Abstract
Purpose
The aim of this retrospective study was to evaluate the clinical outcomes of the patients who underwent primary anterior cruciate ligament (ACL) reconstruction surgery with either hamstring autograft or freeze-dried tibialis anterior allograft, which performed by the same surgeon using the same fixation technique.
Methods
In this retrospective study, patients who had primary ACL reconstruction using either four-strand hamstring autograft (FSH) or freeze-dried irradiated tibialis anterior allograft (FDT) between 2012 and 2015 were evaluated. Patients who were skeletally mature with a minimum follow-up of 24 months and who had no previous surgery from the affected knee were included; patients who had multiple ligament injuries or chondral lesions over Outerbridge grade 2 were excluded from the study. Patients were grouped according to the graft type used in ACL reconstruction. Tegner activity scale and Lysholm knee scoring scale were used to assess patients' activity levels and functional status preoperatively and at the final follow-up. KT-2000 arthrometer measurements were done at the final follow-up to evaluate anterior laxity.
Results
There were 27 patients (mean age 27 ± 8.9 years) in the FSH group and 36 patients (mean age 27.1 ± 6.7 years) in the FDT group. The mean follow-up time was 38.2 ± 3.5 months for the FSH group and 41 ± 6.1 months for the FDT group. There were no statistically significant differences between the groups when preoperative and postoperative Tegner-Lysholm scores were compared (Tegner P = 0.583, 0.742; Lysholm P = 0.592, 0.249). The mean anteroposterior laxity and side-to-side differences measured by KT-2000 were 4.1 mm and 2.1 mm for the FSH group, respectively; 4.2 mm and 2.2 mm for the FDT group, respectively. There was not a statistically significant difference (P = 0.745, 0.562 respectively).
Conclusions
Primary ACL reconstruction with a single loop freeze-dried irradiated tibialis anterior allograft revealed comparable results with four-strand hamstring autograft in non-athlete patients.
Level of evidence
Level III, Therapeutic study.
Keywords: Anterior cruciate ligament, Reconstruction, Freeze-dried, Allograft, Hamstring, Autograft
Introduction
Anterior cruciate ligament (ACL) reconstruction can be performed by different types of grafts and fixation methods.1, 2, 3 Bone-patellar tendon-bone (BPTB) and four-strand (quadruple, double-loop) hamstring (FSH) autografts are the most common graft options currently.1, 2 Bone-tendon type autografts were considered the best for rapid bone-to-bone integration with lower failure rates while having more donor site morbidity. However, the use of FSH autografts seem to have relatively less morbidity than BPTB autografts and recently popularized in ACL reconstruction.2, 3
Allografts are another option in ACL reconstruction. Various systematic reviews had shown no significant differences between allografts and autografts, although there is still concern about graft selection due to lack of prospective, well-controlled studies in the literature.4, 5, 6, 7 Fresh-frozen and cryopreserved allografts for ACL reconstruction were examined well in the literature; however, there are not enough studies about the use of freeze-dried tendon only allografts. Freeze-drying process alters the strength of the bone tissue in BPTB allografts, which cause higher failure rates.8 However, its disadvantage in tendon only graft is not so clear.
In our institute, we used freeze-dried tibialis anterior allograft (FDT) for the reconstruction of ACL for two years and had an opportunity to observe the results. The aim of this retrospective study was to evaluate the clinical outcomes as means of knee laxity test and Tegner-Lysholm score of the patients who underwent primary ACL reconstruction surgery with either FSH autograft or FDT allograft performed by the same surgeon using the same fixation technique.
Material and methods
This retrospective study was performed after having the approval of our institution's ethical review board. Between 2012 and 2015, patients who underwent primary ACL reconstruction with the diagnosis of symptomatic ACL deficiency were participated in this study. Patients who were skeletally mature (between 16 and 50 years old) with a minimum follow-up of 24 months and who had no previous surgery from the affected knee were included in this study. Patients who had multiple ligament injuries or chondral lesions over Outerbridge grade 2 (Outerbridge grade 3–4) were excluded. The patients were grouped depending on the used graft type; either FSH autograft or FDT allograft. We discussed the option of allograft with the patients and decisions were made depending on the patients' demands. There were no professional athletes in both groups. All operations were performed by the same surgeon.
Operative technique
All operations were performed under spinal anesthesia. Patients were placed supine on the operating table with the knee flexed down over the end of the table. Pneumatic tourniquet was used. For the FSH group, a longitudinal incision was made over pes-anserinus. The semitendinosus and gracilis tendons were harvested with a closed loop tendon stripper and the remaining muscle tissues over the harvested graft were cleaned. For the FDT group; allograft rehydrated for 30 min in a warm saline. Free ends of those grafts were prepared with Ethibond (No. 5), using the Krakow technique. Quadruple tendon was prepared using FSH autograft, and double tendon was prepared using FDT allograft FSH group.
Arthroscopic anterior cruciate ligament surgery was done in a trans-tibial technique. Partial meniscectomies were performed when necessary. Femoral fixation was done with a metal TransFix cross-pin (ConMed Linvatec, Largo, FL, USA) sent from the lateral femoral condyle. Then the knee was positioned in full extension, the graft was held tight while an assistant was performing posterior drawer maneuver and the graft was fixed by a bio-absorbable interference screw. The fixation was augmented by a ligament staple.
Rehabilitation
An easy understood self-rehabilitation program, which starts at postoperative first day was taught to all the patients. This consists of some active and passive quadriceps strengthening exercises and limitation of some activities. In the first 4 weeks, partial-weight bearing was allowed with the use of crutches and knee flexion exceeding 90° was avoided. After that, full-weight bearing and gradually increased amount of knee flexion were allowed. At the end of 3 months, plyometric exercises and at the end of 6 months, contact sports were allowed.
Patient evaluation
Tegner activity scale and Lysholm knee scoring scale were used to assess patients' activity levels and functional status preoperatively and at the final follow-up. KT-2000 arthrometer (MEDmetric Corporation, San Diego, CA, USA) measurements were done at the final follow-up. Anteroposterior laxity of the affected side and side-to-side differences were measured in millimeters using the manual maximum test with the knee positioned at 15 degrees of flexion.
Statistical analysis
All data were analyzed by using SPSS Statistics version 22.0 software (SPSS Inc, IBM, Chicago, IL, USA). Differences between two independent groups were evaluated by Mann–Whitney U test and T-test in accordance with Shapiro–Wilk normality test. Categorical variables were compared by non-parametric chi-square test. P values lower than 0.05 (p < 0.05) were considered as statistically significant.
Results
A total of 72 patients who underwent ACL reconstruction between 2012 and 2015 in our institution were found through data. After exclusion, 63 patients were enrolled in this study. The mean follow-up time was 38.2 ± 3.5 months (ranges, 34–50 months) for the FSH group and 41 ± 6.1 months (ranges, 33–54 months) for the FDT group. There were 27 patients (3 females, 24 males) in the FSH group and 36 patients (3 females, 33 males) in the FDT group. The mean age was 27 ± 8.9 years (ranges, 16–49 years) in the FSH group and 27.1 ± 6.7 years (ranges, 18–46 years) in the FDT group. There was not a statistically significant difference when the ages and follow-up time of the groups were compared (p > 0.05). The demographic data and types of the injuries of the patients were shown in Table 1.
Table 1.
The demographic data of the patients and their types of the injuries.
| FSH group (n = 27) | FDT group (n = 36) | P value | |
|---|---|---|---|
| Age (years)a | 27 ± 8.9 (16–49) | 27.1 ± 6.7 (18–46) | 0.415 |
| Genderb | 3 female/24 male | 3 female/33 male | 0.000 |
| Follow-up (months)a | 38.2 ± 3.5 (34–40) | 41 ± 6.1 (33–54) | 0.122 |
| Types of the injuriesb | |||
| Sports | 25 (92.6%) | 32 (88.9%) | |
| Falling | 1 (3.7%) | 4 (11.1%) | |
| Other | 1 (3.7%) | 0 | |
Mean values with "± standard deviation" and (ranges).
Number of the patients and (percent).
There were 14 meniscal tears (51.8%) in the FSH group and 19 (52.7%) in the FDT group (p > 0.05). Meniscal debridement was performed for the meniscal tears. None of the patients had meniscal sutures. Outerbridge grade 1–2 chondral pathologies were found in 5 of 27 (18.5%) patients in the FSH group and 8 of 36 patients (22.2%) in the FDT group (p > 0.05). The mean tunnel diameter, which was prepared according to the diameter of the graft, was 8.5 mm for the FSH group and 7.8 mm for the FDT group. The difference was not statistically significant (p > 0.05) (Table 2).
Table 2.
Arthroscopic findings and tunnel diameters of the patients.
| FSH group (n = 27) | FDT group (n = 36) | P value | |
|---|---|---|---|
| Meniscal teara | 14 (51.8%) | 19 (52.7%) | 0.942 |
| Medial meniscus | 12 (44.4%) | 17 (47.2%) | |
| Lateral meniscus | 1 (3.7%) | 2 (5.5%) | |
| Both | 1 (3.7%) | 0 | |
| Chondral pathologya | 5 (18.5%) | 8 (22.2%) | 0.721 |
| Grade 1 | 3 (11.1%) | 5 (13.9%) | |
| Grade 2 | 2 (7.4%) | 3 (8.3%) | |
| Tunnel diameterb | 8.5 ± 0.6 (7–9) | 7.8 ± 0.8 (7–9) | 0.063 |
Number of the patients and (percent).
Mean values with "± standard deviation" and (ranges).
The mean preoperative and postoperative Tegner activity scale results were 5.7 ± 1.3 (ranges, 4 to 8) and 5.3 ± 1 (ranges, 4 to 7), respectively for the FSH group; 5.1 ± 1.3 (ranges, 4 to 7) and 4.9 ± 1 (ranges, 4 to 7), respectively for the FDT group. The average decrease in Tegner activity scale was 0.4 for the FSH group and 0.2 for the FDT group. The average decrease in FSH group was more than FDT group, and the difference was statistically significant (p < 0.05). The mean preoperative Lysholm score was 50.9 ± 12 (ranges, 25 to 70) for the FSH group and 52.6 ± 12 (ranges, 30 to 81) for the FDT group. There was not a statistically significant difference between the groups (p > 0.05). Postoperative Lysholm scores were; 94.7 ± 3 (ranges, 89 to 100) for the FSH group and 93.9 ± 4 (ranges, 86 to 100) for the FDT group. There was not a statistically significant difference between the groups (p > 0.05). According to Tegner-Lysholm grading scale (84–90 good, >90 excellent), 3 patient had good, and 24 patients had excellent results in the FSH group; 8 patients had good, and 28 patients had excellent results in the FDT group (Table 3).
Table 3.
Tegner activity and Lysholm knee scores of the patients.
| FSH group (n = 27) | FDT group (n = 36) | P value | |
|---|---|---|---|
| Tegner Activity Scorea | |||
| Preoperative | 5.7 ± 1.3 (4–8) | 5.1 ± 1.3 (4–7) | 0.583 |
| Postoperative | 5.3 ± 1 (4–7) | 4.9 ± 1 (4–7) | 0.742 |
| Decrease in Tegner score | 0.4 | 0.2 | 0.047 |
| Lysholm Knee Scorea | |||
| Preoperative | 50.9 ± 12 (25–70) | 52.6 ± 12 (30–81) | 0.592 |
| Postoperative | 94.7 ± 3 (89–100) | 93.9 ± 4 (86–100) | 0.249 |
Bold value indicates there is statistically significant decrease of Tegner activity scale in FSH compared to FDT group.
Mean values with "± standard deviation" and (ranges).
The mean anteroposterior laxity and side-to-side differences measured by KT-2000 were 4.1 mm and 2.1 mm for the FSH group, respectively; 4.2 mm and 2.2 mm for the FDT group, respectively. There was not a statistically significant difference (p > 0.05) (Table 4).
Table 4.
KT-2000 arthrometer measurements of the patients at the final follow-up.
| FSH group (n = 27) | FDT group (n = 36) | P value | |
|---|---|---|---|
| KT-2000a | |||
| Anterior tibial displacement (mm) | 4.1 ± 0.8 (3–6) | 4.2 ± 0.9 (3–6) | 0.745 |
| Side-to-side difference (mm) | 2.1 ± 0.9 (1–4) | 2.2 ± 0.7 (0–4) | 0.562 |
Mean values with "± standard deviation" and (ranges).
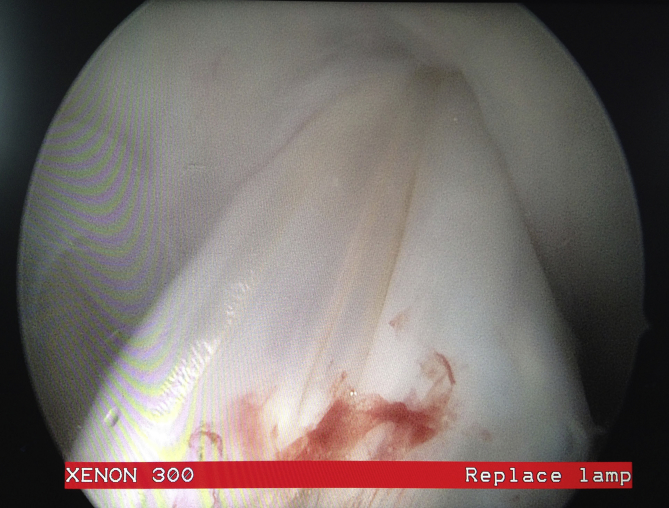
There were no infections, graft failures, implant failures or graft reactions during the follow-up of the patients. Only one patient underwent second-look arthroscopy in the FTD group at postoperative 3rd month because of the limited flexion resistant to physical therapy. Range of motion was gained under anesthesia and the condition of the graft was excellent (Fig. 1).
Fig. 1.
Second look arthroscopic image. The freeze-dried tibialis anterior allograft at 3rd postoperative month.
Discussion
In the reconstruction of the ACL, choice of the fixation technique is primarily based on the training and the experience of the surgeon. However, surgeons and patients still have an opportunity to choose the graft type that is suitable for the technique and the needs of the individual.9 In our institute, we had been routinely using four-strand hamstring autograft with cross-pin for femoral fixation and bio-absorbable interference screw augmented with ligament staple for tibial fixation in ACL reconstruction. Then, we were introduced to freeze-dried tibialis anterior allografts; and began to offer that option to our patients until the social security institution limited its use to only revision ACL surgeries. During this period we discussed pros and cons of the allograft with the patients and majority of them accepted the use of allograft.
There are various options when choosing an allograft for the ACL reconstruction. Besides the origin of the graft, preservation and sterilization methods are variable.10 Each type of graft can be fresh-frozen, cryopreserved or freeze-dried. Sterilization can be done with ethylene-oxide or gamma radiation. Each application has some disadvantages. In the literature, ethylene-oxide was shown to cause synovitis and irradiation was shown to decrease the strength of the graft.2, 3, 6, 8, 11 It was also reported that irradiated fresh-frozen allografts were more likely to fail than non-irradiated ones.2, 12, 13 We did not observe any graft failure during the study despite the freeze-dried allografts that we used were all irradiated (1.05–1.55 Mega Rads). Irradiation alters the tissue by creating free radicals in water containing parts. While freeze dried allografts have less water load they may not be altered by irradiation as much as fresh frozen ones.
Fresh-frozen and cryopreserved allografts have more allogenic tissue load, which may cause undesired immunologic responses.6, 8, 11, 14 Therefore, freeze-dried allografts are less immunogenic compared to fresh-frozen and cryopreserved allografts. Freeze-dried allografts can also be kept at room temperature for 3–5 years. However, fresh-frozen and cryopreserved grafts require expensive storage equipment, which may not be available in many centers. Despite these advantages, there are limited studies in the literature about the clinical use of freeze-dried tendon allografts in the ACL reconstruction. Early studies about the use of freeze-dried allografts had shown unacceptable failure rates. In these studies, all of the authors reported the results of BPTB allografts, which were sterilized with ethylene-oxide.15, [16], 17, 18 It is known that freeze-drying reduces the strength of the bone tissue and in addition to that, ethylene-oxide causes synovitis, which may lead to higher failure rate.13
In this study, we used freeze-dried low-dose irradiated tibialis anterior tendons that have at least 20 cm length and 7 mm width, which are adequate for the ACL reconstruction. We had at least 35 mm femoral tunnel length, and we always had more than enough graft length for the tibial fixation, even for an additional staple. This is an important advantage of the freeze-dried tendon allografts, which ease the operation when compared to freeze-dried bone-tendon-bone allografts with limited length. In the literature, there is still debate about the impact of the graft diameter on the failure rates of ACL reconstructions.19 Failures in ACL reconstruction not only depend on the graft diameter but also the graft type, fixation method and else.19 Mean graft diameter was 7.9 mm in the study group and 8.1 mm in control group in the study of Spragg et al.19 In our study, the mean tunnel diameter of the FTD group was 7.8 mm and 8.5 mm in the FSH group. The mean allograft diameter in our study was comparable to Hamstring autograft group in the study of Spragg et al. The mean graft diameter in the FSH group was relatively larger than the FTD group in our study, however; there was no statistically significant difference between two groups when the graft diameters and clinical results were compared.
One of the advantages of allografts is the shorter operation time.20 However, freeze-dried allografts need to be rehydrated at first, and it takes up to 30 min. Using warm saline decreases the required time. During that period, the graft should not be squeezed or bent because dried fibers crack if not fully rehydrated. Thus, as soon as the initial examination was done, freeze-dried graft should be soaked in warm saline to not to waste time for rehydration.
In this retrospective study, we had no patients who underwent meniscal repair for the meniscal pathologies found during ACL reconstruction. Besides, our study group did not include any patients with accompanying Outerbridge 3–4 chondral pathologies, which need simultaneous surgical intervention. Sofu et al reported that short-term clinical outcomes following ACL reconstruction were not affected by partial meniscectomies performed simultaneously.21 In a prospective, minimum 10-year follow-up clinical study, Bottoni et al reported that the failure rate of ACL reconstruction was independent of meniscal and chondral pathologies.22
When we compared the preoperative and postoperative Tegner activity scores, there were no statistically significant differences between two groups. However, the decrease in the Tegner activity scale was significantly more in FSH group, which can be explained by the protective behavior of the patients. Even though the decrease in the Tegner activity scale was not obvious, many of our patients told us that they become more protective about their knee after ACL reconstruction. The higher failure rate of allografts in patients with higher activity levels was confirmed in the literature.14, 23 The reason for successful results with the absence of allograft failures in our study might be a consequence of relatively sedentary lifestyles of our patients or stopping the activities that caused the injury, such as playing soccer at artificial grass fields. Besides them, all patients were non-athletes in this study. Studies, comparing the results of auto- and allografts in ACL reconstruction mostly could not find significant differences in Lysholm scores.24, 25 When the final Lysholm scores were evaluated, there was an increase within the both groups, but there was no significant difference between the groups.
We used KT-2000 arthrometer to evaluate the anterior knee laxity. Our results revealed that there was not any patient with anterior knee laxity at postoperative 3-year follow-up. In a long-term study (10-year follow-up) with cryopreserved tibialis anterior/posterior tendon allograft in ACL reconstruction, authors reported good clinical results and <3 mm side-to-side difference with KT-1000 measurements.26
The main limitations of this study were relatively short follow-up time and limited study population. Additionally, it was a retrospective evaluation of a prospectively followed patient group. On the other hand, this study is the first study about the use of freeze-dried tibialis anterior allografts in which excellent to good clinical outcomes according to Tegner-Lysholm grading scale were achieved. This study may be a reference to further clinical trials about those allografts, which have some advantages over fresh-frozen and cryopreserved allografts.
Conclusion
Primary ACL reconstruction with a single loop freeze-dried irradiated tibialis anterior allograft revealed comparable results with four-strand hamstring autograft in non-athlete patients in short-term follow-up.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
Contributor Information
Ahmet Issın, Email: ahmet.issin@gmail.com.
Ali Öner, Email: iletisim@alioner.com.tr.
Hakan Sofu, Email: hakansofu@yahoo.com.
Hakan Yurten, Email: hakanyurten@gmail.com.
References
- 1.West R.V., Harner C.D. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13(3):197–207. doi: 10.5435/00124635-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Mehran N., Moutzouros V., Bill, Bedi A. A review of current graft options for anterior cruciate ligament reconstruction. JBJS Rev. 2015;3(11):1. doi: 10.2106/JBJS.RVW.O.00009. [DOI] [PubMed] [Google Scholar]
- 3.Reinhardt K.R., Hetsroni I., Marx R.G. Graft selection for anterior cruciate ligament reconstruction: a level I systematic review comparing failure rates and functional outcomes. Orthop Clin North Am. 2010;41(2):249–262. doi: 10.1016/j.ocl.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Rose M.B., Domes C., Farooqi M., Crawford D.C. A prospective randomized comparison of two distinct allogenic tissue constructs for anterior cruciate ligament reconstruction. Knee. 2016;23(6):1112–1120. doi: 10.1016/j.knee.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Foster T.E., Wolfe B.L., Ryan S., Silvestri L., Krall Kaye E. Does the graft source really matter in the outcome of patients undergoing anterior cruciate ligament reconstruction? Am J Sports Med. 2010;38(1):189–199. doi: 10.1177/0363546509356530. [DOI] [PubMed] [Google Scholar]
- 6.Marrale J., Morrissey M.C., Haddad F.S. A literature review of autograft and allograft anterior cruciate ligament reconstruction. Knee Surg Sport Traumatol Arthrosc. 2007;15(6):690–704. doi: 10.1007/s00167-006-0236-1. [DOI] [PubMed] [Google Scholar]
- 7.Carey J.L., Dunn W.R., Dahm D.L., Zeger S.L., Spindler K.P. A systematic review of anterior cruciate ligament reconstruction with autograft compared with allograft. J Bone Joint Surg Am. 2009;91(9):2242–2250. doi: 10.2106/JBJS.I.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahirogullari M., Ferguson C.M., Whitlock P.W., Stabile K.J., Poehling G.G. Freeze-dried allografts for anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):625–637. doi: 10.1016/j.csm.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Duchman K.R., Lynch T.S., Spindler K.P. Graft selection in anterior cruciate ligament surgery: who gets what and why? Clin Sports Med. 2017;36(1):25–33. doi: 10.1016/j.csm.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Niu Y., Niu C., Wang X. Improved ACL reconstruction outcome using double-layer BPTB allograft compared to that using four-strand hamstring tendon allograft. Knee. 2016;23(6):1093–1097. doi: 10.1016/j.knee.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Park S.S.H., Dwyer T., Congiusta F., Whelan D.B., Theodoropoulos J. Analysis of irradiation on the clinical effectiveness of allogenic tissue when used for primary anterior cruciate ligament reconstruction. Am J Sports Med. 2015;43(1):226–235. doi: 10.1177/0363546513518004. [DOI] [PubMed] [Google Scholar]
- 12.Lawhorn K.W., Howell S.M., Traina S.M., Gottlieb J.E., Meade T.D., Freedberg H.I. The effect of graft tissue on anterior cruciate ligament outcomes: a multicenter, prospective, randomized controlled trial comparing autograft hamstrings with fresh-frozen anterior tibialis allograft. Arthroscopy. 2012;28(8):1079–1086. doi: 10.1016/j.arthro.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Rappé M., Horodyski M., Meister K., Indelicato P.A. Nonirradiated versus irradiated achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35(10):1653–1658. doi: 10.1177/0363546507302926. [DOI] [PubMed] [Google Scholar]
- 14.Singhal M.C., Gardiner J.R., Johnson D.L. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23(5):469–475. doi: 10.1016/j.arthro.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Indelicato P.A., Bittar E.S., Prevot T.J., Woods G.A., Branch T.P., Huegel M. Clinical comparison of freeze-dried and fresh frozen patellar tendon allografts for anterior cruciate ligament reconstruction of the knee. Am J Sports Med. 1990;18(4):335–342. doi: 10.1177/036354659001800401. [DOI] [PubMed] [Google Scholar]
- 16.Roberts T.S., Drez D., Jr., McCarthy W., Paine R. Anterior cruciate ligament reconstruction using freeze-dried, ethylene oxide-sterilized, bone-patellar tendon-bone allografts. Two year results in thirty-six patients. Am J Sports Med. 1991;19(1):35–41. doi: 10.1177/036354659101900106. [DOI] [PubMed] [Google Scholar]
- 17.Jackson D.W., Windler G.E., Simon T.M. Intraarticular reaction associated with the use of freeze-dried, ethylene oxide-sterilized bone-patella tendon-bone allografts in the reconstruction of the anterior cruciate ligament. Am J Sports Med. 1990;18(1):1–11. doi: 10.1177/036354659001800101. [DOI] [PubMed] [Google Scholar]
- 18.Sterling J.C., Meyers M.C., Calvo R.D. Allograft failure in cruciate ligament reconstruction: follow-up evaluation of eighteen patients. Am J Sports Med. 1995;23(2):173–178. doi: 10.1177/036354659502300209. [DOI] [PubMed] [Google Scholar]
- 19.Spragg L., Chen J., Mirzayan R., Love R., Maletis G. The effect of autologous hamstring graft diameter on the likelihood for revision of anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(6):1475–1481. doi: 10.1177/0363546516634011. [DOI] [PubMed] [Google Scholar]
- 20.Steadman J.R., Matheny L.M., Hurst J.M., Briggs K.K. Patient-centered outcomes and revision rate in patients undergoing ACL reconstruction using bone-patellar tendon-bone autograft compared with bone-patellar tendon-bone allograft: a matched case-control study. Arthroscopy. 2015;31(12):2320–2326. doi: 10.1016/j.arthro.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Sofu H., Yildirim T., Gursu S., Issin A., Sahin V. Short-term effects of partial meniscectomy on the clinical results of anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):184–187. doi: 10.1007/s00167-014-2960-2. [DOI] [PubMed] [Google Scholar]
- 22.Bottoni C.R., Smith E.L., Shaha J. Autograft versus allograft anterior cruciate ligament reconstruction. Am J Sports Med. 2015;43(10):2501–2509. doi: 10.1177/0363546515596406. [DOI] [PubMed] [Google Scholar]
- 23.Barrett G.R., Luber K., Replogle W.H., Manley J.L. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593–1601. doi: 10.1016/j.arthro.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Tian S., Wang Y., Wang B. Anatomic double-bundle anterior cruciate ligament reconstruction with a hamstring tendon autograft and fresh-frozen allograft: a prospective, randomized, and controlled study. Arthroscopy. 2016;32(12):2521–2531. doi: 10.1016/j.arthro.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Wei J., Yang H-bin, Qin J-bi, Yang T-bao. A meta-analysis of anterior cruciate ligament reconstruction with autograft compared with nonirradiated allograft. Knee. 2015;22(5):372–379. doi: 10.1016/j.knee.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Almqvist K.F., Willaert P., De Brabandere S., Criel K., Verdonk R. A long-term study of anterior cruciate ligament allograft reconstruction. Knee Surg Sport Traumatol Arthrosc. 2009;17(7):818–822. doi: 10.1007/s00167-009-0808-y. [DOI] [PubMed] [Google Scholar]