Abstract
Objective
To evaluate the polymerase chain reaction (PCR)-reverse-dot-blot (RDB) human papillomavirus (HPV) genotyping test as a feasible assay for the cervical cancer primary screening.
Methods
In a hospital-based cohort, a total of 21,568 women were voluntarily enrolled from March 2009 to November 2016 for evaluating the 3 current cervical cancer screening strategies: co-test, cytology primary and high-risk HPV (HR-HPV) primary by using PCR-RDB HPV genotyping and liquid-based cytology (thinprep cytologic test [TCT]). Women with HR-HPV infection and/or abnormal cytology were referred for colposcopy, and the biopsy or conization was performed according to the American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines.
Results
Overall, 18.20% (3,935/21,568) of the women were detected as HR-HPV-positive, 5.04% (1,088/21,568) were diagnosed with cervical intraepithelial neoplasia 2 or higher (CIN2+), and 3.43% (739/21,568) with CIN3+. The cumulative incidence rates for CIN2+/CIN3+ in patients with HPV-16/18-positive were 48.28%/37.20%, while they were 0.86%/0.38%, 0.30%/0.15% and 0.18%/0.09% in cytology-negative, HR-HPV-negative and co-test-negative population, respectively. Using CIN2+ and CIN3+ as the observed endpoints, the sensitivity and negative predictive value (NPV) of HR-HPV genotyping as a primary screening tool were 90.99%/99.49% and 91.57%/99.80%. Moreover, using HR-HPV genotyping primary screening could detect the same more CIN2+/CIN3+ cases in baseline-detection as co-testing (990/700 vs. 991/701) and far more than cytology primary screening (903/656, p<0.05). It also achieved the lowest misdiagnosis rate (8.01%/5.02%). Although HPV genotyping primary screening required an increased number of colposcopies (2.75/3.89 per CIN2+/CIN3+ case), it yielded an acceptable rate.
Conclusions
The PCR-RDB HPV genotyping test is a cost-effective and beneficial cervical cancer primary screening for hospital-based opportunistic screening.
Keywords: Cervical Cancer, Cytology, Papillomaviridae, Genotype, Cancer Screening
INTRODUCTION
Cervical cancer is the second most common malignancy in women worldwide and a leading cause of death in women, with 529,828 newly diagnosed cases and 275,128 deaths annually [1]. More than 85% of these deaths occur in low- and middle-income countries [2]. As the largest developing country, China has the highest number of cervical cancer patients, with 98,900 newly reported cases and 30,500 deaths in 2015 [3]. However, cervical cancer is a preventable disease. Epidemiology studies have confirmed that persistent infection with high-risk human papillomavirus (HR-HPV) was found in 99.7% of patients with invasive cervical cancer [4,5]. Both HPV-16 and HPV-18 are carcinogenic genotypes responsible for 55%–60% and 10%–15% of cervical cancers, respectively [6].
Subjective interpretation of cytology is associated with a high degree of variability [7], which depends on the quality of the laboratory, experience of the cytologist, adequacy of the sample, and technique of fixation [8]. In contrast to the limitation of cytology, the sensitivity of HR-HPV testing is higher, and the negative predictive value (NPV) is better [9]. Therefore, European guidelines recommend that HPV testing to diagnose women with atypical squamous cells of undetermined significance (ASC-US) and surveillance after treatment of cervical intraepithelial neoplasia (CIN) can serve as an independent primary screening test without cytology for cervical screening [10]. In 2010, the European Research Organization on Genital Infection and Neoplasia (EUROGIN) proposed the implementation of HR-HPV testing in clinical settings [11]. In 2014, World Health Organization declared the HPV genotype test, which can maintain high sensitivity while reducing complexities and waste of unnecessary resources, as a primary screening strategy for cervical cancer [12]. In 2015, the American Society for Colposcopy and Cervical Pathology (ASCCP) put forward the use of HR-HPV testing for primary cervical cancer screening [13].
Four HPV tests, including hybrid capture 2 (HC2®), Cervista® HR-HPV, Cobas® 4800 System, and Aptima® mRNA, have been approved by the Unite State Food and Drug Administration (FDA) for cervical cancer screening. Among them, the Cobas® 4800 System is the only HPV test that was approved for primary screening in 2014 [14]. In our previous study [15], the YaNeng® polymerase chain reaction (PCR) and reverse dot blot (RDB) genotyping HPV assay combined with Thinprep® cytology were evaluated for cervical cancer screening in a hospital-based population, which provided a sensitive and reliable reference for clinical application. This method includes 18 HR-HPV types (HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -53, -56, -58, -59, -66, -68, -73, -82, and -83) and 5 low-risk (LR)-HPV types (HPV-6, -11, -42, -43, and -81). However, there is no adequate research on whether the PCR-RDB HPV test could be a suitable HPV detection test for the primary screening of cervical cancer in clinical settings.
The organizational screening programs aimed at early detection of high-grade CIN have steadily reduced cervical cancer mortality. Nonetheless, individual screening tests can suffer from some shortcomings, particularly when applied indiscriminately to the general screening population [16]. In contrast to most developed countries, the screening coverage in China is relatively low, i.e., approximately 30% in recent years [17]. Because opportunistic cervical cancer screening is commonly performed in China by the different health care provision systems [18], more attention should be paid to the sensitivity and NPV of detection in clinical practice. A large prospective 3-year clinical trial referred to as Addressing the Need for Advanced HPV Diagnostics (ATHENA) used HPV screening as the first-line screening test [19]. Our present study provides evidence on the clinical performance characteristics of different cervical cancer screening strategies with the PCR-RDB HPV test for the detection of high grade squamous intraepithelial lesions (HSIL; CIN2, and worse disease, CIN2+) in a Chinese hospital-based population that underwent opportunistic primary screening of cervical cancer. Moreover, the feasibility of the PCR-RDB HPV assay as a first-line HPV detection test for primary cervical cancer screening was also assessed.
MATERIALS AND METHODS
1. Study population
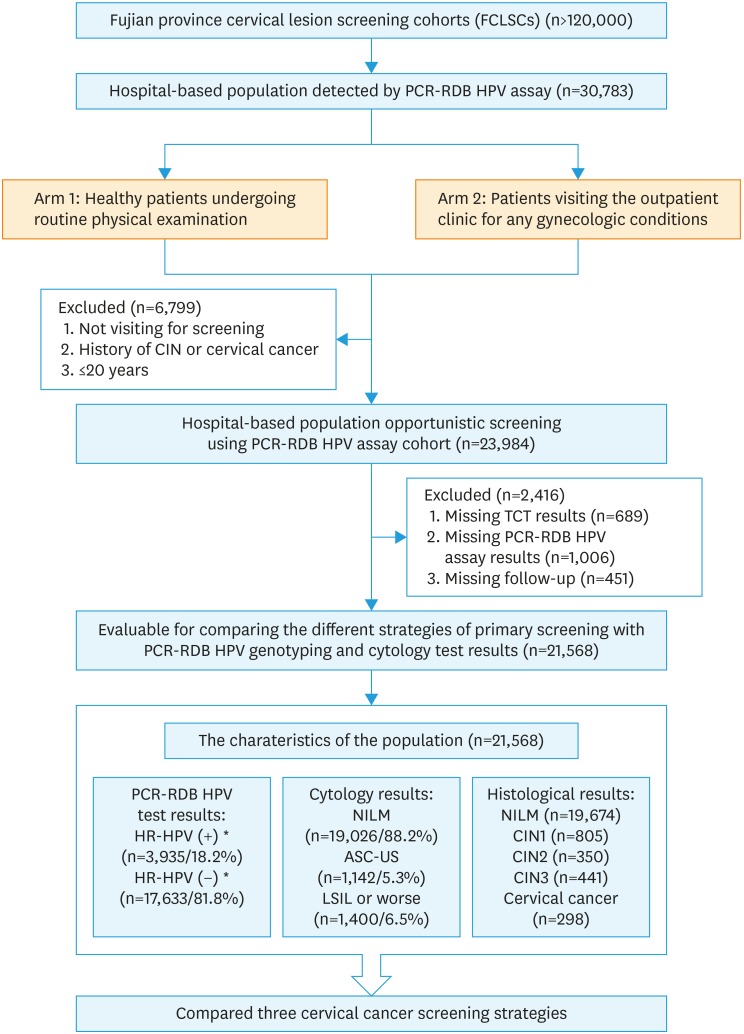
This HPV study was a nested case-control study in a hospital-based population in Fujian, China. This population cohort was one of the Fujian Province Cervical Lesion Screening Cohorts (FCLSCs) with more than 120,000 cases, which involved a community population as well as a hospital-based population. To investigate whether PCR-RDB HPV could be used for primary cervical cancer screening, 30,783 women received PCR-RDB HPV genotyping assay and liquid-based ThinPrep® cytologic test (TCT) were included. The participants were required to meet the following criteria: 1) age above 20 years; 2) sexually active; 3) no history of cervical cancer, CIN, or HIV infection; and 4) did not undergo a cervix surgery or hysterectomy. Two study arms were involved: one comprising healthy patients undergoing routine physical examination and the other comprising patients visiting the outpatient clinic for any gynecological conditions. A total of 6,799 women who did not meet the criteria were excluded. In total, 23,984 women were enrolled into this study, and 2,416 were lost to follow-up. The study flowchart is depicted in Fig. 1. Finally, 21,568 women with complete and detailed clinical information about cytology, HPV, and pathology results for each visitation were included in the evaluation. After the first-round screening, the patients with different results received treatment or follow-up (ranged from 1 to 8 years, median time 5.2 years) according to the ASCCP guidelines. The study was approved by the Ethics Committee of the Affiliated Hospital of Fujian Medical University (No. 59 in 2015), and all individuals in this study provided written informed consent.
Fig. 1. Flowchart of inclusion and exclusion criteria of the study population.
ASC-US, atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LEEP, loop electrosurgical excision procedure; LSIL, low-grade squamous intraepithelial lesion; NILM, negative for intraepithelial lesion or malignancy; PCR-RDB, polymerase chain reaction-reverse dot blot; TCT, thinprep cytologic test.
*HR-HPV (+): including HPV-16/-18 (+) (n=1,336) and HR-HPV non-16/18 (+) (n=2,599).
2. Specimen collection and management
Exfoliated cervical cells were obtained from each participant using a cytobrush. The specimens were obtained from the ecto- or endocervical cervical canals, and were collected in 2-mL vials containing preservation solution for HPV DNA testing or in 20-mL vials of ThinPrep® PreservCyt® solution (Hologic, Waltham, MA, USA) for cytology examination. The samples for HPV genotype testing were stored at −20°C before DNA extraction, and the samples for cytology were stored at 4°C.
3. PCR-RDB HPV genotype testing
The PCR-RDB HPV genotyping kit for 23 types (Yaneng® Limited Corporation, ShenZhen, China) can detect 18 HR-HPV types including HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -53, -56, -58, -59, -66, -68, -73, -82, and -83, and 5 LR-HPV types including HPV-6, -11, -42, -43, and -81. All detection procedures were performed according to the manufacturer's instruction provided by the kit [15]. This method is permitted by the China Food and Drug Administration.
4. Liquid-based cytology
All liquid-based cytology specimens were independently diagnosed by two experienced cytopathologists. If the diagnosis differed between the 2 cytopathologists, the sample was reviewed, and a consensus diagnosis was obtained. The cytology results were analyzed in accordance with the 2001 Bethesda System [20]. Specimens were classified as: negative for intraepithelial lesion or malignancy (NILM); ASC-US, low-grade squamous intraepithelial lesion (LSIL); atypical squamous cells, not possible to exclude high-grade squamous intraepithelial lesion (ASC-H); high-grade squamous intraepithelial lesion (HSIL); squamous cervical cancer; atypical glandular cells (AGCs); and adenocarcinoma in situ (AIS).
5. Histology
Women who were HPV-positive and/or had an abnormal cytological result (with a grade higher than ASC-US) were referred for colposcopy and punch biopsy. Women with a punch biopsy diagnosis greater than HSIL received loop electrosurgical excision procedure cone biopsy (LEEP) or conization by cold knife. Specimens were then fixed in 10% formalin, and march paraffin embedding was performed. Subsequently, 4-μm-thick histological sections were cut and stained with hematoxylin and eosin. In accordance with atypical hyperplasia, cells accounted for the range of squamous epithelium full-thickness and histologically examined and classified according to the CIN system as follows [21]: normal, CIN1, CIN2, CIN3, invasive squamous cell carcinoma, and adenocarcinoma.
6. Comparison of different screening strategies
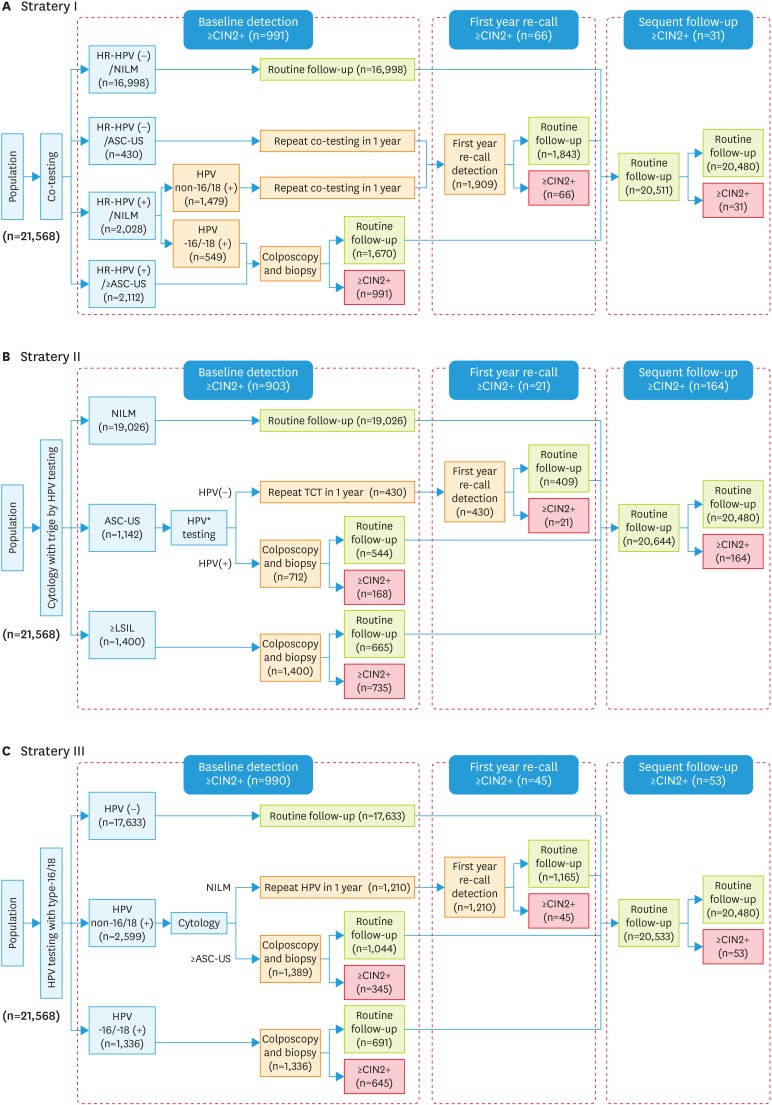
The effectiveness of the three strategies using the PCR-RDB HPV assay to identify CIN2+/CIN3+ in the same evaluated population for cervical cancer screening was compared. In Fig. 2 strategy I, which was co-testing, women were primarily screened (first line screening) by both cytology and HPV testing, and those with cytology ≥LSIL (including AGC) or cytological ASC-US and any HR-HPV (+) were selected for colposcopy. In addition, those who were cytological normal (NILM) but HPV-16/-18 (+) were also directly referred for colposcopy. Strategy II was primary first cytology screening and triage with HR-HPV testing. Women with cytology ≥LSIL only or ASC-US and triaged by reflex testing as HR-HPV (+) were referred for colposcopy. Strategy III was primary first-line HPV genotyping, and women with HPV-16 and/or -18 (+) were referred for colposcopy immediately, and non-HPV-16/-18 HR-positive women were triaged with cytology by referring those with abnormal cytology (≥ASC-US) for colposcopy. In all three strategies, women were directly referred for colposcopy at the baseline round; some of the participants were routinely followed-up for one year. This allowed calculation of how the specific screening strategies would perform, as well as the utilization of clinical resources for how many tests to include, number of colposcopies performed, and number of colposcopies required to detect one CIN2+ case and one CIN3+ case. After analyzing the performance of the different screening strategies incorporating HPV testing alone and in combination with cytology, we decided to perform a post hoc analysis of the performance of these strategies.
Fig. 2. Three cervical screening strategies to detect CIN2+/CIN3+. Effectiveness analysis of cervical screening strategies: primary screening algorithms. (A) Strategy I was co-testing, cytology combined with HPV using as the primary detection. (B) Strategy II was primary cytological detection with triaged by HPV testing. (C) Strategy III was primary HR-HPV testing with HPV-16/-18 genotype. In different strategies, the cases with ≥CIN2+ detected in the baseline screening, first year recall and the subsequent follow-up were listed in Fig. 2.
ASC-US, atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; HR-HPV, high-risk human papillomavirus; NILM, negative for intraepithelial lesion or malignancy.
7. Statistical analysis
Statistical analysis was performed using SPSS 17.0 (IBM, Chicago, IL, USA) for Windows. The performance characteristics of the PCR-RDB HPV test were evaluated by calculating the sensitivity, specificity, positive predictive value (PPV), and NPV for identification of CIN2, CIN3, and invasive cervical cancer, which were determined using standard statistical tests. The 95% confidence intervals (95% CIs) were calculated using exact binomial CIs. Colposcopies to detect 1 case; cases in which colposcopy was performed/cases identified by endpoint. For all analyses, p-values were 2-sided, and statistical significance was accepted if the p<0.05.
RESULTS
1. Clinical characteristics of the studied population
In this study, a total of 30,783 women received PCR-RDB HPV genotyping assay and finally 21,568 cases fulfilled the criteria. In this evaluated population, the average age was 39.9±10.1 years old (ranging from 21 to 78 years). The total percentage of patients positive for HR-HPV at baseline was 18.20% (3,935/21,568), with a baseline value of 11.80% (2,542/21,568) having abnormal cytology results. Furthermore, 44.93% (1,142/2,542) of patients with ASCUS, 3.34% (85/2,542) with ASC-H, 23.68% (602/2,542) with LSIL, 20.14% (512/2,542) with HSIL, and 7.91% (201/2,542) with AGC were observed at the baseline screening. Of the 21,568 cases, 5.04% (1,088/21,568) of the women were diagnosed with CIN2+ and 3.43% (739/21,568) with CIN3+ during the whole screening and follow-up period. Among them, 59.28% (645/1,088) and 67.25% (497/739) of the women were HPV-16/-18-positive while 35.85% (390/1,088) and 29.09% (215/739) of them were non-HPV-16/-18 HR-HPV positive with a diagnosis of CIN2+ and CIN3+, respectively.
2. Cumulative incidence rate for CIN2+/CIN3+
Table 1 presents the cumulative incidence rate (CIR) for all the different combinations of screening test results. For the co-test screening strategy (I), the highest CIR was detected in the HR-HPV-positive and/or TCT ≥ASC-US group, being 23.13% (95% CI=21.91%–24.35%) for CIN2+ cases and 15.84% (95% CI=14.78%–16.90%) for CIN3+ cases, and the lowest CIR in strategy I was detected in the co-test-negative group, being 0.18% (95% CI=0.12%–0.25%) for CIN2+ cases and 0.09% (95% CI=0.04%–0.13%) for CIN3+ cases. For the cytology primary screening strategy (II), the highest CIR was detected in the TCT ≥LSIL group, being 52.50% (95% CI=49.88%–55.12%) for CIN2+ cases and 39.57% (95% CI=37.01%–47.13%) for CIN3+ cases, And the lowest CIR in strategy II was detected in the cytology-negative group, being 0.86% (95% CI=0.73%–0.99%) for CIN2+ cases and 0.38% (95% CI=0.29%–0.47%) for CIN3+ cases. In the HR-HPV primary screening strategy (III), the highest CIR was detected in the HPV-16/-18 (+) group, being 48.28% (95% CI=45.60%–50.96%) for CIN2+ cases and 37.20% (95% CI=34.61%–39.79%) for CIN3+ cases. Further, the lowest CIR in strategy III was detected in the HR-HPV-negative group, being 0.30% (95% CI=0.22%–0.38%) for CIN2+ cases and 0.15% (95% CI=0.10%–0.21%) for CIN3+ cases.
Table 1. CIR of consensus pathology CIN2+ and CIN3+ stratified by different combinations of cervical cytology and HPV results.
| Strategy | Results of screening | No. (% of 21,568) | CIR (95% CI) | |||
|---|---|---|---|---|---|---|
| CIN2+ | CIN3+ | |||||
| Co-testing | Co-testing (negative) | 16,998 (78.81) | 0.18 (0.12–0.25) | 0.09 (0.04–0.13) | ||
| HR-HPV (+) &/or TCT ≥ASC-US | 4,570 (21.19) | 23.13 (21.91–24.35) | 15.84 (14.78–16.90) | |||
| TCT ≥(ASC-US)† & HR-HPV (−) | 636 (2.95) | 3.62 (2.17–5.07) | 2.04 (0.94–3.14) | |||
| TCT (NILM) & HR-HPV (+) | 2,028 (9.40) | 6.56 (5.48–7.64) | 2.82 (2.09–3.53) | |||
| TCT (NILM) & HPV-16/-18 (+) | 549 (2.55) | 16.03 (12.96–19.10) | 8.20 (5.90–10.49) | |||
| TCT (NILM) & HR-HPV non-16/18 (+) | 1,479 (6.86) | 3.04 (2.17–3.92) | 0.81 (0.35–1.27) | |||
| Cytology | TCT(NILM) | 19,026 (88.20) | 0.86 (0.73–0.99) | 0.38 (0.29–0.47) | ||
| TCT ≥ASC-US | 2,542 (11.78) | 36.35 (34.48–38.22) | 26.24 (24.53–27.95) | |||
| TCT (ASC-US) | 1,142 (5.29) | 16.55 (14.39–18.71) | 9.89 (8.16–11.63) | |||
| TCT ≥LSIL | 1,400 (6.49) | 52.50 (49.88–55.12) | 39.57 (37.01–47.13) | |||
| HR-HPV | HR-HPV (−) | 17,633 (81.80) | 0.30 (0.22–0.38) | 0.15 (0.10–0.21) | ||
| HR-HPV (+) | 3,935 (18.20) | 26.30 (24.93–27.68) | 18.09 (16.89–19.30) | |||
| HPV-16/-18 (+)* | 1,336 (6.19) | 48.28 (45.60–50.96) | 37.20 (34.61–39.79) | |||
| HR-HPV non-16/18 (+) | 2,599 (12.05) | 15.01 (13.63–16.38) | 8.27 (7.21–9.33) | |||
ASC-H, atypical squamous cells, not possible to exclude high-grade squamous intraepithelial lesion; ASC-US, atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia; CI, confidence interval; CIR, cumulative incidence rates; HR-HPV, high-risk human papillomavirus; NILM, negative for intraepithelial lesion or malignancy; TCT, thinprep cytologic test.
*Compared to the patients with non-HPV-16/-18 infection, the patients with HPV-16/-18 infection have a significantly increased risk for cancer; †Including ASC-H.
In each screening strategy, the lowest CIR was detected in the participant with negative-result of primary detection. In addition, the CIR for CIN2+ was lower in HR-HPV-positive women (26.30%; 95% CI=24.93%–27.68%) than in women with cytology determined ≥ASC-US (36.35%; 95% CI=34.48%–38.22%), with a CIR ratio of 0.7235 (95% CI=0.6586–0.7890). When abnormal cytology results were added to positive HR-HPV results, the CIR for CIN2+ (23.13%; 95% CI=21.91%–24.35%) was lower than that in HR-HPV-positive women (26.30%; 95% CI=24.93%–27.68%) or women with cytology determined ≥ASC-US (36.35%; 95% CI=34.48%–38.22%) alone. Patients with cytology diagnosed as ≥LSIS or with HPV-16/-18 (+), have the highest CIR to develop into cervical cancer. Compared to the patients with non-HPV-16/-18 infection, the patients with HPV-16/-18 infection have a significant increasing risk for cancer (Table 1). Similar results were obtained when CIN3+ was used as the endpoint.
3. Comparison of different screening strategies for CIN2+/CIN3+
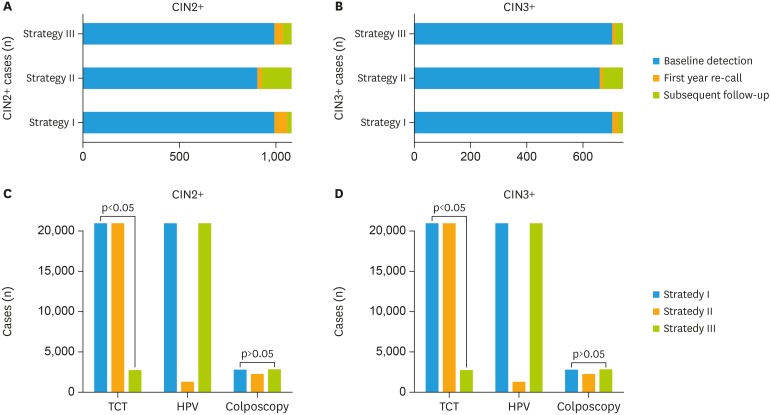
In total, 185 of 1,088 (17.00%) cases of CIN2+ were identified in women with baseline cytology-negative during the study. In contrast, only 98 of 1,088 (9.01%) cases were identified CIN2+ in women who were HR-HPV-negative, which is very similar to the results of co-test negative 8.92% (97/1,088). By using the same HR-HPV assay and cytology detection, three current screening strategies and the detail process were compared (as shown in Fig. 2 and Table 2). Cases in which CIN2+ or CIN 3+ detected in the baseline screening, first-year recall and the subsequent follow-up were calculated respectively. As presented in Table 2 and Fig. 3, for strategy I, the co-test as primary screening, could detect 991 cases with CIN2+ at the baseline, 66 cases in the first-year recall, and 31 cases in the subsequent follow-up. For strategy II, the cytology as primary screening, it detected 903 cases with CIN2+ at the baseline, 21 cases in the first-year recall, and 164 cases in the subsequent follow-up. While the HR-HPV as primary screening (strategy III) could detected almost the same more cases (990 vs 991) CIN2+ at the baseline screening and it detected 45 cases in the first-year recall and 53 cases in the subsequent follow-up round (Fig. 3A and B). And the number of cases detected at baseline round in strategies I and III significantly higher than strategy II (p<0.05). What about the number of colposcopies to detect 1 case, strategy II required the lowest number of colposcopies to detect per case, with 2.34 (2,112/903) for CIN2+ and 3.22 (2,112/656) for CIN3+. And primary HPV-16/-18 test required the highest number of colposcopies to detect per case of CIN2+ compared with the other two strategies, but still yielded an acceptable rate. When the baseline detection was using as the observed point, strategy I and strategy III could detected almost the same more CIN2+ and CIN3+ cases, although the strategy III required an increased number of colposcopies (2.75/3.89 per CIN2+/CIN3+ case) than strategy I (2.69/3.80 per CIN2+/CIN3+ case), but there was no significant difference (p>0.05). However, the strategy I cost far more cytology tests than the strategy III (p<0.001). On the other side, both of the strategies I and III work more effective than strategy II, which could detect more CIN2+ and CIN3+ cases at the baseline screening and first-year recall (Fig. 3C and D).
Table 2. The number of cervical screening examinations and colposcopies required for detecting CIN2+/CIN3+.
| Strategy | No. of total detected cases | No. of detected cases in 1 yr | More than second round | No. of clposcopies | No. of colposcopies to detect 1 case (No.*) | |||
|---|---|---|---|---|---|---|---|---|
| Total | Baseline round | Follow-up 1-yr round | ||||||
| CIN2+ | ||||||||
| Strategy I | 1,088 | 1,057 | 991† | 66 | 31 | 2,661† | 2.69 | |
| Strategy II | 1,088 | 924 | 903 | 21 | 164 | 2,112 | 2.34 | |
| Strategy III | 1,088 | 1,035 | 990† | 45 | 53 | 2,725†,‡ | 2.75 | |
| CIN3+ | ||||||||
| Strategy I | 739 | 724 | 701† | 23 | 15 | 2,661† | 3.80 | |
| Strategy II | 739 | 667 | 656 | 11 | 72 | 2,112 | 3.22 | |
| Strategy III | 739 | 712 | 700† | 12 | 27 | 2,725†,‡ | 3.89 | |
CIN2+, cervical intraepithelial neoplasia grade 2 or worse; CIN3+, cervical intraepithelial neoplasia grade 3 or worse.
*Colposcopies performed/case identified; †Strategy I and strategy III were significantly higher than strategy II (p<0.05). ‡Strategy I was similar to strategy III (p>0.05).
Fig. 3. The examinations consumed by the three cervical screening strategies to detect CIN2+/CIN3+. (A) The cases of CIN2+ detected by three current cervical screening strategies were determined in baseline screening, first year re-call, and subsequent follow-up. (B) The cases of CIN3+ detected by three current cervical screening strategies were calculated in baseline screening, first year re-call, and subsequent follow-up. (C) For baseline screening, according to the different cervical screening strategies, the number of cytologic assays, HPV assays, and colposcopies that should be performed as well as analysis of CIN2+ cases that could be detected. (D) The three cervical screening strategies used for CIN3+ detection, with application of cytology assay, HPV assay, and colposcopy shown.
CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; TCT, thinprep cytologic test.
4. Evaluation of the three cervical screening strategies
As shown in Table 3, the sensitivity and NPV of strategy I (co-testing) were 91.08% and 99.49% for CIN2+ and 91.59% and 99.80% for CIN3+, respectively. The specificity and PPV were 91.85% and 37.24% for CIN2+ and 94.86% and 26.34% for CIN3+, respectively. Misdiagnosis accounted for 8.92% of the CIN2+ cases and 5.14% of the CIN3+ cases. The sensitivity and NPV of strategy II (cytology with triage by HPV test) were the lowest, being 83.00%/99.05% and 88.77%/99.57% for CIN2+ and CIN3+, respectively. But it has the highest specificity and PPV, with 94.10%/42.76% and 93.01%/31.06% for CIN2+/CIN3+, respectively, compared with strategy I and strategy III. Moreover, the proportions of misdiagnosed rate were 17.00% and 11.23% for CIN2+ and CIN3+, respectively, which were significantly higher than those of the others. The sensitivity and NPV of strategy III (HPV testing with type-16/18 genotyping) were similar to those of strategy I, which were 90.99%/99.49% and 91.57%/99.80% for CIN2+ and CIN3+, respectively, and the specificity and PPV were 91.53%/40.31% and 94.72%/28.50% for CIN2+ and CIN3+, respectively. Furthermore, the proportions of misdiagnosed rate were the lowest, which were 8.01% and 5.02% for CIN2+ and CIN3+, respectively.
Table 3. Compare the clinical performance characteristics of different screening strategies in the cervical high-grade lesions.
| Strategy | Strategy I | Strategy II | Strategy III | |
|---|---|---|---|---|
| CIN2+ | ||||
| Sensitivity | 91.08 (89.39–92.78) | 83.00 (80.76–85.23) | 90.99 (89.29–92.69) | |
| Specificity | 91.85 (91.47–92.22) | 94.10 (93.77–94.42) | 91.53 (89.29–92.69) | |
| PPV | 37.24 (35.40–39.08) | 42.76 (40.65–44.87) | 40.31 (38.37–42.25) | |
| NPV | 99.49 (99.39–99.59) | 99.05 (98.91–99.19) | 99.49 (99.39–99.59) | |
| Misdiagnosis | 8.92 (7.22–10.61) | 17.00 (14.77–19.24) | 8.01 (7.01–10.71) | |
| CIN3+ | ||||
| Sensitivity | 91.59 (90.19–90.99) | 88.77 (86.49–91.05) | 91.57 (91.19–91.95) | |
| Specificity | 94.86 (93.27–96.45) | 93.01 (92.67–93.36) | 94.72 (93.11–96.33) | |
| PPV | 26.34 (24.67–28.02) | 31.06 (29.09–33.03) | 28.50 (26.72–30.29) | |
| NPV | 99.80 (99.74–99.86) | 99.57 (99.48–99.66) | 99.80 (99.73–99.86) | |
| Misdiagnosis | 5.14 (3.55–6.73) | 11.23 (8.95–13.51) | 5.02 (3.67–6.89) | |
Data were presented as number of patients/total patients (%, 95% CI).
CI, confidence interval; CIN2+, cervical intraepithelial neoplasia grade 2 or worse; CIN3+, cervical intraepithelial neoplasia grade 3 or worse; NPV, negative predictive value; PPV, positive predictive value.
The performance of the 3 screening strategies was evaluated in HPV-positive women at baseline. The threshold analyses showed that strategies I and III had similar sensitivity for detecting CIN2+ and CIN3+, but only slightly higher than strategy III. Of note, strategy III required more colposcopy examinations to detect each case of CIN2+ and CIN3+ than strategy I.
DISCUSSION
Screening coverage plays an important role in the disease control. As pointed out by Goodman [22], screening performed within 3–36 months at the time of data collection was associated with a significantly reduced risk of death from cervical cancer, with an odds ratio of 0.28–0.60. However, in China, cervical cancer screening coverage is only 29.1% in the urban areas and 16.39% in the rural areas [17]. Even in the relatively developed eastern cities of China, the coverage only reached 31.3% [17], which was still much lower than that in other developed countries such as the United Kingdom and the United States. They have made substantial investments to provide a wide screening coverage [17,23,24]. Therefore, China needs to make further efforts to increase the screening coverage. In recent years, the Chinese government has launched a series of projects to provide free cervical cancer screening in the low-middle income population. On the other hand, most Chinese women were prefer going to the hospital or medical center for health examination, but not visiting a family doctor, for the medical health system different from Western countries. The opportunistic screening plays a major role in the prevention of cervical cancer. Thus, continual efforts should be geared towards finding more suitable screening methods that fit the conditions in China.
In general, cytologic screening based on the Pap smear or liquid thin-preparation test was the first effective screening test to reduce the incidence and mortality of cervical cancer. However, the sensitivity of this test in detecting high grade lesions ranges from 55% to 94%, which depends on the quality control of the laboratory and the experiences of the technologists and cytologists [25,26]. Limited by the shortage of cytologists and quality control, there is a big problem in the cervical cancer screening by using cytology screening alone in the mainland of China [27]. On the other hand, co-test screening with cytology and HPV test is still expensive for both the government and the individual. Recently, several studies have proposed that using the HR-HPV assay for primary screening can be an alternative strategy to prevent cervical cancer [10,11,12,13]. The ATHENA study evaluated the Cobas® HPV test as the primary screening test for cervical cancer and presented a reliable result. Using HR-HPV detection as a primary screening test may provide a safer and more effective alternative. Until now, only Cobas® has been approved by FDA as an HPV test for the primary screening of cervical cancer. In another previous cohort study, we confirmed that other assays such as Cervista® HR-HPV detection could be used as primary screening tests for cervical cancer [28]. Our previous articles have shown that the PCR-RDB HPV genotyping test can provide a reliable and sensitive clinical reference for HPV detection [15,29]. Long-term observational studies showed that HPV-16 and HPV-18 were associated with an elevated risk of high-grade cervical lesions [30,31]. The ARTISTIC study suggested that in unscreened populations, HPV testing provided a higher yield for abnormal cervical findings [32,33]. Hence, based on the same hospital-based population who received opportunistic screening for cervical cancer, three main current strategies detected by using the same HR-HPV assay and cytology detection were evaluated according to the base-line results, first-year-recall results and the follow-up results. Furthermore, the PCR-RDB HPV genotyping test was also evaluated as a potential HPV assay in the primary screening of cervical cancer. To our knowledge, this is the first and largest-scale hospital-based population study investigating the application of PCR-RDB HPV genotyping in the primary screening of cervical cancer among Chinese women. In the present study, the proportions of CIN2+ and CIN3+ cases were 5.04% (1,088/21,568) and 3.43% (739/21,568), respectively. The sensitivity of the HR-HPV test for diagnosing CIN2+ exceeded 80%, which was more preferable than that of cytology. The CIR of CIN2+/CIN3+ in HPV-16/-18-positive women was higher than that in non-HPV-16/-18 HR-HPV-positive women and/or women with abnormal cytology.
In 2015, the 30th International Papillomavirus Society (IPVS) stated that strategies for HPV detection need to balance clinical sensitivity and specificity and maximize the protection of low-risk populations and discrimination of high-risk groups. Comparing strategies I and III, the HPV testing as primary screening can balance screening benefits and harm by maximizing sensitivity (90.99% and 91.57% for CIN2+ and CIN3+, respectively) and NPV (99.49% and 99.80%) and by increasing CIN2+ and CIN3+ detected at the baseline detection because more HPV-16/-18-positive patients may be referred for immediate colposcopy with primary PCR-RDB HPV genotyping. The results of the current analyses also showed that HPV primary screening of strategy III, compared to cytology primary screening in strategy II, provided a higher sensitivity for CIN2+ and a decrease in misdiagnosis. Recently, prospective randomized screening trials in Europe showed that co-testing required more screening tests and offered minimal protection against the subsequent development of cervical diseases compared with HPV primary screening. Moreover, the interpretation of screening results was more complicated, as all women had two test results that had to be considered [17,34,35]. Several cross-sectional and prospective screening trials also documented that HPV screening had superior sensitivity for the detection of cervical histology lesions than screening by TCT test [36]. The ATHENA HPV study was specifically initiated to confirm the performance of the Cobas® HPV test, which could simultaneously detect HPV-16/-18 and 12 HR-HPV genotypes in women in the US and showed the highest sensitivity over 3 years for the detection of CIN2+ and CIN3+ (69.1% and 76.1%, respectively) [19]. Our study indicated that strategy III using PCR-RDB HPV detection provided a 19.28% increase in sensitivity for CIN2+ and a 14.87% increase for CIN3+ compared to the ATHENA trial.
Although strategy III reduced the number of women undergoing colposcopy as opposed to performing colposcopy in all HPV-positive women, it still resulted in a significant increasing in the number of colposcopies compared to cytology primary screening (p<0.05). Very interesting, the increasing compared to the co-testing did not show any significant (p>0.05). A potential harm of over-screening involves the detection of transient HPV infections, which can result in patient anxiety and unnecessary colposcopies [37,38]. When ATHENA is implemented, the value of HPV testing would be outweighed by harm of excess colposcopies, unless some form of triage is used to reduce the number of women with clinically unimportant HPV infections referred for colposcopy [19,34]. Since the HPV primary screening could detected almost the same more CIN2+ and CIN3+ cases as the co-test screening at the baseline round (990 vs. 991, 99.90%) and first-year recall (1,035 vs. 1,057, 97.92%), the HR-HPV primary showed more advantage on the co-effective of screening. Co-testing requires more screening tests than HPV primary screening and interpretation of screening results is also somewhat more complicated. According to our study, in strategy I, all women (n=20,568) were required to undergo both cytology and HPV testing, but in strategy III, all women required HPV testing and only 2,599 women required cytology testing. Strategy III could be a suitable option for cervical cancer screening in Chinese women based on the hospital-based opportunistic screening. What is more, the price of different HPV test methods is different. When considering the cost-effect of the PCR-RDB HPV genotyping test. In mainland China, the price of Cervista® HR-HPV assay currently on the market is about 40–50 US$ per test, Cobas® is about 50–60 US$ per test, and PCR-RDB HPV genotyping is just about 30–35 US$ per test. In general, the PCR-RDB HPV genotyping test is a cost-effective HR-HPV assay for the cervical cancer screening program.
In summary, our study was a large hospital-based opportunistic screening research in China, and the results confirm that the HR-HPV test is an attractive option for primary cervical cancer screening in Chinese women, especially in the opportunistic populations. We compared the performance of three screening triage strategies supported by current guidelines and concluded that the PCR-RDB HPV genotyping test as a primary screening method had far higher sensitivity and NPV and the lowest misdiagnosis rate in detecting CIN2+/CIN3+ when compared to approaches based on either co-testing or cytology. The increase in sensitivity was associated with a significant increase in the number of colposcopies.
ACKNOWLEDGMENTS
The authors would like to thank Mrs. Fen Lin for her excellent assistance in this work.
Footnotes
Funding: The authors wish to thank the funding support from the Fujian Provincial Healthy Innovation Project, China (grant No. 2009-CXB-33) and the Fujian Provincial Key Projects in Science and Technology, China (grant No. 2015YZ0002).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: K.Y., S.P.
- Data curation: K.Y., M.X., D.B., R.G.
- Formal analysis: K.Y., S.P.
- Funding acquisition: S.P.
- Investigation: K.Y., M.X., D.B., R.G., C.L.
- Methodology: K.Y., S.P., R.G.
- Software: K.Y., R.G., C.L.
- Writing - original draft: K.Y.
- Writing - review & editing: S.P.
References
- 1.International Agency for Research on Cancer. GLOBOCAN 2012: cancer fact sheet. Cervical cancer estimated incidence, mortality and prevalence worldwide in 2012 [Internet] Lyon: International Agency for Research on Cancer; 2013. [cited 2013 Dec 12]. Available from: http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp. [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Crosbie EJ, Einstein MH, Franceschi S, Kitchener HC. Human papillomavirus and cervical cancer. Lancet. 2013;382:889–899. doi: 10.1016/S0140-6736(13)60022-7. [DOI] [PubMed] [Google Scholar]
- 5.Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 7.Bhatla N, Maheswari N D. HPV screening for cervical cancer in rural India: do we have an answer? Natl Med J India. 2009;22:183–184. [PubMed] [Google Scholar]
- 8.Soost HJ, Lange HJ, Lehmacher W, Ruffing-Kullmann B. The validation of cervical cytology. Sensitivity, specificity and predictive values. Acta Cytol. 1991;35:8–14. [PubMed] [Google Scholar]
- 9.Zazove P, Reed BD, Gregoire L, Ferenczy A, Gorenflo DW, Lancaster WD. Low false-negative rate of PCR analysis for detecting human papillomavirus-related cervical lesions. J Clin Microbiol. 1998;36:2708–2713. doi: 10.1128/jcm.36.9.2708-2713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arbyn M, Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, et al. European Guidelines for Quality Assurance in Cervical Cancer Screening. Second edition--summary document. Ann Oncol. 2010;21:448–458. doi: 10.1093/annonc/mdp471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franceschi S, Denny L, Irwin KL, Jeronimo J, Lopalco PL, Monsonego J, et al. Eurogin 2010 roadmap on cervical cancer prevention. Int J Cancer. 2011;128:2765–2774. doi: 10.1002/ijc.25915. [DOI] [PubMed] [Google Scholar]
- 12.Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:687–697. doi: 10.7326/0003-4819-155-10-201111150-00376. [DOI] [PubMed] [Google Scholar]
- 13.Huh WK, Ault KA, Chelmow D, Davey DD, Goulart RA, Garcia FA, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178–182. doi: 10.1016/j.ygyno.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Jun SY, Park ES, Kim J, Kang J, Lee JJ, Bae Y, et al. Comparison of the Cobas 4800 HPV and HPV 9G DNA chip tests for detection of high-risk human papillomavirus in cervical specimens of women with consecutive positive HPV tests But negative Pap smears. PLoS One. 2015;10:e0140336. doi: 10.1371/journal.pone.0140336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun P, Song Y, Ruan G, Mao X, Kang Y, Dong B, et al. Clinical validation of the PCR-reverse dot blot human papillomavirus genotyping test in cervical lesions from Chinese women in the Fujian province: a hospital-based population study. J Gynecol Oncol. 2017;28:e50. doi: 10.3802/jgo.2017.28.e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huh WK, Williams E, Huang J, Bramley T, Poulios N. Cost effectiveness of human papillomavirus-16/18 genotyping in cervical cancer screening. Appl Health Econ Health Policy. 2015;13:95–107. doi: 10.1007/s40258-014-0135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B, He M, Chao A, Engelgau MM, Saraiya M, Wang L, et al. Cervical cancer screening among women in China, 2010. Oncologist. 2015;20:627–634. doi: 10.1634/theoncologist.2014-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao K, Eurasian M, Zhang J, Wei Y, Zheng Q, Ye H, et al. Can genomic amplification of human telomerase gene and C-MYC in liquid-based cytological specimens be used as a method for opportunistic cervical cancer screening? Gynecol Obstet Invest. 2015;80:153–163. doi: 10.1159/000371760. [DOI] [PubMed] [Google Scholar]
- 19.Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189–197. doi: 10.1016/j.ygyno.2014.11.076. [DOI] [PubMed] [Google Scholar]
- 20.Verma I, Jain V, Kaur T. Application of Bethesda system for cervical cytology in unhealthy cervix. J Clin Diagn Res. 2014;8:OC26–30. doi: 10.7860/JCDR/2014/9620.4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waxman AG, Chelmow D, Darragh TM, Lawson H, Moscicki AB. Revised terminology for cervical histopathology and its implications for management of high-grade squamous intraepithelial lesions of the cervix. Obstet Gynecol. 2012;120:1465–1471. doi: 10.1097/aog.0b013e31827001d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodman A. HPV testing as a screen for cervical cancer. BMJ. 2015;350:h2372. doi: 10.1136/bmj.h2372. [DOI] [PubMed] [Google Scholar]
- 23.Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S, Bray F. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur J Cancer. 2013;49:3262–3273. doi: 10.1016/j.ejca.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 24.Vaccarella S, Franceschi S, Engholm G, Lönnberg S, Khan S, Bray F. 50 years of screening in the Nordic countries: quantifying the effects on cervical cancer incidence. Br J Cancer. 2014;111:965–969. doi: 10.1038/bjc.2014.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrae B, Andersson TM, Lambert PC, Kemetli L, Silfverdal L, Strander B, et al. Screening and cervical cancer cure: population based cohort study. BMJ. 2012;344:e900. doi: 10.1136/bmj.e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dillner J. Primary human papillomavirus testing in organized cervical screening. Curr Opin Obstet Gynecol. 2013;25:11–16. doi: 10.1097/GCO.0b013e32835c5d10. [DOI] [PubMed] [Google Scholar]
- 27.You W, Li S, Du R, Zheng J, Shen A. Epidemiological study of high-risk human papillomavirus infection in subjects with abnormal cytological findings in cervical cancer screening. Exp Ther Med. 2018;15:412–418. doi: 10.3892/etm.2017.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruan G, Song Y, Dong B, Mao X, Lin F, Kang Y, et al. Cervical cancer screening using the Cervista high-risk human papillomavirus test: opportunistic screening of a hospital-based population in Fujian province, China. Cancer Manag Res. 2018;10:3227–3235. doi: 10.2147/CMAR.S169822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Song Y, Ruan G, Zhang Q, Lin F, Zhang J, et al. Knowledge and attitudes regarding HPV and vaccination among Chinese women aged 20 to 35 years in Fujian province: a cross-sectional study. Cancer Control. 2018;25:1073274818775356. doi: 10.1177/1073274818775356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Wang Y, Liu L, Guo C, Liu Z, Nie S. Prevalence of human papillomavirus infection and genotyping for population-based cervical screening in developed regions in China. Oncotarget. 2016;7:62411–62424. doi: 10.18632/oncotarget.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:553–560. doi: 10.1158/1055-9965.EPI-12-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stoler MH, Wright TC, Jr, Sharma A, Apple R, Gutekunst K, Wright TL, et al. High-risk human papillomavirus testing in women with ASC-US cytology: results from the ATHENA HPV study. Am J Clin Pathol. 2011;135:468–475. doi: 10.1309/AJCPZ5JY6FCVNMOT. [DOI] [PubMed] [Google Scholar]
- 33.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rijkaart DC, Berkhof J, van Kemenade FJ, Coupe VM, Rozendaal L, Heideman DA, et al. HPV DNA testing in population-based cervical screening (VUSA-Screen study): results and implications. Br J Cancer. 2012;106:975–981. doi: 10.1038/bjc.2011.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arbyn M, Ronco G, Anttila A, Meijer CJ, Poljak M, Ogilvie G, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(Suppl 5):F88–F99. doi: 10.1016/j.vaccine.2012.06.095. [DOI] [PubMed] [Google Scholar]
- 36.Khunamornpong S, Settakorn J, Sukpan K, Suprasert P, Srisomboon J, Intaraphet S, et al. Genotyping for human papillomavirus (HPV) 16/18/52/58 has a higher performance than HPV16/18 genotyping in triaging women with positive high-risk HPV test in Northern Thailand. PLoS One. 2016;11:e0158184. doi: 10.1371/journal.pone.0158184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain JM, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis. 2012;16:175–204. doi: 10.1097/LGT.0b013e31824ca9d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol. 2012;120:1222–1238. doi: 10.1097/aog.0b013e318277c92a. [DOI] [PubMed] [Google Scholar]