Abstract
Objective
The aim of this study was to discuss the diagnosis and surgical management and their results according to stage of primary bone tumors at ulna and to share our experience on this exceptional location for bone tumors.
Methods
We have retrospectively reviewed our clinics database and identified 23 cases (14 males and 9 females, mean age was 28.9 (range 4–77)) with primary bone tumors and tumor like lesion involvement of ulna. The patients were evaluated according to complaints, type and grade of tumor, treatment, recurrence and functional status.
Results
The most common first referral complaint was constrictive pain in 52.1% of the cases, benign tumors and tumor like lesions of the bone constituted 73.9% whereas malignant bone tumors were 26.1%, 39.1% of the lesions were located in distal end of ulna and the mean follow up was 33.8 months (range 8–172 months). Local recurrence has unexpectedly occurred in 3 benign lesions (13.1%).
Conclusion
Benign bone lesions tend to involve distal and proximal ends, malign bone lesions involve diaphysis mostly. Both benign and malignant diaphyseal lesions of the ulna have better postoperative results regarding the lesions at both ends of ulna. One should also take care of recurrences even after a decade from the primary surgery.
Level of evidence
Level IV, Therapeutic study
Keywords: Ulna, Ewing sarcoma, Giant cell tumor, Primary bone tumors of the ulna, Recurrence
Introduction
Ulna is an extremely rare location for primary bone tumors and tumor like lesions. Rizzoli Archives have shown that ulnar involvement constitutes only 0,9% of all primary bone tumors and tumor like lesions.1 Exner et al reported that the ulna was the primary site of involvement in 24 cases out of approximately 2000 tumor and tumor like bone lesions which are mostly benign.2 The literature includes several case reports for ulnar bone tumors, especially for distal lesions of the ulna.1, 2, 3, 4, 5, 6, 7, 8 However there is no known large case series or articles comprising primary bone tumors and tumor-like lesions of the ulna.
Various treatment options, from curettage and packing with allografts to resection arthroplasties and amputations have been described.2, 5, 7, 8, 9, 10, 11, 12, 13, 14
Our study aims to discuss the diagnosis and surgical management and their results according to stage of primary bone tumors at ulna and to share our experience on this exceptional location for bone tumors.
Patients and method
We have retrospectively reviewed our clinics bone and soft tissue tumors and tumor like lesions database from 1981 to 2013 and 39 patients with bone tumor of the ulna were identified. Operated cases are included, metastatic and multiple lesions affecting ulna were excluded. Eight cases are excluded for not being primary ulnar lesions of five with hereditary multiple exostosis, two with multiple myeloma and a patient with multiple metastasis lung carcinoma. We have identified 23 cases with primary bone tumors and tumor like lesion involvement of ulna (Table 1). Primary complaints of the patients are categorized as pain, swelling, deformity and incidentally found lesions are indicated. The lesions are grouped according to their sublocalization in ulna by radiologic assessment. Operation records are retrospectively evaluated and recurrences are noted. Further classification was performed by interpretation of radiology, histopathologically revealed diagnosis and Enneking grading of bone and soft tissue tumors. Patients were monitored regarding severity of the tumors. Benign tumors were followed by a standard follow-up protocol of our clinic as; 2 weeks, 6 weeks, 3 months, 6 months, 1 year and annual controls for 5 years postoperatively. Malignant tumors were followed as; 2 weeks, 6 weeks, once in 3 months for 2 years and annual controls after 2 years postoperatively. X rays are evaluated in each visit and magnetic resonance images are controlled at 3rd, 6th months and annually in malignant lesions. Working abilities of the patients and recurrences, if any, were assessed with MSTS (Musculoskeletal Tumor Society Scoring) for upper extremity lesions in postoperative long-term follow-up.15
Table 1.
Demographical data of the patients in this study. Abbreviations used to indicate tumor types; ABC: Aneurysmal bone cyst, GCT: Giant cell tumor of bone, UBC: Unicameral bone cyst, CS: Chondrosarcoma, OC: Osteochondroma, IOGC: Intraosseous ganglion cyst, EWS: Ewing sarcoma, EC: Enchondroma, OS: Osteaosarcoma.
| Patient No. | Age | Gender | Complaint | Location | Tumor type | Enneking Stage | Treatment | Follow-up time (mo) | MSTS score | Recurrence | Management of recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 11 | Male | Swelling | Proximal | ABC | 3 | Curettage, Grafting | 14 | 8 | N/A | N/A |
| 2 | 27 | Female | Swelling | Distal | GCT of Bone | 3 | Curettage, Grafting | 172 | 9 | 14th year | Resection |
| 3 | 4 | Female | Swelling | Distal | ABC | 2 | Curettage, Grafting | 52 | 11 | N/A | N/A |
| 4 | 28 | Male | Pain | Proximal | UBC | 2 | Curettage, Grafting | 19 | 12 | N/A | N/A |
| 5 | 64 | Male | Pain | Mid-diaphysis | CS | IB | Curettage, Grafting | 50 | 16 | N/A | N/A |
| 6 | 16 | Male | Deformity | Distal | OC | 3 | Resection | 30 | 17 | N/A | N/A |
| 7 | 40 | Female | Pain | Mid-diaphysis | CS | IA | Resection, Vascularized Fibula | 31 | 18 | N/A | N/A |
| 8 | 6 | Male | Deformity | Distal | OC | 3 | Resection | 125 | 19 | 10th year | Resection |
| 9 | 56 | Female | Pain | Distal | ABC | 2 | Curettage, Grafting | 16 | 22 | N/A | N/A |
| 10 | 4 | Male | Pain | Proximal | ABC | 3 | Curettage, Cementation | 36 | 23 | N/A | N/A |
| 11 | 24 | Male | Swelling | Distal | GCT of Bone | 2 | Curettage, Grafting | 12 | 24 | N/A | N/A |
| 12 | 30 | Male | Pain | Mid-diaphysis | CS | IA | Resection, Vascularized Fibula | 32 | 25 | N/A | N/A |
| 13 | 42 | Male | Incidental | Distal | IOGC | 1 | Curettage | 10 | 26 | N/A | N/A |
| 14 | 77 | Female | Incidental | Proximal | UBC | 1 | Curettage, Grafting | 8 | 26 | N/A | N/A |
| 15 | 53 | Female | Deformity | Proximal | OC | 2 | Resection | 16 | 26 | N/A | N/A |
| 16 | 13 | Male | Deformity | Mid-diaphysis | OC | 2 | Resection | 16 | 27 | N/A | N/A |
| 17 | 22 | Male | Pain | Mid-diaphysis | EWS | IIB | Resection, Vascularized Fibula | 15 | 27 | N/A | N/A |
| 18 | 13 | Male | Pain | Proximal | ABC | 3 | Curettage, Grafting | 23 | 27 | 15th month | Curettage, Grafting |
| 19 | 18 | Female | Swelling | Distal | OC | 2 | Resection | 48 | 29 | N/A | N/A |
| 20 | 13 | Male | Pain | Mid-diaphysis | EC | 2 | Resection | 20 | 30 | N/A | N/A |
| 21 | 26 | Female | Pain | Mid-diaphysis | EC | 2 | Curettage, Grafting | 13 | 30 | N/A | N/A |
| 22 | 57 | Male | Pain | Distal | OS | III | Amputation | 9 | NA | N/A | N/A |
| 23 | 21 | Female | Pain | Proximal | OS | IIB | Amputation | 12 | NA | N/A | N/A |
Results
There were 14 male and 9 female patients with the mean age of 28,9 (range 4–77) at admittance. Lesions were located on the right side in 13 cases (56,5%) and on the left side in 10 cases (43,5%). 17 of 23 patients had lesions on dominant side. The most common first referral complaint was constrictive pain in 12 of 23 cases (52,1%) in both benign and malignant lesions followed by swelling (21,7%) and deformity (17,3%). Lesions were also encountered incidentally (8,9%). In malignant lesions of our cases pain was the leading complaint. 39,1% of the lesions were located in distal end of ulna whereas, remaining lesions were evenly located in both proximal and diaphyseal regions. Most of the swelling complaints were related with distal involvement however diaphyseal lesions referred with pain mostly. Mean follow up was 33,8 months (range 8–172 months). Patients were called for a final follow-up and evaluation. 2 of the 23 cases were deceased because of grade IIB and III osteosarcoma at 9th and 12th months respectively.
Benign tumors and tumor like lesions of the bone constituted 73,9% whereas malignant bone tumors were 26,1%. Regarding tumor like lesions of bone; 5 cases were aneurysmal bone cyst (ABC) (21,7%), 2 were unicameral bone cysts (8,6%) and a case was intraosseous ganglion cyst (4,3%). For benign bone tumors, 5 patients had osteochondroma (21,7%), 2 had enchondroma (8,6%) and 2 had giant cell tumor (GCT) of bone (8,6%). Considering malignant involvement; 3 cases had chondrosarcoma (13%), 2 cases had osteosarcoma (8,6%) and a case had Ewing sarcoma (4,3%).
According to radiologic and histopathologic assessment, Enneking classification of bone and soft tissue tumors is applied for each lesion. 2 cases were stage 1, 9 lesions were stage 2, 6 lesions were stage 3, 2 lesions were stage IA, a lesion was IB, 2 lesions were IIB and a lesion was stage III. In our series, benign lesions were seem to have slight tendency to affect distal (47,1%) and proximal (26,1%) regions rather than diaphyseal (17,8%) involvement. Except for 2 deceased patients, who had a proximal and a distal involvement of osteosarcoma respectively, all malignant lesions (3 chondrosarcomas and a Ewing sarcoma) were diaphyseally located.
Intralesional excision was the most common treatment method performed in 10 patients (43,4%) of our study, followed by marginal resections in 8 patients (34,8%), wide excision in 3 patients (13,1%) and amputation in 2 patients (8,7%). ABC was the most common indication for intralesional excision constituting 5 of 12 cases (41,7%). Marginal resection was performed for osteochondroma mostly (62,5%). The only malignant tumor that performed marginal excision was grade IB chondrosarcoma. Wide resection was performed for 2 chondrosarcomas and a Ewing sarcoma (Fig. 1). Amputations were performed exceptionally for 2 grade IIB and III osteosarcomas.
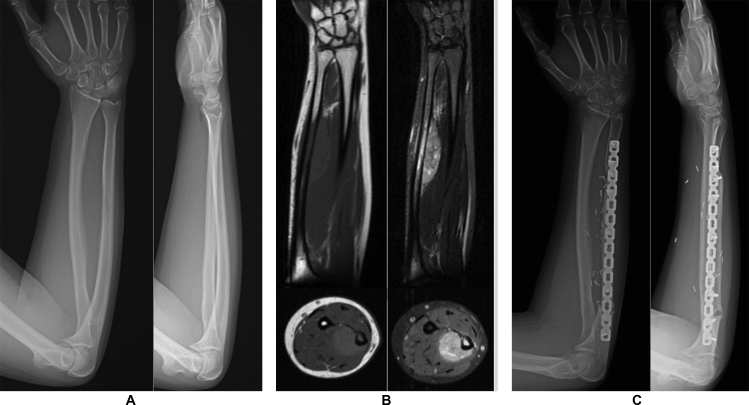
Fig. 1.
A 24-year-old case vignette from our series, who admitted with constricting pain in right forearm. A) Anteroposterior and lateral views of the case was not specifically diagnostic. B) Magnetic resonance images of the case demonstrated a diaphyseal lesion with soft tissue involvement showing low signal intensity on T1 sequences and high signal intensity on T2 sequences. C) Following standard Ewing sarcoma chemotherapy protocole, the patient was applied resection and reconstructed by vascularized fibula.
When working and household abilities are asked to the patients, they have declared the result as whether satisfactory or limited in their last follow up. Mean MSTS score was 21,5 ± 5,9. The best results, with no limitations, were achieved in diaphyseal involvement group, in which 4 of 7 cases were malignant lesions. The worst results were encountered in distal end involvement group, in which 4 of 9 cases declared limited working ability. Consequently, 6 cases (26,1%) had difficulties in either pronation or supination.
Local recurrence has occurred in 3 patients (13,1%) only and unexpectedly the recurred lesions were all benign which consist of a GCT of bone, an ABC and an osteochondroma. Mean recurrence time was 103,6 months (range 15–168 months). The treatment choices were curettage and allograft packing in GCT of bone and ABC cases whereas marginal resection in osteochondroma case. The GCT of bone recurred 14 years after the initial treatment.
Discussion
Ulnar involvement of primary bone tumors and tumor like lesions are extremely rare consisting 1% of all lesions.1, 9 Rizzoli archives, have shown that ulnar involvement constitutes only 0,9% of 19.689 primary bone tumors and tumor like lesions.1 Exner et al reported 24 primary ulnar involvements of bone tumors and tumor like lesions from Balgrist tumor registry.2 Our study indicated 23 primary ulnar involvement cases out of 3500 (0,06%), who had been operated for bone and soft tissue tumors in our clinic between years 1981–2012. When compared with other case series of single center experiences, we believe that this study would make a good contribution to literature.
Ulna is a long bone with a prismatic body, and two ends. Diaphyseal part of ulna has a compact tissue which thickens along the interosseous border and dorsal surface. At both ends this compact layer thins and proximally continues as a plate of close spongy bone.16 The thinned cortices, disturbed continuity of the periosteum and existence of wide spongious bone might be the cause that proximal and distal end tumors become greater in size before they are diagnosed.2 In our study, cases with swelling and deformity complaints and also incidentally found lesions were mostly distal involvement (7 in 11 cases, 63,6%) followed by proximal involvement (3 in 11 cases, 27,3%). Diaphyseal involvements commonly presented with pain (6 in 7 cases, 85,7%), which is thought to be related with thick osseous borders and periosteal distention.
The literature includes few case reports most commonly hereditary multiple exostoses and intraosseous ganglion cysts.4, 17 Besides synovial chondromatosis, desmoplastic fibroma of bone, solitary bone cysts, giant cell tumors, adamantinoma, parosteal osteosarcoma and fibroblastic osteosarcoma are also reported.2, 3, 6, 7, 8, 9 Exner et al have reported a large series about ulna tumors however; the report was only including osseous lesions of the distal ulna.2 Because multiple benign lesions (e.g. hereditary multiple exostoses) and metastatic lesions can affect any bone; our series included primary bone tumors and tumor like lesions of the ulna exclusively.
The two most common benign lesions in our study were solitary osteochondroma and aneurismal bone cysts. Although reported case series indicate that hereditary multiple exostoses are common tumor and tumor like lesions of ulna; solitary involvement is extremely rare.18 Shapiro et al have found an incidence of 80% distal ulnar and 37% proximal ulnar involvement in hereditary multiple exostoses.17 Aneurysmal bone cysts are reported in the literature with few case reports found on either proximal or distal ends as in our study, congruously.10 Rizzoli archives include only one primary chondrosarcoma of the ulna in their 1572-chondrosarcoma series.1 As reported in the literature by Cooper and Schajowicz, different forms of chondrosarcoma in ulna were also available.11, 12 Our study included 3 low-grade diaphyseal chondrosarcoma cases, which was also the most encountered malignancy of bone at ulna. Reported primary ulna bone malignancies indicate that most of the lesions originate from either proximal or distal ends.1, 5, 6, 13 The malignant bone tumors of the ulna in our series were commonly originated from the diaphysis (4 in 6 cases, 66,7%) unlikely.
In the recent literature aggressive benign and malignant lesions are reported mostly.2, 5, 6, 7, 8, 9, 10, 11, 12, 13 Our series indicated 9 of 17 benign lesions as Enneking 2 lesions. And malignancies were mostly low grade (3 of 6 lesions were Enneking IA or IB). Curettage and allograft packing was the most common treatment method in benign tumors and tumor like lesions of ulna in accordance with the literature. However in malignancies; en-bloc resection and vascularized fibular autograft technique was the choice of treatment commonly (3 of 6 lesions). Amputation was performed in 2 high grade, late stage osteosarcoma patients who we consequently lost during follow up period because of lung metastases.
Recurrence rates depend mostly on histopathological type, grade and treatment method of lesions. The local recurrence rates of ABC were reported to be 17–37%.10 The risk of local recurrence is relatively higher in giant cell tumors of distal ulna.18 Poor prognostic results for recurrent giant cell tumors are associated with pathologic fractures, subchondral lesions and malignant transformation.19 Higher recurrence rates are reported with curettage, cauterization and grafting or cementation when compared to resections in GCT of bone.20 Our case recurred at 14th year after the primary treatment. The local recurrence of solitary osteochondroma is most commonly associated with inadequate resection of perichondral ring in skeletally immature patients.21
In conclusion, primary benign or malignant bone tumors of ulna are extremely rare. Due to its close relation with major neurovascular vascular structures intraoperative care should be taken. One should also take care of recurrences even after a decade from the primary surgery. Except giant cell tumors of bone, benign aggressive tumors of the ulna can be managed by curettage and allograft packing, whereas malignant tumors require wide or en bloc resections and reconstructions or amputations regarding histopathologic grade and stage. Benign bone lesions tend to involve distal and proximal ends, whereas malign bone lesions involve diaphysis mostly. Both benign and malignant diaphyseal lesions of the ulna have better postoperative results when compared to the lesions at both ends of ulna.
Conflict of interest
The authors of this study do confirm no conflict of interest.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
References
- 1.Picci P., Manfrini M., Fabbri N. Springer Science & Business Media; 2014. Atlas of Musculoskeletal Tumors and Tumorlike Lesions: The Rizzoli Case Archive; pp. 5–8. [Google Scholar]
- 2.Exner G.U., von Hochstetter A.R., Honegger H. Osseous lesions of the distal ulna: atypical location–unusual diagnosis. Arch Orthop Trauma Surg. 2000;120(3–4):219–223. doi: 10.1007/s004020050049. [DOI] [PubMed] [Google Scholar]
- 3.Erschbamer M., Bode B., Buck F.M. A rare periosteal diaphyseal lesion of the ulna. Open Orthop J. 2012;6:8–10. doi: 10.2174/1874325001206010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein A., Mogan J. Intraosseous ganglion of the distal ulna. J Hand Surg. 1987;12(6):1101–1103. doi: 10.1016/s0363-5023(87)80124-7. [DOI] [PubMed] [Google Scholar]
- 5.Cooney W.P., Damron T.A., Sim F.H. En bloc resection of tumors of the distal end of the ulna. J Bone Joint Surg Am. 1997;79(3):406–412. doi: 10.2106/00004623-199703000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Thirupathi R.G., Vuletin J.C., Wadwa R. Desmoplastic fibroma of the ulna. A case report. Clin Orthop Relat Res. 1983;(179):231–239. [PubMed] [Google Scholar]
- 7.Park Y.K., Ryu K.N., Han C.S. Multifocal, metachronous giant-cell tumor of the ulna. A case report. J Bone Jt Surg Case Connect. 1999;81(3):409–413. doi: 10.2106/00004623-199903000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Gianoutsos M.P., Marsden F.W., McCarthy S.W. Ulnar adamantinoma: en bloc excision and fibular osteoseptocutaneous free flap reconstruction. J Hand Surg. 1994;19(3):495–499. doi: 10.1016/0363-5023(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 9.Maccauro G., Tulli A., Prezioso V. Parosteal osteosarcoma of the ulna: a rare low-grade malignant neoplasm. Case report and review of the literature. J Orthop Traumatol. 2006;7(4):198–200. [Google Scholar]
- 10.Ozger H., Akgul T., Yildiz F. Unusual localization of an aneurysmal bone cyst in ulnar coronoid process. Acta Orthop Traumatol Turc. 2012;46(2):144–147. doi: 10.3944/AOTT.2012.2630. [DOI] [PubMed] [Google Scholar]
- 11.Cooper R.R. Juxtacortical Chondrosarcoma: a case report. J Bone Jt Surg Case Connect. 1965;47(3):524–528. [PubMed] [Google Scholar]
- 12.Schajowicz F. Juxtacortical chondrosarcoma. J Bone Jt Surg Br. 1977;59(4):473–480. doi: 10.1302/0301-620X.59B4.270475. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe S.W., Mih A.D., Hotchkiss R.N. Wide excision of the distal ulna: a multicenter case study. J Hand Surg. 1998;23(2):222–228. doi: 10.1016/s0363-5023(98)80117-2. [DOI] [PubMed] [Google Scholar]
- 14.Sung H.W., Kuo D.P., Shu W.P. Giant-cell tumor of bone: analysis of two hundred and eight cases in Chinese patients. J Bone Joint Surg Am. 1982;64(5):755–761. [PubMed] [Google Scholar]
- 15.Enneking W.F., Dunham W., Gebhardt M.C. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;(286):241–246. [PubMed] [Google Scholar]
- 16.Thompson J.C. Elsevier Health Sciences; 2009. Netter's Concise Orthopaedic Anatomy; p. 89. [Google Scholar]
- 17.Shapiro F., Simon S., Glimcher M.J. Hereditary multiple exostoses. Anthropometric, roentgenographic, and clinical aspects. J Bone Joint Surg. 1979;61(6):815–824. [PubMed] [Google Scholar]
- 18.Bock G.W., Reed M.H. Forearm deformities in multiple cartilaginous exostoses. Skeletal Radiol. 1991;20(7):483–486. doi: 10.1007/BF00194241. [DOI] [PubMed] [Google Scholar]
- 19.Cho H.S., Park I.H., Han I. Giant cell tumor of the femoral head and neck: result of intralesional curettage. Arch Orthop Trauma Surg. 2010;130(11):1329–1333. doi: 10.1007/s00402-009-1026-2. [DOI] [PubMed] [Google Scholar]
- 20.Klenke F.M., Wenger D.E., Inwards C.Y. Giant cell tumor of bone: risk factors for recurrence. Clin Orthop Relat Res. 2011;469(2):591–599. doi: 10.1007/s11999-010-1501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz H.S., editor. OKU, Orthopaedic Knowledge Update: Musculoskeletal Tumors 2. American Academy Of Orthopaedic Surgeons; 2007. p. 107. [Google Scholar]