Abstract
Host-gut microbiota metabolic interactions are closely associated with health and disease. A manifestation of such co-metabolism is the vast structural diversity of bile acids (BAs) involving both oxidative stereochemistry and conjugation. Herein, we describe the development and validation of a LC-MS based method for the analysis of human C24 BA metabolome in serum and urine. The method has high throughput covering the discrimination of oxidative stereochemistry of unconjugated species in a 15-min analytical cycle. The validated quantitative performance provided an indirect way to ascertain the conjugation patterns of BAs via enzyme-digestion protocols that incorporated the enzymes, sulfatase, β-glucuronidase and choloylglycine hydrolase. Application of the method has led to the detection of at least 70 unconjugated BAs including 27 known species and 43 newly found species in the post-prandial serum and urine samples from 7 nonalcoholic steatohepatitis patients and 13 healthy volunteers. Newly identified unconjugated BAs included 3α, 12β-dihydroxy-5β-cholan-24-oic acid, 12α-hydroxy-3-oxo-5β-cholan-24-oic acid and 3α, 7α, 12β-trihydroxy-5β-cholan-24-oic acid. High-definition negative fragment spectra of the other major unknown species were acquired to facilitate future identification endeavors. An extensive conjugation pattern is the major reason for the “invisibility” of the newly found BAs to other common analytical methods. Metabolomic analysis of the total unconjugated BA profile in combination with analysis of their conjugation patterns and urinary excretion tendencies have provided substantial insights into the interconnected roles of host and gut microbiota in maintaining BA homeostasis. It was proposed that the urinary total BA profile may serve as an ideal footprint for the functional status of the host-gut microbial BA co-metabolism. In summary, this work provided a powerful tool for human C24 BA metabolome analysis that bridges the gap between GC-MS techniques in the past age and LC-MS techniques currently prevailing in biomedical researches. Further applications of the present method in clinical, translational research and other biomedical explorations will continue to boost the construction of a host-gut microbial co-metabolism network of BAs and thus facilitate the decryption of BA mediated host-gut microbiota crosstalk in health and diseases.
Keywords: bile acid, host-gut microbial co-metabolism, conjugation, oxidative stereochemistry, liquid chromatography, tandem mass spectrometry
1. Introduction
Host-gut microbiota metabolic interactions are associated with various disorders that are physiologically connected by the gut, liver, muscle, and brain [1]. Bile acids (BAs) are an important class of metabolites that are originally biosynthesized by the host, then further modified by the actions of symbiotic gut microbiota, and play a critical role in shuttling information between them [2–4]. On the side of host, the BA receptors, such as nuclear farnesoid X receptor (FXR) and G protein-coupled membrane receptor 5 (TGR5), have become major targets for studies of metabolic diseases [5,6]. On the other side BAs can modulate gut microbial composition both directly [7–9] and indirectly through activation of the host innate immune signals [10–12]. The in-depth understanding of the host-microbiota crosstalk mechanisms, which will assist in the development of new strategies for disease prevention and therapeutic intervention [13], relies heavily on our ability to analyze the vast structural diversity of the entire BA metabolome.
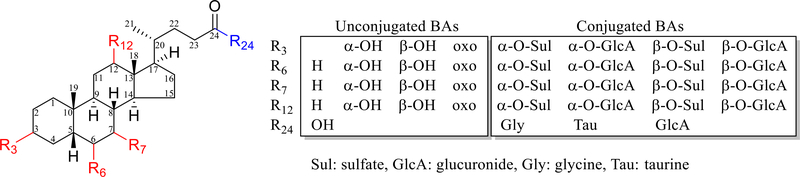
Human BAs are C24 molecules comprised of a cis A/B ring fusioned C19 cyclopentanophenanthrene (steroid) nucleus and a carboxylate side-chain (Fig. 1). The structural diversity of C24 BAs comes primarily from oxidative stereochemistry, with oxidation sites essentially on C-3, commonly on C-6, 7 and 12, and unusually on C-1, 2, 4, 5, 15, 16, 19, 22 and 23 [14]. The diversity is dramatically amplified by metabolic conjugation reactions mainly including sulfation, glucuronidation, and aminoacyl amidates linked with either glycine or taurine [15,16]. Hundreds of unconjugated BAs may be retrieved from the human metabolome database (HMDB) [17] and LIPID MAPS Structure Database (LMSD) [18]. Standards are not available for most of the unusual species, not to mention their conjugated metabolites. As a result, the analysis of the human BA metabolome is challenged by (1) conjugation-derived structural diversity, (2) difficulty in differentiating isomers and epimers, and (3) synthesis of standards especially those with unusual oxidation sites, (4) ethical issues in obtaining access to human samples that are necessary because there are inter-species differences in BA metabolism between humans and animals.
Fig. 1.
Structural diversity of the unconjugated and conjugated C24 bile acids (BAs) with a cis/5β-H A/B ring fusion stereochemistry
The above challenges in BA analysis have been considerably addressed by GC-MS based methods developed in the last century. As summarized in a recent book [19], the sample processing techniques including column separation, chemical hydrolysis, solvolysis and/or enzymes hydrolysis were comprehensively used to cope with conjugation-derived structural diversity. The retention index and fragmentation pattern of the trimethylsilyl and dimethylethylsilyl ethers of BAs determined using GC-MS have been evaluated to facilitate differentiation of isomers and epimers. Some unusual BA species with hydroxylation sites on C-1, 2, 4, 6 and 19 have been detected in biological samples in early human life and in patients with liver diseases. However, these challenges have not yet been addressed by LC-MS techniques currently used in BA analysis [20–24]. Unusual species have seldom been detected using LC-MS, except for a recently-characterized CYP3A-mediated hydroxylated metabolite of DCA, 1β, 3α, 12α-trihydroxy-5β-cholan-24-oic acid (DCA-1β-ol) [25–27]. Lack of standards and underestimation of conjugation-derived structural diversity are the major reasons for the incoherence of the different platforms. This situation has consistently been a bottleneck for BA data analysis thus obstructing a deeper understanding of host-gut microbiota BA co-metabolism.
In this work, our overall objective was to develop a quantitative method for the analysis of human C24 BAs in serum and urine based on enzyme-digestion of conjugated BAs and LC-MS determination of unconjugated BAs. The enzyme-digestion protocols were developed and validated to achieve optimal selectivity and efficacy of the enzymes capable of hydrolyzing conjugated BAs, as well as, achieving acceptable unconjugated BA stability during incubation. Based on our previous work, which focused on negative fragmentation and chromatographic separation of BAs by LC-MS techniques [28,29], the fine-tuned chromatographic gradient and advanced multiple reaction monitor (MRM) were jointly employed to separate and detect the unconjugated BAs in human serum and urine. The validated method developed here allows for analysis of a more complete structural diversity of the human BAs metabolome associated not only with the oxidative stereochemistry but also with varying conjugation patterns. We believe that the knowledge gained in this work will facilitate a deeper understanding of the host-gut microbial co-metabolism of human BAs.
2. Material and methods
2.1. Chemicals and reagents
The abbreviations of BAs were named according to the proposed nomenclature in 1992 [30]. Standards of 33 unconjugated BAs, 27 conjugated BAs and 4 stable isotope labeled internal standards summarized in Table 1 and Electronic Supplementary Material (ESM) Tables S1 and S2 were purchased from Steraloids (Newport, RI, USA), TRC (Toronto, Canada), Santa Cruz Biotechnology (Dallas, TX, USA) or Sigma-Aldrich (St. Louis, MO, USA). Sulfatase from Helix pomatia Type H-1 (SUL-1), sulfatase from abalone entrails Type VIII (SUL-2), sulfatase from Patella vulgata Type IV (SUL-3), β-glucuronidase from Helix pomatia Type H-1 (GLU-1), β-glucuronidase from limpets (GLU-2), β-glucuronidase from Helix aspersa Type HA-4 (GLU-3), choloylglycine hydrolase from clostridium perfringens (CH), activated charcoal, sodium acetate, glacial acetic acid, and LC-MS grade methanol, acetonitrile and formic acid were purchased from Sigma-Aldrich. Ultra-pure water was obtained by using a Milli-Q system (Millipore, Bedford, MA, USA).
Table 1.
The NO., abbreviation, structure, quantitative/validation MRM and retention time of the unconiugated C24 BAs detected in human urine and serum
| NO. | Abbreviation | Structure | MRM |
Retention time (MEAN±SD, min) |
NO. | Abbreviation | Structure | MRM |
Retention time (MEAN±SD, min) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quan | Val | Standard* | Urine | Serum | Quan | Val | Standard* | Urine | Serum | ||||||
| 01 | √ 3-dehydroLCA | 5β-H, 3-oxo | 373>373 | 12.96±0.00 | 12.94±0.02 | 12.96±0.01 | 39 | oxoTBA03 | Unknown | 405>405 | NA | 4.77±0.01 | ND | ||
| 02 | √ alloLCA | 5α-H, 3α | 375>375 | 11.92±0.01 | ND | ND | 40 | oxoTBA04 | Unknown | 405>405 | 405>289 | NA | 5.51±0.01 | 5.49±0.01 | |
| 03 | √ isoLCA | 5β-H, 3β | 375>375 | 12.11±0.01 | ND | 12.11±0.01 | 41 | oxoTBA05 | Unknown | 405>405 | 405>329 | NA | 6.43±0.02 | 6.42±0.02 | |
| 04 | √ LCA | 5β-Η, 3α | 375>375 | 12.76±0.00 | 12.75±0.00 | 12.75±0.00 | 42 | √ 7-oxoDCA | 5β-H, 3α, 7-oxo, 12α | 405>405 | 7.12±0.01 | 7.13±0.01 | 7.12±0.01 | ||
| 05 | dioxoDBA01 | Unknown | 387>387 | NA | 8.97±0.00 | 8.97±0.00 | 43 | oxoTBA06 | Unknown | 405>405 | NA | 7.20±0.02 | 7.20±0.01 | ||
| 06 | dioxoDBA02 | Unknown | 387>387 | NA | 9.21±0.00 | 9.21±0.02 | 44 | oxoTBA07 | Unknown | 405>405 | NA | 7.30±0.01 | 7.29±0.01 | ||
| 07 | √ | 5β-H, 3-oxo, 7-oxo | 387>317 | 387>317 | 10.02±0.01 | 10.02±0.01 | 10.01±0.02 | 45 | oxoTBA08 | Unknown | 405>405 | NA | ND | 7.48±0.01 | |
| 08 | √ | 5β-H, 3-oxo, 12-oxo | 387>343 | 387>343 | 9.99±0.00 | 9.99±0.01 | 9.99±0.02 | 46 | √ 12-oxoCDCA | 5β-H, 3α, 7α, 12-oxo | 405>405 | 7.73±0.01 | ND | ND | |
| 09 | oxoDBA01 | Unknown | 389>389 | NA | 7.94±0.01 | 7.96±0.01 | 47 | oxoTBA09 | Unknown | 405>405 | NA | 7.80±0.02 | 7.79±0.03 | ||
| 10 | oxoDBA02 | Unknown | 389>389 | NA | 8.19±0.01 | 8.19±0.01 | 48 | oxoTBA10 | Unknown | 405>405 | NA | 8.01±0.01 | ND | ||
| 11 | oxoDBA03 | Unknown | 389>389 | NA | 8.83±0.01 | 8.83±0.00 | 49 | √ 3-dehydroCA | 5β-H, 3-oxo, 7α, 12α | 405>405 | 8.36±0.01 | 8.36±0.01 | 8.36±0.01 | ||
| 12 | √ 6-oxoLCA | 5β-H, 3α, 6-oxo | 389>389 | 9.23±0.00 | 9.24±0.01 | 9.22±0.01 | 50 | TBA01 | Unknown | 407>407 | NA | 1.96±0.01 | ND | ||
| 13 | oxoDBA04 (3 -dehydroUDCA?) | 5β-H, 3-oxo, 7β? | 389>389 | NA | 9.30±0.01 | 9.29±0.00 | 51 | TBA02 | Unknown | 407>407 | NA | 2.03±0.02 | ND | ||
| 14 | oxoDBA05 | Unknown | 389>389 | NA | 9.37±0.01 | 9.37±0.01 | 52 | TBA03 | Unknown | 407>407 | NA | 2.13±0.02 | ND | ||
| 15 | √ 7-oxoLCA | 5β-H, 3α, 7-oxo | 389>389 | 9.71±0.01 | 9.71±0.01 | 9.71±0.01 | 53 | √ isoUCA | 5β-H, 3β, 7β, 12α | 407>407 | 407>343 | 2.30±0.00 | 2.30±0.00 | 2.30±0.03 | |
| 16 | √ 12-oxoLCA | 5β-H, 3α, 12-oxo | 389>389 | 389>343 | 9.96±0.00 | 9.96±0.00 | 9.95±0.01 | 54 | TBA04 | Unknown | 407>407 | NA | 2.46±0.00 | ND | |
| 17 | oxoDBA06 | Unknown | 389>389 | NA | 10.23±0.01 | 10.22±0.01 | 55 | TBA05 | Unknown | 407>407 | 407>345 | NA | 2.75±0.01 | 2.74±0.01 | |
| 18 | oxoDBA07 | Unknown | 389>389 | NA | 10.55±0.01 | 10.55±0.01 | 56 | TBA06 | Unknown | 407>407 | 407>317 | NA | 3.96±0.01 | 3.94±0.01 | |
| 19 | √ 3 -dehydroCDC A | 5β-H, 3-oxo, 7α | 389>389 | 10.91±0.00 | 10.92±0.03 | 10.93±0.01 | 57 | TBA07 (12-epiCA) | 5β-H, 3α, 7α, 12β | 407>407 | 407>343 | NA | 4.24±0.01 | 4.23±0.01 | |
| 20 | oxoDBA08 (3-dehydroDCA) | 5β-H, 3-oxo, 12α | 389>343 | 389>343 | NA | 10.92±0.03 | 10.93±0.01 | 58 | √ UCA | 5β-H, 3α, 7β, 12α | 407>407 | 407>345 | 4.36±0.00 | 4.37±0.01 | 4.36±0.02 |
| 21 | √ MDCA | 5β-H, 3α, 6β | 391>391 | 8.15±0.01 | 8.15±0.01 | 8.14±0.01 | 59 | TBA08 | Unknown | 407>407 | NA | 4.49±0.02 | ND | ||
| 22 | √ isoUDCA | 5β-H, 3β, 7β | 391>391 | 8.28±0.01 | 8.28±0.01 | 8.28±0.00 | 60 | TBA09 | Unknown | 407>407 | 407>271 | NA | 4.83±0.01 | 4.82±0.01 | |
| 23 | √ isoHDCA | 5β-H, 3β, 6α | 391>391 | 8.56±0.01 | ND | 8.58±0.02 | 61 | TBA10 | Unknown | 407>407 | 407>345 | NA | 5.02±0.01 | 5.00±0.01 | |
| 24 | √ UDCA | 5β-H, 3α, 7β | 391>391 | 8.85±0.00 | 8.85±0.00 | 8.84±0.00 | 62 | TBA11 | Unknown | 407>407 | NA | 5.20±0.00 | ND | ||
| 25 | √ HDCA | 5β-H, 3α, 6α | 391>391 | 9.00±0.01 | 9.00±0.00 | 9.00±0.00 | 63 | TBA12 | Unknown | 407>407 | NA | 5.58±0.01 | 5.55±0.01 | ||
| 26 | DBA01 (12-epiDCA) | 5β-H, 3α, 12β | 391>391 | 391>345 | NA | 9.57±0.01 | 9.57±0.00 | 64 | √ βHCA | 5β-H, 3α, 6α, 7β | 407>405 | 407>405 | 6.07±0.00 | 6.09±0.01 | 6.07±0.01 |
| 27 | √ isoDCA | 5β-H, 3β, 12α | 391>391 | 391>345 | 9.64±0.01 | 9.65 | 9.64±0.01 | 65 | √ isoCA | 5β-H, 3β, 7α, 12α | 407>289 | 407>289 | 6.20±0.01 | 6.22±0.02 | 6.22±0.02 |
| 28 | DBA02 | Unknown | 391>391 | NA | 10.27±0.01 | 10.26±0.01 | 66 | TBA13 | Unknown | 407>407 | 407>343 | NA | 6.30±0.01 | 6.28±0.01 | |
| 29 | DBA03 | Unknown | 391>391 | NA | 10.58±0.01 | 10.58±0.01 | 67 | TBA14 | Unknown | 407>407 | NA | 6.65±0.01 | 6.63±0.01 | ||
| 30 | √ CDCA | 5β-H, 3α, 7α | 391>391 | 10.79±0.00 | 10.80±0.00 | 10.79±0.00 | 68 | √ aMCA | 5β-H, 3α, 6β, 7α | 407>407 | 407>405 | 6.73±0.01 | ND | ND | |
| 31 | √ DCA | 5β-H, 3α, 12α | 391>391 | 391>345 | 11.02±0.00 | 11.02±0.00 | 11.02±0.00 | 69 | TBA15 | Unknown | 407>407 | NA | 7.09±0.01 | 7.07±0.01 | |
| 32 | √ 3-deoxyCA | 5ß-H, 7α, 12α | 391>391 | 391>345 | 12.19±0.00 | ND | ND | 70 | TBA16 | Unknown | 407>407 | NA | 7.13±0.01 | 7.12±0.01 | |
| 33 | √ 6,7-dioxoLCA | 5β-H, 3α, 6-oxo, 7-oxo | 403>403 | 4.63±0.00 | 4.62±0.02 | ND | 71 | √ βMCA | 5β-H, 3α, 6β, 7β | 407>407 | 7.23±0.00 | ND | ND | ||
| 34 | dioxoTBA01 (3,7-dioxoLCA?) | 5β-H, 3-oxo, 7-oxo, 12α? | 403>403 | 403>341 | NA | 5.53±0.01 | 5.52±0.01 | 72 | TBA17 | Unknown | 407>407 | NA | 7.63±0.01 | 7.63±0.01 | |
| 35 | dioxoTBA02 | Unknown | 403>403 | NA | 7.64 | 7.64±0.00 | 73 | √ aHCA | 5β-H, 3α, 6α, 7α | 407>407 | 8.06±0.00 | 8.06±0.00 | 8.06±0.00 | ||
| 36 | √ 7,12-dioxoLCA | 5β-H, 3α, 7-oxo, 12-oxo | 403>403 | 9.71±0.01 | ND | ND | 74 | TBA18 | Unknown | 407>407 | 407>331 | NA | 8.23±0.00 | 8.23±0.01 | |
| 37 | oxoTBA01 | Unknown | 405>405 | 405>343 | NA | 3.97±0.01 | 3.96±0.01 | 75 | √ alloCA | 5α-H, 3α, 7α, 12α | 407>407 | 407>361 | 8.58±0.00 | 8.58±0.01 | 8.57±0.01 |
| 38 | oxoTBA02 (3-dehydroUCA?) | 5β-H, 3-oxo, 7β, 12α? | 405>405 | 405>271 | NA | 4.07±0.01 | 4.06±0.01 | 76 | √ CA | 5β-H, 3α, 7α, 12α | 407>407 | 407>289 | 8.71±0.00 | 8.71±0.00 | 8.71±0.01 |
Retention time of standards (√) in analytical runs lasting for 8 days. Abbreviations were named according to the proposed nomenclature for BAs [30].
2.2. Human serum and urine
The post-prandial serum and urine were collected from 7 patients with biopsy-confirmed nonalcoholic steatohepatitis (NASH) and 13 healthy volunteers [31]. This study was approved by the UNC-CH Biomedical Institutional Review Board and was published in ClinicalTrials.gov of U.S. National Library of Medicine (NCT01766960). Urine samples were collected and pooled over the 2-h post-prandial period. Blood samples were collected at 0.0, 0.5 h, 1.0 h, 1.5 h and 2.0 h after the meal and allowed to clot for 30–60 min to separate the serum. Serum and urine samples were stored at −80 °C until analysis. The certified human plasma (SRM1950, http://srm1950.nist.gov/) was purchased from the National Institute of Standards and Technology (NIST).
2.3. Preparation of calibration and quality control samples.
The 5 mM stock solutions of unconjugated BAs in methanol were pooled and diluted to final concentrations of 100 μM each with methanol. The blank matrix (serum and urine) was prepared by treating fetal bovine serum (FBS, Omega Scientific Inc., Tarzana, CA) or mixed urine collected from six volunteers with 10% (w/v) with activated charcoal to remove BAs. Calibration samples (4.1, 12.3, 37.0, 111.1, 333.3, 1000 and 3000 nM) and quality control (QC) samples (6.2, 18.5, 55.6, 166.7, 500 and 1500 nM) were prepared by serial dilution of the 100 μM working solution with either the blank serum or the blank urine (charcoal-treated).
2.4. Sample preparations
Urine and serum samples were thawed on ice. Aliquots (50 μL) of each sample were transferred into the 700 μL sample collection plate in quadruplicate. The first aliquot was prepared with treatment-1 (T1), in which 150 uL sodium acetate buffer (pH 5.0) were added to determine the free unconjugated forms. The second aliquot was prepared with treatment-2 (T2), in which 150 uL buffer containing 100 U of CH were added to hydrolyze the aminoacyl amidates. The third aliquot was prepared with treatment-3 (T3), in which 150 uL buffer containing 50 U of SUL1 and 500 U of GLU1 were added to hydrolyze the sulfates and glucuronides. The final aliquot was prepared with treatment-4 (T4), in which 150 uL buffer containing 100 U of CH, 50 U of SUL1 and 500 U of GLU1 were added to determine the total BA levels. The calibration and quality control samples were prepared using the T1 protocol without incubation procedure. The plate was incubated at 37 °C for 6 h and subsequently lyophilized. 200 uL of acetonitrile containing 1% formic acid and 100 nM internal standards were added to each well. The plate was mixed by vortexing at 1500 rpm for 30 min at 10 °C and then centrifuged at 3000 g for 20 min at 4 °C. 200 μL supernatant were transferred to another plate and vacuum-evaporated at 30 °C. The residue was reconstituted with 50 μL of acetonitrile and 50 μL of water and then mixed by vortexing at 900 rpm for 20 min at 10 °C. After centrifugation, the plate was placed into an autosampler for analysis.
2.5. Method Validation
The UPLC-MS/MS method was validated for 32 unconjugated standard species spiked in the blank serum by reference to the FDA criteria for linearity, carry-over, matrix effects, accuracy and precision and stability [32]. All the validation samples except for stability tests were prepared by the T1 protocol without incubation. Linearity was evaluated over a concentration range of 4.1–3000 nM. Carry-over was assessed by comparison of the peak areas detected in the blank matrix vs lower limit of quantification (LLOQ) and upper limit of quantification (ULOQ). Matrix effects were evaluated at QC3 (55.6 nM) and QC6 (1500 nM) by comparing the peak areas of LCA-D4, DCA-D4, UDCA-D4 and CA-D4 that were spiked in solvent and the processed residues of biological samples. Accuracies and precisions were evaluated at six QC levels (4.1, 12.3, 37.0, 111.1, 333.3, 1000 and 3000 nM) on intra-run (5 replicates analyzed on same day) and inter-run (three different days). Stabilities of the unconjugated species were tested at six QC levels with samples prepared by the T4 protocol and incubated at 37 °C for 6 h and 24 h. Method validation for urine samples was abbreviated and focused mainly on linearity, accuracy and precision at three QC levels (30, 300 and 3000 nM).
2.6. Quantitative UPLC-MS/MS analysis
The targeted analysis was performed using an ACQUITY ultra-performance liquid chromatography (UPLC) coupled to a Xevo TQ-S mass spectrometer (Waters, Milford, MA, USA). The mobile phases consisted of 0.01% formic acid in water (mobile phase A) and acetonitrile (mobile phase B). 5 μL of each sample were injected onto an ACQUITY BEH C18 column (1.7 μm, 100 mm × 2.1 mm) (Waters, Milford, MA) maintained at 35 °C. The flow rate was 0.45 mL/min with the following gradient: 0.0–0.5 min (95% A), 0.5–1.0 min (95–64% A), 1.0–2.0 min (64–74% A), 2.0–4.0 min (74–70% A), 4.0–6.0 min (70% A), 6.0–7.0 min (70–62% A), 7.0–9.0 min (62–55% A), 9.0–12.5 min (55–30% A), 12.5–13.0 min (30–0% A), 13.0–14.0 min (0% A) and 14.0–14.1 (0–95% A) and 14.1–15.0 min (95% A). The mass spectrometer was operated in the negative mode with a 3.0 kV capillary voltage. The source and desolvation temperatures were set at 150 and 550 °C with the cone gas flow and desolvation gas flow set at 150 and 950 L/h, respectively. The multiple reaction monitoring (MRM) transitions used for quantification (Quan-MRM) and identification (val-MRM) of unconjugated BAs are summarized in Table 1 and ESM Table S1.
2.7. UPLC-QTOF-MS analysis
The negative fragments spectra of BAs were captured using an ACQUITY UPLC coupled to a Xevo G2-S QToF mass spectrometer with the same chromatographic and source parameters. The data were collected by MS/MS scans (0.036 s/scan) of the [M-H]- anion under various collision energies (CE) in centroid mode. Leucine enkephalin was infused via the reference probe as lockspray to ensure m/z accuracy. The LM and HM resolutions were set at 25.0 and 15.0, respectively. Such settings confined the quadrupole transmission window, excluded isotopic contributors and facilitated data analysis [28].
2.8. Data processing
The UPLC-MS/MS and UPLC-QTOF-MS raw data were processed using MassLynx (V4.1, Waters, Milford, MA, USA) or UNIFI (V1.8, Waters, Milford, MA, USA) software. The QTOF MS/MS spectrum of each BA was obtained by combining the scans within peak width. Principal component analysis (PCA) was carried out on the total BAs levels dataset with Pareto scaling using SIMCA (v14, Umetrics, UmeÅ, Sweden).
3. Results and discussion
3.1. Development of enzyme-digestion protocols
Quantitative enzyme-digestion protocols were developed based on the selectivity and efficacy of enzymes to digest a total of 27 standards of conjugated BAs at 1000 nM (ESM Table S3). CH (50U/50μL sample) showed a high activity (>95%) toward all the tested BA amides, while SULs (10U/50μL sample) and GLUs (50U/50μL sample) demonstrated varying activities (20–100%) toward BA sulfates and glucuronides. The cross-activities of SULs and GLUs to digest sulfates and glucuronides led to the development of T3, in which a combination of SUL1 and GLU1 was employed to investigate the overall sulfation and glucuronidation of BA species. The development of T4 was based on the evidence that the combination of SUL-1, GLU-1 and CH at 50U/50μL sample was able to completely digest the conjugated BAs in a pooled urine sample (ESM Fig. S1). The inclusion of enzymes in sample preparation did not introduce interferences. Because a longer incubation time was necessary for GLU1 to hydrolyse glucuronides linked at C-3 or C-7 [33], we used excess amounts of enzymes, SUL1 50 U, GLU1 500 U and/or CH 100 U/50 μL sample, and a longer incubation time, 37 °C for 6 h, in the final protocols. As illustrated in ESM Fig. S2, the enzyme-digestion protocols were efficient to manipulate the conjugated BAs in urine and serum samples.
3.2. Fine-tuned gradient and advanced MRM techniques discriminated the unconjugated BAs enriched in samples
The enzyme protocols efficiently digested the conjugated BAs and raised some problems relating to the discrimination of the enriched unconjugated BAs. The re-optimization of chromatographic gradient based on our previous work [29] clearly highlighted such challenges. A subtle, segmental gradient was determined to be critical for the resolution power of the tri-hydroxyl BAs eluted within the retention times of isoUCA (53) and αMCA (68) (ESM Fig. S3A/B). A typical example was highlighted in the separation of βHCA (64), isoCA (65) and TBA13 (66). To increase throughput, we did not extend the runtime for a complete resolution of them because βHCA (64), isoCA (65) and TBA13 (66) could be differentiated by their MRM transitions of m/z 407 > 405, m/z 407 > 289 and m/z 407 > 343, respectively (ESM Fig. S3C). Due to lack of standards, the separation power for the oxo species or unsaturated structures was not optimized as thoroughly as the saturated species.
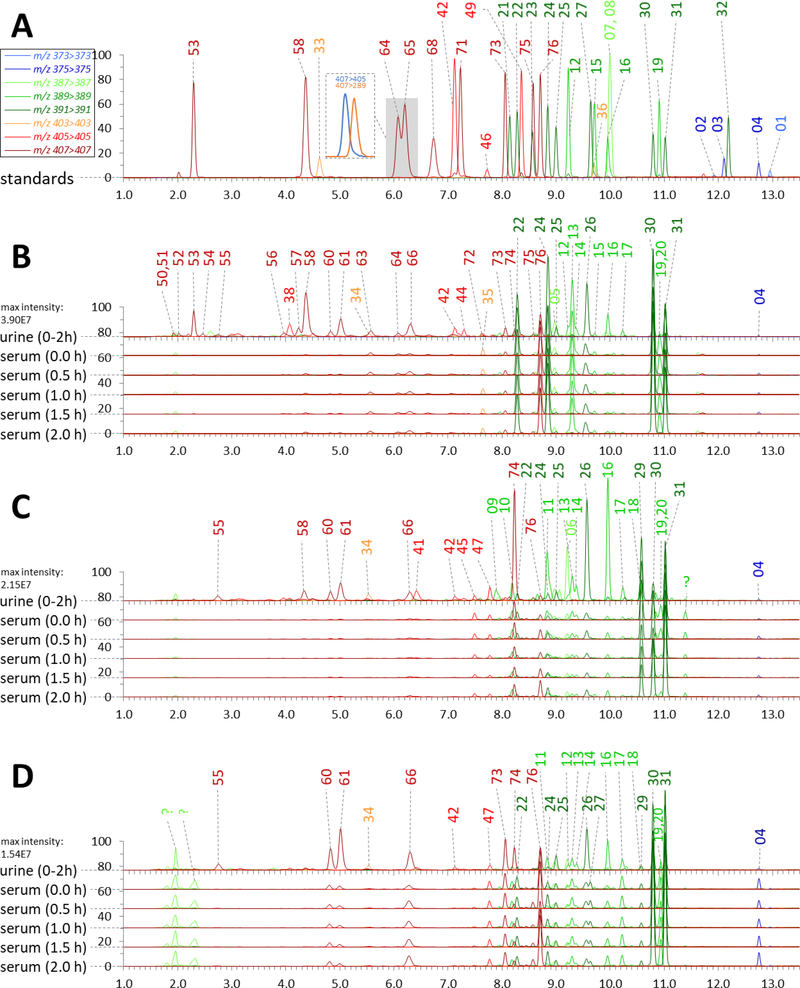
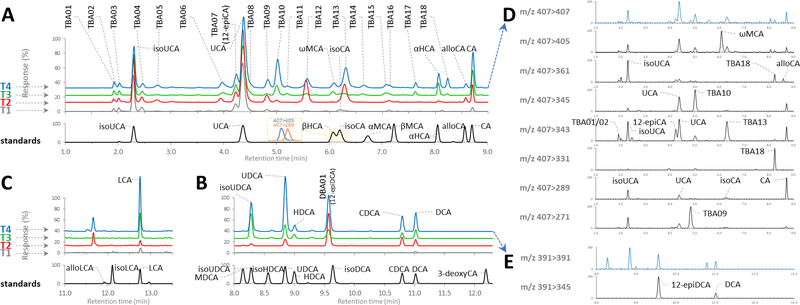
The optimized gradient had a high throughput and acceptable resolution at quan-MRM transients for all the 33 unconjugated standards (Fig. 2A) and the newly found unconjugated species that were enriched in the digested urine and serum samples (Fig. 2B, 2C and 2D). In continuous analytical runs lasting for 8 days the method exhibited strong reproducibility with the retention variations (standard deviation) for all analytes less than ±0.02 min (Table 1). The separation power for mono-hydroxyl, di-hydroxyl and tri-hydroxyl species in a representative urine sample prepared by T1, T2, T3 and T4 is particularly highlighted in Fig. 3A, 3B and 3C. The present method showed clear superiority to previous LC-MS methods because the insufficiently separated unconjugated species were able to be discriminated by the val-MRM transients (Fig. 3D and 3E) [28].
Fig. 2.
Ion chromatograms of unconjugated BAs in the mixed standard samples (A) and the digested urine and serum samples (prepared by T4) from a NASH patient (B) and two healthy subjects (C, D). Peak intensity was normalized to the highest response of MRM transients. Peaks were labeled with the NO. recorded in Table 1
Fig. 3.
Ion chromatograms of BAs detected in the mixed standard sample (black) and a representative urine sample (as seen in Fig. 1B) prepared without enzymes (T1), with choloylglycine hydrolase (T2), with sulfatase and β-glucuronidase (T3), with all three enzymes (T4). (A) Trihydroxy-BAs detected by pseudo-MRM transition m/z 407 > 407, (B) Dihydroxy-BAs by pseudo-MRM transition m/z 391 > 391, (C) Monohydroxy-BAs detected by pseudo-MRM transition m/z 375 > 375. (D) Differentiation of the detected trihydroxy-BAs by the val-MRM transients. (E) Differentiation of DBA01 (12-epiDCA) and DCA from the other detected dihydroxy-BAs by the val-MRM transient of m/z 391>345. The detected BA species were labeled with the abbreviations recorded in Table 1
3.3. Quantitative performances
Beyond previous publications that used standard solutions for calibration, our work prepared the calibration and QC samples by spiking standards in the charcoal-treated blank matrix (serum or urine). A good linearity (R2>0.99) across the concentration range of 4.3–3000 nM was observed for 32 standard species in both serum and urine. LLOQ was verified as 4.1 nM for each standard species in both serum and urine, with signal to noise ratio > 5, the accuracy (percentage bias) < ±20% and the precision (coefficient of variance) < 20% and the carryover lower than 5%. Matrix effects were not observed with coefficient of variance < 15% at QC3 and QC6 for the stable isotope labeled internal standards spiked in solvent and the processed residues of individual serum and urine samples. An acceptable intra-/inter-run accuracy (84%−116%) and precision (no more than 20% for QC1 and no more than 15% for the other QC levels) for 32 standards was demonstrated in serum (ESM Figs. S4 and S5). The quantitative performances for urine samples were similar and the data was shown in ESM Fig. S6. As shown in ESM Fig. S7, all the 32 standard species were stable after being treated with the T4 protocol and incubated for 6 h, however, a significant degradation (< 80%) was observed for some species after 24h of incubation.
3.4. Oxidation stereochemistry of unconjugated BAs in human urine and serum
By using the validated method, we were able to take a closer look at the structural diversity of human C24 BAs derived from oxidation stereochemistry. As listed in Table 1, we detected at least 70 unconjugated species in the postprandial serum and urine samples from 20 adults (7 NASH patients and 13 healthy volunteers). Fig. 2 illustrates the representative ion chromatograms of digested urine and serum samples from one NASH patient (Fig. 2B) and two healthy volunteers (Fig. 2C and 2D). The structural diversity of BAs in urine was much more diverse than that in serum and increased as expected in a rank order based on the steroid nucleus oxidation number, ie., mono-hydroxyl species < di-hydroxyl species < tri-hydroxyl species. Several tetra-hydroxyl species were also detected but not discussed in this work due to lack of standards and much lower responses of them compared to the others.
Among the 33 standards, alloLCA (02), 3-deoxyCA (32), 7, 12-dioxoLCA (36), 12-oxoCDCA (46), αMCA (68) and βMCA (71) were not detected or detected with levels under LLOQ. The absence of αMCA and βMCA in the human BA metabolome confirmed that humans are unable to synthesize αMCA and βMCA as rodents via 6β-hydroxylation of CDCA and UDCA, respectively [34]. Besides the 27 known BA species, the present method detected an additional total of 43 unknown unconjugated species, including 21 saturated species and 22 relevant oxidative or unsaturated structures. The high-definition MS/MS spectra of the detected species in the digested urine samples were captured by UPLC-QTOF-MS analysis and are summarized in the ESM Table S4. The fragmentation data of known species captured in biological samples were well consistent with those captured in the standard samples.
3.5. Structural diversity of mono-hydroxyl species
LCA (04) was the major mono-hydroxyl BA detected in both urine and serum. Low levels of 3-dehydroLCA (01) and isoLCA (03) were also detected in some individuals with a higher LCA level. This observation led us to propose that there was a common pathway involving the oxidation and the epimerization of hydroxyl groups, which is generally acknowledged to be mediated by the hydroxysteroid dehydrogenases (HSDHs) of the gut microbiota [35,36]. Such a pattern became useful for the identification of some newly found unknown species with more than one hydroxyl group on the steroid nucleus.
3.6. Structural diversity of di-hydroxyl species
The di-hydroxyl BAs showed much more diversity than the mono-hydroxyl species. We detected a total of 11 di-hydroxyl species and at least 16 relevant oxidative or unsaturated species. The 11 di-hydroxyl species included 9 known BAs, MDCA (21), isoUDCA (22), isoHDCA (23), UDCA (24), HDCA (25), isoDCA (27), CDCA (30), and DCA (31), along with 3 newly found species, DBA01-DBA03 (26, 28, 29). Among them, isoUDCA, UDCA, HDCA, DBA01, DBA03, CDCA, and DCA were the major di-hydroxyl BAs detected in serum and/or urine (Fig. 2).
DBA01 (26) was detected in both the serum and urine of all individuals. The differentiation of DBA01 from isoDCA (27) was particularly noteworthy because they have only a minor but robust difference in the retention time (9.57 ± 0.01 for DBA01 and 9.64 ± 0.01 min for isoDCA). Interestingly, both DBA01 and isoDCA have almost the same negative fragmentation patterns as that of DCA (ESM Table S4), indicating that DBA01 may be one of the four DCA epimers. Because 12-oxoLCA (16) was detected with consistent levels with respect to DBA01 in individuals, we tentatively identified DBA01 as 3α, 12β-dihydroxy-5β-cholan-24-oic acid (12-epiDCA). The tentative identification matched well with the fragment data of 12-epiDCA retrieved in MASSBANK [37] and was eventually confirmed by comparison to the standard (ESM Fig. S8) prepared from 12-epiDCA methyl ester 3α-benzoate (TRC, Cat D232663). 12-epiDCA is associated with the gut microbial activities of 12-HSDHs that catalyze the epimerization of DCA [35,36]. It was detected in previous studies by GC-MS in human feces [38,39], in the plasma of patients with intestinal bacterial overgrowth [40], in the duodenal bile and urine of diabetic subjects [41], and in the urine of healthy human subjects [42]. Our work has provided the first LC-MS based evidence that 12-epiDCA is a common human BA species.
Unlike 12-epiDCA, DBA03 (29) occurred with an elevated level in some individuals. There were no significant fragments with identification value in the MS/MS spectra of DBA03 (ESM Table S4). The retention time of DBA03 (10.58 min) did not conform to any possible epimers of CDCA and HDCA. An unusual oxidation site other than C-6, C-7 and C-12 was therefore proposed to reside on the skeleton of DBA03. We are currently dealing with the synthesis challenges and the identification result will be reported in a later publication.
Another noteworthy case was the differentiation of oxoDBA08 (20) from 3-dehydroCDCA (19). The retention time variation of the 3-dehydroCDCA standard in runs lasting for 8 days was as narrow as 10.91±0.00 min. However, the retention data of “3-dehydroCDCA” in urine (10.92±0.03 min) and serum (10.93±0.01 min) was much more variable, indicating that another isomer was being co-eluted. They occurred more frequently in serum samples than in urine samples (Fig. 2B, 2C and 2D). OxoDBA08 (20) was hypothesized to be 12α-hydroxy-3-oxo-5β-cholan-24-oic acid (3-dehydroDCA) because isoDCA (27) occurred at higher concentrations in individuals with the retention time of “3-dehydroCDCA” obviously deviated from 10.91 min. Comparison of our data with that of a commercial standard (TRC, Cat O856870) eventually confirmed the identification of 3-dehydroDCA (ESM Fig. S9). In the similar way, we tentatively identified oxoDBA04 (13) as 7β-hydroxy-3-oxo-5β-cholan-24-oic acid (3-dehydroUDCA) because it occurred in individuals consistently with both UDCA (24) and isoUDCA (22). The identification of 3-dehydroUDCA requires confirmation using an standard.
3.7. Structural diversity of tri-hydroxyl species
The tri-hydroxyl BAs showed the most diversity among the detected BA species. We detected a total of 25 tri-hydroxyl BAs and at least 15 relevant oxidative or unsaturated species. The 25 tri-hydroxyl BA species included 7 known BAs, isoUCA (53), UCA (58), βHCA (64), isoCA (65), αHCA (73), alloCA (75), CA (76), and 18 newly found species, TBA01-TBA18 (50–52, 54–57, 58–63, 66, 67, 69, 70, 72, 74).
DioxoTBA01 (34) and oxoTBA02 (38) were detected in individual urine samples in parallel to isoUCA (53) and UCA (58), which was consistent with the patterns for oxoDBA04 (13), UDCA (24) and isoUDCA (22). Based on these data, oxoTBA02 was tentatively identified as 7β, 12α-dihydroxy-3-oxo-5β-cholan-24-oic acid (3-dehydroUCA) and diketoTBA01 was tentatively identified as 12α-hydroxy-3,7-dioxo-5β-cholan-24-oic acid (3, 7-dioxoLCA). The fragmentation spectra of oxoTBA02 and dioxoTBA01 (ESM Table S4) have provided positive supporting evidence for their tentative identification.
Most of the unknown tri-hydroxyl species, such as TBA01 (50), TBA02 (51), TBA05 (55), TBA07 (57), TBA09 (60), TBA10 (61), TBA13 (66) and TBA18 (74) employed distinctive CID dissociation routes including dehydration, loss of carbon monoxide/carbon dioxide, dehydrogenation and cleavages of the steroid skeleton. These fragmentation pathways have been ascribed to 12-hydroxylation that initializes the rotation of the carboxylate side-chain and facilitates the loss of C-24 and ring cleavages [28]. Hydroxylation at both C-3 and C-12 was therefore anticipated on their skeleton. Based on this evidence, TBA07 (57) was eventually identified by a comparison with the commercial standard, (TRC, Cat H943480), as 3α, 7α, 12β-trihydroxy-5β-cholan-24-oic acid (12-epiCA) (ESM Fig. S10). The identification also matched well with the fragments data of 12-epiCA retrieved in MASSBANK [37]. 12-epiCA is also associated with the gut microbial activities of 12-HSDHs that catalyze the epimerization of CA [43]. To the best of our knowledge, there was only one GC-MS based work that ever reported the detection of 12-epiCA in human urine [42]. Our work has provided a strong LC-MS based evidence that 12-epiCA is a common human BA species.
TBA05 (55), TBA09 (60), TBA10 (61), TBA13 (66) and TBA18 (74) were common 12-hydroxylated tri-hydroxyl species detected in human serum and urine (Fig. 2). According to their fragmentation spectra, we proposed an unusual oxidation site besides C-3 and C-12 on their skeletons. One of them was believed to be DCA-1β-ol, which has been characterized as a hydroxylated metabolite of DCA via CYP3A [26,27]. The others were hypothesized to be associated with hydroxylation sites on C-2, C-4, C-5, C-6 and C-19. These tri-hydroxyl BA species have been previously detected and identified in biological samples in early human life and patients with liver diseases [19]. We are currently dealing with the synthesis challenges and the results will be reported later.
3.8. Quantitative metabolomic analysis showed an interplay between the role of host and gut microbiota in maintaining BA homeostasis
By using the validated method, we quantitatively or semi-quantitatively determined the BA species in the postprandial serum and urine from 7 NASH patients and 13 healthy volunteers. The total levels of the major detected BA species in biological samples prepared by T4 are listed in the ESM Table S5. The newly identified or unidentified ones were semi-quantified using the calibration curve of its analogue because they produced equivalent responses at the same level in the same MRM channels. For example, 3-dehydroUDCA (13) was semi-quantified using the calibration curve for 7-oxoLCA (15) at MRM of 389>389, 12-epiDCA (26) was semi-quantified using the calibration curve for isoDCA (27) at MRM of 391>345, and 12-epiCA (57) was semi-quantified using the calibration curve for CA (76) at MRM of 407>407. The other unidentified species were semi-quantified with a similar strategy. For further inter-lab cross-validation purposes we have provided the quantification results of the NIST1950 plasma sample prepared by either T1 or T4 in ESM Table S5.
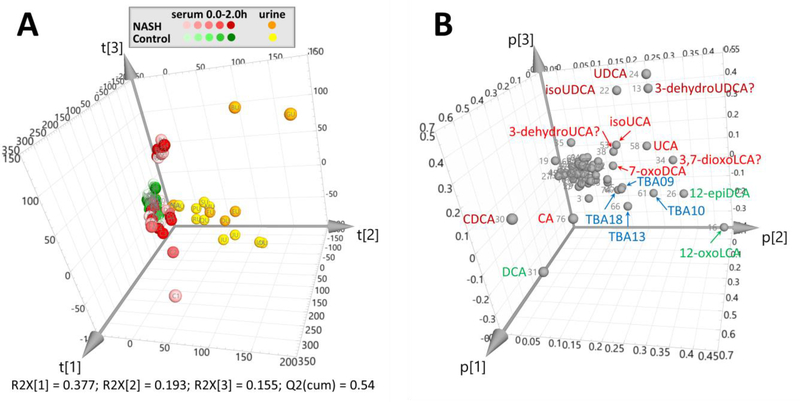
PCA was used to interpret the postprandial changes in the total BA profiles both in serum and urine. The serum BA profiles between NASH patients and healthy volunteers were not fully separated in the PCA scores plot (Fig. 4A and see details in ESM Fig. S11). Nevertheless, the postprandial serum BA profiles of NASH patients changed more than those of healthy controls, reflecting a dysbiosis of NASH patients in contrast to the homeostasis of healthy controls in their host-gut microbial co-metabolism. Because the variances were mainly explained by t[1] and t[3], the BA species contributing the most to p[1] (CDCA, CA, DCA) and p[3] (UDCA, isoUDCA and 3-dehydroUDCA) provided some preliminary evidence for the altered BA metabolism in NASH patients. In detail, two NASH patients (subject-C and -F) showed significantly different post-prandial serum levels of CDCA, CA, DCA, which could possibly be associated with their respective dysfunction of the hepatic BA uptake system. Another two patients (subject-B and -G) demonstrated a significantly elevated and different serum levels of UDCA, isoUDCA and 3-dehydroUDCA, which may be associated with an increased colonization of gut bacteria expressing HSDHs that catalyze the oxidation and epimerization of CDCA [35,36].
Fig. 4.
PCA scores plot (A) and loadings plot (B) of the total levels of the detected BA species in the postprandial serum and urine samples from 7 NASH patients (subject A-G) and 13 healthy volunteers (subject H-T). The total levels of the detected BA species were determined in samples prepared using the enzyme-digestion protocol, T4. The detected BA species were labeled in the loadings plot with the NO. and abbreviations recorded in Table 1
As expected, the PCA scores plot showed a clear separation of the BA profiles in urine and serum. Because the separation of urine and serum was mainly explained by t[2], the variables contributing to p[2] (as labeled in Fig. 4B) highlighted the BA metabolites that have a renal excretion tendency. Except for the unidentified 12-hydroxylated tri-hydroxyl species, TBA09, TBA10, TBA13 and TBA18, most of the highlighted BAs were the oxidized and epimerized metabolites of CDCA (UDCA, isoUDCA and 3-dehydroUDCA), CA (UCA, isoUCA, 7-oxoDCA and 3,7-dioxoLCA) and DCA (12-epiDCA and 12-oxoLCA) [35,36]. In this regard, we proposed that the urinary total BA profile may serve as an ideal footprint manifesting the functional status of the host-gut microbial co-metabolism of BAs.
3.9. Conjugation patterns and urinary excretion tendencies of identified BA species
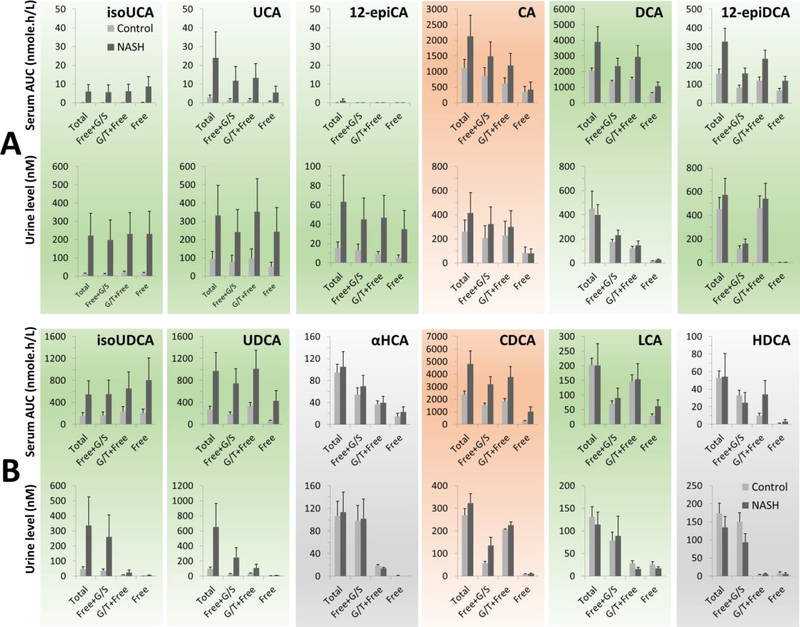
By using the enzyme-digestion protocols, Fig. 5A and 5B demonstrated the conjugation patterns of the downstream metabolites of CA and CDCA in serum and urine of NASH patients and healthy control, respectively. αHCA and HDCA were categorized as the downstream metabolites of CDCA according to the hepatic CYP3A mediated 6α-hydroxylation of CDCA [44] and LCA [45,25,46–48] characterized in vitro. Because CDCA, CA, LCA and DCA exist in serum and urine in various forms but are not inclined to renal excretion, we supposed that renal elimination of BAs requires additional modifications of the steroid nucleus. This hypothesis was tested by analyzing the conjugation patterns and renal excretion tendencies of both the HSDHs-mediated epimerized metabolites and hepatic hydroxylated metabolites.
Fig. 5.
Conjugation patterns and urinary excretion tendencies of the downstream metabolites of CA (A) and CDCA (B) in serum and urine of NASH patients (n=7) and healthy controls (n=13). Data was shown as MEAN ± SEM. The serum level and urine level were given as the area under curve (AUC) of the 0–2h post-prandial serum levels and the urinary concentration in the 0–2h post-prandial urine. Primary BAs were colored in red and secondary BAs were colored in green. The gradient-ramp demonstrated the tendency of certain BAs to undergo renal excretion. The free unconjugated form (Free), free one plus its aminoacyl amidates linked with either glycine or taurine (G/T+Free), free one plus its sulfates and glucuronides (Free+G/S), and the total level of each BA species were determined using the T1, T2, T3 and T4 protocol, respectively
The epimerized metabolites of CA, including isoUCA, UCA and 12-epiCA, existed in the circulation mainly as the free unconjugated form and tended to be excreted in urine. 12-epiDCA, an epimerized metabolite of DCA, also was inclined to be excreted in urine where it was detected mainly as the aminoacyl amidates. These observations revealed that microbial HSDHs-mediated epimerization played a significant role in the renal elimination of CA and DCA. UDCA and isoUDCA, the epimerized metabolites of CDCA, which exist in the circulation mainly as free unconjugated BAs, are not inclined to be excreted in urine, where they present mainly as various conjugated forms. On the contrary, αHCA and HDCA, the hepatic 6α-hydroxylated metabolites of CDCA and LCA, were inclined to be excreted in urine and existed in urine mainly as sulfates and/or glucuronides. This observation was consistent with previous findings that an extensive 6-O-glucuronidation is responsible for the low levels of αHCA and HDCA in the circulation [49–52]. Our data has manifested an interactive role of host and gut microbiota in maintaining BA homeostasis. In this regard, strong interests in the unidentified 12-hydroxylated tri-hydroxyl species, TBA05, TBA09, TBA10, TBA13 and TBA18, have been generated because they are major BA species with a urine excretion tendency (Figs. 2 and 4B).
4. Conclusion
This work described the development and validation of a powerful tool to investigate the human C24 BA metabolome that is associated with a vast structural diversity derived from both oxidative stereochemistry and conjugation. The LC-MS/MS based method has a high throughput, covering a great deal more of unconjugated BA species in a 15-min analytical cycle. It demonstrated acceptable quantitative performances for 32 unconjugated standard species with respect to specificity, accuracy, precision and stability, therefore, providing an indirect way to ascertain BA conjugation patterns through the use of validated enzyme-digestion protocols. Application of the present method in the analysis of post-prandial serum and urine samples from NASH patients and healthy volunteers led to the detection of at least 70 unconjugated C24 BA species and the identification of 12-epiDCA, 3-dehydroDCA and 12-epiCA for the first time by LC-MS techniques. Although the other newly found species remain unidentified, we have provided the high-definition negative fragments spectra of those that were detectable to facilitate further endeavors. Subsequent work following the present method will eventually close the gap between GC-MS techniques in the past age and LC-MS techniques currently prevailing in biomedical research.
Quantitative metabolomic analysis of the total BA spectra in serum and urine also highlighted the fact that the urinary BA profile may serve as an ideal footprint capable of assessing the functional status of the host-gut microbial BA co-metabolism. Preliminary analysis of the conjugation patterns and urinary excretion tendencies of the identified BAs revealed that gut bacteria-mediated epimerization reactions play a significant role in the renal elimination of CA and DCA, while hepatic 6α-hydroxylation of BAs plays a key role in the renal elimination of CDCA and LCA. Based on these findings, this present method has shown an ultra-high performance providing substantial insights into the interplay of host and gut microbiota in maintaining BA homeostasis. In summary, this study provides a powerful tool for human C24 BA metabolome analysis. Further applications in translational and biomedical research will hopefully boost our understanding of host-gut microbial BA co-metabolism and open new therapeutic opportunities for treatment of liver and other metabolic diseases.
Supplementary Material
Acknowledgement
We are grateful to Prof. Dr. Takashi Iida (Nihon University) for the gift of the βUCA synthsized standard. This study was supported, in part, by National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01 GM041935 and R35 GM122576. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Compliance with Ethical Standards
The authors declare that there are no conflicts of interest. The human urine and serum samples used in this work was collected from the clinical trial approved by the UNC-CH Biomedical Institutional Review Board and published in ClinicalTrials.gov of U.S. National Library of Medicine (NCT01766960). Informed consent was obtained from the individual participants who provided the urine and serum samples.
References
- 1.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012:336(6086):1262–1267. doi:science.1223813 [pii] 10.1126/science.1223813 [DOI] [PubMed] [Google Scholar]
- 2.de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17(5):657–669. doi: 10.1016/j.cmet.2013.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wahlstrom A, Sayin SI, Marschall HU, Backhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24(1):41–50. doi: 10.1016/j.cmet.2016.05.005 [DOI] [PubMed] [Google Scholar]
- 4.Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15(2):111–128. doi: 10.1038/nrgastro.2017.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7(8):678–693. doi: 10.1038/nrd2619 [DOI] [PubMed] [Google Scholar]
- 6.Tiwari A, Maiti P. TGR5: an emerging bile acid G-protein-coupled receptor target for the potential treatment of metabolic disorders. Drug Discov Today. 2009;14:(9–10):523–530. doi: 10.1016/j.drudis.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 7.Theriot CM, Koenigsknecht MJ, Carlson PE Jr., Hatton GE, Nelson AM, Li B, Huffnagle GB, ZL J, Young VB. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nature Commun. 2014;5:3114. doi: 10.1038/ncomms4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theriot CM, Bowman AA, Young VB. Antibiotic-induced alterations of the gut microbiota alter secondary bile acid production and allow for clostridium difficile spore germination and outgrowth in the large intestine. mSphere. 2016;1 (1). doi: 10.1128/mSphere.00045-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, Littmann E, van den Brink MR, Jenq RR, Taur Y, Sander C, Cross JR, Toussaint NC, Xavier JB, Pamer EG. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517(7533):205–208. doi: 10.1038/nature13828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103(10):3920–3925. doi: 10.1073/pnas.0509592103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 2009;183(10):6251–6261. doi: 10.4049/jimmunol.0803978 [DOI] [PubMed] [Google Scholar]
- 12.Pavlidis P, Powell N, Vincent RP, Ehrlich D, Bjarnason I, Hayee B. Systematic review: bile acids and intestinal inflammation-luminal aggressors or regulators of mucosal defence? Aliment Pharmacol Ther. 2015;42(7):802–817. doi: 10.1111/apt.13333 [DOI] [PubMed] [Google Scholar]
- 13.Jia W, Li H, Zhao L, Nicholson JK. Gut microbiota: a potential new territory for drug targeting. Nat Rev Drug Discov. 2008;7(2):123–129. doi:nrd2505 [pii] 10.1038/nrd2505 [DOI] [PubMed] [Google Scholar]
- 14.Hofmann AF, Hagey LR, Krasowski MD. Bile salts of vertebrates: structural variation and possible evolutionary significance. J Lipid Res. 2010;51(2):226–246. doi: 10.1194/jlr.R000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trottier J, Milkiewicz P, Kaeding J, Verreault M, Barbier O. Coordinate regulation of hepatic bile acid oxidation and conjugation by nuclear receptors. Mol Pharm. 2006;3(3):212–222. doi: 10.1021/mp060020t [DOI] [PubMed] [Google Scholar]
- 16.Alnouti Y Bile Acid sulfation: a pathway of bile acid elimination and detoxification. Toxicol Sci. 2009;108(2):225–246. doi: 10.1093/toxsci/kfn268 [DOI] [PubMed] [Google Scholar]
- 17.Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, Bouatra S, Sinelnikov I, Arndt D, Xia J, Liu P, Yallou F, Bjorndahl T, Perez-Pineiro R, Eisner R, Allen F, Neveu V, Greiner R, Scalbert A. HMDB 3.0--The Human Metabolome Database in 2013. Nucleic Acids Res. 2013;41 (Database issue):D801–807. doi: 10.1093/nar/gks1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sud M, Fahy E, Cotter D, Brown A, Dennis EA, Glass CK, Merrill AH Jr., Murphy RC, Raetz CR, Russell DW, Subramaniam S. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 2007;35 (Database issue):D527–532. doi: 10.1093/nar/gkl838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sjövall J, Griffiths WJ, Setchell KDR, Mano N, Goto J. Analysis of Bile Acids Steroid Analysis. Springer, 2010. [Google Scholar]
- 20.Garcia-Canaveras JC, Donato MT, Castell JV, Lahoz A. Targeted profiling of circulating and hepatic bile acids in human, mouse, and rat using a UPLC-MRM-MS-validated method. J Lipid Res. 2012;53(10):2231–2241. doi: 10.1194/jlr.D028803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jantti SE, Kivilompolo M, Ohrnberg L, Pietilainen KH, Nygren H, Oresic M, Hyotylainen T. Quantitative profiling of bile acids in blood, adipose tissue, intestine, and gall bladder samples using ultra high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2014;406(30):7799–7815. doi: 10.1007/s00216-014-8230-9 [DOI] [PubMed] [Google Scholar]
- 22.Han J, Liu Y, Wang R, Yang J, Ling V, Borchers CH. Metabolic profiling of bile acids in human and mouse blood by LC-MS/MS in combination with phospholipid-depletion solid-phase extraction. Anal Chem. 2015;87(2):1127–1136. doi: 10.1021/ac503816u [DOI] [PubMed] [Google Scholar]
- 23.Sarafian MH, Lewis MR, Pechlivanis A, Ralphs S, McPhail MJ, Patel VC, Dumas ME, Holmes E, Nicholson JK. Bile acid profiling and quantification in biofluids using ultra-performance liquid chromatography tandem mass spectrometry. Anal Chem. 2015;87(19):9662–9670. doi: 10.1021/acs.analchem.5b01556 [DOI] [PubMed] [Google Scholar]
- 24.Wegner K, Just S, Gau L, Mueller H, Gerard P, Lepage P, Clavel T, Rohn S. Rapid analysis of bile acids in different biological matrices using LC-ESI-MS/MS for the investigation of bile acid transformation by mammalian gut bacteria. Anal Bioanal Chem. 2017;409(5):1231–1245. doi: 10.1007/s00216-016-0048-1 [DOI] [PubMed] [Google Scholar]
- 25.Gustafsson J, Andersson S, Sjovall J. Bile acid metabolism during development: metabolism of taurodeoxycholic acid in human fetal liver. Biol Neonate. 1985;47(1):26–31. [DOI] [PubMed] [Google Scholar]
- 26.Bodin K, Lindbom U, Diczfalusy U. Novel pathways of bile acid metabolism involving CYP3A4. Biochim Biophys Acta. 2005;1687(1–3):84–93. doi: 10.1016/j.bbalip.2004.11.003 [DOI] [PubMed] [Google Scholar]
- 27.Hayes MA, Li XQ, Gronberg G, Diczfalusy U, Andersson TB. CYP3A specifically catalyzes 1beta-hydroxylation of deoxycholic acid: characterization and enzymatic synthesis of a potential novel urinary biomarker for CYP3A activity. Drug Metab Dispos. 2016;44(9):1480–1489. doi: 10.1124/dmd.116.070805 [DOI] [PubMed] [Google Scholar]
- 28.Lan K, Su M, Xie G, Ferslew BC, Brouwer KL, Rajani C, Liu C, Jia W. Key role for the 12-hydroxy group in the negative ion fragmentation of unconjugated C24 bile acids. Anal Chem. 2016;88(14):7041–7048. doi: 10.1021/acs.analchem.6b00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin S, Su M, Xie G, Li X, Wei R, Liu C, Lan K, Jia W. Factors affecting separation and detection of bile acids by liquid chromatography coupled with mass spectrometry in negative mode. Anal Bioanal Chem. 2017;409(23):5533–5545. doi: 10.1007/s00216-017-0489-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofmann AF, Sjovall J, Kurz G, Radominska A, Schteingart CD, Tint GS, Vlahcevic ZR, Setchell KD. A proposed nomenclature for bile acids. J Lipid Res. 1992;33(4):599–604. [PubMed] [Google Scholar]
- 31.Ferslew BC, Xie G, Johnston CK, Su M, Stewart PW, Jia W, Brouwer KL, Sidney Barritt At. Altered bile acid metabolome in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2015;60(11):3318–3328. doi: 10.1007/s10620-015-3776-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine (2013) Guidance for industry: bioanalytical method validation. Rockville, MD, USA [Google Scholar]
- 33.Momose T, Maruyama J, Iida T, Goto J, Nambara T. Comparative abilities and optimal conditions for beta-glycosidase enzymes to hydrolyse the glucuronide, glucoside, and N-acetylglucosaminide conjugates of bile acids. Biol Pharm Bull. 1997;20(8):828–833. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi S, Fukami T, Masuo Y, Brocker CN, Xie C, Krausz KW, Wolf CR, Henderson CJ, Gonzalez FJ. Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans. J Lipid Res. 2016;57(12):2130–2137. doi: 10.1194/jlr.M071183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47(2):241–259. doi: 10.1194/jlr.R500013-JLR200 [DOI] [PubMed] [Google Scholar]
- 36.Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut microbes. 2016;7(1):22–39. doi: 10.1080/19490976.2015.1127483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horai H, Arita M, Kanaya S, Nihei Y, Ikeda T, Suwa K, Ojima Y, Tanaka K, Tanaka S, Aoshima K, Oda Y, Kakazu Y, Kusano M, Tohge T, Matsuda F, Sawada Y, Hirai MY, Nakanishi H, Ikeda K, Akimoto N, Maoka T, Takahashi H, Ara T, Sakurai N, Suzuki H, Shibata D, Neumann S, Iida T, Tanaka K, Funatsu K, Matsuura F, Soga T, Taguchi R, Saito K, Nishioka T. MassBank: a public repository for sharing mass spectral data for life sciences. J Mass Spectrom. 2010;45(7):703–714. doi: 10.1002/jms.1777 [DOI] [PubMed] [Google Scholar]
- 38.Eneroth P, Gordon B, Ryhage R, Sjovall J. Identification of mono- and dihydroxy bile acids in human feces by gas-liquid chromatography and mass spectrometry. J Lipid Res. 1966;7(4):511–523. [PubMed] [Google Scholar]
- 39.Ali SS, Kuksis A, Beveridge JM. Excretion of bile acids by three men on corn oil and butterfat diets. Can J Biochem. 1966;44(10):1377–1388. [DOI] [PubMed] [Google Scholar]
- 40.Setchell KD, Harrison DL, Gilbert JM, Mupthy GM. Serum unconjugated bile acids: qualitative and quantitative profiles in ileal resection and bacterial overgrowth. Clin Chim Acta. 1985;152(3):297–306. [DOI] [PubMed] [Google Scholar]
- 41.Andersen E, Karlaganis G, Sjovall J. Altered bile acid profiles in duodenal bile and urine in diabetic subjects. Eur J Clin Invest. 1988;18(2):166–172. [DOI] [PubMed] [Google Scholar]
- 42.Yamaga N, Ikebuchi J, Kohara H, Ogura Y, Yamada K. Analysis of bile acids in urine specimens from healthy humans: determination of several bile acids with beta-hydroxyl and carbonyl groups. J Biochem. 1996;119(4):725–730. [DOI] [PubMed] [Google Scholar]
- 43.Macdonald IA, Chang FC. The stereospecificity of 3 alpha- and 12 alpha-bile salt hydroxysteroid dehydrogenase systems from four microbial sources. Enzyme. 1982;28(4):392–395. [DOI] [PubMed] [Google Scholar]
- 44.Deo AK, Bandiera SM. Identification of human hepatic cytochrome p450 enzymes involved in the biotransformation of cholic and chenodeoxycholic acid. Drug Metab Dispos. 2008;36(10):1983–1991. doi: 10.1124/dmd.108.022194 [DOI] [PubMed] [Google Scholar]
- 45.Trulzsch D, Roboz J, Greim H, Czygan P, Rudick J, Hutterer F, Schaffner F, Popper H. Hydroxylation of taurolithocholate by isolated human liver microsomes. I. Identification of metabolic product. Biochem Med. 1974;9(2):158–166. [DOI] [PubMed] [Google Scholar]
- 46.Araya Z, Wikvall K. 6alpha-hydroxylation of taurochenodeoxycholic acid and lithocholic acid by CYP3A4 in human liver microsomes. Biochim Biophys Acta. 1999;1438(1):47–54. [DOI] [PubMed] [Google Scholar]
- 47.Deo AK, Bandiera SM. 3-ketocholanoic acid is the major in vitro human hepatic microsomal metabolite of lithocholic acid. Drug Metab Dispos. 2009;37 (9):1938–1947. doi: 10.1124/dmd.109.027763 [DOI] [PubMed] [Google Scholar]
- 48.Deo AK, Bandiera SM. Biotransformation of lithocholic acid by rat hepatic microsomes: metabolite analysis by liquid chromatography/mass spectrometry. Drug Metab Dispos. 2008;36(2):442–451. doi: 10.1124/dmd.107.017533 [DOI] [PubMed] [Google Scholar]
- 49.Parquet M, Pessah M, Sacquet E, Salvat C, Raizman A, Infante R. Glucuronidation of bile acids in human liver, intestine and kidney. An in vitro study on hyodeoxycholic acid. FEBS Lett. 1985;189(2):183–187. [DOI] [PubMed] [Google Scholar]
- 50.Radominska-Pyrek A, Zimniak P, Irshaid YM, Lester R, Tephly TR, St Pyrek J. Glucuronidation of 6 alpha-hydroxy bile acids by human liver microsomes. J Clin Invest. 1987;80(1):234–241. doi: 10.1172/JCI113053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pillot T, Ouzzine M, Fournel-Gigleux S, Lafaurie C, Radominska A, Burchell B, Siest G, Magdalou J. Glucuronidation of hyodeoxycholic acid in human liver. Evidence for a selective role of UDP-glucuronosyltransferase 2B4. J Biol Chem. 1993;268(34):25636–25642. [PubMed] [Google Scholar]
- 52.Sacquet E, Parquet M, Riottot M, Raizman A, Jarrige P, Huguet C, Infante R. Intestinal absorption, excretion, and biotransformation of hyodeoxycholic acid in man. J Lipid Res. 1983;24(5):604–613. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.