Abstract
Glaucoma is a prevalent optic neuropathy characterized by the progressive dysfunction and loss of retinal ganglion cells (RGCs) and their optic nerve axons leading to irreversible visual field loss. Multiple risk factors for the disease have been identified, but elevated intraocular pressure (IOP) remains the primary risk factor amenable to treatment. Reducing IOP however does not always prevent glaucomatous neurodegeneration, and many patients progress with the disease despite having IOP in the normal range. There is increasing evidence that nitric oxide (NO) is a direct regulator of IOP and that dysfunction of the NO- Guanylate Cyclase (GC) pathway is associated with glaucoma incidence. NO has shown promise as a novel therapeutic with targeted effects that: 1) lower IOP; 2) increase ocular blood flow; and 3) confer neuroprotection. The various effects of NO in the eye appear to be mediated through the activation of the GC- guanosine 3:5′-cyclic monophosphate (cGMP) pathway and its effect on downstream targets, such as protein kinases and Ca2+ channels. Although NO-donor compounds are promising as therapeutics for IOP regulation, they may not be ideal to harness the neuroprotective potential of NO signaling. Here we review evidence that supports direct targeting of GC as a novel pleiotrophic treatment for the disease, without the need for direct NO application. The identification and targeting of other factors that contribute to glaucoma would be beneficial to patients, particularly those that do not respond well to IOP-dependent interventions.
1. Introduction
Glaucoma is a neurodegenerative disease characterized by progressive degeneration of retinal ganglion cells (RGCs) and subsequent irreversible loss of vision. Over 60.5 million people worldwide are affected by primary open angle glaucoma (POAG) – a figure projected to increase to 79 million in 2020 and 111.8 million by 2040 1, 2. Glaucoma is often associated with elevated intraocular pressure (IOP), termed ocular hypertension. However, at least a third of patients with glaucomatous vision loss have normotensive IOP (normotensive glaucoma; NTG) 3–6 and disease incidence increases with age, regardless of IOP. This suggests that ocular hypertension is only one mechanism for glaucoma etiology and progression 7. Despite these indications, ocular hypertension remains the only target of current glaucoma therapeutics. Current strategies to lower IOP include topical application of eye drops and surgical intervention. Unfortunately, successful reduction of IOP via these therapies only serves to slow progression of the disease.8 Thus, the identification of novel therapeutics that target other disease mechanisms is important for the evolution of glaucoma treatment.
Nitric oxide (NO) is an endogenous signaling molecule that is emerging as a novel target for therapeutic lowering of IOP 8. NO is produced endogenously in various ocular tissues in both the anterior and posterior segments of the eye and is a potent activator of soluble guanylate cyclase (termed GC, formerly known as sGC). Recent evidence implicates the NO-GC-cyclic guanosine monophosphate (cGMP) pathway in both IOP regulation (see section 6.1) and retinal pathophysiology of glaucoma (see section 6). In this review, we will discuss the evidence that the NO-GC-cGMP pathway may contribute to glaucoma pathophysiology as well as its potential as a novel multi-target approach for glaucoma therapeutics.
2. Pathophysiology of Glaucoma
Glaucoma is a group of optic neuropathies defined by progressive degeneration of RGCs and their axons in the optic nerve, which leads to irreversible loss of vision 3, 8, 9. RGC degeneration is often significantly advanced before changes in visual acuity and evidence of optic nerve cupping are detected in the clinic 10–12. Although the pathogenesis of glaucoma is not well understood, progression correlates with IOP, regardless of whether IOP is normotensive or hypertensive 13. Several clinical trials indicate that IOP-lowering drugs are effective in delaying progression of the disease. In particular, the Early Manifest Glaucoma Trial (EMGT) indicates that the risk of progression decreases by approximately 10% with each 1 mmHg IOP reduction from baseline 4. Similarly, the Ocular Hypertension Treatment study indicates that a 20% reduction in IOP is effective in delaying or preventing the onset of POAG in patients with ocular hypertension 14. Thus, lowering IOP remains the primary course of treatment for glaucoma patients as well as for those with ocular hypertension deemed at-risk for glaucoma.
Our current understanding of the relationship between IOP and RGC degeneration indicates that IOP elevation leads to a corresponding increase in pressure exerted posteriorly at the optic nerve head, where the optic nerve exits the globe of the eye 15, 16. The lamina cribrosa, a band of extracellular matrix in the optic nerve head, marks the beginning of the optic nerve and is prone to compression, deformation, and remodeling induced by mechanical strain related to IOP. This compressive deformation is transferred to RGC axons, which pass through perforations in the lamina cribrosa as they exit the globe. Studies in animal models of glaucoma indicate that ocular hypertension results in the disruption of both anterograde and retrograde transport in RGC axons, particularly near the optic nerve head 17. These studies are corroborated by structural changes in RGC axons of the optic nerve head from human donors with glaucoma 10. Interestingly, studies in animal models indicate that deficits in axon transport occur early in glaucoma progression, prior to structural degeneration of RGC axons and soma 3, 18. These studies suggest that the interval between changes in axon transport and structural degeneration of RGCs may constitute a window for therapeutic intervention. While glaucoma is typically diagnosed in patients already exhibiting 40–50% visual field loss 19, 20, the cellular process of degeneration is occurring at various rates throughout the RGC population. If targetable, this therapeutic window provides the opportunity to interrupt degeneration in RGCs within glaucomatous retina that have not yet progressed to structural degeneration. Thus, there is the possibility of preserving RGCs and preventing further vision loss, independent of or in addition to IOP management.
3. Why the need for new medications?
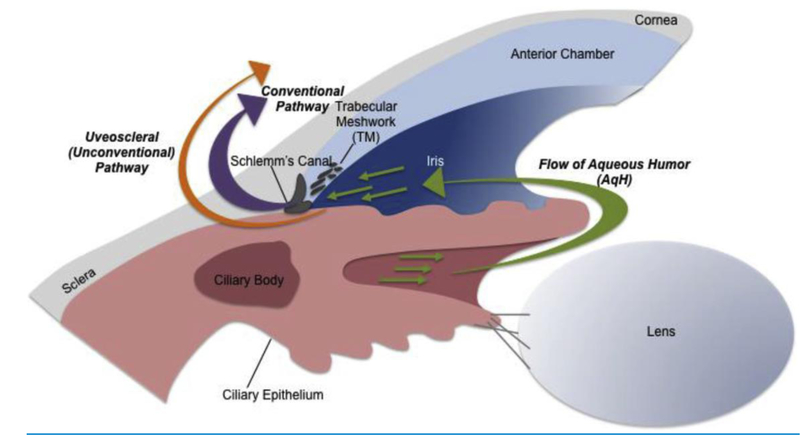
IOP is established by the balance of AqH production and elimination from the anterior chamber. Two independent pathways regulate AqH dynamics: the conventional pathway and the unconventional pathway. In humans, the majority of AqH drainage occurs via the trabecular meshwork (TM) and Schlemm’s canal (SC), which constitute the conventional pathway 21 (Figure 1). However, it has been estimated that around 3–82% of AqH drainage can also occur via the uveoscleral tract of the unconventional pathway across different species 22–27. The first course of treatment to lower IOP is usually through topical application of drugs that modulate AqH dynamics by: 1) reducing AqH production 2) increasing uveoscleral outflow or 3) increasing flow through the conventional pathway via contraction of the ciliary muscle (CM) 8. Issues with patient compliance and side effects can reduce efficacy of topical drugs. Accordingly, sustained delivery platforms, such as the bimatoprost intracemeral slow-release implant, are already in phase III clinical trials 28. Alternative strategies for IOP management are currently surgical, i.e. laser trabeculoplasty or incisional glaucoma surgery 29.
Fig. 1.
Flow of aqueous humor (AqH) in the eye. AqH is produced at the ciliary body and flows (green arrows) through one of two independent pathways that regulate AqH dynamics: the conventional pathway through the trabecular meshwork (TM) and Schlemm's canal (purple arrow) and the non-conventional pathway via the uveoscleral tract (orange arrow). Intraocular pressure (IOP) in the eye is established by the balance of (AqH) production and elimination in the anterior chamber.
As indicated by the wide variety of pharmaceuticals for IOP management (Table 1), each case of glaucoma is unique and requires a unique treatment regimen to effectively lower IOP. This often results in patients utilizing several medications at once and/or combining medications with surgical intervention. Over the long-term, the likelihood of preserving functional vision diminishes and the risk of significant blindness is considerably high. This is likely attributable to both poor patient compliance and the unilateral targeting of only one facet of the disease.
Table 1.
Current IOP-lowering medications for the treatment of Glaucoma.
| Type of Medication | Examples | Mechanism of Action | Adverse Effects |
|---|---|---|---|
| Prostaglandin analogues (PGAs) | Latanoprost Travoprost Tafluprost Bimatoprost Timolol |
Enhanced outflow of AqH through the uveoscleral pathway | Conjunctival hyperemia, lengthening and darkening of eyelashes, uveitis, macular edema, periocular hyperpigmentation, increased iris pigmentation |
| β-Adrenergic blockers | Levobunolol Carteolol Betaxolol Brimonidine |
Reduction of AqH production | Ocular irritation, dry eyes, bronchoconstriction, bradycardia |
| α-Adrenergic agonists | Apraclonidine Dorzolamide |
Decreased AqH production, Enhanced uveoscleral outflow of AqH | Allergic conjunctivitis, dry eyes, contact dermatitis, CNS effects, renal failure |
| Carbonic anhydrase inhibitors | Brinzolamide Acetazolamide (oral) Pilcocaprine |
Decreased AqH production | Ocular irritation, dry eyes, metallic taste, nausea, renal stones |
| Cholinergic agonists | Increased outflow of AqH through conventional pathway | Itching/burning/stinging of the eye, poor vision in dim light, temporary vision loss, headache, brow ache | |
| Rho Kinase Inhibitors Modified PGAs |
Netarsudil Latanoprost bunod (LBN) |
Increased outflow of AqH through conventional pathway Dual mechanism: increased AqH outflow through conventional pathway (via latanoprost acid), and increased outflow of AqH through conventional pathway via nitric oxide release | Conjunctival hyperemia, corneal verticullata Conjunctival hyperemia, punctata keratitis, eye pain, vision loss |
The identification and targeting of other factors that contribute to glaucoma would be beneficial to patients, particularly those that do not respond well to IOP-dependent interventions. Neuroprotection for glaucoma could be an effective strategy, but studies aimed at protecting RGCs have thus far failed to demonstrate efficacy in clinical trials 30. However, recent evidence supports the notion that targeting both neurobiological and IOP regulatory aspects of the disease may be more effective as a treatment strategy. For example, in a study comparing two adrenergic blockers timolol (beta-adrenergic) and brimonidine (alpha-adrenergic), brimonidine was more effective than timolol in stabilizing visual fields 31. Both timolol and brimonidine reduce IOP by decreasing AqH production at the level of the non-pigmented ciliary epithelium 9, 32 and display similar IOP-lowering efficacy 31. However, brimonidine also has neuroprotective qualities, as demonstrated by its use in Alzheimer’s disease and other cognitive impairments 33, 34. Thus, the identification of other pathways that could potentially target both IOP and neurodegeneration is intriguing and potentially beneficial.
NO is emerging as a potential therapeutic target that could impact both IOP regulation and RGC neurodegeneration. Here, we will summarize the potential implications of NO signaling for glaucoma pathophysiology and advocate the NO-GC-cGMP pathway as a putative candidate for a new class of multi-target therapeutics.
4. NO-GC Pathway
NO is a ubiquitous and endogenous signaling molecule. Since its discovery as an endothelium-derived relaxing factor (EDRF) in 1987 35, NO has been implicated in a myriad of physiological processes, including smooth muscle relaxation and vasodilation 35, 36, blood pressure regulation, antimicrobial defense and vascular homeostasis 37, 38. Nitric oxide synthase (NOS) is the enzyme that produces endogenous NO from l-arginine in a two-step oxidation process that also yields l-citrulline 39–41. Molecular oxygen and reduced nicotinamide-adenine-dinucleotide phosphate (NADPH) are co-substrates (reviewed in 42).
There are three isoforms in mammals: neuronal NOS1 (nNOS), endothelial NOS3 (eNOS) and inducible NOS2 (iNOS) 43, 44. Under normal physiological conditions, NO is produced by the two constitutive, Ca2+/calmodulin-regulated isoforms of the enzyme (nNOS and eNOS), which generate relatively small amounts of NO (picomolar to nanomolar range) in response to a variety of stimuli, including elevated calcium and shear stress 45. In pathological conditions (e.g. infection, inflammation or ischemia), there is induction of the third transcriptionally-regulated isoform of NOS (iNOS), which produces higher concentrations NO (micro to millimolar levels) over longer time periods 46. The differential isoforms of NOS, paired with its widespread distribution in most tissues, allows for an array of diverse biological functions of NO.
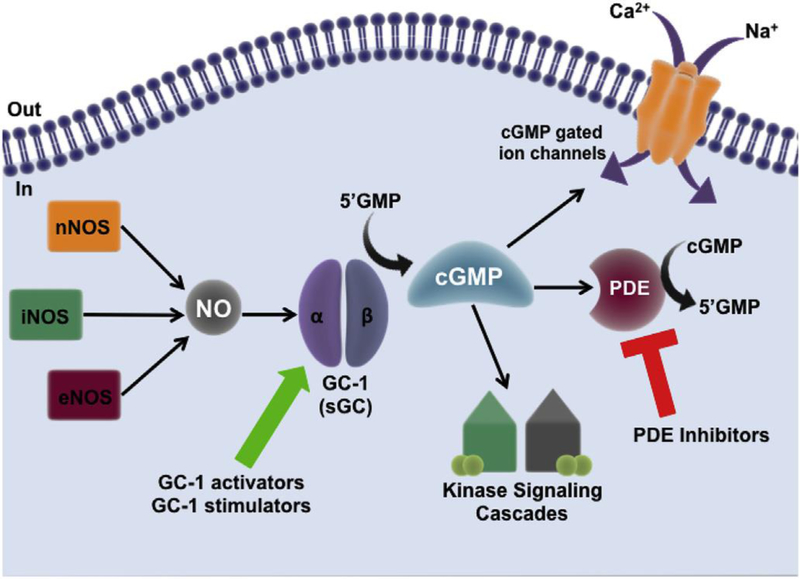
The classic NO pathway starts with the binding of a ligand, i.e. a hormone or first messenger, to its receptor that then induces production of NO by NOS. NO is a lipophilic molecule capable of traversing the phospholipid membranes of cells, where it has numerous targets, reacting typically via thiol groups or transition metal centers 47–50. A major target of NO is the enzyme soluble guanylate cyclase (GC-1 and GC-2, formerly known as sGCα1β1 and sGCα2β1 respectively), the only known receptor of NO 51–53. The GC enzyme is a heme-containing heterodimeric protein, consisting of one α and one β subunit (Figure 2) 52. The GC-α1 and GC- β1 subunits that make up the GC-1 isoform are expressed in most cell types and tissues; however, two other subunits of GC, α2 and β2, have also been identified 54. Although GC-1 is the most abundantly expressed form, other mixed heterodimer combinations of the protein have been identified, such as GC-2 (formerly sGCα2β1), which is expressed in the brain, placenta, spleen, and uterus 54, 55. This review will focus on GC-1, which converts guanosine triphosphate into the secondary messenger cGMP (Figure 2) 56–58. Upon NO binding, the activity of GC-1 increases more than 200-fold 59, 60 producing high concentrations of cGMP that then modulate functions of numerous downstream enzymes, such as cyclic nucleotide phosphodiesterases (PDEs), cGMP-dependent protein kinases and cGMP-gated ion channels 61, 62 (reviewed in 63; Figure 2). Downstream signaling cascades produce different biological effects depending on the location of NO release and the site of cGMP production.
Fig. 2. The NO-GC-1-cGMP pathway.
NO is produced from L-arginine by nitric oxide synthase (NOS) of which there are three isoforms: neuronal NOS1 (nNOS), endothelial NOS3 (eNOS) and inducible NOS2 (iNOS). NO targets guanylate cyclase-1 (GC-1), a heterodimeric protein capable of converting GMP to cGMP. cGMP produced by GC-1 can target cGMP-gated ion channels, and activate downstream kinase signaling cascades. Phosphodiesterase enzymes (PDE) bind to cGMP and catalyse its breakdown into GMP – PDEs act as important regulators of signal transduction mediated by cGMP. cGMP bioavailability in the cell can be modulated in two ways: 1) through the use of GC-1 stimulators and activators, which increase production of cGMP, or, 2) through the use of PDE inhibitors which prevent the breakdown of cGMP in the cell.
4.1. NO-GC-1 Pathway in the Eye
GC-1, the downstream target of NO, is expressed widely in the retina of multiple species, including human 64, 65, rabbit 66, rat 67, turtle 68, and mouse 64, 69. GC-1 expression is evident in RGCs, photoreceptor cells and cells in the vascular smooth muscle layer of retinal arterioles 64, 69 (Table 2). In vitro studies also indicate GC-1 expression in human TM cells, and both human and mouse ciliary muscle (CM) 70,64. Tissues in both the anterior and posterior segments of the eye express the three isoforms of NOS (Table 2). We will review the functional implications of these expression patterns for each isoform.
Table 2.
Ocular localization of nitric oxide synthase (NOS) and soluble guanylate cyclase (GC-1).
| Gene/isoform | Species | Site of expression (cell or tissue) | Reference |
|---|---|---|---|
| NOS1 (nNOS) | Human | Ciliary non-pigmented epithelium | [51] |
| Human | ONH astrocytes, lamina cribrose | [44] | |
| Monkey | Amacrine cells, rod and cone photoreceptors, RGC | [56] | |
| Canine | RGC | [57] | |
| Rabbit | Amacrine cells, rod and cone photoreceptors, RGC | [56] | |
| Rat | Ciliary process epithelium, INL, IPL, RGC layer, photoreceptors | [52,63] | |
| Murine | Retinal amacrine cells | [58] | |
| Murine | Retinal amacrine cells, RGC layer somata; IPL puncta | [59] | |
| Murine | Müller cells | [60] | |
| Guinea pig | RGC layer, INL, IPL | [61] | |
| Chicken | RGC layer, INP, IPL | [62] | |
| NOS2 (iNOS) | Human | Macrophages in stroma and ciliary processes | [46] |
| Human | Astrocytes | [64] | |
| Chicken | RPE | [62] | |
| NOS3 (eNOS) |
Human | Longitudinal CM fibers, TM, SC | [46] |
| Human | Retinal vasculature | [44] | |
| Human | TM | [47] | |
| GC-1 | Human | RGC, IPL, ONL | [66] |
| Human | TM cells | [67] | |
| Rabbit | Amacrine cells, bipolar cells, cone photoreceptors, RGC | [56] | |
| Murine | Somata in the INL, ONL, IPL, and OPL | [59] | |
| Murine | RGC, IPL, ONL | [66] | |
| Turtle | Amacrine cells, bipolar cells, RGC layer, IPL | [69] | |
ONH, optic nerve head; RGC, retinal ganglion cell; IPL, inner plexiform layer; CM, ciliary muscle; TM, trabecular meshwork; SC, Schlemm’s canal; ONL, outer nuclear layer; INL, inner nuclear layer; OPL, outer plexiform layer.
4.1.a. eNOS
The role of eNOS in the cardiovascular system includes regulating vascular tone by inhibiting smooth muscle contraction. In the eye, eNOS is constitutively expressed in sites that are important in the regulation of AqH outflow in the eye, including the endothelium of ciliary and retinal vessels 71, 72, and the ciliary muscle of the uveoscleral pathway 71, 73–75. In the conventional outflow pathway, the trabecular meshwork (TM) was also thought to endogenously produce NO through the activity of eNOS. However, recent data suggests that, in murine eyes, eNOS expression is predominantly found in cells of the SC 76–78. It therefore comes as no surprise that eNOS is an important regulator of IOP through physiological regulation of outflow facility 78. Elevated eNOS expression in eNOS-GFPtg mice leads to reduced IOP and increased outflow facility 78 and conventional AqH outflow is impaired and IOP is increased in eNOS knockout mice 79. Furthermore, eNOS gene polymorphisms are associated with increased risk of developing POAG, including both ocular hypertensive and NTG forms of the disease 80–83.
eNOS also has a central role in ocular blood flow.: NO produced in the endothelium acts as an important physiological mediator to exert vascular smooth muscle relaxation in the eye, as seen in other organs and tissues of mammals. Studies investigating the involvement of endogenous NO on the ocular circulation of healthy subjects show strong evidence for the involvement of endogenous NO derived from either endothelial cells or perivascular nitrergic neurons in the control of vascular smooth muscle tone under resting and stimulated conditions (reviewed extensively in 84).
4.1.b. nNOS
nNOS is constitutively expressed in both the anterior and posterior chambers of the eye. Anteriorly, nNOS is expressed in the ciliary non-pigmented epithelium and is a key factor in controlling ocular blood flow 74, 85. Posteriorly, nNOS is expressed across species in pigment epithelium, optic nerve head and in the neural retina by amacrine cells, rod and cone photoreceptors and RGCs 66, 69, 72, 73, 86–94. It has been suggested that NO production by nNOS may serve as a molecular messenger between cells in the inner layers of the retina (e.g. amacrine cells), astrocytes and cells in the RGC layer 95.
4.1.c. iNOS
iNOS is not constitutively expressed in the eye under physiological conditions. However, upregulation of iNOS expression has been detected in human eyes in macrophages of the stroma and astrocytes 74, 96 and in chicken retinal pigment epithelium (RPE) 94. iNOS activity was discovered in patients with POAG and visual field loss 75. Ex vivo analysis of human donor eyes revealed that iNOS expression in the TM is induced by increasing perfusion pressure in the anterior chamber 97. This increase in iNOS expression is accompanied by NO production, suggesting a functional role for iNOS in mediating pressure-induced NO release 97. Similarly, in vitro studies of ocular tissues and cells indicate that iNOS can also be induced by inflammatory and hypoxic stressors, like those associated with glaucoma 89,73.
5. NO-GC-1 Pathway and Implications for Glaucoma
Direct in vivo measurement of NO in the eye is not yet feasible. However, measurement of nitrate and nitrite levels are routinely used as markers for the activity of NOS and the production of NO radicals 98. Several studies in human glaucoma patients suggest that various components of the NO-GC-1-cGMP pathway and its associated outcomes are linked to disease progression. Glaucoma patients exhibit decreased NO metabolite (nitrate/nitrite) and cGMP levels in AqH and plasma compared to patients without glaucoma 99, 100. This is accompanied by corresponding increases in the AqH level of l-Arginine, the amino acid precursor of NO 101, and serum levels of l-arginine analogs, which are endogenous inhibitors of NOS or l-arginine uptake 102. In eyes harvested posthumously from POAG patients, NADPH-diaphorase (NADPH-d) reactivity, a marker for NO production, is decreased in TM, SC and anterior longitudinal CM fibers 85.
NO production by cells of the SC may have a homeostatic function during IOP elevation, i.e. when the SC narrows and shear stress increases. Cells respond to increased IOP through increases in NO, which increases the permeability of the SC inner wall and decreases contractility of the juxtacanalicular TM in order to normalize IOP levels 77. SC cells isolated from glaucomatous eyes are unresponsive to shear stress 77. This suggests that the homeostatic feedback loop controlling NO synthesis is impaired in glaucoma and may contribute to elevations in IOP.
Finally, NOS inhibition impairs blood flow in the optic nerve head of POAG patients to a greater extent than in healthy controls 103. Taken together, these studies suggest an important role for the NO-GC-1 pathway and its downstream effector, cGMP, in glaucoma pathophysiology 64.
5.1. NO-GC-1-cGMP Pathway as an Etiological Factor in Glaucoma
In recent years, GWAS have identified several genetic loci linked to POAG (reviewed recently in 104). Amongst the genes identified, three are associated with the NO-GC-1-cGMP signaling pathway.
5.1.a. Caveolin 1 and 2 (CAV1/CAV2)
Variants in genes encoding CAV1 and CAV2 are associated with ocular hypertension 104–110. Caveolins are involved in controlling the production of NO by NOS enzymes 111.
5.1.b. NOS3/eNOS
More directly, eNOS gene variants associated with ocular hypertension in females are thought to induce differential expression or modulation of eNOS that effects NO expression in the eye 82. The promoter-region polymorphism T-786C of eNOS may lower local NO concentrations by reducing promoter activity to influence gene transcription 112. This functional polymorphism is associated with POAG and links age and gender with risk of POAG development 113. Similarly, variants in the promoter region of eNOS were identified in 20% of familial POAG patients 114 and a recent study identified the eNOS variant rs2070744 as a significant genetic risk factor for developing disc hemorrhage in NTG patients 83. In the NTG population, additional studies suggest that NO dysregulation may impact blood supply to the optic nerve as well as aqueous humor outflow 100. This is supported by the presence of altered vasodilatory responses in forearm microcirculation of NTG patients 80, and well-documented vascular abnormalities in POAG patients generally (reviewed in 115).
5.1.c. GC-1
A gene candidate association study in the GLAUGEN cohort identified a variant (rs11722059) in GUCY1A3/GUCY1B3, which encodes the α1/β1 subunits of the GC-1 enzyme, in POAG individuals that develop early paracentral visual field loss 108,64. Interestingly, loss of early paracentral visual field loss is predominant in a subsection of POAG patients associated with vascular dysregulation 116. This link between GC-1 and POAG etiology is further confirmed in animal studies, where mice lacking the α1-subunit of GC-1 develop optic neuropathy associated with moderate ocular hypertension 64. This optic neuropathy is accompanied by both retinal and systemic vascular dysfunction 117, 118 and decreased AqH outflow resistance 64.
Together, these studies suggest that genetic mutations leading to impaired NO-GC-1-cGMP signaling are risk factors for glaucoma and reinforce the connection between NO signaling and glaucoma pathogenesis. Furthermore, these studies suggest that the NO-GC-1-cGMP pathway, specifically NO metabolites and cGMP, could be potential biomarkers of glaucoma pathophysiology 64, 98,_99 where early detection could expand the therapeutic window and improve patient outcomes.
6. NO-GC-1-cGMP Pathway and Disease Mechanisms
6.1. IOP regulation
There is increasing evidence that NO is a direct regulator of IOP and that dysfunction of the NO-GC-1 pathway is associated with glaucoma. In healthy human eyes, tissues capable of producing NO include: the ciliary body, the TM, and the SC 119. In many cases, the effect of NO on IOP is linked to the action of its downstream messenger cGMP, particularly in the conventional outflow pathway (see Figure 1). Stimulation of the NO-GC-1-cGMP pathway via administration of NO donor compounds lowers IOP through relaxation of the TM, alteration of TM volume, and an increase in the permeability of cells in the SC 76, 120–127. In addition, increased iNOS is observed following increased perfusion pressure in the anterior segments of human donor eyes 128. In rabbits, a NO donor and a cGMP analogue both decrease IOP 129 and increase outflow facility 130. In mice, eNOS overexpression lowers IOP by increasing pressure-dependent drainage 78. This concurs with previous work suggesting that the ability of NO to lower IOP is mediated by a decrease in the AqH resistance rather than changing the rate of AqH secretion 74. Interestingly, eNOS expression and NO levels are both decreased in the CM, TM, and SC of POAG patients 85, 99, 100.
Intravitreal or intracameral injection of 8-Br-cGMP, a cGMP analog, increased AqH outflow facility in a dose-dependent manner 131. 8-Br-cGMP is cell-permeable, activates cGMP-dependent kinases, and is more resistant to hydrolysis by phosphodiesterases than native cGMP 132. At lower doses (10–30 μg), intravitreal 8-Br-cGMP decreases AqH flow, but does not affect outflow facility 131. In contrast, higher doses of 8-Br-cGMP (100–300 μg), increases outflow facility 131. These results suggest that cGMP may be an important factor in the regulation of IOP by facilitating AqH outflow via the TM and decreasing AqH production by the ciliary epithelium. One potential mechanism that mediates the suppressive effect of NO on AqH production involves inhibition of the Na,K-ATPase pump. In the eye, the Na,K-ATPase is the primary active transporter involved in the establishment of ion gradients that drive AqH formation 133. Inhibition of this pump by ouabain decreases AqH secretion by ~62% 134. Studies indicate that NO donors, such as sodium nitroprusside (SNP), and 8-Br-cGMP reduce AqH secretion and therefore, reduce IOP in bovine and porcine eyes through cGMP- and PKG-dependent inhibition of Na,K-ATPase 135–138.
Relaxation of TM and relaxation of blood vessels occur via similar mechanisms that involve the NO-cGMP pathway 139. The cells of SC share properties with endothelial cells that line blood vessels, which may explain the mechanism of action for NO in IOP regulation. Exploration of the NO-dependent increase in outflow facility in porcine eyes demonstrated that this TM relaxation is GC-1 dependent and prevented by GC inhibitors, such as 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1–1 (ODQ) 70. Additional studies link the NO-GC-1-cGMP pathway directly to AqH outflow capacity. Inhibition of NOS signaling in perfused donor human eyes with LNG-Nitroarginine methyl ester (L-NAME), a non-isoform specific NOS-inhibitor, decreases the rate of AqH outflow 140. Conversely, a NO-donor compound increases AqH outflow rate 140; the AqH outflow rate was directly proportional to increases in cGMP levels in perfused fluid, confirming a role for the NO-GC-1-cGMP pathway in regulation of AqH outflow in humans 140. These findings were corroborated in animal models, where inhaled NO gas lowered IOP in both mice and sheep in a GC-1-dependent manner: in mice, lowering of IOP was attributed to increase in conventional aqueous outflow facility 141, and a small molecule GC-1 stimulator increased cGMP levels and increased AqH outflow in mouse eyes 142. A long-term study on the effect on dietary nitrates on incidence of glaucoma was recently concluded, with the results strongly supporting a role for NO supplementation in the prevention of elevated IOP and thus POAG 143. A greater intake of dietary nitrate was associated with a 20–30% lower risk of POAG; a particularly strong association was seen (40–50%) for those cases of glaucoma with early paracentral vision loss and vascular dysfunction 143. This study further supports a role for impaired NO signaling in the development of glaucoma.
Although these studies strongly implicate the NO-GC-1-cGMP pathway as a regulator of IOP, the mechanism of action for downstream effectors is still poorly understood. However, cGMP-dependent changes in outflow are likely related to contraction and relaxation of the TM. In ex vivo preparations of TM and CM slices that were pre-contracted with carbachol, application of the NOS inhibitor L-nitroarginine led to an increased contraction of both TM and CM. In contrast, application of 8-Br-cGMP to pre-contracted CM and TM strips resulted in relaxation 144. One possible mechanism for cGMP-mediated contraction is activation of protein kinase G (PKG) 70, 73, 125, 130. Activated PKG can phosphorylate numerous targets with multiple downstream effects that relate to contractility, including ion channels and gap junctions 46, 71, 125, 145.
Contractile responses are linked to changes in membrane potential and ultimately, the activity of ion channels. For example, direct application of 8-Br-cGMP has a relaxing effect on bovine TM cells 146 and in CM 144 via activation of BKCa channels. The relaxation effect observed is much more pronounced in TM than in CM 144. Activation of BKCa channels leads to K+ efflux and cell hyperpolarization. This reduces cytosolic Ca2+ through the inhibition of voltage-operated (L-type) Ca2+ channels 147. Similarly, Ca2+ channel blockers, such as topical verapamil, diltiazem, nifedipine, or flunarizine lower IOP in animal models and humans (reviewed in 148). cGMP-dependent changes in Ca2+ dynamics may also involve increased uptake of calcium into the sarcoplasmic reticulum 149.
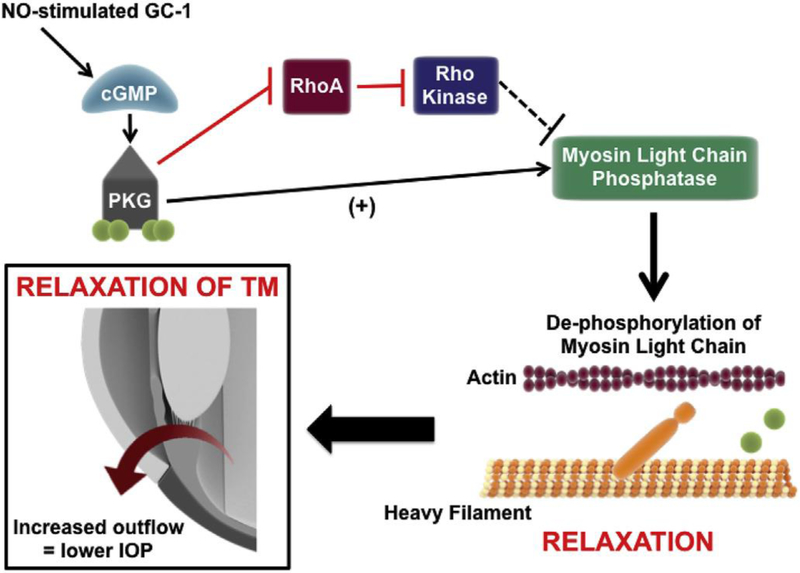
While the mechanism(s) underlying Ca2+-dependent changes in TM relaxation are not well understood, evidence indicates a role for gap junctions 150. One way in which gap junctions are modulated is through rho kinase inhibition. cGMP activates PK-G, which in turn phosphorylates Rho A, leading to its inhibition and subsequent inhibition of Rho Kinase 151. Rho kinase inhibitors, such as netarsudil 152, activate myosin light chain phosphatase (Figure 3; reviewed in 153). Subsequent dephosphorylation of the regulatory light chain of myosin prevents actin–myosin interaction, promoting cell relaxation 154–156. This could lead to a widening of the intercellular spaces in the juxtacanalicular TM and SC, thus facilitate conventional AqH outflow to lower IOP 46, 71, 157, 158 (Figure 1).
Fig. 3. cGMP-mediated modulation of IOP through increase in AqH outflow.
NO triggers production of cGMP by GC-1. cGMP activates protein kinase G (PKG). Activated PKG can phosphorylate numerous targets with multiple downstream effects, including inhibition of Rho A, thus preventing inhibition of myosin phosphatase by Rho Kinase. In addition to inhibition of Rho A, activated PKG can directly activate myosin light chain phosphatase (MLCP). Subsequent dephosphorylation of the regulatory light chain of myosin by MLCP prevents actin–myosin interaction, promoting cell relaxation. This in turn leads to a widening of the intercellular spaces in the juxtacanalicular TM and Schlemm's canal, thus facilitating conventional AqH outflow and relieving IOP.
It is clear that endogenous NO production and subsequent activation of the GC-1-cGMP pathway influence the cellular contractile mechanisms that mediate both AqH outflow and IOP. However, it is not only the TM that is important, the CM also plays a role in IOP regulation. Contraction of the CM causes relaxation of the TM, which increases intratrabecular spaces and increases outflow facility. The relaxation of the CM could possibly decrease conventional outflow. Thus, a functional antagonism between contractility of TM and CM exists and it is the balance between these modalities that may determine total AqH outflow through the conventional route (reviewed in 159). Accordingly, pharmacological agents that preferentially relax the TM rather than the CM may have a beneficial impact on IOP.
6.2. Ocular blood flow
Vascular endothelial dysfunction and impaired blood flow have been associated with POAG, both in ocular hypertensive and normotensive subsets 80, 160–162. The topic of vascular dysfunction and POAG has been recently reviewed (for further details see 115). Briefly, the endothelial monolayer lies between the lumen of blood vessels and underlying smooth muscle tissue and is responsible for modulation of vascular tone, thrombus formation, cell adhesion, and sequestration of inflammatory mediators 115. Impaired endothelial signaling in POAG is observed in tissues relevant for both outflow resistance and RGC support, including: 1) endothelial layers in the inner wall of SC, the ciliary body and the posterior longitudinal muscle (outflow resistance) and 2) vascular endothelial cells that underlie the luminal smooth muscle vessels that supply RGCs 115.
Several studies support vascular dysfunction as a key player in development of glaucoma. Acetylcholine-induced vasodilation, assessed non-invasively in forearm blood vessels, was impaired in NTG patients 80. Several groups have studied flow-mediated vasodilation in brachial arteries of both NTG and POAG patients with elevated IOP, and reported that these patient have abnormal responses compared with controls 160, 163, 164.
Although these studies could explain why glaucoma develops across a range of IOP levels, they do not elucidate the molecular mechanisms underlying vascular dysfunction in glaucoma. However, there is compelling evidence to suggest a role for NO-cGMP signaling in vascular dysfunction associated with glaucoma (see also section 5.1). Both vasoactive and vasoconstrictive factors, NO and endothelin-1 respectively, are produced by the vascular endothelium, and play a major role in the control of ocular blood flow 165–168. In the retina and optic nerve head, endogenous NO helps to maintain basal blood flow 84, 169–171. Blood flow through the optic nerve head is autoregulated. This means that local tissue blood flow (perfusion pressure) is kept constant despite physiological or metabolic changes. It is known that NO is involved in autoregulation at the optic nerve head under glaucoma-related conditions, i.e. ocular hypertension 172, 173. Abnormalities in vascular autoregulation are implicated in glaucomatous optic neuropathy 174, 175, especially in patients with normal tension glaucoma (NTG)176. Several groups have investigated the role of NO in ocular blood flow autoregulation. These studies indicate that NO alters retinal, optic nerve head and choroidal autoregulation of blood flow in rabbits and pigs when IOP is elevated 172, 177. Similarly, NO-donor molecules injected intravitreally enhance tissue oxygenation of the optic nerve head in preclinical animal models 178. These data suggest that NO contributes to blood flow autoregulation in the retina and optic nerve.
Like IOP regulation, blood flow autoregulation is likely mediated by NO and downstream effectors such as cGMP. NO altered choroidal, retinal, and ONH autoregulation during experimental IOP elevations in rabbits and piglets 172, 177. Sildenafil, a phosphodiesterase 5 (PDE5) inhibitor, elevates cGMP levels and increases choroidal blood flow (reviewed in 179) and optic neuropathy in GC-1−/− mice also presents with vascular dysfunction 64. Impairment of the NO-GC-1-cGMP pathway may also impact ocular blood flow and autoregulation indirectly via changes in mean arterial pressure induced by systemic vascular dysfunction.
Blood flow autoregulation in the eye remains a complex phenomenon and the mechanism by which the NO-GC-1-cGMP pathway modulates retinal vascular autoregulation remains to be elucidated. A plausible mechanism may involve the downstream action of cGMP on calcium flux in cells: elevated cGMP leads to calcium efflux from smooth muscle cells, and therefore relaxation of the cell 180. In arterioles (such as those in retinal vessels), this can lead to increased ocular blood flow. Although cGMP effects on calcium flux in cells have beneficial effects on ocular blood flow, a recent retrospective study suggests that use of calcium channel blockers confer a 30% increased risk of POAG progression. Previous studies have reported conflicting data. One study indicates that calcium channel blockers have no effect on the clinical course of glaucoma 181, while another indicates that calcium channel blockers may impede glaucoma progression 182. Additionally, animal studies indicate that calcium channel inhibitors prevent ischemia-induced RGC degeneration by restoring impaired blood flow and directly inhibiting apoptosis pathways 183, 184.
Although there is no direct evidence that improved blood flow via modulation of NO-cGMP signaling leads to preservation of visual field, the studies aforementioned do highlight vascular dysfunction as a likely contributor in disease pathogenesis for both ocular hypertensive and NTG POAG patients. While it is clear that modulation NO-cGMP pathway can improve ocular blood flow, additional studies are required to evaluate any potential efficacy for this strategy in the treatment of glaucoma.
6.3. Neuroprotection
NO signaling is associated with both neuroprotective and neurotoxic outcomes depending on its concentration and the cell types involved. Elevated NO concentrations can lead to oxidative reduction reactions and the production of reactive nitrogen species (RNS) 185–188. RNS, such as peroxynitrite, are implicated in the pathogenesis of many neurodegenerative disorders, such as Alzheimer’s Disease 189. Likewise, some studies indicate that NO may also have neurotoxic effects in retina. In an in vitro study, a NOS inhibitor significantly protected RGCs from anoxia-induced death 190. In rat models of glaucoma, increased expression of NOS1 in retina was associated with RGC degeneration and inhibition of iNOS prevented RGC degeneration 191,192, 193. Finally, the δ-opiod receptor agonist (SNC-121) protects RGCs in a rat model of glaucoma via the suppression of iNOS activity 194.
Conversely, NO can also act in a neuroprotective manner 195–199. Activation of the NO-GC-1-cGMP pathway inhibits apoptotic cell death in a variety of primary neuronal cultures and neural-derived cell lines 200–202. In traumatic brain injury, NO presence is associated with both detrimental secondary damage and neurological recovery 203. The temporal importance of NO in neurological recovery was investigated using iNOS knockout mice. In these studies, oxidative stress markers were more pronounced in iNOS knockout mice than in wild type mice, suggesting a role for NO as an endogenous antioxidant and thus, neuroprotective agent 203. In animal models of RGC degeneration, the neuroprotective effect of a novel NO-releasing beta-blocker (nipradilol) on RGC death is attributable to the released NO 204–208. This neuroprotective effect is NO-dependent. Furthermore, nipradilol promotes regeneration of RGC axon in optic nerve, likely through S-nitrosylation of PTEN and subsequent activation of the mTOR/Akt pathway 209. Further studies are needed to elucidate the neuroprotective capabilities of NO, in particular any involvement of cGMP, in the context of glaucoma.
The neuroprotective properties of NO in cell cultures appear to be mediated, at least in part, by the activation of the GC-cGMP pathway and its effect on downstream targets, such as protein kinases and Ca2+ channels 210, 211,212. As discussed in Section 6.2, cGMP may have an indirect neuroprotective effect via activation of BKCa channels and inhibition of L-type Ca2+ channels 183, 184. In cerebellum, NO acts via the GC-1 pathway to prevent apoptosis through activation of protein kinase-G and Akt 213. In Schwann cells, reduced expression of GC-1 leads to apoptosis of co-cultured neurons and glial cells 212. Furthermore, in primary neuron cultures, direct application of NO or nitrite prevents endoplasmic reticulum-induced apoptosis in a GC-1-cGMP-dependent manner 214. Although excess NO can be neurotoxic, the in vitro and in vivo evidenced outlined here supports a neuroprotective role for the NO-CG-1-cGMP pathway that should be further examined in glaucoma models.
7. The NO-GC-1-cGMP Pathway as a Target for Glaucoma Therapeutics
Given its established role in IOP regulation and ocular blood flow and its potential ability to serve as a neuroprotectant, NO signaling is a prime candidate for the development of novel multi-target therapeutics for glaucoma.
7.1. NO-Donor Compounds
For more than a century, NO-donating compounds, e.g. nitrovasodilators, have been used to successfully treat cardiovascular disease. Previous studies indicate that NO-donation via intravenous or oral administration of nitroglycerin or isosorbide dinitrate effectively lower IOP in both POAG patients and control subjects 215. In animal studies, topical application of these same vasodilators also effectively lowers IOP 121, 122, 127, 216. Accordingly, novel topical NO-releasing compounds for IOP management have recently been developed. For example, latanoprostene bunod (LBN) is a NO-donating prostaglandin F2α analog that is rapidly metabolized in situ (Table 1). Nipradilol is a beta-blocker ligated with a NO-donating moiety. Like their current counterparts, both LBN and nipradilol are effective at lowering IOP 124, 155, 217. LBN is effective at lowering IOP in several animal models of glaucoma, where its parent compound latanoprost had minimal efficacy 158. In Phase 2 and 3 clinical trials, LBN increased latanoprost IOP-lowering capacity by more than 1 mmHg, making it more effective than the preferred drug on the market for the treatment of POAG 218–222. Nipradilol reduces IOP to a similar extent as timolol 223. A recent review of non-clinical studies with LBN highlights that LBN has a dual action, enhancing AqH outflow via both the uveoscleral pathway (due to action of latanoprost) and the TM/SC of the conventional pathway (due to the effect of the NO moiety)224.
Studies in animal models indicate that nipradilol also has neuroprotective qualities 204–206 (see section 6.3). There are conflicting data regarding translation of these neuroprotective qualities to humans; in comparative studies between nipradilol and timolol, no additional beneficial effects were observed in visual field performance of those taking nipradilol vs. timolol 225,226, 227. However, one study has shown that in addition to lowering IOP, nipradilol increases ocular blood flow in both control and NTG eyes 227, which suggests that nipradilol may have an advantage in prevention against glaucomatous damage by increasing ocular blood flow to the optic nerve head, over similar topical therapies. Further research is required to determine its neuroprotective efficacy and the mechanisms involved. There are several additional NO-donor compounds that exhibit pre-clinical efficacy for IOP reduction in animal models, including the prostaglandin analogue NCX 470 and two novel carbonic anhydrase inhibitors: NO-dorzolamide and NO-brinzolamide 228, 229,230. Together, these studies indicate that NO-donor compounds are viable and potentially potent therapeutic agents for IOP lowering in glaucoma patients as well as those with ocular hypertension.
7.2. Alternative NO-GC-1-cGMP Targeting
Although NO-donor compounds are promising as therapeutics for IOP regulation, they may not be ideal neuroprotective agents. As described in this review, the neuroprotective activities of NO can be linked specifically to its activation of GC and subsequent modulation of cGMP. While NO-donation can facilitate activation of GC-1, it also has the potential to induce the production of RNS and promote oxidative stress outcomes. An alternative method of harnessing the neuroprotective properties of NO-GC-1-cGMP, while avoiding the possible GC-independent neurotoxic effects of NO, is the direct targeting of GC or cGMP. As discussed above, NO modulation of both IOP and ocular blood flow is linked to activation of GC-1 and subsequent induction of cGMP-mediated pathways. Targeting GC-1 or cGMP would not only promote the activation of neuroprotection pathways associated with NO, but would likely maintain equivalent IOP lowering efficacy. There are several opportunities for therapeutic activation of either GC-1 or cGMP, including pharmacological activation of GC-1 and inhibition of cGMP degradation. Recent evidence, described below, supports the neuroprotective benefit of these strategies in the CNS.
7.2.a. PDE Inhibitors
cGMP is degraded by PDEs (Figure 2). PDE inhibitors prevent the breakdown of cGMP, thereby increasing cGMP bioavailability and prolonging cGMP-mediated activation of downstream pathways (Figure 2). In a rat model of hypoxic ischemia, elevation of cGMP levels by sildenafil, a PDE5 inhibitor, reduces apoptosis, astrocytosis, and microgliosis in the brain 231. Similarly, tadalafil, another CNS penetrant PDE5 inhibitor, is neuroprotective both in spinal cord injury 232 and in ischemia/reperfusion injury 233. In the eye, PDE6 inhibition prevents hypoxia-induced cell death throughout the whole retina in porcine retinal explants via a cGMP-dependent mechanism 234.
As highlighted in this review, cGMP is involved in AqH dynamics and thus, PDE5 inhibitors have the potential to affect IOP 131, 235. However, findings from several clinical studies indicate that the use of PDE5 inhibitors do not alter IOP. In a Phase I clinical trial, a single dose of PDE5 inhibitor sildenafil does not alter IOP in healthy volunteers either 1 hour or 48 hours post-administration 236. A second study with a larger sample size yielded the same results 237. A similar study also determined that a single dose of sildenafil does not alter IOP either 1 hour or 5 hours post-administration in glaucoma patients 238. Although these studies suggest that PDE5 inhibition does not impact IOP, the findings pertain to only a single administration. To date, there is only one study that examined the long-term effects of sildenafil treatment on IOP. In this study, a small cohort of patients with erectile dysfunction (n=10) received 50mg of sildenafil citrate one or more times a week for a minimum of 3 months and displayed no change in IOP 239. Together, these studies suggest that PDE5 inhibitors likely do not impact IOP. However, a study of long-term use in both healthy volunteers and glaucoma patients would be beneficial..
Although PDE5 inhibitors appear to not influence IOP, visual disturbances have been reported 240, 241. The most common visual disturbances are increased blue tinge in the visual image and an increased sensitivity to light 242–244. The rate of occurrence for these symptoms is low; in 3–11% of men taking sildenafil 25–100 mg 241, 0.3–2% of vardenafil 245, 246, and 0.1% of tadalafil users 247. The symptoms tend to be mild, transient, dose-dependent, and completely reversible. These symptoms likely arise from off-target inhibition of PDE6 in the retina. PDE6 expression in retina is restricted to rod and cone outer segments, where it contributes to phototransduction 248. PDE5 inhibitors currently prescribed having varying selectivity for PDE5 over PDE6: 10-fold for sildenafil 249, 15-fold for vardenafil 250, and 700-fold for tadalafil 251. While these visual disturbances are manageable for intermittent use, they may have greater implications for the use of PDE5 inhibitors in a chronic treatment paradigm, as would be necessary for treatment of glaucoma.
In addition to visual disturbances, a few more serious ocular events have also been noted in male patients prescribed PDE5 inhibitors, including: non-arteritic anterior ischemic optic neuropathy (NAAION) with attendant vision loss, cilio-retinal artery occlusion, central retinal vein occlusion (CRVO), and pupil sparing third nerve palsy (reviewed in 252). However, a direct cause and effect relationship between these conditions and the use of PDE5 inhibitors has not been established and the rate of incidence does not appear higher than that in male populations generally.252
7.2.b. GC Stimulators
Given the potential off-target effects associated with PDE5 inhibition (e.g. PDE6 inhibition in photoreceptors), direct targeting of cGMP production by GC has emerged as a novel therapeutic strategy to lower IOP, and potentially provide neuroprotection in glaucoma 142. GC stimulators, small molecule drugs that synergistically increase GC enzyme activity with NO, are already clinically available or are in clinical trials for a variety of diseases. Riociguat is approved for treatment of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension 253, 254.
Stimulation of GC may have therapeutic benefits outside the cardiovascular system, and particularly in the eye. For example, GC-1 stimulation by IWP-953 increased AqH outflow in enucleated mouse eyes, highlighting the therapeutic potential for GC stimulators as novel ocular hypotensive drugs 142. Safety, tolerability, and efficacy of the GC activator, MGV354, has shown promising IOP-lowering effects in pigmented rabbits and in a cynomolgus monkey model of glaucoma 255. A single topical ocular dose caused a significant dose-dependent IOP reduction of 20% to 40% (versus vehicle), lasting up to 6 hours in pigmented rabbits. The MGV354-induced IOP lowering was sustained for up to 7 days following once-daily dosing in a monkey model of glaucoma and was greater in magnitude compared to travoprost-induced IOP reduction 255. It is not yet clear whether this approach also provides neuroprotection to RGCs beyond that afforded by IOP reduction. Together, these data indicate that pharmacological targeting of GC and cGMP may be a fruitful and beneficial alternative to NO-releasing compounds for glaucoma therapy. However, further studies to evaluate this compound in vivo are necessary.
8. Conclusions
Glaucoma incidence is on the rise, with many more cases expected to surface in the few decades. Despite effective treatments to lower IOP, glaucoma is still a major cause of blindness worldwide. Advances in treatment are impeded by the complex etiology of the disease and lack of understanding of IOP-independent facets of the disease, i.e. neurodegenerative mechanisms. Ideally, novel glaucoma therapeutics would target both IOP-dependent and -independent mechanisms of the disease.
Recent evidence, reviewed here, indicates that impairment in the NO-GC-cGMP pathway is implicated in glaucoma onset and progression. Involvement of the NO-GC-cGMP pathway with both IOP regulation and ocular blood flow and its potential to elicit neuroprotective responses in neural retina make this pathway a strong candidate for therapeutic targeting of multiple pathogenic mechanisms in glaucoma.
The potential of the NO-GC-cGMP pathway to prevent and/or treat glaucoma underlies the development of NO-donor compounds as IOP lowering therapeutics. There are many benefits of targeting the NO-GC-cGMP pathway when developing novel therapeutics for glaucoma. NO increases AqH humor outflow through the conventional pathway, which aids in IOP reduction, whilst also increasing retinal perfusion and having putative neuroprotective effects (Figure 4). The potential benefit in NO-releasing treatments is however offset by the delicate balance necessary to promote beneficial outcomes and avoid negative consequences due, for example, to induction of RNS, and other oxidative stress leading to nitrate tolerance which can ultimately promote insensitivity to long-term NO exposure and inhibition on GC 256. This risk is not alleviated by available NO donor compounds, which activate GC to produce cGMP.
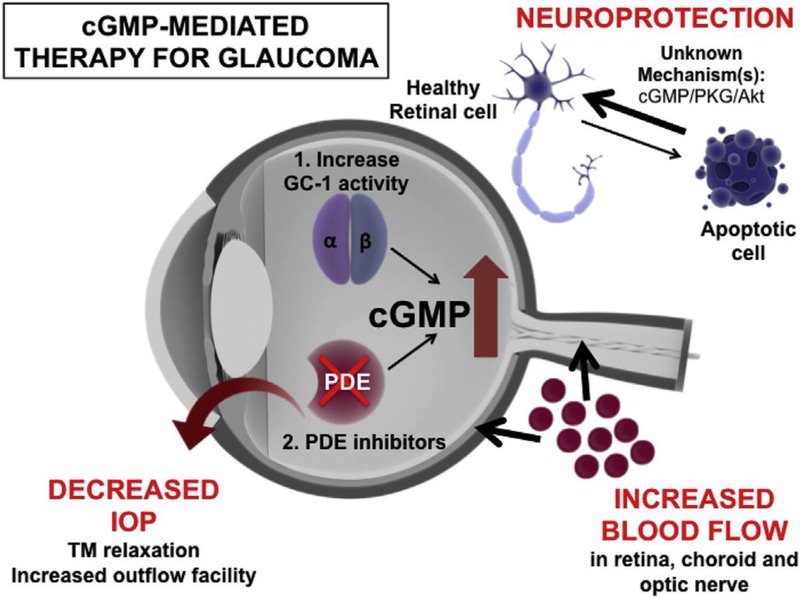
Fig. 4. – GC-1-directed therapy for glaucoma is pleiotrophic in its action.
Increased levels of cGMP have been shown to have pleiotrophic targets that are beneficial in the treatment of glaucoma, including: relaxation of the TM to increase outflow facility which leads to decreases in IOP; increasing blood flow to the retina, choroid and optic nerve head; prevention of degeneration of retinal ganglion cells through mechanisms that may involve downstream kinase pathways. cGMP levels in the eye can be increased in two ways: 1) through the use of GC-1 stimulators and activators, which aim to increase production of cGMP; or 2) through the use of PDE inhibitors which prevent the breakdown of cGMP in the cell to increase bioavailability.
Here, we advocate for the development and further study of compounds that activate the NO-GC-cGMP pathway by targeting GC and cGMP directly. IOP and blood flow regulation by NO is attributable to GC-mediated elevation of cGMP. Likewise, neuroprotective outcomes associated with NO, i.e. anti-apoptotic signaling, are also dependent on GC activation and cGMP modulation. Thus, direct targeting of GC activity or cGMP levels has the potential to promote IOP reduction, increase ocular blood flow and activate neuroprotective mechanisms without the generation of RNS and nitrate (and NO) tolerance. While a few studies suggest that compounds targeting GC activation and cGMP levels are acceptable alternatives to NO-releasing compounds for the treatment of glaucoma, additional research is needed to systematically evaluate the therapeutic efficacy of this approach.
References
- 1.Tham YC, Li X, Wong TY, Quigley HA, Aung T and Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. [DOI] [PubMed] [Google Scholar]
- 2.Quigley HA and Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog Retin Eye Res. 2012;31:702–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M and Early Manifest Glaucoma Trial G. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–79. [DOI] [PubMed] [Google Scholar]
- 5.Iester M, De Feo F and Douglas GR. Visual field loss morphology in high- and normal-tension glaucoma. J Ophthalmol. 2012;2012:327326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shields MB. Normal-tension glaucoma: is it different from primary open-angle glaucoma? Curr Opin Ophthalmol. 2008;19:85–8. [DOI] [PubMed] [Google Scholar]
- 7.Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L and Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–72. [DOI] [PubMed] [Google Scholar]
- 8.Aliancy J, Stamer WD and Wirostko B. A Review of Nitric Oxide for the Treatment of Glaucomatous Disease. Ophthalmol Ther. 2017;6:221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinreb RN, Aung T and Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quigley HA, Addicks EM, Green WR and Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981;99:635–49. [DOI] [PubMed] [Google Scholar]
- 11.Leung CK, Liu S, Weinreb RN, Lai G, Ye C, Cheung CY, Pang CP, Tse KK and Lam DS. Evaluation of retinal nerve fiber layer progression in glaucoma a prospective analysis with neuroretinal rim and visual field progression. Ophthalmology. 2011;118:1551–7. [DOI] [PubMed] [Google Scholar]
- 12.Miglior S, Brigatti L, Lonati C, Rossetti L, Pierrottet C and Orzalesi N. Correlation between the progression of optic disc and visual field changes in glaucoma. Curr Eye Res. 1996;15:145–9. [DOI] [PubMed] [Google Scholar]
- 13.Ekstrom C Elevated intraocular pressure and pseudoexfoliation of the lens capsule as risk factors for chronic open-angle glaucoma. A population-based five-year follow-up study. Acta Ophthalmol (Copenh). 1993;71:189–95. [DOI] [PubMed] [Google Scholar]
- 14.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK 2nd, Wilson MR and Gordon MO. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13; discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 15.Caprioli J, Prum B and Zeyen T. Comparison of methods to evaluate the optic nerve head and nerve fiber layer for glaucomatous change. Am J Ophthalmol. 1996;121:659–67. [DOI] [PubMed] [Google Scholar]
- 16.Heijl A, Bengtsson B, Hyman L, Leske MC and Early Manifest Glaucoma Trial G. Natural history of open-angle glaucoma. Ophthalmology. 2009;116:2271–6. [DOI] [PubMed] [Google Scholar]
- 17.Quigley HA, McKinnon SJ, Zack DJ, Pease ME, Kerrigan-Baumrind LA, Kerrigan DF and Mitchell RS. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000;41:3460–6. [PubMed] [Google Scholar]
- 18.Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL and Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31:152–81. [DOI] [PubMed] [Google Scholar]
- 19.Quigley HA, Addicks EM and Green WR. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol. 1982;100:135–46. [DOI] [PubMed] [Google Scholar]
- 20.Kwon YH, Kim CS, Zimmerman MB, Alward WL and Hayreh SS. Rate of visual field loss and long-term visual outcome in primary open-angle glaucoma. Am J Ophthalmol. 2001;132:47–56. [DOI] [PubMed] [Google Scholar]
- 21.Goel M, Picciani RG, Lee RK and Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmol J. 2010;4:52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alm A and Nilsson SF. Uveoscleral outflow--a review. Exp Eye Res. 2009;88:760–8. [DOI] [PubMed] [Google Scholar]
- 23.Johnson M, McLaren JW and Overby DR. Unconventional aqueous humor outflow: A review. Exp Eye Res. 2017;158:94–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bill A The aqueous humor drainage mechanism in the cynomolgus monkey (Macaca irus) with evidence for unconventional routes. Invest Ophthalmol. 1965;4:911–9. [PubMed] [Google Scholar]
- 25.Bill A and Hellsing K. Production and drainage of aqueous humor in the cynomolgus monkey (Macaca irus). Invest Ophthalmol. 1965;4:920–6. [PubMed] [Google Scholar]
- 26.Bill A The routes for bulk drainage of aqueous humour in the vervet monkey (Cercopithecus ethiops). Exp Eye Res. 1966;5:55–7. [DOI] [PubMed] [Google Scholar]
- 27.Bill A Conventional and uveo-scleral drainage of aqueous humour in the cynomolgus monkey (Macaca irus) at normal and high intraocular pressures. Exp Eye Res. 1966;5:45–54. [DOI] [PubMed] [Google Scholar]
- 28.Lewis RA, Christie WC, Day DG, Craven ER, Walters T, Bejanian M, Lee SS, Goodkin ML, Zhang J, Whitcup SM, Robinson MR and Bimatoprost SRSG. Bimatoprost Sustained-Release Implants for Glaucoma Therapy: 6-Month Results From a Phase I/II Clinical Trial. Am J Ophthalmol. 2017;175:137–147. [DOI] [PubMed] [Google Scholar]
- 29.Prum BE Jr., Lim MC, Mansberger SL, Stein JD, Moroi SE, Gedde SJ, Herndon LW Jr., Rosenberg LF and Williams RD. Primary Open-Angle Glaucoma Suspect Preferred Practice Pattern((R)) Guidelines. Ophthalmology. 2016;123:P112–51. [DOI] [PubMed] [Google Scholar]
- 30.Chang EE and Goldberg JL. Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology. 2012;119:979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krupin T, Liebmann JM, Greenfield DS, Ritch R and Gardiner S. A randomized trial of brimonidine versus timolol in preserving visual function: results from the Low-Pressure Glaucoma Treatment Study. American journal of ophthalmology. 2011;151:671–81. [DOI] [PubMed] [Google Scholar]
- 32.Stewart WC, Konstas AG, Nelson LA and Kruft B. Meta-analysis of 24-hour intraocular pressure studies evaluating the efficacy of glaucoma medicines. Ophthalmology. 2008;115:1117–1122 e1. [DOI] [PubMed] [Google Scholar]
- 33.Semba K, Namekata K, Kimura A, Harada C, Mitamura Y and Harada T. Brimonidine prevents neurodegeneration in a mouse model of normal tension glaucoma. Cell Death Dis. 2014;5:e1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saylor M, McLoon LK, Harrison AR and Lee MS. Experimental and clinical evidence for brimonidine as an optic nerve and retinal neuroprotective agent: an evidence-based review. Arch Ophthalmol. 2009;127:402–6. [DOI] [PubMed] [Google Scholar]
- 35.Palmer RM, Ferrige AG and Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–6. [DOI] [PubMed] [Google Scholar]
- 36.Furchgott RF and Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. [DOI] [PubMed] [Google Scholar]
- 37.Carpenter AW and Schoenfisch MH. Nitric oxide release: part II. Therapeutic applications. Chem Soc Rev. 2012;41:3742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murad F Nitric oxide: the coming of the second messenger. Rambam Maimonides Med J. 2011;2:e0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer RM, Ashton DS and Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–6. [DOI] [PubMed] [Google Scholar]
- 40.Palmer RM and Moncada S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun. 1989;158:348–52. [DOI] [PubMed] [Google Scholar]
- 41.Babu BR, Frey C and Griffith OW. L-arginine binding to nitric-oxide synthase. The role of H-bonds to the nonreactive guanidinium nitrogens. J Biol Chem. 1999;274:25218–26. [DOI] [PubMed] [Google Scholar]
- 42.Griffith OW and Stuehr DJ. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995;57:707–36. [DOI] [PubMed] [Google Scholar]
- 43.Hood JD, Meininger CJ, Ziche M and Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol. 1998;274:H1054–8. [DOI] [PubMed] [Google Scholar]
- 44.Kroll J and Waltenberger J. VEGF-A induces expression of eNOS and iNOS in endothelial cells via VEGF receptor-2 (KDR). Biochem Biophys Res Commun. 1998;252:743–6. [DOI] [PubMed] [Google Scholar]
- 45.Boo YC and Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am J Physiol Cell Physiol. 2003;285:C499–508. [DOI] [PubMed] [Google Scholar]
- 46.Cavet ME, Vittitow JL, Impagnatiello F, Ongini E and Bastia E. Nitric oxide (NO): an emerging target for the treatment of glaucoma. Investigative ophthalmology & visual science. 2014;55:5005–15. [DOI] [PubMed] [Google Scholar]
- 47.Torres J, Darley-Usmar V and Wilson MT. Inhibition of cytochrome c oxidase in turnover by nitric oxide: mechanism and implications for control of respiration. Biochem J. 1995;312 ( Pt 1):169–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stamler JS, Lamas S and Fang FC. Nitrosylation. the prototypic redox-based signaling mechanism. Cell. 2001;106:675–83. [DOI] [PubMed] [Google Scholar]
- 49.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P and Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3:193–7. [DOI] [PubMed] [Google Scholar]
- 50.Davis KL, Martin E, Turko IV and Murad F. Novel effects of nitric oxide. Annu Rev Pharmacol Toxicol. 2001;41:203–36. [DOI] [PubMed] [Google Scholar]
- 51.Knowles RG, Palacios M, Palmer RM and Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989;86:5159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mergia E, Russwurm M, Zoidl G and Koesling D. Major occurrence of the new alpha2beta1 isoform of NO-sensitive guanylyl cyclase in brain. Cell Signal. 2003;15:189–95. [DOI] [PubMed] [Google Scholar]
- 53.Mergia E, Friebe A, Dangel O, Russwurm M and Koesling D. Spare guanylyl cyclase NO receptors ensure high NO sensitivity in the vascular system. J Clin Invest. 2006;116:1731–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Budworth J, Meillerais S, Charles I and Powell K. Tissue distribution of the human soluble guanylate cyclases. Biochem Biophys Res Commun. 1999;263:696–701. [DOI] [PubMed] [Google Scholar]
- 55.Hobbs AJ. Soluble guanylate cyclase: the forgotten sibling. Trends Pharmacol Sci. 1997;18:484–91. [DOI] [PubMed] [Google Scholar]
- 56.Arnold WP, Mittal CK, Katsuki S and Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3':5'-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977;74:3203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murad F Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med. 2006;355:2003–11. [DOI] [PubMed] [Google Scholar]
- 58.Katsuki S, Arnold W, Mittal C and Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977;3:23–35. [PubMed] [Google Scholar]
- 59.Lee YC, Martin E and Murad F. Human recombinant soluble guanylyl cyclase: expression, purification, and regulation. Proc Natl Acad Sci U S A. 2000;97:10763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Humbert P, Niroomand F, Fischer G, Mayer B, Koesling D, Hinsch KD, Gausepohl H, Frank R, Schultz G and Bohme E. Purification of soluble guanylyl cyclase from bovine lung by a new immunoaffinity chromatographic method. Eur J Biochem. 1990;190:273–8. [DOI] [PubMed] [Google Scholar]
- 61.Sharina IG, Jelen F, Bogatenkova EP, Thomas A, Martin E and Murad F. Alpha1 soluble guanylyl cyclase (sGC) splice forms as potential regulators of human sGC activity. J Biol Chem. 2008;283:15104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zagotta WN and Siegelbaum SA. Structure and function of cyclic nucleotide-gated channels. Annu Rev Neurosci. 1996;19:235–63. [DOI] [PubMed] [Google Scholar]
- 63.Francis SH, Busch JL, Corbin JD and Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62:525–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buys ES, Ko YC, Alt C, Hayton SR, Jones A, Tainsh LT, Ren R, Giani A, Clerte M, Abernathy E, Tainsh RE, Oh DJ, Malhotra R, Arora P, de Waard N, Yu B, Turcotte R, Nathan D, Scherrer-Crosbie M, Loomis SJ, Kang JH, Lin CP, Gong H, Rhee DJ, Brouckaert P, Wiggs JL, Gregory MS, Pasquale LR, Bloch KD and Ksander BR. Soluble Guanylate Cyclase alpha1-Deficient Mice: A Novel Murine Model for Primary Open Angle Glaucoma. PloS one. 2013;8:e60156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ellis DZ, Dismuke WM and Chokshi BM. Characterization of soluble guanylate cyclase in NO-induced increases in aqueous humor outflow facility and in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2009;50:1808–13. [DOI] [PubMed] [Google Scholar]
- 66.Haberecht MF, Schmidt HH, Mills SL, Massey SC, Nakane M and Redburn-Johnson DA. Localization of nitric oxide synthase, NADPH diaphorase and soluble guanylyl cyclase in adult rabbit retina. Vis Neurosci. 1998;15:881–90. [DOI] [PubMed] [Google Scholar]
- 67.Kajimura M, Shimoyama M, Tsuyama S, Suzuki T, Kozaki S, Takenaka S, Tsubota K, Oguchi Y and Suematsu M. Visualization of gaseous monoxide reception by soluble guanylate cyclase in the rat retina. Faseb J. 2003;17:506–8. [DOI] [PubMed] [Google Scholar]
- 68.Blute TA, Velasco P and Eldred WD. Functional localization of soluble guanylate cyclase in turtle retina: modulation of cGMP by nitric oxide donors. Vis Neurosci. 1998;15:485–98. [DOI] [PubMed] [Google Scholar]
- 69.Blom J, Giove T, Deshpande M and Eldred WD. Characterization of nitric oxide signaling pathways in the mouse retina. J Comp Neurol. 2012;520:4204–17. [DOI] [PubMed] [Google Scholar]
- 70.Ellis DZ, Dismuke WM and Chokshi BM. Characterization of soluble guanylate cyclase in NO-induced increases in aqueous humor outflow facility and in the trabecular meshwork. Investigative ophthalmology & visual science. 2009;50:1808–13. [DOI] [PubMed] [Google Scholar]
- 71.Becquet F, Courtois Y and Goureau O. Nitric oxide in the eye: multifaceted roles and diverse outcomes. Surv Ophthalmol. 1997;42:71–82. [DOI] [PubMed] [Google Scholar]
- 72.Neufeld AH, Hernandez MR and Gonzalez M. Nitric oxide synthase in the human glaucomatous optic nerve head. Arch Ophthalmol. 1997;115:497–503. [DOI] [PubMed] [Google Scholar]
- 73.Buys ES, Potter LR, Pasquale LR and Ksander BR. Regulation of intraocular pressure by soluble and membrane guanylate cyclases and their role in glaucoma. Frontiers in Molecular Neuroscience. 2014;7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nathanson JA and McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway of the human eye. Invest Ophthalmol Vis Sci. 1995;36:1765–73. [PubMed] [Google Scholar]
- 75.Fernandez-Durango R, Fernandez-Martinez A, Garcia-Feijoo J, Castillo A, de la Casa JM, Garcia-Bueno B, Perez-Nievas BG, Fernandez-Cruz A and Leza JC. Expression of nitrotyrosine and oxidative consequences in the trabecular meshwork of patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2008;49:2506–11. [DOI] [PubMed] [Google Scholar]
- 76.Chang JY, Stamer WD, Bertrand J, Read AT, Marando CM, Ethier CR and Overby DR. Role of nitric oxide in murine conventional outflow physiology. Am J Physiol Cell Physiol. 2015;309:C205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ashpole NE, Overby DR, Ethier CR and Stamer WD. Shear stress-triggered nitric oxide release from Schlemm's canal cells. Invest Ophthalmol Vis Sci. 2014;55:8067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stamer WD, Lei Y, Boussommier-Calleja A, Overby DR and Ethier CR. eNOS, a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci. 2011;52:9438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lei Y, Zhang X, Song M, Wu J and Sun X. Aqueous Humor Outflow Physiology in NOS3 Knockout Mice. Invest Ophthalmol Vis Sci. 2015;56:4891–8. [DOI] [PubMed] [Google Scholar]
- 80.Henry E, Newby DE, Webb DJ and O'Brien C. Peripheral endothelial dysfunction in normal pressure glaucoma. Invest Ophthalmol Vis Sci. 1999;40:1710–4. [PubMed] [Google Scholar]
- 81.Kang JH, Wiggs JL, Rosner BA, Haines J, Abdrabou W and Pasquale LR. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: interactions with hypertension, alcohol intake, and cigarette smoking. Archives of ophthalmology. 2011;129:773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang JH, Wiggs JL, Rosner BA, Hankinson SE, Abdrabou W, Fan BJ, Haines J and Pasquale LR. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: interactions with sex and postmenopausal hormone use. Invest Ophthalmol Vis Sci. 2010;51:971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jeoung JW, Kim DM, Oh S, Lee JS, Park SS and Kim JY. The Relation Between Endothelial Nitric Oxide Synthase Polymorphisms and Normal Tension Glaucoma. J Glaucoma. 2017;26:1030–1035. [DOI] [PubMed] [Google Scholar]
- 84.Toda N and Nakanishi-Toda M. Nitric oxide: ocular blood flow, glaucoma, and diabetic retinopathy. Prog Retin Eye Res. 2007;26:205–38. [DOI] [PubMed] [Google Scholar]
- 85.Nathanson JA and McKee M. Alterations of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci. 1995;36:1774–84. [PubMed] [Google Scholar]
- 86.Yamamoto R, Bredt DS, Snyder SH and Stone RA. The localization of nitric oxide synthase in the rat eye and related cranial ganglia. Neuroscience. 1993;54:189–200. [DOI] [PubMed] [Google Scholar]
- 87.Overby DR, Bertrand J, Schicht M, Paulsen F, Stamer WD and Lutjen-Drecoll E. The structure of the trabecular meshwork, its connections to the ciliary muscle, and the effect of pilocarpine on outflow facility in mice. Invest Ophthalmol Vis Sci. 2014;55:3727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bredt DS, Hwang PM and Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–70. [DOI] [PubMed] [Google Scholar]
- 89.Goureau O, Lepoivre M, Becquet F and Courtois Y. Differential regulation of inducible nitric oxide synthase by fibroblast growth factors and transforming growth factor beta in bovine retinal pigmented epithelial cells: inverse correlation with cellular proliferation. Proc Natl Acad Sci U S A. 1993;90:4276–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Franco-Bourland RE, Guizar-Sahagun G, Garcia GA, Odor-Morales A, Alvarez A, Esquivel F and Rodriguez S. Retinal vulnerability to glutamate excitotoxicity in canine glaucoma: induction of neuronal nitric oxide synthase in retinal ganglion cells. Proc West Pharmacol Soc. 1998;41:201–4. [PubMed] [Google Scholar]
- 91.May CA and Mittag T. Neuronal nitric oxide synthase (nNOS) positive retinal amacrine cells are altered in the DBA/2NNia mouse, a murine model for angle-closure glaucoma. J Glaucoma. 2004;13:496–9. [DOI] [PubMed] [Google Scholar]
- 92.Chen C, Xu Y, Zhang J, Zhu J, Hu N and Guan H. Altered Expression of nNOS/NIDD in the Retina of a Glaucoma Model of DBA/2J Mice and the Intervention by nNOS Inhibition. Journal of molecular neuroscience : MN. 2013;51:47–56. [DOI] [PubMed] [Google Scholar]
- 93.Loeliger M and Rees S. Immunocytochemical development of the guinea pig retina. Exp Eye Res. 2005;80:9–21. [DOI] [PubMed] [Google Scholar]
- 94.Fischer AJ and Stell WK. Nitric oxide synthase-containing cells in the retina, pigmented epithelium, choroid, and sclera of the chick eye. J Comp Neurol. 1999;405:1–14. [DOI] [PubMed] [Google Scholar]
- 95.Shareef S, Sawada A and Neufeld AH. Isoforms of nitric oxide synthase in the optic nerves of rat eyes with chronic moderately elevated intraocular pressure. Invest Ophthalmol Vis Sci. 1999;40:2884–91. [PubMed] [Google Scholar]
- 96.Liu B and Neufeld AH. Nitric oxide synthase-2 in human optic nerve head astrocytes induced by elevated pressure in vitro. Arch Ophthalmol. 2001;119:240–5. [PubMed] [Google Scholar]
- 97.Schneemann A, Leusink-Muis A, van den Berg T, Hoyng PF and Kamphuis W. Elevation of nitric oxide production in human trabecular meshwork by increased pressure. Graefes Arch Clin Exp Ophthalmol. 2003;241:321–6. [DOI] [PubMed] [Google Scholar]
- 98.Moshage H, Kok B, Huizenga JR and Jansen PL. Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem. 1995;41:892–6. [PubMed] [Google Scholar]
- 99.Doganay S, Evereklioglu C, Turkoz Y and Er H. Decreased nitric oxide production in primary open-angle glaucoma. Eur J Ophthalmol. 2002;12:44–8. [DOI] [PubMed] [Google Scholar]
- 100.Galassi F, Renieri G, Sodi A, Ucci F, Vannozzi L and Masini E. Nitric oxide proxies and ocular perfusion pressure in primary open angle glaucoma. Br J Ophthalmol. 2004;88:757–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hannappel E, Pankow G, Grassl F, Brand K and Naumann GO. Amino acid pattern in human aqueous humor of patients with senile cataract and primary open-angle glaucoma. Ophthalmic Res. 1985;17:341–3. [DOI] [PubMed] [Google Scholar]
- 102.Javadiyan S, Burdon KP, Whiting MJ, Abhary S, Straga T, Hewitt AW, Mills RA and Craig JE. Elevation of serum asymmetrical and symmetrical dimethylarginine in patients with advanced glaucoma. Investigative ophthalmology & visual science. 2012;53:1923–7. [DOI] [PubMed] [Google Scholar]
- 103.Polak K, Luksch A, Berisha F, Fuchsjaeger-Mayrl G, Dallinger S and Schmetterer L. Altered nitric oxide system in patients with open-angle glaucoma. Arch Ophthalmol. 2007;125:494–8. [DOI] [PubMed] [Google Scholar]
- 104.Liu Y and Allingham RR. Major review: Molecular genetics of primary open-angle glaucoma. Exp Eye Res. 2017;160:62–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Koolwijk LM, Ramdas WD, Ikram MK, Jansonius NM, Pasutto F, Hysi PG, Macgregor S, Janssen SF, Hewitt AW, Viswanathan AC, ten Brink JB, Hosseini SM, Amin N, Despriet DD, Willemse-Assink JJ, Kramer R, Rivadeneira F, Struchalin M, Aulchenko YS, Weisschuh N, Zenkel M, Mardin CY, Gramer E, Welge-Lussen U, Montgomery GW, Carbonaro F, Young TL, Bellenguez C, McGuffin P, Foster PJ, Topouzis F, Mitchell P, Wang JJ, Wong TY, Czudowska MA, Hofman A, Uitterlinden AG, Wolfs RC, de Jong PT, Oostra BA, Paterson AD, Mackey DA, Bergen AA, Reis A, Hammond CJ, Vingerling JR, Lemij HG, Klaver CC and van Duijn CM. Common genetic determinants of intraocular pressure and primary open-angle glaucoma. PLoS genetics. 2012;8:e1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ozel AB, Moroi SE, Reed DM, Nika M, Schmidt CM, Akbari S, Scott K, Rozsa F, Pawar H, Musch DC, Lichter PR, Gaasterland D, Branham K, Gilbert J, Garnai SJ, Chen W, Othman M, Heckenlively J, Swaroop A, Abecasis G, Friedman DS, Zack D, Ashley-Koch A, Ulmer M, Kang JH, Liu Y, Yaspan BL, Haines J, Allingham RR, Hauser MA, Pasquale L, Wiggs J, Richards JE and Li JZ. Genome-wide association study and meta-analysis of intraocular pressure. Human genetics. 2014;133:41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thorleifsson G, Walters GB, Hewitt AW, Masson G, Helgason A, DeWan A, Sigurdsson A, Jonasdottir A, Gudjonsson SA, Magnusson KP, Stefansson H, Lam DS, Tam PO, Gudmundsdottir GJ, Southgate L, Burdon KP, Gottfredsdottir MS, Aldred MA, Mitchell P, St Clair D, Collier DA, Tang N, Sveinsson O, Macgregor S, Martin NG, Cree AJ, Gibson J, Macleod A, Jacob A, Ennis S, Young TL, Chan JC, Karwatowski WS, Hammond CJ, Thordarson K, Zhang M, Wadelius C, Lotery AJ, Trembath RC, Pang CP, Hoh J, Craig JE, Kong A, Mackey DA, Jonasson F, Thorsteinsdottir U and Stefansson K. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nature genetics. 2010;42:906–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wiggs JL, Kang JH, Yaspan BL, Mirel DB, Laurie C, Crenshaw A, Brodeur W, Gogarten S, Olson LM, Abdrabou W, DelBono E, Loomis S, Haines JL and Pasquale LR. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma in Caucasians from the USA. Human molecular genetics. 2011;20:4707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nunes HF, Ananina G, Costa VP, Zanchin NIT, de Vasconcellos JPC and de Melo MB. Investigation of CAV1/CAV2 rs4236601 and CDKN2B-AS1 rs2157719 in primary open-angle glaucoma patients from Brazil. Ophthalmic Genet. 2018;39:194–199. [DOI] [PubMed] [Google Scholar]
- 110.Rong SS, Chen LJ, Leung CK, Matsushita K, Jia L, Miki A, Chiang SW, Tam PO, Hashida N, Young AL, Tsujikawa M, Zhang M, Wang N, Nishida K and Pang CP. Ethnic specific association of the CAV1/CAV2 locus with primary open-angle glaucoma. Sci Rep. 2016;6:27837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mineo C and Shaul PW. Regulation of eNOS in caveolae. Advances in experimental medicine and biology. 2012;729:51–62. [DOI] [PubMed] [Google Scholar]
- 112.Ghilardi G, Biondi ML, DeMonti M, Bernini M, Turri O, Massaro F, Guagnellini E and Scorza R. Independent risk factor for moderate to severe internal carotid artery stenosis: T786C mutation of the endothelial nitric oxide synthase gene. Clin Chem. 2002;48:989–93. [PubMed] [Google Scholar]
- 113.Magalhaes da Silva T, Rocha AV, Lacchini R, Marques CR, Silva ES, Tanus-Santos JE and Rios-Santos F. Association of polymorphisms of endothelial nitric oxide synthase (eNOS) gene with the risk of primary open angle glaucoma in a Brazilian population. Gene. 2012;502:142–6. [DOI] [PubMed] [Google Scholar]
- 114.Tunny TJ, Richardson KA and Clark CV. Association study of the 5' flanking regions of endothelial-nitric oxide synthase and endothelin-1 genes in familial primary open-angle glaucoma. Clin Exp Pharmacol Physiol. 1998;25:26–9. [DOI] [PubMed] [Google Scholar]
- 115.Pasquale LR. Vascular and autonomic dysregulation in primary open-angle glaucoma. Curr Opin Ophthalmol. 2016;27:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Park SC, De Moraes CG, Teng CC, Tello C, Liebmann JM and Ritch R. Initial parafoveal versus peripheral scotomas in glaucoma: risk factors and visual field characteristics. Ophthalmology. 2011;118:1782–9. [DOI] [PubMed] [Google Scholar]
- 117.Buys ES, Sips P, Vermeersch P, Raher MJ, Rogge E, Ichinose F, Dewerchin M, Bloch KD, Janssens S and Brouckaert P. Gender-specific hypertension and responsiveness to nitric oxide in sGC{alpha}1 knockout mice. Cardiovasc Res. 2008;79:179–86. [DOI] [PubMed] [Google Scholar]
- 118.Nimmegeers S, Sips P, Buys E, Brouckaert P and Van de Voorde J. Functional role of the soluble guanylyl cyclase alpha(1) subunit in vascular smooth muscle relaxation. Cardiovasc Res. 2007;76:149–59. [DOI] [PubMed] [Google Scholar]
- 119.Verkman AS. Role of aquaporin water channels in eye function. Exp Eye Res. 2003;76:137–43. [DOI] [PubMed] [Google Scholar]
- 120.Nathanson JA. Direct application of a guanylate cyclase activator lowers intraocular pressure. European journal of pharmacology. 1988;147:155–6. [DOI] [PubMed] [Google Scholar]
- 121.Nathanson JA. Nitrovasodilators as a new class of ocular hypotensive agents. J Pharmacol Exp Ther. 1992;260:956–65. [PubMed] [Google Scholar]
- 122.Schuman JS, Erickson K and Nathanson JA. Nitrovasodilator effects on intraocular pressure and outflow facility in monkeys. Exp Eye Res. 1994;58:99–105. [DOI] [PubMed] [Google Scholar]
- 123.Behar-Cohen FF, Goureau O, D'Hermies F and Courtois Y. Decreased intraocular pressure induced by nitric oxide donors is correlated to nitrite production in the rabbit eye. Investigative ophthalmology & visual science. 1996;37:1711–5. [PubMed] [Google Scholar]
- 124.Krauss AH, Impagnatiello F, Toris CB, Gale DC, Prasanna G, Borghi V, Chiroli V, Chong WK, Carreiro ST and Ongini E. Ocular hypotensive activity of BOL-303259-X, a nitric oxide donating prostaglandin F2alpha agonist, in preclinical models. Experimental eye research. 2011;93:250–5. [DOI] [PubMed] [Google Scholar]
- 125.Dismuke WM, Mbadugha CC and Ellis DZ. NO-induced regulation of human trabecular meshwork cell volume and aqueous humor outflow facility involve the BKCa ion channel. Am J Physiol Cell Physiol. 2008;294:C1378–86. [DOI] [PubMed] [Google Scholar]
- 126.Dismuke WM, Sharif NA and Ellis DZ. Human trabecular meshwork cell volume decrease by NO-independent soluble guanylate cyclase activators YC-1 and BAY-58–2667 involves the BKCa ion channel. Investigative ophthalmology & visual science. 2009;50:3353–9. [DOI] [PubMed] [Google Scholar]
- 127.Heyne GW, Kiland JA, Kaufman PL and Gabelt BT. Effect of nitric oxide on anterior segment physiology in monkeys. Invest Ophthalmol Vis Sci. 2013;54:5103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.O'Donnell ME, Brandt JD and Curry FR. Na-K-Cl cotransport regulates intracellular volume and monolayer permeability of trabecular meshwork cells. Am J Physiol. 1995;268:C1067–74. [DOI] [PubMed] [Google Scholar]
- 129.Kotikoski H, Alajuuma P, Moilanen E, Salmenpera P, Oksala O, Laippala P and Vapaatalo H. Comparison of nitric oxide donors in lowering intraocular pressure in rabbits: role of cyclic GMP. J Ocul Pharmacol Ther. 2002;18:11–23. [DOI] [PubMed] [Google Scholar]
- 130.Kotikoski H, Vapaatalo H and Oksala O. Nitric oxide and cyclic GMP enhance aqueous humor outflow facility in rabbits. Curr Eye Res. 2003;26:119–23. [DOI] [PubMed] [Google Scholar]
- 131.Kee C, Kaufman PL and Gabelt BT. Effect of 8-Br cGMP on aqueous humor dynamics in monkeys. Investigative ophthalmology & visual science. 1994;35:2769–73. [PubMed] [Google Scholar]
- 132.Zimmerman AL, Yamanaka G, Eckstein F, Baylor DA and Stryer L. Interaction of hydrolysis-resistant analogs of cyclic GMP with the phosphodiesterase and light-sensitive channel of retinal rod outer segments. Proc Natl Acad Sci U S A. 1985;82:8813–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ghosh S, Freitag AC, Martin-Vasallo P and Coca-Prados M. Cellular distribution and differential gene expression of the three alpha subunit isoforms of the Na,K-ATPase in the ocular ciliary epithelium. J Biol Chem. 1990;265:2935–40. [PubMed] [Google Scholar]
- 134.Shahidullah M, Wilson WS, Yap M and To CH. Effects of ion transport and channel-blocking drugs on aqueous humor formation in isolated bovine eye. Invest Ophthalmol Vis Sci. 2003;44:1185–91. [DOI] [PubMed] [Google Scholar]
- 135.Shahidullah M, Yap M and To CH. Cyclic GMP, sodium nitroprusside and sodium azide reduce aqueous humour formation in the isolated arterially perfused pig eye. Br J Pharmacol. 2005;145:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shahidullah M and Delamere NA. NO donors inhibit Na,K-ATPase activity by a protein kinase G-dependent mechanism in the nonpigmented ciliary epithelium of the porcine eye. Br J Pharmacol. 2006;148:871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Millar JC, Shahidullah M and Wilson WS. Intraocular pressure and vascular effects of sodium azide in bovine perfused eye. J Ocul Pharmacol Ther. 2001;17:225–34. [DOI] [PubMed] [Google Scholar]
- 138.Shahidullah M, Mandal A, Wei G and Delamere NA. Nitric oxide regulation of Na, K-ATPase activity in ocular ciliary epithelium involves Src family kinase. J Cell Physiol. 2014;229:343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Thoonen R, Sips PY, Bloch KD and Buys ES. Pathophysiology of hypertension in the absence of nitric oxide/cyclic GMP signaling. Curr Hypertens Rep. 2013;15:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schneemann A, Dijkstra BG, van den Berg TJ, Kamphuis W and Hoyng PF. Nitric oxide/guanylate cyclase pathways and flow in anterior segment perfusion. Graefes Arch Clin Exp Ophthalmol. 2002;240:936–41. [DOI] [PubMed] [Google Scholar]
- 141.Muenster S, Lieb WS, Fabry G, Allen KN, Kamat SS, Guy AH, Dordea AC, Teixeira L, Tainsh RE, Yu B, Zhu W, Ashpole NE, Malhotra R, Brouckaert P, Bloch DB, Scherrer-Crosbie M, Stamer WD, Kuehn MH, Pasquale LR and Buys ES. The Ability of Nitric Oxide to Lower Intraocular Pressure Is Dependent on Guanylyl Cyclase. Invest Ophthalmol Vis Sci. 2017;58:4826–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ge P, Navarro ID, Kessler MM, Bernier SG, Perl NR, Sarno R, Masferrer J, Hannig G and Stamer WD. The Soluble Guanylate Cyclase Stimulator IWP-953 Increases Conventional Outflow Facility in Mouse Eyes. Invest Ophthalmol Vis Sci. 2016;57:1317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kang JH, Willett WC, Rosner BA, Buys E, Wiggs JL and Pasquale LR. Association of Dietary Nitrate Intake With Primary Open-Angle Glaucoma: A Prospective Analysis From the Nurses' Health Study and Health Professionals Follow-up Study. JAMA Ophthalmol. 2016;134:294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wiederholt M, Sturm A and Lepple-Wienhues A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol Vis Sci. 1994;35:2515–20. [PubMed] [Google Scholar]
- 145.Ellis DZ, Sharif NA and Dismuke WM. Endogenous regulation of human Schlemm's canal cell volume by nitric oxide signaling. Invest Ophthalmol Vis Sci. 2010;51:5817–24. [DOI] [PubMed] [Google Scholar]
- 146.Stumpff F, Strauss O, Boxberger M and Wiederholt M. Characterization of maxi-K-channels in bovine trabecular meshwork and their activation by cyclic guanosine monophosphate. Invest Ophthalmol Vis Sci. 1997;38:1883–92. [PubMed] [Google Scholar]
- 147.Stumpff F, Que Y, Boxberger M, Strauss O and Wiederholt M. Stimulation of maxi-K channels in trabecular meshwork by tyrosine kinase inhibitors. Invest Ophthalmol Vis Sci. 1999;40:1404–17. [PubMed] [Google Scholar]
- 148.Araie M and Mayama C. Use of calcium channel blockers for glaucoma. Prog Retin Eye Res. 2011;30:54–71. [DOI] [PubMed] [Google Scholar]
- 149.Twort CH and van Breemen C. Cyclic guanosine monophosphate-enhanced sequestration of Ca2+ by sarcoplasmic reticulum in vascular smooth muscle. Circ Res. 1988;62:961–4. [DOI] [PubMed] [Google Scholar]
- 150.Abelson MB, Gilbert CM and Smith LM. Sustained reduction of intraocular pressure in humans with the calcium channel blocker verapamil. Am J Ophthalmol. 1988;105:155–9. [DOI] [PubMed] [Google Scholar]
- 151.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P and Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275:21722–9. [DOI] [PubMed] [Google Scholar]
- 152.Hoy SM. Netarsudil Ophthalmic Solution 0.02%: First Global Approval. Drugs. 2018;78:389–396. [DOI] [PubMed] [Google Scholar]
- 153.Somlyo AP and Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–58. [DOI] [PubMed] [Google Scholar]
- 154.Dismuke WM, Liang J, Overby DR and Stamer WD. Concentration-related effects of nitric oxide and endothelin-1 on human trabecular meshwork cell contractility. Exp Eye Res. 2014;120:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cavet ME, Vollmer TR, Harrington KL, VanDerMeid K and Richardson ME. Regulation of Endothelin-1-Induced Trabecular Meshwork Cell Contractility by Latanoprostene Bunod. Invest Ophthalmol Vis Sci. 2015;56:4108–16. [DOI] [PubMed] [Google Scholar]
- 156.Pattabiraman PP, Maddala R and Rao PV. Regulation of Plasticity and Fibrogenic Activity of Trabecular Meshwork Cells by Rho GTPase Signaling. Journal of Cellular Physiology. 2014;229:927–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kaufman PL. Enhancing trabecular outflow by disrupting the actin cytoskeleton, increasing uveoscleral outflow with prostaglandins, and understanding the pathophysiology of presbyopia interrogating Mother Nature: asking why, asking how, recognizing the signs, following the trail. Experimental eye research. 2008;86:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cavet ME and DeCory HH. The Role of Nitric Oxide in the Intraocular Pressure Lowering Efficacy of Latanoprostene Bunod: Review of Nonclinical Studies. J Ocul Pharmacol Ther. 2018;34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wiederholt M, Thieme H and Stumpff F. The regulation of trabecular meshwork and ciliary muscle contractility. Prog Retin Eye Res. 2000;19:271–95. [DOI] [PubMed] [Google Scholar]
- 160.Su WW, Cheng ST, Ho WJ, Tsay PK, Wu SC and Chang SH. Glaucoma is associated with peripheral vascular endothelial dysfunction. Ophthalmology. 2008;115:1173–1178 e1. [DOI] [PubMed] [Google Scholar]
- 161.Vallance P and Chan N. Endothelial function and nitric oxide: clinical relevance. Heart. 2001;85:342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Forstermann U and Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–14. [DOI] [PubMed] [Google Scholar]
- 163.Fadini GP, Pagano C, Baesso I, Kotsafti O, Doro D, de Kreutzenberg SV, Avogaro A, Agostini C and Dorigo MT. Reduced endothelial progenitor cells and brachial artery flow-mediated dilation as evidence of endothelial dysfunction in ocular hypertension and primary open-angle glaucoma. Acta Ophthalmol. 2010;88:135–41. [DOI] [PubMed] [Google Scholar]
- 164.Cellini M, Strobbe E, Gizzi C, Balducci N, Toschi PG and Campos EC. Endothelin-1 plasma levels and vascular endothelial dysfunction in primary open angle glaucoma. Life Sci. 2012;91:699–702. [DOI] [PubMed] [Google Scholar]
- 165.Hayreh SS. Blood flow in the optic nerve head and factors that may influence it. Prog Retin Eye Res. 2001;20:595–624. [DOI] [PubMed] [Google Scholar]
- 166.Boltz A, Told R, Napora KJ, Palkovits S, Werkmeister RM, Schmidl D, Popa-Cherecheanu A, Garhofer G and Schmetterer L. Optic nerve head blood flow autoregulation during changes in arterial blood pressure in healthy young subjects. PLoS One. 2013;8:e82351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Haefliger IO, Flammer J, Beny JL and Luscher TF. Endothelium-dependent vasoactive modulation in the ophthalmic circulation. Prog Retin Eye Res. 2001;20:209–25. [DOI] [PubMed] [Google Scholar]
- 168.Schmetterer L and Polak K. Role of nitric oxide in the control of ocular blood flow. Prog Retin Eye Res. 2001;20:823–47. [DOI] [PubMed] [Google Scholar]
- 169.Deussen A, Sonntag M and Vogel R. L-arginine-derived nitric oxide: a major determinant of uveal blood flow. Exp Eye Res. 1993;57:129–34. [DOI] [PubMed] [Google Scholar]
- 170.Harino S, Nishimura K, Kitanishi K, Suzuki M and Reinach P. Role of nitric oxide in mediating retinal blood flow regulation in cats. J Ocul Pharmacol Ther. 1999;15:295–303. [DOI] [PubMed] [Google Scholar]
- 171.Koss MC. Effect of nitric oxide synthesis inhibition on post-occlusive choroidal blood flow in rats. J Ocul Pharmacol Ther. 2000;16:55–64. [DOI] [PubMed] [Google Scholar]
- 172.Okuno T, Oku H, Sugiyama T, Yang Y and Ikeda T. Evidence that nitric oxide is involved in autoregulation in optic nerve head of rabbits. Invest Ophthalmol Vis Sci. 2002;43:784–9. [PubMed] [Google Scholar]
- 173.Takayama J, Tomidokoro A, Tamaki Y and Araie M. Time course of changes in optic nerve head circulation after acute reduction in intraocular pressure. Invest Ophthalmol Vis Sci. 2005;46:1409–19. [DOI] [PubMed] [Google Scholar]
- 174.Feke GT and Pasquale LR. Retinal blood flow response to posture change in glaucoma patients compared with healthy subjects. Ophthalmology. 2008;115:246–52. [DOI] [PubMed] [Google Scholar]
- 175.Feke GT, Bex PJ, Taylor CP, Rhee DJ, Turalba AV, Chen TC, Wand M and Pasquale LR. Effect of brimonidine on retinal vascular autoregulation and short-term visual function in normal tension glaucoma. Am J Ophthalmol. 2014;158:105–112 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Flammer J and Orgul S. Optic nerve blood-flow abnormalities in glaucoma. Prog Retin Eye Res. 1998;17:267–89. [DOI] [PubMed] [Google Scholar]
- 177.Jacot JL, O'Neill JT, Scandling DM, West SD and McKenzie JE. Nitric oxide modulation of retinal, choroidal, and anterior uveal blood flow in newborn piglets. J Ocul Pharmacol Ther. 1998;14:473–89. [DOI] [PubMed] [Google Scholar]
- 178.Khoobehi B, Chiroli V, Ronchetti D, Miglietta D, Thompson H, Ongini E and Impagnatiello F. Enhanced oxygen saturation in optic nerve head of non-human primate eyes following the intravitreal injection of NCX 434, an innovative nitric oxide-donating glucocorticoid. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2011;27:115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Harris A, Kagemann L, Ehrlich R, Ehrlich Y, Lopez CR and Purvin VA. The effect of sildenafil on ocular blood flow. Br J Ophthalmol. 2008;92:469–73. [DOI] [PubMed] [Google Scholar]
- 180.Carvajal JA, Germain AM, Huidobro-Toro JP and Weiner CP. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol. 2000;184:409–20. [DOI] [PubMed] [Google Scholar]
- 181.Liu S, Araujo SV, Spaeth GL, Katz LJ and Smith M. Lack of effect of calcium channel blockers on open-angle glaucoma. J Glaucoma. 1996;5:187–90. [PubMed] [Google Scholar]
- 182.Netland PA, Chaturvedi N and Dreyer EB. Calcium channel blockers in the management of low-tension and open-angle glaucoma. Am J Ophthalmol. 1993;115:608–13. [DOI] [PubMed] [Google Scholar]
- 183.Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M and Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. [DOI] [PubMed] [Google Scholar]
- 184.Osborne NN, Wood JP, Chidlow G, Casson R, DeSantis L and Schmidt KG. Effectiveness of levobetaxolol and timolol at blunting retinal ischaemia is related to their calcium and sodium blocking activities: relevance to glaucoma. Brain Res Bull. 2004;62:525–8. [DOI] [PubMed] [Google Scholar]
- 185.Pacher P, Beckman JS and Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Guix FX, Uribesalgo I, Coma M and Munoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol. 2005;76:126–52. [DOI] [PubMed] [Google Scholar]
- 187.Lipton SA, Singel DJ and Stamler JS. Nitric oxide in the central nervous system. Prog Brain Res. 1994;103:359–64. [DOI] [PubMed] [Google Scholar]
- 188.Kim WK, Choi YB, Rayudu PV, Das P, Asaad W, Arnelle DR, Stamler JS and Lipton SA. Attenuation of NMDA receptor activity and neurotoxicity by nitroxyl anion, NO. Neuron. 1999;24:461–9. [DOI] [PubMed] [Google Scholar]
- 189.Sobrevia L, Ooi L, Ryan S and Steinert JR. Nitric Oxide: A Regulator of Cellular Function in Health and Disease. Oxid Med Cell Longev. 2016;2016:9782346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Morgan J, Caprioli J and Koseki Y. Nitric oxide mediates excitotoxic and anoxic damage in rat retinal ganglion cells cocultured with astroglia. Archives of ophthalmology. 1999;117:1524–9. [DOI] [PubMed] [Google Scholar]
- 191.Park SH, Kim JH, Kim YH and Park CK. Expression of neuronal nitric oxide synthase in the retina of a rat model of chronic glaucoma. Vision Res. 2007;47:2732–40. [DOI] [PubMed] [Google Scholar]
- 192.Neufeld AH, Sawada A and Becker B. Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma. Proc Natl Acad Sci U S A. 1999;96:9944–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Neufeld AH, Das S, Vora S, Gachie E, Kawai S, Manning PT and Connor JR. A prodrug of a selective inhibitor of inducible nitric oxide synthase is neuroprotective in the rat model of glaucoma. J Glaucoma. 2002;11:221–5. [DOI] [PubMed] [Google Scholar]
- 194.Husain S, Abdul Y, Singh S, Ahmad A and Husain M. Regulation of nitric oxide production by delta-opioid receptors during glaucomatous injury. PLoS One. 2014;9:e110397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Weill CL and Greene DP. Prevention of natural motoneurone cell death by dibutyryl cyclic GMP. Nature. 1984;308:452–4. [DOI] [PubMed] [Google Scholar]
- 196.Thippeswamy T and Morris R. Cyclic guanosine 3',5'-monophosphate-mediated neuroprotection by nitric oxide in dissociated cultures of rat dorsal root ganglion neurones. Brain Res. 1997;774:116–22. [DOI] [PubMed] [Google Scholar]
- 197.Thippeswamy T and Morris R. Evidence that nitric oxide-induced synthesis of cGMP occurs in a paracrine but not an autocrine fashion and that the site of its release can be regulated: studies in dorsal root ganglia in vivo and in vitro. Nitric Oxide. 2001;5:105–15. [DOI] [PubMed] [Google Scholar]
- 198.Fiscus RR. Involvement of cyclic GMP and protein kinase G in the regulation of apoptosis and survival in neural cells. Neurosignals. 2002;11:175–90. [DOI] [PubMed] [Google Scholar]
- 199.Nakamizo T, Kawamata J, Yoshida K, Kawai Y, Kanki R, Sawada H, Kihara T, Yamashita H, Shibasaki H, Akaike A and Shimohama S. Phosphodiesterase inhibitors are neuroprotective to cultured spinal motor neurons. J Neurosci Res. 2003;71:485–95. [DOI] [PubMed] [Google Scholar]
- 200.Andoh T, Chock PB and Chiueh CC. Preconditioning-mediated neuroprotection: role of nitric oxide, cGMP, and new protein expression. Ann N Y Acad Sci. 2002;962:1–7. [DOI] [PubMed] [Google Scholar]
- 201.Ciani E, Guidi S, Della Valle G, Perini G, Bartesaghi R and Contestabile A. Nitric oxide protects neuroblastoma cells from apoptosis induced by serum deprivation through cAMP-response element-binding protein (CREB) activation. J Biol Chem. 2002;277:49896–902. [DOI] [PubMed] [Google Scholar]
- 202.Ciani E, Guidi S, Bartesaghi R and Contestabile A. Nitric oxide regulates cGMP-dependent cAMP-responsive element binding protein phosphorylation and Bcl-2 expression in cerebellar neurons: implication for a survival role of nitric oxide. J Neurochem. 2002;82:1282–9. [DOI] [PubMed] [Google Scholar]
- 203.Bayir H, Kagan VE, Borisenko GG, Tyurina YY, Janesko KL, Vagni VA, Billiar TR, Williams DL and Kochanek PM. Enhanced oxidative stress in iNOS-deficient mice after traumatic brain injury: support for a neuroprotective role of iNOS. J Cereb Blood Flow Metab. 2005;25:673–84. [DOI] [PubMed] [Google Scholar]
- 204.Karim MZ, Sawada A, Mizuno K, Kawakami H, Ishida K and Yamamoto T. Neuroprotective effect of nipradilol [3,4-dihydro-8-(2-hydroxy-3-isopropylamino)-propoxy-3-nitroxy-2H-1-benzopyran] in a rat model of optic nerve degeneration. J Glaucoma. 2009;18:26–31. [DOI] [PubMed] [Google Scholar]
- 205.Mizuno K, Koide T, Yoshimura M and Araie M. Neuroprotective effect and intraocular penetration of nipradilol, a beta-blocker with nitric oxide donative action. Invest Ophthalmol Vis Sci. 2001;42:688–94. [PubMed] [Google Scholar]
- 206.Watanabe M, Tokita Y and Yata T. Axonal regeneration of cat retinal ganglion cells is promoted by nipradilol, an anti-glaucoma drug. Neuroscience. 2006;140:517–28. [DOI] [PubMed] [Google Scholar]
- 207.Nakazawa T, Tomita H, Yamaguchi K, Sato Y, Shimura M, Kuwahara S and Tamai M. Neuroprotective effect of nipradilol on axotomized rat retinal ganglion cells. Curr Eye Res. 2002;24:114–22. [DOI] [PubMed] [Google Scholar]
- 208.Kashiwagi K, Iizuka Y and Tsukahara S. Neuroprotective effects of nipradilol on purified cultured retinal ganglion cells. J Glaucoma. 2002;11:231–8. [DOI] [PubMed] [Google Scholar]
- 209.Koriyama Y, Kamiya M, Arai K, Sugitani K, Ogai K and Kato S. Nipradilol promotes axon regeneration through S-nitrosylation of PTEN in retinal ganglion cells. Adv Exp Med Biol. 2014;801:751–7. [DOI] [PubMed] [Google Scholar]
- 210.Kohgami S, Ogata T, Morino T, Yamamoto H and Schubert P. Pharmacological shift of the ambiguous nitric oxide action from neurotoxicity to cyclic GMP-mediated protection. Neurol Res. 2010;32:938–44. [DOI] [PubMed] [Google Scholar]
- 211.Ditlevsen DK, Kohler LB, Berezin V and Bock E. Cyclic guanosine monophosphate signalling pathway plays a role in neural cell adhesion molecule-mediated neurite outgrowth and survival. J Neurosci Res. 2007;85:703–11. [DOI] [PubMed] [Google Scholar]
- 212.Thippeswamy T, McKay JS, Morris R, Quinn J, Wong LF and Murphy D. Glial-mediated neuroprotection: evidence for the protective role of the NO-cGMP pathway via neuron-glial communication in the peripheral nervous system. Glia. 2005;49:197–210. [DOI] [PubMed] [Google Scholar]
- 213.Contestabile A and Ciani E. Role of nitric oxide in the regulation of neuronal proliferation, survival and differentiation. Neurochem Int. 2004;45:903–14. [DOI] [PubMed] [Google Scholar]
- 214.Jeong SO, Son Y, Lee JH, Choi SW, Kim SH, Cheong YK, Chung HT and Pae HO. Both nitric oxide and nitrite prevent homocysteine-induced endoplasmic reticulum stress and subsequent apoptosis via cGMP-dependent pathway in neuronal cells. Biochem Biophys Res Commun. 2017;493:164–169. [DOI] [PubMed] [Google Scholar]
- 215.Wizemann AJ and Wizemann V. Organic nitrate therapy in glaucoma. American journal of ophthalmology. 1980;90:106–9. [DOI] [PubMed] [Google Scholar]
- 216.Nathanson JA. Nitric oxide and nitrovasodilators in the eye: implications for ocular physiology and glaucoma. J Glaucoma. 1993;2:206–10. [PubMed] [Google Scholar]
- 217.Kanno M, Araie M, Masuda K, Takase M, Kitazawa Y, Shiose Y, Azuma I, Ogawa N and Ohdo S. Phase III long-term study and comparative clinical study of nipradilol ophthalmic solution in patients with primary open-angle glaucoma and ocular hypertension. Part 2. Arzneimittelforschung. 2006;56:820–5. [PubMed] [Google Scholar]
- 218.Weinreb RN, Scassellati Sforzolini B, Vittitow J and Liebmann J. Latanoprostene Bunod 0.024% versus Timolol Maleate 0.5% in Subjects with Open-Angle Glaucoma or Ocular Hypertension: The APOLLO Study. Ophthalmology. 2016;123:965–73. [DOI] [PubMed] [Google Scholar]
- 219.Medeiros FA, Martin KR, Peace J, Scassellati Sforzolini B, Vittitow JL and Weinreb RN. Comparison of Latanoprostene Bunod 0.024% and Timolol Maleate 0.5% in Open-Angle Glaucoma or Ocular Hypertension: The LUNAR Study. Am J Ophthalmol. 2016;168:250–259. [DOI] [PubMed] [Google Scholar]
- 220.Kawase K, Vittitow JL, Weinreb RN, Araie M and Group JS. Long-term Safety and Efficacy of Latanoprostene Bunod 0.024% in Japanese Subjects with Open-Angle Glaucoma or Ocular Hypertension: The JUPITER Study. Adv Ther. 2016;33:1612–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Araie M, Sforzolini BS, Vittitow J and Weinreb RN. Evaluation of the Effect of Latanoprostene Bunod Ophthalmic Solution, 0.024% in Lowering Intraocular Pressure over 24 h in Healthy Japanese Subjects. Adv Ther. 2015;32:1128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu JH, Slight JR, Vittitow JL, Scassellati Sforzolini B and Weinreb RN. Efficacy of Latanoprostene Bunod 0.024% Compared With Timolol 0.5% in Lowering Intraocular Pressure Over 24 Hours. Am J Ophthalmol. 2016;169:249–57. [DOI] [PubMed] [Google Scholar]
- 223.Kanno M, Araie M, Tomita K and Sawanobori K. Effects of topical nipradilol, a beta-blocking agent with alpha-blocking and nitroglycerin-like activities, on aqueous humor dynamics and fundus circulation. Invest Ophthalmol Vis Sci. 1998;39:736–43. [PubMed] [Google Scholar]
- 224.Garcia GA, Ngai P, Mosaed S and Lin KY. Critical evaluation of latanoprostene bunod in the treatment of glaucoma. Clin Ophthalmol. 2016;10:2035–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Araie M, Shirato S, Yamazaki Y, Kitazawa Y, Ohashi Y and Nipradilol-Timolol Study G. Clinical efficacy of topical nipradilol and timolol on visual field performance in normal-tension glaucoma: a multicenter, randomized, double-masked comparative study. Jpn J Ophthalmol. 2008;52:255–64. [DOI] [PubMed] [Google Scholar]
- 226.Araie M, Shirato S, Yamazaki Y, Kitazawa Y, Ohashi Y and Nipradilol-Timolol Study G. Visual field loss in patients with normal-tension glaucoma under topical nipradilol or timolol: subgroup and subfield analyses of the nipradilol-timolol study. Jpn J Ophthalmol. 2010;54:278–85. [DOI] [PubMed] [Google Scholar]
- 227.Fukukita M, Ido M, Osawa S, Sasoh M, Furuta M, Ito K, Sugimoto M and Uji Y. Retrobulbar hemodynamic effects of nipradilol in normal and normal-tension glaucoma eyes. J Ophthalmol. 2011;2011:652904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Impagnatiello F, Toris CB, Batugo M, Prasanna G, Borghi V, Bastia E, Ongini E and Krauss AH. Intraocular Pressure-Lowering Activity of NCX 470, a Novel Nitric Oxide-Donating Bimatoprost in Preclinical Models. Invest Ophthalmol Vis Sci. 2015;56:6558–64. [DOI] [PubMed] [Google Scholar]
- 229.Borghi V, Bastia E, Guzzetta M, Chiroli V, Toris CB, Batugo MR, Carreiro ST, Chong WK, Gale DC, Kucera DJ, Jia L, Prasanna G, Ongini E, Krauss AH and Impagnatiello F. A novel nitric oxide releasing prostaglandin analog, NCX 125, reduces intraocular pressure in rabbit, dog, and primate models of glaucoma. J Ocul Pharmacol Ther. 2010;26:125–32. [DOI] [PubMed] [Google Scholar]
- 230.Huang Q, Rui EY, Cobbs M, Dinh DM, Gukasyan HJ, Lafontaine JA, Mehta S, Patterson BD, Rewolinski DA, Richardson PF and Edwards MP. Design, Synthesis, and Evaluation of NO-Donor Containing Carbonic Anhydrase Inhibitors To Lower Intraocular Pressure. Journal of medicinal chemistry. 2015;58:2821–33. [DOI] [PubMed] [Google Scholar]
- 231.Charriaut-Marlangue C, Nguyen T, Bonnin P, Duy AP, Leger PL, Csaba Z, Pansiot J, Bourgeois T, Renolleau S and Baud O. Sildenafil mediates blood-flow redistribution and neuroprotection after neonatal hypoxia-ischemia. Stroke. 2014;45:850–6. [DOI] [PubMed] [Google Scholar]
- 232.Serarslan Y, Yonden Z, Ozgiray E, Oktar S, Guven EO, Sogut S, Yilmaz N and Yurtseven T. Protective effects of tadalafil on experimental spinal cord injury in rats. J Clin Neurosci. 2010;17:349–52. [DOI] [PubMed] [Google Scholar]
- 233.Ozdegirmenci O, Kucukozkan T, Akdag E, Topal T, Haberal A, Kayir H, Oter S, Akyol M and Uzbay T. Effects of sildenafil and tadalafil on ischemia/reperfusion injury in fetal rat brain. J Matern Fetal Neonatal Med. 2011;24:317–23. [DOI] [PubMed] [Google Scholar]
- 234.Olivares-Gonzalez L, Martinez-Fernandez de la Camara C, Hervas D, Marin MP, Lahoz A, Millan JM and Rodrigo R. cGMP-Phosphodiesterase Inhibition Prevents Hypoxia-Induced Cell Death Activation in Porcine Retinal Explants. PLoS One. 2016;11:e0166717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Becker B. Topical 8-bromo-cyclic GMP lowers intraocular pressure in rabbits. Invest Ophthalmol Vis Sci. 1990;31:1647–9. [PubMed] [Google Scholar]
- 236.Yajima T, Yajima Y, Koppiker N, Grunwald JE and Laties AM. No clinically important effects on intraocular pressure after short-term administration of sildenafil citrate (Viagra). Am J Ophthalmol. 2000;129:675–6. [DOI] [PubMed] [Google Scholar]
- 237.Ermis SS, Inan UU, Samli M and Ozturk F. Acute effects of sildenafil on Humphrey visual field and intraocular pressure. Int Ophthalmol. 2004;25:69–72. [DOI] [PubMed] [Google Scholar]
- 238.Grunwald JE, Siu KK, Jacob SS and Dupont J. Effect of sildenafil citrate (Viagra) on the ocular circulation. Am J Ophthalmol. 2001;131:751–5. [DOI] [PubMed] [Google Scholar]
- 239.Oguz HVA, Ozkul Y, Gurkan T, Ciftci H. No effects of long-term sildenafil treatment on ocular functions. Ann Ophthalmol. 2005;37:85–90. [Google Scholar]
- 240.Stockman A, Sharpe LT, Tufail A, Kell PD, Ripamonti C and Jeffery G. The effect of sildenafil citrate (Viagra) on visual sensitivity. J Vis. 2007;7:4. [DOI] [PubMed] [Google Scholar]
- 241.Center for Drug Evaluation and Research: Study 148–232. A randomised, double-blind, placebo-controlled, crossover pilot study to investigate the effects of a single oral tablet dose of sildenafil (200 mg) on visual function (electroretinogram, photostress, visual field and colour discrimination tests) in healthy male volunteers and patients with diabetic retinopathy Viagra (Sildenafil): “Joint Clinical Review” for NDA-20–895. Washington, DC: Center for Drug Evaluation and Research, Food and Drug Administration; 1998:177–178. [Google Scholar]
- 242.Carter JE. Anterior ischemic optic neuropathy and stroke with use of PDE-5 inhibitors for erectile dysfunction: cause or coincidence? J Neurol Sci. 2007;262:89–97. [DOI] [PubMed] [Google Scholar]
- 243.Cunningham AV and Smith KH. Anterior ischemic optic neuropathy associated with viagra. J Neuroophthalmol. 2001;21:22–5. [DOI] [PubMed] [Google Scholar]
- 244.Santaella RM and Fraunfelder FW. Ocular adverse effects associated with systemic medications : recognition and management. Drugs. 2007;67:75–93. [DOI] [PubMed] [Google Scholar]
- 245.Kim CM, Kim YS, Sunwoo S, Cho B, Rho M, Yang YJ, Kim CH, Shin HC, Lee SY, Kim DH and Korea Post-Marketing Surveillance Research G. Post-marketing surveillance study of the efficacy and safety of vardenafil among patients with erectile dysfunction in primary care. Int J Impot Res. 2007;19:393–7. [DOI] [PubMed] [Google Scholar]
- 246.Hellstrom WJ, Gittelman M, Karlin G, Segerson T, Thibonnier M, Taylor T and Padma-Nathan H. Vardenafil for treatment of men with erectile dysfunction: efficacy and safety in a randomized, double-blind, placebo-controlled trial. J Androl. 2002;23:763–71. [PubMed] [Google Scholar]
- 247.Brock GB, McMahon CG, Chen KK, Costigan T, Shen W, Watkins V, Anglin G and Whitaker S. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: results of integrated analyses. J Urol. 2002;168:1332–6. [DOI] [PubMed] [Google Scholar]
- 248.Lagnado L The Wellcome Prize Lecture. Visual signals in the retina: from photons to synapses. Exp Physiol. 2000;85:1–16. [PubMed] [Google Scholar]
- 249.Viagra R. Full prescribing information sildenafil citrate. New York: Pfizer Inc.; 2008. [Google Scholar]
- 250.LEVITRAR. Full prescribing information vardenafil. West Haven, CT: Bayer Pharmaceuticals Corporation; 2008. [Google Scholar]
- 251.CIALISR. Full prescribing information tadalafil. Indianapolis, IN: Lilly ICOS, LLC; 2008. [Google Scholar]
- 252.Azzouni F and Abu samra K. Are phosphodiesterase type 5 inhibitors associated with vision-threatening adverse events? A critical analysis and review of the literature. J Sex Med. 2011;8:2894–903. [DOI] [PubMed] [Google Scholar]
- 253.Makowski CT, Rissmiller RW and Bullington WM. Riociguat: a novel new drug for treatment of pulmonary hypertension. Pharmacotherapy. 2015;35:502–19. [DOI] [PubMed] [Google Scholar]
- 254.Humbert M, Coghlan JG, Ghofrani HA, Grimminger F, He JG, Riemekasten G, Vizza CD, Boeckenhoff A, Meier C, de Oliveira Pena J and Denton CP. Riociguat for the treatment of pulmonary arterial hypertension associated with connective tissue disease: results from PATENT-1 and PATENT-2. Ann Rheum Dis. 2017;76:422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Prasanna G, Ferrara L, Adams C, Ehara T, Li B, Yang L, Xiang C, Ng CTH, Kim S, Towler C, Topley T, McAllister C, Ghosh M, Newton R, Stacy R, Rice DS and Mogi M. A Novel Selective Soluble Guanylate Cyclase Activator, MGV354, Lowers Intraocular Pressure in Preclinical Models, Following Topical Ocular Dosing. Invest Ophthalmol Vis Sci. 2018;59:1704–1716. [DOI] [PubMed] [Google Scholar]
- 256.Munzel T, Daiber A and Mulsch A. Explaining the phenomenon of nitrate tolerance. Circ Res. 2005;97:618–28. [DOI] [PubMed] [Google Scholar]