Abstract
Objectives
Intraoperative tumor manipulation and dissemination may possibly compromise survival of women with early-stage cervical cancer who undergo minimally-invasive radical hysterectomy (RH). The objective of the study was to examine survival related to minimally-invasive RH with a “no-look no-touch” technique for clinical stage IB1 cervical cancer.
Methods
This retrospective study compared patients who underwent total laparoscopic radical hysterectomy (TLRH) with no-look no-touch technique (n=80) to those who underwent an abdominal radical hysterectomy (ARH; n=83) for stage IB1 (≤4 cm) cervical cancer. TLRH with no-look no-touch technique incorporates 4 specific measures to prevent tumor spillage: 1) creation of a vaginal cuff, 2) avoidance of a uterine manipulator, 3) minimal handling of the uterine cervix, and 4) bagging of the specimen.
Results
Surgical outcomes of TLRH were significantly superior to ARH for operative time (294 vs. 376 minutes), estimated blood loss (185 vs. 500 mL), and length of hospital stay (14 vs. 18 days) (all, p<0.001). Oncologic outcomes were similar between the 2 groups, including disease-free survival (DFS) (p=0.591) and overall survival (p=0.188). When stratified by tumor size (<2 vs. ≥2 cm), DFS was similar between the 2 groups (p=0.897 and p=0.602, respectively). The loco-regional recurrence rate following TLRH was similar to the rate after ARH (6.3% vs. 9.6%, p=0.566). Multiple-pelvic recurrence was observed in only 1 patient in the TLRH group.
Conclusion
Our study suggests that the no-look no-touch technique may be a useful surgical procedure to reduce recurrence risk via preventing intraoperative tumor spillage during TLRH for early-stage cervical cancer.
Keywords: Cervical Cancer, Hysterectomy, Minimally Invasive Surgery, Laparoscopic Surgery, Survival
INTRODUCTION
The technical feasibility and oncologic safety of laparoscopic radical hysterectomy (RH) have been demonstrated in many studies [1,2,3,4,5,6,7]. A systematic review of laparoscopic versus open RH by Wang et al. [6], for example, showed that the laparoscopic procedure resulted in a longer operative time but lower blood loss, shorter length of hospital stay, a comparable intraoperative complication rate, and a lower postoperative complication rate in comparison to the open procedure. In another study, the number of lymph nodes harvested, the amount of parametrial tissue excised, the prevalence of positive surgical margins, and the 5-year disease-free survival (DFS) as well as overall survival (OS) rates were similar between the 2 procedures [8]. The reported benefits of laparoscopic RH have led to its widespread acceptance as an alternative to open RH.
In the recently reported Laparoscopic Approach to Cervical Cancer (LACC) trial, a phase III randomized trial of minimally-invasive radical hysterectomy (MIS-RH) versus abdominal radical hysterectomy (ARH) for early-stage cervical cancer, Ramirez et al. [9] found, unexpectedly, that in cases of stage IA1 with lympho-vascular space invasion, IA2, or IB1 (≤4 cm) cervical cancer, the number of disease recurrences following MIS-RH was almost four times higher than the number of recurrences with ARH, and significantly more patients who underwent MIS-RH died during the median follow-up period of 2.5 years (6.0% vs. 1.0%). Importantly, the LACC trial also revealed that the pattern of recurrence following the MIS-RH differs from that following ARH, with the MIS-RH group exhibiting multiple pelvic recurrences [9]. As the results of the LACC trial are contrary to those reported previously, we have questioned whether MIS-RH should be abandoned for early-stage cervical cancer.
When laparoscopic surgery was first adopted for rectal cancer, tumor spillage resulting in peritoneal dissemination or port-site metastasis became a matter of great concern, not only in the context of rectal cancer but also in the broader context of malignancy [10]. Peritoneal dissemination is thought to derive from tumor perforation, excessive manipulation of the tumor, and/or tumor spread via pneumo-peritoneal CO2 [11,12,13,14]. Thus, the problem is thought to be surgeon-related, which in many cases are preventable [15].
MIS-RH includes several procedural steps that can lead to intraoperative tumor spillage. Insertion of a uterine manipulator can lead to tumor perforation and erosion, squeezing of the uterine cervix can result in excessive tumor manipulation, and most importantly intraperitoneal colpotomy can cause tumor exposure to the circulating CO2 and tumor contamination to the pelvic peritoneum.
With the goal of ensuring the safety and advantages of MIS-RH for early-stage cervical cancer, we have incorporated a specific procedure to prevent intraoperative tumor spillage into the surgical field, and we have applied what we refer to as a “no-look no-touch” procedure during total laparoscopic radical hysterectomy (TLRH) consistently since 2014. The objective of the study was to examine the feasibility and oncologic outcome related to TLRH with this no-look no-touch technique for early-stage cervical cancer.
MATERIALS AND METHODS
This study included consecutive cases of women with clinical International Federation of Obstetrics and Gynecologists (FIGO) 1B1 cervical cancer who underwent RH between 2014 and 2017 at the Cancer Institute Hospital (n=163). The patients were grouped based on surgical performance: TLRH with no-look no-touch technique (n=80) and ARH (n=83). In our institution, the surgical approach, whether laparoscopic or open, was chosen by the patients themselves following preoperative counseling. Type III nerve-sparing RH and pelvic lymphadenectomy were performed in all patients in both groups. All TLRHs were performed by the same experienced board-certified laparoscopic surgeon in the same manner, incorporating specific measures designed to prevent tumor spillage. All ARHs were performed by board-certified gynecologic oncologists in the same manner. This study was approved by the institutional review board of our facility.
1. Specific measures adopted to prevent tumor spillage during laparoscopic RH
The TLRH procedure includes four specific measures adopted to prevent tumor spillage (Supplementary Video 1). A Step-by-step approach for the no-look no-touch technique is summarized below. The fundamental concept of the no-look no-touch technique is that the tumor should not be exposed to the surgical field and that direct manipulation of the tumor should be avoided during TLRH as these may cause tumor spillage, dissemination, and contamination of the surgical field.
Creation of a vaginal cuff
Immediately prior to the laparoscopic entry into the abdomen, a vaginal cuff is created. Twelve to 15 sutures are placed circumferentially, approximately 2 cm from the tumor, and the sutures are pulled to reveal the incision line. Adrenaline at a dilution of 1:1,000,000 is injected into the incision line to minimize bleeding. The vaginal mucosa is then incised circumferentially with a monopolar electrocautery device, leaving a vaginal cuff that is closed with a double layer of continuous sutures. The Schiller test (visual inspection) must next be performed to determine the exact tumor margins.
Manipulation of the uterus without insertion of a uterine manipulator
A 5-mm extra-long trocar (150 mm in length) is placed at the posterior vaginal fornix, and forceps are introduced through this port. A 1-0 vicryl suture is placed around the uterine body, and the forceps are used to push and pull the suture to manipulate the uterus.
Minimal handling of the uterine cervix
Essential to RH performance is the creation of wide paravesical and pararectal spaces to allow for exposure and resection of sufficient parametrium. In some cases, as in obese patients or those with bulky tumors, the operative view is poor and the uterine cervix must be squeezed both medially and laterally to expose the parametrium. Sometimes, the parametrium can be sufficiently exposed by suspension of the rectum and umbilical ligament from the abdominal wall, avoiding the need for squeezing the uterine cervix. This suspension also helps develop the paravesical and pararectal spaces. The avascular space between the mesorectal fascia and the presacral fascia is dissected and developed. The inferior hypogastric nerves and the pelvic nerve plexus are identified and preserved as described previously [16]. The rectum is then lifted with the assistant's forceps and peeled away from the surrounding tissue down to the levator ani muscle. To expose the right-side parametrium, the rectum is lifted toward the opposite (left) side, and the umbilical ligament is lifted toward the right side. This suspension technique provides for both safe and sufficient resection of the parametrium without direct handling of the uterine cervix.
Bagging the specimen
A collection bag is always used for retrieval of the surgical specimen(s). Of note, we generally maintain pneumoperitoneal pressure to 8 mmHg and the minimum amount of Trendelenburg position required to visualize the pelvic brim.
2. Postoperative management and surveillance
After either TLRH or RH, patients considered to be at risk for recurrence (i.e., with an intermediate risk factor of lymphovascular space involvement, tumor >4 cm in diameter, and/or >50% myometrial invasion) receive adjuvant chemotherapy as described in the Japan Society of Gynecologic Oncology guidelines [17]. Patients in whom 1 or 2 metastatic lymph nodes are found are also given adjuvant chemotherapy, whereas patients with 3 or more metastatic lymph nodes or a positive surgical margin receive concurrent chemoradiotherapy (CCRT). Upon completion of treatment, patients undergo a follow-up examination every 3 months for post-treatment surveillance, and a 12 month-interval computed tomography scan to evaluate the tumor recurrence. In cases of subjective symptoms or clinical signs of tumor recurrence, a comprehensive diagnostic workup is initiated.
3. Study variables
For the purpose of the study, patients' clinical characteristics and operative outcomes were retrieved from the hospital records, and the following variables were compared between the 2 study groups: age, body mass index (BMI), operation time, blood loss volume, number of lymph nodes harvested, lengths of the excised parametrium and vaginal cuff, intraoperative and/or postoperative complications, length of hospital stay (days), tumor diameter, histologic type (squamous cell carcinoma [SCC] vs. non-SCC), status of the surgical margin, surgical-pathological tumor stage, type of adjuvant therapy, time to recovery of normal bladder function, DFS, OS, and anatomical site of recurrence.
Complications were defined as any event during or after surgery that required a further surgical procedure, interventional radiotherapy, or rehabilitation therapy. Normal bladder function was defined as a post-void residual urine volume of <50 mL. The length of the parametrium and the vaginal cuff were each measured linearly from their attachment to the uterine cervix, and these measurements were taken as the lengths of the excised portions. DFS was defined as the time between the surgery and the time of initial recurrence or death from cervical cancer, and OS was defined as the time between the surgery and the time of death from any cause. Patients known to be disease free or alive at their last contact date were censored. Loco-regional recurrence was defined as cervical cancer recurrence within the pelvis.
4. Statistical analysis
Study variables are shown as median (interquartile range) or mean (standard deviation) values as appropriate. Intra-group differences in continuous variables were analyzed by independent sample t-test or by Mann-Whitney U test depending on normality, and intra-group differences in categorical variables were analyzed by Pearson's χ2 test. DFS and OS were constructed by the Kaplan-Meier method, and intra-group differences were assessed by the log-rank test. All statistical analyses were performed with EZR [18], and p<0.05 was considered statistically significant (2-tailed).
RESULTS
Clinical characteristics of the study patients are shown per group in Table 1. Although patients in the ARH group were older than patients in the TLRH group (mean: 49.0 vs. 44.0 years; p=0.004), BMI (median: 21.4 vs. 20.5 kg/m2; p=0.172) and tumor diameter (median: 2.3 vs. 2.1 cm; p=0.172) were similar. There was no statistically significant difference between the 2 groups in histologic type, surgical-pathological T stage, lymph node metastasis, or adjuvant therapy.
Table 1. Clinical characteristics based on surgical procedure.
| Characteristics | ARH (n=83) | TLRH (n=80) | p | |
|---|---|---|---|---|
| Age | 49.0±11.5 | 44.0±10.2 | 0.004 | |
| BMI (kg/m2) | 21.4 (19.7–23.7) | 20.5 (19.1–23.3) | 0.172 | |
| Tumor size (cm) | 2.3 (1.1–3.0) | 2.1 (1.4–3.0) | 0.769 | |
| Histology | 0.435 | |||
| SCC | 44 (53.0) | 37 (46.3) | ||
| Non-SCC | 39 (47.0) | 43 (53.7) | ||
| pT stage | 0.106 | |||
| 1b1 | 66 (79.6) | 65 (81.3) | ||
| 1b2 | 6 (7.2) | 1 (1.2) | ||
| 2a1 | 4 (4.8) | 4 (5.0) | ||
| 2a2 | 0 (0) | 4 (5.0) | ||
| 2b | 7 (8.4) | 6 (7.5) | ||
| pN stage | 0.642 | |||
| N0 | 71 (85.5) | 71 (88.8) | ||
| N1 | 12 (14.5) | 9 (11.2) | ||
| Adjuvant therapy | 0.117 | |||
| None | 40 (48.2) | 47 (58.8) | ||
| Chemotherapy | 37 (44.6) | 32 (40.0) | ||
| CCRT | 6 (7.2) | 1 (1.2) | ||
Values are shown as median (range), mean±standard deviation, or number (%) as appropriate.
ARH, abdominal radical hysterectomy; BMI, body mass index; CCRT, concurrent chemoradiotherapy; SCC, squamous cell carcinoma; TLRH, total laparoscopic radical hysterectomy.
Operative outcomes are shown in Table 2. The median operative time was shorter in the TLRH group compared to the ARH group (median: 294 vs. 376 minutes, net difference 82 minutes, p<0.001). In addition, the estimated blood loss was lower (median: 185 vs. 500 mL, net difference 315 mL, p<0.001), and the length of hospital stay was shorter (14 vs. 18 days, net difference 4 days, p<0.001) in the TLRH group compared to the ARH group.
Table 2. Operative outcome on surgical approach.
| Characteristics | ARH (n=83) | TLRH (n=80) | p | |
|---|---|---|---|---|
| Operation time (min) | 376 (332.5–419) | 294 (260–326.3) | <0.001 | |
| Blood loss volume | 500 (322.5–817.5) | 185 (100–261.3) | <0.001 | |
| Intraoperative complication | 1 (1.2) | 0 (0) | 0.999 | |
| Postoperative complication | 13 (15.7) | 7 (8.8) | 0.234 | |
| Periods to recover residual volume <50 mL (day) | 10 (9–20.5) | 10 (7–13.8) | 0.056 | |
| Hospital stay (day) | 18 (16–21) | 14 (13–15) | <0.001 | |
| Surgical margin | 0.368 | |||
| R0 | 79 (95.2) | 79 (98.8) | ||
| R1 | 4 (4.8) | 1 (1.2) | ||
| Surgical specimen | ||||
| Number of harvested lymph node | 36 (20–42.3) | 34.5 (28.3–42.3) | 0.865 | |
| Length of parametrium (mm) | 24.0 (18.8–30.3) | 24.5 (20.0–29.5) | 0.745 | |
| Length of vagina (mm) | 18.5 (15.0–22.0) | 20.0 (18.0–26.8) | 0.003 | |
Values are shown as median (range) or number (%) as appropriate.
ARH, abdominal radical hysterectomy; TLRH, total laparoscopic radical hysterectomy.
Intraoperative and postoperative complication rates were similar between the two groups (Table 2). There was only 1 (0.6%) intraoperative complication across both groups, a bladder injury that occurred in an ARH group patient which was able to be intraoperatively repaired. Thirteen (15.7%) patients in the ARH group had postoperative complications: bowel obstruction (n=5), lower limb neuropathy (n=2), peritonitis (n=2), pulmonary embolism (n=1), leg edema (n=1), symptomatic lymphocyst (n=1), and rectal visceral perforation (n=1). Seven (8.8%) patients in the TLRH group had postoperative complications: lymphedema (n=5), bowel obstruction (n=1), and peritonitis (n=1).
Recovery of voiding function occurred at a median of 10 days in both groups (p=0.056). Neither the number of lymph nodes harvested (median: 36 vs. 34.5) nor the length of the excised parametrium (median: 24.0 vs. 24.5 mm) differed between the 2 groups (p=0.865 and p=0.745, respectively). There was, however, a statistically significant difference in the length of the excised vagina (median: ARH vs. TLRH, 18.5 vs. 20 mm, net difference 1.5 mm, p=0.003). The surgical margin was positive in 4 (4.8%) of the ARH group patients and 1 (1.2%) of the TLRH group patients, a difference that did not differ significantly (p=0.368), and cancer was found microscopically at the vaginal stump in all these cases.
The median follow-up, at 31.3 (interquartile range, 23.1–44.2) months in the ARH group and 30.2 (interquartile range, 21.0–37.5) months in the TLRH group, did not differ significantly (p=0.116). Cervical cancer recurrence occurred in eight patients (9.6%) at 11 total anatomical sites in the ARH group and 5 (6.3%) patients 6 total anatomical sites in the TLRH group (Table 3). Two (2.4%) patients in the ARH group died of the disease. Recurrences in patients in the ARH group were located at the vaginal stump (n=1), a single site in the pelvis (n=2), multiple sites in the pelvis (n=3), a regional lymph node (n=2), omentum (n=1), lung (n=1), and liver (n=1). Recurrences in the TLRH group were located at a single site in the pelvis (n=3), multiple sites in the pelvis (n=1), a regional lymph node (n=1), and lung (n=1).
Table 3. Characteristics of recurrence cases.
| Procesure | Tumor size (cm) | pTNM | Adjucant therapy | Recurrence site |
|---|---|---|---|---|
| ARH | 1.8 | pT1b1N1M0 | Chemotherapy | Pelvis (multiple), liver |
| ARH | 2 | pT1b1N0M0 | Chemotherapy | Vaginal stump |
| ARH | 2.7 | pT2a1N1M0 | Chemotherapy | LN |
| ARH | 4.6 | pT1b2N1M0 | Chemotherapy | Pelvis (multiple), lung, LN |
| ARH | 4 | pT2bN1M0 | CCRT | Pelvis (multiple) |
| ARH | 3 | pT1b1N0M0 | Chemotherapy | Pelvis (single) |
| ARH | 4.5 | pT1b2N1M0 | Chemotherapy | Pelvis (single) |
| ARH | 2.1 | pT1b1N1M0 | Chemotherapy | Omentum |
| TLRH | 4.7 | pT1b2N1M0 | Chemotherapy | Pelvis (multiple) |
| TLRH | 1.5 | pT1b1N0M0 | - | Pelvis (single), LN |
| TLRH | 4.5 | pT2a2N0M0 | Chemotherapy | Lung |
| TLRH | 2.2 | pT1b1N0M0 | - | Pelvis (single) |
| TLRH | 4.7 | pT2bN1M0 | Chemotherapy | Pelvis (single) |
ARH, abdominal radical hysterectomy; CCRT, concurrent chemoradiotherapy; LN, regional lymph node; TLRH, total laparoscopic radical hysterectomy.
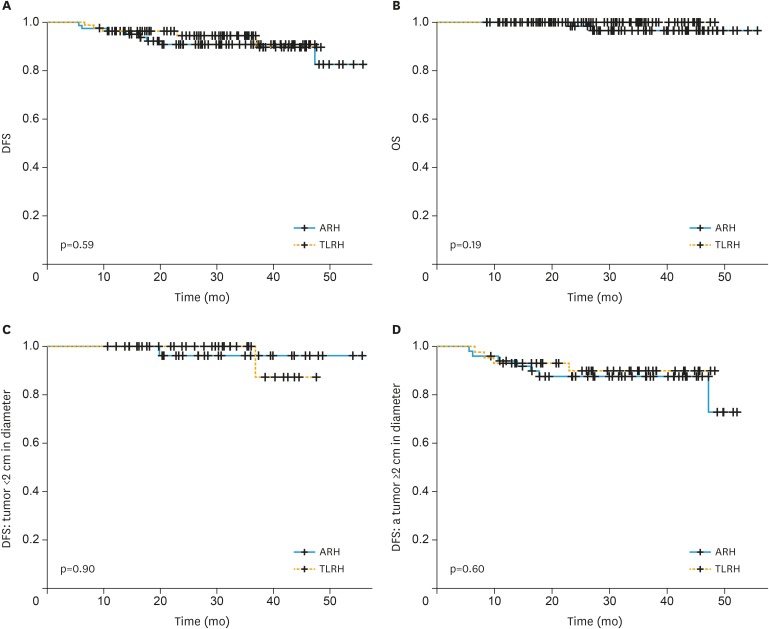
DFS and OS were examined. There was no significant intra-group difference in DFS (TLRH vs. ARH, 2.5-year rates: 94.4% vs. 90.9%, p=0.591; Fig. 1A) or OS (p=0.188; 2.5-year rates: 100% vs. 96.5%, Fig. 1B). In addition, DFS was similar between groups for patients with a tumor <2 cm in diameter (TLRH vs. ARH, 2.5-year rates: 100% vs. 96.3%, p=0.897, Fig. 1C), and those with a tumor ≥2 cm in diameter (2.5-year rates: 89.9% vs. 87.6%, p=0.602; Fig. 1D).
Fig. 1. Survival outcome based on surgical procedure. Kaplan-Meier analysis of (A) DFS and (B) OS for all patients and (C) DFS for patients with a tumor <2 cm in diameter and (D) those with a tumor ≥2 cm in diameter.
DFS, disease-free survival; OS, overall survival.
No significant differences in the anatomical recurrence pattern were seen between the 2 groups. The loco-regional recurrence rate in the ARH group was 9.6% (8/83), and that in the TLRH group was 6.3% (5/80) (p=0.566), while rates of distant recurrence were 3.6% (3/83) and 1.2% (1/80), respectively, (p=0.621).
DISCUSSION
Consistent with previously reported data [8], we found that TLRH, in comparison to ARH, resulted in significantly less blood loss and a shorter hospital stay. Contrary to previously reported data [6], we found that TLRH resulted in an operation time significantly shorter than that with ARH. The outcomes among our patients may be the result of TLRH having been performed by the same surgical team with extensive TLRH experience. Accumulation of surgical experience generally results in shorter operation times, regardless of the procedure. The hospital stay in both of our study groups was longer than those reported in western countries [6]. This could be due, at least in part, to the Japanese medical insurance system. The charge for hospitalization in Japan is very low, and most patients who have undergone cancer surgery prefer to remain in the hospital for a relatively longer period.
Although we showed operation time, estimated blood loss, and hospital stay to be significantly decreased in association with TLRH, we found the intraoperative and postoperative complication rates to be comparable between TLRH and ARH. The LACC trial also demonstrated the same results in that the intraoperative and postoperative complication rates were similar between the two surgical approaches. As the minimally-invasive approach is expected to have lower complication rates than the laparotomy approach in general, the failure to show this advantage in MIS-RH is a concern.
Ramirez et al. [9] noted the routine use of a uterine manipulator and the effect of the CO2 insufflation as two of several possible reasons for the inferior oncologic outcomes associated with minimally-invasive approaches included in the LACC trial. He also noted that the LACC trial was not designed to answer questions regarding the cause of the inferior outcomes with MIS-RH; thus, further investigation is warranted. Among patients included in the LACC trial, the pattern of recurrence in the MIS-RH group differed from the ARH group, exhibiting multiple pelvic recurrences using the MIS-RH approach. This observation led to the hypothesis that some techniques performed during MIS-RH can cause tumor spillage. Therefore, we developed our no-look no-touch TLRH technique, which involves 4 steps to prevent tumor spillage and contamination of the surgical field.
Although a uterine manipulator is useful for exposing the space around the uterus [19], its use risks metastatic spread of tumor cells [20,21,22,23]. Use of a uterine manipulator during robotic-assisted surgery has been reported to cause tumor surface disruption and artifactual parametrial carryover [24]. Its use during laparoscopic hysterectomy has been suggested to result in the transfer of cervical intraepithelial neoplasia III to the fallopian tubes [25]. These findings suggest that use of a uterine manipulator is not appropriate in cases of cancer of the uterine cervix.
The CO2 gas used for pneumo-peritoneum has been suggested as a cause for peritoneal tumor dissemination [26]. A retrospective study of patients with early cervical cancer showed recurrence to be more frequent after colpotomy performed under pneumo-peritoneum than without pneumo-peritoneum [27]. Further, the LACC trial documented recurrence patterns after MIS-RH that were similar to patterns previously reported after such surgical procedure [28,29].
A technique focused on the prevention of tumor spillage during TLRH is of utmost importance, and we now routinely create a vaginal cuff at the beginning of the procedure to prevent the release of tumor cells into the circulating pneumoperitoneal CO2, we also insert an additional trocar into the pouch of Douglas instead of using a uterine manipulator in all patients undergoing TLRH. Lee et al. [12] showed, in a splenic tumor model, that traumatic handling of the tumor results in tumor dissemination. Nishizaki et al. [30] showed in animal experiments that excessive tumor manipulation during surgery can cause tumor cells to spread into the bloodstream (circulating tumor cells), increasing the risk of postoperative metastasis. Findings of these studies suggest that excessive tumor handling during RH can cause tumor spillage into the peritoneal cavity and bloodstream.
In a presentation given at the 2018 American Society of Clinical Oncology annual meeting, MIS-RH was shown not to be inferior to ARH in terms of OS of patients with a tumor <2 cm in diameter, but MIS-RH was shown to be statistically inferior for patients with a tumor ranging 2–4 cm in diameter [31]. An additional recent study identified a similar association [32]. When the cervical tumor is large, exposing the parametrium is more difficult, and thus it becomes necessary to squeeze the uterine cervix to create adequate surgical space for resection of the parametrium. The tips of forceps used during laparoscopic surgery are very small, and squeezing the cervix with these forceps can puncture the tumor, causing spillage of tumor cells into the peritoneal cavity or bloodstream. This may have a negative impact on a patient's oncologic outcome. Thus, we believe that minimal handling of the tumor is particularly important during TLRH, perhaps even more important that during ARH. With our suspension technique, we are able to expose the parametrium with minimal handling of the uterine cervix, even in cases of a large tumor, and this may explain why tumor size did not influence DFS in our study.
Our laparoscopic RH procedure, which incorporates techniques for prevention of tumor cell spillage, yielded an appropriate number of harvested lymph nodes, an appropriate amount of excised parametrial tissue, and an acceptable incidence of positive surgical margins, which implies that the procedure is technically feasible. Performance of the Schiller test at the vaginal cuff during TLRH ensured adequate vaginal margins, and this might explain the significant length of vaginal tissues excised in our TLRH group.
Recurrence patterns did not differ between the two groups, and multiple pelvic recurrences, a salient pattern observed with the MIS-RH approach in the LACC trial, was observed in only 1 (1.2%) case (pT2bN1M0, 4.7 cm tumor size) in our TLRH group. Thirty-three (41.2%) patients in our TLRH group underwent adjuvant treatment, however, CCRT, which greatly decreases the possibility of pelvic recurrence, was performed in only 1 (1.2%) case. CCRT is a more powerful postoperative treatment modality to control pelvic local recurrence in high-risk early-stage cervical cancer when compared to systemic chemotherapy alone [33,34]. The multiple-pelvic recurrence rate was up to 1% in the absence of CCRT in our study which possibly reflect the usefulness of the no-look no-touch technique for reduction of local tumor dissemination.
We found our TLRH procedure including the steps for protection against tumor cell spillage to be technically feasible and oncologically safe. Outcomes were similar to those of ARH among patients with clinical FIGO stage 1B1 cervical cancer who had similar clinical backgrounds and risk factors for recurrence. The strength of this study is that all TLRH cases utilizing the no-look no-touch technique were performed by the same experienced surgical team and in a same manner, which means that neither a learning curve nor variations in surgical techniques influenced the results of the surgery.
The study weakness lies in the limited number of cases and limited follow-up time. A bias inherits to retrospective studies. For example, we were not able to retrieve information regarding the decision-making counseling pertaining to the surgical approach. Our patient population was predominately non-obese, and the generalizability of this technique in different populations remains unknown.
In conclusion, in comparison to open RH, TLRH yielded favorable surgical outcomes including decreased operation time, less blood loss, shorter hospital stays, a similar complication rate, similar urinary recovery time, and similarly sized excised surgical specimens. Oncologic outcomes also were good. Our results indicate that TLRH incorporating specific measures aimed at preventing tumor cell spillage is a reliable alternative procedure to ARH. Surgeons should keep in mind that tumors of the uterine cervix should not be directly exposed to the surgical field (early vaginal cuff closure) and should not be manipulated (avoid uterine manipulator).
ACKNOWLEDGMENTS
We thank Dr. Brendan H. Grubbs, MD, for his scientific input for this manuscript.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: K.H., M.K.
- Data curation: K.H.
- Formal analysis: K.H.
- Investigation: A.Y.
- Methodology: K.H.
- Project administration: K.H., A.Y.
- Resources: A.Y., T.T., N.H., O.S.
- Software: K.H.
- Supervision: T.N.
- Validation: T.N.
- Visualization: K.H.
- Writing - original draft: K.H.
- Writing - review & editing: M.K., T.T., N.H., O.S.
SUPPLEMENTARY MATERIAL
References
- 1.Puntambekar SP, Palep RJ, Puntambekar SS, Wagh GN, Patil AM, Rayate NV, et al. Laparoscopic total radical hysterectomy by the Pune technique: our experience of 248 cases. J Minim Invasive Gynecol. 2007;14:682–689. doi: 10.1016/j.jmig.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Xu H, Li Y, Wang D, Li J, Yuan J, et al. The outcome of laparoscopic radical hysterectomy and lymphadenectomy for cervical cancer: a prospective analysis of 295 patients. Ann Surg Oncol. 2008;15:2847–2855. doi: 10.1245/s10434-008-0063-3. [DOI] [PubMed] [Google Scholar]
- 3.Yan X, Li G, Shang H, Wang G, Han Y, Lin T, et al. Twelve-year experience with laparoscopic radical hysterectomy and pelvic lymphadenectomy in cervical cancer. Gynecol Oncol. 2011;120:362–367. doi: 10.1016/j.ygyno.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 4.Nam JH, Park JY, Kim DY, Kim JH, Kim YM, Kim YT. Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: long-term survival outcomes in a matched cohort study. Ann Oncol. 2012;23:903–911. doi: 10.1093/annonc/mdr360. [DOI] [PubMed] [Google Scholar]
- 5.Yang L, Cai J, Dong W, Shen Y, Xiong Z, Wang H, et al. Laparoscopic radical hysterectomy and pelvic lymphadenectomy can be routinely used for treatment of early-stage cervical cancer: a single-institute experience with 404 patients. J Minim Invasive Gynecol. 2015;22:199–204. doi: 10.1016/j.jmig.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Chu HJ, Shang CL, Gong X, Liu TY, Zhao YH, et al. Long-term oncological outcomes after laparoscopic versus abdominal radical hysterectomy in stage IA2 to IIA2 cervical cancer: a matched cohort study. Int J Gynecol Cancer. 2016;26:1264–1273. doi: 10.1097/IGC.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 7.Shazly SA, Murad MH, Dowdy SC, Gostout BS, Famuyide AO. Robotic radical hysterectomy in early stage cervical cancer: a systematic review and meta-analysis. Gynecol Oncol. 2015;138:457–471. doi: 10.1016/j.ygyno.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Wang YZ, Deng L, Xu HC, Zhang Y, Liang ZQ. Laparoscopy versus laparotomy for the management of early stage cervical cancer. BMC Cancer. 2015;15:928. doi: 10.1186/s12885-015-1818-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895–1904. doi: 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 10.Bertagnolli MM, DeCosse JJ. Laparoscopic colon resection for cancer--an unfavorable view. Adv Surg. 1996;29:155–164. [PubMed] [Google Scholar]
- 11.Lee SW, Gleason NR, Bessler M, Whelan RL. Peritoneal irrigation with povidone-iodine solution after laparoscopic-assisted splenectomy significantly decreases port-tumor recurrence in a murine model. Dis Colon Rectum. 1999;42:319–326. doi: 10.1007/BF02236346. [DOI] [PubMed] [Google Scholar]
- 12.Lee SW, Southall J, Allendorf J, Bessler M, Whelan RL. Traumatic handling of the tumor independent of pneumoperitoneum increases port site implantation rate of colon cancer in a murine model. Surg Endosc. 1998;12:828–834. doi: 10.1007/s004649900723. [DOI] [PubMed] [Google Scholar]
- 13.Reymond MA, Wittekind C, Jung A, Hohenberger W, Kirchner T, Köckerling F. The incidence of port-site metastases might be reduced. Surg Endosc. 1997;11:902–906. doi: 10.1007/s004649900483. [DOI] [PubMed] [Google Scholar]
- 14.Reymond MA, Schneider C, Kastl S, Hohenberger W, Köckerling F. The pathogenesis of port-site recurrences. J Gastrointest Surg. 1998;2:406–414. doi: 10.1016/s1091-255x(98)80030-9. [DOI] [PubMed] [Google Scholar]
- 15.Balli JE, Franklin ME, Almeida JA, Glass JL, Diaz JA, Reymond M. How to prevent port-site metastases in laparoscopic colorectal surgery. Surg Endosc. 2000;14:1034–1036. doi: 10.1007/s004640000223. [DOI] [PubMed] [Google Scholar]
- 16.Kanao H, Fujiwara K, Ebisawa K, Hada T, Ota Y, Andou M. Various types of total laparoscopic nerve-sparing radical hysterectomies and their effects on bladder function. J Gynecol Oncol. 2014;25:198–205. doi: 10.3802/jgo.2014.25.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebina Y, Mikami M, Nagase S, Tabata T, Kaneuchi M, Tashiro H, et al. Japan Society of Gynecologic Oncology guidelines 2017 for the treatment of uterine cervical cancer. Int J Clin Oncol. 2019;24:1–19. doi: 10.1007/s10147-018-1351-y. [DOI] [PubMed] [Google Scholar]
- 18.Jichi Medical University Saitama Medical Center. Windows version of the installation [Internet] Saitama: Jichi Medical University Saitama Medical Center; [cited 2018 Dec 1]. Available from: http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/download.html. [Google Scholar]
- 19.van den Haak L, Alleblas C, Nieboer TE, Rhemrev JP, Jansen FW. Efficacy and safety of uterine manipulators in laparoscopic surgery: a review. Arch Gynecol Obstet. 2015;292:1003–1011. doi: 10.1007/s00404-015-3727-9. [DOI] [PubMed] [Google Scholar]
- 20.Sonoda Y, Zerbe M, Smith A, Lin O, Barakat RR, Hoskins WJ. High incidence of positive peritoneal cytology in low-risk endometrial cancer treated by laparoscopically assisted vaginal hysterectomy. Gynecol Oncol. 2001;80:378–382. doi: 10.1006/gyno.2000.6079. [DOI] [PubMed] [Google Scholar]
- 21.Lim S, Kim HS, Lee KB, Yoo CW, Park SY, Seo SS. Does the use of a uterine manipulator with an intrauterine balloon in total laparoscopic hysterectomy facilitate tumor cell spillage into the peritoneal cavity in patients with endometrial cancer? Int J Gynecol Cancer. 2008;18:1145–1149. doi: 10.1111/j.1525-1438.2007.01165.x. [DOI] [PubMed] [Google Scholar]
- 22.Krizova A, Clarke BA, Bernardini MQ, James S, Kalloger SE, Boerner SL, et al. Histologic artifacts in abdominal, vaginal, laparoscopic, and robotic hysterectomy specimens: a blinded, retrospective review. Am J Surg Pathol. 2011;35:115–126. doi: 10.1097/PAS.0b013e31820273dc. [DOI] [PubMed] [Google Scholar]
- 23.Kitahara S, Walsh C, Frumovitz M, Malpica A, Silva EG. Vascular pseudoinvasion in laparoscopic hysterectomy specimens for endometrial carcinoma: a grossing artifact? Am J Surg Pathol. 2009;33:298–303. doi: 10.1097/PAS.0b013e31818a01bf. [DOI] [PubMed] [Google Scholar]
- 24.Rakowski JA, Tran TA, Ahmad S, James JA, Brudie LA, Pernicone PJ, et al. Does a uterine manipulator affect cervical cancer pathology or identification of lymphovascular space involvement? Gynecol Oncol. 2012;127:98–101. doi: 10.1016/j.ygyno.2012.07.094. [DOI] [PubMed] [Google Scholar]
- 25.McFarland M, Craig E, Lioe TF, Dobbs SP, McCluggage WG. Artefactual displacement of cervical epithelium showing CIN III to fallopian tubes during laparoscopic hysterectomy with intrauterine balloon manipulator. Histopathology. 2014;65:139–141. doi: 10.1111/his.12370. [DOI] [PubMed] [Google Scholar]
- 26.Volz J, Köster S, Spacek Z, Paweletz N. The influence of pneumoperitoneum used in laparoscopic surgery on an intraabdominal tumor growth. Cancer. 1999;86:770–774. [PubMed] [Google Scholar]
- 27.Kong TW, Chang SJ, Piao X, Paek J, Lee Y, Lee EJ, et al. Patterns of recurrence and survival after abdominal versus laparoscopic/robotic radical hysterectomy in patients with early cervical cancer. J Obstet Gynaecol Res. 2016;42:77–86. doi: 10.1111/jog.12840. [DOI] [PubMed] [Google Scholar]
- 28.Cohn DE, Tamimi HK, Goff BA. Intraperitoneal spread of cervical carcinoma after laparoscopic lymphadenectomy. Obstet Gynecol. 1997;89:864. doi: 10.1016/s0029-7844(97)00039-2. [DOI] [PubMed] [Google Scholar]
- 29.Belval CC, Barranger E, Dubernard G, Touboul E, Houry S, Daraï E. Peritoneal carcinomatosis after laparoscopic radical hysterectomy for early-stage cervical adenocarcinoma. Gynecol Oncol. 2006;102:580–582. doi: 10.1016/j.ygyno.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 30.Nishizaki T, Matsumata T, Kanematsu T, Yasunaga C, Sugimachi K. Surgical manipulation of VX2 carcinoma in the rabbit liver evokes enhancement of metastasis. J Surg Res. 1990;49:92–97. doi: 10.1016/0022-4804(90)90116-j. [DOI] [PubMed] [Google Scholar]
- 31.Margul DJ, Yang J, Seagle BL, Kocherginsky M, Shahabi S. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36 Suppl:5502. [Google Scholar]
- 32.Kim SI, Cho JH, Seol A, Kim YI, Lee M, Kim HS, et al. Comparison of survival outcomes between minimally invasive surgery and conventional open surgery for radical hysterectomy as primary treatment in patients with stage IB1–IIA2 cervical cancer. Gynecol Oncol. 2019 doi: 10.1016/j.ygyno.2019.01.008. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 33.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 34.Matsuo K, Shimada M, Aoki Y, Sakamoto M, Takeshima N, Fujiwara H, et al. Comparison of adjuvant therapy for node-positive clinical stage IB–IIB cervical cancer: systemic chemotherapy versus pelvic irradiation. Int J Cancer. 2017;141:1042–1051. doi: 10.1002/ijc.30793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.