Abstract
Objective:
Absence of short-latency cortical median nerve somatosensory evoked potentials (“absent SSEPs”) is considered a nearly perfect predictor of poor outcome after cardiac arrest. However, reports of good outcomes despite absent SSEPs and high rates of withdrawal of life-sustaining therapies (WLST) have raised concerns that estimates of the prognostic value of absent SSEPs may be biased by self-fulfilling prophecies. We aimed to develop an unbiased estimate of the false positive rate (FPR) of absent SSEPs as a predictor of poor outcome after cardiac arrest.
Data Sources:
PubMed.
Study Selection:
We selected 35 studies in cardiac arrest prognostication that reported SSEP results.
Data Extraction:
In each study, we identified rates of WLST and good outcomes despite absent SSEPs. We appraised studies for potential biases using the Quality in Prognostic Studies tool. Using these data, we developed a statistical model to estimate the FPR of absent SSEPs adjusted for WLST rate.
Data Synthesis:
Two thousand, one hundred and thirty-three subjects underwent SSEP testing. Five hundred and ninety-four had absent SSEPs; of these,14 had good functional outcomes. The rate of WLST for subjects with absent SSEP could be estimated in 14 of the 35 studies (mean 80%, median 100%). The FPR for absent SSEP in predicting poor neurologic outcome, adjusted for a WLST rate of 80%, is 7.7% (95% Confidence Interval: 4–13%).
Conclusions:
Absent cortical SSEPs do not infallibly predict poor outcome in patients with coma following cardiac arrest. The chances of survival in subjects with absent SSEPs, though low, may be substantially higher than generally believed.
Keywords: evoked potentials, heart arrest, critical care, prognosis
Introduction
Withdrawal of life-sustaining therapies (WLST) due to poor neurological prognosis is the most common proximate cause of in-hospital death after cardiac arrest.(1, 2) Absent bilateral cortical median nerve somatosensory evoked potentials (SSEP) are considered by many experts an unequivocal predictor of poor outcome in cardiac arrest, with false positive rate (FPR) for making such predictions given as 0.4 and 0.7% in society guidelines (3, 4)
However, recent studies following the introduction of targeted temperature management suggest that, although rare, good neurologic outcomes can occur in cardiac arrest survivors with initially absent SSEPs. Two such examples have been cited in previous reviews.(5, 6) These cases, coupled with reports of high rates of early WLST in patients with absent SSEP, have raised concern that absent: estimates of the FPR for SSEP testing may be confounded by self-fulfilling prophecies.(7, 8)
We hypothesized that estimates in the literature for the FPR of SSEPs as a predictor of poor neurologic outcome after cardiac arrest are biased by the fact that WLST almost invariably leads to death. We conducted a systematic review of studies that report the rate of good and poor outcomes in cardiac arrest subjects who were managed with targeted temperature management and had absent bilateral SSEPs. Finally, we developed a statistical method to adjust for the rate of early WLST, in order to calculate an unbiased estimate of the FPR for absent SSEPs.
Materials and Methods
Search strategy, study selection and data abstraction
A literature search was performed on April 12th, 2017 using PubMed (January 1960 to April 2017) for original and full-text reports in English, German, French, Chinese, and Spanish. The search terms used were: “heart arrest” or “death (sudden cardiac)” or “cardiopulmonary resuscitation” or “hypoxia-ischemia, brain” or “hypoxic-ischemic, brain” or “cardiac arrest” and “targeted temperature management” or “TTM,” “hypothermia” and “prognosis” or “prognostic” or “SSEP” or “N20” or “evoked potentials.” Corresponding authors of the articles selected were contacted whenever there was missing information or if clarification about the data was needed. In cases with potential for subject overlap in publications by the same group of investigators, the corresponding author was contacted for clarification. If overlap was present, only the study with the largest number of subjects enrolled was included. Final study selection and data abstraction were performed by E.A. and M.B.W. using a standardized data collection form. Any disagreements were discussed until reaching consensus. Potential for bias in selected studies was appraised using the Quality in Prognostic Studies (QUIPS) (supplementary materials, Supplemental Digital Content 1, http://links.lww.com/CCM/E10). In an effort to identify all published cases of absent SSEPs with subsequent good neurologic outcome, we performed a secondary review of citations from included studies.
Inclusion criteria
Eligible studies involved comatose adult (age 16 years or above) cardiac arrest survivors with return of spontaneous circulation (ROSC) and out-of-hospital or in-hospital arrest treated with targeted temperature management (normothermia at 36°C or therapeutic hypothermia at 32–34°C). Only studies describing neurologic functional outcomes and SSEP results were included. When studies included a mixed group of subjects managed with or without targeted temperature management, only data from subjects managed with targeted temperature management were included in the final analysis. Case reports describing a single patient good outcome despite initially absent SSEPs were included in order to count the number of such cases reported in the literature to date. However, our subsequent statistical analysis of the FPR of SSEPs was restricted to cohort studies.
Exclusion criteria
Animal studies and clinical studies that involved pediatric subjects (age less than 16 years) or subjects with etiology of coma other than cardiac arrest were excluded. Articles were excluded if they were best categorized as reviews, editorials, letters to the editor, conference abstracts, or unpublished studies.
In the reviewed studies, SSEPs were categorized as “absent” if there were bilaterally absent cortical (N20) responses, “present” if there were unilateral or bilateral cortical responses present, or “indeterminate” in case the presence of a cortical response was indeterminate. For the purposes of this systematic review the term “absent SSEPs” refers to absent short latency cortical (N20) median nerve SSEP responses. Poor outcome was defined as severe neurological deficits, persistent vegetative state, or death.
Statistical Analysis
Estimation of False Positive Rate Correcting for Self-Fulfilling Prophecies
We use binomial probability models to estimate the FPR of absent SSEPs corrected for bias due to self-fulfilling prophecies. We define the natural false positive rate (nFPR) as the proportion of subjects with initially absent SSEPs who would eventually achieve a good neurological recovery in a setting where life-sustaining therapy is continued indefinitely. nFPR cannot be observed directly in the presence of self-fulfilling prophecies. However, nFPR can be estimated from 1) the observed rate of false positive predictions in the literature (oFPR) and 2) the rate of self-fulfilling prophecies, i.e. the rate of WLST in cases of absent SSEPs, denoted WLSTR (withdrawal of life support rate).
Early WLST (i.e. WLSTR > 0) will tend to make the oFPR less than the nFPR, oFPR ≤ nFPR: early WLST reduces the chance of a good outcome in cases that might otherwise have recovered. This is because two independent events need to occur for a subject with negative SSEPs to achieve a good outcome. First, the inciting injury must be survivable, in other words, the negative SSEP result must represent a “false alarm;” by definition the rate at which this occurs is nFPR. Second, the subject must escape becoming the object of a self-fulfilling prophecy; this occurs with probability (1 - WLSTR). The probability of a good outcome despite an absent SSEP result is thus oFPR = (1 - WLSTR) (nFPR). Equivalently, the nFPR (corrected for the bias introduced by self-fulfilling prophecies) is nFPR = oFPR/(1 - WLSTR).
Extending these calculations, in a cohort of n subjects with absent SSEPs, the probability that a “bad” outcome occurs once is thus , and the probability that all outcomes are bad is . We use this latter calculation to estimate the probability that, in any given small cohort, all subjects with absent SSEPs experience poor outcomes.
Results
Literature search yielded 676 manuscripts; 11 additional studies were identified through secondary reference review. Screening for studies restricted to the pediatric population, reviews, or editorials led to exclusion of 509 studies (Figure 1). Duplicate subject inclusion between publications was present in twelve studies from four groups of investigators, and nine studies were excluded. One hundred thirty-four studies did not fulfill inclusion criteria based on lack of SSEP use for prognostication and because the study was not focused on prognostication. Thirty-five studies were included in the final review. The study characteristics of the included studies are summarized in Table s1 (Supplemental Digital Content 2, http://links.lww.com/CCM/E11) (5, 6, 8–39).
Figure 1:

Flow diagram of literature search.
Number with absent SSEPs who had good outcomes
Out of 3,816 subjects included in the 35 studies evaluated, 2,133 had SSEP testing, and 594 of these had bilaterally absent cortical SSEP responses. Fourteen subjects with absent SSEPs survived cardiac arrest with good outcome (Table 1). Two of these subjects had absent SSEPs during hypothermia and recovered cortical responses within 72h from ROSC during normothermia. We included these cases based on claims in the recent literature that therapeutic hypothermia at 32–34°C has no impact on SSEP amplitude or presence. (40, 41) We restrict our subsequent analysis of the FPR associated with absent SSEPs to the nine subjects from cohort studies, to allow accurate determination of the denominator of the estimated rate estimate.
Table 1:
Summary of subject characteristics for cardiac arrest survivors with good outcome and bilaterally absent somatosensory evoked potentials
| Reference | Outcome (timing after ROSC) |
Temperature management | Subject characteristics | Timing absent SSEP after ROSC | Bilateral N20 recovery |
|---|---|---|---|---|---|
| 9 | Ambulation with walker (day 22) |
TH | 31 years-old, VF, ROSC 30 min. GCS 3 at 72h. | 49h (normothermia) | not retested |
| 37 | Barthel 90/100 and EuroQOoL 65% (3 years) | no TTM | 16 years-old, VF, ROSC 28 min, GCS 3 on admission. GCS 5 (M3) at 72h. Head CT at 96h showed slight brain edema and EEG at 72h was consistent with encephalopathy. Admitted to inpatient rehab for seven months. |
day 3 and day 9 (normothermia) | not retested |
| 7 | CPC 1–2 (6 months) | TH | information for the three survivors is unavailable. | during hypothermia | unknown; two subjects in the cohort recovered N20 on normothermia |
| 12 | normal neurological exam (“few” months) | TH | 34 years-old, male, asystole, GCS 4 on admission. |
84h (normothermia) | N20 recovery day 13 |
| 13 | CPC 1 (6 months) | TTM 36°C | GCS M6 at the time of prognostication (>72h from ROSC). |
77h (normothermia) | not retested |
| 18 | GOS 4–5 (10 days) | no TTM | one subject was a 25 years-old male and data on the other subject is unavailable. |
within 24h (normothermia) | day 1 to 3 |
| 20 | GOS 4–5 (8 months) | no TTM | 25 years-old, male; recovered consciousness 10 weeks from initial cardiac arrest. |
while in intensive care unit (exact timing unavailable) | N20 was absent two weeks after hospital discharge |
| 23 | CPC 2 and Barthel 80/100. (20 days) | no TTM | 51years-old, male, PEA, GCS 3, pupils sluggish. Head CT normal, EEG non-convulsive status epilepticus, brain MRI normal. Day 5 GCS 4 (M2), day 6 eye opening, day 7 GCS 8 (M3). |
day 5 and day 6 off sedation for >48h (normothermia) | N20 recovery on day 7 |
| 5 | regained consciousness with normal cognitive function (18 months) | TH | 43 years-old, asystole, ROSC 10 min. At 72h from ROSC and while on midazolam and fentanyl infusions, pupillary reflex was present and motor response to noxious stimuli was absent, NSE 18.2 mcg/L. |
day 3 (normothermia) | N20 recovery 18 months from ROSC |
| 32 | mild dysarthria, minor memory deficits, and ambulation with a cane (6 months) | TH | 36 years-old female, VT, EEG with diffuse slowing and bilateral periodic epileptiform discharges, diffuse myoclonus two days after arrest. Opened eyes on day 29, followed commands on day 31. |
day 20 (normothermia) | not retested |
| 6 | GOS 3–5 (3 months) | no TTM | information for the one survivor is unavailable | day 1 and day 3 (normothermia) | not retested |
TH: therapeutic hypothermia; TTM: Targeted Temperature Management; CPC: Cerebral Performance Category; GOS: Glasgow Outcome Scale; GCS: Glasgow Coma Scale; VF: ventricular fibrillation; VT: ventricular tachycardia; PEA: pulseless electric activity; ROSC: return of spontaneous circulation; SSEP: somatosensory evoked potentials
Withdrawal of life-sustaining therapies
The rate of WLST due to bilateral absent SSEPs was reported explicitly in three studies: 50, 79%, and 82%.(7, 8, 13) Five studies reported a policy of systematic WLST in all cases (100%) of absent SSEPs.(10, 11, 22, 35, 38) Ten studies did not explicitly report the WLST rate but described a “multimodal approach” protocol to prognostication that included absent SSEP results for WLST decisions.(5, 6, 15, 16, 19, 28, 29, 33, 34, 36) Nevertheless, four of these explicitly recommended WLST in subjects who had absent SSEPs, thus we considered their WLST for absent SSEPs as 100%.(19, 28, 29, 36) One study did not disclose SSEP results to the treating team.(31) Two studies did not have any cases of WLST due to neurological reasons.(31, 39) Eight studies did not describe the protocol for WLST.(14, 17, 18, 20, 21, 24, 25, 30) Thus, the estimated mean rate of WLST for cases with absent SSEPs across 14 studies with available information about WLST procedures was 80% (median 100%), with a range between 0 and 100.(7, 8, 10, 11, 13, 19, 22, 28, 29, 31, 35, 36, 38, 39)
Appraisal of risk of bias
The risk of bias ratings is summarized in Figure 2. Attrition and study confounding were the domains with the largest risk of bias, both having 26 studies with high risk of bias. The studies judged to have high bias in these domains did not systematically avoid WLST before one week after ROSC (attrition bias) or had SSEP results available to the treating clinicians responsible for WLST decisions (study confounding bias). Most studies had low bias for study participation, prognostic factor measurement, and outcome measurement (Table s2, Supplemental Digital Content 3, http://links.lww.com/CCM/E12). One study was in the acute rehabilitation setting, and therefore was scored as having high risk for bias in study participation and moderate risk for bias in outcome assessments.(20) One study did not have any cases with WLST (low attrition and low study confounding bias) and one study did not have any WLST for neurological reasons but allowed withdrawal of inotropic support (moderate attrition bias and low study confounding bias).(31, 39)
Figure 2:

Bias risk summary for selected studies using the Quality in Prognosis Studies (QUIPS) tool.
Rate estimates
Figure 3 shows our statistical estimates of the natural false positive rate (nFPR) for absent SSEPs, corrected for the rate of self-fulfilling prophecies, along with the 95% certainty interval. The rate of WLST in response to absent SSEPs, designated WLSTR, varied across various studies in our systematic review, therefore we present nFPR as a function of WLSTR. The estimates are calculated using all subjects identified in our systematic review who had bilaterally absent SSEPs with the exception of case reports.
Figure 3:

Estimated FPR of absent SSEP for predicting poor outcome as a function of the WLST rate.
The observed false positive rate oFPR is 9/589 = 1.5%. This represents the FPR without adjusting for the rate of WLST. In the absence of self-fulfilling prophecies (i.e. if WLSTR = 0), the estimated nFPR coincides with the observed rate (left hand side of the plot). However, in most studies WLSTR is greater than 80% (gray shaded region). In this portion of the plot, the small percentage of observed good outcomes despite high rates of WLST leads to substantially higher estimates of the underlying nFPR At the same time, the estimates necessarily become more uncertain (wider confidence regions). To obtain a single summary number that approximately reflects practices across published studies, we take as an overall (conservative) estimate the value of nFPR resulting when the rate of early WLST is 80%. The nFPR value is 7.7% [CI 4–13].
A possible objection about the foregoing calculations is that multiple independent studies have found the FPR to be zero: shouldn’t the large number of studies reporting oFPR =0 strengthen our confidence that bilaterally absent cortical SSEP results are a near-infallible predictor of poor neurologic outcome after cardiac arrest (Table 1)? To determine the weight of this this objection, we extend the foregoing analysis. Consider a study with n subjects and assume for the sake of argument that nFPR is in fact greater than zero, say 5%. It is possible in any given study that, by chance, no good outcomes will be observed. Furthermore, because most published studies involve small cohorts, such “zero numerator” outcomes should be common. In fact, assuming nFPR= 5%, the probability of zero good outcomes in a study involving n subjects is exactly . To see this, consider that the probability of any one subject with absent SSEPs having a poor outcome is . To obtain the probability that n independent subjects with absent SSEPs in a study all have poor outcomes, we multiply this probability by itself n times. Thus, in a typical study that reports outcomes for n = 20 subjects with absent SSEPs, the probability that all will have a poor outcome (i.e. ‘zero’ good outcomes despite nFPR of 5%) is p =(0.95)20=36%. Now consider the effect that early WLST has on these estimates. In this case, the probability that all observed outcomes are bad is . Based on our systematic review, if we conservatively assume that WLST occurs at a rate of WLSTR = 80%, the probability of observing all poor outcomes in a study of n = 20 is p=(0.99)20 =82%.
Figure 4 illustrates how the probability of a zero-numerator varies with cohort size n and WLST rate, assuming a nFPR for absent SSEPs of 5%. We note that the probability that all observed outcomes after an initial absent SSEPs will be poor increases with higher rates of self-fulfilling prophecies and decreases with larger samples sizes. The probability of a misleading study (zero-numerator result) remains high even for relatively large studies, although small studies are most vulnerable to this problem.
Figure 4:
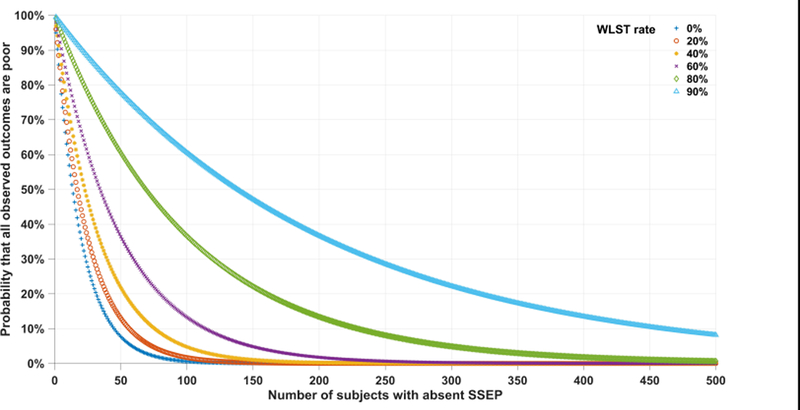
Probability of observing all poor outcomes in a cohort with absent SSEP and incorrectly concluding that FPR is zero
Discussion
Our systematic review adds 12 cases to the two reports found in previous systematic reviews who had good neurological outcome after cardiac arrest despite bilateral absent cortical SSEP N20 responses.(5, 6, 42–45) We find that much of the literature supporting some of the neurology community’s belief in a ‘zero’ false positive prediction rate for SSEPs is biased: the majority of published estimates of the FPR of absent SSEPs is confounded by high rates of WLST or policies in which absent SSEPs systematically results in WLST. Our analysis suggests that, after correcting for early WLST, the FPR of absent SSEPs may be an order of magnitude higher than commonly supposed: 7.7%, rather than 0.7%. (3) This estimate is necessarily tentative given the heterogeneity of the pooled data. Nevertheless, our findings argue that published estimates of the FPR for absent SSEPs as a predictor of poor neurologic outcome should be considered subject to substantial uncertainty. Thus, using absent SSEPs in isolation to predict poor prognosis and systematically recommend WLST is unwarranted.
Evidence-based guidelines recommend a multimodal approach to prognostication in cardiac arrest, and suggest that a combination of poor prognostic signs, such as absent pupillary responses after 72 hours and absent SSEPs during normothermia, have a FPR of 0–0.7%.(3, 4) However, these estimates are based on studies that fall prey to the same potential biases discussed throughout this review. While a multimodal approach should in principle improve predictions, the statistical problems are even more challenging than for absent SSEPs alone as the literature necessarily contains fewer instances of such compound events. Consequently, it is not possible to accurately estimate the FPR of a multimodal assessment from existing studies. Moreover, most neurologic examination findings and test results in subjects with coma after cardiac arrest are correlated rather than independent. The case reports from Karunasekara et al. and Weinstein et al. illustrate how difficult prognostication can be despite multiple poor prognostic markers (Table 1). In the former, the patient had a PEA, status epilepticus, and absent SSEP, however had a normal brain MRI and ultimately had a good outcome. The patient in the Weinstein et al. report had abdominal and upper extremity myoclonus, generalized periodic discharges on the electroencephalogram (EEG), and absent SSEPs. A reasonable approach to multimodal prognostication would be to also focus on the identification of biomarkers of potential for functional recovery such as EEG reactivity and auditory discrimination.(46, 47) Identifying signs of early neurological recovery that precede exam improvement may increase the likelihood to continue intensive supportive care and consequently potentially promote survival with good neurological function. Although tempting, the assumption that a combination of poor prognostic markers is infallible remains unproven and the FPR needs to measured in a prospective study that integrates clinical, neuroimaging, electrophysiology, SSEPs, and serum biomarkers, while also taking measures to minimize self-fulfilling prophecies bias.
Timing, medication use, and technician-reviewer expertise are among the many caveats often overlooked in SSEP interpretation. We identified five cases with good outcome that initially had absent cortical responses and afterwards recovered N20 responses on repeat testing - two recovered cortical responses soon after cooling was completed and two, just a few days after.(5, 12, 18, 23) This observation contrasts with reports suggesting that effects of sedation and therapeutic hypothermia at 32–34°C do not influence SSEP results.(40, 41) Additionally, interrater reliability of SSEP testing is limited, especially for patterns predicting poor outcome.(27, 34) This issue is well illustrated by Bouwes et al., who performed a secondary review of SSEPs from subjects who had good outcome despite absent cortical N20 responses.(7) These authors concluded that the original SSEP assessments for the three survivors with absent SSEPs may have been inaccurate. However, rather than showing that absent SSEPs in the rare cases that do survive are flukes, this reminds us that SSEP testing performed in real-world clinical practice is subject to technical and human error, further reason to regard them as imperfect standalone outcome predictors. This argument also serves to emphasize the need for both high technical and interpretation quality standards in SSEP testing to determine absence of cortical responses with confidence. Repeating SSEP after identifying an absent N20 response or providing the amplitude of the N20 response instead of using a binary classification without an amplitude criterion for presence or absence of a response might help minimize misclassifications.(5, 15, 17)
As for any systematic review, our study has several important limitations. First, the quality of evidence provided in this review is constrained by the inherent variability in local practices and subject characteristics of the studies included. Not all subjects evaluated had evoked potentials tested, and SSEPs were performed at different times and temperature levels using various analgesia, sedation and muscle blockade protocols. More importantly, several studies did not report a specific protocol for when to proceed with it. The lack of uniformity in ancillary testing procedures and WLST decisions highlights prevailing controversies about prognostication best practices in cardiac arrest. Additionally, the specific rate of WLST was not reported in the majority of the studies, therefore our estimation based on protocol descriptions is necessarily approximate. Second, only one study had the treating team blinded to SSEP results, underscoring the risk of self-fulfilling prophecies in the majority of studies. Third, we defined “adequate period of observation” for decisions regarding WLST due to poor neurological prognosis as seven days. While arbitrary, using a specific number was necessary to appraise the literature and attempt to separate studies prone to bias from study attrition related to early WLST. Fourth, the statistical assumption for our FPR analysis is that life-support is withdrawn equally at random amongst patients with absent SSEPs.
There are other limitations of our study regarding statistical issues that should be noted. The formulas for computing joint probabilities of WLST occurrence and outcome assume the two events are essentially independent. This is reasonable to the extent that WLST among patients with absent SSEPs are influenced by factors independent of outcome such as religious or moral reasons, variation in physician biases, and practice variation between hospitals. However, it is also possible that physicians somehow accurately discriminate via “clinical intuition” between patients with bilaterally absent SSEPs who have a lower probability vs. higher probability of survival, and thereupon use this in decisions regarding WLST. Another limitation is that data at the patient level for our study was pooled across studies. This limitation is mitigated to some extent by the fact that the records and events of interest were widely distributed across studies. Lastly, our analysis did not incorporate the time of WLST relative to the time of return of spontaneous circulation or SSEP assessment. All things being equal, WLST earlier in time may have been more likely to censor a possible observation of a good outcome than WLST implemented later. To overcome some of these problems, future work might utilize an “event history” approach using survival analysis in which WLST is regarded as a censored observation, and “studies” are considered a random factor. Relevant demographic and clinical covariates, and prognostic indicators known to be predictive of a bad outcome, could be included in the model. The latter would help control and stratify for the confounding of WLST with an underlying higher probability of a bad outcome. Unfortunately, for our systematic review, we were not able to obtain detailed covariates or time-to-event data at the patient level for most studies examined.
Our findings suggest that treating absent cortical responses on SSEP as a biomarker with near-zero FPR is inadequate. Prognostic uncertainty is more pronounced than commonly appreciated, underscoring the risk that misguided WLST due to absent SSEPs may lead to death in patients who might otherwise have survived and regained independence. An approach to avoid self-fulfilling prophecies and determine the true diagnostic accuracy of SSEPs and other prognostic markers would be to design a study that precludes self-fulfilling prophecies, e.g. by systematically postponing WLST for at least two weeks. Between 380 and 4,898 patients with absent SSEPs would be needed to estimate the FPR of absent SSEPs with a precision margin of error (MOE of ± 1%, if the actual value to be estimated lies between 1% and 15% (the number needed, n, depends on true value of the underlying FPR: . This type of study design could potentially be executed in countries where WLST is not a usual practice.
In the absence of a definitive study, a pragmatic approach would be to avoid the pretense that SSEPs – or any other ancillary test – can, in isolation, predict outcomes with certainty. While for some caregivers a 95%, or even 90% or lower, probability of poor outcome despite intensive medical care is sufficient to support that WLST is the preferred course of action, to others, this degree of uncertainty may dictate continuing life-support despite the presence of poor prognostic markers. In either scenario, informed decisions do not require FPR of 0%, and individualized decision-making can proceed without requiring assurance of absolute certainty.
Conclusion
The 14 reports of good neurologic outcomes in patients with hypoxic-ischemic coma despite lack of bilateral cortical responses on SSEPs demonstrate that absent SSEPs are not infallible predictors of poor outcome. Overconfidence in the prognostic accuracy of absent SSEPs is likely influenced by low estimates of the FPR for absent SSEPs, which are biased by self-fulfilling prophecies involving WLST. Our analysis suggests the that, after adjusting for early WLST, the FPR may be several times higher than generally believed. Similar to other poor prognosis markers previously but no longer believed to be unequivocal, these findings indicate that decisions regarding early WLST should not be solely determined by SSEP results.(3, 4, 7, 36)
Supplementary Material
Acknowledgements
The authors thank Dr. Joseph J. Locascio for the constructive collaboration and assistance with statistical analysis. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Funding
This study was supported by NIH 1K23NS090900, T32HL007901, T90DA22759, T32EB001680; Neurocritical Care Society research training fellowship; American Heart Association postdoctoral fellowship; Andrew David Heitman Neuroendovascular Research Fund; Rappaport Foundation; and Salerno foundation.
Footnotes
Copyright form disclosure: Dr. Amorim’s institution received funding from the National Institutes of Health (NIH), Neurocritical Care Society, and the American Heart Association; he disclosed he is supported by the Andrew David Heitman Neuroendovascular Research Fund, Rappaport Foundation, and Salerno foundation. Drs. Amorim, Ghessemi, Cole, and Westover received support for article research from the NIH. Dr. Lee’s institution received funding from the NIH-NINDS (R03NS091864), and he received funding from SleepMed/DigiTrace and Advance Medical. Dr. Greer received funding from Bard Medical (research grant), and he received funding from medical-legal consultation. Dr. Kaplan received funding from Wiley Blackwell (royalties); Cadwell, lundbeck; and as an expert witness on qEEG. Dr. Cash received support from NIH-NINDS (NINDS RO1-NS062092, and NINDS-K24-NS088568). Dr. Bianchi received support from the Massachusetts General Hospital, the Center for Integration of Medicine and Innovative Technology, the Milton Family Foundation, and the American Sleep Medicine Foundation; he has a patent pending on a home sleep monitoring device; he has received travel funding from Servier; has consulting and research contracts with Foramis, MC10, Insomnisolv, International Flavors and Fragrances, and GrandRounds; and has provided expert testimony in sleep medicine.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
References
- 1.Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. The New England journal of medicine 2013;369(23):2197–2206. [DOI] [PubMed] [Google Scholar]
- 2.Elmer J, Torres C, Aufderheide TP, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation 2016;102:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wijdicks EF, Hijdra A, Young GB, et al. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;67(2):203–210. [DOI] [PubMed] [Google Scholar]
- 4.Sandroni C, Cariou A, Cavallaro F, et al. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Resuscitation 2014;85(12):1779–1789. [DOI] [PubMed] [Google Scholar]
- 5.Leithner C, Ploner CJ, Hasper D, et al. Does hypothermia influence the predictive value of bilateral absent N20 after cardiac arrest? Neurology 2010;74(12):965–969. [DOI] [PubMed] [Google Scholar]
- 6.Young GB, Doig G, Ragazzoni A. Anoxic-ischemic encephalopathy: clinical and electrophysiological associations with outcome. Neurocrit Care 2005;2(2):159–164. [DOI] [PubMed] [Google Scholar]
- 7.Bouwes A, Binnekade JM, Kuiper MA, et al. Prognosis of coma after therapeutic hypothermia: a prospective cohort study. Ann Neurol 2012;71(2):206–212. [DOI] [PubMed] [Google Scholar]
- 8.Maciel CB, Morawo AO, Tsao CY, et al. SSEP in Therapeutic Hypothermia Era. J Clin Neurophysiol 2017. [DOI] [PubMed] [Google Scholar]
- 9.Arch AE, Chiappa K, Greer DM. False positive absent somatosensory evoked potentials in cardiac arrest with therapeutic hypothermia. Resuscitation 2014;85:e97–e98. [DOI] [PubMed] [Google Scholar]
- 10.Bouwes A, Binnekade JM, Zandstra DF, et al. Somatosensory evoked potentials during mild hypothermia after cardiopulmonary resuscitation. Neurology 2009;73(18):1457–1461. [DOI] [PubMed] [Google Scholar]
- 11.Daubin C, Guillotin D, Etard O, et al. A clinical and EEG scoring system that predicts early cortical response (N20) to somatosensory evoked potentials and outcome after cardiac arrest. BMC Cardiovasc Disord 2008;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Codeluppi L, Ferraro D, Marudi A, et al. False positive absent somatosensory evoked potentials in cardiac arrest with therapeutic hypothermia. Resuscitation 2014;85(11):e183–184. [DOI] [PubMed] [Google Scholar]
- 13.Dragancea I, Horn J, Kuiper M, et al. Neurological prognostication after cardiac arrest and targeted temperature management 33°C versus 36°C: Results from a randomised controlled clinical trial. Resuscitation 2015;93:164–170. [DOI] [PubMed] [Google Scholar]
- 14.Eid SM, Albaeni A, Vaidya D, et al. Awakening following cardiac arrest: Determined by the definitions used or the therapies delivered? Resuscitation 2016;100:38–44. [DOI] [PubMed] [Google Scholar]
- 15.Endisch C, Storm C, Ploner CJ, et al. Amplitudes of SSEP and outcome in cardiac arrest survivors: A prospective cohort study. Neurology 2015;85(20):1752–1760. [DOI] [PubMed] [Google Scholar]
- 16.Fugate JE, Wijdicks EF, Mandrekar J, et al. Predictors of neurologic outcome in hypothermia after cardiac arrest. Ann Neurol 2010;68(6):907–914. [DOI] [PubMed] [Google Scholar]
- 17.Grippo A, Carrai R, Scarpino M, et al. Neurophysiological prediction of neurological good and poor outcome in post-anoxic coma. Acta Neurol Scand 2017;135(6):641–648. [DOI] [PubMed] [Google Scholar]
- 18.Guerit JM, de Tourtchaninoff M, Soveges L, et al. The prognostic value of three-modality evoked potentials (TMEPs) in anoxic and traumatic comas. Neurophysiol Clin 1993;23(2–3):209–226. [DOI] [PubMed] [Google Scholar]
- 19.Hofmeijer J, Beernink TM, Bosch FH, et al. Early EEG contributes to multimodal outcome prediction of postanoxic coma. Neurology 2015;85(2):137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howell K, Grill E, Klein AM, et al. Rehabilitation outcome of anoxic-ischaemic encephalopathy survivors with prolonged disorders of consciousness. Resuscitation 2013;84(10):1409–1415. [DOI] [PubMed] [Google Scholar]
- 21.Huntgeburth M, Adler C, Rosenkranz S, et al. Changes in neuron-specific enolase are more suitable than its absolute serum levels for the prediction of neurologic outcome in hypothermia-treated patients with out-of-hospital cardiac arrest. Neurocrit Care 2014;20(3):358–366. [DOI] [PubMed] [Google Scholar]
- 22.Kane N, Oware A. Somatosensory evoked potentials aid prediction after hypoxic-ischaemic brain injury. Pract Neurol 2015;15(5):352–360. [DOI] [PubMed] [Google Scholar]
- 23.Karunasekara N, Salib S, NMacDuff A. A good outcome after absence of bilateral N20 SSEPs post-cardiac arrest. Journal of the Intensive Care Society 2016;17 (2):168–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leão RN, Ávila P, Cavaco R, et al. Therapeutic hypothermia after cardiac arrest: outcome predictors. Rev Bras Ter Intensiva 2015;27(4):322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maia B, Roque R, Amaral-Silva A, et al. Predicting outcome after cardiopulmonary arrest in therapeutic hypothermia patients: clinical, electrophysiological and imaging prognosticators. Acta Med Port 2013;26(2):93–97. [PubMed] [Google Scholar]
- 26.Pardal-Fernández JM, Arciniegas A, Mansilla-López D, et al. Nuevo caso de ausencia de N20 en la evaluación precoz de anoxoisquemia cerebral mediante potenciales evocados somatosensoriales. Med Intensiva 2014;38(3):194–195. [DOI] [PubMed] [Google Scholar]
- 27.Pfeiffer G, Pfeifer R, Isenmann S. Cerebral hypoxia, missing cortical somatosensory evoked potentials and recovery of consciousness. BMC Neurol 2014;14:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossetti AO, Tovar Quiroga DF, Juan E, et al. Electroencephalography Predicts Poor and Good Outcomes After Cardiac Arrest: A Two-Center Study. Crit Care Med 2017;45(7):e674–e682. [DOI] [PubMed] [Google Scholar]
- 29.Samaniego EA, Mlynash M, Caulfield AF, et al. Sedation confounds outcome prediction in cardiac arrest survivors treated with hypothermia. Neurocrit Care 2011;15(1):113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sánchez-Salado JC, Ariza-Solé A, Lorente-Tordera V, et al. Early prognostic evaluation after mild therapeutic hypothermia in sudden cardiac arrest survivors. Rev Esp Cardiol (Engl Ed) 2015;68(2):155–157. [DOI] [PubMed] [Google Scholar]
- 31.Tiainen M, Kovala TT, Takkunen OS, et al. Somatosensory and brainstem auditory evoked potentials in cardiac arrest patients treated with hypothermia. Crit Care Med 2005;33(8):1736–1740. [DOI] [PubMed] [Google Scholar]
- 32.Weinstein J, Mallela AN, Abella BS, et al. Excellent neurologic recovery after prolonged coma in a cardiac arrest patient with multiple poor prognostic indicators. Resuscitation 2017;113:e11–e12. [DOI] [PubMed] [Google Scholar]
- 33.Zanatta P, Linassi F, Mazzarolo AP, et al. Pain-related Somato Sensory Evoked Potentials: a potential new tool to improve the prognostic prediction of coma after cardiac arrest. Crit Care 2015;19:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zandbergen EG, Hijdra A, Koelman JH, et al. Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology 2006;66(1):62–68. [DOI] [PubMed] [Google Scholar]
- 35.Cloostermans MC, van Meulen FB, Eertman CJ, et al. Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest: a prospective cohort study. Crit Care Med 2012;40(10):2867–2875. [DOI] [PubMed] [Google Scholar]
- 36.Cronberg T, Rundgren M, Westhall E, et al. Neuron-specific enolase correlates with other prognostic markers after cardiac arrest. Neurology 2011;77(7):623–630. [DOI] [PubMed] [Google Scholar]
- 37.Bender A, Howell K, Frey M, et al. Bilateral loss of cortical SSEP responses is compatible with good outcome after cardiac arrest. J Neurol 2012;259(11):2481–2483. [DOI] [PubMed] [Google Scholar]
- 38.Bisschops LL, van Alfen N, Bons S, et al. Predictors of poor neurologic outcome in patients after cardiac arrest treated with hypothermia: a retrospective study. Resuscitation 2011;82(6):696–701. [DOI] [PubMed] [Google Scholar]
- 39.Choi SP, Youn CS, Park KN, et al. Therapeutic hypothermia in adult cardiac arrest because of drowning. Acta Anaesthesiol Scand 2012;56(1):116–123. [DOI] [PubMed] [Google Scholar]
- 40.Rothstein TL. Therapeutic hypothermia and reliability of somatosensory evoked potentials in predicting outcome after cardiopulmonary arrest. Neurocrit Care 2012;17(1):146–149. [DOI] [PubMed] [Google Scholar]
- 41.Grippo A, Carrai R, Fossi S, et al. Absent SEP during therapeutic hypothermia did not reappear after re-warming in comatose patients following cardiac arrest. Minerva Anestesiol 2013;79(4):360–369. [PubMed] [Google Scholar]
- 42.Sandroni C, Cavallaro F, Callaway CW, et al. Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: a systematic review and meta-analysis. Part 2: Patients treated with therapeutic hypothermia. Resuscitation 2013;84(10):1324–1338. [DOI] [PubMed] [Google Scholar]
- 43.Kamps MJ, Horn J, Oddo M, et al. Prognostication of neurologic outcome in cardiac arrest patients after mild therapeutic hypothermia: a meta-analysis of the current literature. Intensive Care Med 2013;39(10):1671–1682. [DOI] [PubMed] [Google Scholar]
- 44.Lee YC, Phan TG, Jolley DJ, et al. Accuracy of clinical signs, SEP, and EEG in predicting outcome of hypoxic coma: a meta-analysis. Neurology 2010;74(7):572–580. [DOI] [PubMed] [Google Scholar]
- 45.Golan E, Barrett K, Alali AS, et al. Predicting neurologic outcome after targeted temperature management for cardiac arrest: systematic review and meta-analysis. Crit Care Med 2014;42(8):1919–1930. [DOI] [PubMed] [Google Scholar]
- 46.Amorim E, Rittenberger JC, Zheng JJ, et al. Continuous EEG monitoring enhances multimodal outcome prediction in hypoxic-ischemic brain injury. Resuscitation 2016;109:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tzovara A, Rossetti AO, Juan E, et al. Prediction of awakening from hypothermic post anoxic coma based on auditory discrimination. Ann Neurol 2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


