Abstract
Recently, the human cochlea has been shown to contain numerous resident macrophages under steady-state. The macrophages accumulate in the stria vascularis, among the auditory nerves, and are also spotted in the human organ of Corti. These macrophages may process antigens reaching the cochlea by invasion of pathogens and insertion of CI electrode. Thus, macrophages execute an innate, and possibly an adaptive immunity. Here, we describe the molecular markers CD4 and CD8 of T cells, macrophage markers MHCII and CD11b, as well as the microglial markers TEME119 and P2Y12, in the human cochlea. Immunohistochemistry and the advantageous super-resolution structured illumination microscopy (SR-SIM) were used in the study. CD4+ and CD8+ cells were found in the human cochleae. They were seen in the modiolus in a substantial number adjacent to the vessels, in the peripheral region of the Rosenthal's canal, and occasionally in the spiral ligament. While there are a surprisingly large number of macrophages in the stria vascularis as well as between the auditory neurons, CD4+ and CD8+ cells are hardly seen in these areas, and neither are seen in the organ of Corti. In the modiolus, macrophages, CD4+ and CD8+ cells appeared often in clusters. Interaction between these different cells was easily observed with SR-SIM, showing closely placed cell bodies, and the processes from macrophages reaching out and touching the lymphocytes. Otherwise the CD4+ and CD8+ cells in human cochlear tissue are discretely scattered. The possible roles of these immune cells are speculated.
Keywords: Macrophage, Human cochlea, CD4, CD8, Lymphocyte, T cell
Highlights
-
•
CD4+ and CD8+ cells were found in the human cochleae.
-
•
They were seen in the modiolus in a substantial number adjacent to the vessels, in the peripheral region of the Rosenthal's canal, and occasionally in the spiral ligament.
1. Introduction
Macrophages were found in different regions of the human cochlea, e.g., stria vascularis, spiral ligament, peripheral and central nerve bundles, Rosenthal's canal, tympanic covering layer and even in the organ of Corti in steady state (Liu et al., 2018), with some resident macrophages found to be positive for markers CD163, IBA1, and CD68 (O'Malley et al., 2016). Immunohistochemistry studies have yielded similar findings in the cochleae of animals (Warchol, 1997; Hirose et al., 2005; Okano et al., 2008; Warchol et al., 2012) although anatomic differences exist between human and animals. The fact that macrophages reside in both rodent and human cochleae suggests that the inner ear contains immune-competent cells that may participate in both innate and adaptive immune responses. These macrophages trap and process antigens reaching the inner ear by invasion of pathogens, insertion of CI electrode, abnormally exposed autoantigens, hence serving as an immunity domain in the cochlea together with immune cells in the endolymphatic sac which has direct communication with the endolymph of the cochlea. Lymphocytes were found in guinea pig endolymphatic sac (ES) by using electron microscopy in 80's (Rask-Andersen and Stahle, 1979).
CD4 and CD8 (cluster of differentiation 4 and 8), the transmembrane glycoproteins of T helper and cytotoxic T cells respectively, are co-receptors of the T cell receptor (TCR). CD4 and CD8 assist the TCR in communicating with antigen-presenting cells. The receptor complex binds to distinct regions of the antigen-presenting major histocompatibility complex (MHC) molecules. While CD4 binds to class II MHC molecule (MHCII), CD8 is specific for the class I MHC protein (MHCI) (Gao and Jakobsen, 2000). CD4 positive (CD4+) T helper cells' main role is to send signals to other types of immune cells, including CD8+ killer cells. The latter then destroy the pathogens by releasing toxic granules containing powerful enzymes which kill the pathogen-infected cells.
While we need to know the characteristics of the inner ear resident macrophages and their roles in innate immunoreactivity in the human cochlea by analyzing the molecules expressed, we hope to analyze other cells such as lymphocytes in the cochlea. These lymphocytes in animal cochleae are considered to play roles in adaptive immunity (Yang et al., 2015). However, lymphocytes have not been thoroughly studied in the human cochlea.
In our study we have tried to characterize the molecular markers expressed by inner ear immune cells, CD68 and CD11b by the macrophages, and the CD4 and CD8 by lymphocytes, and to investigate the interaction between these immune cells in the surgically obtained, well-preserved adult human cochlear tissues. The techniques for the study included mainly immunohistochemistry, confocal microscopy and multichannel super-resolution structured illumination microscopy (SR-SIM). Hopefully with further understanding the immune mechanisms associated with the human inner ear, unknown causes for some inner ear diseases that give rise to common and troublesome symptoms such as hearing loss, tinnitus and vertigo could be clarified, and treatment for inner ear disorders, e.g. cochlear implant, anti-inflammation and anti-autoimmune dealings could be improved.
2. Material and methods
2.1. Unique specimen and ethics statement
The human cochleae were collected during the surgery via a trans-cochlear approach for posterior cranial fossa meningioma that had reached the clivus anteriorly. The immediately fixed cochlear tissue was obtained with excellent antigen preservation that has been proved by our previous series studies (Liu et al., 2009, 2010; 2016, 2017; 2018). The study of human cochleae was approved by the local ethics committee (Etikprövningsnämnden Uppsala, no. 99398, 22/9 1999, cont, 2003, no. C254/4; no. C45/7 2007, Dnr. 2013/190), and patient consent was obtained. The age of patients ranged between 40 and 70 years. Their hearing thresholds (pure tone audiometry) were normal except in a few cases where only limited frequencies showed slightly increased thresholds, therefore conceivably normal cochlear tissue was obtained for the study. The study adhered to the rules of the Declaration of Helsinki.
2.2. Obtaining and processing human cochleae
Five cochleae were dissected out using diamond drills of various sizes in standardized surgical procedures. Only experienced surgeons with assistance of instrumental nurses were allowed to handle the specimen delivering it to the fixative. According to the law, no data on age, gender, or audiometric results of the patients were retrieved. There was almost no delay between dissecting the cochleae off the surrounding bones and placing them in 4% paraformaldehyde diluted with 0.1 M phosphate buffered saline (PBS; pH 7.4). The cochleae, transferred from the operating room to the laboratory, were kept in ample fixative fluid for 24-h in a 4 °C fridge. Then the specimens were washed in 0.1 M PBS and then placed in 10% Na-EDTA solution at pH 7.2 for decalcification. The Na-EDTA solution was renewed every two days until the decalcification process was completed, which took approximately three weeks. The decalcified cochleae were rinsed with PBS and placed in 25% sucrose in PBS overnight (4 °C). The cochleae were embedded in Tissue-Tek (OCT Polysciences), rapidly frozen in dry ice, and sectioned at 8–10 μm using a Leica cryostat microtome. The cryo-sections were collected onto gelatin/chrome-alum-coated slides and stored in a freezer of −70 °C before immunohistochemistry was conducted.
2.3. Antibodies and immunohistochemistry
Table 1 shows the antibodies used in the present study. The immunohistochemistry procedures performed on the sections have been described in previous publications (Liu et al., 2012, 2015, 2018). Briefly, the slide-mounted sections were incubated with an antibody solution under a humidified atmosphere at 4 °C for 20 h. After rinsing with PBS three times for 5 min each, the sections were incubated with secondary antibodies conjugated to Alexa Fluor 488, 555 and 647 (Molecular Probes, Carlsbad, CA, USA) and then counter-stained with the nuclear stain DAPI (4′, 6-diamidino-2-phenylindole dihydro-chloride, Thermo Fisher Scientific) for 5–7 min, rinsed with PBS (3 × 5 min), and mounted with ProLong® Gold Antifade Mountant (Thermo Fisher Scientific) and covered with the specified cover-glass required for optically matching the SIM objectives.
Table 1.
Main primary antibodies used in the study.
| Primary antibody | Type | Dilution | Host | Catalog Number | Producer |
|---|---|---|---|---|---|
| IBA1 | polyclonal | 1:100 | rabbit | PA5-27436 | Thermo Fisher, Waltham, USA |
| MHCII | monoclonal | 1:100 | mouse | MA5-11966 | Thermo Fisher, Waltham, USA |
| Collagen IV | polyclonal | 1:10 | goat | AB769 | Millipore, Burlington, USA |
| CX3CL1 | monoclonal | 1:50 | mouse | MAB3651-100 | R&DSystems, Minneapolis, USA |
| CD11b | monoclonal | 1:50 | rabbit | AB52478 | Abcam, Cambridge, UK |
| CD4 | polyclonal | 1:150 | goat | AF-379-NA | R&DSystems, Minneapolis, USA |
| CD8α | monoclonal | 1:100 | mouse | MAB1509 | R&DSystems, Minneapolis, USA |
Primary and secondary antibody controls and labeling controls were performed to exclude endogenous fluorescence or unspecific reaction products. As a routine control, sections were incubated with 2% bovine serum albumin (BSA), omitting the primary antibodies. The control experiment revealed no visible staining in any structure of the cochleae.
2.4. Imaging and photography
The stained sections were first investigated with an inverted fluorescence microscope (Nikon TE2000) equipped with a spot digital camera with three filters (for emission spectra maxima at 358, 461, and 555 nm). Image-processing software (NIS Element BR-3.2, Nikon), including image merging and a fluorescence intensity analyzer, was installed on a computer system connected to the microscope. For laser confocal microscopy, we used the same microscope equipped with a three-channel laser emission system. The optical scanning and image-processing tasks were performed using Nikon EZ-C1 (ver. 3.80) software and included the reconstruction of Z-stack images into projections and 3-D images. Super resolution structured-illumination microscopy (SR-SIM) (Elyra S.1 SIM system with a 63×/1.4 oil Plan-Apochromat objective (Zeiss), sCMOS camera (PCO Edge), ZEN 2012 software (Zeiss)) was performed investigating the structures of interest. Multichannel SR-SIM imaging was achieved with the following laser and filter setup: 405 nm laser of excitation coupled with BP 420–480 + LP 750 filter, 488 nm laser of excitation with BP 495–550 + LP750 filter, 561 nm laser of excitation with BP 570–620 + LP 750 filter, 647 nm laser of excitation with LP 655 filter. To maximize the image quality, five grid rotations and five phases were used for each image plane and channel. The grid size was automatically adjusted by the ZEN software for each wavelength of excitation. SR-SIM images were processed with ZEN software with theoretical point spread function (PSF).
From the SR-SIM dataset, 3-D reconstruction was performed with Imaris 8.2 (Bitplane, Zürich, Switzerland). A bright-field channel is available in the SIM for merging with fluorescence channels facilitating visualization of the cell/tissue borders. The SIM is capable of achieving a lateral (X—Y) resolution of ≈100 nm and an axial (Z) resolution of ≈300 nm (Gustafsson et al., 2008). The resolution of the SIM system (owned by BioVis, Uppsala Universtiy) used in present study was measured with sub-resolution fluorescent beads (40 nm, Zeiss) in the green channel (BP 495–550 + LP750). An average PSF value was obtained from multiple beads with the built-in experimental PSF algorithm of the ZEN software. The typical resolution of the system was 107 nm in the X—Y plane and 394 nm in the Z plane. 3-D reconstructions of collagen IV and IBA1 (Ionized calcium binding adaptor molecule 1) protein expression were made (refer to Liu et al., 2018). Both signals were reconstructed with using surface rendering mode using Imaris 8.2 software.
3. Results
Consistent with our previous study, SR-SIM and confocal microscopy showed a large number of IBA1-expressing cells in all parts of the human cochleae (Fig. 1). IBA1, the microglial and macrophage-specific calcium-binding protein, is involved in the membrane ruffling and phagocytosis of the activated microglia/macrophage. Macrophages having different shapes resided densely in adult human cochleae, along the auditory nerve and in all other cochlear tissues (also refer to Liu et al., 2018). Here, among the molecular markers tested with immunohistochemistry (TMEM119, CD163, P2Y12, CD68, CD11b, MHCII), CD68, CD11b and MHCII were stably expressed in macrophages and they seemed to co-localize in all the macrophages but in different subcellular compartments. TMEM119 and P2Y12 were not seen in our experiments, indicating that the cells labeled with IBA1, MHCII and with other fore-mentioned markers in human cochleae were not microglia.
Fig. 1.
MHCII- and CD11b-immunohistochemistry of macrophages in the human spiral ganglion. In A), variously shaped macrophages are between the spiral ganglion neurons (SGN). Both MHCII and CD11b were expressed in the macrophages. In B), an enlarged cell expressed both markers with CD11b more on the surface (arrow).
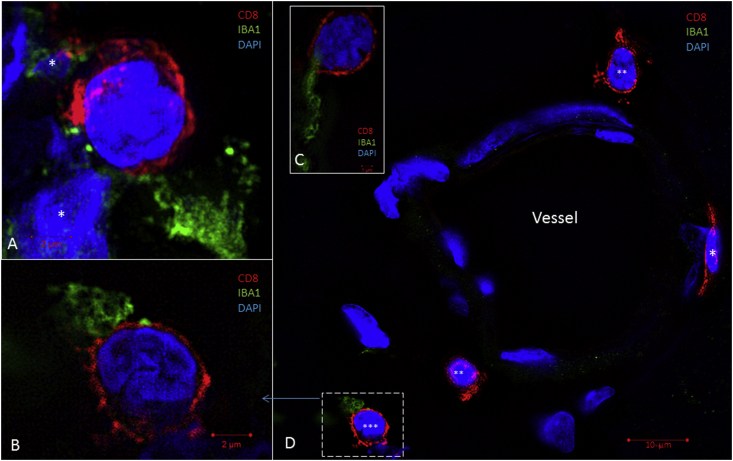
The presence of CD4+ and CD8+ cells and the interactions between these two types of lymphocytes and macrophages in the human cochleae were observed using immunohistochemistry and super-resolution structured-illumination microscopy (SR-SIM) (Fig. 2). In human cochleae, the CD4+ and CD8+ cells were seen around vessels of the modiolus and along the border of the Rosenthal's canal. The T cells were seen in the medial wall of the scala tympani (Fig. 3) that is part of the modiolus between the Rosenthal's canal and the scala tympani. This space contains vessels. The spaces bordering the apical and basal side of the Rosenthal canal contain bigger blood vessels. While a large number of macrophages were seen in the stria vascularis, among the neurons in the Rosenthal's canal and occasionally in organ of Corti, CD4+ and CD8+ cells were not found in these areas. A few isolated CD4+ and CD8+ cells were seen in the spiral ligament and in the peripheral region of the Rosenthal's canal. In the modiolus of the cochleae, macrophages, CD4+ and CD8+ cells were often in clusters. Interaction between these different cells was observed with super-resolution structured illumination microscopy, showing closely located lymphocyte cell bodies to macrophages which often sent processes to reach and touch the CD4+ and CD8+ cells (Fig. 2). The number of macrophages exceeds any other type of the labeled immune cells, in the whole cochlea. The CD4+ and CD8+ cells in human cochleae were mostly separately scattered, and the majority appeared round-shaped except a few that looked flat lying against the outer vessel wall and expressing CD8 (Fig. 2D). These rare cells seemed to have just migrated out of the modiolar blood vessels.
Fig. 2.
IBA1-and CD8-immunohistochemistry of human cochlear modiolus. A), B) and C) show patterns of interaction between the IBA1+ macrophages and the CD8+ T cytotoxic cells. These images were taken either around the modiolar vessels or in the peripheral regions of the Rosenthal canal. The nuclei of the macrophages are not included in the images except for A) where the nuclei with IBA1-labeling (*) are shown close to a CD8+ cell. In D) a blood vessel is seen to be surrounded by several CD8+ T cells, note the one on the right (*) that flattens against the outer vessel wall. The other CD8+ cells (** and ***) appear round-shaped and at a distance from the vessel. One CD8+ cell (***) is enlarged and shown in B).
Fig. 3.
CX3CR1-and CX3CL1-immunohistochemistry of human spiral ganglion (A) and CD4−, CD8−and IBA1-immunohistochemistry of human cochlear modiolus (B and C). In A), spiral ganglion neurons (SGN) express CX3CL1 (Fractalkine) more intense in their perikaryon surface. The receptor CX3CR1-immunostaining appears diffuse in the cytoplasm of both cell bodies (Mɸ) and processes that look like macrophages. B shows several immune cells located along the wall of scala tympani in the modiolus. Two cells positive for CD4 and CD8 respectively are magnified in B); super-resolution SIM microscopy reveals a close relation between these two cells.
4. Discussion
Macrophages are ubiquitously located in the human cochlea including the stria vascularis, organ of Corti, nerve bundles and spiral ganglion (O'Malley et al., 2016; Liu et al., 2018). They could independently accomplish innate immune functions such as phagocytosis of potentially harmful substances including foreign bodies, pathogens, as well as apoptotic cells. In addition they also repair and maintain the intactness of the neurons and hair cells, therefore they are named “multitaskers”. The homeostasis maintenance are going-on activities, not necessarily relying on lymphocytes or other adaptive immune contrivances. This may explain why the number of macrophages exceeds that of T lymphocytes anywhere in the human cochleae although we only compare macrophages with CD4 and CD8 cells. Within the stria vascularis, organ of Corti and spiral ganglion where the densely packed cells have crucial functions for hearing perception, macrophages but not CD4+ or CD8+ cells could be seen in these compartments of the human cochleae. T helper cells and cytotoxic T cells were seen in the modiolus, mostly around the blood vessels, including the space between the Rosenthal's canal and the medial wall of the scala tympani. A few CD4+ and CD8+ cells were scattered in the peripheral region of the Rosenthal's canal and very few in the spiral ligament.
This evidence suggests that the human cochlea is equipped with antigen presenting macrophages as well as T lymphocytes that execute cytotoxic and immune mediation function. In an animal study, following acoustic overstimulation, Yang et al. (2015) found infiltration of macrophages into the region of the basilar membrane that displayed an increased expression of MHCII, and infiltration of CD4+ T cells into the same region. It is assumed that if out of normal range, the immune response could cause autoimmune injuries. Based on a study on cochlin-triggered autoimmune hearing loss (Solares et al., 2004; Baruah, 2014), T lymphocytes can be the cells responsible for autoimmune hearing loss (AIHL), because grafting of the peptide-activated T cells led to reduction in ABR. Among the potential autoimmune antigens that could induce hearing loss, collagen II and myelin basic protein have been thoroughly investigated in human cochleae (Liu et al., 2012, 2015). These two substances exist in human cochleae in a rather large quantity. Claudin-11 might be another important inner ear component that, after certain interaction with immune cells, could lead to autoimmune hearing loss. Claudin-11, also known as oligodendrocyte-specific protein (OSP/claudin-11) and a transmembrane protein intense in central nervous system myelin, is implicated as an autoantigen in the development of autoimmune demyelinating disease (Bronstein et al., 2000). Recent study shows an involvement of Claudin-11 in disruption of blood-brain, blood-spinal cord, and blood-arachnoid barriers in multiple sclerosis (Uchida et al., 2018), an immune-mediated disorder of the central nervous system. In the human cochlea, Claudin-11 forms a massive tight junction barrier between the type I fibrocytes of the spiral ligament, adjacent laterally to the stria vascularis (Liu et al., 2017); macrophages as well as CD4+/CD8+ lymphocytes were hardly seen between the type I fibrocytes in the human cochleae. This characteristic might be related to maintenance of the tight junction or/and prevention from autoimmunity. In other locations of the human cochleae, these two types of immune cells appeared not consistent in number indicating a diverse function of macrophages vs. lymphocytes in the cochlear tissues considered to be from normal hearing cochleae.
Tissue lymphocytes and the circulating ones may exchange. Our study has demonstrated a remarkable organization of immune cells and their interaction in the cochlear tissue. T cells in the human cochlear tissue may include T memory cells that remember each specific pathogen encountered, and are able to mount a strong and rapid response if the same pathogen is detected again. In the study by Souter et al. (2012), when systemically increasing the immune reactivity by using Keyhole hemocyanin, the inner ear immunoreaction was enhanced. This was reflected in increased inflammatory activity after insertion of an electrode array into the cochlea, and the over-active inflammation led to hearing decline.
The chemokine fractalkine (CX3CL1) was found in both inner and outer hair cells, as well as in the spiral ganglion neurons in human cochleae in our previous and present studies (Liu et al., 2018, Fig. 3). Its receptor CX3CR1 was seen in the macrophages among human spiral ganglion neurons (Fig. 3). This chemokine and its receptor in human cochleae as seen in our study lay the bases for interaction between macrophages and other important cell types (Kaur et al., 2015).
Cluster of differentiation molecule 11B (CD11b) is one protein subunit that forms the heterodimeric integrin alpha-M beta-2 (αMβ2) molecule. AlphaMβ2 is expressed on the surface of many leukocytes involved in the innate immunity, such as macrophages and natural killer cells (Solovjov et al., 2005). It mediates inflammation by regulating cell adhesion and migration and has been implicated in several immune processes such as phagocytosis, cell-mediated cytotoxicity and chemotaxis (Solovjov et al., 2005). CD11b, IBA1, and MHCII are frequently used as markers for macrophages and microglia (Frick et al., 2013). However, human cochleae seemed to lack microglia since the markers for microglia, TMEM119 and P2Y12, were negative in human cochleae (detailed data not shown here). TMEM119 is a cell-surface protein and a specific microglial marker in humans. TMEM119 is not expressed by macrophages or other immune or neural cell types. P2Y12 was expressed in parenchymal microglia of the human brain but absent in perivascular or meningeal macrophages (Mildner et al., 2017).
Taken together, specific antibodies and SR-SIM used in the study helped uniquely visualize the distribution of lymphocytes and macrophages with their molecular expression in human cochleae. Using SR-SIM, we verified that resident IBA1-positive macrophages are widely distributed in human cochlea (Liu et al., 2018). Some of these macrophages form contact with CD4+ and CD8+ lymphocytes, especially in the modiolus of human cochleae. Our study is the first revealing T lymphocyte-macrophage interaction using super-resolution immunohistochemistry in freshly fixed, healthy adult human cochleae. The results strengthen the view that, in the human cochlea, antigen presenting cells as well as T lymphocytes form part of the immune network to mediate immune function. Further studies will be performed to identify other immune cells in human cochleae (e.g., T memory cells, T regulatory cells and B cells). Such studies will help more comprehensively understand the immune network in human cochlea and its role in diseases.
Acknowledgments
This study was supported by ALF and private funds from Börje Runögård, Sweden. We are grateful to SciLiyfe Laboratories and the BioVis Platform at the Uppsala University for providing SR-SIM microscope equipment and for personal support throughout the study. This study was partly supported by MED-EL, Inc., Innsbruck, Austria.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Contributor Information
Wei Liu, Email: wei.liu@surgsci.uu.se.
Helge Rask-Andersen, Email: helge.rask-andersen@surgsci.uu.se.
References
- Baruah P. Cochlin in autoimmune inner ear disease: is the search for an inner ear autoantigen over? Auris Nasus Larynx. 2014;41:499–501. doi: 10.1016/j.anl.2014.08.014. Epub 2014 Sep 8. [DOI] [PubMed] [Google Scholar]
- Bronstein J.M., Tiwari-Woodruff S., Buznikov A.G., Stevens D.B. Involvement of OSP/claudin-11 in oligodendrocyte membrane interactions: role in biology and disease. J. Neurosci. Res. 2000;59:706–711. doi: 10.1002/(SICI)1097-4547(20000315)59:6<706::AID-JNR2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Frick L.R., Williams K., Pittenger C. Microglial dysregulation in psychiatric disease. Clin. Dev. Immunol. 2013;2013:608654. doi: 10.1155/2013/608654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson M.G., Shao L., Carlton P.M., Wang C.J., Golubovskaya I.N., Cande W.Z. Three-dimensional resolution doubling in wide-field fluorescence microscopy by structured illumination. Biophys. J. 2008;94:4957–4970. doi: 10.1529/biophysj.107.120345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G., Jakobsen B. Molecular interactions of coreceptor CD8 and MHC class I: the molecular basis for functional coordination with the T-cell receptor. Immunol. Today. 2000;21:630–636. doi: 10.1016/s0167-5699(00)01750-3. PMID 11114424. [DOI] [PubMed] [Google Scholar]
- Hirose K., Discolo C.M., Keasler J.R., Ransohoff R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J. Comp. Neurol. 2005;489:180–194. doi: 10.1002/cne.20619. [DOI] [PubMed] [Google Scholar]
- Kaur T., Zamani D., Tong L., Rubel E.W., Ohlemiller K.K., Hirose K. Fractalkine signaling regulates macrophage recruitment into the cochlea and promotes the survival of spiral ganglion neurons after selective hair cell lesion. J. Neurosci. 2015;35:15050–15061. doi: 10.1523/JNEUROSCI.2325-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Boström M., Kinnefors A., Rask-Andersen H. Unique expression of connexins in the human cochlea. Hear. Res. 2009;250:55–62. doi: 10.1016/j.heares.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Liu W., Kinnefors A., Boström M., Rask-Andersen H. Expression of peripherin in human cochlea. Cell Tissue Res. 2010;342:345–351. doi: 10.1007/s00441-010-1081-6. [DOI] [PubMed] [Google Scholar]
- Liu W., Edin F., Blom H., Magnusson P., Schrott-Fischer A., Glueckert R., Santi P.A., Li H., Laurell G., Rask-Andersen H. Super-resolution structured illumination fluorescence microscopy of the lateral wall of the cochlea: the Connexin 26/30 proteins are separately expressed in man. Cell Tissue Res. 2016;365:13–27. doi: 10.1007/s00441-016-2359-0. Epub 2016 Mar 4. [DOI] [PubMed] [Google Scholar]
- Liu W., Molnar M., Garnham C., Benav H., Rask-Andersen H. Macrophages in the human cochlea: saviors or predators-A study using super-resolution immunohistochemistry. Front. Immunol. 2018;9:223. doi: 10.3389/fimmu.2018.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Schrott-Fischer A., Glueckert R., Benav H., Rask-Andersen H. The human “cochlear battery” - claudin-11 barrier and ion transport proteins in the lateral wall of the cochlea. Front. Mol. Neurosci. 2017;10:239. doi: 10.3389/fnmol.2017.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Bostrom M., Kinnefors A., Linthicum F., Rask-Andersen H. Expression of myelin basic protein in the human auditory nerve—an immunohistochemical and comparative study. Auris Nasus Larynx. 2012;39:18–24. doi: 10.1016/j.anl.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Liu W., Atturo F., Aldaya R., Santi P., Cureoglu S., Obwegeser S., Glueckert R., Pfaller K., Schrott-Fischer A., Rask-Andersen H. Macromolecular organization and fine structure of the human basilar membrane - RELEVANCE for cochlear implantation. Cell Tissue Res. 2015;360:245–262. doi: 10.1007/s00441-014-2098-z. Epub 2015 Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A., Huang H., Radke J., Stenzel W., Priller J. P2Y12 receptor is expressed on human microglia under physiological conditions throughout development and is sensitive to neuroinflammatory diseases. Glia. 2017;65:375–387. doi: 10.1002/glia.23097. Epub 2016 Nov 12. [DOI] [PubMed] [Google Scholar]
- Okano T., Nakagawa T., Kita T., Kada S., Yoshimoto M., Nakahata T. Bone marrow-derived cells expressing Iba1 are constitutively present as resident tissue macrophages in the mouse cochlea. J. Neurosci. Res. 2008;86:1758–1767. doi: 10.1002/jnr.21625. [DOI] [PubMed] [Google Scholar]
- O'Malley J.T., Nadol J.B., Jr., McKenna M.J. Anti CD163+, Iba1+, and CD68+ cells in the adult human inner ear: normal distribution of an unappreciated class of macrophages/microglia and implications for inflammatory otopathology in humans. Otol. Neurotol. 2016;37:99–108. doi: 10.1097/MAO.0000000000000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask-Andersen H., Stahle J. Lymphocyte-macrophage activity in the endolymphatic sac. An ultrastructural study of the rugose endolymphatic sac in the Guinea pig. ORL J. Otorhinolaryngol. Relat. Spec. 1979;41:177–192. doi: 10.1159/000275458. [DOI] [PubMed] [Google Scholar]
- Solovjov D.A., Pluskota E., Plow E.F. Distinct roles for the alpha and beta subunits in the functions of integrin alphaMbeta2. J. Biol. Chem. 2005;280:1336–1345. doi: 10.1074/jbc.M406968200. [DOI] [PubMed] [Google Scholar]
- Solares C.A., Edling A.E., Johnson J.M., Baek M.J., Hirose K., Hughes G.B., Tuohy V.K. Murine autoimmune hearing loss mediated by CD4+ T cells specific for inner ear peptides. J. Clin. Invest. 2004;113:1210–1217. doi: 10.1172/JCI18195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souter M., Eastwood H., Marovic P., Kel G., Wongprasartsuk S., Ryan A.F., O'Leary S.J. Systemic immunity influences hearing preservation in cochlear implantation. Otol. Neurotol. 2012;33:532–538. doi: 10.1097/MAO.0b013e31824bac44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y., Sumiya T., Tachikawa M., Yamakawa T., Murata S., Yagi Y., Sato K., Stephan A., Ito K., Ohtsuki S., Couraud P.O., Suzuki T., Terasaki T. Involvement of claudin-11 in disruption of blood-brain, -spinal cord, and -arachnoid barriers in multiple sclerosis. Mol. Neurobiol. 2018 doi: 10.1007/s12035-018-1207-5. July 8. [DOI] [PubMed] [Google Scholar]
- Warchol M.E. Macrophage activity in organ cultures of the avian cochlea: demonstration of a resident population and recruitment to sites of hair cell lesions. J. Neurobiol. 1997;33:724–734. [PubMed] [Google Scholar]
- Warchol M.E., Schwendener R.A., Hirose K. Depletion of resident macrophages does not alter sensory regeneration in the avian cochlea. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Vethanayagam R.R., Dong Y., Cai Q., Hu B.H. Activation of the antigen presentation function of mononuclear phagocyte populations associated with the basilar membrane of the cochlea after acoustic overstimulation. Neuroscience. 2015;303:1–15. doi: 10.1016/j.neuroscience.2015.05.081. Epub 2015 Jun 20. [DOI] [PMC free article] [PubMed] [Google Scholar]