Abstract
Zinc is an abundant trace metal in the hippocampus nerve terminals. Previous studies demonstrate the ability of zinc to selectively block neurosteroid-sensitive, extrasynaptic GABA-A receptors in the hippocampus (Carver et al, 2016). Here we report that zinc prevents the seizure protective effects of the synthetic neurosteroid ganaxolone (GX) in an experimental model of epilepsy. GABA-gated and tonic currents were recorded from dissociated dentate gyrus granule cells (DGGCs), CA1 pyramidal cells (CA1PCs), and hippocampal slices from adult mice. Antiseizure effects of GX and the reversal of these effects by zinc were evaluated in fully-kindled mice expressing generalized (stage 5) seizures. In electrophysiological studies, zinc blocked the GABA-evoked and GX-potentiated GABA-gated chloride currents in DGGCs and CA1PCs in a concentration-dependent fashion similar to the competitive GABA-A receptor antagonists bicuculline and gabazine. Zinc completely blocked GX potentiation of extrasynaptic tonic currents, but not synaptic phasic currents. In hippocampus kindling studies, systemic administration of GX produced a dose-dependent suppression of behavioral and electrographic seizures in fully-kindled mice with complete seizure protection at the 10 mg/kg dose. However, the antiseizure effect of GX was significantly prevented by intrahippocampal administration of zinc (ED50, 150 µM). The zinc antagonistic response was reversible as animals responded normally to GX administration 24 h post-zinc blockade. These results demonstrate that zinc reduces the antiseizure effects of GX by selectively blocking extrasynaptic δGABA-A receptors in the hippocampus. These pharmacodynamic interactions have clinical implications in neurosteroid therapy for brain conditions associated with zinc fluctuations.
Keywords: Zinc, ganaxolone, GABA-A receptors, tonic inhibition, hippocampus, seizures
1. Introduction
The hippocampus is a key region associated with the pathophysiology of epilepsy (Jutila et al., 2002). There are two distinct categories of GABA-A receptors in the hippocampus. Synaptic receptors and extrasynaptic receptors exhibit different characteristics in their affinity and efficacy to GABA, desensitization rate, and drug sensitivity (Bianchi and Macdonald, 2002; 2003; Brown et al., 2002; Mortensen et al., 2011; Wohlfarth et al., 2002). Synaptic receptors, mainly γ- containing receptors, produce rapid and transient phasic currents in response to presynaptic release of GABA (~1 mM), whereas extrasynaptic receptors, mostly δ-containing receptors in dentate gyrus and α5β3γ2 receptors in CA1, generate persistent, non-desensitizing tonic currents by ambient GABA (~0.3‒2 µM) (Mody et al., 1994; Brickley et al., 1996; Farrant and Nusser, 2005). Tonic currents contribute to the overall basal tone and shunting inhibition via continuous channel conductance in neurons expressing δ-containing receptors. Hippocampus granule cells (DGGCs), which express high density of δ-subunit-containing extrasynaptic receptors, promote tonic inhibition and thereby regulate network excitability (Coulter and Carlson, 2007; Glykys et al., 2008). GABA-A receptors are modulated by many compounds including benzodiazepines, neurosteroids, and zinc (Zn2+).
Zn2+ is the second most abundant trace metal in many cells and neurons in the brain. It regulates cellular functions such as gene expression, epigenetic enzymatic activity, protein structural stability, and neuronal plasticity. Zn2+ is particularly abundant in the cortex and limbic system, including hippocampus and amygdala (Frederickson, 1989; Frederickson et al., 2000; 2005; Gower-Winter and Levenson, 2012; Szewczyk, 2013). Zn2+ homeostasis in the brain is tightly controlled and primarily regulated by Zn2+ transporters and binding proteins. High level of “chelatable” Zn2+ is sequestered in the synaptic vesicles of glutamatergic neurons through Zn2+ transporter protein ZnT3. Other transporters like ZIP proteins promote the efflux of vesicular Zn2+ and increase the concentration of cytoplasmic Zn2+ (Cole et al., 1999; Kambe et al., 2004; Lee et al., 2011). Zn2+ is synaptically co-released with glutamate from presynaptic vesicles of the hippocampal mossy fibers, the axons of DGGCs (Assaf and Chung, 1984; Frederickson et al., 1983; Molnar and Nadler, 2001; Paoletti et al., 2009). Zn2+ has been shown to modulate both excitatory and inhibitory transmission (Westbrook and Mayer, 1987; Harrison and Gibbons, 1994; Vogt et al., 2000). Zn2+ inhibits GABA-A receptors and has distinct sensitivities at different receptor subtypes partly due to the accessibility of Zn2+ binding sites (Smart et al., 1991; Barberis et al., 2000; Ruiz et al., 2004). The δ-containing extrasynaptic receptors exhibit greater sensitivity to Zn2+ blockade than γ-containing synaptic receptors (Hosie et al., 2003). Alterations in Zn2+ homeostasis have implications in several neurological disorders (Frederickson et al., 2005; Bitanihirwe and Cunningham, 2009; Qian et al., 2011; Szewczyk, 2013; Prakash et al., 2015). Aberrant Zn2+-rich mossy fiber sprouting is a classical feature in limbic epileptogenesis (Cavazos et al., 1991). Excessive release of Zn2+ drastically alters the excitability of hippocampal circuits and plays an important role in the pathophysiology of epilepsy (Takeda et al., 1999; Coulter, 2000; Foresti et al., 2008; Elsas et al., 2009).
Allopregnanolone (3α-hydroxy-5α-pregnan-20-one, AP) and related neurosteroids are endogenous modulators of neuronal excitability and seizure susceptibility (Chuang and Reddy, 2018; Younus and Reddy, 2018). Neurosteroids modulate GABA-A receptors primarily via allosteric potentiation of GABA at nanomolar concentrations and through direct activation of the channel at micromolar concentrations (Hosie et al., 2007). AP has powerful antiseizure activity (Carver et al., 2016; Kaminski et al., 2003; Kaminski et al., 2004; Kokate et al., 1996; Reddy and Estes, 2016). Ganaxolone (3α-hydroxy-3β-methyl-5α-pregnan-20-one, GX), a synthetic analog of AP, is designed to treat epilepsy and related seizure conditions. GX produces robust positive allosteric modulation of GABA-A receptors, with preferential sensitivity at δ-subunit-containing extrasynaptic receptors (Chuang and Reddy, 2018b). GX has a more favorable biopharmaceutical profile than AP due to its higher bioavailability and less hormonal side effects (Carter et al., 1997; Reddy and Woodward, 2004; (Carver and Reddy, 2016). GX produces powerful antiseizure activity in a wide range of experimental models, and is being evaluated in clinical trials for epilepsy (Bialer et al., 2015; Braat et al., 2015; Kerrigan et al., 2000; Laxer et al., 2000; Nohria and Giller, 2007; Pieribone et al., 2007; Reddy and Rogawski, 2012; Sperling et al., 2017). Recently, Zn2+ has been shown to selectively block neurosteroid-sensitive, extrasynaptic δGABA-A receptors in the hippocampus (Carver et al, 2016). Therefore, it is likely that Zn2+ may exert antagonistic effects on GX modulation of tonic inhibition and seizure protection.
In the present study, we investigated the pharmacodynamic interactions of Zn2+ and GX at extrasynaptic GABA-A receptors and its functional relevance on the anticonvulsant activity of GX in the kindling model. Our results demonstrate that Zn2+ diminishes the antiseizure effects of GX by selectively blocking the extrasynaptic δGABA-A receptors in the hippocampus.
2. Materials and methods
2.1. Animals
Young adult (2–3 months-old) male mice of C57BL/6–129SV background strain were used in this study. Animals were group housed in an environmentally controlled facility with a 12 h light/dark cycle. The animals were cared for in compliance with the guidelines in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal procedures were performed in a protocol approved by the university’s Institutional Animal Care and Use Committee.
2.2. Hippocampal slice preparation
Coronal slices (320 µm thickness) of hippocampus were prepared from mice using standard techniques (Carver et al., 2016). Mice were anesthetized with isoflurane and brains were excised rapidly and placed in 3.5˚C artificial cerebrospinal fluid (aCSF) buffer that composed of (in mM): 126 NaCl, 3 KCl, 0.5 CaCl2, 5 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, 11 glucose, and 0.3 mM kynurenic acid (pH adjusted to 7.35–7.40 with 95% O2 - 5% CO2, 305–315 mOsm/kg). Hippocampal slices were cut with a Vibratome in 3.5˚C aCSF (model 1500 with 900 Refrigeration System; Leica Microsystems, Inc., Bannockburn, IL). For each electrophysiology experiment, 2–4 animals were used for each group and concentration tested.
2.3. Dissociation of neurons
Acute neuron dissociation was performed by an enzymatic technique as described previously (Kay and Wong, 1986; Reddy and Jian, 2010). The hippocampal pieces of the DG and CA1 regions were microdissected carefully under a microscope (model SMZ 647; Nikon, Tokyo, Japan) and incubated in aCSF for 1 h at 28°C. The isolated sections were transferred into an enzymatic solution consisting of aCSF with protease XXIII (3 mg/ml, Sigma-Aldrich, St. Louis, MO). The slices were then incubated for precisely 23 to 25 min at 28°C. The remaining slices were rinsed twice with aCSF and gently triturated through Pasteur pipettes with three distinct diameters (fire-polished) to yield single cells. For each batch, slices were triturated six to nine times with each pipette in approximately 1.5 ml of aCSF. Trituration is proceeded from largest diameter to smallest diameter pipette. Then, the solution with tissue was allowed to settle for 1 min, and the suspension of freshly dispersed cells was carefully plated onto the recording chamber (Warner Instruments, Hamden, CT) for electrophysiology experiments.
2.4. Recording of GABA-gated currents
Whole-cell patch-clamp configuration was used for electrophysiological recordings in dissociated cells (Reddy and Jian, 2010). Experiments were performed at room temperature (22–24 °C). The recording chamber was fixed into the stage of an inverted microscope with phase-contrast and differential interference contrast optics (model IX71; Olympus, Tokyo, Japan). The physiological bath solution for the recording of dissociated neurons was composed of (in mM): 140 NaCl, 3 KCl, 10 HEPES, 2 MgCl2, 2 CaCl2, and 16 glucose (pH adjusted to 7.4 with NaOH, osmolarity, 315–325 mOsm/kg). Cells were visualized and images were acquired through video camera CCD-100 (Dage-MTI, Michigan City, IN) with FlashBus Spectrim 1.2 software (Pelco, Clovis, CA). Recording pipettes were pulled from capillary glass tubes (King Precision Glass, Claremont, CA) using a P-97 Flaming-Brown horizontal puller (Sutter Instrument Company, Novato, CA). The pipette tip resistances were 4 to 6 MΩ for recording. The recording pipettes were filled with a cesium pipette solution containing (in mM): 124 CsCl, 20 tetraethylammonium, 2 MgCl2, 10 EGTA, 10 HEPES, 0.1 GTP, 4 ATP (pH adjust to 7.2 with CsOH, osmolarity, 295–305 mOsm/kg). Currents were recorded using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). The membrane capacitance, series resistance, and input resistance of the recordings were monitored by applying a 5-mV (100-ms) depolarizing voltage step from a holding potential of −70 mV for dissociated cells. Signals were low-pass filtered at 2 kHz and digitized at 10 kHz with Digidata 1440A system. The current values were normalized to cell capacitance (an index of cell size) and expressed as current density (pA/pF). For whole cell current from isolated single cells, current response was expressed as the percentage of potentiation produced by GABA or GABA+GX. For fast application of test drugs, the perfusion pipette was positioned <200 µm away from the cell in the chamber. GABA, GX, and Zn2+ (1–30 μM) were applied using a multi-channel perfusion system (Automate Scientific, Berkeley, CA). A two-minute wash with bath solution was instituted after each drug trial in order to prevent receptor desensitization.
2.5. Slice patch-clamp electrophysiology
Electrophysiological recordings in hippocampal slices were performed in the whole-cell patch- clamp configuration (Carver et al., 2014; Wu et al., 2013). Hippocampal neurons were visually identified with an Olympus BX51 microscope equipped with a 40x water-immersion objective, infrared-differential interference contrast optics, and video camera. Hippocampal slices (320 µm) were maintained in continuously oxygenated aCSF at 28°C in a holding chamber for 60 min, and experiments were performed at room temperature (22–24 °C). The physiological bath solution for whole-cell recording from slice were composed of (in mM): 124 NaCl, 3 KCl, 1.5 MgSO4, 2.4 CaCl2, 1.25 NaH2PO4, 26 NaHCO3 and 10 glucose (pH adjusted to 7.4 with NaOH, osmolarity, 295–305 mOsm/kg). The pipette tip resistances were 5 to 7 MΩ for slice recordings, and were filled with a cesium pipette solution containing (in mM): 124 CsCl, 20 tetraethylammonium-chloride, 2 MgCl2, 10 EGTA, 10 HEPES, 0.1 GTP, 4 ATP, and 5 lidocaine N-ethyl bromide (QX-314), (pH adjusted to 7.2 with CsOH, osmolarity, 295–305 mOsm/kg). Currents were recorded using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). The membrane capacitance, series resistance, and input resistance of the recordings were monitored by applying a 5-mV (100-ms) depolarizing voltage step from a holding potential of −65 mV for slice recordings. Signals were low-pass filtered at 2 kHz and digitized at 10 kHz with Digidata 1440A system.
2.6. Tonic current recording and analysis
Tonic current and phasic, miniature inhibitory postsynaptic currents (mIPSCs) of GABA-A receptors were recorded in the presence of tetrodotoxin (TTX, 0.5 µM, Na+ channel blocker and inhibition of action potential-evoked neurotransmitter release), D,L-2-amino-5-phosphonovaleric acid (APV, 40 µM, N-methyl-D-aspartate channel blocker), and 6,7-dinitroquinoxaline-2,3-dione (DNQX, 10 µM, non-N-methyl-D-aspartate glutamate receptor blocker). The competitive antagonist gabazine (SR-95531, 50 µM) was added to perfusion at the conclusion of slice recordings to confirm block of GABAergic currents. Drugs were delivered to the bath chamber using a multi-channel perfusion system (Automate Scientific, Berkeley, CA). Perfusion rate was maintained at 2 ml/min. Drugs were allowed 2–4 min to perfuse into the bath chamber and slice before measurements were obtained. Off-line current analysis was performed with pClamp 10.2 software (Molecular Devices, Sunnyvale CA) and in-house software. To study the tonic inhibitory currents, transient events were manually removed from the current trace, so that the recording consisted only of the holding current in the voltage-clamp mode (Wu et al., 2013). Averaged amplitude of tonic current shift in conductance and root-mean-square (RMS) noise amplitude were measured. The tonic current was expressed as the difference in holding current before and after application of gabazine (50 µM) or Zn2+ (0.1–10 mM). IRMS is the noise conductance from chloride ions passing through the opened channels and in proportion to the chloride driving force. Tonic current was measured and averaged in 100 ms per epoch with 1 sec interval between epochs for 30 epochs. The measurements were taken 30 s before and 2–3 min after application of a drug. IRMS was studied in 50 ms each epoch with 500 ms interval between epochs for 30 epochs before and after drug application in each cell. To assess the effect of a drug on IRMS in an individual neuron, the distribution of IRMS in 30 epochs before the application of a drug (during the baseline period) was compared with that after drug application by a Student’s independent t-test. To compare data obtained from a group of neurons, IRMS values in individual epochs before and after drug application were averaged. Changes in IRMS are expressed in pA of current. Currents for a single cell were normalized to membrane capacitance (pA/pF) as tonic current density. Synaptic currents were recorded and analyzed as previously described (Carver et al., 2016). mIPSCs were acquired for 3 min for each drug response and condition. The amplitude and kinetics of mIPSCs were measured using MiniAnalysis software (Synaptosoft). Nonoverlapping events with single peaks were used to create an ensemble average mIPSC. A mean weighted decay time constant was determined from biexponential fitting function as . For each electrophysiology experiment, 2–4 animals were used per group.
2.7. Zinc administration
Zinc doses were given acutely via intrahippocampal microinfusion in mice, as previously described (Carver et al., 2016). The sterile saline was used as a control. Apart from a bipolar electrode in the hippocampus, a 26 gauge guide cannula (Plastics One) was stereotaxically implanted into the left ventral hippocampus (2.9 mm posterior, 3.0 mm lateral, and 3.0 mm below dura). After a period of at least 1 week of recovery, dual-implanted animals were subjected to kindling. Zinc was dissolved in sterile saline. Mice received zinc doses (10–300 µM) by slow intrahippocampal microinfusion 10 min prior to GX administration and 25 min before kindling stimulations. The efficiency of zinc delivery was confirmed by the correct placement of the guide cannula by histology (Carver et al., 2016).
2.8. Hippocampus kindling model of epilepsy
A hippocampus kindling model was used in seizure experiments (Reddy and Mohan, 2011; Reddy et al., 2015). Mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). A bipolar electrode fixed to a guide cannula (Plastics One) was stereotaxically implanted in the right ventral hippocampus (2.9 mm posterior, 3.0 mm lateral, and 3.0 mm below dura). After postoperative recovery, animals were subjected to kindling stimulation (Reddy and Mohan, 2011). The electrographic afterdischarge (AD) threshold was determined by application of 1 ms biphasic rectangular pulses at 60 Hz for 1 s, in increments of 25 µA using an isolated pulse stimulator (A-M Systems). AD duration was the total duration of electrographic spike activity (amplitude >2X baseline) occurring in a rhythmic pattern at a frequency >1 Hz. Mice were stimulated at 125% AD threshold once per day until Stage 5 seizures were elicited on 3 consecutive days, considered the fully kindled state. The electrographic activity was recorded using Axoscope 8.0 software with Digidata 1322A interface (Molecular Devices) through a Grass CP511 preamplifier (Astro-Med). Behavioral seizures were rated according to Racine’s scale as modified for mouse (Racine, 1972). One week after kindling, ZnCl2 (10 –300 µM) was dissolved in sterile saline and microinfused in 5 µl volume directly into the hippocampus using a perfusion pump at 0.2 µl/min. Mice were monitored for seizures and electrographic activity for at least 10 min. GX (s.c.) was administered 15 min after infusion of Zn2+. GX was dissolved in 20% β-cyclodextrin solution for subcutaneous injections. The control group received this vehicle 15 min prior to kindling stimulations. Mice were scored for protection based on the behavioral motor seizures and AD duration after each kindling session.
2.9. Drugs and reagents
All chemicals used in electrophysiology studies were purchased from Sigma-Aldrich unless otherwise specified. Ganaxolone was prepared as 2 mM stock solutions in dimethyl sulfoxide for electrophysiology experiments. Stock solutions were diluted in the external perfusion solution to the desired concentration for electrophysiological use. The concentration of dimethyl sulfoxide in final solution was less than 1%. GX and kynurenic acid were acquired from Tocris. TTX was purchased from Calbiochem (Billerica, MA).
2.10. Statistical analysis
Data was expressed as the mean ± standard error of mean (SEM). In electrophysiology studies, concentration-response curve data were subjected to non-linear, logistic fitting. Statistical comparisons of parametric measures including electrophysiology data were performed using one-way analysis of variance followed by post hoc Tukey’s test. In kindling epilepsy studies, differences in seizure stages and AD duration between groups were compared with the nonparametric Kruskal-Wallis test followed by the Mann-Whitney U-test. For patch-clamp data with small sample size, nonparametric tests were used to check the statistical significance especially when the data points were not normally distributed. In all statistical tests, the criterion for statistical significance was p<0.05, unless otherwise specified.
3. Results
3.1. Concentration-dependent inhibition of GX potentiation of GABA-gated currents by Zn2+ in hippocampal neurons
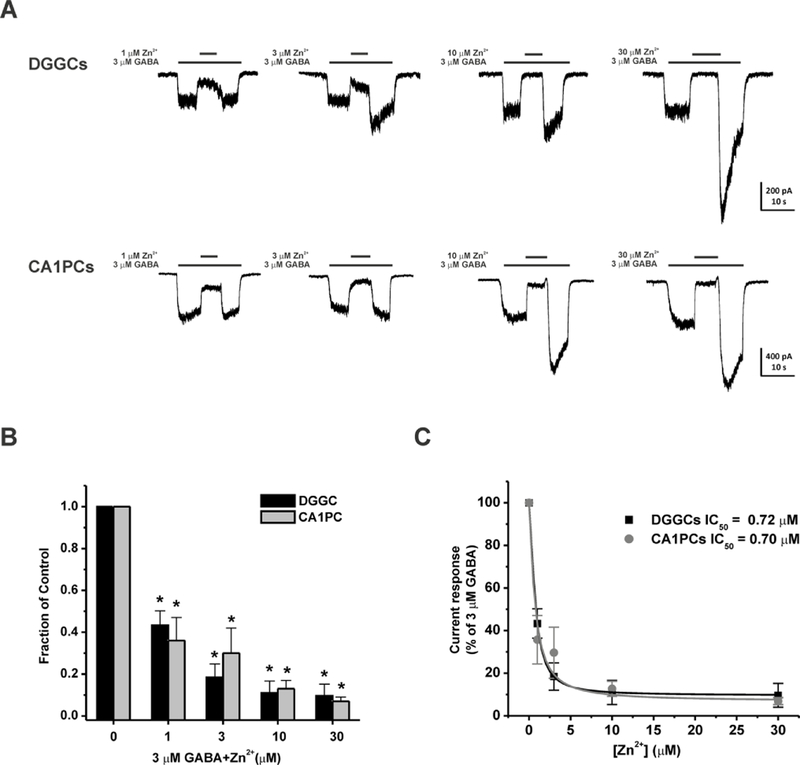
We first examined the blockade of GABAergic chloride currents with Zn2+, a negative allosteric modulator of GABA-A receptors (Barberis et al., 2000; Carver et al., 2016; Kapur and Macdonald, 1997; Smart et al., 1991). To examine the effects of subunit composition on Zn2+ inhibition of GABA-A receptor, concentration-response profiles were compiled in two cell types: δ-abundant DGGCs and δ-sparse CA1 pyramidal cells (CA1PCs). 3 µM GABA (EC10) was applied for 5 seconds followed by the application of Zn2+ for 5 seconds (Fig. 1A‒C). GABA at 3 µM induced 249.5 ± 16.5 and 538.1 ± 34.3 pA of currents in DGGCs and CA1PCs, respectively. Zn2+ blocked inhibitory GABAergic chloride currents concentration-dependently in both cell types. The Zn2+ at 1, 3, 10, and 30 µM application blocked a mean 56.7 ± 6.9 %, 81.6 ± 6.4 %, 89.0 ± 5.7 %, and 90.4 ± 5.6 % of GABA-induced currents in DGGCs, respectively. While the Zn2+ at 1, 3, 10, and 30 µM blocked a mean 64.3 ± 11.4 %, 70.4 ± 12.0 %, 87.3 ± 3.6 %, and 93.1 ± 1.6 % of GABA-induced currents in γ2-containing CA1PCs, respectively. GABA- modulated currents fully returned to peak amplitude after the removal of Zn2+ application. The half maximal inhibitory concentration (IC50) for Zn2+ block of 3 µM GABA-induced currents were 0.72 and 0.70 µM in DGGCs and CA1PCs, respectively. These results showed a similar concentration-dependent profile of Zn2+ blockade of GABA-gated chloride currents in DGGCs and CA1PCs.
Fig. 1.
Zn2+ blockade of GABA-gated currents is concentration-dependent in dissociated DGGCs and CA1PCs. (A) Representative whole-cell GABAergic current recording from DGGCs and CA1PCs. Zn2+ (1–30 μM) inhibited GABA currents in a concentration-dependent fashion. (B) Fractional block of currents by Zn2+ (1–30 μM). (C) Inhibitory concentration response curves of DGGCs and CA1PCs by Zn2+. DGGCs IC50 = 0.72 μM; CA1PCs IC50 = 0.70 μM. *p<0.05 vs. control. Each bar represents mean ± SEM (n = 4–6 cells per group).
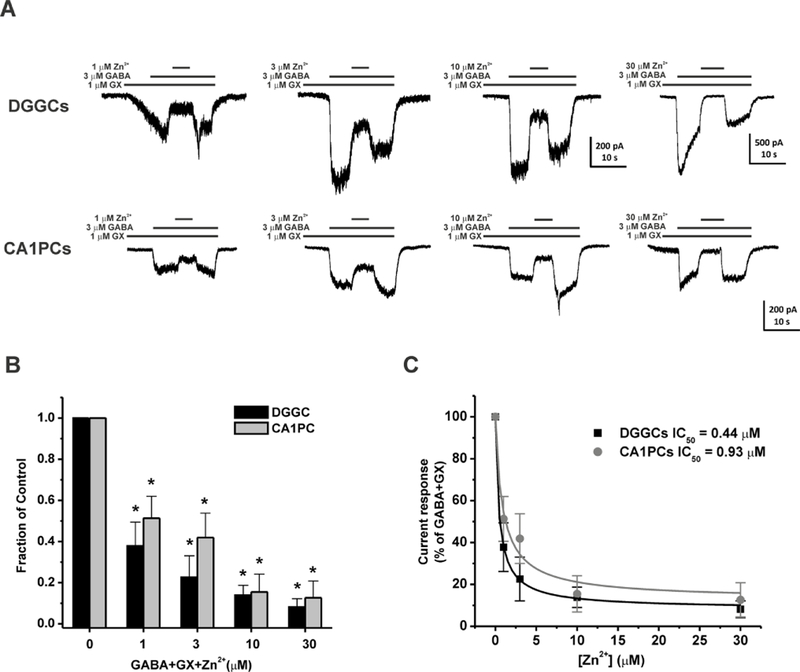
Next, we studied the modulatory effects of Zn2+ on GX-potentiated GABA-gated currents. GX at 1 µM was co-applied with 3 µM GABA to obtain the fractional potentiation of GABAergic currents mediated by GX. Subsequent application of Zn2+ (1–30 µM) blocked whole-cell inhibitory currents in a concentration-dependent manner (Fig. 2A‒C). In δ-containing DGGCs, the IC50 for Zn2+ block of 3 µM GABA + 1 µM GX currents was 0.44 µM. The 1 µM Zn2+ application blocked a mean 62.2 ± 11.6 % of whole-cell current modulation by GX. The 3 µM Zn2+ application blocked a mean 77.4 ± 10.5 % of whole-cell current modulation by GX. The 10 µM Zn2+ application blocked a mean 86.1 ± 4.8 % of whole-cell current modulation by GX. A 30 µM concentration of Zn2+ completely blocked GABA-A receptor whole-cell currents, similar to that of the competitive antagonism of channels by bicuculline and gabazine. In γ-containing CA1PCs, the sensitivity of Zn2+ inhibition on GX-induced currents was modestly reduced compared to that of Zn2+ in DGGCs (Fig. 2B). The IC50 for Zn2+ block of 3 µM GABA + 1 µM GX currents was 0.93 µM, which is 2.1-fold higher than that of Zn2+ in DGGCs (0.44 µM). The 1 µM Zn2+ application blocked a mean 48.7 ± 10.7 % of whole-cell current modulation by GX. The 3 µM Zn2+ application blocked a mean 58.1 ± 11.9 % of whole-cell current modulation by GX. The 10 µM Zn2+ application blocked a mean 84.5 ± 8.7 % of whole-cell current modulation by GX. A 30 µM concentration of Zn2+ blocked a mean 87.3 ± 8.2 % of whole-cell currents. Overall, our results demonstrated that concentration-dependent blockade of Zn2+ on GX-potentiated GABA- gated chloride currents has higher sensitivity at neurons that have a higher expression of δ- containing GABA-A receptors.
Fig. 2.
Zn2+ blockade of GX-sensitive GABA-gated currents is concentration-dependent in dissociated DGGCs and CA1PCs. (A) Representative whole-cell GX-activated GABAergic current recording from DGGCs and CA1PCs. Zn2+ (1–30 μM) inhibited GX-potentiated GABA currents in a concentration-dependent fashion. (B) Fractional block of currents by Zn2+ (1–30 μM). (C) Inhibitory concentration response curves of DGGCs and CA1PCs by Zn2+. DGGCs IC50 = 0.44 μM; CA1PCs IC50 = 0.93 μM. *p<0.05 vs. control. Each bar represents mean ± SEM (n = 4–5 cells per group).
3.2. Selective antagonism of GX potentiation of tonic currents by Zn2+ in hippocampal slices
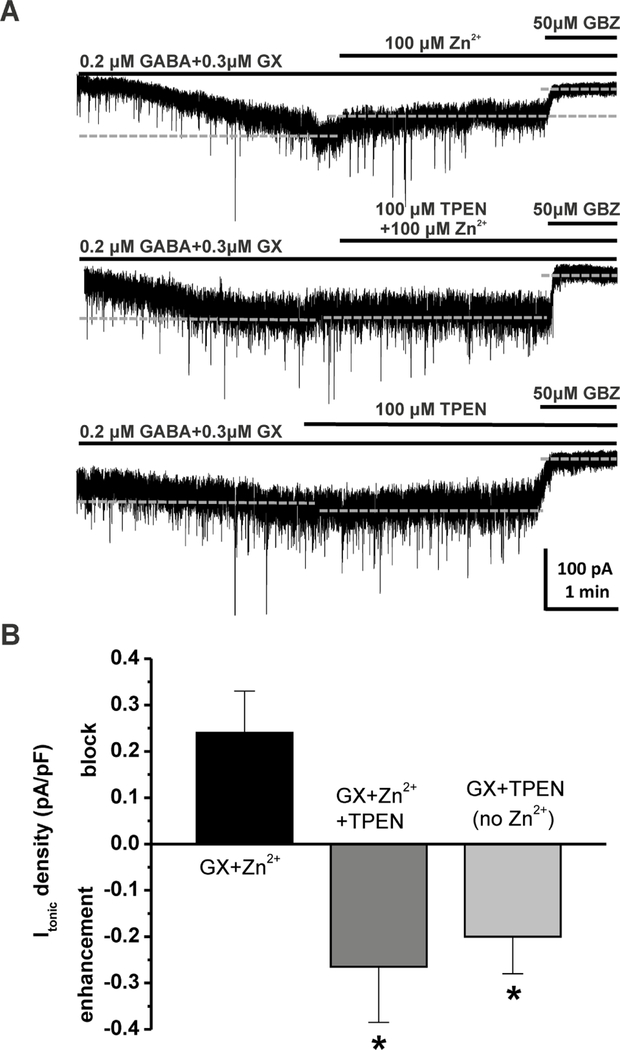
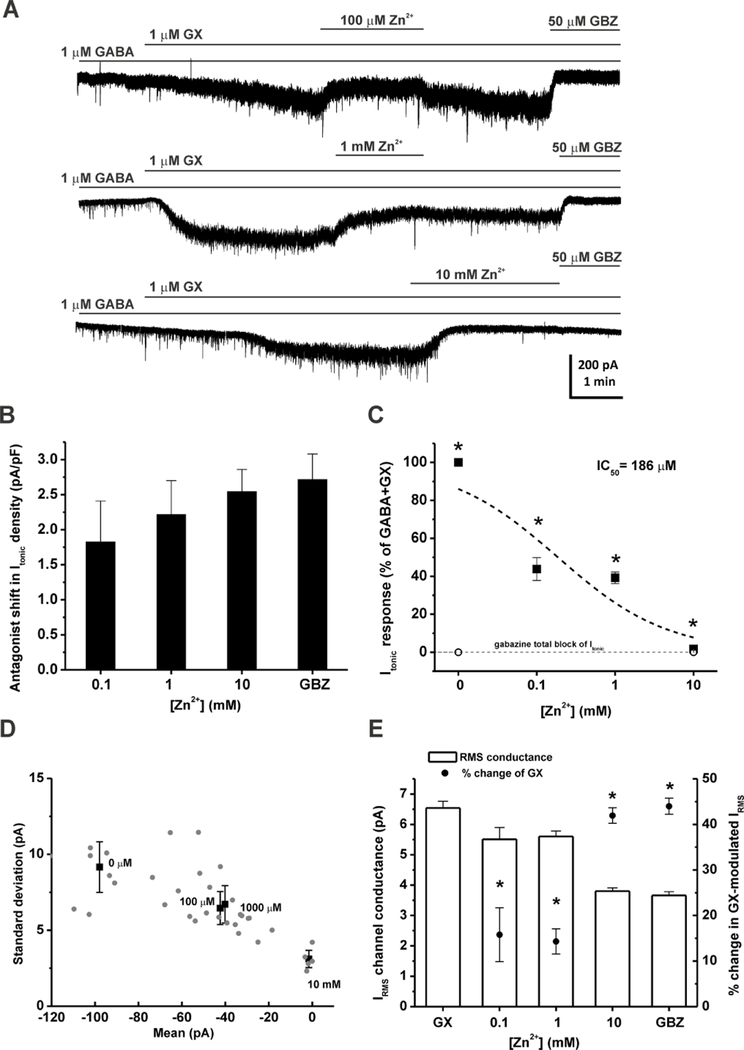
To examine the interactions of Zn2+ with GX at extrasynaptic δ-containing GABA-A receptors, we recorded GX-activated tonic currents from DGGCs in the hippocampus slice using whole-cell voltage-clamp (−65 mV) recording. At the end of each recording, 50 µM gabazine was perfused to determine the total tonic current shift. Tonic current of each cell was normalized to the cell capacitance as a measure of current density (pA/pF). A physiological concentration of GABA (0.2 µM) was used to examine GX allosteric potentiation (Wlodarczyk et al., 2013). Our results showed that application of 0.2 µM GABA + 0.3 µM GX resulted in a negative shift in the holding current level and an increase in the RMS channel conductance (Fig. 5A). However, Zn2+ (100 µM) perfusion positively shifted GX-potentiated tonic currents (current density: GABA + GX, 0.96 ± 0.18 pA/pF; GABA + GX + Zn2+, 0.72 ± 0.11 pA/pF, n = 5 cells). To further demonstrate the pharmacological sensitivity of Zn2+ blockade of GX-induced tonic current potentiation, 1 µM of GABA and GX were used to record tonic responses (Fig. 3A). Subsequent application of Zn2+ (0.1–10 mM) induced a concentration-dependent blockade of tonic current density, measured as positive shift of the holding currents (Fig. 3B). Zn2+ (100 µM) wash-out reversed the holding currents to the previously enhanced level by GX. However, with the application of 1 mM Zn2+, Zn2+ wash-out was not able to eliminate the effects induced by Zn2+. The IC50 value for Zn2+ blockade of tonic currents was 0.186 mM (Fig. 3C). The mean and the standard deviation (s.d.) of the tonic currents for different Zn2+ concentrations were plotted in Fig. 3D. Higher the mean of tonic currents was accompanied with higher the s.d. of tonic currents. Zn2+ concentration- dependently reduced both the mean and s.d. of GX-potentiated tonic currents. Zn2+ at 0.1, 1, and 10 mM caused 56.2 ± 6.0 %, 60.8 ± 3.0 %, and 98.3 ± 0.7 % reduction of GX-potentiated tonic currents, respectively. Zn2+ also significantly reduced GX-potentiated RMS channel conductance at all concentration tested (Fig. 3E). Zn2+ at 10 mM caused 42.0 ± 1.7 % reduction of the RMS channel conductance, similar to that of the competitive antagonist gabazine (44.0 ± 1.8 %). Overall, these results demonstrate a concentration-dependent blockade of Zn2+ on GX- potentiated tonic currents.
Fig. 5.
Zn2+ chelator TPEN inhibits the Zn2+ antagonism of GX-sensitive tonic currents. (A) Representative GABAergic Itonic recording from DGGCs before and after the application of Zn2+ (100 µM) or Zn2+ chelator TPEN (100 µM), or in coapplication of both in modulation of 0.2 µM GABA + 0.3 µM GX. TPEN prevented Zn2+ blockade of GX-sensitive current, and Itonic exhibited significant enhancement by TPEN in hyperpolarization of the holding current level. Quantification of Itonic was achieved relative to complete block by gabazine (GBZ). Dashed lines indicate average holding current level throughout each drug application. (B) Itonic density shift (pA/pF) in the presence of Zn2+ and/or TPEN, with positive values as block of GX-sensitive Itonic and negative values as enhancement of Itonic. Data are mean ± SEM (n = 5 cells per group). *p<0.05 vs. GX + Zn2+ combination (Mann-Whitney U-test).
Fig. 3.
GX-potentiated tonic currents are sensitive to Zn2+ blockade in DGGCs in hippocampus slices. (A) Representative GABAergic Itonic recording from DGGCs in the presence of GABA + GX, Zn2+, and 50 μM gabazine (GBZ). (B) Concentration-response of GX-modulated, normalized Itonic (pA/pF) to block by Zn2+ (0.1–10 mM). (C) Fractional response of GX-modulated Itonic due to Zn2+ (*p<0.05 vs. maximal block due to saturating 50 μM gabazine (GBZ). (D) Zn2+ reduced the mean and standard deviation of GX-potentiated tonic currents in a concentration- dependent fashion. (E) IRMS channel conductance (pA) and % change of Zn2+ blockade of GX- dependent IRMS. GX denotes 1 μM GABA + 1 μM GX condition without Zn2+. *p<0.05 vs. GX. Each bar represents mean ± SEM (n = 6–21 cells per group).
3.3. GX potentiation of phasic currents are insensitive to Zn2+ antagonism
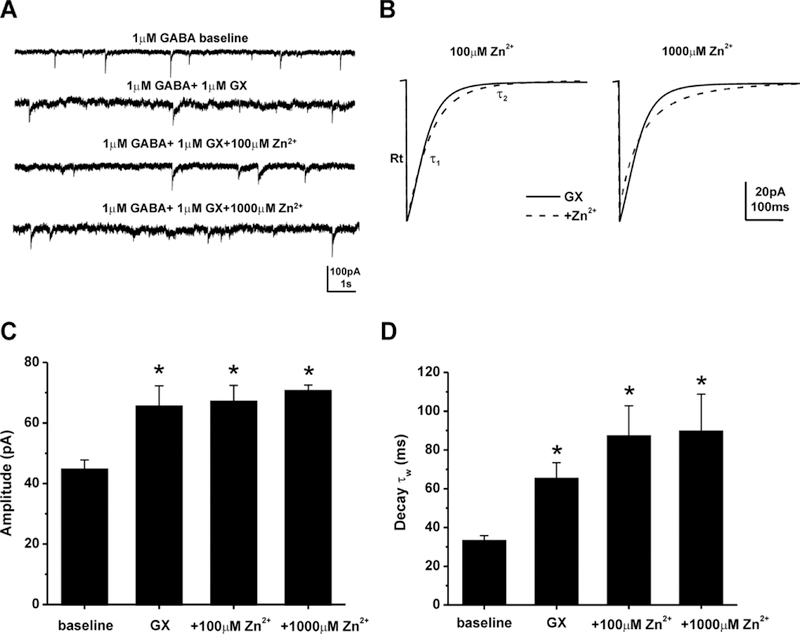
Next, to examine the block of Zn2+ on GX-potentiated phasic currents in DGGCs, we recorded GX-activated mIPSCs, which primarily reflect the activation of synaptic GABA-A receptors, in the presence of the NMDA receptor antagonist (APV, 40 µM), the AMPA receptor antagonist (DNQX, 10 µM), and the sodium channel blocker (TTX, 0.5 µM) (Fig. 4). Representative traces and ensemble average mIPSCs for each condition are shown in Figure 4A-B. The average mIPSC from each cell was best fit with a double-exponential decay curve, depicted as τ1 and τ2. A mean weighted decay constant τw was also derived from τ1 and τ2 (see Materials and Methods). Table 1 showed the characteristics of mIPSCs modulated by GABA, GX, or Zn2+. GX significantly potentiated the amplitude, decay time τ2, and the mean weighted decay time τw of mIPSCs from DGGCs (Fig. 4C‒D). However, Zn2+ perfusion did not change GX-modulated mean peak amplitude or decay time constant. These findings indicate that Zn2+ blockade of GX- induced GABAergic currents is highly selective for extrasynaptic δGABA-A receptor-mediated tonic currents.
Fig. 4.
GX-activated mIPSCs are not sensitive to Zn2+ blockade. (A) Representative traces of mIPSCs. GABAA receptor mIPSC activity was isolated by using TTX, APV, and DNQX, and was completely blocked by gabazine. GX (1 µM) potentiated synaptic current but Zn2+ did not significantly alter the GX-potentiated mIPSCs. (B) Averaged mIPSCs in the presence of GX (solid line) and addition of Zn2+ (dashed line). (C) Amplitudes were not significant different between GX and 100 μM Zn2+ (p = 0.65), or GX and 1000 μM Zn2+ (p = 0.70). (D) Zn2+did not significantly alter mean weighted decay kinetics (τw) of GX modulation. Data bars represent mean ± SEM (n = 4–9 cells per group). *p<0.05 vs. baseline. GX denotes GABA + GX; baseline denotes GABA alone; Rt denotes rise time.
Table 1.
GABA-A receptor-mediated mIPSC characteristics in DGGCs.
| Group (n) | Amplitude (pA) | Rise10–90% (ms)a | Decay τ1 (ms)a | Decay τ2 (ms)a | Decay τw (ms) | Frequency (Hz) |
|---|---|---|---|---|---|---|
| 1μM GABA (13) | 43.1 ± 1.5 | 1.1 ± 0.1 | 10.6 ± 1.3 | 48.0 ± 1.9 | 30.2 ± 1.4 | 0.56 ± 0.08 |
| GABA+1μM GX (9) | 65.4 ± 6.9* | 1.6 ± 0.3 | 14.5 ± 1.5 | 104.8 ± 10.7* | 65.5 ± 7.9* | 0.43 ± 0.16 |
| GABA+GX+100μM Zn2+ (5) | 67.0 ± 5.4* | 1.0 ± 0.3 | 22.4 ± 4.7*# | 114.1 ± 16.1* | 87.4 ± 15.4* | 0.30 ± 0.17 |
| GABA+GX+1000μM Zn2+ (4) | 70.5 ± 2.0* | 1.0 ± 0.1 | 18.7 ± 3.9*# | 144.9 ± 18.9* | 89.9 ± 18.9* | 0.10 ± 0.02* |
p< 0.05 vs. GABA
p< 0.05 vs. GABA+GX.
3.4. The Zn2+ chelator TPEN reverses Zn2+ blockade of GX-sensitive tonic currents
To further confirm the Zn2+ blockade of extrasynaptic δGABA-A receptors, we investigated tonic current response in the presence of a membrane-permeable, high affinity Zn2+ chelator, N,N,N’,N’-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN). Zn2+ (100 µM) perfusion blocked GX-potentiated tonic currents in DGGCs, resulting in a positive shift of tonic current density (0.24 ± 0.09 pA/pF). Such blockade by Zn2+ was prevented when TPEN (100 µM) was added to the perfusion, and DGGCs displayed −0.27 ± 0.11 pA/pF negative shift in tonic current density (Fig.5). The difference between Zn2+-antagonized and TPEN-enhanced tonic currents achieves statistical significance (p = 0.008, n = 5 cells per group). Without exogenous Zn2+ added to perfusion, TPEN sustained the negative shift of current density modulated by GX (−0.2 ± 0.08 pA/pF, n = 5 cells), indicating significant modulation of endogenous Zn2+ within the hippocampus.
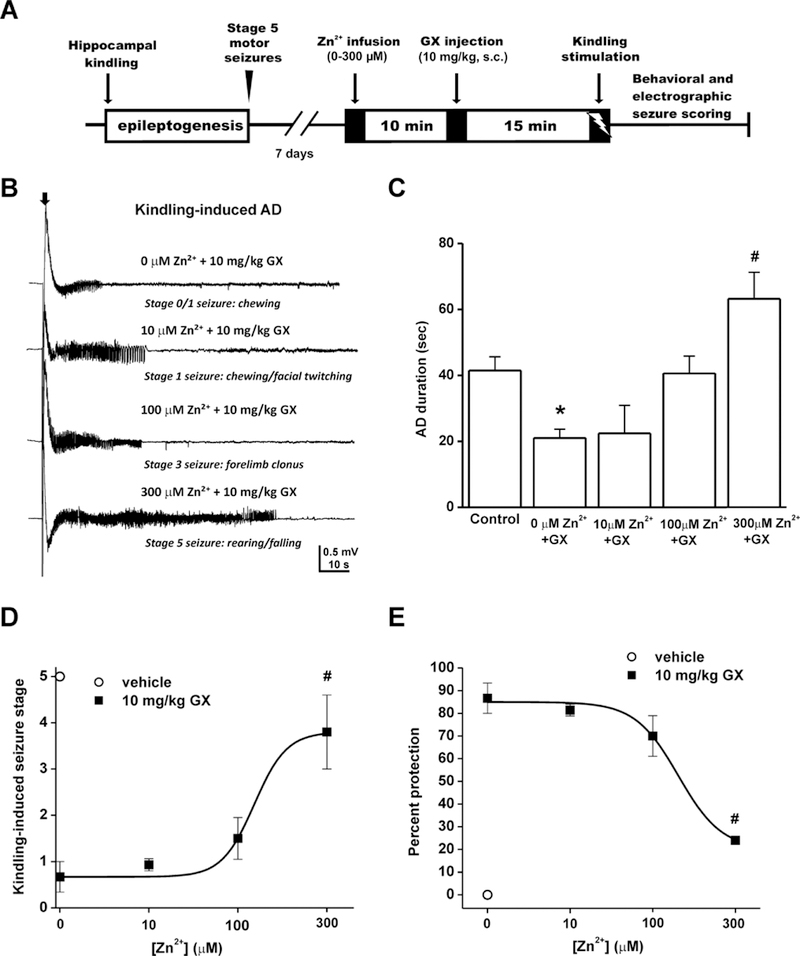
3.5. Intrahippocampal Zn2+ antagonizes the seizure protective effect of GX in fully-kindled mice
To directly investigate the interactions of Zn2+ and GX on hippocampal excitability and seizures, we examined the antiseizure activity of GX with or without intrahippocampal Zn2+ infusion in a mouse kindling model, in which the fully-kindled mice exhibit consistent, generalized stage 5 seizures. The experimental paradigm was shown in Fig. 6A. Intrahippocampal Zn2+ (10–300 µM) infusion was performed 10 min before GX (10 mg/kg, s.c.) treatment followed by kindling stimulation. GX (10 mg/kg, s.c.) significantly suppressed kindling-induced seizures (Fig. 6D-E). Zn2+ infusion blocked the antiseizure activity of GX dose-dependently, in which Zn2+ at 300 µM achieves a statistical significance. The electrograph recordings of electrically-induced, kindling afterdischarge (AD) were shown in Fig. 6B. GX-treated mice (10 mg/kg, s.c.) displayed significantly reduced AD durations compared to vehicle-treated mice (Fig. 6C). Despite GX treatment, 300 µM Zn2+-infused mice exhibited significantly greater AD durations and higher incidence of seizures (Fig. 6C‒E). The reversible effect of Zn2+ on kindling seizures was demonstrated since all animals exhibited stage 5 seizures after 24-hr wash-out of drug. Overall, these results suggest that Zn2+ plays a key role in the modulation of seizure susceptibility by anticonvulsant neurosteroids like GX that are potent activators of δGABA-A receptors.
Fig. 6.
Intrahippocampal Zn2+ infusion completely prevents the antiseizure effect of the neurosteroid GX in fully-kindled mice. (A) Experimental paradigm and infusion protocol for saline or Zn2+ delivery (0–300 μM) prior to GX (10 mg/kg, s.c.) treatment and kindling stimulation. (B) Electrograph recordings of electrically-induced, kindling afterdischarge (AD). The black arrow denotes stimulation-onset artifact. (C) AD duration of electrograph activity. Control denotes saline infusion (Zn2+ -free) and vehicle injection (GX-free) condition upon kindling. (D) Kindling- induced behavioral seizure score after Zn2+ infusion. ED50 = 150 µM. (E) Dose-response curve of percent seizure protection. Anticonvulsant effect of 10 mg/kg GX was inhibited by 300 μM Zn2+. After 24 hr wash-out, mice displayed stage 5 seizures and similar AD duration to the control group. Data represent mean ± SEM (n = 5–6 mice per group). *p<0.05 vs. control; #p<0.05 vs. GX without Zn2+ (Mann-Whitney U-test).
4. Discussion
Zn2+ is an extremely abundant transition metal in the synaptic vesicles of hippocampal glutamatergic mossy fibers and remains a key factor in the modulation of neuronal plasticity (Assaf and Chung, 1984). Disruption of Zn2+ homeostasis is associated with many neurological disorders, including seizures, epilepsy, and conditions with compromised brain functions. Previous studies show that Zn2+ modulates kinetics of synaptic GABA-A receptors and alters the balance of inhibition and excitability (Barberis et al., 2000; Lambert and Belelli, 2002). Zn2+ exhibits higher sensitivity to extrasynaptic δGABA-A receptors than synaptic γGABA-A receptors (Hosie et al., 2003; Wei et al., 2003). However, its inhibition on extrasynaptic receptor-mediated tonic currents and interactions with neurosteroids are not fully understood. Our current study shows that Zn2+ pretreatment prevents the anticonvulsant activity of neurosteroids, an effect most likely due to the Zn2+blockade of neurosteroid-potentiated extrasynaptic δGABA-A receptors. These receptors represent the main contributors in maintaining the tonic inhibition in the dentate gyrus, which is involved in a number of seizure and memory disorders (Carver et al., 2016).
Positive allosteric modulators targeting extrasynaptic δGABA-A receptors, such as neurosteroids, are being evaluated as potential therapeutic agents for the treatment of hyperexcitable brain disorders (Reddy and Rogawski, 2001; 2010; Reddy et al., 2018; Younus and Reddy, 2018). GX is a neurosteroid analog developed as a more favorable therapeutic compound with superior bioavailability and pharmacokinetic profile compared to its prototype neurosteroid AP. Synthetic GX serves as a robust antiseizure agent as well as a powerful allosteric modulator of GABA-A receptors with higher selectivity for extrasynaptic δGABA-A receptors (Reddy and Rogawski, 2000; 2010; Clossen and Reddy, 2017a; b; Chuang and Reddy, 2018). Elucidating the neuroprotective effects of GX as well as its interactions with other molecules at the δGABA extrasynaptic receptors is of critical importance for clinical use as an antiepileptic drug. In the present study, we demonstrate that Zn2+ selectively blocks GX-induced tonic inhibition, but not phasic inhibition in DGGCs. Chelation of endogenous Zn2+ sustains the enhancement of tonic inhibition by GX. Furthermore, intrahippocampal infusion of Zn2+ significantly blocked the antiseizure activity of GX in the hippocampus kindling model of epilepsy. Zn2+ selective antagonistic interactions with GX at the extrasynaptic δGABA-A receptors in the hippocampus may contribute to the blockade of GX-induced antiseizure activity by Zn2+; this drug-drug interaction provides clinical implications in the therapeutic use of GX. Overall, these findings are compatible with an excitability-facilitating and proconvulsant role of Zn2+ in seizure- related disorders (Buhl et al., 1996; Cavazos et al., 1991; Coulter, 2000).
Receptor subunit composition plays a critical role not only in neurosteroid sensitivity but also in Zn2+ inhibition on GABA-A receptors (Draguhn et al., 1990; Smart et al., 1991). Although, in recombinant systems, Zn2+ inhibition on GABA-A receptors exhibits similar sensitivity in human α4β3γ2 and α4β3δ GABA-A receptors (Brown et al., 2002), Zn2+ significantly shortens the decay time constant of spontaneous IPSCs as well as dendritically-evoked IPSCs in wildtype δ-rich DGGCs but not δ-subunit knockout DGGCs from hippocampus slices (Wei et al., 2003). The absence of sensitivity to Zn2+ inhibition in δ-subunit knockout DGGCs may be due to the compensatory upregulation of γ2 subunits and thus the reduction of Zn2+ binding sites (Peng et al., 2002; Hosie et al., 2003; Carver and Reddy, 2013). In the present study, we compared the sensitivity of GABA-induced currents and GX-potentiated GABA chloride currents to Zn2+ inhibition in native δ-abundant DGGCs and δ-sparse CA1PCs. We found increased inhibition of neurosteroid-potentiated GABA currents by Zn2+ in DGGCs compared to CA1PCs, displaying the selectivity of Zn2+ inhibition based on receptor subunit composition. In addition, Zn2+ at 30 µM completely blocks GX-potentiated GABAergic chloride currents, similar to that of the competitive antagonist, bicuculline and gabazine. Due to its preferential modulation to δ- containing receptors, Zn2+ represents a potential noncompetitive antagonist of extrasynaptic δGABA-A receptors and would aide in pharmacological investigations of extrasynaptic GABA-A receptors.
In single channel studies, Zn2+ primarily inhibits GABA-A receptors through the reduction of channel opening probability (Smart, 1992; Smart et al., 1994). In slice recordings, Zn2+ significantly reduces phasic mIPSC event amplitude and kinetics, as well as desensitization kinetics (Barberis et al., 2000; Ruiz et al., 2004). However, we demonstrate that GX-enhanced phasic mIPSCs were unaltered by Zn2+. It could be that GX potentiation overcomes Zn2+ depression of synaptic receptors so that the overall effects of Zn2+ on GX-potentiated phasic currents were undetectable. However, in tonic current potentiation, Zn2+ blocks GX-potentiated tonic response concentration-dependently in δ-rich DGGCs, probably due to its greater sensitivity towards δ-containing receptors. Unexpectedly, Zn2+ prolongs the fast decay time constant τ1 of mIPSCs in the presence of GX. One possibility might be due to the biphasic effects of Zn2+ on GABA-B receptors that tonically enhance GABA-A receptor currents (Turgeon and Albin, 1992; Khatri et. al., 2018). In addition, receptor combinations with different subunits respond to Zn2+ and neurosteroids differently. Finally, Zn2+ may have different effects on fast- closing GABA-A receptor subtypes (Draguhn et al., 1990; Smart et al., 1991).
Zn2+ has been shown to act on multiple neurotransmission systems in the brain. In addition to its inhibitory effects on GABA-A receptor function (Smart et al., 1991;1994; Barberis et. al., 2000; Carver et al., 2016), Zn2+ also antagonizes excitatory glutamate NMDA receptor channels and decreases the activation and surface expression of NR2A-contaning NMDA receptors in hippocampal neurons (Westbrook and Mayer, 1987; Vogt et al., 2000; Zhu et al., 2012). Additionally, Zn2+ enhances the activity of AMPA receptors (Timofeeva and Nadler, 2006) and increases glycinergic neurotransmission. Therefore, it is possible that the excitability-facilitating and proconvulsant role of Zn2+ against GX-induced protective effects in the kindling seizure model may be resulted from the overall outcome of disrupted neurotransmission by Zn2+. Nevertheless, we have previously shown a critical role of extrasynaptic δGABA-A receptor in seizure susceptibility as mice lacking δ subunit have significantly reduced tonic currents and greater seizure susceptibility (Carver et al., 2014). Thus, Zn2+ selective antagonistic interactions with GX at the extrasynaptic δGABA-A receptors in the hippocampus may contribute to the inhibition of GX-induced antiseizure activity by Zn2+.
Zn2+ exhibits antagonistic activity at α4βγ-and α1βδ-containing GABA-A receptors, albeit at lower sensitivities than α4βδ subtypes (Brown et al., 2002). Specific deletion of δ subunits in α1βδ- containing DG interneurons leads to the reduced GABAergic tonic inhibition and elevated firing rate of interneurons that decrease DGGC excitability and in vivo seizure susceptibility (Lee and Maguire, 2013). Therefore, Zn2+ may also contribute to the disinhibition of α1βδ-containing interneurons. In the seizure model of hippocampal kindling, the composition and expression of GABA-A receptors in the hippocampus has been largely altered (Nishimura et al., 2005). In hippocampus DG, the mRNA levels of δ subunit were significantly reduced with an increase in α2 subunits seven days after kindling completion. In CA3 pyramidal neurons, α2 and β3 subunits were significantly upregulated after hippocampal kindling. While in CA1 pyramidal neurons, no significant changes of GABA-A receptor subunits were observed. As a result, the sensitivity of each cell types to Zn2+ modulation may be different from animals without kindling, which may also involve in the Zn2+ antagonism of GX-induced seizure protection. Nevertheless, we observed the net outcome of Zn2+ in hippocampal kindling to be proconvulsant.
Zn2+ increases the permeability of blood-brain barrier and proconvulsant activity in rats (Yorulmaz et al., 2013). Severe blood-brain barrier damage is also found in PTZ-induced epileptic seizures. The Zn2+ level in the brain may fluctuate due to the contribution of peripheral Zn2+ that penetrates the damaged blood-brain barrier. Zn2+ concentrations are particularly high in some brain regions including hippocampus and amygdala, which are also the most vulnerable regions for the focal point of seizures (Frederickson et al., 2005). The effective concentrations of Zn2+ in the inhibition of GX-potentiated tonic currents are within the levels that may occur in synaptic clefts following the Zn2+ release from the vesicles of presynaptic neurons during neuronal activity. Therefore, Zn2+ may disrupt the neurosteroid-augmented basal inhibitory tone and hinder the balance of hippocampal neural circuits. In physiological condition, free zinc cations released from glutamatergic synapses in the hippocampus may block the inhibition of extrasynaptic activity of neurosteroids, hindering the antiseizure effects of neurosteroids. Therefore, combination therapies of neurosteroids with Zn2+ chelators may be potential avenues for the treatment of seizure-related disorders. We have attempted to examine the effects of Zn2+ chelators on the seizure activity of hippocampal kindling animals. However, a previous study shows that TPEN exerts notable toxic effects in in vivo animal studies, which was the reason these studies were not conducted further (Elsas et al., 2009). In Foresti et al., 2008, they used another Zn2+ chelator and found that pretreatment of Zn2+ chelator DEDTC (700 mg/kg) diminishes the duration of behavioral seizures and electrical afterdischarges, and EEG spikes, without altering seizure severity progression in a rat model of amygdala kindling, showing the antiseizure effects of the Zn2+ chelator (Foresti et al., 2008). Although their experiments show an antiseizure role of the zinc chelator, they cannot exclude the potential toxic effects of DEDTC. In their studies, they selected a dose that did not cause significant behavioral changes but still may be high enough to cause toxic effects in cellular and molecular levels since animals experienced severe ocular and nasal bleeding and reduced locomotor activity with the treatment of DEDTC. Future studies of zinc chelators on the seizure activity are feasible when nontoxic Zn2+ chelators become available.
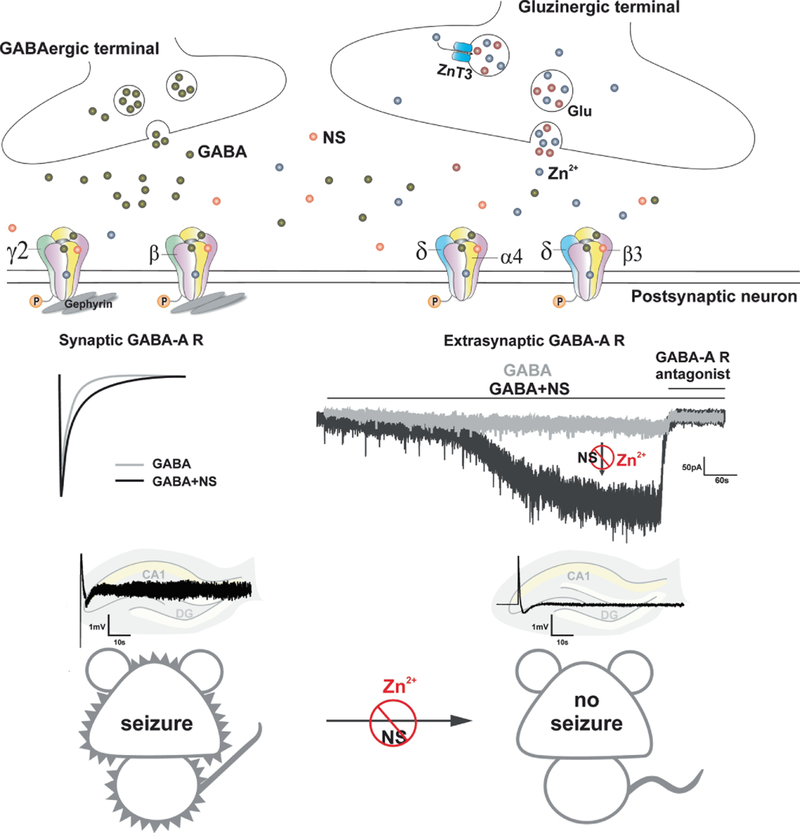
The schematic diagram of the antagonistic interactions of Zn2+ and neurosteroids (NSs) at GABA-A receptors is summarized in Fig. 7. Neurosteroids exhibit powerful seizure protective effects against experimental seizures probably through the potentiation of synaptic and extrasynaptic GABA-A receptor-mediated inhibitory currents. However, Zn2+ selectively hinders neurosteroid-augmented tonic inhibition but not phasic inhibition, which may partially contribute to the Zn2+ antagonism of neurosteroid-induced antiseizure activity. Previous studies show that Zn2+ blocks the frequency of mIPSCs in DGGCs from kindled but not controls (Buhl et al., 1996). Reduced Zn2+ sensitivity in GABA currents in DGGCs from rats with status epilepticus (Kapur and Macdonald, 1997). Zn2+ inhibition of GABA-A receptor function is also decreased in pilocarpine-induced status epilepticus (Banerjee et al., 1999). Overall, Zn2+ greatly contributes to the balance of neuronal excitation and inhibition in both physiological and pathophysiological conditions. There are many questions that remain unclear, including whether posttranslational modifications affect Zn2+ sensitivity of GABA-A receptors.
Fig. 7.
Schematic diagram of pharmacodynamic interactions of Zn2+ and neurosteroids at GABA-A receptors. Neurosteroids exhibit powerful seizure protective effects against kindling- induced limbic seizures probably through the potentiation of synaptic and more preferably extrasynaptic GABA-A receptor-mediated inhibitory currents. Zn2+ selectively hinders neurosteroid-sensitive tonic inhibition but not phasic inhibition, which may partially contribute to the Zn2+ antagonism of antiseizure activity of neurosteroids, including endogenous neurosteroids
In conclusion, the present study demonstrates selective antagonistic interactions of Zn2+ and GX at extrasynaptic δGABA-A receptors in the hippocampus. Zn2+ chelation sustains the potentiation of tonic inhibition by GX. Furthermore, intrahippocampal infusion of Zn2+ significantly blocked the antiseizure activity of GX in the mouse kindling model of epilepsy, indicating that Zn2+ reduces the antiseizure effects of neurosteroids by selective blockade of extrasynaptic δGABA-A receptors. These pharmacodynamic interactions may have clinical implications in neurosteroid therapy of brain disorders susceptible to zinc fluctuations.
Highlights:
Zinc modulates hippocampus neuronal excitability.
Neurosteroids are powerful anticonvulsants via modulation of GABA-A receptors
Zinc selectively blocked extrasynaptic tonic inhibition.
Zinc prevented the protective effects of ganaxolone, an antiepileptic synthetic neurosteroid.
Zinc‒neurosteroid interactions have therapeutic implications in epilepsy.
Acknowledgements
The author’s research was supported by the CounterACT Program, National Institutes of Health, Office of the Director and the National Institute of Neurologic Disorders and Stroke [Grant U01 NS083460].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None
References
- Assaf SY, Chung SH, 1984. Release of endogenous Zinc from brain tissue during activity. Nature 308, 734–736. [DOI] [PubMed] [Google Scholar]
- Banerjee PK, Olsen RW, Snead OC, 1999. Zinc inhibition of gamma-aminobutyric acid A receptor function is decreased in the cerebral cortex during pilocarpine-induced status epilepticus. J. Pharmacol. Exp. Ther 291, 361–366. [PubMed] [Google Scholar]
- Barberis A, Cherubini E, Mozrzymas JW, 2000. Zinc inhibits miniature GABAergic currents by allosteric modulation of GABA-A receptor gating. J. Neurosci 20, 8618–8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS, 2015. Progress report on new antiepileptic drugs: A summary of the Twelfth Eilat Conference (EILAT XII). Epilepsy Res 111, 85–141. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL, 2002. Slow phases of GABA-A receptor desensitization: structural determinants and possible relevance for synaptic function. J. Physiol 544, 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL, 2003. Neurosteroids shift partial agonist activation of GABA-A receptor channels from low- to high-efficacy gating patterns. J. Neurosci 23, 10934–10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Cunningham MG, 2009. Zinc: the brain’s dark horse. Synapse 63, 1029–1049. [DOI] [PubMed] [Google Scholar]
- Braat S, D’Hulst C, Heulens I, De Rubeis S, Mientjes E, Nelson DL, Willemsen R, Bagni C, Van Dam D, De Deyn PP, Kooy RF, 2015. The GABA-A receptor is an FMRP target with therapeutic potential in fragile X syndrome. Cell Cycle 14, 2985–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M, 1996. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol 497, 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA, 2002. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA-A receptors. Br. J. Pharmacol 136, 965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl EH, Otis TS, Mody I, 1996. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science 271, 369–373. [DOI] [PubMed] [Google Scholar]
- Carver CM, Chuang SH, Reddy DS, 2016. Zinc selectively blocks neurosteroid-sensitive extrasynaptic deltaGABA-A receptors in the hippocampus. J. Neurosci 36, 8070–8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, Reddy DS, 2013. Neurosteroid interactions with synaptic and extrasynaptic GABA-A receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology (Berl) 230, 151–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, Reddy DS, 2016. Neurosteroid structure-activity relationships for functional activation of extrasynaptic deltaGABA-A receptors. J. Pharmacol. Exp. Ther 357, 188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, Wu X, Gangisetty O, Reddy DS, 2014, Perimenstrual-like hormonal regulation of extrasynaptic delta-containing GABA-A receptors mediating tonic inhibition and neurosteroid sensitivity. J. Neurosci 34, 14181–14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazos JE, Golarai G, Sutula TP, 1991. Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J. Neurosci 11, 2795–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SH, Reddy DS, 2018a. Genetic and molecular regulation of extrasynaptic GABA-A receptors in the brain: therapeutic insights for epilepsy. J. Pharmacol. Exp. Ther 364, 180–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SH, Reddy DS, 2018b. 3beta-methyl-neurosteroid analogs are preferential positive allosteric modulators and direct activators of extrasynaptic deltaGABA-A receptors in the hippocampus dentate gyrus subfield. J. Pharmacol. Exp. Ther 365, 583–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clossen BL, Reddy DS, 2017a. Catamenial-like seizure exacerbation in mice with targeted ablation of extrasynaptic deltaGABA-A receptors in the brain. J. Neurosci. Res 95, 1906–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clossen BL, Reddy DS, 2017b. Novel therapeutic approaches for disease-modification of epileptogenesis for curing epilepsy. Biochim. Biophys. Acta 1863, 1519–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD, 1999. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl. Acad. Sci. U.S.A 96, 1716–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DA, 2000. Mossy fiber zinc and temporal lobe epilepsy: pathological association with altered “epileptic” gamma-aminobutyric acid A receptors in dentate granule cells. Epilepsia 41 (Suppl. 6), 96–99. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Carlson GC, 2007. Functional regulation of the dentate gyrus by GABA-mediated inhibition. Prog. Brain. Res 163, 235–243. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B, 1990. Functional and molecular distinction between recombinant rat GABA-A receptor subtypes by Zn2+. Neuron 5, 781–788. [DOI] [PubMed] [Google Scholar]
- Elsas SM, Hazany S, Gregory WL, Mody I, 2009. Hippocampal zinc infusion delays the development of afterdischarges and seizures in a kindling model of epilepsy. Epilepsia 50, 870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z, 2005. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci 6, 215–229. [DOI] [PubMed] [Google Scholar]
- Foresti ML, Arisi GM, Fernandes A, Tilelli CQ, Garcia-Cairasco N, 2008. Chelatable zinc modulates excitability and seizure duration in the amygdala rapid kindling model. Epilepsy Res 79, 166–172. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, 1989. Neurobiology of zinc and zinc-containing neurons. Int. Rev. Neurobiol 31,145–238. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Klitenick MA, Manton WI, Kirkpatrick JB, 1983. Cytoarchitectonic distribution of zinc in the hippocampus of man and the rat. Brain Res 273, 335–339. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Suh SW, Silva D, Frederickson CJ, Thompson RB, 2000. Importance of zinc in the central nervous system: the zinc-containing neuron. J. Nutr 130,1471S–1483S. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Koh JY, Bush AI, 2005. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci 6, 449–462. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I, 2008. Which GABA-A receptor subunits are necessary for tonic inhibition in the hippocampus? J. Neurosci 28, 1421–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower-Winter SD, Levenson CW, 2012. Zinc in the central nervous system: From molecules to behavior. BioFactors 38,186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NL, Gibbons SJ, 1994. Zn2+: an endogenous modulator of ligand- and voltage- gated ion channels. Neuropharmacology 33, 935–952. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Dunne EL, Harvey RJ, Smart TG, 2003. Zinc-mediated inhibition of GABA-A receptors: discrete binding sites underlie subtype specificity. Nat. Neurosci 6, 362–369. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Smart TG, 2007. Neurosteroid binding sites on GABA-A receptors. Pharmacol. Ther 116, 7–19. [DOI] [PubMed] [Google Scholar]
- Jutila L, Immonen A, Partanen K, Partanen J, Mervaala E, Ylinen A, Alafuzoff I, Paljarvi L, Karkola K, Vapalahti M, Pitkanen A, 2002. Neurobiology of epileptogenesis in the temporal lobe. Adv. Tech. Stand. Neurosurg 27, 5–22. [PubMed] [Google Scholar]
- Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M, 2004. Overview of mammalian zinc transporters. Cell. Mol. Life Sci 61, 49–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski RM, Gasior M, Carter RB, Witkin JM, 2003. Protective efficacy of neuroactive steroids against cocaine kindled-seizures in mice. Eur. J. Pharmacol 474, 217–222. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Livingood MR, Rogawski MA, 2004. Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia 45, 864–867. [DOI] [PubMed] [Google Scholar]
- Kapur J, Macdonald RL, 1997. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABA-A receptors. J. Neurosci 17, 7532–7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AR, Wong RK, 1986. Isolation of neurons suitable for patch-clamping from adult mammalian central nervous systems. J. Neurosci. Methods 16, 227–238. [DOI] [PubMed] [Google Scholar]
- Khatri SN, Wu WC, Pugh JR, 2018. Enhancement of extrasynaptic GABA-A receptors by GABA- B receptors and L-type calcium channels. Program No. 200.10. 2018 Neuroscience Meeting Planner San Diego, CA: Society for Neurosci. Online. [Google Scholar]
- Kerrigan JF, Shields WD, Nelson TY, Bluestone DL, Dodson WE, Bourgeois BF, Pellock JM, Morton LD, Monaghan EP, 2000. Ganaxolone for treating intractable infantile spasms: a multicenter, open-label, add-on trial. Epilepsy Res 42, 133–139. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Cohen AL, Karp E, Rogawski MA, 1996. Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology 35, 1049–1056. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D 2002. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA-A receptors: commentary on Brown et al. Br. J. Pharmacol 136, 957–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxer K, Blum D, Abou-Khalil BW, Morrell MJ, Lee DA, Data JL, Monaghan EP, 2000. Assessment of ganaxolone’s anticonvulsant activity using a randomized, double-blind, presurgical trial design. Ganaxolone Presurgical Study Group. Epilepsia 41, 1187–1194. [DOI] [PubMed] [Google Scholar]
- Lee JY, Kim JS, Byun HR, Palmiter RD, Koh JY, 2011. Dependence of the histofluorescently reactive zinc pool on zinc transporter-3 in the normal brain. Brain Res 1418, 12–22. [DOI] [PubMed] [Google Scholar]
- Lee V, Maguire J, 2013. Impact of inhibitory constraint of interneurons on neuronal excitability. J. Neurophysiol 110, 2520–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, De Koninck Y, Otis TS, Soltesz I, 1994. Bridging the cleft at GABA synapses in the brain. Trends Neurosci 17, 517–525. [DOI] [PubMed] [Google Scholar]
- Molnar P, Nadler JV, 2001. Synaptically-released zinc inhibits N-methyl-D-aspartate receptor activation at recurrent mossy fiber synapses. Brain Res 910, 205–207. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Patel B, Smart TG, 2011. GABA Potency at GABA-A Receptors Found in Synaptic and Extrasynaptic Zones. Front. Cell Neurosci 6, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Schwarzer C, Gasser E, Kato N, Vezzani A, Sperk G 2005. Altered expression of GABA(A) and GABA(B) receptor subunit mRNAs in the hippocampus after kindling and electrically induced status epilepticus. Neuroscience 134, 691–704. [DOI] [PubMed] [Google Scholar]
- Nohria V, Giller E, 2007. Ganaxolone. Neurotherapeutics 4, 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Vergnano AM, Barbour B, Casado M, 2009. Zinc at glutamatergic synapses. Neuroscience 158, 126–136. [DOI] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR, 2002. GABA-A receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J. Comp. Neurol 446, 179–197. [DOI] [PubMed] [Google Scholar]
- Pieribone VA, Tsai J, Soufflet C, Rey E, Shaw K, Giller E, Dulac O, 2007. Clinical evaluation of ganaxolone in pediatric and adolescent patients with refractory epilepsy. Epilepsia 48, 1870–1874. [DOI] [PubMed] [Google Scholar]
- Prakash A, Bharti K, Majeed AB, 2015. Zinc: indications in brain disorders. Fundam. Clin. Pharmacol 29, 131–149. [DOI] [PubMed] [Google Scholar]
- Qian J, Xu K, Yoo J, Chen TT, Andrews G, Noebels JL, 2011. Knockout of Zn transporters Zip-1 and Zip-3 attenuates seizure-induced CA1 neurodegeneration. J. Neurosci 31, 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Estes WA, 2016. Clinical Potential of Neurosteroids for CNS Disorders. Trends Pharmacol. Sci 37, 543–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Jian K, 2010. The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABA-A receptors. J. Pharmacol. Exp. Ther 334, 1031–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Mohan A, 2011. Development and persistence of limbic epileptogenesis are impaired in mice lacking progesterone receptors. J. Neurosci 31, 650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA, 2000. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J. Pharmacol. Exp. Ther 295, 1241–1248. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA, 2001. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia 42, 337–344. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA, 2010. Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model. Epilepsy Res 89, 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA, 2012. Neurosteroids - endogenous regulators of seizure susceptibility and role in the treatment of epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (Eds.), Jasper’s Basic Mechanisms of the Epilepsies National Center for Biotechnology Information (US), Bethesda (MD). [PubMed] [Google Scholar]
- Reddy DS, Yoshimura RF, Ramanathan G, Carver C, Johnstone TB, Hogenkamp DJ, Gee KW, 2018. Role of beta2/3-specific GABA-A receptor isoforms in the development of hippocampus kindling epileptogenesis. Epilepsy Behav 82, 57–63. [DOI] [PubMed] [Google Scholar]
- Reddy SD, Younus I, Clossen BL, Reddy DS, 2015. Antiseizure Activity of Midazolam in Mice Lacking delta-Subunit Extrasynaptic GABA-A Receptors. J. Pharmacol. Exp. Ther 353, 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Walker MC, Fabian-Fine R, Kullmann DM, 2004. Endogenous zinc inhibits GABA-A receptors in a hippocampal pathway. J. Neurophysiol 91, 1091–1096. [DOI] [PubMed] [Google Scholar]
- Smart TG, 1992. A novel modulatory binding site for zinc on the GABA-A receptor complex in cultured rat neurones. J. Physiol 447, 587–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart TG, Moss SJ, Xie X, Huganir RL, 1991. GABA-A receptors are differentially sensitive to zinc: dependence on subunit composition. Br. J. Pharmacol 103, 1837–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart TG, Xie X, Krishek BJ, 1994. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Prog. Neurobiol 42, 393–441. [DOI] [PubMed] [Google Scholar]
- Sperling MR, Klein P, Tsai J, 2017. Randomized, double-blind, placebo-controlled phase 2 study of ganaxolone as add-on therapy in adults with uncontrolled partial-onset seizures. Epilepsia 58, 558–564. [DOI] [PubMed] [Google Scholar]
- Szewczyk B, 2013. Zinc homeostasis and neurodegenerative disorders. Front. Aging Neurosci 5, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A, Hanajima T, Ijiro H, Ishige A, Iizuka S, Okada S, Oku N, 1999. Release of zinc from the brain of El (epilepsy) mice during seizure induction. Brain Res 828, 174–178. [DOI] [PubMed] [Google Scholar]
- Timofeeva O, Nadler JV, 2006. Facilitation of granule cell epileptiform activity by mossy fiber- released zinc in the pilocarpine model of temporal lobe epilepsy. Brain Res 1078, 227–234. [DOI] [PubMed] [Google Scholar]
- Turgeon SM, Albin RL, 1992. Zinc modulates GABA-B binding in rat brain. Brain Res 596, 30–34. [DOI] [PubMed] [Google Scholar]
- Vogt K, Mellor J, Tong G, Nicoll R, 2000. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron 26, 187–196. [DOI] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I, 2003. Perisynaptic localization of delta subunit-containing GABA-A receptors and their activation by GABA spillover in the mouse dentate gyrus. J. Neurosci 23, 10650–10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook GL, Mayer ML, 1987. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature 328, 640–643. [DOI] [PubMed] [Google Scholar]
- Wlodarczyk AI, Xu C, Song I, Doronin M, Wu YW, Walker MC, Semyanov A, 2013. Tonic GABAA conductance decreases membrane time constant and increases EPSP-spike precision in hippocampal pyramidal neurons. Front. Neural Circuits 7, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL, 2002. Enhanced neurosteroid potentiation of ternary GABA-A receptors containing the delta subunit. J. Neurosci 22, 1541–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gangisetty O, Carver CM, Reddy DS, 2013. Estrous cycle regulation of extrasynaptic delta-containing GABA-A receptor-mediated tonic inhibition and limbic epileptogenesis. J. Pharmacol. Exp. Ther 346, 146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorulmaz H, Seker FB, Demir G, Yalcin IE, Oztas B, 2013. The effects of zinc treatment on the blood-brain barrier permeability and brain element levels during convulsions. Biol. Trace Elem. Res 151, 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younus I, Reddy DS, 2018. A resurging boom in new drugs for epilepsy and brain disorders. Expert Rev. Clin. Pharmacol 11, 27–45. [DOI] [PubMed] [Google Scholar]
- Zhu J, Shao CY, Yang W, Zhang XM, Wu ZY, Zhou L, Wang XX, Li YH, Xia J, Luo JH, Shen Y 2012. Chronic zinc exposure decreases the surface expression of NR2A- containing NMDA receptors in cultured hippocampal neurons. PLoS One 7, e46012. [DOI] [PMC free article] [PubMed] [Google Scholar]