Abstract
Objective
The aim of this study was to define histopathological features of giant cell tumor of bone, especially accompanying fibrohistiocytic or aneurysmal bone cyst like components, in the light of our institutions experience.
Methods
A total of 120 cases (64 females and 56 males; mean age: 36.2 (12–80)) with ‘GCT’ diagnosed between the years 1996–2016 were included in this retrospective analysis. Cases were evaluated according to clinical features such as age, gender, localization, recurrence, metastasis and histopathological features.
Results
Tumors were localized most frequently at proximal tibia and distal femur, respectively. In 11 cases areas rich in fibrohistiocytic component and in 20 cases aneurysmal bone cyst like component were observed. In 2 cases both components were present. Twenty three cases recurred. In 1 case which was primarily located at calcaneus, tumor metastasized to lung 4 years later during follow-up.
Conclusion
GCT can be confused with other tumor or tumor-like lesions involving giant cells. Secondary changes such as fibrohistiocytic or aneurysmal bone cyst-like components and coagulation necrosis were frequently seen in conventional giant cell tumor of bone. For tumors having prominent fibrohistiocytic and/or aneurysmal bone cyst-like components, in order to detect characteristic areas representing GCT, additional sampling is essential. Although secondary histopathological changes do not appear to affect clinical outcome, these features are important in differential diagnosis. Approximately one fifth of GCT cases show recurrence and sacrum and foot bones were the most frequent sites for recurrence.
Level of evidence
Level IV, diagnostic study.
Keywords: Giant cell tumor of bone, Osteoclastoma, Bone tumors, Pathology, Bone
Introduction
Giant cell tumor of bone (GCT) is a rare neoplasm. The entity was first described by Jaffe in 1940.1 The peak incidence is in the third and the fourth decades of life.2, 3, 4 Clinically it is usually seen as a lytic lesion of the epiphyseal region of bone. It most often occurs in the distal femur and proximal tibia. Radiologically usually a well-circumscribed lytic lesion over the epiphyseal region is found. Histopathologically, these tumors are comprised of mononuclear cells, macrophages and uniformly distributed multinuclear giant cells.3, 5, 6 GCT is regarded as a predominantly osteoclastogenic stromal tumor. It has been shown that the giant cells in GCT were reactive osteoclasts.7, 8, 9 The mononuclear stromal cells were claimed to be the neoplastic and proliferative component of GCT's and it has been reported that these neoplastic stromal cells had been capable of inducing osteoclast-like differentiation.6, 10, 11 Mononuclear monocytes were thought to be the osteoclast precursor cells.12, 13 Mononuclear stromal cells may show rare mitotic figures, however atypical mitosis is absent.9 Mitotic figures are not seen in the multinucleated giant cells.3, 6 Marked cytologic atypia is not present in mononuclear stromal cells.3, 9
They frequently display secondary changes complicating characteristic histopathological appearance. We evaluated our GCT cases diagnosed within 20 years period in a single institute retrospectively and discussed the histopathological findings.
Materials and methods
Archival material of the cases diagnosed as "Giant cell tumor of bone" between the years 1996–2016 were retrieved and included in this retrospective analysis. Cases were evaluated according to radiological features, clinical features such as age, gender, localization, recurrence, metastasis and histopathological features including accompanying fibrohistiocytic or aneurysmal bone cyst like components.
Results
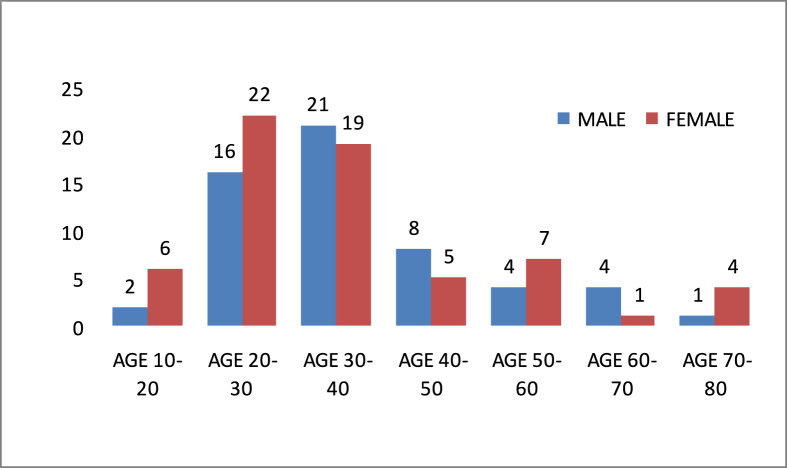
The case series included 120 patients, 64 female (53,3%) and 56 male (46,7%). Age range was between 12 and 80 (Table 1) with a mean age of 36.2 years. Tumors were localized most frequently at tibia (all 28 cases at the proximal part) and femur (21 of 25 cases at the distal part). In Table 2, localizations of the tumors are shown.
Table 1.
Age distribution according to gender.
Table 2.
Localization.
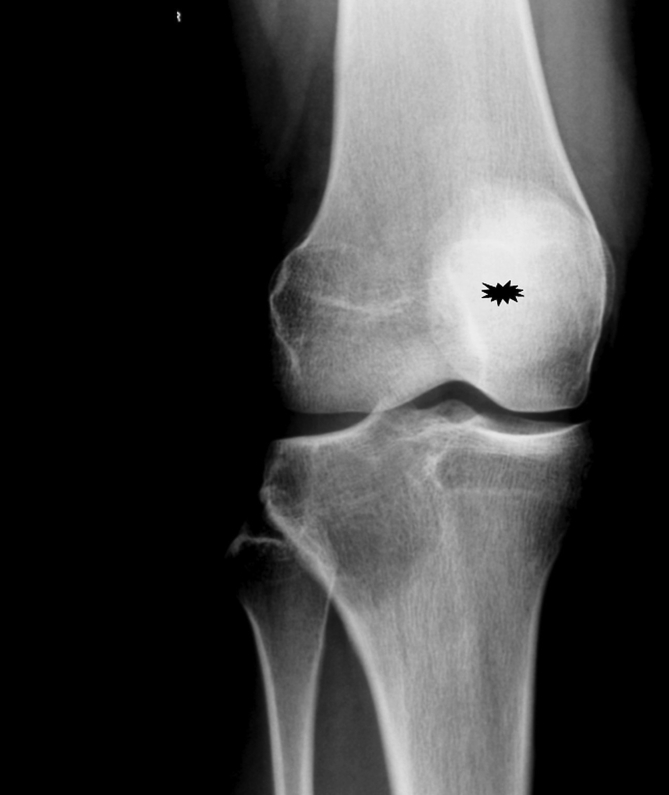
Radiological findings of 62 patients were available. Radiological consultation was done for these cases. A characteristic plain roentgenogram of GCT located at proximal metaphysis of tibia was shown in Fig. 1.
Fig. 1.
Plain roentgenogram of a well defined lucent lesion of proximal tibia.
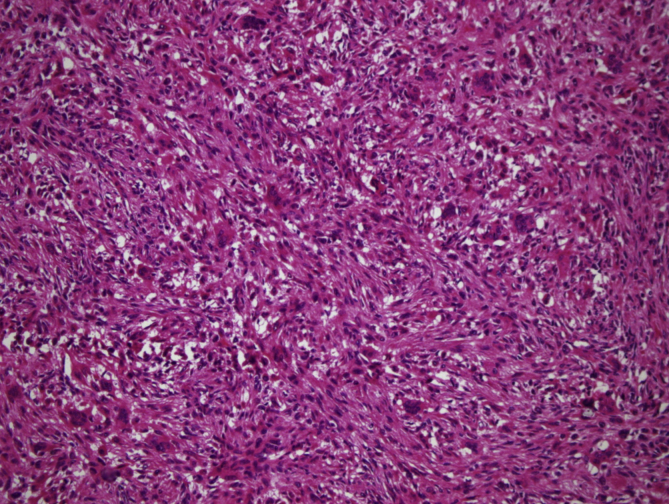
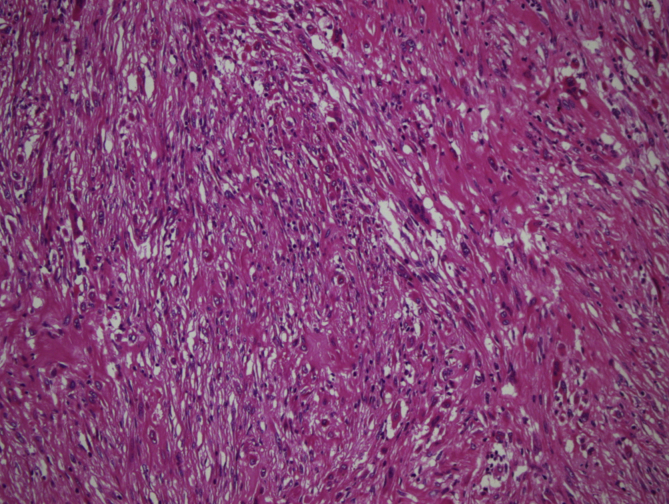
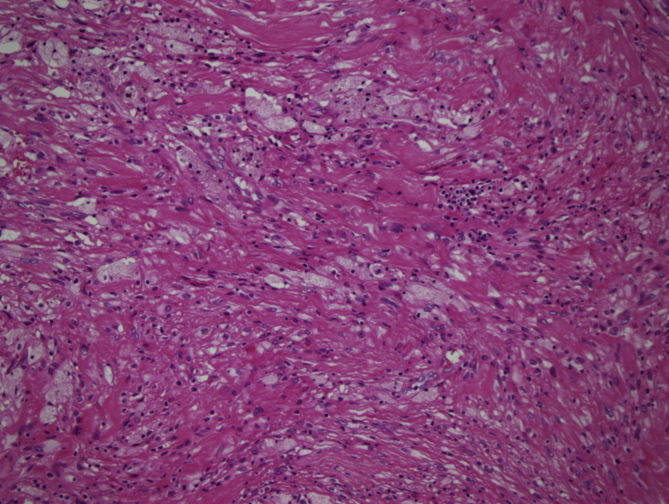
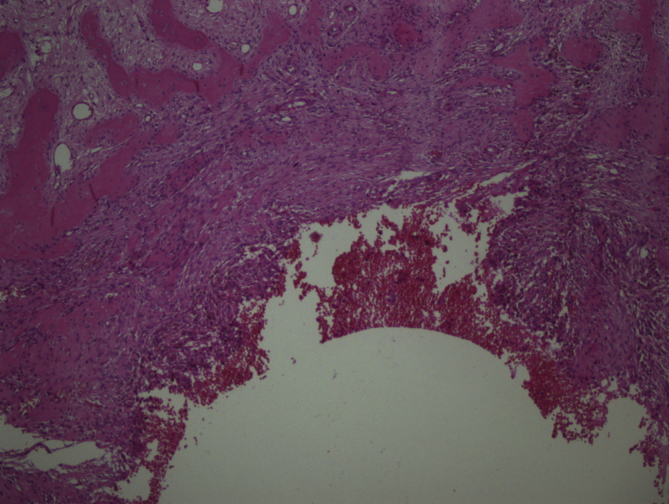
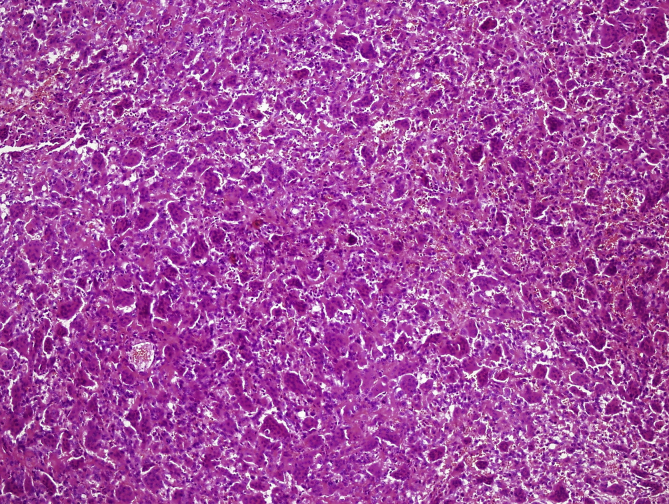
In 11 cases, areas rich in fibrohistiocytic component were detected (9,2% of the cases) (Fig. 2, Fig. 3). In six of these tumors, this component was essentially fibroxanthomatous (Fig. 4). In 20 cases secondary aneurysmal bone cyst like component were observed (16,7% of the cases) (Fig. 5). In 2 cases both components were present. In all these cases, with additional sampling (2 samples for every 1 cm of the maximum diameter of the tumor, instead of 1 sample per 1 cm), characteristic areas consisting of mononuclear stromal cells and a second population of mononuclear monocytes and multinucleated giant cells, representing GCT were detected2 (Fig. 6, Fig. 7).
Fig. 2.
Areas rich in fibrohistiocytic component (H-E, x100).
Fig. 3.
Areas rich in fibrohistiocytic component (H-E, x100).
Fig. 4.
Fibroxanthomatous areas (H-E, x100).
Fig. 5.
Secondary aneurysmal bone cyst like areas (H-E, x40).
Fig. 6.
Mononuclear cells and multinucleated giant cells (H-E, x100).
Fig. 7.
Mononuclear cells and multinucleated giant cells (H-E, x200).
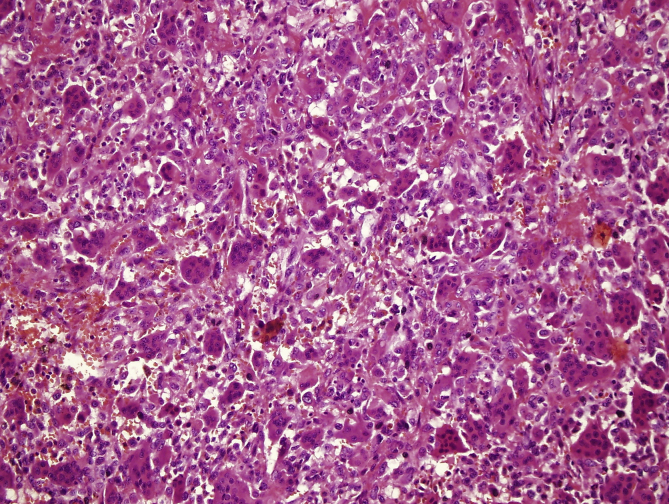
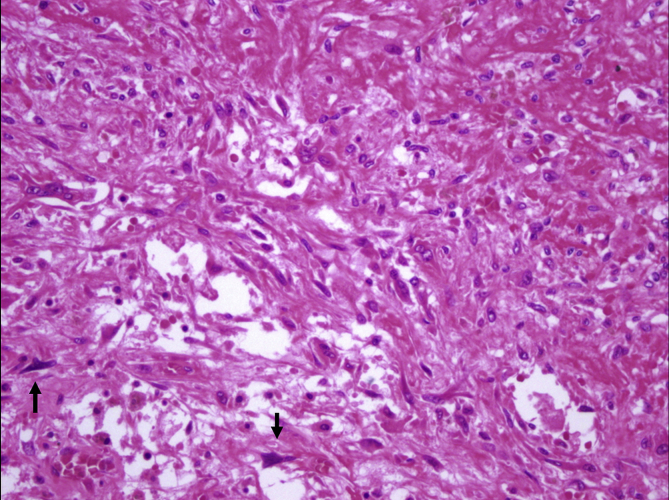
Coagulation necrosis were observed in 6 of the 23 recurrent cases (26%) and 10 of the remaining 97 cases (10,3%). One of these cases, displaying extensive necrosis, had been diagnosed as giant cell rich osteosarcoma in another pathology institute. Tumor had brisk mitotic activity and slight cytologic atypia adjacent to necrotic areas (Fig. 8), however atypical mitosis and malignant osteoid formation could not be detected.
Fig. 8.
Slight cytologic atypia adjacent to necrotic areas (arrows) (H-E, x200).
Twenty three cases recurred (19,2% of the cases). Recurrence interval for 17 cases was known. Fourteen of these 17 cases showed recurrence within 3 years and 2 cases after 4 years. In one of our cases, the tumor, which was located at proximal tibia, showed recurrence 16 years later. Six cases showed multiple recurrences (2 or 3 occurrence). Sacrum was the most frequent site showing recurrence. Five of the 23 recurrent cases were located at sacrum (21,7%).
Metastasis to lung, 4 years after the primary diagnosis was detected in one case. Tumor was primarily located at calcaneus. During follow-up, tumor showed recurrence 3 times in 8 year-period following metastasis. Three months later following excision of the multiple metastases, new pulmonary metastatic nodules were detected and they were excised also.
The treatment modalities of 20 of 23 recurrent cases and of 48 of the nonrecurrent 97 cases were known. In recurrent cases, curettage and cementing for 14 patients, resection for 4 patients, firstly curettage and then resection in recurrence for 2 patients were performed. For 1 patient radiotherapy, for 4 patients RANKL inhibitor (Denosumab) and for 1 patient chemotherapy were used. In 1 patient endoprosthetic replacement were used for her recurrent tumor and since this replacement tumor did not recur. Twenty eight cases of nonrecurrent 48 cases were treated by curettage and cementing, 18 cases by resection, 2 cases by firstly curettage and then resection in recurrence. For 1 patient radiotherapy and for 2 patients RANKL inhibitor (Denosumab) were used.
Discussion
GCT is a locally aggressive neoplasm with an unpredictable course. Although it has been reported that the occurrence of GCT in patients younger than 20 years and older than 55 years was unusual,3 in our case series 8 patients were younger than 20 (6,7% of the cases) and 13 patients were older than 55 (10,8% of the cases) (These incidences are consistent with the literature).14, 15
Nonepiphyseal GCT has been reported to be extremely rare.3 In our series we have observed 1 case at metaphysis.
The most common involved site was reported to be the distal end of the femur and the second was the proximal end of the tibia.2, 3, 4, 5 In our case series, proximal tibia was the most frequent site for the tumor and the second most common site was distal femur.
It has been claimed that approximately 3–4% of GCT cases had been localized at small bones of hands and feet. In our series in 3 patients tumor was located at feet bones and 2 cases in hand (Total 5/120; 4,2%). GCT of small bones may be confused with giant cell reparative granuloma. Histopathologically, in giant cell reparative granuloma, mononuclear stromal cells are absent. It has been suggested that GCT of these bones had shown higher recurrence rate than the one in the long tubular bones.2 In 3 of our cases located at foot bones showed recurrence supporting this observation. A tumor of calcaneus bone, in addition to recurrences, displayed pulmonary metastases also.
The well defined histopathologic pattern of GCT is frequently lost by secondary reactive changes such as fibrohistiocytic proliferation, hemorrhage, necrosis and aneurysmal bone cyst formation. Fibrohistiocytic reaction sometimes become prominent and therefore in such cases differential diagnosis between nonossifying fibroma (NOF) and benign fibrous histiocytoma should be made. NOF occurs in metaphysis. Stroma is more fibroblastic in NOF than that of GCT, sometimes forming storiform pattern, and giant cells are more irregularly scattered in NOF. It has been observed that at the end stage of reparative processes following hemorrhage and necrosis; lipoidization, scarring and fibrohistiocytic proliferation had been formed.1, 16 Benign fibrous histiocytomas do not contain broad sheets of mononuclear cells.17 In our case series we observed areas rich in fibrohistiocytic component in 11 cases. In six of these tumors, fibroxanthomatous pattern was dominant. In all cases characteristic areas representing GCT were found. We could not detect any adverse effect of these histopathological features on clinical outcome.
Aneurysmal bone cyst-like areas are frequently detected in GCT. Especially solid areas in aneurismal bone cyst may be misdiagnosed as GCT. In ABC, giant cells are smaller and giant cells are unevenly distributed. In solid type of ABC, stroma is more fibrotic than that of GCT.17 With additional sampling in all cases, areas showing typical GCT were detected except for 1 case. In this case, tumor presented with typical solid type ABC areas and the definitive diagnosis could not be made, GCT could not be excluded. Five months later tumor recurred and this time it displayed typical GCT areas with secondary ABC-like areas.
Necrosis with or without hemorrhage can be observed occasionally in conventional GCT. Adjacent to the necrotic areas, mononuclear stromal cells may show cytologic atypia focally, mimicking malignancy.2 However no atypical mitosis is present, supporting the benign nature of these changes. In our case series, although 16 cases showed necrosis, in no case marked cytologic atypia or atypical mitosis were present. Giant-cell rich osteosarcoma is a tumor that should be differentiated from GCT. In giant-cell rich osteosarcoma, nuclear pleomorphism, abnormal mitotic figures and malignant osteoid formation is characteristic. For only one case – 28 year-old male patient – which was located at metaphysis of fibula, evaluated in our institute within this 20 year-period, showing these histopathological findings, a diagnosis of ‘osteosarcoma rich in giant cells’ was given. This tumor showed soft tissue invasion also. A case which had been sent to our institute for consultation with a diagnosis of giant cell rich osteosarcoma, was revised and the diagnosis was changed as GCT, because no atypical mitosis or malignant osteoid formation had been detected.
Malignant GCT is a rarely observed entity. Related with differential diagnosis, it was reported that in primary malignant GCT, the tumor gradually progressed into the sarcomatous area.18 Although Bertoni et al had reported that in secondary malignant GCT, no residual conventional GCT had been found, Gong et al had observed in 4 of their 11 cases.18, 19 We have not detected malignant transformation in any of our GCT cases.
Up to 50% of GCTs shows recurrence after curettage within 3 years.1,3,20 Rarely recurrence may occur after more than 3 years. In our series, one tumor showed recurrence 16 years later. Six cases showed multiple recurrences. Interestingly, sacrum was the site displaying recurrence most frequently (5 of the 23 recurrent cases – 21,7%). Wide surgical excision was reported to reduce recurrence rate.1 In our case series recurrence was seen in 19,2% of patients.
Pulmonary metastases are reported to occur in approximately 1–2% of GCT patients.3 These nodules are amenable to surgical excision and they have a relatively good prognosis, however sometimes pulmonary spread can lead to death of the patient.2 In our series we have observed only 1 patient with pulmonary metastases.
Multifocal GCT is reported to occur very rarely without preexisting Paget's disease.3 In our series we have seen this situation in one case. Primary tumor was located at distal femur. It showed recurrence at the same localization and 4 months later second recurrence was presented at distal tibia. Interestingly, at the same time with the second recurrence, a fibrohistiocytic focus was detected at talus bone. Hyperparathyroidism was not detected, therefore this fibrohistiocytic focus was thought to represent a burned out GCT.
Main treatment option in GCT is surgery (usually curettage and cementing) and complementary radiotherapy. RANKL inhibitors (denosumab) and biphosphonates are new agents generally used for unresectable or metastatic disease. In our series RANKL inhibitors (denosumab) was only used in 6 patients and 2 of these patients showed recurrence in spite of therapy.
Conclusions
-
-
Proximal tibia was the most frequent site for GCT.
-
-
Secondary changes such as fibrohistiocytic or aneurysmal bone cyst-like components and coagulation necrosis were frequently seen in conventional GCT.
-
-
For tumors having prominent fibrohistiocytic and aneurysmal bone cyst-like components, in order to detect characteristic areas representing GCT, additional sampling is essential.
-
-
Secondary histopathological changes do not appear to affect clinical outcome, however recognizing these patterns is important in differential diagnosis and correct diagnosis of GCT.
-
-
Sacrum and foot bones were the most frequent sites for recurrence.
-
-
Approximately one fifth of GCT cases show recurrence.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
Contributor Information
Nil Çomunoğlu, Email: nilustundag@yahoo.com.
Nuray Kepil, Email: nuraykepil@yahoo.com.
Sergülen Dervişoğlu, Email: sergulen.dervisoglu@gmail.com.
References
- 1.Mirra J.M. Giant cell tumors. In: Mirra J.M., Picci P., Gold R.H., editors. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Lea & Febiger; Philadelphia: 1989. pp. 942–1020. [Google Scholar]
- 2.Unni K.K., Inwards C.Y. Giant cell tumour (Osteoclastoma) In: Unni K.K., Inwards C.Y., editors. Dahlin's Bone Tumors. Wolters Kluwer, Lippincott Williams & Wilkins; Philadelphia: 2010. pp. 225–242. [Google Scholar]
- 3.Czerniak B. Giant cell lesions. In: Dorfman H.D., Czerniak B., editors. Dorfman and Czerniak's Bone Tumors. Elsevier Saunders; Philadelphia: 2016. pp. 692–759. [Google Scholar]
- 4.Niu X., Xu H., Inwards C.Y. Primary bone tumors: epidemiologic comparison of 9200 patients treated at Beijing Ji Shui Tan Hospital, Beijing, China, with 10165 patients at Mayo clinic, Rochester, Minnesota. Arch Pathol Lab Med. 2015;139:1149–1155. doi: 10.5858/arpa.2014-0432-OA. [DOI] [PubMed] [Google Scholar]
- 5.Thomas D.M., Skubitz K.M. Giant cell tumour of bone. Curr Opin Oncol. 2009;21:338–344. doi: 10.1097/CCO.0b013e32832c951d. [DOI] [PubMed] [Google Scholar]
- 6.Wülling M., Engels C., Jesse N., Werner M., Delling G., Kaiser E. The nature of giant cell tumor of bone. J Cancer Res Clin Oncol. 2001;127:467–474. doi: 10.1007/s004320100234. [DOI] [PubMed] [Google Scholar]
- 7.Athanasou N.A., Bliss E., Gatter K.C., Heryet A., Woods C.G., McGee J.O. An immunohistological study of giant-cell tumour of bone: evidence for an osteoclast origin of the giant cells. J Pathol. 1985;147:153–158. doi: 10.1002/path.1711470302. [DOI] [PubMed] [Google Scholar]
- 8.Chambers T.J., Fuller K., McSheehy P.M., Pringle J.A. The effects of calcium regulating hormones on bone resorption by isolated human osteoclastoma cells. J Pathol. 1985;145:297–305. doi: 10.1002/path.1711450403. [DOI] [PubMed] [Google Scholar]
- 9.Zheng M.H., Robbins P., Xu J., Huang L., Wood D.J., Papadimitriou J.M. The histogenesis of giant cell tumour of bone: a model of interaction between neoplastic cells and osteoclasts. Histol Histopathol. 2001;16:297–307. doi: 10.14670/HH-16.297. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y., Nizami S., Goto H., Lee F.Y. Modern interpretation of giant cell tumor of bone: predominantly osteoclastogenic stromal tumor. Clin Orthop Surg. 2012;4:107–116. doi: 10.4055/cios.2012.4.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wülling M., Delling G., Kaiser E. The origin of the neoplastic stromal cell in giant cell tumor of bone. Hum Pathol. 2003;34:983–993. doi: 10.1053/s0046-8177(03)00413-1. [DOI] [PubMed] [Google Scholar]
- 12.Goldring S.R., Roelke M.S., Petrison K.K., Bhan A.K. Human giant cell tumors of bone identification and characterization of cell types. J Clin Invest. 1987;79:483–491. doi: 10.1172/JCI112838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brecher M.E., Franklin W.A., Simon M.A. Immunohistochemical study of mononuclear phagocyte antigens in giant cell tumor of bone. Am J Pathol. 1986;125:252–257. [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Ibraheemi A., Inwards C.Y., Zreik R.T. Histologic spectrum of giant cell tumor (GCT) of bone in patients 18 Years of age and below: a study of 63 patients. Am J Surg Pathol. 2016;40:1702–1712. doi: 10.1097/PAS.0000000000000715. [DOI] [PubMed] [Google Scholar]
- 15.Broehm C.J., Inwards C.Y., Al-Ibraheemi A. Giant cell tumor of bone in patients 55 Years and older: a study of 34 patients. Am J Clin Pathol. 2018;149:222–233. doi: 10.1093/ajcp/aqx155. [DOI] [PubMed] [Google Scholar]
- 16.Errani C., Ruggieri P., Asenzio M.A. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36:1–7. doi: 10.1016/j.ctrv.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Greenspan A., Jundt G., Remagen W. Miscellaneous tumors and tumor-like lesions. In: Greenspan A., Jundt G., Remagen W., editors. Differential Diagnosis in Orthopedic Pathology. Wolters Kluwer, Lippincott Williams & Wilkins; Philadelphia: 2006. pp. 387–400. [Google Scholar]
- 18.Gong L., Liu W., Sun X. Histological and clinical characteristics of malignant giant cell tumor of bone. Virchows Arch. 2012;460:327–334. doi: 10.1007/s00428-012-1198-y. [DOI] [PubMed] [Google Scholar]
- 19.Bertoni F., Bacchini P., Staals E.L. Malignancy in giant cell tumor of bone. Cancer. 2003;97:2520–2529. doi: 10.1002/cncr.11359. [DOI] [PubMed] [Google Scholar]
- 20.Campanacci M. Giant cell tumor. In: Campanacci M., editor. Bone and Soft Tissue Tumors. 2nd ed. Piccin Nuova Libraria S.P.A.; Padova-Italy: 1999. pp. 117–151. [Google Scholar]