Abstract
Axotomy results in permanent loss of function after brain and spinal cord injuries due to the minimal regenerative propensity of the adult central nervous system (CNS). To identify pharmacological enhancers of axon regeneration, 960 compounds were screened for cortical neuron axonal regrowth using an in vitro cortical scrape assay. Diltiazem, verapamil and bromopride were discovered to facilitate axon regeneration in rat cortical cultures, in the presence of chondroitin sulfate proteoglycans (CSPGs). Diltiazem, an L-type calcium channel blocker (L-CCB), also promotes axon outgrowth in adult primary mouse dorsal root ganglion (DRG) and induced human sensory (iSensory) neurons.
Keywords: Axon Regeneration, Cortical Neurons, Scrape Assay, Sensory Neurons, Chondroitin Sulfate Proteoglycans, Diltiazem
INTRODUCTION
Long-distance axon regeneration does not occur within the adult mammalian CNS. As a result, functional deficits typically persist after brain and spinal cord trauma. Clinically approved therapeutic options for CNS injuries are limited, and none target the regrowth of severed axons. One reason for this lack of effective treatments may be the use of in vitro models of axon regeneration with limited relevance to common neurological conditions. For example, although cortical neurons are damaged in adult traumatic brain injury, spinal cord injury and stroke, mature cortical neurons are not readily isolated for neuronal cultures. Embryonic or early postnatal cortical neurons, which are more feasible to culture, do not resemble mature cortical neurons with regard to receptor expression or responsiveness to axon regeneration inhibitors (ARIs), such as CNS myelin-associated inhibitors (MAIs) and CSPGs [1].
To facilitate the study of mature cerebral cortical axon regeneration in vitro, we developed a cortical scrape assay in which embryonic neurons are maintained in culture for several weeks and acquire a more mature phenotype [1, 2]. Rodent cortical neurons are most easily isolated during embryonic development, when their expression of receptors with demonstrated roles in adult CNS regeneration failure is minimal or absent [1]. Therefore, these embryonic neurons likely have limited utility in the modeling of adult CNS axon regeneration. However, as maturation proceeds in culture, protein levels of Nogo receptor 1 (NgR1) and Paired immunoglobulin-like receptor B (PirB) increase over several weeks [1], resembling their developmental upregulation in vivo [3]. This time-course parallels an enhancement of sensitivity to ARIs, and by 21 days in vitro (DIV), cortical neuron axonal regeneration is potently inhibited by CNS myelin, Nogo-22 and CSPGs after a scrape injury [1, 2]. This provides rationale for using the cortical scrape assay to screen for compounds that increase axon growth. In this assay, cortical cultures are wounded at 21 DIV, followed by 5 days during which axons regenerate into the lesion. To model CNS physiological conditions, regeneration can be inhibited by the addition of ARIs to the culture medium. In addition, small molecules can be evaluated for enhancement of axonal growth.
CSPGs associated with the glial scar, including brevican, neurocan, aggrecan, phosphacan and versican, as well as MAIs, such as Nogo-A, myelin-associated glycoprotein (MAG) and oligodendrocyte myelin glycoprotein (OMgp), have been implicated in CNS axon regeneration failure [4]. Numerous studies have demonstrated that CSPGs and their receptors restrict axonal growth following CNS trauma. For example, enzymatic removal of inhibitory CSPG glycosaminoglycan side-chains enhances sensory and corticospinal tract (CST) axon regeneration and promotes functional recovery after SCI [5]. Genetic deficiency of the CSPG receptors leukocyte common antigen-related phosphatase (Lar) and receptor protein tyrosine phosphatase σ (Ptpσ) also promote CST regeneration [6, 7]. NgR1 and Nogo receptor 3 (NgR3) are additional CSPG receptors which mediate growth inhibition in vivo [8]. These studies indicate that CSPGs and their receptors limit axonal regrowth and may represent important targets for drug discovery.
Using the cortical scrape assay, a compound library (Gen-Plus, Microsource) was screened to identify regeneration-promoting drugs which attenuate CSPG inhibition of axon growth.
METHODS
Cortical scrape assay and compound screen
The cortical axon regeneration (cortical scrape) assay has been described previously [1, 2]. Dissociated cortical cultures were established from embryonic day 18 Sprague Dawley rat embryos at a density of 120,000 cells/cm2 (40,000 cells/well in 120 μl) in poly-D-lysine (PDL)-coated 96-well plates (BD BioCoat, Corning 354461). At 21 DIV, cultures were scraped using a floating pin tool [1], creating an area devoid of cellular material through the center of each well and injuring many axons. Immediately after the scrape injury, 50 percent of the culture medium was replaced with fresh medium containing CSPG and/or small molecules, which remained in the medium for the 5 day regeneration period. CSPG was included to inhibit axon growth, thereby increasing the opportunity for growth enhancement by small molecules. To visualize regenerated axons, cultures were fixed at 26 DIV and immunostained for βIII-tubulin (Promega, G7121, 1:1,000) with an Alexa Fluor 488 secondary antibody (Invitrogen, A11029, 1:500).
Compounds (Gen-Plus library, MicroSource, Supplementary Table 1) were evaluated for enhancement of cortical axon regeneration, in the presence of CSPGs. The compound library was obtained from the Yale Center for Molecular Discovery and was rearrayed from 384-well to 96-well format. To minimize edge effects, only the inner 48-wells of 96-well plates were used for neuronal cultures, and the outer wells contained culture media only. Therefore, compounds were rearrayed a second time during transfer to the cultures. Compounds were tested, in duplicate, at a concentration of 10 μM in 0.1% DMSO, in the presence of CSPGs (10.5 μg/ml, Millipore, CC117). Each plate contained two wells per control condition: vehicle (0.1% DMSO, i.e. “no inhibitor”), CSPG, and CSPG + Y27632 (25 μM).
Axon regeneration was assessed microscopically, blind to treatment conditions, and compounds which noticeably enhanced regeneration in both replicates were considered preliminary hits. In addition, axon regeneration was quantified for control wells from 21 randomly selected plates. To quantify axon regeneration in control wells and for several concentrations of preliminary hits (Fig. 1), images were acquired with an ImageXpress 5000A High Content Imaging System (Molecular Devices), using a 10X objective. Images were cropped to the center ~75% of the lesion using Adobe Photoshop. The area covered by axons was measured with MetaXpress Version 1.7 image analysis software, using an angiotube formation algorithm, and data were normalized to the no inhibitor control.
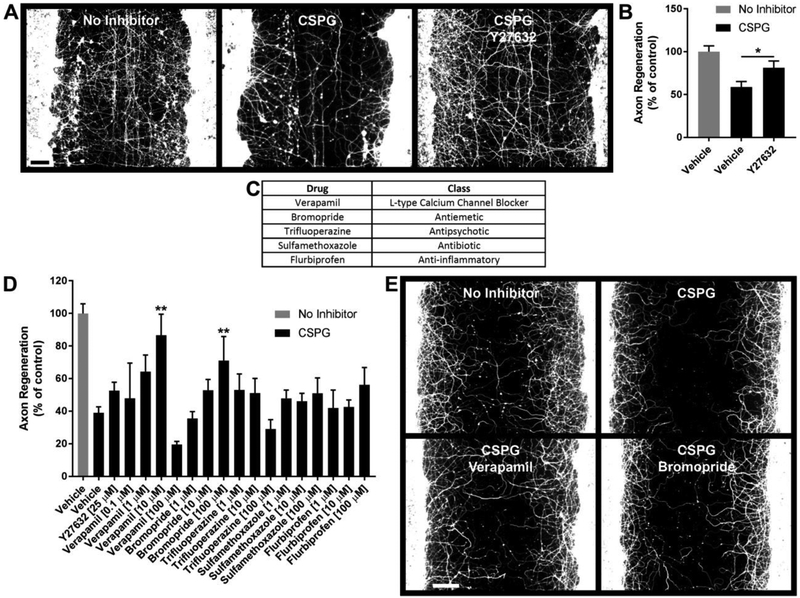
Fig. 1.
Cortical Axon Regeneration Compound Screen. 960 compounds (MicroSource Gen-Plus compound library) were screened in duplicate to identify compounds which promote cortical axon regeneration in the presence of CSPG. A, Photomicrographs of control wells immunostained for βIII-tubulin. Cortical cultures were scraped at 21 DIV and regenerated for 5 days under the indicated conditions. B, Quantification of axon regeneration for control conditions. CSPG inhibits cortical axon regeneration and this inhibition is attenuated by Y27632 (positive control). C, Preliminary hits. D, Concentration-responses for preliminary hits. Verapamil (10 μM) and bromopride (100 μM) significantly promote axon regeneration in the presence of CSPG. E, Photomicrographs demonstrating enhanced regeneration in the presence of verapamil (10 μM) and bromopride (100 μM). Data are mean ± S.E. n = 3-42. Scale bars, 100 μm. *, p < 0.05; **, p < 0.01 vs. CSPG control, Student’s two-tailed t-test (B) or one-way ANOVA, post hoc Dunnett (D). CSPG, chondroitin sulfate proteoglycan.
For subsequent scrape assay experiments (Figs. 2 through 5, Supplementary Fig. 1), images were acquired with an ImageXpressmicro High Content Imaging System (Molecular Devices), using a 10X objective, and axon regeneration was quantified using an automated image analysis protocol [1]. In some experiments, DAPI (1 μg/ml) was used to evaluate the number of nuclei in a 0.6 mm2 region centered approximately 1 mm from one edge of the scrape. This region was not directly injured by the scrape and permitted nuclei to be counted to rule out any overtly toxic effects of compounds or CSPG on cell survival. Individual compounds were purchased from Sigma-Aldrich, Tocris or EMD Millipore. n = 3-42 wells per condition.
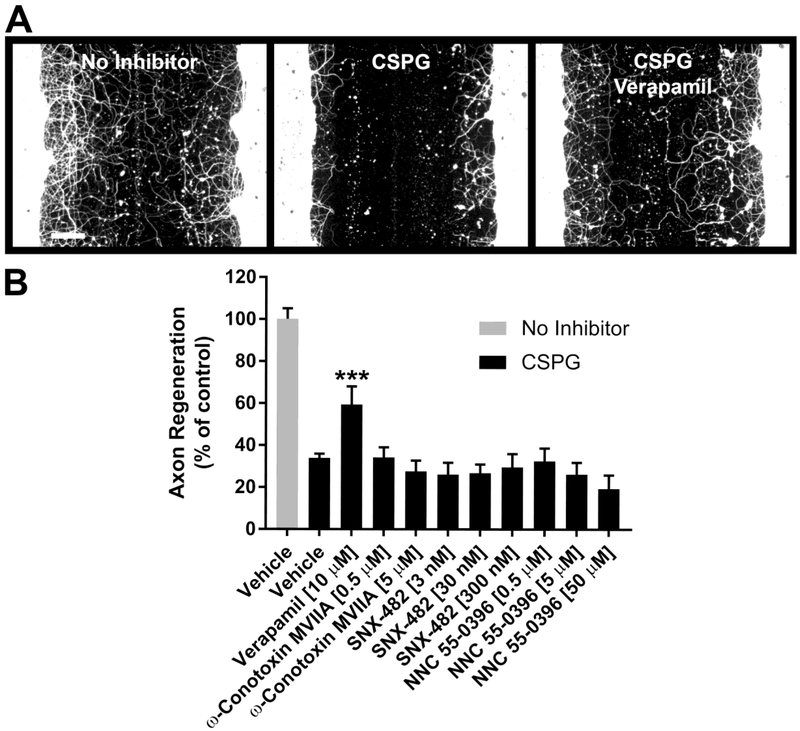
Fig. 2.
Specificity of L-type calcium channel blockade. A, βIII-tubulin immunostaining of cortical cultures after five days of regeneration under the indicated conditions. B, Quantification of axon regeneration. Verapamil (L-type calcium channel blocker) significantly enhances axon regeneration in the presence of CSPG. Inhibitors of other calcium channel sub-types (ω-conotoxin MVIIA, N-type; SNX-482, R-type; NNC 55-0396, T-type) do not affect cortical axon regeneration at the indicated concentrations. Data are mean ± S.E. n = 6-29. Scale bar, 100 μm. ***, p < 0.001 vs. CSPG control, one-way ANOVA, post hoc Dunnett.
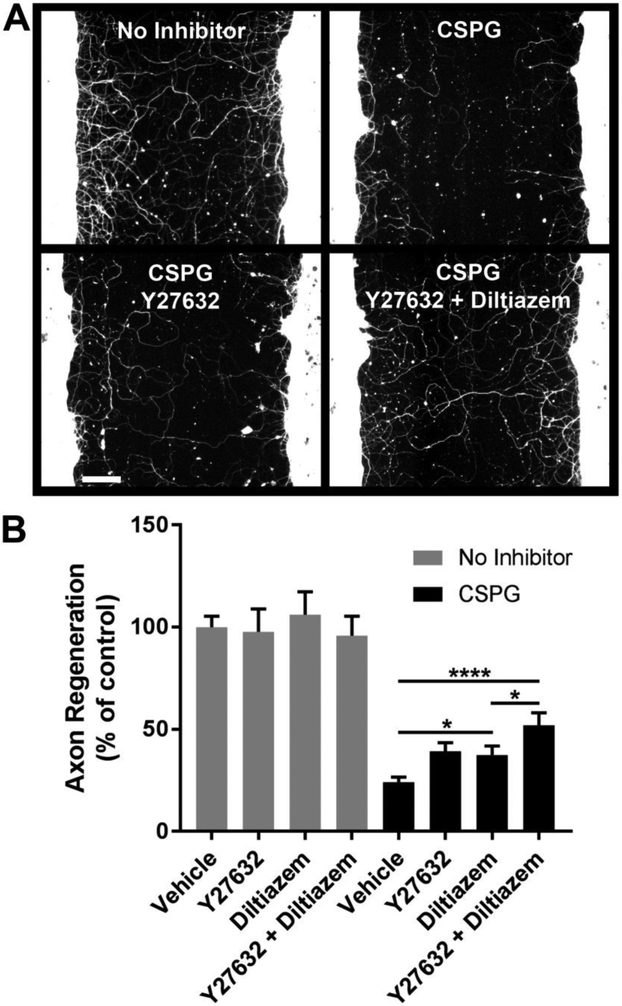
Fig. 5.
Y27632 and diltiazem selectively promote axon regeneration in the presence of CSPG. A, βIII-tubulin immunostaining of cortical cultures after five days of regeneration under the indicated conditions. B, Quantification of axon regeneration. The combination of Y27632 + diltiazem increases axon regeneration 2.2-fold compared with the CSPG control. Y27632 and diltiazem, alone or in combination, do not affect cortical axon regeneration in the absence of inhibitor. Data are mean ± S.E. n = 11-24. Scale bar, 100 μm. *, p < 0.05; ****, p < 0.0001, two-way ANOVA, post hoc Tukey.
Dissociated dorsal root ganglion (DRG) neuronal cultures
DRG cultures were prepared as described previously [9–11]. 8-10 week old C57BL/6J mouse DRGs were dissociated and cultured for 24 hours in 96-well PDL plates (BD BioCoat, Corning 354461) coated with laminin (Sigma L2020, 10 μg/ml in PBS, one hour at room temperature) or CSPG (Millipore, CC117). CSPG (3.3 ng/well in 50 μl PBS) was immobilized by drying overnight in a tissue culture hood and then rinsed with 50 μl of water to remove excess salt. DRG neurons were plated at a density of 2,500 neurons/cm2 (833 neurons/well). Small molecules were added to the culture medium at the time of cell plating. After 24 hours of axon outgrowth, cultures were fixed and immunostained for βIII-tubulin (Sigma-Aldrich, T8660, 1:800) with an Alexa Fluor 488 secondary antibody (Invitrogen, A-11029, 1:500). Images were acquired using an ImageXpressmicro High Content Imaging System (Molecular Devices) with a 10X objective (four sites per well). Axon outgrowth was quantified using MetaXpress (Molecular Devices). The total axon length per neuron (axon length) is reported. n = 12-96 wells per condition.
Induced sensory (iSensory) neurons
Human sensory neurons were generated using a version of the Studer protocol [12] that we have optimized to generate neurofilament-expressing sensory neurons. Differentiated neurons were replated into 96-well plates at a density of 6,000 cells/cm2 (2,000 cells/well). Neurons were treated with 2 μM diltiazem immediately after plating. After 24 hours, cultures were fixed and immunostained for βIII-tubulin (Sigma, T8660). Images were acquired, and the total neurite length per neuron was determined, using a Cellomics Arrayscan XTI (ThermoScientific). n = 3-4 wells per condition.
Statistical Analysis
Statistical analyses were performed with GraphPad Prism version 7.02 using Student’s two-tailed t tests, one-way ANOVA and two-way ANOVA as described in the figure legends. Pre-specified post hoc comparisons were performed to test the differences between the factors after adjusting for multiple comparisons using Dunnett, Tukey or Bonferroni methods.
RESULTS
Cortical axon regeneration compound screen
The cortical scrape assay [1] was used to screen 960 compounds, in duplicate, to discover molecules which promote cortical axon regeneration in the presence of CSPGs. The compound library (Gen-Plus, MicroSource) was selected because it includes small molecules with known bioactivity and drugs currently in clinical use. Compounds were tested at a standard concentration of 10 μM, to balance the chance of identifying hits, which is more likely at higher concentrations, with goals of minimizing DMSO toxicity and avoiding the discovery of hits with low potency and selectivity.
Cortical cultures sustained a scrape injury at 21 DIV, followed by a 5 day regeneration period during which CSPGs and/or compounds were included in the culture medium. The Rock2 inhibitor Y27632 [13, 14] was used as a positive control (Fig. 1A and B). Axon growth was significantly inhibited by CSPG exposure and increased by Y27632 in the presence of CSPG (Fig. 1B).
Five preliminary hits were identified from the screen (Fig. 1C). Quantification of axon regeneration for several concentrations of these drugs revealed that bromopride (100 μM) and verapamil (10 μM) significantly enhance cortical axon regeneration (Fig. 1D and E).
L-type, but not N-, R- or T-type calcium channel blockers promote cortical axon regeneration
One of the regeneration-promoting hits, verapamil (Fig. 1D and E; Fig. 2A and B), is an L-type calcium channel blocker (L-CCB). To determine if antagonism of other calcium channel subtypes affects cortical axon regeneration, ω-conotoxin, SNX-482 and NNC 55-0396 were used to inhibit N-type, R-type and T-type calcium channels, respectively (Fig. 2B). In contrast to verapamil, these calcium channel antagonists did not stimulate axon growth at the concentrations tested. However, diltiazem, an L-CCB structurally distinct from verapamil, also promoted cortical axon regeneration (Fig. 4B; Fig. 5B), suggesting that it is L-type calcium channel blockade (as opposed to off-target activity) that mediates the growth enhancement by this drug class in this assay.
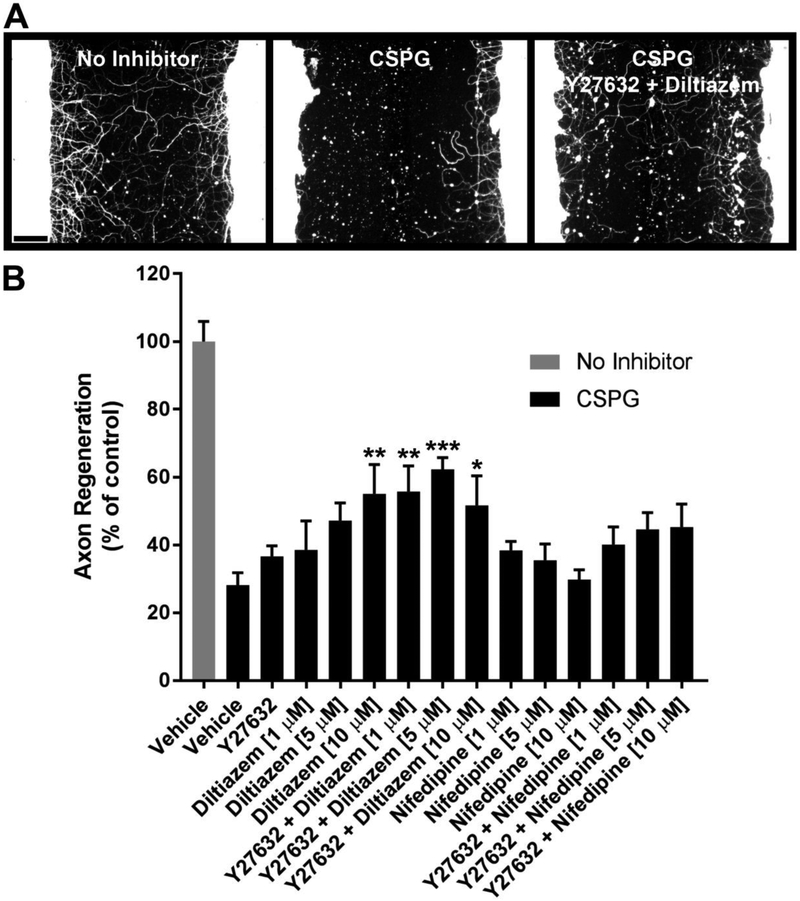
Fig. 4.
Concentration-responses for L-type calcium channel blockers combined with Y27632. A, βIII-tubulin immunostaining of cortical cultures after five days of regeneration under the indicated conditions (Y27632, 25 μM; diltiazem, 5 μM). B, Quantification of axon regeneration. Data are mean ± S.E. n = 5-12. Scale bar, 100 μm. *, p < 0.05; **, p < 0.01; ***, p < 0.001 vs. CSPG control, one-way ANOVA, post hoc Dunnett.
Lack of drug synergy
To investigate potential additive or synergistic effects, verapamil, bromopride, flurbiprofen and sulfamethoxazole (10 μM each) were tested alone and in all possible two-drug combinations (Supplementary Fig. 1). No enhancement of axon regeneration occurs for combinations of drugs compared to the drugs individually (Supplementary Fig. 1B).
To rule out an effect of drugs or CSPG on cell survival, the number of DAPI-positive nuclei in a 0.6 mm2 region adjacent to the scrape was quantified. No difference in nuclear number was detected for any of the conditions compared to the no inhibitor control (data not shown).
L-type calcium channel blockers combined with Y27632 enhance cortical axon regeneration
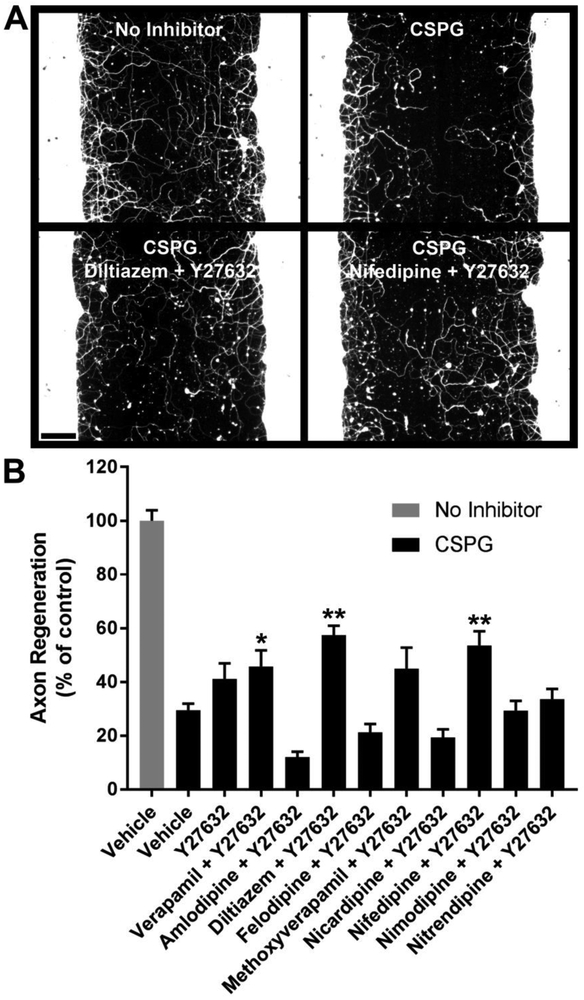
To screen for combinations of molecules which enhance regeneration in the presence of CSPG, Y27632 (25 μM) was tested alone or in combination with various L-CCBs at a standard screening concentration of 10 μM (Fig. 3). Combinations which significantly enhanced axon regeneration relative to the CSPG control are verapamil + Y27632, diltiazem + Y27632, and nifedipine + Y27632 (Fig. 3B), although the contribution of each small molecule to the overall growth-promoting effects of the combinations was not addressed in this experiment. The two most robust growth-promoting combinations implicated in this focused screen (diltiazem + Y27632, and nifedipine + Y27632) were further evaluated for individual drug effects and optimal concentrations (Fig. 4). The combination of diltiazem (5 μM) + Y27632 (25 μM) significantly enhanced regeneration by 2.2-fold compared with the CSPG control (Fig. 4B).
Fig. 3.
L-type calcium channel blockers combined with Y27632 enhance cortical axon regeneration. A, βIII-tubulin immunostaining of cortical cultures after five days of regeneration under the indicated conditions. B, Quantification of axon regeneration. Y27632 (25 μM) plus verapamil, diltiazem or nifedipine (10 μM each) significantly enhance axon regeneration in the presence of CSPG. Data are mean ± S.E. n = 5-36. Scale bar, 100 μm. *, p < 0.05; **, p < 0.01 vs. CSPG control, one-way ANOVA, post hoc Dunnett.
Specificity of growth-promoting effects for overcoming CSPG inhibition was evaluated by testing Y27632 (25 μM) and diltiazem (5 μM) in the absence or presence of CSPG (Fig. 5). Neither small molecule influences axon regeneration in the absence of inhibitor. In contrast, in the presence of CSPG, diltiazem and Y27632 each individually enhance axon regeneration by 1.6-fold (Fig. 5B). The combination of Y27632 + diltiazem increases axon regeneration by 2.2- fold. Thus, the combined effect is additive. The growth-promoting effect of the combination (Y27632 + diltiazem) is significantly greater than that of diltiazem alone, but not of Y27632 alone. Two-way ANOVA indicates significant growth-promoting effects of Y27632 (p = 0.003) and diltiazem (p = 0.001) with no significant interaction. These results indicate that Y27632 and diltiazem additively enhance axonal growth in the presence of CSPGs.
Diltiazem promotes DRG axon outgrowth
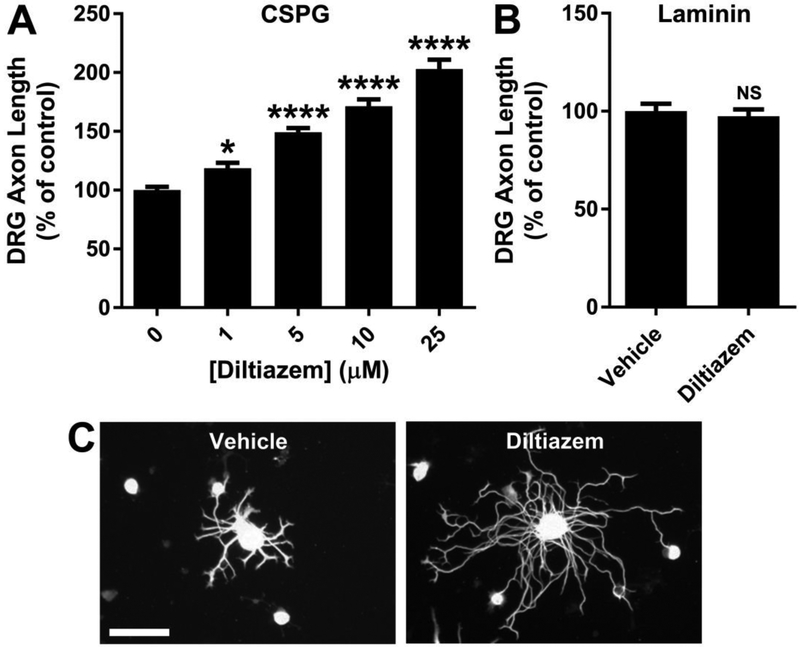
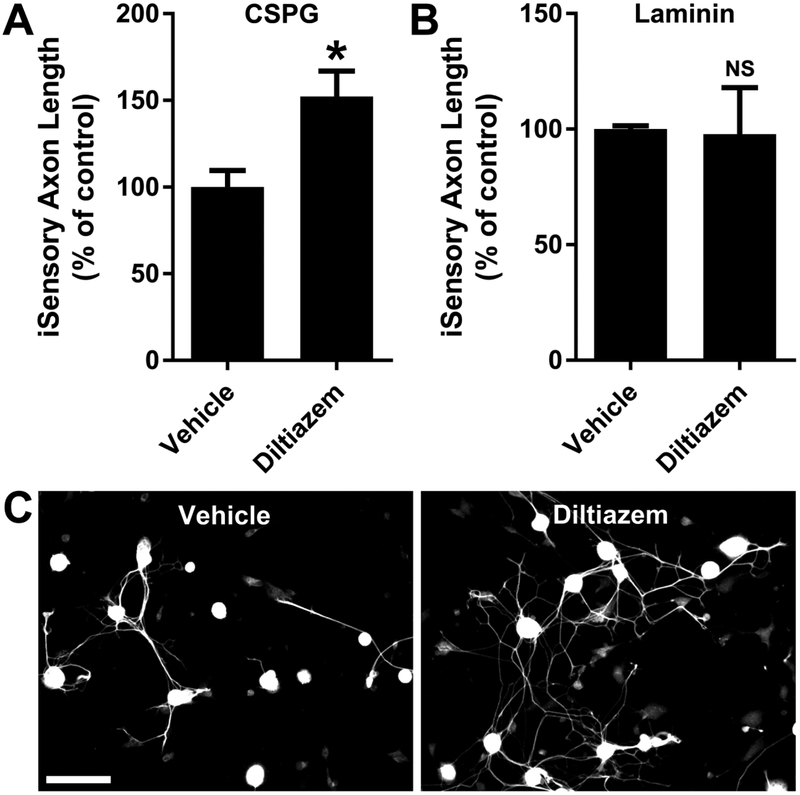
The most effective growth-promoting concentrations of Y27632 (Supplementary Fig. 2) and diltiazem (Fig. 6) were determined in dissociated adult mouse DRG neurons cultured for 24 hours on CSPG. Y27632 increases DRG axon length up to 2.0-fold on CSPG (Supplementary Fig. 2A), with an EC50 of 5.9 ± 1.2 μM. Similarly, diltiazem enhances axon length up to 2.0-fold on CSPG (Fig. 6A), with an EC50 of 4.4 ± 0.7 μM. Diltiazem (2 μM) also enhances replated induced human sensory (iSensory) neuron axon length by 1.5-fold on CSPG (Fig. 7).
Fig. 6.
Diltiazem promotes DRG axon outgrowth. A, Diltiazem concentration-response for DRG axon outgrowth on CSPG. Diltiazem (25 μM) increases the total axon length per neuron by 2.0-fold. B, No effect of diltiazem (5 μM) on DRG axon length is detected on laminin. C, βIII-tubulin immunostaining of DRG neurons cultured on CSPG for 24 hours in the presence of diltiazem (25 μM) or vehicle. Data are mean ± S.E. n = 12-36. Scale bar, 100 μm. *, p < 0.05; ****, p < 0.0001, one-way ANOVA, post hoc Dunnett; NS, not significant, Student’s two-tailed t-test.
Fig. 7.
Diltiazem promotes human induced sensory (iSensory) neuron axon outgrowth. A, Diltiazem (2 μM) enhances iSensory neuron axon length by ~50 percent on CSPG. B, No effect on axon length is detected on laminin. C, βIII-tubulin immunostaining of iSensory neurons cultured on CSPG for 24 hours in the presence of diltiazem or vehicle. Data are mean ± S.E. n = 3-4. Scale bar, 100 μm. *, p < 0.05, Student’s two-tailed t-test.
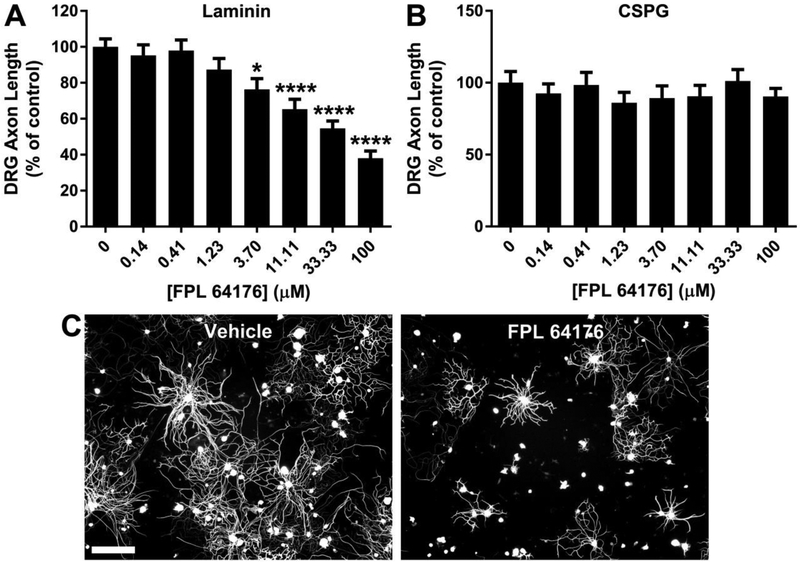
Since L-CCBs promote axon outgrowth, L-type calcium channel agonists would be predicted to have the opposite effect. To investigate this possibility, FPL 64176, an L-type calcium channel agonist, was tested in DRG neurons at various concentrations on laminin and CSPG (Fig. 8). FPL 64176 significantly inhibits axon outgrowth on laminin, in a concentration-dependent manner (Fig. 8A), with an EC50 of 58.3 ± 13.4 μM. Axon outgrowth is minimal on CSPG, precluding further inhibition of growth. Thus, no effect of FPL 64176 is detected on CSPG (Fig. 8B).
Fig. 8.
The L-type calcium channel agonist FPL 64176 inhibits DRG axon outgrowth. A, FPL 64176 inhibits DRG axon outgrowth on laminin in a concentration-dependent manner. B, FPL 64176 has no effect on axon length of DRG neurons cultured on CSPG. C, βIII-tubulin immunostaining of DRG neurons cultured on laminin for 24 hours in the presence of FPL 64176 (33.33 μM) or vehicle. Data are mean ± S.E. n = 12-36. Scale bar, 100 μm. *, p < 0.05; ****, p < 0.0001, one-way ANOVA, post hoc Dunnett.
These results indicate that L-type calcium channel agonism inhibits axon outgrowth on a permissive substrate, whereas antagonism of these channels promotes growth on CSPG.
DISCUSSION
L-CCBs have previously been implicated in axon regeneration. For example, nimodipine enhances neurite outgrowth in dopaminergic brain slice co-cultures [15]. Nifedipine and nimodipine increase axon outgrowth in developing cortical neurons [16], and a mixture of calcium channel inhibitors (amiloride, amlodipine and NBQX) promotes retinal ganglion cell survival and regeneration after optic nerve crush [17]. The current study, using an unbiased phenotypic screen, extends previous findings to relatively mature cortical neurons and identifies verapamil and diltiazem as regeneration-promoting L-CCBs, which alleviate CSPG-mediated inhibition of axon growth.
Diltiazem is a benzothiazepine L-CCB, whereas verapamil is a phenylalkylamine L-CCB. The structural dissimilarity of these CCBs reinforces the conclusion that L-type calcium channel antagonism is the likely mechanism by which these drugs enhance axonal growth in the presence of CSPGs. Additional evidence that modulation of L-type calcium channel activity influences neurite extension is provided by the L-type calcium channel agonist FPL 64176, which inhibits DRG axon outgrowth. Intracellular calcium elevation [18] and calcium signaling through protein kinase C [19] have been implicated in CSPG inhibition of axon growth. Therefore, the growth inhibition by FPL 64176 on laminin likely reflects the elevation of intracellular calcium this compound produces, which simulates the inhibitory effect of CSPG. Saturated calcium signaling and minimal growth on CSPG may explain the lack of further inhibition of DRG axon outgrowth by FPL 64176 on this inhibitory substrate.
The growth-promoting effects of diltiazem and verapamil in the presence of CSPG suggest that L-CCBs may facilitate axon regeneration after CNS injury. The antihypertensive activity of L-CCBs reduces the risk of stroke in hypertensive individuals [20], and the L-CCB nimodipine is used to treat cerebral vasospasm after hemorrhagic stroke [21]. Other potential beneficial effects of calcium blockade have been theorized to include direct neuroprotection, based on an involvement of intracellular calcium elevation in cytotoxic neuronal injury. Consistent with this hypothesis are pre-clinical studies showing that calcium channel blockade improves neuronal survival and functional outcomes in rodents after ischemic stroke [22, 23] and TBI [24, 25]. However, clinical trials thus far have failed to demonstrate benefit of calcium channel blockade in humans after ischemic stroke [26] or TBI [27, 28].
Achievement and maintenance of effective L-CCB levels within the CNS represents a therapeutic challenge. Diltiazem crosses the blood-brain barrier but is a p-glycoprotein (P-gp) substrate and is rapidly effluxed from the CNS [29, 30]. Other L-CCBs are also P-gp substrates [29], thus limiting their utility in the treatment of CNS disorders. Supratherapeutic diltiazem concentrations can transiently be achieved in mouse retina 1 hour after intravitreal or subcutaneous injection but decline substantially by 12 hours (data not shown), complicating evaluation of the action of the drug on injured CNS.
Phenotypic screens offer the opportunity to discover compounds suitable for repurposing as regeneration-promoters and to identify targets like L-CCBs for which small molecules with more favorable drug-like properties and distribution into the CNS can be developed. Utilization of primary neurons in the cortical scrape assay allows axon growth to be modeled in vitro with a cell type likely to be relevant for regeneration in vivo. Induced neurons permit validation in human cells, which may optimize translating discoveries into effective therapies by eliminating those that have no activity on human neurons.
We conclude that antagonism of L-type calcium channels facilitates axon growth in the presence of CSPG of primary cortical, adult DRG and induced human sensory neurons, suggesting exploration of whether L-CCBs can be exploited therapeutically to promote axon regeneration.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grants T32GM07205 and T32NS007473 (E.A.H.), R01NS42304, R01NS39962 and R01NS33020 (S.M.S.), the Falk Medical Research Trust (S.M.S.) and the Dr. Miriam and Sheldon M. Adelson Medical Research Foundation (C.J.W.). This work was conducted with partial support from the Yale Center for Molecular Discovery, Harvard Catalyst ∣ The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
REFERENCES
- 1.Huebner EA, Kim BG, Duffy PJ, Brown RH, Strittmatter SM (2011) A multi-domain fragment of Nogo-A protein is a potent inhibitor of cortical axon regeneration via Nogo receptor 1. J Biol Chem 286:18026–36. doi: 10.1074/jbc.M110.208108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zai L, Ferrari C, Dice C, Subbaiah S, Havton LA, Coppola G, Geschwind D, Irwin N, Huebner E, Strittmatter SM, Benowitz LI (2011) Inosine augments the effects of a Nogo receptor blocker and of environmental enrichment to restore skilled forelimb use after stroke. J Neurosci 31:5977–88. doi: 10.1523/JNEUROSCI.4498-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Chun S-J, Treloar H, Vartanian T, Greer C a, Strittmatter SM (2002) Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J Neurosci 22:5505–5515. doi: 20026582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quraishe S, Forbes LH, Andrews MR (2018) The Extracellular Environment of the CNS: Influence on Plasticity, Sprouting, and Axonal Regeneration after Spinal Cord Injury. Neural Plast 2018:2952386. doi: 10.1155/2018/2952386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradbury EJ, Moon LDF, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB (2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416:636–40. doi: 10.1038/416636a [DOI] [PubMed] [Google Scholar]
- 6.Xu B, Park D, Ohtake Y, Li H, Hayat U, Liu J, Selzer ME, Longo FM, Li S (2015) Role of CSPG receptor LAR phosphatase in restricting axon regeneration after CNS injury. Neurobiol Dis 73:36–48. doi: 10.1016/j.nbd.2014.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fry EJ, Chagnon MJ, López-Vales R, Tremblay ML, David S (2010) Corticospinal tract regeneration after spinal cord injury in receptor protein tyrosine phosphatase sigma deficient mice. Glia 58:423–33. doi: 10.1002/glia.20934 [DOI] [PubMed] [Google Scholar]
- 8.Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, Katagiri Y, Benowitz LI, Geller HM, Giger RJ (2012) NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci 15:703–712. doi: 10.1038/nn.3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omura T, Omura K, Tedeschi A, Riva P, Painter MW, Rojas L, Martin J, Lisi V, Huebner EA, Latremoliere A, Yin Y, Barrett LB, Singh B, Lee S, Crisman T, Gao F, Li S, Kapur K, Geschwind DH, Kosik KS, Coppola G, He Z, Carmichael ST, Benowitz LI, Costigan M, Woolf CJ (2015) Robust Axonal Regeneration Occurs in the Injured CAST/Ei Mouse CNS. Neuron 86:1215–1227. doi: 10.1016/j.neuron.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma CHE, Omura T, Cobos EJ, Latrémolière A, Ghasemlou N, Brenner GJ, van Veen E, Barrett L, Sawada T, Gao F, Coppola G, Gertler F, Costigan M, Geschwind D, Woolf CJ (2011) Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J Clin Invest 121:4332–47. doi: 10.1172/JCI58675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costigan M, Mannion RJ, Kendall G, Lewis SE, Campagna JA, Coggeshall RE, Meridith-Middleton J, Tate S, Woolf CJ (1998) Heat shock protein 27: developmental regulation and expression after peripheral nerve injury. J Neurosci 18:5891–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambers SM, Qi Y, Mica Y, Lee G, Zhang X-J, Niu L, Biisland J, Cao L, Stevens E, Whiting P, Shi S-H, Studer L (2012) Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol 30:715–20. doi: 10.1038/nbt.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watzlawick R, Sena ES, Dirnagl U, Brommer B, Kopp M a, Macleod MR, Howells DW, Schwab JM (2014) Effect and reporting bias of RhoA/ROCK-blockade intervention on locomotor recovery after spinal cord injury: a systematic review and meta-analysis. JAMA Neurol 71:91–9. doi: 10.1001/jamaneurol.2013.4684 [DOI] [PubMed] [Google Scholar]
- 14.Fournier AE, Takizawa BT, Strittmatter SM (2003) Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci 23:1416–23. doi: 23/4/1416 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sygnecka K, Heine C, Scherf N, Fasold M, Binder H, Scheller C, Franke H (2015) Nimodipine enhances neurite outgrowth in dopaminergic brain slice co-cultures. Int J Dev Neurosci 40:1–11. doi: 10.1016/j.ijdevneu.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 16.Tang F, Dent EW, Kalil K (2003) Spontaneous calcium transients in developing cortical neurons regulate axon outgrowth. J Neurosci 23:927–936. doi: 23/3/927 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribas VT, Koch JC, Michel U, Bähr M, Lingor P (2017) Attenuation of Axonal Degeneration by Calcium Channel Inhibitors Improves Retinal Ganglion Cell Survival and Regeneration After Optic Nerve Crush. Mol Neurobiol 54:72–86. doi: 10.1007/s12035-015-9676-2 [DOI] [PubMed] [Google Scholar]
- 18.Snow DM, Atkinson PB, Hassinger TD, Letourneau PC, Kater SB (1994) Chondroitin sulfate proteoglycan elevates cytoplasmic calcium in DRG neurons. Dev Biol 166:87–100. doi: 10.1006/dbio.1994.1298 [DOI] [PubMed] [Google Scholar]
- 19.Sivasankaran R, Pei J, Wang KC, Zhang YP, Shields CB, Xu X-M, He Z (2004) PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci 7:261–268. doi: 10.1038/nn1193 [DOI] [PubMed] [Google Scholar]
- 20.Chaugai S, Sherpa LY, Sepehry AA, Kerman SRJ, Arima H (2018) Effects of Long- and Intermediate-Acting Dihydropyridine Calcium Channel Blockers in Hypertension. J Cardiovasc Pharmacol Ther 23:433–445. doi: 10.1177/1074248418771341 [DOI] [PubMed] [Google Scholar]
- 21.Tallarico RT, Pizzi MA, Freeman WD (2018) Investigational drugs for vasospasm after subarachnoid hemorrhage. Expert Opin Investig Drugs 27:313–324. doi: 10.1080/13543784.2018.1460353 [DOI] [PubMed] [Google Scholar]
- 22.Gupta S, Sharma U, Jagannathan NR, Gupta YK (2017) Neuroprotective effect of lercanidipine in middle cerebral artery occlusion model of stroke in rats. Exp Neurol 288:25–37. doi: 10.1016/J.EXPNEUROL.2016.10.014 [DOI] [PubMed] [Google Scholar]
- 23.Babu CS, Ramanathan M (2011) Post-ischemic administration of nimodipine following focal cerebral ischemic-reperfusion injury in rats alleviated excitotoxicity, neurobehavioural alterations and partially the bioenergetics. Int J Dev Neurosci 29:93–105. doi: 10.1016/j.ijdevneu.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 24.Berman RF, Verweij BH, Muizelaar JP (2000) Neurobehavioral protection by the neuronal calcium channel blocker ziconotide in a model of traumatic diffuse brain injury in rats. J Neurosurg 93:821–8. doi: 10.3171/jns.2000.93.5.0821 [DOI] [PubMed] [Google Scholar]
- 25.Gurkoff G, Shahlaie K, Lyeth B, Berman R (2013) Voltage-gated calcium channel antagonists and traumatic brain injury. Pharmaceuticals (Basel) 6:788–812. doi: 10.3390/ph6070788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Yang J, Zhang C, Jiang X, Zhou H, Liu M (2012) Calcium antagonists for acute ischemic stroke. Cochrane Database Syst Rev CD001928. doi: 10.1002/14651858.CD001928.pub2 [DOI] [PubMed] [Google Scholar]
- 27.Xu G-Z, Wang M-D, Liu K-G, Bai Y-A, Wu W, Li W (2013) A meta-analysis of treating acute traumatic brain injury with calcium channel blockers. Brain Res Bull 99:41–47. doi: 10.1016/j.brainresbull.2013.09.011 [DOI] [PubMed] [Google Scholar]
- 28.McConeghy KW, Hatton J, Hughes L, Cook AM (2012) A Review of Neuroprotection Pharmacology and Therapies in Patients with Acute Traumatic Brain Injury. CNS Drugs 26:613–636. doi: 10.2165/11634020-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 29.Wessler JD, Grip LT, Mendell J, Giugliano RP (2013) The P-Glycoprotein Transport System and Cardiovascular Drugs. J Am Coll Cardiol 61:2495–2502. doi: 10.1016/j.jacc.2013.02.058 [DOI] [PubMed] [Google Scholar]
- 30.Naito K, Nagao T, Otsuka M, Harigaya S, Nakajima H (1986) Penetration into and elimination from the cerebrospinal fluid of diltiazem, a calcium antagonist, in anesthetized rabbits. Arzneimittelforschung 36:25–8 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.