Introduction
Drug transporters are critically important for the absorption, distribution, metabolism, and excretion of many drugs and endogenous compounds. Therefore, disruption of these pathways by inhibition, induction, genetic polymorphisms or disease can have profound effects on overall physiology, drug pharmacokinetics, drug efficacy and toxicity. This white paper provides a review of changes in transporter function associated with acute and chronic disease states, describes regulatory pathways affecting transporter expression, and identifies opportunities to advance the field.
Why a White Paper?
The importance of transporters in drug absorption, distribution, metabolism and excretion (ADME) has been widely recognized for over 30 years, and drug candidates are routinely tested as substrates and inhibitors for a panel of drug transporters during development (1). Our understanding of the role of drug transporters in humans is largely based on experience in healthy subjects. However, emerging data indicate that transporter activity may be altered in various diseases, which could impact drug efficacy and/or toxicity in patients. Unfortunately, much of the knowledge regarding disease alterations in transporters has been generated in preclinical species, and the translation of such findings to clinical practice is presently limited. At the third International Transporter Consortium (ITC) Workshop, organized in Washington DC in March 2017, several examples were presented illustrating the current understanding of the effects of disease on transporter function in humans. Subsequently, a group of expert scientists from academia and pharmaceutical industry convened to discuss current knowledge about drug transporter function in acute and chronic disease states in humans, potential approaches to reduce drug-mediated organ toxicity, and mechanisms controlling transporter expression and activity. This white paper reviews the current status of the field and discusses potential implications from a drug discovery and development perspective.
Complexities in the physiological roles of transporters and the interplay between transporters and metabolic enzymes
Historically, the field of drug transport has focused on interactions of exogenous chemicals (i.e., drugs) with transporters, either as substrates or inhibitors, often in a very linear process where one drug and one transporter are examined at a time under normal physiological conditions. However, the contribution of membrane transporters to physiological and biological processes is vast. One of the most important principles of membrane transporters is that they often work in pairs or groups with other proteins determining the fate of an endogenous or exogenous chemical (e.g., creatinine, uric acid, bilirubin, drugs). For instance, in polarized cells, drugs may be transported into the cells by basolateral uptake transporters and exported out of the cells by apically-localized efflux transporters.
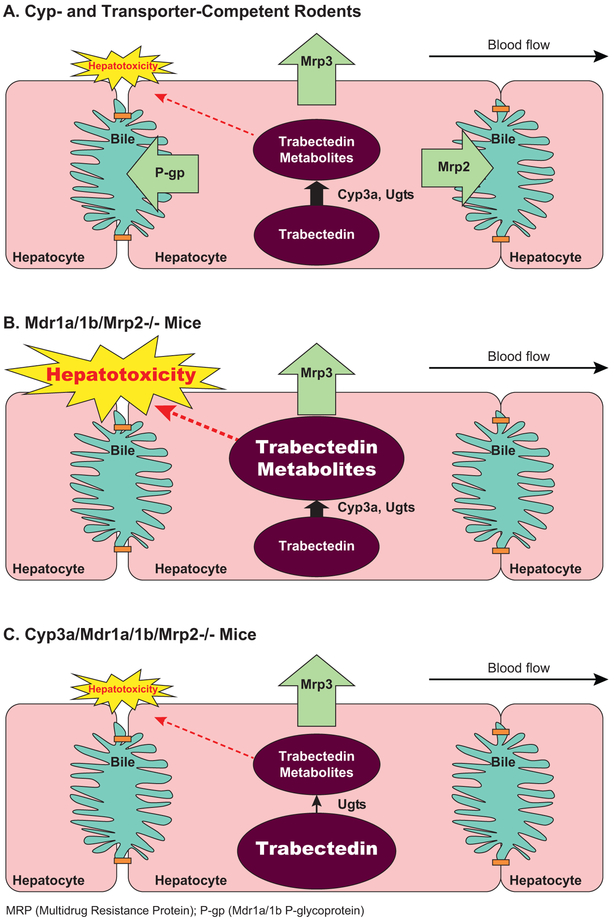
Disease-mediated alterations in transporter-mediated processes cannot be considered in isolation because of the extensive sharing of pathways and redundancy within the transporter families, and interplay with metabolic enzymes. This point is nicely illustrated with the anti-cancer agent trabectedin and the observed clinical hepatotoxicity (Figure 1) (2, 3). Trabectedin undergoes extensive hepatic cytochrome P450 (CYP)-mediated biotransformation, and metabolites are eliminated into bile; trabectedin is a multidrug resistance 1 P-glycoprotein (MDR1 P-gp), multidrug resistance protein 2 (MRP2) and MRP3 substrate. Data from sandwich-cultured hepatocytes (SCH) and experiments with knockout mice highlighted the importance of Cyp3a-generated metabolites as hepatotoxicants as opposed to trabectedin itself, and demonstrated that transporters are pivotal in protecting the cell by removing hepatotoxicant(s). Loss-of-function of two transporters (e.g., Mrp2 and Mrp3; P-gp and Mrp2) resulted in severe hepatotoxicity, whereas the loss of one efflux pathway only produced a modest insult. This example emphasizes the importance of the interplay between transporters and drug metabolizing enzymes and the built-in redundancy in excretion pathways.
Figure 1. Interplay of enzymes and transporters in trabectedin-mediated hepatotoxicity.
Panel A. Trabectedin is metabolized by Cyp3a and both parent and metabolites are substrates for P-gp, Mrp2 and Mrp3. Pretreatment with dexamethasone, a CYP3a inducer, reduced hepatotoxicity, suggesting that CYP3A was protective. Hepatoprotection from trabectedin-mediated cytotoxicity in rat sandwich-cultured hepatocytes by dexamethasone treatment was attributed to enhanced metabolism and biliary excretion due to protein induction of Cyp3a and Mrp2, respectively, although the anti-inflammatory effect of dexamethasone could have contributed to the effect as well (3). Panel B. In Mdr1a/1b/Mrp2−/− mice, hepatotoxicity was severe, with liver transaminase levels >100-fold relative to the wild-type treated mice (2). This finding suggested that transporters are pivotal in protecting the cell by removing hepatotoxicant(s). Panel C. Only mild hepatotoxicity was observed in Cyp3a/Mdr1a/1b/Mrp2−/− mice, highlighting the importance of Cyp3a-generated metabolites as hepatotoxicants and not trabectedin itself. Loss-of-function of two transporters (e.g., Mrp2 and Mrp3; P-gp and Mrp2) caused severe hepatotoxicity, whereas the loss of one efflux pathway only produced a modest insult (2).
Exogenous molecules can alter the transport of endogenous compounds, including important clinical biomarkers such as creatinine, glucose, bilirubin, and hormones. The mechanism(s) of interaction include inhibition, induction or a combination of these processes. An interesting example of transporter induction is the case of DMP 904, a corticotropin releasing factor receptor 1 antagonist that disrupts thyroid hormone homeostasis in rats (4). Generally, alterations in thyroid hormone levels occur either by a direct action on the thyroid gland or by increased clearance of the hormone itself due to induction of thyroxine (T4) glucuronidation, with excretion of the conjugate into bile. Historically, transporters were not considered to be a key component of the clearance pathway. Following DMP 904 administration to rats, serum T4 levels were below detectable limits (<1 μg/dL) at 72 hr, with tri-iodothyronine (T3) levels reduced ~70%, and T4 total body clearance increased 6-fold. Even though Cyp and glucuronosyl transferase (Ugt) enzymes were induced, T4 clearance into bile was enhanced 80-fold due to increased functional activity of Oatps and Mrp2, the transporters that respectively mediate the hepatic uptake and biliary excretion of thyroxine.
These examples serve to highlight the complexity of transporter interactions among these proteins as well as with metabolic enzymes and nuclear receptors, a topic that is addressed at the end of this white paper. These complexities must be considered when evaluating the effects of disease on drug disposition.
Transporter changes associated with acute disease and altered physiological states
Over the past several decades, numerous studies have reported increased plasma drug concentrations in patients with infectious or acute inflammatory conditions, a phenomenon replicated in animal models. This was attributed primarily to changes in drug metabolism and plasma protein binding. However, more recent studies have provided evidence that ischemic injury and inflammation-mediated changes in the expression and activity of drug transporters also may contribute to altered drug concentrations.
In the following section, changes in transporter activity related to acute illness or acute relapse of chronic disease are reviewed (Table 1). The main focus is on the impact of infectious- and auto-immune-mediated inflammation or ischemia on human transporter expression and activity. In several diseases, inflammation results in the release of pro-inflammatory cytokines, especially interleukins (e.g., IL-1, IL-6 and IL-8), tumor necrosis factor (TNF-α) and interferon gamma (IFN-γ) as an acute phase response (5). These pro-inflammatory cytokines are known to impact the expression and activity of drug-metabolizing enzymes, but can also alter membrane transporters (5).
Table 1.
Changes in transporter expression levels in various acute disease states versus healthy subjects*
| Disease | Organ | Transporter mRNA (Species) |
Transporter Protein (Species) |
Clinical or
Preclinical ‘phenotype’ |
References |
|---|---|---|---|---|---|
| Inflammation | Liver | Human: postmortem
sepsis: ↓BSEP ↑ MRP3, MRP4 |
(9) | ||
| ICG: ↓ CL in sepsis non-survivors and IL-6 plasma levels correlated with ICG CL |
(8) | ||||
| LPS treated liver slices: ↔ MRP2 and BSEP |
LPS treated liver slices: ↓MRP2 and BSEP |
(7) | |||
| Oncostatin M treated
hepatocytes: ↓NTCP, OATP 1B1, OATP1B3, OATP2B1, OCT1, OAT2, MRP2, BCRP ↔ P-gp, MRP3, MRP4, BSEP |
Oncostatin M treated
hepatocytes: ↓NTCP, OATP1B1, OATP2B1, BCRP |
Oncostatin M treated
hepatocytes: ↓NTCP, OATP |
(99) | ||
| IL-6 treated hepatocytes: ↓P-gp, MRP2, BCRP, NTCP, OATB1B1, OATP1B3, OATP2B1, OCT1 |
IL-6 treated hepatocytes: ↓MRP2, BCRP, NTCP, OATP1B1 ↑MRP3 ↔P-gp |
IL-6 treated hepatocytes: ↓NTCP, OATP, OCT1 |
(6) | ||
| TNF-α treated
hepatocytes: ↓BSEP, NTCP, OATP1B1, OATP1B3, OCT1, OAT2 |
TNF-α treated
hepatocytes: ↓NTCP, OATP1B1, ↑MRP3, BCRP ↔MRP2, P-gp |
TNF-α treated
hepatocytes: ↓NTCP, OATP, OCT1 |
(6) | ||
| IFN-ϒ treated
hepatocytes: ↓P-gp, MRP2,MRP3, BCRP, BSEP, OATP1B1, OATP1B3, OATP2B1 |
(6) | ||||
| Intestine | TNF-α, IL-1β,
INF-ϒ mixture treated Intestinal biopsies: ↓P-gp |
(100) | |||
| ↓ P-gp activity in patients with diarrhea | (16) | ||||
| Ischemic Kidney Injury | Kidney | Rat: ↓Oat1, Oat3, Oct2, Mate |
Human: ↓OAT1, OAT3 Rat: ↓Oat1, Oat3 ↑ P-gp (endotoxemia) |
(23,24,29) | |
| Stroke | Blood brain barrier | Human: ↑P-gp Rat: ↑P-gp, Bcrp, Mrp5, Oatp2 |
Human: ↑P-gp Mouse: ↑P-gp (↓)Mrp1 |
(32-35) | |
| Chorioamnionitis | Placenta | TNF-α or IL-1β treated primary
human term trophoblasts: ↓ P-gp, BCRP |
TNF-α or IL-1β treated primary
human term trophoblasts: ↓ P-gp, BCRP |
TNF-α treated primary human term
trophoblasts: ↓ BCRP activity |
(36) |
| Human: choriamniotic placenta: ↑ or ↓BCRP ↑ or ↔ P-gp ↓OATP2B1 |
Human: choriamniotis placenta: ↓BCRP ↔ P-gp ↓OATP2B1 |
(38,39) | |||
| Intra-uterine growth retardation | ↓P-gp | ↓P-gp | (101) |
ICG = Indocyanine green; CL = plasma clearance; ↓, decreased expression; ↑, increased expression; ↔, no effect
Data obtained in preclinical species were only included in cases where human data were not available
Acute Inflammation and liver transporters
Inflammation-induced changes in the expression and function of membrane transporters have been documented in various models of acute inflammation in vitro and in vivo (reviewed in (5, 6)). Most often, studies use IL-6, IFN, IL-1β, TNF-α, and liposaccharides (LPS) as inducers of inflammation in human hepatocytes or human liver slices. In inflammation-induced disease states, the general picture is a decrease in mRNA expression, protein levels and activity for most, but not all transporters (Table 1). Variation in the reported correlations between inflammation and transporter expression and activity are likely due to differences in study design, experimental conditions, and/or end-point measurements. For example, it was observed that MRP2 and bile salt efflux pump (BSEP) transcript levels were largely unchanged in LPS-treated human liver slices, but protein levels of these transporters were virtually obliterated, suggesting that post-transcriptional regulatory mechanisms may play a role in humans (7).
Human ex vivo or clinical studies investigating the impact of critical illness and inflammation on transporter expression and/or function are scarce. In patients with sepsis, the clearance of indocyanine green (ICG), which was used as a combined marker of liver blood flow, hepatic uptake by organic anion transporting polypeptide 1B3 (OATP1B3) and sodium-taurocholate co-transporting polypeptide (NTCP), biliary excretion by the phospholipid translocator MDR3 P-gp (MDR3), and energy metabolism was significantly higher in sepsis survivors on days 1 and 3 after diagnosis than in non-survivors (8). Weak, but significant positive correlations were found between IL-6 levels and ICG plasma disappearance rates. Moreover, in liver transplant recipients with a biliary t-tube, ICG plasma clearance was reduced in all patients, but ICG biliary excretion was almost negligible in patients with an unfavorable course after liver transplant (8). The authors speculated that these findings point to a significant inflammatory response with local cytokine production along with glutathione depletion initiated by cold ischemia and reperfusion in the patients with the unfavorable response. Data from these clinical studies are consistent with data from human liver slices, where the canalicular transporters, MDR3 and MRP2, were indeed more sensitive to down-regulation than the basolateral transporters, OATP1A2 and OATP1B1, by either inflammation induced by LPS, IL-1β or TNF-α, or glutathione depletion induced by phorone (8). Interestingly, in contrast to ICG, bilirubin excretion was less affected in this inflammation/hypoxia-related downregulation of transporter function.
In addition, VanWijngaerden et al. (9) studied critical illness-related hyperbilirubinemia and the relationship with bile acid metabolism and transport. In postmortem liver biopsies from prolonged critically ill patients, expression of CYP7A1 protein, the rate limiting enzyme in bile acid synthesis, was similar to controls. In contrast, BSEP protein staining was reduced, while BSEP mRNA expression was increased 2-fold. Furthermore, MRP3 staining was increased, in concert with a 3-fold higher mRNA expression. MRP4 mRNA expression was also 3-fold increased, but staining data were not reported. In accord with these changes in transporter levels, serum conjugated bile acid concentrations were increased (9). Increased bile acid serum concentrations were also reported in a large cohort of intensive care unit (ICU) patients with a prolonged length of ICU stay, and effects were similar in patients with and without sepsis (10). The similar changes in non-sepsis patients may be explained by ischemia/reperfusion-related inflammation and cytokine release, because a large proportion of non-sepsis patients were admitted to the ICU post-cardiac surgery. These findings also are consistent with hypoxia-associated increases in hepatic basolateral organic anion transporter α and β (OSTα/β) expression, which may contribute to increased efflux of bile acids from hepatocytes into the systemic circulation (11).
Overall, emerging data suggest that the pharmacokinetics (PK) of a multitude of drugs administered to critically ill patients may be altered in severe sepsis or ischemia/reperfusion states (e.g., directly after liver transplant) due to changes in hepatic transporters. Unfortunately, definitive clinical data to support this claim have not yet been reported.
Acute inflammation and intestinal transporters
Frequent reports of increased plasma concentrations of tacrolimus in transplant patients suffering from infectious or chronic diarrhea (12) (13), have been linked to decreased intestinal P-gp activity in these patients. Consistent with these observations, incubation of human intestinal Caco-2 cells with TNF-α or IL-2 produced a strong time-dependent attenuation of P-gp mRNA levels along with significant decreases in Rhodamine 123 transport (14,15). Cytokine-dependent decreases in P-gp expression have been reported in human intestinal biopsy samples treated with the pro-inflammatory cytokines TNF-α, IL-1β or IFN-ϒ (16). On the other hand, Bertilsson et al. (17) reported induction of P-gp mRNA levels and increased apparent permeability in Caco-2 cells after incubation with IL-1β, IL-6 or IFN-ϒ. Infection with the enteropathogenic Escherichia coli (E. coli), an important causative pathogen for infantile diarrhea, correlated with down-regulation of the intestinal apical monocarboxylate transporter 1 (MCT1) expression in Caco-2 cells (18). The bioactive soluble factors secreted by the probiotic Lactobacillus acidophilus upregulated MCT1 and abolished the MCT1 down-regulation induced by E. coli in Caco-2 cells (19). MCT1 expression and activity also were decreased in mucosal inflammation and colon cancer ((19) and references therein). Exposure of healthy adults to high-altitude (>4500 meters) hypoxia increases serum pro-inflammatory markers. In duodenal biopsies from volunteers exposed to acute high altitude, mRNA levels of most transporters were significantly decreased (20); exceptions were the equilibrative nucleoside transporter 2 (ENT2) and the glucose transporter 1 (GLUT1), which showed a trend towards higher expression.
As the intestine plays a central role in absorption, first pass metabolism, enterohepatic recycling of drug (metabolites) and bile salts, an integrated translational approach is needed to explore the complexity and interplay of intestinal transporters and enzymes. In particular, drug induced diarrhea, the composition of the microbiome, levels of pro-inflammatory cytokines, and bile acid homeostasis may all modulate expression levels of drug transporters and thereby drug disposition.
Ischemic kidney injury
Ischemia reperfusion (I/R) injury is a major cause of acute kidney injury (AKI). In addition to a rapid decline in glomerular filtration rate (GFR), tissue damage results in altered expression of several drug transporters in the basolateral and apical membranes of proximal tubular cells. I/R injury to human renal allografts resulted in an 8.6-fold reduced clearance of the organic anion transporter 1 (OAT1) substrate para-aminohippurate (PAH) in patients with sustained acute renal failure (21). Immunohistochemical analysis of transplanted kidneys, one hour after reperfusion, demonstrated aberrant distribution to the cytoplasm and/or diminution of OAT1, which is normally localized in the basolateral membrane of proximal tubular cells (22). This was confirmed by increased urinary levels of OAT1 and OAT3 protein in patients with AKI, which can be explained by the translocation of these basolateral transporters to the apical membrane and their subsequent loss in urine (23).
In I/R rat kidney, the protein expression of Oat1 and Oat3 was down-regulated. GFR and PAH secretion were initially reduced, but gradually improved after 24–48 hours, which was paralleled by a recovery of the expression of Oat1 and Oat3 (24). A proposed underlying mechanism is the inflammatory response to tissue damage and increased systemic levels of the uremic toxin indoxyl sulfate because of Oat1 and Oat3 dysfunction (25). This results in excessive synthesis of prostaglandin E2 and subsequent prostaglandin E receptor (EP) 4-mediated inhibition of Oat transcription (26). Non-steroidal anti-inflammatory COX-1 inhibitors, including indomethacin, appeared to protect rat kidneys against ischemia reperfusion injury (27). The urinary excretion of cationic drugs was decreased to a similar extent in I/R rat kidney injury. The expression of basolateral organic cation transporter 2 (Oct2) and apical multidrug and toxin extrusion 1 transporter (Mate1) were more than 4 to 5-fold down-regulated after 48 hours, whereas apical expression of P-gp was not changed (28). In contrast, upregulation of P-gp has been found during endotoxemic AKI in rats (29).
Stroke
Imaging studies have shown that the permeability of the human blood brain barrier (BBB) is rapidly increased after ischemic stroke (30). Rodent models demonstrated that P-gp is upregulated in brain capillary endothelium within three hours after stroke, which may limit the entrance of neuroprotective drugs (31). Increased luminal expression of P-gp is likely mediated by hypoxia-inducible factor-1, as was observed in human microvascular endothelial cells (32). Even when BBB permeability was increased, P-gp efflux maintained low drug concentrations in ischemic mouse brain, and inhibition of P-gp by tariquidar enhanced the penetration and efficacy of two neuroprotective substrate drugs (33). The abluminal BBB transporter Mrp1 was found to be down-regulated by ~50% in mice at 3 hr after cerebral artery occlusion, and expression partly recovered within 24 to 72 hr after stroke. MRP1 downregulation was accompanied by a lower efficacy of neuroprotective and neurotoxic compounds, indicating that Mrp1 may act as a gateway for drugs to treat the ischemic brain (34). Limited information is available on the effect of stroke on other relevant drug transporters in the BBB. Following cerebral artery occlusion in rats, after 14 days a maximum increase in mRNA and protein expression of the breast cancer resistance protein (Bcrp; 4 to 5-fold) and Mrp5 (1.5 to 2.5-fold) was found, and a 2.0 to 2.5-fold upregulation of Oatp2 mRNA (35).
Chorioamnionitis and placental transport
In vitro studies with primary term trophoblasts demonstrated that treatment of cells with the pro-inflammatory cytokines TNF-α or IL-1β significantly decreased P-gp and BCRP expression at the gene and protein level (36). Incubation of first but not third trimester placental villous explants with endotoxin reduced mRNA and protein expression of P-gp and BCRP (37). In contrast, incubations with polyinosinic:polycytidylic acid, an immunostimulant that interacts with toll-like receptor 3, decreased P-gp expression in the third trimester, but not in the first trimester explants. These data suggest that inflammatory triggers may be dependent on gestational age. Studies examining human placental tissues isolated from women with chorioamnionitis, a bacterial infection of the placental membranes, have shown contradictory results. As compared to healthy preterm controls, placentas collected from preterm women with chorioamnionitis demonstrated 2.5 to 3-fold increases in mRNA levels of IL-6, IL-1β and TNF-α in one study, which highly and positively correlated with mRNA levels of P-gp, although significant differences in P-gp mRNA or protein expression were not detected between groups (38). A significant 2-fold decrease of BCRP and OATP2B1 also was associated with this inflammation. In a similar study in preterm chorioamnionitis and controls, a 1.7-fold increase in P-gp mRNA levels highly correlated to a 4-fold increase in IL-8 expression while no change in IL-6, IL-1β and TNF-α expression was found (39).
Similar inconsistencies regarding the effect of disease on BCRP regulation in the placenta exist in the literature. Incubation of placental villous explants with polyinosinic:polycytidylic acid did not have a significant effect on BCRP expression (37). Furthermore, both an induction and reduction of BCRP have been reported in human placental tissues isolated from women with chorioamnionitis, as compared to healthy controls. In one study, a 2-fold reduction in BCRP transcript and protein levels was observed when placentas were obtained at either term or preterm parturition alongside markedly elevated levels of IL-6, IL-1β and TNF-α (38). In contrast, 2-fold increased BCRP mRNA levels were observed in human placentas obtained from chorioamnionitis cases with preterm labor (39). As IL-6 and TNF-α levels were not altered in this study, it is plausible that differences in disease severity, drug administration or gestational stage could be responsible for the apparent discrepancies. Indeed, treatment of placental cells with prostaglandin E2 resulted in an increase in mRNA expression and activity of BCRP (40). Interestingly, P-gp protein and mRNA levels in the placenta, as well as cytokine mRNA expression of IL-1a, IL-1b, and IL-10 were decreased in infants born “small for gestational age” compared to normal infants, independent of time from exposure to exogenous corticosteroids, which were administered to prevent preterm labor (101).
Overall, these data suggest that chorioamnionitis and intrauterine growth retardation result in altered placental transporter expression, and represent a theoretical risk of increased fetal exposure to P-gp and BCRP substrates. Exposure in the first trimester to P-gp, but not BCRP substrates, has been associated with a higher risk of congenital anomalies (41). The lack of an association with BCRP substrates likely stems from the low number of exposed women. These studies suggest that altered placental transport later in pregnancy due to chorioamnionitis may similarly alter the risk of fetal exposure and direct drug effects in the full grown term neonate. To our knowledge, however, clinical data showing impact on placental transport late in pregnancy is lacking and, therefore, medication necessary for women with chorioamnionitis should not be withheld or adjusted.
Changes in transporters in chronic disease states
Human Immunodeficiency Virus (HIV)
Inflammation and increased levels of pro-inflammatory cytokines, which could cause transporter dysregulation, are found in numerous chronic illnesses, including HIV (42). Preclinical studies in HIV (+) transgenic rats and in viral protein-treated cultured astrocytes demonstrated HIV-associated alterations in the expression and function of several ABC transporters that are thought to occur primarily through activation of inflammatory responses (42). Indeed, treatment of primary cultures of human astrocytes with the HIV viral protein gp120 stimulated the release of TNF-α, IL-6, and IL-1β and decreased P-gp expression and activity, resulting in a 1.6-fold increase in digoxin accumulation (42). Corresponding changes may be observed clinically; P-gp and MRP2 protein expression were decreased by 2.5 to 3.3-fold in recto-sigmoid colon tissues from antiretroviral-naïve HIV infected patients, compared to uninfected controls (Table 2) (42). Likewise, protein expression of MRP2 and BCRP was 2.2 to 3.3-fold lower in the upper intestinal tissues of treatment naïve HIV-infected patients compared to control subjects (42). As MRP2, BCRP and P-gp limit the intestinal absorption of numerous antiretroviral agents, decreased expression of these transporters could affect oral bioavailability. For example, although the effect was modest, Cmax of the P-gp substrate digoxin increased 1.3-fold in treatment naïve patients (43). However, the intestinal and colonic expression of these transporters was increased to uninfected control levels in patients on antiretroviral therapy (43). Clinical PK studies with transporter probe substrates are warranted to investigate potential changes in drug transporter activity in HIV infected patients.
Table 2.
Changes in transporter expression levels in various chronic disease states versus healthy subjects*
| Disease | Organ | Transporter mRNA |
Transporter Protein |
References |
|---|---|---|---|---|
| Chronic Kidney Disease | Kidney | Rat: ↑Mrp2, Mrp3, Mrp4, Oatp2, Oatp3 ↓Oatk2, Oatp1, OATP4c1, Oatp5, P-gp, Urat1, Npt1 |
Rat: ↑Mrp2, Mrp3, Mrp4, Oatp2, Oatp3 ↓Oat1, Oat2, Oat3, Oatp1, Oatp4c1, P-gp, Urat1, Npt1 |
(102) |
| Liver | Rat: ↑P-gp, Mrp2 |
Rat: ↑P-gp ↓Oatp2 |
(103) | |
| Intestine | Rat: ↓P-gp, Mrp2, Mrp3 |
(104) | ||
| Nonalcoholic Fatty Liver Disease/Nonalcoholic steatohepatitis | Liver | Human: ↓BSEP (males and females), MRP2 (females), NTCP (males and females) |
(105) | |
| Human: ↓MRP6 ↑MRP1, MRP3, MRP4, MRP5, P-gp, BCRP |
Human: ↑MRP1, MRP3, MRP4, MRP5, MRP6, P-gp, BCRP |
(57) | ||
| Human: ↔OATP1B1, OATP2B1 ↓OATP1B3 |
Human: ↔OATP1A2 ↓OATP1B3 ↑OATP1B1 |
(54) | ||
| Hepatitis C Cirrhosis | Liver | Humana: ↓NTCP, OATP1B3, OCT1, BSEP, MRP2, P-gp ↑ MATE1 ↔ OATP1B1, OATP2B1, BCRP, MRP3 |
(46) | |
| Human: ↓OCT1, OAT2, OAT7, OATP1B1, OATP1B3, OATP2B1, MATE1, MRP2, MRP3, MRP6, BCRP ↑P-gp, MRP1, MRP4, MRP5 |
Human: ↑MRP4 |
(48) | ||
| Alcoholic Cirrhosis | Liver | Humana: ↓NTCP, OATP1B1, OATP1B3, OCT1, BCRP, BSEP ↑MATE1, MRP3 ↔ OATP2B1, MATE1, MRP2, P-gp |
(46) | |
| Human: ↓OATP1B3, ↑MRP4, MRP5, BCRP, OATP2B1, MRP1, MRP3, MRP5 |
(47) | |||
| HCV Hepatocellular Carcinoma | Liver | Human cancerous tissuea: ↓NTCP, OATP1B1, OATP1B3, OATP2B1, OCT1, BSEP, MRP2, MRP3, P-gp ↔ MATE1 Human non-cancerous tissuea: ↓OATP1B1, OATP1B3, OATP2B1, OCT1, BSEP, MATE1, MRP3, P-gp ↑NTCP ↔ MRP2 |
(106) | |
| Human Immuno-Deficiency Virus | Intestine | Human: ↓MRP2, BCRP |
Human: ↓MRP2, BCRP |
(107) |
| Colon | Human: ↓P-gp, MRP4 |
Human: ↓P-gp, MRP2 |
(108) | |
| Inflammatory Bowel Disease | Colon | Human: ↓P-gp, BCRP, MRP3, MRP4, OCTN2, OSTα/β ↑OATP2B1, OATP4A1, ENT1, ENT2, CNT2, PEPT1 |
Human: ↓P-gp, BCRP ↑MRP1 |
(65,67-69) |
| Ileum | Human: ↓ASBT, BCRP, OCTN2 ↑OATP2B1, ENT1, ENT2 |
(67,68) |
↑, expression increased in disease state relative to controls; ↓, expression decreased in disease state relative to controls; mg, milligram
Data obtained in preclinical species were only included in cases where human data were not available
Data were normalized to picomole/gram of liver
Liver fibrosis and cirrhosis
Chronic liver injury due to ethanol abuse, cholestasis, hepatitis C (HCV) infection, or the metabolic syndrome can result in hepatic fibrosis (see (44) for an excellent review on this topic). After acute liver injury, parenchymal cells regenerate and replace necrotic or apoptotic cells. If injury persists, the liver’s ability to regenerate eventually fails and hepatocytes will be replaced with extracellular matrix, including fibrillar collagen, by activated hepatic stellate cells, Kupffer cells and myofibroblasts. The activation of immune cells, hepatocytes and cholangiocytes, results in the excretion of a wide range of immunomodulatory molecules such as pro-inflammatory cytokines, resulting in an inflammatory response. Initially, patients with fibrosis show no phenotype, but after a period of 15–20 years, fibrosis may transition to cirrhosis and ultimately hepatocellular carcinoma. Cirrhosis is characterized by major clinical complications such as ascites, renal failure, hepatic encephalopathy, and variceal bleeding (44).
The effect of cirrhosis on transporter expression has been studied in different patient populations. Liver disease is a complication in children with parenteral nutrition-dependent intestinal failure. In these patients, portal inflammation correlated with increased expression of IL-6 and TNF-α, repression of FXR and decreased protein expression of BSEP and MRP4, although effects were relatively modest (<2-fold) (45).
Transport protein amounts, measured by quantitative proteomics in liver tissue from patients with HCV-cirrhosis was significantly altered (up to ~2-fold, depending on the protein; see Table 2). Interestingly, the effects observed were different in patients with alcoholic cirrhosis (Table 2) (46). In general, transporter protein expression was reduced in liver tissue from patients with alcoholic cirrhosis, with the exception of MATE1 and MRP3, which were increased. The results of protein quantification were different from mRNA analysis of alcoholic (47) or HCV (48) cirrhotic livers, suggesting that changes in protein levels do not correlate with mRNA, but rather are affected by post-transcriptional events, or that effects depend on disease state, ethnicity, and/or other intrinsic and extrinsic factors.
In cirrhotic liver tissue from patients with chronic HCV, protein expression of NTCP, OATP1B3, OCT1, BSEP, MRP2 and P-gp decreased when expressed per gram liver tissue relative to healthy control liver tissue (Table 2) (46). In patients with chronic HCV and varying degrees of fibrosis, reduced hepatic uptake of 99mTc-mebrofenin, an OATP1B1, OATP1B3 and MRP2 substrate, and increased 99mTc-mebrofenin hepatic exposure, measured by gamma scintigraphy, were consistent with impaired function of OATPs and MRP2, respectively (49).
In general, most drugs can be used safely in cirrhosis, although lower doses are often needed due to changes in PK (111). For example, AUC0-inf and Cmax values of pitavastatin acid were increased 3.6- and 2.5-fold, respectively, in patients with cirrhosis Child-Pugh B score relative to healthy controls (Table 3), whereas these values were decreased 0.7- and 0.5-fold, respectively, for pitavastatin lactone (50). Most likely, these changes are due to reduced transport and UGT-mediated metabolism in this patient population. The OATP1B1 substrate repaglinide is another example demonstrating altered PK of a drug in cirrhotic patients compared to control (51), likely due to reduced amounts of transporter protein. Incorporation of OATP1B1 protein quantitation data into a physiologically-based PK/pharmacodynamics (PD) model improved the prediction of repaglinide disposition for patients with alcoholic liver cirrhosis (46).
Table 3.
Examples of the effect of disease state on the exposure of transporter substrates in human
| Disease State |
Drug | Transporter/Enzyme Substrate |
AUC0-last
Patients |
AUC0-last
Healthy Subjects |
Mean Ratio |
Cmax
Patients |
Cmax
Healthy Subjects |
Mean Ratio |
Cl/F Patients |
CL/F Healthy Subjects |
Mean Ratio |
Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLD (majority with Child-Pugh grade B or C) | Repaglinide (4 mg P.O.) | OATP1B1, CYP2C8, CYP3A4 | 368.9 ng*h/mL | 91.6 ng*h/mL | 4.34 | 105.4 ng/mL | 46.7 ng/mL | 2.46 | - | - | - | (51) |
| CLD (Child-Pugh A) | Pitavastatin (2 mg P.O.) | OATP1B1, OATP1B3, BCRP, MRP2, UGT1A3, UGT2B7 | 174.1 ng*h/mL | 135.9 ng*h/mL | 1.28 | 70.7 ng/mL | 59.5 ng/mL | 1.19 | - | - | - | (50) |
| CLD (Child-Pugh B) | Pitavastatin (2 mg P.O.) | OATP1B1, OATP1B3, BCRP, MRP2, UGT1A3, UGT2B7 | 481.1 ng*h/mL | 135.9 ng*h/mL | 3.54 | 147.1 ng/mL | 59.5 ng/mL | 2.47 | - | - | - | (50) |
| HD-CKD | Fexofenadine (120 mg P.O.) | P-gp, OATP1B3, OATP2B1 | 2,327 ng*h/mL | 1,008 ng*h/mL | 2.3 | 531.1 ng/mL | 246.7 ng/mL | 2.2 | 60 mL/min | 135 mL/min | 0.44 | (109) |
| CKD (severe, non-dialysis patients) | Pitavastatin (4 mg P.O.) | OATP1B1, OATP1B3, BCRP, MRP2, UGT1A3, UGT2B7 | 164 ng*h/mL | 125.9 ng*h/mL | 1.3 | 74.28 ng/mL | 63.12 ng/mL | 1.18 | - | - | - | (110) |
| NASH | Morphine-glucuronidesa | MRP3 | 58.8 μM*min | 37.2 μM*min | 1.6 | 343 nM | 225 nM | 1.5 | - | - | - | (60) |
| NASH | 99mTc-mebrofeninb | OATP1B1, OATP1B3, MRP2, MRP3 | 2,440 µCi*min/L | 1,780 µCi*min/L | 1.4 | 286 µCi/L | 136 µCi/L | 2.1 | (56) | |||
| Pediatric NASH | Acetaminophen-glucuronidec | MRP3 | 207.7 nmol*h/mL | 143 nmol*h/mL | 1.4 | - | - | - | - | - | - | (59) |
CLD: Chronic Liver Disease; CKD; Chronic Kidney Disease; HD-CKD: hemodialysis-Chronic Kidney Disease; -, no data reported
No significant changes for morphine parent drug were observed
AUC0-180,liver: 277 vs. 433 kcounts*min/sec (liver activity-time curves measured by gamma scintigraphy) in NASH patients vs. healthy subjects (1.6-fold mean ratio)
No significant changes for acetaminophen parent drug were observed
Nonalcoholic Steatohepatitis (NASH)
NASH is an advanced form of nonalcoholic fatty liver disease characterized by hepatic steatosis, hepatocyte ballooning, and lobular inflammation. NASH may progress to advanced fibrosis, cirrhosis, hepatocellular carcinoma (HCC), and fulminant liver failure in some patients, and is a major public health concern due to the growing obesity epidemic. Altered expression and function of transport proteins associated with NASH may have important implications for drug therapy in these patients (see Table 3) (52).
Global gene expression analysis revealed that hepatic uptake transporter genes (53) were downregulated, consistent with a significant decrease in OATP1B3 protein expression in liver tissue from NASH patients (Table 2) (54). In contrast, OATP1B1 protein expression was significantly increased in NASH patients, although no change in OATP2B1 protein was noted (54). A significant increase in the amount of unglycosylated OATP1B1, OATP1B3, OATP2B1 and NTCP protein was detected in liver tissue from NASH patients (55), which may contribute to decreased function of these transporters. Indeed, decreased hepatic uptake of 99mTc-mebrofenin measured by gamma scintigraphy, was reported in patients with biopsy-confirmed NASH (56), consistent with NASH-mediated impairment of OATP function.
Liver samples from patients with NASH exhibited increased protein amounts of MRP1–6, P-gp and BCRP. Interestingly, although MRP2 protein expression was increased in NASH liver tissue, a significant increase in unglycosylated protein was observed (57) (55). Reduced glycosylation of transporter proteins (i.e., Mrp2) typically decreases membrane localization and function (58). These findings are consistent with internalization of MRP2, as assessed by immunohistochemistry, in liver tissue from adult (57) and pediatric (59) NASH patients. Increased hepatic exposure (~2-fold) to 99mTc-mebrofenin in NASH patients was consistent with impaired MRP2 function (56). Additionally, impaired MRP2 function, as well as induction of the basolateral efflux transporter MRP3, may explain increased systemic exposure to morphine glucuronides in adult NASH patients (60), and elevated serum and urine concentrations of acetaminophen glucuronide in pediatric NASH patients (59) (Table 3).
Diabetes and metabolic syndrome
Diabetes and metabolic syndrome are complex heterogeneous disorders, and the potential for altered transporter expression and function in humans warrants investigation considering the growing number of patients affected by these diseases. Unfortunately, to date, the majority of studies examining changes in transporter function have been limited to rodent models (See Supplemental Material). However, there are a few examples demonstrating how the onset of diabetes and metabolic syndrome could alter transporter function in humans. The most familiar case is associated with insulin, which tightly controls blood glucose concentrations and is a well-known stimulator of GLUT4 translocation to the membrane (61). Insulin exposure also induces organic cation-carnitine transporter 2 (OCTN2) mRNA 2.3-fold in human muscle (62). Changes in other transporters have been identified, such as in patients with diabetic nephropathy who exhibit reduced OAT1 and OAT3 mRNA levels (63), and diabetic patients who have altered absorption of PEPT1 substrates (64).
The studies described above represent a starting point in characterizing the effects of diabetes and metabolic syndrome on regulation of transporter function. However, a substantial effort is needed to address all changes and the clinical impact. As clinical data emerge, it will be important to determine whether changes in transporters noted in preclinical models or in cell-based systems translate to patients. Additionally, these findings highlight another important question that needs to be addressed: are such changes due to diabetes-induced tissue damage, or are they the result of altered metabolic and/or cell signaling activity?
Inflammatory Bowel Disease (IBD)
A marked decrease in the amount of P-gp (6.7-fold) and BCRP (2.7-fold) protein has been observed in intestinal samples obtained from patients with IBD, as compared to samples from healthy control subject (65). This change was associated with disease activity; transporter dysregulation occurs in active disease and is significantly correlated with elevated intestinal levels of IL-6 (65). A pronounced increase in Cmax (4.2-fold) and AUC0–6h (4.2-fold) of the P-gp and CYP3A substrate propranolol has been reported in patients with active Crohn’s disease (66), related to altered CYP3A4 and perhaps P-gp activity.
Many of the major bile acid transporters are dysregulated in IBD. Samples obtained from patients with IBD demonstrated a pronounced downregulation of mRNA encoding the apical sodium-dependent bile salt transporter (ASBT) in ileal tissues (67,68), and mRNA downregulation of MRP3, MRP4, P-gp and OSTα/β in colonic tissues (67). In contrast, MRP1 protein was induced in colonic tissue from IBD patients (69).
Dysregulation of solute carier superfamily (SLC) drug transporters also appears to occur in IBD. Transcript levels of OATP2B1, OATP4A1, ENT1, ENT2, concentrative nucleoside transporter 2 (CNT2), and PEPT1 were significantly increased in colonic samples of IBD patients as compared to healthy controls, while the mRNA level of OCTN2 was decreased (68). Dysregulation of these transporters appears to be modulated by the degree of inflammation as differences were observed between inflamed and non-inflamed tissues from the same patients.
Rheumatoid Arthritis (RA)
RA is a chronic inflammatory disease that is associated with active episodes of symptomatic flares along with periods of remission. To date, there is limited information on the impact of RA on the expression and activity of drug transporters, and PK studies with specific in vivo transporter substrates have not been performed. However, there is some, albeit limited, preclinical and clinical evidence of transporter dysregulation. Systemic exposure of the OATP1B1, OATP1B3, and CYP3A4 substrate, simvastatin, was approximately 4-fold higher in RA patients with active disease, and simvastatin plasma concentrations significantly decreased after initiation of treatment with the anti-IL-6 receptor antibody tocilizumab (70). Decreased hepatic mRNA expression of Oatp1a1, Oatp1b2 and Oatp1a4 along with decreased hepatic uptake clearance of simvastatin was observed in preclinical models of RA, indicating potential involvement of transporters in this disease-drug interaction. Further clinical studies are warranted, and disease activity will be an important consideration in the study design. Elevated levels of pro-inflammatory cytokines (e.g., IL-6) are found in the systemic circulation of patients with active disease and these have been linked to down regulation of drug metabolizing enzyme and transporter activity in patients and in preclinical animal models. Indeed several in vivo and in vitro studies have shown a direct link between elevated IL-6 levels and CYP3A and transporter mRNA suppression (5). This led to the development of physiologically-based PK models that incorporate IL-6 levels in the in silico prediction of disease-drug interactions involving drug metabolizing enzymes (71). Treatment of primary cultures of human hepatocytes with IL-6 results in concentration- and time-dependent decreases in transcript levels of numerous uptake (NTCP, OCT1, OAT2, OATP1B1, OATP1B3 and OATP2B1) and efflux (MDR1, MRP2, MRP4 and BCRP) transporters (72). Therefore, future work should expand on these prediction models to allow incorporation of transporter changes.
Chronic kidney disease (CKD)
CKD is characterized by a progressive decline in kidney function for a period of at least three months, and is categorized by decreased GFR and albuminuria; in many cases, CKD transitions into end-stage renal disease. The prevalence of CKD is high, and can be complicated by comorbidities such as diabetes, hypertension and glomerular diseases (www.usrds.org/adr.aspx). Management of patients with CKD is complex because of their high median daily pill burden. To further complicate matters, the PK of drugs that undergo renal as well as hepatic clearance may be altered in patients with CKD. An extensive analysis of the effect of kidney disease on drug metabolizing enzymes and transporters was published recently (73). A complex example is fexofenadine; a 40–60% decrease in the oral clearance of fexofenadine was observed in CKD patients. Fexofenadine is a substrate for P-gp in the intestine, liver and kidney, OAT3 in the kidney, OATP2B1 in the intestine, and OATP1B3 in the liver (73). Therefore, a decrease in the expression and/or function of these transporters could result in decreased intestinal efflux and/or absorption, decreased hepatic uptake and decreased biliary and renal excretion. A decrease in the activity of OATP1B1 and OATP1B3 in patients with CKD is further supported by a decrease in the clearance ratio of a number of statins (73). Assessing the direct effect of CKD on transporter-mediated drug clearance by the kidney is difficult, as changes in renal clearance will be confounded by a decrease in GFR. However, the active secretion of most drugs that are known OAT1 and OAT3 substrates decreases faster than renal filtration (74), whereas the active secretion of OCT2 substrates is comparable with changes in GFR (75). In another recent analysis of the correlation between the decline of GFR in patients with CKD and renal clearance, the measure of GFR failed to accurately predict renal clearance for 48% of the compounds analyzed. In general, secretory clearance declined more rapidly than filtration clearance, although for some drugs the reverse was found (76).
The mechanisms by which transporter activity is altered in CKD are not fully elucidated, but there are some recent insights; a systematic evaluation of the inhibitory effect of 72 uremic toxins identified as many as 13 as OAT1 and OAT3 inhibitors at clinically relevant concentrations (74). Additionally, a limited number of uremic toxins were identified as inhibitors of OATP1B1, OATP1B3 and/or OATP2B1 in transfected cell lines or human hepatocytes (77). Hypotheses that need further testing include whether uremic toxins affect transporter expression or whether the increased level of inflammation in kidney disease, and associated increased levels of pro-inflammatory cytokines and their effects on injury-causing and protective genes, affect expression levels of transporters in kidney and/or intestinal epithelial cells or hepatocytes (78,79).
Regulatory pathways for transporters
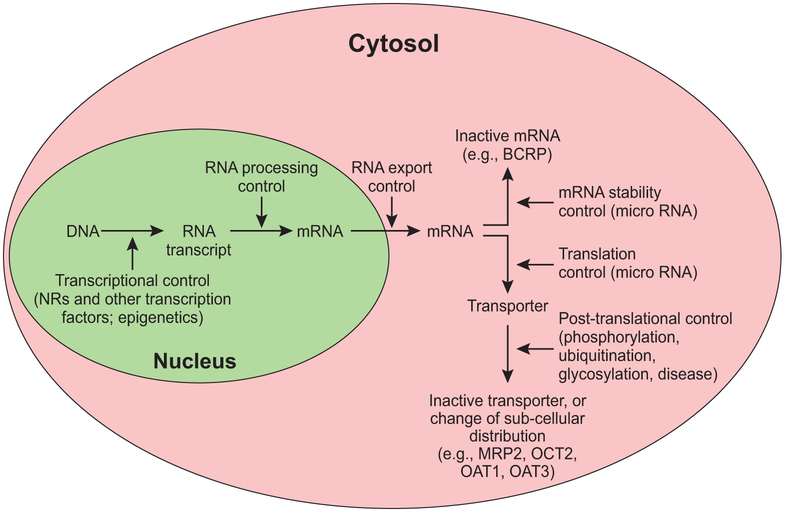
The expression of drug transporters is subject to transcriptional regulation by nuclear receptors (NRs). NRs belong to a superfamily of DNA-binding transcription factors. Most, if not all, NRs contain a DNA-binding domain and a ligand-binding domain that can be recognized and activated by steroids, thyroid hormones and many other lipophilic compounds. Ligand activated NRs regulate the expression of their target genes by binding to DNA response elements present in the target gene promoters. A significant number of drug transporters, such as P-gp and OATPs, are transcriptional targets of NRs, including the xenobiotic receptors pregnane X receptor (PXR) and constitutive androstane receptor (CAR). Both human and rodent drug transporters are regulated by PXR/Pxr and CAR/Car (80). In addition, the expression of human transporters, such as the hepatic uptake transporters OATP1B1, OATP1B3, and OATP2B1, has been reported to be regulated by several other NRs, including hepatocyte nuclear factor 1α (HNF1α) (81), farnesoid X receptor (FXR) (81), liver X receptor α (LXRα) (82), and HNF3β (83). In addition to transcriptional regulation, the expression of drug transporters is also subject to epigenetic regulation, such as DNA methylation and histone modification, nucleosome remodeling, and noncoding RNA expression (80).
Since transporter function is highly dependent on the subcellular distribution of the protein, another layer of regulation is the subcellular redistribution of transporters in response to disease, or post-translational modifications such as phosphorylation, ubiquitination, glycosylation, disulfide bonds and S-nitrosylation (84). As an example of the disease effect on transporter redistribution, NASH is known to cause the redistribution of MRP2 from the canalicular membrane to intracellular compartments such as the Rab11-containing endosomes (85), which results in reduced transport activity, as discussed above in the section on NASH. Recent results by Dzierlenga et al. suggested that in a methionine and choline-deficient diet-induced rat model of NASH, the membrane protein retrieval and insertion processes were affected through the regulation of expression or activity of protein kinase A (PKA), PKCα, PKCδ, and PKCε and their substrates radixin, myristoylated alanine-rich C-kinase and Rab11, all of which are known to regulate membrane protein retrieval and insertion (86).
Among examples of the regulation of human transporters by post-translational mechanisms, human OCT2 was subject to tyrosine kinase-induced phosphorylation, which influenced transport activity (87). In the case of ubiquitination, a critical step preceding OAT1 internalization is the ubiquitination of the transporter (84). There are three important ubiquitin-accepting lysine residues (Lys297, Lys303 and Lys315) within the large intracellular loop between transmembrane domains 6 and 7 of OAT1. These lysine residues play a synergistic role in PKC-regulated OAT1 ubiquitination, because mutating any one of the three lysine residues prevented the ubiquitination of the other two (88). Glycosylation has complex effects on transporters, ranging from plasma membrane targeting of the human OATs and ENT2 (89) (90), to transporter turnover of rabbit Oct2 (91).
The regulation of drug transporters has clear implications for therapeutic outcomes and drug development, especially in cancer therapies. Cancer chemoresistance is rather common in the clinic. A mechanism for cancer chemoresistance is decreased expression and/or activity of uptake transporters, or increased expression and/or activity of efflux transporters in cancer cells due to genetic or epigenetic mechanisms. As an example of chemoresistance due to a decreased expression of an uptake transporter, oxaliplatin, a platinum-based chemotherapy, is ineffective against advanced renal cell carcinoma (RCC) because inadequate amounts of platinum reach the target DNA (92). Liu et al. reported that OCT2 repression is a potential factor contributing to oxaliplatin resistance in RCC. The authors’ epigenetic analysis revealed that the repressed OCT2 promoter in RCC is characterized by hypermethylated CpG islands and the absence of histone H3K4 methylation. The authors went on to show that epigenetic activation of OCT2 by decitabine, an anti-cancer drug and DNA demethylation agent, sensitizes RCC cells to oxaliplatin, both in vitro and in xenografts (92). Interestingly, in the same study, MATE-2K, another transporter that normally pumps oxaliplatin out of renal cells into the urine, was repressed in cancer cells regardless of decitabine treatment, leading to oxaliplatin accumulation in treated cancer cells, but not in the surrounding normal tissues (92).
Human prostate cancer tissue expresses PXR. As an example of chemoresistance due to increased efflux transporter expression, pretreatment with SR12813, a potent and selective agonist of human PXR, led to nuclear translocation of PXR in PC-3 prostate cancer cells and increased expression of P-gp and CYP3A4 (93). P-gp was the first drug transporter that was reported to be regulated by human PXR (94). SR12813 pretreatment increased resistance of PC-3 cells to paclitaxel and vinblastine. In contrast, the sensitivity of PC-3 cells to paclitaxel and vinblastine was improved when PXR expression was knocked-down (93). These results suggest that PXR, and its positive regulation of efflux transporters, may play an important role in prostate cancer resistance to chemotherapeutics.
Because NRs are key regulators of drug transporters and drug-metabolizing enzymes, alterations in the molecular activities of NRs may explain changes in drug clearance. For example, Synold et al. showed that paclitaxel, a commonly used chemotherapeutic agent, activated PXR and enhanced P-gp-mediated drug clearance. In contrast, docetaxel, a closely related antineoplastic agent, did not activate PXR and displayed superior PK properties (94). Of course, there may be other reasons for the differences in PK between these two antineoplastic agents, in addition to the activation or inhibition of PXR.
Among many challenges, there are gaps in understanding of how transporters are regulated: (i) What is the relative contribution of transcriptional regulation, posttranslational modification and subcellular redistribution to the expression and activity of transporters?; (ii) What if a drug affects the transcription and activity of a transporter and is also a substrate of that transporter?; and (iii) How do species differences in transporter regulation impact data interpretation when preclinical models are routinely used in drug development?
Summary and conclusions
It has become increasingly clear that some transporters like P-gp may be involved primarily in the transport of xenobiotics (95), but that others, such as OATP1B, MATEs, and OCT2, also play an important role in the excretion of endogenous compounds (e.g., bilirubin and creatinine) (see the “State of the Art” by Chu et al. elsewhere in this issue). In general, there is redundancy in critical transporter pathways, and overlapping substrate specificity between transporters provides compensatory mechanisms if the activity of one transporter is impaired. As discussed, regulatory pathways can affect either expression levels or transporter activity by a range of transcriptional and post-translational mechanisms (Figure 2). Therefore, inhibition of individual transporters by drugs typically does not result in overt toxicity. There are exceptions, however, such as the inhibition by febratinib of the thiamine transporter THTR1 in the intestine, which in rare cases can result in Wernicke’s encephalopathy (96).
Figure 2: Mechanisms of regulation of transporter gene expression, post-translational modification and intracellular distribution.
Examples of transporters affected by specific regulatory pathways include Breast Cancer Resistance Protein (BCRP), Multidrug Resistance Protein 2 (MRP2), Organic Cation Transporter 2 (OCT2), Organic Anion Transporter 1 (OAT1) and OAT3.
Various acute and chronic disease states alter the mRNA and/or protein levels of transporters (Tables 1 and 2). Based on the data presented in this paper, these changes often correlate with increased levels of pro-inflammatory cytokines (e.g., IL-6). Other factors also may play a role, as in the case of liver cirrhosis where differences in the effects on transporters were observed based on the etiology of the disease. In interpreting protein or mRNA quantification data, it is often inferred that differences measured between the tissues from healthy subjects versus subjects with disease are caused by down-regulation of expression. Although in some cases an increase in transporter levels has been observed, an alternative explanation that should be considered is that changes are due to tissue infiltration by, for instance in the liver, Kupffer cells, Stellate cells, and/or myofibroblasts that don’t express hepatocyte specific transporters such as BSEP and OATP1B family members. The latter could affect the relative amount of hepatocytes in liver tissue samples of patients with liver disease. This is an area where additional scientific insights would be valuable.
The extent to which disease-mediated changes in transporter expression and activity translates into altered drug disposition has not been investigated in great detail. In several disease states (e.g., RA, liver cirrhosis), the effects on the PK of drugs that are transporter substrates have been described (Table 3). For example, decreased OATP1B1 protein in patients with liver cirrhosis correlated with increased plasma levels of pitavastatin or repaglinide. In the case of CKD and critical illnesses, several examples have been described where the PK of CYP substrates were altered, but such information is not available for drug transporter substrates. There is also a paucity of data regarding the clinical relevance of changes in drug transporters in various tissues in patients with diabetes and metabolic disease. Program announcements, fiscal appropriations, set-aside funding for RFPs (i.e., requests for proposals), and other mechanisms are needed from funding agencies to stimulate systematic and in-depth mechanistic and clinical studies to evaluate the impact of disease-associated changes in drug transporters on medication efficacy and toxicity.
So how does the global transporter research community move forward to address the broader questions of changes in transporter expression in disease states? The ITC encourages a unified international collaboration focused on application of broad “multiomics” approaches, establishment of public tissue banks, and clinical phenotyping/genotyping of disease populations. First, we need to apply advanced “multiomics” technologies to enhance our ability to integrate and understand the multiple dependencies among and between the SLC and ABC families. The examples of trabectedin (Figure 1) and DMP 904 highlight some of the best mechanistic work. However, the ability to unravel these complex associations often takes years of research as it requires the generation and integration of data from models that are representative of human physiology. Current technology is available that can accelerate our ability to map, integrate and understand the extensive physiological and toxicological transporter networks, as illustrated by the high-throughput “multiomics” platform developed by the Caprioli laboratory to elucidate the cellular response and mechanisms of action of cisplatin within 30 days (97). This platform included transcriptomics, proteomics, metabolomics, imaging, and data analytics to quantify over 10,000 unique molecular changes following cisplatin dosing, and extended knowledge regarding cisplatin’s mechanisms of action by identifying new pathways. Expression data on dozens of SLC and ABC transporters following cisplatin treatment were included by the authors so that scientists could explore other questions on the role and/or impact of transporters in cisplatin efficacy, disposition and toxicity. This work highlights that seemingly unrelated associations can be made using these approaches, but data informatics tools are needed to interpret and integrate the vast amount of data into plausible hypotheses. Second, a community BioBank needs to be established where researchers have access to high quality tissue, protein and mRNA samples. This BioBank would help supply samples to the “multiomics” studies. It could also lead to a coordinated effort to measure absolute or relative amounts of transporter proteins, in relatively large numbers of tissues from patients with disease versus controls to established scaling factors essential for building physiologically-based PK/PD models. Finally, a large consortium effort should be put in place to phenotype/genotype patients with different diseases using current transporter/metabolic drug cocktails (98). These data could be complemented by measuring endogenous biomarkers that have been identified as transporter substrates. The objective would be to identify patients with prevalent diseases and matching control subjects and characterize transporters/metabolic function, proteomics and genotype; this effort needs to be on the same scale as the 1000 Genomes Project (http://www.internationalgenome.org/about). The conduct of clinical DDI studies in various patient populations by industry or academic investigators in isolation is a step in the right direction, but will not generate the large database required to comprehensively understand disease-mediated changes in transporter expression and PK differences in patients; a collaborative effort is needed. This clinical information needs to be curated into a public transporters database similar to Transcelerate (http://www.transceleratebiopharmainc.com/what-we-do/work-clinical-development/).
If appropriately enacted, such a global effort would yield new insights and move the transporter field forward. Moreover, a database would be a valuable resource for research, drug developers, clinicians, and regulatory agencies to refine current predictions of drug PK and drug-drug interactions (DDIs) in patient populations. DDI studies are not typically conducted in patients; thus, the availability of qualified models could help ensure selection of appropriate dosage regimens for patients with comorbidities. Collaboration across disciplines and innovative approaches will advance our understanding of how disease-mediated alterations in drug transporters affect the PK and toxicity of drugs, and ultimately will lead to improved therapeutic outcomes for patients.
Supplementary Material
Acknowledgements:
We thank Drs. Conrad Raab, Iain Martin, Xiaoyan Chu, Frank Sistare, Donald Tweedie, Pär Matsson, and Nick McMahon for their constructive comments in the preparation of this manuscript.
Funding:
K.L.R. Brouwer is supported by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under Award Numbers R01 GM041935 and R35 GM122576. W. Xie is supported in part by the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health (NIH) under Award Numbers R01 ES023438. M. Piquette-Miller is supported by an operating grant from the Canadian Institutes of Health Research [MOP 13346]. F.G.M. Russel is partly supported by EFRO (European Fund for Regional Development) under grant number PROJ-00722.
Abbreviations:
- ADME
Absorption, Distribution, Metabolism, Excretion
- BBB
blood-brain-barrier
- CAR
constitutive androstane receptor
- EP
prostaglandin E receptor
- FXR
farnesoid X receptor
- HIF
hypoxia-inducible factor
- HCC
hepatocellular carcinoma
- HCV
hepatitis C
- HIV
human immunodeficiency virus
- IBD
inflammatory bowel disease
- ICG
indocyanine green
- ICU
intensive care unit
- IL
interleukin
- ITC
International Transporter Consortium
- IR
ischemia reperfusion
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NR
nuclear (hormone) receptor
- PK
pharmacokinetics
- PKA/C/α/δ/ε
protein kinase A/C/α/δ/ε
- PD
pharmacodynamics
- PAH
para-amino hippurate
- PXR
pregnane X receptor
- T
thyroxine
- TNF
tumor necrosis factor
- UGT
UDP-glucuronosyl transferase
Footnotes
References
- (1).Giacomini KM et al. Membrane transporters in drug development. Nature reviews 9, 215–36 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).van Waterschoot RA et al. ABCC2, ABCC3, and ABCB1, but not CYP3A, Protect against Trabectedin-Mediated Hepatotoxicity. Clin Cancer Res 15, 7616–23 (2009). [DOI] [PubMed] [Google Scholar]
- (3).Lee JK, Leslie EM, Zamek-Gliszczynski MJ & Brouwer KL Modulation of trabectedin (ET-743) hepatobiliary disposition by multidrug resistance-associated proteins (Mrps) may prevent hepatotoxicity. Toxicology and applied pharmacology 228, 17–23 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Wong H et al. Increased hepatobiliary clearance of unconjugated thyroxine determines DMP 904-induced alterations in thyroid hormone homeostasis in rats. Toxicol Sci 84, 232–42 (2005). [DOI] [PubMed] [Google Scholar]
- (5).Morgan ET et al. Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug metabolism and disposition: the biological fate of chemicals 36, 205–16 (2008). [DOI] [PubMed] [Google Scholar]
- (6).Fardel O & Le Vee M Regulation of human hepatic drug transporter expression by pro-inflammatory cytokines. Expert opinion on drug metabolism & toxicology 5, 1469–81 (2009). [DOI] [PubMed] [Google Scholar]
- (7).Elferink MG et al. LPS-induced downregulation of MRP2 and BSEP in human liver is due to a posttranscriptional process. American journal of physiology 287, G1008–16 (2004). [DOI] [PubMed] [Google Scholar]
- (8).Kortgen A et al. Prospective assessment of hepatic function and mechanisms of dysfunction in the critically ill Shock (Augusta, Ga: 32, 358–65 (2009). [DOI] [PubMed] [Google Scholar]
- (9).Vanwijngaerden YM et al. Critical illness evokes elevated circulating bile acids related to altered hepatic transporter and nuclear receptor expression Hepatology (Baltimore, Md: 54, 1741–52 (2011). [DOI] [PubMed] [Google Scholar]
- (10).Jenniskens M et al. On the Role of Illness Duration and Nutrient Restriction in Cholestatic Alterations that Occur During Critical Illness Shock (Augusta, Ga, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Schaffner CA, Mwinyi J, Gai Z, Thasler WE, Eloranta JJ & Kullak-Ublick GA The organic solute transporters alpha and beta are induced by hypoxia in human hepatocytes. Liver Int 35, 1152–61 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Eades SK, Boineau FG & Christensen ML Increased tacrolimus levels in a pediatric renal transplant patient attributed to chronic diarrhea. Pediatr Transplant 4, 63–6 (2000). [DOI] [PubMed] [Google Scholar]
- (13).Maezono S et al. Elevated blood concentrations of calcineurin inhibitors during diarrheal episode in pediatric liver transplant recipients: involvement of the suppression of intestinal cytochrome P450 3A and P-glycoprotein. Pediatr Transplant 9, 315–23 (2005). [DOI] [PubMed] [Google Scholar]
- (14).Belliard AM, Lacour B, Farinotti R & Leroy C Effect of tumor necrosis factor-alpha and interferon-gamma on intestinal P-glycoprotein expression, activity, and localization in Caco-2 cells. Journal of pharmaceutical sciences 93, 1524–36 (2004). [DOI] [PubMed] [Google Scholar]
- (15).Belliard AM, Tardivel S, Farinotti R, Lacour B & Leroy C Effect of hr-IL2 treatment on intestinal P-glycoprotein expression and activity in Caco-2 cells. The Journal of pharmacy and pharmacology 54, 1103–9 (2002). [DOI] [PubMed] [Google Scholar]
- (16).Blokzijl H et al. Decreased P-glycoprotein (P-gp/MDR1) expression in inflamed human intestinal epithelium is independent of PXR protein levels. Inflammatory bowel diseases 13, 710–20 (2007). [DOI] [PubMed] [Google Scholar]
- (17).Bertilsson PM, Olsson P & Magnusson KE Cytokines influence mRNA expression of cytochrome P450 3A4 and MDRI in intestinal cells. Journal of pharmaceutical sciences 90, 638–46 (2001). [DOI] [PubMed] [Google Scholar]
- (18).Borthakur A, Gill RK, Hodges K, Ramaswamy K, Hecht G & Dudeja PK Enteropathogenic Escherichia coli inhibits butyrate uptake in Caco-2 cells by altering the apical membrane MCT1 level. American journal of physiology 290, G30–5 (2006). [DOI] [PubMed] [Google Scholar]
- (19).Kumar A, Alrefai WA, Borthakur A & Dudeja PK Lactobacillus acidophilus counteracts enteropathogenic E. coli-induced inhibition of butyrate uptake in intestinal epithelial cells. American journal of physiology 309, G602–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wojtal KA et al. Downregulation of duodenal SLC transporters and activation of proinflammatory signaling constitute the early response to high altitude in humans. American journal of physiology 307, G673–88 (2014). [DOI] [PubMed] [Google Scholar]
- (21).Corrigan G et al. PAH extraction and estimation of plasma flow in human postischemic acute renal failure. Am J Physiol 277, F312–8 (1999). [DOI] [PubMed] [Google Scholar]
- (22).Kwon O, Hong SM & Blouch K Alteration in renal organic anion transporter 1 after ischemia/reperfusion in cadaveric renal allografts. J Histochem Cytochem 55, 575–84 (2007). [DOI] [PubMed] [Google Scholar]
- (23).Kunin M, Holtzman EJ, Melnikov S & Dinour D Urinary organic anion transporter protein profiles in AKI. Nephrol Dial Transplant 27, 1387–95 (2012). [DOI] [PubMed] [Google Scholar]
- (24).Huo X & Liu K Renal organic anion transporters in drug-drug interactions and diseases. Eur J Pharm Sci 112, 8–19 (2018). [DOI] [PubMed] [Google Scholar]
- (25).Matsuzaki T et al. Downregulation of organic anion transporters in rat kidney under ischemia/reperfusion-induced acute [corrected] renal failure. Kidney international 71, 539–47 (2007). [DOI] [PubMed] [Google Scholar]
- (26).Preising C, Schneider R, Bucher M, Gekle M & Sauvant C Regulation of Expression of Renal Organic Anion Transporters OAT1 and OAT3 in a Model of Ischemia/Reperfusion Injury. Cell Physiol Biochem 37, 1–13 (2015). [DOI] [PubMed] [Google Scholar]
- (27).Schneider R et al. Oat1/3 restoration protects against renal damage after ischemic AKI. Am J Physiol Renal Physiol 308, F198–208 (2015). [DOI] [PubMed] [Google Scholar]
- (28).Matsuzaki T et al. Altered pharmacokinetics of cationic drugs caused by down-regulation of renal rat organic cation transporter 2 (Slc22a2) and rat multidrug and toxin extrusion 1 (Slc47a1) in ischemia/reperfusion-induced acute kidney injury. Drug metabolism and disposition: the biological fate of chemicals 36, 649–54 (2008). [DOI] [PubMed] [Google Scholar]
- (29).Masereeuw R & Russel FG Regulatory pathways for ATP-binding cassette transport proteins in kidney proximal tubules. The AAPS journal 14, 883–94 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Warach S & Latour LL Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke 35, 2659–61 (2004). [DOI] [PubMed] [Google Scholar]
- (31).Hermann DM, Kilic E, Spudich A, Kramer SD, Wunderli-Allenspach H & Bassetti CL Role of drug efflux carriers in the healthy and diseased brain. Annals of neurology 60, 489–98 (2006). [DOI] [PubMed] [Google Scholar]
- (32).Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC & Colgan SP Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer research 62, 3387–94 (2002). [PubMed] [Google Scholar]
- (33).Spudich A et al. Inhibition of multidrug resistance transporter-1 facilitates neuroprotective therapies after focal cerebral ischemia. Nature neuroscience 9, 487–8 (2006). [DOI] [PubMed] [Google Scholar]
- (34).Kilic E et al. ABCC1: a gateway for pharmacological compounds to the ischaemic brain. Brain 131, 2679–89 (2008). [DOI] [PubMed] [Google Scholar]
- (35).Dazert P et al. Differential regulation of transport proteins in the periinfarct region following reversible middle cerebral artery occlusion in rats. Neuroscience 142, 1071–9 (2006). [DOI] [PubMed] [Google Scholar]
- (36).Evseenko DA, Paxton JW & Keelan JA Independent regulation of apical and basolateral drug transporter expression and function in placental trophoblasts by cytokines, steroids, and growth factors. Drug metabolism and disposition: the biological fate of chemicals 35, 595–601 (2007). [DOI] [PubMed] [Google Scholar]
- (37).Lye P, Bloise E, Javam M, Gibb W, Lye SJ & Matthews SG Impact of bacterial and viral challenge on multidrug resistance in first- and third-trimester human placenta. The American journal of pathology 185, 1666–75 (2015). [DOI] [PubMed] [Google Scholar]
- (38).Petrovic V, Kojovic D, Cressman A & Piquette-Miller M Maternal bacterial infections impact expression of drug transporters in human placenta. International immunopharmacology 26, 349–56 (2015). [DOI] [PubMed] [Google Scholar]
- (39).Mason CW, Buhimschi IA, Buhimschi CS, Dong Y, Weiner CP & Swaan PW ATP-binding cassette transporter expression in human placenta as a function of pregnancy condition. Drug metabolism and disposition: the biological fate of chemicals 39, 1000–7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Mason CW, Lee GT, Dong Y, Zhou H, He L & Weiner CP Effect of prostaglandin E2 on multidrug resistance transporters in human placental cells. Drug metabolism and disposition: the biological fate of chemicals 42, 2077–86 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Daud AN et al. P-Glycoprotein-Mediated Drug Interactions in Pregnancy and Changes in the Risk of Congenital Anomalies: A Case-Reference Study. Drug Saf 38, 651–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Alam C, Whyte-Allman SK, Omeragic A & Bendayan R Role and modulation of drug transporters in HIV-1 therapy. Advanced drug delivery reviews 103, 121–43 (2016). [DOI] [PubMed] [Google Scholar]
- (43).Jetter A et al. Do activities of cytochrome P450 (CYP)3A, CYP2D6 and P-glycoprotein differ between healthy volunteers and HIV-infected patients? Antivir Ther 15, 975–83 (2010). [DOI] [PubMed] [Google Scholar]
- (44).Bataller R & Brenner DA Liver fibrosis. The Journal of clinical investigation 115, 209–18 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Mutanen A, Lohi J, Heikkila P, Jalanko H & Pakarinen MP Liver Inflammation Relates to Decreased Canalicular Bile Transporter Expression in Pediatric Onset Intestinal Failure. Annals of surgery, (2017). [DOI] [PubMed] [Google Scholar]
- (46).Wang L et al. Transporter Expression in Liver Tissue from Subjects with Alcoholic or Hepatitis C Cirrhosis Quantified by Targeted Quantitative Proteomics. Drug metabolism and disposition: the biological fate of chemicals 44, 1752–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).More VR et al. Alcohol cirrhosis alters nuclear receptor and drug transporter expression in human liver. Drug metabolism and disposition: the biological fate of chemicals 41, 1148–55 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Ogasawara K et al. Hepatitis C virus-related cirrhosis is a major determinant of the expression levels of hepatic drug transporters. Drug metabolism and pharmacokinetics 25, 190–9 (2010). [DOI] [PubMed] [Google Scholar]
- (49).Kula M, Karacavus S, Baskol M, Deniz K, Abdulrezzak U & Tutus A Hepatobiliary function assessed by 99mTc-mebrofenin cholescintigraphy in the evaluation of fibrosis in chronic hepatitis: histopathological correlation. Nucl Med Commun 31, 280–5 (2010). [DOI] [PubMed] [Google Scholar]
- (50).Hui CK, Cheung BM & Lau GK Pharmacokinetics of pitavastatin in subjects with Child-Pugh A and B cirrhosis. British journal of clinical pharmacology 59, 291–7 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Hatorp V, Walther KH, Christensen MS & Haug-Pihale G Single-dose pharmacokinetics of repaglinide in subjects with chronic liver disease. Journal of clinical pharmacology 40, 142–52 (2000). [DOI] [PubMed] [Google Scholar]
- (52).Thakkar N, Slizgi JR & Brouwer KLR Effect of Liver Disease on Hepatic Transporter Expression and Function. Journal of pharmaceutical sciences 106, 2282–94 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Lake AD et al. Analysis of global and absorption, distribution, metabolism, and elimination gene expression in the progressive stages of human nonalcoholic fatty liver disease. Drug metabolism and disposition: the biological fate of chemicals 39, 1954–60 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Clarke JD et al. Synergistic interaction between genetics and disease on pravastatin disposition. Journal of hepatology 61, 139–47 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Clarke JD, Novak P, Lake AD, Hardwick RN & Cherrington NJ Impaired N-linked glycosylation of uptake and efflux transporters in human non-alcoholic fatty liver disease. Liver Int 37, 1074–81 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Ali I et al. Transporter-mediated Alterations in Patients with NASH Increase Systemic and Hepatic Exposure to an OATP and MRP2 Substrate. Clinical pharmacology and therapeutics, 104:749–756, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Hardwick RN, Fisher CD, Canet MJ, Scheffer GL & Cherrington NJ Variations in ATP-binding cassette transporter regulation during the progression of human nonalcoholic fatty liver disease. Drug metabolism and disposition: the biological fate of chemicals 39, 2395–402 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Zhang P, Tian X, Chandra P & Brouwer KL Role of glycosylation in trafficking of Mrp2 in sandwich-cultured rat hepatocytes. Molecular pharmacology 67, 1334–41 (2005). [DOI] [PubMed] [Google Scholar]
- (59).Canet MJ et al. Altered regulation of hepatic efflux transporters disrupts acetaminophen disposition in pediatric nonalcoholic steatohepatitis. Drug metabolism and disposition: the biological fate of chemicals 43, 829–35 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Ferslew BC et al. Altered morphine glucuronide and bile acid disposition in patients with nonalcoholic steatohepatitis. Clinical pharmacology and therapeutics 97, 419–27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Huang S & Czech MP The GLUT4 glucose transporter. Cell metabolism 5, 237–52 (2007). [DOI] [PubMed] [Google Scholar]
- (62).Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ & Greenhaff PL Insulin stimulates L-carnitine accumulation in human skeletal muscle. FASEB J 20, 377–9 (2006). [DOI] [PubMed] [Google Scholar]
- (63).Sharma K et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol 24, 1901–12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Brandsch M Drug transport via the intestinal peptide transporter PepT1. Current opinion in pharmacology 13, 881–7 (2013). [DOI] [PubMed] [Google Scholar]
- (65).Gutmann H et al. Breast cancer resistance protein and P-glycoprotein expression in patients with newly diagnosed and therapy-refractory ulcerative colitis compared with healthy controls. Digestion 78, 154–62 (2008). [DOI] [PubMed] [Google Scholar]
- (66).Schneider RE, Babb J, Bishop H, Hoare AM & Hawkins CF Plasma propranolol levels in coeliac disease and Crohn’s disease. Br Med J 2, 1324 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Jahnel J, Fickert P, Hauer AC, Hogenauer C, Avian A & Trauner M Inflammatory bowel disease alters intestinal bile acid transporter expression. Drug metabolism and disposition: the biological fate of chemicals 42, 1423–31 (2014). [DOI] [PubMed] [Google Scholar]
- (68).Wojtal KA et al. Changes in mRNA expression levels of solute carrier transporters in inflammatory bowel disease patients. Drug metabolism and disposition: the biological fate of chemicals 37, 1871–7 (2009). [DOI] [PubMed] [Google Scholar]
- (69).Blokzijl H et al. Up-regulation and cytoprotective role of epithelial multidrug resistance-associated protein 1 in inflammatory bowel disease. The Journal of biological chemistry 283, 35630–7 (2008). [DOI] [PubMed] [Google Scholar]
- (70).Schmitt C, Kuhn B, Zhang X, Kivitz AJ & Grange S Disease-drug-drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis. Clinical pharmacology and therapeutics 89, 735–40 (2011). [DOI] [PubMed] [Google Scholar]
- (71).Machavaram KK et al. A physiologically based pharmacokinetic modeling approach to predict disease-drug interactions: suppression of CYP3A by IL-6. Clinical pharmacology and therapeutics 94, 260–8 (2013). [DOI] [PubMed] [Google Scholar]
- (72).Yang Q, Doshi U, Li N & Li AP Effects of culture duration on gene expression of P450 isoforms, uptake and efflux transporters in primary hepatocytes cultured in the absence and presence of interleukin-6: implications for experimental design for the evaluation of downregulatory effects of biotherapeutics. Current drug metabolism 13, 938–46 (2012). [DOI] [PubMed] [Google Scholar]
- (73).Miners JO, Yang X, Knights KM & Zhang L The Role of the Kidney in Drug Elimination: Transport, Metabolism, and the Impact of Kidney Disease on Drug Clearance. Clinical pharmacology and therapeutics 102, 436–49 (2017). [DOI] [PubMed] [Google Scholar]
- (74).Hsueh CH et al. Identification and Quantitative Assessment of Uremic Solutes as Inhibitors of Renal Organic Anion Transporters, OAT1 and OAT3. Molecular pharmaceutics 13, 3130–40 (2016). [DOI] [PubMed] [Google Scholar]
- (75).Cheung KWK et al. The Effect of Uremic Solutes on the Organic Cation Transporter 2. Journal of pharmaceutical sciences 106, 2551–7 (2017). [DOI] [PubMed] [Google Scholar]
- (76).Chapron A, Shen DD, Kestenbaum BR, Robinson-Cohen C, Himmelfarb J & Yeung CK Does Secretory Clearance Follow Glomerular Filtration Rate in Chronic Kidney Diseases? Reconsidering the Intact Nephron Hypothesis. Clinical and translational science 10, 395–403 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Reyes M & Benet LZ Effects of uremic toxins on transport and metabolism of different biopharmaceutics drug disposition classification system xenobiotics. Journal of pharmaceutical sciences 100, 3831–42 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Ruiz-Andres O et al. Downregulation of kidney protective factors by inflammation: role of transcription factors and epigenetic mechanisms. Am J Physiol Renal Physiol 311, F1329–F40 (2016). [DOI] [PubMed] [Google Scholar]
- (79).Dreisbach AW The influence of chronic renal failure on drug metabolism and transport. Clinical pharmacology and therapeutics 86, 553–6 (2009). [DOI] [PubMed] [Google Scholar]
- (80).Amacher DE The regulation of human hepatic drug transporter expression by activation of xenobiotic-sensing nuclear receptors. Expert opinion on drug metabolism & toxicology 12, 1463–77 (2016). [DOI] [PubMed] [Google Scholar]
- (81).Jung D et al. Human organic anion transporting polypeptide 8 promoter is transactivated by the farnesoid X receptor/bile acid receptor. Gastroenterology 122, 1954–66 (2002). [DOI] [PubMed] [Google Scholar]
- (82).Meyer Zu Schwabedissen HE, Bottcher K, Chaudhry A, Kroemer HK, Schuetz EG & Kim RB Liver X receptor alpha and farnesoid X receptor are major transcriptional regulators of OATP1B1 Hepatology (Baltimore, Md: 52, 1797–807 (2010). [DOI] [PubMed] [Google Scholar]
- (83).Vavricka SR, Jung D, Fried M, Grutzner U, Meier PJ & Kullak-Ublick GA The human organic anion transporting polypeptide 8 (SLCO1B3) gene is transcriptionally repressed by hepatocyte nuclear factor 3beta in hepatocellular carcinoma. Journal of hepatology 40, 212–8 (2004). [DOI] [PubMed] [Google Scholar]
- (84).Xu D & You G Loops and layers of post-translational modifications of drug transporters. Advanced drug delivery reviews 116, 37–44 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Wang W et al. Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology 131, 878–84 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Dzierlenga AL, Clarke JD & Cherrington NJ Nonalcoholic Steatohepatitis Modulates Membrane Protein Retrieval and Insertion Processes. Drug metabolism and disposition: the biological fate of chemicals 44, 1799–807 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Sprowl JA et al. A phosphotyrosine switch regulates organic cation transporters. Nature communications 7, 10880 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Li S, Zhang Q & You G Three ubiquitination sites of organic anion transporter-1 synergistically mediate protein kinase C-dependent endocytosis of the transporter. Molecular pharmacology 84, 139–46 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Tanaka K, Xu W, Zhou F & You G Role of glycosylation in the organic anion transporter OAT1. The Journal of biological chemistry 279, 14961–6 (2004). [DOI] [PubMed] [Google Scholar]
- (90).Ward JL, Leung GP, Toan SV & Tse CM Functional analysis of site-directed glycosylation mutants of the human equilibrative nucleoside transporter-2. Arch Biochem Biophys 411, 19–26 (2003). [DOI] [PubMed] [Google Scholar]
- (91).Pelis RM, Suhre WM & Wright SH Functional influence of N-glycosylation in OCT2-mediated tetraethylammonium transport. Am J Physiol Renal Physiol 290, F1118–26 (2006). [DOI] [PubMed] [Google Scholar]
- (92).Liu Y et al. Epigenetic activation of the drug transporter OCT2 sensitizes renal cell carcinoma to oxaliplatin. Science translational medicine 8, 348ra97 (2016). [DOI] [PubMed] [Google Scholar]
- (93).Chen Y, Tang Y, Wang MT, Zeng S & Nie D Human pregnane X receptor and resistance to chemotherapy in prostate cancer. Cancer research 67, 10361–7 (2007). [DOI] [PubMed] [Google Scholar]
- (94).Synold TW, Dussault I & Forman BM The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nature medicine 7, 584–90 (2001). [DOI] [PubMed] [Google Scholar]
- (95).Borst P & Schinkel AH P-glycoprotein ABCB1: a major player in drug handling by mammals. The Journal of clinical investigation 123, 4131–3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Zhang Q et al. The Janus kinase 2 inhibitor fedratinib inhibits thiamine uptake: a putative mechanism for the onset of Wernicke’s encephalopathy. Drug metabolism and disposition: the biological fate of chemicals 42, 1656–62 (2014). [DOI] [PubMed] [Google Scholar]
- (97).Norris JL et al. Integrated, High-Throughput, Multiomics Platform Enables Data-Driven Construction of Cellular Responses and Reveals Global Drug Mechanisms of Action. Journal of proteome research 16, 1364–75 (2017). [DOI] [PubMed] [Google Scholar]
- (98).Prueksaritanont T et al. Validation of a microdose probe drug cocktail for clinical drug interaction assessments for drug transporters and CYP3A. Clinical pharmacology and therapeutics 101, 519–30 (2017). [DOI] [PubMed] [Google Scholar]
- (99).Le Vee M, Jouan E, Stieger B, Lecureur V & Fardel O Regulation of drug transporter expression by oncostatin M in human hepatocytes. Biochemical pharmacology 82, 304–11 (2011). [DOI] [PubMed] [Google Scholar]
- (100).Lemahieu W, Maes B, Verbeke K, Rutgeerts P, Geboes K & Vanrenterghem Y Cytochrome P450 3A4 and P-glycoprotein activity and assimilation of tacrolimus in transplant patients with persistent diarrhea. Am J Transplant 5, 1383–91 (2005). [DOI] [PubMed] [Google Scholar]
- (101).Hodyl NA, Stark MJ, Butler M & Clifton VL Placental P-glycoprotein is unaffected by timing of antenatal glucocorticoid therapy but reduced in SGA preterm infants. Placenta 34, 325–30 (2013). [DOI] [PubMed] [Google Scholar]
- (102).Naud J et al. Effects of chronic renal failure on kidney drug transporters and cytochrome P450 in rats. Drug metabolism and disposition: the biological fate of chemicals 39, 1363–9 (2011). [DOI] [PubMed] [Google Scholar]
- (103).Naud J, Michaud J, Leblond FA, Lefrancois S, Bonnardeaux A & Pichette V Effects of chronic renal failure on liver drug transporters. Drug metabolism and disposition: the biological fate of chemicals 36, 124–8 (2008). [DOI] [PubMed] [Google Scholar]
- (104).Naud J et al. Down-regulation of intestinal drug transporters in chronic renal failure in rats. The Journal of pharmacology and experimental therapeutics 320, 978–85 (2007). [DOI] [PubMed] [Google Scholar]
- (105).Okushin K et al. The intrahepatic expression levels of bile acid transporters are inversely correlated with the histological progression of nonalcoholic fatty liver disease. J Gastroenterol 51, 808–18 (2016). [DOI] [PubMed] [Google Scholar]
- (106).Billington S et al. Transporter expression in non-cancerous and cancerous liver tissue from donors with hepatocellular carcinoma and chronic hepatitis C infection quantified by LC-MS/MS proteomics. Drug metabolism and disposition: the biological fate of chemicals, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Kis O, Sankaran-Walters S, Hoque MT, Walmsley SL, Dandekar S & Bendayan R HIV-1 Alters Intestinal Expression of Drug Transporters and Metabolic Enzymes: Implications for Antiretroviral Drug Disposition. Antimicrob Agents Chemother 60, 2771–81 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).De Rosa MF et al. Expression of membrane drug efflux transporters in the sigmoid colon of HIV-infected and uninfected men. Journal of clinical pharmacology 53, 934–45 (2013). [DOI] [PubMed] [Google Scholar]
- (109).Thomson BK et al. Effect of CKD and dialysis modality on exposure to drugs cleared by nonrenal mechanisms. Am J Kidney Dis 65, 574–82 (2015). [DOI] [PubMed] [Google Scholar]
- (110).Morgan RE, Campbell SE, Yu CY, Sponseller CA & Muster HA Comparison of the safety, tolerability, and pharmacokinetic profile of a single oral dose of pitavastatin 4 mg in adult subjects with severe renal impairment not on hemodialysis versus healthy adult subjects. J Cardiovasc Pharmacol 60, 42–8 (2012). [DOI] [PubMed] [Google Scholar]
- (111).Lewis JH & Stine JG Review article: prescribing medications in patients with cirrhosis - a practical guide. Aliment Pharmacol Ther 37, 1132–1156 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.