The introduction of Janus kinase (JAK) 1/2 inhibitor ruxolitinib into the armamentarium of myelofibrosis therapies has allowed to effectively control systemic symptoms, reduce splenomegaly, and improve the quality of life of myelofibrosis patients [1,2]. Long-term follow-up of patients treated with ruxolitinib suggested an additional benefit of improved survival, with median overall survival in patients with Intermediate-2 and High risk myelofibrosis treated with ruxolitinib reaching as high as ~8 years and 4.2 years, respectively[3]. The clinical benefits of ruxolitinib have raised the question of whether ruxolitinib can effectively delay (or even replace) allogeneic stem cell transplantation for selected higher risk patients [4].
Importantly, how ruxolitinib may lead to improved survival in myelofibrosis is not well understood, with most evidence pointing to the reduction in inflammatory cytokines. Whether ruxolitinib has a true disease-modifying effect is less clear, as ruxolitinib therapy leads to only a partial hematologic response and a modest reduction of JAK2 V617F allele burden[1,2]. Histomorphologic improvements on ruxolitinib are also modest, with a recent report that ruxolitinib may act to slow down or stabilize the progression of fibrosis compared to best available therapy [5]. Ruxolitinib does not appear to change the rates of transformation to acute leukemia or leukemia cutis [6,7], which serves as a cautionary reminder of the opportunity cost of delaying allogeneic transplantation in transplant-eligible Intermediate-2 and High risk myelofibrosis patients. In this manuscript, we expand the spectrum of end-stage extramedullary complications of myelofibrosis that can emerge and progress during prolonged ruxolitinib therapy and add to the risk/benefit analysis of selecting an optimal treatment strategy for myelofibrosis patients.
A 52-year-old previously healthy male was diagnosed with a JAK2 V617F mutation-positive primary myelofibrosis after presenting with weight loss, night sweats, and abdominal distention caused by massive splenomegaly. Hematologic studies showed a white blood cell count of 32.9 × 109/L, with a leucoerythroblastic smear and 2% circulating blasts, a hemoglobin of 13.0 g/dL, and a platelet count of 475 × 109/L. Serum lactic dehydrogenase was elevated at 1,857 units/L. Bone marrow histopathology was notable for marked fibrosis and osteosclerosis, decreased hematopoiesis, and megakaryocytic atypia. Cytogenetic studies showed a normal male karyotype. A JAK2 V617F mutation was detected. According to the Dynamic International Prognostic Scoring System-Plus (DIPSS-Plus), he had Intermediate-2 risk myelofibrosis, based on leukocytosis, systemic symptoms, and circulating blasts ≥1%. An allogeneic stem cell transplantation was recommended, but donor search failed to identify suitably matched allogeneic donors. Because of severe symptoms, the patient was started on ruxolitinib 20mg twice daily and quickly increased to 25mg twice daily, resulting in a 35% reduction in spleen volume and a significant improvement in systemic symptoms within ten months of starting therapy.
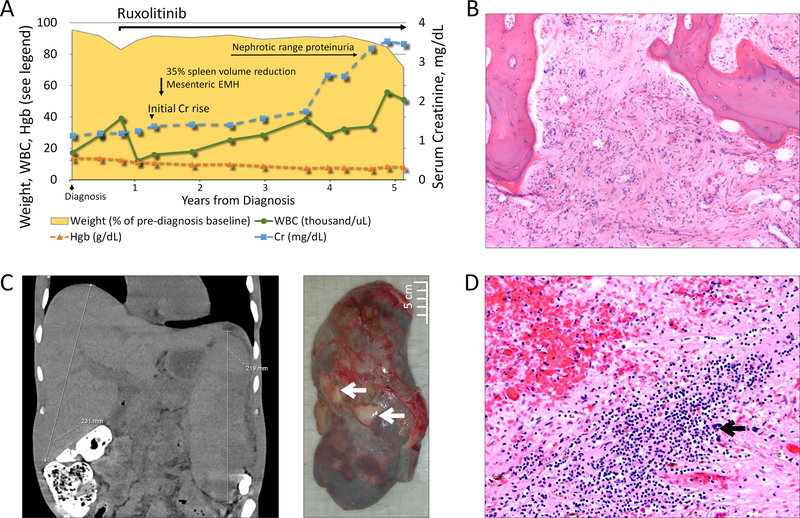
However, despite these objective improvements, the patient continued to experience chronic diffuse abdominal discomfort, a sensation of fullness, and early satiety, which was attributed to his hepatosplenomegaly. A high-calorie diet with thrice-daily nutritional supplementation was started to maintain his nutritional status. Abdominal imaging obtained ten months after ruxolitinib initiation showed improving but still massive splenomegaly, also noting an apparent infiltration of omental fat by small irregular soft tissue opacities, felt to represent omental infarction or extramedullary hematopoiesis (Fig. 1A–C). Additionally, six months after starting ruxolitinib therapy, the patient developed a rise in serum creatinine, initially rising modestly from 1.2 mg/dL to 1.4mg/dL, but progressively worsening over time, peaking at 4.5 mg/dL, in conjunction with nephrotic range proteinuria of 6.86 grams of protein per day. Renal biopsy revealed interstitial fibrosis, and showed extensive subendothelial deposition of Congo red positive fibrils. There was no evidence of monoclonal gammopathy or paraprotein. Lesions associated with myeloproliferative neoplasm-associated glomerulopathies[8] were not seen. Liquid chromatography-mass spectrometry, performed on the Congo-red positive, microdissected areas revealed an abundance of amyloid-associated proteins supporting the diagnosis of amyloidosis, but, in agreement with myelofibrosis-related secondary amyloidosis [9–12], did not identify a known amyloid subtype.
Figure 1. Patient’s disease course and extramedullary complications.
A. A schematic diagram of the patient’s disease course in relation to diagnosis and JAK1/2 inhibitor therapy. Plotted on the left vertical axis are the patients white blood cell count (WBC in thousand cells/µL, solid green line with circles), hemoglobin (Hgb in g/dL, dashed orange line with triangles), and the patient’s weight (shown as a % of pre-diagnosis weight, yellow shaded area). Serum creatinine in mg/dL is plotted on the right vertical axis, with X-axis showing the duration of disease in years from diagnosis. B. Bone marrow with diffuse fibrosis and minimal hematopoietic precursors (H&E, 10x). C. The panel on the left shows computed tomography examination of the patient’s abdomen and pelvis with massive hepatosplenomegaly. The panel on the right shows massive splenic enlargement, with multiple infarctions and cortical adhesions (black arrows). D. Hematoxylin and eosin (H&E) stained sections of the spleen with focal areas of extramedullary hematopoiesis (20x).
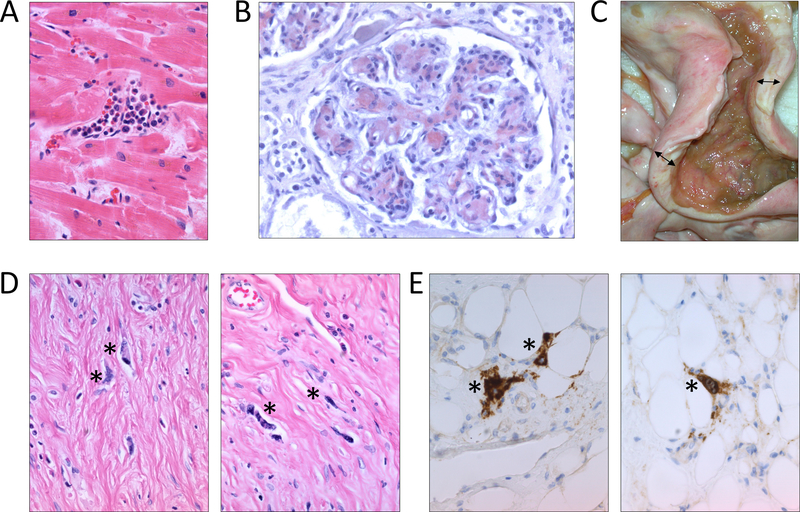
Four years after his initial presentation, the patient developed progressive leukocytosis, transfusion-dependent anemia, and worsening splenomegaly (Fig 1A). He had severe ruxolitinib withdrawal symptoms with any delays or accidentally missed ruxolitinib doses. A bone marrow biopsy showed fibrosis and hypocellular marrow (Fig 1B), with normal cytogenetics, and molecular studies notable for a JAK2 V617F mutation in 94% of cells, and two frameshift mutations in ASXL1 (p.G626Pfs*10 and p.G646Wfs*12), in 37% and 10% of cells, respectively. Because of accelerating disease, the patient was started on a combination of ruxolitinib and decitabine. Unfortunately, his condition continued to progress, and he died after a prolonged hospital stay from hypoxemic respiratory failure due to pneumonia. An autopsy, performed at his death after 4.5 years of high dose ruxolitinib therapy, revealed multiple end-stage extramedullary sequelae of myelofibrosis. He had marked splenomegaly with several infarcts (Fig 1C) and extramedullary hematopoiesis in the spleen (Fig 1D), liver and heart (Fig 2A). There was diffuse glomerular deposition of amyloid in the kidneys (Fig 2B). There were no other sites of amyloid identified, and Congo red staining of the patient’s bone marrow, liver, spleen, and pituitary gland were negative. Strikingly, the patient was also found to have a marked thickening of serosa almost completely encircling small and large bowel (Fig 2C) with diffuse mesenteric sclerosing fibrosis and scattered large atypical megakaryocytes (Fig 2D, 2E), consistent with sclerosing extramedullary hematopoietic tumor[13,14].
Figure 2. End-stage extramedullary complications of myelofibrosis in a patient on long-term ruxolitinib therapy.
A. Heart with focal areas of extramedullary hematopoiesis and nuclear features of myocyte hypertrophy (H&E, 40x). B. Glomeruli with mesangial and capillary wall amyloid deposits, highlighted by Congo red stain (Congo red, 400x). C. Terminal ileum and cecum with extensive mesenteric and serosal fibrosis resulting in markedly thickened bowel wall indicated by the double-headed arrows (gross). D. Bowel wall with markedly atypical cells scattered among dense fibrosis, indicated by asterisks (H&E, 400x). E. The atypical cells scattered in the fibrosis are megakaryocytes and show positive staining for CD61 by immunohistochemistry, indicated by asterisks (400x).
Extramedullary hematopoiesis is a well-known complication of myelofibrosis, most commonly presenting as hepatosplenomegaly, and, less frequently, as hematopoietic cell deposits in other organs. Rarer sequela of myelofibrosis, such as renal amyloidosis and mesenteric sclerosing fibrosis in our patient, present more insidiously and can go unrecognized. Interestingly, these complications developed in our patient during high-dose ruxolitinib therapy, despite having objective measures of response (improvement in spleen size and clinical symptoms). The emergence of extramedullary complications during ruxolitinib therapy may reflect differences in cell trafficking or drug distribution or metabolism at extramedullary sites. It is also possible that, as patients are surviving longer, extended time with aggressive disease and its associated hypercytokinemia allow for the development and progression of these otherwise rare end-stage complications. Although these complications are rare, the conundrums brought up by this case are not. This case highlights potential risks of delaying transplant in patients with aggressive myelofibrosis because organ-compromising extramedullary complications can arise early despite ruxolitinib therapy, potentially compromising future transplant options.
Acknowledgments
We are grateful to Dr. Martin P. Carroll for establishing the Penn IRB-approved hematologic malignancies patient registry. We thank the patient for participation in the registry. This work was supported by the NHLBI K08 HL132101 to D.B.
Footnotes
Conflict of interest statement: The authors have no conflicts of interests to disclose.
References
- 1.Verstovsek S, Mesa RA, Gotlib J, et al. . A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012;366:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison C, Kiladjian JJ, Al-Ali HK, et al. . JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 2012;366:787–798. [DOI] [PubMed] [Google Scholar]
- 3.Verstovsek S, Gotlib J, Mesa RA, et al. . Long-term survival in patients treated with ruxolitinib for myelofibrosis: COMFORT-I and -II pooled analyses. J Hematol Oncol 2017;10:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lestang E, Peterlin P, Le Bris Y, et al. . Is allogeneic stem cell transplantation for myelofibrosis still indicated at the time of molecular markers and JAK inhibitors era? Eur J Haematol 2017;99:60–69. [DOI] [PubMed] [Google Scholar]
- 5.Kvasnicka HM, Thiele J, Bueso-Ramos CE, et al. . Long-term effects of ruxolitinib versus best available therapy on bone marrow fibrosis in patients with myelofibrosis. J Hematol Oncol 2018;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verstovsek S, Mesa RA, Gotlib J, et al. . Efficacy, safety, and survival with ruxolitinib in patients with myelofibrosis: results of a median 3-year follow-up of COMFORT-I. Haematologica 2015;100:479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kremyanskaya M, Mascarenhas J, Rampal R, Hoffman R. Development of extramedullary sites of leukaemia during ruxolitinib therapy for myelofibrosis. Br J Haematol 2014;167:144–146. [DOI] [PubMed] [Google Scholar]
- 8.Said SM, Leung N, Sethi S, et al. . Myeloproliferative neoplasms cause glomerulopathy. Kidney Int 2011;80:753–759. [DOI] [PubMed] [Google Scholar]
- 9.Leblebisatan G, Sasmaz I, Antmen B, Ergin M, Kilinc Y. Concomitance of idiopathic myelofibrosis and amyloidosis. Hematol Oncol Stem Cell Ther 2010;3:206–207. [DOI] [PubMed] [Google Scholar]
- 10.Chan KW, Ho CP. Amyloidosis complicating idiopathic myelofibrosis. Am J Kidney Dis 1999;34:E27. [DOI] [PubMed] [Google Scholar]
- 11.Akikusa B, Komatsu T, Kondo Y, Yokota T, Uchino F, Yonemitsu H. Amyloidosis complicating idiopathic myelofibrosis. Arch Pathol Lab Med 1987;111:525–529. [PubMed] [Google Scholar]
- 12.Ferhanoglu B, Erzin Y, Baslar Z, Tuzuner HA. Secondary amyloidosis in the course of idiopathic myelofibrosis. Leukemia research 1997;21:897–898. [DOI] [PubMed] [Google Scholar]
- 13.Kwon Y, Yu E, Huh J, Lee SK, Ro JY. Sclerosing extramedullary hematopoietic tumor involving lesser omentum and ligamentumteres in liver explant. Ann Diagn Pathol 2004;8:227–232. [DOI] [PubMed] [Google Scholar]
- 14.Remstein ED, Kurtin PJ, Nascimento AG. Sclerosing extramedullary hematopoietic tumor in chronic myeloproliferative disorders. Am J Surg Pathol 2000;24:51–55. [DOI] [PubMed] [Google Scholar]