Abstract
Emerging research suggests that adiponectin, a cytokine produced by adipose tissue, may be implicated in ASD. In this prospective birth cohort study (n=847), we assessed the association between cord, early childhood plasma adiponectin and the risk of developing ASD. ASD was defined based on ICD codes of physician diagnosis. Cord adiponectin levels were inversely associated with ASD risk (aOR: 0.50; 95% CI: 0.33, 0.77), independent of preterm birth, early childhood adiponectin and other known ASD risk factors. Early childhood adiponectin, assessed prior to ASD diagnosis, was associated with lower risk of ASD, which attenuated after adjusting for cord adiponectin, indicating the relative importance of cord adiponectin in ASD risk. Further research is warranted to confirm our findings and elucidate biological mechanisms.
Keywords: Autism, adiponectin, preterm birth, cytokines
Autism Spectrum Disorder (ASD) is a neurodevelopmental condition characterized by impairments in social interaction and communication, and by the presence of restrictive, repetitive behaviors and interests (American Psychiatric Association 2013). Recent epidemiological studies suggest that one in 59 children are diagnosed with ASD (Baio et al. 2018). While genetic, environmental, and prenatal/perinatal factors have been implicated in ASD (Fiorentino et al. 2016; Wozniak et al. 2017), the etiology of the disorder remains largely unknown and no convincing biomarkers of ASD have been identified (Molloy et al. 2006; Lyall et al. 2017; Akintunde et al. 2015). Disturbances in immunoinflammatory factors and adipocytokines have been observed in subjects with ASD (Molloy et al. 2006; Ghaffari et al. 2016). Aberrant immune activity during vulnerable periods of neurodevelopment could potentially play a role in neural dysfunction associated with ASD (Ashwood et al. 2006). Emerging evidence suggests that immune dysfunction and neuroinflammation may be a common mechanism across the individual ASD risk factors (Erdei and Dammann 2014).
Beyond its energy storing capabilities, adipose tissue has emerged as an endocrine organ that orchestrates inflammatory response through the production of adipocytokines (Hansen-Pupp et al. 2015; Mazaki-Tovi et al. 2009; Villarreal-Molina and Antuna-Puente 2012). The family of adipocytokines includes a variety of highly active molecules including interleukin (IL)-6, tumor necrosis factor (TNF)-α, leptin, adiponectin, resistin and vistafin, all of which are known to play a critical role in the regulation of inflammatory responses (Hansen-Pupp et al. 2015; Mazaki-Tovi et al. 2009). Adiponectin is the most abundant adipocytokine, mainly produced by brown and white adipose tissues (Hansen-Pupp et al. 2015).
The role of adiponectin in ASD deserves attention for at least two important reasons. First, as an anti-inflammatory cytokine, adiponectin functions as a mediator of inflammatory response and has a protective role against metabolic disturbances (Mazaki-Tovi et al. 2009; Lenz and Diamond 2012; Fujita-Shimizu et al. 2010). Emerging evidence suggests that adiponectin may be implicated in neurological conditions (Lisik et al. 2016; Mansur et al. 2016; Hu et al. 2015) (Burd et al. 2012). To our knowledge, three studies have assessed adiponectin levels in children with ASD (Fujita-Shimizu et al. 2010; Blardi et al. 2010; Rodrigues et al. 2014), of which only one found altered adiponectin levels in subjects with ASD (Fujita-Shimizu et al. 2010). The second study observed no significant difference in adiponectin levels between the ASD subjects and controls (Blardi et al. 2010). Similarly, Rodrigues et al., noted unaltered adiponectin levels; however, they reported a negative correlation between adiponectin levels and severity of symptoms (Rodrigues et al. 2014). In addition to these inconsistent findings, limited sample size, lack of adjustment of numerous confounders, and cross-sectional study designs have precluded elucidation of the role of adiponectin in the development of ASD.
Second, preterm birth has become an established risk factor for ASD (Darcy-Mahoney et al. 2016) (Schendel and Bhasin 2008; Kuzniewicz et al. 2014; Movsas and Paneth 2012; Fezer et al. 2017; Angelidou et al. 2012). Lower serum adiponectin levels are noted in mothers who had preterm labor and delivery (Mazaki-Tovi et al. 2009) as well as in children born preterm when compared to their term counterparts (Saito et al. 2011; Oberthuer et al. 2012; Kajantie et al. 2004; Lindsay et al. 2003; Nakano et al. 2013; Terrazzan et al. 2014; Yoshida et al. 2009; Siahanidou et al. 2007). While the role of adiponectin in prematurity is well characterized (Saito et al. 2011; Oberthuer et al. 2012; Kajantie et al. 2004; Lindsay et al. 2003; Nakano et al. 2013; Terrazzan et al. 2014; Yoshida et al. 2009; Siahanidou et al. 2007), existing research has not jointly looked at adiponectin and gestational age at birth in the context of ASD risk.
To fill these gaps, we conducted a study to longitudinally assess the association between plasma adiponectin, measured in cord blood at birth and early childhood venous blood, and subsequent ASD risk. In addition, we set out to understand whether preterm birth and cord adiponectin had joint effects on ASD risk. We sought to clarify these questions by analyzing longitudinal data from the Boston Birth Cohort (BBC), a predominantly urban low-income minority birth cohort, enriched with preterm births.
Methods
Participants and data collection procedure
This study included 847 mother-child pairs from the BBC, of which 792 children were considered neurotypical and 55 received an ASD diagnosis. Online Resource Figure 1 outlines the enrollment, postnatal follow-up, inclusions and exclusions. Between 1998 and 2009, mothers who delivered a singleton live birth at the Boston Medical Center (BMC), were invited to participate in this study. After obtaining informed consent, mothers were interviewed 24–72 hours after delivery using a standardized postpartum questionnaire. Exclusion criteria for the initial enrollment were multiple-gestation pregnancies, chromosomal abnormalities, major birth defects and preterm deliveries as a result of maternal trauma. Children that continued to receive pediatric care at the BMC were included in this study, and they were followed-up until 2015 (Online Resource Figure 1). The study was approved by the Institutional Review Boards of the Johns Hopkins Bloomberg School of Public Health and the Boston University Medical Center.
Exposure measures
Plasma adiponectin in the children was measured at two time points: (1) Umbilical cord blood sample, collected at delivery, and (2) early childhood venous blood, collected during a pediatric visit (median time of measurement: 19.03 months; Interquartile range: 10.4–49.2 months). Plasma samples were stored in a freezer at −80°C. Adiponectin was measured using an immunoassay (ELISA) and had an inter-assay variation of <5.8% (G. Wang et al. 2016a). The assays were run according to the manufacturer’s recommendation. Children that had adiponectin assessed after ASD diagnosis were excluded from the analysis. Unlikely adiponectin levels, defined as greater than 3SD above the mean, were observed in 15 and 18 subjects for cord and early childhood adiponectin, respectively and were reassigned a value of 3 SD.
Gestational age at birth was characterized based on the first day of the last menstrual period data and early ultrasound data (X. Wang et al. 2002; G. Wang et al. 2014). Children with gestational age ≥ 37 completed weeks of gestation were categorized as full-term and those <37 weeks were grouped into late- (≥ 34 to <37 weeks) and early-preterm (<34 weeks).
Outcome measure
Electronic Medical Records (EMR) data were used to identify children with ASD. Children were classified as an ASD case if they were ever diagnosed with autism (ICD-9 code 299.00), Asperger syndrome (299.80) and/or pervasive developmental disorder not otherwise specified (299.90). Children that were never diagnosed with ASD, ADHD (314.0 – 314.9), intellectual disabilities (317 – 319) or other developmental disabilities (315.0 – 315.9) were classified as ‘neurotypical.’ When children with ASD also had ADHD, intellectual disabilities, or other developmental disabilities, they were still categorized as ASD. Two separate sensitivity analyses were performed using the following criteria: 1) a stringent ASD definition that included only those that were diagnosed with ASD on two separate occasions, and had at least one visit with a specialist such as a developmental behavioral pediatrician, pediatric neurologist or child psychologist; and 2) a stringent definition for neurotypical children which excluded those with competing diagnoses such as ADHD (314.0 – 314.9), intellectual disabilities (317 – 319), other developmental disabilities (315.0 – 315.9), Conduct Disorder (312.0 – 312.9), emotional disturbances of childhood or adolescence including Oppositional Defiant Disorder (313.0 – 313.9) and Congenital Anomalies (740 – 759.9).
Covariates
Based on the existing literature and our earlier work in the BBC, we selected the covariates for adjustment a priori (G. Wang et al. 2016a; G. Wang et al. 2014; Raghavan et al. 2017; Li et al. 2016), including maternal pre-pregnancy BMI, maternal diabetes status, maternal race/ethnicity, maternal age at the time of delivery, smoking during pregnancy (ever smoked 3 months before pregnancy/during pregnancy vs. not smoked during preconception/ pregnancy), parity (not including the index pregnancy), maternal education (high school or less vs. some college or more), child’s sex (female vs. male), year of baby’s birth (1998–2006 vs. 2007–2013), mode of feeding (formula only, both formula and breast feeding and breastfeeding only), age at which early childhood blood was drawn and follow up time for each participant. Maternal pre-pregnancy weight and height were collected using a standardized questionnaire 2–3 days after delivery, which was used to calculate maternal BMI, defined as weight in kilograms divided by height in meters squared. Mother’s diabetes status was categorized into the following groups: 1) normal (without a pregestational or gestational diabetes diagnosis); 2) gestational diabetes (ever diagnosed with diabetes mellitus complicating pregnancy); and 3) pregestational diabetes (ever diagnosed with diabetes). Race/ethnicity was categorized into black, white, Hispanic and Other. Plasma insulin and leptin, in cord blood and in venous blood during childhood, were measured using a sandwich immunoassay and the interassay coefficient of variation was 4.0% and 4.5%, respectively (G. Wang et al. 2016b). Adiponectin/leptin ratio was calculated and was log-transformed. Based on the WHO reference values, weight-for-age z-score was used to calculate first year weight gain and was categorized into slow (weight gain z-score <−0.67), on track (−0.67 to 0.67), rapid (>0.67 to 1.28), and extremely rapid (>1.28) (G. Wang et al. 2016b).
Statistical Analyses
Distributional assumptions such as normality of cord and plasma adiponectin levels were assessed using the Shapiro-Wilk test. Since adiponectin distributions were skewed, they were log-transformed for both cord and early childhood adiponectin levels (Online Resource Figure 2). Preliminary data analysis was conducted to compare neurotypical and ASD children using a chi-squared test for categorical variables and ANOVA for continuous variables. Logistic regression was used to assess the relationship between log-transformed cord and early childhood plasma adiponectin levels (independent variables) and ASD (dependent variable), with adjustment for potential confounders listed above. The results are presented as odds ratio. Baron and Kenny mediation analysis was used to explore the relationships between preterm birth, cord adiponectin and ASD risk (Baron and Kenny 1986). The Baron and Kenny approach assessed the indirect association between the exposure (i.e. preterm birth) and outcome (i.e. ASD status), through the mediating variable (i.e. cord adiponectin), by comparing four regression models. The first model assessed the total association between the primary exposure and primary outcome. The second model assessed the association between the primary exposure and potential mediator. The third model assessed the association between the potential mediator and primary outcome. Finally, the fourth model assessed the association between the primary exposure and primary outcome, after accounting for the potential mediator. All 4 models adjusted for the confounders listed above. All statistical analyses were performed using STATA version 13.0 (StataCorp, College Station, TX). Differences were considered statistically significant if the p value was <0.05.
Results
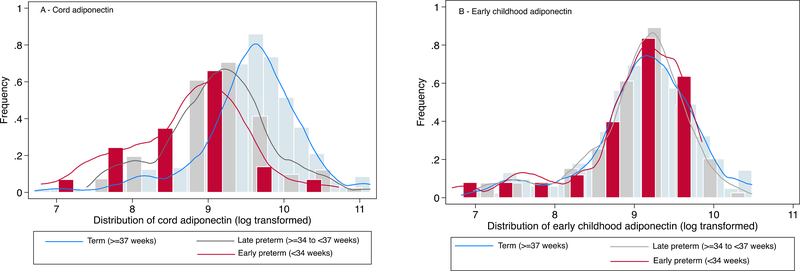
The demographic and clinical characteristics of mothers and children in this study are presented in the Table 1, and have also been documented in earlier studies from this cohort (Li et al. 2016; Raghavan et al. 2017; Brucato et al. 2017; G. Wang et al. 2014; G. Wang et al. 2016a). We found that risk factors such as advanced maternal age, maternal diabetes, male sex and preterm birth were more common among children with ASD. In addition, ASD children were more likely to have lower birth weight and lower cord adiponectin levels (Table 1). Mean and median cord adiponectin levels were 15.54 μg/mL and 13.52 μg/mL (interquartile range: 8.76–19.21 μg/mL), and mean and median early childhood adiponectin levels were 10.61 μg/mL and 9.68 μg/mL (interquartile range: 6.75–13.5 μg/mL), respectively. Mean cord adiponectin levels were significantly different across groups defined by gestational age at birth, with early preterm children (8.29 μg/mL) having the lowest levels, followed by late preterm (11.51 μg/mL) and term infants (16.90 μg/mL) (p value <0.001). Consistent with other studies (Lenz and Diamond 2012; Mantzoros et al. 2009; Nakano et al. 2013; Yeung et al. 2015; Martos-Moreno et al. 2009; Hellgren et al. 2015), we observed that cord adiponectin was associated with the duration of gestation (Online Resource Table 1). Figure 1 shows the distribution of cord adiponectin by preterm status. As observed in panel A, the cord adiponectin distribution is shifted to the left for early preterm (<34 weeks) and late preterm (≥ 34 to <37 weeks) babies, when compared to term infants. However, there were no differences in the distribution of early childhood adiponectin levels based on gestational age at birth (Figure 1, panel B). Cord adiponectin levels were also correlated with birth weight. Children with lower cord adiponectin levels were likely to have rapid or extremely rapid weight gain (Online Resource Table 2), but the association attenuated after adjusting for gestational age at birth (data not shown). Correlation between cord and early childhood adiponectin was weak (r=0.18), although children whose cord adiponectin were in low, medium or high tertiles likely continued to stay in their respective categories at early childhood (Online Resource Figure 3).
Table 1:
Maternal and child characteristics by child’s case status (neurotypical vs. ASD) in the Boston Birth Cohort
| Neurotypical (n=792) | ASD (n=55) | p value | |
|---|---|---|---|
| Characteristics | |||
| Mothers | |||
| Age at birth (yrs), mean (SD) | 28.19 (6.56) | 30.35 (6.16) | 0.02 |
| Parity (%) | 0.89 | ||
| 0 | 333 (42.05) | 21 (38.18) | |
| 1 or more | 458 (57.83) | 34 (61.82) | |
| Missing | 1 (0.16) | 0 (0.00) | |
| Mother’s education (%) | 0.85 | ||
| High School or less | 510 (64.39) | 33 (60.00) | |
| Some college or more | 275 (34.72) | 21 (38.18) | |
| Missing | 7 (0.88) | 1 (1.82) | |
| Maternal BMI (%) | 0.21 | ||
| Underweight (<18.5) + Normal Weight (≥18.5-<25) | 385 (48.61) | 20 (36.36) | |
| Overweight (25–29.9) | 245 (30.93) | 19 (34.55) | |
| Obesity (>=30) | 162 (20.45) | 16 (29.09) | |
| Diabetes (%) | 0.04 | ||
| No | 718 (90.66) | 47 (85.45) | |
| Gestational | 50 (6.31) | 2 (3.64) | |
| Diabetes mellitus | 24 (3.03) | 6 (10.91) | |
| Smoking during & 3 months prior to pregnancy (%) | 0.45 | ||
| No | 677 (85.48) | 42 (76.36) | |
| Yes | 109 (13.76) | 12 (21.82) | |
| Missing | 6 (0.76) | 1 (1.82) | |
| Child | |||
| Gender (%) | <0.001 | ||
| Male | 337 (42.55) | 40 (72.73) | |
| Female | 455 (57.45) | 15 (27.27) | |
| Race-ethnicity (%) | 0.83 | ||
| Black | 453 (57.20) | 34 (61.82) | |
| White | 48 (6.06) | 4 (7.27) | |
| Hispanic | 186 (23.48) | 11 (20.00) | |
| Other | 100 (12.63) | 6 (10.91) | |
| Missing | 5 (0.63) | 0 (0.00) | |
| Gestational age (%) | 0.04 | ||
| Term | 601 (75.88) | 34 (61.82) | |
| Late preterm (≥34 - <37 weeks) | 128 (16.16) | 10 (18.18) | |
| Early preterm (<34 weeks) | 63 (7.95) | 11 (20.00) | |
| Birth weight (g) | 3017.85 (713.20) | 2803.73 (886.24) | 0.03 |
| Year of birth (%) | 0.66 | ||
| 1998–2006 | 429 (54.17) | 30 (54.55) | |
| 2007–2013 | 363 (45.83) | 25 (45.45) | |
| Mean cord blood adiponectin (SD)a (μg/mL) | 15.80 (10.84) | 11.37 (88.27) | 0.01 |
| Mean early childhood adiponectin (SD)b (μg/mL) | 10.67 (62.15) | 10.11 (73.59) | 0.61 |
| First year weight gain pattern (%)c | 0.05 | ||
| On target | 182 (33.96) | 9 (18.75) | |
| Slow | 65 (12.13) | 6 (12.50) | |
| Rapid weight gain | 92 (17.16) | 6 (12.50) | |
| Extremely rapid weight gain | 197 (36.75) | 27 (56.25) | |
| Mode of feeding (%) | 0.86 | ||
| Formula | 183 (23.11) | 11 (20.00) | |
| Both | 549 (69.32) | 37 (67.27) | |
| Breastfeeding | 54 (6.82) | 6 (10.91) | |
| Missing | 6 (0.76) | 1 (1.82) |
n=674 (Neurotypical n=634; ASD n=40)
n=638 (Neurotypical n=602; ASD n=36)
n=584 (Neurotypical n=536; ASD n=48)
Fig 1.
Distribution of plasma adiponectin levels (μg/mL) in cord blood (panel A) and in early childhood venous blood (panel B), stratified by preterm status in the Boston Birth Cohort (Total n for cord adiponectin =674; Total n for early childhood adiponectin= 638)
Adiponectin and ASD risk
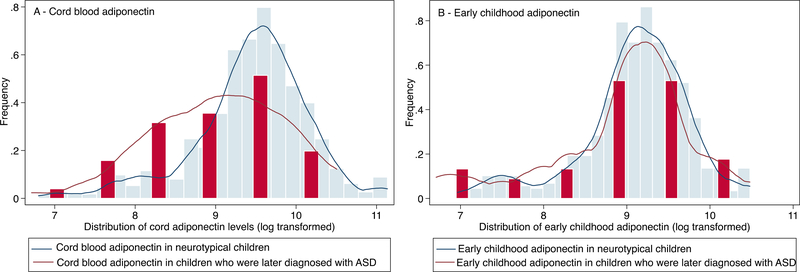
A total of 674 children, including 634 neurotypical and 40 ASD children, were part of this analysis. Figure 2 shows the distribution of cord and early childhood adiponectin levels by ASD status. The mean cord adiponectin level is reduced among children later diagnosed with ASD (Table 1) and this is evident by the distinct lower cord adiponectin distribution observed in Figure 2, panel A. On the other hand, there was no difference in the mean early childhood adiponectin levels between neurotypical and ASD children (Figure 2, panel B).
Fig 2.
Distribution of plasma adiponectin levels (μg/mL) in cord blood (panel A) and in early childhood venous blood (panel B), stratified by neurotypical children vs. those with ASD in the Boston Birth Cohort (Total n for cord adiponectin =674; Total n for early childhood adiponectin= 638)
Table 2 presents the association between cord adiponectin and subsequent ASD risk. Higher cord adiponectin levels were associated with a lower risk of ASD (OR: 0.51; 95% CI: 0.35, 0.75). When cord adiponectin levels were categorized into quartiles, an inverse dose-response relationship was observed. Compared to children with the lowest cord adiponectin levels (quartile 1), the highest cord adiponectin (ORquartile 4: 0.23, 95% CI: 0.08, 0.62) was associated with reduced risk of ASD in unadjusted analyses. This association persisted after adjustment for potential confounders (including child’s sex, race, maternal education, maternal age, parity, smoking status, pre-pregnancy BMI, maternal diabetes status and follow-up time), (aORquartile 4: 0.14, 95% CI: 0.04, 0.46; model 1). Sequentially adjusting for gestational age, early childhood adiponectin (Table 2) and additional covariates such as cord insulin, cord leptin and weight gain during first year of life (Online Resource Table 3) did not alter the association between cord adiponectin and ASD. This finding was robust irrespective of how adiponectin was categorized (tertiles, quintiles, extreme levels) (Online Resource Table 4).
Table 2:
Association between cord plasma adiponectin levels and ASD risk in children in the Boston Birth Cohorta
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total n | ASD n | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Continuous | 674 | 40 | 0.51 (0.35, 0.75) | 0.001 | 0.50 (0.33, 0.77) | 0.002 | 0.47 (0.30, 0.74) | 0.001 | 0.36 (0.21, 0.62) | <0.001 |
| Quartile | ||||||||||
| Q1 | 167 | 20 | Ref | Ref | Ref | Ref | ||||
| Q2 | 170 | 8 | 0.36 (0.16, 0.85) | 0.02 | 0.39 (0.15, 1.01) | 0.05 | 0.38 (0.14, 1.03) | 0.06 | 0.32 (0.11, 0.91) | 0.03 |
| Q3 | 169 | 7 | 0.32 (0.13, 0.77) | 0.01 | 0.40 (0.15, 1.10) | 0.08 | 0.38 (0.13, 1.12) | 0.08 | 0.23 (0.07, 0.77) | 0.02 |
| Q4 | 168 | 5 | 0.23 (0.08, 0.62) | 0.004 | 0.14 (0.04, 0.46) | 0.001 | 0.13 (0.04, 0.45) | 0.001 | 0.08 (0.02, 0.34) | 0.001 |
Adiponectin was entered as a continuous variable in one model and categorical variable in another model
Model 1: Adjusted for child’s sex, race, maternal education, maternal age, parity, smoking status, pre-pregnancy BMI, diabetes status, follow-up time
Model 2: Model 1 + gestational age
Model 3: Model 1 + early childhood adiponectin
A total of 638 children had data on early childhood adiponectin, of which 36 were subsequently diagnosed with ASD. As seen in Online Resource Figure 4, there was no difference in the timing of adiponectin measurement between neurotypical children and those that had ASD. In an unadjusted model, early childhood adiponectin was not associated with ASD risk (OR: 0.71; 95% CI: 0.45, 1.13) (Table 3). After adjusting for potential confounders (including maternal age, diabetes status, pre-pregnancy BMI, sex, race, preterm birth, age of measurement, follow-up time and breastfeeding status; model 1), higher early childhood adiponectin levels, modeled as a continuous variable, was inversely associated with the risk of ASD (aOR: 0.54; 95% CI: 0.33, 0.90). When each of the covariates were added stepwise to the model, age of biomarker measurement turned the association from non-significant to significant (data not shown). This is likely because the age distribution is younger in ASD children compared to neurotypical children.
Table 3:
Association between early childhood plasma adiponectin levels and ASD risk in children in the Boston Birth Cohorta
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total n | ASD n | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Continuous | 638 | 36 | 0.71 (0.45, 1.13) | 0.15 | 0.54 (0.33, 0.90) | 0.02 | 0.59 (0.35, 0.99) | 0.05 | 0.67 (0.34, 1.33) | 0.25 |
| Quartile | ||||||||||
| Q1 | 160 | 11 | Ref | Ref | Ref | Ref | ||||
| Q2 | 159 | 8 | 0.72 (0.28, 1.83) | 0.49 | 0.68 (0.25, 1.85) | 0.45 | 0.74 (0.26, 2.05) | 0.56 | 0.77 (0.19, 3.14) | 0.71 |
| Q3 | 160 | 10 | 0.90 (0.38, 2.19) | 0.82 | 0.71 (0.27, 1.90) | 0.50 | 0.84 (0.31, 2.29) | 0.73 | 0.85 (0.23, 3.11) | 0.80 |
| Q4 | 159 | 7 | 0.62 (0.24, 1.65) | 0.34 | 0.30 (0.09, 0.98) | 0.05 | 0.32 (0.10, 1.08) | 0.07 | 0.68 (0.15, 3.10) | 0.61 |
Adiponectin was entered as a continuous variable in one model and categorical variable in another model
Model 1: Adjusted for maternal age, diabetes status, maternal BMI, sex, race, age of measurement, follow-up time and breastfeeding status
Model 2: Model 1 + gestational age at birth
Model 3: Model 1 + cord adiponectin
Similar to cord adiponectin, when early childhood adiponectin was categorized as quartiles, those with the highest levels (quartile 4) were associated with the lowest ASD risk (aORquartile 4: 0.30; 95% CI: 0.09, 0.98). The association approached significance when adjusted for gestational age at birth (model 2). However, after adjusting for cord adiponectin levels (model 3), the association between early childhood adiponectin and ASD was no longer significant. We repeated the analysis by adjusting for additional covariates such as early childhood insulin, early childhood leptin and weight gain during first year of life (Online Resource Table 5) and categorizing early childhood adiponectin as tertiles, quintiles and extreme values (Online Resource Table 6), and observed similar findings.
Sensitivity analyses using stringent ASD case and neurotypical definitions yielded consistent and stronger associations for both cord and early childhood adiponectin (Online Resource Tables 7–10). Repeating the analysis using ASD subjects that were diagnosed >2 years did not alter the findings.
Since leptin is linked to ASD in several studies (Ashwood et al. 2008; Blardi et al. 2010; Rodrigues et al. 2014; Al-Zaid et al. 2014; Essa M.M. et al. 2011), including ours, and adiponectin-leptin ratio is considered as a marker of metabolic syndrome (Inoue et al. 2005), we assessed the association between log-transformed adiponectin-leptin ratio and subsequent ASD risk. We conducted this analysis for both cord and early childhood ratios (Online Resource Tables 11–12). While the associations were statistically significant, the ratio did not predict ASD risk beyond the independent effects of leptin and/or adiponectin.
Next, the joint effects of preterm birth and adiponectin levels on the risk of ASD were assessed. Children that had term birth (≥37 completed weeks of gestation) and high cord adiponectin levels (defined as ≥50th percentile) were the reference group (Table 4). After adjusting for confounders, the risk of ASD was not different between preterm (<37 completed weeks of gestation) and term children, as long as they had high cord adiponectin. ASD risk was elevated among term children with low adiponectin (defined as < 50th percentile) in both unadjusted and adjusted models (aOR: 3.06; 95% CI: 1.27, 7.40). Among preterm children with low adiponectin, the ASD risk was significantly higher in the unadjusted model and approached statistical significance, after adjusting for confounders (aOR: 2.62; 95% CI: 0.99, 6.99).
Table 4:
Joint effects of preterm birth and cord adiponectin in predicting ASD risk in the Boston Birth Cohort
| Joint effects of preterm birtha and adiponectinb | Total n | ASD n | Unadjusted | P value | Adjustedc | P value |
|---|---|---|---|---|---|---|
| Full term + High adiponectin | 309 | 11 | Ref | Ref | ||
| Preterm + High adiponectin | 28 | 1 | 1.0 (0.12, 8.07) | 1.0 | 1.22 (0.14, 10.87) | 0.86 |
| Full term + Low adiponectin | 221 | 17 | 2.26 (1.04, 4.92) | 0.04 | 3.06 (1.27, 7.40) | 0.01 |
| Preterm + Low adiponectin | 116 | 11 | 2.84 (1.20, 6.74) | 0.02 | 2.62 (0.99, 6.99) | 0.05 |
Full term defined as ≥ 37 completed weeks of gestation; preterm defined as <37 completed weeks of gestation
High cord adiponectin – defined as ≥50th percentile; Low cord adiponectin – defined as <50th percentile
Adjusted for child’s sex, maternal education, maternal age, parity, maternal BMI, maternal smoking status, diabetes, race ethnicity and follow-up time
Mediation analysis: An exploration
Since the temporal ordering of preterm birth and altered adiponectin levels is unclear (Mazaki-Tovi et al. 2009), mediation analysis was explored in two ways: 1) Cord adiponectin as a mediator in the relationship between preterm birth (independent variable) and ASD risk (dependent variable); and 2) Preterm birth as a mediator in the relationship between cord adiponectin (independent variable) and ASD risk (dependent variable). As seen in Online Resource Table 13, early preterm (independent variable) was associated with ASD risk (dependent variable) (step 1), both early and late preterm birth was associated with adiponectin (mediator) (step 2), and cord adiponectin was associated with ASD risk (step 3). In the last regression model (step 4), the association attenuated suggesting that cord adiponectin mediated the relationship between early preterm birth and ASD risk (Online Resource Table 13). In the second mediation analysis, preterm birth was considered as mediator in the association between cord adiponectin levels and ASD. Here, cord adiponectin was associated with ASD risk (step 1), preterm birth was associated with cord adiponectin (step 2) and early preterm birth was associated with ASD risk (step 3). In the regression model, adjusting for preterm birth did not alter the association between cord adiponectin and ASD risk, suggesting that there was no mediation (Online Resource Table 14). The role of early childhood adiponectin mediating the association between preterm birth and ASD risk is presented in Online Resource Table 15.
Discussion
To our knowledge, this is the first study to prospectively assess adiponectin levels in cord blood (a proxy of fetal adiponectin) and in early childhood venous blood in relation to ASD risk. We find cord blood adiponectin levels to be inversely associated with the risk of ASD. In comparison, the association between early childhood adiponectin and ASD was less robust and was further weakened after adjustment of cord adiponectin. These associations remained consistent after additional analyses, including sequential adjustment for potential confounders and other metabolic biomarkers (such as leptin, insulin) and after more stringent ASD and neurotypical classifications of outcome.
Our findings are in line with the observations of Fujita-Shimizu et al. who noted that adiponectin levels are lower in subjects with ASD (Fujita-Shimizu et al. 2010). Specifically, they reported a negative correlation between adiponectin and social development in children, characterized by the Autism Diagnostic Interview Revised (Fujita-Shimizu et al. 2010). Consistent with these findings, Rodrigues et al., noted that adiponectin levels were inversely correlated with the severity of autism symptoms (Rodrigues et al. 2014). Lisik et al. showed that adiponectin levels were lower in those with Fragile X Syndrome (Lisik et al. 2016). These findings suggest that adiponectin may have a larger role to play in neurodevelopmental processes, rather than merely regulate energy expenditure or serve as a biomarker for the onset of metabolic syndrome (Inami et al. 2007; Blardi et al. 2009).
Adiponectin is detected in cord serum as early as 24 weeks of gestation, after which the concentration rises 20-fold until term (Kajantie et al. 2004). This increase during third trimester mirrors the increase in adiposity (Lenz and Diamond 2012) and is in stark contrast with children and adults in whom adiponectin concentration is inversely associated with body fat percentage (Kajantie et al. 2004; Siahanidou et al. 2007). Cord adiponectin does not correlate with maternal adiponectin suggesting the fetal origins of cord adiponectin (Lenz and Diamond 2012; Saito et al. 2011; Brochu-Gaudreau et al. 2010; Dawczynski et al. 2014; Mazaki-Tovi et al. 2007). In the fetus, adiponectin is secreted by muscle and vascular cells, in addition to adipocytes (Z. Q. Zhang et al. 2016).
While cord adiponectin is associated with ASD, it is intriguing that early childhood adiponectin demonstrates a less robust association. Adiponectin expression and its relationship to growth parameters and fat distribution seem to evolve temporally, from birth to childhood (Meyer et al. 2017; Kotani et al. 2004; Inami et al. 2007). At birth, adiponectin is positively correlated with growth parameters and fat mass (Kotani et al. 2004; Tsai et al. 2004; Pardo et al. 2004; Weyermann et al. 2006; Inami et al. 2007). Subsequent to this initial time window, there is possibly a shift in this relationship noting an inverse association between adiponectin and fat mass (Kotani et al. 2004; Stefan et al. 2002; Inami et al. 2007; Mantzoros et al. 2009). Taken together, these findings suggest that the role of adiponectin in-utero may be fundamentally different than early childhood adiponectin (Meyer et al. 2017; Mantzoros et al. 2009). The discrepancy between adiponectin at different time points has been hypothesized to the differential origination of adiponectin, variation in fat storage, secretion and modulation of adiponectin (Z. Q. Zhang et al. 2016; Mantzoros et al. 2009). While the evidence discussed here is in the context of obesity, adiponectin’s effect may not be different for neurodevelopmental outcomes such as ASD.
Adiponectin has been previously shown to be altered in preterm babies, however, for the first time we jointly assessed adiponectin and preterm birth in the context of ASD and showed that lower adiponectin levels could increase ASD risk, irrespective of the gestational age. As a next step, we conducted a preliminary mediation analysis. Given the uncertainty in the biological temporal relationship between preterm birth and cord adiponectin (meaning whether preterm birth alters adiponectin levels, or changes in adiponectin triggers preterm birth) (Mazaki-Tovi et al. 2009), our analysis assessed both as mediators. In this exploratory analysis, cord adiponectin mediated the relationship between preterm birth and ASD; whereas, preterm birth did not seem to mediate the association between cord adiponectin and ASD. These findings are preliminary and suggestive of a potential mediating role of adiponectin in relationship between preterm birth and ASD. More research in animal models and human studies is warranted to further clarify this relationship.
Biological plausibility
Several lines of evidence support the biological plausibility of adiponectin’s role in ASD. Adiponectin suppresses the macrophage production of pro-inflammatory cytokines such as TNF- α, IL-6 (Brochu-Gaudreau et al. 2010), Interferon (IFN)-γ (Mazaki-Tovi et al. 2009), which are noted to be elevated in children with ASD (Chez et al. 2007; Wei et al. 2013; Ashwood et al. 2011). Consistent findings suggest that IL-6 was significantly increased in the anterior cingulated gyrus, frontal cortices and cerebellum(Wei et al. 2013) and TNF was increased almost 50 times in the cerebrospinal fluid (Angelidou et al. 2012). Similarly, IFN-γ is elevated in the brain of ASD subjects, when compared to controls (Wei et al. 2013). Adiponectin signaling inhibits NF-κB, an essential transcription factor that mediates and regulates inflammatory and stress-related protein expression (Villarreal-Molina and Antuna-Puente 2012), which is aberrantly expressed in subjects with ASD (Young et al. 2011).
Beyond its role in influencing other cytokines, emerging evidence suggests that adiponectin may be implicated in brain functions (Thundyil et al. 2012). Adiponectin enters the brain from circulation and directly targets neurons (Ng and Chan 2017; Thundyil et al. 2012; D. Zhang et al. 2016; Zhang et al. 2017; Song et al. 2015). Its receptors are widely expressed in the dentate gyrus of hippocampus (D. Zhang et al. 2016), hypothalamus, cortex and pituitary glands (Thundyil et al. 2012; Brochu-Gaudreau et al. 2010). Given this broad distribution of adiponectin receptors in different brain regions, adiponectin’s role may be broader than the regulation of metabolic homeostasis and could plan an important role in the ‘adipocyte-brain cross-talk’ (Thundyil et al. 2012). Adiponectin is involved in neurogenesis, dendritic spine remodeling, hippocampal neural stem cell proliferation and dentate gyrus neuronal excitability (Zhang et al. 2017; Ng and Chan 2017; Song et al. 2015; D. Zhang et al. 2016). It also promotes adaptive neuroplasticity and may possess cerebra-protective role, likely mediated through the eNOS signal pathway (Thundyil et al. 2012; Brochu-Gaudreau et al. 2010; Machado-Vieira et al. 2017). Abnormal development of dentate gyrus of hippocampus, an important center for learning and memory, is likely implicated in the pathophysiology of ASD (Ito et al. 2017). Adiponectin may also possess higher brain functions. For example, diminished adiponectin levels are associated with clinically significant affective episodes and subjects with major depressive symptoms are known to have lower adiponectin levels (Machado-Vieira et al. 2017). Adiponectin knockout mice have exhibited depressive-like behavior (Ng and Chan 2017). Further, hypoadiponectinemia is thought to increase sympathetic nervous system activity – which is observed in depression (Lehto et al. 2010).
Limitations and strengths
There are limitations to highlight. First, only total adiponectin and not the distinct forms such as low-molecular weight trimmers, medium-molecular-weight hexamers and high-molecular-weight oligomers (HMW) was examined. It is possible that these individual components could have different roles and should be further explored in the context of ASD (Mazaki-Tovi et al. 2009). However, research in pediatric populations has found no difference in the role of total vs. HMW adiponectin (Meyer et al. 2017). Second, ASD assessment relied on EMR data, which can introduce outcome misclassification in unpredictable ways. However, the results of our sensitivity analysis showed consistent associations when using more stringent outcome classification for both cases and neurotypical children. Third, because of the relatively small sample of ASD children in the cohort, our estimates may be subject to random variation. Fourth, although well-known ASD risk factors were adjusted for, there is a possibility of residual confounding. Finally, this study consisted primarily of urban, low-income minority population with high risk for preterm births. Thus, the findings may not be generalizable to other U.S. populations with different characteristics, although, few ASD studies have focused on this important group.
Despite these limitations, our study has a number of strengths. This study is based on a well-designed prospective birth cohort in an urban under-represented minority population. This is one of the first longitudinal studies to measure adiponectin at two-time points (birth and early childhood) prior to ASD diagnosis. Among studies that have looked at adipocytokines in the context of ASD, most of them were cross-sectional, and very few have used cord and/or newborn samples (Zerbo et al. 2014). Additionally, using a preterm enriched cohort, our study was uniquely poised to explore a novel question on the joint effect and mediating role of adiponectin in explaining the relationship between preterm birth and ASD risk and highlighted research gaps that can be explored by future studies.
Conclusions
In summary, our study found an inverse association between cord adiponectin levels and subsequent ASD risk in childhood. The effects of lower adiponectin on increased ASD risk were demonstrated independent of gestational age, early childhood adiponectin, and other important covariables. However, we emphasize that our findings be regarded as hypothesis generating; additional research is needed to replicate these findings. If confirmed, subsequent research can determine whether cord and/or early childhood adiponectin, along with other known risk factors, can be considered a biomarker for identifying children that are at high risk for ASD and thereby serve as a potential molecular target for developing novel interventions.
Supplementary Material
Acknowledgments:
Funding Source: This study is supported in part by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under grant number R40MC27443, Autism Field-initiated Innovative Research Studies Program; and grant number UJ2MC31074, Autism Single Investigator Innovation Program. This information or content and conclusions are those of the author and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS or the U.S.
Government. The Boston Birth Cohort (the parent study) was supported in part by the March of Dimes PERI grants (20-FY02-56, #21-FY07-605); and the National Institutes of Health (NIH) grants (R21ES011666, R01HD041702, R21HD066471, U01AI090727, R21AI079872, and 5R01HD086013). Ramkripa Raghavan is supported by John and Alice Chenoweth-Pate Fellowship in her current training.
Footnotes
Conflict of interest: None of the authors have a conflict of interest pertaining to this work.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Contributor Information
Ramkripa Raghavan, Center on the Early Life Origins of Disease, Department of Population, Family and Reproductive Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA, 615 N. Wolfe Street, Baltimore, MD 21205-2179, Ramkripa@gmail.com.
M. Daniele Fallin, Wendy Klag Center for Autism and Developmental Disabilities, Department of Mental Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA, 624 N. Broadway, Baltimore, MD 21205, USA, Phone 410-955-3463, Fax 410-955-0863, dfallin@jhu.edu.
Xiumei Hong, Center on the Early Life Origins of Disease, Department of Population, Family and Reproductive Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA, 615 N. Wolfe Street, Baltimore, MD 21205-2179, Phone: 410-502-8919, Xhong3@jhu.edu.
Guoying Wang, Center on the Early Life Origins of Disease, Department of Population, Family and Reproductive Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA, 615 N. Wolfe Street, Baltimore, MD 21205-2179, Phone: 410-502-8973, gwang24@jhu.edu.
Yuelong Ji, Center on the Early Life Origins of Disease, Department of Population, Family and Reproductive Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA, 615 N. Wolfe Street, Baltimore, MD 21205-2179, yji7@jhu.edu.
Elizabeth A. Stuart, Department of Biostatistics, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA, 624 N. Broadway, Baltimore, MD 21205, USA, Phone: 410-502-6222, Fax: 410-955-9088, estuart@jhu.edu
David Paige, Department of Population, Family and Reproductive Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA, 615 N. Wolfe Street, Baltimore, MD 21205-2179, Phone: 410-502-5445, dpaige@jhu.edu.
Xiaobin Wang, Center on the Early Life Origins of Disease, Department of Population, Family and Reproductive Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA, Department of Pediatrics, Johns Hopkins University School of Medicine, MD, USA, 615 N. Wolfe Street, Baltimore, MD 21205-2179, Phone: 410-955-5824, Fax: 410-502-5831, xwang82@jhu.edu.
References
- Akintunde ME, Rose M, Krakowiak P, Heuer L, Ashwood P, Hansen R, et al. (2015). Increased production of IL-17 in children with autism spectrum disorders and co-morbid asthma. J Neuroimmunol, 286, 33–41, doi: 10.1016/j.jneuroim.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zaid FS, Alhader AA, & Al-Ayadhi LY (2014). Altered ghrelin levels in boys with autism: a novel finding associated with hormonal dysregulation. Sci Rep, 4, 6478, doi: 10.1038/srep06478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelidou A, Asadi S, Alysandratos KD, Karagkouni A, Kourembanas S, & Theoharides TC (2012). Perinatal stress, brain inflammation and risk of autism-review and proposal. BMC Pediatr, 12, 89, doi: 10.1186/1471-2431-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, & Van de Water J (2011). Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun, 25(1), 40–45, doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Kwong C, Hansen R, Hertz-Picciotto I, Croen L, Krakowiak P, et al. (2008). Brief report: plasma leptin levels are elevated in autism: association with early onset phenotype? J Autism Dev Disord, 38(1), 169–175, doi: 10.1007/s10803-006-0353-1. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Wills S, & Van de Water J (2006). The immune response in autism: a new frontier for autism research. J Leukoc Biol, 80(1), 1–15, doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- Association, A. P. (2013). Diagnostic and Statistical Manual of Mental Disorders. (5th edition ed.). Washington, D.C. [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. (2018). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ, 67(6), 1–23, doi: 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, & Kenny DA (1986). The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol, 51(6), 1173–1182. [DOI] [PubMed] [Google Scholar]
- Blardi P, de Lalla A, Ceccatelli L, Vanessa G, Auteri A, & Hayek J (2010). Variations of plasma leptin and adiponectin levels in autistic patients. Neurosci Lett, 479(1), 54–57, doi: 10.1016/j.neulet.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Blardi P, de Lalla A, D’Ambrogio T, Vonella G, Ceccatelli L, Auteri A, et al. (2009). Long-term plasma levels of leptin and adiponectin in Rett syndrome. Clin Endocrinol (Oxf), 70(5), 706–709, doi: 10.1111/j.1365-2265.2008.03386.x. [DOI] [PubMed] [Google Scholar]
- Brochu-Gaudreau K, Rehfeldt C, Blouin R, Bordignon V, Murphy BD, & Palin MF (2010). Adiponectin action from head to toe. Endocrine, 37(1), 11–32, doi: 10.1007/s12020-009-9278-8. [DOI] [PubMed] [Google Scholar]
- Brucato M, Ladd-Acosta C, Li M, Caruso D, Hong X, Kaczaniuk J, et al. (2017). Prenatal exposure to fever is associated with autism spectrum disorder in the boston birth cohort. Autism Res, 10(11), 1878–1890, doi: 10.1002/aur.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd I, Balakrishnan B, & Kannan S (2012). Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am J Reprod Immunol, 67(4), 287–294, doi: 10.1111/j.1600-0897.2012.01110.x. [DOI] [PubMed] [Google Scholar]
- Chez MG, Dowling T, Patel PB, Khanna P, & Kominsky M (2007). Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr Neurol, 36(6), 361–365, doi: 10.1016/j.pediatrneurol.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Darcy-Mahoney A, Minter B, Higgins M, Guo Y, Williams B, Head Zauche LM, et al. (2016). Probability of an Autism Diagnosis by Gestational Age. Newborn Infant Nurs Rev, 16(4), 322–326, doi: 10.1053/j.nainr.2016.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawczynski K, de Vries H, Beck JF, Schleussner E, Wittig S, & Proquitte H (2014). Adiponectin serum concentrations in newborn at delivery appear to be of fetal origin. J Pediatr Endocrinol Metab, 27(3–4), 273–278, doi: 10.1515/jpem-2013-0218. [DOI] [PubMed] [Google Scholar]
- Erdei C, & Dammann O (2014). The Perfect Storm: Preterm Birth, Neurodevelopmental Mechanisms, and Autism Causation. Perspect Biol Med, 57(4), 470–481, doi: 10.1353/pbm.2014.0036. [DOI] [PubMed] [Google Scholar]
- Essa MM, Braidy N, Al-Sharbati MM, Al-Farsi YM, Ali A, Waly MI, et al. (2011). Elevated plasma leptin levels in autisic children of Sultanate of Oman. International Journal of Biological & Medical Research, 2(3), 803–805. [Google Scholar]
- Fezer GF, Matos MB, Nau AL, Zeigelboim BS, Marques JM, & Liberalesso PBN (2017). Perinatal Features of Children with Autism Spectrum Disorder. Rev Paul Pediatr, 35(2), 130–135, doi: 10.1590/1984-0462/;2017;35;2;00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino M, Sapone A, Senger S, Camhi SS, Kadzielski SM, Buie TM, et al. (2016). Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism, 7, 49, doi: 10.1186/s13229-016-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita-Shimizu A, Suzuki K, Nakamura K, Miyachi T, Matsuzaki H, Kajizuka M, et al. (2010). Decreased serum levels of adiponectin in subjects with autism. Prog Neuropsychopharmacol Biol Psychiatry, 34(3), 455–458, doi: 10.1016/j.pnpbp.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Ghaffari MA, Mousavinejad E, Riahi F, Mousavinejad M, & Afsharmanesh MR (2016). Increased Serum Levels of Tumor Necrosis Factor-Alpha, Resistin, and Visfatin in the Children with Autism Spectrum Disorders: A Case-Control Study. Neurol Res Int, 2016, 9060751, doi: 10.1155/2016/9060751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen-Pupp I, Hellgren G, Hard AL, Smith L, Hellstrom A, & Lofqvist C (2015). Early Surge in Circulatory Adiponectin Is Associated With Improved Growth at Near Term in Very Preterm Infants. J Clin Endocrinol Metab, 100(6), 2380–2387, doi: 10.1210/jc.2015-1081. [DOI] [PubMed] [Google Scholar]
- Hellgren G, Engstrom E, Smith LE, Lofqvist C, & Hellstrom A (2015). Effect of Preterm Birth on Postnatal Apolipoprotein and Adipocytokine Profiles. Neonatology, 108(1), 16–22, doi: 10.1159/000381278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Dong X, & Chen J (2015). Adiponectin and depression: A meta-analysis. Biomed Rep, 3(1), 38–42, doi: 10.3892/br.2014.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inami I, Okada T, Fujita H, Makimoto M, Hosono S, Minato M, et al. (2007). Impact of serum adiponectin concentration on birth size and early postnatal growth. Pediatr Res, 61(5 Pt 1), 604–606, doi: 10.1203/pdr.0b013e3180459f8a. [DOI] [PubMed] [Google Scholar]
- Inoue M, Maehata E, Yano M, Taniyama M, & Suzuki S (2005). Correlation between the adiponectin-leptin ratio and parameters of insulin resistance in patients with type 2 diabetes. Metabolism, 54(3), 281–286, doi: 10.1016/j.metabol.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Ito H, Morishita R, & Nagata KI (2017). Autism spectrum disorder-associated genes and the development of dentate granule cells. Med Mol Morphol, 50(3), 123–129, doi: 10.1007/s00795-017-0161-z. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Hytinantti T, Hovi P, & Andersson S (2004). Cord plasma adiponectin: a 20-fold rise between 24 weeks gestation and term. J Clin Endocrinol Metab, 89(8), 4031–4036, doi: 10.1210/jc.2004-0018. [DOI] [PubMed] [Google Scholar]
- Kotani Y, Yokota I, Kitamura S, Matsuda J, Naito E, & Kuroda Y (2004). Plasma adiponectin levels in newborns are higher than those in adults and positively correlated with birth weight. Clin Endocrinol (Oxf), 61(4), 418–423, doi: 10.1111/j.1365-2265.2004.02041.x. [DOI] [PubMed] [Google Scholar]
- Kuzniewicz MW, Wi S, Qian Y, Walsh EM, Armstrong MA, & Croen LA (2014). Prevalence and neonatal factors associated with autism spectrum disorders in preterm infants. J Pediatr, 164(1), 20–25, doi: 10.1016/j.jpeds.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Lehto SM, Huotari A, Niskanen L, Tolmunen T, Koivumaa-Honkanen H, Honkalampi K, et al. (2010). Serum adiponectin and resistin levels in major depressive disorder. Acta Psychiatr Scand, 121(3), 209–215, doi: 10.1111/j.1600-0447.2009.01463.x. [DOI] [PubMed] [Google Scholar]
- Lenz AM, & Diamond F (2012). The Importance of the Adiponectin and Leptin Relationship in In Utero and Infant Growth. New York, NY: Springer. [Google Scholar]
- Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, et al. (2016). The Association of Maternal Obesity and Diabetes With Autism and Other Developmental Disabilities. Pediatrics, 137(2), e20152206, doi: 10.1542/peds.2015-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay RS, Walker JD, Havel PJ, Hamilton BA, Calder AA, Johnstone FD, et al. (2003). Adiponectin is present in cord blood but is unrelated to birth weight. Diabetes Care, 26(8), 2244–2249. [DOI] [PubMed] [Google Scholar]
- Lisik MZ, Gutmajster E, & Sieron AL (2016). Plasma Levels of Leptin and Adiponectin in Fragile X Syndrome. Neuroimmunomodulation, 23(4), 239–243, doi: 10.1159/000452336. [DOI] [PubMed] [Google Scholar]
- Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, et al. (2017). The Changing Epidemiology of Autism Spectrum Disorders. Annu Rev Public Health, 38, 81–102, doi: 10.1146/annurev-publhealth-031816-044318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Gold PW, Luckenbaugh DA, Ballard ED, Richards EM, Henter ID, et al. (2017). The role of adipokines in the rapid antidepressant effects of ketamine. Mol Psychiatry, 22(1), 127–133, doi: 10.1038/mp.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur RB, Rizzo LB, Santos CM, Asevedo E, Cunha GR, Noto MN, et al. (2016). Adipokines, metabolic dysfunction and illness course in bipolar disorder. J Psychiatr Res, 74, 63–69, doi: 10.1016/j.jpsychires.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, & Gillman MW (2009). Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics, 123(2), 682–689, doi: 10.1542/peds.2008-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martos-Moreno GA, Barrios V, Saenz de Pipaon M, Pozo J, Dorronsoro I, Martinez-Biarge M, et al. (2009). Influence of prematurity and growth restriction on the adipokine profile, IGF1, and ghrelin levels in cord blood: relationship with glucose metabolism. Eur J Endocrinol, 161(3), 381–389, doi: 10.1530/EJE-09-0193. [DOI] [PubMed] [Google Scholar]
- Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Efraty Y, Schiff E, et al. (2007). Determining the source of fetal adiponectin. J Reprod Med, 52(9), 774–778. [PubMed] [Google Scholar]
- Mazaki-Tovi S, Romero R, Vaisbuch E, Erez O, Mittal P, Chaiworapongsa T, et al. (2009). Dysregulation of maternal serum adiponectin in preterm labor. J Matern Fetal Neonatal Med, 22(10), 887–904, doi: 10.1080/14767050902994655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DM, Brei C, Stecher L, Much D, Brunner S, & Hauner H (2017). Cord blood and child plasma adiponectin levels in relation to childhood obesity risk and fat distribution up to 5 y. Pediatr Res, 81(5), 745–751, doi: 10.1038/pr.2016.275. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Morrow AL, Meinzen-Derr J, Schleifer K, Dienger K, Manning-Courtney P, et al. (2006). Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol, 172(1–2), 198–205, doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Movsas TZ, & Paneth N (2012). The effect of gestational age on symptom severity in children with autism spectrum disorder. J Autism Dev Disord, 42(11), 2431–2439, doi: 10.1007/s10803-012-1501-4. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Itabashi K, Sakurai M, Aizawa M, Dobashi K, & Mizuno K (2013). Preterm infants have altered adiponectin levels at term-equivalent age even if they do not present with extrauterine growth restriction. Horm Res Paediatr, 80(3), 147–153, doi: 10.1159/000354037. [DOI] [PubMed] [Google Scholar]
- Ng RC, & Chan KH (2017). Potential Neuroprotective Effects of Adiponectin in Alzheimer’s Disease. Int J Mol Sci, 18(3), doi: 10.3390/ijms18030592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberthuer A, Donmez F, Oberhauser F, Hahn M, Hoppenz M, Hoehn T, et al. (2012). Hypoadiponectinemia in extremely low gestational age newborns with severe hyperglycemia--a matched-paired analysis. PLoS One, 7(6), e38481, doi: 10.1371/journal.pone.0038481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo IM, Geloneze B, Tambascia MA, & Barros-Filho AA (2004). Hyperadiponectinemia in newborns: relationship with leptin levels and birth weight. Obes Res, 12(3), 521–524, doi: 10.1038/oby.2004.59. [DOI] [PubMed] [Google Scholar]
- Raghavan R, Riley AW, Volk H, Caruso D, Hironaka L, Sices L, et al. (2017). Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr Perinat Epidemiol, doi: 10.1111/ppe.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues DH, Rocha NP, Sousa LF, Barbosa IG, Kummer A, & Teixeira AL (2014). Changes in adipokine levels in autism spectrum disorders. Neuropsychobiology, 69(1), 6–10, doi: 10.1159/000356234. [DOI] [PubMed] [Google Scholar]
- Saito M, Nishimura K, Nozue H, Miyazono Y, & Kamoda T (2011). Changes in serum adiponectin levels from birth to term-equivalent age are associated with postnatal weight gain in preterm infants. Neonatology, 100(1), 93–98, doi: 10.1159/000322654. [DOI] [PubMed] [Google Scholar]
- Schendel D, & Bhasin TK (2008). Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics, 121(6), 1155–1164, doi: 10.1542/peds.2007-1049. [DOI] [PubMed] [Google Scholar]
- Siahanidou T, Mandyla H, Papassotiriou GP, Papassotiriou I, & Chrousos G (2007). Circulating levels of adiponectin in preterm infants. Arch Dis Child Fetal Neonatal Ed, 92(4), F286–290, doi: 10.1136/adc.2006.106112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Kang SM, Kim E, Kim CH, Song HT, & Lee JE (2015). Adiponectin receptor-mediated signaling ameliorates cerebral cell damage and regulates the neurogenesis of neural stem cells at high glucose concentrations: an in vivo and in vitro study. Cell Death Dis, 6, e1844, doi: 10.1038/cddis.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan N, Bunt JC, Salbe AD, Funahashi T, Matsuzawa Y, & Tataranni PA (2002). Plasma adiponectin concentrations in children: relationships with obesity and insulinemia. J Clin Endocrinol Metab, 87(10), 4652–4656, doi: 10.1210/jc.2002-020694. [DOI] [PubMed] [Google Scholar]
- Terrazzan AC, Procianoy RS, & Silveira RC (2014). Neonatal cord blood adiponectin and insulin levels in very low birth weight preterm and healthy full-term infants. J Matern Fetal Neonatal Med, 27(6), 616–620, doi: 10.3109/14767058.2013.823939. [DOI] [PubMed] [Google Scholar]
- Thundyil J, Pavlovski D, Sobey CG, & Arumugam TV (2012). Adiponectin receptor signalling in the brain. Br J Pharmacol, 165(2), 313–327, doi: 10.1111/j.1476-5381.2011.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PJ, Yu CH, Hsu SP, Lee YH, Chiou CH, Hsu YW, et al. (2004). Cord plasma concentrations of adiponectin and leptin in healthy term neonates: positive correlation with birthweight and neonatal adiposity. Clin Endocrinol (Oxf), 61(1), 88–93, doi: 10.1111/j.1365-2265.2004.02057.x. [DOI] [PubMed] [Google Scholar]
- Villarreal-Molina MT, & Antuna-Puente B (2012). Adiponectin: anti-inflammatory and cardioprotective effects. Biochimie, 94(10), 2143–2149, doi: 10.1016/j.biochi.2012.06.030. [DOI] [PubMed] [Google Scholar]
- Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, et al. (2014). Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA, 311(6), 587–596, doi: 10.1001/jama.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Hu FB, Mistry KB, Zhang C, Ren F, Huo Y, et al. (2016a). Association Between Maternal Prepregnancy Body Mass Index and Plasma Folate Concentrations With Child Metabolic Health. JAMA Pediatr, 170(8), e160845, doi: 10.1001/jamapediatrics.2016.0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Johnson S, Gong Y, Polk S, Divall S, Radovick S, et al. (2016b). Weight Gain in Infancy and Overweight or Obesity in Childhood across the Gestational Spectrum: a Prospective Birth Cohort Study. Sci Rep, 6, 29867, doi: 10.1038/srep29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, et al. (2002). Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA, 287(2), 195–202. [DOI] [PubMed] [Google Scholar]
- Wei H, Alberts I, & Li X (2013). Brain IL-6 and autism. Neuroscience, 252, 320–325, doi: 10.1016/j.neuroscience.2013.08.025. [DOI] [PubMed] [Google Scholar]
- Weyermann M, Beermann C, Brenner H, & Rothenbacher D (2006). Adiponectin and leptin in maternal serum, cord blood, and breast milk. Clin Chem, 52(11), 2095–2102, doi: 10.1373/clinchem.2006.071019. [DOI] [PubMed] [Google Scholar]
- Wozniak RH, Leezenbaum NB, Northrup JB, West KL, & Iverson JM (2017). The development of autism spectrum disorders: variability and causal complexity. Wiley Interdiscip Rev Cogn Sci, 8(1–2), doi: 10.1002/wcs.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung EH, McLain AC, Anderson N, Lawrence D, Boghossian NS, Druschel C, et al. (2015). Newborn Adipokines and Birth Outcomes. Paediatr Perinat Epidemiol, 29(4), 317–325, doi: 10.1111/ppe.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Nagasaki H, Asato Y, & Ohta T (2009). The ratio of high-molecular weight adiponectin and total adiponectin differs in preterm and term infants. Pediatr Res, 65(5), 580–583, doi: 10.1203/PDR.0b013e3181995103. [DOI] [PubMed] [Google Scholar]
- Young AM, Campbell E, Lynch S, Suckling J, & Powis SJ (2011). Aberrant NF-kappaB expression in autism spectrum condition: a mechanism for neuroinflammation. Front Psychiatry, 2, 27, doi: 10.3389/fpsyt.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo O, Yoshida C, Grether JK, Van de Water J, Ashwood P, Delorenze GN, et al. (2014). Neonatal cytokines and chemokines and risk of Autism Spectrum Disorder: the Early Markers for Autism (EMA) study: a case-control study. J Neuroinflammation, 11, 113, doi: 10.1186/1742-2094-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Wang X, & Lu XY (2016). Adiponectin Exerts Neurotrophic Effects on Dendritic Arborization, Spinogenesis, and Neurogenesis of the Dentate Gyrus of Male Mice. Endocrinology, 157(7), 2853–2869, doi: 10.1210/en.2015-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Wang X, Wang B, Garza JC, Fang X, Wang J, et al. (2017). Adiponectin regulates contextual fear extinction and intrinsic excitability of dentate gyrus granule neurons through AdipoR2 receptors. Mol Psychiatry, 22(7), 1044–1055, doi: 10.1038/mp.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZQ, Lu QG, Huang J, Jiao CY, Huang SM, & Mao LM (2016). Maternal and cord blood adiponectin levels in relation to post-natal body size in infants in the first year of life: a prospective study. BMC Pregnancy Childbirth, 16(1), 189, doi: 10.1186/s12884-016-0978-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.