadalafil-treated WT mice (p = 0.0009; n=7, Figure 1B). In addition to blood plasma, cGMP levels in retinal tissue was also analyzed; however, levels were below the minimal level of detection (<20pmol cGMP/mg protein). Regardless, our results demonstrate target engagement of tadalafil and indicate that 4 weeks of daily dosing with tadalafil is sufficient to increase cGMP levels in both the presence and absence of GC-1 activity.
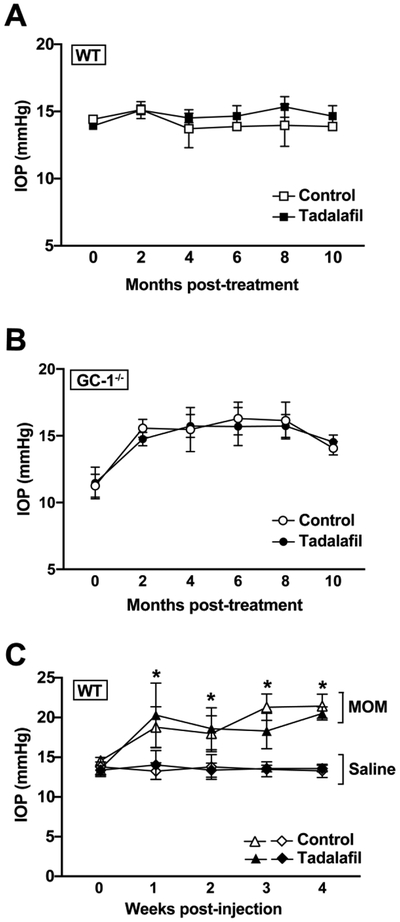
Tadalafil does not alter IOP in either murine model of glaucoma
Recent clinical trials demonstrate that a novel class of NO-donating drugs may be effective as IOP lowering agents for glaucoma 33, 34. To determine whether tadalafil treatment alters IOP, we measured IOP as a function of tadalafil treatment in GC-1−/− mice, a murine model of POAG and in WT mice using the microbead model of PACG. For POAG studies, we maintained GC-1−/− and WT mice on control or tadalafil diet for 10 months (beginning at 3 months of age) and measured IOP monthly. Comparisons between naïve WT and GC-1−/− mice determine the success of the POAG model and comparisons between GC-1−/− mice on control and tadalafil diets determines efficacy of tadalafil as an interventional treatment for POAG. For PACG, we maintained saline- and microbead-injected mice on control or tadalafil diet for 4 weeks (beginning 3 days prior to injection) and measured IOP three times per week for 4 weeks. Similarly, comparison between saline-injected and microbead-injected mice in the control diet group determines the success of the model. Comparison between control and tadalafil diet groups in microbead-injected mice determines the efficacy of tadalafil as an interventional treatment for PACG and finally, comparison of saline-injected animals determines baseline effects of tadalafil in healthy mice.
Treatment with tadalafil did not alter IOP in either WT (Figure 2A) or GC-1−/− mice (Figure 2B) over the 10-month treatment period (p >0.05 for all). In PACG studies, at it’s peak, IOP was 60 % higher in microbead-injected eyes (21.7 ± 0.8 mmHg, n=10) than in saline-injected eyes (13.5 ± 0.5 mmHg, n=7) of mice maintained on control diet (p>0.0001; Figure 2C) 49. In tadalafil-treated mice, mean peak IOP was 51% higher in microbead-injected eyes (20.8 ± 0.9 mmHg, n=9) than in saline-injected eyes (13.8± 0.9 mmHg, n=7, p = <0.0002; Figure 2C). There was no significant difference in IOP between mice maintained on control and tadalafil diets for either saline- or microbead-injected eyes (p>0.05, Figure 2C). These data indicate that these tadalafil exposures did not significantly affect IOP elevation in either POAG or PACG murine models. Together, our findings suggest that daily administration of 10mg/kg/day tadalafil enhances systemic cGMP levels without altering either systemic blood pressure or IOP.
Figure 2. Tadalafil does not affect IOP measurements in either POAG or PACG murine models of glaucoma.
IOP was measured over time in WT or GC-1−/− mice in the POAG model of glaucoma, and in WT mice with and without microbead injection in the PACG model. A) IOP in WT mice does not change with tadalafil treatment, B) IOP in GC-1−/− mice does not change with tadalafil treatment and C) tadalafil treatment does not alter IOP in the PACG murine model of glaucoma in WT mice. Data expressed as mean ± S.D. Statistical significances between saline and MOM (microbead occlusion model) groups are p<0.0002 for all comparisons.
Tadalafil protects RGCs in a murine model of POAG
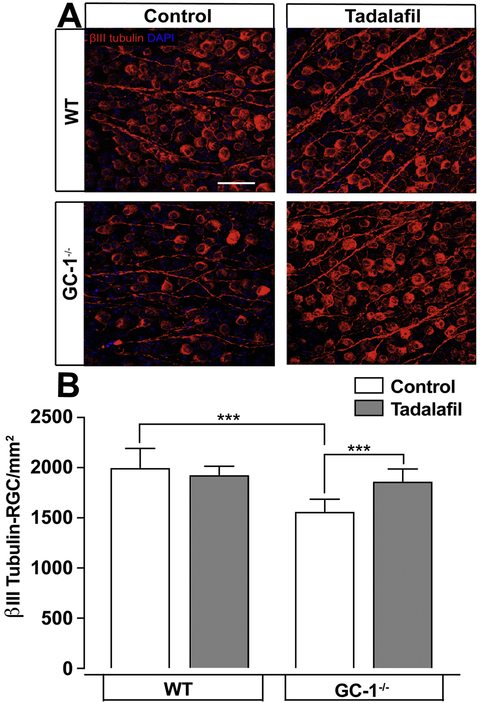
Female GC-1−/− mice develop glaucoma characterized by age-dependent loss of RGCs and ON axons 12. To investigate whether tadalafil can prevent optic neuropathy associated with GC-1-deficiency, age-matched WT and GC-1−/− mice were maintained on control or tadalafil chow for 10 months. After 10 months of treatment, we examined RGC degeneration by quantifying the density of β-tubulin-stained RGC soma in the retina and density of RGC axons in the optic nerve. Consistent with previous findings 12, βtub+ RGC density was 28% lower in GC-1−/− mice (1555 ± 49.69 RGC/mm2, n=6) than in WT mice (1991 ± 76.71 RGC/mm2, n=6) maintained on control diet (p = 0.0007; Figure 3A, B). In contrast, density of βtub+ RGCs was 19% higher in retina from GC-1−/− mice maintained on tadalafil chow (1855 ± 45.4 RGC/mm2, n=7) than GC-1−/− mice maintained on control chow. This higher βtub+ RGC density in GC-1−/− mice treated with tadalafil was similar to that in WT mice maintained on the tadalafil diet (1855 ± 45.4 RGC/mm2 n=7 vs. 1918 ± 36.61 RGC/mm2, n=5, p=0.34 Figure 3A, B). Tadalafil chow did not a lter the density of βtub+ RGCs in WT mice (1918 ± 36.61 RGC/mm2, n=5), as compared to control chow (1991 ± 76.71 RGC/mm2, n=6, p > 0.05; Figure 3A, B). These data indicate that tadalafil treatment preserves βtub+ RGC soma in retina from a POAG murine model.
Fig. 3. Tadalafil prevents RGC loss in the murine POAG model of glaucoma.
Retina were flatmounted and stained for βIII tubulin. A) Representative βIII tubulin-stained RGC micrographs in WT and GC-1−/− mice maintained on control or tadalafil chow for 10 months are shown, scale bar = 50μM. B) Quantitative analysis of the mean density of βIII tubulin-positive RGCs (y-axis; mean RGCs/mm2 ± S.D) in retina reveals decreased RGC density in GC-1−/− mice that is reversed by tadalafil treatment. *** p<0.0009. MOM = microbead occlusion model.
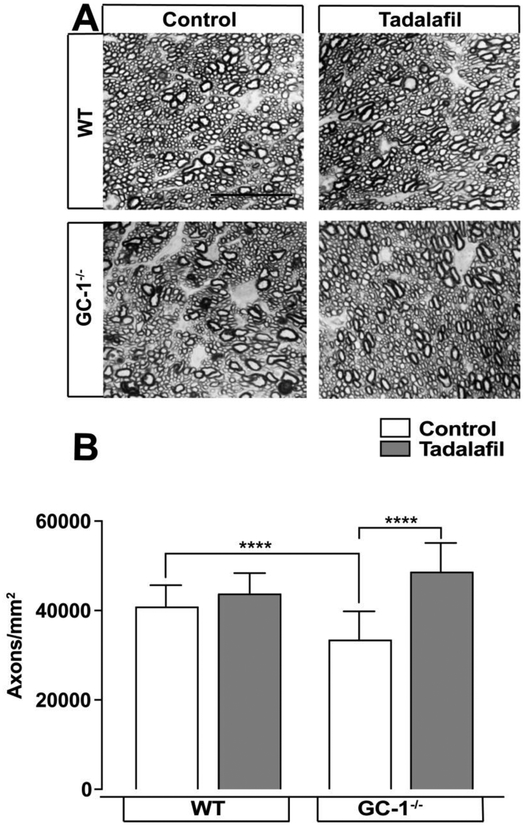
RGC degeneration in POAG occurs in a retrograde fashion, where RGC axons in the optic nerve degenerate prior to RGC soma in the retina 50, 51. Thus, we quantified the RGC axon density in optic nerve from both control and GC-1−/− mice with and without tadalafil treatment at 10 months. In mice maintained on control chow, axon density in optic nerves from GC-1−/− mice (33,629 ± 822.1 axons/mm2 n=6) was 18% lower than in WT mice (41,016 ± 540.2 axons/mm2, n=6, p<0.0001; Figure 4A, B). This is consistent with previously reported findings in this model 12. Tadalafil treatment led to 7% higher axon counts in WT optic nerves (41,016± 540.2 axons/mm2, n=6 control chow vs. tadalafil chow at 43,936 ± 522.6 axons/mm2 n=5, *p = 0.002; Figure 4B). In GC-1−/− mice, axons counts were 45% higher in mice fed tadalafil vs. control diet (48,828 ± 707.4 axons/mm2, n=7 vs. 33,629 ± 822.1 axons/mm2, n=6, p < 0.0001; Figure 4B). These data indicate that tadalafil treatment markedly attenuates degeneration of RGC axons in the optic nerve of mice from a POAG murine model.
Figure 4. Tadalafil prevents ON axon depletion in WT and GC-1−/− mice in the murine POAG model of glaucoma.
A) Representative paraphenylenediamine -stained ON axon micrographs in WT and GC-1−/− mice maintained on control or tadalafil chow, scale bar = 10μM. B) Quantitative analysis of the mean density of RGC axons (y-axis; mean axons/mm2 ± S.D) in optic nerve reveals decreased RGC axon density in GC-1−/− mice that is reversed by tadalafil treatmern ***, p<0.0001.
Tadalafil protects RGCs in a murine model of PACG
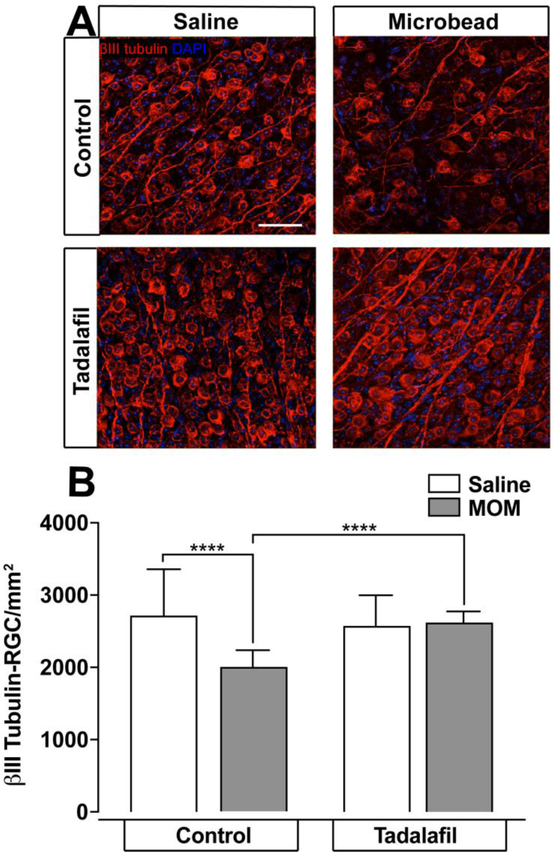
To determine whether the apparent neuroprotective properties of tadalafil are specific to POAG or could also translate to PACG-induced RGC degeneration, we examined the impact of tadalafil on RGC degeneration in the microbead occlusion model (MOM) 49. In the MOM, intracameral injection of polystyrene microbeads leads to elevated IOP via blockade of aqueous drainage canals 26. WT mice received intracameral injection of either microbeads or an equivalent volume of saline (experimental control). Three days prior to model induction, mice were fed tadalafil chow or control chow. After 4 weeks, β-tub+ RGC soma and axons were quantified in the retina and the optic nerve, respectively. Consistent with previous findings 49, microbead-induced ocular hypertension in mice maintained on control chow resulted in a 26% lower β-tub+ RGC density (2007 ± 48.01 RGC/mm2, n=10), as compared to saline-injected controls (2726 ± 132 RGC/mm2, n=7; p <0.0001; Figure 5A, B). In contrast, the density of β-tub+ RGCs was similar in saline- (2580 ± 86.9 RGC/mm2, n=7) and microbead-injected eyes (2630 ± 28.1 RGC/mm2, n=9) from mice maintained on tadalafil diet (p>0.05; Figure 5A, B). Across microbead-injected mice, the density of β-tub+ RGCs in microbead-injected eyes was 31% higher in tadalafil-treated mice than in mice maintained on control chow (2630 ± 86.9 RGC/mm2, n=9 vs. 2007 ± 48 RGC/mm2, n=10, p < 0.0001, Figure 5A, B). In contrast, there was no significant difference in the density of β-tub+ RGCs in saline-injected mice treated with tadalafil or maintained on control chow (2726 ± 132 RGC/mm2, n=7 vs. 2580 ± 86.9 RGC/mm2, n=7, p>0.05; Figure 5A, B).
Figure 5. Tadalafil prevents RGC loss in the murine PACG model of glaucoma.
Retina were flatmounted and stained for βIII tubulin. A) Representative βIII tubulin-stained RGC micrographs in WT mice maintained on control or tadalafil chow for 1 month after saline or microbead injection are shown, scale bar = 50μM. B) Quantitative analysis of the mean density of βIII tubulin-positive RGCs (y-axis; mean RGCs/mm2 ± S.D) in retina reveals decreased RGC density in mice injected with microbeads that is reversed by tadalafil treatment. *** p<0.0001. MOM = microbead occlusion model.
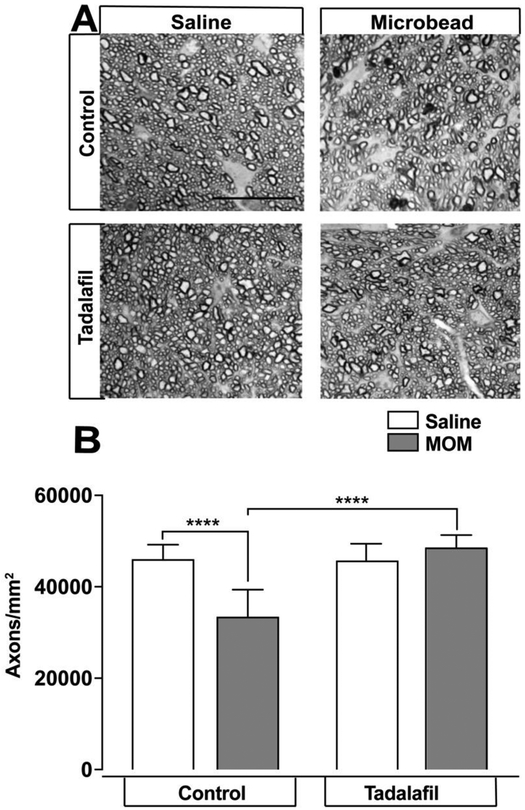
In mice maintained on control chow, RGC axon density in optic nerves from microbead-injected eyes (33,563 ± 1045 axons/mm2, n=10) was 27% lower than that of saline-injected eyes (46,178 ± 501.1 axons/mm2, n=7, p < 0.0001; Figure 6A, B). Treatment with tadalafil prevented the loss of RGC axons: RGC axon density was similar in optic nerves from microbead-injected eyes (48,747 ± 517.1 axons/mm2, n=9) and saline-injected eyes (45,855 ± 654.9 axons/mm2, n=7 (p = 0.001; Figure 6A, B) and RGC axon density was 45% higher in mice treated with tadalafil chow (48,747 ± 517.1 axons/mm2, n=9) than in microbead-injected mice maintained on control chow (33,563 ± 1045 axons/mm2, n=10, p<0.0001, Figure 6A, B). RGC axon density did not differ significantly between optic nerves of saline-injected mice treated with tadalafil or maintained on control chow (45,855 ± 654.9 axons/mm2, n=7, vs. 46178 ± 501.1 axons/mm2, n=7, p=0.7 Figure 6A,B). Together, these data indicate that tadalafil treatment preserves β-tub+ RGC soma in retina and RGC axon density in a PACG-like murine model.
Figure 6. Tadalafil prevents ON axon depletion in the murine PACG model of glaucoma.
A) Representative PPD-stained ON axon micrographs in WT mice on control or tadalafil chow for 1 month after saline or microbead injection, scale bar = 10μM. B) Quantitative analysis of the mean density of RGC axons (y-axis; mean axons/mm2 ± S.D) in optic nerve reveals decreased RGC axon density in microbead-injected mice that is reversed by tadalafil treatment. Data expressed as mean ± S.D. **** p<0.0001. MOM = microbead occlusion model.
cGMP directly influences necrotic and apoptotic cell death in retina
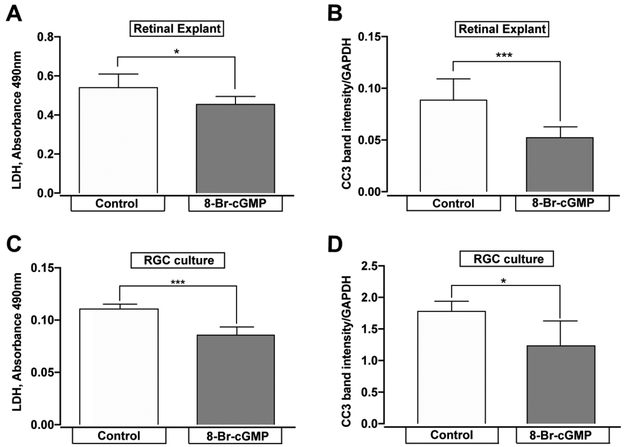
Our data indicate that daily administration of 10mg/kg/day of tadalafil prevents RGC degeneration in both POAG (Figures 3, 4) and PACG models (Figure 5, 6), despite continued IOP elevation (Figure 2). These results suggest that tadalafil influences RGC degeneration despite an IOP insult. Since tadalafil increases the bioavailability of cGMP, we sought to determine whether cGMP can directly impact the survival of RGCs. We examined necrotic and apoptotic cell death in organotypic culture of whole retina 72 hours after axotomy in the presence (48 hours) or absence of 100μM 8-Br-cGMP, a cell-membrane permeable cGMP analogue. Necrotic cell death was assessed by LDH assay and apoptotic death was assessed by caspase-3 cleavage (CC3). LDH release was 16 % lower in whole retina explants treated with 8-Br-cGMP, than in those treated with vehicle (p = 0.02; Figure 7A). Similarly, CC3 cleavage was 38% lower in retina explants treated with 100μM 8-Br-cGMP than in those treated with vehicle (p = 0.002; Figure 7B). These data indicate that increased cGMP levels attenuate cell death of retinal tissue.
Figure 7. 8-Br-cGMP impedes necrotic and apoptotic pathways.
A) Necrotic cell death, measured by LDH absorbance, was less pronounced in retinal explants incubated with 100 μM 8-Br-cGMP than in explants incubated with vehicle, n=6, *p=0.02 B) Cleaved-caspase-3 (CC3) expression was attenuated in retinal explants when incubated with 100 μM 8-Br-cGMP, n= 6, **p=0.002, C) Necrotic cell death, measured by LDH absorbance, was less pronounced in primary RGCs incubated with 100 μM 8-Br-cGMP than in RGCs incubated with vehicle, n=4, ***p =0.0008 and D) CC3 expression was attenuated in primary RGCs when incubated with 100 μM 8-Br-cGMP, n=4, *p=0.04. Data expressed as mean ± S.D.
cGMP directly impacts necrotic and apoptotic cell death pathways in RGCs
Like organotypic culture, production of primary RGC cultures requires transection of RGC axons. Although there is some initial recovery from axotomy, cell survival depreciates with time in culture. This depreciation is attributable to both necrotic and apoptotic mechanisms 52. Therefore we examined the ability of cGMP to impact post-axotomy cell death using primary cultures of purified rat RGCs. We treated RGC cultures with 100μM 8-Br-cGMP or vehicle for 48 hours and measured baseline levels of necrotic and apoptotic cell death after 4 days in culture. Consistent with our results in whole retina explants, treatment with 8-Br-cGMP reduced LDH release by 22% (p = 0.0008; Figure 7C). Similarly, caspase-3 cleavage was also reduced by 30%, as compared to vehicle (p = 0.04; Figure 7D). These data indicate that increasing cGMP bioavailability improves survival of RGCs specifically and does so, in part, by mitigating both necrotic and apoptotic cell death.
Discussion
Multiple studies implicate impaired NO-cGMP signaling as a possible pathogenic mechanism in POAG 11, 14, 53-55. It is known that NO and cGMP regulate AqH outflow and IOP in pre-clinical models and clinical models 29-34, and that NO has been attributed to both neurotoxic and neuroprotective roles, which appear to stem from concentration effects in vivo. Excess endogenous NO is noxious, since it can undergo oxidative reduction reactions to produce reactive nitrogen species 56, 57, which have been implicated in the pathogenesis of neurodegenerative disorders such as Alzheimer’s Disease 58. However, several studies have revealed neuroprotective properties of NO through its downstream activation of the cGMP pathway and subsequent downstream effectors, such as protein kinases and Ca2+ channels 59-61. Our previous work established female GC-1−/− mice as a new animal model for POAG, suggesting a central role for NO/GC-1 signaling in the regulation of IOP and RGC homeostasis 12.
Despite an important role for the NO-cGMP pathway emerging in the pathophysiology of glaucoma 55, there have been little preclinical work done to explore the therapeutic potential of harnessing this pathway in glaucoma treatment. We show in two murine models of glaucoma (POAG and PACG) that systemic delivery of tadalafil prevents IOP-induced degeneration of RGCs. This apparent neuroprotection was independent of IOP, as we detected no change in the magnitude or duration of IOP elevation with tadalafil treatment in either the POAG or PACG model. There are some reports that the PDE5 inhibitors tadalafil and sildenafil cause transient increases in IOP in sheep 62 and also in humans 63. However, our results align well with a long-term study in humans with POAG that demonstrated no significant change in IOP when sildenafil, also a PDE5 inhibitor, was administered at a dose of 100 mg 64.
While tadalafil treatment did not impact IOP elevation in our POAG and PACG models, systemic enhancement of cGMP has the potential to affect other relevant parameters, such as blood pressure. We determined that our treatment paradigm for tadalafil was sufficient to enhance systemic cGMP levels, illustrating target engagement, without elevating systemic blood pressure, a potential confounding aspect discussed below. It may seem surprising that genetic ablation of GC-1 is not associated with reduced cGMP levels in mice maintained on control chow; however, in addition to GC-1, cGMP is generated by other guanylyl cyclase isoforms, such as GC-2 and natriuretic peptide receptors 65. Despite this, plasma cGMP levels were lower in GC-1−/− mice than WT mice fed tadalafil diet, confirming that ablation of GC-1 is associated with impaired cGMP signaling.
Given that tadalafil treatment in our study was sufficient to enhance cGMP levels and protect RGCs independently of IOP, we explored whether cGMP enhancement directly impacted survival of retinal cells generally and RGCs specifically. In ex vivo retinal explants, incubation with 8-Br-cGMP led to a decrease in extracellular LDH, an easily quantifiable indicator of necrotic cell death. Levels of CC3 were also lower in tadalafil-than in vehicle-treated explants, suggesting a reduction in the activation of pro-apoptotic pathways. In vitro studies in primary purified RGCs yielded similar results, indicating that enhancement of cGMP levels has the potential to mitigate both necrotic and apoptotic pathways of cell death in retina and more specifically, RGCs. Together, these data suggest that the apparent neuroprotective effect of tadalafil in our POAG and PACG models arises, at least in part from cGMP-dependent attenuation of pro-apoptotic pathways, known to underlie RGC degeneration in both murine glaucoma models 66-70 and in human patients 66-70. Consistent with our findings in primary RGCs, anti-apoptotic roles of NO and cGMP have already been implicated in RGC survival 71, 72 and in other neuronal cell lines, such as motor and sympathetic neurons 73, 74 and hippocampal neurons 75.
The encouraging results reported in this study are likely to trigger additional research. Firstly, to show a true neuroprotective role of tadalafil in glaucoma, assessment of the effect of tadalafil on visual function in the glaucoma models studied will be required. Also, while no effect of 6-month treatments with tadalafil or sildenafil on electroretinography was detected in healthy humans 76 chronic tadalafil treatment was associated with toxic effects on photoreceptors in rat studies 77. Thus, studies of the effects of chronic tadalafil on ultrastructural features should be executed when further exploring a potential therapeutic role of tadalafil in glaucoma management. Any side effects of the use of PDE5 inhibiters may be circumvented by taking advantage of other compounds under clinical development that increase cGMP bioavailability 78. In addition, other dosing strategies for tadalafil and other PDE inhibitors have not yet been explored, and were beyond the scope of this study. Other PDE5 inhibitors have been used in the clinic and previous studies have shown that they are effective as neuroprotective agents. In a rat model of hypoxic ischemia, activation of the NO-cGMP pathway by sildenafil, a PDE5 inhibitor similar to tadalafil but with reduced half-life and specificity, reduced apoptosis, astrocytosis and microgliosis in the brain 79. We selected the PDE5 inhibitor tadalafil to elevate cGMP levels in vivo due to its longer half life 80, proven safety in chronic treatments 81, and ability to cross the blood-brain barrier 82. Tadalafil has also been proven to be neuroprotective in spinal chord injury83 and in ischemia/reperfusion injury in the fetal rat brain 84.
Visual disturbances have been reported in patients taking PDE5 inhibitors85, 86, the most common being an increased sensitivity to light 87-89. These symptoms likely arise from off-target inhibition of PDE6 in the retina. However, the selectivity of PDE5 over PDE6 is 700-fold for tadalafil 90 compared with 10-fold for sildenafil 91 and 15-fold for vardenafil 92, so off-target effects when using tadalafil are likely limited. In fact, the rate of occurrence for these symptoms is low at only 0.1% of tadalafil users 93. The decision to dose tadalafil so as not to impact MAP was to prevent the potentially confounding effect of MAP on ocular blood flow. Although we did not identify changes in MAP and there are conflicting data on the role of systemic blood pressure in glaucoma etiology and progression94-97, we cannot rule out a tadalafil-mediated effect on retinal vascular function as a contributor to the apparent neuroprotection we noted in vivo.
Conclusions
Overall, our results indicate that increasing cGMP bioavailability promotes RGC survival in murine models of POAG and PACG, likely through direct modulation of pro- and anti-apoptotic pathways. Furthermore, our data identify tadalafil and related compounds that enhance cGMP bioavailability and that are already clinically available or being tested in clinical trials for a variety of indications 78 as potential therapeutics for direct RGC neuroprotection. Such therapeutics could serve to increase efficacy of treatment in glaucoma patients with continued disease progression despite IOP lowering interventions. Further experiments are warranted to explore the possible effects of tadalafil on retinal vasculature and other putative mechanisms of RGC survival in glaucoma models.
Highlights.
Glaucoma is a common intraocular pressure-related optic neuropathy characterized by the degeneration of retinal ganglion cells (RGCs) and their axons in the optic nerve
The nitric oxide – guanylyl cyclase-1 – cyclic guanylate monophosphatepathway has emerged as a potential pathogenic mechanism for glaucoma
Tadalafil is a highly specific phosphodiesterase type 5 inhibitor
Treatment with tadalafil prevents degeneration of RGC soma and axons in two murine models of glaucoma
PDE5 inhibitors may be a novel neuroprotective strategy in the treatment of glaucoma
Acknowledgments
Funding Sources
These studies were supported by the National Eye Institute awards R01EY022746 (ESB), R01EY020496 (RMS), R01EY015473 (LRP) and P30EY08126 (Vanderbilt Vision Research Center), Unrestricted (Vanderbilt Eye Institute) awards from Research to Prevent Blindness, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quigley HA and Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. The Br Journal Ophthalmol. 2006;90:262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tham YC, Li X, Wong TY, Quigley HA, Aung T and Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. [DOI] [PubMed] [Google Scholar]
- 3.Shaikh Y, Yu F and Coleman AL. Burden of undetected and untreated glaucoma in the United States. Am J Ophthalmol. 2014;158:1121–1129 e1. [DOI] [PubMed] [Google Scholar]
- 4.Friedman DS, Wolfs RC, O'Colmain BJ, Klein BE, Taylor HR, West S, Leske MC, Mitchell P, Congdon N, Kempen J and Eye Diseases Prevalence Research G. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122:532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mabuchi F, Aihara M, Mackey MR, Lindsey JD and Weinreb RN. Optic nerve damage in experimental mouse ocular hypertension. Invest Ophthalmol Vis Sci. 2003;44:4321–30. [DOI] [PubMed] [Google Scholar]
- 6.Yucel YH, Zhang Q, Weinreb RN, Kaufman PL and Gupta N. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res. 2003;22:465–81. [DOI] [PubMed] [Google Scholar]
- 7.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M and Early Manifest Glaucoma Trial G. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–79. [DOI] [PubMed] [Google Scholar]
- 8.Garway-Heath DF, Crabb DP, Bunce C, Lascaratos G, Amalfitano F, Anand N, Azuara-Blanco A, Bourne RR, Broadway DC, Cunliffe IA, Diamond JP, Fraser SG, Ho TA, Martin KR, McNaught AI, Negi A, Patel K, Russell RA, Shah A, Spry PG, Suzuki K, White ET, Wormald RP, Xing W and Zeyen TG. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385:1295–304. [DOI] [PubMed] [Google Scholar]
- 9.The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J of Ophthalmol. 1998;126:498–505. [DOI] [PubMed] [Google Scholar]
- 10.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR and Gordon MO. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13; discussion 829-30. [DOI] [PubMed] [Google Scholar]
- 11.Kang JH, Wiggs JL, Rosner BA, Hankinson SE, Abdrabou W, Fan BJ, Haines J and Pasquale LR. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: interactions with sex and postmenopausal hormone use. Invest Ophthalmol Vis Sci. 2010;51:971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buys ES, Ko YC, Alt C, Hayton SR, Jones A, Tainsh LT, Ren R, Giani A, Clerte M, Abernathy E, Tainsh RE, Oh DJ, Malhotra R, Arora P, de Waard N, Yu B, Turcotte R, Nathan D, Scherrer-Crosbie M, Loomis SJ, Kang JH, Lin CP, Gong H, Rhee DJ, Brouckaert P, Wiggs JL, Gregory MS, Pasquale LR, Bloch KD and Ksander BR. Soluble Guanylate Cyclase alpha1-Deficient Mice: A Novel Murine Model for Primary Open Angle Glaucoma. PloS one. 2013;8:e60156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emam WA, Zidan HE, Abdulhalim BE, Dabour SA, Ghali MA and Kamal AT. Endothelial nitric oxide synthase polymorphisms and susceptibility to high-tension primary open-angle glaucoma in an Egyptian cohort. Mol Vis. 2014;20:804–11. [PMC free article] [PubMed] [Google Scholar]
- 14.Magalhaes da Silva T, Rocha AV, Lacchini R, Marques CR, Silva ES, Tanus-Santos JE and Rios- Santos F. Association of polymorphisms of endothelial nitric oxide synthase (eNOS) gene with the risk of primary open angle glaucoma in a Brazilian population. Gene. 2012;502:142–6. [DOI] [PubMed] [Google Scholar]
- 15.Ayub H, Khan MI, Micheal S, Akhtar F, Ajmal M, Shafique S, Ali SH, den Hollander AI, Ahmed A and Qamar R. Association of eNOS and HSP70 gene polymorphisms with glaucoma in Pakistani cohorts. Mol Vis. 2010;16:18–25. [PMC free article] [PubMed] [Google Scholar]
- 16.Awadalla MS, Thapa SS, Hewitt AW, Craig JE and Burdon KP. Association of eNOS polymorphisms with primary angle-closure glaucoma. Invest Ophthalmol Vis Sci. 2013;54:2108–14. [DOI] [PubMed] [Google Scholar]
- 17.Rong SS, Tang FY, Chu WK, Ma L, Yam JC, Tang SM, Li J, Gu H, Young AL, Tham CC, Pang CP and Chen LJ. Genetic Associations of Primary Angle-Closure Disease: A Systematic Review and Meta-analysis. Ophthalmology. 2016;123:1211–21. [DOI] [PubMed] [Google Scholar]
- 18.Kang JH, Wiggs JL, Haines J, Abdrabou W and Pasquale LR. Reproductive factors and NOS3 variant interactions in primary open-angle glaucoma. Mol Vis. 2011;17:2544–51. [PMC free article] [PubMed] [Google Scholar]
- 19.Tunny TJ, Richardson KA and Clark CV. Association study of the 5' flanking regions of endothelial-nitric oxide synthase and endothelin-1 genes in familial primary open-angle glaucoma. Clin Exp Pharmacol Physiol. 1998;25:26–9. [DOI] [PubMed] [Google Scholar]
- 20.Jeoung JW, Kim DM, Oh S, Lee JS, Park SS and Kim JY. The Relation Between Endothelial Nitric Oxide Synthase Polymorphisms and Normal Tension Glaucoma. J Glaucoma. 2017;26:1030–1035. [DOI] [PubMed] [Google Scholar]
- 21.Ozel AB, Moroi SE, Reed DM, Nika M, Schmidt CM, Akbari S, Scott K, Rozsa F, Pawar H, Musch DC, Lichter PR, Gaasterland D, Branham K, Gilbert J, Garnai SJ, Chen W, Othman M, Heckenlively J, Swaroop A, Abecasis G, Friedman DS, Zack D, Ashley-Koch A, Ulmer M, Kang JH, Liu Y, Yaspan BL, Haines J, Allingham RR, Hauser MA, Pasquale L, Wiggs J, Richards JE and Li JZ. Genome-wide association study and meta-analysis of intraocular pressure. Human Genet. 2014;133:41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorleifsson G, Walters GB, Hewitt AW, Masson G, Helgason A, DeWan A, Sigurdsson A, Jonasdottir A, Gudjonsson SA, Magnusson KP, Stefansson H, Lam DS, Tam PO, Gudmundsdottir GJ, Southgate L, Burdon KP, Gottfredsdottir MS, Aldred MA, Mitchell P, St Clair D, Collier DA, Tang N, Sveinsson O, Macgregor S, Martin NG, Cree AJ, Gibson J, Macleod A, Jacob A, Ennis S, Young TL, Chan JC, Karwatowski WS, Hammond CJ, Thordarson K, Zhang M, Wadelius C, Lotery AJ, Trembath RC, Pang CP, Hoh J, Craig JE, Kong A, Mackey DA, Jonasson F, Thorsteinsdottir U and Stefansson K. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nature Genet. 2010;42:906–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiggs JL, Kang JH, Yaspan BL, Mirel DB, Laurie C, Crenshaw A, Brodeur W, Gogarten S, Olson LM, Abdrabou W, DelBono E, Loomis S, Haines JL and Pasquale LR. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma in Caucasians from the USA. Human Mol Genet. 2011;20:4707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunes HF, Ananina G, Costa VP, Zanchin NIT, de Vasconcellos JPC and de Melo MB. Investigation of CAV1/CAV2 rs4236601 and CDKN2B-AS1 rs2157719 in primary open-angle glaucoma patients from Brazil. Ophthalmic Genet. 2018;39:194–199. [DOI] [PubMed] [Google Scholar]
- 25.Khawaja AP, Cooke Bailey JN, Wareham NJ, Scott RA, Simcoe M, Igo RP Jr., Song YE, Wojciechowski R, Cheng CY, Khaw PT, Pasquale LR, Haines JL, Foster PJ, Wiggs JL, Hammond CJ, Hysi PG, Eye UKB, Vision C and Consortium N. Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat Genet. 2018;50:778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mineo C and Shaul PW. Regulation of eNOS in caveolae. Advances in experimental medicine and biology. 2012;729:51–62. [DOI] [PubMed] [Google Scholar]
- 27.Lei Y, Zhang X, Song M, Wu J and Sun X. Aqueous Humor Outflow Physiology in NOS3 Knockout Mice. Invest Ophthalmol Vis Sci. 2015;56:4891–8. [DOI] [PubMed] [Google Scholar]
- 28.Chang JY, Stamer WD, Bertrand J, Read AT, Marando CM, Ethier CR and Overby DR. Role of nitric oxide in murine conventional outflow physiology. Am J Physiol Cell Physiol. 2015;309:C205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis DZ, Dismuke WM and Chokshi BM. Characterization of soluble guanylate cyclase in NO-induced increases in aqueous humor outflow facility and in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2009;50:1808–13. [DOI] [PubMed] [Google Scholar]
- 30.Khoobehi B, Chiroli V, Ronchetti D, Miglietta D, Thompson H, Ongini E and Impagnatiello F. Enhanced oxygen saturation in optic nerve head of non-human primate eyes following the intravitreal injection of NCX 434, an innovative nitric oxide-donating glucocorticoid. J Ocul Pharmacol Ther. 2011;27:115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotikoski H, Vapaatalo H and Oksala O. Nitric oxide and cyclic GMP enhance aqueous humor outflow facility in rabbits. Curr Eye Res. 2003;26:119–23. [DOI] [PubMed] [Google Scholar]
- 32.Krauss AH, Impagnatiello F, Toris CB, Gale DC, Prasanna G, Borghi V, Chiroli V, Chong WK, Carreiro ST and Ongini E. Ocular hypotensive activity of BOL-303259-X, a nitric oxide donating prostaglandin F2alpha agonist, in preclinical models. Exp Eye Res. 2011;93:250–5. [DOI] [PubMed] [Google Scholar]
- 33.Weinreb RN, Ong T, Scassellati Sforzolini B, Vittitow JL, Singh K, Kaufman PL and group Vs. A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: the VOYAGER study. Br J Ophthalmol. 2015;99:738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinreb RN, Scassellati Sforzolini B, Vittitow J and Liebmann J. Latanoprostene Bunod 0.024% versus Timolol Maleate 0.5% in Subjects with Open-Angle Glaucoma or Ocular Hypertension: The APOLLO Study. Ophthalmology. 2016;123:965–73. [DOI] [PubMed] [Google Scholar]
- 35.Kawase K, Vittitow JL, Weinreb RN, Araie M and Group JS. Long-term Safety and Efficacy of Latanoprostene Bunod 0.024% in Japanese Subjects with Open-Angle Glaucoma or Ocular Hypertension: The JUPITER Study. Adv Ther. 2016;33:1612–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muenster S, Lieb WS, Fabry G, Allen KN, Kamat SS, Guy AH, Dordea AC, Teixeira L, Tainsh RE, Yu B, Zhu W, Ashpole NE, Malhotra R, Brouckaert P, Bloch DB, Scherrer-Crosbie M, Stamer WD, Kuehn MH, Pasquale LR and Buys ES. The Ability of Nitric Oxide to Lower Intraocular Pressure Is Dependent on Guanylyl Cyclase. Invest Ophthalmol Vis Sci. 2017;58:4826–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buys ES, Sips P, Vermeersch P, Raher MJ, Rogge E, Ichinose F, Dewerchin M, Bloch KD, Janssens S and Brouckaert P. Gender-specific hypertension and responsiveness to nitric oxide in sGCalpha1 knockout mice. Cardiovascular Res. 2008;79:179–86. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Wei X, Cho KS, Chen G, Sappington R, Calkins DJ and Chen DF. Optic neuropathy due to microbead-induced elevated intraocular pressure in the mouse. Invest Ophthalmol Vis Sci. 2011;52:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sappington RM, Sidorova T, Ward NJ, Chakravarthy R, Ho KW and Calkins DJ. Activation of transient receptor potential vanilloid-1 (TRPV1) influences how retinal ganglion cell neurons respond to pressure-related stress. Channels (Austin). 2015;9:102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sappington RM, Chan M and Calkins DJ. Interleukin-6 protects retinal ganglion cells from pressure-induced death. Invest Ophthalmol Vis Sci. 2006;47:2932–42. [DOI] [PubMed] [Google Scholar]
- 41.Shoge K, Mishima HK, Mukai S, Shinya M, Ishihara K, Kanno M and Sasa M. Rat retinal ganglion cells culture enriched with the magnetic cell sorter. Neurosci Lett. 1999;259:111–4. [DOI] [PubMed] [Google Scholar]
- 42.Mukai S, Mishima HK, Shoge K, Shinya M, Ishihara K and Sasa M. Existence of ionotropic glutamate receptor subtypes in cultured rat retinal ganglion cells obtained by the magnetic cell sorter method and inhibitory effects of 20-hydroxyecdysone, a neurosteroid, on the glutamate response. Jpn J Pharmacol. 2002;89:44–52. [DOI] [PubMed] [Google Scholar]
- 43.Levin LA. Retinal ganglion cells and supporting elements in culture. J Glaucoma. 2005;14:305–7. [DOI] [PubMed] [Google Scholar]
- 44.Dordea AC, Bray MA, Allen K, Logan DJ, Fei F, Malhotra R, Gregory MS, Carpenter AE and Buys ES. An open-source computational tool to automatically quantify immunolabeled retinal ganglion cells. Exp Eye Res. 2016;147:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zarei K, Scheetz TE, Christopher M, Miller K, Hedberg-Buenz A, Tandon A, Anderson MG, Fingert JH and Abramoff MD. Automated Axon Counting in Rodent Optic Nerve Sections with AxonJ. Sci Rep. 2016;6:26559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao D, Cho J, Kim MH and Guallar E. The association of blood pressure and primary open-angle glaucoma: a meta-analysis. Am J Ophthalmol. 2014;158:615–27 e9. [DOI] [PubMed] [Google Scholar]
- 47.Lee NY, Jung Y, Han K and Park CK. Fluctuation in systolic blood pressure is a major systemic risk factor for development of primary open-angle glaucoma. Scientific Reports. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buys ES, Raher MJ, Kirby A, Mohd S, Baron DM, Hayton SR, Tainsh LT, Sips PY, Rauwerdink KM, Yan Q, Tainsh RE, Shakartzi HR, Stevens C, Decaluwe K, Rodrigues-Machado Mda G, Malhotra R, Van de Voorde J, Wang T, Brouckaert P, Daly MJ and Bloch KD. Genetic modifiers of hypertension in soluble guanylate cyclase alpha1-deficient mice. J Clinl Invest. 2012;122:2316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sappington RM, Carlson BJ, Crish SD and Calkins DJ. The microbead occlusion model: a paradigm for induced ocular hypertension in rats and mice. Invest Ophthalmol Vis Sci. 2010;51:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buckingham BP, Inman DM, Lambert W, Oglesby E, Calkins DJ, Steele MR, Vetter ML, Marsh-Armstrong N and Horner PJ. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci. 2008;28:2735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog Retin Eye Res. 2012;31:702–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL and Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31:152–81. [DOI] [PubMed] [Google Scholar]
- 53.Doganay S, Evereklioglu C, Turkoz Y and Er H. Decreased nitric oxide production in primary open-angle glaucoma. Eur J Ophthalmol. 2002;12:44–8. [DOI] [PubMed] [Google Scholar]
- 54.Galassi F, Renieri G, Sodi A, Ucci F, Vannozzi L and Masini E. Nitric oxide proxies and ocular perfusion pressure in primary open angle glaucoma. Br J Ophthalmol. 2004;88:757–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wareham LK, Buys ES and Sappington RM. The nitric oxide-guanylate cyclase pathway and glaucoma. Nitric Oxide. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pacher P, Beckman JS and Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guix FX, Uribesalgo I, Coma M and Munoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol. 2005;76:126–52. [DOI] [PubMed] [Google Scholar]
- 58.Sobrevia L, Ooi L, Ryan S and Steinert JR. Nitric Oxide: A Regulator of Cellular Function in Health and Disease. Oxid Med Cell Longev. 2016;2016:9782346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kohgami S, Ogata T, Morino T, Yamamoto H and Schubert P. Pharmacological shift of the ambiguous nitric oxide action from neurotoxicity to cyclic GMP-mediated protection. Neurol Res. 2010;32:938–44. [DOI] [PubMed] [Google Scholar]
- 60.Ditlevsen DK, Kohler LB, Berezin V and Bock E. Cyclic guanosine monophosphate signalling pathway plays a role in neural cell adhesion molecule-mediated neurite outgrowth and survival. J Neurosci Res. 2007;85:703–11. [DOI] [PubMed] [Google Scholar]
- 61.Thippeswamy T, McKay JS, Morris R, Quinn J, Wong LF and Murphy D. Glial-mediated neuroprotection: evidence for the protective role of the NO-cGMP pathway via neuron-glial communication in the peripheral nervous system. Glia. 2005;49:197–210. [DOI] [PubMed] [Google Scholar]
- 62.Gerometta R, Alvarez LJ and Candia OA. Effects of sildenafil and tadalafil on intraocular pressure in sheep: implications for aqueous humor dynamics. Invest Ophthalmol Vis Sci. 2010;51:3139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gerometta R, Alvarez LJ and Candia OA. Effect of sildenafil citrate on intraocular pressure and blood pressure in human volunteers. Exp Eye Res. 2011;93:103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grunwald JE, Jacob SS, Siu K, Piltz J and Dupont J. Acute effects of sldenafil ctrate (Viagra) on intraocular pressure in open-angle glaucoma. Am J Ophthalmol. 2001;132:872–4. [DOI] [PubMed] [Google Scholar]
- 65.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP and Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- 66.Berkelaar M, Clarke DB, Wang YC, Bray GM and Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994;14:4368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia-Valenzuela E, Gorczyca W, Darzynkiewicz Z and Sharma SC. Apoptosis in adult retinal ganglion cells after axotomy. J Neurobiol. 1994;25:431–8. [DOI] [PubMed] [Google Scholar]
- 68.Garcia-Valenzuela E, Shareef S, Walsh J and Sharma SC. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp Eye Res. 1995;61:33–44. [DOI] [PubMed] [Google Scholar]
- 69.Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ and Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–86. [PubMed] [Google Scholar]
- 70.Reichstein D, Ren L, Filippopoulos T, Mittag T and Danias J. Apoptotic retinal ganglion cell death in the DBA/2 mouse model of glaucoma. Exp Eye Res. 2007;84:13–21. [DOI] [PubMed] [Google Scholar]
- 71.Olivares-Gonzalez L, Martinez-Fernandez de la Camara C, Hervas D, Marin MP, Lahoz A, Millan JM and Rodrigo R. cGMP-Phosphodiesterase Inhibition Prevents Hypoxia-Induced Cell Death Activation in Porcine Retinal Explants. PLoS One. 2016;11:e0166717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schallner N, Romao CC, Biermann J, Lagreze WA, Otterbein LE, Buerkle H, Loop T and Goebel U. Carbon monoxide abrogates ischemic insult to neuronal cells via the soluble guanylate cyclase-cGMP pathway. PLoS One. 2013;8:e60672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Farinelli SE, Park DS and Greene LA. Nitric oxide delays the death of trophic factor-deprived PC12 cells and sympathetic neurons by a cGMP-mediated mechanism. J Neurosci. 1996;16:2325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Estevez AG, Spear N, Thompson JA, Cornwell TL, Radi R, Barbeito L and Beckman JS. Nitric oxide-dependent production of cGMP supports the survival of rat embryonic motor neurons cultured with brain-derived neurotrophic factor. J Neurosci. 1998;18:3708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barger SW, Fiscus RR, Ruth P, Hofmann F and Mattson MP. Role of cyclic GMP in the regulation of neuronal calcium and survival by secreted forms of beta-amyloid precursor. J Neurochem. 1995;64:2087–96. [DOI] [PubMed] [Google Scholar]
- 76.Cordell WH, Maturi RK, Costigan TM, Marmor MF, Weleber RG, Coupland SG, Danis RP, McGettigan JW Jr., Antoszyk AN, Klise S, Sides GD and Consortium ERGTDCPIA. Retinal effects of 6 months of daily use of tadalafil or sildenafil. Arch Ophthalmol. 2009;127:367–73. [DOI] [PubMed] [Google Scholar]
- 77.Sarhan NR and Omar NM. An immunohistochemical and ultrastructural analysis of the retina in tadalafil (Cialis) treated rats. Acta Histochem. 2018;120:312–322. [DOI] [PubMed] [Google Scholar]
- 78.Buys ES, Zimmer DP, Chickering J, Graul R, Chien YT, Profy A, Hadcock JR, Masferrer JL and Milne GT. Discovery and development of next generation sGC stimulators with diverse multidimensional pharmacology and broad therapeutic potential. Nitric Oxide. 2018;78:72–80. [DOI] [PubMed] [Google Scholar]
- 79.Charriaut-Marlangue C, Nguyen T, Bonnin P, Duy AP, Leger PL, Csaba Z, Pansiot J, Bourgeois T, Renolleau S and Baud O. Sildenafil mediates blood-flow redistribution and neuroprotection after neonatal hypoxia-ischemia. Stroke. 2014;45:850–6. [DOI] [PubMed] [Google Scholar]
- 80.Coward RM and Carson CC. Tadalafil in the treatment of erectile dysfunction. Ther Clin Risk Manag. 2008;4:1315–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Forgue ST, Patterson BE, Bedding AW, Payne CD, Phillips DL, Wrishko RE and Mitchell MI. Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2006;61:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garcia-Barroso C, Ricobaraza A, Pascual-Lucas M, Unceta N, Rico AJ, Goicolea MA, Salles J, Lanciego JL, Oyarzabal J, Franco R, Cuadrado-Tejedor M and Garcia-Osta A. Tadalafil crosses the blood-brain barrier and reverses cognitive dysfunction in a mouse model of AD. Neuropharmacology. 2013;64:114–23. [DOI] [PubMed] [Google Scholar]
- 83.Serarslan Y, Yonden Z, Ozgiray E, Oktar S, Guven EO, Sogut S, Yilmaz N and Yurtseven T. Protective effects of tadalafil on experimental spinal cord injury in rats. J Clin Neurosci. 2010;17:349–52. [DOI] [PubMed] [Google Scholar]
- 84.Ozdegirmenci O, Kucukozkan T, Akdag E, Topal T, Haberal A, Kayir H, Oter S, Akyol M and Uzbay T. Effects of sildenafil and tadalafil on ischemia/reperfusion injury in fetal rat brain. J Matern Fetal Neonatal Med. 2011;24:317–23. [DOI] [PubMed] [Google Scholar]
- 85.Stockman A, Sharpe LT, Tufail A, Kell PD, Ripamonti C and Jeffery G. The effect of sildenafil citrate (Viagra) on visual sensitivity. J Vis. 2007;7:4. [DOI] [PubMed] [Google Scholar]
- 86.Center for Drug Evaluation and Research: Study 148-232. A randomised, double-blind, placebo-controlled, crossover pilot study to investigate the effects of a single oral tablet dose of sildenafil (200 mg) on visual function (electroretinogram, photostress, visual field and colour discrimination tests) in healthy male volunteers and patients with diabetic retinopathy Viagra (Sildenafil): “Joint Clinical Review” for NDA-20-895; Washington, DC: Center for Drug Evaluation and Research, Food and Drug Administration. 1998:177–178. [Google Scholar]
- 87.Carter JE. Anterior ischemic optic neuropathy and stroke with use of PDE-5 inhibitors for erectile dysfunction: cause or coincidence? J Neurol Sci. 2007;262:89–97. [DOI] [PubMed] [Google Scholar]
- 88.Cunningham AV and Smith KH. Anterior ischemic optic neuropathy associated with viagra. J Neuroophthalmol. 2001;21:22–5. [DOI] [PubMed] [Google Scholar]
- 89.Santaella RM and Fraunfelder FW. Ocular adverse effects associated with systemic medications : recognition and management. Drugs. 2007;67:75–93. [DOI] [PubMed] [Google Scholar]
- 90.CIALISR. Full prescribing information tadalafil. Indianapolis, IN: Lilly ICOS, LLC; 2008. [Google Scholar]
- 91.Viagra R. Full prescribing information sildenafil citrate. New York: Pfizer Inc; 2008. [Google Scholar]
- 92.LEVITRAR. Full prescribing information vardenafil. West Haven, CT: Bayer Pharmaceuticals Corporation; 2008. [Google Scholar]
- 93.Brock GB, McMahon CG, Chen KK, Costigan T, Shen W, Watkins V, Anglin G and Whitaker S. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: results of integrated analyses. J Urol. 2002;168:1332–6. [DOI] [PubMed] [Google Scholar]
- 94.Bonomi L, Marchini G, Marraffa M, Bernardi P, Morbio R and Varotto A. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology. 2000;107:1287–93. [DOI] [PubMed] [Google Scholar]
- 95.Mitchell P, Lee AJ, Rochtchina E and Wang JJ. Open-angle glaucoma and systemic hypertension: the blue mountains eye study. J Glaucoma. 2004;13:319–26. [DOI] [PubMed] [Google Scholar]
- 96.Orzalesi N, Rossetti L, Omboni S, Group OS and Conproso. Vascular risk factors in glaucoma: the results of a national survey. Graefes Arch Clin Exp Ophthalmol. 2007;245:795–802. [DOI] [PubMed] [Google Scholar]
- 97.Leske MC. Ocular perfusion pressure and glaucoma: clinical trial and epidemiologic findings. Curr Opin Ophthalmol. 2009;20:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]