Abstract
Rationale.
Synthetic cathinones constitute a class of abused drugs that can act at dopamine, norepinephrine, and serotonin transporters (DAT, NET, and SERT, respectively). Intracranial self-stimulation (ICSS) is a preclinical procedure that can be used to evaluate abuse potential of drugs, and prior studies have indicated that abuse-related ICSS effects of monoamine transporter substrates, including some synthetic cathinones, is positively correlated with drug selectivity for DAT vs. SERT. Abuse potential of drugs can also be influenced by regimens of repeated drug exposure, but the role of repeated exposure on abuse-related ICSS effects of synthetic cathinones has not been examined.
Objectives.
This study used ICSS to evaluate effects of repeated treatment with the DAT>SERT substrate methcathinone, the DAT<SERT substrate fenfluramine, and the DAT≈SERT substrate mephedrone.
Methods.
Male Sprague-Dawley rats were trained in a frequency-rate ICSS procedure, and different groups were used to evaluate effects of methcathinone, mephedrone, and fenfluramine before, during, and after regimens of repeated treatment with the designated drug.
Results.
Before repeated treatment, methcathinone produced dose-dependent and abuse-related ICSS facilitation, fenfluramine produced dose-dependent ICSS depression, and mephedrone produced mixed effects that included both facilitation and depression. Chronic treatment produced no change in effects of methcathinone, but complete tolerance to effects of fenfluramine. For mephedrone, chronic treatment produced partial tolerance to ICSS depression and enhanced expression of ICSS facilitation.
Conclusions.
Repeated exposure to mixed-action DAT≈SERT substrates such as mephedrone can result in increased abuse potential due to sustained expression of DAT-mediated abuse-related effects and tolerance to SERT-mediated abuse-limiting effects.
INTRODUCTION
Synthetic cathinones constitute a family of abused drugs that share a structural similarity to the parent compound cathinone and that produce their pharmacological effects by acting primarily on dopamine (DA), norepinephrine (NE), and/or serotonin (5-HT) transporters (DAT, NET, and SERT, respectively) (Baumann et al. 2014; De Felice et al. 2014). Like other families of monoamine transporter ligands, synthetic cathinones can vary both in their selectivity for DAT, NET, and/or SERT, and in their function at each transporter as either a substrate or inhibitor. Substrates (e.g. methcathinone) act like amphetamine to pass through the transporter and trigger a series of intracellular events that culminates in neurotransmitter release. Inhibitors (e.g. methylenedioxypyrovalerone, MDPV) act like cocaine to block the transporter and prevent uptake of released neurotransmitter. Intracranial self-stimulation (ICSS) is one preclinical behavioral procedure that has been used to investigate the expression, mechanisms, and treatment of abuse-related effects produced by synthetic cathinones (Bonano et al. 2015; Bonano et al. 2014; Robinson et al. 2012; Watterson et al. 2012; Watterson et al. 2014). In “frequency-rate” ICSS procedures, experimental subjects are implanted with microelectrodes in brain reward areas such as the medial forebrain bundle and trained to press a lever for pulses of electrical brain stimulation delivered at different frequencies (Carlezon and Chartoff 2007; Negus and Miller 2014). Increasing frequencies of brain stimulation maintain increasing ICSS response rates, and drug effects are evaluated on these frequency-rate curves. Many drugs of abuse increase or “facilitate” low ICSS rates maintained by low frequencies of brain stimulation, resulting in leftward or upward shifts in frequency-rate curves, whereas many drugs that lack abuse liability in humans fail to facilitate ICSS up to doses that depress high ICSS rates maintained by high frequencies of brain stimulation, resulting in rightward or downward shifts in frequency-rate curves.
We have shown previously that levels of abuse-related ICSS facilitation produced by some synthetic cathinones and other monoamine transporter ligands in ICSS are governed in part by their relative potency to act at DAT vs. SERT (Bauer et al. 2013; Negus and Banks 2017). For example, DAT>SERT-selective transporter substrates (e.g. methamphetamine and methcathinone) produce dose-dependent and robust ICSS facilitation, mixed-action compounds with similar potencies at DAT and SERT (e.g. methylenedioxymethamphetamine (MDMA) and mephedrone) produce weaker ICSS facilitation, and DAT<SERT-selective compounds (e.g. fenfluramine and 4-trifluoromethylmethcathinone) produce only ICSS depression. The degree of maximal ICSS facilitation produced by monoamine transporter substrates correlates with other preclinical measures of abuse potential, such as maximal breakpoints maintained by progressive-ratio drug self-administration procedures, and also corresponds to metrics of abuse by humans (Bauer et al. 2013; Negus and Miller 2014).
Drug history is one factor that can influence abuse potential of drugs (Schenk 2009; Young et al. 1981), and one advantage of ICSS as a preclinical procedure for abuse potential assessment is that evolving expression of abuse-related effects can be examined in drug-naive subjects and during the earliest phases of drug exposure (Negus and Miller 2014). For example, morphine often produces primarily ICSS depression in opioid-naive rats, but repeated daily morphine administration for as little as one week can produce tolerance to ICSS rate-decreasing effects and increased expression of abuse-related ICSS facilitation (Miller et al. 2015a; Reid 1987). The goal of the present study was to examine the degree to which repeated exposure might modify the effects of synthetic cathinones on ICSS. Three monoamine transporter substrates were selected for study: the DAT>SERT-selective substrate methcathinone, the DAT≈SERTsubstrate mephedrone, and the SERT>DAT-selective substrate fenfluramine (Rothman et al. 2001,Bonano, 2014 #53). The transporter selectivities of these compounds were determined by comparing in vitro potencies to promote DAT- or SERT-mediated monoamine release from rat-brain synaptosomes, and these potencies expressed as the concentration required to elicit 50% of maximal release are as follows: methcathione (DAT=12.5 nM, SERT=3860 nM, DAT selectivity = 309), mephedrone (DAT=49.1 nM, SERT=118 nM, DAT selectivity = 2.41), and fenfluramine (DAT>10,000 nM, SERT=79.3 nM, DAT selectivity<0.01) (Rothman et al. 2001,Bonano, 2014 #53). Additionally, we have shown previously that, after acute treatment, methcathinone produces a dose-dependent, robust, and abuse-related facilitation of ICSS, whereas fenfluramine only depresses ICSS, and mephedrone produces a mixed profile that includes both ICSS facilitation and ICSS depression (Bauer et al. 2013; Bonano et al. 2014). We were especially interested to evaluate the degree to which repeated exposure might increase expression of ICSS facilitation by these compounds.
MATERIALS AND METHODS
Subjects
Seventeen adult male Sprague-Dawley rats (Harlan, Frederick, MD, USA) weighing 313 to 415 g at the time of surgery were individually housed and maintained on a 12 h light/dark cycle with lights on from 6:00 a.m. to 6:00 p.m. Rats had free access to food and water except during testing. Animal maintenance and research adhered to guidelines for the care and use of laboratory animals (National Research Council, 2011) as adopted and promulgated by the National Institutes of Health. All animal use protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Intracranial self-stimulation (ICSS)
Surgery.
Experimental procedures were similar to those used previously to evaluate repeated treatment effects with opioids, nicotine, and Δ9-tetrahydrocannabinol on ICSS (Altarifi et al. 2012; Freitas et al. 2016; Kwilasz and Negus 2012). Briefly, rats were anesthetized with isoflurane (2.5–3% in oxygen; Webster Veterinary, Phoenix, AZ, USA) for implantation of stainless steel electrodes (Plastics One, Roanoke, VA, USA) into the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to bregma, 1.7 mm lateral to the midsagittal suture, and 8.8 mm ventral to the skull). The electrode was secured to the skull with skull screws and dental acrylic. Ketoprofen (5 mg/kg; Spectrum Chemical, New Brunswick, NJ) was used for post-operative analgesia immediately and 24 h after surgery, and training was initiated at least seven days after surgery.
Apparatus.
Sound-attenuating boxes contained modular acrylic and metal test chambers (29.2 × 30.5 × 24.1 cm) equipped with a response lever, three stimulus lights located above the lever, a house light, and an ICSS stimulator (Med Associates, St. Albans, VT, USA). Electrodes were connected to the stimulator via bipolar cables routed through a swivel commutator (Model SL2C, Plastics One, Roanoke, VA, USA). Control of stimulus events in the test chamber, recording of lever press responses, and initial data analysis were accomplished by a computer and interface operated by custom software (Med Associates).
Training.
During behavioral sessions, the house light was on and each lever press resulted in the delivery of a 0.5 s train of square wave cathodal pulses (0.1 ms pulse duration) accompanied by illumination of the stimulus lights over the lever. Responding during stimulation delivery did not result in additional stimulations. Initially, the stimulation intensity and frequency were set at 150 μA and 126 Hz, respectively, during 60-min training sessions. Stimulation intensity was individually adjusted for each rat to the lowest value that maintained a rate of reinforcement >30 stimulations/min. This intensity (110–250 μA across rats; no difference between groups as indicated by one-way ANOVA: F(2,14)=2.136, p=0.155) was then held constant for the remainder of the study, and frequency manipulations were introduced during sessions lasting 30 min and composed of three consecutive 10-min components. During each component, a descending series of 10 frequencies (158 to 56 Hz in 0.05 log increments) was presented, with each frequency available for a 1-min trial. Each frequency trial consisted of a 10-s time-out, during which responding had no scheduled consequences and five non-contingent “priming” stimulations were delivered at the frequency available during that trial, followed by a 50-s “response” phase, during which each response produced electrical stimulation. Training continued until rats reliably responded for only the first three to six frequency trials of each component over a period of at least three consecutive training days.
Testing.
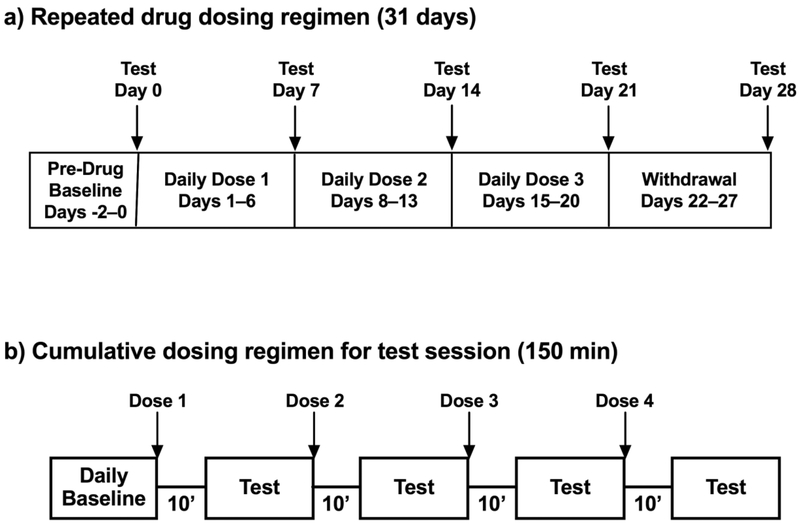
Once training was complete, testing was initiated using protocols illustrated in Figure 1. Racemic methcathinone, fenfluramine, and mephedrone were tested in separate groups of rats using a 31-day protocol (Figure 1a). All ICSS test sessions began with three baseline components as described above, and data from these baseline components on Days −2 to 0 were averaged to yield pre-drug baseline data (see “Data Analysis” below). On Days 0, 7, 14, 21, and 28, baseline components were followed by a series of four test cycles, during which increasing doses of methcathinone (0.032–1.0 mg/kg), fenfluramine (0.1–3.2 mg/kg) or mephedrone (0.32–10 mg/kg) were administered at 30-min intervals using a cumulative-dosing procedure (Figure 1b). Each dose increased the total, cumulative dose by 0.5 log units, and two test ICSS components (lasting a total of 20 min) began 10 min after each injection. Cumulative dosing test sessions always occurred on Wednesdays. Between these sessions, rats received daily injections of the test drug on Days 1–6, 8–13, and 15–20, and the daily dose of test drug increased in 0.5 log unit increments from week to week (methcathinone 0.1–1.0 mg/kg; fenfluramine 0.32–3.2 mg/kg; mephedrone 1.0–10 mg/kg). On weekdays, drug doses were administered in the context of an ICSS test session consisting of three baseline components followed first by a 10-min timeout period when drug was administered and then by two test components. On weekends, drug doses were administered at approximately the same time each day but without ICSS. On Days 22–27, daily dosing was terminated to examine any effects associated with drug withdrawal. Doses of each drug were selected based on previous studies of acute drug effects on ICSS (Bauer et al. 2013; Bonano et al. 2014).
Figure 1. Experimental design.
(a) Protocol for repeated dosing over the course of the 31-day study in each group of rats. The three “Daily Doses” for each test drug in each group were as follows: methcathinone (0.1, 0.32, and 1.0 mg/kg/day), fenfluramine (0.32, 1.0, and 3.2 mg/kg/day), and mephedrone (1.0, 3.2, and 0 mg/kg/day). (b) Protocol for cumulative drug dosing on test days 0, 7, 14, 21, and 28. The cumulative doses for each test drug were as follows: methcathinone (0.032–1.0 mg/kg), fenfluramine (0.1–3.2 mg/kg) and mephedrone (0.32–10 mg/kg). Cumulative doses were administered in 0.5 log increments.
Data Analysis.
The primary dependent variable was the reinforcement rate in stimulations per minute during each frequency trial. The first component of each session was considered an acclimation component, and data from this component were discarded throughout the study. To normalize the remaining data, raw reinforcement rates from each trial in each rat were converted to percent maximum control rate (%MCR) in that rat, with the MCR defined as the mean of the maximal rates observed during the second and third “baseline” components of the three pre-drug baseline sessions (Days −2 to 0 in Figure 1). Thus, %MCR values for each trial were calculated as (reinforcement rate during a frequency trial ÷ maximum control rate) × 100. On each cumulative-dosing test day (i.e. Days 0, 7, 14, 21, and 28), ICSS was evaluated both before drug administration on that day (daily baseline components), and after drug doses (test components) (see Figure 1b). Data from the second and third daily-baseline components were averaged to yield a daily-baseline frequency-rate curve for that day in each rat, and data from each pair of test components after each cumulative drug dose were averaged to generate a test frequency-rate curve for that dose. Baseline and test curves were then averaged across rats to yield mean baseline and test curves for each manipulation. For statistical analysis, pre-drug baseline MCR values and frequency-rate curves across groups were compared by one- and two-way ANOVA, respectively. Drug effects within each group were compared by repeated-measures two-way ANOVA, with ICSS frequency as one factor and either treatment day (for comparison of daily baselines across test days) or dose (for dose-effect analysis within a test day) as the other factor. A significant interaction between main effects in the two-way ANOVA was followed by the Holm-Sidak post hoc test, and the criterion for significance was set at p < 0.05.
To provide an additional summary measure of ICSS performance, the total number of stimulations per component was calculated as the sum of stimulations delivered across all 10 frequency-trials for each component. Test data were then normalized to individual pre-drug baseline data using the equation % baseline total stimulations per component = (mean total stimulations per test component ÷ mean total stimulations per pre-drug baseline component) × 100. The pre-drug numbers of stimulations per component across groups were compared by one-way ANOVA. Summary data from subsequent test days within a group were averaged across rats for each cumulative drug dose on each test day and compared across test days by two-way ANOVA with dose and test day as the two factors. As with frequency-rate curve analysis, a significant interaction between main effects in the two-way ANOVA was followed by the Holm-Sidak post hoc test, and the criterion for significance was set at p < 0.05.
Drugs
(±)-Methcathinone HCl and (±)-mephedrone HCl were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). (±)-Fenfluramine HCl was purchased from Sigma Chemical Co. (St. Louis, MO). All compounds were prepared in sterile saline for i.p. administration and all drug doses are expressed as the salt forms listed above.
RESULTS
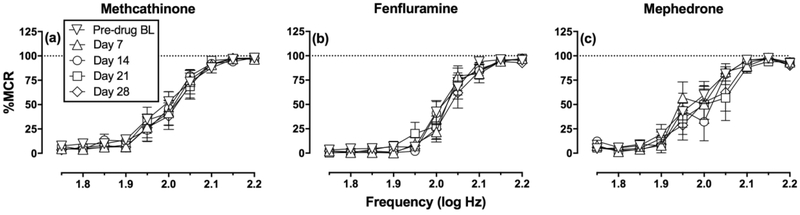
Figure 2 shows that electrical brain stimulation maintained a frequency-dependent increase in ICSS rates under pre-drug baseline conditions. Generally, low stimulation frequencies (1.75–1.90 log Hz) maintained low rates of ICSS, intermediate frequencies (1.90–2.05 log Hz) supported frequency-dependent increases in ICSS rates, and high frequencies (2.05–2.20 log Hz) maintained high asymptotic ICSS rates. One-way ANOVA indicated no significant difference between groups for pre-drug baseline values of either maximum control rate (MCR; F(2,14)=0.323, p=0.729) or total stimulations per component (F=(2,14)=2.409, p=0.126). Across all 17 rats used in these studies, the average ± SEM baseline MCR was 61.9 ± 1.87 stimulations per trial. The mean ± SEM number of total baseline stimulations per component was 289 ± 13.6.
Figure 2. Baseline ICSS responding before, during, and after repeated treatment with (a) methcathinone, (b) fenfluramine and (c) mephedrone.
Abscissae: frequency of electrical brain stimulation in log Hz. Ordinates: percent maximum control reinforcement rate (% MCR). Data are shown for the pre-drug baseline (Pre-drug BL; determined on Days −2 to 0 before any drug administration) and for daily baselines determined on test days 7, 14, 21, and 28. Twoway ANOVA results indicated a significant main effect of frequency in all panels (p<0.01). However, there was no difference between groups in pre-drug baseline frequency-rate curves (i.e. no main effect of group [F (2, 14) = 2.043, p=0.1665], and no frequency x group interaction [F (18, 126) = 1.58, p=0.075]). Within each group, there was no significant main effect of day, and no significant frequency x day interactions. Interaction results were as follows: (a) Methcathinone (N=6): F(36,180)=0.43, p=1.00; (b) Fenfluramine (N=6): F(36,180)=0.99, p=0.50; (c) Mephedrone (N=5): F(36,144)=1.27, p=0.17.
Figure 2 also compares the baseline frequency-rate curves determined before any drug administration [Pre-drug Baseline (BL), Days −2 to 0] and prior to each cumulative-dosing test session in each group of rats (Days 7, 14, 21, and 28). There was no difference between groups in pre-drug baseline frequency-rate curves, and there were no significant changes in baseline frequency-rate curves across the course of the study within any group. Statistical results for this and all other figures are reported in the figure legends. Thus, repeated exposure to and withdrawal from these monoamine releasers did not significantly alter baseline ICSS performance.
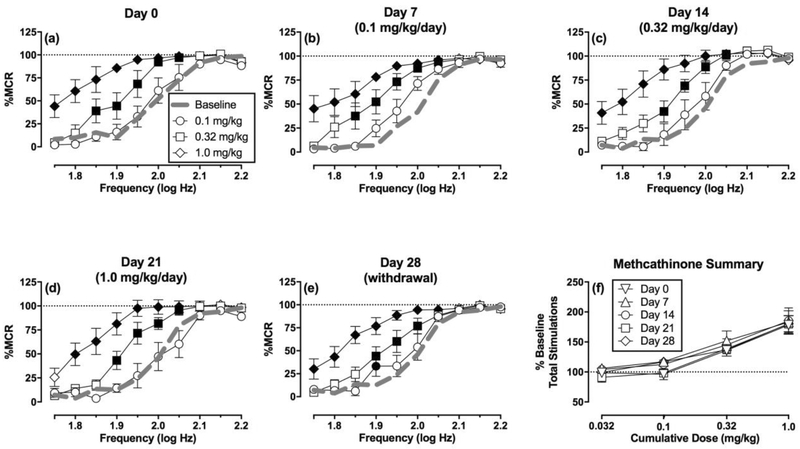
Figure 3 (a–e) shows the effects of cumulative methcathinone administration across the course of the study. Methcathinone produced similar dose-dependent leftward shifts in ICSS frequency-rate curves before, during, and after repeated methcathinone administration. Figure 3f shows that there was no change across days in the methcathinone dose-effect curves for the summary measure of Percent Baseline Stimulations per Component. (For methcathinone and for the other two drugs discussed below, note that effects of the lowest cumulative dose are not shown in panels a-e for clarity, but results with this low dose were included in statistical analysis, and summary results are presented in the panel f.)
Figure 3. Effects of cumulative methcathinone administration on ICSS before, during, and after repeated daily methcathinone treatment (N=6).
Panels a-e show full frequency-rate curves on a given test day. The title of each panel shows the test day and the methcathinone treatment administered daily for the six days preceding that test day. Abscissae: frequency of electrical brain stimulation in log Hz. Ordinates: percent maximum control reinforcement rate (% MCR) ± SEM. Note that error for the Daily Baseline (Baseline) on each test day is shown in Figure 2 but is not included here for clarity. Filled symbols show significant differences in response rates relative to the baseline on that day as determined by repeated measure two-way analysis of variance (ANOVA) followed by Holm-Sidak post hoc test, p<0.05. Two-way ANOVA results indicated significant main effects of frequency and dose in all panels (p<0.01), and interaction results were as follows: (a) Day 0: F(36,180)=5.07, p<0.01, (b) Day 7: F(36,180)=5.22, p<0.01, (c) Day 14: F(36,180)=4.29, p<0.01, (d) Day 21: F(36,180)=4.72, p<0.01, (e) Day 28: F(36,180)=4.19, p<0.01. Panel f compares methcathinone effects across test days on the summary measure of ICSS. Abscissa: cumulative methcathinone dose in mg/kg. Ordinate: Percent Total Baseline Stimulations per Component ± SEM. There was a significant main effect of methcathinone dose [F(3,15)=24.35, p<0.01] but not of test day [F(4,20)=0.59, p=0.67], and the interaction was not significant [F(12,60)=0.96, p=0.50].
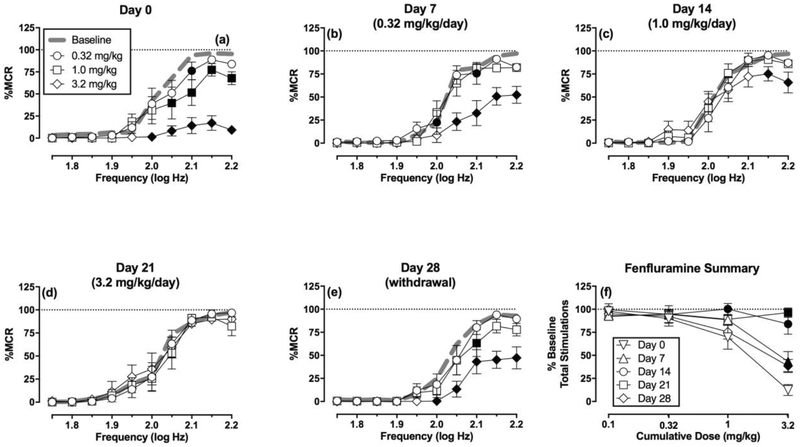
Figure 4 (a–e) shows the effects of cumulative fenfluramine administration. Fenfluramine produced dose-dependent downward shifts in the ICSS frequency-rate curve on Day 0. Repeated treatment with increasing fenfluramine doses produced increasing levels of tolerance to fenfluramine effects, such that fenfluramine had no effect on ICSS on Day 21. Expression of fenfluramine-induced rate-decreasing effects partially recovered by Day 28. Figure 4f shows summary data for this profile of tolerance to fenfluramine rate-decreasing effects followed by partial recovery after one week of withdrawal from repeated fenfluramine.
Figure 4. Effects of cumulative fenfluramine administration on ICSS before, during, and after repeated daily fenfluramine treatment (N=6).
Panels a-e show full frequency-rate curves on a given test day. The title of each panel shows the test day and the fenfluramine treatment administered daily for the six days preceding that test day. Abscissae: frequency of electrical brain stimulation in log Hz. Ordinates: percent maximum control reinforcement rate (% MCR) ± SEM (note that Baseline error is shown in Figure 2 but is not shown here for clarity). Filled symbols show significant differences relative to the baseline on that day as determined by repeated measure two-way analysis of variance (ANOVA) followed by Holm-Sidak post hoc test, p<0.05. Two-way ANOVA results indicated significant main effects of frequency in all panels (p<0.01). Statistical results for the main effect of fenfluramine dose and the frequency x dose interaction results were as follows: (a) Day 0: Dose [F(4,20)=25.17, p<0.01], Interaction [F(36,180)=9.67, p<0.01]; (b) Day 7: Dose [F(4,20)=25.20, p<0.01], Interaction [F(36,180)=4.22, p<0.01]; (c) Day 14: Dose [F(4,20)=0.69, p=0.61], Interaction [F(36,180)=1.80, p<0.01]; (d) Day 21: Dose [F(4,20)=0.90, p=0.48], Interaction [F(36,180)=0.81, p=0.76]; (e) Day 28: Dose [F(4,20)=20.28, p<0.01], Interaction [F(36,180)=3.09, p<0.01]. Panel f compares fenfluramine effects across test days on the summary measure of ICSS. Abscissa: cumulative fenfluramine dose in mg/kg. Ordinate: Percent Total Baseline Stimulations per Component ± SEM. There were significant main effects of fenfluramine dose [F(3,15)=25.76, p<0.01] and test day [F(4,20)=7.42, p<0.01], and the interaction was also significant [F(12,60)=7.80, p<0.01].
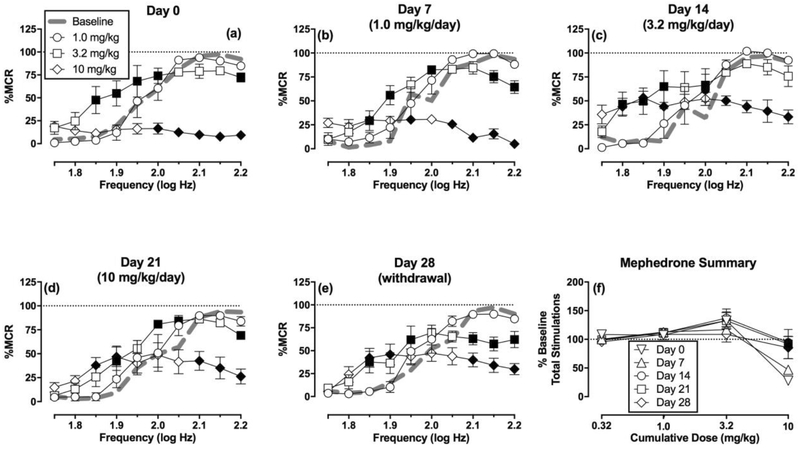
Figure 5 (a–e) shows the effects of cumulative mephedrone administration. On Day 0, mephedrone produced mixed effects on ICSS, such that low doses of 0.32 and 1.0 mg/kg did not alter ICSS. A higher dose of 3.2 mg/kg facilitated ICSS at four intermediate frequencies (1.85–1.95 log Hz) and depressed ICSS at the highest frequency (2.2 log Hz), and 10 mg/kg only depressed ICSS at the highest five frequencies (2.0–2.2 log Hz). During exposure to and withdrawal from mephedrone treatment, low doses of 0.32 and 1.0 mg/kg mephedrone continued to produce no effect, and the higher dose of 3.2 mg/kg mephedrone generally continued to produce mixed effects that included facilitation of ICSS at two to four intermediate frequencies and depression of ICSS at higher frequencies. However, 10 mg/kg mephedrone produced less ICSS depression and more ICSS facilitation after periods of repeated mephedrone (Days 7, 14, and 21) than on Day 0, and this change largely persisted after seven days of mephedrone withdrawal (Day 28). Figure 5f shows summary data for this profile of tolerance to rate-decreasing effects of 10 mg/kg mephedrone.
Figure 5. Effects of cumulative mephedrone administration on ICSS before, during, and after repeated daily mephedrone treatment (N=5).
Panels a-e show full frequency-rate curves on a given test day. The title of each panel shows the test day and the mephedrone treatment administered daily for the six days preceding that test day. Abscissae: frequency of electrical brain stimulation in log Hz. Ordinates: percent maximum control reinforcement rate (% MCR) ± SEM (note that Baseline error is shown in Figure 2 but is not shown here for clarity). Filled symbols show significant differences relative to the baseline on that day as determined by repeated measure two-way analysis of variance (ANOVA) followed by Holm-Sidak post hoc test, p<0.05. Two-way ANOVA results indicated significant main effects of frequency in all panels (p<0.01). Statistical results for the main effect of mephedrone dose and the frequency x dose interaction results were as follows: (a) Day 0: Dose [F(4,16)=43.17, p<0.01], Interaction [F(36,144)=11.49, p<0.01]; (b) Day 7: Dose [F(4,16)=17.95, p<0.01], Interaction [F(36,144)=10.77, p<0.01]; (c) Day 14: Dose [F(4,16)=5.02, p<0.01], Interaction [F(36,144)=8.50, p<0.01]; (d) Day 21: Dose [F(4,16)=1.98, p=0.15], Interaction [F(36,144)=5.94, p<0.01]; (e) Day 28: Dose [F(4,16)=1.18, p=0.35], Interaction [F(36,144)=6.98, p<0.01]. Panel f compares mephedrone effects across test days on the summary measure of ICSS. Abscissa: cumulative mephedrone dose in mg/kg. Ordinate: Percent Total Baseline Stimulations per Component ± SEM. There was not significant main effect of test day [F(4,16)=1.44, p=0.27], but there was a significant main effect of mephdrone dose [F(3,12)=14.64, p<0.01], and the interaction was also significant [F(12,48)=3.92, p<0.01].
DISCUSSION
This study evaluated effects of repeated treatment with methcathinone, mephedrone, and fenfluramine in a “frequency-rate” ICSS procedure in rats. There were three main findings. First, upon initial treatment, methcathinone produced dose-dependent ICSS facilitation, fenfluramine produced dose-dependent ICSS depression, and mephedrone produced a mixed profile of effects that included both ICSS facilitation and depression. Second, repeated treatment with escalating daily doses produced no change in ICSS facilitating effects of methcathinone but complete tolerance to the ICSS depressant effects of fenfluramine. For mephedrone, repeated treatment produced partial tolerance to ICSS depressing effects and increased expression of ICSS facilitating effects, especially at the highest mephedrone dose. Lastly, termination of repeated treatment produced no changes in baseline ICSS performance and recovery of ICSS depressant effects of fenfluramine but not mephedrone. These results are consistent with the interpretation that repeated exposure may increase the abuse potential of mixed-action synthetic cathinones like mephedrone, possibly due to development of tolerance to serotonergic effects that initially limit abuse potential of these compounds.
The initial effects of methcathinone, fenfluramine, and mephedrone in drug-naïve rats (i.e. on Day 0 of the experiment) agree with previous reports describing effects of these and related compounds on ICSS (Bauer et al. 2013; Bonano et al. 2014). Thus, as in previous studies, the DAT>SERT-selective substrate methcathinone produced dose-dependent and robust ICSS facilitation, the DAT<SERT-selective substrate fenfluramine produced dose-dependent ICSS depression, and the DAT≈SERT substrate mephedrone produced a mixed profile of ICSS facilitation and depression depending on the mephedrone dose and the brain-stimulation frequency. Repeated treatment with the doses of each compound tested here also did not alter baseline ICSS frequency-rate curves determined approximately 22 hr after each repeated dose. One implication of this finding is that effects of each daily dose dissipated before the next daily session. This conclusion is consistent with previous evidence from acute time-course studies that effects of the highest doses tested here for methcathinone (1.0 mg/kg) and mephedrone (10 mg/kg) peak within 10 min and are no longer apparent after 300 min (Bonano et al. 2014). In similar time-course studies, the highest fenfluramine dose tested here (3.2 mg/kg) had a slower onset (peak effects at 30 min) and longer duration of action, with some ICSS depression still apparent after 24 hr (Bauer et al. 2013). The failure of this high fenfluramine dose to produce evidence of long-term ICSS depression during repeated dosing is one source of evidence for tolerance to fenfluramine effects.
A second implication of stable ICSS baselines during repeated dosing is that these dosing regimens did not produce protracted withdrawal. Termination of chronic dosing with psychostimulants and some other drugs of abuse (e.g. mu opioid receptor agonists) can produce abstinence signs that include ICSS depression lasting for a day or more (Altarifi et al. 2013; Bauer et al. 2014; Cryan et al. 2003; Schulteis et al. 1994); however, the dosing regimens used here were not sufficient to produce these levels of dependence. In a related point, termination of chronic fenfluramine treatment did not result in withdrawal-induced enhancement of ICSS even though tolerance to fenfluramine-induced ICSS depression did develop. This suggests that although pharmacologically elevated 5-HT levels can depress ICSS, basal 5-HT levels may not be an important determinant of ICSS. In support of this conclusion, we found previously that fenfluramine and other SERT substrates depressed ICSS only at doses sufficient to increase 5-HT levels by at least 200%, and statistically significant but smaller increases in 5-HT levels did not alter ICSS (Bauer et al. 2013; Bonano et al. 2015; Suyama et al. 2016). Similarly, the 5-HT2c receptor antagonist SB 242,084 completely blocked fenfluramine-induced ICSS depression but had no significant effect on ICSS when it was administered alone (Bauer et al. 2015).
The stability of methcathinone effects during repeated administration of escalating daily doses agrees with other evidence to suggest that ICSS facilitation by DAT substrates and inhibitors is sustained during chronic treatment. For example, continuous infusion of amphetamine via minipump at doses of 2.4–10 mg/kg day in rats produced dose-dependent and sustained ICSS facilitation for periods of up to two weeks (Bauer et al. 2014; Cryan et al. 2003; Johnson et al. 2018). Similarly, repeated daily cocaine administration produced consistent levels of ICSS facilitation that neither sensitized nor tolerated in mice despite concurrent evidence for sensitization to the locomotor stimulant effects of cocaine (Riday et al. 2012). The ICSS facilitating effects of some other drugs, such as mu opioid receptor agonists and nicotine, are also sustained with little evidence of tolerance during regimens of repeated daily dosing, although tolerance may develop during treatment with very high doses (Altarifi and Negus 2011; Freitas et al. 2016; Kornetsky and Esposito 1979; Reid 1987). Taken together, these results provide evidence to suggest that neural mechanisms that underlie abuse-related ICSS facilitation are relatively resistant to tolerance during chronic treatment.
In contrast to the sustained ICSS facilitation by methcathinone, robust tolerance developed to the ICSS depressing effects of fenfluramine. This report of dose-dependent tolerance to fenfluramine doses up to 3.2 mg/kg confirms and extends a previous report describing modest tolerance to ICSS rate-decreasing effects of a very high fenfluramine dose (20 mg/kg) in rats that had received the same dose one week earlier (Olds and Yuwiler 1992). Moreover, tolerance to fenfluramine-induced ICSS depression is consistent with evidence for tolerance to some other fenfluramine effects in rats, such as depression of food intake and heroin self-administration, possibly due to a depletion of releasable pools of serotonin (Kleven et al. 1988; Rowland and Carlton 1983; Rowland and Carlton 1986; Wang et al. 1995).
Previous studies have identified three general profiles of ICSS effects that can occur during chronic treatment with drugs that produce primarily ICSS depression in drug-naïve subjects: (1) tolerance to ICSS depression and emergence of ICSS facilitation [e.g. with mu opioid receptor agonists like morphine (Legakis and Negus 2018; Miller et al. 2015a; Reid 1987; Wiebelhaus et al. 2016) or high nicotine doses (Freitas et al. 2016)], (2) tolerance to ICSS depression without emergence of ICSS facilitation [e.g. with cannabinoid receptor agonists like Δ9-tetrahydrocannabinol (Grim et al. 2015; Kwilasz and Negus 2012), the N-methyl-D-aspartate glutamate receptor antagonist ketamine (Hillhouse et al. 2014), and the delta opioid receptor agonist SNC80 (Negus et al. 2012)], or (3) sustained ICSS depression [e.g. with the kappa agonist salvinorin A (Potter et al. 2011), the dopamine D2 agonist quinpirole (Negus et al. 2012), and the serotonin 5HT1A/2A receptor agonist flibanserin (Lazenka et al. 2016)]. In general, drugs that produce ICSS facilitation initially and/or after repeated treatment (Profile 1) also display other signs of preclinical abuse potential (e.g. reinforcing effects in drug self-administration procedures) and have abuse liability in humans, whereas drugs that produce sustained ICSS depression do not (Negus and Miller 2014). However, drugs with the second profile of effects during repeated treatment (i.e. tolerance to initial ICSS depression without emergence of ICSS facilitation) may or may not function as effective reinforcers in drug self-administration studies and/or maintain some level of abuse in humans (John et al. 2017; Negus et al. 1998; Young and Woods 1981). In the present study, fenfluramine displayed this second profile of effects during repeated treatment, but fenfluramine does not maintain drug self-administration in preclinical studies (Dahl and Gotestam 1989; Wee and Woolverton 2006) and displays low abuse liability in humans (Kintz and Mangin 1992; Vivero et al. 1998).
For mephedrone, repeated treatment resulted in partial tolerance to ICSS depression and a modest increase in ICSS facilitation, especially with the highest mephedrone dose (10 mg/kg). This increase in ICSS facilitation during repeated dosing suggests that repeated mephedrone exposure may increase mephedrone abuse potential. The mechanisms that underlie this change remain to be determined; however, one possibility suggested by results above with methcathinone and fenfluramine is that repeated mephedrone exposure results in sustained DAT-mediated ICSS facilitation accompanied but at least partial tolerance to SERT-mediated ICSS depression. This conclusion is also consistent with a similar mechanism of increasing abuse potential that has been proposed for MDMA, another mixed-action DAT≈SERT substrate (Schenk 2009). Specifically, MDMA often functions as an unreliable reinforcer during initial training in laboratory animals, but reinforcing effectiveness increases either over time as self-administered MDMA exposure increases or with non-contingent MDMA pretreatment (Schenk et al. 2007; van de Wetering and Schenk 2017). Moreover, MDMA self-administration can decrease SERT density and brain 5-HT levels (Do and Schenk 2013; Schenk et al. 2007), and acquisition of MDMA self-administration can be enhanced either by a genetic mutation that impairs SERT function or by prior treatment with the serotonin-depleting agent 5,7-dihydroxytripatmine (Bradbury et al. 2014; Schenk 2009). Also, we found previously that pretreatment with the 5-HT2c receptor antagonist SB 242,084 increased MDMA-induced ICSS facilitation (Bauer et al. 2015). These results have been interpreted to suggest that 5-HT limits initial sensitivity to abuse-related MDMA effects, but repeated MDMA exposure can reduce 5-HT-mediated effects of MDMA and increase expression of DAT-mediated abuse-related effects. Taken together, these results provide convergent evidence to suggest that abuse-related effects of mixed-action DAT≈SERT substrates can be initially limited by SERT-mediated effects, but tolerance to these effects may occur during repeated exposure and result in increased abuse potential.
Although repeated treatment produced tolerance to the rate-decreasing effects of both fenfluramine and mephedrone, there were differences in the degrees and durations of that tolerance. Specifically, for the dosing regimens used here, tolerance to fenfluramine-induced ICSS depression was more complete but shorter acting than tolerance to mephedrone-induced ICSS depression. The reason for these differences remain to be determined. The greater degree of tolerance to fenfluramine might be related to the longer duration of action of fenfluramine in comparison to MDMA (Bauer et al. 2013; Bonano et al. 2014); however, this would not explain the shorter duration of tolerance. Future studies to examine cross-tolerance between these compounds could help to clarify their respective mechanisms of action. Of relevance to abuse-potential assessment, the sustained tolerance to ICSS depression and expression of ICSS facilitation by mephedrone suggests that repeated exposure can produce a relatively sustained increase in mephedrone abuse potential.
The present study evaluated effects of chronic treatment with monoamine-transporter substrates that varied in DAT vs. SERT selectivity and found evidence for tolerance to SERT-mediated ICSS depression but not to DAT-mediated ICSS facilitation. Drugs that function as inhibitors of monoamine transporters (e.g. cocaine) also vary in DAT vs. SERT selectivity in a way that correlates with expression of abuse-related effects, although inhibitors can tolerate higher proportions of SERT activity than substrates. For example, MDPV has DAT≫SERT selectivity and produces robust ICSS facilitation (like the DAT-selective substrate methcathinone in the present study), whereas citalopram has SERT≫DAT selectivity and produces only ICSS depression (like the SERT-selective substrate fenfluramine in the present study) (Bonano et al. 2014; Rosenberg et al. 2013). Cocaine is a nonselective DAT≈SERT inhibitor, roughly analogous to mephedrone as a nonselective DAT≈SERT substrate, but unlike mephedrone, cocaine produces only ICSS facilitation across a broad range of doses (Johnson et al. 2018; Rothman et al. 2001). Thus, for inhibitors, DAT≈SERT selectivity is not sufficient to reveal SERT-mediated ICSS depression; however, SERT-mediated ICSS depression does become apparent with slightly more SERT-selective inhibitors such as amitifadine (5- to 10-fold SERT>DAT selective) (Miller et al. 2015b). The ICSS effects of repeated treatment with monoamine transporter inhibitors like amitifadine, which have mixed DAT and SERT activity and produce a mixed profile of ICSS facilitation and depression, have not been tested, but results of the present study suggest that, as with mephedrone, repeated treatment might produce tolerance to SERT-mediated ICSS suppression and a resulting increase in DAT-mediated ICSS facilitation.
ACKNOWLEDGEMENTS
This work was supported by grants R01 DA033930 and F30 DA037649.
REFERENCES
- Altarifi AA, Miller LL, Negus SS (2012) Role of micro-opioid receptor reserve and micro-agonist efficacy as determinants of the effects of micro-agonists on intracranial self-stimulation in rats. Behav Pharmacol 23: 678–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Negus SS (2011) Some determinants of morphine effects on intracranial self-stimulation in rats: dose, pretreatment time, repeated treatment, and rate dependence. Behav Pharmacol 22: 663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Rice KC, Negus SS (2013) Abuse-related effects of micro-opioid analgesics in an assay of intracranial self-stimulation in rats: modulation by chronic morphine exposure. Behav Pharmacol 24: 459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS (2013) Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br J Pharmacol 168: 850–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS (2015) Role of 5-HT(2)C receptors in effects of monoamine releasers on intracranial self-stimulation in rats. Psychopharmacology (Berl) 232: 3249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Negus SS (2014) The effect of chronic amphetamine treatment on cocaine-induced facilitation of intracranial self-stimulation in rats. Psychopharmacology (Berl) 231: 2461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Solis E Jr., Watterson LR, Marusich JA, Fantegrossi WE, Wiley JL (2014) Baths salts, spice, and related designer drugs: the science behind the headlines. J Neurosci 34: 15150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Banks ML, Kolanos R, Sakloth F, Barnier ML, Glennon RA, Cozzi NV, Partilla JS, Baumann MH, Negus SS (2015) Quantitative structure-activity relationship analysis of the pharmacology of para-substituted methcathinone analogues. Br J Pharmacol 172: 2433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS (2014) Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology (Berl) 231: 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury S, Bird J, Colussi-Mas J, Mueller M, Ricaurte G, Schenk S (2014) Acquisition of MDMA self-administration: pharmacokinetic factors and MDMA-induced serotonin release. Addict Biol 19: 874–84. [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr., Chartoff EH (2007) Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc 2: 2987–95. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Hoyer D, Markou A (2003) Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol Psychiatry 54: 49–58. [DOI] [PubMed] [Google Scholar]
- Dahl CB, Gotestam KG (1989) Lack of self-administration of different fenfluramine isomers in rats. Addict Behav 14: 239–47. [DOI] [PubMed] [Google Scholar]
- De Felice LJ, Glennon RA, Negus SS (2014) Synthetic cathinones: chemical phylogeny, physiology, and neuropharmacology. Life Sci 97: 20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do J, Schenk S (2013) Self-administered MDMA produces dose- and time-dependent serotonin deficits in the rat brain. Addict Biol 18: 441–7. [DOI] [PubMed] [Google Scholar]
- Freitas K, Carroll FI, Negus SS (2016) Comparison of effects produced by nicotine and the alpha4beta2-selective agonist 5-I-A-85380 on intracranial self-stimulation in rats. Exp Clin Psychopharmacol 24: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim TW, Wiebelhaus JM, Morales AJ, Negus SS, Lichtman AH (2015) Effects of acute and repeated dosing of the synthetic cannabinoid CP55,940 on intracranial self-stimulation in mice. Drug Alcohol Depend 150: 31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse TM, Porter JH, Negus SS (2014) Dissociable effects of the noncompetitive NMDA receptor antagonists ketamine and MK-801 on intracranial self-stimulation in rats. Psychopharmacology (Berl) 231: 2705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John WS, Martin TJ, Nader MA (2017) Behavioral Determinants of Cannabinoid Self-Administration in Old World Monkeys. Neuropsychopharmacology 42: 1522–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AR, Banks ML, Selley DE, Negus SS (2018) Amphetamine maintenance differentially modulates effects of cocaine, methylenedioxypyrovalerone (MDPV), and methamphetamine on intracranial self-stimulation and nucleus accumbens dopamine in rats. Neuropsychopharmacology 43: 1753–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintz P, Mangin P (1992) Toxicological findings after fatal fenfluramine self-poisoning. Hum Exp Toxicol 11: 51–2. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Schuster CR, Seiden LS (1988) Effect of depletion of brain serotonin by repeated fenfluramine on neurochemical and anorectic effects of acute fenfluramine. J Pharmacol Exp Ther 246: 822–8. [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU (1979) Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc 38: 2473–6. [PubMed] [Google Scholar]
- Kwilasz AJ, Negus SS (2012) Dissociable effects of the cannabinoid receptor agonists Delta9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J Pharmacol Exp Ther 343: 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenka MF, Blough BE, Negus SS (2016) Preclinical Abuse Potential Assessment of Flibanserin: Effects on Intracranial Self-Stimulation in Female and Male Rats. J Sex Med 13: 338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legakis LP, Negus SS (2018) Repeated Morphine Produces Sensitization to Reward and Tolerance to Antiallodynia in Male and Female Rats with Chemotherapy-Induced Neuropathy. J Pharmacol Exp Ther 365: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LL, Altarifi AA, Negus SS (2015a) Effects of repeated morphine on intracranial self-stimulation in male rats in the absence or presence of a noxious pain stimulus. Exp Clin Psychopharmacol 23: 405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LL, Leitl MD, Banks ML, Blough BE, Negus SS (2015b) Effects of the triple monoamine uptake inhibitor amitifadine on pain-related depression of behavior and mesolimbic dopamine release in rats. Pain 156: 175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Banks ML (2017) Decoding the Structure of Abuse Potential for New Psychoactive Substances: Structure-Activity Relationships for Abuse-Related Effects of 4-Substituted Methcathinone Analogs. Curr Top Behav Neurosci 32: 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Gatch MB, Mello NK, Zhang X, Rice K (1998) Behavioral effects of the delta-selective opioid agonist SNC80 and related compounds in rhesus monkeys. J Pharmacol Exp Ther 286: 362–75. [PubMed] [Google Scholar]
- Negus SS, Miller LL (2014) Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev 66: 869–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Rosenberg MB, Altarifi AA, O’Connell RH, Folk JE, Rice KC (2012) Effects of the delta opioid receptor agonist SNC80 on pain-related depression of intracranial self-stimulation (ICSS) in rats. J Pain 13: 317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds ME, Yuwiler A (1992) Effects of acute and chronic fenfluramine on self-stimulation and its facilitation by amphetamine. Eur J Pharmacol 216: 363–72. [DOI] [PubMed] [Google Scholar]
- Potter DN, Damez-Werno D, Carlezon WA Jr., Cohen BM, Chartoff EH(2011) Repeated exposure to the kappa-opioid receptor agonist salvinorin A modulates extracellular signal-regulated kinase and reward sensitivity. Biol Psychiatry 70: 744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid LD (1987) Tests involving pressing for intracranial stimulation as an early procedure for screening the likelihood of addiction of opioids and other drugs. In: Bozarth MJ (ed) Methods of assessing the reinforcing properties of abused drugs. Springer-Verlag, Berlin, pp 391–420 [Google Scholar]
- Riday TT, Kosofsky BE, Malanga CJ (2012) The rewarding and locomotor-sensitizing effects of repeated cocaine administration are distinct and separable in mice. Neuropharmacology 62: 1858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JE, Agoglia AE, Fish EW, Krouse MC, Malanga CJ (2012) Mephedrone (4-methylmethcathinone) and intracranial self-stimulation in C57BL/6J mice: comparison to cocaine. Behav Brain Res 234: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg MB, Carroll FI, Negus SS (2013) Effects of monoamine reuptake inhibitors in assays of acute pain-stimulated and pain-depressed behavior in rats. J Pain 14: 246–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS (2001) Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39: 32–41. [DOI] [PubMed] [Google Scholar]
- Rowland N, Carlton J (1983) Different behavioral mechanisms underlie tolerance to the anorectic effects of fenfluramine and quipazine. Psychopharmacology (Berl) 81: 155–7. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Carlton J (1986) Tolerance to fenfluramine anorexia: fact or fiction? Appetite 7 Suppl: 71–83. [DOI] [PubMed] [Google Scholar]
- Schenk S (2009) MDMA self-administration in laboratory animals: a summary of the literature and proposal for future research. Neuropsychobiology 60: 130–6. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hely L, Lake B, Daniela E, Gittings D, Mash DC (2007) MDMA self-administration in rats: acquisition, progressive ratio responding and serotonin transporter binding. Eur J Neurosci 26: 3229–36. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Gold LH, Stinus L, Koob GF (1994) Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. J Pharmacol Exp Ther 271: 1391–8. [PubMed] [Google Scholar]
- Suyama JA, Sakloth F, Kolanos R, Glennon RA, Lazenka MF, Negus SS, Banks ML (2016) Abuse-Related Neurochemical Effects of Para-Substituted Methcathinone Analogs in Rats: Microdialysis Studies of Nucleus Accumbens Dopamine and Serotonin. J Pharmacol Exp Ther 356: 182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering R, Schenk S (2017) Repeated MDMA administration increases MDMA-produced locomotor activity and facilitates the acquisition of MDMA self-administration: role of dopamine D2 receptor mechanisms. Psychopharmacology (Berl) 234: 1155–1164. [DOI] [PubMed] [Google Scholar]
- Vivero LE, Anderson PO, Clark RF (1998) A close look at fenfluramine and dexfenfluramine. J Emerg Med 16: 197–205. [DOI] [PubMed] [Google Scholar]
- Wang Y, Joharchi N, Fletcher PJ, Sellers EM, Higgins GA (1995) Further studies to examine the nature of dexfenfluramine-induced suppression of heroin self-administration. Psychopharmacology (Berl) 120: 134–41. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Hood L, Sewalia K, Tomek SE, Yahn S, Johnson CT, Wegner S, Blough BE, Marusich JA, Olive MF (2012) The Reinforcing and Rewarding Effects of Methylone, a Synthetic Cathinone Commonly Found in “Bath Salts”. J Addict Res Ther Suppl 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF (2014) Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Addict Biol 19: 165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Woolverton WL (2006) Self-administration of mixtures of fenfluramine and amphetamine by rhesus monkeys. Pharmacol Biochem Behav 84: 337–43. [DOI] [PubMed] [Google Scholar]
- Wiebelhaus JM, Walentiny DM, Beardsley PM (2016) Effects of Acute and Repeated Administration of Oxycodone and Naloxone-Precipitated Withdrawal on Intracranial Self-Stimulation in Rats. J Pharmacol Exp Ther 356: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AM, Herling S, Woods JH (1981) History of drug exposure as a determinant of drug self-administration. NIDA Res Monogr 37: 75–88. [PubMed] [Google Scholar]
- Young AM, Woods JH (1981) Maintenance of behavior by ketamine and related compounds in rhesus monkeys with different self-administration histories. J Pharmacol Exp Ther 218: 720–7. [PubMed] [Google Scholar]