Abstract
The human circadian system regulates hunger independently of behavioral factors, resulting in a trough in the biological morning and a peak in the biological evening. However, the role of the only known orexigenic hormone ghrelin in this circadian rhythm is unknown. Furthermore, although shift work is an obesity risk factor, the separate effects of the endogenous circadian system, the behavioral cycle, and circadian misalignment on ghrelin has not been systematically studied. Here we show—by using two 8-d laboratory protocols—that circulating active (acylated) ghrelin levels are significantly impacted by endogenous circadian phase in healthy adults. Active ghrelin levels were higher in the biological evening than the biological morning (fasting +15.1%, P=0.0001; postprandial +10.4%, P=0.0002), consistent with the circadian variation in hunger (P=0.028). Moreover, circadian misalignment itself (12-h behavioral cycle inversion) increased postprandial active ghrelin levels (+5.4%; P=0.04). While not significantly influencing hunger (P>0.08), circadian misalignment increased appetite for energy-dense foods (all P<0.05). Our results provide possible mechanisms for the endogenous circadian rhythm in hunger as well as for the increased risk of obesity among shift workers.
Introduction
There is a large endogenous circadian rhythm in hunger (17%), with a peak in the biological evening and the trough in the biological morning[1]. This circadian rhythm in hunger may explain why, despite the extended overnight fast, people often feel least hungry in the morning and often skip breakfast. However, the neuroendocrine mechanisms through which the circadian system regulates hunger and appetite remain unclear. Ghrelin, a peptide secreted primarily by the stomach, is the only known circulating orexigenic hormone and a key element in the complex signaling network of energy balance. Acylation of ghrelin is essential for its appetite-stimulating activity[2]. The circulating level of active (acylated) ghrelin is mainly regulated by nutritional status, rising by fasting and decreasing after food ingestion[2]. While some rodent and human studies have reported a diurnal rhythm in ghrelin under fasted conditions[3–5], none accounted for the contribution of other behavioral factors such as sleep/wake and rest/activity cycles. Thus, it remains to be elucidated whether there is an endogenous circadian rhythm in circulating active ghrelin (AG) levels, independent of behavioral cycles.
Furthermore, shift workers have an increased risk of obesity[6] and may experience hyperphagia and high desire for energy-dense food[7, 8]. This raises the question whether misalignment between the endogenous circadian system and the 24-h environmental/behavioral cycles (i.e., circadian misalignment), that is typical of night work, may contribute to increased energy intake in shift workers through neuroendocrine changes. We are aware of no study to date that systematically tested the separate and relative effects of circadian phase and circadian misalignment, independent of the behavioral cycles, on AG, hunger and appetite.
To address the above questions, we assessed AG levels and hunger/appetites before and after identical test meals in a randomized, crossover design with two 8-day laboratory protocols (aligned vs. misaligned; Fig S1). Together, these two protocols allow us to separate the impacts of behavioral cycle, circadian phase, and circadian misalignment. We report fasting and postprandial levels separately, since they have different implications: the former is related to meal initiation, the latter to the satiety cascade[9].
Methods
Other aspects of this study—which was designed to test separate hypotheses—have been published[10,11,21–24].
Participants and Experimental Design.
Fourteen healthy young adults completed this study [mean age±SD, 28±9y; BMI, 25.4±2.6kg/m2; 8 men]. For details on subject recruitment, screening methods and pre-inpatient study conditions, see Supplementary Methods and [11].
Diet.
Participants consumed an isocaloric diet per 24h. Identical test meals were given at 1h (breakfast) and 13h (dinner) following scheduled wake time on the 1st and 3rd test days. Blood samples for AG assay (n=14) were collected 7 minutes before the test meal (fasting), 60 minutes and 120 minutes after each test meal started (postprandial). Details see Supplementary Methods.
Hunger and appetite ratings.
Participants used computerized, visual analog scales (VAS; 0 as “not at all” and 100 as “very much”/“extremely”) to rate hunger, appetite, and food preferences hourly (n=14), including 5 minutes before the test meal (fasting), and 55 minutes and 115 minutes after the start of each test meal (postprandial).
Data analysis.
Using linear mixed model analysis with participants as random factor, we tested the independent effect of circadian phase (biological morning vs. biological evening), alignment condition (circadian alignment vs. circadian misalignment), the behavioral cycle (breakfast vs. dinner) on fasting and postprandial AG levels and hunger/appetites. We also tested whether the separate effects of the circadian system and circadian misalignment on the outcomes would change upon repeated exposure to circadian misalignment by including their interaction terms with test day (first vs. third). To account for time-course influences, time since meal (postprandial: 1-h and 2-h) and its interaction with the main effects were added into the linear mixed model. To assess whether AG was associated with the circadian effect on hunger, we added AG as a covariate into the linear mixed model. Statistical significance was accepted as P < 0.05. To represent the independent effects of the circadian phase, circadian misalignment and the behavioral cycle, in the table, we (i) averaged 8:00 AM (BA and DM, annotation as shown in Fig. 1) and 8:00 PM (DA and BM) test meal values separately across both protocols (circadian phase effect); (ii) averaged alignment (BA and DA) and misalignment (BM and DM) test meal values within each protocol (circadian misalignment effect); and (iii) averaged breakfast time (BA and BM) and dinner time (DA and DM) test meal values separately across both protocols (behavioral cycle effect).
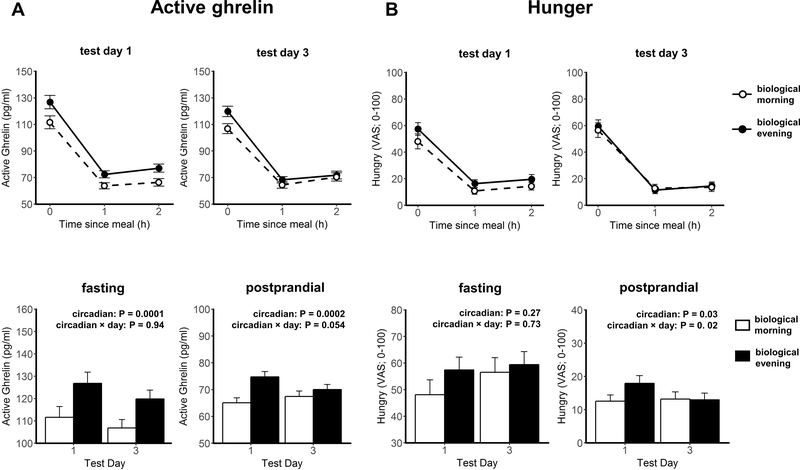
Figure 1.
Fasting and postprandial AG level (A) and hunger (B) in the biological morning (white) and in the biological evening (black). Fasting AG levels were measured 7 min before the test meal (meal start = 0h), and postprandial AG levels were measured at 60min (1h) and 120min (2h) after test meal start. Hunger ratings were measured 5 min before the test meal (fasting), and 55 min and 115 min after test meal start (postprandial). In the bottom panels, postprandial data are averaged across the two postprandial assessments. Data are shown in mean±SEM.
Results
Table 1 shows comparisons of fasting and postprandial AG levels, hunger and appetite ratings between circadian phases, alignment conditions, and behavioral cycles.
Table 1 -.
Mean fasting and postprandial active ghrelin levels and hunger and appetites in the biological morning or in the biological evening (circadian phase), when circadian aligned or misaligned (alignment condition), at breakfast or at dinner (behavioral cycle).
| circadian phase |
alignment condition |
behavioral cycle |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | morning | evening | P | P (× day) | aligned | misaligned | P | P (× day) | breakfast | dinner | P | P (× day) |
| AG (pg/mL) | ||||||||||||
| fasting | 109.20±22.45 | 123.30±23.98 | <0.001 | 0.94 | 115.79±28.11 | 117.00±19.57 | 0.53 | 0.38 | 115.05±23.11 | 117.76±25.41 | 0.62 | 0.75 |
| postprandial | 66.26±14.28 | 72.41±14.73 | <0.001 | 0.05 | 67.83±15.67 | 70.90±13.78 | 0.04 | 0.18 | 71.56±15.42 | 67.13±13.85 | 0.02 | 0.67 |
| Hunger | ||||||||||||
| fasting | 52.31±6.61 | 58.44±5.02 | 0.08 | 0.36 | 58.42±5.19 | 52.33±6.63 | 0.08 | 0.77 | 51.02±6.65 | 59.73±6.14 | 0.01 | 0.86 |
| postprandial | 12.87±3.55 | 15.43±3.91 | 0.03 | 0.02 | 14.15±4.02 | 14.15±3.54 | 1.00 | 0.16 | 15.86±4.02 | 12.44±3.49 | <0.01 | 0.12 |
| Full | ||||||||||||
| fasting | 23.02±3.76 | 20.28±3.74 | 0.27 | 0.73 | 21.66±3.43 | 21.64±4.07 | 0.99 | 0.26 | 20.43±3.77 | 22.86±4.08 | 0.32 | 0.64 |
| postprandial | 70.61±4.62 | 65.41±4.06 | 0.01 | 0.28 | 68.56±4.92 | 67.46±3.79 | 0.59 | 0.39 | 66.21±4.18 | 69.81±4.64 | 0.08 | 0.01 |
| Strong | ||||||||||||
| fasting | 53.13±6.79 | 59.76±5.94 | 0.06 | 0.09 | 59.57±5.49 | 53.32±7.24 | 0.08 | 0.68 | 53.18±6.96 | 59.71±6.89 | 0.07 | 0.97 |
| postprandial | 13.79±3.83 | 15.78±3.98 | 0.08 | 0.04 | 14.76±4.13 | 14.81±3.75 | 0.96 | 0.99 | 16.81±4.22 | 12.76±3.66 | <0.001 | 0.12 |
| Much | ||||||||||||
| fasting | 57.29±5.35 | 61.28±4.82 | 0.09 | 0.14 | 61.39±4.25 | 57.18±5.79 | 0.08 | 0.58 | 58.79±4.84 | 59.78±5.72 | 0.68 | 0.93 |
| postprandial | 20.54±3.70 | 23.86±4.13 | 0.02 | 0.40 | 22.25±3.79 | 22.14±4.14 | 0.94 | 0.17 | 23.38±3.94 | 21.01±3.87 | 0.10 | 0.19 |
| Sweet | ||||||||||||
| fasting | 33.41±7.42 | 34.33±6.42 | 0.72 | 0.37 | 36.38±6.76 | 31.37±7.05 | 0.0496 | 0.81 | 28.58±6.07 | 39.16±7.93 | <0.0001 | 0.24 |
| postprandial | 14.83±3.85 | 18.09±4.05 | 0.02 | 0.98 | 15.99±3.69 | 16.94±4.47 | 0.48 | 0.82 | 17.94±4.32 | 14.98±3.61 | 0.03 | 0.32 |
| Starch | ||||||||||||
| fasting | 47.27±8.27 | 52.04±7.65 | 0.12 | 0.96 | 52.10±7.09 | 47.21±8.80 | 0.11 | 0.41 | 48.46±8.37 | 50.84±8.27 | 0.44 | 0.91 |
| postprandial | 16.83±4.47 | 19.38±4.78 | 0.15 | 0.19 | 15.12±3.84 | 21.09±5.97 | <0.001 | 0.87 | 19.99±4.76 | 16.23±4.55 | 0.03 | 0.47 |
| Salty | ||||||||||||
| fasting | 41.49±7.95 | 43.14±8.04 | 0.45 | 0.16 | 42.56±7.23 | 42.07±8.76 | 0.82 | 0.32 | 38.08±7.57 | 46.55±8.51 | <0.001 | 0.37 |
| postprandial | 17.51±4.62 | 20.67±4.73 | 0.09 | 0.51 | 16.30±3.77 | 21.88±6.14 | <0.001 | 0.90 | 21.15±4.92 | 17.03±4.45 | 0.03 | 0.45 |
| Meat | ||||||||||||
| fasting | 48.32±6.50 | 52.31±6.92 | 0.29 | 0.28 | 50.99±6.15 | 49.64±8.05 | 0.72 | 0.66 | 49.08±7.26 | 51.55±6.91 | 0.51 | 0.93 |
| postprandial | 14.43±4.11 | 17.24±4.05 | 0.03 | 0.27 | 14.72±3.98 | 16.95±4.17 | 0.08 | 0.15 | 18.37±4.39 | 13.30±3.67 | <0.001 | 0.01 |
| Dairy | ||||||||||||
| fasting | 44.35±8.29 | 48.09±7.82 | 0.21 | 0.90 | 45.60±7.78 | 46.84±8.46 | 0.68 | 0.76 | 46.56±8.46 | 45.88±8.45 | 0.82 | 0.76 |
| postprandial | 16.36±4.69 | 18.39±4.96 | 0.26 | 0.18 | 15.15±4.28 | 19.59±5.93 | 0.02 | 0.83 | 19.63±5.20 | 15.11±4.44 | 0.01 | 0.32 |
| Vegetable | ||||||||||||
| fasting | 32.75±5.34 | 37.04±5.74 | 0.18 | 0.75 | 35.98±5.06 | 33.82±5.97 | 0.50 | 0.38 | 29.79±5.52 | 40.00±6.70 | <0.01 | 0.16 |
| postprandial | 11.47±3.09 | 13.37±3.21 | 0.28 | 0.49 | 9.94±2.13 | 14.91±4.63 | 0.01 | 0.37 | 14.34±3.46 | 10.51±2.85 | 0.03 | 0.38 |
| Fruit | ||||||||||||
| fasting | 45.70±7.76 | 50.42±7.45 | 0.08 | 0.57 | 49.83±7.00 | 46.29±8.25 | 0.19 | 0.92 | 47.97±8.36 | 48.15±7.43 | 0.95 | 0.62 |
| postprandial | 20.03±5.04 | 21.89±5.14 | 0.24 | 0.66 | 19.01±4.36 | 22.91±6.06 | 0.01 | 0.66 | 23.23±5.31 | 18.69±4.92 | <0.01 | 0.79 |
Data are presented as mean ± SE. See Methods for details of linear mixed effect model. P-values of main effect and their interaction effects with test day are listed above as P and P (× day), respectively Significant effects (P<0.05) are shown in bold.
There was a significant circadian phase effect, independent of the behavioral cycles, on fasting (P=0.0001) and postprandial (P=0.0002) AG levels with higher values in the biological evening than the biological morning (Fig 1A). Similar circadian phase effects were also present for postprandial hunger (P=0.03, Figure 1B), estimates of how much food participants could eat (much; P=0.02), appetites for sweet (P=0.02) and meat (P=0.03), all with higher scores in the biological evening than the biological morning. Consistently, fullness was lower in the biological evening (P=0.01). However, there were no significant circadian effects on fasting hunger and appetite ratings (all P≥0.06). The circadian phase effect on postprandial hunger was significantly damped upon repeated exposure to circadian misalignment (P=0.02 for the interaction term: circadian phase × test day), with a similar trend for postprandial AG (P=0.054). This circadian effect on postprandial hunger lost significance (P=0.13) if AG was added as a covariate (p=0.058), indicating this effect may be mediated through AG.
Circadian misalignment, independent of the circadian phase and behavioral cycle, significantly increased postprandial AG level as compared to circadian alignment (P=0.04), without significant differences in hunger and fullness (all P≥0.08). Of interest, postprandial appetites for starch (P<0.001), salty (P<0.001), diary (P=0.02), vegetable (P=0.01), and fruits (P=0.01) were all significantly higher under misaligned conditions than aligned conditions. These findings were not influenced by exposure duration to circadian misalignment (all P≥0.16 for interaction term: circadian misalignment × test day).
Postprandial AG (P=0.02), together with hunger (P<0.01), desire to eat (strong; P<0.001), and all appetites (all P<0.04) were affected by the behavioral cycle while controlling for circadian effects, with higher levels at breakfast than at dinner. The behavioral cycle also influenced fasting hunger, appetites for sweet, salty, and vegetable, though contrary to the postprandial scores, fasting ratings were higher at dinner than at breakfast.
Discussion
We reveal for a strong endogenous circadian effect on AG concentrations, with higher fasting and postprandial levels in the biological evening than the biological morning. Postprandial (but not fasting) hunger also showed a consistent circadian variation. Furthermore, postprandial fullness, and appetites for energy-dense food (i.e., sweet and meat) show significant circadian effects, in directions that support the central observation for AG and hunger. The circadian effects on postprandial hunger was blunted following consecutive days of circadian misalignment, with a similar trend for AG. The attenuation in the biological morning/evening differences may be due to blunted, shifted or otherwise disrupted circadian control, and is consistent with evidence from melatonin, cortisol, glucose, and diet-induced thermogenesis[10, 11].
Current results are consistent with a previous study demonstrating an endogenous circadian rhythm in hunger with a trough at a circadian phase equivalent to ~8AM and a peak at ~8PM[1]. The present study unraveled a potential neuroendocrine mechanism for the circadian rhythm in hunger—a circadian control of AG levels. This result is in line with prior research showing that ghrelin continues to oscillate under fasted conditions[3–5]. However, our study furthermore minimized the influence of sleep/wake and rest/activity cycles, relevant because growth hormone release, which is stimulated during sleep, inhibits ghrelin secretion[12]. Our result is also consistent with a recent study showing the circadian rhythm in fasting AG using a forced desynchrony protocol [25], while our study further 1) distinguished the separate influences from circadian phase and circadian misalignment; 2) revealed circadian variation not only in fasting but also postprandial AG levels; 3) indicated that AG might mediate the circadian effects on hunger. The circadian variation in AG levels may be mediated by the circadian clock in ghrelin-secreting stomach cells which can be entrained to prior feeding schedules[4, 5].
Using a simulated night shift protocol, we found that circadian misalignment, independent of circadian phase and behavioral cycle, increased postprandial AG levels. This effect was sustained upon repeated exposure. This indicated that increase in AG levels did not subside as an adaptation to circadian misalignment, which may lead to a lasting positive energy balance in shift workers. Circadian misalignment also increased many postprandial appetites, despite no changes in hunger and fullness. This contrast suggests that misalignment may alter eating primarily via the food reward system (related to appetites) rather than the homeostatic pathway (related to hunger and fullness)[13]. Prior studies on the effect of circadian disruption in the form of experimental night shifts or jet lag on ghrelin levels have been controversial, with either elevated[14, 15] or unchanged[16, 17] ghrelin levels. The inconsistency may be attributed to the variations in diet condition (e.g. ad libitum caloric intake, calorie-balanced diet, fixed-calorie meal), the timing and frequency of blood sampling, or the form of ghrelin measured (active vs. total). It is possible that the effect of night shift on appetite-regulating hormones is obscured by uncontrolled caloric intake. The present study circumvented such limitation by providing each participant the same isocaloric diet per 24-h in both protocols.
There were also significant behavioral cycle effects on postprandial AG, independent of circadian phase and circadian misalignment, with lower levels at dinner than at breakfast. This may be due to the higher postprandial glucose levels at dinner[11] as glucose can markedly inhibit ghrelin secretion[18]. We also detected significant behavioral influences on postprandial hunger and most appetite ratings, consistent with the observation for AG. In contrast, despite the longer fasting duration before breakfast (4-h longer than dinner), the fasting hunger, desire for sweet, salty, and vegetable were lower at breakfast than at dinner. This seeming discrepancy may be because, as compared to postprandial appetites, fasting appetites can be largely shaped by culture norms for which dinner is usually the largest meal of the day in US.
Limitations of the study included the relative small sample size. Also, we were only able to measure the circulating level of ligand in human, but not downstream actions including ghrelin receptor expression in vagal afferent neurons, or ghrelin-sensitivity in the arcuate nucleus―the center of appetite regulation. Both sites are also under circadian control[19, 20].
In conclusion, our findings show the separate effects of circadian phase and circadian misalignment, independent of behavioral cycle, on AG and hunger/appetites in healthy humans. These results provide possible mechanisms for the endogenous circadian rhythm in hunger as well as for the increased risk of obesity among shift workers.
Supplementary Material
Acknowledgements
We thank the research volunteers and Brigham and Women’s Hospital’s Center for Clinical Investigation nursing and technical staff. This study was supported by NIH Grant R01HL094806 (F.A.J.L.S.) and UL1RR025758 (to Harvard University and Brigham and Women’s Hospital). J.Q. was supported in part by American Diabetes Association grant #1–17-PDF-103 (J.Q.). R.C. was supported in part by the Minerva Scholarship and the Trustee Fund. M.G. was supported in part by The Spanish Government of Investigation, Development and Innovation (SAF2017–84135-R) including FEDER co-funding, and NIDDK R01DK105072. F.A.J.L.S. was supported in part by NIH grants R01HL094806, R01HL118601, R01DK099512, R01DK102696, and R01DK105072 (F.A.J.L.S.).
Footnotes
Conflict of Interest
C.J.M. reports receiving salary from Grünenthal Ltd, UK., which relationship is not related to the present article. F.A.J.L.S. received speaker fees from Bayer Healthcare, Sentara Healthcare, Philips, Kellogg Company, Vanda Pharmaceuticals, and Pfizer.
References
- 1.Scheer FA, Morris CJ, and Shea SA (2013). The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity 21, 421–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spiegel K, Tasali E, Leproult R, Scherberg N, and Van Cauter E (2011). Twenty-four-hour profiles of acylated and total ghrelin: relationship with glucose levels and impact of time of day and sleep. J Clin Endocrinol Metab 96, 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Espelund U, Hansen TK, Hojlund K, Beck-Nielsen H, Clausen JT, Hansen BS, Orskov H, Jorgensen JO, and Frystyk J (2005). Fasting unmasks a strong inverse association between ghrelin and cortisol in serum: studies in obese and normal-weight subjects. J Clin Endocrinol Metab 90, 741–746. [DOI] [PubMed] [Google Scholar]
- 4.LeSauter J, Hoque N, Weintraub M, Pfaff DW, and Silver R (2009). Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci U S A 106, 13582–13587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Natalucci G, Riedl S, Gleiss A, Zidek T, and Frisch H (2005). Spontaneous 24-h ghrelin secretion pattern in fasting subjects: maintenance of a meal-related pattern. Eur J Endocrinol 152, 845–850. [DOI] [PubMed] [Google Scholar]
- 6.Pan A, Schernhammer ES, Sun Q, and Hu FB (2011). Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med 8, e1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cain SW, Filtness AJ, Phillips CL, and Anderson C (2015). Enhanced preference for high-fat foods following a simulated night shift. Scand J Work Environ Health 41, 288–293. [DOI] [PubMed] [Google Scholar]
- 8.Bonnell EK, Huggins CE, Huggins CT, McCaffrey TA, Palermo C, and Bonham MP (2017). I nfluences on Dietary Choices during Day versus Night Shift in Shift Workers: A Mixed Methods Study. Nutrients 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benelam B (2009). Satiation, satiety and their effects on eating behaviour. Nutrition Bulletin 34, 126–173. [Google Scholar]
- 10.Morris CJ, Garcia JI, Myers S, Yang JN, Trienekens N, and Scheer FA (2015). The Human Circadian System Has a Dominating Role in Causing the Morning/Evening Difference in Diet-Induced Thermogenesis. Obesity (Silver Spring) 23, 2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, and Scheer FA (2015). Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A 112, E2225–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seoane LM, Al-Massadi O, Barreiro F, Dieguez C, and Casanueva FF (2007). Growth hormone and somatostatin directly inhibit gastric ghrelin secretion. An in vitro organ culture system. J Endocrinol Invest 30, RC22–25. [DOI] [PubMed] [Google Scholar]
- 13.Rogers PJ, and Hardman CA (2015). Food reward. What it is and how to measure it. Appetite 90, 1–15. [DOI] [PubMed] [Google Scholar]
- 14.Schiavo-Cardozo D, Lima MM, Pareja JC, and Geloneze B (2013). Appetite-regulating hormones from the upper gut: disrupted control of xenin and ghrelin in night workers. Clin Endocrinol (Oxf) 79, 807–811. [DOI] [PubMed] [Google Scholar]
- 15.Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, Czeisler CA, and Shea SA (2012). Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med 4, 129ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McHill AW, Melanson EL, Higgins J, Connick E, Moehlman TM, Stothard ER, and Wright KP Jr. (2014). Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc Natl Acad Sci U S A 111, 17302–17307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonnissen HK, Rutters F, Mazuy C, Martens EA, Adam TC, and Westerterp-Plantenga MS (2012). Effect of a phase advance and phase delay of the 24-h cycle on energy metabolism, appetite, and related hormones. Am J Clin Nutr 96, 689–697. [DOI] [PubMed] [Google Scholar]
- 18.Baldelli R, Bellone S, Castellino N, Petri A, Rapa A, Vivenza D, Bellone J, Broglio F, Ghigo E, and Bona G (2006). Oral glucose load inhibits circulating ghrelin levels to the same extent in normal and obese children. Clin Endocrinol (Oxf) 64, 255–259. [DOI] [PubMed] [Google Scholar]
- 19.Kentish SJ, Frisby CL, Kennaway DJ, Wittert GA, and Page AJ (2013). Circadian variation in gastric vagal afferent mechanosensitivity. J Neurosci 33, 19238–19242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buijs FN, Guzman-Ruiz M, Leon-Mercado L, Basualdo MC, Escobar C, Kalsbeek A, and Buijs RM (2017). Suprachiasmatic Nucleus Interaction with the Arcuate Nucleus; Essential for Organizing Physiological Rhythms. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian Jingyi, Man Chiara Dalla, Morris Christopher J., Cobelli Claudio, Scheer Frank A. J. L., (2018) Differential effects of the circadian system and circadian misalignment on insulin sensitivity and insulin secretion in humans. Diabetes, Obesity and Metabolism 20 (10):2481–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chellappa Sarah L., Morris Christopher J., Scheer Frank A. J. L., (2018) Daily circadian misalignment impairs human cognitive performance taskdependently. Scientific Reports 8 (1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Peng, Morris Christopher J., Patxot Melissa, Yugay Tatiana, Mistretta Joseph, Purvis Taylor E., Scheer Frank A. J. L., Hu Kun, (2017) Reduced Tolerance to Night Shift in Chronic Shift Workers: Insight From Fractal Regulation. Sleep 40 (7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris Christopher J., Purvis Taylor E., Hu Kun, Scheer Frank A. J. L., (2016) Circadian misalignment increases cardiovascular disease risk factors in humans. Proceedings of the National Academy of Sciences 113 (10):E1402–E1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McHill Andrew W., Hull Joseph T., McMullan Ciaran J., Klerman Elizabeth B., (2018) Chronic Insufficient Sleep Has a Limited Impact on Circadian Rhythmicity of Subjective Hunger and Awakening Fasted Metabolic Hormones. Frontiers in Endocrinology 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.