Abstract
A rat model of chronic tympanic membrane perforation was developed to be used in the search of new materials for the sealing of these perforations. A longitudinal study was carried out in rats subjected to incisional myringotomy followed by the application of mitomycin C alone or with dexamethasone. Rats were checked at days 3, 7, 10, 14 and weekly thereafter until perforation closure, for up to 6 months. The addition of dexamethasone is a key component in order to obtain a chronic opening. Myringotomies treated with saline had a mean healing time of 8.5 days. At 8 weeks, between 62.5% and 77.7% of tympanic membranes treated with mitomycin C and dexamethasone remained perforated and at 6 months this number fell to 21.4%. This technique is able to maintain most tympanic membrane perforations patent for at least 8 weeks. This rat model is adequate for its use in preclinical or translational research.
Keywords: Animal model, Chronic tympanic membrane perforation, Mitomycin C, Myringotomy, Dexamethasone
1. Introduction
Otitis media is a middle ear pathology that includes a wide range of inflammatory diseases, such as otitis media with effusion, chronic suppurative otitis media with and without cholesteatoma and adhesive otitis media. In chronic suppurative otitis media (CSOM), a tympanic membrane (TM) perforation with persistent drainage from the middle ear is the most common pathology (Tran Ba Huy, 2005). One of the treatments is a surgical repair by placing an underlay support material medial to the TM. This is done after refreshing the edges of the perforation to activate healing and migration of the epithelial layer. Multiple substances have been used as a scaffold in the treatment of TM perforation such as temporalis muscle fascia, perichondrium, cartilage, fat, and spongostan (haemostatic gel foam). Although the rates of closures after the initial attempt are not bad (Sakagami et al., 2007), there is a continuous search for new materials that are more effective in the healing of these TM perforations. Among these, the amniotic membrane could be of interest since it contains stem cells which are endowed with anti-inflammatory, anti-infective and immuno-modulatory properties (Insausti et al., 2011). However, prior to launching human clinical trials to test new materials, a preclinical study is needed using animal models of chronic TM perforation.
Preclinical studies have been performed so far in animal models of TM perforation which involve laser myringotomy and treatment with hydrocortisone or mitomycin C (Jassir et al., 2003; Spandow and Hellström, 1993; Kaftan and Hosemann, 2006), but laser equipment is not a common tool in research centers. Therefore, we aimed to develop a new model to create a chronic tympanic membrane perforation (CTMP) by myringotomy and instillation of mitomycin C. Previous methods to create a CTMP without a laser equipment, such as amputation of the handle of the malleus and the application of substances such as dexamethasone (Kaftan and Hosemann, 2006; Wang et al., 2015), have not obtained conclusive results. Thus, the main objective of the present paper was to identify whether incisional myringotomy with the application of mitomycin C alone or in combination with dexamethasone may produce a chronic tympanic perforation model in rats. As a secondary objective, we have analyzed whether the amputation of the handle of the malleus with application of mitomycin C before or after incisional myringotomy could influence the duration of tympanic membrane perforation patency in rats.
2. Materials and methods
All the research was conducted in the Centre for Experimentation and Biomedical Research (CEIB) of the Universidad de Murcia (Murcia, Spain). All experiments and procedures were approved by the local ethics committee and followed the ethical principles and current legislation on protection of animals for research (Real Decreto, 2005; BOE, 2007; Directiva, 2010/63/UE). The study was performed in 34 healthy male Sprague Dawley rats of 8 weeks of age and weights ranging from 310 to 370 g. The environmental conditions were kept constant throughout the study with cycles of 12 h of light and darkness, a constant temperature of 20 °C and a humidity of 48% (Baumans, 2007). The animals were anesthetized with a mixture of ketamine (40–90 mg/kg, ip) and Xylazine (5–10 mg/kg) (ULAM, 2005).
To study the patency of a perforation and the influence of different agents in creating a chronic perforation we used two substances: mitomycin C and dexamethasone. Mitomycin C is an aminoglycoside antibiotic that has been used as a cytostatic due to its ability to disrupt DNA replication, inhibiting mitosis, and protein synthesis, thus preventing the replication of fibroblast and epithelial cells and eventually prolonging the healing time (Marquet, 1977). Dexamethasone is a classical steroid anti-inflammatory drug that inhibits the production of collagen from fibroblasts. The group of controls rats was treated with a 0.9% saline solution.
2.1. Experimental procedure
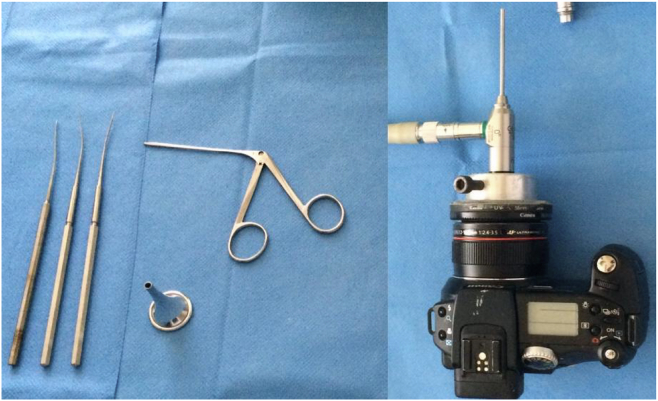
Procedures were performed under direct vision with a Zeiss microscope (Germany) with TM photographs being acquired with a digital camera (Canon Power Shot Pro 1, Japan) attached to a 0° and 3 mm diameter rigid endoscope via an adaptor (GAES audio test HD) and a Karl Storz cold light source, model 482 (Germany). The surgical instruments consisted of a 4 mm diameter ear speculum, a Hartman clip and a Tympanoplasty set (blunt and sharp straight needle, and a small Rosen ring) as shown in Fig. 1. All myringotomies (size of 2.4 × 2.4 mm) were performed in the pars tensa using a sharp punch, amputating the eardrum handle with the same instrument and extracting the bone fragment with a Hartman clip. The concentration of mitomycin C was 0.4 mg/ml and the time of instillation of mitomycin and 0.9% saline solution was always of 10 min.
Fig. 1.
Camera with optical adapter (right) and tympanoplasty instruments used (left).
Rats were checked afterwards to assess the state of healing of the tympanic perforations at days 3, 7, 10, 14 and weekly thereafter until perforation closure, for up to 6 months. The perforations were measured with a small Rosen ring (0.8 mm diameter). The date of the complete closure of the perforation was obtained by averaging the last day that the perforation was seen and the first day that it was found to be closed. The amount of anesthesia was 0.7 ml of ketamine-Xylazine mixture in the first intervention and 0.4 ml in the revision days. Photographs of the eardrums were taken at every intervention and in revisions. Mitomycin C was applied to the TM on spongostan that had been soaked in it (0.4 mg/ml). Dexamethasone (4 mg/ml) was applied directly in drops given through the external auditory canal until fully filling the middle and the external ear.
The statistical analysis was performed using the software R Core Team 2015 (R Core Team, 2014), by using an ANOVA test and a p value lower than 0.05 was considered to express a significant difference.
2.2. Experimental groups
A total of 34 rats were used in the present study. They were divided into three groups (A, B and C) to compare the different interventions and their results (Table 1). In summary, in group A (n = 10), all rats were treated with amputation of the hammer handle. Those of subgroup A1 with application of mitomycin C and subgroup A2 with saline. In all of them, mitomycin or saline was applied after performing myringotomy and amputation of the hammer handle. In group B (n = 12), mitomycin C or saline was applied, according to the subgroups, before performing myringotomy. In subgroup B1, mitomycin was applied before myringotomy without amputating the handle of the hammer. In subgroup B2, mitomycin was applied before myringotomy and the hammer handle was amputated. And in subgroups B3 and B4, saline was applied before the myringotomies. Finally, in group C (n = 12), we proceeded similarly as in group B, but in addition to mitomycin C, dexamethasone was also applied.
Table 1.
Experimental groups.
| Groups | n | Subgroups | Number of Eardrums | 1st intervention | 2nd intervention | 3rd intervention |
|---|---|---|---|---|---|---|
| A | 10 | A1 | 10 Left ears | Myringotomy + HHA | Mitomycin C | |
| A2 | 10 Right ears | Myringotomy + HHA | Saline solution | |||
| B | 12 | B1 | 10 Alternate ears | Mitomycin C | Myringotomy | |
| B2 | 10 Alternate ears | Mitomycin C | Myringotomy + HHA | |||
| B3 | 2 Alternate ears | Saline solution | Myringotomy | |||
| B4 | 2 Alternate ears | Saline solution | Myringotomy + HHA | |||
| C | 14 | C1 | 10 Alternate ears | Mitomycin C | Myringotomy | Dexamethasone |
| C2 | 10 Alternate ears | Mitomycin C | Myringotomy + HHA | Dexamethasone | ||
| C3 | 2 Alternate ears | Saline solution | Myringotomy | Saline solution | ||
| C4 | 2 Alternate ears | Saline solution | Myringotomy + HHA | Saline solution |
HHA: Hammer handle amputation.
3. Results
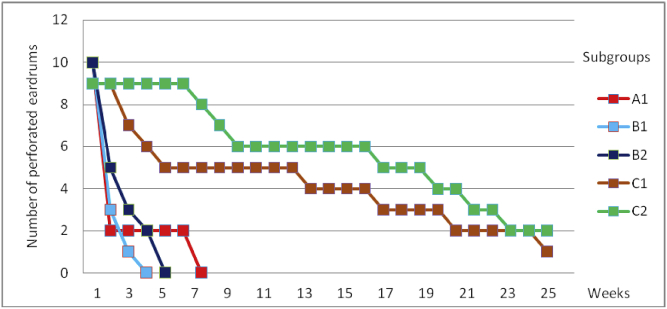
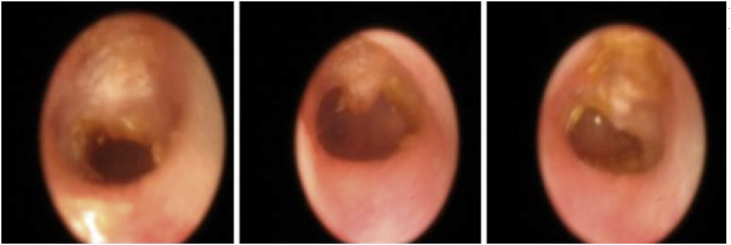
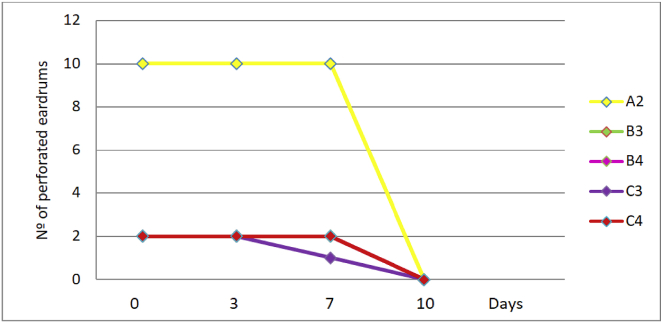
Myringotomies treated with saline solution (Table 2) had a mean healing time of 8.5 days. In group C3, one ear healed at 8.5 days and the other one, at 5 days. Those treated with mitomycin C with or without dexamethasone are shown in Table 3 and Fig. 2. In subgroup C1, at 8 weeks, there were 8 ears under study, of which 5 were perforated (62.5%). In subgroup C2, at 8 weeks, there were 9 ears in the study, of which 7 remained perforated (77.7%). At 6 months, 12% remained perforated in C1 and 22% in C2 (Fig. 3). Fig. 3 shows the healing results of the eardrums treated with mitomycin C alone (A1, B1 and B2) and with mitomycin C plus dexamethasone (C1 and C2). Only the ears of group C remain perforated at 8 weeks and 3 eardrums (1 of subgroup C1 and 2 of subgroup C2) remain open for 6 months (Fig. 4). Fig. 5 shows the duration of tympanic perforations of the ears treated with saline (subgroups A2, B3, B4, C3 and C4) that healed before day 10.
Table 2.
Tympanic permeability duration in myringotomies treated with saline solution.
| Subgroups | Number of eardrums | Treatment | Permeability (days) |
|---|---|---|---|
| A2 | 10 | Saline solution | 8.5 |
| B3 | 2 | Saline solution | 8.5 |
| B4 | 2 | Saline solution | 8.5 |
| C3 | 2 | Saline solution | 8.5 (n = 1), 5.0 (n = 1) |
| C4 | 2 | Saline solution | 8.5 |
Table 3.
Tympanic permeability duration in myringotomies treated with Mitomycin C.
| Subgroups | Treatment | Number of eardrums | Permeability (days) | Unhealed Myringotomies at 6 months |
Mean permeability by subgroups (days) | Discarded eardrums | Mean permeability by groups (days) |
|---|---|---|---|---|---|---|---|
| A1 | Miringotomy + HHA + Mitomycin C | 8 2 |
12.0 45.5 |
18.6 | |||
| B1 | Mitomycin C + Miringotomy | 5 2 2 1 |
8.5 12.0 17.5 24.5 |
12.6 | 15.1 | ||
| B2 | Mitomycin C + Miringotomy + HHA | 2 3 2 1 2 |
8.5 12.0 17.5 24.5 31.5 |
17.5 | |||
| C1 | Mitomycin C + Miringotomy + Dexamethasone | 1 1 1 1 1 1 |
17.5 24.5 31.5 87.5 143.5 178.5 |
1 | 95.0 | 1 Exitus 2 Otorrhea |
112.7 |
| C2 | Mitomycin C + Miringotomy + HHA + Dexamethasone | 1 1 1 1 1 |
45.5 52.5 136.5 150.5 164.5 |
2 | 130.5 | 1 Exitus 2 Otorrhea |
HHA: Hammer handle amputation.
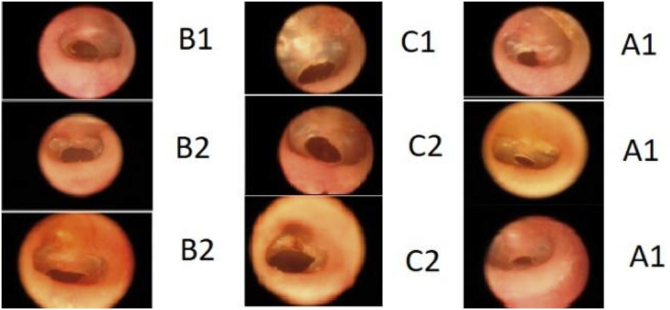
Fig. 2.
Perforated eardrums of groups A, B and C at 8 weeks.
Fig. 3.
Healing time of tympanic membranes treated with mitomycin C.
Fig. 4.
Perforated eardrums at 6 months (one of subgroup C1 (left image) and two of C2).
Fig. 5.
Healing time of tympanic membranes treated with saline solution.
Laterality (the influence of treating the right or the left ear of the rat) did not significantly affect healing time (p > 0.6). With regard to healing time, the comparison of subgroups A1 and B2 did not show a significant difference when mitomycin C was applied before or after myringotomy (p > 0.8). We subsequently compared groups A1 and A2, showing a significant prolongation of the healing time with mitomycin C compared to saline (p < 0.03). There were significant differences (p < 0.001) when comparing the eardrums treated only with Mitomycin C (group B, subgroups B1 and B2) with the eardrums treated with Mitomycin C plus Dexametasone (group C, subgroups C1 and C2). No significant differences were found between the amputation of the hammer handles (B2) and myringotomy without amputation (B1). There were also no differences between the subgroups with (C2) or without amputation (C1). Finally, a significant difference (p < 0.006) was also observed between the eardrums treated with Mitomycin C plus Dexamethasone (groups C1 and C2) and those treated with saline (C3 and C4).
4. Discussion
The tympanic membrane perforations in experimental animals are considered chronic when they are maintained open between 8 and 15 weeks (Santa Maria et al., 2007). Since the 1990s, numerous studies have attempted to establish an animal model of chronic TM perforations using different animals and substances. Wang et al. (2014) performed a review of the literature and, out of 37 studies, only 23 of them were able to achieve chronic TM perforations. The chinchilla was the animal most commonly used in these studies, followed by the rat and guinea pig. In our study, we used Sprague Dawley rats because they were easy to handle and have eardrums with histological characteristics similar to humans (Santa Maria et al., 2010).
Also we decided to use a dose of 0.4 mg/ml of mitomycin C. This was based in the study of Jassir et al. (2003) that compared mitomycin doses of 0.2, 0.4 and 2 mg/ml showing that at 8 weeks 50% of myringotomies treated with 0.4 mg/ml remained patent. Babu et al. (2005) recommended the use of gel foam soaked in mitomycin C. These authors performed a comparative study between the direct application of mitomycin C and application with a gel foam, evaluating the toxicity by measuring auditory brainstem responses. The application with gel foam completely prevented toxicity, justifying its use.
Strem et al. (Strem and Batra, 1999) conducted a study in 60 rats, producing myringotomies by laser and mitomycin C at a concentration of 2 mg/ml. They established several groups with different times of application of mitomycin C and concluded that the patency rate did not improve with an application longer than 10 min nor by repeating the dose. In the study by Jang et al. (2003) on the effects of mitomycin C in tympanic membrane fibroblasts in vitro, using different concentrations and times of exposure, the lowest fibroblasts viability was obtained when applying mitomycin C for 10 min at a concentration of 0.4 mg/ml. According to these studies, we applied mitomycin C by spongostan at a concentration of 0.4 mg/ml for 10 min.
Our data shows that the addition of dexamethasone to mitomycin C treatment prolongs the patency rate of myringotomies. These results agree with those of Kaftan H et al. (Wang et al., 2015) in a rat model in which they used mitomycin C at a concentration of 2 mg/ml applied for 10 min before producing the tympanic perforation. In the revisions, they applied dexamethasone at a concentration of 4 mg/ml, with a mean patency of the perforation of 17.5 days when they used mitomycin C alone and 32 days when associated with dexamethasone. In our study we obtained a similar average patency of 15.1 days when treating rats with myringotomies after application of mitomycin C alone (group B), while in Group C (application of mitomycin C and dexamethasone) we obtained an average permeability of 112.7 days, significantly higher than that achieved by Kaftan et al. (Wang et al., 2015). However, these authors (Wang et al., 2015) used a mitomycin C concentration 5 times greater than the concentration recommended, which we used. Previously published studies of incisional myringotomy with mitomycin C did not succeed in obtaining enough chronic tympanic membrane perforations. Only the laser myringotomy associated with mitomycin C treatment was able to produce chronic perforations (Jassir et al., 2001). However, the laser technology is not available to the majority of experimental laboratories.
Recent studies by Wang et al. (2017) have used a similar method although they obtained perforations of insufficient duration to establish and constitute a chronicity model. The difference is probably related to the method used since these authors employed a higher dose of mitomycin, different from the one recommended in the Jassir et al. (2003) work. Another important difference, in our opinion, is the application of dexamethasone, which they applied only once. We think that the application of dexamethasone in every revision is essential to succeed with the chronic lesion.
5. Conclusion
In this study, we have established a rat model of chronic tympanic membrane perforation by combining mitomycin C (0.4 mg/ml for 10 min) prior to incisional myringotomy and adding dexamethasone (4 mg/ml) on four additional doses. The addition of dexamethasone to mitomycin C is a key component for obtaining a good rate of tympanic opening in this experimental animal model. Our technique is able to maintain, between 62 and 77% of tympanic membrane perforations created by incisional myringotomy and mitomycin C treatment, patent for at least 8 weeks. This model performed in Sprague Dawley rats is robust, reproducible and convenient for preclinical as well as translational research of this pathology.
Acknowledgements
This work was supported by funding from the Instituto de Salud Carlos III Spanish Net of Cell Therapy (TerCel), RETICS subprogram of the I+D+I 2013–2016 Spanish National Plan, projects “RD12/0019/0001“, “RD12/0019/0023” and “RD16/0011/0001” funded by ISCIII and co-founded by ERDF.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Contributor Information
Tomás Esteban, Email: tomas.esteban@carm.es.
Noemí M. Atucha, Email: ntma@um.es, jgestan@um.es.
José María Moraleda, Email: jmoraled@um.es.
Joaquín García-Estañ, Email: jgestan@um.es.
Carmen L. Insausti, Email: caymed@gmail.es.
Javier Moraleda-Deleyto, Email: jmoraleda@nhs.net.
References
- Babu S.C., Kartush J.M., Patni A. Otologic effects of topical mitomycin C: phase I-evaluation of ototoxicity. Otol. Neurotol. 2005;26(2):140–144. doi: 10.1097/00129492-200503000-00002. [DOI] [PubMed] [Google Scholar]
- Baumans V. The welfare of laboratory mice. In: En: Laliste E., editor. The Welfare of Laboratory Animals. Springer; Dordrecht: 2007. pp. 119–152. [Google Scholar]
- BOE Ley 32/2007 para el cuidado de los animales en su explotación, transporte, experimentación y sacrificio. BOE. 2007;268(8 Noviembre 2007):45914–45920. https://www.boe.es/buscar/doc.php?id=BOE-A-2007-19321 2007. [Google Scholar]
- Directiva 2010/63/UE del Parlamento Europeo y del Consejo de 22 de Septiembre, relativa a la protección de los animales utilizados para fines científicos, (http://eur-lex.europa.eu/legal-content/ES/TXT/?uri=LEGISSUM%3Asa0027).
- Insausti C.L., Blanquer M., Majado M.J., Insausti A., Moraleda J.M. Utilidad terapéutica potential de las células madre de la membrana amniótica. Revista de Hematología mexicana. 2011;11(4):276–286. [Google Scholar]
- Jang C.H., Song C.H., Pak S.C. Effect of exposure to mitomycin C on cultured tympanic membrane fibroblasts. Int. J. Pediatr. Otorhinolaryngol. 2003;67(2):173–176. doi: 10.1016/s0165-5876(02)00367-1. [DOI] [PubMed] [Google Scholar]
- Jassir D., Buchman C.A., Gomez-Marin O. Safety and efficacy of topical mitomycin C in myringotomy patency. Otolaryngol. Head Neck Surg. 2001;124(4):368–373. doi: 10.1067/mhn.2001.114255. [DOI] [PubMed] [Google Scholar]
- Jassir D., Odabasi O., Gomez-Marin O., Buchman C.A. Dose-response relationship of topically applied mitomycin C for the prevention of laser myringotomy closure. Otolaryngol. Head Neck Surg. 2003;129(5):471–474. doi: 10.1016/S0194-59980301394-9. [DOI] [PubMed] [Google Scholar]
- Kaftan H., Hosemann W. Topical application of mitomycin C in combination with dexamethasone: effective delay of myringotomy closure. ORL J Otorhinolaryngol Relat Spec. 2006;68(4):185–188. doi: 10.1159/000091393. [DOI] [PubMed] [Google Scholar]
- Marquet J.F. Twelve years' experience with homograft tympanoplasty. Otolaryngol. Clin. 1977;10(3):581–593. [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2014. R: a Language and Environment for Statistical Computing.http://www.R-project.org/ [Google Scholar]
- Real Decreto Real Decreto 1201/2005 de 10 de Octubre sobre protección de los animales utilizados para experimentación y otros fines científicos. BOE. 2005;252(21 Octubre 2005):34367–34391. https://www.boe.es/buscar/doc.php?id=BOE-A-2005-17344 [Google Scholar]
- Sakagami M., Yuasa R., Yuasa Y. Simple underlay myringoplasty. J. Laryngol. Otol. 2007;121:840–844. doi: 10.1017/S0022215106005561. [DOI] [PubMed] [Google Scholar]
- Santa Maria P.L., Atlas M.D., Ghassemifar R. vol. 15. 2007. pp. 450–458. (Wound Repair and Regeneration: Oficial Publication of the Wound Healing Society and the European Tissue Repair Society). (4) [DOI] [PubMed] [Google Scholar]
- Santa Maria P.L., Redmond S.L., Atlas M.D., Ghassemifar R. Histology of the healing tympanic membrane following perforation in rats. Laryngoscope. 2010;120(10):2061–2070. doi: 10.1002/lary.20998. [DOI] [PubMed] [Google Scholar]
- Spandow O., Hellström S. Animal model for persistent tympanic membrane perforations. Ann. Otol. Rhinol. Laryngol. 1993;102(6):467–472. doi: 10.1177/000348949310200611. [DOI] [PubMed] [Google Scholar]
- Strem S.A., Batra P.S. Preventing myringotomy closure with topical mitomycin C in rats. Otolaryngol. Head Neck Surg. 1999;120(6):794–798. doi: 10.1016/S0194-5998(99)70316-5. [DOI] [PubMed] [Google Scholar]
- Tran Ba Huy P. Chronic otitis media. Natural history and clinical forms. EMC Otolaryngology. 2005;2:26–61. [Google Scholar]
- ULAM . University of Michigan; 2005. Guidelines for Anesthesia and Analgesia of Rats.https://wiki.med.umich.edu/display/ULAMGSOP/Guidelines+on+Anesthesia+and+Analgesia+in+Rats [Google Scholar]
- Wang A.Y., Shen Y., Wang J.T., Friedland P.L., Atlas M.D., Dilley R.J. Animal models of chronic tympanic membrane perforation: a “time-out” to review evidence and standardize design. Int. J. Pediatr. Otorhinolaryngol. 2014;78(12):2048–2055. doi: 10.1016/j.ijporl.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Wang A.Y., Shen Yi, Liew L.J., Wang J.T., von Unge M., Atlas M.D., Dilley R.J. Searching for a rat model of chronic tympanic membrane perforation: healing delayed by mitomycin C/dexamethasone but not paper implantation or iterative myringotomy. Int. J. Pediatr. Otorhinolaryngol. 2015;79:1240–1247. doi: 10.1016/j.ijporl.2015.05.020. [DOI] [PubMed] [Google Scholar]
- Wang A.Y., Shen Yi, Liew L.J., Wang J.T., Unge M., Atlas M.D., Dilley R.J. Rat model of chronic tympanic membrane perforation: a longitudinal histological evaluation of underlying mechanisms. Int. J. Pediatr. Otorhinolaryngol. 2017;93:88–96. doi: 10.1016/j.ijporl.2016.12.028. [DOI] [PubMed] [Google Scholar]