Abstract
There is controversy regarding the surgical route selection for tuberculum sellae meningiomas (TSMs): the transsphenoidal (TS) or transcranial (TC) approach? We conducted a systematic review and meta-analysis to compare clinical outcomes and postoperative complications between two surgical approaches. Literature search was performed. Relevant articles were selected and evaluated. Data were extracted and analyzed. Eight articles comprising 550 patients met the inclusion criteria. Traditionally, the rates of gross total resection, tumor recurrence, visual improvement, and cerebrospinal fluid leakage were the most common outcomes of interest. We demonstrated that the TS approach was significantly associated with better visual outcomes but more frequent cerebrospinal fluid leakage, while the rates of tumor resection and recurrence showed no significant difference between groups. In addition to surgical results that were consistent with previous studies, we further evaluated the impact of approach selection on common postoperative complications, which were closely related to the recovery course and quality of life. We revealed that the risk of dysosmia was significantly higher in the TS group. There was no significant difference between groups regarding infection, intracranial hemorrhage, and endocrine disorders. Because of the relatively low evidence levels of included retrospective studies, it was difficult to reach a categorical conclusion about the optimal surgical approach for TSMs. Finally, we recommended that the TS approach was an alternative option in patients with smaller TSMs (<30 mm) and limited invasion of optic canals in experienced neurosurgical centers.
Introduction
Tuberculum sellae meningiomas (TSMs) represent 5 to 10% of all intracranial meningiomas, invading the optic canals and displacing the optic nerves upward and laterally1. Therefore, despite of the relatively small proportion, such lesions are deeply concerned because of visual impairment in most cases2. Tumor resection and visual restoration are the two primary goals of the surgical treatment of TSMs. Traditionally, the transcranial approach (TC) has been the standard surgical route of removing TSMs and has achieved good outcomes3–5. In recent years, with the accumulation of endoscopic techniques and experiences, the transsphenoidal approach (TS) has been proposed for the resection of TSMs because of its minimal invasive nature6–10. Compared with TC, TS has some inherent advantages, such as minimized brain retraction, little optic apparatus manipulation, and direct removal of affected bone and dura. However, TS is more frequently associated with cerebrospinal fluid (CSF) leakage, which leads to a high risk of infection and may require a secondary repair. Nevertheless, with remarkable advances in skull base reconstruction, TS is considered as an important option for TSM resection8,10–12. Thus, there is a debate about the approach selection for TSMs13–15.
Although previous systematic reviews provided valuable conclusions in the approach selection for TSMs, each review had some limitations, of which the greatest one was the inability of calculation of overall odds ratio (OR) because of including non-comparative case series16–20. As the endoscopic technology matures, practitioners have reported more cases to directly compare the two approaches15,21,22. Herein, we performed a systematic review and meta-analysis of comparative studies regarding the approach selection for TSMs.
Methods
Search strategy
Our review is in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement23. We performed a systematic review of literature using Pubmed and Embase from inception to June 21, 2018. The following search terms in various combinations were used: tuberculum sellae, meningioma, endoscopy, endoscopic, endonasal, minimal invasive, transsphenoidal, transcranial, and craniotomy. Only the English-language articles were included. Two independent researchers (C.Y. & Z.S.) performed the literature searches separately. If there was any discrepancy regarding the eligibility of an article, consensus was reached with the guidance of the senior authors (X.B. & R.W.).
Inclusion criteria, data extraction, and quality assessment
The goals of the literature search were to find articles that met the following inclusion criteria: (1) described a comparative study including TSM patients treated by TS or TC approaches; (2) reported the number of patients and included at least three patients for each group; (3) reported the number of events for each group. Therefore, conference abstracts, non-comparative studies, and case reports were excluded. A flow chart of study selection process is shown in Fig. 1. Two authors (C.Y. & Z.S.) extracted relevant data from the selected studies independently and created a meta-analysis database with the following categories: (1) fundamental literature information including first author, publication year, and country; (2) characteristics of TSM patients including the number of total and female patients, age, tumor size, the number of patients with optic canal invasion and visual disturbance, and duration of follow-up; (3) data of clinical outcomes including the number of patients with gross total resection, improved visual outcomes, and recurrence for each group; (4) data of postoperative complications including the number of patients with CSF leakage, dysosmia, infection, intracranial hemorrhage, and endocrine disorders for each group. The 9-star Newcastle–Ottawa Scale (NOS) was used to assess the quality of eligible publications24. In this meta-analysis, studies with scores of 6 or more were considered as high-quality.
Figure 1.
Flow diagram of search strategy.
Data analysis
We performed the statistical analyses of pooled data to compare the surgical outcomes and postoperative complications between TS and TC groups using Review Manager, version 5.3.5 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The overall OR was computed using the method of Mantel-Haenszel test. The random-effects model was performed. Study heterogeneity was determined using the Cochrane Q and I2 statistics. Heterogeneity was considered significant when the p value from Cochran Q was <0.1 or I2 > 50%. A subgroup analysis and a sensitivity analysis were used to find the main source of between-study heterogeneity. Publication bias was assessed by visually inspecting the funnel plots25–27.
Results
Literature search
Our search strategy initially identified 306 articles, of which 59 duplicated articles were excluded. After the initial screening of titles and abstracts, 204 articles were excluded because of unrelated subject matter, inappropriate article types and none-English languages. The remaining 43 articles were reviewed in full text and assessed for eligibility. Another 35 articles were excluded for insufficient data and non-comparative study designs. The selection process yielded 8 articles comprising 550 patients for inclusion15,21,22,28–32. Among the general population, 2 patients underwent both approaches, and the relevant data of TS and TC surgeries were separately analyzed in each group.
Baseline data of included studies
The TS group comprised 220 patients, and the TC group comprised 332 patients. The number of female patients was available in 7 studies with an overall female proportion of 79.8%, indicating that women are predisposed to TSMs. The mean age in each group was reported in 6 studies, with age ranging from 23–82 years in the general patients. The detailed characteristics of included studies are presented in Table 1.
Table 1.
Characteristics of included studies.
| Authors & Year | Country | Years | No. of Ptx | TS | TC | Female | Age (mean ± SD) | Age (range) | No. of visual disturbance | Tumor size (p value) | Optic canal invasion (p value) | Follow-up (median) | Follow-up (range) | NOS scores |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Song et al. 2018 | Korea | 2004–2015 | 84 | 44 | 40 | 72 | TS: 53; TC: 54 | 24–76 | 78 | TS: 25 ± 6 mm; TC: 26 ± 8 mm (p = 0.570) | TS: 34/44; TC: 32/40 (p = 0.832) | TS: 27; TC: 44 | 0–147 | 9 |
| Magill et al. 2018 | US | 1997–2016 | 139 | 44 | 95 | NA | NA | NA | 121 | NA | TS: 26/44; TC: 86/95 (p < 0.001; p = 0.177 regarding severity) | 29; 46 (mean) | 0–174 | 7 |
| Kong et al. 2018 | Korea | 2010–2016 | 178 | 84 | 94 | 136 | TS: 54 ± 14; TC: 54 ± 11 | 31–79 | 157 | TS: 24 ± 7 mm; TC: 21 ± 8 mm (p > 0.05) | TS: 60/84; TC: 51/94 (p = 0.013) | 28 (mean) | 3–71 | 9 |
| Linsler et al. 2017 | Germany | 2011–2016 | 22 | 6 | 16 | 17 | TS: 66 ± 12; TC: 60 ± 12 | 46–82 | 8 | TS: 2.1 ± 0.8 cm3; TC: 14.9 ± 8.2 cm3 (p < 0.05) | NA | 18 ± 14 (mean ± SD) | 3–60 | 8 |
| Bowers et al. 2011 | US | 2002–2010 | 27 | 5 | 22 | 22 | TS: 58 ± 17; TC: 53 ± 13 | 23–77 | 23 | TS: 25 ± 7 mm; TC: 31 ± 13 mm (p = 0.945) | NA | NA | 12–120 | 9 |
| Fatemi et al. 2009 | US | 2000–2008 | 21 | 14 | 9 | 16 | TS: 51 ± 15; TC: 49 ± 7 | 31–77 | 19 | TS: 25 ± 8 mm; TC: 33 ± 10 mm (p = 0.008) | NA | TS: 28; TC: 14 | TS: 6–65; TC: 3–28 | 8 |
| Divitiis et al. 2008 | Italy | 1983–2006 | 51 | 7 | 44 | 41 | NA | NA | 51 | TC: < 2 cm 6 cases; 2–4 cm 33 cases; > 4 cm 5 cases; TS: < 2 cm 2 cases; 2–4 cm 5 cases | TS: 1/7; TC: 2/44 (p = 0.364) | NA | TS: 0.75–20; TC: 9–252 | 8 |
| Kitano et al. 2007 | Japan | 1994–2006 | 28 | 16 | 12 | 24 | TS: 54 ± 10; TC: 61 ± 9 | 42–76 | 26 | TC: 8.9 ± 9.4 mm3; TS: 7.5 ± 5.4 mm3 (p = 0.435) | NA | NA | TS: 3–96; TC: 108–156 | 9 |
Pts = patients; TS = transsphenoidal; TC = transcranial; NA = not available; NOS = Newcastle-Ottawa Scale; Unit of age = years; Unit of follow-up = months; Fatemi et al.: Two patients underwent both approaches; Kitano et al.: tumor volume = length × height × width/2; Linsler et al.: tumor volume = 3/4 × π × length × height × width.
Meta-analyses of clinical outcomes
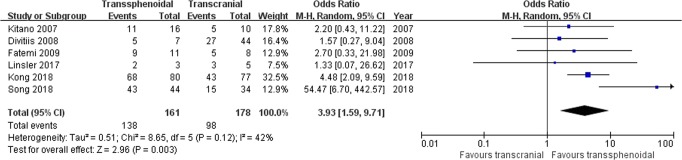
Visual outcome: Six studies comprising 339 patients were included for the random-effects meta-analysis15,21,28,29,31,32. The rate of visual improvement in the TS group was 138/161 (85.7%), and it was 98/178 (55.1%) in the TC group. The meta-analysis of pooled data showed a significant benefit from the TS approach in the rate of improved visual function (OR 3.93, 95% CI 1.59–9.71; p = 0.003; Fig. 2). The I2 statistic of 42% indicated no significant heterogeneity among included studies.
Figure 2.
Forest plot of all studies with their respective OR and 95% CI, the number of events (visual improvement), and overall OR. OR = odds ratio; CI = confidence interval; M-H = Mantel-Haenszel.
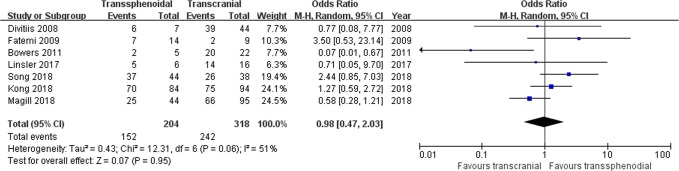
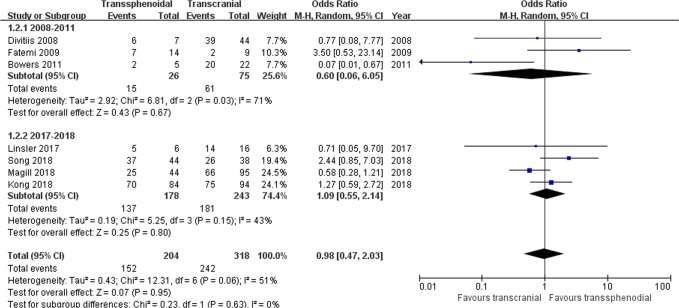
Tumor resection: Seven studies comprising 522 patients were included for the random-effects meta-analysis15,21,22,28–31. The rate of gross total resection (GTR) in the TS group was 152/204 (74.5%), and it was 242/318 (76.1%) in the TC group. No significant difference was detected in the rate of GTR between the two groups (OR 0.98, 95% CI 0.47–2.03; p = 0.95; Fig. 3). The I2 statistic of 51% indicated significant heterogeneity among included studies. In the subgroup analysis, we divided the included studies into two groups according to the publication year range: group 1 (2008–2011) and group 2 (2017–2018). Significant between-study heterogeneity was detected in group 1 (p = 0.03, I2 = 71%), but not in group 2 (p = 0.15, I2 = 43%) (Fig. 4). The further sensitivity analysis in Table 2 revealed that the study by Bowers et al. was the main resource of heterogeneity. After removing the study by Bowers et al., there was still no significant difference in the rate of GTR (OR 1.16, 95% CI 0.66–2.07; p = 0.23), and then the I2 statistic of 27% indicated no significant heterogeneity among remaining studies.
Figure 3.
Forest plot of all studies with their respective OR and 95% CI, the number of events (gross total resection), and overall OR.
Figure 4.
Subgroup analysis of gross total resection by the publication year.
Table 2.
Sensitivity analysis comparison of TS and TC approaches regarding gross total resection.
| Removed study | Overall effect of remaining studies (TS versus TC) | Study heterogeneity | ||
|---|---|---|---|---|
| OR (95% CI) | P value | I2 | P value | |
| Divitiis 2008 | 0.99 (0.44–2.21) | 0.97 | 59% | 0.03 |
| Fatemi 2009 | 0.85 (0.39–1.81) | 0.67 | 52% | 0.06 |
| Bowers 2011 | 1.16 (0.66–2.07) | 0.23 | 27% | 0.60 |
| Linsler 2017 | 0.99 (0.44–2.20) | 0.98 | 59% | 0.03 |
| Song 2018 | 0.79 (0.36–1.71) | 0.55 | 44% | 0.11 |
| Kong 2018 | 0.87 (0.33–2.34) | 0.79 | 57% | 0.04 |
| Magill 2018 | 1.14 (0.48–2.72) | 0.77 | 46% | 0.10 |
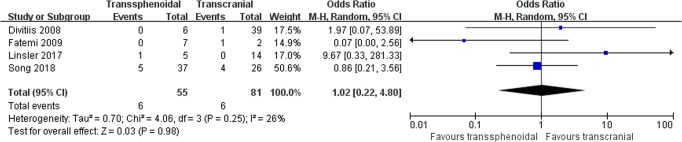
Recurrence: Four studies comprising 136 patients were included for the random-effects meta-analysis15,21,29,31. The rate of recurrence in the TS group was 6/55 (10.9%), and it was 6/81 (7.4%) in the TC group. No significant difference was detected in the rate of recurrence between the two groups (OR 1.02, 95% CI 0.22–4.80; p = 0.98; Fig. 5). The I2 statistic of 26% indicated no significant heterogeneity among included studies.
Figure 5.
Forest plot of all studies with their respective OR and 95% CI, the number of events (tumor recurrence), and overall OR.
Meta-analyses of postoperative complications
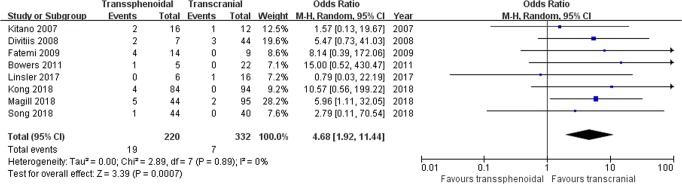
Cerebrospinal fluid leakage: Eight studies comprising 552 patients were included for the random-effects meta-analysis15,21,22,28–32. The rate of CSF leakage in the TS group was 19/220 (8.6%), and it was 7/332 (2.1%) in the TC group. The meta-analysis of pooled data showed a significantly higher risk from the TS approach with respect to the rate of CSF leakage (OR 4.68, 95% CI 1.92–11.44; p = 0.0007; Fig. 6). The I2 statistic of 0% indicated no significant heterogeneity among included studies.
Figure 6.
Forest plot of all studies with their respective OR and 95% CI, the number of events (cerebrospinal fluid leakage), and overall OR.
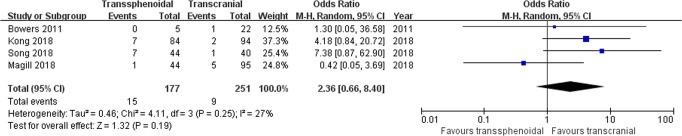
Infection: Four studies comprising 428 patients were included for the random-effects meta-analysis22,28,30,31. The rate of infection in the TS group was 15/177 (8.5%), and it was 9/251 (3.6%) in the TC group. The meta-analysis of pooled data showed a higher risk from the TS approach in the rate of infection (OR 2.36, 95% CI 0.66–8.40; p = 0.19; Fig. 7), but the difference was not statistically significant. The I2 statistic of 27% indicated no significant heterogeneity among included studies.
Figure 7.
Forest plot of all studies with their respective OR and 95% CI, the number of events (infection), and overall OR.
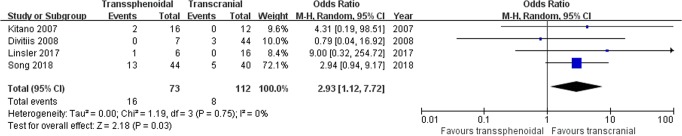
Dysosmia: Four studies comprising 185 patients were included for the random-effects meta-analysis15,29,31,32. The rate of dysosmia in the TS group was 16/73 (21.9%), and it was 8/112 (7.1%) in the TC group. The meta-analysis of pooled data showed a significantly higher risk from the TS approach in the rate of hyposmia (OR 2.93, 95% CI 1.12–7.72; p = 0.03; Fig. 8). The I2 statistic of 0% indicated no significant heterogeneity among included studies.
Figure 8.
Forest plot of all studies with their respective OR and 95% CI, the number of events (dysosmia), and overall OR.
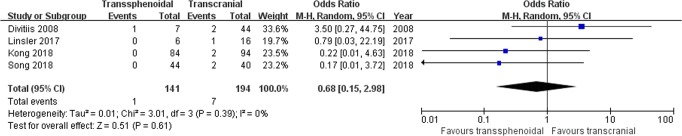
Intracranial hemorrhage: Four studies comprising 335 patients were included for the random-effects meta-analysis15,28,29,31. The rate of intracranial hemorrhage in the TS group was 1/141 (0.7%), and it was 7/194 (3.6%) in the TC group. No significant difference was detected in the rate of intracranial hemorrhage between the two groups (OR 0.68, 95% CI 0.15–2.98; p = 0.61; Fig. 9). The I2 statistic of 0% indicated no significant heterogeneity among included studies.
Figure 9.
Forest plot of all studies with their respective OR and 95% CI, the number of events (intracranial hemorrhage), and overall OR.
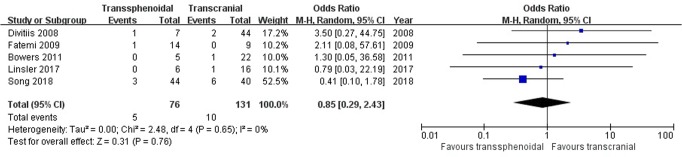
Endocrine disorders: Five studies comprising 207 patients were included for the random-effects meta-analysis15,21,22,29,31. The rate of endocrine disorders in the TS group was 5/76 (6.6%), and it was 10/131 (7.6%) in the TC group. No significant difference was detected in the rate of endocrine disorders between the two groups (OR 0.85, 95% CI 0.29–2.43; p = 0.76; Fig. 10). The I2 statistic of 0% indicated no significant heterogeneity among included studies.
Figure 10.
Forest plot of all studies with their respective OR and 95% CI, the number of events (endocrine disorders), and overall OR.
Publication bias and quality assessment: The funnel plots for clinical outcomes and postoperative complications were overall symmetrical, suggesting no obvious publication bias (see Supplementary Fig. S1–8). The NOS sore of included studies ranged from 7 to 9.
Discussion
Given the proximity to vital neurovascular structures, resection of TSMs remains a substantial challenge despite the remarkable advances in surgical techniques and approaches for skull base tumors.
Previous studies provided various levels of evidence about the approach selection in TSMs. Clark et al.16 performed a meta-analysis that included 6 studies involving 49 patients in the TS group and 11 studies involving 412 patients in the TC group demonstrating the association between the TS approach and higher rates of CSF leakage and visual improvement as well as no difference in extent of resection and morbidity. Graffeo et al.20 and Muskens et al.19 conducted meta-analyses involving more case series yielding similar results. In addition, Graffeo et al.20 revealed a higher risk of recurrence associated with the TS approach. Muskens et al.19 revealed that TS approach was associated with higher rates of intraoperative arterial injury. The reliability of these reviews was diminished because of including non-comparative studies.
In the past, with respect to the TS surgery, there were some apparent drawbacks, such as unclear tumor exposure, limited operation experiences under an endoscopic view, and lack of reliable sellar reconstruction. These operative limitations gave rise to the debate regarding the application of the TS approach in resection of TSMs. However, with advances in related techniques, neurosurgeons performed the TS approach surgery in selected patients with TSMs, achieving a remarkable rate of gross resection with an acceptable rate of CSF leakage12,33,34. Thus, many centers started to alternatively use the TS approach in selected patients. In this study, we enrolled data from comparative studies. The purpose of this meta-analysis was to comprehensively compare the clinical outcomes and postoperative complications of removing TSMs between the TS and TC approaches.
Traditionally, tumor resection, tumor recurrence, visual improvement, and CSF leakage were the most focused surgical results. In the present study, we further evaluated the impact of approach selection on common postoperative complications, which drew little attentions previously but were closely related to the recovery course and quality of life.
The extent of tumor resection is the strongest independent prognostic factor for meningioma35. With respect to the rate of GTR, our meta-analysis did not show a significant difference between the TS and TC groups. The result was consistent to previous studies. However, in the early years of the endoscopic surgery, the selection bias existed resulting in the inclusion of patients with smaller tumor size and less complicated anatomical relationships into the TS group. Tumor size, lateral extension, and neurovascular encasement contribute to the extent of tumor resection rather than the surgical approach alone. In three studies22,28,31,32, baseline tumor size was balanced between groups, mostly being less than 30 mm in diameter. Optic canal invasion was compared at baseline in four studies15,28,30,31, of which three studies showed no significant difference between groups15,30,31. The TS surgery is more suitable for tumors with small size (<30 mm) and limited extension whereas the complex anatomy is the indication for the TC surgery for sure. In our perspective, along with constant improvement of the endoscopic instruments and skills, endoscopic visualization can detect lateral extension easily and offer high-definition intraoperative images, allowing for more delicate surgical manipulation for complex TSMs in the future.
The most common initial presentation of TSMs is visual disturbance due to tumor extension to the optic canal36. Therefore, the visual change is considered as a major surgical indication for TSMs, and the restoration and preservation of visual function are the primary goals of surgical treatment. Visual improvement is defined as either or both of improved visual acuity and visual field in our study. Tumor features including size, extension and duration of preoperative visual dysfunction are critical predictive factors for visual recovery. Tumor size and extension were balanced in almost half of included studies as we mentioned above. Unfortunately, included studies didn’t bring much attention to detailed neuroimaging characteristics and ophthalmologic examination results. Therefore, we proposed that the ophthalmologist should be a member of the research team for TSMs. Considering the growth pattern of TSMs, prompt and complete optical canal decompression is associated with better visual outcomes. The results of visual outcomes in different studies are more favorable in the TS approach. Two key explanations are given to elucidate the phenomenon as following: (1) minimized manipulation of the optic nerves and optic chiasm; (2) well protection of blood supply to optic nerves. In contrast, in the TC approach, brain retraction and manipulation of neurovascular tissues are indispensable for tumor exposure and are likely to cause iatrogenic injuries to the optic nerves. Meanwhile, Julien et al.37 also suggested that contralateral transcranial approach could allow preservation of vascular supply and less mobilization of the compromised optic nerve. Apart from the approach selection, tumor size must be taken into consideration because tumor size is the most important factor affecting preoperative visual function. In the present study, six studies were included for the analysis of visual outcomes in each group. Among three over six included studies28,31,32, tumor size was compared at baseline and showed no significant difference between groups. When including the three high-level studies into a meta-analysis, the results were also in favor of the TS approach. Thus, it is believed that visual recovery is more favored in the TS group for patients with small tumor size (<30 mm) and limited extension.
In our study, recurrence is defined as tumor regrowth after complete resection. Pathologically, TSMs are commonly WHO I meningiomas classified as benign tumors. Therefore, considering similar rate of GTR between groups, recurrence is the most reliable indicator to evaluate the long-term clinical outcome. Theoretically, the TS approach is more convenient to remove affected bone and dura, achieving Simpson I resection and decreasing the risk of recurrence. On one hand, for a relatively new technique for tumor resection, the mean follow-up period in prior studies is not long enough for each individual to completely estimate its effect on tumor recurrence. On the other hand, the learning curve may undermine the real long-term outcome of the TS approach for TSM resection in experienced hands. The present data showed no significant difference between groups. From our view, the impact of approach selection on TSM recurrence will be better clarified with the data accumulation regarding longer follow-up results and more experienced TS resection of TSMs.
Though the TS approach has many advantages, its drawbacks are non-negligible, such as the difficulty in reconstructing cranial base dura and bone defects. CSF leakage is a significant complication of adopting the TS approach though it is commonly not associated with additional mortality unless surgical repairing is required. In our meta-analysis, the TS approach is associated with a significant higher rate of CSF leakage. The finding is consistent to previous studies. However, there is an obvious decreasing risk of CSF leakage as the neurosurgeons become more experienced in TS approach and skull base closure techniques.
Postoperative infection is generally associated with many factors such as blood loss, operation time, CSF leakage and surgical incision. The TS approach is associated with significant shorter operation time and less amount of bleeding compared with the TC approach. The higher risk of CSF leak and intranasal bacteria may account for higher rate of infection in the TS group though there is no significant difference between groups. It is commonly viewed that the most important factor of infection is CSF leakage in patients receiving a TS surgery. As the technology of skull base reconstruction improves, the rate of CSF leakage-associated infection in the TS group is bound to be low in the future.
Dysosmia is a notable complication because diminished olfaction after surgery is associated with a poor quality of life. Olfaction dysfunction is attributed to damage of olfactory nerve in the TC approach. In the TS approach, the superior trajectory frequently skirts the ethmoid sinuses and damages olfaction. In addition, excessive abrasion of the nasal mucosa also accounts for olfactory disturbance.
The risks of intracranial hemorrhage and endocrine disorders are not affected by the approach selection according to our result of data synthesis. The rate of intracranial hemorrhage is higher in the TC group with no significant statistical difference. Intracranial hemorrhage, which is extremely rare in a TS procedure, is generally considered as a craniotomy-specific complication. As the craniotomy skill improves, the risk of intracranial hemorrhage has been lowered to an acceptable level in clinical practice38. Endocrine disorders after resection of TSMs mainly consist of diabetes insipidus and hypopituitarism. The symptoms were transient or persistent requiring replacement therapy.
Study Limitations
The present study had several limitations. First, only 8 studies were included in the meta-analysis. More studies are warranted to compare the surgical results of different approaches. Second, the pooled data were all from retrospective studies with inherent selection and treatment bias. The evaluation of clinical symptoms, selection of patients, tumor size, and definition of gross total resection were methodologically variable among studies. Tumor location and extension, which were important for further stratification of results, were not well documented or balanced at baseline in all included studies. Thus, it is difficult to make a categorical conclusion for surgical route selection. Third, the time span of patient inclusion was long, ranging from 1983 to 2016, during which both the TS and TC techniques developed rapidly. In addition, as a novel technique, the learning curve existed in the early era of TS surgery. The above drawbacks may limit the application of our conclusions in clinical practice. Despite these limitations diminishing the comparability of the two surgical routes, to date, this meta-analysis provides the most convincing evidence in selection of surgical approach for TSMs. More prospective researches are needed to further determine the optimal use of the two approaches in specific patients.
Conclusions
Our findings recommend that the TS approach with minimal invasive features serves as a safe and effective alternative to the TC approach for TSM treatment in selected cases with small tumor size and limited optic canal invasion. The major risk of TS approach is CSF leakage, which is deemed to decrease in parallel with ongoing improvements of surgeon experiences and skull base reconstruction techniques.
Supplementary information
Author Contributions
X.B. designed the study and revised the article. C.Y. searched the articles, collected and analyzed the data, and drafted the manuscript. Y.F. analyzed the data and prepared all the figures and tables. Z.S. collected the data. R.W. revised the article. All authors approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-41292-0.
References
- 1.Gokalp HZ, Arasil E, Kanpolat Y, Balim T. Meningiomas of the tuberculum sella. Neurosurgical review. 1993;16:111–114. doi: 10.1007/BF00258241. [DOI] [PubMed] [Google Scholar]
- 2.Andrews BT, Wilson CB. Suprasellar meningiomas: the effect of tumor location on postoperative visual outcome. Journal of neurosurgery. 1988;69:523–528. doi: 10.3171/jns.1988.69.4.0523. [DOI] [PubMed] [Google Scholar]
- 3.Karsy M, Raheja A, Eli I, Guan J, Couldwell WT. Clinical Outcomes with Transcranial Resection of the Tuberculum Sellae Meningioma. World neurosurgery. 2017;108:748–755. doi: 10.1016/j.wneu.2017.09.090. [DOI] [PubMed] [Google Scholar]
- 4.Telera S, et al. Supraorbital keyhole approach for removal of midline anterior cranial fossa meningiomas: A series of 20 consecutive cases. Neurosurgical review. 2012;35:67–83. doi: 10.1007/s10143-011-0340-7. [DOI] [PubMed] [Google Scholar]
- 5.Mortazavi MM, et al. Planum Sphenoidale and Tuberculum Sellae Meningiomas: Operative Nuances of a Modern Surgical Technique with Outcome and Proposal of a New Classification System. World neurosurgery. 2016;86:270–286. doi: 10.1016/j.wneu.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 6.Makarenko S, Carreras EM, Akagami R. Craniotomy for perisellar meningiomas: comparison of simple (appropriate for endoscopic approach) versus complex anatomy and surgical outcomes. Journal of neurosurgery. 2017;126:1191–1200. doi: 10.3171/2016.3.jns152307. [DOI] [PubMed] [Google Scholar]
- 7.Gardner PA, et al. Endoscopic endonasal resection of anterior cranial base meningiomas. Neurosurgery. 2008;63:36–52. doi: 10.1227/01.NEU.0000335069.30319.1E. [DOI] [PubMed] [Google Scholar]
- 8.de Divitiis E, Cavallo LM, Esposito F, Stella L, Messina A. Extended endoscopic transsphenoidal approach for tuberculum sellae meningiomas. Neurosurgery. 2008;62:1192–1201. doi: 10.1227/01.neu.0000333785.04435.2c. [DOI] [PubMed] [Google Scholar]
- 9.Ceylan S, Koc K, Anik I. Extended endoscopic transphenoidal approach for tuberculum sellae meningiomas. Acta neurochirurgica. 2011;153:1–9. doi: 10.1007/s00701-010-0788-1. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhury FH, Haque MR, Goel AH, Kawsar KA. Endoscopic endonasal extended transsphenoidal removal of tuberculum sellae meningioma (TSM): an experience of six cases. British journal of neurosurgery. 2012;26:692–699. doi: 10.3109/02688697.2012.673648. [DOI] [PubMed] [Google Scholar]
- 11.Laufer I, Anand VK, Schwartz TH. Endoscopic, endonasal extended transsphenoidal, transplanum transtuberculum approach for resection of suprasellar lesions. Journal of neurosurgery. 2007;106:400–406. doi: 10.3171/jns.2007.106.3.400. [DOI] [PubMed] [Google Scholar]
- 12.Liu JK, Christiano LD, Patel SK, Shane Tubbs R, Eloy JeanAnderson. Surgical nuances for removal of tuberculum sellae meningiomas with optic canal involvement using the endoscopic endonasal extended transsphenoidal transplanum transtuberculum approach. Neurosurgical focus. 2011;30:E2. doi: 10.3171/2011.3.focus115. [DOI] [PubMed] [Google Scholar]
- 13.Soni RS, et al. From above or below: the controversy and historical evolution of tuberculum sellae meningioma resection from open to endoscopic skull base approaches. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2014;21:559–568. doi: 10.1016/j.jocn.2013.03.043. [DOI] [PubMed] [Google Scholar]
- 14.Bohman, L. E. et al. Decision analysis: Endoscopic versus open resection of tuberculum sellae meningiomas. Journal of Neurological Surgery, Part B: Skull Base73, 10.1055/s-0032-1312073 (2012).
- 15.de Divitiis E, Esposito F, Cappabianca P, Cavallo LM, de Divitiis O. Tuberculum sellae meningiomas: high route or low route? A series of 51 consecutive cases. Neurosurgery. 2008;62:556–563. doi: 10.1227/01.neu.0000317303.93460.24. [DOI] [PubMed] [Google Scholar]
- 16.Clark AJ, et al. Endoscopic surgery for tuberculum sellae meningiomas: A systematic review and meta-analysis. Neurosurgical review. 2013;36:349–359. doi: 10.1007/s10143-013-0458-x. [DOI] [PubMed] [Google Scholar]
- 17.Turel MK, et al. Endonasal endoscopic transsphenoidal excision of tuberculum sellae meningiomas: a systematic review. Journal of neurosurgical sciences. 2016;60:463–475. [PubMed] [Google Scholar]
- 18.Van Gompel JJ, et al. Expanded endonasal endoscopic resection of anterior fossa meningiomas: report of 13 cases and meta-analysis of the literature. Neurosurgical focus. 2011;30:E15. doi: 10.3171/2011.1.FOCUS118. [DOI] [PubMed] [Google Scholar]
- 19.Muskens IS, et al. The endoscopic endonasal approach is not superior to the microscopic transcranial approach for anterior skull base meningiomas—a meta-analysis. Acta neurochirurgica. 2018;160:59–75. doi: 10.1007/s00701-017-3390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graffeo CS, et al. A panoramic view of the skull base: systematic review of open and endoscopic endonasal approaches to four tumors. Pituitary. 2014;17:349–356. doi: 10.1007/s11102-013-0508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fatemi, N., Dusick, J. R., de Paiva Neto, M. A., Malkasian, D. & Kelly, D. F. Endonasal versus supraorbital keyhole removal of craniopharyngiomas and tuberculum sellae meningiomas. Neurosurgery64, 269–284; discussion 284–266, 10.1227/01.neu.0000327857.22221.53 (2009). [DOI] [PubMed]
- 22.Bowers CA, Altay T, Couldwell WT. Surgical decision-making strategies in tuberculum sellae meningioma resection. Neurosurgical focus. 2011;30:E1. doi: 10.3171/2011.2.focus1115. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/S0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 27.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong, D. S. et al. Selection of endoscopic or transcranial surgery for tuberculum sellae meningiomas according to specific anatomical features: a retrospective multicenter analysis (KOSEN-002). Journal of neurosurgery, 1–10, 10.3171/2017.11.jns171337 (2018). [DOI] [PubMed]
- 29.Linsler S, Fischer G, Skliarenko V, Stadie A, Oertel J. Endoscopic Assisted Supraorbital Keyhole Approach or Endoscopic Endonasal Approach in Cases of Tuberculum Sellae Meningioma: Which Surgical Route Should Be Favored? World neurosurgery. 2017;104:601–611. doi: 10.1016/j.wneu.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 30.Magill ST, et al. Tuberculum sellae meningiomas: grading scale to assess surgical outcomes using the transcranial versus transsphenoidal approach. Neurosurgical focus. 2018;44:E9. doi: 10.3171/2018.1.focus17753. [DOI] [PubMed] [Google Scholar]
- 31.Song SW, et al. Outcomes After Transcranial and Endoscopic Endonasal Approach for Tuberculum Meningiomas-A Retrospective Comparison. World neurosurgery. 2018;109:e434–e445. doi: 10.1016/j.wneu.2017.09.202. [DOI] [PubMed] [Google Scholar]
- 32.Kitano M, Taneda M, Nakao Y. Postoperative improvement in visual function in patients with tuberculum sellae meningiomas: results of the extended transsphenoidal and transcranial approaches. Journal of neurosurgery. 2007;107:337–346. doi: 10.3171/jns-07/08/0337. [DOI] [PubMed] [Google Scholar]
- 33.Kulwin C, Schwartz TH, Cohen-Gadol AA. Endoscopic extended transsphenoidal resection of tuberculum sellae meningiomas: nuances of neurosurgical technique. Neurosurgical focus. 2013;35:E6. doi: 10.3171/2013.8.focus13338. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa Y, Tominaga T. Extended transsphenoidal approach for tuberculum sellae meningioma-What are the optimum and critical indications? Acta neurochirurgica. 2012;154:621–626. doi: 10.1007/s00701-011-1266-0. [DOI] [PubMed] [Google Scholar]
- 35.Kotecha RS, et al. Meningiomas in children and adolescents: a meta-analysis of individual patient data. The Lancet Oncology. 2011;12:1229–1239. doi: 10.1016/s1470-2045(11)70275-3. [DOI] [PubMed] [Google Scholar]
- 36.Nimmannitya P, et al. Characteristic of optic canal invasion in 31 consecutive cases with tuberculum sellae meningioma. Neurosurgical review. 2016;39:691–697. doi: 10.1007/s10143-016-0735-6. [DOI] [PubMed] [Google Scholar]
- 37.Engelhardt J, et al. Contralateral Transcranial Approach to Tuberculum Sellae Meningiomas: Long-Term Visual Outcomes and Recurrence Rates. World neurosurgery. 2018;116:e1066–e1074. doi: 10.1016/j.wneu.2018.05.166. [DOI] [PubMed] [Google Scholar]
- 38.Morales-Valero SF, Van Gompel JJ, Loumiotis I, Lanzino G. Craniotomy for anterior cranial fossa meningiomas: historical overview. Neurosurgical focus. 2014;36:E14. doi: 10.3171/2014.1.FOCUS13569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.