Abstract
Protein tyrosine phosphatase, receptor type N2 (PTPRN2) encodes a major islet autoantigen in type-1 diabetes. Previous genetic studies have shown its significant association with obesity. PTPRN2 plays an important role in epigenetic regulation of metabolic diseases and cancers. We investigated CpG methylations (cg17429772 and cg158269415) in PTPRN2 by pyrosequencing from blood samples of childhood obesity (n = 638). cg158269415 had significant positive correlations with body mass index (BMI) and waist-hip ratio (WHR). Case-control analysis showed that cg158269415 methylation in blood sample was significantly more hypermethylated in obese cases (n = 252), an average of 2.93% more than that that in controls (n = 386). The cg158269415 methylation has a trimodal distribution pattern with strong dependency on nearby located rs1670344 G > A genotype. Correlations of cg158269415 with BMI and WHR were significant and strong in major G allele carriers (GG + GA). Our study showed that an epigenetic association of PTPRN2 gene with childhood obesity was under certain genetic background. The genetic and epigenetic interplay of PTPRN2 gene may implicate a mechanism of childhood obesity. Whether these small changes in DNA methylation from whole blood are causally or consequently related to childhood obesity outcome and their clinical relevance remains to be determined. However, this study presents a promising obesity risk marker that warrants further investigation.
Introduction
Obesity is a prevalent health problem affecting modern societies. It is particularly severe for children in developed countries. Overweight children are more likely to have risk factors of chronic diseases including cardiovascular disease and type 2 diabetes later in life than other children1.
Protein Tyrosine phosphatase, receptor type N2 (PTPRN2) encodes a major islet autoantigen in type-1 diabetes. It is localized to the membrane of insulin-containing dense-core vesicles2,3. PTPRN2 plays an important role in insulin secretion in response to glucose stimuli. Its specific substrates and binding partners suggest a variety of its function for metabolism including obesity and type 2 diabetes4,5.
Previous genetic linkage study of body mass index (BMI) has shown strong signals from 7q36.3. Sixty-five independent loci associated with obesity have been found (with q < 0.05) by targeted resequencing of chromosome 7q36.3 region in Northern Han Chinese, and twenty-two of them, which located at introns of PTPRN2, have significant associations with obesity6. Novel candidate loci including PTPRN2 associated with pediatric obesity have been identified by genome-wide copy number variation (CNV) analysis7. By investigating rare (<1%) CNV, PTPRN2 has been found to be a biologically relevant candidate gene for pediatric obesity.
PTPRN2 gene is epigenetically regulated in various biological processes including tumor pathogenesis8–12. Chen et al. have reported that different gene expression and DNA methylation of PTPRN2 in the human placenta are related to the intrauterine environment8. Prenatal growth patterns and birthweight are significantly associated with the epigenetic signature of PTPRN2 in human placentas. PTPRN2 is up-regulated in highly metastatic breast cancer cells9. It can enzymatically change activity in metastatic breast cancer cells and enhance cellular migration and metastatic capacity. Gentilini et al. have found that PTPRN2 is potentially involved in hepatocellular carcinoma pathogenesis by investigating genome-wide DNA methylation profile for 69 pairs of hepatocellular carcinoma cancer and adjacent non-cancerous liver tissues10. Hypomethylated PTPRN2 is significantly over-expressed in tumor tissue compared to that in normal liver tissue11. In naive CD4+ T cell, PTPRN2 cis-acting genetic variants account for some ethnicity-specific variability in DNA methylation12. PTPRN2 is hypomethylated in African-American lupus patients compared to European-American decent.
The objective of this study was to investigate epigenetic variations of the PTPRN2 gene by using target pyrosequencing of up to 638 Korean childhood obesity subjects. We examined obesity-related differentially methylated CpG site (cg158269415) by pyrosequencing to investigate their associations with obesity related traits. The potential role of epigenetic mechanism to individual risk of childhood obesity was explored and background genetic contribution for obesity specific CpG methylation was also determined. Our results revealed a crosstalk between genetic and epigenetic events of PTPRN2 gene in childhood obesity.
Experimental Procedures
All methods are approved by the committee of Korea National Institute of Health (KNIH) and carried out in accordance with relevant guidelines and regulations of KNIH.
Study subjects
Subjects of this study were selected from the Korean Child-Adolescent Cohort Study13–15. The overall objective of the cohort study was to identify early risk factors for obesity and associated metabolic diseases in Korean children. Obesity is defined as a BMI at or above the 95th percentile for children and teens of the same age and sex according to the recently proposed definition by the Centers for Disease Control and Prevention. Informed consent was obtained from children’s parents. The Institutional Review Board (IRB) of the Korea National Institute of Health approved this study (IRB approval number: 2014-08EXP-05-P-A). Detailed subject characteristics are presented in Table 1.
Table 1.
Characteristics of subjects with childhood obesity.
| Variables | Controls | Cases | P |
|---|---|---|---|
| Age | 13.93 ± 0.77 | 13.94 ± 0.84 | 0.6805 |
| N (M/F) | 386 (192/194) | 252 (138/114) | |
| BMI | 20.23 ± 2.58 | 32.69 ± 3.60 | <0.0001 |
| WHR | 0.77 ± 0.05 | 0.90 ± 0.06 | <0.0001 |
| FPG | 92.79 ± 7.03 | 94.93 ± 12.58 | <0.0001 |
| AST | 19.11 ± 3.98 | 27.10 ± 16.30 | <0.0001 |
| ALT | 12.85 ± 11.27 | 35.59 ± 33.94 | <0.0001 |
| TC | 158.05 ± 25.62 | 175.06 ± 28.29 | <0.0001 |
| TG | 73.14 ± 37.96 | 122.87 ± 65.98 | <0.0001 |
| HDL | 56.25 ± 10.25 | 46.12 ± 8.19 | <0.0001 |
| LDL | 87.17 ± 21.98 | 104.48 ± 24.95 | <0.0001 |
N(M/F): Number(Male/Female); BMI (kg/m2): Body Mass Index; WHR: Waist to Hip Ratio; FPG (mg/dL): Fasting Plasma Glucose; AST(IU/L): Aspartate transaminase; ALT(IU/L): alanine aminotransferase; TC (mg/dL): Total Cholesterol; TG (mg/dL): Triglyceride; HDL (mg/dL): High-density lipoproteins-Cholesterol. Raw P values < 0.05 are shown in bold.
Quantitation of CpG DNA methylation and genotyping by pyrosequencing analyses
Polymerase chain reactions (PCRs) of PTPRN2 were performed for the purpose of pyrosequencing16. PCR cycling conditions and sequences of all primers designed with MethPrimer (Biotage AB, Uppsala, Sweden) for cg17429172 and cg158269415 are shown in Fig. 1. Pyrosequencing was conducted using bisulfite treated DNA (Zymo Research, CA). Technical controls for pyrosequencing revealed median methylations of 0% ~ 10% (unmethylated control), and >90% (methylated control), thereby defining detection limits of EpiTect PCR control DNA set (QIAGEN, USA). Pyrosequencing assays were designed, optimized, and performed on a PSQ HS 96A Pyrosequencing System (Pyrosequencing, Westborough, MA, USA) according to the manufacturer’s specifications.
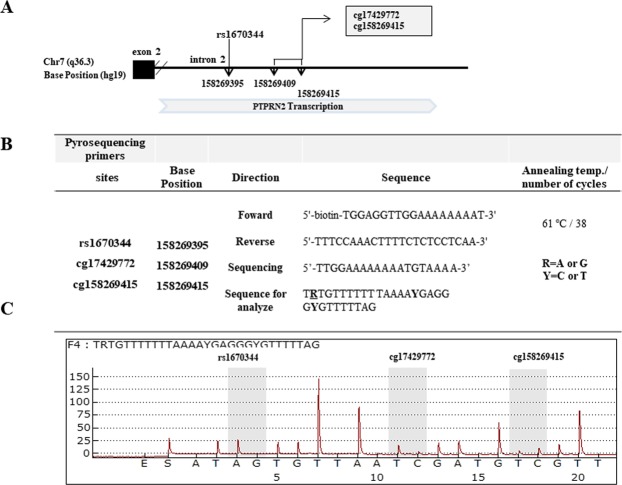
Figure 1.
Schematic diagrams of PTPRN2 locus and pyrosequencing. (A) Schematic diagrams of PTPRN2 locus with rs1670344 and two CpG sites (cg17429772 and cg158269415) in intron 2. The biological position of each site is indicated by arrows(hg19). (B) Primer sequences and PCR conditions for pyrosequencing. (C) Pyrogram for the experiment.
With one assay, quantitation of cg17429772 and cg158269415 was performed with rs1670344 genotyping. Genomic position and gene positions of these two target CpG sites and rs1670344 were analyzed via pyrosequencing. Results are described in Fig. 1. These two CpG methylation sites (cg17429772 and cg158269415) are located downstream from rs1670344 (cg17429772: 14 bp and cg158269415: 20 bp). Finally, we measured DNA methylations in 638 samples (controls, n = 386; cases, n = 252). Pyrosequencing with genotyping was performed using bisulfite-treated DNA. We determined genotyping and CpG methylations at the same time.
Statistical analyses
Independent sample t-tests were used to compare physical and metabolic characteristics between control and obese case groups. Data are presented as mean ± standard deviation of the mean for ease of interpretation. Multiple regression analyses were performed after adjusting for age and sex.
Statistical analysis for DNA methylation was performed by non-parametric methods because of its bimodal data distribution. Wilcoxon signed-rank test, Kruskal–Wallis test, and Spearman’s correlation test were adopted. Age and sex were used as covariates for the study performed with regression analysis. We used R scripts for all other analytical and graphic processing. Data were visualized using ggplot2 (http://www.r-project.org/).
Results
General characteristics of obese children with age- and sex-matched controls are summarized in Table 1. We categorized children (n = 252) with an average body mass index (BMI) of 32.69 ± 3.60 as obese and those (n = 386) with an average BMI of 20.23 ± 2.58 as lean controls. There was no significant difference in age (P > 0.05) between the two groups. Table 1 also shows averages of BMI, waist-hip-ratio (WHR), fasting glucose levels (FPG), hepatotoxicity measurements, AST (aspartate transaminase), and ALT (alanine aminotransferase), lipid, TC (total cholesterol), TG (triglyceride), HDL (high-density lipoprotein-cholesterol), and LDL (low-density lipoproteins-cholesterol) levels, including significant differences between obese children and controls.
CpG methylation of childhood obesity
Two CpG methylation sites, cg17429772 and cg158269415, are located at intron 2 of PTPRN2. We examined these two CpG sites by pyrosequencing using bisulfite-treated DNA. cg17429772 is located six bases after cg158269415. It was named by chromosome base pair location (hg19). Table 2 shows average distributions of these two CpG methylations in all subjects with childhood obesity (n = 638). Only cg158269415 was particularly differentially methylated between control and obese groups. The methylation was 2.93% higher in cases. It was significantly associated with childhood obesity (P = 0.004).
Table 2.
CpG methylations of PTPRN2 gene in children with childhood obesity.
| CpG sites | Position | methylation % of Controls (n = 386) | methylation % of Cases (n = 252) | P |
|---|---|---|---|---|
| cg17429772 | chr7:158269409 | 54.19 ± 11.5 | 54.05 ± 11.22 | 0.85 |
| cg158269415 | chr7:158269415 | 21.13 ± 15.21 | 24.06 ± 14.17 | 0.004 |
Methylation percentage values are indicated by mean ± SD, P values < 0.05 are described in bold. Position: base positions.
To investigate the potential biological significance of cg158269415 methylation, we interrogated CpG methylation with obesity related traits (n = 638). Results of linear regression analysis with beta and P values of the methylation of cg158269415 with nine obesity related traits (BMI, WHR, FPG, AST, ALT, TC, TG, HDL, and LDL) are listed by totals, controls, and cases (Table 3). Age and sex are used as covariates for regression analysis.
Table 3.
Linear regression analysis of cg158269415 methylation with obesity related traits.
| Traits | Total subjects (n = 638) | Control subjects (n = 386) | Case subjects (n = 252) | |
|---|---|---|---|---|
| BMI (kg/m2) | beta | 0.04452 | −0.016610 | 0.05306 |
| P | 0.0355 | 0.04839 | 0.0002972 | |
| WHR | beta | 0.0003438 | −0.0003633 | 0.0005827 |
| P | 1.924e-08 | <2.2e-16 | 2.237e-08 | |
| FPG (mg/dL) | beta | −0.003988 | 0.02541 | −0.07162 |
| P | 2.555e-05 | 9.788e-06 | 0.06202 | |
| AST (IU/L) | beta | 0.07789 | −0.00276 | 0.17742 |
| P | 4.274e-08 | 1.322e-05 | 6.276e-05 | |
| ALT (IU/L) | beta | 0.17692 | −0.04406 | 0.4342 |
| P | 1.113e-06 | 0.02282 | 5.324e-06 | |
| TC (mg/dL) | beta | 0.07938 | −0.01786 | 0.1567 |
| P | 0.000319 | 2.95e-07 | 0.2636 | |
| TG (mg/dL) | beta | 0.1800 | −0.1078 | 0.2467 |
| P | 0.048 | 0.03833 | 0.1714 | |
| HDL (mg/dL) | beta | −0.006262 | 0.05648 | −0.02699 |
| P | 0.01464 | 0.009061 | 0.4422 | |
| LDL (mg/dL) | beta | 0.04963 | −0.05277 | 0.1344 |
| P | 0.006204 | 4.32e-06 | 0.08108 | |
BMI (kg/m2): Body Mass Index; FPG (mg/dL): Fasting Plasma Glucose; WHR: Waist to Hip Ratio; AST(IU/L): Aspartate transaminase; ALT(IU/L): alanine aminotransferase; TC (mg/dL): Total Cholesterol; TG (mg/dL): Triglyceride; HDL (mg/dL): High-density lipoproteins-Cholesterol; LDL(mg/dL): Low-density lipoproteins-Cholesterol. Raw P values < 0.05 are described in bold characters. Age and sex are used as covariates.
The cg158269415 methylation was significantly correlated with all nine obesity-related traits in total and control groups (P < 0.05), but only significant with BMI, WHR, AST, and ALT in the case group (P < 0.05). These correlations were positive for seven of nine traits, excluding FPG and HDL. For every 1% increase in cg158269415 in blood cells, BMI increased by 0.04 kg/m2 in total subjects, −0.02 kg/m2 in the control group, and 0.05 kg/m2 in the case group. WHR increased by 0.0003 in the total subjects, −0.0003 in the control group, and 0.0005 in the case group for every 1% increase in cg158269415.
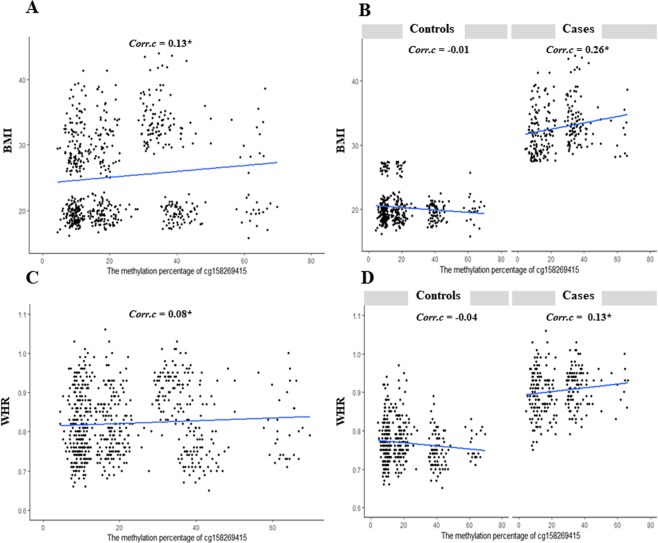
We graphed correlations of cg158269415 with BMI (Fig. 2AB) and WHR (Fig. 2CD) for the total subjects (Fig. 2AC) and control-case groups (Fig. 2BD). cg158269415 methylation was significantly correlated with BMI (Spearman test, Corr.c = 0.13*) and WHR (Spearman test, Corr.c = 0.08*) in total subjects. In control and case groups, correlation directions for BMI and WHR were different: negative in controls but positive in case subjects. Correlation directions of cg158269415 with BMI and WHR were significantly different depending on the group. Correlations were insignificantly negative in the control group, but significantly positive in the case group (Spearman test).
Figure 2.
Correlations of cg158269415 with (A) BMI in total subjects, (B) BMI in controls and obese cases, (C) WHR in total subjects, and (D) WHR in controls and obese cases. The correlation line is graphed. Spearman correlation coefficients (Corr.c) are indicated at the top right with statistical significance. P < 0.05 is marked with a star (*).
Relation of rs1670344 genotype with cg158269415 methylation
Mechanistic studies for the association of cg158269415 methylation with obesity related traits were performed by investigating nearby rs1670344 genotype which might be important for epigenetic regulation. We conducted pyrosequencing with genotyping of rs1670344 at the same time of CpG methylation measurement. We found 54.4% of GG, 40.4% of AG, and 5.2% of AA genotypes in the total of 638 subjects. By analyzing 386 controls and 252 obese cases, rs1670344 was not associated with childhood obesity, with an odd ratio of 0.98 (raw P values > 0.05). By linear regression analysis with age and sex as covariates, rs1670344 had significant associations with WHR (beta = −0.0001, P = 5.73e-08), FPG (beta = −0.07, P = 2.52e-05), AST (beta = 1.11, P = 3.068e-07), ALT (beta = 2.13, P = 1.486e-05), TC (beta = 3.09, P = 1.518e-04), HDL (beta = 0.6826, P = 9.597e-03), and LDL (beta = 2.328, P = 3.252e-03). However, it had no significant association with BMI (beta = 0.09, P > 0.05) or TG (beta = 0.42, P > 0.05). Correlations were positive for seven of the nine traits, excluding WHR and FPG. Logistic and linear regression analyses showed no significant association of rs1670344 with BMI.
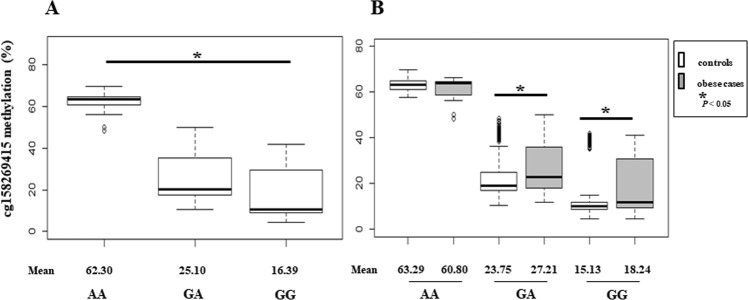
To investigate genetic and epigenetic interactions in PTPRN2 gene, we analyzed rs1670344 and cg158269415 genotypes in total, control, and case subjects. Figure 3 shows boxplots of relationships of cg158269415 methylations with genotype. The relationship of CpG methylation with rs1670344 genotypes (GG: homozygous for the major allele, GA: heterozygous, AA: homozygous for the minor allele) is graphed in Fig. 3A. cg158269415 methylation had trimodal distribution patterns that depended on the genotype. The cg158269415 methylation was low at GG, middle at GA, and high at AA (Kruskal–Wallis test, P < 0.05). Changes of cg158269415 methylation between AA and GG genotypes were 45.91% on average. Mean values of cg158269415 methylations are indicated under the graph.
Figure 3.
Boxplots of cg158269415 methylation of childhood obesity (n = 638). (A) cg158269415 methylations by rs1670344 genotype. AA: homozygous minor genotype; GA: heterozygous genotype; GG: homozygous major genotype. (B) cg158269415 methylations between controls and obese cases by rs1670344 genotype. Gray boxes indicate obese cases. P values < 0.05 are marked with a star (*).
cg158269415 methylations in controls and obese cases are graphed by each genotype (Fig. 3B). The degree of CpG methylation of obese cases was significantly higher than that of controls by an average of 3.46% at GA (Wilcoxon test, P < 0.05), 3.11% at GG (Wilcoxon test, P < 0.05), and −2.49% at AA genotype (Wilcoxon test, P > 0.05) (Fig. 3B). Finally, cg158269415 methylations in whole blood cells were strongly associated with childhood obesity under certain genetic background of rs1670344.
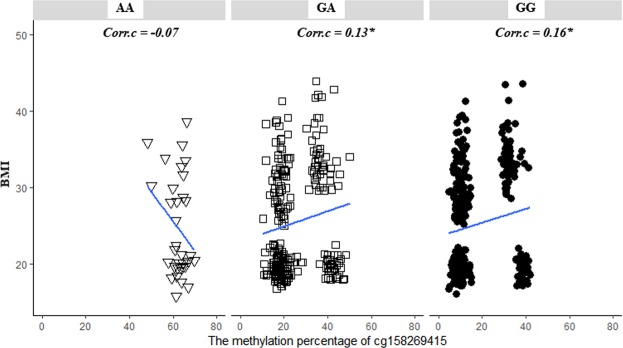
Our data showed that the correlation of cg158269415 with BMI was different depending on subjects’ rs1670344 genotype. Therefore, we graphed cg158259415 methylations with BMI based on each genotype (Fig. 4). Correlation coefficients for each genotype (AA, AG, GG) are described at the top. Statistic significances with P < 0.05 are marked with a star (Spearman test). cg158269415 had a bimodal distribution pattern in GA and GG groups, but not in AA group. Correlation directions and strengths differed depending on genotypes. In AA genotype, cg158269415 showed negative correlation with BMI (Spearman test, Corr.c = −0.07). However, the correlation was positive in major G allele carriers (Spearman test, Corr.c = 0.13* in GA and 0.16* in GG).
Figure 4.
Correlations of cg158269415 methylation with BMI by rs1670344 genotype. AA: homozygous minor genotype, GA: heterozygous genotype, GG: homozygous major genotype. Correlation lines fitted on graphs with spearman correlation coefficients (Corr.c) indicated in the top of each graph. Statistic significances with P value < 0.05 are marked with a star (*).
Discussion
Results of this study showed that cg158269415 was significantly hypermethylated in the blood of obese children. This study is the first to validate the association of cg158269415 methylation with childhood obesity. Interestingly, the significance and strength of correlations with BMI were different between control and case groups. The correlation of cg158269415 methylation with BMI was strong and significant in case group, suggesting that cg158269415 hypermethylation of obese children might not be directly functional or causal. It might be epigenetic mark left by another process. In addition to BMI and WHR, cg158269415 methylation had positive correlations with five biochemical traits (AST, ALT, TC, TG, and LDL), but negative correlations with two traits (FPG and HDL). DNA methylation associations with BMI may be driven by co-associations with other factors such as hyperlipidemia (TC, TG and LDL).
Previous BMI-related CpG methylation studies in blood showed that increased methylation at the CpG sites located at the HIF3A locus is associated with increases or changes in BMI15,17,18. The average of the difference in BMI-related CpG methylation between lean and obese children was found to be less than 3% by pyrosequencing or by Sequenom’s MassARRAY. cg158269415 of PTPRN2 was hypermethylated by an average of 2.93% in cases. Whether this small changes in DNA methylation from whole blood are causally or consequently related to childhood obesity outcome remains to be determined.
The rs1670344 is located in intron 2. Its functional consequences are currently unclear. The genetic variant, rs1670344, had close effects on nearby obesity-associated cg158269415. This likely reflects the regulation of PTPRN2 gene by epigenetic process. rs1670344 was significantly associated with obesity-related traits and cg158259415 methylation, but not directly with BMI. Our case-control and regression study showed no genetic association of rs1670344 with BMI. rs1670344 genotype might have triggered epigenetic changes of closely located cg158269415 or other CpG sites that had a dynamic role in gaining weight. Environmental factors also may drive epigenetic perturbation of PTPRN2 based on the rs1670344 genetic background.
The correlation of cg158259415 methylation with BMI was different according to each genotype of rs1670344. It was especially significant and strong in major G allele carriers (GG and GA group). High and positive correlation of CpG methylation with BMI might have come from G allele specific which had bimodal methylation patterns. In AA group, the average of cg158269415 methylation was 62.45% whereas it was 16.39% in GG group. Hypermethylation of cg158269415 in AA group might have a small room for additional DNA methylation by environmental factors.
We investigated differential cg158269415 methylation in blood for obesity. However, the effect of epigenetic perturbation of PTPRN2 remains to be determined depending on cell type. Obesity is an important component of the pathophysiology of immune diseases. Obesity specific cg158269415 in peripheral blood may come from obesity derived from different immune-related functions or immune cell composition in blood between lean and obese children. Epigenome-wide analysis of obesity in peripheral blood cells has shown that dysregulated DNA methylation is associated with inflammation and immune disease12,19,20. Especially, the differential methylation of PTPRN2 in naive CD4+ T cell was associated with autoimmune disease, which suggested its role for immune response12.
cg158269415 methylation was also significantly associated with FPG. The correlation of cg158269415 methylation with FPG was significant and negative (beta = −0.004, P = 2.55e-05). rs1670344 also had significant correlation with FPG (beta = −0.07, P = 2.52e-05). PTPRN2 as a major autoantigen in insulin-dependent diabetes mellitus may explain its genetic and epigenetic associations with FPG. PTPRN2 shares >90% sequence identities with rat phogrin and mouse Islet antigen 2 beta (IA-2 beta)21. IA-2 beta (−/−) knockout mice show mild glucose intolerance and impaired glucose-stimulated insulin secretion22.
In general, a genetic context of the PTPRN2 locus might be important for epigenetic modification of cg158269415 in high-BMI children. Further analysis of these newly-identified genetic and epigenetic factors may be necessary to explain where and how these sophisticated DNA methylation changes come from.
Acknowledgements
This work was supported by intramural grants (2014-NI73002-00, 2014-ND73001-00, 2016-NI73004-00, and 2017-NI73003-00) from the Korean National Institute of Health.
Author Contributions
S.L. conceived this study and secured funding. S.L. analyzed the data and wrote the manuscript.
Competing Interests
The author declares no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weihrauch-Bluher S, Wiegand S. Risk Factors and Implications of ChildhoodObesity. Curr Obes Rep. 2018;7:254–259. doi: 10.1007/s13679-018-0320-0. [DOI] [PubMed] [Google Scholar]
- 2.Bian X, et al. Tracking the Antibody Immunome in Type 1 Diabetes Using Protein Arrays. J Proteome Res. 2017;16:195–203. doi: 10.1021/acs.jproteome.6b00354. [DOI] [PubMed] [Google Scholar]
- 3.Carmona GN, Nishimura T, Schindler CW, Panlilio LV, Notkins AL. The dense core vesicle protein IA-2, but not IA-2beta, is required for active avoidance learning. Neuroscience. 2014;269:35–42. doi: 10.1016/j.neuroscience.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui L, Yu WP, DeAizpurua HJ, Schmidli RS, Pallen CJ. Cloning and characterization of islet cell antigen-related protein-tyrosine phosphatase (PTP), a novel receptor-like PTP and autoantigen in insulin-dependent diabetes. J Biol Chem. 1996;271:24817–24823. doi: 10.1074/jbc.271.40.24817. [DOI] [PubMed] [Google Scholar]
- 5.Bence, K. K. Protein tyrosine phosphatase control of metabolism. (Springer, 2013).
- 6.Wu Y, Wang W, Jiang W, Yao J, Zhang D. An investigation of obesity susceptibility genes in Northern Han Chinese by targeted resequencing. Medicine (Baltimore) 2017;96:e6117. doi: 10.1097/MD.0000000000006117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selvanayagam T, et al. Genome-wide copy number variation analysis identifies novel candidate loci associated with pediatric obesity. Eur J Hum Genet. 2018;26:1588–1596. doi: 10.1038/s41431-018-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen PY, et al. Prenatal Growth Patterns and Birthweight Are Associated With Differential DNA Methylation and Gene Expression of Cardiometabolic Risk Genes in Human Placentas: A Discovery-Based Approach. Reprod Sci. 2018;25:523–539. doi: 10.1177/1933719117716779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sengelaub CA, Navrazhina K, Ross JB, Halberg N, Tavazoie SF. PTPRN2 and PLCbeta1 promote metastatic breast cancer cell migration through PI(4,5)P2-dependent actin remodeling. EMBO J. 2016;35:62–76. doi: 10.15252/embj.201591973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gentilini D, et al. Epigenome-wide association study in hepatocellular carcinoma: Identification of stochastic epigenetic mutations through an innovative statistical approach. Oncotarget. 2017;8:41890–41902. doi: 10.18632/oncotarget.17462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen J, et al. Integrative epigenomic and genomic filtering for methylation markers in hepatocellular carcinomas. BMC Med Genomics. 2015;8:28. doi: 10.1186/s12920-015-0105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coit P, et al. Ethnicity-specific epigenetic variation in naive CD4+ T cells and the susceptibility to autoimmunity. Epigenetics Chromatin. 2015;8:49. doi: 10.1186/s13072-015-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo S, Lee SY, Kim KN, Sung E. Obesity in Korean pre-adolescent school children: comparison of various anthropometric measurements based on bioelectrical impedance analysis. Int J Obes (Lond) 2006;30:1086–1090. doi: 10.1038/sj.ijo.0803327. [DOI] [PubMed] [Google Scholar]
- 14.Koh IU, Lee HJ, Hwang JY, Choi NH, Lee S. Obesity-related CpG Methylation (cg07814318) of Kruppel-like Factor-13 (KLF13) Gene with Childhood Obesity and its cis-Methylation Quantitative Loci. Sci Rep. 2017;7:45368. doi: 10.1038/srep45368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S, et al. Positive correlation of cg16672562 methylation with obesity-related traits in childhood obesity, and its independence with underlying HIF3A (hypoxia-inducible factor 3a) genetic background. Oncotarget. 2017;8:67473–67481. doi: 10.18632/oncotarget.18707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song MA, et al. Quantification of CpG methylation at the 5′-region of XIST by pyrosequencing from human serum. Electrophoresis. 2007;28:2379–2384. doi: 10.1002/elps.200600852. [DOI] [PubMed] [Google Scholar]
- 17.Dick, K. J. et al. DNA methylation and body-mass index: a genome-wide analysis. Lancet (2014). [DOI] [PubMed]
- 18.Wang S, et al. HIF3A DNA Methylation Is Associated with Childhood Obesity and ALT. Plos One. 2016;10:e0145944. doi: 10.1371/journal.pone.0145944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demerath EW, et al. Epigenome-wide association study (EWAS) of BMI, BMI change and waist circumference in African American adults identifies multiple replicated loci. Hum Mol Genet. 2015;24:4464–4479. doi: 10.1093/hmg/ddv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rastogi D, Suzuki M, Greally JM. Differential epigenome-wide DNA methylation patterns in childhood obesity-associated asthma. Sci Rep. 2013;3:2164. doi: 10.1038/srep02164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawasaki E, Hutton JC, Eisenbarth GS. Molecular cloning and characterization of the human transmembrane protein tyrosine phosphatase homologue, phogrin, an autoantigen of type 1 diabetes. Biochem Biophys Res Commun. 1996;227:440–447. doi: 10.1006/bbrc.1996.1526. [DOI] [PubMed] [Google Scholar]
- 22.Cai T, et al. Deletion of Ia-2 and/or Ia-2beta in mice decreases insulin secretion by reducing the number of dense core vesicles. Diabetologia. 2011;54:2347–2357. doi: 10.1007/s00125-011-2221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]