Abstract
To identify intestinal bacteria that produce phenols (phenol and p-cresol), we screened 153 strains within 152 species in 44 genera by culture-based assay using broth media supplemented with 200 µM each of tyrosine and its predicted microbial metabolic intermediates (4-hydroxyphenylpyruvate, DL-4-hydroxyphenyllactate, 3-(p-hydroxyphenyl)propionate, 4-hydroxyphenylacetate and 4-hydroxybenzoate). Phenol-producing activity was found in 36 strains and p-cresol-producing activity in 55 strains. Fourteen strains had both types of activity. Phylogenetic analysis based on the 16S rRNA gene sequences of strains that produced 100 µM or more of phenols revealed that 16 phenol producers belonged to the Coriobacteriaceae, Enterobacteriaceae, Fusobacteriaceae and Clostridium clusters I and XIVa; four p-cresol-producing bacteria belonged to the Coriobacteriaceae and Clostridium clusters XI and XIVa; and one strain producing both belonged to the Coriobacteriaceae. A genomic search for protein homologs of enzymes involved in the metabolism of tyrosine to phenols in 10 phenol producers and four p-cresol producers, the draft genomes of which were available in public databases, predicted that phenol producers harbored tyrosine phenol-lyase or hydroxyarylic acid decarboxylase, or both, and p-cresol producers harbored p-hydroxyphenylacetate decarboxylase or tyrosine lyase, or both. These results provide important information about the bacterial strains that contribute to production of phenols in the intestine.
Keywords: phenol, p-cresol, intestinal bacteria, tyrosine, metabolite, phylogenetic analysis
We newly identified phenol- and p-cresol-producing bacteria by culture-based screening, and elucidated phylogenetic distribution of phenol- andp-cresol-producers.
INTRODUCTION
The more than 100 trillion bacteria in the human intestinal tract form a complicated ecosystem (Bäckhed et al. 2005). These bacteria produce many metabolites that can either harm or benefit host health (Nicholson et al. 2012). Short-chain fatty acids, which are produced mainly through the fermentation of carbohydrates, not only are used as energy sources for the host’s colonocytes but also have anti-inflammatory effects (Verbeke et al. 2015). Polyamines in the intestinal lumen enhance longevity and delay senescence (Kibe et al. 2014). Equol produced by intestinal microbiota reduces the risk of prostate cancer (Sugiyama et al. 2013). In contrast to these beneficial metabolites, intestinal secondary bile acid concentrations are closely related to the incidence of colorectal cancer (Ajouz, Mukherji and Shamseddine 2014), and indole, which is a uremic toxin, promotes the progression of chronic kidney disease (Evenepoel et al. 2009; Ito and Yoshida 2014). Because of the increasing importance of metabolites to host health, many metabolomic analyses have been performed to identify novel factors. For example, it has been found that trimethylamine is a risk factor for cardiovascular disease (Wang et al. 2011). As shown in these studies, we are aware of the role of metabolites in host health, but few studies have attempted to identify the bacteria involved in producing each type of metabolite in the colon. Obtaining information about the bacteria producing these metabolites would provide new clues to our understanding of disease from the perspectives of morbidity risk evaluation and the establishment of prevention methods.
Phenols (phenol and p-cresol) are microbial metabolites produced from tyrosine (Windey, De Preter and Verbeke 2012). Phenol exhibits cytotoxicity and increases paracellular permeability in vitro (Verbeke et al. 2015); it acts as a promoter of skin cancer in an animal model (Boutwell and Bosch 1959). p-cresol exhibits cytotoxicity and genotoxicity and reduces endothelial barrier function in vitro (Andriamihaja et al. 2015; Verbeke et al. 2015). p-cresyl sulfate, a sulfate-conjugate of p-cresol, suppresses Th1-type cellular immune responses in mice (Shiba et al. 2014); an increase in its levels is associated with chronic kidney disease-associated events such as cardiovascular disease (Meyer and Hostetter 2012; Ito and Yoshida 2014). Furthermore, phenol and p-cresol suppress the differentiation of keratinocytes in humans and cause dermal disorders in mice (Iizuka et al. 2009a,b). Although studies focusing on the relationship between phenols and various diseases have been accumulating, to our knowledge there has been no comprehensive study to identify the bacteria contributing to phenol- andp-cresol-production, with the exception of reports focused only on the genus Clostridium or on limited species (Bone, Tamm and Hill 1976; Elsden, Hilton and Waller 1976; Smith and Macfarlane 1996).
Here, we screened bacteria producing phenol or p-cresol, or both, using 153 strains within 152 species in 44 genera—mainly of intestinal bacteria—to determine which strains had the ability to produce phenol or p-cresol or both. Strains that screened positive were analyzed to determine the relationship between the ability to produce phenols and phylogenetic classification. They were then genetically analyzed to predict their metabolic pathways from tyrosine to phenols.
MATERIALS AND METHODS
Chemicals
DL-4-hydroxyphenyllactic acid, 4-hydroxyphenylpyruvic acid, 4-hydroxyphenylacetic acid and 4-hydroxybenzoic acid were obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Tyrosine and 3-(p-hydroxyphenyl)propionate were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Substrate solution was prepared by dissolving these compounds together in 18 mM NaOH solution (final 2 mM each) and filtered for sterilization through a 0.20 µm cellulose acetate filter (Toyo Roshi Kaisha, Ltd., Tokyo, Japan).
Bacterial strains and culture conditions
The 153 bacterial strains and culture conditions used for screening are listed in Table 1. The 153 strains represented 152 species found in the human gut habitat and their phylogenetic relatives; they accounted for about 70% of the common species detected in human feces (Qin et al. 2010). Two types of media (rich medium and poor medium) were used for culture. Rich medium was used for its growth efficiency: modified Gifu anaerobic medium broth (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) supplemented with 1% glucose; MRS broth (Nissui Pharmaceutical Co., Ltd.); Trypticase soy broth (Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA); or peptone–yeast extract (PY) broth supplemented with 1% glucose was used. The PY broth (1 L) contained 5.0 g peptone, 5.0 g trypticase peptone, 10.0 g yeast extract, 0.5 g L-cysteine HCl • H2O, 4.0 g Na2CO3, 7 mL 0.07% hemin solution, 1.0 mL 0.1% resazurin solution, 0.04 g K2HPO4, 0.04 g KH2PO4, 0.4 g NaHCO3, 0.08 g NaCl, 8 mg CaCl2, 19 mg MgSO4 • 7H2O and 1 mg vitamin K1 (pH 6.9). Basal Medium (Bone, Tamm and Hill 1976), which contains Tripticase Peptone (Becton, Dickinson and Company) instead of casein hydrolysate, was used as poor medium. As glucose supplementation can have critical effects on the production of phenols (Smith and Macfarlane 1996), basal medium that did not contain glucose as a carbon source was selected. The substrate solution described above was added to rich medium or poor medium to prepare test medium (final 200 µM each). Bacterial strains were pre-cultured in 4 mL of rich medium, and aliquots (40 µL) were inoculated into 4 mL of test media and incubated statically at 37°C for 6 days. An anaerobic chamber (N2:CO2:H2 = 88:5:7) was used for culture, except in the case of three strains: Cl. perfringens YIT 6050T and Cl. difficile YIT 10084Twere cultured under O2 free N2 gas, and Staphylococcus epidermidis YIT 6049Twas cultured aerobically.
Table 1.
Bacterial strains used in this study, and culture conditions
| No. | Species | Registration No. | Medium for culture |
|---|---|---|---|
| 1 | Acidaminococcus fermentans | YIT 6071T = ATCC 25085T | modified GAM + 1% Glucose broth |
| 2 | Acinetobacter baumannii | YIT 12295T = JCM 6841T | Trypticase Soy broth |
| 3 | Akkermansia muciniphila | YIT 11774T = ATCC BAA-835T | modified GAM + 1% Glucose broth |
| 4 | Anaerococcus hydrogenalis | YIT 12837T = JCM 7635T | modified GAM + 1% Glucose broth |
| 5 | Anaerococcus vaginalis | YIT 11698T = DSM 7457T | modified GAM + 1% Glucose broth |
| 6 | Anaerostipes caccae | YIT 10168T = DSM 14662T | modified GAM + 1% Glucose broth |
| 7 | Anaerostipes hadrus | YIT 10092T = DSM 3319T | modified GAM + 1% Glucose broth |
| 8 | Bacteroides caccae | YIT 10226T = JCM 9498T | modified GAM + 1% Glucose broth |
| 9 | Bacteroides dorei | YIT 12192 | modified GAM + 1% Glucose broth |
| 10 | Bacteroides eggerthii | YIT 10227T = DSM 20697T | modified GAM + 1% Glucose broth |
| 11 | Bacteroides fragilis | YIT 6158T = ATCC 25285T | modified GAM + 1% Glucose broth |
| 12 | Bacteroides ovatus | YIT 6161T = ATCC 8483T | modified GAM + 1% Glucose broth |
| 13 | Bacteroides plebeius | YIT 12661 | modified GAM + 1% Glucose broth |
| 14 | Bacteroides stercoris | ATCC 43183T | modified GAM + 1% Glucose broth |
| 15 | Bacteroides thethaiotaomicron | YIT 6163T = JCM 5827T | modified GAM + 1% Glucose broth |
| 16 | Bacteroides uniformis | YIT 6164T = JCM 5828T | modified GAM + 1% Glucose broth |
| 17 | Bacteroides vulgatus | YIT 6159T = ATCC 8482T | modified GAM + 1% Glucose broth |
| 18 | Bifidobacterium adolescentis | YIT 4011T = ATCC 15703T | modified PYG broth |
| 19 | Bifidobacterium animalis subsp. lactis | YIT 4121T = DSM 10140T | modified PYG broth |
| 20 | Bifidobacterium angulatum | YIT 4012T = ATCC 27535T | modified PYG broth |
| 21 | Bifidobacterium bifidum | YIT 4039T = DSM 20456T | modified PYG broth |
| 22 | Bifidobacterium breve | YIT 4014T = ATCC 15700T | modified PYG broth |
| 23 | Bifidobacterium catenulatum | YIT 4016T = ATCC 27539T | modified PYG broth |
| 24 | Bifidobacterium longumsubsp. infantis | YIT 4018T = ATCC 15697T | modified PYG broth |
| 25 | Bifidobacterium longum subsp. longum | YIT 4021T = ATCC 15707T | modified PYG broth |
| 26 | Bifidobacterium pseudocatenulatum | YIT 4072T = JCM 1200T | modified PYG broth |
| 27 | Blautia coccoides | YIT 6035T = JCM 1395T | modified GAM + 1% Glucose broth |
| 28 | Blautia hansenii | YIT 12129T = DSM 20583T | modified GAM + 1% Glucose broth |
| 29 | Blautia hydrogenotrophica | YIT 10080T = DSM 10507T | modified GAM + 1% Glucose broth |
| 30 | Blautia producta | YIT 6141T = JCM 1471T | modified GAM + 1% Glucose broth |
| 31 | Blautia schinkii | YIT 6177T = DSM 10518T | modified GAM + 1% Glucose broth |
| 32 | Butyrivibrio crossotus | YIT 10152T = DSM 2876T | modified GAM + 1% Glucose broth |
| 33 | Citrobacter freundii | YIT 6045T = JCM 1657T | Trypticase Soy broth |
| 34 | Citrobacter koseri | YIT 10117T = JCM 1658T | Trypticase Soy broth |
| 35 | Clostridium aminophilum | YIT 6167T = DSM 10710T | modified GAM + 1% Glucose broth |
| 36 | Clostridium aminovalericum | YIT 10174T = JCM 11016T | modified GAM + 1% Glucose broth |
| 37 | Clostridium asparagiforme | YIT 12840T = DSM 15981T | modified GAM + 1% Glucose broth |
| 38 | Clostridium bifermentans | YIT 6053T = JCM 1386T | modified GAM + 1% Glucose broth |
| 39 | Clostridium butyricum | YIT 10073T = JCM 1391T | modified GAM + 1% Glucose broth |
| 40 | Clostridium celerecrescens | YIT 6168T = DSM 5628T | modified GAM + 1% Glucose broth |
| 41 | Clostridium clostridioforme | YIT 6051T = JCM 1291T | modified GAM + 1% Glucose broth |
| 42 | Clostridium cochlearium | YIT 12837T = JCM 1396T | modified GAM + 1% Glucose broth |
| 43 | Clostridium cocleatum | YIT 6036T = JCM 1397T | modified GAM + 1% Glucose broth |
| 44 | Clostridium difficile | YIT 10084T = JCM 1296T | modified GAM + 1% Glucose broth |
| 45 | Clostridium ghonii | YIT 11479T = JCM 1400T | modified GAM + 1% Glucose broth |
| 46 | Clostridium glycolicum | YIT 6058T = JCM 1401T | modified GAM + 1% Glucose broth |
| 47 | Clostridium hathewayi | YIT 12259T = DSM 13479T | modified PYG broth |
| 48 | Clostridium hylemonae | YIT 12258T = DSM 15053T | modified PYG broth |
| 49 | Clostridium indolis | YIT 10077T = JCM 1380T | modified GAM +1% Glucose broth |
| 50 | Clostridium innocuum | YIT 10151T = DSM 1286T | modified GAM + 1% Glucose broth |
| 51 | Clostridium leptum | YIT 6169T = DSM 753T | modified GAM + 1% Glucose broth |
| 52 | Clostridium limosum | YIT 6061T = JCM 1427T | modified GAM + 1% Glucose broth |
| 53 | Clostridium malenominatum | YIT 12839T = JCM 1405T | modified GAM + 1% Glucose broth |
| 54 | Clostridium nexile | YIT 6170T = ATCC 27757T | modified GAM + 1% Glucose broth |
| 55 | Clostridium orbiscindens | YIT 10060T = DSM 6740T | modified GAM + 1% Glucose broth |
| 56 | Clostridium oroticum | YIT 6037T = JCM 1429T | modified GAM + 1% Glucose broth |
| 57 | Clostridium paraputrificum | YIT 10074T = JCM 1293T | modified GAM + 1% Glucose broth |
| 58 | Clostridium perfringens | YIT 6050T = JCM 1290T | modified GAM + 1% Glucose broth |
| 59 | Clostridium ramosum | YIT 10062T = JCM 1298T | modified GAM + 1% Glucose broth |
| 60 | Clostridium saccharolyticum | YIT 12747T = DSM 2544T | modified GAM + 1% Glucose broth |
| 61 | Clostridium scindens | YIT 6171T = JCM 6567T | modified GAM + 1% Glucose broth |
| 62 | Clostridium sordellii | YIT 6065T = JCM 3814T | modified GAM + 1% Glucose broth |
| 63 | Clostridium sphenoides | YIT 6059T = JCM 1415T | modified GAM + 1% Glucose broth |
| 64 | Clostridium spiroforme | YIT 10342T = JCM 1432T | modified GAM + 1% Glucose broth |
| 65 | Clostridium sporogenes | YIT 6060T = JCM 1416T | modified GAM + 1% Glucose broth |
| 66 | Clostridium symbiosum | YIT 11480T = JCM 1297T | modified GAM + 1% Glucose broth |
| 67 | Clostridium tetanomorphum | YIT 12841T = DSM 4474T | modified GAM + 1% Glucose broth |
| 68 | Clostridium xylanovorans | YIT 12130T = DSM 12503T | modified PYG broth |
| 69 | Collinsella aerofaciens | YIT 10235T = DSM 3979T | modified GAM + 1% Glucose broth |
| 70 | Coprococcus eutactus | YIT 10160T = ATCC 27759T | modified GAM + 1% Glucose broth |
| 71 | Cronobacter sakazakii | YIT 10246T = JCM 1233T | Trypticase Soy broth |
| 72 | Dorea formicigenerans | YIT 10093T = DSM 3992T | modified GAM + 1% Glucose broth |
| 73 | Edwardsiella tarda | YIT 10118T = JCM 1656T | Trypticase Soy broth |
| 74 | Eggerthella lenta | YIT 6077T = ATCC 25559T | modified GAM + 1% Glucose broth |
| 75 | Enterobacter aerogenes | YIT 6042T = JCM 1235T | Trypticase Soy broth |
| 76 | Enterobacter cloacae | YIT 6041T = JCM 1232T | Trypticase Soy broth |
| 77 | Enterococcus avium | YIT 10255T = JCM 8722T | MRS broth |
| 78 | Enterococcus durans | YIT 2036T = GIFU 9960T | MRS broth |
| 79 | Enterococcus faecalis | YIT 2031T = ATCC 19433T | MRS broth |
| 80 | Enterococcus faecium | YIT 2032T = ATCC 19434T | MRS broth |
| 81 | Enterococcus gilvus | YIT 11114T = DSM 15689T | MRS broth |
| 82 | Enterococcus hirae | YIT 2004T = ATCC 8043T | MRS broth |
| 83 | Enterococcus malodoratus | YIT 11175T = JCM 8730T | MRS broth |
| 84 | Enterococcus mundtii | YIT 11176T = JCM 8731T | MRS broth |
| 85 | Enterococcus pseudoavium | YIT 11177T = JCM 8732T | MRS broth |
| 86 | Enterococcus raffinosus | YIT 11178T = JCM 8733T | MRS broth |
| 87 | Escherichia coli | YIT 6044T = JCM 1649T | Trypticase Soy broth |
| 88 | Eubacterium biforme | YIT 6076T = ATCC 27806T | modified GAM + 1% Glucose broth |
| 89 | Eubacterium cellulosolvens | YIT 12261T = ATCC 43171T | modified GAM + 1% Glucose broth |
| 90 | Eubacterium cylindroides | YIT 10236T = DSM 3983T | modified GAM + 1% Glucose broth |
| 91 | Eubacterium dolichum | YIT 10081T = DSM 3991T | modified GAM + 1% Glucose broth |
| 92 | Eubacterium eligens | YIT 10078T = DSM 3376T | modified GAM + 1% Glucose broth |
| 93 | Eubacterium hallii | YIT 10064T = DSM 3353T | modified GAM + 1% Glucose broth |
| 94 | Eubacterium rectale | YIT 6082T = ATCC 33656T | modified GAM + 1% Glucose broth |
| 95 | Eubacterium siraeum | YIT 10049T = DSM 3996T | modified GAM + 1% Glucose broth |
| 96 | Eubacterium uniforme | YIT 12318T = ATCC 35992T | modified GAM + 1% Glucose broth |
| 97 | Eubacterium ventriosum | YIT 10066T = ATCC 27560T | modified GAM + 1% Glucose broth |
| 98 | Faecalibacterium prausnitzii | YIT 10067T = ATCC 27768T | modified PYG broth |
| 99 | Fusobacterium necrogenes | YIT 10362T = ATCC 25556T | modified GAM + 1% Glucose broth |
| 100 | Fusobacterium necrophorum subsp. necrophorum | YIT 10343T = JCM 3718T | modified GAM + 1% Glucose broth |
| 101 | Fusobacterium nucleatum subsp. nucleatum | YIT 6069T = JCM 8532T | modified GAM + 1% Glucose broth |
| 102 | Fusobacterium russii | YIT 10363T = ATCC 25533T | modified GAM + 1% Glucose broth |
| 103 | Fusobacterium varium | YIT 11855 = JCM 3722 | modified GAM + 1% Glucose broth |
| 104 | Hafnia alvei | YIT 10121T = JCM 1666T | Trypticase Soy broth |
| 105 | Holdemania filiformis | YIT 12717 | modified GAM + 1% Glucose broth |
| 106 | Klebsiella oxytoca | YIT 10122T = JCM 1665T | Trypticase Soy broth |
| 107 | Klebsiella pneumoniae | YIT 6046T = JCM 1662T | Trypticase Soy broth |
| 108 | Lactobacillus acidophilus | YIT 0070T = ATCC 4356T | MRS broth |
| 109 | Lactobacillus brevis | YIT 0076T = ATCC 14869T | MRS broth |
| 110 | Lactobacillus casei | YIT 0180T = ATCC 334T | MRS broth |
| 111 | Lactobacillus fermentum | YIT 0081T = ATCC 14931T | MRS broth |
| 112 | Lactobacillus fructivorans | YIT 0235T = JCM 1117T | MRS broth |
| 113 | Lactobacillus gasseri | YIT 0192T = DSM 20243T | MRS broth |
| 114 | Lactobacillus plantarum | YIT 0102T = ATCC 14917T | MRS broth |
| 115 | Lactobacillus reuteri | YIT 0197T = JCM 1112T | MRS broth |
| 116 | Lactobacillus ruminis | YIT 0221T = JCM 1152T | MRS broth |
| 117 | Lactobacillus sakei subsp. sakei | YIT 0247T = JCM 1157T | MRS broth |
| 118 | Lactococcus garvieae | YIT 2071T = NCFB 2155T | MRS broth |
| 119 | Lactococcus lactis subsp. lactis | YIT 2008T = ATCC 19435T | MRS broth |
| 120 | Lactococcus plantarum | YIT 2061T = ATCC 43199T | MRS broth |
| 121 | Lactococcus raffinolactis | YIT 2062T = ATCC 43920T | MRS broth |
| 122 | Megasphaera elsdenii | YIT 6063T = JCM 1772T | modified GAM + 1%Glucose broth |
| 123 | Morganella morganii | YIT 10124T = JCM 1672T | Trypticase Soy broth |
| 124 | Olsenella uli | YIT 12014T = JCM 12494T | modified GAM + 1%Glucose broth |
| 125 | Parabacteroides distasonis | YIT 6162T = JCM 5825T | modified GAM + 1%Glucose broth |
| 126 | Parabacteroides johnsonii | YIT 12680 | modified GAM + 1%Glucose broth |
| 127 | Parabacteroides merdae | ATCC 43184T | modified GAM + 1%Glucose broth |
| 128 | Peptoniphilus asaccharolyticus | YIT 10026T = GIFU 7656T | modified GAM + 1%Glucose broth |
| 129 | Porphyromonas gingivalis | YIT 12766T = JCM 12257T | modified GAM + 1%Glucose broth |
| 130 | Prevotella denticola | YIT 6131 = JCM 8528 | modified GAM + 1%Glucose broth |
| 131 | Prevotella intermedia | YIT 12886T = JCM 11150T | modified GAM + 1%Glucose broth |
| 132 | Prevotella melaninogenica | YIT 6039T = ATCC 25845T | modified GAM + 1%Glucose broth |
| 133 | Prevotella oris | YIT 6134T = JCM 8540T | modified GAM + 1%Glucose broth |
| 134 | Proteus mirabilis | YIT 6047T = JCM 1669T | Trypticase Soy broth |
| 135 | Proteus penneri | YIT 10252T = JCM 3948T | Trypticase Soy broth |
| 136 | Proteus vulgaris | YIT 10335T = DSM 13387T | Trypticase Soy broth |
| 137 | Providencia alcalifaciens | YIT 10128T = JCM 1673T | Trypticase Soy broth |
| 138 | Providencia rettgerii | YIT 10108T = DSM 4542T | Trypticase Soy broth |
| 139 | Pseudomonas aeruginosa | YIT 6108T = IFO 12689T | Trypticase Soy broth |
| 140 | Romboutsia lituseburensis | YIT 10059T = JCM 1404T | modified GAM + 1% Glucose broth |
| 141 | Roseburia faecis | YIT 11921T = DSM 16840T | modified GAM + 1% Glucose broth |
| 142 | Roseburia hominis | YIT 11920T = DSM 16839T | modified GAM + 1% Glucose broth |
| 143 | Roseburia intestinalis | YIT 10172T = DSM 14610T | modified GAM + 1% Glucose broth |
| 144 | Ruminococcus bromii | YIT 6078T = ATCC 27255T | modified GAM + 1% Glucose broth |
| 145 | Ruminococcus gnavus | YIT 6176T = ATCC 29149T | modified GAM + 1% Glucose broth |
| 146 | Ruminococcus lactaris | YIT 10225T = ATCC 29176T | modified GAM + 1% Glucose broth |
| 147 | Ruminococcus obeum | YIT 6085T = ATCC 29174T | modified GAM + 1% Glucose broth |
| 148 | Ruminococcus torques | YIT 10159T = ATCC 27756T | modified GAM + 1% Glucose broth |
| 149 | Staphylococcus epidermidis | YIT 6049T = ATCC 14990T | Trypticase Soy broth |
| 150 | Streptococcus mitis | YIT 2069T = GIFU 12458T | MRS broth |
| 151 | Streptococcus salivarius | YIT 10260T = JCM 5707T | MRS broth |
| 152 | Streptococcus thermophilus | YIT 2037T = ATCC 19258T | MRS broth |
| 153 | Veillonella parvula | YIT 6072T = GIFU 7884T | modified GAM + 1% Glucose broth |
Extraction and preparation of phenols from culture
Phenols were extracted by using a previously reported method, with partial modification (Niwa 1993). The bacterial culture was centrifuged at 20,400g for 5 min at 4°C, and the supernatant was filtered through a 0.20 µm cellulose acetate filter. Filtrates were diluted if necessary, and 225 µL of filtrate was mixed with 0.3 g sodium chloride, 180 µL of 1 N hydrochloride, 45 µL of 200 µM 4-isopropylphenol as an internal control and 450 µL of ethyl acetate, then vigorously vortexed for 30 s. The mixture was centrifuged at 2,350g for 5 min at room temperature. The ethyl acetate layer was filtered by using 0.45 µm PTFE filter vials (Thomson Instrument Company, Oceanside, California, USA), and the filtrate was subjected to HPLC analysis.
HPLC conditions
HPLC analysis was performed under the following conditions: pump: PU-2080 Plus (JASCO Corporation, Tokyo, Japan); column: L-column (Chemicals Evaluation and Research Institute, Tokyo, Japan); detector: FP-2025 Plus (excitation wavelength 260 nm and emission wavelength 305 nm); column temperature: 40°C; mobile phase: 0.1% phosphoric acid: acetonitrile (75:25) mixture; flow rate: 1 mL/min; sample injection volume: 6 µL.
Statistical analysis
Bacterial culture was performed three times independently. Bacterial strains were judged positive on screening if the concentrations of phenols in their cultures were significantly higher than those in uninoculated controls as background levels. Results were analyzed by using Student’s t-test, and strains were considered positive if the P-value was less than 0.05.
Phylogenetic analysis
Sequences of the 16S rRNA genes of bacterial strains identical to, or the same species as, the strains used in this study were collected from the Ribosomal Database Project (http://rdp.cme.msu.edu/index.jsp) or GenBank (http://www.ncbi.nlm.nih.gov/genbank/). Sequences were aligned by using Clustal X 2.1 (Larkin et al. 2007) and analyzed by using the Neighbor Joining method (Saitou and Nei 1987). The phylogenetic tree was visualized by using the TreeView 32 program (ver.1.6.6) (Page 1996). The 16S rRNA sequence of Desulfovibrio desulfuricans ATCC 29577T was used as an outgroup.
Search for homologous protein
Files on the proteins that phenol- or p-cresol-producing bacteria were expected to have were obtained from the National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov/); the accession numbers of the derived genomes are listed in Tables 2 and 3. The amino acid sequences of tyrosine phenol-lyase (TPL) from Citrobacter freundii MT-10419 (Iwamori et al. 1991), TyrB (tyrosine aminotransferase) from Escherichia coli K-12 substr. MG1655 (Accession No. NP_418478), and ThiH (tyrosine lyase) from E. coli K-12 (Accession No. NP_418417) were used as queries. Homology searches between queries and obtained protein lists were performed by using GENETYX ver.11. Searches for proteins homologous to KpdB, KpdC and KpdD (Klebsiella pneumoniae decarboxylase) from K. pneumoniae NCTC 418 (Accession Nos. AAY57854, AAY57855 and AAY57856, respectively); HpdA, HpdB and HpdC (p-hydroxyphenylacetate decarboxylase) from Cl. difficile DSM 1296T (Accession Nos. AJ543427, AJ543425 and AJ543426, respectively); FldH (phenyllactate dehydrogenase); FldBC (phenyllactate dehydratase); AcdA (acyl-CoA dehydrogenase) and PorA (pyruvate:ferredoxin oxidoreductase A) were performed by using MultiGeneBlast (Medema, Takano and Breitling 2013) with the default parameters. Amino acid sequences encoded by gene clusters consisting of fldL, fldA, fldI, fldB, fldC, acdA, etfB, etfA, permease and fldH from Cl. sporogenes ATCC 15579T (Accession Nos. EDU39251 to 39261) were used as queries to identify homologs of FldH, FldBC and AcdA. Similarly, amino acid sequences encoded by porA from Cl. sporogenes ATCC 15579T (Accession Nos. EDU39094 to 39096) were used to search for homologous proteins.
Table 2.
Predicted proteins homologous to enzymes involved in metabolic pathways from tyrosine to phenol
| % of identity / E-value | |||||||
|---|---|---|---|---|---|---|---|
| Species | Strains | Genome (Accession No.) | TPLa) | TyrBb) | HadBc) | HadCd) | HadDe) |
| Citrobacter freundii | YIT 6045T | NZ_JMTA00000000 | 99/0.0 | 90/0.0 | 83/6e−98 | 97/0.0 | 87/3e−37 |
| Clostridium saccharolyticum | YIT 12747T | NC_014376 | 70/0.0 | – | – | – | – |
| Cronobacter sakazakii | YIT 10246T | NZ_CP011047 | – | 85/0.0 | 82/3e−91 | 93/0.0 | 88/7e−38 |
| Enterobacter aerogenes | YIT 6042T | NC_015663 | – | 88/0.0 | 92/2e−106 | 98/0.0 | 92/7e−40 |
| Enterobacter cloacae | YIT 6041T | NC_014121 | – | 87/0.0 | 90/4e−103 | 96/0.0 | 94/6e−38 |
| Fusobacterium necrophorum | YIT 10343T | NZ_FMXX00000000 | 76/0.0 | – | – | – | – |
| Fusobacterium russii | YIT 10363T | NZ_ARMK00000000 | 82/0.0 | – | – | – | – |
| Klebsiella pneumoniae | YIT 6046T | NZ_AJJI00000000 | – | 84/0.0 | 100/2e−114 | 100/0.0 | 100/5e−42 |
| Morganella morganii | YIT 10124T | NZ_BCZU00000000 | 90/0.0 | 66/0.0 | – | – | – |
| Olsenella uli | YIT 12014T | NC_014363 | – | – | 48/6e−127 | 40/9e−12 | 48/1e−48 |
Table 3.
Predicted proteins homologous to enzymes involved in metabolic pathways from tyrosine to p-cresol
| % of identity / E-value | |||||||
|---|---|---|---|---|---|---|---|
| Species | Strains | Genome (Accession No.) | ThiHa) | TyrBb) | HpdAc) | HpdBd) | HpdCe) |
| Blautia hydrogenotrophica | YIT 10080T | NZ_ACBZ00000000 | – | – | 57/2e−108 | 55/0.0 | 42/5e−17 |
| Clostridium difficile | YIT 10084T | NZ_AUOX00000000 | 36/8e−85 | – | 99/0.0 | 100/0.0 | 100/9e−47 |
| Olsenella uli | YIT 12014T | NC_014363 | – | – | 56/6e−109 | 55/0.0 | 34/4e−8 |
| Romboutsia lituseburensis | YIT 10059T | NZ_FNGW00000000 | 35/2e−82 | – | 68/9e−133 | 76/0.0 | 59/2e−28 |
RESULTS
Evaluation of phenol-producing ability
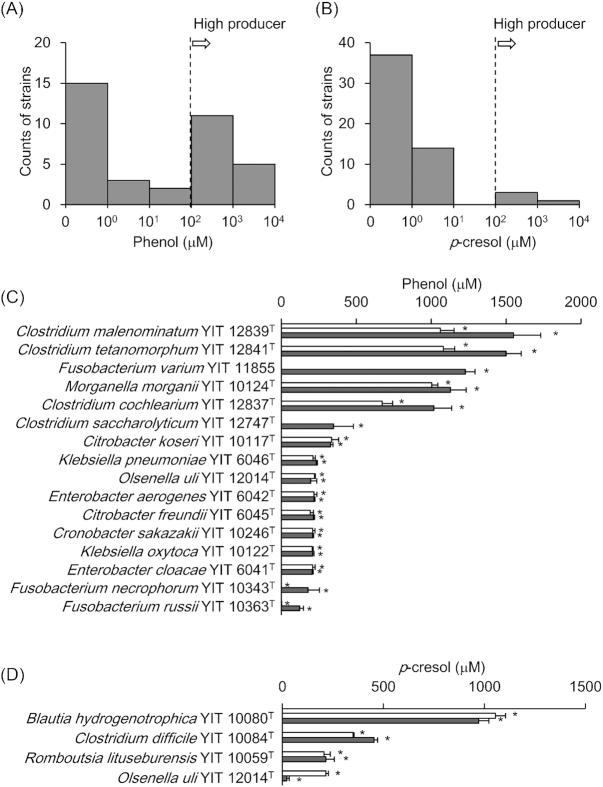
We determined the phenol concentrations in cultures of the 153 strains. The cultures of 36 strains had higher phenol concentrations than the background level (Fig. 1A). Of these 36 strains, 16 (Cl. malenominatum YIT 12839T, Cl. tetanomorphum YIT 12841T, Fusobacterium varium YIT 11855, Morganella morganii YIT 10124T, Cl. cochlearium YIT 12837T, Cl. saccharolyticum YIT 12747T, Citrobacter koseri YIT 10117T, K. pneumoniae YIT 6046T, Olsenella uli YIT 12014T, Enterobacter aerogenes YIT 6042T, Citrobacter freundii YIT 6045T, Cronobacter sakazakii YIT 10246T, K. oxytoca YIT 10122T, En. cloacae YIT 6041T, F. necrophorum subsp. necrophorum YIT 10343T and F. russii YIT 10363T) exhibited phenol production at 100 µM or more in their cultures (Fig. 1C). They were calculated to convert at least half of the supplemented substrates, even if only one of the substrates were metabolized. The remaining 20 strains produced less than 100 µM of phenol in their cultures (Fig. 2A, blue).
Figure 1.
Evaluation of phenol and p-cresol production ability in 153 screened strains
One-hundred fifty three strains were cultured in rich or poor medium for 6 days. The counts of (A) phenol-positive strains and (B)p-cresol-positive strains are shown as histograms. The concentrations of (C) phenol and (D)p-cresol produced in culture by high producers are shown. White bars indicate results using rich-medium; gray bars indicate those using basal medium. Error bars indicate standard deviations. Asterisks represent P < 0.05 as analyzed by Student’s t-test (increased compared with uncultured control medium).
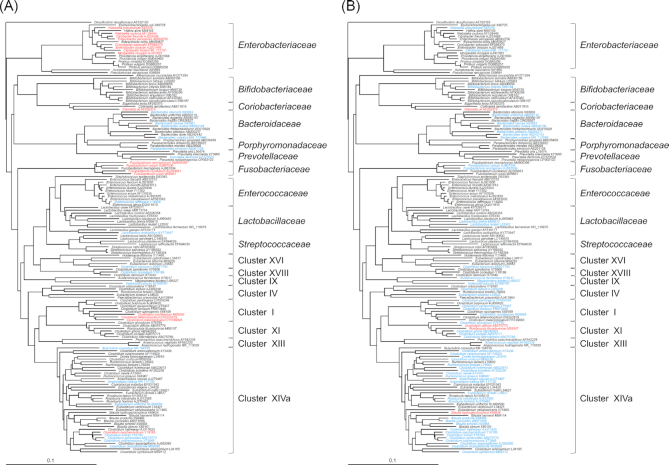
Figure 2.
Phylogenetic analysis of phenol or p-cresol producing bacteria
DNA sequences of 16S rRNA from 153 strains were subjected to phylogenetic analysis using Clustal X 2.1 and phylogenetic trees were constructed. (A) Phenol- or (B)p-cresol-producing strains are colored red (strains that produced at least 100 µM product) or blue (strains that produced less than 100 µM product). Strains in black font are phenol non-producers. Cluster no. represents the Clostridium 16S rRNA phylogenic cluster number (Collins et al.1994). Accession numbers used for analysis are displayed according to the name of each species, respectively.
We then determined the p-cresol concentrations in the cultures of the 153 strains. The p-cresol concentrations in the cultures of 55 strains were higher than the background level (Fig. 1B). Blautia hydrogenotrophica YIT 10080T, Cl. difficile YIT 10084T, O. uli YIT 12014T and Romboutsia lituseburensis YIT 10059T produced at least 100 µM of p-cresol (Fig. 1D). These four strains had markedly higher p-cresol production than the other 51, which produced less than 10 µM (Fig. 2B, blue).
Fourteen strains produced both phenol and p-cresol (Anaerostipes hadrus YIT 10092T, Bacteroides caccae YIT 10226T, B. ovatus YIT 6161T, B. vulgatus YIT 6159T, Cl. celerecrescens YIT 6168T, Cl. clostridioforme YIT 6051T, Cl. cochlearium YIT 12837T, Cl. indolis YIT 10077T, Cl. innocuum YIT 10151T, Cl. saccharolyticum YIT 12747T, Cl. sphenoides YIT 6059T, F. varium YIT 11855, O. uli YIT 12014T and Veillonella parvula YIT 6072T). Of these strains, only O. uli YIT 12014T produced both products at more than 100 µM; the others produced phenol or p-cresol, or both, at less than 10 µM.
Phylogenetic analysis of phenol-producing strains
All strains used in the screening were phylogenetically analyzed on the basis of the DNA sequences of the 16S rRNA gene. Phylogenetic tree analysis indicated that the phenol-producing strains were widely distributed in the Enterobacteriaceae, Coriobacteriaceae, Bacteroidaceae, Prevotellaceae, Porphyromonadaceae, Fusobacteriaceae, Enterococcaceae and Lactobacillaceae, as well as Clostridium clusters XVIII, XVI, IX, I and XIVa (Fig. 2A). The 16 strains that produced high levels of phenol (100 µM or more) belonged to specific families, namely the Coriobacteriaceae, Enterobacteriaceae and Fusobacteriaceae, along with Clostridium clusters I and XIVa. p-cresol-producing strains were dispersed across the Bifidobacteriaceae, Coriobacteriaceae, Bacteroidaceae, Fusobacteriaceae and Lactobacillaceae, along with Clostridium clusters XVI, IX, IV, I, XI, XIII and XIVa (Fig. 2B). Among them, four high p-cresol producers (100 µM or more) belonged to the specific family Coriobacteriaceae, or to Clostridium clusters XI and XIVa. The 14 strains that produced both phenol and p-cresol fell into the Fusobacteriaceae, Coriobacteriaceae or Bacteroidaceae, or Clostridium clusters XVI, IX, I and XIVa (Fig. 2). O. uli YIT 12014T, which had strong ability to produce phenol and p-cresol, belonged to the Coriobacteriaceae.
Prediction of metabolic pathways in phenol-producing strains
Three enzymes are involved in the initial or final steps of metabolic pathways from tyrosine to phenol: TPL, which metabolizes tyrosine to phenol in one step; TyrB, which metabolizes tyrosine to 4-hydroxyphenylpyruvate; and Had (hydroxyarylic acid decarboxylase), which metabolizes 4-hydroxybenzoate to phenol (Fig. 3A and B). Their activities were examined by using TPL from C. freundii MT-10419 (Iwamori et al. 1991), TyrB from E. coli strain K-12 (Kuramitsu et al. 1985), and Had from K. pneumoniae NCTC 418 (Lupa 2005), respectively. We then analyzed 10 strains with high phenol-producing ability, namely C. freundii YIT6045T, Cl. saccharolyticum YIT 12747T, C. sakazakii YIT 10246T, En. aerogenes YIT 6042T, En. cloacae YIT 6041T, F. necrophorum subsp. necrophorum YIT 10343T, F. russii YIT 10363T, K. pneumoniae YIT 6046T, M. morganii YIT 10124T and O. uli YIT 12014T, the draft genomes of which had already been sequenced, to determine whether homologous proteins of TPL, TyrB or Had were encoded in their genomes. A search for homologs of TPL derived from C. freundii MT-10419 revealed that homologs were encoded in the genomes of C. freundii YIT 6045T (99% identity of amino acid sequences), Cl. saccharolyticum YIT 12747T(70%), F. necrophorumsubsp.necrophorum YIT 10343T(76%), F. russii YIT 10363T(82%) and M. morganii YIT 10124T (90%) (Table 2). Similarly, we found that homologs of TyrB from E. coli strain K-12 were encoded in the genomes of C. freundii YIT 6045T (90% identity of amino acid sequences), C. sakazakii YIT 10246T (85%), En. aerogenes YIT 6042T(88%), En. cloacae YIT 6041T (87%), K. pneumoniae YIT 6046T (84%) and M. morganii YIT 10124T (66%) (Table 2). Had activity depended on three clusters encoded in the hadBCD operon and a cell lysate of E. coli transformed with kpdBCD; the hadBCD operon derived from K. pneumoniae NCTC 418 can metabolize 4-hydroxybenzoate to phenol (Lupa 2005). Thus, homologs of KpdBCD were found to be encoded in the genome of C. freundii YIT 6045T, C. sakazakii YIT 10246T, En. aerogenes YIT 6042T, En. cloacae YIT 6041T and K. pneumoniae YIT 6046T with more than 80% identity of amino acid sequences; in the case of O. uli YIT 12014T there was 40% to 48% identity (Table 2). The three homologs were encoded on these genomes in the order of HadB, HadC and HadD, except in the case of O. uli YIT 12014T, the three homologs of which were encoded on the genome in the order of hadC, hadD and hadB; the ORF encoding cation transporter was inserted between hadD and hadB (Fig. S1A, Supporting Information). FldBC homologs and AcdA homologs were not detected in the genomes of these six hadBCD-operon-positive strains (data not shown).
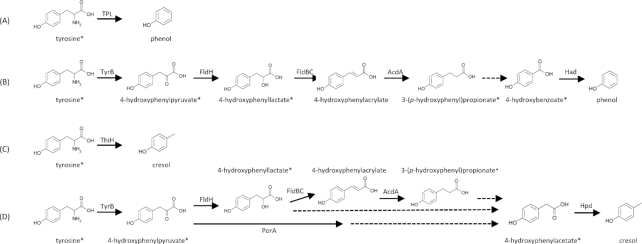
Figure 3.
Metabolic pathways from tyrosine to phenol and p-cresol
Metabolic pathways from tyrosine to phenol (A, B) and p-cresol (C, D) are shown as indicated by previous reports (Enei et al.1973; Gelfand and Steinberg 1977; Kriek et al.2007; Windey, De Preter and Verbeke 2012; Dodd et al.2017). Known enzymes—tyrosine phenol-lyase (TPL), tyrosine aminotransferase B (TyrB), phenyllactate dehydrogenase (FldH), phenyllactate dehydratase (FldBC), acyl-CoA dehydrogenase (AcdA), hydroxyarylic acid decarboxylase (Had), tyrosine lyase (ThiH), pyruvate:ferredoxin oxidoreductase A (PorA) and hydroxyphenylacetate decarboxylase (Hpd)—are shown near the arrows for each step. Steps with unidentified enzymes are indicated by dotted lines. Compounds used in this study are marked with asterisks.
Prediction of metabolic pathways in p-cresol-producing bacteria
TyrB and Hpd, which metabolize 4-hydroxyphenylacetate to p-cresol, and ThiH, which metabolizes tyrosine to p-cresol in one step, are metabolic enzymes that act in metabolic pathways from tyrosine to p-cresol (Fig. 3C and D). We therefore examined whether TyrB, Hpd or ThiH homologous proteins were found in all four strains (B. hydrogenotrophica YIT 10080T, Cl. difficile YIT 10084T, O. uli YIT 12014T and R. lituseburensis YIT 10059T) with high p-cresol-producing ability. We used information already reported on their draft genome sequences. No proteins with more than 30% amino acid sequence identity to TyrB of E. coli strain K-12 were found. In Cl. difficile DSM 1296T, three enzymes—HpdA, an activating enzyme; HpdC, a large subunit; and HpdC, a small subunit—are responsible for Hpd activity and are encoded in the hpdBCA operon (Andrei et al. 2004). Homologs of HpdBCA were identified in all four strains, with more than 30% identity of amino acid sequences (Table 3). In all four strains, the three homologs were encoded in a line in the order of hpdB, hpdC and hpdA (Fig. S1B, Supporting Information). ThiH from E. coli strain K-12 metabolizes tyrosine to dehydroglycine as the first step of the thiamine synthesis pathway, and p-cresol is formed as a by-product of this step (Kriek et al. 2007). We then found ThiH homologs encoded by the genome of Cl. difficile YIT 10084T (36% amino acid sequence identity) and R. lituseburensis YIT 10059T (35%) (Table 3). Analysis of homologs of other enzymes involved revealed that all four strains harbored FldH or PorA or both (data not shown). FldBC homologs were identified in B. hydrogenotrophica YIT 10080T and O. uli YIT 12014T. No AcdA homologs were identified in any strain (data not shown).
DISCUSSION
Screening conditions
To identify phenol- and p-cresol-producing bacteria, we used two major strategies. First, we supplemented the culture media with metabolic intermediates. Some of the supplemented intermediates—for example, 4-hydroxyphenyllactate and 4-hydroxyphenylacetate—are formed by intestinal bacteria in vitro (Smith and Macfarlane 1996; Beloborodova et al. 2012), suggesting that phenol- and p-cresol-producing bacteria further metabolize these intermediates in the intestinal environment. Cl. difficile YIT 10084T and O. uli YIT 12014T, which lacked a gene encoding TyrB in their genomes, might produce phenols from 4-hydroxyphenylpyruvate, 4-hydroxyphenyllactate, 3-(p-hydroxyphenyl)propionate, 4-hydroxybenzoate or 4-hydroxyphenylacetate as initial substrates in the intestine (Fig. 3B and D). Considering the complicated nature of the intestinal ecosystem, adding predicted metabolic intermediates to the culture media for screening was an effective strategy.
Second, we considered that other factors in the media might have affected phenol-production ability. Enei et al. (1973) reported that the presence of glucose in culture media suppressed TPL production in Erwinia herbicola ATCC 21434. Indeed, some TPL-positive strains, as represented by Cl. saccharolyticum YIT 12747T and F. russii YIT 10363T, produced much more phenol in glucose-limited media (poor media) than glucose-supplemented media (rich media) (Fig. 1C). On the other hand, glucose-limited media might be disadvantageous to growth. For example, O. uli YIT 12014T produced less p-cresol in poor medium than in rich medium (Fig. 1D), possibility because of this growth limitation. Thus it is a reasonable strategy to use both rich and poor media supplemented with tyrosine metabolites.
Identification of strains producing phenols
This study newly found 29 strains with phenol-producing potential and 51 with p-cresol-producing potential. Of the 36 phenol-positive strains, three—Cl. malenominatum YIT 12839T, Cl. tetanomorphum YIT 12841T and Cl. cochlearium YIT 12837T—have already been reported to produce phenol (Elsden, Hilton and Waller 1976). Moreover, K. pneumoniae YIT 6046T, En. cloacae YIT 6041T and M. morganii YIT 10124T are known as phenol-producing bacteria at the species level (Patel and Grant 1969; Valkova et al. 2001; Matsui et al. 2006; Iizuka et al. 2009b). The phenol-producing ability of C. freundii YIT 6045T had not been reported but had been surmised, because the phenol-forming activity of the purified TPL gene product from C. freundii species has been well characterized (Chandel and Azmi 2013). To our knowledge, the remaining 29 strains were identified here for first time as phenol producing. Among the 55 p-cresol-producing strains identified in this study, B. longumsubsp.infantis YIT 4018T, Cl. difficile YIT 10084T, Cl. paraputrificum YIT 10074T and F. necrogenes YIT 10362T have already been examined for their ability to produce p-cresol (Bone, Tamm and Hill 1976; Elsden, Hilton and Waller 1976; Smith and Macfarlane 1996). Here, we identified, for the first time, the remaining 51 strains as p-cresol-producing bacteria.
An abundance of strong producers of phenols in the intestine could affect the host’s health. The 16 phenol producers with high activity belonged to the Fusobacteriaceae, Enterobacteriaceae or Coriobacteriaceae, or to Clostridium clusters I and XIVa, and the four p-cresol producers with high activity belonged to the Coriobacteriaceae or to Clostridium cluster XI or XIVa. Kaur, Das and Mande (2017) have reported a relationship between the abundance of specific bacterial groups or specific putrefaction pathways in the intestine and the host’s stage of colorectal cancer. The information from our study could be a new clue to understanding diseases associated with phenols (Boutwell and Bosch 1959; Iizuka et al. 2009a; b, Windey, De Preter and Verbeke 2012; Ito and Yoshida 2014; Shiba et al. 2014; Andriamihaja et al. 2015; Verbeke et al. 2015). For this purpose, we need to examine whether fecal concentrations of phenols are related to the intestinal counts of phenol- and p-cresol-producing clusters. Furthermore, clinical studies are needed to investigate whether the occurrence of diseases associated with phenols is relevant to the abundance of intestinal producers of phenols.
Metabolic pathways from tyrosine to phenols
The metabolic pathways by which bacteria produce phenols are linked to the possession of pathway-related metabolic enzymes. In the genomes of 10 of the strong phenol producers analyzed here (Table 2; genome information for the remaining six was not available in the public database), homologs of TPL or Had were encoded, suggesting that each strain used pathways relevant to the enzymes they possessed (Fig. 3A and B). Cl. saccharolyticum YIT 12747T, F. necrophorum subsp. necrophorum YIT 10343T, F. russii YIT 10363T, and M. morganii YIT 10124T used TPL-dependent pathways; C. sakazakii YIT 10246T, En. aerogenes YIT 6042T, En. cloacae YIT 6041T, K. pneumoniae YIT 6046T and O. uli YIT 12014T used Had-dependent pathways, and C. freundii YIT 6045T used both TPL- and Had-dependent pathways. None of the Had-positive strains harbored FldBC homologs, indicating that these strains could use 3-(p-hydroxyphenyl)propionate or 4-hydroxybenzoate as initial metabolic substrates. More detailed analysis is needed to clarify the enzymes involved in the unknown parts of the Had-dependent pathways (Fig. 3B).
All four strong p-cresol-producing bacteria are predicted to harbor homologs of ThiH or Hpd that are involved in the final steps of p-cresol production. (Fig. 3C and D). This result suggests that ThiH or Hpd, or both, are key enzymes in producing p-cresol in these strains. We can predict from the genomic analysis that B. hydrogenotrophica YIT 10080T and O. uli YIT 12014T could utilize Hpd-dependent pathways, whereas Cl. difficile YIT 10084T and R. lituseburensis YIT 10059T could use both Hpd- and ThiH-dependent pathways. The lack of TyrB homologs and the presence of Hpd homologs in the four abovementioned strains suggest that these strains utilize tyrosine metabolites such as 4-hydroxyphenylpyruvate, 4-hydroxyphenyllactate, 3-(p-hydroxyphenyl)propionate or 4-hydroxyphenylacetate as initial substrates (Fig. 3D). This information could be a clue to identifying the metabolic scheme of p-cresol formation.
Revealing overall metabolic pathways is important for understanding intestinal microbial ecology. Draft genome sequencing of six strains not analyzed in this study (Cl. malenominatum YIT 12839T, Cl. tetanomorphum YIT 12841T, F. varium YIT 11855, Cl. cochlearium YIT 12837T, C. koseri YIT 10117T and K. oxytoca YIT 10122T) is needed. We also need to identify the currently unknown enzymes involved in the metabolism of phenols.
Limitations of this study
This screening took into account the intestinal environment, but there were three major limitations. First, the number of strains examined was limited from the perspective of the diversity of intestinal bacteria. Second, because the ability to produce phenols was evaluated in only one representative strain of each species, we did not consider variations in the ability to produce phenols among strains within a species. Third, the results of this in vitro screening might not always reflect the ability to produce phenols in the intestinal environment. Despite these limitations, this study was meaningful in that we were able to relate producers of phenols to clusters by phylogenetic analysis. This should give new insights into production of phenols in the intestine from the perspective of molecular genetics.
CONCLUSION AND FUTURE PERSPECTIVES
We identified 36 phenol-producing bacteria and 55 p-cresol-producing bacteria. Strong phenol producers belonged to the Coriobacteriaceae, Enterobacteriaceae, Fusobacteriaceae and Clostridium clusters I and XIVa, and strong p-cresol producers belonged to the Coriobacteriaceae and Clostridium clusters XI and XIVa. Such information on phenol- and p-cresol-producing bacteria should help identify the relationships between microbiota and host disease, as well as the underlying mechanisms.
Supplementary Material
ACKNOWLEDGEMENTS
All of the authors are employed by Yakult Honsha Co., Ltd. The authors thank Dr. Koji Kawakami from our institute for their technical help and useful advice and discussions.
Conflict of interest. None declared.
REFERENCES
- Ajouz H, Mukherji D, Shamseddine A. Secondary bile acids: an underrecognized cause of colon cancer. World J Surg Oncol. 2014;12:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei PI, Pierik AJ, Zauner S et al.. Subunit composition of the glycyl radical enzyme p-hydroxyphenylacetate decarboxylase. A small subunit, HpdC, is essential for catalytic activity. Eur J Biochem. 2004;71:2225–30. [DOI] [PubMed] [Google Scholar]
- Andriamihaja M, Lan A, Beaumont M et al.. The deleterious metabolic and genotoxic effects of the bacterial metabolite p-cresol on colonic epithelial cells. Free Radic Biol Med. 2015;85:219–27. [DOI] [PubMed] [Google Scholar]
- Bäckhed F, Ley RE, Sonnenburg JL et al.. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. [DOI] [PubMed] [Google Scholar]
- Beloborodova N, Bairamov I, Olenin A et al.. Effect of phenolic acids of microbial origin on production of reactive oxygen species in mitochondria and neutrophils. J Biomed Sci. 2012;19:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone E, Tamm A, Hill M. The production of urinary phenols by gut bacteria and their possible role in the causation of large bowel cancer. Am J Clin Nutr. 1976;29:1448–54. [DOI] [PubMed] [Google Scholar]
- Boutwell RK, Bosch DK. The tumor-promoting action of phenol and related compounds for mouse skin. Cancer Res. 1959;19:413–24. [PubMed] [Google Scholar]
- Chandel M, Azmi W. Purification and characterization of tyrosine phenol lyase from Citrobacter freundii. Appl Biochem Biotechnol. 2013;171:2040–52. [DOI] [PubMed] [Google Scholar]
- Collins MD, Lawson PA, Willems A et al.. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44:812–26. [DOI] [PubMed] [Google Scholar]
- Dodd D, Spitzer MH, Van Treuren W et al.. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551:648–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsden SR, Hilton MG, Waller JM. The end products of the metabolism of aromatic amino acids by Clostridia. Arch Microbiol. 1976;107:283–8. [DOI] [PubMed] [Google Scholar]
- Enei H, Yamashiti K, Okumura S et al.. Culture conditions for the preparation of cells containing high tyrosine phenol lyase activity. Agr Biol Chem. 1973;37:485–92. [Google Scholar]
- Evenepoel P, Meijers BK, Bammens BR et al.. Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl. 2009;114:S12–9. [DOI] [PubMed] [Google Scholar]
- Gelfand DH, Steinberg RA. Escherichia coli mutants deficient in the aspartate and aromatic amino acid aminotransferases. J Bacteriol. 1977;130:429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka R, Kawakami K, Chiba K. Gut bacteria producing phenols disturb keratinocyte differentiation in human skin. Microbial Ecol Health Dis. 2009;21:221–7. [Google Scholar]
- IIzuka R, Kawakami K, Izawa N et al.. Phenols produced by gut bacteria affect the skin in hairless mice. Microbial Ecol Health Dis. 2009;21:50–6. [Google Scholar]
- Ito S, Yoshida M. Protein-bound uremic toxins: new culprits of cardiovascular events in chronic kidney disease patients. Toxins (Basel). 2014;6:665–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamori S, Yoshino S, Ishiwata K et al.. Structure of tyrosine phenol-lyase genes from Citrobacter freundii and structural comparison with tryptophanase from Escherichia coli. J Ferment Bioeng. 1991;72:147–51. [Google Scholar]
- Kaur H, Das C, Mande SS. In Silico Analysis of putrefaction pathways in bacteria and its implication in colorectal cancer. Front Microbiol. 2017;8:2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibe R, Kurihara S, Sakai Y et al.. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci Rep. 2014;4:4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriek M, Martins F, Challand MR et al.. Thiamine biosynthesis in Escherichia coli: identification of the intermediate and by-product derived from tyrosine. Angew Chem Int Ed Engl. 2007;46:9223–6. [DOI] [PubMed] [Google Scholar]
- Kuramitsu S, Inoue K, Ogawa T et al.. Aromatic amino acid aminotransferase of Escherichia coli: nucleotide sequence of the tyrB gene. Biochem Biophys Res Commun. 1985;133:134–9. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP et al.. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–8. [DOI] [PubMed] [Google Scholar]
- Lupa B, Lyon D, Gibbs MD et al.. Distribution of genes encoding the microbial non-oxidative reversible hydroxyarylic acid decarboxylases/phenol carboxylases. Genomics. 2005;86:342–51. [DOI] [PubMed] [Google Scholar]
- Matsui T, Yoshida T, Hayashi T et al.. Purification, characterization, and gene cloning of 4-hydroxybenzoate decarboxylase of Enterobacter cloacae P240. Arch Microbiol. 2006;186:21–9. [DOI] [PubMed] [Google Scholar]
- Medema MH, Takano E, Breitling R. Detecting sequence homology at the gene cluster level with MultiGeneBlast. Mol Biol Evol. 2013;30:1218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TW, Hostetter TH. Uremic solutes from colon microbes. Kidney Int. 2012;81:949–54. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Holmes E, Kinross J et al.. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–7. [DOI] [PubMed] [Google Scholar]
- Niwa T. Phenol and p-cresol accumulated in uremic serum measured by HPLC with fluorescence detection. Clin Chem. 1993;39:108–11. [PubMed] [Google Scholar]
- Page RD. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–8. [DOI] [PubMed] [Google Scholar]
- Patel JC, Grant DJ. The formation of phenol in the degradation of p-hydroxybenzoic acid by Klebsiella aerogenes (Aerobacter aerogenes). Antonie Van Leeuwenhoek. 1969;35:53–64. [DOI] [PubMed] [Google Scholar]
- Qin J, Li R, Raes J et al.. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25. [DOI] [PubMed] [Google Scholar]
- Shiba T, Kawakami K, Sasaki T et al.. Effects of intestinal bacteria-derived p-cresyl sulfate on Th1-type immune response in vivo and in vitro. Toxicol Appl Pharmacol. 2014;274:191–9. [DOI] [PubMed] [Google Scholar]
- Smith EA, Macfarlane GT. Enumeration of human colonic bacteria producing phenolic and indolic compounds: effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J Appl Bacteriol. 1996;81:288–302. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Masumori N, Fukuta F et al.. Influence of isoflavone intake and equol-producing intestinal flora on prostate cancer risk. Asian Pac J Cancer Prev. 2013;14:1–4. [DOI] [PubMed] [Google Scholar]
- Valkova N, Lépine F, Valeanu L et al.. Hydrolysis of 4-hydroxybenzoic acid esters (parabens) and their aerobic transformation into phenol by the resistant Enterobacter cloacae strain EM. Appl Environ Microbiol. 2001;67:2404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke KA, Boobis AR, Chiodini A et al.. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr Res Rev. 2015;28:42–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Klipfell E, Bennett BJ et al.. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windey K, De Preter V, Verbeke K. Relevance of protein fermentation to gut health. Mol Nutr Food Res. 2012;56:184–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.