Abstract
Catecholamine transmitters dopamine (DA) and norepinephrine (NE) regulate prefrontal cortical (PFC) circuit activity and PFC-mediated executive functions. Accordingly, pharmacological agents that influence catecholamine neurotransmission exert prominent effects on cognition. Many such agents are used clinically to treat attention disorders. For example, methylphenidate blocks DA and NE reuptake and is the leading choice for attention deficit hyperactivity disorder (ADHD) treatment. Recently, we have designed SK609 – a selective small molecule agonist of the DA D3 receptor (D3R). In this study, we further characterized SK609’s ability to selectively inhibit the reuptake of NE by NE transporters (NET). Our results indicate SK609 selectively inhibits NET with a Ki value of ~500nM and behaves as a NET substrate. Systemic dosing of SK609 (4 mg/kg; i.p.) in naïve rats produced a 300% and 160% increase in NE and DA, respectively, in the PFC as measured by microdialysis. Based on these neurochemical results, SK609 was tested in a PFC-dependent, visually-guided sustained attention task in naïve rats. SK609 improved performance in a dose-dependent manner with a classical inverted-U dose response function with a peak effect at 4 mg/kg. SK609’s peak effect was blocked by a pre-treatment with either the D2/D3R antagonist raclopride (0.05 mg/kg; i.p) or the alpha-1 adrenergic receptor antagonist prazosin (0.25 mg/kg; i.p), confirming a role for both DA and NE in promoting sustained attention. Additionally, SK609 improved sustained attention more prominently among low-performing animals. Doses of SK609 (2, 4, and 8 mg/kg) associated with cognitive enhancement did not produce an increase in spontaneous locomotor activity, suggesting a lack of side effects mediated by DA transporter (DAT) activity. These results demonstrate that the novel catecholaminergic modulator SK609 has the potential to treat sustained attention deficits without affecting DAT activity, distinguishing it from amphetamines and methylphenidate.
Introduction
The dopamine (DA) and norepinephrine (NE) transmitter systems have prominent projections to the prefrontal cortex (PFC) and influence PFC-dependent tasks. The PFC has extensive neuronal projections to cortical and subcortical structures positioning it in a prime location to regulate various cognitive control processes, such as sustained attention (Selemon & Goldman-Rakic, 1988; Szczepanski & Knight, 2014). Sustained attention is the process of readily detecting and responding to unpredictable yet relevant stimuli over a sustained period of time (Sarter, Givens, & Bruno, 2001). It has been demonstrated that lesions of the medial PFC in rodents impair sustained attention with observed reductions in performance accuracy (Kahn et al., 2012). Further, evaluation of neuronal activity within the anterior cingulate cortex of rats exhibited a correlation between firing rate and sustained attention (Wu et al., 2017). This cognitive function is impaired in several psychiatric and neurodegenerative disorders such as Alzheimer’s disease, schizophrenia, attention deficit hyperactivity disorder (ADHD), and Parkinson’s disease (Dimitrov, Grafman, Soares, & Clark, 1999; Koerts et al., 2010; Liu et al., 2002; Owen et al., 1992; Park, Hood, Shah, Fogg, & Wyatt, 2012; Sagvolden, 2011).
Current treatments for ADHD target catecholaminergic systems and, it has been shown that agents that modulate NE and DA in the PFC have therapeutic effects. Psychostimulants such as methylphenidate (MPH) and amphetamine exert their beneficial effects for ADHD treatment in patients and animal models through disruption of DA and NE reuptake (Bedard et al., 2015; Faraone, Biederman, & Roe, 2002; Kishikawa et al., 2014; Manos, Short, & Findling, 1999; Sagvolden, 2011). Additionally, atomoxetine, a selective reuptake inhibitor of the NE transporter (NET), and guanfacine, an alpha-2a receptor agonist, are successfully used to treat ADHD symptoms (Sikirica et al., 2013). Further, it has been demonstrated that the beneficial effects of MPH on sustained attention performance also involve alpha-1 adrenergic receptor activity (Berridge et al., 2012). The cognitive effects of MPH facilitate sustained attention by preferentially increasing catecholaminergic efflux in the PFC over other brain regions (Berridge et al., 2012; Chu, Shumsky, & Waterhouse, 2016). However, higher doses of MPH utilized in rodent self-administration studies increase surface expression of the DA transporter (DAT) and subsequently facilitate MPH potency in drug seeking behaviors (Calipari et al., 2014; Calipari, Ferris, Salahpour, Caron, & Jones, 2013). Hence, despite having relatively good therapeutic effects - existing treatments suffer from non-specific effects or potential abuse liability. Therefore, investigation into new cognitive enhancing catecholaminergic treatments that lack non-specific, deleterious effects is warranted.
DA receptors play an integral role in PFC-mediated cognitive tasks (Trantham-Davidson et al., 2014). Changes in D1 receptor (D1R) and D2 receptor (D2R) expression produce persistent alterations in cognitive function, particularly in sustained attention performance (Briand et al., 2008). Reductions in D4 receptor (D4R) expression impaired attentional performance in the 5-choice serial reaction time task (Young, Powell, Scott, Zhou, & Geyer, 2011). Further, treatment with a D4R agonist improved sustained attention performance in low attentive rodents (Tomlinson et al., 2015). The D3 receptor (D3R) has specifically been implicated in cognition, with modulation of D3R resulting in alterations in cognition in both healthy individuals and those suffering from neuropsychiatric disorders (Nakajima et al., 2013). Administration of either a D3R agonist (7-OH-PIPAT) or antagonist (nafadotride) increased reaction time and decreased accuracy in rodents performing a sustained attention task (Bari & Robbins, 2013). Further, the D2/D3R antagonist sulpiride ameliorated attentional impairments observed in PFC-lesioned rats (Pezze, Dalley, & Robbins, 2009).
Based on these evidence, it is clear that modulation of both DA and NE can improve performance in sustained attention tasks. However, inhibition of DAT function presents a risk for abuse liability that may outweigh therapeutic efficacy. To overcome this problem, we have developed a unique approach to pharmacologically combine the effects of D3R agonism with NET inhibition. We recently designed and characterized a novel series of selective, biased D3R agonists (Xu et al., 2017). SK609, the lead molecule of this series, effectively improved motor deficits seen in hemi-parkinsonian rats while demonstrating efficacious brain penetrability (Simms, Huettner, & Kortagere, 2016). In the present study, we examined the effects of SK609 on monoamine transporters and in a rodent visual discrimination task of sustained attention.
MATERIALS AND METHODS
Drugs
SK609 was synthesized in house as previously described (Xu et al., 2017). Raclopride, prazosin, and methylphenidate were purchased from Sigma Aldrich (St. Louis, Missouri). All drugs were dissolved in 0.9% sterile saline solution and prepared 10 minutes prior to injection.
Uptake experiment
Uptake experiments were performed two days after transfecting COS-7 cells with cDNAs for hSERT, hNET, and hDAT and plating the cells in 96-well plates. Using an automatic plate washer, cells were washed with room temperature phosphate buffered saline containing 0.1 mM CaCl2 and 1 mM MgCl2 (PBSCM). The cells were pre-incubated for 5 min with varying concentrations of SK609 at room temperature. The uptake assays were initiated by the addition of 100 nM tritiated substrate (5HT, NE, or DA) and incubated for 10 min. Uptake was terminated by two washes and cells were solubilized in scintillation liquid cocktail. Accumulated radiolabel was determined using liquid scintillation counting. All assays were performed as four independent experiments and each experiment was performed in quadruplicate repeats. The concentration-curves generated were fitted to a Hill’s equation by non-linear regression analysis using GraphPad Prism (version 5.01) to determine the IC50 value. Ki values were calculated from IC50 values using the Cheng-Prusoff equation (Cheng and Prusoff, 1973) and are denoted as mean ± standard error of the mean from four independent experiments.
Efflux experiment
COS-7 cells were transfected as above and plated in 24-well plates. Efflux experiments were performed two days after cell transfection. Cells were washed with room temperature phosphate buffered saline supplemented with 0.1 mM CaCl2 and 1 mM MgCl2 (PBSCM) and then loaded with 50 nM tritiated substrate (5HT, NE, or DA) for 1 h. After pre-loading, cells were washed twice with buffer and then incubated for 10 min with substrates (10 µM), SK609 (50 µM), or a known non-transported inhibitor of the respective transporters, desipramine (1 µM) (SERT and NET) or cocaine (100 µM) (DAT), at room temperature. After the incubation, the supernatant was collected, liquid scintillation added, and accumulated radiolabel was determined using liquid scintillation counting. The cells were solubilized in a solution of 0.1% SDS and 0.1 M NaOH, liquid scintillation was added, and radiolabel determined as above. All assays were performed as four independent experiments and each experiment was performed in quadruplicate repeats. To calculate efflux, the supernatant counts were divided by the total (sum of supernatant and remaining cellular radioactivity).
Animals
Care and use of all laboratory animals followed NIH guidelines and all experimental procedures were approved by the Drexel University Institutional Animal Care and Use Committee (IACUC #20595). Male Sprague Dawley rats were housed in standard ventilated cages. Animals were maintained on a 12 h light/dark cycle (lights on at 7AM) and experimental sessions occurred between the hours of 9AM and 12PM. For microdialysis, twelve male Sprague Dawley rats (300–400g, Taconic) were housed by pairs and maintained on food and water ad libitum. For sustained attention, singly housed animals (n=16, 450–550g, Taconic) were maintained on food ad libitum. Each rate received access to one water bottle for 10 min/day, reducing animals to 90% ad libitum body weight as previously described (Chu et al., 2016). An additional cohort of rodents (n=8, 250–350g, Taconic) was used for locomotor experiments. This group of animals were house in pairs and received free access to food and water.
Microdialysis
Microdialysis surgeries were performed as previously described (Brodnik, Bongiovanni, Double, & Jaskiw, 2012; Brodnik, Double, & Jaskiw, 2013). Rats were anesthetized with 4% isoflurane, weighed, shaved, and then placed in a stereotaxic frame where anesthesia was maintained via 2% isoflurane. A guide cannula and a microdialysis probe (PAN 30 kDa, MWCO, 320-µm OD, 4-mm active membrane; Bioanalytical Science) were surgically implanted into the medial PFC (probe terminating: ML ±0.70 mm, AP +3.20 mm, DV −7.00 mm; relative to bregma). One probe was implanted in each animal with the side (L vs. R) alternating between rats. Following implantation, the probe was connected to polyethylene tubing (0.965-mm OD, 0.58-mm ID; length, 2 m), a swivel, and a perfusion pump. Each rat was then placed singly in a Plexiglas experimental chamber (14×14×18) with hardwood shavings and food and water ad libitum.
All microdialysis experiments were conducted during the rats’ light cycle between 10:30AM and 4:30PM. On the morning of experimentation, microdialysis probes were first flushed at a perfusion rate of 10 µl/min for 15 min and then perfused at 1 µl/min for the duration of experimentation. Probes were perfused at 1 µl/min for at least 2 hr before the collection of baseline samples. Samples were collected every 30 min, and three baseline samples were collected before vehicle or SK609 (4 mg/kg; i.p.) injections.
The microdialysates (22 µl) were analyzed with HPLC coupled with electrochemical detection according to Brodnik and Jaskiw (2015). Separation was achieved using a 100×3.0-mm reversed-phase C18 column with 3-µm particles (Phenominex Luna C-18(2), CA, USA). The mobile phase consisted of 12.5 mM citrate, 20.0 mM acetate, and 0.1 mM EDTA, with 10 % (v/v) acetonitrile adjusted to pH 4.5 with sodium hydroxide and 2.0–4.0 mM octylsulfonic acid. The mobile phase was pumped at a rate of 0.5 mL/min. NE and DA were measured using an electrochemical detector with a glassy carbon electrode and maintained at a relative potential of 0.60 V to an Ag/AgCl reference electrode (Antex Decade Elite, NV, The Netherlands). The detection limit for both NE and DA was 50 fg/10 µl at a 3:1 signal to noise ratio.
Sustained Attention
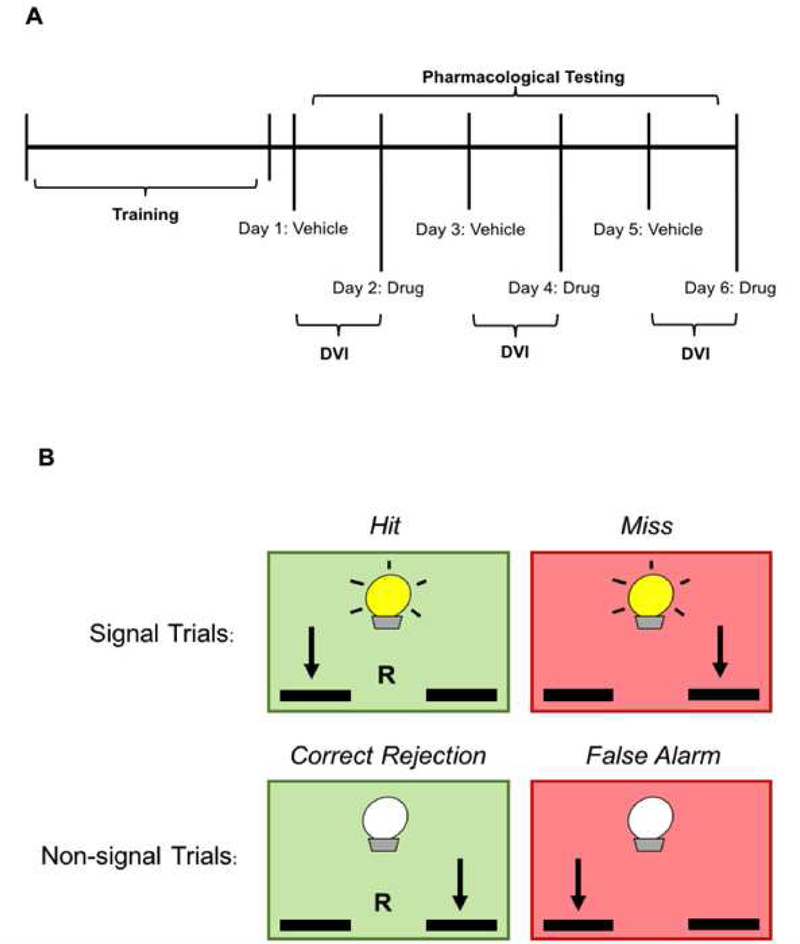
The sustained attention task was performed as previously described using a sound attenuated operant chamber (Med Associates Inc.) (Berridge et al., 2006). The experimental design for this behavior task is demonstrated in Figure 1A. Briefly, animals were water restricted while food was provided ad libitum. Animals were trained to press a lever in response to a stimulus cue light during the signal trials or press the opposite lever in the absence of a cue light during the non-signal trials. Five phases of training occurred over the course of 2–3 months (Table 1). Pharmacological studies commenced once the rodents successfully completed phase 5. Task performance is demonstrated in Figure 1B. Correct responses in signal trials were designated as hits; correct responses in non-signal trials were classified as correct rejections. Both these correct responses yielded a water reward. Incorrect responses during the signal or non-signal trials were categorized as a miss or false alarm, respectively, and did not result in a reward. Failure to respond to the levers after 5 sec resulted in an omission, retraction of the levers, and a 5 sec timeout period of darkness. The signal trial length was variable, randomly selected from the list: 0.125, 0.25, 0.5, 0.75, 1.0, 1.5, and 2.0-sec with replacement as described in Berridge et al (Berridge et al., 2012). A variable inter-trial interval of 13-sec (minimum 5-sec), was adapted before the start of new trial. Trials with no response did not occur on a regular basis and if they did the data was excluded from the analyses. Performance was determined based on an animal’s Vigilance Index (VI), which is a relative measure of stimulus detectability and is calculated as:
This formula derives from the sensitivity index calculation commonly used in signal detection theory (Sahgal, 1988). Performance as measured by VI assesses the relative number of hits and misses, as opposed to the probability measured by signal sensitivity. Therefore, this equation has been modified to yield a score that does not involve errors of omission (McGaughy & Sarter, 1995). Behavioral sessions were 46 min, including one min of habituation. Trials commenced after 60 sec and the two trial types were presented to animals in a pseudo-randomized order. Animals were also equally presented with both signal and non-signal trials. Upon completion of training, animals were administered various doses of SK609 (2–20 mg/kg; i.p), raclopride (0.05 mg/kg; i.p), MPH (2 mg/kg; i.p), prazosin (0.25 mg/kg; i.p), and vehicle, or a combination of these drugs 10 min prior to the commencement of trials. All animals received all the drugs but with wash out days between drug treatments. This design was previously adapted and well tested by others and we used similar design to reduce the number of animals and reuse well trained animals since it takes ~3 months to establish a stable baseline in this behavioral task. Experimental sessions occurred on consecutive days, but drug treatments were administered on alternate days to avoid any influence of previous exposure. Vehicle treatments were performed between drug exposures to maintain baseline conditions. Due to the variability in individual and daily VI scores, performance was measured based on difference VI, defined as the difference in drug-induced VI score from the previous day’s vehicle VI score. In addition, each dose or combination drug treatment was tested in three independent trials (Figure 1A). For drug combination studies, animals were given two-week washout period between drug treatment regimens because rats were required to establish new performance baselines to avoid any residual effects of antagonists. Positive values were therefore characterized as an increase in performance, while negative values reflected a decrease.
Figure 1. Sustained attention experimental and operant paradigm.
(A) After animals were trained to criteria they underwent three independent trials of systemic drug testing (SK609 – 2, 4, 6, 8 10, 20 mg/kg; MPH, 2 mg/kg; raclopride, 0.05 mg/kg; prazosin, 0.25 mg/kg; or a combination of treatments administered i.p.). These trials consisted of three vehicle treatments each followed by the administration of a drug the next day, facilitating a six-day treatment schedule per drug. Sustained attention performance was defined by either the Vigilance Index (VI), which corresponds to scores obtained on drug testing days (Days 2, 4, 6), or by Difference in VI (DVI), which was calculated as the difference between drug and vehicle testing days (i.e. Day 2 – Day 1). (B) Illustration of rules corresponding to each type of sustained attention response. Signal trials are indicated by a cue light in the top row, whereas non-signal trials are demonstrated on the bottom row without an illumination of the cue light. Correct responses to either signal or non-signal trials are characterized by green boxes, and red boxes signify incorrect responses during both trial types. Arrows and ‘R’ represent lever presses and water rewards, respectively.
Table 1: Training phases utilized for sustained attention performance.
Criteria for each behavioral phase during sustained attention training. Animal commences experimental trials after completion of phase 5.
| Phase | Criteria to advance in phases |
|---|---|
| 1 | 30 presses 2 days in a row |
| 2 | 200 presses 2 days in a row |
| 3 | ≥ 75% accuracy on both signal and non-signal trials 3 days in a row |
| 4 | Signal and non-signal responses ≥ 59%, omissions ≤ 35%, and VI score ≥ 0.35 |
| 5 | Signal and non-signal responses ≥ 59%, omissions ≤ 35%, and VI score ≥ 0.35 |
Locomotor Activity
Locomotor activity sessions were measured using an open field Plexiglas chamber whose subject field measured 17” wide x 17” long x 12” high (Med Associates Inc.) and consisted of 32 infrared photo beams (16X & 16Y) that recorded ambulatory count, distance traveled, and vertical count. Ambulatory count was defined as the number of beam breaks independent of angle, whereas distance traveled reflected differences in angular movements. Vertical count measured the number of times the animal reared. Locomotor behavior was recorded using Activity Monitor Version 5 software. Experimental sessions were 60 min and started 10 min post-vehicle or SK609 (2, 4, and 8 mg/kg; i.p.) treatment which was performed according to a latin square design. Each drug concentration was repeated in triplicates, with a 24 h wash out period of vehicle treatment between doses of SK609.
Data Analyses
Statistical analyses for the uptake and efflux assays included one-way analyses of variance (ANOVA). Behavioral effects of various drug treatments during the sustained attention task were analyzed with either one-way or two-way ANOVAs as well as repeated measures ANOVAs where applicable. All ANOVAs were followed by Bonferroni post-hoc analysis for multiple comparisons and all data are presented as mean ± s.e.m. If the variance from repeated measures ANOVA was non-homogenous,we have used a non-parametric approach of Wilcoxon signed ranks test for planned comparisons. Outliers in any of the behavioral assays were removed using the Grubbs outlier test (Jain, 2010). Details of specific statistical methods and comparisons performed are listed in the figure legends.
RESULTS
SK609 demonstrates selectivity for NET
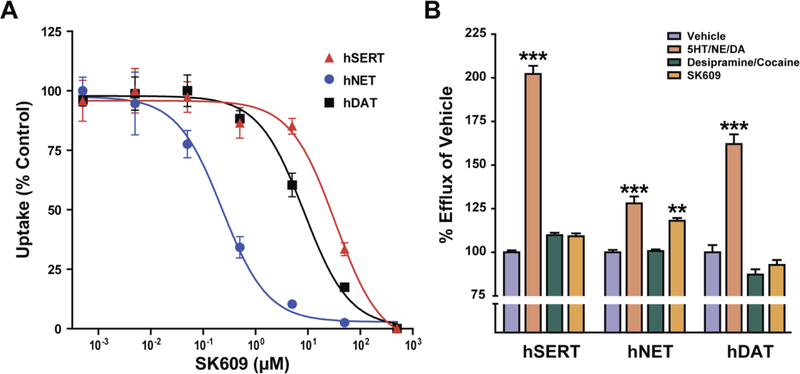
SK609 was screened against hDAT, hNET, and hSERT using uptake and efflux assays in COS-7 cells overexpressing each of the three transporters. In uptake assays, SK609 selectively inhibited hNET (Ki value: 0.57±0.11 µM) and demonstrated weak inhibitory effects on hDAT (Ki value: 5.7±1.3 µM) and hSERT (Ki value: 24.0±4.9 µM). SK609’s effect on hNET significantly differed from its effects on both hDAT and hSERT (Figure 2A). In efflux assays, endogenous substrates (DA, 5HT, and NE; 10 uM) robustly elicited efflux, whereas known non-transported inhibitors (desipramine, 1 uM; cocaine, 100 uM) did not elicit efflux. SK609 (50 uM) triggered hNET efflux (release) comparable to its endogenous substrate NE (Figure 2B) but did not trigger efflux in hDAT or hSERT (Figure 2B). This indicates that SK609 is a selective inhibitor and substrate of hNET and a non-transported inhibitor of hSERT and hDAT at higher concentrations of the compound.
Figure 2. Effect of SK609 on monoamine transporter function.
SK609 was screened for inhibitory and substrate effects in COS-7 cells expressing either hSERT, hDAT or hNET transporters (A) One way ANOVA revealed a significant effect of SK609 on uptake inhibition expressed as area under the curve (F(2,11)=201.14, p=0.00000003). Post hoc analysis indicated that SK609 effects on uptake inhibition for hNET were significantly different from hSERT (p = 0.00000003; 95% CI [5040.33, 6810.16]) and hDAT(p = 0.0004; 95% CI [5040.33, 6810.16]). These results suggest SK609 is a selective inhibitor of hNET when compared to hDAT and hSERT (B) Individual one-way ANOVAS for the three transporters indicated significant effects of treatments for hSERT (F(3,11)=354.01; p = 0.000000008) hNET (F(3,11)=36.19; p = 0.000053), and hDAT (F(3,11)=74.49; p = 0.000003). Post-hoc analyses using Dunnett’s tests relative to vehicle treatment indicate that 10uM substrate significantly elicited efflux from their respective transporters compared to vehicle (5HT: p = 0.00000005; 95% CI[91.82, 112.62]: NE: p = 0.000064; 95% CI[18.72, 37.29]:; DA: p = 0.000012; 95% CI[45.64, 78.44]). Further, SK609 (50 µM) acted as a substrate of hNET, eliciting significant efflux from hNET (p = 0.001319; 95% CI[8.79, 27.36] but not hSERT (p = 0.062968; 95% CI[−0.56, 20.24]:or hDAT (p = 0.479; 95% CI[−23.66, 9.14]). Data represents mean ± s.e.m. of three independent experiments: **p<0.01, ***p<0.001.
SK609 improves sustained attention by decreasing incorrect responses
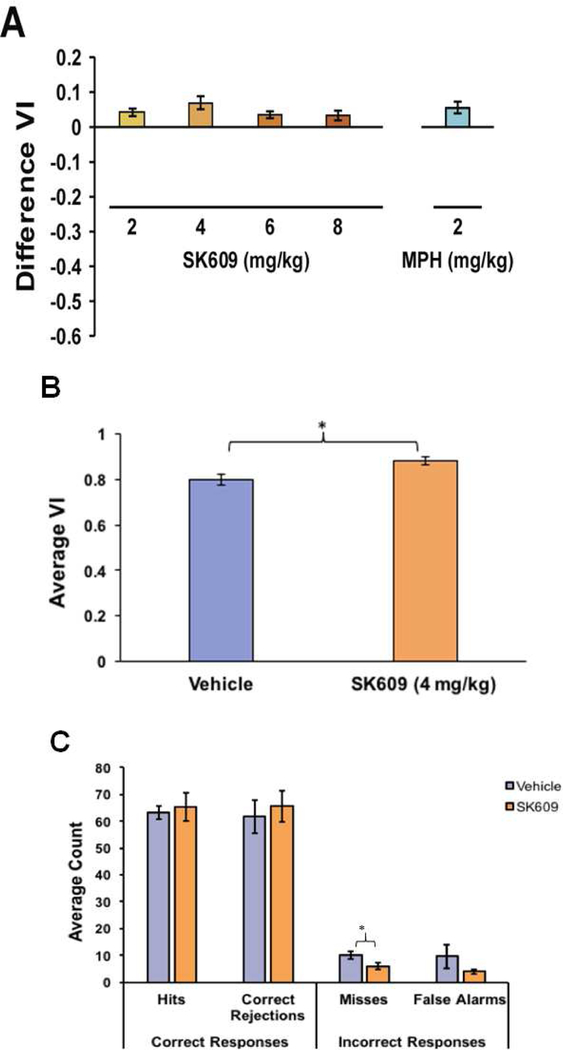
Dose-dependent effects of SK609 (2–8 mg/kg; i.p) on sustained attention were assessed in naïve rats (n=16). A therapeutic dose of MPH (2mg/kg; i.p) was used as a positive control and 0.9% saline was used as vehicle control. Vehicle treatment was used to calculate the difference in VI (see Methods section for details). This study demonstrated that SK609 improved sustained attention with an inverted-U shaped dose response similar to MPH although repeated measures ANOVA did not reveal a significant effect of the various doses of SK609 on Difference VI (Figure 3A). Paired t-test indicated that 4 mg/kg SK609 significantly increased sustained attention performance as measured by average VI score relative to vehicle treatment (Figure 3B). Paired t-tests also revealed that SK609 (4 mg/kg; i.p.) specifically affected the selection of incorrect answers, significantly reducing the average number of executed misses compared to vehicle conditions in the operant task (Figure 3C).
Figure 3. SK609 improved sustained attention by decreasing incorrect responses.
(A) Difference VI measurements calculated against previous day vehicle performance in rats (n=16) showed SK609 improved sustained attention performance and produced an inverted dose response with peak effect at 4mg/kg which was comparable to MPH’s (2mg/kg) performance in the assay. Repeated measures ANOVA did not reveal a significant effect of SK609 on Difference VI (F(3,42) = 0.923, p = 0.438). The effects of MPH are shown for comparison, but no statistics were conducted on this group. (B) Paired t-test indicated that 4 mg/kg SK609 significantly increased sustained attention performance as measured by average VI score relative to vehicle treatment (t(7)=3.1, p = 0.017; 95% CI[0.14, 0.19]). (C) Paired t-tests revealed that SK609 (4 mg/kg; i.p.) specifically affected the selection of incorrect answers, significantly reducing the average number of executed misses compared to vehicle conditions (t(6)=3.27, p = 0.017; 95% CI[1.02, 7.11]). Data represents mean ± s.e.m. of three independent trials: (*p<0.05).
Cognitive enhancing effects of SK609 require DA and NE activity
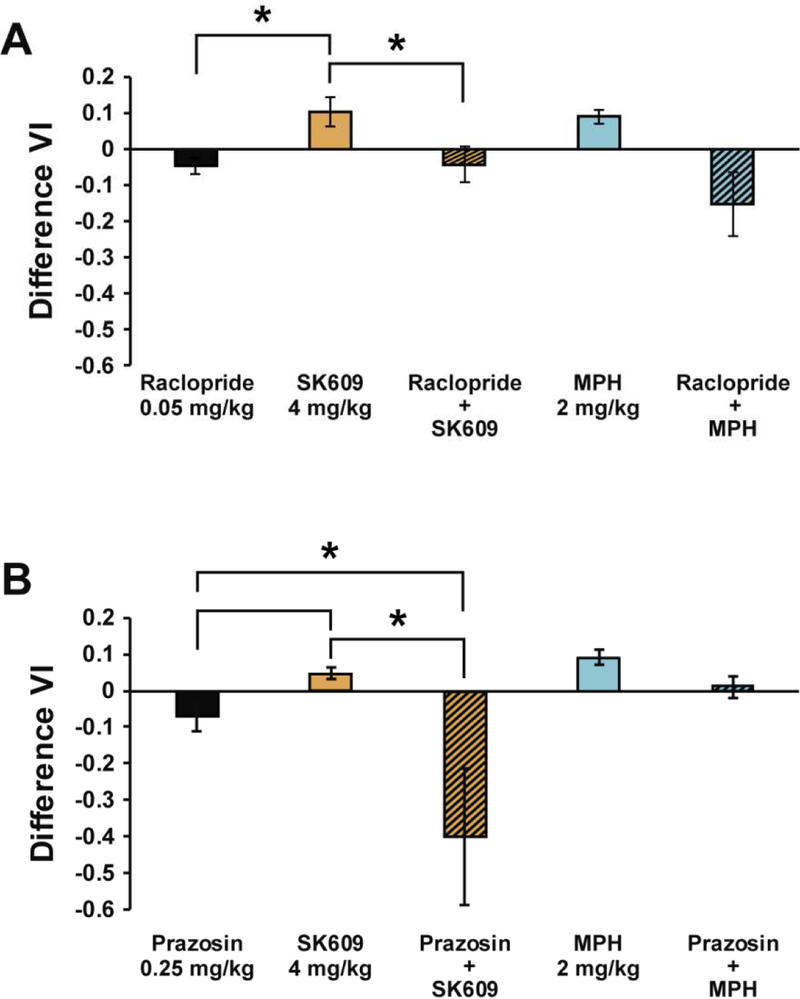
To determine DA and NE contributions in SK609-induced improvement in sustained attention performance, the cognitive effects of the D2/D3R antagonist raclopride and alpha1-adrenergic receptor antagonist prazosin were evaluated. One-way repeated measures ANOVA indicated a significant of drug treatments wherein SK609 (4 mg/kg; i.p) and MPH (2 mg/kg; i.p) improved performance, raclopride (0.05 mg/kg; i.p) and prazosin (0.25 mg/kg; i.p) impaired performance. In addition, Wilcoxon signed rank post hoc tests across select comparisons indicated that the effects of D2/D3 antagonist raclopride pretreatment significantly blocked the effects of SK609 on performance but had no significant effect on MPH (Figure 4A). Further, prazosin pretreatment significantly reduced the performance of animals and blocked the effects of SK609 (Figure 4B). Results from both these agonist-antagonist combination studies suggest that SK609 mediated improvement in sustained attention may be mediated by both DA and NE.
Figure 4. SK609-induced attentional improvement is blocked by D2/D3R or alpha-1 adrenergic receptor antagonists.
Rats were treated with SK709 (4mg/kg), raclopride (0.05mg/kg), MPH (2mg/kg), prazosin (0.25mg/kg) or a combination of SK609 with raclopride or prazosin or MPH with raclopride or prazosin and their effects on sustained attention performance was evaluated as difference VI measurements (see methods for details). (A) One-way repeated measures ANOVA indicated a significant of drug (F(4,24)=3.891, p=0.014 corrected for non-homogeneity of variance using Huyn-Feldt correction). Wilcoxon signed rank post hoc tests across select comparisons indicated that the effects of D2/D3 antagonist raclopride significantly differed from SK609 on Difference VI (p = 0.018) and that raclopride pretreatment significantly blocked the effects of SK609 on Difference VI (p = 0.043). No significant differences were observed when comparing the effects of raclopride vs raclopride pretreatment with SK609 (p = 0.735), raclopride vs methylphenidate (p = 0.128), methylphenidate vs raclopride pretreatment with methylphenidate (p = 0.128), or raclopride vs raclopride pretreatment with methylphenidate (p = 0.237). (B) One-way repeated measures ANOVA indicated a significant of drug (F(4,24)=5.312, p=0.048 corrected for non-homogeneity of variance using Huyn-Feldt correction). Wilcoxon signed rank post-hoc tests across select comparisons indicated that the effects of alpha 1 antagonist prazosin significantly differed from SK609 (p = 0.018) and methylphenidate (p = 0.028) on Difference VI and that prazosin pretreatment significantly blocked the effects of SK609 (p = 0.028). No significant differences were observed when comparing the effects of prazosin vs prazosin pretreatment with SK609 (p = 0.108), methylphenidate vs prazosin pretreatment with methylphenidate (p = 0.206), or prazosin vs prazosin pretreatment with methylphenidate (p = 0.063). Data represent mean ± s.e.m: *p<0.05.
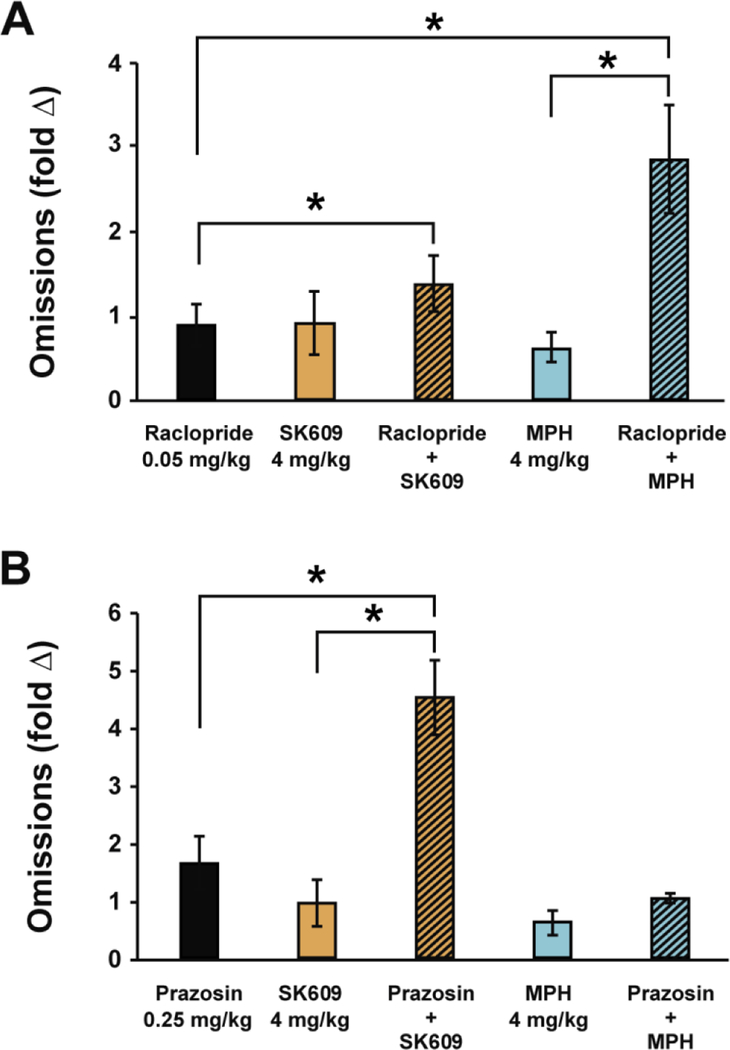
SK609 does not affect omissions
Omissions, or the failure to select a lever within 5 sec of its presentation, were also measured for all drug treatments (Figure 5). The results suggest that the drug treatments do not individually have any statistically significant changes in omissions when compared to baseline omissions. However, Wilcoxon signed rank post-hoc tests across select comparisons showed treatment combinations of raclopride with either SK609 or MPH increased omissions when compared to SK609 or MPH treatments respectively. Similarly, prazosin pretreatment with SK609 increased omissions when compared to SK609 but prazosin pretreatment had no effect on omissions when combined with MPH in the sustained attention task.
Figure 5. SK609 does not increase omissions.
Errors of omissions executed after various drug treatments (n=7/gp) were analyzed against the baseline omissions and plotted as fold change against baseline omissions (A) One-way repeated measures ANOVA indicated a significant of drug (F(4,24)= 8.05, p= 0.0026 corrected for non-homogeneity of variance using Huyn-Feldt correction). Wilcoxon signed rank post-hoc tests across select comparisons indicated that the effects of raclopride did not significantly differ from SK609 on Omissions (p = 0.866) nor did raclopride pretreatment with SK609 vs SK609 (p = 0.176). However, the effects of raclopride vs raclopride pretreatment with SK609 did significantly differ on Omissions (p = 0.018). Although raclopride vs methylphenidate (p = 0.310) did not differ on Omissions, methylphenidate vs raclopride pretreatment with methylphenidate (p = 0.018) and raclopride vs raclopride pretreatment with methylphenidate (p = 0.018) both significantly differed on Omissions. (B) One-way repeated measures ANOVA indicated a significant of drug (F(4,24)=15.582, p= 0.00087) corrected for non-homogeneity of variance using Huyn-Feldt correction). Wilcoxon signed rank post-hoc tests across select comparisons indicated that the effects of the alpha 1 antagonist prazosin did not differ from SK609 (p = 0.310), but that SK609 vs prazosin pretreatment with SK609 (p = 0.028) and prazosin vs prazosin pretreatment with SK609 did significantly differ on Omissions (p = 0.018). No significant differences were observed when comparing the effects of prazosin vs methylphenidate (p = 0.128), methylphenidate vs prazosin pretreatment with methylphenidate (p = 0.128), or prazosin vs prazosin pretreatment with methylphenidate (p = 0.499). Data represents mean ± s.e.m. of three independent trials: *p<0.05.
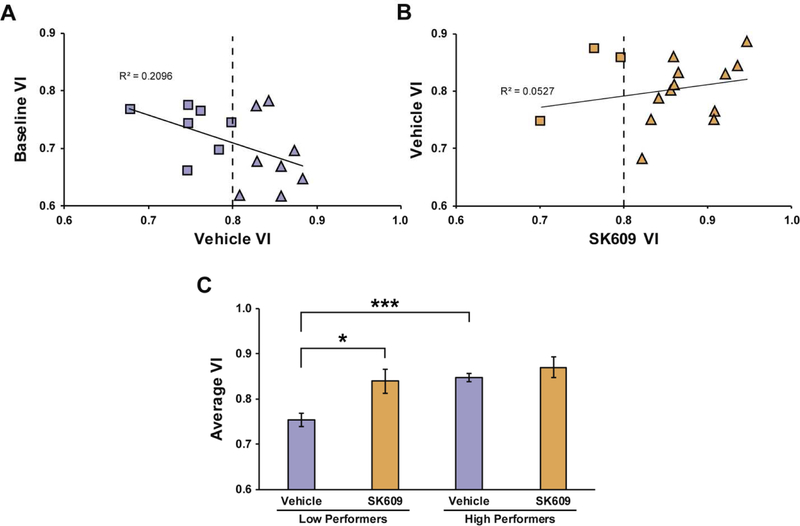
SK609-induced cognitive enhancement depends on baseline performance
Correlating baseline and vehicle treatment performance demonstrated that animals could be classified as low or high performers as defined by a median split VI value of 0.8 and a R2 value of 0.20 (Figure 6A). Following SK609 (4 mg/kg; i.p.) treatment, low performers exhibited an improvement in attentional performance that re-categorized 5 of these animals as high performers (Figure 6B). Planned comparisons using paired or independent samples t-tests demonstrated that low performers displayed a significant cognitive deficit compared to high performers under vehicle conditions but this difference in performance was not observed between low and high performers following 4mg/kg SK609. Thus SK609-induced changes in performance were unique to low performers, and had no effect on high performers (Figure 6C).
Figure 6. SK609 significantly improved sustained attention performance in low performing animals.
(A) Correlation of VI values between performance under baseline conditions and vehicle treatment (n=15). Using a median cut-off of 0.8, animals were grouped as low performers (squares; n=7) and high performers (triangles; n=8) (R2=0.209). (B) Correlation between VI values from vehicle and SK609 (4 mg/kg; i.p.) treatments displayed a shift from low to high performer status among 5 of 7 low performing animals (R2=0.052). (C) Two-way ANOVA with performance as the between subjects variable and treatment as the within subjects variable indicated a significant effect of performance (F(1,13)=9.995, p=0.0075), a significant effect of treatment (F(1,13)=8.339, p=0.0127) but no significant performance x treatment interaction (F(1,13)=2.884, p=0.1132) on sustained attention performance. Planned comparisons using paired or independent samples t-tests demonstrated that low performers displayed a significant cognitive deficit compared to high performers under vehicle conditions (p 0.00007, 95% CI[−0.12, −0.05]) but this difference in performance was not observed between low and high performers following 4mg/kg SK609 (p = 0.399, 95% CI[−0.11, 0.04]). This difference between SK609 effects on performance was due to SK609 significantly increasing Difference VI in low performers (p = 0.0196, 95% CI[−0.15, −0.02]) but not high performers (p=0.4148, 95% CI[−0.08, 0.04]). Data represent mean ± s.e.m. of three independent trials: *p<0.05, ***p<0.001.
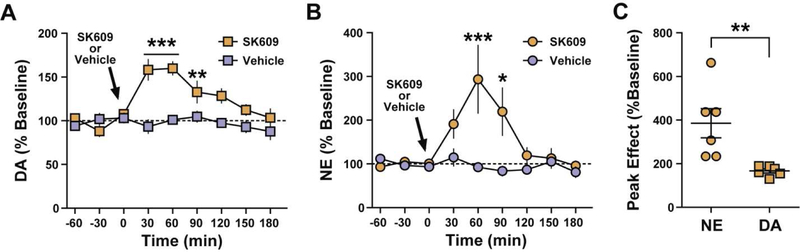
SK609 increases catecholaminergic efflux in the PFC
We used microdialysis to examine whether SK609-induced enhancement of sustained attention may be driven by elevations in extracellular catecholamine in the PFC. A two-way ANOVA, with time as a repeated measure and treatment as a between subjects’ measure, revealed that SK609 (4 mg/kg) elevated DA levels to ~160% of baseline (Figure 7A; n=6/group) and NE levels to approximately 300% of baseline (Figure 7B; n=6/group), which significantly differed from vehicle treatment. SK609 also produced a larger peak effect on NE than on DA (Figure 7C).
Figure 7. SK609 increases catecholaminergic efflux in the PFC.
(A) Two-way ANOVA with treatment as the between subjects variable and time as the repeated measures variable revealed a significant effect of treatment (F(1,8)=30.32, p=0.00057), time (F(6,48)=7.95, p=0.00000056) and treatment x time Interaction (F(6,48)=8.85, p=0.0000016) on extracellular DA levels in PFC. Bonferroni post-hoc analyses revealed that SK609 significantly increased DA levels at the 30 (p = 0.000000087, %95 CI[−100.1, −40.98]), 60 (p = 0.0000076, 95% CI[−87.50, - 28.37]) and 90 min (p = 0.0075, 95% CI[−66.11, −6.981]) time points relative to vehicle. (B) Two-way ANOVA with treatment as the between subjects variable and time as the repeated measures variable revealed a significant effect of treatment (F(1,9)=9.24, p=0.014), Time (F(6,54)=3.14, p=0.0102) and treatment x time Interaction (F(6,54)=3.60, p=0.0045) on extracellular NE levels in PFC. Bonferroni post-hoc analyses revealed that SK609 significantly increased NE levels at the 60 (p = 0.0002, %95 CI[−325.3, −79.83]) and 90 min (p = 0.0217, 95% CI[−258.7, - 13.29]) time points relative to vehicle. (C) Independent samples t-test revealed that increases in extracellular NE levels following SK-609 were significantly greater than increases in DA levels (t(10) = 3.24, p = 0.0089; 95% CI [68.30, 369.1]). Results are expressed as a percent of baseline. Data represents mean ± s.e.m: *p<0.05, **p<0.01, ***p<0.001.
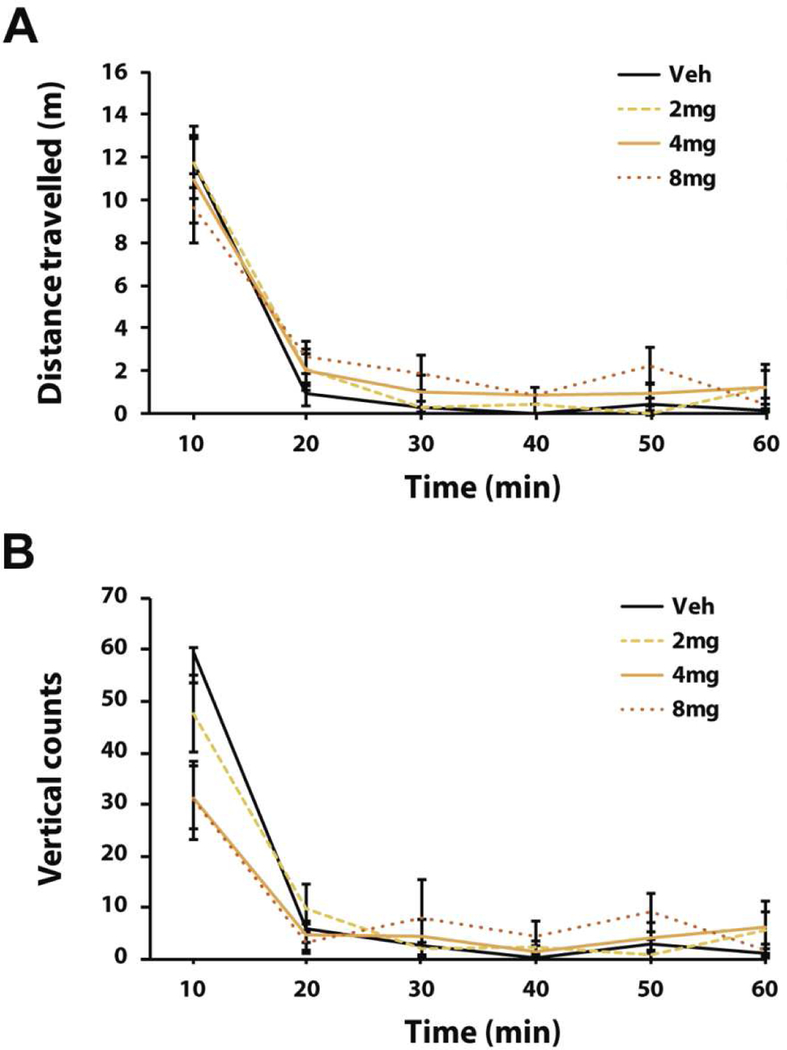
SK609 does not induce spontaneous locomotor effects
We assessed SK609’s effects on locomotor activity to determine if the observed improvement in sustained attention performance could be attributed to hyperlocomotion. In an open field task, SK609 (2, 4, and 8 mg/kg; i.p.) did not increase ambulatory count, distance traveled, or vertical counts when compared to vehicle treatment. Interestingly, most of the locomotor activity was observed during the first 10 minutes since these animals were well habituated to the apparatus to remove the novelty induced effects (Figure 8). Two-way ANOVA with treatment and time as repeated measures indicated a significant effect of time, but no treatment or treatment vs. time interaction for either distance traveled or vertical counts. The results suggest that SK609 does not induce hyper locomotor effects in rats at any of the doses tested and hence the increase in performance observed under SK609 treatment in sustained attention was not due to an increase in locomotor effects.
Figure 8. SK609 does not affect locomotor activity.
(A) Two-way ANOVA with treatment and time as repeated measures indicated a significant effect of time (F(5,25)=61.988, p = 0.000011 corrected for non-homogeneity of variance using Huyn-Feldt correction), but no treatment (F(3,15)=0.882, p = 0.472) or treatment x time interaction (F(15,75)=1.238, p = 0.264) for distance traveled. (B) Two-way ANOVA with treatment and time as repeated measures indicated a significant effect of time (F(5,25)=107.564, p = 0.00000000000000045) and a significant treatment x time interaction (F(15,75)=1.238, p = 0.000019), but no significant effect of treatment (F(3,15)=0.387, p = 0.764) for vertical counts. Dunnett’s post hoc analyses comparing each drug dose to vehicle treatment across the various time points revealed no significant differences of treatment at any of the time points (stats not shown). Data represents mean ± s.e.m.
DISCUSSION
Low doses of MPH elicit selective increases in DA and NE levels in the PFC leading to cognitive enhancement (Berridge et al., 2006; Berridge et al., 2012). In addition, high doses of MPH induce increases in catecholamine efflux throughout the brain, resulting in reinforcing effects mediated by DAT (Berridge et al., 2006; Teuns, Geys, Geuens, Stinissen, & Meert, 2014). Hence, MPH is often abused for recreational purposes at doses much higher than the therapeutic dose. Recent studies have highlighted the alarming abuse rate of MPH (Babcock & Byrne, 2000; Busardo, Kyriakou, Cipolloni, Zaami, & Frati, 2016). In this study, we propose a novel approach to improving sustained attention without the risk of increasing DAT-mediated efflux of DA. SK609 was designed as a biased D3R agonist and was recently demonstrated to improve motor deficits in a rodent model of Parkinson’s disease (Simms et al., 2016; Xu et al., 2017). In this study, we further characterized SK609 for its effects on monoamine transporters. Our results demonstrate that SK609, in addition to its D3R agonist effects, possesses selective NET effects as both an inhibitor and substrate. SK609’s unique dual DA and NE pharmacological effects led us to investigate its effects on PFC-mediated cognitive tasks.
In a sustained attention task, SK609 significantly improved performance in a dose-dependent manner with peak effects at 4 mg/kg. Further, SK609 produced an inverted-U shaped dose response, similar to that of MPH (Berridge et al., 2012). Specifically, 6 of the 7 low performing rats significantly improved in performance when treated with SK609, thereby transitioning into high performers. SK609 did not elicit an improvement in sustained attention performance among high performers that scored above 0.8 under vehicle conditions. Subject-specific cognitive effects in naïve rodents were also observed after MPH treatment, as behaviorally relevant doses do not improve sustained attention in high performing animals (Chu et al., 2016). Additionally, MPH did not improve sustained attention in high performing healthy individuals (Finke et al., 2010). Taken together, this suggests that measurement of the PFC-mediated task of sustained attention may yield ceiling effects in performance that may influence the detection of SK609-induced cognitive improvement in some animals. This potential ceiling effect is also observed in the way SK609 enhances sustained attention. MPH improves sustained attention by specifically increasing the average count of hits (Chu et al., 2016). Although SK609 similarly influences signal trial responses, this novel treatment decreased misses in low performing rodents. Accordingly, a ceiling effect may have prohibited an increase in correct responses after SK609 treatment, facilitating an improvement in sustained attention by decreasing incorrect responses.
Both D2/D3R antagonist raclopride and the alpha-1 adrenergic receptor antagonist prazosin individually impair sustained attention performance (Bari & Robbins, 2013; Hillhouse & Prus, 2013; Puumala, Riekkinen, & Sirvio, 1997; Shoaib & Bizarro, 2005). The peak effects produced by SK609 was blocked by a pretreatment with the D2/D3R antagonist raclopride, indicating that its cognitive-enhancing effects are mediated by D3Rs. The cognitive effect of SK609 was also blocked by prazosin. These behavioral results indicate that the PFC-mediated task of sustained attention may be mediated by both catecholamines. To further validate SK609’s effects on catecholaminergic neurotransmission within the PFC, we measured prefrontal DA and NE efflux levels using microdialysis assays. The peak dose of SK609 (4mg/kg) significantly increased both DA and NE levels in the PFC to greater than 160% and 300% of baseline values, respectively. This provides further evidence for the role of both catecholamines in SK609-mediated improvements in sustained attention.
It is likely that SK609’s cognitive effect could be mediated by D3Rs in the PFC, which are known to be significantly distributed in the medial and orbitofrontal cortex (Nakajima et al., 2013; Sokoloff, Giros, Martres, Bouthenet, & Schwartz, 1990). It is also likely that the improvement seen in the sustained attention task could be solely mediated by NET in the PFC. Several studies suggest that the beneficial effects of MPH are NET- and DAT-mediated in the PFC (Berridge et al., 2006; Hannestad et al., 2010). There is significant evidence supporting an increased distribution of NET in the PFC of rodents, relative to DAT concentrations (Carboni, Tanda, Frau, & Di Chiara, 1990). Consequently, NET often facilitates both DA and NE release, which could likely explain the 1.6-fold increase in DA efflux we observed in our prefrontal microdialysate samples (Bymaster et al., 2002). Low doses of MPH (2 mg/kg) produce a similar trend in catecholaminergic release in the PFC, stimulating a greater increase in NE than DA (Berridge et al., 2006; Rowley et al., 2014). The NET inhibitors desipramine and atomoxetine also elevate DA levels in addition to NE in the PFC, suggesting that prefrontal NET plays a role in the uptake of both DA and NE (Ago et al., 2014; Carboni et al., 1990). Further, the NET inhibitor atomoxetine does not yield an increase in DA efflux in the nucleus accumbens, where DAT is abundant (Bymaster et al., 2002).
Our in vitro results demonstrate that SK609 has very weak affinity for DAT, even at higher concentrations, suggesting the doses used in vivo in this study are unlikely to produce a DAT-mediated effect in the sustained attention behavioral assay. Rodents lacking the DAT gene do not display an increase in locomotion after treatment with amphetamine contrary to what is observed in control animals (Giros, Jaber, Jones, Wightman, & Caron, 1996). Within a therapeutically relevant range, MPH dose-dependently increases locomotor activity, which linearly correlates with MPH-evoked increases in extracellular DA (Rowley et al., 2014). SK609 (2, 4, and 8 mg/kg) did not induce spontaneous locomotor activity in an open field task, providing further in vivo evidence that SK609 lacks inhibitory effects on DAT. Our recent studies have also demonstrated that SK609 has no functional or binding effects on D2Rs, further validating the lack of spontaneous locomotor effects (Xu et al., 2017). Taken together, these results suggest SK609 may be better suited for treating attention deficits than conventional psychostimulants that possess a liability for abuse.
In conclusion, SK609 is a novel D3R agonist with selective inhibitory and releasing effects at NET. SK609 readily penetrates the brain and produces a 160% and 300% increase in DA and NE levels, respectively, in the PFC. This increase in extracellular catecholamine concentrations could be responsible for the significant, dose-dependent improvement observed in a PFC-mediated sustained attention task. The peak effect of SK609 was similar to the improvement observed with MPH. Most importantly, SK609 has a low affinity for DAT, as it did not trigger efflux in DAT in vitro or produce hyperlocomotion in vivo. These unique properties of SK609 differentiate it from existing therapies for ADHD, many of which are misused for recreational purposes or for their cognitive and performance-enhancing properties.
Highlights.
SK609 specifically improved sustained attention performance in low performing rats
SK609’s produced an inverted-U shaped dose response similar to methylphenidate
SK609’s peak dose increased the levels of Dopamine by 160% and Norepinephrine by 300% in the prefrontal cortex suggesting a role for both these catecholamines in sustained attention behaviors.
Acknowledgments
We acknowledge funding support by NIH-R01 MH106912 to SK, OVM and CM, NIH-R01 DA019676 to MEAR, NIH- R01 DA017960 to BDW and NIH-R01 DA031900 to RAE. We thank Dr. Andreia C.K. Fontana and Lauren Keibel for their assistance in pharmacological studies and animal training, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure for S. Kortagere: SK609 is a novel compound that was discovered in our laboratory and is currently listed as a lead molecule in two patent applications 13/764,623 and 15/028,654 filed by Drexel University. Both the patents are licensed to Polycore Therapeutics LLC. Polycore Therapeutics did not fund or influence any aspect of this study. SK is a co-founder and holds equity in Polycore Therapeutics LLC.
FINANCIAL DISCLOSURE FOR OTHER AUTHORS: CM, ZBD, OVM, MEAR, JSS, BDW and RAE have no financial conflicts of interest and nothing to disclose.
REFERENCES
- Ago Y, Umehara M, Higashino K, Hasebe S, Fujita K, Takuma K, & Matsuda T (2014). Atomoxetine-induced increases in monoamine release in the prefrontal cortex are similar in spontaneously hypertensive rats and Wistar-Kyoto rats. Neurochem Res, 39(5), 825–832. doi: 10.1007/s11064-014-1275-5 [DOI] [PubMed] [Google Scholar]
- Babcock Q, & Byrne T (2000). Student perceptions of methylphenidate abuse at a public liberal arts college. J Am Coll Health, 49(3), 143–145. doi: 10.1080/07448480009596296 [DOI] [PubMed] [Google Scholar]
- Bari A, & Robbins TW (2013). Noradrenergic versus dopaminergic modulation of impulsivity, attention and monitoring behaviour in rats performing the stop-signal task: possible relevance to ADHD. Psychopharmacology (Berl), 230(1), 89–111. doi: 10.1007/s00213-013-3141-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard AC, Stein MA, Halperin JM, Krone B, Rajwan E, & Newcorn JH (2015). Differential impact of methylphenidate and atomoxetine on sustained attention in youth with attention-deficit/hyperactivity disorder. J Child Psychol Psychiatry, 56(1), 40–48. doi: 10.1111/jcpp.12272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, … Spencer RC (2006). Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry, 60(10), 1111–1120. doi: 10.1016/j.biopsych.2006.04.022 [DOI] [PubMed] [Google Scholar]
- Berridge CW, Shumsky JS, Andrzejewski ME, McGaughy JA, Spencer RC, Devilbiss DM, & Waterhouse BD (2012). Differential sensitivity to psychostimulants across prefrontal cognitive tasks: differential involvement of noradrenergic alpha(1) - and alpha(2)-receptors. Biol Psychiatry, 71(5), 467–473. doi: 10.1016/j.biopsych.2011.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, & Robinson TE (2008). Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology, 33(12), 2969–2980. doi: 10.1038/npp.2008.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodnik Z, Bongiovanni R, Double M, & Jaskiw GE (2012). Increased tyrosine availability increases brain regional DOPA levels in vivo. Neurochem Int, 61(7), 1001–1006. doi: 10.1016/j.neuint.2012.07.012 [DOI] [PubMed] [Google Scholar]
- Brodnik Z, Double M, & Jaskiw GE (2013). Presynaptic regulation of extracellular dopamine levels in the medial prefrontal cortex and striatum during tyrosine depletion. Psychopharmacology (Berl), 227(2), 363–371. doi: 10.1007/s00213-013-2977-0 [DOI] [PubMed] [Google Scholar]
- Busardo FP, Kyriakou C, Cipolloni L, Zaami S, & Frati P (2016). From Clinical Application to Cognitive Enhancement: The Example of Methylphenidate. Curr Neuropharmacol, 14(1), 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, … Perry KW (2002). Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology, 27(5), 699–711. doi: 10.1016/S0893-133X(02)00346-9 [DOI] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Melchior JR, Bermejo K, Salahpour A, Roberts DC, & Jones SR (2014). Methylphenidate and cocaine self-administration produce distinct dopamine terminal alterations. Addict Biol, 19(2), 145–155. doi: 10.1111/j.1369-1600.2012.00456.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Salahpour A, Caron MG, & Jones SR (2013). Methylphenidate amplifies the potency and reinforcing effects of amphetamines by increasing dopamine transporter expression. Nat Commun, 4, 2720. doi: 10.1038/ncomms3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Tanda GL, Frau R, & Di Chiara G (1990). Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem, 55(3), 1067–1070. [DOI] [PubMed] [Google Scholar]
- Chu R, Shumsky J, & Waterhouse BD (2016). Differentiation of rodent behavioral phenotypes and methylphenidate action in sustained and flexible attention tasks. Brain Res, 1641(Pt B), 306–319. doi: 10.1016/j.brainres.2015.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov M, Grafman J, Soares AH, & Clark K (1999). Concept formation and concept shifting in frontal lesion and Parkinson’s disease patients assessed with the California Card Sorting Test. Neuropsychology, 13(1), 135–143. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, & Roe C (2002). Comparative efficacy of Adderall and methylphenidate in attention-deficit/hyperactivity disorder: a meta-analysis. J Clin Psychopharmacol, 22(5), 468–473. [DOI] [PubMed] [Google Scholar]
- Finke K, Dodds CM, Bublak P, Regenthal R, Baumann F, Manly T, & Muller U (2010). Effects of modafinil and methylphenidate on visual attention capacity: a TVA- based study. Psychopharmacology (Berl), 210(3), 317–329. doi: 10.1007/s00213-010-1823-x [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, & Caron MG (1996). Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature, 379(6566), 606–612. doi: 10.1038/379606a0 [DOI] [PubMed] [Google Scholar]
- Hannestad J, Gallezot JD, Planeta-Wilson B, Lin SF, Williams WA, van Dyck CH, … Ding YS (2010). Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatry, 68(9), 854–860. doi: 10.1016/j.biopsych.2010.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse TM, & Prus AJ (2013). Effects of the neurotensin NTS(1) receptor agonist PD149163 on visual signal detection in rats. Eur J Pharmacol, 721(1–3), 201–207. doi: 10.1016/j.ejphar.2013.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RB (2010). A recursive version of Grubbs’ test for detecting multiple outliers in environmental and chemical data. Clin Biochem, 43(12), 1030–1033. doi: 10.1016/j.clinbiochem.2010.04.071 [DOI] [PubMed] [Google Scholar]
- Kahn JB, Ward RD, Kahn LW, Rudy NM, Kandel ER, Balsam PD, & Simpson EH (2012). Medial prefrontal lesions in mice impair sustained attention but spare maintenance of information in working memory. Learn Mem, 19(11), 513–517. doi: 10.1101/lm.026302.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishikawa Y, Kawahara Y, Yamada M, Kaneko F, Kawahara H, & Nishi A (2014). The spontaneously hypertensive rat/Izm (SHR/Izm) shows attention deficit/hyperactivity disorder-like behaviors but without impulsive behavior: therapeutic implications of low-dose methylphenidate. Behav Brain Res, 274, 235–242. doi: 10.1016/j.bbr.2014.08.026 [DOI] [PubMed] [Google Scholar]
- Koerts J, Borg MA, Meppelink AM, Leenders KL, van Beilen M, & van Laar T (2010). Attentional and perceptual impairments in Parkinson’s disease with visual hallucinations. Parkinsonism Relat Disord, 16(4), 270–274. doi: 10.1016/j.parkreldis.2010.01.003 [DOI] [PubMed] [Google Scholar]
- Liu SK, Chiu CH, Chang CJ, Hwang TJ, Hwu HG, & Chen WJ (2002). Deficits in sustained attention in schizophrenia and affective disorders: stable versus state-dependent markers. Am J Psychiatry, 159(6), 975–982. doi: 10.1176/appi.ajp.159.6.975 [DOI] [PubMed] [Google Scholar]
- Manos MJ, Short EJ, & Findling RL (1999). Differential effectiveness of methylphenidate and Adderall in school-age youths with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry, 38(7), 813–819. [DOI] [PubMed] [Google Scholar]
- McGaughy J, & Sarter M (1995). Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl), 117(3), 340–357. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Gerretsen P, Takeuchi H, Caravaggio F, Chow T, Le Foll B, … Graff-Guerrero A (2013). The potential role of dopamine D(3) receptor neurotransmission in cognition. Eur Neuropsychopharmacol, 23(8), 799–813. doi: 10.1016/j.euroneuro.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, … Robbins TW (1992). Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain, 115 ( Pt 6), 1727–1751. [DOI] [PubMed] [Google Scholar]
- Park M, Hood MM, Shah RC, Fogg LF, & Wyatt JK (2012). Sleepiness, parkinsonian features and sustained attention in mild Alzheimer’s disease. Age Ageing, 41(6), 765–770. doi: 10.1093/ageing/afs084 [DOI] [PubMed] [Google Scholar]
- Pezze MA, Dalley JW, & Robbins TW (2009). Remediation of attentional dysfunction in rats with lesions of the medial prefrontal cortex by intra-accumbens administration of the dopamine D(2/3) receptor antagonist sulpiride. Psychopharmacology (Berl), 202(1–3), 307–313. doi: 10.1007/s00213-008-1384-4 [DOI] [PubMed] [Google Scholar]
- Puumala T, Riekkinen P Sr., & Sirvio J (1997). Modulation of vigilance and behavioral activation by alpha-1 adrenoceptors in the rat. Pharmacol Biochem Behav, 56(4), 705–712. [DOI] [PubMed] [Google Scholar]
- Rowley HL, Kulkarni RS, Gosden J, Brammer RJ, Hackett D, & Heal DJ (2014). Differences in the neurochemical and behavioural profiles of lisdexamfetamine methylphenidate and modafinil revealed by simultaneous dual-probe microdialysis and locomotor activity measurements in freely-moving rats. J Psychopharmacol, 28(3), 254–269. doi: 10.1177/0269881113513850 [DOI] [PubMed] [Google Scholar]
- Sagvolden T (2011). Impulsiveness, overactivity, and poorer sustained attention improve by chronic treatment with low doses of l-amphetamine in an animal model of Attention-Deficit/Hyperactivity Disorder (ADHD). Behav Brain Funct, 7, 6. doi: 10.1186/1744-9081-7-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahgal A (1988). Vasopressin and amphetamine, but not desglycinamide vasopressin, impair positively reinforced visual attention performance in rats. Behav Brain Res, 29(1–2), 35–42. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, & Bruno JP (2001). The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev, 35(2), 146–160. [DOI] [PubMed] [Google Scholar]
- Selemon LD, & Goldman-Rakic PS (1988). Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci, 8(11), 4049–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, & Bizarro L (2005). Deficits in a sustained attention task following nicotine withdrawal in rats. Psychopharmacology (Berl), 178(2–3), 211–222. doi: 10.1007/s00213-004-2004-6 [DOI] [PubMed] [Google Scholar]
- Sikirica V, Findling RL, Signorovitch J, Erder MH, Dammerman R, Hodgkins P, … Wu EQ (2013). Comparative efficacy of guanfacine extended release versus atomoxetine for the treatment of attention-deficit/hyperactivity disorder in children and adolescents: applying matching-adjusted indirect comparison methodology. CNS Drugs, 27(11), 943–953. doi: 10.1007/s40263-013-0102-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms SL, Huettner DP, & Kortagere S (2016). In vivo characterization of a novel dopamine D3 receptor agonist to treat motor symptoms of Parkinson’s disease. Neuropharmacology, 100, 106–115. doi: 10.1016/j.neuropharm.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, & Schwartz JC (1990). Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature, 347(6289), 146–151. doi: 10.1038/347146a0 [DOI] [PubMed] [Google Scholar]
- Szczepanski SM, & Knight RT (2014). Insights into human behavior from lesions to the prefrontal cortex. Neuron, 83(5), 1002–1018. doi: 10.1016/j.neuron.2014.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuns GB, Geys HM, Geuens SM, Stinissen P, & Meert TF (2014). Abuse liability assessment in preclinical drug development: predictivity of a translational approach for abuse liability testing using methylphenidate in four standardized preclinical study models. J Pharmacol Toxicol Methods, 70(3), 295–309. doi: 10.1016/j.vascn.2014.02.002 [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Grayson B, Marsh S, Hayward A, Marshall KM, & Neill JC (2015). Putative therapeutic targets for symptom subtypes of adult ADHD: D4 receptor agonism and COMT inhibition improve attention and response inhibition in a novel translational animal model. Eur Neuropsychopharmacol, 25(4), 454–467. doi: 10.1016/j.euroneuro.2014.11.016 [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Burnett EJ, Gass JT, Lopez MF, Mulholland PJ, Centanni SW, … Chandler LJ (2014). Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex. J Neurosci, 34(10), 3706–3718. doi: 10.1523/JNEUROSCI.0623-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Deng H, Xiao X, Zuo Y, Sun J, & Wang Z (2017). Persistent Neuronal Activity in Anterior Cingulate Cortex Correlates with Sustained Attention in Rats Regardless of Sensory Modality. Sci Rep, 7, 43101. doi: 10.1038/srep43101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Wang X, Tocker AM, Huang P, Reith ME, Liu-Chen LY, … Kortagere S (2017). Functional Characterization of a Novel Series of Biased Signaling Dopamine D3 Receptor Agonists. ACS Chem Neurosci, 8(3), 486–500. doi: 10.1021/acschemneuro.6b00221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Scott CN, Zhou X, & Geyer MA (2011). The effect of reduced dopamine D4 receptor expression in the 5-choice continuous performance task: Separating response inhibition from premature responding. Behav Brain Res, 222(1), 183–192. doi: 10.1016/j.bbr.2011.03.054 [DOI] [PMC free article] [PubMed] [Google Scholar]