Abstract
Objective
To analyze the outcomes of sequential or sandwich chemotherapy (ChT) and radiotherapy (RT) in patients with node-positive endometrial cancer (EC).
Methods
Data from 4 centers were collected retrospectively for 179 patients with stage IIIC EC treated with postoperative RT and ChT (paclitaxel and carboplatin). Patients were either treated with 6 cycles of ChT followed by RT (sequential arm; 96 patients) or with 3 cycles of ChT, RT, and an additional 3 cycles of ChT (sandwich arm; 83 patients). Prognostic factors affecting overall survival (OS) and progression-free survival (PFS) were analyzed.
Results
The 5-year OS and PFS rates were 64% and 59%, respectively, with a median follow-up of 41 months (range, 5–167 months). The 5-year OS rates were significantly higher in the sandwich than sequential arms (74% vs. 56%; p=0.03) and the difference for 5-year PFS rates was nearly significant (65% vs. 54%; p=0.05). In univariate analysis, treatment strategy, age, International Federation of Gynecology and Obstetrics (FIGO) stage, pathology, rate of myometrial invasion, and grade were prognostic factors for OS and PFS. In multivariate analysis, non-endometrioid histology, advanced FIGO stage, and adjuvant sequential ChT and RT were negative predictors for OS, whereas only non-endometrioid histology was a prognostic factor for PFS.
Conclusion
Postoperative adjuvant ChT and RT for stage IIIC EC patients, either given sequentially or sandwiched, offers excellent clinical efficacy and acceptably low toxicity. Our data support the superiority of the sandwich regimen compared to the sequential strategy in stage IIIC EC patients for OS.
Keywords: Endometrial Cancer, Surgery, Radiotherapy, Chemotherapy, Lymphatic Metastasis
INTRODUCTION
Endometrial cancer (EC) is the most common gynecological cancer and the fourth most common cancer in the female population [1,2]. Most patients with EC are diagnosed at an early stage and have a good prognosis with 5-year survival rates of 80%–85% [3,4]. However, patients with lymph node metastasis (stage IIIC) represent the most common subgroup of locally advanced disease, which accounts for 8%–10% of all EC cases [5,6].
The primary treatment option for stage IIIC disease is total hysterectomy with bilateral salpingo-oophorectomy and pelvic/para-aortic lymph node dissection. The recurrence rates are high in locally advanced disease with survival rates of 30%–89% for patients with stage III and 0%–10% for those with stage IV diseases [7,8]. Because local recurrence and distant metastasis rates are higher in patients with stage IIIC disease, many efforts have been made to improve the prognosis in these patients. Adjuvant radiotherapy (RT) is a common approach to treat patients with locally advanced EC [9,10]. However, higher rates of distant metastasis increase concerns regarding insufficiency of RT for distant disease control. Although chemotherapy (ChT) reduces the risk of distant metastasis, local and regional relapse rates are higher with adjuvant ChT alone [7,11]. The use of a combination of ChT and RT is more effective compared to either modality alone [12]. Results of a pooled analysis of 2 randomized trials demonstrated the efficacy of ChT and RT delivered sequentially for high-risk EC patients [13].
The major concern regarding combined ChT and RT is the optimal timing of ChT and RT to alleviate toxicity. For example, patients receiving ChT initially may develop toxicity, and their capacity to complete RT may be compromised [14]. Conversely, when RT is administered first, patients may be more susceptible to tumor progression located away from irradiation fields prior to ChT [15]. Therefore, studies have evaluated if the particular sequence in which adjuvant therapy is administered can mitigate toxicity and improve patient outcomes [12,14,15,16,17,18,19]. By modifying the timing of ChT and RT, the toxicities may theoretically be more manageable, thus facilitating the administration of both treatments properly [17,19,20,21]. Adjuvant ChT and RT may be delivered sequentially. One such approach, the “sandwich” method, involves adjuvant ChT followed by RT and subsequent ChT. The sandwich approach potentially confers a prognostic benefit compared to conventional sequential administration of ChT and RT with high efficacy and manageable toxicity. A series of studies support the sandwich method for the treatment of stage III EC and report 3-year progression-free survival (PFS) rates of 53%–80% and 3-year overall survival (OS) rates of 52%–90% [14,18,20,21,22]. However, most of these studies evaluated heterogeneous patient population including EC patients with high risk features with different pathologies, treatment modalities and with relatively limited patient number. There is limited data about the feasibility of adjuvant combined ChT and RT, and also sequencing the treatment modalities in EC patients with lymph node metastasis.
The optimal sequence of administering ChT and RT for stage IIIC EC patients, either sequentially or sandwiched, remains controversial. No prospective study has compared outcomes across these available sequences of ChT and RT in the adjuvant treatment of stage IIIC EC patients. In the absence of available prospective data, we sought to analyze the outcomes of sequential or sandwich ChT and RT in node-positive EC patients. Additionally, prognostic factors for stage IIIC patients treated with combined ChT and RT were assessed.
MATERIALS AND METHODS
1. Patient selection
Electronically stored clinical data for 259 EC patients with International Federation of Gynecology and Obstetrics (FIGO) stage IIIC disease treated between 2000 and 2016 at 4 different university hospitals were retrospectively reviewed. All patients were surgically staged with total hysterectomy, bilateral salpingo-oophorectomy, and bilateral pelvic and para-aortic lymph node dissection. The inclusion criteria were FIGO stage IIIC (IIIC1 with pelvic lymph node metastasis and IIIC2 with para-aortic lymph node metastasis), postoperative adjuvant ChT and RT, and ChT regimen including paclitaxel and carboplatin. After initial analysis, 66 patients were excluded because they were treated with adjuvant RT only and 14 patients were excluded because they received an adjuvant ChT protocol other than paclitaxel and carboplatin. Finally, 179 patients were evaluated to assess the effects of sequential versus sandwich treatment protocols.
2. Treatment protocol
All patients were treated according to their clinicians' practices and department policies. Patients commenced the ChT regimen within 2–3 weeks of surgery. The ChT regimen included carboplatin (area under curve 5 or 6) and paclitaxel (175 mg/m2) every 21 days. The sequence of adjuvant ChT and RT was determined using the difference between the time to initiation of adjuvant ChT and RT. Two groups were created: those with ChT followed by RT (sequential arm) and those with sandwiched ChT and RT (sandwich arm). In the sandwich protocol, patients received 3 cycles of ChT followed by RT and an additional 3 cycles of ChT. In the sequential arm, patients completed 6 cycles of ChT followed by RT. All patients received adjuvant TC in both the sequential and sandwich protocols.
All patients were treated with external beam RT using a total dose of 50.4 Gy to the pelvis over 5 weeks with a daily fraction size of 1.8 Gy using 18 or 25 MV photon energies. RT was delivered with 3-dimensional conformal technique (3DCRT) in 118 patients (66%) or intensity-modulated RT in 61 patients (34%). In 3DCRT technique, RT was administered via a 4-field technique employing an 18-MV linear accelerator with custom multi-leaf collimation blocking with conventional fractionation delivers in 25–28 days. The treatment fields either encompassed the pelvic lymphatics or pelvic and paraaortic (PA) lymphatics starting from T12–L1 interspace cranially. In patients with PA lymph node metastasis, PA fields were irradiated routinely. The addition of vaginal cuff brachytherapy (BRT) was left to the discretion of the treating physician. Median total external RT dose was 50.4 Gy (range, 40–50.4 Gy). The median total BRT and fraction BRT doses for those who received vaginal BRT were 18 Gy (range, 12–28 Gy) and 6 Gy (range, 3–7 Gy), respectively.
Treatment toxicities were assessed according to “Common Terminology Criteria for Adverse Events” version 4.0. Radiation related gastrointestinal system (GIS), genitourinary system (GUS) [23], and hematologic toxicities were analyzed. Additionally, treatment breaks during RT delivery were assessed.
3. Statistical analysis
All statistical analyses were performed using standard software (SPSS version 20; SPSS Inc., Chicago, IL, USA). The primary outcomes of interest were OS and PFS. Time to death or progression was calculated as the period from date of diagnosis to date of death or first clinical or imaging evidence of disease recurrence. Both OS and PFS rates were estimated by the Kaplan-Meier method. Age (<60 years vs. ≥60 years), FIGO stage (IIIC1 vs. IIIC2), tumor grade (G1–2 vs. G3), histology (endometrioid vs. non-endometrioid), myometrial invasion (<50% vs. ≥50%), lymphovascular space invasion (absent vs. present), adjuvant treatment (sequential vs. sandwich), and vaginal cuff BRT (absent vs. present) were analyzed for association with OS and PFS. The χ2 test or student's t-test were used to analyze the differences in clinical and pathological factors between the sequential and sandwich arms. Univariate analysis was performed via the log-rank test. Multivariate analysis was performed using the Cox proportional hazards model, using covariates with a p-value less than 0.10 based on univariate analysis. All p-values <0.05 were statistically significant.
RESULTS
1. Patient characteristics
Patient characteristics of the entire cohort according to treatment arms are presented in Table 1. The median age of patients in the entire cohort was 61 years (range, 36–88 years). In most instances, the disease was at FIGO stage IIIC1. The predominant histology was endometrioid adenocarcinoma (72%), and non-endometrioid histology (papillary serous carcinoma [23 patients, 13%], sarcoma [11 patients, 6%], mixed tumor [11 patients, 6%], and clear cell carcinoma [5 patients, 3%]) was seen in 28% of patients. The median number of dissected and metastatic lymph nodes were 39 (range, 5–162 nodes) and 2 (1–61 nodes), respectively.
Table 1. Patient and tumor characteristics according to ChT regimen.
| Patient characteristics | All patients | Sequential ChT | Sandwich ChT | p-value | |
|---|---|---|---|---|---|
| Patient age | 0.02 | ||||
| <60 yr | 89 (50) | 55 (31) | 34 (23) | ||
| ≥60 yr | 90 (50) | 41 (19) | 49 (27) | ||
| Stage | 0.27 | ||||
| IIIC1 | 120 (67) | 62 (35) | 58 (32) | ||
| IIIC2 | 59 (33) | 34 (19) | 25 (14) | ||
| Pathology | 0.12 | ||||
| Endometrioid | 129 (72) | 65 (36) | 64 (36) | ||
| Non-endometrioid | 50 (28) | 31 (17) | 19 (11) | ||
| LVSI | 0.67 | ||||
| Present | 117 (65) | 61 (34) | 56 (31) | ||
| Absent | 44 (25) | 26 (15) | 18 (10) | ||
| Unknown | 18 (10) | 9 (5) | 9 (5) | ||
| Myometrial invasion | 0.07 | ||||
| <50% | 44 (24) | 20 (11) | 24 (13) | ||
| ≥50% | 123 (69) | 66 (37) | 57 (32) | ||
| Unknown | 12 (7) | 10 (6) | 2 (1) | ||
| Grade | 0.79 | ||||
| I | 33 (18) | 16 (9) | 17 (9) | ||
| II | 59 (33) | 32 (18) | 27 (15) | ||
| III | 87 (49) | 48 (27) | 39 (22) | ||
| Vaginal BRT | 0.07 | ||||
| Absent | 91 (51) | 55 (31) | 36 (20) | ||
| Present | 88 (49) | 41 (23) | 47 (26) | ||
Values are presented as number (%).
BRT, brachytherapy; ChT, chemotherapy; LVSI, lymphovascular space invasion.
Ninety-six patients (54%) were treated with the sequential protocol and 83 patients (46%) were treated with the sandwich protocol. The patients in the sequential and sandwich arms were equally distributed, except younger patients (<60 years) who were primarily treated with the sequential method (p=0.02).
All patients received planned RT doses, but 144 patients (80%) completed ChT regimens as planned. In the sandwich arm, all patients received 3 initial cycles of ChT, whereas 67 patients (81%) received 3 additional cycles after completion of RT, 12 patients (14%) had 2 cycles, and 4 patients (2%) received 1 cycle. In the sequential arm, 77 patients (80%) received all 6 cycles, 10 patients (12%) received 5 cycles, 5 patients (6%) received 4 cycles, and 1 patient (1%) received 3 cycles of ChT before RT delivery.
2. Treatment outcomes
Median follow-up times for the entire cohort and survivors were 41 months (range, 5–167 months) and 60 months (range, 6–167 months), respectively. The 3- and 5-year OS rates were 79% and 64%, respectively, and PFS rates were 68% and 59%, respectively.
Of the 179 patients studied, 60 (34%) had disease progression, including 30 (50%) with distant metastases, 12 (20%) with pelvic recurrences, and 18 (30%) with both locoregional and distant metastasis at the last visit. Patients treated in the sequential arm had higher rates of disease progression than patients in the sandwich arm did (37 patients, 38% vs. 23 patients, 28%; p=0.05). In sandwich arm distant metastasis rate was higher (20 patients, 54%) compared to patients in sequential arm (10 patients, 43%), while local recurrence was more frequent in sequential arm (8 patients, 35%) compared to sandwich arm (4 patients, 11%). At final follow-up, 121 patients (68%) were alive (9, 5% with disease) and 58 patients (32%) had died (52, 29% from EC; 6, 3% from other causes).
3. Prognostic factors for OS and PFS
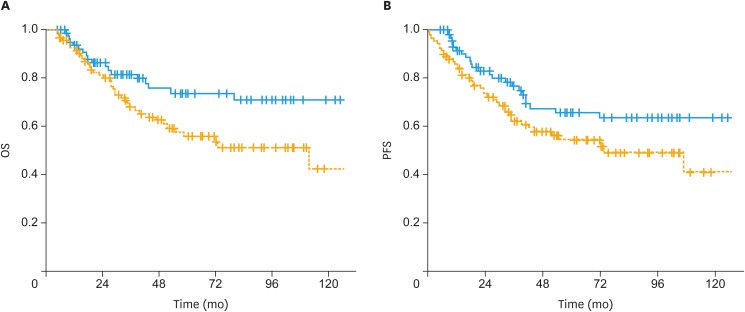
The 5-year OS rates were significantly higher in the sandwich arm than the sequential arm (74% vs. 56%; p=0.03) (Fig. 1A). However, the difference between the sandwich and sequential arms for 5-year PFS rates was nearly significant (65% vs. 54%; p=0.05) (Fig. 1B). In univariate analysis, treatment strategy, age, FIGO stage, pathology, rate of myometrial invasion, and grade were significant prognostic factors for OS (Table 2). Similarly, age, FIGO stage, pathology, myometrial invasion rate, and grade were identified as significant predictors of PFS.
Fig. 1. (A) The OS and (B) PFS of patients treated with postoperative sandwich ChT (solid line) and adjuvant sequential ChT and RT (dashed line) in the entire cohort.
ChT, chemotherapy; OS, overall survival; PFS, progression-free survival; RT, radiotherapy.
Table 2. Univariate analysis of prognostic factors for OS and PFS.
| Patient characteristics | 5-year OS (%) | p-value | 5-year PFS (%) | p-value | |
|---|---|---|---|---|---|
| Patient age | 0.04 | 0.04 | |||
| <60 yr | 72 | 68 | |||
| ≥60 yr | 56 | 50 | |||
| Stage | 0.001 | 0.004 | |||
| IIIC1 | 74 | 68 | |||
| IIIC2 | 45 | 45 | |||
| Pathology | <0.001 | <0.001 | |||
| Endometrioid | 72 | 67 | |||
| Non-endometrioid | 43 | 40 | |||
| LVSI | 0.1 | 0.18 | |||
| Absent | 72 | 67 | |||
| Present | 58 | 55 | |||
| Myometrial invasion | 0.03 | 0.03 | |||
| <50% | 82 | 78 | |||
| ≥50% | 62 | 56 | |||
| Grade | <0.001 | 0.003 | |||
| I–II | 74 | 67 | |||
| III | 53 | 51 | |||
| Vaginal BRT | 0.2 | 0.84 | |||
| Absent | 67 | 60 | |||
| Present | 60 | 59 | |||
| ChT | 0.03 | 0.05 | |||
| Sequential | 56 | 54 | |||
| Sandwich | 74 | 65 | |||
BRT, brachytherapy; ChT, chemotherapy; LVSI, lymphovascular space invasion; OS, overall survival; PFS, progression-free survival.
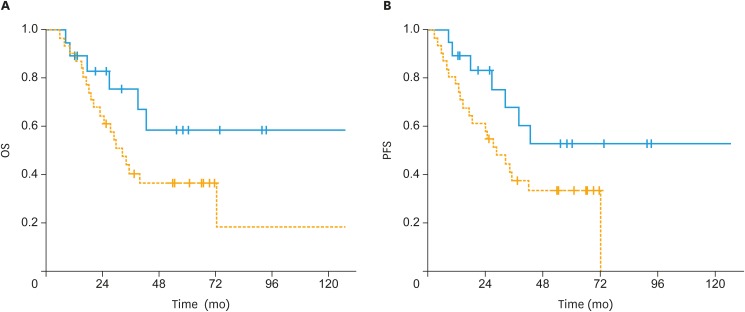
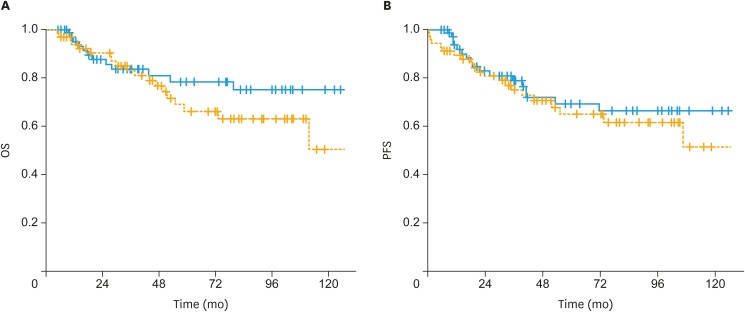
In patients with non-endometrioid histology, 5-year OS rates were 37% in the sequential arm and 59% in the sandwich arm (p=0.1) (Fig. 2A). The 5-year PFS rates for the sequential and sandwich arms were 33% and 53%, respectively (p=0.06) (Fig. 2B). There was no significant difference in terms of OS (66% vs. 78%; p=0.2) and PFS (65% vs. 69%; p=0.5) between the sequential and sandwich arms in patients with endometrioid histology (Fig. 3A and B).
Fig. 2. (A) The OS and (B) PFS of patients treated with postoperative sandwich ChT (solid line) and adjuvant sequential ChT and RT (dashed line) for patients with non-endometrioid histology.
ChT, chemotherapy; OS, overall survival; PFS, progression-free survival; RT, radiotherapy.
Fig. 3. (A) The OS and (B) PFS of patients treated with postoperative sandwich ChT (solid line) and adjuvant sequential ChT and RT (dashed line) for patients with endometrioid histology.
ChT, chemotherapy; OS, overall survival; PFS, progression-free survival; RT, radiotherapy.
In multivariate analysis, non-endometrioid histology, FIGO stage IIIC2 disease, and adjuvant sequential ChT and RT were negative predictors for OS (Table 3). For PFS, only non-endometrioid histology was a significant prognostic factor in multivariable analysis.
Table 3. Multivariate analysis of prognostic factors for OS and PFS.
| Variables | Risk factors | HR (95% CI) | p-value | |
|---|---|---|---|---|
| OS | ||||
| Age (yr) | >60 vs. ≤60 | 1.69 (0.93–3.06) | 0.08 | |
| Pathology | Non-endometrioid vs. endometrioid | 2.16 (1.08–4.32) | 0.03 | |
| Stage | IIIC2 vs. IIIC1 | 2.01 (1.15–3.53) | 0.02 | |
| Grade | III vs. I and II | 1.39 (0.67–2.86) | 0.36 | |
| Myometrial invasion | ≥50% vs <50% | 2.19 (0.93–5.18) | 0.07 | |
| ChT | Sequential vs. sandwich | 1.89 (1.04–3.45) | 0.04 | |
| PFS | ||||
| Age (yr) | >60 vs. ≤60 | 1.67 (0.97–2.89) | 0.07 | |
| Pathology | Non-endometrioid vs. endometrioid | 2.44 (1.28–4.65) | 0.007 | |
| Stage | IIIC2 vs. IIIC1 | 1.53 (0.90–2.58) | 0.09 | |
| Grade | III vs. I and II | 1.02 (0.53–1.97) | 0.64 | |
| Myometrial invasion | ≥50% vs. <50% | 2.00 (0.94–4.26) | 0.07 | |
| ChT | Sequential vs. sandwich | 1.57 (0.92–2.69) | 0.09 | |
ChT, chemotherapy; CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
4. Toxicity
Acute grade I–II GIS and GUS toxicities for the entire group were 55% and 38%, respectively. Grade III GIS toxicity was observed in 7 patients (4%), 3 patients in the sequential arm and 4 patients in the sandwich arm. The incidence of acute grade I–II GIS toxicity in the sequential and sandwich arms were 56% and 54% (p=0.5), whereas the GUS toxicities were 40% and 36% (p=0.4), respectively. Undesired treatment breaks during RT were seen in 35 patients (20%), 19 patients (11%) in the sequential arm and 16 patients (9%) in the sandwich arm (p=0.5). The median duration of treatment break was 5 days (range, 1–30 days). The incidence of grade >II neutropenia was 12% in the sequential arm and 9% in the sandwich arm (p=0.7). There were no treatment-related deaths in the cohort.
DISCUSSION
Our data supports that the sandwich therapy is feasible and efficacious in EC patients with lymph node metastasis. Non-endometrioid histology is an important prognosticator for both OS and PFS; additional negative prognostic factors for OS were para-aortic lymph node metastasis and sequential treatment strategy. All patients received the planned RT schedule, and 80% of patients completed the ChT regimens as planned. The tolerability of combined ChT and RT was high in either sequential or sandwich fashion, with no significant difference in acute GIS and GUS toxicity or neutropenia between the 2 different treatment modalities. No treatment-related mortality was observed.
The high incidence of local and distant recurrence in patients with stage IIIC EC requires adjuvant treatment strategies. Adjuvant RT and ChT are widely used for patients with locally advanced EC, but no optimal adjuvant therapy exists in this clinical setting [7,9,10,12,13,15,21,24,25]. Although many studies have analyzed the role of postoperative adjuvant RT in stage IIIC disease, the role of adjuvant RT remains controversial [9,10,25,26]. Mariani et al. [10] found that treatment with both adequate lymphadenectomy and RT is a significant independent predictor of pelvic failure. In a multi-institutional study with 116 stage IIIC EC patients, Brown et al. [26] found the 5-year OS rates were significantly higher in patients who received RT compared to patients who did not (57% vs. 42%; p=0.001). The authors concluded that adjuvant RT may be specifically indicated in patients with endometrioid histology, high-grade tumors, and para-aortic lymph node metastasis. In a recent Surveillance, Epidemiology, and End Results database study including 2,177 stage IIIC EC patients, the 3-year OS rates with and without RT were 81% vs. 68% (p<0.001) and cause-specific survival rates were 83% vs. 73% (p<0.001), respectively. In addition, the use of adjuvant RT was an independent prognostic factor for OS in multivariate analysis [9].
Although adjuvant RT demonstrates promising results for OS and better local and locoregional control, high rates of distant recurrences led to concerns about the insufficiency of RT alone for postoperative treatment of stage IIIC EC patients. Thus, numerous studies evaluated the role of adjuvant ChT in this group of patients, and the 5-year survival rates in these studies range from 48% to 76% [7,27,28,29,30]. In a meta-analysis with 1,326 patients comparing postoperative ChT and RT, ChT was associated with a relative risk (RR) of death at 5 years of 0.87 [31]. Although ChT reduces the risk of developing the first recurrence outside the pelvis (RR=0.79, 0.68–0.92), ChT may be less effective than RT in pelvic recurrences (RR=1.28, 0.97–1.68). Because ChT reduces the risk of developing a metastasis, it adds value when used with RT. Thus, combining ChT and RT might potentially improve both local control and distant metastasis.
Recent prospective studies exploring the efficacy of combination of ChT and RT in advanced EC report improved survival with acceptable toxicity profiles [13,21,32,33]. Lupe et al. [21] analyzed the feasibility of 4 cycles of adjuvant paclitaxel and carboplatin interposed with RT and an additional 2 cycles of ChT for 33 advanced EC patients of all histologies in a phase II study. The 3-year OS and PFS rates were 53% and 68%, respectively, with 3% pelvic relapse rates. A combined analysis of 2 randomized trials was recently pooled in response to poor accrual [13]. The entire cohort included disease stages II–IIIC with mostly endometrioid histology. The analysis of pooled data demonstrated a significant risk reduction in cancer-specific survival, a trend towards benefit in OS, and a 36% reduction in the risk of death in patients receiving multimodality therapy versus RT alone. Using the National Cancer Database (NCDB) with 6,720 patients, the combination of RT and ChT was the strongest predictor for improved OS compared to RT or ChT alone [34]. In another NCDB study, Boothe et al. [12] found that the median survival for patients treated with ChT alone, RT alone, and combined ChT and RT were 5.6, 7.1, and 10.3 years, respectively (p<0.001). In addition to prospective trials, several retrospective trials support the efficacy of multimodality treatment compared to ChT or RT alone [26,35]. In a study with 66 EC patients with lymph node metastasis, Lee and Viswanathan [34] found that PFS and OS are significantly higher in patients treated with ChT and RT than in patients receiving RT alone.
The use of multimodality adjuvant treatment with ChT and RT in the treatment of stage III EC increases by 1.8% per year, compared to a 3.4% decrease per year in treatment with RT alone [9]. However, the optimal timing of ChT and RT and the optimal number of ChT cycles is still under debate because of the potential for alleviating toxicities. Initial ChT before subsequent RT may increase ChT-related hematological toxicities, so the capacity to complete the planned RT can be compromised [14]. In contrast, when RT is delivered before ChT, patients may be more susceptible to disease progression at sites outside the radiation fields [15]. Alternatively, a “sandwich” method involving 3–4 cycles of ChT followed by pelvic RT and 2–3 additional cycles of ChT theoretically diminishes hematologic toxicities and facilitates administration of both planned treatments [20,21]. This method may also provide a therapeutic benefit, with ChT helping to stave off distant metastasis and RT mitigating the local recurrence risk. Two prospective studies investigating the feasibility of the sandwich method in high-risk EC patients demonstrated the efficacy of this method, with 3-year OS rates in patients receiving sequential and sandwich treatment of 52% and 68%, and 3-year PFS rates of 54% and 53%, respectively [20,21]. In a phase II trial conducted in 42 patients with stage III, IV, and recurrent EC treated with adjuvant carboplatin and docetaxel followed by RT, Geller et al. [22] found that 3-year OS and PFS rates in patients receiving sandwich and sequential treatment were 90% and 71%, respectively. Other studies also reported the efficacy of the sandwich method over single modality treatment with 5-year OS ranging from 68% to 88% and 5-year PFS rates ranging from 53% to 72% [15,16,17,21,34].
Although the efficacy and safety of the sandwich method over RT or ChT only has been demonstrated in numerous studies, the optimal sequence of administration of ChT and RT for patients with stage IIIC EC remains controversial. An NCDB study with 1,218 node-positive EC patients demonstrated that 5-year OS rates are better in sequential ChT and RT compared to concurrent chemoradiotherapy (CRT) (67% vs. 62%; p=0.004) [18]. Secord et al. [15] published a retrospective multicenter analysis with 109 advanced stage EC patients treated with a sandwich protocol, RT followed by ChT, or ChT followed by RT. The authors concluded the sandwich method is associated with improved survival in women with advanced stage disease compared to other sequencing modalities. Lu et al. [17] found no statistically significant difference in OS and PFS rates in 51 stage III EC patients with endometrioid histology treated with sequential or sandwich modality. In the present study, we found a significant difference in 5-year OS (56% vs. 74%; p=0.03) and a trend for 5-year PFS rates (54% vs. 65%; p=0.05) in favor of the sandwich modality compared to the sequential modality. Additionally, in multivariate analysis, the sandwich method is associated with improved OS compared to the sequential method (HR=1.89, 1.04–3.45; p=0.04). In Secord et al.'s study [15], only 48% of patients had endometrioid histology, whereas Lu et al. [17] evaluated only patients with endometrioid histology. In this study, although there was a statistically significant difference in OS and a trend for PFS between sandwich and sequential modalities in the entire cohort (72% with endometrioid histology), the significant difference between these 2 treatment modalities was lost when further analysis was performed for only endometrioid or non-endometrioid histology.
The addition of a vaginal cuff BRT boost to external beam radiation therapy (EBRT) is often based on institutional preference with limited guidelines in this setting. Although early stage EC patients are often recommended vaginal cuff BRT because of the high rates of vaginal cuff recurrence, the risk of pelvic relapse is greater than vaginal vault recurrence for patients with stage IIIC disease, thus potentially reducing the therapeutic benefit of vaginal cuff BRT [9]. There is relatively little data regarding the role of BRT for stage III EC patients [9,36]. Huddleston et al. [35] found borderline significance for pelvic control (92% vs. 70%; p=0.056) but not for vaginal control (94% vs. 90%; p=0.50) in 100 patients (82 treated with a vaginal BRT and 10 not treated with vaginal BRT). Shaikh et al. [9] found the 3-year OS rates with or without BRT were 80.8% and 79.5%, respectively (p=0.6). Current guidelines leave the use of BRT to the discretion of the treating physician [37]. In our analysis, there was no improvement in OS or PFS with the addition of BRT to EBRT; thus, our results suggest that BRT should not be routinely added to EBRT for stage IIIC EC patients. Although patients with a positive vaginal cuff margin may benefit from aggressive local therapy including vaginal cuff BRT boost, we were unable to make a recommendation because data regarding local control according to margin status is lacking in our series.
The present study had clear limitations, primarily because of its retrospective nature. First, appreciation of overall toxicity may be confounded because toxicity analysis was performed retrospectively using patient charts. We could only analyze the acute toxicities seen during ChT and RT delivery; however, neurotoxicities caused by systemic ChT seen as late complications were not analyzed. Second, the surgical procedures may vary according to surgeons' preferences. However, to overcome surgical procedure bias, we only analyzed patients receiving a hysterectomy with systemic pelvic and para-aortic lymph node dissection. Lastly, although most of the patients (72%) had endometrioid histology, patients with non-endometrioid histology were also included in analyzes. However, our study had strengths in the large number of patients, long follow-up time, and homogeneous patient population that included only EC patients with lymph node metastasis. Only patients receiving adjuvant paclitaxel and carboplatin ChT either using the sequential or sandwich protocol were analyzed, because the efficacy of paclitaxel and carboplatin on OS and PFS had been demonstrated in a phase III trial [37].
Our study demonstrates that postoperative adjuvant ChT and RT for stage IIIC EC patients given in either sequential or sandwich fashion offers excellent clinical efficacy and acceptably low toxicity. Together, our data support the significant efficacy of the sandwich regimen compared to the sequential strategy for OS of stage IIIC EC patients. However, the significant improvement was lost for OS in a separate analysis for patients with endometrioid or non-endometrioid histology. Besides sequential ChT and RT delivery, FIGO stage IIIC2 disease and non-endometrioid histology proved independently predictive of worse OS, whereas only non-endometrioid histology was a negative predictor for PFS. Further prospective randomized trials conducted on larger scales with well-defined patient populations are needed to better elucidate the impact of various sequencing schedules on clinical outcomes.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: O.C., Y.B.A.
- Data curation: S.S.Y., Y.B.A., Y.G.
- Formal analysis: O.C., S.S.Y., Y.B.A., A.S., Y.F.
- Funding acquisition: Y.G.
- Investigation: S.S.Y., Y.G., G.M.
- Methodology: O.C., G.M., A.S., Y.F.
- Project administration: O.C., Y.G.
- Resources: S.S.Y., Y.B.A., G.M., A.S.
- Software: Y.G.
- Supervision: O.C., A.S., Y.F.
- Validation: G.M., Y.F.
- Visualization: S.S.Y., Y.B.A., Y.G., G.M., Y.F.
- Writing - original draft: O.C.
- Writing - review & editing: O.C., A.S., Y.F.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J Natl Cancer Inst. 2017;109:djx030. doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC study group. Post operative radiation therapy in endometrial carcinoma. Lancet. 2000;355:1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 4.Papanikolaou A, Kalogiannidis I, Goutzioulis M, Misailidou D, Makedos A, Vergote I, et al. Pelvic lymphadenectomy as alternative to postoperative radiotherapy in high risk early stage endometrial cancer. Arch Gynecol Obstet. 2006;274:91–96. doi: 10.1007/s00404-006-0138-y. [DOI] [PubMed] [Google Scholar]
- 5.Lewin SN, Herzog TJ, Barrena Medel NI, Deutsch I, Burke WM, Sun X, et al. Comparative performance of the 2009 international federation of gynecology and obstetrics' staging system for uterine corpus cancer. Obstet Gynecol. 2010;116:1141–1149. doi: 10.1097/AOG.0b013e3181f39849. [DOI] [PubMed] [Google Scholar]
- 6.McMeekin DS, Lashbrook D, Gold M, Johnson G, Walker JL, Mannel R. Analysis of FIGO stage IIIc endometrial cancer patients. Gynecol Oncol. 2001;81:273–278. doi: 10.1006/gyno.2001.6157. [DOI] [PubMed] [Google Scholar]
- 7.Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a gynecologic oncology group study. J Clin Oncol. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 8.Smith RS, Kapp DS, Chen Q, Teng NN. Treatment of high-risk uterine cancer with whole abdominopelvic radiation therapy. Int J Radiat Oncol Biol Phys. 2000;48:767–778. doi: 10.1016/s0360-3016(00)00724-0. [DOI] [PubMed] [Google Scholar]
- 9.Shaikh T, Churilla TM, Mantia-Smaldone GM, Chu C, Rubin SC, Anderson PR. The role of adjuvant radiation in lymph node positive endometrial adenocarcinoma. Gynecol Oncol. 2016;141:434–439. doi: 10.1016/j.ygyno.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Mariani A, Dowdy SC, Cliby WA, Haddock MG, Keeney GL, Lesnick TG, et al. Efficacy of systematic lymphadenectomy and adjuvant radiotherapy in node-positive endometrial cancer patients. Gynecol Oncol. 2006;101:200–208. doi: 10.1016/j.ygyno.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 11.van Wijk FH, van der Burg ME, Burger CW, Vergote I, van Doorn HC. Management of surgical stage III and IV endometrioid endometrial carcinoma: an overview. Int J Gynecol Cancer. 2009;19:431–446. doi: 10.1111/IGC.0b013e3181a1a04f. [DOI] [PubMed] [Google Scholar]
- 12.Boothe D, Orton A, Odei B, Stoddard G, Suneja G, Poppe MM, et al. Chemoradiation versus chemotherapy or radiation alone in stage III endometrial cancer: patterns of care and impact on overall survival. Gynecol Oncol. 2016;141:421–427. doi: 10.1016/j.ygyno.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Hogberg T, Signorelli M, de Oliveira CF, Fossati R, Lissoni AA, Sorbe B, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer--results from two randomised studies. Eur J Cancer. 2010;46:2422–2431. doi: 10.1016/j.ejca.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geller MA, Ivy J, Dusenbery KE, Ghebre R, Isaksson Vogel R, Argenta PA. A single institution experience using sequential multi-modality adjuvant chemotherapy and radiation in the “sandwich” method for high risk endometrial carcinoma. Gynecol Oncol. 2010;118:19–23. doi: 10.1016/j.ygyno.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 15.Secord AA, Havrilesky LJ, O'Malley DM, Bae-Jump V, Fleming ND, Broadwater G, et al. A multicenter evaluation of sequential multimodality therapy and clinical outcome for the treatment of advanced endometrial cancer. Gynecol Oncol. 2009;114:442–447. doi: 10.1016/j.ygyno.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Lan C, Huang X, Cao X, Huang H, Feng Y, Huang Y, et al. Adjuvant docetaxel and carboplatin chemotherapy administered alone or with radiotherapy in a “sandwich” protocol in patients with advanced endometrial cancer: a single-institution experience. Expert Opin Pharmacother. 2013;14:535–542. doi: 10.1517/14656566.2013.778243. [DOI] [PubMed] [Google Scholar]
- 17.Lu SM, Chang-Halpenny C, Hwang-Graziano J. Sequential versus “sandwich” sequencing of adjuvant chemoradiation for the treatment of stage III uterine endometrioid adenocarcinoma. Gynecol Oncol. 2015;137:28–33. doi: 10.1016/j.ygyno.2015.01.546. [DOI] [PubMed] [Google Scholar]
- 18.Modh A, Ghanem AI, Burmeister C, Hanna RK, Elshaikh MA. What is the optimal adjuvant treatment sequence for node-positive endometrial cancer? Results of a national cancer database analysis. Int J Gynecol Cancer. 2018;28:248–253. doi: 10.1097/IGC.0000000000001162. [DOI] [PubMed] [Google Scholar]
- 19.Gao H, Zhang Z. Sequential chemotherapy and radiotherapy in the sandwich method for advanced endometrial cancer: a meta-analysis. Medicine (Baltimore) 2015;94:e672. doi: 10.1097/MD.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fields AL, Einstein MH, Novetsky AP, Gebb J, Goldberg GL. Pilot phase II trial of radiation “sandwiched” between combination paclitaxel/platinum chemotherapy in patients with uterine papillary serous carcinoma (UPSC) Gynecol Oncol. 2008;108:201–206. doi: 10.1016/j.ygyno.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Lupe K, Kwon J, D'Souza D, Gawlik C, Stitt L, Whiston F, et al. Adjuvant paclitaxel and carboplatin chemotherapy with involved field radiation in advanced endometrial cancer: a sequential approach. Int J Radiat Oncol Biol Phys. 2007;67:110–116. doi: 10.1016/j.ijrobp.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Geller MA, Ivy JJ, Ghebre R, Downs LS, Jr, Judson PL, Carson LF, et al. A phase II trial of carboplatin and docetaxel followed by radiotherapy given in a “sandwich” method for stage III, IV, and recurrent endometrial cancer. Gynecol Oncol. 2011;121:112–117. doi: 10.1016/j.ygyno.2010.12.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer. 2016;26:2–30. doi: 10.1097/IGC.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klopp AH, Jhingran A, Ramondetta L, Lu K, Gershenson DM, Eifel PJ. Node-positive adenocarcinoma of the endometrium: outcome and patterns of recurrence with and without external beam irradiation. Gynecol Oncol. 2009;115:6–11. doi: 10.1016/j.ygyno.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 25.Gadducci A, Guerrieri ME, Cosio S, Fabrini MG, Laliscia C, Attianese D, et al. Rates, sites and times of recurrence and clinical outcome of endometrial cancer patients with histologically-positive nodes: an Italian two-center retrospective study. Anticancer Res. 2018;38:1695–1703. doi: 10.21873/anticanres.12403. [DOI] [PubMed] [Google Scholar]
- 26.Brown AP, Gaffney DK, Dodson MK, Soisson AP, Belnap TW, Alleman K, et al. Survival analysis of endometrial cancer patients with positive lymph nodes. Int J Gynecol Cancer. 2013;23:861–868. doi: 10.1097/IGC.0b013e3182915c3e. [DOI] [PubMed] [Google Scholar]
- 27.Maggi R, Lissoni A, Spina F, Melpignano M, Zola P, Favalli G, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266–271. doi: 10.1038/sj.bjc.6603279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Susumu N, Sagae S, Udagawa Y, Niwa K, Kuramoto H, Satoh S, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese gynecologic oncology group study. Gynecol Oncol. 2008;108:226–233. doi: 10.1016/j.ygyno.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 29.Wolfson AH, Brady MF, Rocereto T, Mannel RS, Lee YC, Futoran RJ, et al. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I–IV carcinosarcoma (CS) of the uterus. Gynecol Oncol. 2007;107:177–185. doi: 10.1016/j.ygyno.2007.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson N, Bryant A, Miles T, Hogberg T, Cornes P. Adjuvant chemotherapy for endometrial cancer after hysterectomy. Cochrane Database Syst Rev. 2011;(10):CD003175. doi: 10.1002/14651858.CD003175.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Einstein MH, Frimer M, Kuo DY, Reimers LL, Mehta K, Mutyala S, et al. Phase II trial of adjuvant pelvic radiation “sandwiched” between combination paclitaxel and carboplatin in women with uterine papillary serous carcinoma. Gynecol Oncol. 2012;124:21–25. doi: 10.1016/j.ygyno.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T. Final analysis of RTOG 9708: adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancer. Gynecol Oncol. 2006;103:155–159. doi: 10.1016/j.ygyno.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Wong AT, Rineer J, Lee YC, Schwartz D, Safdieh J, Weiner J, et al. Utilization of adjuvant therapies and their impact on survival for women with stage IIIC endometrial adenocarcinoma. Gynecol Oncol. 2016;142:514–519. doi: 10.1016/j.ygyno.2016.07.091. [DOI] [PubMed] [Google Scholar]
- 34.Lee LJ, Viswanathan AN. Combined chemotherapy and radiation improves survival for node-positive endometrial cancer. Gynecol Oncol. 2012;127:32–37. doi: 10.1016/j.ygyno.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huddleston A, Zhen S, Qi L, Rash D, Leiserowitz G, Mayadev J. The impact of a vaginal brachytherapy boost to pelvic radiation in stage III endometrial cancer. J Contemp Brachytherapy. 2015;7:122–127. doi: 10.5114/jcb.2015.50877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koh WJ, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Chan J, et al. Uterine neoplasms, version 1.2014. J Natl Compr Canc Netw. 2014;12:248–280. doi: 10.6004/jnccn.2014.0025. [DOI] [PubMed] [Google Scholar]
- 37.Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a gynecologic oncology group study. J Clin Oncol. 2004;22:2159–2166. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]