SUMMARY
Collective migration of epithelial cells is essential for morphogenesis, wound repair, and the spread of many cancers, yet how individual cells signal to one another to coordinate their movements is largely unknown. Here we introduce a tissue-autonomous paradigm for semaphorin-based regulation of collective cell migration. Semaphorins typically regulate the motility of neuronal growth cones and other migrating cell types by acting as repulsive cues within the migratory environment. Studying the follicular epithelial cells of Drosophila, we discovered that the transmembrane semaphorin, Sema-5c, promotes collective cell migration by acting within the migrating cells, themselves, not the surrounding environment. Sema-5c is planar polarized at the basal epithelial surface, such that it is enriched at the leading edge of each cell. This location places it in a prime position to send a repulsive signal to the trailing edge of the cell ahead to communicate directional information between neighboring cells. Our data show that Sema-5c can signal across cell-cell boundaries to suppress protrusions in neighboring cells and that Plexin A is the receptor that transduces this signal. Finally, we present evidence that Sema-5c antagonizes the activity of Lar, another transmembrane guidance cue that operates along leading-trailing cell-cell interfaces in this tissue, via a mechanism that appears to be independent of Plexin A. Together our results suggest that multiple transmembrane guidance cues can be deployed in a planar polarized manner across an epithelium and work in concert to coordinate individual cell movements for collective migration.
Keywords: collective cell migration, epithelium, semaphorin, plexin, Lar, Fat2, guidance cue, egg chamber, follicle cell, Drosophila, contact inhibition of locomotion, planar cell polarity, actin
eTOC Blurb
Stevenson et al. examine how Semaphorin-5c and Plexin A promote epithelial migration in the context of the Drosophila egg chamber. They show that Semaphorin-5c is planar polarized across the epithelium at the leading edge of each cell, and that it directs cell motility by acting within the migrating cohort, not the surrounding environment.
INTRODUCTION
Collective migration of epithelial cells underlies numerous tissue remodeling events [1,2]. In embryos, epithelial migration shapes organs including the mammary gland, vasculature, kidney, and eye [3–6]. In adults, it closes wounds in the skin and cornea, and facilitates metastasis [7–9]. For epithelial cells to migrate collectively, each cell must coordinate its movements with those of its neighbors. It is likely that both mechanical and biochemical signals are used to achieve this goal [10]. To date, however, few biochemical signals have been identified.
The Drosophila egg chamber provides a tractable system in which to identify these coordinating biochemical signals and the principles underlying their activity [11]. Egg chambers are organ-like structures that will each develop into one egg (Figure 1A). They have an inner germ cell cluster surrounded by follicular epithelial cells (follicle cells), whose basal surfaces contact the basement membrane (BM) ECM that ensheaths the organ. From the time an egg chamber forms through stage 8 of oogenesis, the follicle cells collectively migrate along the BM [12,13]. This motion causes the egg chamber to rotate within the BM (Figure 1B), and helps to create the ellipsoid shape of the egg. Each migrating follicle cell extends leading edge protrusions and has a parallel array of stress fibers along its basal surface that mediates adhesion to the BM. These actin-based structures all align in the direction of tissue movement, revealing a high degree of coordination among the cells (Figure 1C).
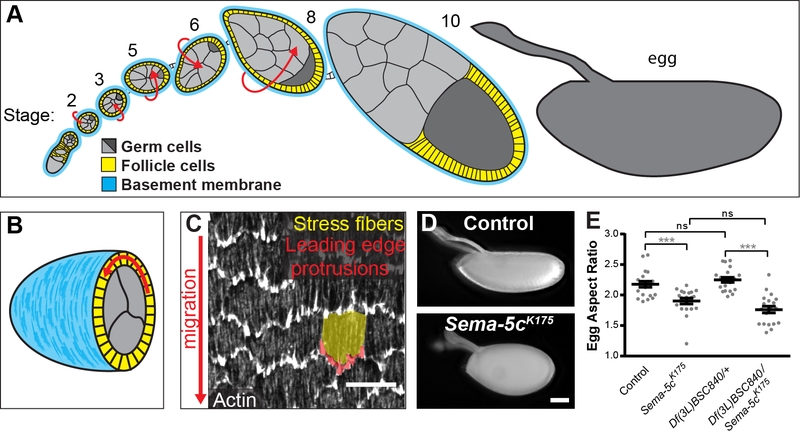
Figure 1. Sema-5c is required for egg chamber elongation.
(A) Illustration of a sagittal section through a developmental array of egg chambers. Arrows indicate rotation stages.
(B) Illustration of a transverse section through an egg chamber. Arrow indicates rotation.
(C) Image of the basal epithelial surface highlighting protrusions and stress fibers in one cell.
(D) Images of eggs from control and Sema-5cK175 females.
(E) Quantification of egg aspect ratio. Eggs from Sema-5cK175 females are rounder than controls.
Data in (E) represent mean ± SEM. Unpaired t-test. ns, not significant (p > 0.05), ***p < 0.001. Scale bars, 10 μm (C); 100 μm (D). See also Figure S1.
The migration of the follicular epithelium requires the receptor protein tyrosine phosphatase (RPTP) Lar and the cadherin Fat2, which are planar polarized at the basal epithelial surface along leading-trailing cell-cell interfaces [14–17]. Lar localizes to each cell’s leading edge and Fat2 localizes to the trailing edge, allowing them to mediate signaling between the leading and trailing edges of neighboring cells [14]. Whether other signaling systems also operate along these critical cell-cell interfaces is unknown.
The Semaphorins are a family of both secreted and membrane-associated proteins that activate plexin receptors [18,19]. They were first identified as repulsive cues for axon guidance, but also regulate the motility of other cell types, including collectively migrating neural crest and endothelial cells [20,21]. Typically, the plexin is expressed by the migrating cells and the semaphorin is expressed by cells within the migratory environment. When a plexin-expressing cell encounters a source of semaphorin, it is repelled, and thus confined to a particular migration path. Drosophila have three classes of semaphorins (Sema-1a/1b, Sema-2a/2b, and Sema-5c) and two plexins (PlexA and PlexB) [19]. It is conceivable that a transmembrane semaphorin and a plexin could be coexpressed within an epithelium, similar to Lar and Fat2, to allow each cell to influence the migratory behavior of its neighbors. However, no such signaling system involving a semaphorin has yet been found.
Here we show that the transmembrane semaphorin, Sema-5c, functions within the follicle cells, not the migratory environment, to promote their collective motility. We further show that Sema-5c is planar polarized at the basal epithelial surface, and enriched at each cell’s leading edge. This location places it in a prime position to signal to the trailing edge of the cell ahead, which could coordinate migration direction between neighboring cells. Indeed, we find that Sema-5c can signal across cell-cell boundaries to suppress protrusions and that Plexin A appears to transduce this signal. Finally, we present evidence that Sema-5c also interacts with Lar. Altogether, these results show that diverse guidance cues can be deployed within an epithelium to coordinate cellular movements for collective motility.
RESULTS
Semaphorin-5c is required for egg chamber elongation
We previously performed a genetic screen to identify genes required in the follicle cells to produce the elongated shape of the egg [22]. The K175 allele found in this screen is homozygous viable. However, in homozygous females, the aspect ratio (length/width) of egg chambers and eggs is reduced (Figures 1D, 1E and S1). Using deficiency mapping to identify the mutated gene, we found that females with K175 in trans to Df(3L)BSC840 produce rounded eggs similar to those of K175 homozygotes (Figure 1E). This deficiency contains the Semphorin-5c (Sema-5c) gene, which is non-essential for viability [23]. Sequencing Sema-5c coding regions in K175 animals identified a point mutation, T393-to-A, which produces a premature stop codon near the protein’s N-terminus. Together, these data suggest that K175 is a nonsense mutation in Sema-5c (Sema-5cK175), and that Sema-5c is required for egg chamber elongation.
Loss of Semaphorin-5c slows the rate and onset of epithelial migration
Defects in egg chamber elongation are often associated with impaired follicle cell migration [24], so we investigated if Sema-5c is required for this process. Ex vivo live imaging showed that Sema-5c epithelia appear to be non-migratory (Figures 2A and 2B, and Video S1). However, careful analysis of the actin cytoskeleton in fixed tissue, as discussed below, indicated that Sema-5c epithelia might have cryptic migratory ability.
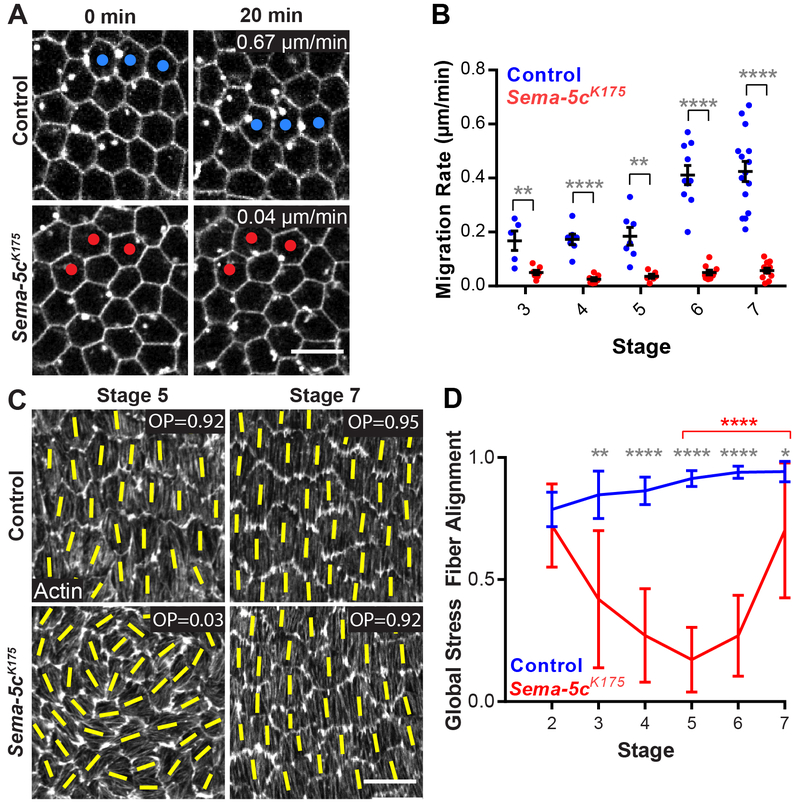
Figure 2. Loss of Sema-5c disrupts epithelial migration and global stress fiber alignment.
(A) Still images from videos of control and Sema-5c K175 epithelia, stage 7. Dots mark the same cells over time.
(B) Quantification of migration rates for control and Sema-5c K175 epithelia.
(C) Images of global stress fiber alignment in control and Sema-5c K175 epithelia. Yellow lines show the primary stress fiber direction in each cell. OP = order parameter.
(D) Quantification of global stress fiber alignment in control and Sema-5c K175 epithelia. Grey asterisks compare control to Sema-5c K175. Red asterisks compare Sema-5cK175 stage 5 to stage 7. n ≥ 8 for all conditions.
Data represent mean ± SEM in (B) and mean ± SD in (D). Unpaired t-test. *p < 0.05, **p < 0.01, ****p < 0.0001. Scale bars, 10 μm. See also Video S1.
The stress fibers at the basal surfaces of the follicle cells are aligned globally across the tissue, such that they all run parallel to the direction of movement [12,25]. We quantify this global alignment with an order parameter, where zero represents no alignment and one represents perfect alignment. Global stress fiber alignment is high in wild-type epithelia when migration begins, and increases until the end of migration at stage 8. By contrast, when migration is blocked, the alignment starts at the same high level, but then decreases until the order parameter is near zero at stage 8 [12].
Because ex vivo live imaging revealed no obvious migration in Sema-5c epithelia, we expected to see a consistent decrease in global stress fiber alignment, similar to other non-migratory conditions. Instead, the alignment decreases until stage 5, but then recovers, such that many Sema-5c epithelia are indistinguishable from controls at stage 7 (Figures 2C and 2D). This observation suggested that Sema-5c epithelial might migrate extremely slowly, and that the onset of motility might be delayed to stage 5/6. Given that ex vivo live imaging can only be performed for hours, and follicle cell migration lasts ~2 days [11,12], these subtle tissue dynamics could be missed by live imaging alone.
To test this, we developed an in vivo method to track epithelial migration over longer time periods, which leverages the fact that these cells secrete new matrix proteins into the BM as they migrate [13,26]. We induced small clones of cells to express an mRFP-tagged version of the BM protein type IV collagen (Col IV-mRFP), and identified them by the fluorescent collagen within the secretory pathway of the expressing cells (Figure 3A). In a migratory epithelium, these clones deposit stripes of Col IV-mRFP into the BM behind them, creating a permanent record that migration occurred (Figures 3A–3E). By contrast, clones in a non-migratory fat2N103−2 epithelium only deposit spots of Col IV-mRFP into the BM directly adjacent to the expressing cells (Figures 3F and 3G). We call the BM stripes COMETtails (Clonal Overexpression of Matrix proteins for Epithelial cell Tracking). A similar method was recently published [27].
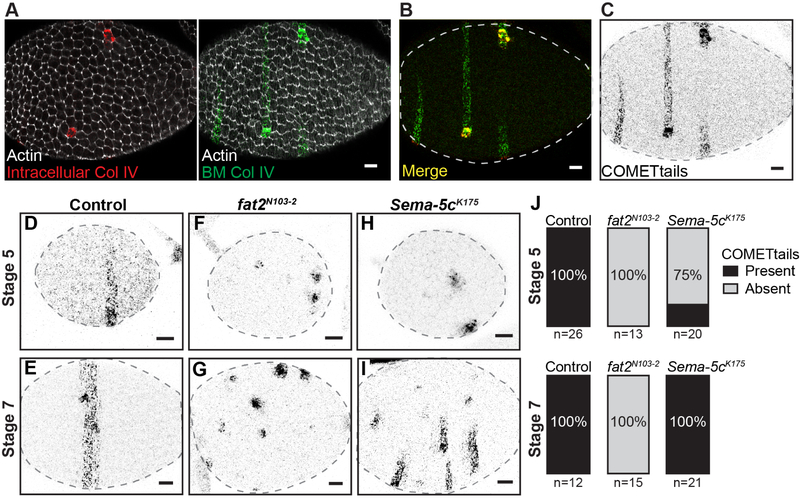
Figure 3. Loss of Sema-5c slows the onset and rate of epithelial migration.
(A-C) The COMETtail method. Clones expressing Col IV-mRFP are detected by the intracellular signal. Clones deposit stripes of Col IV-mRFP into the BM as they migrate (false-colored green). (B) Overlay of Col IV-mRFP images in (A). (C) Image from (B) in grayscale.
(D-I) Images of COMETtails from control, non-migratory fat2N103−2, and Sema-5c K175 epithelia.
(J) Quantification of COMETtail data. n = number of egg chambers examined.
Scale bars, 10 μm.
Using COMETtails, we found that Sema-5c epithelia display a phenotype that is intermediate between control and fat2N103−2 epithelia. At stage 7, when global stress fiber alignment has largely recovered, all egg chambers have COMETtails (Figures 3I and 3J), which indicates that these Sema-5c epithelia do migrate. However, because Sema-5c COMETails are shorter than controls, and ex vivo live imaging reveals almost no movement (Figure 2B), the migration must be extremely slow. At stage 5, when stress fibers are maximally mis-aligned, only 25% of Sema-5c epithelia have COMETtails (Figures 3H and 3J). This observation is consistent with migration initiating in the majority of Sema-5c epithelia around stage 5/6. Altogether, these data show that loss of Sema-5c slows the rate of epithelial migration and often delays its onset.
Semaphorin-5c localizes to each cell’s leading edge
To explore how Sema-5c promotes epithelial migration, we determined its localization in the egg chamber. We used the CRISPR-Cas9 system to insert three copies of GFP into the Sema-5c locus (Sema-5c-3xGFP). The resulting C-terminal protein fusion appears to be functional, as eggs from Sema-5c-3xGFP females elongate normally (Figure S2A). Sema-5c-3xGFP is expressed strongly in the follicle cells through stage 7, with little signal in the germ cells (Figure 4A). Optical sections through the epithelium reveal that Sema-5c-3xGFP is on apical and lateral cell membranes, and intracellular puncta that may represent endosomes (Figure 4B).
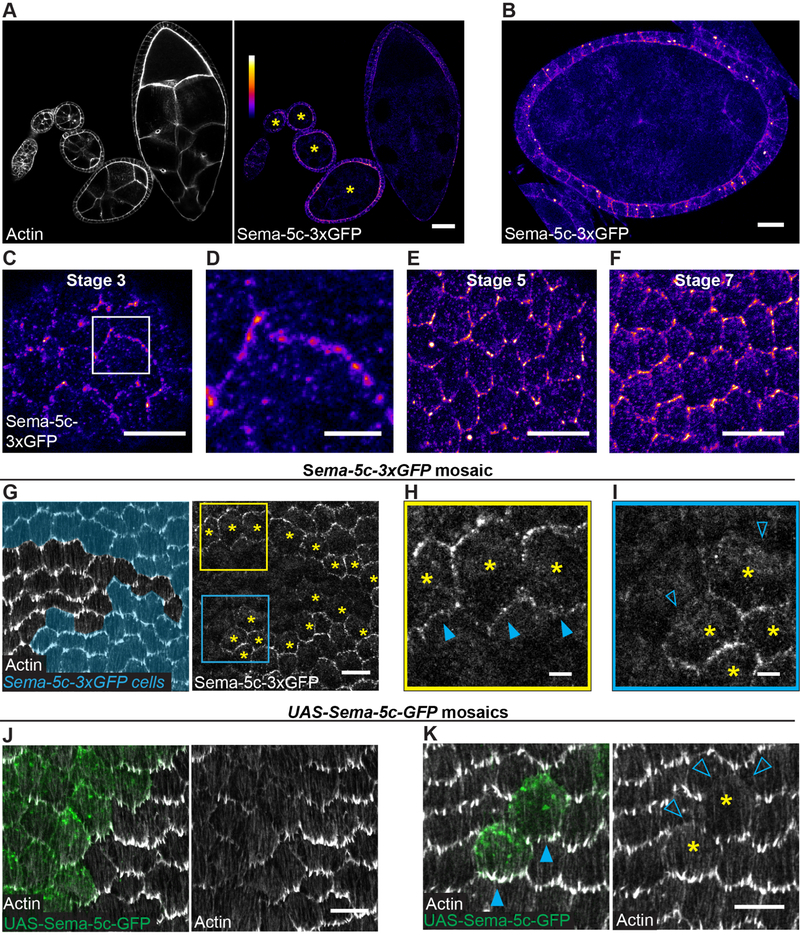
Figure 4. Sema-5c localizes to the leading edge and can suppress protrusions in neighboring cells.
(A) Images of a sagittal section through an ovariole expressing Sema-5c-3xGFP. Asterisks mark migratory stages. Heat map index applies to (A-F).
(B) Image of a sagittal section through an egg chamber, stage 6. Sema-5c-3xGFP is on apical and lateral cell membranes, and intracellular puncta.
(C-F) Images of the basal epithelial surface at indicated stages. Sema-5c-3xGFP is punctate along leading-trailing cell-cell interfaces. (D) Zoom of boxed region in (C).
(G-I) Images of the basal surface of a Sema-5c-3xGFP mosaic epithelium, stage 7. (G) Cells expressing Sema-5c-3xGFP are pseudocolored cyan. Asterisks mark Sema-5c-3xGFP-expressing cells at the clone boundary. (H) Zoom of the yellow boxed region in (G). Sema-5c-3xGFP-expressing cells have GFP at their leading edge (solid triangles). (I) Zoom of the blue boxed region in (G). Sema-5c-3xGFP-expressing cells lack GFP at their trailing edge (open triangles).
(J-K) Images of the basal surface of UAS-Sema-5c-GFP mosaic epithelia, stage 7. (J) Protrusions are reduced in the clone. (K) Protrusions are present at the front of the clone (closed triangles) but are lost from cells directly behind the clone (open triangles). Asterisks mark the clone.
Scale bars, 100 μm (A); 10 μm (B, C, E-G, J and K); 3 μm (D, H and I). See also Figures S2 and S3.
Because the migration machinery is at the tissue’s basal surface, we were particularly interested in Sema-5c’s localization along this plane. We found that Sema-5c-3xGFP is both punctate and planar polarized along leading-trailing cell-cell interfaces (Figures 4C–4F). To determine if this localization corresponds to the leading edge, trailing edge, or both, we generated mosaic epithelia wherein some cells express Sema-5c-3xGFP and the remainder express untagged Sema-5c. Using leading edge protrusions to indicate migration direction, we found that Sema-5c-3xGFP-expressing cells migrating directly behind unmarked cells have GFP at their leading edges, whereas Sema-5c-3xGFP-expressing cells migrating directly ahead of unmarked cells largely lack GFP at their trailing edges (Figures 4G–I, S2B and S2C). Hence, Sema-5c is enriched at each cell’s leading edge.
Semaphorin-5c can suppress protrusions in neighboring cells
One way that semaphorins regulate cell motility is by signaling non-cell-autonomously to suppress protrusions [28]. This activity was first identified in axon guidance, where semaphorins “collapse” the protrusive growth cone that pioneers the migration of an axon toward its target, but it also applies to other migratory cell types [29–31]. Although the level and location of protrusions appear normal in Sema-5c clones at stage 7 (Figure S2D), there is an abundance of mis-oriented protrusions in Sema-5c epithelia at stage 6 (Figures S2E–S2L). Moreover, the presence of short COMETtails in Sema-5c epithelia at this stage indicates that the mis-oriented protrusions can occur in conjunction with the early stages of epithelial motility. Together with the localization data above, these observations suggest that Sema-5c might signal from each cell’s leading edge to regulate the site of protrusion formation in the cell ahead.
To examine whether Same-5c can suppress protrusions in neighboring cells, we overexpressed Sema-5c (UAS-Sema-5c-GFP) in follicle cell clones, which causes Sema-5c’s localization to expand to the trailing edge (Figure S3A). In this overexpression condition, Sema-5c signals non-cell-autonomously to suppress leading edge protrusions in the cells directly behind the overexpressing cells (Figures 4J and 4K). The leading edge localization of the actin assembly factor SCAR is also reduced (Figures S3B and S3C). These phenotypes are only seen along cellular interfaces that directly contact the overexpressing cell, denoting an extremely short-range signal. Although Sema-5c is not normally localized to the trailing edge, this overexpression system demonstrates that Sema-5c can suppress protrusions, and that it does so by signaling across cell-cell boundaries.
Plexin A mediates Semaphorin-5c signaling
Our data suggest that Sema-5c promotes epithelial migration via intercellular signaling. To identify Sema-5c’s receptor, we expressed RNAi against the Drosophila plexins. Two PlexB RNAi transgenes, previously validated in other tissues, do not affect egg elongation (Figure S4A). By contrast, two independent PlexA RNAi transgenes strongly deplete PlexA from the follicle cells and produce round eggs (Figures 5A, S4B and S4C). Moreover, global stress fiber alignment in PlexA-RNAi epithelia decreases until stage 5, but then recovers through stage 7, and we detected no obvious migration in PlexA RNAi epithelia by ex vivo live imaging (Figures 5B–5D and Video S2). Thus, depletion of PlexA causes migration-associated phenotypes that are strikingly similar to those caused by loss of Sema-5c.
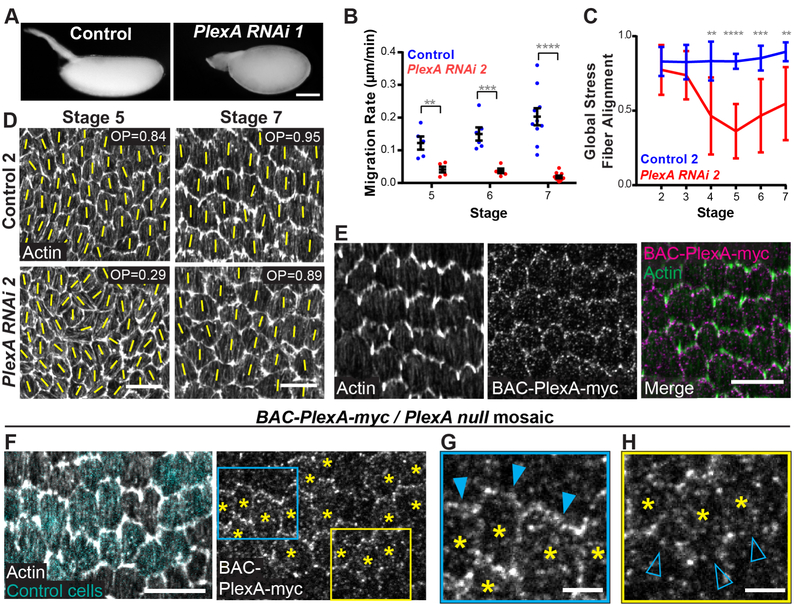
Figure 5. Loss of PlexA phenocopies loss of Sema-5c and PlexA is enriched at the trailing edge.
(A) Images of eggs from a control female and a female expressing PlexA RNAi in the follicular epithelium.
(B) Quantification of migration rates from control and PlexA RNAi epithelia.
(C) Quantification of global stress fiber alignment in control and PlexA RNAi epithelia. n ≥ 8 for all conditions.
(D) Images of global stress fiber alignment in control and PlexA RNAi epithelia. Yellow lines show the primary stress fiber direction in each cell. OP = order parameter.
(E) Images of the basal surface of a BAC-PlexA-myc epithelium, myc immunostaining, stage 7. PlexA is punctate along leading-trailing cell-cell interfaces.
(F-H) Images of the basal surface of a mosaic epithelium with BAC-PlexA-myc clones in a PlexA null background, PlexA and myc immunostaining, stage 7. (F) Control cells are marked in cyan. (F-H) Asterisks mark cells expressing BAC-PlexA-myc at the clone boundary. (G) Zoom of the blue boxed region in (F). BAC-PlexA-myc-expressing cells primarily have PlexA enriched at their trailing edge (solid triangles). (H) Zoom of the yellow boxed region in (F). BAC-PlexA-myc-expressing cells also have some PlexA at their leading edge (open triangles).
Data represent mean ± SEM in (B) and mean ± SD in (C). Unpaired t-test. **p < 0.01; ***p < 0.001; ****p < 0.0001. Scale bars, 100 μm (A); 10 μm (D-F); 3 μm (G and H) See also Figure S4 and Video S2.
We then mapped PlexA’s localization pattern. Using both a BAC transgene wherein PlexA is tagged with myc (BAC-PlexA-myc) and a PlexA antibody, we found that PlexA is punctate at the basal epithelial surface, and planar polarized along leading-trailing cell-cell interfaces (Figures 5E and S4D). To determine PlexA’s subcellular localization, we then used a mosaic method similar to that described for Sema-5c above (STAR Methods). This experiment revealed that, although some PlexA is at the leading edge, most PlexA is at the trailing edge (Figures 5F–5H and S4E–S4G). Thus, PlexA is in the right position to receive a Sema-5c signal from the leading edge of the cell behind.
To determine if PlexA binds to Sema-5c, we probed their interaction in vitro and in vivo. First, we employed an ELISA-type assay using the ectodomains of PlexA and the five Drosophila semaphorins (Figures S5A and S5B). This assay revealed robust binding between PlexA and Sema-5c. Additionally, PlexA binds to its known ligands, Sema-1a and 1b [32], but not to Sema-2a and 2b, which are PlexB ligands [33,34]. Second, we asked whether Sema-5c and PlexA are required for each other’s localization. Although PlexA levels appear normal in Sema-5c epithelia (Figure S5C), Sema-5c-3xGFP is reduced at the basal surface of PlexA-RNAi epithelia (Figures 6A, 6B, and S5D). Despite this stabilizing effect of PlexA on Sema-5c, PlexA puncta are sparser and only sometimes colocalize with Sema-5c puncta (Figures 6C–6G) Altogether, these data indicate that PlexA and Sema-5c do interact, but that their interaction in vivo may be transient.
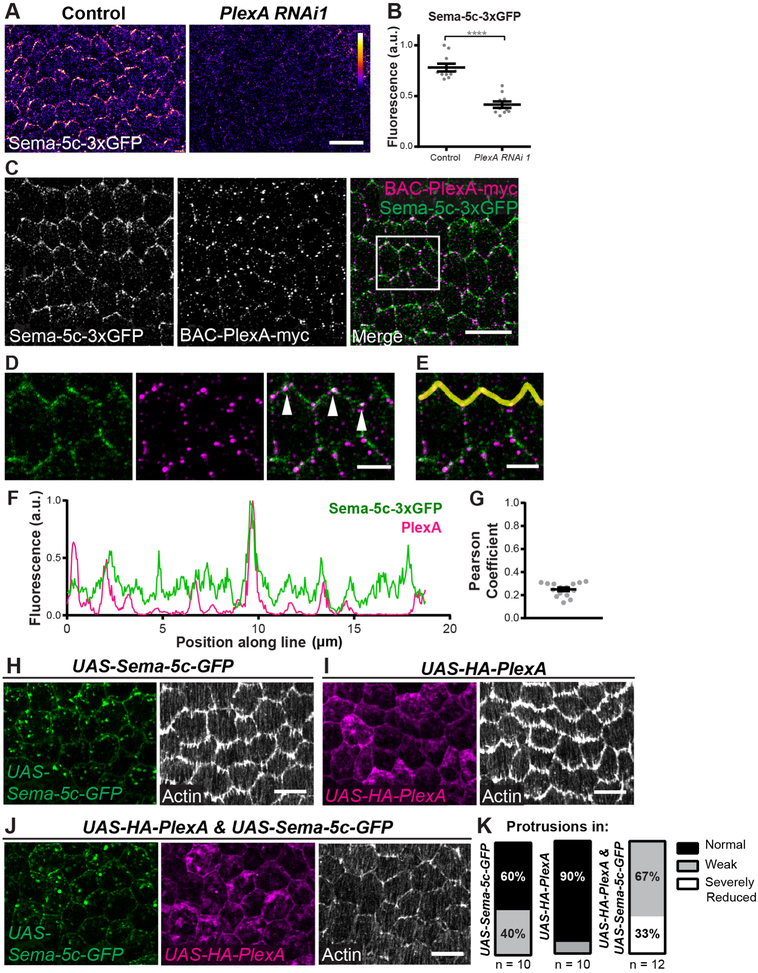
Figure 6. PlexA is the likely receptor for Sema-5c.
(A) Images of the basal epithelial surface, stage 7. PlexA RNAi reduces Sema-5c-3xGFP levels.
(B) Quantification of Sema-5c-3xGFP levels at cell-cell interfaces in control and PlexA RNAi epithelia, stage 7.
(C-E) Images of the basal surface of an epithelium expressing Sema-5c-3xGFP and BAC PlexA-myc, myc immunostaining, stage 7. (D and E) Zoom of boxed region in (C). White triangles indicate colocalization between Sema-5c and PlexA. (F) Quantification of fluorescence intensities of Sema-5c-3xGFP and BAC-PlexA-myc along the cell-cell interfaces marked by the yellow line in (E).
(G) Pearson correlation coefficient for Sema-5c-3xGFP and BAC-PlexA-myc fluorescence intensities along leading-trailing cell-cell interfaces, stage 7. Each point is one epithelium in which a line scan was performed along 5–8 cell-cell interfaces.
(H-J) Coexpression of PlexA enhances the Sema-5c overexpression phenotype. Images are of the basal epithelial surface, stage 8. (H) A low level of UAS-Sema-5c-GFP mildly reduces protrusions. (I) UAS-HA-PlexA has little effect on protrusions. (J) Coexpressing both transgenes strongly reduces protrusions.
(K) Quantification of data in (H-J). n = number of egg chambers examined.
Data in (B) represent mean ± SEM. Unpaired t-test. ****p < 0.0001. Scale bars, 10 μm (A, C and H-J); 3 μm (D and E). See also Figure S5.
Finally, we revisited our Sema-5c overexpression condition to ask if PlexA mediates Sema-5c’s ability to suppress protrusions. Because there is some PlexA at each cell’s leading edge, overexpressed Sema-5c may inhibit protrusions in the cell behind by signaling through this population of the receptor. Indeed, depleting PlexA from UAS-Sema-5c-GFP clones abrogates Sema-5c’s ability to suppress protrusions (Figures S5E–S5H), which shows that PlexA is required for Sema-5c activity. We further found that expressing either UAS-HA-PlexA or a low level of UAS-Sema-5c-GFP in the entire epithelium has only a minor effect on protrusions, whereas expressing both transgenes together strongly suppresses protrusions (Figures 6H–6K). Thus, overexpressed PlexA can also enhance Sema-5c’s ability to signal. Altogether, these data suggest that PlexA acts as a receptor for Sema-5c in the follicular epithelium.
Semaphorin-5c interacts with Lar
The localization patterns of Sema-5c and PlexA resemble those of Lar and Fat2, which suggests that these four proteins might work together to promote epithelial motility. However, neither Sema-5c clones (Figure S2D), nor PlexA RNAi clones (Figure S5F), show the defects in leading edge protrusions and/or trailing edge retraction caused by loss of Lar or Fat2 [14]. This observation argues against Sema-5c and PlexA being part of the Lar/Fat2 signaling system; yet, there may still be crosstalk between the two pathways.
We therefore explored if Sema-5c/PlexA and Lar/Fat2 interact at the genetic level. Removing one copy of Lar substantially rescues the egg shape defect in Sema-5c females (Figures 7A, S6A and S6B). Reduced Lar dosage may allow migration to begin earlier in Sema-5c epithelia, as global stress fiber alignment is higher in the rescued epithelia than in Sema-5c epithelia (Figures 7B–7D and S6C). Reduced Lar dosage does not rescue the migration rate, however, as we detected no directional motility in rescued epithelia by ex vivo live imaging (Figure S6D). Removing one copy of fat2 does not rescue the egg shape defect in Sema-5c females (Figure S6E), nor does removing one copy of Lar in PlexA-RNAi females (Figure S6F). Altogether, these data suggest that Sema-5c antagonizes Lar activity, and that this interaction may be independent of Fat2 and PlexA.
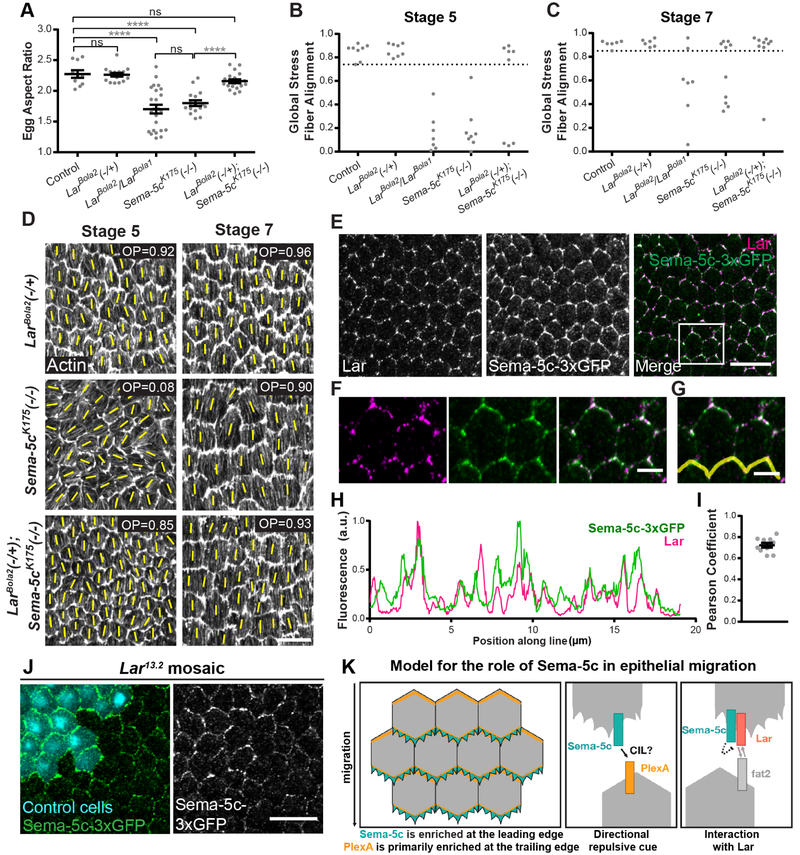
Figure 7. Sema-5c interacts with Lar.
(A) Quantification of egg aspect ratios. Removing one copy of Lar rescues the Sema-5cK175 egg shape defect.
(B and C) Quantification of global stress fiber alignment. Removing one copy of Lar partially rescues the defect in Sema-5cK175 epithelia.
(D) Images of global stress fiber alignment for some conditions analyzed in (B and C). Yellow lines show the primary stress fiber direction in each cell. OP = order parameter.
(E-G) Images of the basal surface of a Sema-5c-3xGFP epithelium with Lar immunostaining, stage 7. Sema-5c and Lar colocalize. (F-G) Zoom of the boxed region in (E).
(H) Quantification of fluorescence intensities of Sema-5c-3xGFP and Lar along the cell-cell interfaces marked by the yellow line in (G).
(I) Pearson correlation coefficient for Sema-5c-3xGFP and Lar along leading-trailing cell-cell interfaces, stage 7. Each point represents one epithelium in which a line scan was performed along 5–8 cell-cell interfaces.
(J) Images of the basal surface of a Lar13.2 mosaic epithelium, stage 7. Sema-5c-3xGFP is reduced in mutant cells.
(K) Illustration of our working model for Sema-5c’s role in follicle cell migration.
Data in (A) represent mean ± SEM. Unpaired t-test. ns, not significant (p > 0.05); ****p < 0.0001.
Scale bars, 10 μm (D, E and J); 3μm (F and G). See also Figure S6.
Since Sema-5c and Lar both localize to the leading edge, we next asked if they colocalize. The density of Sema-5c and Lar puncta is similar along the leading-trailing cell-cell interfaces, and they consistently overlap (Figures 7E–7I). Moreover, although Lar levels appear normal in Sema-5c and PlexA RNAi clones, Sema-5c levels are reduced in Lar clones (Figures 7J, S6G and S6H). This reduction is not due to an effect on PlexA, as PlexA levels are normal in Lar clones (Figure S6I). Altogether, these data suggest that Sema-5c has a second function that involves a cis interaction with Lar.
DISCUSSION
Here we introduce a tissue-autonomous model for semaphorin signaling in collective cell migration (Figure 7K). Our data show that Sema-5c acts as a migratory cue within the collectively migrating epithelial cells, themselves, instead of acting in the migratory environment. Sema-5c is planar polarized at the basal epithelial surface, localizing to each cell’s leading edge. We envision that this placement allows Sema-5c to activate PlexA on the trailing edge of the cell ahead, and thus communicate directional information between neighboring cells. We further identify an interaction between Sema-5c and Lar, which suggests that Sema-5c may play a second role in the Lar/Fat2 signaling pathway. Together these results highlight how multiple guidance cues work in concert within an epithelium to coordinate cell movements for collective motility.
Semaphorin-5c may promote collective motility by acting as a repulsive cue for neighboring cells
Given that overexpressing Sema-5c collapses protrusions in neighboring cells, we propose that Sema-5c may promote collective motility via contact inhibition of locomotion (CIL). CIL describes a set of behaviors exhibited by a migrating cell when it collides with another cell [35]. Specifically, the cell retracts its protrusions from the point of contact and repolarizes to migrate away in a new direction. CIL is mediated by signaling proteins on the cells’ surfaces, including semaphorins and plexins [28,36]. Although CIL is often used to disperse cells, cases exist where CIL appears to organize cells for collective movement [35]. We propose that the Sema-5c at each cell’s leading edge could signal to suppress protrusions at the trailing edge of the cell ahead, and thus direct the signal-receiving cell to polarize away from this point of contact, such that both cells then migrate in the same direction. In this scenario, the semaphorin is a repulsive cue, similar to the most common function of semaphorins in axon guidance. Supporting this notion, we see an abundance of mis-oriented protrusions in Sema-5c epithelia at stages 5 and 6. Determining if Semaphorin-based CIL is operating in the follicular epithelium will be an important area for future work.
The role we have identified for Sema-5c in promoting collective migration may be conserved. Sema-5c is the only Drosophila semaphorin with direct homologs in vertebrates (Sema5A and Sema5B). Moreover, similar to Sema-5c, the vertebrate class 5 semaphorins promote the migration of multiple cell types [30,37–39], and can collapse protrusions non-cell-autonomously [30,37]. Given that three of the five classes of vertebrate semaphorins are integral membrane proteins (classes 4, 5, and 6), any one of these family members could promote collective migration similarly to Sema-5c, which opens the possibility that Sema-5c’s mechanism of action could be widely used.
Semaphorin-5c likely signals through Plexin A
Four pieces of evidence suggest that PlexA is a Sema-5c receptor. First, the follicle cells require PlexA to migrate normally. Second, the dynamics of the global stress fiber pattern in PlexA RNAi epithelia is similar to that of Sema-5c epithelia. Third, PlexA and Sema-5c interact both in vitro and in vivo. Fourth, PlexA primarily localizes to each cell’s trailing edge, placing it in the right position to receive a Sema-5c signal from the leading edge of the cell behind. This observation is particularly intriguing, as a vertebrate homolog of PlexA, Plexin A1, functions at the trailing edge of migrating dendritic cells [40].
Given that PlexA is required for Sema-5c’s localization, we were surprised to find that they only rarely colocalize. We are not aware of other studies that report colocalization of a semaphorin-plexin pair at the sub-cellular level, so the dynamics of the ligand-receptor interaction in vivo are mysterious. It is interesting to speculate that the repulsive nature of the semaphorin signal may necessitate a transient interaction with its receptor.
Although our data strongly suggest that Sema-5c signals through PlexA, more complex models are possible. For example, vertebrate class 5 semaphorins signal through both A- and B-type plexins [30,37,41,42]. Thus, Sema-5c could also signal through PlexB. There may also be reverse signaling through Sema-5c’s intracellular domain [43]. Future work will determine if these alternate modes of Sema-5c signaling also contribute to epithelial motility.
Multiple guidance cues work in concert to promote epithelial migration
The Sema-5c/PlexA and Lar/Fat2 signaling systems both operate along leading-trailing cell-cell interfaces to promote collective motility. Although phenotypic differences argue against all four proteins acting in one pathway, three pieces of evidence indicate that Sema-5c interacts with Lar. First, Sema-5c and Lar colocalize at the basal epithelial surface. Second, Lar is required for Sema-5c’s localization. Third, reducing Lar dosage rescues the global stress fiber alignment and egg shape phenotypes caused by loss of Sema-5c. These data suggest that Sema-5c antagonizes Lar activity, and further imply that Lar may inhibit cell migration under some circumstances. It is possible that Sema-5c plays two roles in the follicle cells – one with PlexA and one with Lar. Alternatively, the interaction between Sema-5c and Lar may represent a point of convergence between two otherwise separate signaling pathways.
Given that Sema-5c and Lar both localize to the leading edge, they likely interact in cis. We envision two non-mutually-exclusive models by which this could occur. Class 5 semaphorins and Lar both bind heparin sulfate proteoglycans [44,45], making it possible that glycan chains could bridge an interaction between their ectodomains. Alternatively, the interaction could occur downstream of their intracellular domains [43]. A recent study showed that semaphorins interact with Lar-family RPTPs in the nervous systems of C. elegans and mice [46]. However, the mechanism described therein is different, with Lar functioning in cis with the plexin to amplify a secreted semaphorin signal. Thus, there may be crosstalk between Lar and semaphorins in multiple contexts.
The protein families to which Sema-5c, PlexA, Lar, and Fat2 belong are all known for their roles in nervous system development. Semaphorins and plexins are one of the canonical families of axon guidance cues [47], and Lar-family RPTPs function in both axon guidance and synapse formation [48]. Although less studied, Fat-like cadherins also help to wire the nervous system [49]. The steering of a growth cone toward its target represents a system in which a guidance cue from the cellular environment modulates the behavior of a migrating cell. We have now identified a situation wherein these same guidance cues are planar polarized across an epithelium to allow each cell within the tissue to modulate the migratory behavior of their neighbors for collective motility. A recent study noted that the appearance of the semaphorin and plexin families predates the evolution of the nervous system in metazoans [50]. The same is true for Fat-like cadherins [51]. Thus, the ancestral role for these guidance cues may be to regulate epithelial dynamics, with their role in guiding axons arising later.
Collective migration of the follicle cells can begin late in development from a disordered state
Finally, this work elucidates the cellular parameters required for the follicular epithelium to migrate. The follicle cells typically begin migrating shortly after an egg chamber forms, and their stress fibers always show a high degree of global alignment [12]. We have discovered that neither of these features is strictly required for epithelial motility. Moreover, our observations that epithelial migration can begin later in development, and can do so after the stress fiber pattern has become disordered, show that the ability of this epithelium to break symmetry and polarize is more robust than previously appreciated.
There is a fat2 partial-loss-of-function condition that is phenotypically similar to Sema-5c [27,52]. In this condition, epithelial migration is so slow that it cannot be detected by ex vivo live imaging. Moreover, migration is likely delayed, as the global stress fiber pattern is disordered at stage 6, but recovers by stage 8. Future work will determine the extent to which these two mutant conditions phenocopy, and whether these similarities indicate yet more points of convergence between the Sema-5c/PlexA and Lar/Fat2 signaling systems.
The observation that some mutant epithelia polarize and begin migrating at stage 5/6 suggests that there may be developmentally programmed changes in the egg chamber that create a more favorable environment for epithelial motility. We previously noted that global stress fiber alignment and migration rate both increase in wild-type epithelia around this time [12]. This improved migratory ability could be due to higher BM stiffness, as stiffer matrices promote cell migration [53], and the stiffness of the follicular BM increases over time [54,55]. Determining if the cells are responding to mechanical changes in the BM, and how they do so, will be fertile areas for future research.
Conclusion
This work introduces a tissue-autonomous role for semaphorins in guiding the collective migration of epithelial cells. Moreover, our observation that Sema-5c and PlexA act alongside Lar and Fat2 to promote epithelial motility highlights the complex signaling interactions that occur along leading-trailing cell-cell interfaces to allow these cells to coordinate their individual movements for collective motility.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Sally Horne-Badovinac (shorne@uchicago.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Drosophila genetics
D. melanogaster was cultured on cornmeal molasses agar food using standard techniques. All experiments were performed on adult females. For most experiments, crosses were raised at 25°C and experimental females were aged on yeast with males for 2–3 days at the same temperature. Experimental genotypes for each figure panel are in Table S1. The culturing conditions for the females in each experiment are detailed in Table S2.
Clones of either Sema-5cK175 mutant cells or Sema-5c-3xGFP expressing cells were produced using FRT80B with e22c-Gal4 driving FLP recombinase expression [56]. For Flp out clones, UAS lines were crossed to flies with FLP recombinase under a heat shock promoter and an Act5c>>Gal4 Flp out cassette with or without UAS-GFP or UAS-RFP. Heat shock was induced by incubating pupae and adults at 37°C for 1 hour, followed by 1 hour of recovery at 25°C, and then another hour at 37°C. This heat shock procedure was performed 3 times over the course of 2 days. Females that eclosed during the period were placed on yeast with males overnight and dissected the next day.
For the studies of the Plexin receptors, the following RNAi lines were used: UAS-PlexA-RNAi 1 (v27240, GD14483), UAS-PlexA-RNAi 2 (TRiP.HM05221), UAS-PlexB-RNAi 1 (v27220, GD14473), and UASPlexB-RNAi 2 (v12167, GD3150) [57].
Stocks are from Bloomington Drosophila Stock Center with the following exceptions. UAS-PlexARNAiGD14483, UAS-PlexB-RNAiGD14473, and UAS-PlexB-RNAiGD3150 are from the Vienna Drosophila Resource Center. Traffic jam-Gal4 (104055) is from the Drosophila Genetic Resource Center in Kyoto. LarBola1 and LarBola2 are a gift from Allan Spradling [58]. UAS-Col4a1-mRFP is a gift from Jose Pastor-Pareja [59]. Lar13.2, FRT40A is a gift from David Van Vactor [15]. The BAC-PlexA-myc strain is a gift from Alex Kolodkin [50,60]. UAS-HA-PlexA is a gift from Johnathan Terman [61]. Both w;; BAC-PlexA-myc, ubi-GFP, h, FRT80B/TM6; Df(4)C3/CiD and yw, hsFLP;; FRT80B, e, ca/TM6; PlexAMB/CiD are gifts from Trudi Schüpbach. Sema-5cK175 and fat2N103−2 are from [22] and Fat2–3xGFP is from [14].
METHOD DETAILS
Time lapse video acquisition and microscopy
Ex vivo live imaging of egg chambers was performed largely as described [62,63], with the exact procedure outlined below. Experimental females were collected 1–2 days after eclosion and aged on yeast for 2 days. Ovaries were dissected in live imaging media (Schneider’s Drosophila medium containing 15% FBS and 200 μg/ml insulin) containing CellMask Deep Red Plasma Membrane Stain (Thermo-Fisher; 1:1000). After carefully removing the muscle sheathes with forceps, individual ovarioles were transferred to fresh live imaging media to wash out excess CellMask. The ovarioles and media were transferred to a glass slide; 51 μm Soda Lime Glass beads (Cospheric LLC) were added to support a 22 × 22 μm No. 1.5 coverslip. The edges of the coverslip were sealed with Vaseline to prevent evaporation. Each slide was used for no more than 2 hours. All egg chambers were examined for damage prior to imaging, using CellMask to highlight damaged areas, as damaged egg chambers do not rotate. Egg chambers were imaged with one of two laser-scanning confocal microscopes running Zen 2.3 acquisition software, either a Zeiss LSM 800 with a 40x/1.3 NA EC Plan-NEOFLUAR objective, or a Zeiss LSM 880 with 40x/1.3 Plan-APOCHROMAT objective. Time-lapse movies were performed by capturing single confocal slices near the basal epithelial surface every 60 seconds. To calculate epithelial migration rates, kymographs were generated from the time-lapse image stacks in Fiji (ImageJ) [64,65] by drawing a single line across several cell diameters in the direction of migration. The migration rate for each epithelium was then determined by measuring the slope of 3–4 kymograph lines and averaging the values. Please see [14] for an illustration of this technique.
Fixed image acquisition and microscopy
Ovaries were dissected in live imaging media, as described above. To isolate individual ovarioles, the muscle sheaths were either removed with forceps during dissection or by gentle pipetting post-fixation. In all cases, egg chambers at stage 10 or older were discarded. Egg chambers were fixed for 15 minutes in 4% EM grade formaldehyde (Polysciences) in PBT (PBS + 0.1% Triton X-100), the one exception is the use of 4% formaldehyde in Schneider’s medium when immunostaining for Lar. To stain F-actin, egg chambers were washed 3x in PBT, incubated in TRITC Phalloidin (1:200 for 25 minutes, Sigma) or AlexaFluor-647 phalloidin (1:50 overnight or 1:30 for 3 hours, Invitrogen), then washed 3x in PBT and mounted with one drop of SlowFade Antifade (Invitrogen) onto a slide with a 22 × 50mm No. 1.5 coverslip. For antibody staining, egg chambers were fixed as above, washed 4x in either PBT, PBT2 or PBT3 (PBS + 0.1%, 0.2% or 0.3% Triton X-100, respectively), and incubated at 4°C overnight with the primary antibody. Then egg chambers were washed 4x, incubated with secondary antibodies conjugated to AlexaFluor-555, or −647 (Invitrogen, 1:200) for 2–3 hours at room temperature, washed 4X and mounted as above. Anti-Lar (9D82B3, 1:200 concentrate) and anti-SCAR (P1C1, 1:200 concentrate) are from the Developmental Studies Hybridoma Bank, anti-ß-Galactosidase is from Promega (Z378A, 1:200), anti-myc is from Cell Signaling Technologies (71D10, 1:200) and anti-HA is from Rockland Inc. (600-401-384, 1:250). Anti-PlexA is a gift from Takaki Komiyama [66], and was pre-absorbed overnight on egg chambers expressing UAS-PlexA-RNAi to reduce background signal prior to tissue staining and used at 1:1000. Tissue was imaged with one of the two scanning confocal microscopes described above, using the same objectives or a 63x/1.4 NA Plan-APOCHROMAT objective. For all images a single confocal slice is shown. All image processing was done in Fiji (ImageJ) [64,65] and utilized the ScientiFig Plugin [67] for image overlays.
Quantification of egg aspect ratio
Ovaries were dissected in freshly made Robb’s minimal saline and fixed in Robb’s containing 8% EM grade formaldehyde (Polysciences) for 5 minutes. The tissue was washed 3x in PBT, then disrupted with pipetting to remove the muscle sheath. Tissue was stained with phalloidin (1:200) and DAPI (1 μg/ml, Sigma) for 20 minutes. Stage 14 egg chambers and mature eggs were mounted and imaged on one of the two scanning confocal microscopes described above, using a 20x/0.8 NA Plan-APOCHROMAT objective. Aspect ratios were calculated by dividing the length of each egg by its width, which were both measured in Fiji (ImageJ) [64,65]; dorsal appendages were not included in the measurements. To obtain brightfield images of eggs, ovaries were dissected as described above, and eggs were imaged in Robb’s media on a Leica MZ FLIII microscope with a Canon Rebel Camera. Image processing was done in Fiji (ImageJ) [64,65].
Measurements of global stress fiber alignment
Egg chambers were fixed, stained with phalloidin, and imaged as described above. The orientation of stress fibers in an individual cell was determined by using the Measure function of the OrientationJ plugin [68] for Fiji (ImageJ) [64,65] after manually selecting a circular region of interest at the basal surface of each cell, excluding cell boundaries. To determine the tissue-level stress fiber alignment (the order parameter) for a given egg chamber, a single imaging plane was used and the orientation of the stress fibers in each cell was compared to all neighboring cells using a custom Python script as previously described [12,69].
COMETtail analysis
Flp out clones, as described above, were used to express UAS-Col4a1-mRFP in either control or mutant backgrounds. For COMETtail analysis, however, only adult flies were heat shocked (incubated on yeast at 37°C for 1 hour, followed by a 1 hour recovery period at 25°C followed by another hour at 37°C) and dissected 12 hours after the start of the incubation period. Clones were identified by intracellular accumulation of UAS-Col4a1-mRFP within the secretory pathway of expressing cells. A COMETtail was scored as being “present” in the BM if it extended more than one cell length away from the clone. Some diffusion can occur even when there is no migration, as was occasionally seen in the fat2N103−2 condition, which is non-migratory [14].
in vitro binding assay
To test binding between Plexin A and the five Drosophila semaphorins, we applied the Extracellular Interactome Assay (ECIA) [70], which is an avidity-based high-throughput interaction detection assay. The ectodomains of PlexA and the five semaphorins were cloned into expression plasmids that produce soluble bait and prey, with an Fc tag for capture on Protein A-coated plates and with a pentamerized Alkaline Phosphatase (AP5) tag for detection of binding, respectively. For higher expression levels, the original metallothionein promoter in the ECIA expression plasmids [70,71] were replaced with the highly active, constitutive Actin5c promoter. The bait and prey were expressed in and secreted from Drosophila S2 cells, and the binding of the prey to bait was detected using the BluePhos colorigenic substrate (KPL) for Alkaline Phosphatase with absorbance at 650 nm.
Quantification of protein localization
Relative levels of Sema-5c-3xGFP and BAC-PlexA-myc at the leading versus trailing edges of cells were quantified in Fiji (ImageJ) [64,65]. A 10-pixel wide line was drawn along leading-trailing cell-cell interfaces at the basal surface of epithelia that were mosaic for the tagged protein. The Measure function was then used to obtain an average fluorescence intensity over each line. The leading versus trailing edge of each clone was determined using leading-edge protrusions, which were marked by Phalloidin. Lines were drawn over 3 categories of cell-cell interfaces: leading edges of the clones, trailing edges of the clones, and cell-cell interfaces outside of the clone (background staining). For each epithelium, the intensities from all the measurements within a single category were averaged together to obtain a single value for each epithelium in each category (leading edge, trailing edge, and background); the background level was used to normalize the data. In an individual epithelium, the same number of cell-cell interfaces was quantified across all categories.
BAC-PlexA-myc mosaic epithelia were generated by creating Flp FRT clones of BAC-PlexA-myc in an epithelium that was null for PlexA. It is necessary to use a PlexA null background when examining clones of BAC-PlexA-myc in order to maintain the endogenous level of PlexA in the cells being analyzed (when extra copies of PlexA are expressed, its planar polarity is lost). The mosaic tissue was then simultaneously immunostained with anti-PlexA and anti-myc to boost the signal.
Quantification of protein colocalization
Line scans were generated by manually drawing a 10-pixel wide line over leading-trailing cell-cell interfaces at the basal epithelial surface using Fiji (ImageJ) [64,65]. Each line scan spanned 5–8 cells. The PlotProfile function was used to obtain fluorescence intensities for each point along the line, and the data were normalized. Prism (GraphPad) was used to plot them against each other and calculate a Pearson correlation coefficient. BAC-PlexA-myc was always used with a PlexA deficiency in the background to maintain endogenous levels of PlexA, for the reason described above.
Quantification of protein levels
Levels of Sema-5c-3xGFP in PlexA RNAi epithelia were quantified at cell-cell interfaces at the basal surface of stage 7 epithelia within a region of uniform size across all samples. Each cell was manually outlined with a 10-pixel wide line in Fiji (ImageJ) [64,65]; Phalloidin was used to mark cell outlines. The Convert to Mask function was used to create a mask from these outlines. The Multiply function within Image Calculator was then used on the mask and the original image. This creates a new image in which only the pixels contained within the mask are present. The Measure function was used to obtain average fluorescence intensity across the multiplied image and data were normalized.
Quantification of protrusions
For quantification of protrusion levels, analysis was performed on stage 8 epithelia stained with Phalloidin, as described above. Protrusions were scored by eye and binned into one of three categories based on comparison to controls: normal, weak, or severely depleted. Protrusions were scored as weak if they were reduced in either number or intensity.
For quantification of protrusion orientation, analysis was performed on stage 6 epithelia stained for Phalloidin. A 48 × 38 μm region of the basal surface (approximately 70 cells) was chosen for each sample, and a 10-pixel wide line was manually drawn over all leading-trailing cell-cell interfaces in that region using FIJI (ImageJ) [64, 65]. The average fluorescence intensity along each line was then calculated using the Measure function. For a single epithelium, the measurements from all leading-trailing interfaces were averaged together to generate a single value. Keeping the lines along the leading-trailing interfaces as a guide, a 10-pixel wide line was then manually drawn over all intervening, lateral interfaces. The average fluorescence intensity of these lines was measured and averaged in the same way as the leading-trailing interfaces to produce a single value for a given epithelium. Epithelia whose protrusions were not well preserved though dissection and fixation protocols were excluded from the analysis.
The analysis of protrusion orientation was performed at stage 6 because most Sema-5c epithelial are migrating at this stage as shown by COMETtail analysis, which allows a leading-trailing cell interface to be defined by tissue movement. An egg chamber had to have a COMETtail to be included in the analysis. It is important to realize, however, that our quantification method underestimates the extent to which protrusions are mis-oriented in the mutant condition; the measurements along leading-trailing cell-cell interfaces cannot distinguish between a normally oriented protrusion emanating from the leading edge and a mis-oriented protrusion emanating from the trailing edge.
Generation of Sema-5c-3xGFP transgenic line
The Sema-5c-3xGFP line was generated by using CRISPR-Cas9 mediated homologous recombination following the general design strategies described by [72,73]. The target sequence selected for gRNA production was 5’-GTTGCCTAGCGGGTCACGACCGG-3’, where the underlined sequence represents the region that was cloned into the pU6-Bbsl-chiRNA plasmid, which contains the Drosophila snRNA:U6:96Ab promoter for in vivo transcription, and the bold sequence represents the adjacent PAM motif.
For homologous recombination, the donor plasmid contained three tandem copies of GFP coding sequence followed by a floxed 3xP3-DsRed module [73] for screening insertion events. This entire cassette was flanked by approximately 2 kb homology arms that contained sequence that matched either side of the target locus. The insertion was made after position 13,696 in the Sema-5c gene, immediately before the stop codon, which corresponds to amino acid 1093 of the Sema-5c protein (according to isoform A sequence).
Injections were performed by Genetivision Inc. into transgenic embryos expressing Cas9 under the nanos promoter. Resulting adults were mated to balancer flies and progeny were screened for founders using DsRed expression in the adult eye with a Leica MZ FL III microscope. Founders were used to establish stocks and crossed to Cre-expressing flies to excise the DsRed module. Insertions were verified by sequencing. Two independent lines were derived and no differences were observed between them.
Generation of UAS-Sema-5c-GFP transgenic line
Sema5c full-length cDNA without a stop codon was amplified from clone RE68041 and inserted into pENTR™/D-TOPO™ and then transferred to the Drosophila Gateway Collection™ vector pTWG for C-terminal GFP tagging. UASt-Sema5c-GFP flies were generated using P element injection by GenetiVision. Insertions were verified by sequencing. Two independent lines were derived and no differences were observed between them.
QUANTIFICATION AND STATISTICAL ANALYSIS
All data were obtained from at least two independent experiments, and several females were analyzed each time. All data were highly reproducible. No statistical method was used to predetermine sample size. The sample size for each experiment can be found in the figure panel or in the figure legend. A Student’s t-test was used to determine if two data sets were significantly different. This analysis was performed using Prism software, version 6 (GraphPad). These tests are appropriate because all data obtained follow an approximately normal distribution. These experiments were not randomized, nor was the data analysis performed blind. Egg chambers damaged by the dissection process were not included in the analysis.
Supplementary Material
Methods S1. Plasmid sequences used in the generation of transgenic flies, related to STAR Methods.
Video S1. Loss of Sema-5c disrupts follicle cell migration, related to Figure 2. Time lapse sequences of control and Sema-5cK175 epithelia, stage 7. Cell membranes labeled. Sequences are 20 frames, elapsed time displayed in minutes. Scale bar, 10 μm.
Video S2. Knockdown of PlexA disrupts follicle cell migration, related to Figure 5. Time lapse sequences of control and PlexA RNAi 2 epithelia, stage 7. Cell membranes labeled. Sequences are 20 frames, elapsed time displayed in minutes. Scale bars, 10 μm
Highlights.
Semaphorin-5c and Plexin A promote the collective migration of epithelial cells
Semaphorin-5c is at each cell’s leading edge and Plexin A is at the trailing edge
Semaphorin-5c can signal across cell-cell boundaries to suppress protrusions
Semaphorin-5c appears to signal through Plexin A, and also interacts with Lar
ACKNOWLEDGEMENTS
We thank Trudi Schüpbach, Allan Spradling, Jose Pastor-Pareja, Alex Kolodkin, Johnathan Terman, David Van Vactor and Takaki Komiyama for Drosophila stocks and reagents, Adam Isabella for illustrations and Python code, Maureen Cetera and Kari Barlan for experimental advice, and Darcy Andersen, Rich Wang and Mitch Anderson for experimental assistance. Kristin Sherrard and Robert Carrillo provided valuable comments on the manuscript. Funding provided by the following grants: NIH T32 GM007183 to C.G.S., NIH T32 HD055164 to A.M.W., American Heart Association 16POST2726018 and American Cancer Society 132123-PF-18-025-01-CSM postdoctoral fellowships to A.L.Z., NIH R01 NS097161 and a Klingenstein-Simons Fellowship Award in the Neurosciences to E. Ö., and American Cancer Society RSG-14–176 and NIH R01 GM126047 to S. H-B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Friedl P, and Gilmour D (2009). Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol 10, 445–457. [DOI] [PubMed] [Google Scholar]
- 2.Mayor R, and Etienne-Manneville S (2016). The front and rear of collective cell migration. Nat. Rev. Mol. Cell Biol 17, 97–109. [DOI] [PubMed] [Google Scholar]
- 3.Ewald AJ, Brenot A, Duong M, Chan BS, and Werb Z (2008). Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell 14, 570–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michaelis UR (2014). Mechanisms of endothelial cell migration. Cell. Mol. Life Sci 71, 4131–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sidhaye J, and Norden C (2017). Concerted action of neuroepithelial basal shrinkage and active epithelial migration ensures efficient optic cup morphogenesis. eLife 6, e22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasilyev A, Liu Y, Mudumana S, Mangos S, Lam P-Y, Majumdar A, Zhao J, Poon K-L, Kondrychyn I, Korzh V, et al. (2009). Collective cell migration drives morphogenesis of the kidney nephron. PLoS Biol. 7, e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedl P, Locker J, Sahai E, and Segall JE (2012). Classifying collective cancer cell invasion. Nat. Cell Biol 14, 777–783. [DOI] [PubMed] [Google Scholar]
- 8.Liu C-Y, and Kao WW-Y (2015). Corneal epithelial wound healing. Prog. Mol. Biol. Transl. Sci 134, 61–71. [DOI] [PubMed] [Google Scholar]
- 9.Shaw TJ, and Martin P (2016). Wound repair: a showcase for cell plasticity and migration. Curr. Opin. Cell Biol 42, 29–37. [DOI] [PubMed] [Google Scholar]
- 10.Ladoux B, and Mège R-M (2017). Mechanobiology of collective cell behaviours. Nat. Rev. Mol. Cell Biol 18, 743–757. [DOI] [PubMed] [Google Scholar]
- 11.Horne-Badovinac S, and Bilder D (2005). Mass transit: Epithelial morphogenesis in the Drosophila egg chamber. Dev. Dyn 232, 559–574. [DOI] [PubMed] [Google Scholar]
- 12.Cetera M, Ramirez-San Juan GR, Oakes PW, Lewellyn L, Fairchild MJ, Tanentzapf G, Gardel ML, and Horne-Badovinac S (2014). Epithelial rotation promotes the global alignment of contractile actin bundles during Drosophila egg chamber elongation. Nat. Commun 5, 5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haigo SL, and Bilder D (2011). Global tissue revolutions in a morphogenetic movement controlling elongation. Science 331, 1071–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barlan K, Cetera M, and Horne-Badovinac S (2017). Fat2 and Lar define a basally localized planar signaling system controlling collective cell migration. Dev. Cell 40, 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bateman J, Reddy RS, Saito H, and Van Vactor D (2001). The receptor tyrosine phosphatase Dlar and integrins organize actin filaments in the Drosophila follicular epithelium. Curr. Biol 11, 1317–1327. [DOI] [PubMed] [Google Scholar]
- 16.Viktorinova I, Konig T, Schlichting K, and Dahmann C (2009). The cadherin Fat2 is required for planar cell polarity in the Drosophila ovary. Development 136, 4123–4132. [DOI] [PubMed] [Google Scholar]
- 17.Viktorinová I, and Dahmann C (2013). Microtubule polarity predicts direction of egg chamber rotation in Drosophila. Curr. Biol 23, 1472–1477. [DOI] [PubMed] [Google Scholar]
- 18.Jongbloets BC, and Pasterkamp RJ (2014). Semaphorin signaling during development. Development 141, 3292–3297. [DOI] [PubMed] [Google Scholar]
- 19.Yazdani U, and Terman JR (2006). The semaphorins. Genome Biol. 7, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu C, and Giraudo E (2013). The role of semaphorins and their receptors in vascular development and cancer. Exp. Cell Res 319, 1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theveneau E, and Mayor R (2012). Neural crest migration: interplay between chemorepellents, chemoattractants, contact inhibition, epithelial-mesenchymal transition, and collective cell migration. Wiley Interdiscip. Rev. Dev. Biol 1, 435–445. [DOI] [PubMed] [Google Scholar]
- 22.Horne-Badovinac S, Hill J, Gerlach G, Menegas W, and Bilder D (2012). A screen for round egg mutants in Drosophila identifies tricornered, furry, and misshapen as regulators of egg chamber elongation. G3 2, 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bahri SM, Chia W, and Yang X (2001). Characterization and mutant analysis of the Drosophila sema 5c gene. Dev. Dyn 221, 322–330. [DOI] [PubMed] [Google Scholar]
- 24.Cetera M, and Horne-Badovinac S (2015). Round and round gets you somewhere: collective cell migration and planar polarity in elongating Drosophila egg chambers. Curr. Opin. Genet. Dev 32, 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutzeit HO (1990). The microfilament pattern in the somatic follicle cells of midvitellogenic ovarian follicles of Drosophila. Eur. J. Cell Biol 53, 349–356. [PubMed] [Google Scholar]
- 26.Isabella AJ, and Horne-Badovinac S (2016). Rab10-mediated secretion synergizes with tissue movement to build a polarized basement membrane architecture for organ morphogenesis. Dev. Cell 38, 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen D-Y, Crest J, and Bilder D (2017). A cell migration tracking tool supports coupling of tissue rotation to elongation. Cell Rep. 21, 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung R-J, and Terman JR (2011). Extracellular inhibitors, repellents, and Semaphorin/Plexin/MICAL-mediated Actin filament disassembly. Cytoskeleton 68, 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barberis D, Artigiani S, Casazza A, Corso S, Giordano S, Love CA, Jones EY, Comoglio PM, and Tamagnone L (2004). Plexin signaling hampers integrin-based adhesion, leading to Rho-kinase independent cell rounding, and inhibiting lamellipodia extension and cell motility. FASEB J. 18, 592–594. [DOI] [PubMed] [Google Scholar]
- 30.Li X, and Lee AYW (2010). Semaphorin 5A and Plexin-B3 inhibit human glioma cell motility through RhoGDIα-mediated inactivation of Rac1 GTPase. J. Biol. Chem 285, 32436–32445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Rijn A, Paulis L, te Riet J, Vasaturo A, Reinieren-Beeren I, van der Schaaf A, Kuipers AJ, Schulte LP, Jongbloets BC, Pasterkamp RJ, et al. (2016). Semaphorin 7A promotes chemokine-driven dendritic cell migration. J. Immunol 196, 459–468. [DOI] [PubMed] [Google Scholar]
- 32.Winberg ML, Noordermeer JN, Tamagnone L, Comoglio PM, Spriggs MK, Tessier-Lavigne M, and Goodman CS (1998). Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell 95, 903–916. [DOI] [PubMed] [Google Scholar]
- 33.Ayoob JC (2006). Drosophila Plexin B is a Sema-2a receptor required for axon guidance. Development 133, 2125–2135. [DOI] [PubMed] [Google Scholar]
- 34.Wu Z, Sweeney LB, Ayoob JC, Chak K, Andreone BJ, Ohyama T, Kerr R, Luo L, Zlatic M, and Kolodkin AL (2011). A combinatorial semaphorin code instructs the initial steps of sensory circuit assembly in the Drosophila CNS. Neuron 70, 281–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stramer B, and Mayor R (2016). Mechanisms and in vivo functions of contact inhibition of locomotion. Nat. Rev. Mol. Cell Biol [DOI] [PubMed] [Google Scholar]
- 36.Deb Roy A, Yin T, Choudhary S, Rodionov V, Pilbeam CC, and Wu YI (2017). Optogenetic activation of Plexin-B1 reveals contact repulsion between osteoclasts and osteoblasts. Nat. Commun 8, 15831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Artigiani S, Conrotto P, Fazzari P, Gilestro GF, Barberis D, Giordano S, Comoglio PM, and Tamagnone L (2004). Plexin-B3 is a functional receptor for Semaphorin 5A. EMBO Rep. 5, 710–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Law JWS, and Lee AYW (2012). Semaphorin 5A and Plexin-B3 regulate human glioma cell motility and morphology through Rac1 and the Actin cytoskeleton. Oncogene 31, 595–610. [DOI] [PubMed] [Google Scholar]
- 39.Sadanandam A, Rosenbaugh EG, Singh S, Varney M, and Singh RK (2010). Semaphorin 5A promotes angiogenesis by increasing endothelial cell proliferation, migration, and decreasing apoptosis. Microvasc. Res 79, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takamatsu H, Takegahara N, Nakagawa Y, Tomura M, Taniguchi M, Friedel RH, Rayburn H, Tessier-Lavigne M, Yoshida Y, Okuno T, et al. (2010). Semaphorins guide the entry of dendritic cells into the lymphatics by activating Myosin II. Nat. Immunol 11, 594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duan Y, Wang S-H, Song J, Mironova Y, Ming G, Kolodkin AL, and Giger RJ (2014). Semaphorin 5A inhibits synaptogenesis in early postnatal- and adult-born hippocampal dentate granule cells. eLife 3, e.04390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuoka RL, Chivatakarn O, Badea TC, Samuels IS, Cahill H, Katayama K, Kumar SR, Suto F, Chédotal A, Peachey NS, et al. (2011). Class 5 transmembrane semaphorins control selective mammalian retinal lamination and function. Neuron 71, 460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Battistini C, and Tamagnone L (2016). Transmembrane semaphorins, forward and reverse signaling: have a look both ways. Cell. Mol. Life Sci 73, 1609–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson KG, Tenney AP, Ghose A, Duckworth AM, Higashi ME, Parfitt K, Marcu O, Heslip TR, Marsh JL, Schwarz TL, et al. (2006). The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron 49, 517–531. [DOI] [PubMed] [Google Scholar]
- 45.Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, et al. (2004). Semaphorin 5A Is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron 44, 961–975. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura F, Okada T, Shishikura M, Uetani N, Taniguchi M, Yagi T, Iwakura Y, Ohshima T, Goshima Y, and Strittmatter SM (2017). Protein tyrosine phosphatase δ mediates the Sema3A-induced cortical basal dendritic arborization through the activation of Fyn tyrosine kinase. J. Neurosci. Off. J. Soc. Neurosci 37, 7125–7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolodkin AL, and Tessier-Lavigne M (2011). Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb. Perspect. Biol 3, a001727–a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han KA, Jeon S, Um JW, and Ko J (2016). Emergent synapse organizers: LAR-RPTPs and their companions In International Review of Cell and Molecular Biology, Jeon, Kwang W, ed. (Elsevier; ), pp. 39–65. [DOI] [PubMed] [Google Scholar]
- 49.Avilés EC, and Goodrich LV (2017). Configuring a robust nervous system with Fat cadherins. Semin. Cell Dev. Biol 69, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoo SK, Pascoe HG, Pereira T, Kondo S, Jacinto A, Zhang X, and Hariharan IK (2016). Plexins function in epithelial repair in both Drosophila and zebrafish. Nat. Commun 7, 12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hulpiau P, and van Roy F (2011). New insights into the evolution of metazoan cadherins. Mol. Biol. Evol 28, 647–657. [DOI] [PubMed] [Google Scholar]
- 52.Aurich F, and Dahmann C (2016). A mutation in fat2 uncouples tissue elongation from global tissue rotation. Cell Rep. 14, 2503–2510. [DOI] [PubMed] [Google Scholar]
- 53.Mason BN, Califano JP, and Reinhart-King CA (2012). Matrix stiffness: a regulator of cellular behavior and tissue formation In Engineering Biomaterials for Regenerative Medicine, Bhatia SK, ed. (New York, NY: Springer; ), pp. 19–37. [Google Scholar]
- 54.Chlasta J, Milani P, Runel G, Duteyrat J-L, Arias L, Lamiré L-A, Boudaoud A, and Grammont M (2017). Variations in basement membrane mechanics are linked to epithelial morphogenesis. Development 144, 4350–4362. [DOI] [PubMed] [Google Scholar]
- 55.Crest J, Diz-Muñoz A, Chen D-Y, Fletcher DA, and Bilder D (2017). Organ sculpting by patterned extracellular matrix stiffness. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duffy JB, Harrison DA, and Perrimon N (1998). Identifying loci required for follicular patterning using directed mosaics. Development 125, 2263–2271. [DOI] [PubMed] [Google Scholar]
- 57.Meltzer S, Yadav S, Lee J, Soba P, Younger SH, Jin P, Zhang W, Parrish J, Jan LY, and Jan Y-N (2016). Epidermis-derived semaphorin promotes dendrite self-avoidance by regulating dendrite-substrate adhesion in Drosophila sensory neurons. Neuron 89, 741–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frydman HM, and Spradling AC (2001). The receptor-like tyrosine phosphatase Lar is required for epithelial planar polarity and for axis determination within Drosophila ovarian follicles. Development 128, 3209–3220. [DOI] [PubMed] [Google Scholar]
- 59.Zang Y, Wan M, Liu M, Ke H, Ma S, Liu L-P, Ni J-Q, and Carlos Pastor-Pareja J (2015). Plasma membrane overgrowth causes fibrotic collagen accumulation and immune activation in Drosophila adipocytes. eLife 4, e.07187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pecot MY, Tadros W, Nern A, Bader M, Chen Y, and Zipursky SL (2013). Multiple interactions control synaptic layer specificity in the Drosophila visual system. Neuron 77, 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Terman JR, Mao T, Pasterkamp RJ, Yu H-H, and Kolodkin AL (2002). MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell 109, 887–900. [DOI] [PubMed] [Google Scholar]
- 62.Cetera M, Lewellyn L, and Horne-Badovinac S (2016). Cultivation and live imaging of Drosophila ovaries. In Dahmann Drosophila, C., ed. (New York, NY: Springer; ), pp. 215–226. [DOI] [PubMed] [Google Scholar]
- 63.Prasad M, Jang AC-C, Starz-Gaiano M, Melani M, and Montell DJ (2007). A protocol for culturing Drosophila melanogaster stage 9 egg chambers for live imaging. Nat. Protoc 2, 2467–2473. [DOI] [PubMed] [Google Scholar]
- 64.Schneider CA, Rasband WS, and Eliceiri KW (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sweeney LB, Couto A, Chou Y-H, Berdnik D, Dickson BJ, Luo L, and Komiyama T (2007). Temporal target restriction of olfactory receptor neurons by Semaphorin-1a/PlexinA-mediated axon-axon interactions. Neuron 53, 185–200. [DOI] [PubMed] [Google Scholar]
- 67.Aigouy B, and Mirouse V (2013). ScientiFig: a tool to build publication-ready scientific figures. Nat. Methods 10, 1048–1048. [DOI] [PubMed] [Google Scholar]
- 68.Rezakhaniha R, Agianniotis A, Schrauwen JTC, Griffa A, Sage D, Bouten CVC, van de Vosse FN, Unser M, and Stergiopulos N (2012). Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech. Model. Mechanobiol 11, 461–473. [DOI] [PubMed] [Google Scholar]
- 69.Isabella AJ, and Horne-Badovinac S (2015). Dynamic regulation of basement membrane protein levels promotes egg chamber elongation in Drosophila. Dev. Biol 406, 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Özkan E, Carrillo RA, Eastman CL, Weiszmann R, Waghray D, Johnson KG, Zinn K, Celniker SE, and Garcia KC (2013). An extracellular interactome of immunoglobulin and LRR proteins reveals receptor-ligand networks. Cell 154, 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carrillo RA, Özkan E, Menon KP, Nagarkar-Jaiswal S, Lee P-T, Jeon M, Birnbaum ME, Bellen HJ, Garcia KC, and Zinn K (2015). Control of synaptic connectivity by a network of Drosophila IgSF cell surface proteins. Cell 163, 1770–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gratz SJ, Cummings AM, Nguyen JN, Hamm DC, Donohue LK, Harrison MM, Wildonger J, and O’Connor-Giles KM (2013). Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics 194, 1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gratz SJ, Ukken FP, Rubinstein CD, Thiede G, Donohue LK, Cummings AM, and O’Connor-Giles KM (2014). Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics 196, 961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods S1. Plasmid sequences used in the generation of transgenic flies, related to STAR Methods.
Video S1. Loss of Sema-5c disrupts follicle cell migration, related to Figure 2. Time lapse sequences of control and Sema-5cK175 epithelia, stage 7. Cell membranes labeled. Sequences are 20 frames, elapsed time displayed in minutes. Scale bar, 10 μm.
Video S2. Knockdown of PlexA disrupts follicle cell migration, related to Figure 5. Time lapse sequences of control and PlexA RNAi 2 epithelia, stage 7. Cell membranes labeled. Sequences are 20 frames, elapsed time displayed in minutes. Scale bars, 10 μm