Abstract
Mucosal associated invariant T (MAIT) have properties of both the innate and adaptive immune systems but are an understudied population within exercise immunology. These lymphocytes aggregate at the mucous membranes, but it is unknown if submaximal exercise alters their circulating numbers or function.
PURPOSE:
To determine the MAIT cell response to submaximal exercise on activation and homing marker expression and stimulated cytokine production.
METHODS:
Twenty healthy, young, recreationally active males cycled for 40 min at 86% of VT following an overnight fast. Peripheral blood mononuclear cells were isolated and labelled to identify specific MAIT cell populations using flow cytometry. Cytokine production following stimulation was also determined.
RESULTS:
MAIT cells were 2.9% of T-cells and increased to 3.9% after exercise and with recovery whereas cell numbers significantly increased by 91.5% following exercise before returning to resting levels. Chemokine and activation marker absolute cell number significantly increased while expression levels remained constant but the high levels of CCR5 may help direct MAIT cells to sites of inflammation. Following stimulation, TNFα expression significantly increased after exercise before returning to baseline with a similar trend for IFNγ.
CONCLUSIONS:
MAIT cell numbers undergo a partial biphasic response following submaximal exercise and appear to be preferentially mobilized within T-cells; however, the magnitude of the submaximal response was attenuated relative to maximal exercise. Stimulated MAIT cells increase TNFα expression, indicating greater responsiveness to pathogens following acute exercise.
Keywords: TCR Vα7.2, exercise immunology, MAIT cells, cytokines
INTRODUCTION
Mucosal associated invariant T (MAIT) cells, identified by the semi-invariant Vα7.2-Jα33/12/20 T-cell receptor (TCR) and high expression levels of CD161 (1, 2), display both innate and acquired immune systems characteristics. These MR1-expressing hematopoietic cells are selected in the thymus, triggering the development of the MAIT cell TCR (2) and recognition of antigens presented on the major histocompatibility complex (MHC) class I-related (MR1) molecule (3). After thymic egress, MAIT cells express homing markers (e.g. CCR4, CCR5, and CCR6) that direct them to mucosal tissues, including the liver, gut, spleen and lungs (4) and inflammation sites (5).
MAIT cells interact with commensal flora and B cells, initiating TCR adaptations, proliferation, and accumulation in the mucosal tissue (2, 6, 7). MAIT cells respond primarily to bacteria (4, 8) via riboflavin biosynthetic pathway intermediates bound to MR1 on antigen presenting cells (9). MAIT TCR recognition of MR1 with accompanying riboflavin ligand stimulates the upregulation of early activation marker CD69 and the release of IL-2, IL-17, IFNγ, and TNFα (1, 4). Thus, MAIT cells have the acquired characteristic of interacting with their environment to cause adaptations, yet also contribute to the gut innate immune response (10).
MAIT cell counts and function are adversely affected in several pathological conditions. Compared to healthy controls, individuals with irritable bowel disease have a reduced circulating MAIT cell frequency with elevations within injured ileum tissue and higher IL-17 secretion, (11), though this finding has been recently debated (12). Similarly, obese and diabetic individuals possess lower MAIT cell numbers in the blood with a subsequent increase in the adipose tissue and higher IL-17 production (13, 14). Severe asthma reduces MAIT cell counts in both the blood and lung tissue (15), with the magnitude of MAIT cell loss being correlated with clinical severity and indicating a potential relationship between MAIT cells and respiratory health as well. Viral infections (e.g. HIV) also impact MAIT cell counts yet these cells remain functional and remain capable of producing cytokines (16).
Acute exercise has been shown to rapidly mobilize the immune system and potentially alter cytokine production (17–21) in an intensity-dependent manner (22, 23). MAIT cells are understudied within exercise immunology, with only a single study demonstrating that maximal aerobic exercise increases circulating MAIT cell number and frequency (24). However, maximal exercise is not always practical or possible in clinical populations and the recovery response of these cells has yet to be determined. Acute exercise has been shown to alter tissue homing expression, IFNγ and TNFα secretion, and the number of cells expressing pro- and anti-inflammatory markers (22, 25–27), but none of these have not been examined in MAIT cells. Higher levels of cytokine production following stimulation and tissue homing expression suggest these cells are more responsive to pathogens and will better egress into the respective tissues, indicating greater cell function. An understanding of MAIT cell function during moderate intensity exercise in healthy individuals may permit expansion into clinical populations as a potential means of reducing symptoms through changes in MAIT cell function.
The purpose of this study is to determine the MAIT cell response to 40 minutes of aerobic exercise performed at 90–98% of ventilatory threshold (VT) and to examine the effects on early activation (CD69) and tissue homing marker (CCR4, CCR5, CCR6) expression. Additionally, cryopreserved MAIT cells were stimulated and the pro-inflammatory cytokine (TNFα, IFNγ, and IL-17) response was investigated. We hypothesized that acute exercise would induce a biphasic response for MAIT cell counts and frequencies, with increases immediately after exercise followed by a decline below baseline during recovery. It was expected that CD69 and CCR4, CCR5, and CCR6 expression would follow a response similar to the counts and frequencies. Finally, stimulated MAIT cells would have higher cytokine production immediately after exercise and cytokine production would follow the levels of activation marker expression.
METHODOLOGY
Participants
Twenty males, ages 18–35 completed the study. All participants were non-smokers who were recreationally active, having participated in vigorous activity as outlined by the American College of Sports Medicine for 30 minutes at least three times per week. Exclusion criteria included: any contraindications for exercise testing, a body mass index > 30, and therapeutic corticosteroid medication. Written informed consent was obtained from participants prior to any testing and all procedures were approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
Study Design
Three visits were required for this study. The first visit was a screening and familiarization session for the graded exercise test (GXT), the second visit entailed a GXT on the cycle ergometer to determine VT, and the final visit was a 40-minute moderate intensity exercise trial completed at 90–98% of VT with immune cells being isolated and analyzed. All participants were asked to adhere to the following pre-assessment guidelines: 1) no exercise in the previous 12 hours; 2) no alcohol consumption in the previous 48 hours; 3) no diuretic medication 7 days prior or 4) therapeutic corticosteroid medication 4 weeks prior to testing. These guidelines were verbally confirmed at each visit.
Visit 1: Body Composition and Familiarization Session
At the onset of visit one, participants completed a medical history questionnaire, physical activity readiness questionnaire (PAR-Q) and informed consent was obtained. Body composition was assessed using dual energy X-ray absorptiometry (Hologic Inc., Bedford, MA, USA; Apex Software Version 3.3), as performed previously (24). A resting 12-lead electrocardiogram (GE CASE Cardiosoft V. 6.6 ECG diagnostic system; General Electric, Palatine, IL, USA) was obtained and sent to the study physician, along with all screening questionnaires, for clearance to perform maximal exercise.
Participants then completed a familiarization session on an electro-magnetic breaking cycle ergometer (Lode, Groningen, Netherlands). Cycling was selected as the mode of exercise to allow for comparisons with previous work investigating MAIT cells (24). Ergometer seat height was adjusted to ensure proper cycling mechanics and participants were fitted with a heart rate monitor (Polar FT1, Polar USA, Port Washington, NY, USA) and a respiratory mask. Participants sat quietly on the cycle ergometer for 3 minutes, completed a 2 minute warm up with no resistance, and then performed 2 minute stages with increases of 50 watts (W) per stage. After 250 W, stages were reduced to 1 minute and resistance increased by 30 W. The GXT familiarization ended when 70% heart rate reserve was reached as determined by the Karvonen formula.
Visit 2: Graded Exercise Test (GXT)
Within 1 week of the familiarization session, participants returned to complete the GXT. Baseline heart rate and blood pressure were obtained after 5 minutes of seated rest. Respiratory gasses were collected continuously with 5 second averages (TrueOne 2400, Parvo Medics, Sandy, UT, USA) and heart rate was monitored every 30 seconds. Rating of perceived exertion were recorded 30 second before the end of each stage. Gas analyzers were calibrated prior to each test using known gas concentrations (21.0% O2 and 0.03% CO2, 16.0% O2 and 4.0% CO2).
The GXT protocol was identical to familiarization protocol except that termination of the test was volitional fatigue. At the conclusion, participants moved to a seated recovery position and lactate levels were determined after 3 minutes (Lactate Plus Analyzer, Nova Biomedical, Waltham, MA, USA). Participants returned to the cycle ergometer to complete a brief cool down with minimal resistance (~ 25 W). Maximal oxygen uptake (VO2max) was determined using standard criteria (28). The VT was determined by plotting the O2 ventilatory equivalent (VE/VO2) and CO2 ventilatory equivalent (VE/VCO2), as described previously (29).
Visit 3: Sub-maximal Exercise Trial
Approximately 3 to 7 days after the GXT and between the hours of 0600 and 1000, participants returned to the laboratory in a fasted state and having followed all other pre-assessment guidelines. Following 10 minutes of supine rest, a venous catheter was inserted for repeat blood sampling. Venous blood (24 mL) was drawn into EDTA collection tubes, inverted several times, and placed on ice. The catheter was flushed with sterile saline. Participants were again fitted with a heart rate monitor and a respiratory mask. The protocol used was modified from Bishop et al. (17) and included a warm up of 2 minutes at 25 W and 2 minutes at 50 W. If the trial workload exceeded 150 Watts, an additional 30 seconds at 100 W was included. Resistance was then increased to elicit 90% of VT, based on results from the GXT. Participants cycled at this resistance until oxygen uptakes corresponding to 90% of VT were observed twice. Participants then maintained this resistance for 10 minutes while oxygen uptake and pulmonary ventilation were monitored, attempting to achieve 90–98% VT. Respiratory gases were collected from the start of exercise to 10 minutes, from 20–25 minutes and 35–40 minutes and resistance was adjusted accordingly to keep participants within 5% of the desired VT, ensuring that all subjects would complete the trial at a submaximal effort. Participants were allowed ad libitum access to water while not wearing the respiratory mask during the trial. Immediately following the trial, an additional blood sample was obtained (0 h) and participants then completed 60 minutes of seated recovery before the final blood sample was obtained (1 h).
Hematology Analysis
Complete blood counts from whole blood were obtained in duplicate from each time point (Beckman Coulter AcT Diff, Brea, CA, USA) with a maximal white blood cell difference of 0.1 cells/μL and the values were averaged. Plasma volume shifts with exercise were calculated as described previously (30).
Peripheral Blood Mononuclear Cells (PBMC) Isolation and Immunofluorescence Labeling
Peripheral bound mononuclear cell (PBMC) isolation and cell labelling were completed as performed previously (24). Briefly, whole blood was diluted in PBS and isolated using SepMate™-50 (Stemcell, Vancouver, BC Canada) as specified by the manufacturer. PBMCs were washed, counted via hemocytometer, and 2×10^6 cells were aliquoted for immunofluorescence labeling. Remaining PBMCs were aliquoted into a minimum of 1×10^6 cells and were cryopreserved in 90% fetal bovine serum (FBS; VWR, Atlanta, GA, USA) and 10% DMSO (VWR, Atlanta, GA, USA) for future analysis.
MAIT cell phenotyping was determined using direct immunofluorescence labeling of cell surface markers with mouse anti-human monoclonal antibodies [CD3 (APC-Cy7); CD4 (BV510); CD8 (AF700); CD45 (PerCP-Cy5.5); CD69 (AF488); CD161 (BV605); TCR Vα7.2 (PE); CCR5 (BV421); CCR6 (BV650); CCR4 (PE-Cy7), Biolegend, San Diego, CA, USA)] in 100 μL of cell staining buffer (Biolegend, San Diego, CA, USA) for 15 minutes at 4°C in the dark. Cells were washed to remove excess antibody prior to being suspended in 200 μL of cell staining buffer for flow cytometry analysis. Titrations were performed to determine optimal antibody concentrations.
Stimulation and Intracellular Staining
Cryopreserved PBMCs were available for a subset of all participants (n=17). PBMCs were thawed, washed with complete media (90% RPMI, 10% FBS, 1.0% penicillin-streptomysin; (Sigma Aldrich, St. Louis, MO, USA)), and rested overnight at 37°C and 5% CO2. The next morning, cell viability (trypan blue) was determined using an automated cell counter (TC-20, BioRad, Hercules, CA, USA) with viability >90%. PBMCs were then stimulated for 4 hours with 2 ng/μL of phorbol 12-myristate 13-acetate (PMA) and 1 ug/μL ionomycin (Sigma Aldrich, St. Louis, MO, USA) at 37°C and 5% CO2. Following the stimulation, cells were washed and surface markers were labelled as described above, except that CCR4, CCR5 and CCR6 were omitted. Cells were washed in 1x phosphate buffered saline and were treated with a fixation and permeabilization kit (BD Biosciences Cytofix/Cytoperm, San Jose, CA, USA) following manufacturer instructions. Intracellular staining was performed using mouse anti-human monoclonal antibodies in 100 μL of permeabilization buffer to quantify cytokine production [IFNγ (APC); TNFα (BV650); IL-17A (BV711), Biolegend, San Diego, CA, USA)] for 30 minutes at 4°C in the dark. Cells were washed to remove excess antibody prior to being suspended in 200 μL of cell staining buffer for flow cytometry analysis.
Flow Cytometry
All cellular events were analyzed via flow cytometry on a BD Fortessa running FACSDIVA v6.1 (BD, San Jose, CA, USA) software. Our gating strategy has been described elsewhere (24), with Vα7.2 and CD161 cells being used to identified MAIT cells from the CD3+ population with gating on CD8+ and CD4+ to identify specific subpopulations. Additional markers were used to determine the activation status (CD69), homing marker expression (CCR4, CCR5, and CCR6), or intracellular cytokines of MAIT cells with exercise, along with fluorescence minus one (FMO) controls. CD14 (PE-Dazzle594; Biolegend) and CD66 (APC, Novus Biologicals, LLC, Littleton, CO, USA) were used initially to confirm the desired lymphocyte population did not consist of monocytes or neutrophils. Unlabeled, single color compensation and AbC™ Total Antibody Compensation Bead Kit (Thermo Fisher Scientific, Hampton, NH, USA) for markers with low expression (i.e. CD69, CCR4, CCR5, CCR6, IL-17, TNFα, and IFNγ) were used for every experiment in addition to fluorescence minus one (FMO) controls for PE as this channel was used to detect Vα7.2. Gating analyses were completed using FlowJo version 10.1r7 (Ashland, OR, USA). Circulating cell number was determined by multiplying the percentage of lymphocytes expressing the markers of interest with the hematology total lymphocyte count for each specific cell population.
Statistical Analyses
Descriptive statistics were used to summarize participant characteristics and data were reported as mean and standard deviation (SD). One-way, repeated measures ANOVA with a Bonferroni post-hoc analysis was used to compare MAIT cell count and frequencies changes for activation marker expression, homing marker expression, and intracellular cytokine production between baseline and 0 h and 1 h after exercise. Statistical significance was set at p<0.05. Normality and sphericity were assessed using the Shapiro Wilk Test and Mauchly’s Test of Sphericity, respectively, with p values for the main effect of time adjusted using Greenhouse-Geisser when appropriate. In situations where both sphericity and normality were violated, nonparametric Friedman and Wilcoxon Signed-Rank Tests were used alternatively. All analyses were performed using SPSS version 21 (SPSS, Inc., Chicago, IL, USA). All figures were made using GraphPad Prism version 7 (GraphPad Software, Inc., La Jolla, CA, USA).
Sample size was determined using G*Power 3.1 based on the effects of maximal exercise on MAIT cell counts. The MAIT cell count effect size from previously data was 0.94 (24), which was reduced to 0.8 due to an expected lower lymphocytosis with submaximal exercise. With an effect size of 0.8 and 80% power, a sample size of 15 was required. The sample size was inflated to 20 to account for unknown effects of submaximal exercise and to increase the likelihood of seeing differences in activation, homing, and cytokine markers.
RESULTS
Participants
Twenty young, healthy males with moderate aerobic fitness completed the study (Table 1). The 40-minute acute exercise bout was completed with an average power output of 152 (30 W), which corresponded to 63.5 (5.3 %) of VO2max and 86.0 (18.0%) of VT, which was slightly under the targeted range.
Table 1.
Participant characteristics (n=20).
| Mean (SD) | Range | |
|---|---|---|
| Age (y) | 22 (4) | 18 – 34 |
| Height (cm) | 181.1 (5.4) | 169.6 – 190.3 |
| Mass (kg) | 78.2 (9.6) | 61.2 – 92.5 |
| Fat Mass (kg) | 13.6 (4.3) | 9.2 – 23.0 |
| Lean Mass (kg) | 60.7 (8.3) | 46.5 – 74.4 |
| Body Fat (%) | 17.6 (4.8) | 11.3 – 27.8 |
| Absolute VO2max (L/min) | 4.0 (0.8) | 2.56 – 5.51 |
| Relative VO2max (mL/kg/min) | 51.3 (9.9) | 38.2 – 67.1 |
| % of VO2max during moderate intensity trial (%) | 63.5 (5.3) | 50.1 – 74.1 |
| Ventilatory Threshold (L/min) | 2.6 (0.6) | 2.0 – 3.9 |
| % VT during moderate intensity trial (%) | 86.0 (18.0) | 61.3 – 130.0 |
Percent changes in leukocyte and lymphocyte counts
All leukocyte populations significantly increased following exercise (all p<0.05). Specifically, lymphocyte counts increased by 44.0% (33.9) from baseline to 0 h (p<0.001) before decreasing to −33.7% (19.3) below baseline at 1 h (p<0.001 Table 2). Plasma volume shifts at 0 h were −15.6% (7.0) but returned to resting values by 1 h.
Table 2.
Complete blood count performed at baseline and after 0h and 1h of recovery following acute exercise.
| Baseline | 0h | 1h | |
|---|---|---|---|
| Erythrocytes (x106 cells/μL) | 5.1 (0.4) | 5.5 (0.4) α | 5.2 (0.4) |
| Leukocytes (x103 cells/μL) | 6.5 (1.6) | 8.7 (2.1) α | 7.4 (2.4) |
| Lymphocytes (x103 cells/μL) | 1.9 (0.6) | 2.7 (0.7) α | 1.3 (0.3) α |
| Monocytes (x103 cells/μL) | 0.5 (0.3) | 0.8 (0.3) α | 0.4 (0.2) |
| Granulocytes (x103 cells/μL) | 4.2 (1.5) | 5.4 (1.7) α | 5.6 (2.3) α |
| Hemoglobin (g/dL) | 14.3 (1.0) | 15.8 (1.0) α | 14.7 (1.1) |
| Hematocrit (%) | 45.1 (2.8) | 49.1 (3.0) α | 45.7 (3.3) |
| Plasma volume shifts (%) | − | −15.6 (7.0) α | −3.2 (8.7) b |
Mean (SD).
= Significantly different from baseline (p < 0.05)
= Significantly different from 0h (p < 0.05)
T Cell Proportions
The percent of CD3+ T-cells relative to their parent population decreased from baseline to 0 h (p=0.001), increased from 0 h to 1 h (p=0.028) and increased from baseline to 1 h (p=0.001; Figure 1A, left panel and SDC Figure 1, representative T cell gating image). Cytotoxic T-lymphocytes (CD3+CD8+) comprised a significantly smaller percent of CD3+ cells at 1 h compared to baseline (p = 0.020; Figure 1B) whereas the proportion of helper T cells (CD3+CD4+) was unchanged (Figure 1C).
Figure 1.
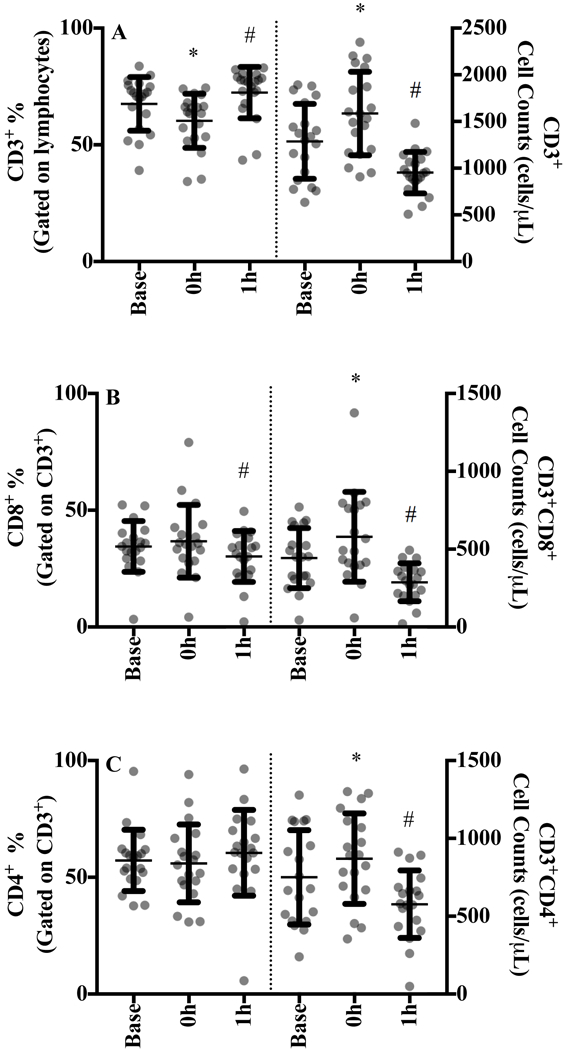
A) CD3+ T-cells, B) CD3+CD8+ T cells, and C) CD3+CD4+ T-cells at baseline, 0 h and 1 h after sub-maximal aerobic exercise for n=20. T-cells are expressed as a percentage of their parent population in the left panel and the absolute cell counts are displayed in the right panel.
* p<0.05 from all time points, # p<0.05 from baseline
T Cell Counts
Exercise induced a significant biphasic response for all conventional T cell counts. CD3+ counts increased by 27% (25.6) at 0 h (p<0.001) and decreased by 22.1% (19.5) from baseline to 1 h (p=0.001; Figure 1A, right panel). CTL and helper T-cell counts increased by 32.8% (30.8) and 21.8% (28.2) at 0 h (both p<0.001; Figure 2B and 2C) followed by a 32.4% (23.7) and 15.9% (29.2) drop below baseline values at 1 h, respectively.
Figure 2.
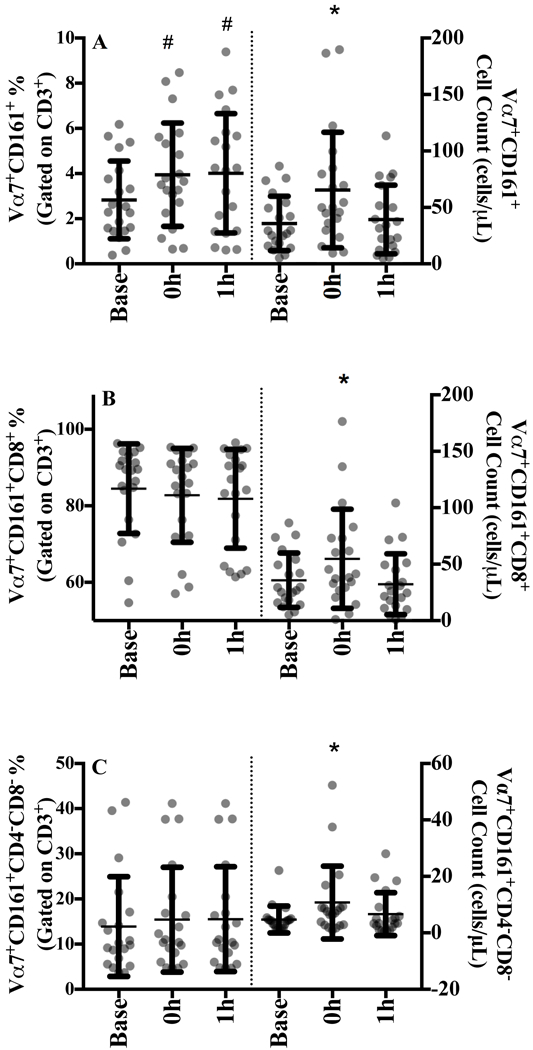
A) Vα7.2+CD161+, B) Vα7.2+CD161+CD8+, and C) Vα7.2+CD161+CD4-CD8- MAIT cells at baseline, 0 h and 1 h after sub-maximal aerobic exercise for n=20. MAIT cells are expressed as a percentage of their parent population in the left panel and the absolute cell counts are displayed in the right panel.
* p<0.05 from all time points, # p<0.05 from baseline
MAIT Cell Proportions
MAIT cells were defined as CD3+Vα7.2+CD161+. There was a significant increase in the proportion of circulating MAIT cells from baseline to 0 h (p=0.002) that was maintained at 1 h (p=0.03; Figure 2A, left panel and SDC Figure 2, representative MAIT cell gating image). MAIT cell subpopulations were predominately CD8+ (Figure 2B), accounting for ~80% of all MAIT cells at baseline with no significant change in either distribution over time (Figure 2B and 2C).
MAIT Cell Counts
Moderate intensity acute exercise significantly increased MAIT cell counts increased by 91.5% (100.1) at 0 h (p=0.003) and returned to baseline during recovery (Figure 2A, right panel). Similarly, CD8+ MAIT cell counts increased by 84.1% (70.6) at 0 h (p=0.002; Figure 2B) and CD4-CD8- counts increased by 186% (475) before returning to baseline values at 1 h (p=0.036; Figure 2C).
Chemokine Receptor and Activation Marker Proportions
At baseline, MAIT cells expression was 37.3% (25.8) for chemokine receptor CCR4, 81.3% (31.2) for CCR5, and 31.6% (31.2) for CCR6 and none of these proportions changed with exercise (Figure 3A, 3B and 3C, left panels and SDC Figure 3, representative chemokine gating image). Because of the high percentage of MAIT cells expressing CCR5 (potential ceiling effect), mean fluorescence intensities were determined but were unaltered with acute exercise (data not shown). MAIT cell expression of CD69 at baseline was low, 7.3% (7.4) and remained constant over time (Figure 3D and SDC Figure 3, representative activation marker gating image).
Figure 3.
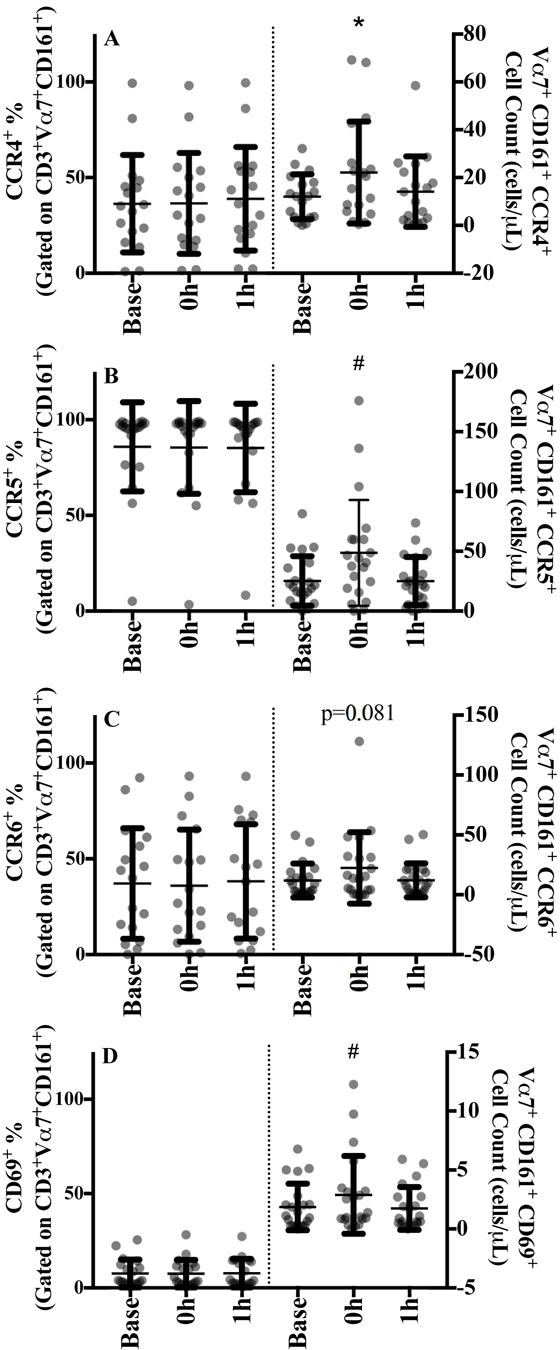
MAIT cells expressing the cell surface markers A) CCR4, B) CCR5, C) CCR6, and D) CD69 at baseline, 0 h and 1 h after sub-maximal aerobic exercise for n=20. The proportion of MAIT cell expressing each specific chemokine or activation marker are displayed in the left panel and the absolute cell counts are displayed in the right panel.
# p<0.05 from baseline
Chemokine Receptor and Activation Marker Cell Counts
Absolute CCR4+ and CCR5+ MAIT cell counts significantly increased by 104% (139) and 86.8% (100.7) at 0 h, respectively (both p<0.001; Figure 3A and B, right panel) before returning to baseline at 1 h. CCR6+ MAIT cells showed a trend towards increasing at 0 h but did not reach statistical significance (p=0.081; Figure 3C). Significant increases in CD69+ MAIT cell counts were observed at 0 h [89.7% (117.9), p=0.031; Figure 4D] that returned to baseline values during recovery.
Figure 4.
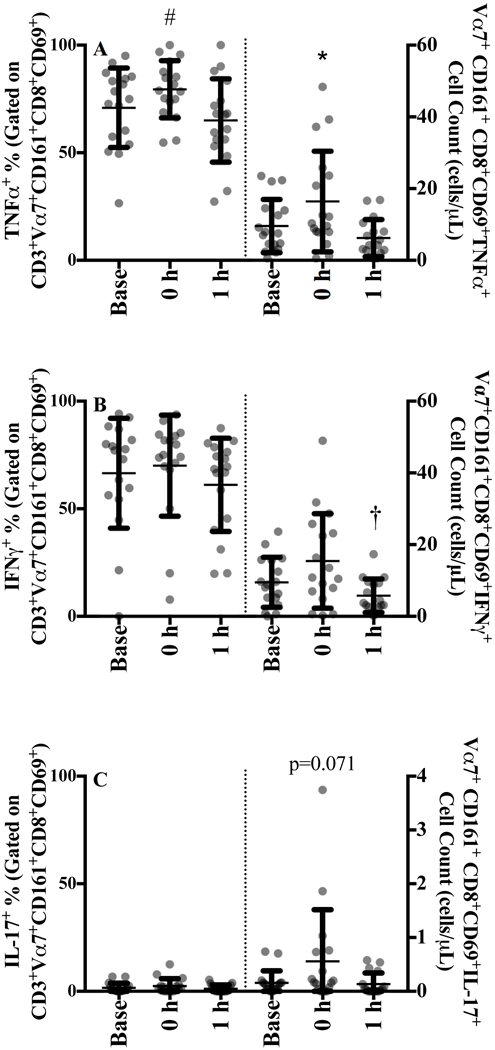
MAIT cells expressing intracellular A) TNFα, B) INFɣ, and C) IL-17 following 4 hours of stimulation with 2 ng/μL of phorbol 12-myristate 13-acetate and 1 ug/μL ionomycin at baseline, 0 h and 1 h after sub-maximal aerobic exercise for n=17. The proportion of MAIT cell expressing each cytokine are displayed in the left panel and the absolute cell counts are displayed in the right panel.
* p<0.05 from all time points, # p<0.05 from baseline, † p<0.05 from 0 h
Stimulated Intracellular Cytokine Proportions
MAIT cells expression of CD69 following the 4 h of stimulation was 76.0 (13.4%) and remained constant with exercise (data not shown). The proportion of CD8+CD69+ MAIT cells expressing TNFα significantly increased from 71% to 79% (p=0.017) with exercise before returning to baseline (65%) at 1 h (Figure 4A, left panel and SDC Figure 4, intracellular cytokine representative gating image). MAIT cell IFNγ expression tended to increase at 0 h but did not reach statistical significant (p=0.126, Figure 4B), whereas IL-17 was unchanged (Figure 4C).
Stimulated Intracellular Cytokine Cell Counts
The absolute number of MAIT cells expressing TNFα significantly increased by 89.5% (137.3) at 0 h (p=0.018, Figure 4A, right panel). IFNγ counts did not significantly increase at 0 h [64.5% (127.5), p=0.119; Figure 4B]. However, there was a 62.1% decrease from 0 h to 1 h (p=0.15) and a tendency for counts at 1 h to be significantly lower than baseline (p=0.061). Despite the greatest percent change in cell number immediately following exercise [275.4% (370.5), Figure 4C], IL-17 counts showed only a trend (p=0.071) at 0 h but these cells were rare.
DISCUSSION
The purpose of this study was to examine changes in Vα7.2+CD161+ MAIT cell frequency and absolute number for tissue homing (CCR4, CCR5, CCR6) and activation markers (CD69) expression along with stimulated pro-inflammatory cytokine production following 40 minutes of moderate intensity aerobic exercise. Significant increases in MAIT cell frequency and number support and extend our previous work (24) by demonstrating a preferential mobilization of MAIT cells within total T-cells, indicating these cells are rapidly mobilized within the exercise-induced lymphocytosis but may egress more slowly in recovery. This finding is consistent with the constant chemokine expression levels that were observed. Exercise transiently increased the percentage and total number of MAIT cells expressing TNFα, which may aid in activation and recruitment of additional immune cells, and indicates greater responsiveness to pathogens. The absolute counts for the majority of MAIT cell population increased immediately after exercise before returning to baseline with 1 h of recovery. Overall, increased pro-inflammatory cytokine production and cell numbers are part of the enhanced immune response seen immediately following exercise and suggests that MAIT cells play an important role within this response.
Elevated MAIT cell counts after exercise were expected, as both the proportion of T-cells and total lymphocyte numbers increased at 0 h. The 72% increase in cell counts immediately following submaximal exercise was smaller than the 116% increase observed with maximal exercise (24). This difference is attributed exercise intensity, as maximal efforts induce lymphocytosis that is several fold greater (18, 21, 24) than the current study but the changes reported in the current study are consistent with other submaximal trials (17, 19). These MAIT cell changes at different exercise intensities support literature where exercise intensity has been shown to determine the extent of lymphocyte response (22, 23). After 1 h of recovery, MAIT cell counts were similar to baseline and do not follow the traditional biphasic response (decline below baseline), which failed to support our hypothesis and contrasts findings from our classical T-cell populations and others (17, 21).
MAIT cells comprised 2.8% of all T-cells at rest, which is consistent with prior work (11, 13, 24). The increases to 3.9% following moderate intensity exercise is also similar to changes with maximal exercise (24). Interestingly, we show for the first time that MAIT cell proportions remain elevated (4% of T-cells) during recovery and this increase likely offsets the decline in lymphocyte numbers such that absolute counts return to baseline levels, rather than declining below it. Several possibilities exist for the higher MAIT cell proportion in circulation at 1 h. It is well established that during moderate intensity exercise, activation of the sympathetic nervous system occurs and blood flow to the small intestine and kidney are reduced to facilitate oxygen delivery to active tissue (31, 32). With less blood being directed to their resident tissues, MAIT cells would remain in the blood longer and may account for the increased percentage during recovery from exercise. An additional hypothesis for the kinetic difference between MAIT cells and other CD3+ subpopulations may be due to the relative expression of adhesion proteins on MAIT cells. The high responsiveness of natural killer (NK) cells to exercise has been reported to potentially be due to modulation of adhesion molecules such as CD44 by exercise-induced catecholamine release (33). Both murine MAIT cells and human MAIT cells have been shown to express CD44 (2, 34). Alternately, IL-18 stimulated MAIT cells upregulate very late antigen-4, an integrin that mediates T-cell migration through its interaction with vascular cell adhesion molecule-1 (35). Therefore, CD44+ MAIT cell and other adhesion marker and the response to acute aerobic exercise are potential targets for future research to examine the mechanism behind elevated MAIT cell proportions during recovery.
Growing evidence indicates MAIT cell numbers and frequency increase with moderate and maximal exercise, but functional changes have not previously been examined. MAIT cell migration to the mucosal tissues does not appear to be influenced by exercise, as no changes in the percentage of MAIT cells expressing chemokine receptors (CCR4, CCR5, and CCR6) or antigen density were found. This was contrary to our hypothesis but supports recent work indicating that NK cell CCR5 expression remains constant immediately following acute resistance exercise and decreases only after 24h (25). Expression levels for CCR5+ in the current study was high (81.3%) and was similar to previous work (36, 37). A recent review suggests that CCR5 expression is critical to directing cells to sites of inflammation (5). In healthy individuals, MAIT cell chemokine receptor expression may be optimal and it is only during co-infections with HIV or tuberculosis that decreased expression levels are observed (37). Acute exercise may be able to alter chemokine expression with MAIT cell depletion, but this remains to be tested in future studies. Alternatively, moderate intensity exercise may be insufficient to alter chemokine expression or 1 h after exercise is possibly too soon to observe alterations in these markers.
As part of the MAIT cell response to exercise, early activation markers (CD69) were assessed as T-cells usually need to be activated in order to mediate their functions. CD69+ MAIT cells produce an array of cytokines including IL-2, IL-17, IFNγ, and TNFα, among others (1, 4, 38), which allow MAIT cells to combat different types of pathogens. CD69 is upregulated on MAIT cells in vivo in certain disease states but also when cultured with bacteria or PMA and ionomycin (2, 4, 38, 39). While CD69+ MAIT cell numbers increased after exercise in the current study, there was no change in the percentage of MAIT cells expressing CD69 or the total amount of CD69 being expressed, consistent with previous work in T- and NK cells, respectively (19, 20). As all of these studies used recovery windows of 1 h or less, changes in CD69 expression with exercise alone may occur outside of this timeframe, similar to the lack of change in chemokine expression. Instead, any improvements in immunity attributed to MAIT cells is likely due to increased cell counts from exercise-induced lymphocytosis and preferential mobilization in unstimulated cells, rather than changes in intrinsic cell function.
Ex vivo stimulation models are one means of investigating cellular function. In this study, MAIT cells stimulated with PMA and ionomycin demonstrated large increases in intracellular cytokine production compared to unstimulated cells at all time points (data not shown). Acute exercise induces a biphasic response for absolute cell number for CD3+CD8+ CTLs expressing IFNγ in an intensity-dependent manner (22). In the current study, only a trend (p=0.061) for an increase in stimulated MAIT cell number was observed at 0 h and was most likely due to using moderate exercise intensity. However, it is important to note that MAIT cells only make up a small proportion (~10%) of T cells that express CD8 (24), which could also may have contributed to lower absolute increase in cell number. When examining the expression levels of IFNγ within MAIT cells, this study reports for the first time an increasing trend immediately after exercise. This potential rise in the proportion of IFNγ+ MAIT cells may be part of the shift toward a Th1 profile that is observed with moderate (22) but not strenuous exercise. Contrary to moderate intensity exercise, high intensity or long duration exercise decreases T-cell IFNγ secretion and immune cell mobility and activation (26). In this study, moderate intensity exercise appears to increase IFNγ expression and absolute cell number, providing preliminary evidence that MAIT cells may be involved in the enhanced immunity following moderate but not intense exercise (23).
TNFα was expressed by the majority of MAIT cells, similar to previously reported (13), and supports high TNFα secretion rates seen in MAIT cells with levels that are comparable to CTLs (4). TNFα expression that increases from 71 to 79% at 0 h is a key finding, indicating greater sensitivity to pathogens by initiating the inflammatory response. Importantly, this response is transient as expression levels return to baseline by 1 h. Exercise alone alters TNFα+ lymphocyte number in a biphasic manner with a similar but non-significant results in CTLs (27), with both cell counts being slightly greater than MAIT cell counts in the current study. However, as indicated earlier, MAIT cells are a minor population within CD8+ T-cells yet have a greater proportional of TNFα+ cells that suggests that stimulation and exercise are synergistic. Collectively, the increased cell number and frequency of TNFα and IFNγ within MAIT cells suggests improved function with acute moderate intensity exercise.
Asthma, HIV, obesity and diabetes are among several conditions that decrease circulating MAIT cell counts and elevate IL-17 levels (11, 13, 15, 36), a pro-inflammatory cytokine that stimulates chemokine release (40). This study showed negligible MAIT cell expression of IL-17 that was lower than previous work (4, 14), but differences with the stimulation length and populations utilized may contribute to this discrepancy. Acute exercise increases plasma IL-17 levels (40), whereas we found no change in IL-17 expression in MAIT cells. This potentially argues against MAIT cells using IL-17 to recruit additional immune cells after exercise. To the contrary, exercise training reduces plasma levels and PBMC secretion of IL-17 in individuals with multiple sclerosis (41). Strenuous exercise disrupts the cytokine milieu, with reductions in pro-inflammatory markers while anti-inflammatory markers remain constant (26). As such, exercise training may promote a shift in cytokine profiles that, when combined with transient increases in MAIT cell numbers and proportions, are hypothesized to alleviate MAIT cell deficiencies and elevated pro-inflammatory levels in clinical populations despite no change in activation or homing. In support of this, HIV infections reduce MAIT cell numbers but not functional capacity as cytokine production remains intact (16). Increased cytokine expression with exercise may allow for a normal inflammatory response by increasing the efficiency of MAIT cells even as absolute number declines. Moreover, other changes associated with regular exercise (e.g. body composition, glycemic control, inflammation) may also influence MAIT cell number and function and need to be explored as simple, cost-effective therapeutic options.
While we present several novel findings that expand our knowledge of MAIT cells with acute exercise, there are limitations in this study. Only healthy men were used in this study, as estrogen status may alter the immune response (42). Circulating MAIT cell number and frequency were assessed and systemic changes may not reflect events at the mucosa (11, 12). Acute exercise increases both pro- and anti-inflammatory markers (26) but because MAIT cells are pro-inflammatory (9), we focused on these markers. Limited sample availability prevented further analysis. MAIT cell function in females and after exercise training are important next steps in determining the role of these cells and how they contribute to immunity during and after exercise.
In conclusion, Vα7.2+CD161+ MAIT cells increased in frequency relative to total T-cells and cell numbers followed a biphasic response to 40 minutes of moderate intensity exercise. The absolute number of MAIT cells expressing homing marker CCR5 and activation marker CD69 increased after exercise but there was no change in the frequency or the expression level of either marker. Increased pro-inflammatory cytokine levels after exercise suggests higher pathogen responsiveness, indicating improved function. These finding support our previous work but expand the MAIT cell and exercise field by profiling the response to sub-maximal, acute exercise and provide new hypotheses to further investigate how MAIT cells respond to exercise in healthy individuals.
Supplementary Material
Representative FACS plots for classical CD3+ T-cells gated on lymphocytes at A) baseline, B) 0 h and C) 1 h after sub-maximal aerobic exercise. CD4+ and CD8+ T-cells gated on CD3+ T-cells at D) baseline, E) 0 h and F) 1 h after sub-maximal aerobic exercise. The representative FACS plots were selected from the participant with percent of T-cells closest to mean baseline value for the respective population while the 0 h and 1 h plots are from the same participant.
Representative FACS plots for Vα7.2+CD161+ MAIT cells gated on CD3+ T-cells at A) baseline, B) 0 h and C) 1 h after sub-maximal aerobic exercise. CD8+ and CD4−CD8−MAIT cell subpopulations gated on CD3+ T-cells at D) baseline, E) 0 h and F) 1 h after sub-maximal aerobic exercise. The representative FACS plots were selected from the participant with percent of MAIT cells closest to mean baseline value for the respective population while the 0 h and 1 h plots are from the same participant.
Representative FACS plots for MAIT cell chemokine receptor expression for CCR4 at A) baseline, B) 0 h and C) 1 h post-exercise, CCR5 at D) baseline, E) 0 h and F) 1 h post-exercise, CCR6 at G) baseline, H) 0 h and I) 1 h post-exercise, and MAIT cells expressing the early activation maker CD69 at J) baseline, K) 0 h and L) 1 h post-exercise. The representative plots were selected from the participant with percent of the respective chemokine expression that was closest to mean baseline value while the 0 h and 1 h plots are from the same participant.
Representative FACS plots for stimulated MAIT cells expressing intracellular TNFα at A) baseline, B) 0 h and C) 1 h post-exercise, IFNγ D) baseline, E) 0 h and F) 1 h post-exercise, and IL-17 at G) baseline, H) 0 h and I) 1 h post-exercise. The representative plots were selected from the participant with percent of the respective chemokine expression that was closest to mean baseline value while the 0 h and 1 h plots are from the same participant.
ACKNOWLEDGMENTS
The authors wish to thank the participants for being involved in the study, Andrew McCrae, Krista Rosenquest, Brianna Leibeck, and Cody Hayslette for assistance with data collection, Janet Dow of the UNC Flow Cytometry Core for assistance with data acquisition and analysis, and the following funding sources: Junior Faculty Development Award (EH); University Research Council Award (EH) from the University of North Carolina. The UNC Flow Cytometry Core Facility is supported in part by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center. Research reported in this publication was supported by the Center for AIDS Research award number 5P30AI050410. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation, and do not constitute endorsement by ACSM.
Footnotes
The authors have no conflict of interest to report.
REFERENCES
- 1.Billerbeck E, Kang YH, Walker L, et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci U S A. 2010;107(7):3006–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin E, Treiner E, Duban L, et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7(3):e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tilloy F, Treiner E, Park SH, et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999;189(12):1907–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dusseaux M, Martin E, Serriari N, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117(4):1250–9. [DOI] [PubMed] [Google Scholar]
- 5.Vangelista L, Vento S. The Expanding Therapeutic Perspective of CCR5 Blockade. Front Immunol. 2017;8:1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koay HF, Gherardin NA, Enders A, et al. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat Immunol. 2016;17(11):1300–11. [DOI] [PubMed] [Google Scholar]
- 7.Treiner E, Duban L, Bahram S, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422(6928):164–9. [DOI] [PubMed] [Google Scholar]
- 8.Ussher JE, Klenerman P, Willberg CB. Mucosal-associated invariant T-cells: new players in anti-bacterial immunity. Front Immunol. 2014;5:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z, Wang H, D’Souza C, et al. Mucosal-associated invariant T-cell activation and accumulation after in vivo infection depends on microbial riboflavin synthesis and co-stimulatory signals. Mucosal Immunol. 2017;10(1):58–68. [DOI] [PubMed] [Google Scholar]
- 10.Kawachi I, Maldonado J, Strader C, Gilfillan S. MR1-restricted V alpha 19i mucosal-associated invariant T cells are innate T cells in the gut lamina propria that provide a rapid and diverse cytokine response. J Immunol. 2006;176(3):1618–27. [DOI] [PubMed] [Google Scholar]
- 11.Serriari NE, Eoche M, Lamotte L, et al. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol. 2014;176(2):266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiejima E, Kawai T, Nakase H, et al. Reduced Numbers and Proapoptotic Features of Mucosal-associated Invariant T Cells as a Characteristic Finding in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21(7):1529–40. [DOI] [PubMed] [Google Scholar]
- 13.Magalhaes I, Pingris K, Poitou C, et al. Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. J Clin Invest. 2015;125(4):1752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carolan E, Tobin LM, Mangan BA, et al. Altered distribution and increased IL-17 production by mucosal-associated invariant T cells in adult and childhood obesity. J Immunol. 2015;194(12):5775–80. [DOI] [PubMed] [Google Scholar]
- 15.Hinks TS, Zhou X, Staples KJ, et al. Innate and adaptive T cells in asthmatic patients: Relationship to severity and disease mechanisms. J Allergy Clin Immunol. 2015;136(2):323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez CS, Amarasena T, Kelleher AD, et al. MAIT cells are depleted early but retain functional cytokine expression in HIV infection. Immunol Cell Biol. 2015;93(2):177–88. [DOI] [PubMed] [Google Scholar]
- 17.Bishop NC, Hayashida H, Clark M, Coombs C, Miller S, Stensel DJ. Effect of acute and regular exercise on growth hormone secretagogue receptor-1a expression in human lymphocytes, T cell subpopulation and monocytes. Brain Behav Immun. 2014;39:172–9. [DOI] [PubMed] [Google Scholar]
- 18.Campbell JP, Guy K, Cosgrove C, Florida-James GD, Simpson RJ. Total lymphocyte CD8 expression is not a reliable marker of cytotoxic T-cell populations in human peripheral blood following an acute bout of high-intensity exercise. Brain Behav Immun. 2008;22(3):375–80. [DOI] [PubMed] [Google Scholar]
- 19.Green KJ, Rowbottom DG, Mackinnon LT. Acute exercise and T-lymphocyte expression of the early activation marker CD69. Med Sci Sports Exerc. 2003;35(4):582–8. [DOI] [PubMed] [Google Scholar]
- 20.Millard AL, Valli PV, Stussi G, Mueller NJ, Yung GP, Seebach JD. Brief exercise increases peripheral blood NK cell counts without immediate functional changes, but impairs their responses to ex vivo stimulation. Frontiers in Immunology. 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson RJ, Cosgrove C, Ingram LA, et al. Senescent T-lymphocytes are mobilised into the peripheral blood compartment in young and older humans after exhaustive exercise. Brain Behav Immun. 2008;22(4):544–51. [DOI] [PubMed] [Google Scholar]
- 22.LaVoy EC, Hussain M, Reed J, et al. T-cell redeployment and intracellular cytokine expression following exercise: effects of exercise intensity and cytomegalovirus infection. Physiol Rep. 2017;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nieman DC, Miller AR, Henson DA, et al. Effect of high- versus moderate-intensity exercise on lymphocyte subpopulations and proliferative response. Int J Sports Med. 1994;15(4):199–206. [DOI] [PubMed] [Google Scholar]
- 24.Hanson ED, Danson E, Nguyen-Robertson CV, et al. Maximal exercise increases mucosal associated invariant T cell frequency and number in healthy young men. Eur J Appl Physiol. 2017;117(11):2159–69. [DOI] [PubMed] [Google Scholar]
- 25.Dorneles GP, Colato AS, Galvao SL, et al. Acute response of peripheral CCr5 chemoreceptor and NK cells in individuals submitted to a single session of low-intensity strength exercise with blood flow restriction. Clin Physiol Funct Imaging. 2016;36(4):311–7. [DOI] [PubMed] [Google Scholar]
- 26.Shaw DM, Merien F, Braakhuis A, Dulson D. T-cells and their cytokine production: The anti-inflammatory and immunosuppressive effects of strenuous exercise. Cytokine. 2018;104:136–42. [DOI] [PubMed] [Google Scholar]
- 27.Zaldivar F, Wang-Rodriguez J, Nemet D, et al. Constitutive pro- and anti-inflammatory cytokine and growth factor response to exercise in leukocytes. J Appl Physiol (1985). 2006;100(4):1124–33. [DOI] [PubMed] [Google Scholar]
- 28.Howley ET, Bassett DR Jr., Welch HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc. 1995;27(9):1292–301. [PubMed] [Google Scholar]
- 29.Gaskill SE, Ruby BC, Walker AJ, Sanchez OA, Serfass RC, Leon AS. Validity and reliability of combining three methods to determine ventilatory threshold. Med Sci Sports Exerc. 2001;33(11):1841–8. [DOI] [PubMed] [Google Scholar]
- 30.Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37(2):247–8. [DOI] [PubMed] [Google Scholar]
- 31.Tidgren B, Hjemdahl P, Theodorsson E, Nussberger J. Renal Neurohormonal and Vascular-Responses to Dynamic Exercise in Humans. Journal of Applied Physiology. 1991;70(5):2279–86. [DOI] [PubMed] [Google Scholar]
- 32.van Wijck K, Lenaerts K, Grootjans J, et al. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: strategies for evaluation and prevention. Am J Physiol-Gastr L. 2012;303(2):G155–G68. [DOI] [PubMed] [Google Scholar]
- 33.Nagao F, Suzui M, Takeda K, Yagita H, Okumura K. Mobilization of NK cells by exercise: downmodulation of adhesion molecules on NK cells by catecholamines. Am J Physiol-Reg I. 2000;279(4):R1251–R6. [DOI] [PubMed] [Google Scholar]
- 34.Sugimoto C, Konno T, Wakao R, Fujita H, Fujita H, Wakao H. Mucosal-Associated Invariant T Cell Is a Potential Marker to Distinguish Fibromyalgia Syndrome from Arthritis. Plos One. 2015;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiba A, Tamura N, Yoshikiyo K, et al. Activation status of mucosal-associated invariant T cells reflects disease activity and pathology of systemic lupus erythematosus. Arthritis research & therapy. 2017;19(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosgrove C, Ussher JE, Rauch A, et al. Early and nonreversible decrease of CD161++ /MAIT cells in HIV infection. Blood. 2013;121(6):951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saeidi A, Tien VLT, Al-Batran R, et al. Attrition of TCR Va7.2+CD161++ MAIT Cells in HIV-Tuberculosis Co-Infection Is Associated with Elevated Levels of PD-1 Expression. Plos One. 2015;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Bourhis L, Dusseaux M, Bohineust A, et al. MAIT Cells Detect and Efficiently Lyse Bacterially-Infected Epithelial Cells. Plos Pathog. 2013;9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Wilgenburg B, Scherwitzl I, Hutchinson EC, et al. MAIT cells are activated during human viral infections. Nature communications. 2016;7:11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugama K, Suzuki K, Yoshitani K, Shiraishi K, Kometani T. IL-17, neutrophil activation and muscle damage following endurance exercise. Exerc Immunol Rev. 2012;18:116–27. [PubMed] [Google Scholar]
- 41.Golzari Z, Shabkhiz F, Soudi S, Kordi MR, Hashemi SM. Combined exercise training reduces IFN-gamma and IL-17 levels in the plasma and the supernatant of peripheral blood mononuclear cells in women with multiple sclerosis. Int Immunopharmacol. 2010;10(11):1415–9. [DOI] [PubMed] [Google Scholar]
- 42.Giefing-Kroll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14(3):309–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative FACS plots for classical CD3+ T-cells gated on lymphocytes at A) baseline, B) 0 h and C) 1 h after sub-maximal aerobic exercise. CD4+ and CD8+ T-cells gated on CD3+ T-cells at D) baseline, E) 0 h and F) 1 h after sub-maximal aerobic exercise. The representative FACS plots were selected from the participant with percent of T-cells closest to mean baseline value for the respective population while the 0 h and 1 h plots are from the same participant.
Representative FACS plots for Vα7.2+CD161+ MAIT cells gated on CD3+ T-cells at A) baseline, B) 0 h and C) 1 h after sub-maximal aerobic exercise. CD8+ and CD4−CD8−MAIT cell subpopulations gated on CD3+ T-cells at D) baseline, E) 0 h and F) 1 h after sub-maximal aerobic exercise. The representative FACS plots were selected from the participant with percent of MAIT cells closest to mean baseline value for the respective population while the 0 h and 1 h plots are from the same participant.
Representative FACS plots for MAIT cell chemokine receptor expression for CCR4 at A) baseline, B) 0 h and C) 1 h post-exercise, CCR5 at D) baseline, E) 0 h and F) 1 h post-exercise, CCR6 at G) baseline, H) 0 h and I) 1 h post-exercise, and MAIT cells expressing the early activation maker CD69 at J) baseline, K) 0 h and L) 1 h post-exercise. The representative plots were selected from the participant with percent of the respective chemokine expression that was closest to mean baseline value while the 0 h and 1 h plots are from the same participant.
Representative FACS plots for stimulated MAIT cells expressing intracellular TNFα at A) baseline, B) 0 h and C) 1 h post-exercise, IFNγ D) baseline, E) 0 h and F) 1 h post-exercise, and IL-17 at G) baseline, H) 0 h and I) 1 h post-exercise. The representative plots were selected from the participant with percent of the respective chemokine expression that was closest to mean baseline value while the 0 h and 1 h plots are from the same participant.


