Abstract
Primary vitreoretinal lymphoma (PVRL) is a rare ocular lymphoid malignancy, which consists a subset of primary central system lymphoma (PCNSL) and the most common type of intraocular lymphoma. The involvement of eyes is estimated to be approximately 20% of PCNSL, but the brain involvement may be up to 80% of PVRL. Typically, PVRL is a high grade B-cell malignancy of the retina and needs to be assorted from choroidal low-grade B-cell lymphomas. Very often PVRL masquerades and can be erroneously diagnosed as chronic uveitis, white dot syndromes or other neoplasms. Establishing an accurate diagnosis may involve cytology/pathology, immunohistochemistry, flow cytometry, molecular pathology and cytokine profile analysis. There is inadequate information about PVRL’s true incidence, ethnic/geographical variation and pathogenetic mechanisms. The therapeutic approach of PVRL involves aggressive chemotherapy and radiation therapy. Although PVRL tends to have a good response to the initial treatment, the prognosis is poor and the survival restricted due to the high relapse rates and CNS involvement.
Keywords: B-cell lymphoma, Primary vitreoretinal lymphoma, Intraocular lymphoma, Primary CNS lymphoma, Masquerade syndrome
Introduction
Intraocular lymphomas are a heterogeneous group of malignant lymphoid neoplasms, which are subcategorized into 2 main groups: (1) those deriving from the vitreoretinal tissue and (2) those that arise in the uveal tract.1 The lymphomas of the retina and/or vitreous are primary tumors and often correlated with central nervous system (CNS) disease. Conversely, uveal lymphomas are subdivided to those that present as a primary disease or as a secondary localization of systemic non-Hodgkin lymphoma (NHL).1, 2 This review focuses only on primary vitreoretinal lymphomas (PVRL), formerly defined as primary intraocular lymphoma (PIOL), and consists an analysis of the current literature and our experience [Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7].
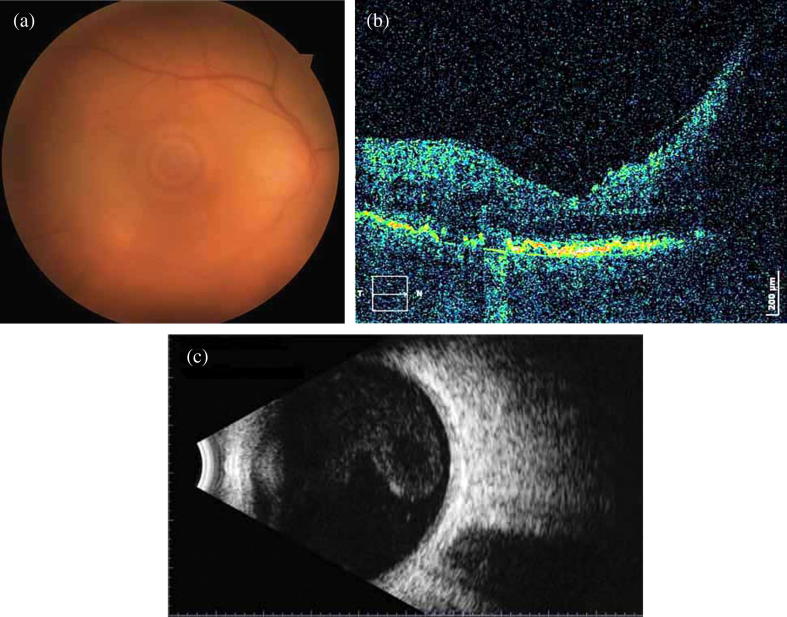
Fig. 1.
(a) Fundus image with optic disc edema and obscured details due to vitreous haze in a 65 year-old lady (affected right eye), (b) SD-OCT: papillomacular bundle edema, nodular hyper-reflective infiltrations on the level of retinal pigment epithelium (RPE) along with partial destruction of RPE, c) B-mode of the same eye: moderately severe vitritis.
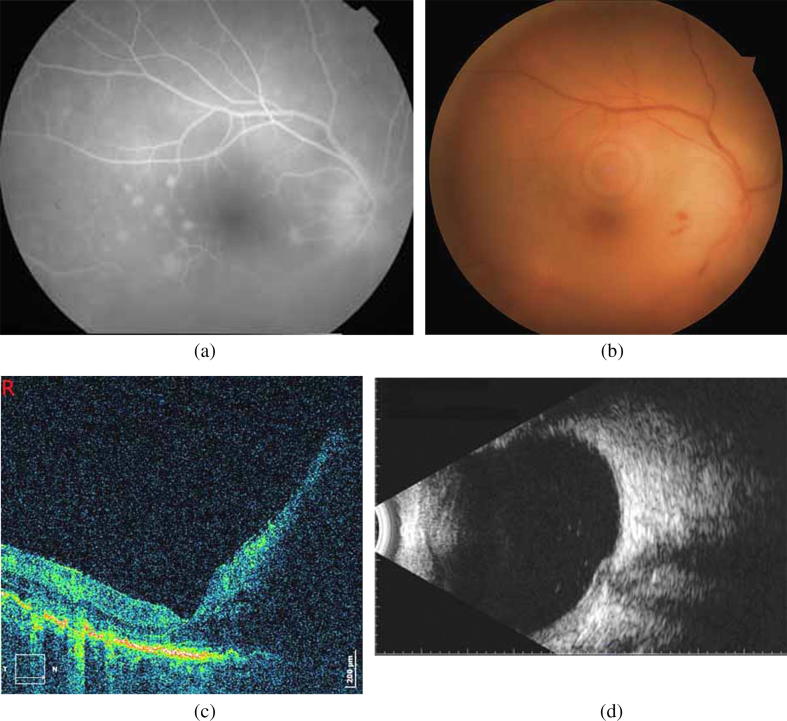
Fig. 2.
(a) Fluorescein angiography: optic disc edema and hyperfluorescent dots in the posterior pole, (b) Signs of retinal vasculitis (hemorrhages) and optic disc edema extended mainly to the nasal area of the posterior pole, (c) SD-OCT: increased papillomacular bundle edema compared to the initially documented (Fig. 1b) (d) B-mode: remnants of vitritis.
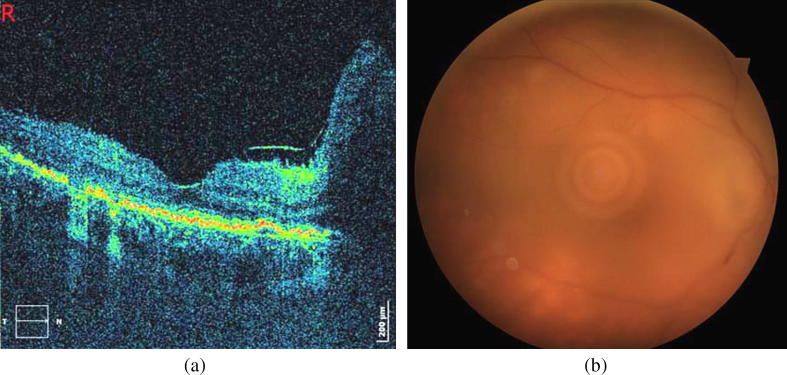
Fig. 3.
(a) SD-OCT 1 week before the end of the treatment: significant decrease of papillomacular bundle edema, along with reduction of number of hyper-reflective infiltrations. (b) Clouding of fundus details (VA: counting fingers at 0.5 m) due to recurrence of vitritis, with a deterioration of optic disc edema and signs of vasculitis. Yellow retinal infiltrates are increased in number.
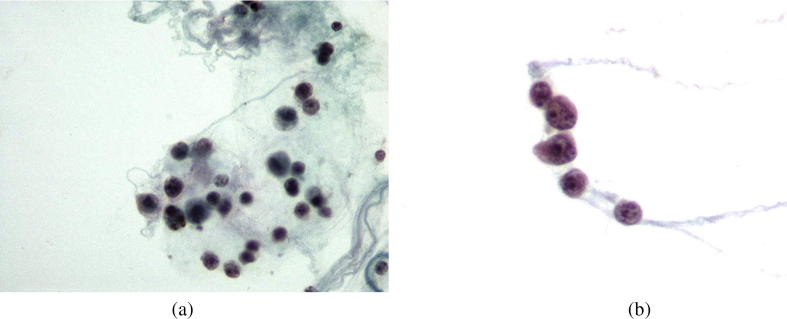
Fig. 4.
(a) Lymphoid cells of medium size admixed with histiocytes. (Thin Prep smear, Papanicolaou stain; X 600), (b) Lymphoid cells with atypical morphologic features. (Thin Prep smear, Papanicolaou stain; X 600).
Fig. 5.
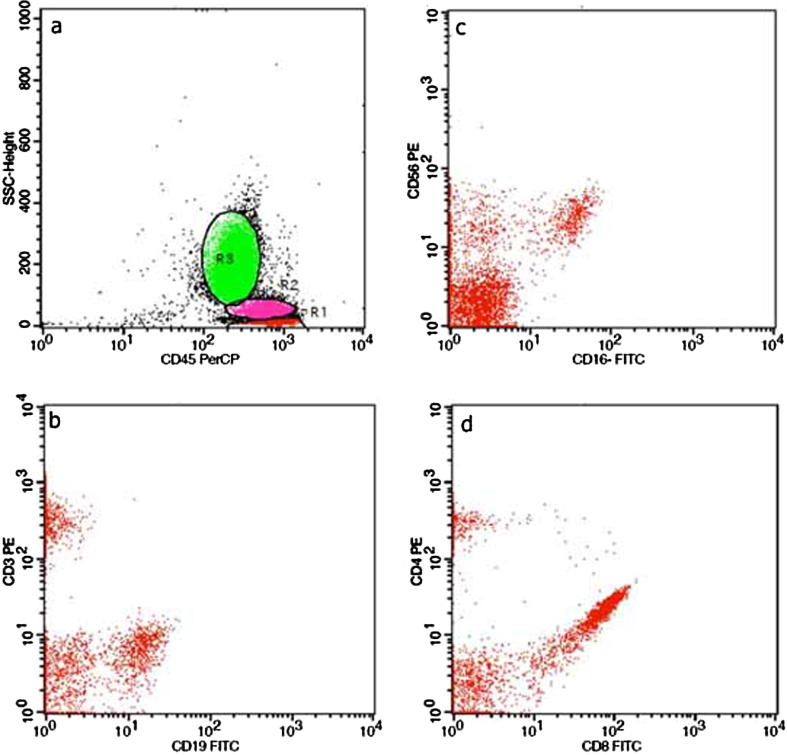
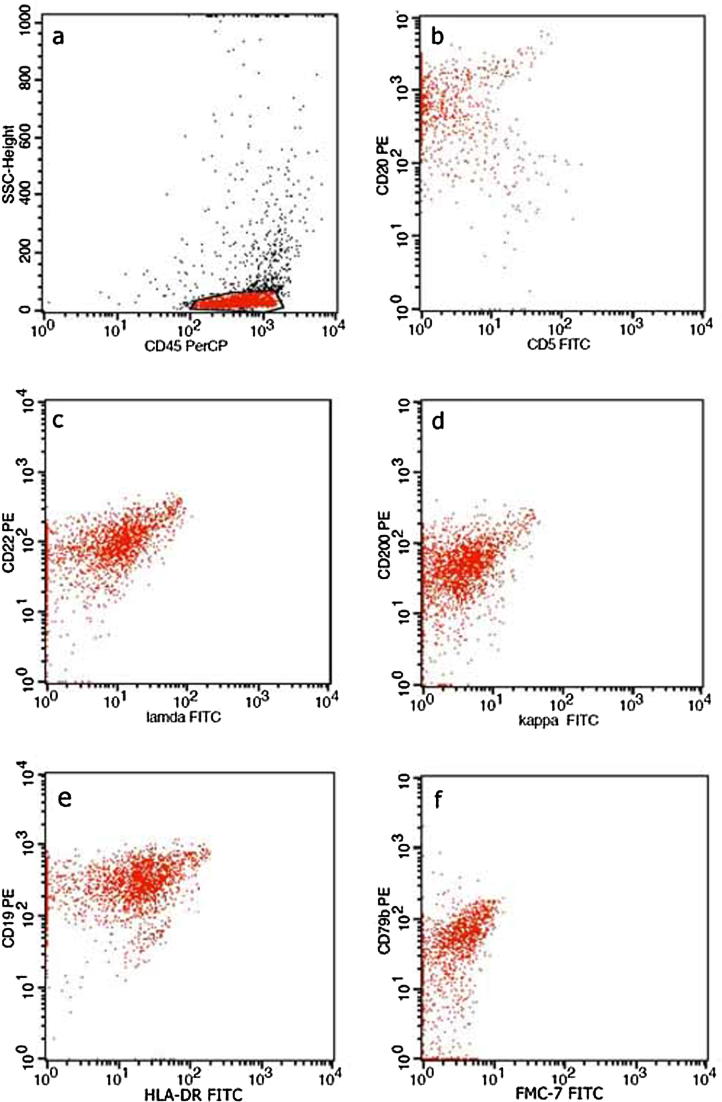
(a) Characteristic dot plots of CDs expression on peripheral blood lymphocytes subpopulations T- lymphocytes (CD3+) (b), B-lymphocytes (CD19+) (b), NK cells (CD16+56 positive) (c), T -helper cells (CD4+) and T -cytotoxic cells (CD8+) (d). Fig. 5b. (a) Characteristic dot plots of CDs expression on vitreous aspirations, showing the characteristic phenotypic profile CD20+ CD5- (b), CD22+ CD λ dim+ (c), CD200+, CDK- (d), CD19+ HLADR+ (e), FMC7- CD79b+ (f). The above findings consist of B lymphoma cells.
Fig. 6.
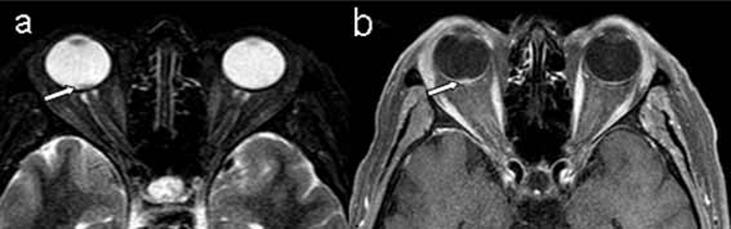
(a) STIR-weighted transverse image, (b) T1-weighted transverse image after contrast injection reveal a small enhanced mass lesion in the dorsal portion of the right eye ball (arrows).
Fig. 7.
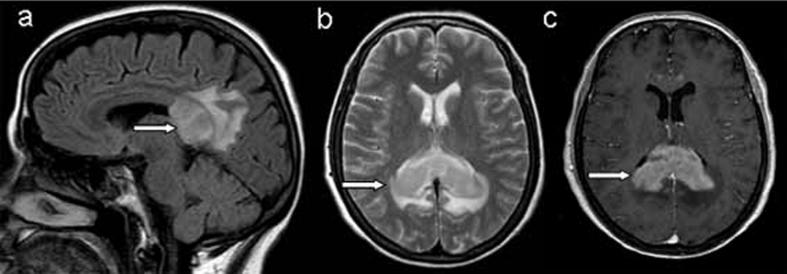
(a) Sagittal T2-weighted FLAIR image in the midline shows an extensive mass lesion in splenium of the corpus callosum, (b) Axial T2-weighted image reveals an infiltrating mass lesion without evidence of necrosis, (c) Axial T1-weighted image after contrast injection (Gd-DTPA 0.1 mmol/kg) shows intensely and diffusely enhancement of the lesion (arrows). The magnetic resonance imaging findings are compatible with lymphoma.
PVRL are rare tumors but still the most frequent type of intraocular lymphoma. The estimated annual incidence is 0.46 per 100,000 person.4 It is an aggressive high-grade NHL and has a strong relation to primary CNS lymphoma (PCNSL). According to the current World Health Organization (WHO) classification for lymphomas the majority of PVRL are diffuse large B-cell lymphomas.5 It has been estimated that about 80% of PVRL individuals will finally develop PCNSL and 20% of PCNSL cases present with PVRL. Therefore, PVRL is usually fatal due to the ultimate CNS correlation.2 Despite its rare occurrence PVRL remains a diagnostic and therapeutic challenge. The lack of effective therapeutic tools and delay in diagnosis may lead to poor prognosis.5
Epidemiology and risk factors
Most patients with PVRL are over the age of 40 years with the most expected age of onset being at the end of the 5th and 6th decade of life with no clear gender predilection.5 Only a few cases were recorded in infants and teenagers, particularly in those who are immunocompromised either as a result of a treatment or because of human immunodeficiency virus (HIV) infection.1, 2, 3, 5 Immunodeficiency/immunosuppression and Epstein Barr virus (EBV) infection are the only recognized risk factor for the development of PVRL.1, 2, 3, 5 Over the last years, a substantial rise in the incidence of primary CNS lymphomas (PCNSL) has been reported. This trend is probably related not only to the elevated numbers of individuals with immunodeficiency and immunosuppression but also to the improvement of the diagnostic methods.1, 2, 3, 5 At present no racial or regional variations have been documented in PVRL.
Pathogenesis
The etiology of PVRL remains obscure. Some of the potential etiopathogenic factors and mechanisms include infectious agents, homing of neoplastic lymphocytes to the ocular tissue and clonal transformation of polyclonal inflammatory cells.6 It has been suggested that PVRL cells could derive from neoplastic modification of a lymphocyte sub-populations from lymphoid organs. On the other hand, polyclonal reactive lymphocytic proliferations in the CNS might evolve in an aberrant monoclonal malignant cell population that can subsequently lead to malignant lymphoma.7 The analysis of homing mechanisms is important for the understanding of the origin of the lymphoma cells in PVRL. In this respect, chemokines and chemokine receptors have a wide spectrum of biologic activities, such as regulation of leukocyte trafficking, adhesion to extracellular matrix molecules and modulation of hematopoietic cell proliferation.6 Chan et al.,6 investigated the following molecules in eyes with PIOL: stromal cell-derived factor-1 (SDF-1, CXCL12, SCYB12) and B-lymphocyte chemoattractant (BLC); BCA-1; CXCL13, SCYB13) that are chemotactic for human B-cells, and their ligands CXCR4 and CXCR5 are differentially expressed on B-cells, including malignant B cells. High expression levels of CXCR4 and CXCR5 were detected limited to the lymphoma cells.6 In contrast, BLC protein was expressed in the RPE but not in other ocular resident cells. SDF-1 was hardly recognized only in a few RPE cells. CXCR4 and CXCR5 transcripts were found in abundance in lymphoma cells, whereas SDF-1 and BLC transcripts were detected only in the RPE and not the malignant cells. No chemokine expression was detected on the RPE cells in the normal control eye.6 They concluded that chemokines and chemokine receptors selective for B-cells were identified in RPE and malignant B cells, respectively. BLC, and possibly SDF-1, attracts both normal and malignant B-cells while promoting migration of only small numbers of T-cells and macrophages. They proposed that B-cell chemokines may be involved in the pathogenesis of PIOL by selectively attracting lymphoma cells to the RPE from the choroidal circulation.6 On the basis of their data, they suggested that inhibition of B-cell chemoattractants could be a future strategy for the treatment of PIOL.6 Moreover, in PCNSL, interaction of the adhesion molecules lymphocyte function-associated antigen-1 (LFA-1) and intercellular adhesion molecule (ICAM-1) may play a role in lymphoma cells homing to the brain.8
Clinical findings
The onset of PVRL symptoms is often subtle leading to a delayed diagnosis,2, 3, 5 whereas the mean duration of symptoms prior to setting a final diagnosis is approximately 6 months. Interestingly, symptoms may precede diagnosis even by 2 to 3 years before lymphoma is included in the differential diagnosis. It has been emphasized that there is a wide spectrum of non-specific symptoms for patients with PVRL that include blurred vision (40–50%), reduced visual acuity (25–30%) and floaters (20–25%).1, 2 PVRL symptoms can imitate a wide range of other ocular entities and has therefore often been described as a masquerade syndrome. The clinical signs are bilateral, in approximately 60–90% of individuals but may also be initially asymmetrical and uneven, thereby giving the impression of unilateral involvement.1, 2
In many cases, PVRL also masquerades as chronic posterior uveitis.2 The vitreous floaters mentioned above are frequently thought to occur in terms of vitritis or degenerative changes. Steroids are used to treat the suspected vitritis, causing temporary masking of symptoms and consequently delaying the diagnosis of PVRL.2 Inflammation of the anterior segment is not a frequent finding.2, 9 The lymphoma cells, however, may surround the lens and spread into the anterior chamber, causing keratic precipitates, cells and flare.10 Lymphoma cells may aggregate on the iris or the trabecular meshwork, resulting in heterochromia and secondary angle closure, respectively.1, 2 Pseudohypopyon has also been identified but it is a rare manifestation and its pathogenesis is unclear.11 Notably, sheets and clusters of lymphoma cells can be observed in the vitreous cavity. The tumor cells are bigger than the inflammatory cells and do not congregate with the reactive inflammatory cells, leading to the formation of a clinical feature that has been described as “aurora borealis” appearance due to the lining of cells along the vitreous fibrils.2 Some mild to moderate haze, which is more prominent peripherally or superiorly, might also exist.2, 3 Deposits of lymphoma cells may primarily gather around the blood vessels of the retina presenting as perivascular sheathing and therefore imitating vasculitis.12 A bigger number of creamy deposits can also emerge over the course of time, mimicking a white-dot syndrome or drusen.3, 12 These deposits can also lead to the development of “punched-out” or atrophic RPE lesions.12 With the progression of the disease, the lymphoma cells fuse together and grow at a level that they gradually substitute the whole thickness of the retina that becomes gradually opaque in the corresponding areas. Lymphoma cells are able to proliferate under the RPE along the inner side of the Bruch’s membrane (i.e. subretinal space). However, PVRL extends into the choroid only in rare cases, as the Bruch’s membrane appears to be impermeable by the lymphoma cells.12 The lymphoma cells near the retinal vessels and choriocapillaris tend to survive, but those that are not close to the blood supply undergo apoptotic cell death. The development of PVRL close to the macula may appear as disciform scar. Orbital, extrascleral and sclerochoroidal involvement are regarded as infrequent but may be also observed. Involvement of the optic nerve is also rare and causes optic disc oedema that must discerned from papilledema.12
A wide range of secondary conditions related to PVRL have been reported. For example, retinal infarcts and hemorrhages may be caused due to vascular occlusions, mimicking acute retinal necrosis or retinitis. The retinal vasculopathy can lead to macular edema, exudation and serous retinal detachment.1, 2, 3 Painful and red eye, secondary glaucoma and hyphema can also develop as a result of iris neovascularization.1 These features might be falsely attributed to uveitis making the diagnostic approach complicated and challenging.
CNS lymphoma can precede, follow or occur at the same time with PVRL. CNS lymphoma can be focal and/or diffuse and is typically observed in the frontal lobes, causing behavioral changes or even new-onset seizures.1, 2, 3, 12 Neurological semiology such as hemiparesis (40–50%) and cerebral signs (15–40%) can be observed. Moreover, diffuse leptomeningeal entanglement may lead to several motor and sensory deficits. Consequently, PVRL should always be incorporated in the differential diagnosis in cases with uveitis and neurological symptoms.12
Imaging findings
Optical coherence tomography (OCT)
OCT facilitates the detection of retinal abnormalities related to PVRL.13 Lymphoma cells invade the retinal tissue and proliferate in the subretinal space. These lymphomatous infiltrates are illustrated as homogenous semi-opaque greyish spots in fundus photography and can be observed as hyper-reflective signals (nodules, bands and nods) at the level of RPE on OCT scan.13, 14 Interestingly, Casady et al. reported nodular or band hyper-reflective spots in 43% of eyes with PVRL.15 Nevertheless, it is important to differentiate these hyper-reflective spots from those which can be detected in other clinical entities (e.g. diabetic retinopathy, age-related macular degeneration etc.).16, 17, 18 Barry et al. summarized a range of spectral domain (SD) OCT findings consistent with PVRL, including hyper-reflective subretinal infiltration, hyper-reflective infiltrates in inner retinal layers, RPE undulation, clumps of vitreous cells and sub-RPE deposits. Interestingly, the hyper-reflective subretinal infiltrates present with a distinct appearance to PVRL, with characteristics not observed in any other clinical entities.15 Cystoid macular edema is a rare finding.19 OCT scan is non-invasive imaging method that can be used for monitoring and record the resolution of PVRL or potential relapses.1, 2, 3
Fundus fluorescein angiography (FFA) and indocyanine green angiography (ICGA)
The most characteristic feature on FA is hypofluorescent spots that present with the so-called “leopard-spot” appearance (43%).3, 14, 20 In cases with outer retinal involvement FFA indicates early and late hypofluorescence.24 In a study of 44 PVRL patients, punctate hyperfluorescent window defects (55%), round hypofluorescent lesions (34%) and vasculitis (14%) were reported. Cystoid macular edema did not exceed 2%.21 Another study of 53 individuals with PVRL detected clusters of round hypofluorescent areas on FFA, compatible with punctate whitish lesions and round-clustered hypofluorescent lesions that are very rarely revealed by ICGA.20 The positive and negative predictive value of the combined use of FFA and ICGA is 89% and 85%, respectively.20 In addition, fluorescein leakage along retinal vessels and periarterioral staining may also be seen in eyes with PVRL.16 Small hypofluorescent lesions can be seen in ICGA in the early stages of PVRL, but the same lesions become less obvious at later stages of the disease.3 Venkatesh et al. reported a novel FA finding of capillary dropout in a patient diagnosed with PVRL, suggesting that this feature is probably attributed to the occlusion of the retinal vasculature by the tumor cells and their invasion into the inner retinal layers.22
Fundus autofluorescence (FAF)
FAF is non-invasive imaging technique that is used for the topographic illustration of the lipofuscin distribution in the RPE in several disorders of the retina (e.g. AMD).40 FAF may facilitate the detection of the active status of PVRL. Areas of hypofluorescence on FAF images are suggestive of lymphoma cells above the RPE or RPE atrophy, whereas spots of hyperfluorescence represent the overlapping of RPE and neoplastic cells.3, 38, 40 One of the most typical features of lymphoma is a granular pattern of hyperautofluorescent spots encircled by a hypoautofluorescent ring.15 Granular patterns were detected in several retinal areas, but this finding was not restricted to visible tumor location. It is noteworthy, that the corresponding transitions from hyperautofluorescent to hypoautofluorescent spots are observed after intravitreal administration of methotrexate.13, 16 Most probably, the spots of hyperautofluorescence on FAF are associated with the spots of hypofluorescence on FFA (36%) and the hyper-reflective spots on OCT (43%).16 This conformity was recorded at a percentage of 62% among patients with PVRL.12 In 11% of eyes with PVRL blockage by mass lesion has also been described.16
Ultrasound B-scan
There are not specific features for PVRL in ultrasound B-scan. However, B-scan can be very useful when there is limited view and the visualization of the posterior segment is difficult.3 Some of the findings that can be emphasized include elevated chorioretinal lesions, retinal detachment, vitreous debris and enlargement of the optic nerve shadow.3
Neuro-imaging
CNS involvement is present in approximately 16–34% of cases at the time of diagnosis.2, 7, 9, 18 Involvement of the CNS may happen at any stage of the disease, especially at the later stages during the extensive dissemination of PCNSL (7–8% of cases). Taking into consideration that PVRL is a subset of PCNSL, the existence of CNS lesions needs to be excluded. This can be achieved with contrast enhanced magnetic resonance imaging (MRI). A percentage between 42% and 92% of PVRL patients are diagnosed with intracranial lymphoma within a mean interval of 8–29 months. CNS lesions can be detected in 62% of patients with PVRL (especially these that had a relapse).11 Uni- or multi-focal tumorous lesions with either discrete or diffuse margins appear hypodense on T1-weighted and hyperdense on T2-weighted images.3 Consequently, contrast-enhanced MRI is imperative every 3 months after setting a diagnosis in order to detect potential CNS involvement.2 Interestingly, positron emission tomography (PET) and computed tomography (CT) have facilitated the identification of CNS lesions and ocular activity.23 Moreover, as it will be discussed later on, neuro-imaging is very useful when an image-guided stereotactic brain biopsy is required.17
Histological and immunophenotypical findings
Histological features
Most PVRL (95%) are histologically classified as diffuse large B-cell lymphoma (DLBCL).24 Nevertheless, PVRL is a specific type of lymphoma, not only because of its distinct biological and clinical characteristics but also due to its association with PCNSL. There is strong immunophenotypic and genetic evidence that 80–90% of PVRL cases belong to the activated B-cell (ABC) subtype of DLBCL.24
Immunophenotypical features
Apart from the so-called pan-B-cell markers (i.e. CD20, CD79a and PAX5), PVRL express MUM1/IRF4, very often BCL-2 and −6, and are usually negative for CD10 which is GC marker and plasma cell markers (e.g. CD138, Vs38c etc.).25 These immunophenotypical features indicate that PVRL are derived from lymphoid cells at a late stage of the B-cell differentiation in the germinal center (GC).25, 26 Immunoglobulin heavy chains (either IgM or both IgM and IgD) and light chains are monotypically expressed.25 Cellular proliferation and apoptosis are notably high and this feature can be confirmed by the lytic cellular background which is often observed in vitrectomy specimens.12
Cytogenetic and molecular features
In many cases an increased number of somatic hypermutations (SHM) of the re-arranged immunoglobulin (IG) genes can be detected, but there is no evidence for ongoing IG gene hypermutations. Likewise PCNSL, PVRL commonly exhibit immunoglobulin re-arrangements with limited usage of the gene segments IGHV4-34.25, 27 Studies of PVRL based on polymerase chain reaction (PCR) findings have reported an elevated frequency of IGH/BCL-2 re-arrangements due to the t(14;18) chromosomal translocation that defines 85–90% of follicular lymphoma and approximately 30% of DLBCL of GC B-cell type.28 However, this appears to be contradictory to the findings that the majority of PVRL are of ABC type (that normally does not have BCL-2 re-arrangements) and that BCL-2 translocations are infrequent in PCNSL.29 It has been suggested that these findings are associated with a less aggressive PVRL subset.5
BCL-6 translocations occur in 17–47% of PCNSL and it is possible that the activation of this regulator of the GC-reaction may be related to some extend for the arrest in the terminal B-cell differentiation stage in both PCNSL and PVRL.29, 30 Frequent gains on chromosomes 1q, 18q, and 19q and common loses on 6q have been reported in PVRL by using a high resolution single nucleotide polymorphism (SNP) array.31 Notably, the same alterations are also frequently detected in PCNSL.31
Researchers that used conventional techniques or next generation sequencing have detected high frequencies of mutations in MYD88, the gene encoding Myeloid Differentiation Factor 88 (MYD88), a member of the toll-like receptor pathway, and members of the B-cell receptor signaling pathway, including CD79B, leading to a constitutive activation of NF-kB signaling.32, 33 Results from whole exome sequencing indicate a major impact of aberrant somatic hypermutation (SHM) on the mutational profile of PCNSL. SHM is the process by which mutations are introduced into the rearranged immunoglobulin genes of germinal centre B-cells in order to increase the binding affinity of the B-cell receptor. Apart from targets, such as MYC, PAX5 and PIM1, aberrant SHM appears to aim to additional genes in PCNSL, some of which are associated with the CNS development.33
Tuo et al. analyzed the microRNA (miRNA) expression profiles in PVRL with the use Real-Time PCR-based miRNA panel in order to compare findings from uveitic and PVRL vitreous aspirates.34 They reported that miR155 was systematically downregulated in specimens from eyes with PVRL, suggesting that miR155 could be a novel biomarker for PVRL.34 Baraniskin et al. found an upregulation of mir-19b, mir-21 and mir-92 in the cerebrospinal fluid (CSF) of patients with PCNSL in comparison with the CSF of patients with other inflammatory and neoplastic diseases of the CNS.35, 36. Kakkassery et al. showed that the same miRNA panel had reproducible specificity and sensitivity for the establishment of a PVRL diagnosis.37
Intraocular lymphoma animal models
Aronow et al. 2015 et al. in a recent review paper reported that studies in humans may not be suitable for the study of the pathogenetic mechanisms of PVRL because of the rarity of the disease, its inconstant clinical presentation, restricted volume of available ocular fluids and fragility of sampled lymphoma cells.75 Therefore, Aronow et al., 2015 reported that animal models ARE important for gaining further insight in the pathogenesis of intraocular lymphoma and for the development of novel therapeutic modalities.38 Indeed, in a B-cell murine model for intraocular lymphoma, Li et al. (2006)39 carried out intravitreal injection of a human B-cell lymphoma (cell line CA46) in severe combined immunodeficient mice. Flow cytometry showed that the cell line CA46 express important markers for the pathogenesis of PVRL such as C-X-C chemokine receptor type 4 (CXCR4, which binds to stromal cell-derived factor-1), CXCR5 (which binds to B-cell chemoattractant), and CD22 (surface marker on mature and some immature B cells).38 CA46 cells also express the B-cell growth factor/anti-inflammatory cytokine IL-10, which is both a pathogenic factor and an important diagnostic marker for human PVRL.38 Histology revealed tumor development in day 10 after inoculation and lymphoma cells were found in the retinal surface, the subretinal space and into the CNS.39 In this model a single intravitreal injection of immunotoxin HA22 in murine eyes with established lymphoma could result in complete regression lymphoma. This murine model of intraocular lymphoma clinically mimics human PVRL in terms of tissue distribution, expression of important markers and cytokine profile. Moreover, this model showed that B-cell-specific immunotoxin therapy may be significant for treating human. In another study, researchers40 created a murine model using intravitreal inoculation to investigate the immune responses in the microenvironment of intraocular lymphomas. Histology revealed intraocular lymphoma involvement on day 19, mainly within the retina and the vitreous.40 Up to 15% of viable cells were T-cells after the establishment of intraocular lymphoma on day 19. Analysis of the cytokine profile of the supernatant of intraocular cells verified the existence of IL-10, IL-6, IFN-γ, and TNF-α. Stimulation of these intraocular cells with anti-CD3 and anti-CD28 antibodies increased the IFN-γ level and led to a subsequent production IL-2, consistent with a type 1 (Th1/Tc1-like) pattern of cytokine expression.40 These findings showed that TIL from intraocular B lymphoma are defined by a Th1/Tc1-like profile, which was partially inhibited in vivo by a lack of IL-2 in the absence of T-cell stimulation. This potentially indicates a therapeutic role for in situ T-cell stimulation to reactivate Th1/Tc1 lymphocytes and improve intraocular immunity against the progression of lymphoma. Interestingly, Mineo et al. (2008)41 developed a murine model to explore the efficiency of intravitreal and intracerebral anti-CD20 monoclonal antibody (rituximab). In this model, human CD20-transfected murine B-lymphoma cells (38C13 CD20+) were inoculated in the vitreous or in the brain caudate nucleus of immunocompetent syngeneic mice. Inoculation of lymphoma cells into the vitreous or CNS resulted in tumor occurrence after a median of 15 or 22 days, respectively. In more than half of the mice the administration of local rituximab (a chimeric monoclonal antibody against CD20) injections eradicated the lymphoma resulting in a substantial inhibition of tumor progression in the remainder. On the contrary, a) mice that were inoculated with a control cell line (38C13 CD20 negative) showed progression of lymphoma regardless of the rituximab injection and b) mice that were inoculated with 38C13 CD20+ lymphoma cells and subsequently treated with local injection of trastuzumab, an irrelevant antibody against human epithelial receptor type 2, also showed progression of the disease.41 This model of intravitreal and cerebral inoculation of human lymphoma cells in immunocompetent syngeneic mice consists a method for assessing both the pathogenesis and potential treatments of PVRL and PCNSL using local injections of rituximab. More recently, Ben Abdelwahed et al. (2013)42 created a combined intravitreal and cerebral murine model of intraocular lymphoma to investigate the effects of ublituximab, a glycoengineered chimeric monoclonal anti-human CD20 monoclonal antibody that may enhance antibody-dependent cell-mediated cytotoxicity compared to rituximab. A murine lymphoma B-cell line A20.IIA-GFP-hCD20 (H-2 d) was injected into either the cerebral striatum or the vitreous cavity of immunocompetent adult BALB/c (H-2 d) mice. Neoplastic infiltration was detected in the vitreous, CNS and cervical lymph nodes. After 4–7 days, ublituximab (1, 5, or 20 μg) was injected either intracerebrally or intravitreally. A single dose of ublituximab, injected either into the brain or vitreous, caused an important decrease in tumor burden and an increase in survival compared to mice treated with rituximab (20 μg).42 These early results recommend a potential role for ublituximab as treatment for PVRL and PCNSL.
Diagnostic approach
Vitreous biopsy remains the gold-standard for setting a diagnosis of PVRL but if any diagnostic difficulties arise chorioretinal biopsy or subretinal aspirate can be performed simultaneously or at a later stage.3 Surgical intervention for obtaining a sufficient sample may also require aqueous aspiration (e.g. cases with pseudohypopyon) and rarely diagnostic enucleation.43, 44 Prior to the biopsy, a communication between the surgeons and the pathologists is critical in order to confirm the accurate use of the correct containers and preservative solutions.2 The obtained material can be sent for performing a wide range of laboratory examinations including cytological and histopathological examination, immunocytochemistry, flow cytometry, molecular investigations and exploration of cytokine profile.3, 11, 12
Moreover, it is recommended that cytological specimens should be used within 60 minutes after aspiration.45, 46 In cases that a prolonged delay of transportation is expected, the material should be placed into culture medium or a mild cytofixative solution [e.g. Hepes-glutamic acid buffer-mediated organic solvent protection effect (HOPE) solution or Cytolyt].45, 46
Cytological material is routinely prepared onto slides with the cytospin technique, using the May-Grunewald-Giemsa (MGG) morphological stain and unstained cytospins are also prepared for immunocytochemistry.12 Otherwise, the cell-block technique using diluted vitreous humor with embedding in agar or paraffin can be used for cell-rich specimens.47 A study that analyzed cell-block specimens from diluted vitreous fluid showed high sensitivity and low pseudo-positive rate for the cytological diagnosis of VRL. This is a very promising method especially in the differential diagnosis between vitreoretinal lymphoma and idiopathic uveitis with vitreous opacities.47
Vitreous aspiration and pars plana vitrectomy
Vitreous humor can be acquired either by pars plana vitrectomy (PPV) or vitreous aspiration.3, 11, 12 Lymphoma cells are extremely fragile and can easily undergo necrosis. Moreover, when systemic steroids are being used for the treatment of a presumed uveitis, they must be stopped at least two weeks before the vitreous biopsy in order to avoid having false negative results.3
Pars plana vitrectomy is the mostly preferred technique in PVRL-suspected cases, because its diagnostic value is higher than that of vitreous aspiration.43 Particularly, a twenty-five gauge transconjunctival sutureless vitrectomy has been shown to be safe and effective.44 The effect of various vitrectomy cut-rates on viability of lymphoma cells and diagnostic yield was evaluated and a cut-rate of 600 cpm is suggested for stablishing a diagnosis of PVRL.19 A retinal biopsy is rarely performed and, if so, retinal samples should be obtained from the deeper part of the suspicious lesion, in close proximity with the choriocapillaris area where viable neoplastic cells are mostly expected to be detected.43
Tumor seeding via the sclerotomy port to the epibulbar space consists one of the complications of vitreous biopsy.48, 49 Very often more than one biopsy may be needed for establishing a definitive diagnosis, especially when it comes to paucicellular specimens mixed with necrotic lymphoma cells.50
Cyto-histopatholoy, immunohistochemistry and flow cytometry
For the histological diagnosis of PVRL, the use of histochemical stainings such as Giemsa, Hematoxylin-eosin stain, or Diff-Quick staining is a standard practice.1 The MGG-stained cytospins identifies medium to large atypical lymphoid cells with large, irregular nuclei and one to several prominent nucleoli, basophilic cytoplasm, rare mitoses and increased nuclear/cytoplasmic ratio.12 The presence of smaller, round, reactive lymphocytes, macrophages and lytic cells are often interpreted as inflammatory cells (as they may outnumber the tumor cells) and may complicate the diagnosis. It is important to note, that atypical monocytes may also exist in a reactive status (e.g. acute viral infection) and therefore a CD68 stain together with B-cell (CD20, CD79a, PAX5) or T-cell (e.g. CD2 or CD3) markers should be incorporated in the initial work-up.50, 51 The main advantage of the PAX5 immunocytochemical stain is the potential detection of lymphoma B-cells that have breached and do not have any more a distinct membrane that could be highlighted by CD20 stain.51, 52
The sensitivity and specificity rates of cytological examination with regard to the diagnosis of PRVL may differ. Cytological examination alone can establish a diagnosis of PVRL in approximately 45–60% of cases and false positive results are relatively infrequent.51, 52
A diagnosis of PVRL can be enhanced by the detection of monoclonality, which is defined as a population of B-cells displaying κ (kappa) or λ (lambda) light chain restriction.53 Notably, some very rare cases of anaplastic large cell and T-cell types of PVRL have been reported.54, 55
Multicolor flow cytometry may also be used in addition to immunocytochemistry and immunohistochemistry, since there is strong evidence that can be reliable for phenotyping of vitreous aspirates. The advantage of flow cytometry method is that it can simultaneously analyze large numbers of different markers of the cell surface, providing reliable information for the distinction between uveitis and lymphoma. The use of immunoglobulin light chains can facilitate the detection of monotypical expression.56 A ratio of κ:λ light chains of >3 or <0.6 is considered as a reliable and useful marker for clonality. Moreover, ratios of T-cell subsets can often be determined by multiple gating.56, 57
Molecular analysis
Molecular investigations of vitreous samples with PCR using consensus primer sets (e.g. those developed by the BIOMED-2 consortium) for the detection of clonal immunoglobulin gene rearrangements can be very helpful for the identification of clonal population of lymphocytes that can contribute substantially in the confirmation of PVRL diagnosis.58, 59, 60, 61, 62, 63 Some factors, such as the choice of primer sets, laboratory’s experience or the quality of the material may affect the sensitivity of the detection of clonality, which has been reported to vary from 65% to 95%.45, 50, 60, 61, 62 The immunoglobulin heavy chain (IgH; FR2, FR3, and/or CDR3 primers) together with T-cell receptor gamma chain gene rearrangements (TCR-γ) can contribute to the molecular diagnosis of B-cell and T-cell lymphoma, respectively.63 Importantly, a relevant study reported positive IgH re-arrangements in 80.6% in a cohort of 67 individuals with PIOL.60 However, sampling of an excessively small number of cells from microdissection may result in false-positive results.29, 63 False-negative results may also occur in PVRL.64, 63 Moreover, and, in order to avoid misinterpretation of minor clonal expansions as evidence of lymphoma, the results should be evaluated in the context of clinical and morphological features.12
Bonzheim et al.32 reported the common occurrence of MYD88 mutations in PCNSL for the detection of the canonical L265P mutation in approximately 70% of cases. At present, MYD88 mutational analysis is being used by some centers as an alternative to IgH-PCR, especially in cases of low DNA yield.12 However, the absence of MYD88 mutation does not certainly exclude lymphoma if the rest of the pathological findings strongly support such a diagnosis.12
In a very important recent study Cani AK, used next generation sequencing (NGS) and showed MYD88 gain-of-function mutations and loss of CDKN2A in 3 PVRL specimens.64 In the same study, further findings, such as low level gain of chromosome 19 (1 case) or focal loss of AKT1 (1 case) were also described. The results indicate that the design of a NGS-targeted panel could be very useful, particularly in cases of lower quantity samples.65 More specifically, Cani et al.66 demonstrated the feasibility of targeted NGS on intraocular liquid biopsies underlining that their approach does not compromise the volumes needed for cytology-based and other diagnostics (e.g. flow cytometry). Their NGS method can contribute to overcoming difficulties associated with high vitreous viscosity, low cellularity, poor cellular preservation, false-negative and false-positive PCR-based results, which can often delay the diagnosis and therapy.62, 66
MYD88 Gain Of Function (GOF) mutations were detected in 3 out of 4 samples (75%) and high level copy number loss of CDKN2A were detected in all 4 samples (100%).66 All MYD88 mutations occurred in the TIR domain, and 2/4 samples (50%) harbored the p.L265P point mutation.66 Cani et al.66 detected MYD88 p.S243N mutation (1/4 samples, 25%), high level CDKN2A loss (4 out of 4 samples, 100%), and low level PTEN loss (1/4 samples, 25%) for the first time in VRL. Almost all of these alterations are potentially targetable. For example, currently recruiting clinical trials in DLBCLs assessing TLR inhibitors require the MYD88 p.L265P mutation as an entry criterion.66 Both p.L265P and p.S243N MYD88 mutations demonstrate high levels of NF-κB transactivation, which can potentially be targeted by the Bruton’s kinase inhibitor ibrutinib, and IRAK1/4 antagonists.67, 68, 69 MYD88 mutations other than p.L265P make up a quarter of all MYD88 mutations in patients with DLBCLs and unlike other studies that solely evaluated for the p.L265P mutation (and one study that reported p.P258L),63, 70, 71 the comprehensive NGS-based approach of Cani et al.66 reveals other potentially actionable, and diagnostic, GOF MYD88 alterations in VRL. In addition, high level CDKN2A loss is considered potentially targetable, as ilorasertib (an inhibitor of Aurora, VEGF, and PDGF tyrosine kinase families) and palbociclib (a CDK4/6 inhibitor) are being tested in advanced CDKN2A-deficient tumors.66 Although not prioritized, an annotated one-copy loss in the oncogene AKT1 was observed in case 101.66 While oncogenic AKT1 GOF mutations and amplifications have been linked to a variety of cancers,72 another report has found recurrent one-copy losses of AKT1 in DLBCLs.73
Cytokine profile
The measurement of cytokine levels in aqueous and vitreous humor is an adjunct for the diagnosis of PVRL.2, 21, 61, 74, 75, 76, 77 The techniques for this measurement include multiplex-based cytometric bead array and enzyme-linked immunosorbent assay (ELISA). An elevated interleukin-10 (IL-10) level in pure aqueous or vitreous humor specimens or an IL-10:IL-6 ratio higher than 1 is considered as indicative of PVRL.74, 75, 76, 77 The precise cut-off for the IL10 concentration or IL10:IL6 ratio may differ among several laboratories, due to various reasons (type and sensitivity of the techniques and methods applied, conditions of specimen harvesting and storage, equipment etc.).12, 75, 76, 77 A recent study reported that a cutoff of 65 pg/mL and 30 pg/mL IL-10 in the vitreous and aqueous humor, respectively, was correlated with sensitivity of 93% and 78%, respectively, and specificity of 100% and 97%, respectively.78 Moreover, ratio higher than 1 in the vitreous showed sensitivity of 93% and specificity of 100%.78 Another study, showed that patients with primary intraocular lymphoma had higher levels of IL-10 in their aqueous humor in comparison with uveitic patients, as the mean values were 543.4 pg/mL and 21.9 pg/mL, respectively.79 Furthermore, the measurement of interleukins of intraocular fluids has also been suggested as a potential tool for monitoring the response to treatment of patients with PVRL.75, 76, 77
Cerebrospinal fluid (CSF) examination and brain biopsy
Since PVRL is considered as a subset of PCNSL, it is always vital to examine the possibility of CNS involvement. In spite of the fact that the yield of lymphoma cells in CSF may be up to 25% of patients with CNS lesions are found with a positive CSF cytology.23, 79 Detection of lymphoma cells in CSF together with simultaneous ocular involvement precludes the necessity for histological diagnosis of ocular lymphoma and consequently spares the patient from having an invasive procedure (e.g. vitrectomy). On the other hand, image-guided stereotactic brain biopsy should be carried out in existence of suspicious MRI findings but negative CSF cytology.
Differential diagnosis
Very often PVRL presents as a masquerade syndrome making its diagnosis extremely challenging and complicated. The differential diagnosis includes a wide spectrum of infectious and non-infectious conditions such as uveitis, toxoplasmosis, syphilis, tuberculosis, viral retinitis, sarcoidosis, Adamantiades-Behcet’s disease, idiopathic uveitis, endophthalmitis, amelanotic melanoma and metastasis.1, 3 In addition, degenerative diseases of the eye (e.g. polypoidal choroidal vasculopathy and acute macular degeneration) should also be considered as a differential diagnosis.3
Therapeutic approach
There is no established treatment for PVRL so far and many aspects of the management remain controversial. A multidisciplinary assessment of patients with PVRL and/or PCNSL80, 81 is required and, in 2011, the importance of a collaboration between ophthalmologists, pathologists and oncologists (either hemato-oncologists or neuro-oncologists) was highlighted leading to the following guidelines2:
-
(A)
Without systemic or CNS involvement: local treatment (i.e. intravitreal methotrexate, intravitreal rituximab) or low dose stereotactic external beam radiotherapy (30–35 Gy) to the affected eye and follow-up. In case of bilateral involvement, they suggested intravitreal medications together with systemic chemotherapy.2, 82
-
(B)
With CNS involvement: high dose of systemic methotrexate (probably together with systemic rituximab) in combination with local therapy. In case of systemic therapy failure and if patient is not fit for a more aggressive treatment, such as autologous stem cell transplantation (ASCT), ocular and integral brain radiotherapy should be considered.
The Neuro-Oncology Society (National Cancer Action Team Rare Tumor Guidelines, June 2011) recommended a different approach, proposing that administration of high-dose systemic methotrexate followed by whole-globe radiotherapy for ocular only or concurrent individuals and intravitreal methotrexate IS an effective therapeutic approach in cases of isolated ocular recurrences.80, 81
Chemotherapy
The response rates of high-dose methotrexate reach up to 72% as a monotherapy and up to 94–100% when methotrexate is used in combination with other therapeutic modalities.2, 3, 23. Combination of intravitreal methotrexate with high-dose systemic methotrexate was effective in 19 patients with PIOL with a 5-year survival rate of 55.8%.83 Moreover, in individuals with relapsed PIOL and PCNSL chemotherapy with cyclophosphamide, busulfan and thiotepa that was followed by hematopoietic stem cell rescue increased the 5-year survival probability to 62% and achieved complete remission of the disease in 66 out of 79 cases.3 However, according to the 17-Center European collaborative study, systemic chemotherapy in isolated PVRL individuals was associated with various severe complications such as acute renal failure and did not prevent CNS lymphoma.84 Maculopathy is also one of the potential adverse effects of chemotherapy but it does not appear to affect visual acuity significantly.3
Radiotherapy
The low-dose (30–35 Gy) stereotactic external beam radiotherapy is the recommended local treatment for PVRL patients with ocular only involvement.2 Remarkably, no ophthalmic relapses were reported in an overall of 12 individuals that received radiotherapy (30–35 Gy in 15 fractions) in a mean 19-months follow-up.85 In patients with concurrent CNS involvement who did not respond to systemic chemotherapy and are not eligible for more aggressive therapy, whole brain and ocular radiotherapy is suggested.86 However, radiation of the brain may often induce delayed neurotoxicity, ataxia, decline in cognitive function or even death. Ocular radiation can result in early cataract, radiation retinopathy and dry eyes. At present, it remains controversial of whether eye radiation or intravitreal chemotherapy should be applied as a first-line therapy.85
Ocular therapy
-
(a)
Intravitreal methotrexate
Treatment with intravitreal methotrexate is extremely successful in achieving clinical remission of ocular involvement in PVRL with sustainable morbidity. A recent study reported that there were no intraocular recurrences in an overall of 26 PVRL patients that were treated with intravitreal methotrexate as a first-line therapy.87 Intravitreal methotrexate injections at a dose of 0.4 mg/0.1 were administered two times per week for 4 week, once per week for 8 weeks and once monthly for the next 9 months. Remission was recorded after a mean of 6.4 injections. The most frequent complications include transient elevation of intraocular pressure and corneal epitheliopathy that subsided by increasing the intervals between consecutive injections.2, 23
-
(b)
Intravitreal rituximab
Rituximab is an anti-CD20 monoclonal antibody that has been used intravitreally for the treatment of CD20-positive PVRL.88 An overall of 20 eyes with CD20-positive PVRL that developed severe corneal epitheliopathy, secondarily to intravitreal methotrexate, were switched to intravitreal rituximab. These patients had intravitreal rituximab injections at the dose of 1 mg/0.1 mL once weekly for 4 weeks in the terms of a one-course protocol. In spite of the facts that the results were generally auspicious, half of the patients had recurrence of the disease. Thus, rituximab may be an alternative option to methotrexate due to the lower levels of toxicity.89
Emerging therapeutic options
A small number of studies report on the efficacy of high-dose chemotherapy and autologous stem cell transplantation in the treatment of refractory or recurrent PVRL or PCNSL with favorable outcomes.90 However, it remains debatable of whether this approach should be facilitated as consolidation in primary treatment of PVRL.
Thalidomide-related agents have been previously used in the treatment of systemic diffuse large B cell lymphoma.91 Pomalidomide, which is known to have a better penetration into the CNS, has demonstrated efficacy in CNS lymphoma animal models.92
Ibrutinib is a Bruton’s tyrosine kinase (BTK) and HCK tyrosine kinase protein inhibitor that was initially used for the treatment of chronic lymphocytic leukemia (CLL).93 Both of these pathways are upregulated by the MYD88 L265P mutation, which is prevalent in many cases of vitreoretinal lymphoma.94 Ibrutinib has already received an FDA approval for the treatment of Waldenström’s macroglobulinemia WM.95 Due to the fact that both Waldenström’s and PVRL have a similar MYD88 mutation, it might be reasonable to consider this agent in clinical trials for patients with VRL. It must be underlined though, that the simultaneous use of ibrutinib and high-dose steroids has been correlated with invasive fungal and pneumocystis infections.96, 97
The use of lenalidomide plus rituximab for relapsed or refractory PCNSL or PVRL has been reported by an abstract at the American Society of Hematology Annual Meeting in 2016 from the French LOC Network. Results deriving from a phase II trial showed very promising results in the management of VRL.98
Finally, it has been suggested that vitrectomy could also have a therapeutic effect, but there are not adequate data to support that debulking could be considered as a monotherapy. However, it may be beneficial as an adjunct approach in cases with increased cellular load.99
Prognosis
Due to the rarity and the diversity of the disease, there are no consistent reports available with regard to the mortality related to PVRL.3 The mortality rates vary from 9% to 81%.3 Since the 5-year overall survival rate of PVRL is less than 25%, it is obvious that the prognosis of this entity is poor. A multicenter study among 16 centers from 7 countries showed that ocular therapy contributed to an improved control of the local tumor but did not influence survival.100 This study reported an overall survival and median progression-free survival of 31 and 18 months, respectively. The overall mortality was 67.9% (150 of 221 patients).100
Conclusions and future perspectives
The diagnosis and management of PVRL is a challenge, because the rates of morbidity and mortality remain high. As PVRL IS a subset of PCNSL and can be misdiagnosed as uveitis or intraocular inflammation, it is possible that the correct diagnosis cannot be made thereby leading to inappropriate management. However, new advances in imaging and molecular techniques (e.g. PCR detection of monoclonality, detection of cell surface markers by immunocytochemistry and flow cytometry and examination of cytokine profile) have contributed to a more accurate diagnostic approach of PVRL. A more detailed molecular profiling of lymphoma cells from individual patients could probably improve the understanding of pathogenetic mechanisms and the clinical behavior of the tumor. A detailed exploration of the epidemiology, pathophysiology, molecular and cellular biology and genetics of PVRL can potentially improve the management of this highly aggressive disease. Hopefully, new targeted therapies will ameliorate the prognosis of PVRL, especially if diagnosed at an early stage.
Conflicts of interest
All authors declare that there is no conflict of interest.
Funding sources
All authors declare that they received no funding for this study.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Coupland S.E., Damato B. Understanding intraocular lymphomas. Clin Experiment Ophthalmol. 2008;36:564–578. doi: 10.1111/j.1442-9071.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- 2.Chan C.C., Rubenstein J.L., Coupland S.E. Primary vitreoretinal lymphoma: a report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. Oncologist. 2011;16:1589–1599. doi: 10.1634/theoncologist.2011-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sagoo M.S., Mehta H., Swampillai A.J. Primary intraocular lymphoma. Surv Ophthalmol. 2014;59:503–516. doi: 10.1016/j.survophthal.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Aziz H.A., Peereboom D.M., Singh A.D. Primary central nervous system lymphoma. Int Ophthalmol Clin. 2015;55:111–121. doi: 10.1097/IIO.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 5.Fend F., Ferreri A.J., Coupland S.E. How we diagnose and treat vitreoretinal lymphoma. Br J Haematol. 2016;173:680–692. doi: 10.1111/bjh.14025. [DOI] [PubMed] [Google Scholar]
- 6.Chan C.C., Shen D., Hackett J.J., Buggage R.R., Tuaillon N. Expression of chemokine receptors, CXCR4 and CXCR5, and chemokines, BLC and SDF-1, in the eyes of patients with primary intraocular lymphoma. Ophthalmology. 2003;110:421–426. doi: 10.1016/S0161-6420(02)01737-2. [DOI] [PubMed] [Google Scholar]
- 7.Buggage R.R., Chan C.C., Nussenblatt R.B. Ocular manifestations of central nervous system lymphoma. Curr Opin Oncol. 2001;13(3):137–142. doi: 10.1097/00001622-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Bashir R., Coakham H., Hochberg F. Expression of LFA-1/ ICAM-1 in CNS lymphomas: possible mechanism for lymphoma homing into the brain. J Neurooncol. 1992;12(2):103–110. doi: 10.1007/BF00172658. [DOI] [PubMed] [Google Scholar]
- 9.Cassoux N., Merle-Beral H., Leblond V. Ocular and central nervous system lymphoma: Clinical features and diagnosis. Ocul Immunol Inflamm. 2000;8:243–250. doi: 10.1076/ocii.8.4.243.6463. [DOI] [PubMed] [Google Scholar]
- 10.Al Qahtani A., Touitou V., Cassoux N. More than a masquerade syndrome: atypical presentations of vitreoretinal lymphomas. Ocul Immunol Inflamm. 2014;22:189–196. doi: 10.3109/09273948.2013.835427. [DOI] [PubMed] [Google Scholar]
- 11.Zhou M., Xu G. Recent progress in the diagnosis and treatment of primary vitreoretinal lymphoma. Taiwan J Ophthalmol. 2016;6:170–176. doi: 10.1016/j.tjo.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Araujo I., Coupland S.E. Primary vitreoretinal lymphoma - a review. Asia Pac J Ophthalmol (Phila) 2017;6:283–289. doi: 10.22608/APO.2017150. [DOI] [PubMed] [Google Scholar]
- 13.Egawa M., Mitamura Y., Hayashi Y., Naito T. Spectral-domain optical coherence tomographic and fundus autofluorescence findings in eyes with primary intraocular lymphoma. Clin Ophthalmol. 2014;8:335–341. doi: 10.2147/OPTH.S58114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang H.S., Sepah Y.J., Sophie R. Longitudinal spectral domain optical coherence tomography changes in eyes with intraocular lymphoma. J Ophthalmic Inflamm Infect. 2013;3:59. doi: 10.1186/1869-5760-3-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casady M., Faia L., Nazemzadeh M., Nussenblatt R., Chan C.C., Sen H.N. Fundus autofluorescence patterns in primary intraocular lymphoma. Retina. 2014;34:366–372. doi: 10.1097/IAE.0b013e31829977fa. [DOI] [PubMed] [Google Scholar]
- 16.Folgar F.A., Chow J.H., Farsiu S. Spatial correlation between hyperpigmentary changes on color fundus photography and hyperreflective foci on SDOCT in intermediate AMD. Invest Ophthalmol Vis Scis. 2012;53:4626–4633. doi: 10.1167/iovs.12-9813. [DOI] [PubMed] [Google Scholar]
- 17.Ota M., Nishijima K., Sakamoto A. Optical coherence tomographic evaluation of foveal hard exudates in patients with diabetic maculopathy accompanying macular detachment. Ophthalmology. 2010;117:1996–2002. doi: 10.1016/j.ophtha.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 18.Barry R.J., Tasiopoulou A., Murray P.I., Patel P.J., Sagoo M.S., Denniston A.K., Keane P.A. Characteristic optical coherence tomography findings in patients with primary vitreoretinal lymphoma: a novel aid to early diagnosis. Br J Ophthalmol. 2018 doi: 10.1136/bjophthalmol-2017-311612. pii: bjophthalmol-2017-311612, [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Jiang T., Zhao Z., Chang Q. Evaluation of cytologic specimens obtained during experimental vitreous biopsy using B-cell lymphoma line. Eur J Ophthalmol. 2014;24:911–917. doi: 10.5301/ejo.5000488. [DOI] [PubMed] [Google Scholar]
- 20.Fardeau C., Lee C.P., Merle-Beral H. Retinal fluorescein, indocyanine green angiography, and optic coherence tomography in non-Hodgkin primary intraocular lymphoma. Am J Ophthalmol. 2009;147:886–894. doi: 10.1016/j.ajo.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Cassoux N., Giron A., Bodaghi B. IL-10 measurement in aqueous humor for screening patients with suspicion of primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2007;48:3253–3259. doi: 10.1167/iovs.06-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatesh R., Gurav P., Abhishek Dave P., Gandhi A. Capillary dropout: A novel fluorescein angiography finding in primary vitreoretinal lymphoma. Ocul Oncol Pathol. 2017;3(4):324–327. doi: 10.1159/000472152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan C.C., Sen H.N. Current concepts in diagnosing and managing primary vitreoretinal (intraocular) lymphoma. Discov Med. 2013;15:93–100. [PMC free article] [PubMed] [Google Scholar]
- 24.Coupland S.E., Hummel M., Stein H. Demonstration of identical clonal derivation in a case of “oculocerebral” lymphoma. Br J Ophthalmol. 2005;89:238–239. doi: 10.1136/bjo.2004.047001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coupland S.E., Loddenkemper C., Smith J.R. Expression of immunoglobulin transcription factors in primary intraocular lymphoma and primary central nervous system lymphoma. Invest Ophthalmol Vis Sci. 2005;46:3957–3964. doi: 10.1167/iovs.05-0318. [DOI] [PubMed] [Google Scholar]
- 26.Camilleri-Broet S., Criniere E., Broet P. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006;107:190–196. doi: 10.1182/blood-2005-03-1024. [DOI] [PubMed] [Google Scholar]
- 27.Malumbres R., Davis J., Ruiz P. Somatically mutated immunoglobulin IGHV@ genes without intraclonal heterogeneity indicate a postgerminal centre origin of primary intraocular diffuse large B-cell lymphomas. Br J Haematol. 2007;138:749–755. doi: 10.1111/j.1365-2141.2007.06744.x. [DOI] [PubMed] [Google Scholar]
- 28.Wallace D.J., Shen D., Reed G.F. Detection of the bcl-2 t(14;18) translocation and proto-oncogene expression in primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2006;47:2750–2756. doi: 10.1167/iovs.05-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montesinos-Rongen M., Zuhlke-Jenisch R., Gesk S. Interphase cytogenetic analysis of lymphoma-associated chromosomal breakpoints in primary diffuse large B-cell lymphomas of the central nervous system. J Neuropathol Exp Neurol. 2002;61:926–933. doi: 10.1093/jnen/61.10.926. [DOI] [PubMed] [Google Scholar]
- 30.Cady F.M., O’Neill B.P., Law M.E. Del(6)(q22) and BCL6 rearrangements in primary CNS lymphoma are indicators of an aggressive clinical course. J Clin Oncol. 2008;26:4814–4819. doi: 10.1200/JCO.2008.16.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L., Sato-Otsubo A., Sugita S. High-resolution genomic copy number profiling of primary intraocular lymphoma by single nucleotide polymorphism microarrays. Cancer Sci. 2014;105:592–599. doi: 10.1111/cas.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonzheim I., Giese S., Deuter C. High frequency of MYD88 mutations in vitreoretinal B-cell lymphoma: a valuable tool to improve diagnostic yield of vitreous aspirates. Blood. 2015;126:76–79. doi: 10.1182/blood-2015-01-620518. [DOI] [PubMed] [Google Scholar]
- 33.Vater I., Montesinos-Rongen M., Schlesner M. The mutational pattern of primary lymphoma of the central nervous system determined by whole-exome sequencing. Leukemia. 2015;29:677–685. doi: 10.1038/leu.2014.264. [DOI] [PubMed] [Google Scholar]
- 34.Tuo J., Shen D., Yang H.H. Distinct microRNA-155 expression in the vitreous of patients with primary vitreoretinal lymphoma and uveitis. Am J Ophthalmol. 2014;157:728–734. doi: 10.1016/j.ajo.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baraniskin A., Kuhnhenn J., Schlegel U. Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood. 2011;117:3140–3146. doi: 10.1182/blood-2010-09-308684. [DOI] [PubMed] [Google Scholar]
- 36.Baraniskin A., Kuhnhenn J., Schlegel U. MicroRNAs in cerebrospinal fluid as biomarker for disease course monitoring in primary central nervous system lymphoma. J Neurooncol. 2012;109:239–244. doi: 10.1007/s11060-012-0908-2. [DOI] [PubMed] [Google Scholar]
- 37.Kakkassery V., Schroers R., Coupland S.E. Vitreous microRNA levels as diagnostic biomarkers for vitreo-retinal lymphoma. Blood. 2017 doi: 10.1182/blood-2017-01-765180. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Aronow M.E., Shen D., Hochman J., Chan C.C. Intraocular lymphoma models. Ocul Oncol Pathol. 2015;1(3):214–222. doi: 10.1159/000370158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Z., Mahesh S.P., Shen de F., Liu B., Siu W.O., Hwang F.S. Eradication of tumor colonization and invasion by a B cell-specific immunotoxin in a murine model for human primary intraocular lymphoma. Cancer Res. 2006;66:10586–10593. doi: 10.1158/0008-5472.CAN-06-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Touitou V., Daussy C., Bodaghi B. Impaired th1/tc1 cytokine production of tumor-infiltrating lymphocytes in a model of primary intraocular B-cell lymphoma. Invest Ophthalmol Vis Sci. 2007;4 8:3223–3229. doi: 10.1167/iovs.07-0008. [DOI] [PubMed] [Google Scholar]
- 41.Mineo J.F., Scheffer A., Karkoutly C., Nouvel L., Kerdraon O., Trauet J. Using human CD20-transfected murine lymphomatous B cells to evaluate the efficacy of intravitreal and intracerebral rituximab injections in mice. Invest Ophthalmol Vis Sci. 2008;49:4738–4745. doi: 10.1167/iovs.07-1494. [DOI] [PubMed] [Google Scholar]
- 42.Ben Abdelwahed R., Donnou S., Ouakrim H. Preclinical study of ublituximab, a glycoengineered anti-human CD20 antibody, in murine models of primary cerebral and intraocular B-cell lymphomas. Invest Ophthalmol Vis Sci. 2013;54:3657–3665. doi: 10.1167/iovs.12-10316. [DOI] [PubMed] [Google Scholar]
- 43.Gonzales J.A., Chan C.C. Biopsy techniques and yields in diagnosing primary intraocular lymphoma. Int Ophthalmol. 2007;27:241–250. doi: 10.1007/s10792-007-9065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeh S., Weichel E.D., Faia L.J. 25-Gauge transconjunctival sutureless vitrectomy for the diagnosis of intraocular lymphoma. Br J Ophthalmol. 2010;94:633–638. doi: 10.1136/bjo.2009.167940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coupland S.E., Perez-Canto A., Hummel M. Assessment of HOPE fixation in vitrectomy specimens in patients with chronic bilateral uveitis (masquerade syndrome) Graefes Arch Clin Exp Ophthalmol. 2005;243:847–852. doi: 10.1007/s00417-005-1166-1. [DOI] [PubMed] [Google Scholar]
- 46.Coupland S.E. Analysis of intraocular biopsies. Dev Ophthalmol. 2011;49:96–116. doi: 10.1159/000328266. [DOI] [PubMed] [Google Scholar]
- 47.Kase S., Namba K., Iwata D. Diagnostic efficacy of cell block method for vitreoretinal lymphoma. Diagn Pathol. 2016;11:29. doi: 10.1186/s13000-016-0479-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mudhar H.S., Sheard R. Diagnostic cellular yield is superior with full pars plana vitrectomy compared with core vitreous biopsy. Eye (Lond) 2013;27:50–55. doi: 10.1038/eye.2012.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cursiefen C., Holbach L.M., Lafaut B., Heimann K., Kirchner T., Naumann G.O. Oculocerebral non-Hodgkin's lymphoma with uveal involvement: Development of an epibulbar tumor after vitrectomy. Arch Ophthalmol. 2000;118:1437–1440. doi: 10.1001/archopht.118.10.1437. [DOI] [PubMed] [Google Scholar]
- 50.Coupland S.E., Chan C.C., Smith J. Pathophysiology of retinal lymphoma. Ocul Immunol Inflamm. 2009;17:227–237. doi: 10.1080/09273940903168696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kimura K., Usui Y., Goto H. Japanese Intraocular Lymphoma Study Group. Clinical features and diagnostic significance of the intraocular fluid of 217 patients with intraocular lymphoma. Jpn J Ophthalmol. 2012;56:383–389. doi: 10.1007/s10384-012-0150-7. [DOI] [PubMed] [Google Scholar]
- 52.Wittenberg L.A., Maberley D.A., Ma P.E. Contribution of vitreous cytology to final clinical diagnosis: fifteen-year review of vitreous cytology specimens from one institution. Ophthalmology. 2008;115:1944–1950. doi: 10.1016/j.ophtha.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 53.Davis J.L. Intraocular lymphoma: a clinical perspective. Eye (Lond) 2013;27:153–162. doi: 10.1038/eye.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coupland S.E., Anastassiou G., Bornfeld N. Primary intraocular lymphoma of T-cell type: report of a case and review of the literature. Graefes Arch Clin Exp Ophthalmol. 2005;243:189–197. doi: 10.1007/s00417-004-0890-2. [DOI] [PubMed] [Google Scholar]
- 55.Park C.Y., Hwang S.W., Kim do Y., Huh H.J., Oh J.H. Anaplastic large cell lymphoma involving anterior segment of the eye. Korean J Ophthalmol. 2014;28:108–112. doi: 10.3341/kjo.2014.28.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davis J.L., Ruiz P., Jr, Shah M. Evaluation of the reactive T-cell infiltrate in uveitis and intraocular lymphoma with flow cytometry of vitreous fluid (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2012;110:117–129. [PMC free article] [PubMed] [Google Scholar]
- 57.Missotten T., Tielemans D., Bromberg J.E. Multicolor flowcytometric immunophenotyping is a valuable tool for detection of intraocular lymphoma. Ophthalmology. 2013;120:991–996. doi: 10.1016/j.ophtha.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 58.Langerak A.W., Groenen P.J., Bruggemann M. EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia. 2012;26:2159–2171. doi: 10.1038/leu.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coupland S.E., Hummel M., Muller H.H. Molecular analysis of immunoglobulin genes in primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2005;46:3507–3514. doi: 10.1167/iovs.05-0401. [DOI] [PubMed] [Google Scholar]
- 60.Baehring J.M., Androudi S., Longtine J.J. Analysis of clonal immunoglobulin heavy chain rearrangements in ocular lymphoma. Cancer. 2005;104:591–597. doi: 10.1002/cncr.21191. [DOI] [PubMed] [Google Scholar]
- 61.Merle-Beral H., Davi F., Cassoux N. Biological diagnosis of primary intraocular lymphoma. Br J Haematol. 2004;124:469–473. doi: 10.1046/j.1365-2141.2003.04800.x. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y., Shen D., Wang V.M. Molecular biomarkers for the diagnosis of primary vitreoretinal lymphoma. Int J Mol Sci. 2011;12:5684–5697. doi: 10.3390/ijms12095684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sugita S., Takase H., Sugamoto Y. Diagnosis of intraocular lymphoma by polymerase chain reaction analysis and cytokine profiling of the vitreous fluid. Jpn J Ophthalmol. 2009;53:209–214. doi: 10.1007/s10384-009-0662-y. [DOI] [PubMed] [Google Scholar]
- 64.Cani A.K., Soliman M., Hovelson D.H. Comprehensive genomic profiling of orbital and ocular adnexal lymphomas identifies frequent alterations in MYD88 and chromatin modifiers: new routes to targeted therapies. Mod Pathol. 2016;29:685–697. doi: 10.1038/modpathol.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aronow M.E., Singh A.D. The use of imaging in the diagnosis and management of intraocular lymphoma. Int Ophthalmol Clin. 2012;52:199–208. doi: 10.1097/IIO.0b013e318265d4e3. [DOI] [PubMed] [Google Scholar]
- 66.Cani A.K., Hovelson D.H., Demirci H., Johnson M.W., Tomlins S.A., Rao R.C. Next generation sequencing of vitreoretinal lymphomas from small-volume intraocular liquid biopsies: new routes to targeted therapies. Oncotarget. 2017;8(5):7989–7998. doi: 10.18632/oncotarget.14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ngo V.N., Young R.M., Schmitz R. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Treon S.P., Xu L., Hunter Z. MYD88 mutations and response to Ibrutinib in Waldenstrom's Macroglobulinemia. N Engl J Med. 2015;373:584–586. doi: 10.1056/NEJMc1506192. [DOI] [PubMed] [Google Scholar]
- 69.Rhyasen G.W., Starczynowski D.T. IRAK signalling in cancer. Br J Cancer. 2015;112:232–237. doi: 10.1038/bjc.2014.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raja H., Salomao D.R., Viswanatha D.S., Pulido J.S. Prevalence of Myd88 L265p mutation in histologically proven, diffuse large B-Cell vitreoretinal lymphoma. Retina. 2016;36:624–628. doi: 10.1097/IAE.0000000000000996. [DOI] [PubMed] [Google Scholar]
- 71.Pulido J.S., Salomao D.R., Frederick L.A., Viswanatha D.S. MyD-88 L265P mutations are present in some cases of vitreoretinal lymphoma. Retina. 2015;35:624–627. doi: 10.1097/IAE.0000000000000589. [DOI] [PubMed] [Google Scholar]
- 72.Carpten J.D., Faber A.L., Horn C. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 73.Cui W., Cai Y., Wang W. Frequent copy number variations of PI3K/AKT pathway and aberrant protein expressions of PI3K subunits are associated with inferior survival in diffuse large B cell lymphoma. J Transl Med. 2014;12:10. doi: 10.1186/1479-5876-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Costopoulos M., Touitou V., Golmard J.L. ISOLD: a new highly sensitive interleukin score for intraocular lymphoma diagnosis. Ophthalmology. 2016;123:1626–1628. doi: 10.1016/j.ophtha.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 75.Fisson S., Ouakrim H., Touitou V. Cytokine profile in human eyes: contribution of a new cytokine combination for differential diagnosis between intraocular lymphoma or uveitis. PLoS One. 2013;8:e52385. doi: 10.1371/journal.pone.0052385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raja H., Snyder M.R., Johnston P.B. Effect of intravitreal methotrexate and rituximab on interleukin-10 levels in aqueous humor of treated eyes with vitreoretinal lymphoma. PLoS One. 2013;8:e65627. doi: 10.1371/journal.pone.0065627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saleh M., Nikolitch K., Bourcier T. Repeated IL-10 measurement in aqueous humor and OCT imaging are valuable tools to monitor intraocular lymphoma treated with intravitreal injections of methotrexate. Graefes Arch Clin Exp Ophthalmol. 2012;250:761–764. doi: 10.1007/s00417-011-1718-5. [DOI] [PubMed] [Google Scholar]
- 78.Pochat-Cotilloux C., Bienvenu J., Nguyen A.M. Use of a threshold of interleukin-10 and IL-10/IL-6 ratio in ocular samples for the screening of vitreoretinal lymphoma. Retina. 2017 doi: 10.1097/IAE.0000000000001922. [DOI] [PubMed] [Google Scholar]
- 79.Ramkumar H.L., Shen de F., Tuo J. IL-10-1082 SNP and IL-10 in primary CNS and vitreoretinal lymphomas. Graefes Arch Clin Exp Ophthalmol. 2012;250:1541–1548. doi: 10.1007/s00417-012-2037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoang-Xuan K., Bessell E., Bromberg J. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: guidelines from the European Association for Neuro-Oncology. Lancet Oncol. 2015;16:e322–e332. doi: 10.1016/S1470-2045(15)00076-5. [DOI] [PubMed] [Google Scholar]
- 81.Nguyen D.T., Houillier C., Choquet S. Primary oculocerebral lymphoma: MTX polychemotherapy alone on intraocular disease control. Ophthalmology. 2016;123:2047–2050. doi: 10.1016/j.ophtha.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 82.Pe’er J., Hochberg F.H., Foster C.S. Clinical review: treatment of vitreoretinal lymphoma. Ocul Immunol Inflamm. 2009;17:299–306. doi: 10.3109/09273940903370755. [DOI] [PubMed] [Google Scholar]
- 83.Ma W.L., Hou H.A., Hsu Y.J. Clinical outcomes of primary intraocular lym-phoma patients treated with front-line systemic high-dose methotrexate and intravitreal methotrexate injection. Ann Hematol. 2016;95:593–601. doi: 10.1007/s00277-015-2582-x. [DOI] [PubMed] [Google Scholar]
- 84.Riemens A., Bromberg J., Touitou V. Treatment strategies in primary vit-reoretinal lymphoma: a 17-center European collaborative study. JAMA Ophthalmol. 2015;133:191–197. doi: 10.1001/jamaophthalmol.2014.4755. [DOI] [PubMed] [Google Scholar]
- 85.Berenbom A., Davila R.M., Lin H.S., Harbour J.W. Treatment outcomes for primary intraocular lymphoma: implications for external beam radiotherapy. Eye (Lond) 2007;21:1198–1201. doi: 10.1038/sj.eye.6702437. [DOI] [PubMed] [Google Scholar]
- 86.Rosenfeld M.R., Pruitt A.A. Management of malignant gliomas and primary CNS lymphoma: standard of care and future directions. Continuum. 2012;18:406–415. doi: 10.1212/01.CON.0000413666.88539.0b. [DOI] [PubMed] [Google Scholar]
- 87.Chan C.C. Primary intraocular lymphoma: clinical features, diagnosis, and treatment. Clin Lymphoma. 2003;4:30–31. doi: 10.1016/s1526-9655(11)70005-7. [DOI] [PubMed] [Google Scholar]
- 88.Hashida N., Ohguro N., Nishida K. Efficacy and complications of intravitreal rituximab injection for treating primary vitreoretinal lymphoma. Transl Vis Scie Technol. 2012;1:1. doi: 10.1167/tvst.1.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hashida N., Nakai K., Saitoh N., Nishida K. Association between ocular findings and preventive therapy with onset of central nervous system involvement in patients with primary vitreoretinal lymphoma. Graefes Arch Clin Exp Ophthalmol. 2014;252:687–693. doi: 10.1007/s00417-014-2584-8. [DOI] [PubMed] [Google Scholar]
- 90.Illerhaus G., Marks R., Ihorst G. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol. 2006;24:3865–3870. doi: 10.1200/JCO.2006.06.2117. [DOI] [PubMed] [Google Scholar]
- 91.Witzig T.E., Vose J.M., Zinzani P.L. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2011;22:1622–1627. doi: 10.1093/annonc/mdq626. [DOI] [PubMed] [Google Scholar]
- 92.Li Z., Qiu Y., Personett D. Pomalidomide shows significant therapeutic activity against CNS lymphoma with a major impact on the tumor microenvironment in murine models. PLoS One. 2013;8:e71754. doi: 10.1371/journal.pone.0071754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Byrd J.C., Furman R.R., Coutre S.E. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang G., Buhrlage S.J., Tan L. HCK is a survival determinant transactivated by mutated MYD88, and a direct target of ibrutinib. Blood. 2016;127:3237–3252. doi: 10.1182/blood-2016-01-695098. [DOI] [PubMed] [Google Scholar]
- 95.Treon S.P., Tripsas C.K., Meid K. Ibrutinib in previously treated Waldenstrom’s macroglobulinemia. N Engl J Med. 2015;372:1430–1440. doi: 10.1056/NEJMoa1501548. [DOI] [PubMed] [Google Scholar]
- 96.Ahn I.E., Jerussi T., Farooqui M., Tian X., Wiestner A., Gea-Banacloche J. Atypical Pneumocystis jirovecii pneumonia in previously untreated patients with CLL on single-agent ibrutinib. Blood. 2016;128:1940–1943. doi: 10.1182/blood-2016-06-722991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Okamoto K., Proia L.A., Demarais P.L. Disseminated cryptococcal disease in a patient with chronic lymphocytic leukemia on ibrutinib. Case Rep Infect Dis. 2016;2016:4642831. doi: 10.1155/2016/4642831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghesquieres H., Houillier C., Chinot O. Rituximab-Lenalidomide (REVRI) in relapse or refractory primary central nervous system (PCNSL) or vitreo retinal lymphoma (PVRL): results of a “proof of concept” phase II study of the French LOC network (Abstract 785) Blood. 2016;128:785. [Google Scholar]
- 99.Iaccheri B., Fiore T., Cerquaglia A., Lupidi M., Cagini C. Transient therapeutic effect of vitrectomy in primary intraocular lymphoma. Int Ophthalmol. 2017;37:1333–1335. doi: 10.1007/s10792-016-0405-2. [DOI] [PubMed] [Google Scholar]
- 100.Grimm S.A., Pulido J.S., Jahnke K. Primary intraocular lymphoma: an International Primary Central Nervous System Lymphoma Collaborative Group report. Ann Oncol. 2007;18:1851–1855. doi: 10.1093/annonc/mdm340. [DOI] [PubMed] [Google Scholar]