Abstract
Yersinia enterocolitica causes a severe enteric infection in infants and young children. There is no vaccine approved for use in humans. We investigated the immunogenicity and protective capacity of Yersinia YopB, a conserved type III secretion system protein, alone or combined with LcrV in adult mice immunized intranasally. YopB or LcrV (5 μg) administered with the E. coli double mutant heat labile toxin (dmLT) adjuvant afforded modest (10–30%) protection against lethal Y. enterocolitica oral infection. The combination of YopB and LcrV (5 μg each) dramatically improved vaccine efficacy (70–80%). Additionally, it afforded complete protection against Y. pestis pulmonary infection. Immunization with YopB/LcrV+dmLT resulted in antigen-specific serum IgG, systemic and mucosal antibody secreting cells, as well as IFN-γ, TNF-α, IL-2, IL-6, IL-17A and KC production by spleen cells. Serum antibodies elicited by YopB/LcrV+dmLT had enhanced bactericidal and opsonophagocytic killing activity. After Y. enterocolitica challenge, YopB/LcrV+dmLT vaccinated mice exhibited intact intestinal tissue, active germinal centers in mesenteric lymph nodes, IgG+ and IgA+ plasmablasts in the lamina propria, and antibodies in intestinal fluid. On the contrary, complete tissue destruction and abscesses were seen in placebo recipients that succumbed to infection. Mice immunized as infants with YopB+dmLT or LcrV+dmLT achieved 60% protection against lethal Y. enterocolitica infection, and vaccine efficacy increased to 90–100% when they received YopB/LcrV+dmLT. YopB+dmLT also afforded substantial (60%) protection when administered intradermally to infant mice. YopB/LcrV+dmLT is a promising subunit vaccine candidate with the potential to elicit broad protection against Yersinia spp.
Keywords: Yersinia, mucosal vaccines, enteric pathogens, antibody secreting cells, intestinal pathology
Introduction
Yersinia is a zoonotic human pathogen, usually transmitted through contaminated food and water. It causes an acute gastrointestinal diarrheal illness known as yersiniosis. Clinical manifestations are broad and include enteritis, enterocolitis, and mesenteric lymphadenitis, and severe cases can develop septicemia and result in death (1). Individuals recovering from infection often suffer from reactive arthritis or other chronic inflammatory diseases (2). The majority of Yersinia infection cases in the US (~100,000 annually) are attributed to Y. enterocolitica, which affects primarily infants and children under 5 years of age (3). Y. enterocolitica is also the third cause of bacterial diarrheal infection in Europe (4). Although information is limited, a high prevalence of Yersinia-associated enteric disease has also been reported in developing countries (5, 6). Because it is often misdiagnosed and underreported, the real burden is likely higher than officially estimated and despite efforts to improve hygiene and food control, incidence remains stagnant. Antibiotic treatment, the standard of care for symptomatic cases, has harmful side effects in young children (7). Antibiotic therapy is also constrained by biofilm formation, which reduces bacterial susceptibility and promotes the selection of antibiotic resistant strains (8). Prophylactic immunization of young children, the most affected group, would help prevent Y. enterocolitica infection in a practical and cost-effective manner. Unfortunately, there is no approved vaccine available.
Molecularly engineered live attenuated strains of Y. enterocolitica and Y. pestis have been efficacious against Y. enterocolitica infection in proof-of-concept mouse studies (9–12). Such vaccines are intended for oral immunization in humans and expected to engender protective immunity through the natural route of exposure. A drawback of this approach is the limited success met by oral vaccines when given to children living in poor countries as compared to those from industrialized nations (13, 14). On the other hand, parenterally delivered subunit-based vaccines have a strong record of safety and effectiveness in preventing infectious diseases through routine infant immunization. Subunit vaccines based on Yersinia HSP60 virulence factor (15), type III secretion system (T3SS) needle tip protein LcrV (16) and the outer membrane effector protein YopE (17, 18) formulated as single or fusion proteins have been shown to protect mice against Y. enterocolitica infection. While LcrV and the Yersinia fraction 1 (F1) capsular protein have been tested in humans as components of a prospective plague vaccine, none of the existing candidates for prevention of yersiniosis have advanced into human clinical studies.
LcrV is considered a leading Yersinia vaccine antigen due to its role in bacterial pathogenesis and ability to induce strong immune responses. However, because the lcrV gene is highly polymorphic and even absent in recent clinical isolates, a vaccine relying solely on LcrV might have limited applicability (19, 20). LcrV is also reportedly immunosuppressive (21), which raises a safety concern when administered in high doses.
Herein, we investigated the immunogenicity and protective efficacy of YopB, a T3SS translocator protein that participates in the formation of pores in the host cell membrane (22) and the injection of microbial effector proteins that lead to cell damage and bacterial dissemination (23). The rationale for selecting of YopB as a vaccine candidate was the successful protection afforded by Shigella IpaB (24, 25) and P. aeruginosa PopB (26), both of which share significant sequence homology with YopB. YopB is essential for virulence and less likely to be lost or modified from evolutionary adaptation. The conserved structure of YopB is particularly appealing due to its potential for eliciting broad protection across Yersinia spp. Current models of the Yersinia T3SS injectisome assume that LcrV and YopB work in synergy forming a pore in the host cell membrane, with LcrV sitting at the tip of the needle and connecting with the target cell through the YopB/D pore complex (27). Thus, we further hypothesized that protection could be enhanced by simultaneously blocking YopB and LcrV. In a similar manner, the combination of T3SS IpaB and IpaD had been pursued to elicit broad protective immunity against shigellosis (25).
Hence, we examined the immune responses and protective efficacy induced by YopB alone or combined with LcrV in adult mice immunized via the intranasal route and challenged with different Yersinia strains. The E. coli double mutant heat-labile toxin (dmLT), a clinically advanced adjuvant, was co-administered with the vaccine antigens or given alone as placebo. Mice were also immunized as infants to confirm the suitability of the vaccine for younger hosts. Systemic and mucosal immunity were examined after vaccination. Assays were established to determine the functional, antimicrobial activity of vaccine-induced antibodies. Survival rates, intestinal tissue damage, and recall mucosal and systemic immunity were determined post-challenge as indicators of vaccine efficacy.
Materials and Methods
Vaccine preparation.
YopB was produced as previously described (28). Briefly, the YopB gene from Y. enterocolitica was amplified by PCR, cloned into pET15b, and used to transform E. coli NovaBlue. The translocator chaperone sycD was also cloned into pACYDuet-1 and used to transform E. coli Tuner for co-expression. Bacteria containing both plasmids were selected and YopB/SycD co-expressed and purified using standard immobilized metal affinity chromatography (IMAC). The chaperone was removed through a second IMAC step using buffer containing lauryldimethylamine N-oxide (LDAO). The YopB used for vaccination was resuspended in PBS containing 0.05% (w/v) LDAO and determined to be >90% pure by SDS-PAGE. For purification of LcrV, the LcrV gene from Y. pseudotuberculosis was amplified by PCR from plasmid pSEC91-LcrV, cloned into the Lactococcus lactis expression vector pMUC003 and plasmid pMUC056 was the result. This plasmid was used to transform L. lactis PA1001 and LcrV was purified using standard IMAC. LcrV was confirmed to be >95% pure by SDS-PAGE. The E. coli dmLT was purified by affinity chromatography (29); it was produced by the Walter Reed Army Institute of Research and obtained through an agreement with PATH-EVI. The YopB/LcrV+dmLT used for immunization had negligible endotoxin content: 3.8 EU/ml, determined by Endosafe PTS® chromogenic Limulus Amebocyte Lysate assay (Charles River, MA); values <20 EU/ml are recommended for recombinant subunit vaccines (30). The same individual antigen lots were used for in vitro cell stimulation in cytokine production assays.
Mice, vaccination, tissue collection, and experimental challenge.
Adult (7–8 wks old) female BALB/c mice (10 per group) obtained from Charles River Laboratories (Wilmington, MA) were immunized via the intranasal (i.n.) route on days 0, 14, and 28 with increasing amounts (5, 10, or 20 μg) of YopB and/or LcrV, with or without dmLT (2.5 μg), in a 20 μl volume. Control mice received dmLT alone or PBS. Serum was collected prior to each vaccination, and on days 42 and 56 (immediately before challenge). Lung, bronchoalveolar and intestinal lavage fluid, spleen, and bone marrow were collected from subgroups of 5 mice on days 35 and/or 56 after initial vaccination, as previously described (24, 31). Mice bred in house (7–12 pups from 2–3 litters per group) were vaccinated i.n. on days 7, 14, 21, and 28 after birth with YopB and LcrV (5 μg of each) with or without dmLT (1 μg). Control groups received 1 μg of dmLT or remained unvaccinated (naïve). In a separate experiment, infant mice were vaccinated on days 14, 21, 28, and 35 after birth with YopB (50, 100, or 200 ng) and dmLT (50 ng) via the intradermal (i.d.) route, as described previously (24); controls received 50 ng of dmLT alone or PBS. Blood samples were collected from age-matched pups on day 7 or 14 (prior to vaccination) and from vaccinated mice every week thereafter until day 28 and every two weeks until day 63 after birth. Twenty eight days after the last immunization, mice were challenged via oral gavage with 100 μl containing 9.7–10.3×107 CFU (15–16 week old adult mice) and 10.9–12.0×107 CFU (8–9 week old young mice) of Y. enterocolitica strain Ye8081 (provided by Dr. Virginia Miller, University of North Carolina School of Medicine); organisms were grown as previously described (32). The challenge dose corresponded to ~10 times the dose at which 50% death was achieved (LD50) for both age groups. A group of vaccinated and a group of control adult mice were challenged i.n. with of 4×104 CFU (in 30 μl) of Y. pestis CO92 Δpgm, corresponding to ~30 LD50, grown under conditions previously described (33). Studies were approved by the University of Maryland Institutional Animal Care and Use Committee.
Antibody measurements.
LcrV- and dmLT-specific antibodies were measured by ELISA as previously described (25, 34). For YopB-specific antibodies, plates were coated with 0.1 μg/ml of YopB. Serum bactericidal activity (SBA) and opsonophagocytic killing activity (OPKA) assays were adapted and optimized using Y. enterocolitica Ye8081 as target strain based on published methods (35). For SBA, heat-inactivated mouse serum samples were added to 96-well U bottom plates in duplicate (Fisher Scientific, Hampton, NH) and serially diluted two-fold in PBS (initial serum dilution of 1:200). 25 μl of baby rabbit complement (BRC, Pel Biologicals, Rogers, AR) followed by 2×104 CFU in 10 μl of Y. enterocolitica Ye8081 were added to the wells, and plates incubated for 1 h at 30°C, shaking at 225 rpm. Viable CFU were determined by plating 10 μl from reaction mix on animal product free LB agar and counting colonies after 48 h incubation at room temperature; a total of 4 independent CFU counts per serum dilution were averaged for titer calculation. Controls in each assay included: bacteria and BRC (no serum or antibody) and a positive serum from mice vaccinated with YopB/LcrV+dmLT and challenged with virulent Ye8081 (mean SBA titer ± SD = 5,460 ± 639). SBA titer was defined as the reciprocal of the highest serum dilution that produced > 50% bacterial killing in relation to the killing observed for the bacteria and BRC control. For OPKA, 1×105 J774 murine macrophage cells were seeded overnight to form monolayers in supplemented Dulbecco modified Eagle medium (DMEM). The following morning, 4×104 CFU (in 20 μl) of Y. enterocolitica Ye8081 were mixed with 10 μl of heat-inactivated serially diluted serum along with 10 μl BRC in DMEM in a 96-well U bottom plate, in duplicate, and incubated at room temperature, with shaking, for 20 min. Then 10 μl of the opsonized bacteria mixture was added to J774 cell monolayers containing 90 μl of fresh DMEM. Cells and opsonized bacteria were incubated for 45 min at 37°C, 5% CO2. Viable CFU were determined as described above. A negative control included bacteria (no serum), BRC, and J774 cells. The same hyperimmune serum mentioned above (mean OPKA titer ± SD = 3,518 ± 125) was included as positive control. OPKA titers were determined as the reciprocal of the serum dilution that produced 50% bacterial killing based in relation to the killing observed for the bacteria, BRC, and cells control.
Antibody secreting cells (ASC) and cytokine measurements.
Frequencies of IgG and IgA ASC in the spleen, bone marrow, and lungs were measured by ELISpot as previously described (31); plates were coated with 5 μg/ml of YopB, LcrV, or dmLT. For cytokine measurements, spleen cells (2×105 in 100 μl) were stimulated for 48 h with 5 μg/ml of YopB or LcrV, and cytokine levels were measured in culture supernatants using the V-PLEX Plus Proinflammatory Panel 1 and IL-17A kits from Meso Scale Discovery (MSD), Gaithersburg, MD, as described (31).
Tissue histology and immunofluorescence.
A subgroup of 5 adult mice were euthanized one week after challenge (before deaths occurred in control groups), and mesenteric lymph nodes (MLN) and intestinal tissue were collected and preserved in 4% paraformaldehyde. Tissue samples were embedded in paraffin, sectioned, mounted on slides, and stained with H&E. For immunofluorescence, sections were de-paraffinized, incubated for 1 h with blocking buffer (PBS containing 10% goat serum and 1% BSA) and incubated overnight at 4°C in a humidified chamber with primary antibodies diluted in blocking buffer as follows: anti-CD138 (BioLegend, San Diego, CA) diluted at 1:1000, anti-CD19 (Cell Signaling Technology, Danvers, MA) diluted at 1:50, and biotin-labeled IgG or biotin-labeled IgA (KPL, Gaithersburg, MD) diluted at 1:50. Alexa 568-labeled goat anti-rat IgG, Alexa 633-labeled goat anti-rabbit IgG and Alexa 488-labeled streptavidin (all from Life Technologies, Carlsbad, CA) secondary antibodies were added for 30 min in the dark at room temperature. Cells were counterstained with DAPI (Life Technologies) and mounted in VectaShield antifade medium (Vector Laboratories, Burlingame, CA). Histology Images were captured using an Olympus BH-2 microscope with a SPOT 4 Mega Pixel RT color camera and imaging software (SPOT Imaging, Sterling Heights, MI). Fluorescent images were acquired on a Nikon A1 confocal microscope and brightness and contrast adjusted equivalently using NIS Elements software (Nikon Instruments, Melville, NY).
Statistical Analyses.
ELISA IgG and IgA titers were analyzed using 2-way ANOVA with Tukey’s multiple comparisons test. SBA and OPKA titers were compared using 1-way ANOVA with Tukey’s multiple comparisons test. Survival post-challenge was compared by Log-rank (Mantel-Cox) tests. Pre- and post-challenge SBA titers were compared using Wilcoxon matched-pairs signed rank test. ASC numbers and cytokine levels were compared using 2-way ANOVA with Dunnet’s multiple comparisons test. For all analyses, differences were considered statistically significant at P ≤ 0.05. GraphPad Prism 6 software (GraphPad Software, Inc., La Jolla, CA) was used for all statistical analyses.
Results
Mucosal immunization with YopB and LcrV conferred broad protection against lethal Yersinia infection.
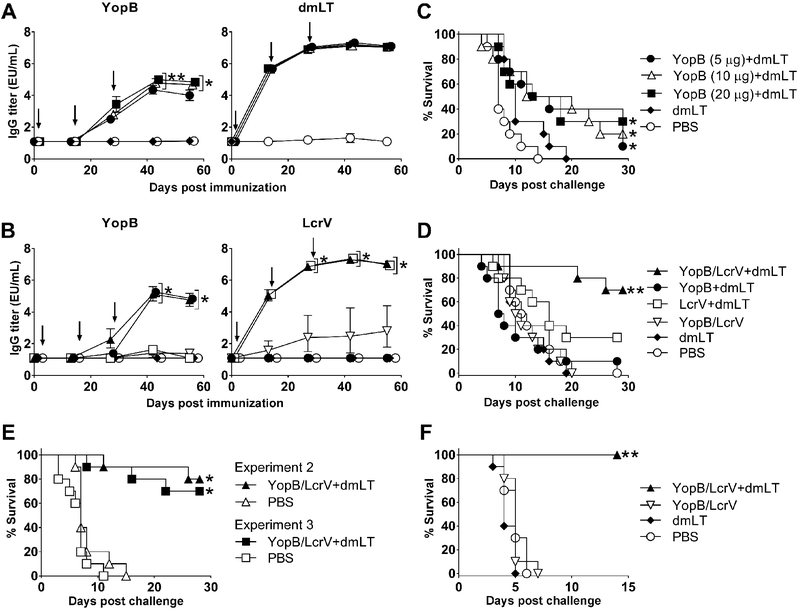
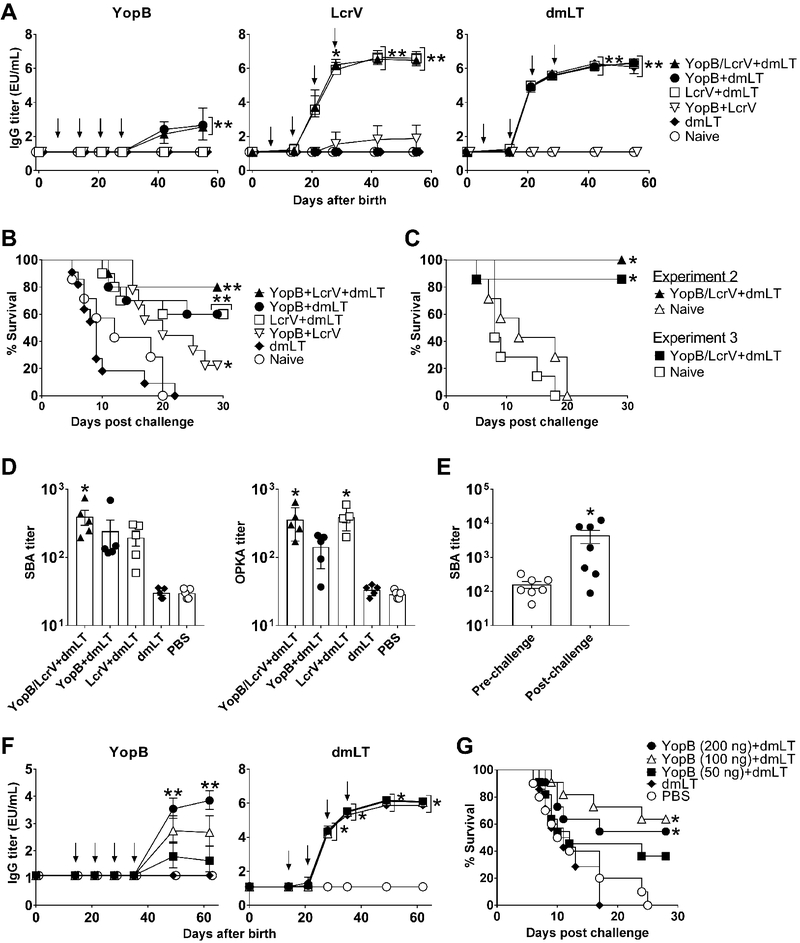
To test the hypothesis that the T3SS translocator YopB could elicit a protective immunity against Y. enterocolitica, increasing amounts of YopB (5, 10, and 20 μg) along with 2.5 μg of the E. coli dmLT adjuvant were administered i.n. to adult mice on 3 occasions, 2 weeks apart (Fig. 1). Control groups received dmLT alone or PBS. All YopB-vaccinated mice developed antigen-specific serum IgG antibodies. Titers increased after the second and third immunizations, and reached a plateau 2 weeks after the last vaccine dose (Fig. 1A). Mice receiving 10 and 20 μg of YopB had the highest serum IgG titers from all groups. YopB-vaccinated mice also developed high levels of dmLT-specific serum IgG; the kinetics of antibody response for all groups that received dmLT were almost identical (Fig. 1A). No responses were seen in the unvaccinated controls. One month after the third vaccination, all animals were exposed to lethal oral Y. enterocolitica infection. Control mice succumbed 15–20 days post-challenge. In contrast, modest protection (up to 30%) and prolonged survival were observed in YopB+dmLT recipients as compared to the unvaccinated (PBS) controls (Fig. 1C).
Figure 1. A combined YopB and LcrV vaccine induced broad protective immunity against Yersinia infection.
Kinetics of serum IgG titers measured by ELISA in adult mice immunized i.n. with 5 μg, 10 μg, or 20 μg of YopB along with 2.5 μg dmLT (A) or with YopB and/or LcrV (5 μg each) with or without 2.5 μg dmLT (B). Data points represent the geometric mean titer of 10 mice per group for YopB and pool titers for dmLT; error bars represent 95% confidence interval on a log10 scale. Arrows indicate vaccination. Significant differences in IgG titers between groups were determined by 2-way ANOVA with Tukey’s multiple comparisons test; *, P<0.05 compared to YopB (5μg)+dmLT, dmLT and PBS, **, P<0.05 compared to all groups (A). *, P <0.05 compared to YopB/LcrV, dmLT and PBS groups (B). (C & D) Survival following oral lethal challenge with Y. enterocolitica YE8081 (10 LD50) four weeks after the final immunization. (E) Survival rates of YopB/LcrV+dmLT vaccinated and control mice in two subsequent independent experiments (n=10 mice per group in each experiment). (F) Survival rates of YopB and LcrV vaccinated mice and controls (10 per group) exposed to lethal pulmonary infection with Y. pestis CO92 Δpgm (30 LD50) four weeks after the final immunization. Significant differences in survival curves were determined by log-rank test; *, P<0.05 compared to PBS (C and E), ** P<0.05 compared to all groups (D and F).
We next tested the hypothesis that co-administration of Y. enterocolitica YopB and LcrV would improve the protection afforded by YopB alone. To this end, 5 μg of YopB (the lowest dosage level tested in the experiment described above) was combined with 5 μg LcrV and with 2.5 μg of dmLT (the same amount of adjuvant given before), and administered to mice as described above. The 5 μg antigen dose was chosen to discern any synergistic effect of the combined proteins, which could have been missed using a higher dose. Additional groups received YopB or LcrV admixed with dmLT, or YopB and LcrV in the absence of dmLT. This last group was included to distinguish the adjuvant contribution. Controls received dmLT or PBS.
Mice immunized with YopB and LcrV, either alone or combined, in the presence of dmLT, developed high levels of YopB- and LcrV-specific serum IgG (Fig. 1B). The magnitude of YopB IgG responses was the same regardless of whether it was given alone or combined with LcrV; it was also similar across experiments (~105 EU/mL). In contrast, negligible YopB- and LcrV-specific IgG titers were produced when the antigens were given without dmLT. IgG responses induced by LcrV were more robust (~2-logs higher) than those against YopB and readily detectable after a single immunization, while three doses of YopB were required to achieve similar antibody levels. Protection against lethal oral Y. enterocolitica infection remarkably improved (to 70%) when mice were immunized with both antigens (YopB/LcrV) plus dmLT, while YopB+dmLT and LcrV+dmLT groups exhibited 10 and 30% survival, respectively (Fig. 1D). All mice that received only dmLT or PBS succumbed to the infection. The superior protection elicited by YopB/LcrV+dmLT was confirmed in two subsequent independent experiments (Fig. 1E); the mean survival rate from all experiments was 73%. Importantly, the YopB and LcrV combination not only afforded a high level of protection against oral Y. enterocolitica but also complete protection against lethal pulmonary infection with Y. pestis (Fig. 1F).
Combined YopB and LcrV immunization enhanced serum functional antibody activity.
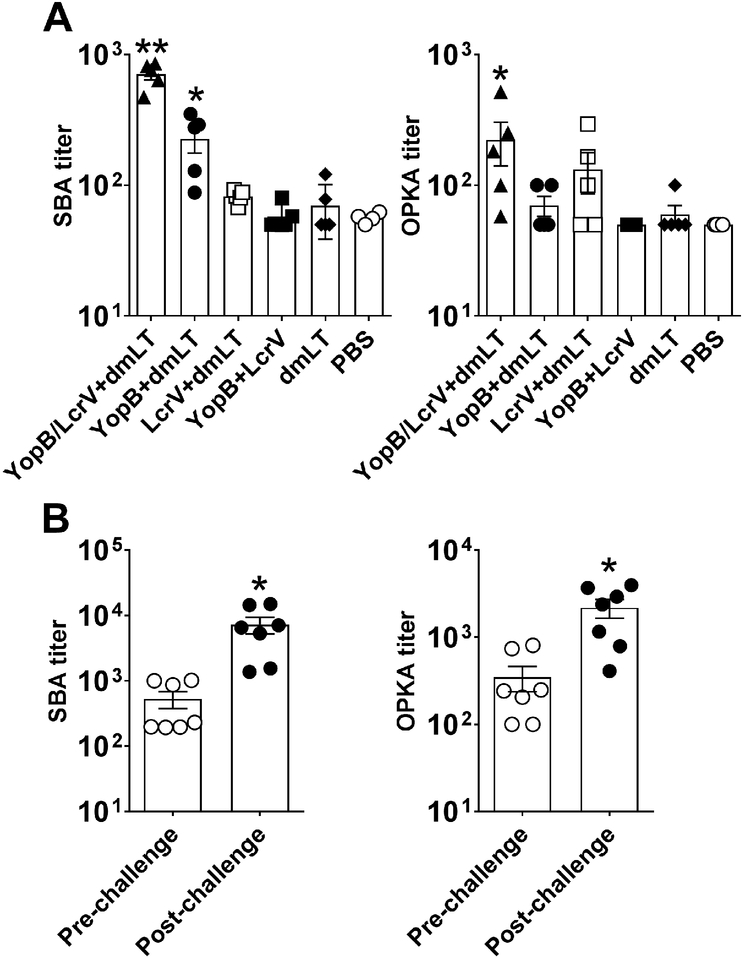
To understand the mechanism underlying the heightened protective efficacy of the YopB and LcrV combination, we examined the functional, antimicrobial capacity of serum antibodies induced post-vaccination. The antigen combination did not result in increased antigen-specific serum IgG levels compared to immunization with the single antigens (Fig. 1B). However, we reasoned that the concerted action of both antibody specificities could enhance antimicrobial activity. To test this hypothesis, we measured SBA and OPKA activity against Y. enterocolitica in serum from YopB and LcrV vaccinated groups. Assays were developed and established for this purpose, and end-point SBA and OPKA titers were determined in individual mice prior to and after challenge. Higher SBA and OPKA titers were detected in mice immunized with YopB/LcrV+dmLT at the time of challenge as compared to the remaining groups (Fig. 2A). SBA activity (4-fold above unvaccinated controls) was also seen in mice that received YopB+dmLT. Individual titers in the remaining groups were mostly below the limit of detection of the assays. The higher SBA and OPKA activity in the group that received the YopB/LcrV combination was recreated in vitro by mixing pooled sera from YopB and LcrV+dmLT vaccinated mice (Supplemental Fig. 1). SBA and OPKA titers increased significantly in YopB/LcrV+dmLT vaccine recipients that survived the challenge (Fig. 2B); this was the only group for which the measurement could be performed.
Figure 2. The YopB and LcrV combined vaccine induced serum functional antimicrobial antibodies.
Adult mice were immunized as described in Fig. 1. (A) SBA or OPKA individual and mean titers ± SEM from 5 mice per group. Significant differences among groups were determined by 1-way ANOVA with Tukey’s multiple comparisons test; *, P<0.05 compared to PBS and **, P<0.05 compared to all groups. (B) SBA and OPKA titers in YopB/LcrV+dmLT vaccinated mice before and 30 days Y. enterocolitica post-challenge; individual and mean titers ± SEM from 7 mice per group (survivors). Significant difference between groups was determined by Wilcoxon matched-pairs signed rank test; *, P<0.05 compared to pre-challenge.
YopB and LcrV immunization induced systemic and mucosal ASC.
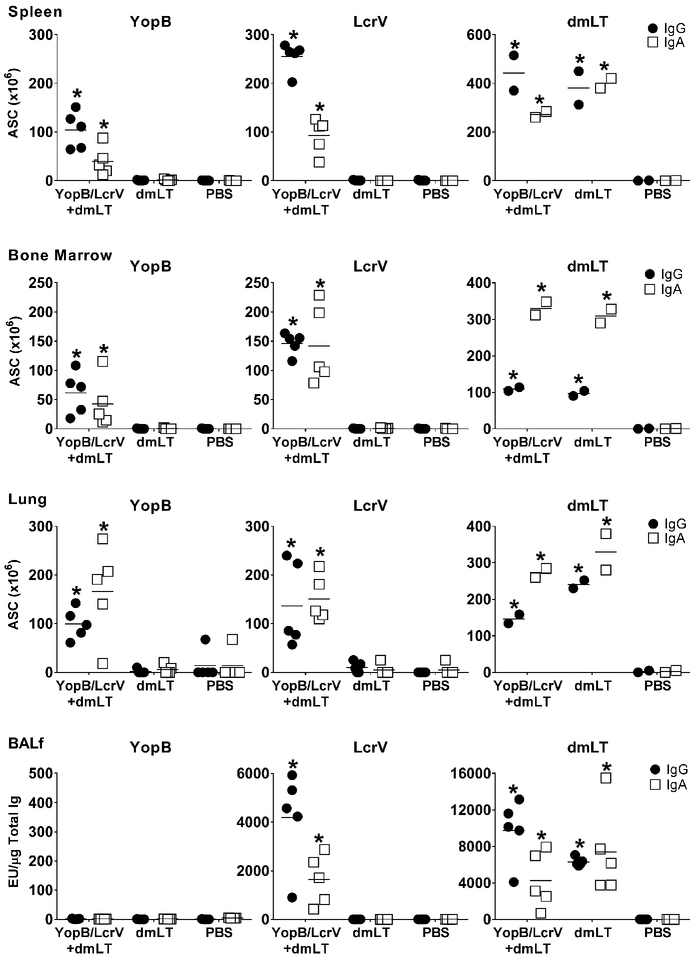
To further characterize the B cell responses induced by YopB/LcrV+dmLT, ASC were measured in spleen and bone marrow at the time of challenge (Fig. 3). During pathogen exposure, antigen-specific B cells that reside in (reservoir) lymphoid organs may reactivate, enter circulation to replenish the systemic B cell pools, and/or migrate to the gut for local antibody production. YopB- and LcrV-specific IgG and IgA ASC were detected in both tissues. LcrV-specific ASC frequencies were higher than those against YopB, and IgG secreting cells more abundant in the spleen. Vaccinated mice also developed a strong dmLT-specific ASC response; the (3 times) higher frequency of dmLT-specific IgA (as compared to IgG) ASC in the bone marrow was noteworthy. ASC were likewise measured in the lung to demonstrate the presence of vaccine-induced mucosal B cells (i.e plasmablasts). Mice that received YopB/LcrV+dmLT had high numbers of YopB- and LcrV-specific IgG and IgA ASC in the lungs, revealing a robust local B cell immunity. dmLT also induced a lung ASC response, particularly IgA secreting cells. Furthermore, LcrV- and dmLT-specific IgG and IgA were detected in bronchoalveolar fluid (BALf) of YopB/LcrV+dmLT vaccinated mice one week after the last immunization (Fig. 3). YopB-specific antibodies were not detected. Similarly, YopB antibodies were not found in intestinal fluid after vaccination, but were readily detected in immunized mice post challenge (see below).
Figure 3. Vaccination with YopB/LcrV+dmLT induces systemic and mucosal IgG and IgA antibody secreting cells (ASC) and antibodies.
Adult mice were immunized as described in Fig. 1B. Spleen and bone marrow were collected four weeks after the last immunization. Lung tissue and Bronchoalveolar lavage fluid (BALf) were collected one week after the last immunization. YopB, LcrV and dmLT-specific IgG and IgA ASC were measured by ELISpot. Graphs depict ASC counts per 106 cells (mean from duplicate wells) measured in 5 individual mice in each group for YopB and LcrV and in pooled tissue from the same 5 mice (in duplicate) for dmLT. Antigen-specific IgG and IgA were also measured by ELISA in BALf. Data correspond to individual titers from 5 mice per group. Mean response is shown as a line. Significant differences were determined by 2-way ANOVA with Dunnet’s multiple comparisons test; *, P<0.05 compared to PBS.
YopB and LcrV immunization induced antigen-specific cytokine-secreting cells.
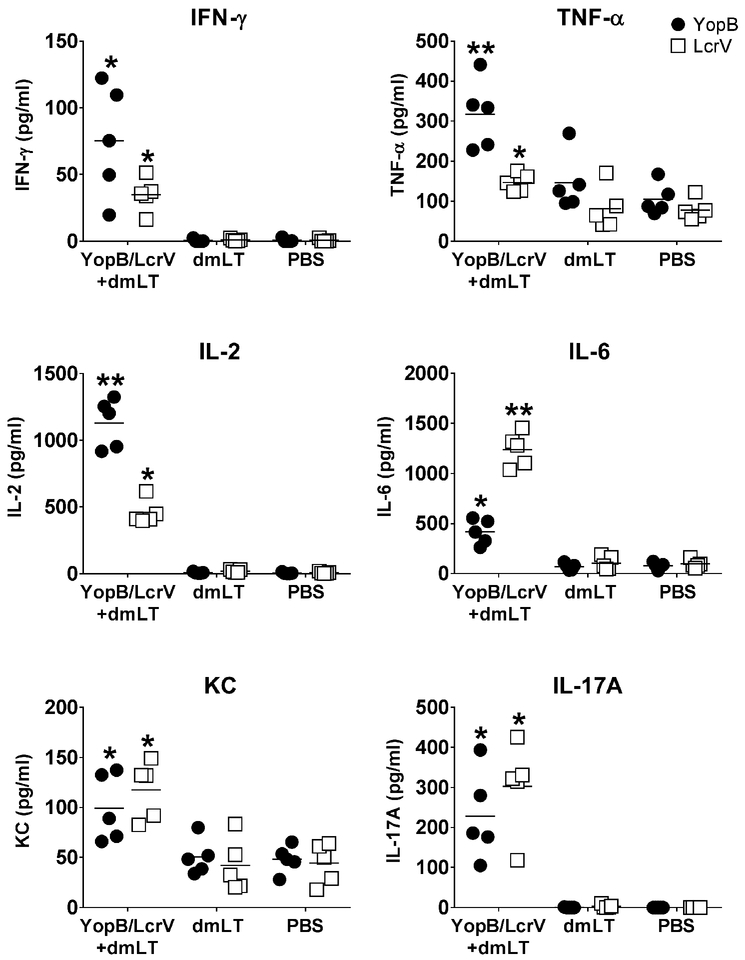
Cell-mediated immunity was evaluated through the production of Th1, Th2 and Th17 cytokines by spleen cells from YopB/LcrV+dmLT-vaccinated and control mice in response to YopB and LcrV in vitro recall stimulation. IFN-γ, IL-10, IL-12p70, IL-1β, IL-2, IL-4, IL-5, IL-6, KC/GRO, IL-17A, and TNF-α were quantified by MSD multiplex technology in culture supernatants from antigen stimulated and non-stimulated cells. IFN-γ, TNF-α, IL-2, IL-6, IL-17A and KC were the only cytokines produced at levels above those of non-stimulated cells (Fig. 4). Mice that received YopB/LcrV+dmLT exhibited recall cytokine responses above those of dmLT and PBS controls. YopB evoked particularly robust production of TNF-α and IL-2, whereas LcrV elicited high levels IL-6. Both YopB and LcrV stimulated production of IFN-γ, IL-2, IL-6, and IL-17A in vaccinated mice above the controls. Despite higher baseline production, TNF-α and KC responses were clearly increased in spleen supernatants of vaccine recipients in response to YopB and LcrV stimulation.
Figure 4. Cell-mediated immune responses induced by YopB/LcrV+dmLT.
Adult mice were immunized as described in Fig.1B and spleens were obtained four weeks after the last vaccination. Cytokines produced by spleen cells following 48 h stimulation with YopB or LcrV were measured by MSD multiarray technology. Data represent mean concentration from duplicate wells for 5 mice per group. The mean of each group is depicted as a line. Significant differences were determined by 2-way ANOVA with Dunnet’s multiple comparisons; *, P<0.05 compared to PBS and **, P<0.05 compared to all groups.
YopB and LcrV immunization prevented gastrointestinal tissue damage induced by oral Yersinia infection.
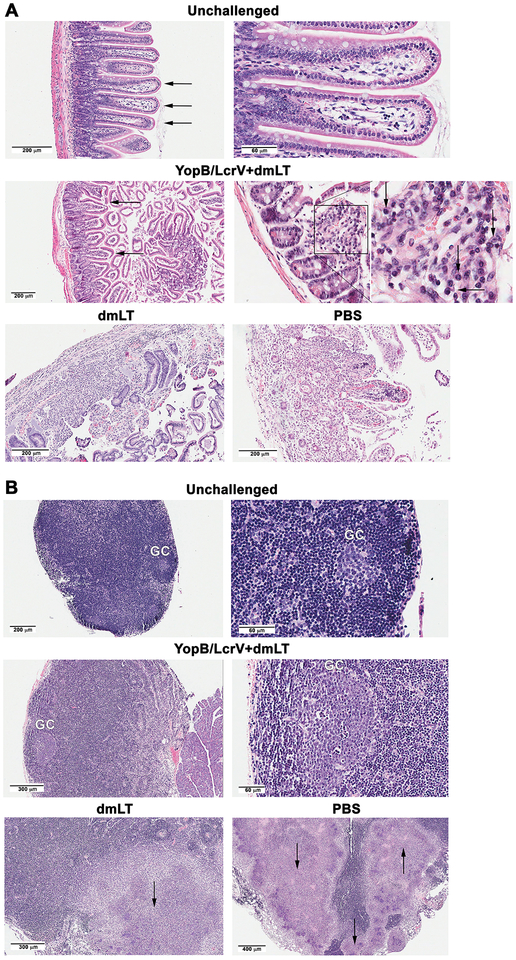
Inflammation and tissue damage within gut and draining lymph nodes were investigated post-challenge as indicators of vaccine-induced protection. Small intestine sections and MLN were obtained from YopB/LcrV+dmLT vaccinated and controls one week after Y. enterocolitica challenge - at the time when signs of disease (diarrhea, dehydration, lethargy, anorexia, and weight loss) begin to appear, and were examined by histopathology (Fig. 5). Normal intestinal tissue and intact villous-crypt architecture are depicted in unchallenged mice; the villi appeared slender (Fig 5A, top panel, arrows) and mononuclear infiltrates in the lamina propria were sparse, as expected. After challenge, YopB/LcrV+dmLT vaccinated mice exhibited mild blunting of villi (Fig 5A middle panel, arrows) with the expansion of the lamina propria due to some interstitial edema. The magnified image and expanded insert show an accumulation of inflammatory cells (predominantly mononuclear) with a significant proportion of plasma cells, characterized by a perinuclear Hof “halo” (Fig. 5A middle panel insert, arrows). Conversely, tissues from unvaccinated mice (dmLT and PBS) exhibited a complete loss of intestinal glandular structures (Fig. 5A, bottom panel). Some areas showed partial architectural disruption with prominent inflammatory infiltrates, patchy necrosis, and edema. Scattered areas of full thickness intestinal necrosis and bacteria were also observed.
Figure 5. Small intestine and MLN histology from YopB/LcrV+dmLT vaccinated mice after oral Y. enterocolitica challenge.
Mice were vaccinated and challenged as described in Fig. 1B. Small intestine (A) and MLN (B) were collected one week post-infection and tissues stained with H&E. Images (representative of tissues from 3 animals) are shown with scale bars as indicated. (A) The top panels show normal villus architecture (arrows) and the expected sparse lymphoid cells in the lamina propria of unvaccinated and unchallenged control mice (right panel). The middle panels show intestinal villi with partial blunting in YopB/LcrV+dmLT vaccinated and challenge mice (left image, arrows). The right images show marked increased in inflammatory cells in the lamina propria (square) with numerous plasma cells. The enlarged square shows plasma cells with the characteristic perinuclear halo (arrows). The lower panel shows tissue destruction in dmLT and PBS (placebo groups) post infection. (B) The upper panels show an inactive MLN with small germinal center (GC) in control mice; the mantle zone with small lymphocytes is appreciated in the right image. The middle panels, show enlarged, activated GC with several tangible body macrophages in YopB/LcrV+dmLT vaccinated mice post challenge. There is no abscess formation. The lower panels show partial destruction of the lymph node by confluent abscesses (arrows) in dmLT and PBS groups post infection.
The MLN from unchallenged mice showed a normal, resting lymphoid tissue with small germinal centers (GC) (Fig. 5B top panel). The magnified image (right picture) shows a typical GC cuffed by the lymphocytic mantle. On the other hand, MLN from YopB/LcrV+dmLT vaccinated mice were notably enlarged after challenge, representing a viable and reactive tissue with multiple and prominent GC. The magnified image (Fig 5B, middle panel, right picture) depicts an active GC with scattered tingible body macrophages amidst immunoblasts and centroblasts. No abscesses were seen in this tissue. In contrast, multiple necrotic abscesses (round, clear, white patches, indicated by an arrow) were readily detected in the unvaccinated, challenged controls (Fig 5B, bottom panel). This extensive destruction of MLN architecture is indicative of extra-intestinal bacterial spread and systemic infection (all unvaccinated mice died by day 21 post-infection).
Mucosal recall responses in vaccinated mice after challenge.
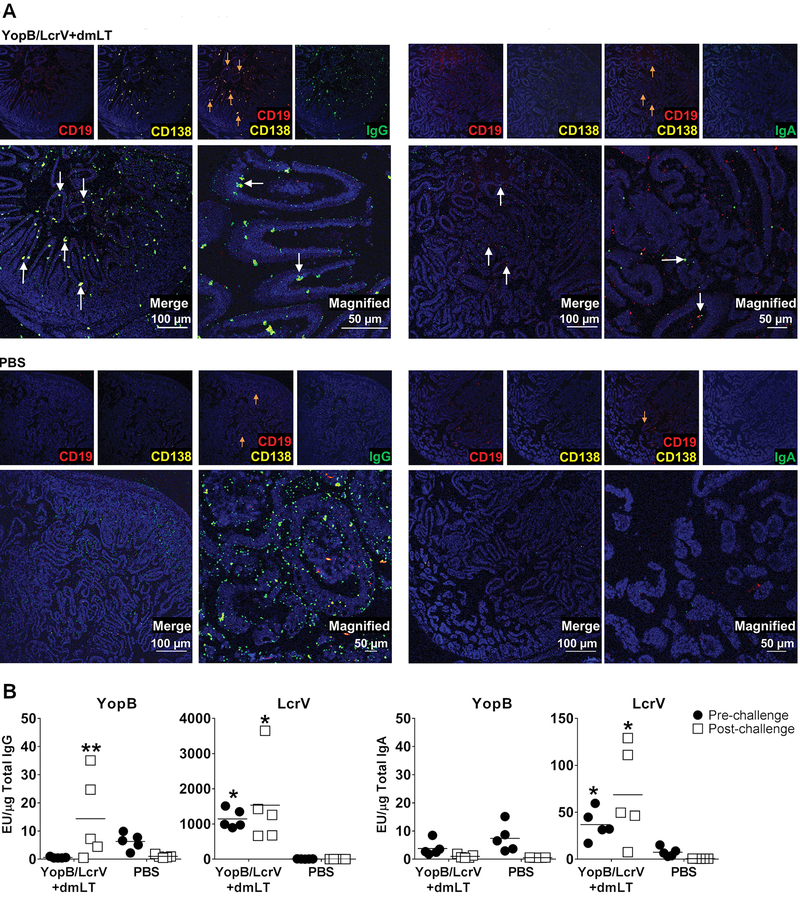
Mucosal B cell responses were evaluated in small intestine from vaccinated and control groups one week post-challenge. Tissue sections were stained with fluorescent antibodies to detect CD138+CD19+ plasmablasts (36). IgG or IgA staining and co-localization with CD138+CD19+ staining allowed identification of committed plasmablasts. CD138+CD19+ IgG+ cells lined the villi of YopB/LcrV+dmLT vaccinated mice (Fig. 6A arrows). In contrast, a few CD138+CD19+ cells were seen in unvaccinated (PBS) controls with diffuse (non-specific) IgG staining. IgA+ CD138+CD19+ plasmablasts were also detected in YopB/LcrV+dmLT vaccinated mice, albeit at much lower levels (Fig. 6A, right panels, arrows). Intestinal antibody responses were also investigated one week after vaccination and one week post-challenge (in the same mice used for immunofluorescent staining). YopB- and LcrV-specific IgG and LcrV-specific IgA were detected in the intestinal fluid from YopB/LcrV+dmLT recipients, but not in unvaccinated controls (Fig. 6B). YopB-specific IgG significantly increased post-challenge whereas levels of LcrV-specific IgG and IgA were detected before and after challenge.
Figure 6. Intestinal ASC and antibodies produced in YopB/LcrV+dmLT vaccinated mice after infection.
Mice were vaccinated and challenged as described in Fig. 1B and intestinal tissue collected one week post-challenge. Tissue sections were stained with antibodies specific for CD138, CD19, IgG, or IgA, followed by fluorescent secondary antibodies. Cells were counterstained with DAPI (blue). (A) IgG and IgA ASC lining the small intestine of YopB/LcrV+dmLT vaccinated and PBS mice post-challenge. Orange arrows signify CD138+CD19+ plasmablasts. White arrows on merge panels indicate co-localized staining for CD139, CD19, and IgG or IgA. (B) YopB- and LcrV-specific IgG and IgA in intestinal lavage fluid obtained one week after vaccination (pre-challenge) and after challenge. Data represent antibody titers (from 5 mice per group) normalized by total IgG or IgA content. Line shows arithmetic mean titers. Significant differences between vaccinated and control mice were determined by 2-way ANOVA with Tukey’s multiple comparisons; *, P<0.05 compared to PBS and **, P<0.05 compared to PBS and pre-challenge titer.
Mice immunized as infants with YopB/LcrV+dmLT were protected against lethal oral Y. enterocolitica challenge.
Considering that young children are the most susceptible to Yersinia infection and are primary targets for vaccination, we investigated the immunogenicity and protective capacity of YopB and LcrV in mice vaccinated as infants. YopB and LcrV (5 μg each, alone or combined) with or without dmLT (1 μg) were administered i.n. on days 7, 14 and 21 after birth (infancy) followed by a booster dose on day 28. Control mice were given dmLT alone or remained unvaccinated (naïve). Similar to adults, young mice immunized with YopB/LcrV+dmLT developed YopB-, LcrV-, and dmLT-specific serum IgG (Fig. 7A). Antibody responses to LcrV were again stronger compared to those against YopB; while the latter appeared only after the 4th dose, LcrV-specific IgG was detected after the 2nd vaccination and reached >3-log higher peak titers. Compared to adults, mice vaccinated as infants had a lower-fold increase in antibody titers, which appeared to be antigen dependent: YopB < dmLT< LcrV (Table I). Notably, immunization of infant mice with YopB/LcrV+dmLT afforded 80–100% protection against lethal Y. enterocolitica oral challenge, which was confirmed in multiple independent experiments (Fig. 7B–C). Different from what was observed in adult mice, YopB and LcrV given to infant mice as single antigens along with dmLT elicited 60% protection, which reached statistical significance above the non-vaccinated controls. Serum antibodies with bactericidal and OPKA activity were also detected in mice vaccinated as infants (Fig. 7D and E). As observed in adults, SBA and OPKA titers were significantly increased in YopB/LcrV+dmLT vaccine recipients (Fig. 7D) and, intriguingly, OPKA titers were increased in young mice that had received LcrV+dmLT. The YopB/LcrV+dmLT group exhibited a strong SBA recall response after challenge, reaching levels comparable to those of vaccinated and challenged adults (Fig. 7E).
Figure 7. Antibody responses and protection against Y. enterocolitica oral infection in mice immunized as infants with YopB/LcrV+dmLT.
Mice were immunized i.n. with YopB and/or LcrV (5 μg each) with or without 1 μg dmLT on days 7, 14, 21, and 28 after birth. (A) Kinetics of serum IgG against YopB, LcrV, and/or dmLT measured by ELISA. Data represent the geometric mean titer of 9–12 mice per group in a log10 scale with 95% CI error bars. (B) Survival after oral challenge with Y. enterocolitica YE8081 four weeks after the final immunization. (C) Survival of mice vaccinated as infants with YopB/LcrV+dmLT in two subsequent independent experiments. (D) SBA and OPKA activity were measured on day 55 after birth. Data represent individual and mean titers ± SEM from 5 mice per group. Significant differences among groups were determined by 1-way ANOVA with Tukey’s multiple comparisons test; *, P<0.05 compared to dmLT and PBS controls. (E). SBA titers in YopB/LcrV+dmLT vaccinated mice immediately before and 30 days after oral lethal challenge with Y. enterocolitica. Data represent individual and mean titers ± SEM from 7 mice per group. Significant differences were determined by Wilcoxon matched-pairs signed rank test; *, P<0.05 compared to pre-challenge. (F) Infant mice were also immunized i.d. with 50 ng, 100 ng, or 200 ng of YopB given with 50 ng of dmLT on days 14, 21, 28, and 35 after birth (arrows). (G) Survival after Y. enterocolitica YE8081 challenge four weeks after the final immunization. Significant differences in IgG titers between groups were determined by 2-way ANOVA with Tukey’s multiple comparisons test; *, P<0.05 compared to controls (dmLT and PBS). **, P<0.05 compared to all groups. Significant differences in survival curves were determined by log-rank test; *, P<0.05 compared to controls (naïve, dmLT, and PBS, where applicable), ** P<0.05 compared to YopB+LcrV and control groups.
Table I.
Ag-specific IgG responses in mice vaccinated i.n. with YopB/LcrV+dmLT as adult or infants
| Fold-increase IgG Titera | |||
|---|---|---|---|
| Age Group | YopB | LcrV | dmLT |
| Adults | 8,645.5 | 982,905.3 | 956,460.0 |
| Infants | 263.5 | 357,067.7 | 188,103.8 |
Similarly, high levels of protection (80–90%) were observed in a subsequent experiment that involved mice immunized i.n. with YopB/LcrV+dmLT (same doses as indicated above) on days 14 and 21 (infancy), followed by two booster immunizations on days 28 and 35 after birth. The same immunization schedule was used to investigate the efficacy of YopB when administered parenterally. Infant mice were immunized intradermally (id) with increasing amounts of YopB (50, 100, and 200 ng) with 50 ng of dmLT on days 14 and 21, and two boosters were given on days 28 and 35. Vaccinated mice developed YopB-specific serum IgG that increased proportionally with the given dose; recipients of the 200-ng dose had the highest titers, comparable to those elicited in mice immunized i.n. under the same schedule, and despite receiving a fraction of the dose (data not shown). Strong serum IgG responses were induced by dmLT as well (Fig. 7F). YopB (100 ng and 200 ng) + dmLT conferred 64% and 55% protection against Y. enterocolitica infection, respectively, which were significantly higher than the unvaccinated controls (Fig. 7G). Protective rates remained similar (50–60%) when YopB and LcrV were combined at the 50 ng dose (the only combination dose tested, data not shown).
Discussion
It would be highly advantageous from the public health standpoint to have access to a safe and effective vaccine that can prevent Yersinia-induced gastroenteritis. As a prophylactic tool, a vaccine would reduce burden of disease and mortality, and prevent long-term sequelae and disabilities associated with Yersinia infection. Important attributes of such a vaccine include practical administration, the ability to induce strong and durable protective immunity, particularly in young hosts, and the capacity to prevent infection caused by multiple serotypes and molecular variants. In this study, we report a novel subunit vaccine candidate consisting of Yersinia T3SS proteins YopB and LcrV, given with the E. coli dmLT adjuvant. This vaccine was immunogenic and highly protective in mice immunized as adults or as infants. Importantly, it was effective not only against lethal oral Y. enterocolitica infection but also against lethal pulmonary Y. pestis infection. Consequently, a vaccine based T3SS proteins that are conserved across Yersinia species could also be useful for purposes of biodefense.
To determine whether YopB/LcrV+dmLT could induce protective immunity when administered through a (practical) mucosal route, we employed a mouse model of intranasal vaccination. Experiments were conducted in mice immunized as adults (7–12 wks. of age) or as infants (1–4 wks. of age) to establish the effectiveness of this vaccine when given early in life. Antibody responses in 1–3 wks. old mice have been shown to reflect those of human newborns and infants (37). A similar approach allowed us to demonstrate the protective capacity of Shigella T3SS proteins, IpaB (homologue of YopB) and IpaD at different ages (25, 38) and was therefore adopted for this work. Oral vaccination was not utilized due the low efficiency of this route in rodents (31, 39). Intranasal vaccination, on the other hand, has been widely used to evaluate enteric vaccine candidates in multiple animal models [reviewed in (40, 41)].
As in previous studies, the presence of dmLT was critical to augment immune responses to the protein antigens (24, 31). The most important feature of the YopB/LcrV vaccine was its capacity to engender high levels of protective immunity in mice immunized as adults or as infants. While the individual proteins (YopB or LcrV+dmLT) failed to protect adult mice against lethal Y. enterocolitica infection, survival greatly improved in recipients of the YopB and LcrV combination. A synergistic protective effect was observed whereby the efficacy of the YopB/LcrV group surpassed the added efficacy of the individual antigens. Intriguingly, the individual proteins conferred substantial protection (≥60%) when administered mucosally to younger mice. This was also the case for YopB+dmLT given intradermally. The stringency of challenge was similar for all age groups (~10 MLD50, as determined for specific ages at the time of challenge), and therefore unlikely to have biased the protection results. One possible explanation for the age-associated difference in vaccine efficacy is that requirements for protection might be different in adults as compared to young hosts. They may also develop distinct responses to the vaccine antigens (not captured by our analysis). These results highlight the impact of age, as well as antigenic composition, in vaccine-induced protective immunity. A mechanistic understanding of the susceptibility and protective immunity in infants vs. adults is important in designing effective vaccines and requires further investigation.
Our work provides the first evidence of YopB being a protective antigen for use in a Yersinia vaccine candidate. Antibodies against Yersinia Yops have been detected in serum from convalescent individuals and in mice following Y. pestis infection (42–46). Similarly, a recent study reported Yop-specific serum IgG in mice immunized with an engineered live-attenuated Y. pseudotuberculosis strain and protected against plague (47). In a small-scale experiment from an early publication, mice immunized subcutaneously with YopB+alum produced serum antibodies detected by immunoblot analysis (48). Possibly, the difficulty of purifying YopB in sufficient quantities prevented its evaluation in larger pre-clinical studies. The Picking lab was able to circumvent this challenge by co-expressing hydrophobic YopB with its chaperone, SycD, then removing the chaperone with the detergent LDAO and retaining the protein in LDAO to maintain solubility (28).
The literature reports YopB being less immunogenic compared to YopD (42). In our studies, YopB was generally less immunogenic than LcrV, whether it was given alone or combined or to adult or infant mice. The reasons for this observation are unclear and might reflect intrinsic features of the protein (i.e. aminoacid composition, folding). Nonetheless, a sufficient YopB-specific response was generated that either contributed to protection or prevented infection on its own. Young mice immunized with YopB i.d. developed a robust YopB-IgG response and substantial protection even though they received 1/25th of the dose given i.n. This suggests the possibility of further improving the immunization strategy by selecting the optimal route and dose combination.
Adult mice immunized with YopB/LcrV (the group with highest survival rates) had enhanced capacity of serum antibody-mediated complement-dependent bactericidal and opsonophagocitic killing activity compared to mice immunized with the individual antigens. Like the protective outcome, the functional antimicrobial antibody activity (SBA titers) also showed a synergistic effect. The greater antimicrobial activity achieved in the YopB/LcrV group was recreated in vitro by mixing serum from mice immunized with each individual antigen. This could be attributed to the formation of higher density (multivalent) Ag-Ab complexes when both specificities are present, which facilitates complement deposition and phagocytic uptake, and killing. Curiously, sera from adult mice immunized with YopB exhibited enhanced SBA activity above that of mice immunized with LcrV or of unvaccinated controls. In a published study, YopB antibody treatment of mice orally infected with Y. enterocolitica resulted in reduced intestinal colonization and recovery of live organisms (49), which concur with our findings of anti-YopB serum antibodies exhibiting antimicrobial activity in vitro. Sera from mice immunized as infants with LcrV exhibited enhanced OPKA activity above those immunized with YopB or unvaccinated controls; a similar trend was seen in adult mice. Antibodies to LcrV are known to be protective in vivo and have been shown to promote phagocytosis in vitro (50).
Attempting to explain the mechanism of action of anti-YopB antibodies, others have proposed that serum proteins could trigger YopB localization to the tip of the T3SS needle, where it is subsequently recognized by antibodies (48). Similarly, it has been hinted that antibodies specific for LcrV could neutralize surface accessible LcrV. Our observation that both YopB and LcrV can prevent infection on their own (in the younger mice), confirms that immune responses elicited by both antigens (independently) contribute to protection. LcrV-specific antibodies capable of neutralizing macrophage toxicity have been implicated as a correlate of protection against Y. pestis infection (51) and by the same mechanism, they may protect against Y. enterocolitica. Similar to the enhanced antimicrobial activity detected in vitro, YopB and LcrV antibodies may have a concerted protective role in vivo, allowing for a more robust neutralization of T3SS translocation of effector proteins.
In addition to serum antibodies, systemic and mucosal IgG and IgA ASC were elicited following YopB/LcrV vaccination. Gut-homing ASC are likely a primary source of local antibodies that can block bacterial adherence and invasion, and/or promote the killing of enteric pathogens. The YopB/LcrV vaccine induced a strong mucosal B cell response, as demonstrated by the rapid expansion of intestinal IgG ASC soon upon challenge, as discussed in more detail below. It is possible that the functional activity detected in serum of YopB/LcrV-vaccinated mice actually reflects the antimicrobial activity of IgG produced by intestinal ASC activated post-challenge.
Cell-mediated immunity (particularly CD4 Th1 and Th17 lymphocytes) is also required for elimination of Y. enterocolitica (52, 53). In our study, YopB and LcrV induced distinct cytokine secretion profiles. YopB elicited a Th1-type response, with higher production of TNF-α, IFN-γ, and IL-2), whereas LcrV favored a Th2-type response with elevated levels of IL-6, which is in agreement with the robust antibody levels against this antigen. IL-6 has been implicated in down-regulation of the inflammatory response during Y. enterocolitica infection (54). TNF-α and IFN-γ are critical for antibody-mediated protection against Y. pestis (55). Therefore, the induction of a balanced Th1/Th2 immune response is advantageous to reduce inflammation while promoting functional antibody activity against Yersinia spp. Significant IL-17A recall responses were produced by spleen cells from YopB/LcrV+dmLT vaccinated mice. IL-17 has been implicated in protection against oral Y. enterocolitica infection by promoting the production of secretory IgA (56) and against pulmonary Y. pestis infection by stimulating recruitment of neutrophils (57). The increased LcrV-specific IgA levels in intestinal lavage fluid after challenge may be a downstream effect of L-17 production in our model.
To better understand the protective immunity induced by YopB/LcrV+dmLT, we measured immune effectors at the time of challenge and those recalled locally promptly after challenge. Intestinal ASC [IgG+ and IgA+ CD138+CD19+ plasmablasts] were detected in the villi of vaccinated animals following infection, but not in unvaccinated controls. This B cell expansion coincided with secretion of YopB- and LcrV-specific antibodies in the intestinal lumen. It was surprising to see an abundance of IgG as opposed to IgA intestinal ASC. Vaccinated mice exhibited a strong GC reaction in the MLN post challenge indicative of recall immune stimulation. The integrity of the intestinal mucosal barrier was preserved in vaccinated animals post-challenge, which contrasted with the widespread tissue destruction and abscesses in those unvaccinated. These findings suggest that local B cell responses, particularly IgG+ ASC, IgG antibodies, and MLN activation are critical in preventing oral Yersinia infection. The post-challenge analysis was valuable in uncovering additional vaccine immunological priming that was otherwise missed, as was the case with YopB intestinal antibodies, which could not be detected after vaccination but were readily found after challenge (and exclusively in the vaccinated group).
In summary, we demonstrated for the first time that Yersinia YopB is a protective antigen, and that a dmLT-adjuvanted vaccine containing both YopB and LcrV was highly effective in preventing oral Y. enterocolitica in adult as well as in mice immunized during infancy. Furthermore, the vaccine fully protected adult mice against Y. pestis pulmonary infection. Several immunological insights helped understand the underlying protective immunity: the induction of antibodies that facilitate complement-mediated bactericidal activity and opsonophagocytic killing, systemic ASC, expansion of local plasma cells, secretion of antigen-specific antibodies to the intestinal lumen, and production of Th1, Th2 and Th17-type cytokines. This is a promising vaccine candidate for broad protection against Yersinia infection that warrants further investigation.
Supplementary Material
Acknowledgements.
We would like to acknowledge Henry S. Heine of the Institute for Therapeutic Innovation, University of Florida, for his expert advice and critical reading of the manuscript. Members of the CVD Applied Immunology Section at the University of Maryland, School of Medicine, Mucosis, and the Picking laboratory at the University of Kansas, provided technical assistance. We also thank Esther Ndungo (CVD Applied Immunology) for critical reading of the manuscript.
This work was supported by the National Institutes of Health Grant R01 AI089519.
Abbreviations
- dmLT
E. coli double mutant labile toxin
- T3SS
type III secretion system
- F1
Y. pestis fraction 1 capsule
- IMAC
immobilized metal affinity chromatography
- LDAO
lauryldimethylamine N-oxide
- i.n.
intranasal
- i.d.
intradermal
- SBA
serum bactericidal activity
- OPKA
opsonophagocytic killing activity
- BRC
baby rabbit complement
- ASC
Ab secreting cells
- BALf
bronchoalveolar lavage fluid
- MSD
Meso Scale Discovery
- MLN
mesenteric lymph node
- GC
germinal center
References
- 1.Bottone EJ. 1999. Yersinia enterocolitica: overview and epidemiologic correlates. Microbes Infect 1: 323–33 [DOI] [PubMed] [Google Scholar]
- 2.Hill Gaston JS, Lillicrap MS. 2003. Arthritis associated with enteric infection. Best practice & research Clinical rheumatology 17: 219–39 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2016. Yersinia enterocolitica (Yersiniosis). [Google Scholar]
- 4.European Food Safety Authority, Control ECfDPa. 2016. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA journal 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saraka D, Savin C, Kouassi S, Cisse B, Koffi E, Cabanel N, Bremont S, Faye-Kette H, Dosso M, Carniel E. 2017. Yersinia enterocolitica, a Neglected Cause of Human Enteric Infections in Cote d’Ivoire. PLoS Negl Trop Dis 11: e0005216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman A, Bonny TS, Stonsaovapak S, Ananchaipattana C. 2011. Yersinia enterocolitica: Epidemiological Studies and Outbreaks. Journal of pathogens 2011: 239391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulfer A, Blaser MJ. 2015. Risks of Antibiotic Exposures Early in Life on the Developing Microbiome. PLoS Pathog 11: e1004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ioannidis A, Kyratsa A, Ioannidou V, Bersimis S, Chatzipanagiotou S. 2014. Detection of biofilm production of Yersinia enterocolitica strains isolated from infected children and comparative antimicrobial susceptibility of biofilm versus planktonic forms. Mol Diagn Ther 18: 309–14 [DOI] [PubMed] [Google Scholar]
- 9.Dorrell N, Li SR, Everest PH, Dougan G, Wren BW. 1998. Construction and characterisation of a Yersinia enterocolitica O:8 ompR mutant. FEMS microbiology letters 165: 145–51 [DOI] [PubMed] [Google Scholar]
- 10.Li SR, Dorrell N, Everest PH, Dougan G, Wren BW. 1996. Construction and characterization of a Yersinia enterocolitica O:8 high-temperature requirement (htrA) isogenic mutant. Infection and immunity 64: 2088–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igwe EI, Russmann H, Roggenkamp A, Noll A, Autenrieth IB, Heesemann J. 1999. Rational live oral carrier vaccine design by mutating virulence-associated genes of Yersinia enterocolitica. Infection and immunity 67: 5500–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zauberman A, Flashner Y, Levy Y, Vagima Y, Tidhar A, Cohen O, Bar-Haim E, Gur D, Aftalion M, Halperin G, Shafferman A, Mamroud E. 2013. YopP-expressing variant of Y. pestis activates a potent innate immune response affording cross-protection against yersiniosis and tularemia [corrected]. PloS one 8: e83560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qadri F, Bhuiyan TR, Sack DA, Svennerholm A-M. 2013. Immune responses and protection in children in developing countries induced by oral vaccines. Vaccine 31: 452–60 [DOI] [PubMed] [Google Scholar]
- 14.Parker EP, Ramani S, Lopman BA, Church JA, Iturriza-Gomara M, Prendergast AJ, Grassly NC. 2018. Causes of impaired oral vaccine efficacy in developing countries. Future Microbiol 13: 97–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noll A, Autenrieth Ib. 1996. Immunity against Yersinia enterocolitica by vaccination with Yersinia HSP60 immunostimulating complexes or Yersinia HSP60 plus interleukin-12. Infection and immunity 64: 2955–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt A, Schaffelhofer S, Muller K, Rollinghoff M, Beuscher HU. 1999. Analysis of the Yersinia enterocolitica 0:8 V antigen for cross protectivity. Microbial pathogenesis 26: 221–33 [DOI] [PubMed] [Google Scholar]
- 17.Singh AK, Kingston JJ, Gupta SK, Batra HV. 2015. Recombinant Bivalent Fusion Protein rVE Induces CD4+ and CD8+ T-Cell Mediated Memory Immune Response for Protection Against Yersinia enterocolitica Infection. Frontiers in microbiology 6: 1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh AK, Kingston JJ, Murali HS, Batra HV. 2014. A recombinant bivalent fusion protein rVE confers active and passive protection against Yersinia enterocolitica infection in mice. Vaccine 32: 1233–9 [DOI] [PubMed] [Google Scholar]
- 19.Miller NC, Quenee LE, Elli D, Ciletti NA, Schneewind O. 2012. Polymorphisms in the lcrV gene of Yersinia enterocolitica and their effect on plague protective immunity. Infection and immunity 80: 1572–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roggenkamp A, Geiger AM, Leitritz L, Kessler A, Heesemann J. 1997. Passive immunity to infection with Yersinia spp. mediated by anti-recombinant V antigen is dependent on polymorphism of V antigen. Infection and immunity 65: 446–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brubaker RR. 2003. Interleukin-10 and inhibition of innate immunity to Yersiniae: roles of Yops and LcrV (V antigen). Infect Immun 71: 3673–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montagner C, Arquint C, Cornelis GR. 2011. Translocators YopB and YopD from Yersinia enterocolitica form a multimeric integral membrane complex in eukaryotic cell membranes. Journal of bacteriology 193: 6923–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordfelth R, Wolf-Watz H. 2001. YopB of Yersinia enterocolitica is essential for YopE translocation. Infect Immun 69: 3516–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heine SJ, Diaz-McNair J, Andar AU, Drachenberg CB, van de Verg L, Walker R, Picking WL, Pasetti MF. 2014. Intradermal delivery of Shigella IpaB and IpaD type III secretion proteins: kinetics of cell recruitment and antigen uptake, mucosal and systemic immunity, and protection across serotypes. Journal of immunology (Baltimore, Md : 1950) 192: 1630–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Becerra FJ, Kissmann JM, Diaz-McNair J, Choudhari SP, Quick AM, Mellado-Sanchez G, Clements JD, Pasetti MF, Picking WL. 2012. Broadly protective Shigella vaccine based on type III secretion apparatus proteins. Infection and immunity 80: 1222–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu W, Huang J, Duan B, Traficante DC, Hong H, Risech M, Lory S, Priebe GP. 2012. Th17-stimulating protein vaccines confer protection against Pseudomonas aeruginosa pneumonia. American journal of respiratory and critical care medicine 186: 420–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broz P, Mueller CA, Muller SA, Philippsen A, Sorg I, Engel A, Cornelis GR. 2007. Function and molecular architecture of the Yersinia injectisome tip complex. Mol Microbiol 65: 1311–20 [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Choudhari SP, Kumar P, Toth RTt, Kim JH, Van Roosmalen ML, Leenhouts K, Middaugh CR, Picking WL, Picking WD. 2015. Biophysical Characterization of the Type III Secretion System Translocator Proteins and the Translocator Proteins Attached to Bacterium-Like Particles. J Pharm Sci 104: 4065–73 [DOI] [PubMed] [Google Scholar]
- 29.Summerton NA, Welch RW, Bondoc L, Yang HH, Pleune B, Ramachandran N, Harris AM, Bland D, Jackson WJ, Park S, Clements JD, Nabors GS. 2010. Toward the development of a stable, freeze-dried formulation of Helicobacter pylori killed whole cell vaccine adjuvanted with a novel mutant of Escherichia coli heat-labile toxin. Vaccine 28: 1404–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brito LA, Singh M. 2011. Acceptable levels of endotoxin in vaccine formulations during preclinical research. J Pharm Sci 100: 34–7 [DOI] [PubMed] [Google Scholar]
- 31.Heine SJ, Diaz-McNair J, Martinez-Becerra FJ, Choudhari SP, Clements JD, Picking WL, Pasetti MF. 2013. Evaluation of immunogenicity and protective efficacy of orally delivered Shigella type III secretion system proteins IpaB and IpaD. Vaccine 31: 2919–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner NJ, Lin CP, Borst LB, Miller VL. 2013. YaxAB, a Yersinia enterocolitica pore-forming toxin regulated by RovA. Infect Immun 81: 4208–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galen JE, Wang JY, Carrasco JA, Lloyd SA, Mellado-Sanchez G, Diaz-McNair J, Franco O, Buskirk AD, Nataro JP, Pasetti MF. 2015. A bivalent typhoid live vector vaccine expressing both chromosome- and plasmid-encoded Yersinia pestis antigens fully protects against murine lethal pulmonary plague infection. Infect Immun 83: 161–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mellado-Sanchez G, Ramirez K, Drachenberg CB, Diaz-McNair J, Rodriguez AL, Galen JE, Nataro JP, Pasetti MF. 2013. Characterization of systemic and pneumonic murine models of plague infection using a conditionally virulent strain. Comp Immunol Microbiol Infect Dis 36: 113–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimanovich AA, Buskirk AD, Heine SJ, Blackwelder WC, Wahid R, Kotloff KL, Pasetti MF. 2016. Functional and Antigen-Specific Serum Antibody Levels as Correlates of Protection Against Shigellosis in a Controlled Human Challenge Study. Clin Vaccine Immunol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tellier J, Nutt SL. 2017. Standing out from the crowd: How to identify plasma cells. Eur J Immunol 47: 1276–9 [DOI] [PubMed] [Google Scholar]
- 37.Siegrist CA. 2001. Neonatal and early life vaccinology. Vaccine 19: 3331–46 [DOI] [PubMed] [Google Scholar]
- 38.Heine SJ, Franco-Mahecha OL, Chen X, Choudhari S, Blackwelder WC, van Roosmalen ML, Leenhouts K, Picking WL, Pasetti MF. 2015. Shigella IpaB and IpaD displayed on L. lactis bacterium-like particles induce protective immunity in adult and infant mice. Immunology and cell biology 93: 641–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirabayashi Y, Kurata H, Funato H, Nagamine T, Aizawa C, Tamura S, Shimada K, Kurata T. 1990. Comparison of intranasal inoculation of influenza HA vaccine combined with cholera toxin B subunit with oral or parenteral vaccination. Vaccine 8: 243–8 [DOI] [PubMed] [Google Scholar]
- 40.Higginson EE, Simon R, Tennant SM. 2016. Animal Models for Salmonellosis: Applications in Vaccine Research. Clin Vaccine Immunol 23: 746–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mani S, Wierzba T, Walker RI. 2016. Status of vaccine research and development for Shigella. Vaccine 34: 2887–94 [DOI] [PubMed] [Google Scholar]
- 42.Benner GE, Andrews GP, Byrne WR, Strachan SD, Sample AK, Heath DG, Friedlander AM. 1999. Immune response to Yersinia outer proteins and other Yersinia pestis antigens after experimental plague infection in mice. Infection and immunity 67: 1922–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolin I, Portnoy DA, Wolf-Watz H. 1985. Expression of the temperature-inducible outer membrane proteins of yersiniae. Infection and immunity 48: 234–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li B, Du C, Zhou L, Bi Y, Wang X, Wen L, Guo Z, Song Z, Yang R. 2012. Humoral and cellular immune responses to Yersinia pestis infection in long-term recovered plague patients. Clinical and vaccine immunology : CVI 19: 228–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazza G, Karu AE, Kingsbury DT. 1985. Immune response to plasmid- and chromosome-encoded Yersinia antigens. Infection and immunity 48: 676–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nemeth J, Straley SC. 1997. Effect of Yersinia pestis YopM on experimental plague. Infection and immunity 65: 924–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Demeure CE, Derbise A, Guillas C, Gerke C, Cauchemez S, Carniel E, Pizarro-Cerda J. 2019. Humoral and cellular immune correlates of protection against bubonic plague by a live Yersinia pseudotuberculosis vaccine. Vaccine 37: 123–9 [DOI] [PubMed] [Google Scholar]
- 48.Ivanov MI, Noel BL, Rampersaud R, Mena P, Benach JL, Bliska JB. 2008. Vaccination of mice with a Yop translocon complex elicits antibodies that are protective against infection with F1- Yersinia pestis. Infection and immunity 76: 5181–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burdack S, Schmidt A, Knieschies E, Rollinghoff M, Beuscher HU. 1997. Tumor necrosis factor-alpha expression induced by anti-YopB antibodies coincides with protection against Yersinia enterocolitica infection in mice. Medical microbiology and immunology 185: 223–9 [DOI] [PubMed] [Google Scholar]
- 50.Cowan C, Philipovskiy AV, Wulff-Strobel CR, Ye Z, Straley SC. 2005. Anti-LcrV antibody inhibits delivery of Yops by Yersinia pestis KIM5 by directly promoting phagocytosis. Infect Immun 73: 6127–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zauberman A, Cohen S, Levy Y, Halperin G, Lazar S, Velan B, Shafferman A, Flashner Y, Mamroud E. 2008. Neutralization of Yersinia pestis-mediated macrophage cytotoxicity by anti-LcrV antibodies and its correlation with protective immunity in a mouse model of bubonic plague. Vaccine 26: 1616–25 [DOI] [PubMed] [Google Scholar]
- 52.Autenrieth IB, Beer M, Hantschmann P, Preger S, Vogel U, Heymer B, Heesemann J. 1993. The cellular immune response against Yersinia enterocolitica in different inbred strains of mice: evidence for an important role of T lymphocytes. Zentralbl Bakteriol 278: 383–95 [DOI] [PubMed] [Google Scholar]
- 53.Kempf VA, Bohn E, Noll A, Bielfeldt C, Autenrieth IB. 1998. In vivo tracking and protective properties of Yersinia-specific intestinal T cells. Clin Exp Immunol 113: 429–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dube PH, Handley SA, Lewis J, Miller VL. 2004. Protective role of interleukin-6 during Yersinia enterocolitica infection is mediated through the modulation of inflammatory cytokines. Infect Immun 72: 3561–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parent MA, Wilhelm LB, Kummer LW, Szaba FM, Mullarky IK, Smiley ST. 2006. Gamma interferon, tumor necrosis factor alpha, and nitric oxide synthase 2, key elements of cellular immunity, perform critical protective functions during humoral defense against lethal pulmonary Yersinia pestis infection. Infect Immun 74: 3381–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DePaolo RW, Kamdar K, Khakpour S, Sugiura Y, Wang W, Jabri B. 2012. A specific role for TLR1 in protective T(H)17 immunity during mucosal infection. J Exp Med 209: 1437–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin JS, Kummer LW, Szaba FM, Smiley ST. 2011. IL-17 contributes to cell-mediated defense against pulmonary Yersinia pestis infection. J Immunol 186: 1675–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.