Abstract
Objective
Human papillomavirus (HPV) infection is the most important risk factor for cervical cancer, which progresses from precursor lesions with no symptom if left untreated. We compared the risk of cervical dysplasia among HPV-positive Korean women based on HPV types and infection patterns.
Methods
We observed participants of a 5-year multicenter prospective cohort study, comprising HPV-positive women with either atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion of the cervix at their enrollment. Follow-ups, comprising cytology and HPV DNA testing results, were included in the final analysis. Incidence was calculated for each infection pattern (persistent infection, incidental infection, and clearance). To investigate cervical dysplasia risk, we used Cox proportional hazard models adjusted for variables that were significantly different among infection patterns. From April 2010 to September 2017, 71 of 1,027 subjects developed cervical dysplasia more severe than high-grade squamous intraepithelial lesion of the cervix.
Results
Of these 71 subjects, persistent infection, incidental infection, and clearance were noted in 30, 39, and 2 individuals, respectively. Based on changes in DNA results during follow-up, cumulative incidence was 27.2%, 10.4%, and 0.5% for persistent infection, incidental infection, and clearance, respectively. Compared to clearance, the adjusted hazard ratios for cervical dysplasia were 51.6 and 24.1 for persistent and incidental infections, respectively (p<0.001).
Conclusion
Individuals persistently infected with the same HPV types during the follow-up period had the highest risk of severe cervical dysplasia. Hence, it is necessary to monitor HPV types and infection patterns to prevent severe cervical precancerous lesions.
Keywords: Papillomavirus Infections, Uterine Cervical Cancer, Uterine Cervical Dysplasia, Cohort Studies, Republic of Korea
INTRODUCTION
Cervical cancer is a malignancy originating in the transformation zone of the cervix that involves abnormal cellular changes. In Korea, cervical cancer is the sixth most common cancer in women, after excluding thyroid cancer, which tends to have a high incidence as it is overdiagnosed. Despite an average annual decrease of 3.7% in cervical cancer incidence from 1999 to 2015, a total of 3,582 women were newly diagnosed with cervical cancer in 2015. This resulted in an age-standardized incidence rate of 9.1 per 100,000, calculated using the Segi's world standard population [1]. It is well known that human papillomavirus (HPV) infection is a necessary as well as the most common cause of cervical cancer, which can progress from precursor lesions (if left untreated) even after several years [2]. This precancerous stage, irrespective of severity, is very challenging to detect owing to the absence of symptoms in the infected individual.
More than 100 different HPV genotypes have been characterized so far, and approximately 40 of these are known to infect basal epithelial cells, causing benign and malignant lesions in the genital regions [3]. Many epidemiological studies led to the classification of oncogenic types that confer a high risk for progression of cervical intraepithelial lesions to cervical cancer. In many countries, HPV-16 and HPV-18 are considered the most common high-risk HPV types that cause most of cervical cancers that develop from precancerous cervical lesions [4]. Based on the risk associated with the HPV types, 3 kinds of vaccines are currently available for use: a bivalent vaccine targeting HPV-16 and HPV-18; a quadrivalent vaccine targeting the 2 most carcinogenic types (HPV-16/18) and 2 low-risk types (HPV-6/11); and a nonavalent vaccine targeting 5 more carcinogenic types (HPV-31/33/45/52/58) in addition to the 4 types (HPV-16/18/6/11) [5].
The geographical variations observed among the common HPV types is still unclear. Therefore, recognizing the prevalent types among specific populations is necessary for designing prophylactic HPV vaccines for effectively preventing cervical cancer [6,7]. The risk of cervical carcinogenesis may depend on the type of HPV and the diversity of infection patterns [8]. Determining the contribution of HPV genotypes to cervical carcinogenesis will help estimate the potential impact of vaccines and cancer screening programs.
Hence, in this study comprising HPV-positive women from a Korean HPV cohort study, we aimed to evaluate the risk of cervical dysplasia based on infection patterns and types of HPV.
MATERIALS AND METHODS
This cohort study was conducted at 7 different university obstetrics and gynecology clinics nationwide, since 2010 [9]. We enrolled women aged 20 to 60 years who were HPV-positive, with either atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL) of the cervix at the time of enrollment. Women who had undergone hysterectomy previously, were treated for cervical intraepithelial neoplasia (CIN) within the last 6 months of enrollment, were diagnosed with any cancer type or coexisting malignancies, had been affected by psychological disease and were currently undergoing treatment, or were pregnant at the time of enrollment were excluded from the study. In this study, participants who did not develop the severe precancerous stage during the follow-up period (maximum 5 years) were categorized as having non-progressive disease. The study was approved by the Institutional Review Boards (Cheil General Hospital and Women's Healthcare Center of the Dankook University: CGH-IRB-2010-13, Busan Paik Hospital of the Inje University: 11-117, Seoul St. Mary's Hospital of the Catholic University: KC10ONME0075, Dongsan Medical Center of the Keimyung University: 2011-11-099, Guro Hospital of the Korea University: KUGH16066-001, and Chonnam University Hospital: CNUHH-2015-026) and informed consent was obtained from each study participant.
To evaluate the presence of CIN, colposcopy-directed biopsies were performed at initial presentation and during follow-up. At the time of enrollment, patients having a diagnosis with chronic inflammation or CIN 1 were included in this study, and those having a lesion as greater than CIN 2 were not included although their cytologic results were either ASCUS or LSIL. During follow-up, patients having cytologic results indicative of conditions worse than high-grade squamous intraepithelial lesion were confirmed the presence of CIN by biopsy. If patients diagnosed with a lesion worse than CIN 2, those were terminated from this study while the patients diagnosed having CIN 1 remained as follow-up participants. For HPV genotyping at the initial visit, two different DNA microarray techniques were used based on the participating hospital. Cheil General Hospital used the Cheil HPV DNA Chip Kit (Cheil General Hospital, Seoul, Korea) and all of other participating hospitals used the method named Anyplex™ II HPV 28 detection (Seegene Inc., Seoul, Korea). During the follow-up examinations, Cheil General Hospital served as a central laboratory and performed HPV genotyping uniformly with the collected samples from the participating hospitals using a Cheil HPV DNA Chip Kit.
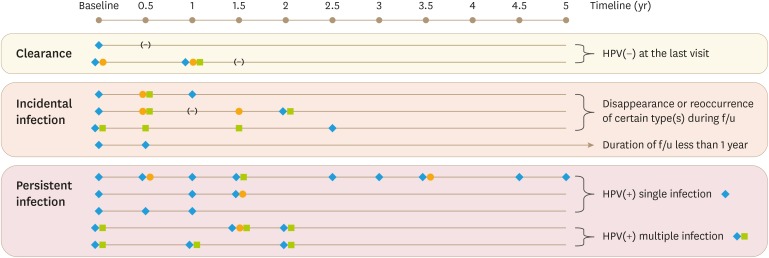
Among the 1,409 subjects recruited, from April 2010 to September 2017, 382 subjects were excluded owing to the following: the lack of information about HPV genotype at enrollment (n=188), HPV DNA test results obtained only once at the time of enrollment (n=186), and lack of response to surveys (n=8). A total of 1,027 subjects were selected in the final analysis: 578 (56.3%) with ASCUS and 449 (43.7%) with LSIL. The follow-ups, with results of cytology and HPV DNA testing, were included in the final analysis. We analyzed the cytological and histological data obtained from each follow-up, which took place at 6-month intervals. Demographic and behavioral information was obtained via self-administered questionnaires and interview surveys every year. Enrolled participants who were diagnosed with a lesion classified as more severe than cervical intraepithelial neoplasia 2 (CIN 2+) by colposcopically directed biopsy were not followed up further in the course of this study. Based on oncogenicity in humans, the genotypes of HPV were classified as high-risk (19 HPV types: HPV-16, HPV-18, HPV-26, HPV-31, HPV-33, HPV-35, HPV-39, HPV-45, HPV-51, HPV-52, HPV-53, HPV-56, HPV-58, HPV-59, HPV-66, HPV-68, HPV-69, HPV-73, and HPV-82) or low-risk (9 HPV types: HPV-6, HPV-11, HPV-40, HPV-42, HPV-43, HPV-44, HPV-54, HPV-61, and HPV-70) [4]. Based on the results of HPV DNA testing at each visit, the HPV infection patterns were classified into 3 types: persistent infection, incidental infection, and clearance (Fig. 1). Cases in which infection at least 1 HPV genotype persisted during the follow-up period were classified as persistent infections. Cases where patients had undergone HPV DNA test only twice could not be categorized as persistent infections. Cases with changes in, disappearance of, or reoccurrence of HPV types during the follow-up period were categorized as incidental infections. This infection pattern also included participants who had been persistently infected with certain HPV genotype(s) until their second visit, but had not yet visited a third time. Lastly, clearance of infection was defined as a reversion to the uninfected state, without detection of any HPV type at the latest visit [9].
Fig. 1. Examples of infection patterns and their definitions.
The symbols (eg. diamond, square, or circle) stand for different HPV genotype detected at the time of visit, and the notation of (−) stands for being negative without detecting any of HPV genotype.
HPV, human papillomavirus.
The descriptive statistics were summarized using the t-test or analysis of variance for continuous data, and the χ2 test for categorical data. The incidences were calculated for each HPV infection pattern (persistent infection, incidental infection, and clearance). To estimate the risk of cervical dysplasia, hazard ratios were calculated using Cox proportional hazard models, with adjustment for covariates, including age, marital status, drinking, menopause, oral contraceptive use, and the cumulative number of sex partners. The data were analyzed using SAS version 9.4 (SAS Institute, Cary, NC, USA), and a p-value of less than 0.05 was considered statistically significant.
RESULTS
The general characteristics of the study population (n=1,027) are described in Table 1. The average follow-up period was 3.1 years, and the average age at the time of enrollment was 40.6 years. Majority of the subjects were married and lived with their spouses (63.4%), were current drinkers (70.0%), had not reached menopause (82.9%), and used oral contraceptives (83.8%).
Table 1. Epidemiologic characteristics of study subjects based on HPV infection patterns, the Korea HPV cohort 2010–2017.
| Variables | All (n=1,027) | Persistent infection (n=144) | Incidental infection (n=474) | Clearance (n=409) | p-value* | |||
|---|---|---|---|---|---|---|---|---|
| Follow-up (yr) | 3.06±1.54 | 3.01±1.37 | 2.63±1.56 | 3.58±1.41 | <0.001 | |||
| Age (yr) | 40.57±9.64 | 42.93±9.90 | 40.58±9.96 | 39.73±9.04 | 0.003 | |||
| Age group | 0.001 | |||||||
| 20–29 | 167 (16.26) | 18 (12.50) | 86 (18.14) | 63 (15.40) | ||||
| 30–39 | 297 (28.92) | 33 (22.92) | 122 (25.74) | 142 (34.72) | ||||
| 40–49 | 350 (34.08) | 51 (35.42) | 159 (33.54) | 140 (34.23) | ||||
| ≥50† | 213 (20.74) | 42 (29.17) | 107 (22.57) | 64 (15.65) | ||||
| BMI | 21.77±2.81 | 21.97±2.82 | 21.75±2.76 | 21.72±2.85 | 0.637 | |||
| Marital status | 0.024 | |||||||
| Co-habitation | 650 (63.35) | 102 (71.33) | 277 (58.44) | 271 (66.26) | ||||
| Separation | 123 (11.99) | 14 (9.79) | 68 (14.35) | 41 (10.02) | ||||
| Single | 253 (24.66) | 27 (18.88) | 129 (27.22) | 97 (23.72) | ||||
| Education | 0.184 | |||||||
| ≤highschool level | 408 (39.73) | 66 (45.83) | 190 (40.08) | 152 (37.16) | ||||
| ≥college level | 619 (60.27) | 78 (54.17) | 284 (59.92) | 257 (62.84) | ||||
| Job | 0.356 | |||||||
| Manager/head | 250 (24.34) | 32 (22.22) | 118 (24.89) | 100 (24.45) | ||||
| Professionals | 186 (18.11) | 28 (19.44) | 78 (16.46) | 80 (19.56) | ||||
| Business/service | 211 (20.55) | 24 (16.67) | 108 (22.78) | 79 (19.32) | ||||
| Housekeeping | 331 (32.23) | 53 (36.81) | 142 (29.96) | 136 (33.25) | ||||
| Unemployed/student | 49 (4.77) | 7 (4.86) | 28 (5.91) | 14 (3.42) | ||||
| Monthly income ($) | 0.219 | |||||||
| <2,000 | 133 (12.95) | 20 (13.89) | 64 (13.50) | 49 (11.98) | ||||
| 2,000–5,000 | 448 (43.62) | 66 (45.83) | 208 (43.88) | 174 (42.54) | ||||
| ≥5,000 | 382 (37.20) | 50 (34.72) | 164 (34.60) | 168 (41.08) | ||||
| Don't know | 64 (6.23) | 8 (5.56) | 38 (8.02) | 18 (4.40) | ||||
| Smoking‡ | 0.310 | |||||||
| No | 881 (85.78) | 128 (88.89) | 399 (84.18) | 354 (86.55) | ||||
| Yes | 146 (14.22) | 16 (11.11) | 75 (15.82) | 55 (13.45) | ||||
| Drinking | 0.041 | |||||||
| No | 244 (23.73) | 41 (28.47) | 103 (21.73) | 100 (24.51) | ||||
| Not currently (yes) | 64 (6.24) | 5 (3.47) | 24 (5.06) | 35 (8.58) | ||||
| Currently (yes) | 718 (69.98) | 98 (68.06) | 347 (73.21) | 273 (66.91) | ||||
| Regular exercise | ||||||||
| No | 583 (56.77) | 75 (52.08) | 262 (55.27) | 246 (60.15) | ||||
| Yes | 444 (43.23) | 69 (47.92) | 212 (44.73) | 163 (39.85) | ||||
| Pregnancy | 0.437 | |||||||
| No | 224 (21.81) | 27 (18.75) | 111 (23.42) | 86 (21.03) | ||||
| Yes | 803 (78.19) | 117 (81.25) | 363 (76.58) | 323 (78.97) | ||||
| No. of offspring(s) | 0.094 | |||||||
| None | 93 (11.58) | 15 (12.82) | 43 (11.85) | 35 (10.84) | ||||
| 1 | 188 (23.41) | 18 (15.38) | 77 (21.21) | 93 (28.79) | ||||
| 2 | 429 (53.42) | 68 (58.12) | 198 (54.55) | 163 (50.46) | ||||
| 3+ | 93 (11.58) | 16 (13.68) | 45 (12.40) | 32 (9.91) | ||||
| Menopause | 0.001 | |||||||
| No | 851 (82.94) | 106 (73.61) | 390 (82.28) | 355 (87.01) | ||||
| Yes | 175 (17.06) | 38 (26.39) | 84 (17.72) | 53 (12.99) | ||||
| Contraceptive use | 0.908 | |||||||
| No | 633 (61.64) | 91 (63.19) | 292 (61.60) | 250 (61.12) | ||||
| Yes | 394 (38.36) | 53 (36.81) | 182 (38.40) | 159 (38.88) | ||||
| Oral contraceptive use | 0.011 | |||||||
| No | 861 (83.84) | 127 (88.19) | 380 (80.17) | 354 (86.55) | ||||
| Yes | 166 (16.16) | 17 (11.81) | 94 (19.83) | 55 (13.45) | ||||
| Cumulative No. of sex partner(s) | 0.049 | |||||||
| 1 | 407 (39.71) | 68 (47.22) | 173 (36.58) | 166 (40.69) | ||||
| 2–3 | 368 (35.90) | 44 (30.56) | 164 (34.67) | 160 (39.22) | ||||
| 4–5 | 157 (15.32) | 22 (15.28) | 80 (16.91) | 55 (13.48) | ||||
| 6+ | 61 (5.95) | 7 (4.86) | 38 (8.03) | 16 (3.92) | ||||
| No response | 32 (3.12) | 3 (2.08) | 18 (3.81) | 11 (2.70) | ||||
| HPV vaccination | 0.651 | |||||||
| No | 752 (73.22) | 110 (76.39) | 345 (72.78) | 297 (72.62) | ||||
| Yes | 275 (26.78) | 34 (23.61) | 129 (27.22) | 112 (27.38) | ||||
| No. of HPV vaccination | 0.568 | |||||||
| 1 | 53 (5.16) | 8 (5.56) | 20 (4.22) | 25 (6.11) | ||||
| 2 | 40 (3.89) | 7 (4.86) | 20 (4.22) | 13 (3.18) | ||||
| 3 | 182 (17.72) | 19 (13.19) | 89 (18.78) | 74 (18.09) | ||||
Data are presented as the mean±standard deviation for continuous variables and number (percentage) for categorical variables.
BMI, body mass index; HPV, human papillomavirus.
*p-values were obtained by using the analysis of variance test for continuous variables and the χ2 test for categorical variables; p<0.05 at significance in bold letters; †Age group of ≥50 included 3 subjects aged 60 years; ‡Smoking status (yes) refers to a person who has smoked 100 cigarettes in her lifetime.
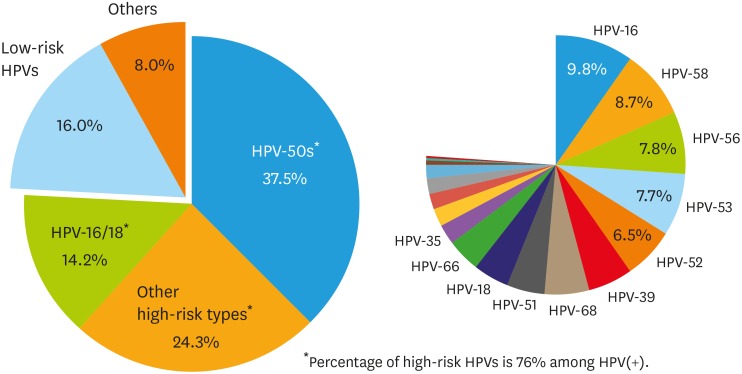
The distribution of HPV genotypes in the study subjects is summarized in Fig. 2. The percentage values for prevalence of each HPV type included cases of single infection as well as cases of multiple infections in which the particular HPV type was one of the infective agents. The most prevalent genotype was HPV-16 (9.8%), followed by HPV-58 (8.7%), HPV-56 (7.8%), HPV-53 (7.7%), and HPV-52 (6.5%). At the time of enrollment, the majority of identified genotypes were classified as high-risk HPV (76%), and the remaining genotypes were classified as low-risk HPV (16%) or other HPV types (8%). High-risk HPV-50s (HPV-51/52/53/56/58/59) were found to be most common among the high-risk genotypes, followed by other high-risk types (HPV-26/31/33/35/39/45/66/68/69/73/82), and HPV-16/18.
Fig. 2. Distribution of HPVs among ASCUS or LSIL cases.
ASCUS, atypical squamous cells of undetermined significance; HPV, human papillomavirus; LSIL, low-grade squamous intraepithelial lesion.
*Detailed percentages of high-risk HPVs: HPV-16 (9.8%), HPV-58 (8.7%), HPV-56 (7.8%), HPV-53 (7.7%), HPV-52 (6.5%), HPV-39 (5.6%), HPV-68 (5.3%), HPV-51 (4.9%), HPV-18 (4.4%), HPV-66 (4.1%), HPV-35 (2.5%), HPV-31 (2.3%), HPV-33 (2.0%), HPV-59 (2.0%), HPV-45 (1.5%), HPV-82 (0.6%), HPV-69 (0.3%), HPV-26 (0.2%), and HPV-73 (0.1%).
The cases of a total of 1,027 study subjects were classified into 3 infection patterns, according to the maintenance or change in HPV genotypes during the follow-up period. There were 144 subjects with persistent infection (14.0%), 474 subjects with incidental infection (46.2%), and 409 subjects who showed clearance (39.8%). Comparison of the 3 groups showed that women with persistent infections were the oldest, with an average age of 42.9 years. This group had a relatively large proportion of women aged 50–60 years (29.2%), as well as women living with their spouses (71.3%), and who had reached menopause (26.4%). With respect to drinking status, more women with incidental infections were current drinkers (73.2%) (Table 1).
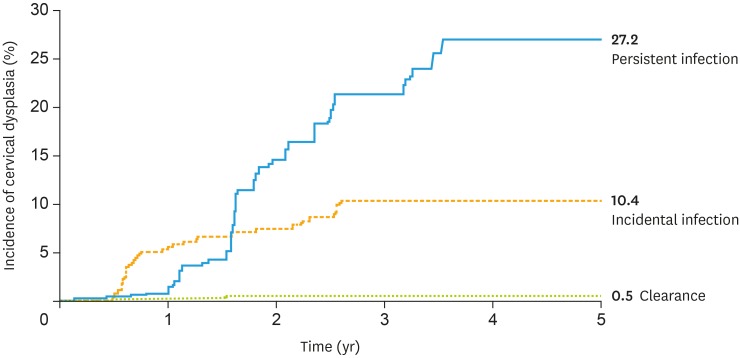
A total of 71 of the 1,027 subjects developed precancerous cervical dysplasia CIN 2+. Based on HPV infection patterns during the follow-up period, the incidence rates (cumulative incidence, incidence density) of progression to CIN 2+ were as follows: persistent infection, 27.2% (76.2 per 1,000 person-years); incidental infection, 10.4% (31.2 per 1,000 person-years); and clearance, 0.5% (1.4 per 1,000 person-years) (Fig. 3 and Table 2). Individuals persistently infected with the same HPV type(s) during the follow-up period had the highest risk of severe cervical dysplasia. Out of 144 subjects having persistent infection, the lesions more severe than CIN2 developed in 30 subjects. The mean of survival time was 1.95 years until developing CIN 2+ in the 30 subjects whereas that of 114 persistently infected subjects without developing CIN 2+ was 3.29 years.
Fig. 3. Cumulative incidence of cervical dysplasia based on HPV infection patterns during follow-up in the Korea HPV cohort, 2010–2017.
HPV, human papillomavirus.
Table 2. Incidence rate of cervical dysplasia per 1,000 person-years (n=71), the Korea HPV cohort 2010–2017.
| Covariates* | HPV infection patterns (No. per 1,000 person-years) | |||
|---|---|---|---|---|
| Persistent infection (n=30) | Incidental infection (n=39) | Clearance (n=2) | ||
| Incidence of cervical dysplasia | 76.2 | 31.2 | 1.4 | |
| Age group (yr) | ||||
| 20–29 | 75.4 | 21.9 | 4.8 | |
| 30–39 | 46.3 | 29.9 | 2.0 | |
| 40–49 | 76.6 | 50.9 | 0 | |
| ≥50 | 62.2 | 13.1 | 0 | |
| Marital status | ||||
| Co-habitation | 65.9 | 38.1 | 0 | |
| Separation | 93.3 | 24.6 | 0 | |
| Single | 61.9 | 20.8 | 6.1 | |
| Drinking experience | ||||
| Currently (yes) | 78.1 | 25.8 | 0 | |
| Not currently (yes) | 0 | 111.5 | 0 | |
| No | 56.5 | 35.2 | 5.7 | |
| Menopause | ||||
| Yes | 56.4 | 17.0 | 0 | |
| No | 74.4 | 34.6 | 1.6 | |
| Oral contraceptive use | ||||
| Yes | 141.1 | 11.3 | 0 | |
| No | 61.4 | 34.2 | 1.6 | |
| Cumulative number of sex partner(s) | ||||
| 1 | 59.0 | 43.5 | 0 | |
| 2–3 | 81.4 | 25.2 | 1.8 | |
| 4–5 | 74.2 | 18.9 | 5.4 | |
| 6+ | 0 | 21.3 | 0 | |
On comparison of the incidence rate for each risk factor, we found that the order of risk remained the highest for patients with persistent infections, followed by that for patients with incidental infection and clearance (Table 2). Compared to clearance, the risk of developing CIN 2+ was approximately 50 times higher in patients with persistent infection (hazard ratio [HR]=51.6) and approximately 25 times higher in patients with incidental infection in which the HPV types were not found to persist during the follow-up (HR=24.1). Specifically, for the persistent infection pattern, the risk was the highest for infections with HPV-16/18 (HR=83.0), whereas it was relatively low for infections with high-risk HPV-50s (HR=42.3). In both the persistent and incidental patterns, subjects infected with more than 2 HPV types showed higher risk than those infected with a single HPV type (Table 3).
Table 3. HRs for the development of cervical dysplasia among HPV-positive women, the Korea HPV cohort 2010–2017.
| HPV infection patterns* | Subjects | CIN 2+ | Crude | Adjusted‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||||||
| Clearance | 409 | 2 | 1.0 | |||||||
| Incidental infection† | 474 | 39 | 21.5 | 5.1–88.9 | 24.1 | 5.7–100.2 | ||||
| Low-risk HPV detected at last visit | 100 | 3 | 1.7 | 0.1–18.6 | 1.7 | 0.1–19.3 | ||||
| High-risk HPV detected at last visit | 238 | 35 | 40.9 | 9.8–169.8 | 47.2 | 11.2–197.6 | ||||
| Single infection | 196 | 27 | 38.1 | 9.1–160.3 | 44.6 | 10.5–188.5 | ||||
| Multiple infection | 42 | 8 | 54.1 | 11.4–254.7 | 61.1 | 12.6–294.8 | ||||
| High/low-risk HPV detected at last visit (mixed) | 136 | 1 | 8.2 | 1.3–49.1 | 1.8 | 0.2–19.3 | ||||
| Persistent infection | 144 | 30 | 47.0 | 11.2–196.8 | 51.6 | 12.2–217.5 | ||||
| Persistent with high-risk HPV | 126 | 29 | 51.8 | 12.3–217.1 | 57.1 | 13.5–240.8 | ||||
| Single infection | 77 | 17 | 49.1 | 11.3–212.6 | 52.9 | 12.0–232.1 | ||||
| Multiple infection | 49 | 12 | 56.2 | 12.5–251.0 | 63.5 | 14.1–285.3 | ||||
| HPV type 16/18 | 31 | 10 | 74.2 | 16.2–338.8 | 83.0 | 17.6–390.1 | ||||
| Single infection | 17 | 6 | 80.9 | 16.3–400.8 | 77.2 | 14.6–405.8 | ||||
| Multiple infection | 14 | 4 | 66.1 | 12.1–360.9 | 95.1 | 16.8–536.9 | ||||
| HPV type 50s | 60 | 10 | 38.4 | 8.4–175.2 | 42.3 | 9.1–195.0 | ||||
| Single infection | 40 | 7 | 40.7 | 8.4–195.7 | 44.8 | 9.1–218.5 | ||||
| Multiple infection | 20 | 3 | 34.0 | 5.6–203.4 | 36.7 | 6.0–223.3 | ||||
| Other types | 35 | 9 | 54.8 | 11.8–253.7 | 61.6 | 13.2–287.1 | ||||
| Single infection | 20 | 4 | 40.2 | 7.3–219.6 | 49.2 | 8.8–274.0 | ||||
| Multiple infection | 15 | 5 | 77.3 | 14.9–398.5 | 77.2 | 14.8–401.9 | ||||
| Persistent with low-risk HPV | 18 | 1 | 12.5 | 1.1–137.5 | 13.0 | 1.1–144.9 | ||||
CI, confidence interval; CIN, cervical intraepithelial neoplasia; CIN 2+, more severe than cervical intraepithelial neoplasia 2; HPV, human papillomavirus; HR, hazard ratio.
*The definition of HPV infection patterns can be found in Fig. 1; †Low-risk HPV including 9 HPV types including HPV-6, HPV-11, HPV-40, HPV-42, HPV-43, HPV-44, HPV-54, HPV-61, and HPV-70 and high-risk HPV including 19 HPV types including HPV-16, HPV-18, HPV-26, HPV-31, HPV-33, HPV-35, HPV-39, HPV-45, HPV-51, HPV-52, HPV-53, HPV-56, HPV-58, HPV-59, HPV-66, HPV-68, HPV-69, HPV-73, and HPV-82; ‡Adjustment for covariates including age, marital status, drinking, menopause, oral contraceptive, and cumulative number of sex partners.
DISCUSSION
In the present study, both the incidence and risk of progression to CIN 2+ were the highest in subjects persistently infected with at least one type of high-risk HPV throughout the follow-up period, followed by the incidence and risk in cases of incidental infection and clearance. The risk of developing CIN 2+ was the highest in subjects persistently infected with HPV-16/18, and it increased if the subjects were infected with more than two types of high-risk HPVs at the same time.
Our findings were consistent with those of previous studies with respect to the following: persistent infection with high-risk HPV is necessary for the development of precancerous lesions [10], and infections with HPV-16 and/or HPV-18 are associated with the highest risk of CIN [11].Furthermore, the strength of risk for cervical dysplasia also depends on whether the subject is infected with single or multiple HPV types. For both persistent and incidental infection patterns, subjects infected with multiple high-risk HPVs had a higher risk than those infected with a single high-risk HPV. Many reports have indicated that multiple HPV infections are associated with higher grades of cervical abnormalities, and the cervical cancer risk is higher in patients infected with multiple HPV types than in those infected with a single HPV type. Prospective studies have shown that infection with multiple high-risk HPV types acts synergistically in cervical carcinogenesis [12]. Moreover, cancers in patients infected with multiple HPV types could be more resistant to therapy than in those infected with a single HPV type. A related study reported that the treatment failure rate of cervical cancer patients infected with multiple HPV types was 5 times higher than that of patients infected with a single HPV type [13]. However, some studies have reported that infection with multiple HPV types does not affect either the incidence or severity of cervical cancer [14,15].
Some strengths of the present study are as follows: the study participants were recruited from 4 metropolitan cities in Korea rather than from only 1 area. To our knowledge, this is the first Korean HPV cohort study including a relatively large-scale assessment of the distribution of HPV types among HPV-positive women. Furthermore, the risk of developing severe cervical dysplasia was compared based on the prevalent genotypes and infection patterns. There have been various studies demonstrating the differences in HPV distribution between the Western and Eastern population [16]. HPV-58, while commonly found in East Asia, is a rare HPV type worldwide that accounts for only 3.3% of cervical cancer cases globally [17,18]. In Asia, a larger proportion of cervical cancers are associated with HPV-58 and HPV-52 among rarer types excluding HPV-16 and HPV-18 [19]. In this study population, the genotypes of HPV-50s (HPV-58/56/53/52/51/59) were most commonly found. However, the high frequency of these genotypes was not associated with an increased risk for cervical precancerous lesions. With respect to the natural course leading to severe cervical dysplasia, the risk was relatively higher in subjects persistently infected with HPV-16/18 than in those infected with the HPV-50s.
Previous studies have demonstrated that immunological deficiency was a factor that induces development of malignant lesions, allowing higher rates of HPV persistence, suggesting that the presence of chronic inflammation with weakened cellular immunity may play a decisive role in accelerating the rate of progression, leading to cancer [20]. Therefore, variations in the immunity level of the patients may affect the acquisition or clearance of infection [21]. However, this study did not consider immunologic factors. Any measurements indicating the immunity level of the participants were not used in the present study.
Further study is required to determine the risk factors associated with progression of HPV infection to cervical cancer, specifically, among subjects with persistent infection. A detailed evaluation of subjects with persistent infections during the follow-up period may be a further step in the investigation of potential risk factors for HPV persistence, such as alcohol consumption, female-specific conditions including parity or long-time use of oral contraceptives, and sexual habits. For instance, a previous study, conducted in Korea, suggested that the synergistic effect of high-risk HPV and alcohol consumption increased the risk of viral persistence [22].
In conclusion, the current study demonstrates that women persistently infected with multiple HPV genotypes during their follow-up periods had the highest risk of showing progression to the severe stage of cervical dysplasia. Among the high-risk genotypes, HPV-16 and HPV-18 were the most likely to cause CIN 2+, despite the HPV-50s being the most common in HPV-positive women in this Korean cohort study. Our results suggest that it is necessary to monitor infection patterns in addition to the HPV type, in order to prevent severe cervical precancerous lesions.
Footnotes
Presentation: This study was presented at the 70th Annual Meeting of the Korean Society for Preventive Medicine in Busan, Korea during October 18-20, 2017.
Funding: This study was supported by a grant for the Chronic Infectious Disease Cohort Study (Korea HPV Cohort Study) from the Korea Centers for Disease Control and Prevention (2016-E51002-02) and a research grant from the National Cancer Center of Korea (NCC-1710141).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Funding acquisition: K.M.K., S.J., K.M.
- Investigation: K.T.J., H.C.S., C.C.H., J.D.H., S.S.J., L.J.K., H.S.
- Methodology: P.Y., K.M.
- Resources: K.M.K., S.J., K.M.
- Supervision: K.M.
- Writing - original draft: P.Y.
References
- 1.Jung KW, Won YJ, Kong HJ, Lee ES Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat. 2018;50:303–316. doi: 10.4143/crt.2018.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trottier H, Franco EL. Human papillomavirus and cervical cancer: burden of illness and basis for prevention. Am J Manag Care. 2006;12:S462–S472. [PubMed] [Google Scholar]
- 3.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens--part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer. A review of human carcinogens. Part B: Biological agents [Internet] Lyon: International Agency for Research on Cancer; 2012. [cited 2018 Feb 20]. Available from: http://monographs.iarc.fr/ENG/Monographs/vol100B/index.php. [Google Scholar]
- 5.de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Wang P, Ren Y, Du J, Jiang J, Jia X, et al. Prevalence of high-risk human papillomavirus (HR-HPV) genotypes and multiple infections in cervical abnormalities from Northern Xinjiang, China. PLoS One. 2016;11:e0160698. doi: 10.1371/journal.pone.0160698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutts FT, Franceschi S, Goldie S, Castellsague X, de Sanjose S, Garnett G, et al. Human papillomavirus and HPV vaccines: a review. Bull World Health Organ. 2007;85:719–726. doi: 10.2471/BLT.06.038414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wheeler CM. Natural history of human papillomavirus infections, cytologic and histologic abnormalities, and cancer. Obstet Gynecol Clin North Am. 2008;35:519–536. doi: 10.1016/j.ogc.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Lee WC, Lee SY, Koo YJ, Kim TJ, Hur SY, Hong SR, et al. Establishment of a Korea HPV cohort study. J Gynecol Oncol. 2013;24:59–65. doi: 10.3802/jgo.2013.24.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Travassos AG, Netto E, Xavier-Souza E, Nóbrega I, Adami K, Timbó M, et al. Predictors of HPV incidence and clearance in a cohort of Brazilian HIV-infected women. PLoS One. 2017;12:e0185423. doi: 10.1371/journal.pone.0185423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huh WK, Ault KA, Chelmow D, Davey DD, Goulart RA, Garcia FA, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178–182. doi: 10.1016/j.ygyno.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Trottier H, Mahmud S, Costa MC, Sobrinho JP, Duarte-Franco E, Rohan TE, et al. Human papillomavirus infections with multiple types and risk of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2006;15:1274–1280. doi: 10.1158/1055-9965.EPI-06-0129. [DOI] [PubMed] [Google Scholar]
- 13.Munagala R, Donà MG, Rai SN, Jenson AB, Bala N, Ghim SJ, et al. Significance of multiple HPV infection in cervical cancer patients and its impact on treatment response. Int J Oncol. 2009;34:263–271. [PubMed] [Google Scholar]
- 14.Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 15.Kay P, Soeters R, Nevin J, Denny L, Dehaeck CM, Williamson AL. High prevalence of HPV 16 in South African women with cancer of the cervix and cervical intraepithelial neoplasia. J Med Virol. 2003;71:265–273. doi: 10.1002/jmv.10479. [DOI] [PubMed] [Google Scholar]
- 16.Crosbie EJ, Einstein MH, Franceschi S, Kitchener HC. Human papillomavirus and cervical cancer. Lancet. 2013;382:889–899. doi: 10.1016/S0140-6736(13)60022-7. [DOI] [PubMed] [Google Scholar]
- 17.Chan PK. Human papillomavirus type 58: the unique role in cervical cancers in East Asia. Cell Biosci. 2012;2:17. doi: 10.1186/2045-3701-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao YP, Li N, Smith JS, Qiao YL ACCPAB members. Human papillomavirus type distribution in women from Asia: a meta-analysis. Int J Gynecol Cancer. 2008;18:71–79. doi: 10.1111/j.1525-1438.2007.00959.x. [DOI] [PubMed] [Google Scholar]
- 19.Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88:63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brickman C, Palefsky JM. Human papillomavirus in the HIV-infected host: epidemiology and pathogenesis in the antiretroviral era. Curr HIV/AIDS Rep. 2015;12:6–15. doi: 10.1007/s11904-014-0254-4. [DOI] [PubMed] [Google Scholar]
- 21.Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24(Suppl 1):S1–15. doi: 10.1016/j.vaccine.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 22.Min KJ, Lee JK, Lee S, Kim MK. Alcohol consumption and viral load are synergistically associated with CIN1. PLoS One. 2013;8:e72142. doi: 10.1371/journal.pone.0072142. [DOI] [PMC free article] [PubMed] [Google Scholar]