Abstract
Chronic pain is associated with neuroplastic changes in the amygdala that may promote hyper-responsiveness to mechanical and thermal stimuli (allodynia and hyperalgesia) and/or enhance emotional and affective consequences of pain. Stress promotes dynorphin-mediated signaling at the kappa opioid receptor (KOR) in the amygdala and mechanical hypersensitivity in rodent models of functional pain. Here, we tested the hypothesis that KOR circuits in the central nucleus of the amygdala (CeA) undergo neuroplasticity in chronic neuropathic pain resulting in increased sensory and affective pain responses. After spinal nerve ligation (SNL) injury in rats, pretreatment with a long-acting KOR antagonist, nor-binaltorphimine (nor-BNI), subcutaneously or through microinjection into the right CeA, prevented conditioned place preference (CPP) to intravenous gabapentin, suggesting that nor-BNI eliminated the aversiveness of ongoing pain. By contrast, systemic or intra-CeA administration of nor-BNI had no effect on tactile allodynia in SNL animals. Using whole-cell patch-clamp electrophysiology, we found that nor-BNI decreased synaptically evoked spiking of CeA neurons in brain slices from SNL but not sham rats. This effect was mediated through increased inhibitory postsynaptic currents, suggesting tonic disinhibition of CeA output neurons due to increased KOR activity as a possible mechanism promoting ongoing aversive aspects of neuropathic pain. Interestingly, this mechanism is not involved in SNL-induced mechanical allodynia. Kappa opioid receptor antagonists may therefore represent novel therapies for neuropathic pain by targeting aversive aspects of ongoing pain while preserving protective functions of acute pain.
Keywords: Neuropathic pain, Amygdala central nucleus, Kappa opioid antagonist, Conditioned place preference, Synaptic transmission, Disinhibition, Slice physiology
1. Introduction
Chronic neuropathic pain is characterized by peripheral and central sensitization in the spinal cord and the brain that may amplify nociceptive signaling and promote aversive behavior. The amygdala has emerged as a key brain region that modulates aversive responses to stress, fear, and pain.2,41,57 Neuroimaging studies have demonstrated that amygdala function and connectivity are altered in patients with chronic pain,10,23,62 suggesting that amygdala hypersensitivity may contribute to chronic pain. However, neural mechanisms underlying amygdala sensitization are not well understood.
The amygdala encompasses several functionally and structurally distinct nuclei. The lateral subdivision of the central nucleus (CeL) receives direct nociceptive input from calcitonin gene-related peptide (CGRP)-expressing neurons in the parabrachial area (PB) as well as contextual pain information from the cortex and lateral–basolateral amygdala. The CeA contains mostly gamma-aminobutyric acid neurons, some of which also coexpress neuropeptides such as corticotropin-releasing factor (CRF) and dynorphin, an endogenous agonist at kappa opioid receptors (KORs).14 Direct projections from CeA CRF1 neurons to brainstem areas including the PB and the periaqueductal grey area24,54,59 promote aversive and anxiety-like behaviors.4,12,35,52
We have demonstrated in rodent models that chronic pain promotes neuroplasticity in the amygdala that is linked to affective pain behaviors (for reviews see Refs. 41, 42, and 44). Multiple mechanisms of synaptic plasticity in the amygdala have been identified, including enhanced excitatory neurotransmission at the PB-CeL and BLA-CeL synapses,13,17,22,43,51 decreased intercalated cell-mediated feedforward inhibition from the prefrontal cortex,27,50 and increased excitability of the CeL neurons.13,43 These neuroplastic changes ultimately increase the activity of the CeA output neurons and amplify pain responses in chronic pain states. Therefore, the CeA may be a primary brain region sustaining chronic pain through descending pain facilitatory outputs.35,49
Many chronic functional pain conditions such as migraine, fibromyalgia, irritable bowel syndrome, and others are exacerbated by stress, and pain itself can be a stressor.6,34 Stress can elicit release of CRF and dynorphin in a number of brain regions including the amygdala. Moreover, dynorphin/KOR signaling in the amygdala has been shown to be critical for behavioral responses to stress and stress-induced reinstatement of drug seeking.7 Our previous reports using a rat model of medication overuse headache demonstrate that a brief exposure of sensitized animals to stress promotes dynorphin release and KOR phosphorylation in the amygdala, as well as enhanced mechanical allodynia and a loss of diffuse noxious inhibitory controls (DNIC).38,68 Importantly, the stress-induced allodynia and loss of DNIC can be prevented by systemic or intra-amygdala pretreatment with a KOR antagonist, nor-binaltorphimine (nor-BNI).38,68 Therefore, enhanced descending facilitation from the amygdala may promote stress-induced pain.
Here, we tested the hypothesis that chronic neuropathic pain increases the activity of CeA output neurons through KOR-mediated disinhibition. We also hypothesized that blockade of dynorphin/KOR signaling in the CeA would decrease excitability of the output neurons and block nerve injury–induced allodynia and aversive behavior.
2. Methods
2.1. Animals
Male, Sprague-Dawley rats, 250 to 350 g at time of testing (Harlan Laboratories, Indianapolis, IN) were housed 3 per cage (unless otherwise stated) on a 12-hour light–dark cycle (lights on at 7:00 am) with food and water ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Arizona and Texas Tech University. All studies were conducted in accordance with the policies and recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animals were monitored throughout the studies to reduce unnecessary stress or pain. All behavioral studies were performed with the experimenter blinded to the treatment conditions.
2.2. Spinal nerve ligation surgery
L5/L6 ligation surgeries were performed as described by Kim and Chung.25 Briefly, under anesthesia (5% induction, 2% maintenance isoflurane at 1 L/minute), an approximately 2-cm incision was made to the left of the midline, and the L5 and L6 were exposed and tightly ligated with 4–0 silk suture (Henry Shein Inc., Melville, NY). Muscle was closed with 4–0 silk suture, and the skin closed with wound clips (MikRon Precision Inc., Gardena, CA). Sham surgery was the same, except the L5 and L6 were not ligated. For electrophysiology experiments, only the L5 nerve was ligated, consistent with our previous studies in this model.22 After surgery, all rats were given 1 dose of gentamycin (8 mg/kg, subcutaneously (s.c.); VetOne, Boise, ID) and were monitored for any sign of infection or distress.
2.3. Stereotaxic intracranial cannula implantation
For behavioral experiments requiring intracranial cannula implantation, stereotaxic surgeries were performed at approximately 7 days after spinal nerve ligation (SNL). Animals were anesthetized by intraperitoneal ketamine/xylazine (80/12 mg/kg; Western Medical Supply, Arcadia, CA/Sigma-Aldrich), and the head was fixed in ear bars. Single-guide cannula (26 gauge; PlasticsOne, Roanoke, VA) was inserted into the right central nucleus of the amygdala using brain loci coordinates obtained from Paxinos and Watson’s brain atlas47 (−4.0-mm mediolateral, −2.0-mm anteroposterior, and −7.0-mm dorsoventral from Bregma). Rats were then housed individually and allowed to recover for at least 7 days before behavioral testing. All cannula placements were verified postmortem, and 6 rats with incorrect placement or clogged cannulas were excluded from all analysis. Verification entailed injection of black dye through the cannula, harvesting of brain and then fixation in 10% formalin for 24 hours. Thirty-micrometer coronal sections were cut in the area of interest, and placement was verified with reference to Paxinos and Watson’s brain atlas.47
2.4. Drug administration
For all experiments, nor-BNI (Tocris, Minneapolis, MN) was dissolved in saline. For systemic dosing, nor-BNI was administered subcutaneously at 3 mg/kg/mL (dose/volume). For intracranial administration, unilateral injection cannulae extending 1 mm beyond the end of the guide cannulae were connected to a 2-μL Hamilton syringe, and 2.5 μg in a volume of 0.5 μL was injected into the right central nucleus of the amygdala as previously described.38,68 Gabapentin (GBP; Spectrum Chemical MFG, Gardena, CA), dissolved in distilled water, was administrated by intravenous injection (50 mg/kg).
2.5. Tactile sensory thresholds
Mechanical sensory thresholds were measured before and after SNL or sham surgeries to verify development of neuropathic pain and then following drug administration. Rats were tested with a series of von Frey filaments (Touch Test sensory evaluators; Stoelting, Wood Dale, IL), and withdrawal threshold was calculated using the Dixon up–down method.23 Hindpaw withdrawal threshold was tested by perpendicular application of the filaments to the plantar surface of the left (ipsilateral to SNL) paw; the cutoff filament was 15 g.
2.6. Conditioned place preference
To test affective pain behavior, a single-trial place-conditioning protocol was used as previously described.26 On preconditioning day, rats were placed into the CPP boxes with free access to all 3 chambers (2 conditioning chambers and a middle chamber). To verify whether a preexisting chamber bias existed, automated software (Anymaze or San Diego Instruments Photobeam Activity System) was used, and the time spent in each chamber was determined across 15 minutes (900 seconds). Animals spending more than 80% (720 seconds) or less than 20% (180 seconds) of the total time in either conditioning chamber were eliminated from further testing. After the baseline CPP evaluation, the animals received nor-BNI treatment either subcutaneously or in the right CeA and were returned to the animal facility. The next day (conditioning day), rats received a vehicle treatment and were immediately placed into the assigned conditioning chamber for 30 minutes. Four hours later, rats received gabapentin treatment (50 mg/kg, intravenously [i.v.]) and were placed into the opposite pairing chamber for 30 minutes. On test day, rats were placed in the middle chamber with access to all chambers and were allowed to explore freely for 15 minutes while the time spent in each chamber was automatically recorded using the Anymaze software or the San Diego Instruments Photobeam Activity System. In this procedure, rats received no drug treatment on test day–eliminating concerns of locomotor/sedative effects of gabapentin. Difference scores were calculated as test time minus preconditioning time spent in the drug-paired chamber. Time heat maps in the chambers were generated in the Anymaze software.
2.7. Brain slice preparation for electrophysiology
Coronal (400 μm) brain slices containing the CeA of the right hemisphere were obtained from neuropathic (SNL) or sham rats 4 weeks after surgery. As described previously,19,20,50 brains were quickly removed and immersed in oxygenated ice-cold sucrose-based physiological solution containing the following (in mM): 87 NaCl, 75 sucrose, 25 glucose, 5 KCl, 21 MgCl2, 0.5 CaCl2, and 1.25 NaH2PO4. Brain slices were prepared using a Vibratome (Series 1000 Plus; The Vibratome Company St. Louis, MO). Brain slices were then incubated in oxygenated artificial cerebrospinal fluid at physiological temperature (35°C) for at least 1 hour before patch recordings. Artificial cerebrospinal fluid, pH 7.4, contained the following (in mM): 117 NaCl, 4.7 KCl, 1.2 NaH2PO4, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, and 11 glucose. A single brain slice was transferred to the recording chamber and submerged in artificial cerebrospinal fluid (35 ± 1°C) superfusing the slice at ~2 mL/minute. Only 1 or 2 brain slices per animal were used. Only 1 neuron was recorded in each slice.
2.8. Patch-clamp recording
Whole-cell voltage- and current-clamp recordings were made from visually identified neurons in the lateral–capsular CeA (CeLC) using DIC-IR videomicroscopy as described previously.20,50 Patch electrodes had tip resistances of 3 to 6 MΩ. The following internal solution was used (in mM): 122 K-gluconate, 5 NaCl, 0.3 CaCl2, 2 MgCl2, 1 EGTA, 10 HEPES, 5 Na2-ATP, and 0.4 Na3-GTP, pH adjusted to 7.2 to 7.3 with KOH and osmolarity to 280 mOsm/kg with sucrose. Data acquisition and analysis was performed using a low-noise Digidata 1440A interface (Axon Instruments, Molecular Devices, San Jose, CA), a dual 4-pole Bessel filter (Warner Instruments), an Axoclamp-2A amplifier (Axon Instruments), and pClamp10 software (Axon Instruments). If series resistance (monitored with pClamp10 software) changed >20%, the neuron was discarded.
2.9. Synaptic transmission
Action potentials (spikes) and inhibitory postsynaptic currents (IPSCs) were evoked in CeLC neurons by focal electrical stimulation (150-μs square-wave pulses; using an S88 stimulator; Grass Technologies, West Warwick, RI) in the fiber tract that runs dorsomedial to the CeA and outside of the caudateputamen as described in our previous studies13,43,50 using a concentric bipolar stimulation electrode (Kopf Instruments, Tujunga, CA). Synaptically evoked spiking (E-S coupling) was recorded in current-clamp mode. Stimulus intensity was adjusted for 50% spiking probability (spikes evoked in 5 of 10 consecutive trials). Increased IPSCs were recorded at 0 mV in voltage-clamp mode and were blocked by bicuculline (10 μm) or NBQX (10 μM), consistent with glutamate-driven feedforward inhibition. Input–output (I-O) functions of IPSCs were obtained by measuring peak amplitudes as a function of stimulation intensity (200 μA steps).
2.10. Statistical analysis
All graphs were created, and t tests were performed in GraphPad Prism (7.0). Evoked pain time-course experiments were analyzed using a 2-way repeated-measures analysis of variance (ANOVA) using SPSS Statistics (24; IBM). Between-subject factor was GROUP, and within-subject factor was TIME and/or TREATMENT. Where significance was seen, a Bonferroni post hoc test was performed. For CPP experiments, data are presented as difference scores (ie, the difference between the time spent in the drug-paired chamber on testing day and on baseline day). Previous experiments confirmed that the used CPP procedure is unbiased. Thus, a positive CPP score represents place preference, a negative score indicates aversion, and zero no preference.30,36 To evaluate whether the animals show significant preference or aversion, differences from a hypothetical value 0 (ie, no preference) were determined for each group’s difference score using a 1-sample t test. Subsequently, to compare between the 2 treatment groups, an unpaired t test was used. Two-way ANOVA with Bonferroni post-test was used to analyze the time course of nor-BNI effects on synaptically evoked spiking. Two-way repeated-measures ANOVA with Tukey’s post-test was used when comparing the drug effects on input–output functions of evoked IPSCs. One-way repeated-measures ANOVA with Tukey’s post-test were used to analyze the effect of nor-BNI on IPSC amplitude at 600-μA stimulation intensity. Time course of drug effects on IPSCs evoked at 600 μA was analyzed using a 2-way ANOVA with Tukey’s post hoc tests. All data are shown as mean ± SEM. Significance was set at P < 0.05.
3. Results
In this study, we tested the hypothesis that KOR antagonist nor-BNI would inhibit neuroplastic changes in the amygdala and pain-related behaviors induced by SNL in rats. Because the central nucleus of the right amygdala is the main amygdala output region and because of reports of hemispheric lateralization of pain modulation to the right amygdala, we focused on targeting the right CeA.
3.1. Blockade of kappa opioid receptor with systemic administration of nor-binaltorphimine relieves aversiveness of ongoing pain but not tactile allodynia in spinal nerve ligation rats
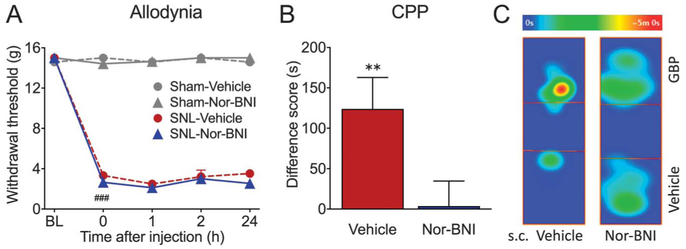
Rats with SNL surgery developed long-lasting mechanical allodynia in the ipsilateral paw, demonstrated as reduced tactile paw withdrawal thresholds to probing with von Frey filaments (Fig. 1A; P < 0.0001). Systemic administration of nor-BNI (3 mg/kg, s.c.) or vehicle (saline, s.c.) had no effect on tactile allodynia in either sham or SNL animals during a 24-hour time course (Fig. 1A). Because of the long-lasting effects of nor-BNI, the indirect CPP paradigm39 was used. With this approach, we assessed whether pretreatment with nor-BNI can relieve the aversiveness of ongoing neuropathic pain and prevent the ability of an effective, short-acting analgesic, gabapentin, to elicit CPP. SNL animals were pretreated with either nor-BNI (3 mg/kg, s.c.) or vehicle (saline, s.c.) 1 day before CPP conditioning. We have reported previously that intravenous GBP (50 mg/kg, i.v.) promotes CPP in SNL rats.3 Consistent with those results, administration of gabapentin (GBP, 50 mg/kg, i.v.) in saline-pretreated rats produced significant place preference for the GBP-paired chamber, shown as a positive difference score (Fig. 1B). This suggests the presence of ongoing pain in saline-pretreated SNL rats that is relieved by gabapentin treatment (N = 16; P = 0.0062, 1-sample t test). By contrast, nor-BNI pretreated animals showed no chamber preference, indicating that nor-BNI relieved ongoing pain (Fig. 1B). The difference between saline- and nor-BNI–treated groups was statistically significant (P = 0.027; t = 2.33; df = 27; unpaired t test).
Figure 1.
Systemic nor-BNI relieves aversiveness of ongoing pain but not tactile allodynia in SNL rats. (A) After SNL surgery, rats develop long-lasting mechanical allodynia in the injured paw, demonstrated as reduced tactile paw withdrawal thresholds. Systemic administration of nor-BNI (3 mg/kg, s.c.) or vehicle (saline, s.c.) had no effect on tactile allodynia in either sham or SNL animals during a 24-hour time course. N = 4–8; ###P < 0.001. (B) SNL animals were pretreated with either nor-BNI (3 mg/kg, s.c.) or vehicle (saline, s.c.) 1 day before CPP conditioning. In vehicle pretreated rats, administration of gabapentin (50 mg/kg, i.v.) produced significant place preference for the GBP-paired chamber, shown as a positive difference score, suggesting the presence of ongoing pain that is relieved by gabapentin treatment. N = 16, **P = 0.006. By contrast, nor-BNI pretreated animals show no chamber preference, indicating that nor-BNI relieved ongoing pain. N = 13. (C) Representative heat maps demonstrating time spent in different locations in the CPP box on test day. SNL rat pretreated with subcutaneous (s.c.) saline spent more time in the GBP-paired chamber, whereas nor-BNI pretreated rat spent equivalent time in both chambers. Data are expressed as mean ± SEM. CPP, conditioned place preference; nor-BNI, nor-binaltorphimine; SNL, spinal nerve ligation.
3.2. Blockade of kappa opioid receptor in the CeA relieves aversiveness of ongoing neuropathic pain in spinal nerve ligation rats
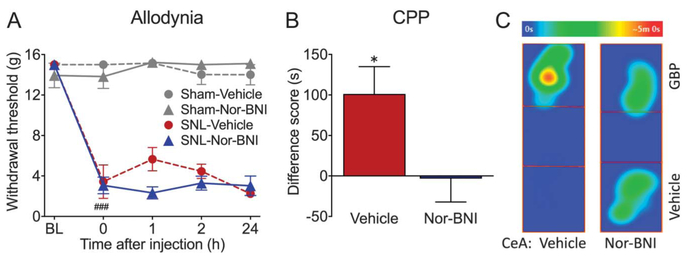
Another cohort of animals was used to investigate whether direct blockade of KOR in the right CeA would block sensory or affective pain behavior. Spinal nerve ligation rats displayed significantly reduced paw withdrawal thresholds compared with their pre-surgery baselines, indicating presence of tactile allodynia (Fig. 2A, P < 0.0001). Microinjection of nor-BNI (2.5 μg/0.5 μL) or vehicle (saline, 0.5 μL) into the right CeA had no effect on tactile allodynia in either sham or SNL animals during a 24-hour time course (Fig. 2A). Gabapentin (50 mg/kg; i.v.) produced CPP in SNL rats pretreated 24 hours prior with vehicle into the right CeA (N = 13, P = 0.011, 1-sample t test). However, gabapentin had no effect in SNL rats microinjected with nor-BNI (2.5 μg/0.5 μL), suggesting that pain aversiveness had already been relieved by nor-BNI. The difference between CeA saline- and nor-BNI–treated groups was statistically significant (P = 0.026; t = 2.35; df = 29; unpaired t test).
Figure 2.
Blockade of KOR in the CeA relieves aversiveness of ongoing neuropathic pain in SNL rats. (A) SNL rats displayed significantly reduced paw withdrawal thresholds, indicating presence of tactile allodynia. Microinjection of nor-BNI (2.5 μg/0.5 μL) or vehicle (saline, 0.5 μL) into the right CeA had no effect on tactile allodynia in either sham or SNL animals during a 24-hour time course. N = 3–5; ###P < 0.001. (B) Gabapentin (50 mg/kg; i.v.) produces CPP in SNL rats pretreated 24 hours before conditioning with vehicle into the right CeA (N = 13, *P = 0.011), but not in SNL rats microinjected with nor-BNI (2.5 μg/0.5 μL) (N = 18). (C) Representative heat maps demonstrating time spent in different locations in the CPP box on test day. SNL rat pretreated with saline in the CeA spent more time in the GBP-paired chamber on test day. By contrast, CeA nor-BNI pretreated rat spent equivalent time in both chambers. Data are expressed as mean ± SEM. CPP, conditioned place preference; KOR, kappa opioid receptor; nor-BNI, nor-binaltorphimine; SNL, spinal nerve ligation.
3.3. Synaptically evoked spiking of CeA neurons is reduced by nor-binaltorphimine in slices from spinal nerve ligation rats
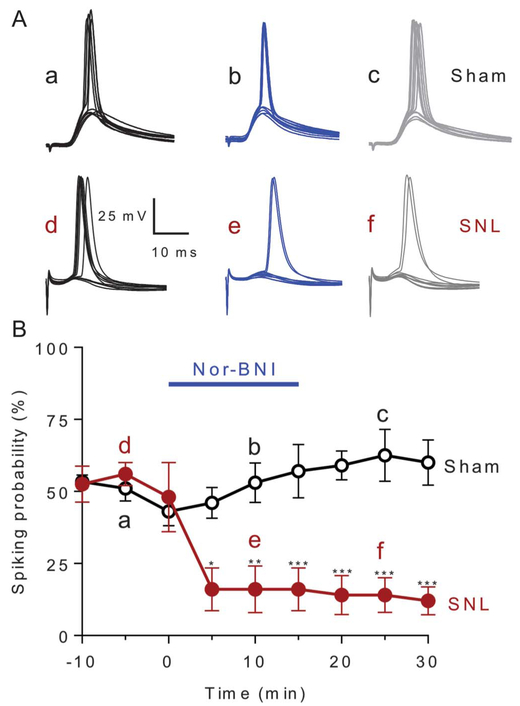
Slice electrophysiology was performed to determine whether neuropathic pain–induced plasticity in the amygdala is reversed by nor-BNI. Brain slices were obtained from SNL or sham rats 4 weeks after surgery. Recordings were made from regular spiking CeLC neurons as in our previous studies41 because they typically are peptidergic projection neurons.16 As a measure of neuronal output, excitatory postsynaptic potential-spike coupling (E-S coupling) was measured. To do so, action potentials (spikes) were evoked by electrical stimulation of the dorsomedial fiber tract containing presumed axons from the PB, and action potentials were recorded from neurons in the lateral-capsular division of the central nucleus of the amygdala (CeLC) in current-clamp mode. Stimulus intensity was adjusted to evoke spikes in 5 of 10 consecutive trials. The stimulation currents (mean ± SEM) required to obtain 50% excitatory postsynaptic potential-spike coupling for SNL and sham neurons were 1.020 ± 0.097 mA (n = 5) and 0.908 ± 0.063 mA (n = 10), respectively. These 2 values are not significantly different (P = 0.335, unpaired t test). Application of nor-BNI (1 μM, 15 minutes) to the slice by superfusion significantly decreased synaptically evoked spiking (E-S coupling) in CeLC neurons from SNL but not sham rats, as compared to predrug baselines at −5 minutes (Fig. 3; sham: n = 10 neurons; SNL: n = 5 neurons; P < 0.001; F8,110 = 4.926; 2-way ANOVA with Bonferroni post hoc tests). Because of long-lasting effects of nor-BNI, no changes were observed after a washout, and thus, the difference between SNL and sham groups was significant during the 5- to 30-minute period. Voltage traces show action potentials of 1 neuron from sham and 1 from SNL rat recorded at −60 mV in response to synaptic stimulation (Fig. 3A). Averaged spiking probability values (±SEM) for the sample of 5 neurons from SNL rats and 10 neurons from sham rats are shown in Figure 3B.
Figure 3.
Synaptically evoked spiking. Nor-BNI (1 μM, 15 minutes) decreased synaptically evoked spiking (E-S coupling) in neurons in the laterocapsular division of the central nucleus of the amygdala (CeLC) in brain slices from SNL but not sham rats, 4 weeks after surgery. (A) Current-clamp recordings of action potentials (spikes) evoked by electrical stimulation of the dorsomedial fiber tract into CeLC. Voltage traces (a–f) show responses of 1 neuron recorded at −60 mV (a and d) before, (b and e) during, and (c and f) after washout of nor-BNI in slices from sham or SNL rats. Each trace is the response to one synaptic stimulation. (B) Time course of drug effects. Graph shows averaged values (±SEM) for the sample of neurons from sham (n = 10 neurons) and SNL rats (n = 5 neurons). Each symbol is the mean value for all neurons in each sample. In each neuron, a series of 10 synaptically evoked responses was measured per time point to determine the spiking probability. Bars represent SEM. Two-way ANOVA with Bonferroni post hoc test’s comparison between sham and SNL groups (*P < 0.05, **P < 0.01, ***P < 0.001; F8,110 = 4.926). ANOVA, analysis of variance; nor-BNI, norbinaltorphimine; SNL, spinal nerve ligation.
3.4. Synaptic inhibition is increased by nor-binaltorphimine in slices from spinal nerve ligation rats
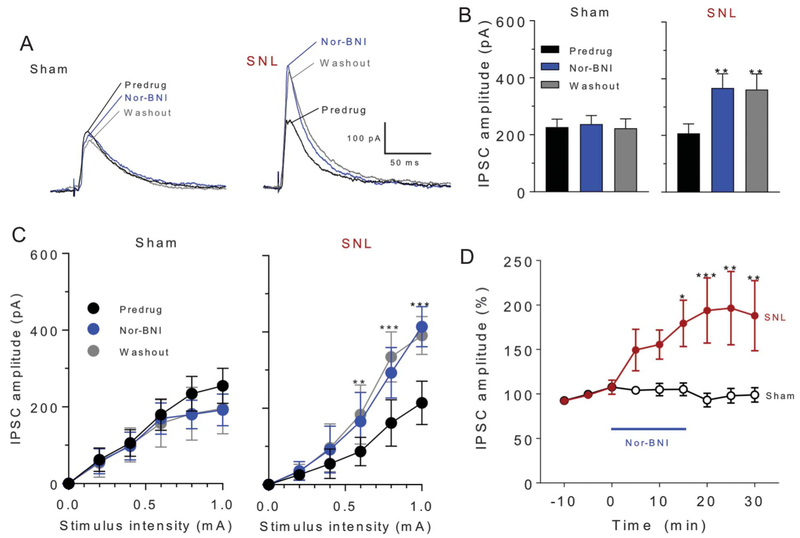
Next, we tested the hypothesis that the inhibitory effect of nor-BNI on neuronal output involved blockade of KOR-mediated disinhibition in the CeA. Nor-BNI (1 μM, 15 minutes) increased inhibitory synaptic currents evoked by electrical synaptic stimulation of the dorsomedial fiber tract into CeLC in brain slices from SNL rats but not sham controls, 4 weeks after surgery (individual neuron example shown in Fig. 4A). Increased IPSCs could be blocked with bicuculline and NBQX (data not shown; see Methods), which is consistent with glutamate-driven feedforward inhibition. Input–output functions of evoked IPSCs recorded at 0 mV in brain slices from SNL rats are increased by the application of nor-BNI to the brain slice, and this effect persists upon washout (Fig. 4C; sham, n = 5 neurons, F2,10 = 0.725, P = 0.488; SNL, n = 6 neurons, F2,10 = 13.95, P < 0.01; repeated-measures 2-way ANOVA with Tukey’s multiple comparison test). Analysis of mean IPSC amplitudes (±SEM) evoked at 600 μA in the sample of neurons recorded in brain slices from sham and SNL rats showed a significant increase of synaptic inhibition by nor-BNI in SNL rats but not in sham controls (Fig. 4B; SNL: n = 7 neurons; F2,14 = 10.98, P < 0.05; Sham: n = 9 neurons; F2,23 = 0.060, P = 0.942; repeated-measures ANOVA with Tukey’s post-tests). Time course of drug effects on IPSCs evoked at 600 μA demonstrated that the difference between SNL and sham groups was significant during the 15- to 30-minute period. (Fig. 4D; F8,130 = 2.384, P < 0.05; 2-way ANOVA with Tukey’s post hoc tests).
Figure 4.
Synaptic inhibition. Nor-BNI (1 μM) increased inhibitory synaptic currents (IPSCs) in neurons in the CeLC in brain slices from SNL rats but not sham controls, 4 weeks after surgery. Electrophysiological recordings were performed from regular spiking neurons after electrical stimulation of the dorsomedial fiber tract. (A) Current traces from an individual CeLC neuron from a sham rat and another neuron from an SNL rat show voltage-clamp recordings of IPSCs (at 0 mV) evoked by electrical synaptic stimulation (600 μA) before and during drug application and during washout. (B) Mean IPSC amplitudes (±SEM) evoked at 600 μA in the sample of neurons from sham and SNL rats (sham, n = 9 neurons, F2,23 = 0.060, P = 0.942; SNL, n = 7 neurons, F2,14 = 10.98, *P < 0.01; repeated-measures ANOVA with Tukey’s post hoc tests). Each symbol is the mean value for all neurons in each sample. In each neuron, a series of 3 synaptically evoked responses was measured per time point. (C) Input–output function of IPSCs in brain slices from sham and SNL rats shows significant effects of nor-BNI in the neuropathic pain model but not in sham control conditions (sham, n = 5 neurons, F2,10 = 0.725, P = 0.488; SNL, n = 6 neurons, F2,10 = 13.95, **, *** P < 0.01, 0.001, repeated-measures 2-way ANOVA with Tukey’s post hoc tests). (D) Time course of drug effects on IPSCs evoked at 600 μA. Nor-BNI increased IPSCs in CeLC neurons from SNL rats (n = 7 neurons) but not sham rats (n = 9 neurons). Graph shows averaged values (±SEM). F8,130 = 2.384, *P < 0.05, ** P < 0.01, ***P < 0.001; 2-way ANOVA with Tukey’s post hoc tests. ANOVA, analysis of variance; nor-BNI, nor-binaltorphimine; SNL, spinal nerve ligation.
4. Discussion
The National Institutes of Health has recently proposed that a cluster of prevalent and often overlapping pain conditions including migraine, medication overuse headache, tension-type headache, fibromyalgia, irritable bowel syndrome, temporomandibular disorders, myalgic encephalomyelitis/chronic fatigue syndrome, chronic low-back pain, and others be termed chronic overlapping pain conditions (COPCs). The frequent co-occurrence of these conditions raises the possibility of shared underlying mechanisms.63 Although the nature of pain varies widely in COPCs, a common feature is a strong relationship with self-identified stress events in precipitating episodes of pain.6,18,58 In addition, a major risk factor for the chronification of pain in many of the COPCs is the frequency of pain attacks.6,48 Repeated stress events may therefore represent a common sensitization mechanism by which pain is produced in vulnerable patients and a process by which episodic pain is transformed to chronic pain.
Although response to stress is essential for survival, repeated or uncontrolled stress is maladaptive and promotes states of allostatic overload characterized by neural adaptations in brain circuits that amplify nociceptive signaling to promote pain.5 Work from multiple laboratories has revealed a role for dynorphin/KOR signaling in brain circuits that characterizes a part of the stress response.7,28 Our previous studies have used a 2-hit model of functional pain to investigate the role of KOR signaling in the CeA in promoting stress-related pain.38,68 After sensitization by exposure to opioids or triptans that are known to promote medication overuse headache,61 stress increased responses to normally innocuous touch stimuli that are accompanied by changes in circulating CGRP and disruption of descending modulation (ie, the DNIC response). Such stress-induced pain behaviors and increased CGRP levels are prevented by pretreating animals (before stress) with systemic nor-BNI, suggesting a critical importance of KOR signaling in sensitized states.38,68 We have further demonstrated that blockade of KOR signaling in the right, but not left, CeA blocks both stress-related evoked pain responses and CGRP levels in sensitized animals.38,68 Finally, microinjection of U69,593 into the right, but not left, CeA promotes allodynia more robustly in sensitized animals.68
The requirement for a state of sensitization in the amplification of KOR signaling and pain in these preclinical studies is consistent with positive correlation of frequent pain episodes as a risk factor in the transformation of episodic to chronic pain in COPCs.32 These observations suggest that repeated pain episodes may decrease thresholds to elicit pain from triggers including stress. Chronic injury–related pains are believed to result from increased afferent discharge to the nervous system that promotes and sustains a state of central sensitization.67 Increasing evidence suggests that injury-related chronic pain can be regarded as a stress state. Fillingham et al. have studied the relationship of stress and chronic pain in patients with osteoarthritis and reported that individuals with high pain have shorter telomere lengths, a marker of cellular aging.56 Leukocyte telomere length was suggested as a “downstream” marker of cumulative, persistent, biological, or psychosocial stress, and these authors noted that over time, osteoarthritis pain extends to other body regions and is associated with hyperalgesia, allodynia, and decreased efficiency of endogenous inhibitory mechanisms. Consistent with these observations of chronic pain–related adaptations, preclinical studies have repeatedly observed increased expression of dynorphin in models of chronic pain in multiple regions of the neuraxis correlating in time with expression of pain.33,65 These findings led us to investigate the possible role of dynorphin/KOR signaling in a model of neuropathic pain in rats induced by SNL.
After experimental nerve injury, we observed the expected decrease in sensory threshold responses to von Frey filaments. Treatment with nor-BNI, at a dose that we have previously shown to be effective in blocking KOR,68 did not alter these responses. We have previously shown that rats with SNL injury show motivated behaviors to seek relief that can be captured using a place conditioning assay.26,39,40 Rats with neuropathic injury will seek contexts that are paired with systemic administration of gabapentin.3 As nor-BNI has an extremely long half-life in vivo that makes assessment of CPP difficult, we pretreated rats with nor-BNI to determine possible effects on motivation to seek the context paired with gabapentin-induced pain relief. We found that nor-BNI prevented gabapentin-induced pain relief suggesting that the aversive qualities of experimental neuropathic pain may be associated with increased signaling at the KOR. The mechanism of action of gabapentin remains uncertain, but our previous studies have suggested that gabapentin may act at spinal and supraspinal sites to modulate both the sensory and affective qualities of pain. The present studies suggest that blockade of KOR in animals with neuropathic pain may preferentially modulate affective qualities of pain, so that the actions of gabapentin are no longer sufficient to produce motivated behaviors for pain relief.
As noted above, in models of functional pain, stress drives KOR signaling within central circuits relevant to the right, but not left, central nucleus of the amygdala (CeA).38,68 This is consistent with previous findings that pain is encoded in the right but not left CeA.9,21 Therefore, consequences of blockade of KOR signaling in animals with SNL were evaluated only in the right CeA. Similar to effects observed after systemic nor-BNI, this treatment did not affect nerve injury–induced allodynia. However, intra-CeA nor-BNI blocked the CPP that was observed with systemic gabapentin suggesting that neural adaptations that occur with chronic neuropathic pain may involve increased KOR signaling in the right CeA to promote aversive qualities of pain. These results are consistent with interpretation of pain as a stress response within the amygdala circuit but also point out differences in acute models of stress-related functional pain and chronic neuropathic pain. In our 2-hit priming model of functional pain, we observed that driving KOR receptors with an agonist could promote amplification of pain signaling measured as tactile allodynia.38,68 In addition, after priming, we found that stress could produce allodynia that was prevented by systemic or intra-CeA nor-BNI. By contrast, the qualities of SNL-induced aversive responses were prevented by blocking KOR signaling without altering evoked hypersensitivity. The reasons for these discrepancies are not clear and may have to do with differential activation of KOR signaling by acute episodes of stress in injury-free primed animals and after continuous nerve injury–induced afferent input in nerve-injured animals. These questions remain to be explored in future studies.
Based on the behavioral observations, we determined the effects of nerve injury on synaptic plasticity within the amygdala circuit using brain slices from nerve-injured rats and sham controls. Electrical stimulation was used to activate synaptic inputs to CeLC neurons. Although the specific pathway was not determined here, the placement of the electrode dorsomedial to the CeA and ventral to but outside of the caudate-putamen suggests that the fiber tract containing afferents from the PB was stimulated.13,37,43,66 The trajectory of PB fibers and their termination in the CeLC have been described in detail (see for example Ref. 53), and only afferents to the CeA from the PB have been reported in the vicinity of this tract.15,55 Stimulation of these afferent fibers produced an increase in evoked spiking in CeLC neurons. Neurons recorded here were nonaccommodating regular spiking, characteristic of peptidergic neurons containing CRF and/or dynorphin41; they are distinct from late-firing PKCδ+ CeA neurons that inhibit amygdala output.8,11,16,35 The neuronal subtype was not determined in this study. Administration of nor-BNI inhibited increased output suggesting dynorphinergic/KOR tone that was established in the nerve-injured animal remains within the slice preparation to drive activity in the amygdala output region. Recording of IPSCs revealed that nor-BNI increased inhibitory synaptic currents in CeLC neurons from SNL rats but not sham controls, suggesting that nerve injury reduced synaptic inhibition and this is restored by treatment with nor-BNI. Thus, a likely mechanism for nerve injury–induced excitability within the CeA is decreased tonic inhibitory control of neurons in the amygdala output region (disinhibition). Blockade of endogenous dynorphinergic tone with KOR antagonists may restore the inhibitory control to normalize output. Taken together with the observed behavioral responses, it seems possible that increased pronociceptive output from the CeA in the nerve-injured rat promotes pain aversiveness while non-KOR–dependent mechanisms, possibly within spinal circuits, promote enhanced tactile hypersensitivity.
These findings may also have significant therapeutic implications. Selective KOR agonists have previously advanced to clinical trials,45,46 but whether these compounds produce clinically significant pain relief remains uncertain. Mixed and suboptimal outcomes were observed in 2 trials with enadoline, a highly selective and efficacious KOR agonist.45,46 In addition, nalfurafine, a high-efficacy KOR agonist, is used clinically as an antipruritic agent29 and has not been reported to produce analgesia,31 consistent with the lack of analgesic reports associated with salvinorin A that also acts selectively at the KOR.60 Highly selective and short-acting KOR antagonists, however, are currently in development for numerous stress-related functional pain disorders and may soon progress to clinical trials.64 The present results suggest that blockade of KOR signaling within the right CeA may provide benefit in inhibiting the affective qualities of pain while preserving physiologically relevant nociceptive signaling. Finally, it should be noted that blockade of KOR may also be beneficial in preventing the anxiety that is associated with surgery and the consequences of increased preoperative stress that may promote the development of chronic postsurgical pain.1
Acknowledgements
This work was supported by NIH grants R01DA041809 to F. Porreca and R01NS106902 to F. Porreca and V. Neugebauer.
Footnotes
Conflict of interest statement
The authors have no conflict of interest to declare.
References
- [1].Arora V, Martin TJ, Aschenbrenner CA, Hayashida K, Kim SA, Parker RA, Eisenach JC, Peters CM. Psychosocial stress delays recovery of postoperative pain following incisional surgery in the rat. Neuroscience 2018;382:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baliki MN, Apkarian AV. Nociception, pain, negative moods, and behavior selection. Neuron 2015;87:474–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bannister K, Qu C, Navratilova E, Oyarzo J, Xie JY, King T, Dickenson AH, Porreca F. Multiple sites and actions of gabapentin-induced relief of ongoing experimental neuropathic pain. PAIN 2017;158:2386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beckerman MA, Van Kempen TA, Justice NJ, Milner TA, Glass MJ. Corticotropin-releasing factor in the mouse central nucleus of the amygdala: ultrastructural distribution in NMDA-NR1 receptor subunit expressing neurons as well as projection neurons to the bed nucleus of the stria terminalis. Exp Neurol 2013;239:120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Borsook D, Maleki N, Becerra L, McEwen B. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron 2012;73:219–34. [DOI] [PubMed] [Google Scholar]
- [6].Bourke JH, Langford RM, White PD. The common link between functional somatic syndromes may be central sensitisation. J Psychosom Res 2015; 78:228–36. [DOI] [PubMed] [Google Scholar]
- [7].Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res 2010;1314:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC-delta(+) neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci 2014;17:1240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carrasquillo Y, Gereau RW IV. Hemispheric lateralization of a molecular signal for pain modulation in the amygdala. Mol Pain 2008;4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen Z, Chen X, Liu M, Dong Z, Ma L, Yu S. Altered functional connectivity of amygdala underlying the neuromechanism of migraine pathogenesis. J Headache Pain 2017;18:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 2010;468: 277–82. [DOI] [PubMed] [Google Scholar]
- [12].Fendt M, Koch M, Schnitzler HU. Corticotropin-releasing factor in the caudal pontine reticular nucleus mediates the expression of fear-potentiated startle in the rat. Eur J Neurosci 1997;9:299–305. [DOI] [PubMed] [Google Scholar]
- [13].Fu Y, Neugebauer V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J Neurosci 2008;28:3861–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol 2002;87:251–8. [DOI] [PubMed] [Google Scholar]
- [15].Harrigan EA, Magnuson DJ, Thunstedt GM, Gray TS. Corticotropin releasing factor neurons are innervated by calcitonin gene-related peptide terminals in the rat central amygdaloid nucleus. Brain Res Bull 1994;33:529–34. [DOI] [PubMed] [Google Scholar]
- [16].Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 2010;468:270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ikeda R, Takahashi Y, Inoue K, Kato F. NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. PAIN 2007;127:161–72. [DOI] [PubMed] [Google Scholar]
- [18].Iliopoulos P, Damigos D, Kerezoudi E, Limpitaki G, Xifaras M, Skiada D, Tsagkovits A, Skapinakis P. Trigger factors in primary headaches subtypes: a cross-sectional study from a tertiary centre in Greece. BMC Res Notes 2015;8:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ji G, Fu Y, Adwanikar H, Neugebauer V. Non-pain-related CRF1 activation in the amygdala facilitates synaptic transmission and pain responses. Mol Pain 2013;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ji G, Li Z, Neugebauer V. Reactive oxygen species mediate visceral pain-related amygdala plasticity and behaviors. PAIN 2015;156:825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ji G, Neugebauer V. Hemispheric lateralization of pain processing by amygdala neurons. J Neurophysiol 2009;102:2253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ji G, Zhang W, Mahimainathan L, Narasimhan M, Kiritoshi T, Fan X, Wang J, Green TA, Neugebauer V. 5-HT2C receptor knockdown in the amygdala inhibits neuropathic-pain-related plasticity and behaviors. J Neurosci 2017;37:1378–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jiang Y, Oathes D, Hush J, Darnall B, Charvat M, Mackey S, Etkin A. Perturbed connectivity of the amygdala and its subregions with the central executive and default mode networks in chronic pain. PAIN 2016; 157:1970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jongen-Relo AL, Amaral DG. Evidence for a GABAergic projection from the central nucleus of the amygdala to the brainstem of the macaque monkey: a combined retrograde tracing and in situ hybridization study. Eur J Neurosci 1998;10:2924–33. [DOI] [PubMed] [Google Scholar]
- [25].Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. PAIN 1992;50:355–63. [DOI] [PubMed] [Google Scholar]
- [26].King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci 2009;12:1364–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kiritoshi T, Ji G, Neugebauer V. Rescue of impaired mGluR5-driven endocannabinoid signaling restores prefrontal cortical output to inhibit pain in arthritic rats. J Neurosci 2016;36:837–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Knoll AT, Carlezon WA Jr. Dynorphin, stress, and depression. Brain Res 2010;1314:56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kozono H, Yoshitani H, Nakano R. Post-marketing surveillance study of the safety and efficacy of nalfurafine hydrochloride (Remitch((R)) capsules 2.5 mug) in 3,762 hemodialysis patients with intractable pruritus. Int J Nephrol Renovasc Dis 2018;11:9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kuo CC, Yen CT. Comparison of anterior cingulate and primary somatosensory neuronal responses to noxious laser-heat stimuli in conscious, behaving rats. J Neurophysiol 2005;94:1825–36. [DOI] [PubMed] [Google Scholar]
- [31].Lazenka ML, Moerke MJ, Townsend EA, Freeman KB, Carroll FI, Negus SS. Dissociable effects of the kappa opioid receptor agonist nalfurafine on pain/itch-stimulated and pain/itch-depressed behaviors in male rats. Psychopharmacology (Berl) 2018;235:203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping chronic pain conditions: implications for diagnosis and classification. J Pain 2016;17(9 suppl):T93–T107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Malan TP, Ossipov MH, Gardell LR, Ibrahim M, Bian D, Lai J, Porreca F. Extraterritorial neuropathic pain correlates with multisegmental elevation of spinal dynorphin in nerve-injured rats. PAIN 2000;86:185–94. [DOI] [PubMed] [Google Scholar]
- [34].Maleki N, Becerra L, Borsook D. Migraine: maladaptive brain responses to stress. Headache 2012;52(suppl 2):102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, Bruchas MR. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 2015;87:605–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mitchell JM, Margolis EB, Coker AR, Allen DC, Fields HL. Intra-VTA deltorphin, but not DPDPE, induces place preference in ethanol-drinking rats: distinct DOR-1 and DOR-2 mechanisms control ethanol consumption and reward. Alcohol Clin Exp Res 2014;38:195–203. [DOI] [PubMed] [Google Scholar]
- [37].Nakao A, Takahashi Y, Nagase M, Ikeda R, Kato F. Role of capsaicin-sensitive C-fiber afferents in neuropathic pain-induced synaptic potentiation in the nociceptive amygdala. Mol Pain 2012;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nation KM, De Felice M, Hernandez PI, Dodick DW, Neugebauer V, Navratilova E, Porreca F. Lateralized kappa opioid receptor signaling from the amygdala central nucleus promotes stress-induced functional pain. PAIN 2018;159:919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Navratilova E, Xie J, King T, Porreca F. Evaluation of reward from pain relief. Ann N Y Acad Sci 2013;1282:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Navratilova E, Xie JY, Meske D, Qu C, Morimura K, Okun A, Arakawa N, Ossipov M, Fields HL, Porreca F. Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. J Neurosci 2015;35: 7264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Neugebauer V Amygdala pain mechanisms. Handb Exp Pharmacol 2015;227:261–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res Rev 2009;60:226–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Neugebauer V, Li W, Bird GC, Bhave G, Gereau RW IV. Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci 2003;23: 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist 2004;10:221–34. [DOI] [PubMed] [Google Scholar]
- [45].Pande AC, Pyke RE, Greiner M, Cooper SA, Benjamin R, Pierce MW. Analgesic efficacy of the kappa-receptor agonist, enadoline, in dental surgery pain. Clin Neuropharmacol 1996;19:92–7. [DOI] [PubMed] [Google Scholar]
- [46].Pande AC, Pyke RE, Greiner M, Wideman GL, Benjamin R, Pierce MW. Analgesic efficacy of enadoline versus placebo or morphine in postsurgical pain. Clin Neuropharmacol 1996;19:451–6. [DOI] [PubMed] [Google Scholar]
- [47].Paxino G, Watson C. The rat brain in stereotaxic coordinates. New York, NY: Academic Press, 2007. [Google Scholar]
- [48].Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states—maybe it is all in their head. Best Pract Res Clin Rheumatol 2011;25:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, Blasio A, Dadgar J, Kharazia V, De Guglielmo G, Crawford E, Janak PH, George O, Rice KC, Messing RO. A transgenic rat for investigating the anatomy and function of corticotrophin releasing factor circuits. Front Neurosci 2015;9:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ren W, Kiritoshi T, Gregoire S, Ji G, Guerrini R, Calo G, Neugebauer V. Neuropeptide S: a novel regulator of pain-related amygdala plasticity and behaviors. J Neurophysiol 2013;110:1765–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ren W, Neugebauer V. Pain-related increase of excitatory transmission and decrease of inhibitory transmission in the central nucleus of the amygdala are mediated by mGluR1. Mol Pain 2010;6:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Reyes BA, Carvalho AF, Vakharia K, Van Bockstaele EJ. Amygdalar peptidergic circuits regulating noradrenergic locus coeruleus neurons: linking limbic and arousal centers. Exp Neurol 2011;230:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sarhan M, Freund-Mercier MJ, Veinante P. Branching patterns of parabrachial neurons projecting to the central extended amgydala: single axonal reconstructions. J Comp Neurol 2005;491:418–42. [DOI] [PubMed] [Google Scholar]
- [54].Schiess MC, Callahan PM, Zheng H. Characterization of the electrophysiological and morphological properties of rat central amygdala neurons in vitro. J Neurosci Res 1999;58:663–73. [PubMed] [Google Scholar]
- [55].Schwaber JS, Sternini C, Brecha NC, Rogers WT, Card JP. Neurons containing calcitonin gene-related peptide in the parabrachial nucleus project to the central nucleus of the amygdala. J Comp Neurol 1988;270: 416–26, 398–419. [DOI] [PubMed] [Google Scholar]
- [56].Sibille KT, Chen H, Bartley EJ, Riley J III, Glover TL, King CD, Zhang H, Cruz-Almeida Y, Goodin BR, Sotolongo A, Petrov ME, Herbert M, Bulls HW, Edberg JC, Staud R, Redden D, Bradley LA, Fillingim RB. Accelerated aging in adults with knee osteoarthritis pain: consideration for frequency, intensity, time, and total pain sites. Pain Rep 2017;2: e591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D. The human amygdala and pain: evidence from neuroimaging. Hum Brain Mapp 2014;35:527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016;338:114–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol 1993;330:381–404. [DOI] [PubMed] [Google Scholar]
- [60].Taylor GT, Manzella F. Kappa opioids, salvinorin A and major depressive disorder. Curr Neuropharmacol 2016;14:165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Tepper SJ. Medication-overuse headache. Continuum (Minneap Minn) 2012;18:807–22. [DOI] [PubMed] [Google Scholar]
- [62].Vachon-Presseau E, Tetreault P, Petre B, Huang L, Berger SE, Torbey S, Baria AT, Mansour AR, Hashmi JA, Griffith JW, Comasco E, Schnitzer TJ, Baliki MN, Apkarian AV. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain 2016; 139(Pt 7):1958–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Veasley C, Clare D, Clauw DJ, Cowley T, Nguyen RHN, Reinecke P, Vernon SD, Williams D. Impact of chronic overlapping pain conditions on public health and the urgent need for safe and effective treatment: chronic pain research alliance, The TMJ Association, Ltd., Milwaukee, WI, 2015. [Google Scholar]
- [64].Wallace TL. Proceedings of the Society for Neuroscience, 2017. November 11–15, 2017, Washington, DC. [Google Scholar]
- [65].Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP Jr, Lai J, Porreca F. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci 2001;21:1779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Watabe AM, Ochiai T, Nagase M, Takahashi Y, Sato M, Kato F. Synaptic potentiation in the nociceptive amygdala following fear learning in mice. Mol Brain 2013;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Woolf CJ. Central sensitization: uncovering the relation between pain and plasticity. Anesthesiology 2007;106:864–7. [DOI] [PubMed] [Google Scholar]
- [68].Xie JY, De Felice M, Kopruszinski CM, Eyde N, LaVigne J, Remeniuk B, Hernandez P, Yue X, Goshima N, Ossipov M, King T, Streicher JM, Navratilova E, Dodick D, Rosen H, Roberts E, Porreca F. Kappa opioid receptor antagonists: a possible new class of therapeutics for migraine prevention. Cephalalgia 2017;37:780–94. [DOI] [PMC free article] [PubMed] [Google Scholar]