Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) has emerged as a significant contributor to morbidity and mortality associated with influenza infection. Here we show in a mouse model that preceding influenza infection promotes S. aureus resistance to killing by antibiotics. This resistance coincides with influenza-induced accumulation of inflammatory monocytes in the lung. C-C chemokine receptor type 2 (CCR2) is responsible for pulmonary monocyte recruitment after influenza infection. We found that antibiotic-treated Ccr2-deficient (Ccr2−/−) mice exhibit significantly improved bacterial control and survival from influenza and MRSA coinfection, despite a delay in viral clearance. Mechanistically, our results from in vivo studies indicate that influenza-induced monocytes serve as reservoirs for intracellular S. aureus survival, thereby promoting bacterial resistance to antibiotic treatment. Blocking CCR2 with a small molecular inhibitor (PF-04178903), in conjunction with antibiotic treatment, enhanced lung bacterial clearance and significantly improved animal survival. Collectively, our study demonstrates that inflammatory monocytes constitute an important and hitherto underappreciated mechanism of the conflicting immune requirements for viral and bacterial clearance by hosts, which subsequently leads to exacerbated outcomes of influenza and S. aureus coinfection.
Introduction
Secondary bacterial infections, commonly associated with Streptococcus pneumoniae, Staphylococcus aureus and Haemophilus influenzae, are known to be frequent causes of severe morbidity and mortality after influenza infection (1–3). These influenza-complicated bacterial infections are more difficult to treat than bacterial pneumonia alone. Our knowledge of influenza-induced susceptibility and exacerbation of bacterial infection is improving, but still very limited (4–8). There is an urgent need to identify critical determinants of this influenza and bacterial synergy before the development of effective treatments.
Inflammatory monocytes express C-C chemokine receptor type 2 (CCR2), a chemokine receptor that promotes monocyte emigration from the bone marrow to infiltrate sites of infection (9, 10). This population had a variety of names, including exudate macrophages (9), TNF-/iNOS-producing DCs (11), or TRAIL+ monocytes (10). Ccr2−/− mice have increased susceptibility to many microbial infections due to defective monocyte/macrophage responses (12). However, airway accumulation of inflammatory monocytes has been linked with exacerbated lung damage during severe influenza infection (9, 11, 13, 14). Furthermore, it has been shown that this monocyte-induced lung damage facilitates bacterial systemic invasion, thereby leading to increased mortality during influenza and S. pneumoniae coinfection (10). Nonetheless, it remains unclear whether influenza-induced monocyte infiltration contributes to the exacerbation of secondary S. aureus infection, particularly the resistance of influenza/S. aureus coinfection to antibiotic therapy (15).
We have previously reported that influenza-induced suppression of antibacterial immunity coincides with accumulation of inflammatory monocytes in the lower respiratory tract (16). Thus, we hypothesized that influenza-induced dysregulation of inflammatory cell infiltration contributes to the influenza/S. aureus synergy in mortality. Indeed, in this study, we demonstrate that CCR2-dependent immune response facilitates viral clearance but impairs the bactericidal effect of antibiotics. Specifically, influenza-induced inflammatory monocytes serve as reservoirs for intracellular S. aureus survival, thereby promoting bacterial persistence after antibiotic treatment. As a result, inhibition of monocyte recruitment, by pharmacologically blocking CCR2 (14), significantly improves the therapeutic effect of antibiotics against post-influenza S. aureus infection.
Materials and Methods
Murine model of viral and bacterial infections
Specific pathogen-free, C57BL/6 WT and Ccr2−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and bred at University of Nebraska Medical Center following IACUC guidelines. Viral challenge was performed with a sublethal, i.e., 50 PFU/female and 75 PFU/male, of PR8 administered i.n. to anesthetized, sex and age-matched adult mice in 50 μl of sterile PBS. Titers of virus stocks and viral levels in the bronchoalveolar lavage fluids (BALF) and lungs of infected mice were determined by plaque assays on MDCK cell monolayers (16). Bacterial super-infection was performed seven days later. To induce bacterial pneumonia, anesthetized mice were inoculated i.n. with ATCC MRSA strain BAA-1695 or DsRed-expressing BAA-1695 in 50 μl of sterile PBS (17). Bacterial burdens were measured by sacrificing infected mice at the indicated time points, and plating serial 10-fold dilutions of each sample onto blood agar plates.
Bronchoalveolar lavage (BAL) and lung cell analysis
BAL fluid (BALF) samples were collected by making an incision in the trachea and lavaging the lung twice with 0.8 ml PBS, pH 7.4. Single lung cell suspensions were obtained by lung digestion with 2.5 mg/ml collagenase D and 0.25 mg/ml DNase I (Roche Diagnostics) for 1 h at 37°C under constant agitation, followed by passage through a nylon mesh. Total cell counts were determined using a hemacytometer.
For flow cytometry analysis, BALF or lung cells were incubated with 2.4G2 mAb against FcγRII/III, and stained with APC conjugated anti-CD11c (Biolegend), BUV395-conjugated or PE-Cy7-conjugated anti-CD11b (BD Biosciences), FITC-conjugated or PE-Cy7-conjugated anti-Ly6G (clone 1A8, Biolegend), PerCP-Cy5.5-conjugated (eBiosciences) or FITC-conjugated anti-Ly6C (BD Biosciences), and BV421-conjugated or PE-conjugated anti-Siglec-F (BD Biosciences) mAbs. The stained cells were analyzed on a BD LSRII-green using BD FACSDiva and FlowJo software analysis.
Quantification of live bacteria within phagocytes
Seven days after inoculation of PR8 virus, mice were infected with MRSA and treated with gentamicin 4 h later. At 24 h BALF cells were collected and sorted using FACSAria (BD Biosciences). Cell populations in the airway were classified using these surface markers: AM (CD11chiCD11blow), inflammatory monocytes (CD11b+Ly6C+), and neutrophils (CD11b+Ly6G+). Extracellular bacteria were lysed by lysostaphin. Cytospins of cells were prepared and Diff-Quick stained. The sorted cells were washed with PBS and lysed in sterile water, and then plated on blood agar plates. The number of CFU was expressed per 1000 cells for each myeloid cell subset.
In vitro phagocytosis assay
Mice were sacrificed seven days after PR8 infection. Lung cells were harvested, and erythrocytes were lysed using Ammonium-Chloride-Potassium (ACK) lysing buffer. Single-cell suspensions were then co-incubated with DsRed-expressing S. aureus BAA-1695 at multiplicity of infection (MOI) 5 for 3 h at 37°C in RPMI containing 10% FBS. The cells were harvested by centrifugation, and re-suspended with 2% BSA for cell surface marker staining and flow cytometry analysis.
Treatment with antibiotics
Mice were i.p. injected with a therapeutic dose (100 mg/kg/day) of gentamicin beginning 4 h after MRSA infection and then followed by 50 mg/kg/day (15). In some experiments, mice were treated i.p. with vancomycin (150 mg/kg/day) beginning 2 h after MRSA infection and then followed by 75 mg/kg/day. Control mice received PBS. All antibiotic treatment and sham injections continued through day 10 after MRSA infection.
Treatment with CCR2 antagonist
The CCR2 antagonist (1,5-anhydro-2,3-dideoxy-3-{[(1R,3S)-3-isopropyl-3-({4-[4-(trifluoromethyl)pyridin-2-yl]piperazin-1-yl}carbonyl)cyclopentyl]amino}−4-O-methylpentitol) was provided by Pfizer (Groton, CT) and is designated PF-04178903. Mice were treated s.c. twice daily at a dose of 50 mg/kg in PBS until the day of MRSA infection.
Statistics
Significant differences between experimental groups were determined using a two-tailed Student t-test (to compare two samples), an ANOVA analysis followed by Tukey’s multiple comparisons test (to compare multiple samples) or Mann Whitney test (nonparametric test) in GraphPad Prism 7 (La Jolla, CA). Survival analyses were performed using the log-rank test. For all analyses, a P value <0.05 was considered to be significant.
Results
Influenza infection impairs the bactericidal effect of antibiotics during secondary S. aureus infection
To evaluate the impact of influenza infection on lung bacterial control by antibiotics, C57BL/6 wild-type (WT) mice were inoculated with a sublethal dose of PR8 virus, followed by a high dose of MRSA infection seven days later. Starting at 4 h after MRSA super-infection, all animals were treated with gentamicin or PBS control, including mice infected with MRSA alone (15). As expected, antibiotic treatment significantly reduced bacterial burden in MRSA single-infected and super-infected mice (Fig. 1A–B). Of note, in this lethal coinfection model (15), all PR8/MRSA coinfected mice died within 24 h in the absence of antibiotic treatment. Conversely, at 8 h after bacterial infection, lung bacterial burdens were comparable between MRSA single-infected and super-infected mice treated with PBS control (Fig. 1A). This result suggests that the acute impact of influenza infection on immune clearance of S. aureus is overwhelmed after this high dose of bacterial infection. This lethal coinfection model thus allowed us to investigate the impact of influenza infection on antibiotic-mediated bacterial control, in an attempt to mimic the poor responsiveness of pneumonic patients to antibiotic treatments at the later phase of influenza and S. aureus coinfection.
Figure 1. Preceding influenza infection impairs the bactericidal effect of gentamicin during MRSA super-infection.
(A) Lung bacterial burdens (mean±SD, n=5) at 8 h after infection of naïve (MRSA) and day seven PR8-infected (PR8/MRSA) WT mice with 4×108 CFU MRSA. P<0.05, two-way ANOVA; *P< 0.05, ***P< 0.001, t test. (B) Lung bacterial burdens (mean±SD, n≥8), (C) numbers of live bacteria associated with lung cells (mean±SD, n=5) at 24 h, and (D) animal survival after infection of naïve (MRSA) and day seven PR8-infected (PR8/MRSA) WT mice with 4×108 CFU MRSA. In (C), Single-cell suspensions were plated for bacterial CFUs before (−) and after (+) lysostaphin treatment. Mice were treated with gentamicin (+Gent) or PBS control (+PBS) 4 h after MRSA infection. P<0.05, one-way ANOVA; *P< 0.05, ***P< 0.001, t test. Data shown were combined (B, D) or representative (A, C) of 2–3 independent experiments.
Indeed, after gentamicin treatment, PR8/MRSA coinfected mice exhibited significantly increased bacterial burden as compared with MRSA single-infected animals at 24 h, suggesting that influenza infection impairs the bactericidal effect of antibiotics (Fig. 1B). In vitro lysostaphin treatment had no significant effect on the increased viable bacteria in lung cells isolated from coinfected mice, suggesting intracellular S. aureus survival (Fig. 1C). In agreement with that, while daily gentamicin treatment provided sufficient protection against MRSA single infection, all animals still succumbed to influenza/MRSA coinfection. (Fig. 1D). Together, these results indicate that influenza infection promotes bacterial resistance to antibiotic killing during secondary S. aureus lung infection.
Influenza-induced monocyte recruitment coincides with S. aureus resistance to the bactericidal effect of antibiotics
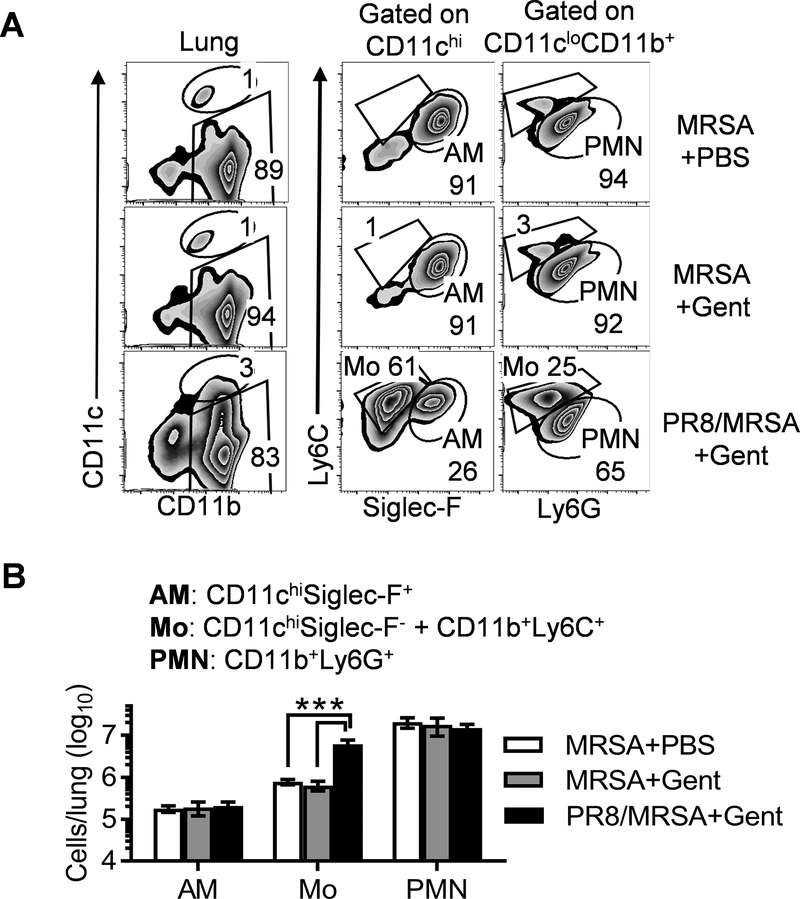
To determine cellular reservoirs for bacterial survival from antibiotics, we next challenged mice with DsRed-expressing MRSA and determined bacteria-containing lung cells by flow cytometry analysis (17). Resident alveolar macrophages (AMs) are the primary immune cells in the airway of naïve mice (17). However, due to intensive neutrophil recruitment, AMs (CD11c+Siglec-F+) only represented a minor population (~1%) in both MRSA single-infected and super-infected mice (Fig. 2A). Ly6C+ monocytes are circulating mononuclear cells that enter lungs after influenza infection (18). This surface marker was used to distinguish inflammatory monocytes (Mo) from CD11b+Ly6G+ neutrophils (PMN). In agreement with our previous findings (16), PR8/MRSA coinfected mice exhibited a significant increase in lung inflammatory monocytes (Fig. 2). The increased monocytes in coinfected mice led to a corresponding decrease in the proportion of neutrophils (Fig. 2A). Nonetheless, the actual number of neutrophils in PR8/MRSA coinfected mice was comparable to that in MRSA single-infected controls (Fig. 2B).
Figure 2. Preceding influenza infection promotes lung monocyte recruitment.
Naïve (MRSA) and day seven PR8-infected (PR8/MRSA) WT mice were infected with 4×108 CFU MRSA and treated with gentamicin (+Gent) or PBS control (+PBS) 4 h after MRSA infection. At 24 h lungs were analyzed for (A) the percentages (mean of 5 mice/group) and (B) numbers (mean±SD, n=5) of AMs, monocytes (Mo) and neutrophils (PMN). P<0.05, one-way ANOVA; ***P< 0.001, Tukey’s multiple comparisons test. Data shown are representative of two independent experiments.
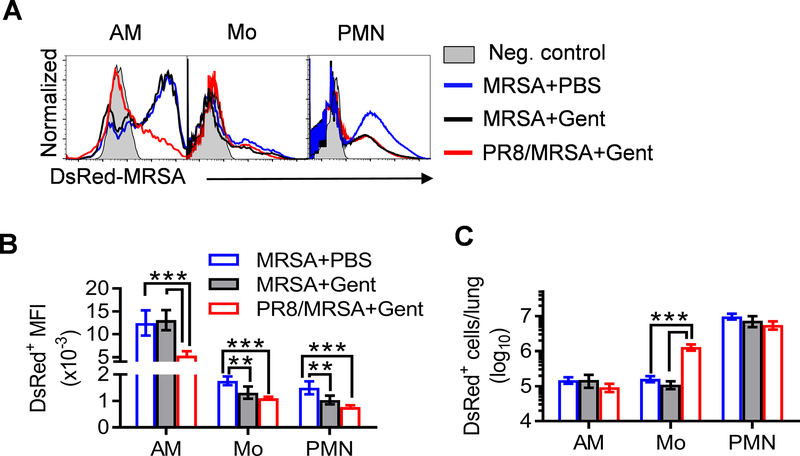
Interestingly, during MRSA infection alone, although there were 100-fold increased bacterial burdens in PBS-treated controls as compared with gentamicin-treated mice (Fig. 1B), the numbers of DsRed+ monocytes and neutrophils were not affected by antibiotic treatment, except that their mean fluorescence intensity (MFI) were significantly decreased (Fig. 3). In contrast, after gentamicin treatment, the number and MFI of DsRed+ neutrophils were comparable between MRSA single-infected and super-infected mice. Only the number of DsRed-MRSA+ monocytes significantly increased after coinfection (Fig. 3C). Considering these findings, we proposed that inflammatory monocytes trafficking into influenza-infected lungs protect bacteria from killing by antibiotics, and thereby leading to impaired lung bacterial control during S. aureus super-infection.
Figure 3. Influenza-induced monocyte recruitment coincides with increased intracellular S. aureus survival from antibiotics.
Naïve (MRSA) and day seven PR8-infected (PR8/MRSA) WT mice were infected with 4×108 CFU DsRed-MRSA and treated with gentamicin (+Gent) or PBS control (+PBS) 4 h after MRSA infection. At 24 h lungs were analyzed for (A) the representative histograms, (B) mean fluorescence intensity (MFI), and (C) numbers (mean±SD, n=5) of DsRed-MRSA+ AMs, monocytes (Mo) and neutrophils (PMN). P<0.05, one-way ANOVA; **P< 0.01, ***P< 0.001, Tukey’s multiple comparisons test. Data shown are representative of two independent experiments.
Ccr2−/− mice are defective in inflammatory monocyte recruitment following influenza infection
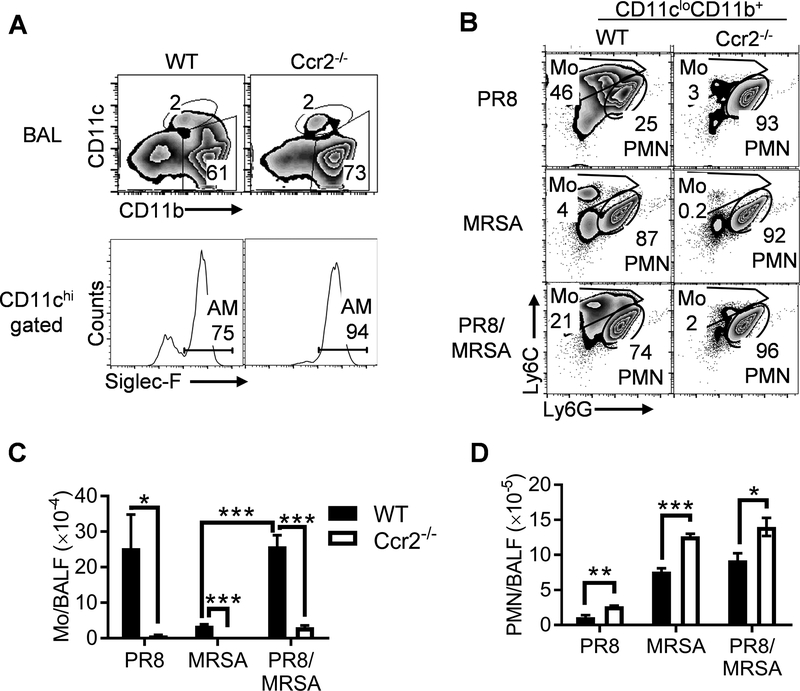
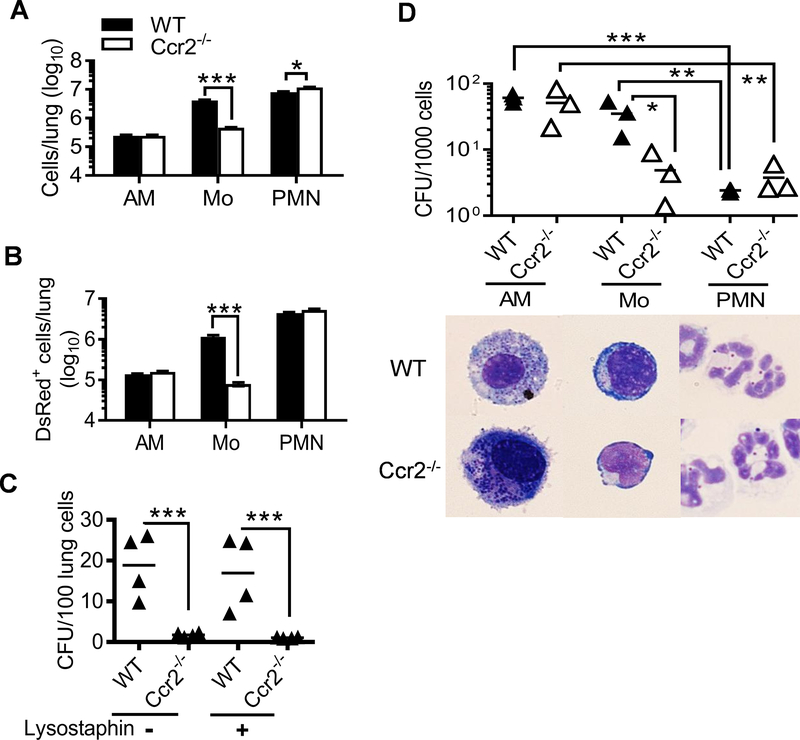
Based on the previous reports demonstrating that lung monocyte accumulation is CCR2-dependent (9, 11, 13, 14), we expected that the recruitment of monocytes would be diminished in Ccr2−/− mice. Indeed, few inflammatory monocytes were recovered from PR8-infected or PR8/MRSA coinfected Ccr2−/− airways (Fig. 4). As a result, neutrophils were the single most inflammatory cell population in Ccr2−/− airways after coinfection, similar to MRSA single-infected WT and Ccr2−/− mice (Fig. 4B–D).
Figure 4. CCR2 mediates airway monocyte recruitment following influenza infection.
Mice were infected with PR8 and seven days later super-challenged with 4×108 CFU MRSA or PBS control. At 24 h BALF cells from WT and Ccr2−/− mice were analyzed for (A) airway myeloid cell profiles (mean of 4–5 mice/group), (B) compositions of CD11c–CD11b+ inflammatory cells (mean of 4–5 mice/group), and (C-D) numbers (mean±SEM) of CD11b+Ly6C+ monocytes (Mo) (C), and CD11b+Ly6G+ neutrophils (PMN) (D). P<0.05, two-way ANOVA; *P< 0.05, **P< 0.01, ***P< 0.001, Tukey’s multiple comparisons test. All mice were treated with gentamicin 4 h after MRSA infection. Data shown are representative of two independent experiments.
CCR2 deficiency restores the bactericidal effect of antibiotics during post-influenza S. aureus infection
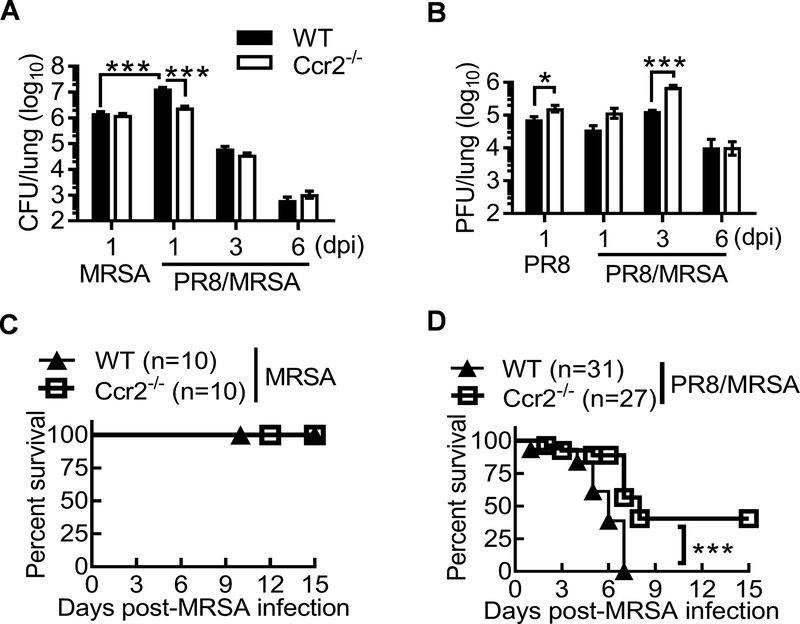
We next evaluated the impact of influenza-induced monocyte recruitment on S. aureus resistance to antibiotics using Ccr2−/− mouse model. Due to the high-dose of MRSA infection, even MRSA single-infected WT and Ccr2−/− mice displayed substantial lung bacterial burden at 24 h, despite of gentamicin treatment (Fig. 5A). Nonetheless, we detected ~10-fold decreased bacterial burdens in coinfected Ccr2−/− mice, as compared with coinfected WT controls. Importantly, bacterial burdens in coinfected Ccr2−/− mice were comparable to MRSA single-infected WT or Ccr2−/− mice. These results indicate that in contrast to WT animals, prior influenza infection does not inhibit acute bactericidal effect of gentamicin in Ccr2−/− mice. In other words, it suggests that CCR2-mediated immune mechanism is responsible for influenza-induced S. aureus resistance to killing by antibiotics. No significant difference in bacterial burden was detected at the later time points of coinfection, likely due to the dominant bactericidal effect of antibiotics.
Figure 5. Monocyte recruitment promotes bacterial resistance to antibiotic therapy during influenza/S. aureus coinfection.
(A) Lung bacterial and (B) viral burdens (mean±SEM, n ≥ 9 mice/group) at various time points after inoculation of naïve (MRSA) or day seven postinfluenza infected (PR8/MRSA) WT and Ccr2−/− mice with 4×108 CFU of MRSA or PBS (PR8). All PR8 and/or MRSA infected mice were treated daily with gentamicin until 24 h before harvesting the samples. P<0.05, two-way ANOVA; ***P< 0.001, Mann Whitney test. (C-D) Survival of WT and Ccr2−/− mice after infection of naïve (C) or day seven postinfluenza infected (D) WT and Ccr2−/− mice with 4×108 CFU of MRSA and then daily treatment with gentamicin. ***P< 0.001, log-rank test. Data shown were combined from 2–4 independent experiments.
Despite of improved bacterial control at the acute phase, CCR2 deficiency led to a significant delay in viral clearance, indicated as ~10-fold increased viral burdens in Ccr2−/− mice 3 days after coinfection (dpi) (Fig. 5B). This finding is consistent with previous reports demonstrating that inflammatory monocytes are required for adequate antiviral T cell responses (18, 19). Together, our results provide novel evidence that there is a CCR2-dependent functional confliction between antiviral and antibacterial immune responses during host defense against influenza and S. aureus coinfection.
On the other hand, although Ccr2−/− mice exhibited significant increases in neutrophil recruitment compared with corresponding WT controls (Fig. 4D), the impaired bacterial clearance only coincides with prominent increases of inflammatory monocytes in coinfected WT mice, as revealed by a comparative analysis between WT and Ccr2−/− mice, without or without preceding PR8 infection (Fig. 5A). This adverse correlation between monocyte recruitment and bacterial control implies that inflammatory monocytes promote S. aureus resistance to antibiotic treatment during coinfection.
Furthermore, daily gentamicin treatment resulted in 100% survival of WT and Ccr2−/− mice infected with MRSA alone (Fig. 5C). However, antibiotic treatment was not as efficacious in protection against influenza and S. aureus coinfection. Nonetheless, compared with WT controls, Ccr2−/− mice exhibited significantly increased survival from coinfection after gentamicin treatment (Fig. 5D). These differences in influenza-induced exacerbation of S. aureus infection between WT and Ccr2−/− mice were observed for both males and females. Together, these results establish a detrimental effect of CCR2-mediated monocyte recruitment on antibacterial defense.
Bacterial uptake by inflammatory monocytes promotes intracellular survival of S. aureus
Compared with their relatively limited ability to bind and uptake pneumococci, macrophages and neutrophils are proficient in innate uptake of S. aureus (Fig. 3). As a result, phagocytic clearance of S. aureus is mainly restricted by intracellular killing process (16). On the other hand, due to its weak permeability into host cells, gentamicin has a limited intracellular bactericidal activity. We thus investigated whether CCR2-recruited monocytes promote intracellular S. aureus survival, thereby enabling bacteria to escape from killing by antibiotics.
To quantitate bacterial phagocytosis in vivo, we challenged WT and Ccr2−/− mice with DsRed-expressing MRSA after influenza. There were no apparent differences in the number of DsRed-MRSA+ AMs and neutrophils between WT and Ccr2−/− mice (Fig. 6A–B &Supplemental Fig. 1); however, the numbers of both total and DsRed-MRSA+ monocytes were ~10-fold lower in Ccr2−/− mice after coinfection (Fig. 6A–B). Importantly, this decrease in DsRed-MRSA+ monocytes was associated with a comparable reduction in viable S. aureus within Ccr2−/− lung cells (Fig. 6C).
Figure 6. Bacterial uptake by inflammatory monocytes leads to increased intracellular S. aureus survival from antibiotics.
Lungs were analyzed for the numbers (mean±SEM) of (A) total and (B) DsRed+ AMs (CD11c+Siglec-F+), monocytes (Mo, CD11c+Siglec-F− plus CD11b+Ly6C+), and neutrophils (PMN, CD11b+Ly6G+), and (C) numbers of live bacteria within lung cells 24 h after super-challenge of day seven PR8-infected WT and Ccr2−/− mice with 4×108 CFU DsRed-MRSA. All mice were treated with gentamicin 4 h after bacterial infection. In (C), total lung cells were plated for bacterial CFU before (−) and after (+) lysostaphin treatment, and the number of CFU was expressed per 100 cells. *P< 0.05, ***P< 0.001, t test. Data shown are representative of two independent experiments. (D) Numbers of live bacteria within AMs, Mo, and PMN isolated from WT and Ccr2−/− airways 24 h after super-challenge of PR8-infected WT and Ccr2−/− mice with 4×108 CFU MRSA. Cytospins of sorted cells were prepared and Diff-Quick stained (down panel). The number of CFU was expressed per 1000 cells for each phagocyte subset. Data shown were combined from three independent experiments. *P< 0.05, **P< 0.01, ***P< 0.001, paired t test.
Considering the differential antimicrobial capacity of macrophages and neutrophils (20, 21), we hypothesized that influenza-induced monocytes are not as potent in intracellular bacterial killing as neutrophils. To evaluate this possibility, we sorted out individual phagocyte subsets after coinfection by flow cytometry, and quantified the numbers of viable bacteria within (Fig. 6D). Indeed, on a per cell basis, monocytes contained ~15-fold more viable bacteria than neutrophils (Fig. 6D). This finding suggests that rather than facilitating bacterial clearance, phagocytosis by inflammatory monocytes protects S. aureus from more potent intracellular killing by neutrophils. Collectively, these results suggest that internalization by inflammatory monocytes enables S. aureus to escape from the bactericidal effect of antibiotics, thereby leading to increased intracellular bacterial survival during coinfection.
Inflammatory monocytes from influenza-infected mice are predominantly responsible for phagocytosis of S. aureus in vitro
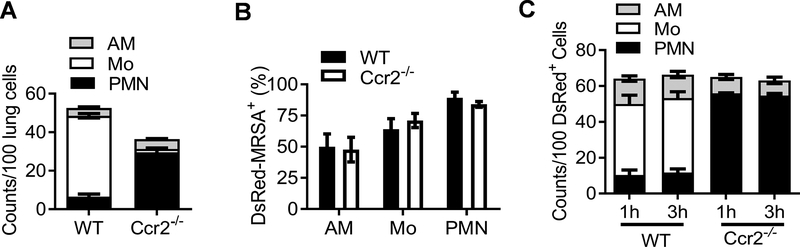
We next determined whether inflammatory monocytes have a regulatory effect on phagocytic bacterial killing in vitro. As expected, influenza infection primarily induced lung recruitment of monocytes in WT but neutrophils in Ccr2−/− mice (Fig. 7A). After in vitro incubation of single lung cells with DsRed-MRSA, the percentages of DsRed+ cells within each phagocyte population, including AM and neutrophils, were comparable between WT and Ccr2−/− mice, suggesting that CCR2 does not directly regulate the efficiency of bacterial uptake (Fig. 7B). Nonetheless, consistent with differential lung cell composition (Fig. 7A), inflammatory monocytes were the predominant DsRed-MRSA+ population in PR8-infected WT lung cells, whereas neutrophils were primarily responsible for bacterial uptake by Ccr2−/− lung cells in vitro (Fig. 7C).
Figure 7. Inflammatory monocytes are predominantly responsible for in vitro phagocytosis of S. aureus by influenza-induced lung cells.
(A) The relative numbers of AM, Mo and PMN in the single-cell suspensions prepared from PR8-infected WT and Ccr2−/− lungs. (B) The percentages and (C) relative numbers of DsRed+ AMs, Mo and PMN after co-incubation of WT and Ccr2−/− lung cells with DsRed-MRSA at MOI 5 for 1 (C) and 3 h at 37°C. In (A&C), the relative numbers were expressed as per 100 lung cells. Data (mean±SEM) shown are combined from three independent experiments.
CCR2 antagonist in conjunction with antibiotic treatment protects against lethal influenza and MRSA coinfection
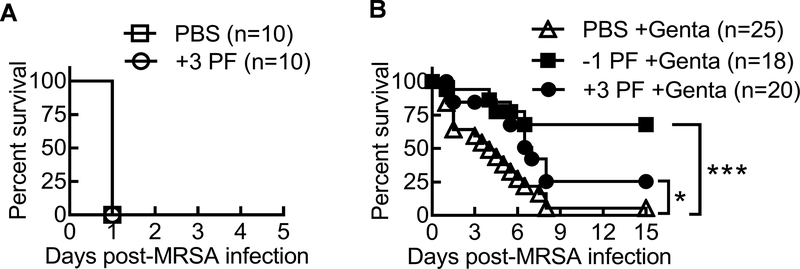
To investigate the practical application of our findings, we evaluated the effect of the CCR2 antagonist PF-04178903 (14), in conjunction with antibiotics for treatment of post-influenza MRSA infection. Initiating PF-04178903 treatment before or after influenza infection significantly reduced airway monocytes but did not alter neutrophil recruitment during coinfection (Fig. 8A–B). In addition, production of pro-inflammatory cytokines such as TNF-α and IFN-γ, was comparable between CCR2 antagonist or PBS-treated mice (Fig. 8C–D). Similar to reported findings in influenza infection models (14), pharmacologically blocking CCR2 did not change viral burden in coinfected mice (Fig. 8E). Nonetheless, lung bacterial burden significantly decreased in PF-04178903-treated mice (Fig. 8F). Although treatment with CCR2 antagonist alone was not sufficient to improve animal survival (Fig. 9A), in conjunction with antibiotic gentamicin, PF-04178903 significantly reduced the mortality rate of coinfected WT mice (Fig. 9B). Together, our results demonstrate that pharmacologically blocking monocyte recruitment enhances the bactericidal effect of antibiotics, and thereby improves host protection against otherwise lethal influenza and MRSA coinfection.
Figure 8. Pharmacologically blocking monocyte recruitment enhances the bactericidal effect of antibiotics after coinfection.
Mice were inoculated with PR8 and seven days later super-infected with 4×108 CFU MRSA. (A) Numbers of BALF inflammatory monocytes (Mo) and (B) neutrophils (PMN), (C-D) TNF-α and IFN-γ levels, (E-F) lung viral and bacterial burdens at 24 h after coinfection. Mice were treated twice daily with PBS or CCR2 antagonist PF-04178903 beginning one day before (−1 PF) or three days after (+3 PF) PR8 infection. All mice were treated with gentamicin 4 h after bacterial infection. P<0.05, one-way ANOVA; *P< 0.05, **P< 0.01, Mann Whitney test. Data shown are representative (A-E) or combined (F) of two independent experiments.
Figure 9. Antibiotic treatment in conjunction with CCR2 antagonist improves protection against coinfection.
Mice were inoculated with PR8 and seven days later super-infected with 4×108 CFU MRSA. (A) Survival of PR8/MRSA coinfected mice after treatments with PBS or PF-04178903 (+3 PF) beginning three days after PR8 infection. (B) Survival of PR8/MRSA coinfected mice after treatments with gentamicin (PBS+Genta), or PF-04178903 plus gentamicin beginning one day before (−1 PF+Genta) or three days after (+3 PF+Genta) PR8 infection. *P< 0.05, ***P< 0.001, log-rank test. Data shown were combined from 2–3 independent experiments.
Discussion
The effect of influenza-induced monocytes on lung antiviral defense has been well documented (9, 11, 13, 14). However, it is not clear how these inflammatory cells regulate lung defense against subsequent S. aureus infection. In this study, we selected this phagocytic cell population as a target to investigate influenza-induced resistance to antibiotic therapy during secondary S. aureus infection. CCR2 is required for monocyte emigration from the bone marrow to infiltrate afflicted tissues. As expected, we found that monocytes in Ccr2−/− mice had an impaired ability to accumulate in the lung following influenza infection. Importantly, Ccr2−/− mice exhibited improved protection against influenza/S. aureus coinfection after antibiotic treatment. Mechanistically, we show that inflammatory monocytes recruited during influenza infection serve as reservoirs to promote intracellular S. aureus survival from antibiotics, and thereby promote bacterial persistence in the lung. Together, our findings establish that influenza-induced monocytes significantly contribute to the resistance of secondary S. aureus infection to antibiotic therapy.
During respiratory bacterial infection alone, it has been shown in Ccl2−/− mice that lung exudate macrophage and conventional DC recruitment critically contributes to lung protective immunity against S. pneumoniae (22). However, we did not observe a significant effect of CCR2 on lung antibacterial defense during S. aureus infection alone. On the other hand, contradictory results have been reported regarding the overall impact of inflammatory monocytes on antiviral defense during primary influenza infection. Specifically, several reports demonstrated that CCR2-dependent recruitment of monocytes exacerbates lung injury after influenza infection (9, 11, 13, 14). As a result, CCR2 deficiency is associated with attenuated alveolar leakage and improved animal survival. On the other hand, the contribution of inflammatory monocytes to protective antiviral immunity is less clear. While some studies suggested a protective role of inflammatory monocytes in T cell-mediated viral clearance and animal survival, others found no effect (11, 23, 24). The discrepancy may be explained by differences in these infectious models, such as doses and strains of influenza virus, usage of Ccr2−/− or Ccl2−/− model, and different genetic backgrounds of mice (23, 25).
Of particular note, these previous findings were achieved in lethal influenza infection models. In our current coinfection model, after a low dose of PR8 infection, Ccr2−/− mice exhibited delayed viral clearance; however, influenza-infected both WT and Ccr2−/− mice exhibited 100% survival after a low dose of S. aureus super-infection (Supplemental Fig. 2). This observation implies that defective bacterial control and subsequent lung inflammation is the primary cause of animal death after a high dose of S. aureus super-infection (Supplemental Fig. 2C). Indeed, despite the delay in viral clearance, Ccr2−/− mice exhibited significantly improved bacterial clearance, and subsequently increased survival from influenza/S. aureus coinfection after antibiotic treatment.
It has been reported that CCR2 recruitment of monocytes increases lung injury during a low-dose of influenza infection (25). However, we found no evidence of CCR2-enhanced lung histopathology during influenza/MRSA coinfection (Supplemental Fig. 2D). Given the known detrimental contribution of neutrophils to inflammatory lung damage (26, 27), it is possible that the increases in neutrophils and viral loads offset the beneficial effects of reduced monocytes and bacterial burdens on lung pathology in Ccr2−/− mice. Therefore, although we provide evidence for the detrimental contribution of inflammatory monocytes to bacterial control, their direct impact on lung inflammatory damage during coinfection requires further study.
Investigations in influenza and bacterial coinfection models have identified multiple influenza-induced regulatory factors contributing to host susceptibility to secondary bacterial infection, typically by inhibition of phagocyte-mediated antibacterial immunity (28, 29). However, majority of these previous studies were accomplished in influenza/S. pneumoniae coinfection models (30–32). Unlike S. pneumoniae (33), phagocyte-mediated S. aureus clearance is mainly restricted by intracellular killing process. This is a possible reason why S. aureus vaccines targeting antibody-mediated opsonophagocytosis have been unsuccessful thus far, although S. aureus is considered to be an extracellular pathogen. In line with that, it has been shown that T cell-derived IFN-γ increases neutrophil recruitment that can actually perpetuate infection in a S. aureus wound infection model (34). In other words, in the absence of competent intracellular killing, bacterial uptake by phagocytes could actually lead to increased intracellular survival of S. aureus (35).
Resident AMs constitute the first line of defense against inhaled bacteria (36–38). Interestingly, our data showed that compared with bacterial infection alone, the MFI of DsRed-MRSA+ AMs significantly decreased after influenza/bacterial coinfection, suggesting that influenza infection inhibits bacterial phagocytosis by AMs (Fig. 3A–B). Nonetheless, due to intensive inflammatory monocyte and neutrophil recruitment, AMs only represented a minor population during influenza and S. aureus coinfection (Fig. 3C). Therefore, the direct contribution of AMs to S. aureus killing is limited in this lethal infection model.
Considering the relative antimicrobial capacity of each phagocyte subset, neutrophils are likely most important for phagocytic killing of S. aureus in the lung (39, 40). Indeed, on a per cell basis, AMs and monocytes in influenza-infected WT lungs contain 10-fold more viable bacteria than neutrophils (Fig. 6D), suggesting that they are not as effective as neutrophils in intracellular bacterial killing. On the other hand, Ly6C+ monocytes are the primary inflammatory cell population recruited into WT lungs after influenza infection, while neutrophils are predominantly recruited in Ccr2−/− mice (Fig. 7). Therefore, in the absence of antibiotic treatment, the diminished monocytes but increased neutrophils is likely the reason of improved bacterial control in Ccr2−/− mice during sublethal influenza/S. aureus coinfection (Supplemental Fig. 2A).
Our findings above were achieved with the aminoglycoside antibiotic gentamicin. We have also examined the therapeutic effect of vancomycin that is commonly applied in MRSA patients. Of note, the minimum inhibitory concentration (MIC) values of antibiotics for MRSA BAA-1695 are ≤ 12.5 μg/ml for gentamicin, and ≤ 1.17 μg/ml for vancomycin in vitro (15). Despite that, gentamicin exhibited advantageous bactericidal effect as compared with vancomycin in vivo, especially during MRSA single infection (Supplemental Fig. 3A). This is likely due to the limited ability of vancomycin to penetrate into lung tissue (41). On the other hand, although bacterial burdens were similar between coinfected WT and Ccr2−/− mice after vancomycin treatment, intracellular viable bacteria significantly decreased in Ccr2−/− lung cells (Supplemental Fig. 3B–C), in line with findings with gentamicin treatment (Supplemental Fig. 1C). Furthermore, compared with WT controls, Ccr2−/− mice exhibited significantly increased survival from coinfection after vancomycin treatment (Supplemental Fig. 3C).
To summarize, we show that inflammatory monocytes trafficking into influenza-infected lungs promote intracellular S. aureus survival from antibiotics and more potent neutrophils, and thereby impairing lung bacterial control by antibiotic therapy. At the same time, CCR2 deficiency increases viral loads, suggesting that CCR2-recruited monocytes/macrophages contribute to protective antiviral immunity. Importantly, pharmacologically blocking CCR2 improves bacterial control but does not affect viral clearance, and thereby significantly increases the therapeutic effect of antibiotics against influenza and S. aureus infection. Collectively, our findings in this study provide not only a mechanistic explanation for influenza-induced resistance of S. aureus to the bactericidal effect of antibiotics, but also experimental evidence for targeting monocytes as therapeutic intervention during influenza and S. aureus coinfection.
Supplementary Material
Acknowledgements
We would like to acknowledge Drs. Vinai Chittezham Thomas for providing DsRed-expressing S. aureus. We would also like to thank University of Nebraska Medical Center (UNMC) Flow Cytometry Research Facility for assistance with FACS analysis. The UNMC Flow Cytometry Research Facility is supported by state funds from the Nebraska Research Initiative (NRI) and The Fred and Pamela Buffett Cancer Center’s National Cancer Institute Cancer Support Grant.
Footnote: This work was supported by National Institutes of Health/National Heart, Lung, and Blood Institute R01 HL118408 to K.S.
References
- 1.Randolph AG, Vaughn F, Sullivan R, Rubinson L, Thompson BT, Yoon G, Smoot E, Rice TW, Loftis LL, Helfaer M, Doctor A, Paden M, Flori H, Babbitt C, Graciano AL, Gedeit R, Sanders RC, Giuliano JS, Zimmerman J, Uyeki TM, I. Pediatric Acute Lung, N. Sepsis Investigator’, L. the National Heart, and A. C. T. N. Blood Institute. 2011. Critically ill children during the 2009–2010 influenza pandemic in the United States. Pediatrics 128: e1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah NS, Greenberg JA, McNulty MC, Gregg KS, Riddell J. t., Mangino JE, Weber DM, Hebert CL, Marzec NS, Barron MA, Chaparro-Rojas F, Restrepo A, Hemmige V, Prasidthrathsint K, Cobb S, Herwaldt L, Raabe V, Cannavino CR, Hines AG, Bares SH, Antiporta PB, Scardina T, Patel U, Reid G, Mohazabnia P, Kachhdiya S, Le BM, Park CJ, Ostrowsky B, Robicsek A, Smith BA, Schied J, Bhatti MM, Mayer S, Sikka M, Murphy-Aguilu I, Patwari P, Abeles SR, Torriani FJ, Abbas Z, Toya S, Doktor K, Chakrabarti A, Doblecki-Lewis S, Looney DJ, and David MZ. 2016. Bacterial and viral co-infections complicating severe influenza: Incidence and impact among 507 U.S. patients, 2013–14. J Clin Virol 80: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams DJ, Hall M, Brogan TV, Farris RW, Myers AL, Newland JG, and Shah SS. 2011. Influenza coinfection and outcomes in children with complicated pneumonia. Arch Pediatr Adolesc Med 165: 506–512. [DOI] [PubMed] [Google Scholar]
- 4.Small CL, Shaler CR, McCormick S, Jeyanathan M, Damjanovic D, Brown EG, Arck P, Jordana M, Kaushic C, Ashkar AA, and Xing Z. 2010. Influenza infection leads to increased susceptibility to subsequent bacterial superinfection by impairing NK cell responses in the lung. J Immunol 184: 2048–2056. [DOI] [PubMed] [Google Scholar]
- 5.Kudva A, Scheller EV, Robinson KM, Crowe CR, Choi SM, Slight SR, Khader SA, Dubin PJ, Enelow RI, Kolls JK, and Alcorn JF. 2011. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol 186: 1666–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson KM, Lee B, Scheller EV, Mandalapu S, Enelow RI, Kolls JK, and Alcorn JF. 2015. The role of IL-27 in susceptibility to post-influenza Staphylococcus aureus pneumonia. Respir Res 16: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson KM, McHugh KJ, Mandalapu S, Clay ME, Lee B, Scheller EV, Enelow RI, Chan YR, Kolls JK, and Alcorn JF. 2014. Influenza A virus exacerbates Staphylococcus aureus pneumonia in mice by attenuating antimicrobial peptide production. J Infect Dis 209: 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson KM, Choi SM, McHugh KJ, Mandalapu S, Enelow RI, Kolls JK, and Alcorn JF. 2013. Influenza A exacerbates Staphylococcus aureus pneumonia by attenuating IL-1beta production in mice. J Immunol 191: 5153–5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin KL, Suzuki Y, Nakano H, Ramsburg E, and Gunn MD. 2008. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol 180: 2562–2572. [DOI] [PubMed] [Google Scholar]
- 10.Ellis GT, Davidson S, Crotta S, Branzk N, Papayannopoulos V, and Wack A. 2015. TRAIL+ monocytes and monocyte-related cells cause lung damage and thereby increase susceptibility to influenza-Streptococcus pneumoniae coinfection. EMBO Rep 16: 1203–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aldridge JR Jr., Moseley CE, Boltz DA, Negovetich NJ, Reynolds C, Franks J, Brown SA, Doherty PC, Webster RG, and Thomas PG. 2009. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci U S A 106: 5306–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serbina NV, Jia T, Hohl TM, and Pamer EG. 2008. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 26: 421–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herold S, Steinmueller M, von Wulffen W, Cakarova L, Pinto R, Pleschka S, Mack M, Kuziel WA, Corazza N, Brunner T, Seeger W, and Lohmeyer J. 2008. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med 205: 3065–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin KL, Sweeney S, Kang BD, Ramsburg E, and Gunn MD. 2011. CCR2-antagonist prophylaxis reduces pulmonary immune pathology and markedly improves survival during influenza infection. J Immunol 186: 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun K, Yajjala VK, Bauer C, Talmon GA, Fischer KJ, Kielian T, and Metzger DW. 2016. Nox2-derived oxidative stress results in inefficacy of antibiotics against post-influenza S. aureus pneumonia. J Exp Med 213: 1851–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun K, and Metzger DW. 2014. Influenza infection suppresses NADPH oxidase-dependent phagocytic bacterial clearance and enhances susceptibility to secondary methicillin-resistant Staphylococcus aureus infection. J Immunol 192: 3301–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yajjala VK, Thomas VC, Bauer C, Scherr TD, Fischer KJ, Fey PD, Bayles KW, Kielian T, and Sun K. 2016. Resistance to Acute Macrophage Killing Promotes Airway Fitness of Prevalent Community-Acquired Staphylococcus aureus Strains. J Immunol 196: 4196–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo SU, Kwon HJ, Ko HJ, Byun YH, Seong BL, Uematsu S, Akira S, and Kweon MN. 2011. Type I interferon signaling regulates Ly6C(hi) monocytes and neutrophils during acute viral pneumonia in mice. PLoS Pathog 7: e1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, and Gunn MD. 2009. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol 10: 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathan C, and Shiloh MU. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A 97: 8841–8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva MT, and Correia-Neves M. 2012. Neutrophils and macrophages: the main partners of phagocyte cell systems. Front Immunol 3: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winter C, Herbold W, Maus R, Langer F, Briles DE, Paton JC, Welte T, and Maus UA. 2009. Important role for CC chemokine ligand 2-dependent lung mononuclear phagocyte recruitment to inhibit sepsis in mice infected with Streptococcus pneumoniae. J Immunol 182: 4931–4937. [DOI] [PubMed] [Google Scholar]
- 23.Dawson TC, Beck MA, Kuziel WA, Henderson F, and Maeda N. 2000. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am J Pathol 156: 1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dessing MC, van der Sluijs KF, Florquin S, and van der Poll T. 2007. Monocyte chemoattractant protein 1 contributes to an adequate immune response in influenza pneumonia. Clin Immunol 125: 328–336. [DOI] [PubMed] [Google Scholar]
- 25.Wareing MD, Lyon A, Inglis C, Giannoni F, Charo I, and Sarawar SR. 2007. Chemokine regulation of the inflammatory response to a low-dose influenza infection in CCR2−/− mice. J Leukoc Biol 81: 793–801. [DOI] [PubMed] [Google Scholar]
- 26.Stifter SA, Bhattacharyya N, Pillay R, Florido M, Triccas JA, Britton WJ, and Feng CG. 2016. Functional Interplay between Type I and II Interferons Is Essential to Limit Influenza A Virus-Induced Tissue Inflammation. PLoS Pathog 12: e1005378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellani G, Messa C, Guerra L, Spagnolli E, Foti G, Patroniti N, Fumagalli R, Musch G, Fazio F, and Pesenti A. 2009. Lungs of patients with acute respiratory distress syndrome show diffuse inflammation in normally aerated regions: a [18F]-fluoro-2-deoxy-D-glucose PET/CT study. Crit Care Med 37: 2216–2222. [DOI] [PubMed] [Google Scholar]
- 28.Metzger DW, and Sun K. 2013. Immune dysfunction and bacterial coinfections following influenza. J Immunol 191: 2047–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker D 2017. Impact of Type I and III Interferons on Respiratory Superinfections Due to Multidrug-Resistant Pathogens. J Infect Dis 215: S58–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun K, and Metzger DW. 2008. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med 14: 558–564. [DOI] [PubMed] [Google Scholar]
- 31.Ghoneim HE, and McCullers JA. 2014. Adjunctive corticosteroid therapy improves lung immunopathology and survival during severe secondary pneumococcal pneumonia in mice. J Infect Dis 209: 1459–1468. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura S, Davis KM, and Weiser JN. 2011. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Invest 121: 3657–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bansal S, Yajjala VK, Bauer C, and Sun K. 2018. IL-1 Signaling Prevents Alveolar Macrophage Depletion during Influenza and Streptococcus pneumoniae Coinfection. J Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLoughlin RM, Lee JC, Kasper DL, and Tzianabos AO. 2008. IFN-gamma regulated chemokine production determines the outcome of Staphylococcus aureus infection. J Immunol 181: 1323–1332. [DOI] [PubMed] [Google Scholar]
- 35.Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, and Lindberg FP. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol 164: 3713–3722. [DOI] [PubMed] [Google Scholar]
- 36.Califano D, Furuya Y, and Metzger DW. 2018. Effects of Influenza on Alveolar Macrophage Viability Are Dependent on Mouse Genetic Strain. J Immunol 201: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith AM, and Smith AP. 2016. A Critical, Nonlinear Threshold Dictates Bacterial Invasion and Initial Kinetics During Influenza. Sci Rep 6: 38703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu M, Gibbons JG, DeLoid GM, Bedugnis AS, Thimmulappa RK, Biswal S, and Kobzik L. 2017. Immunomodulators targeting MARCO expression improve resistance to postinfluenza bacterial pneumonia. Am J Physiol Lung Cell Mol Physiol 313: L138–L153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson KM, Ramanan K, Clay ME, McHugh KJ, Rich HE, and Alcorn JF. 2018. Novel protective mechanism for interleukin-33 at the mucosal barrier during influenza-associated bacterial superinfection. Mucosal Immunol 11: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shepardson KM, Larson K, Morton RV, Prigge JR, Schmidt EE, Huber VC, and Rynda-Apple A. 2016. Differential Type I Interferon Signaling Is a Master Regulator of Susceptibility to Postinfluenza Bacterial Superinfection. MBio 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moise-Broder PA, Forrest A, Birmingham MC, and Schentag JJ. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 43: 925–942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.