More than 230 million patients undergo major surgical procedures every year worldwide (1), among whom 16% to 18% will develop major postoperative complications (2,3), which negatively affect morbidity, mortality, healthcare cost, and quality of life. Patients undergoing thoracic surgery are at a higher risk of postoperative complications, especially those involving the respiratory system. Potential pathophysiology leading to postoperative pulmonary complications (PPCs) includes depressed central respiratory drive, changes in end-expiratory muscle tone, reduced lung volume (including functional residual capacity), abnormal regional distribution of ventilation, and atelectasis (4).
Among all the modifiable risk factors for postoperative complications, inappropriate intraoperative ventilation strategies may lead to tidal hyperinflation and tidal recruitment; i.e., major mechanisms associated with ventilator-induced lung injury. This is particularly true during one-lung ventilation for thoracic surgery, when more than one-third of anesthesiologists will not actively minimize tidal volume (5), which is inevitably associated with increased stress and strain. As a result, the concept of lung-protective ventilation (LPV) has been introduced from intensive care settings to the operating theater.
In the Pulmonary Surgery with Protective Ventilation (PPV) trial (6), which was a prospective, double-blind, randomized controlled trial in 13 thoracic surgical centers over a 33-month study period, patients undergoing lobectomy or pneumonectomy for lung cancer were randomly assigned to either LPV [tidal volume 5 mL/kg ideal body weight (IBW), and positive end-expiratory pressure (PEEP) 5 to 8 cmH2O] or conventional ventilation (tidal volume 10 mL/kg IBW without PEEP) during anesthesia. Lung recruitment maneuvers (RMs) were performed in both groups at the discretion of the anesthesiologists in charge. The primary outcome included major pulmonary and non-pulmonary complications or death within 30 days after surgery. The study was stopped prematurely after enrollment of only 346 patients (about one-third of the predicted sample size of 900 patients) due to slow recruitment. In a modified intention-to-treat analysis, 23 patients (13.4%) in the LPV group developed major postoperative complications, compared with 38 patients (22.2%) in the control group [(odds ratio (OR) 0.54, 95% confidence interval (CI): 0.31 to 0.95, P=0.03)]. In addition, duration of hospital length of stay was significantly shorter in the LPV group (median 11 vs. 12 days, P=0.048). Despite limitations such as underestimation of complication rate in the control group, premature termination of the trial, and non-standardized RMs, the authors concluded that the PPV trial provided preliminary evidence supporting LPV during one-lung ventilation.
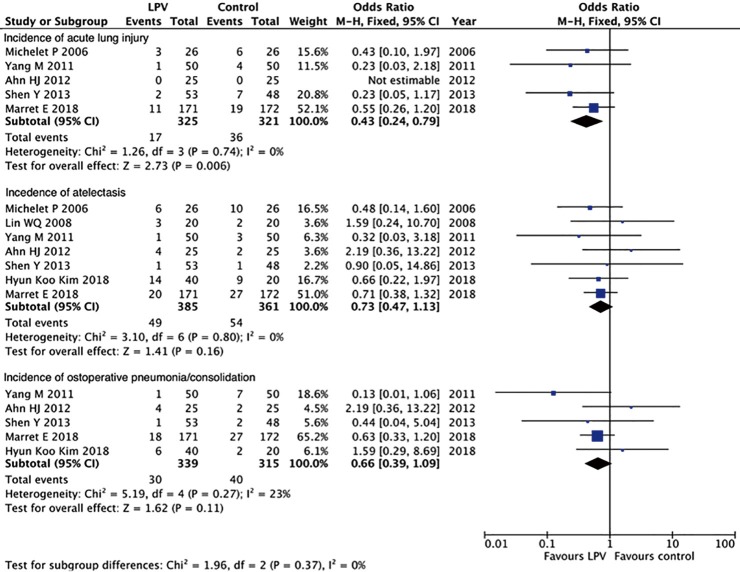
Apart from the PPV trial, there have been several other studies comparing LPV and conventional ventilation during one-lung ventilation. We have searched PubMed with the following strategy: ((low tidal [Title/Abstract] OR protective [Title/Abstract])) AND (one-lung [Title/Abstract] OR thoracic surgery [Title/Abstract]). This search identified 9 trials (6-14) (Table 1). A meta-analysis of these 9 trials using a fixed-effects model showed a significant decrease in acute lung injury (OR 0.43, 95% CI: 0.24 to 0.79, P=0.006), a nonsignificant reduction in atelectasis (OR 0.73, 95% CI: 0.47 to 1.13, P=0.16) and postoperative pneumonia/consolidation (OR 0.66, 95% CI: 0.39 to 1.09, P=0.11) (Figure 1).
Table 1. Characteristics and ventilator settings of included studies.
| Studies | Sample size | Age | Male (%) | LPV group | Control group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Vt | PEEP | RR | n | Vt | PEEP | RR | |||||
| Ahn 2012 (7) | 50 | 58 | NA | 25 | 6 | 5 | NA | 25 | 10 | 0 | NA | |
| Cai 2013 (8) | 60 | 22.8 | 53 | 30 | 3-5 | NA | NA | 30 | 8–10 | NA | NA | |
| Lin 2008 (9) | 40 | 55 | 78 | 20 | 5–6 | 3–5 | 13 | 20 | 10 | 0 | 11 | |
| Maslow 2013 (10) | 32 | 65.4 | 41 | 16 | 5 | 5 | 14 | 16 | 10 | 0 | 7 | |
| Michelet 2006 (11) | 52 | 60.5 | 83 | 26 | 5 | 5 | 15 | 26 | 9 | 0 | 12 | |
| Shen 2013 (12) | 101 | 58.9 | 71.3 | 53 | 5 | 5 | NA | 48 | 8 | 0 | NA | |
| Yang 2011 (13) | 100 | 59 | 62 | 50 | 6 | 5 | 12.8 | 50 | 10 | 0 | 9.4 | |
| Kim 2018 (14) | 60 | 57 | 45 | 40 | 6 | 5 | 12 | 20 | 10 | 0 | 10 | |
| Marret 2018 (6) | 343 | 63 | 71.9 | 171 | 5 | 5 | 17 | 172 | 10 | 0 | 10.8 | |
LPV, lung-protective ventilation; NA, not available; PEEP, positive end-expiratory pressure; RR, respiratory rate; Vt, tidal volume.
Figure 1.
Forest plot of the meta-analyses.
Despite all these efforts to delineate the effects of LPV to prevent postoperative complications, especially PPCs, the importance of individual components of LPV (low tidal volume, PEEP, and RM) remains to be addressed. Interestingly, an international prospective randomized controlled trial enrolling 900 patients at risk for PPCs undergoing elective open abdominal surgery compared high PEEP (12 cmH2O) with RM and low PEEP (2 cmH2O) without RM during low tidal volume (8 mL/kg IBW) ventilation (15). The study showed a similar prevalence of PPCs between the two groups, suggesting that the beneficial effects of LPV might be attributable to low tidal volume ventilation rather than high PEEP. This viewpoint was also supported by findings of an individual patient data meta-analysis (16). In contrast, a retrospective cohort study of 1,019 patients undergoing thoracic surgery with one-lung ventilation concluded that, without adequate PEEP, low tidal volume did not prevent PPCs (17).
There are even more questions to be answered. First, for those who cannot be extubated in the operating theatre, how should LPV be continued in the intensive care unit? Second, with regards to the ventilator settings, should we adopt a one-size-fits-all approach (as tested in almost all studies) or an individualized approach? And, if the latter approach is appropriate, how should we determine the appropriateness of tidal volume and/or PEEP? For example, how should we set PEEP level during one-lung ventilation, and why? From the previous experience in ventilating ARDS patients, we understand that gas exchange may not be the answer as it is very often, if not always, dissociated from lung protection under these circumstances (18). Therefore, can lung mechanics or imaging investigations provide convincing answers? Although it is true that further studies are needed to address the above questions, we would rather believe that these studies should be designed based on a robust and well-defined pathophysiology.
Acknowledgements
None.
Provenance: This is an invited Editorial commissioned by the Section Editor Shuangjiang Li (Department of Thoracic Surgery and West China Medical Center, West China Hospital, Sichuan University, Chengdu, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008;372:139-44. 10.1016/S0140-6736(08)60878-8 [DOI] [PubMed] [Google Scholar]
- 2.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med 2009;361:1368-75. 10.1056/NEJMsa0903048 [DOI] [PubMed] [Google Scholar]
- 3.International Surgical Outcomes Study group Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Br J Anaesth 2016;117:601-9. 10.1093/bja/aew316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth 2017;118:317-34. 10.1093/bja/aex002 [DOI] [PubMed] [Google Scholar]
- 5.Kidane B, Choi S, Fortin D, et al. Use of lung-protective strategies during one-lung ventilation surgery: a multi-institutional survey. Ann Transl Med 2018;6:269. 10.21037/atm.2018.06.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marret E, Cinotti R, Berard L, et al. Protective ventilation during anaesthesia reduces major postoperative complications after lung cancer surgery: a double-blind randomised controlled trial. Eur J Anaesthesiol 2018;35:727-35. 10.1097/EJA.0000000000000804 [DOI] [PubMed] [Google Scholar]
- 7.Ahn HJ, Kim JA, Yang M, et al. Comparison between conventional and protective one-lung ventilation for ventilator-assisted thoracic surgery. Anaesth Intensive Care 2012;40:780-8. 10.1177/0310057X1204000505 [DOI] [PubMed] [Google Scholar]
- 8.Cai K, Wang X, Ye J, et al. Laryngeal mask anesthesia in video-assisted thoracoscopic surgery for pulmonary bulla: comparison with intubation anesthesia. Nan Fang Yi Ke Da Xue Xue Bao 2013;33:756-60. [PubMed] [Google Scholar]
- 9.Lin WQ, Lu XY, Cao LH, et al. Effects of the lung protective ventilatory strategy on proinflammatory cytokine release during one-lung ventilation. Ai Zheng 2008;27:870-3. [PubMed] [Google Scholar]
- 10.Maslow AD, Stafford TS, Davignon KR, et al. A randomized comparison of different ventilator strategies during thoracotomy for pulmonary resection. J Thorac Cardiovasc Surg 2013;146:38-44. 10.1016/j.jtcvs.2013.01.021 [DOI] [PubMed] [Google Scholar]
- 11.Michelet P, D'Journo XB, Roch A, et al. Protective ventilation influences systemic inflammation after esophagectomy: a randomized controlled study. Anesthesiology 2006;105:911-9. 10.1097/00000542-200611000-00011 [DOI] [PubMed] [Google Scholar]
- 12.Shen Y, Zhong M, Wu W, et al. The impact of tidal volume on pulmonary complications following minimally invasive esophagectomy: a randomized and controlled study. J Thorac Cardiovasc Surg 2013;146:1267-73. 10.1016/j.jtcvs.2013.06.043 [DOI] [PubMed] [Google Scholar]
- 13.Yang M, Ahn HJ, Kim K, et al. Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery?: a randomized controlled trial. Chest 2011;139:530-7. 10.1378/chest.09-2293 [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Seo JH, Park KU, et al. Effect of combining a recruitment maneuver with protective ventilation on inflammatory responses in video-assisted thoracoscopic lobectomy: a randomized controlled trial. Surg Endosc 2018. [Epub ahead of print]. 10.1007/s00464-018-6415-6 [DOI] [PubMed] [Google Scholar]
- 15.Hemmes SN, Gama de Abreu M, Pelosi P, et al. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 2014;384:495-503. 10.1016/S0140-6736(14)60416-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serpa Neto A, Hemmes SN, Barbas CS, et al. Protective versus conventional ventilation for surgery: a systematic review and individual patient data meta-analysis. Anesthesiology 2015;123:66-78. 10.1097/ALN.0000000000000706 [DOI] [PubMed] [Google Scholar]
- 17.Blank RS, Colquhoun DA, Durieux ME, et al. Management of one-lung ventilation: impact of tidal volume on complications after thoracic surgery. Anesthesiology 2016;124:1286-95. 10.1097/ALN.0000000000001100 [DOI] [PubMed] [Google Scholar]
- 18.Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]