Abstract
Nickel norcorrole provides an unusual example of a molecule that is strongly antiaromatic according to the magnetic criterion, but which exhibits, according to high-quality DFT calculations, a symmetric, delocalized structure with no difference in bond length between adjacent Cmeso-Cα bonds. A fragment molecular orbital analysis suggests that these discordant observations are a manifestation of the high stability of the dipyrrin fragments, which retain their electronic and structural integrity even as part of the norcorrole ring system.
Introduction
Over a dozen years ago, one of us conceptualized norcorrole (H2Nc) as the smallest, realistic, fully conjugated tetrapyrrole ring system as part of a theoretical exercise (Fig. 1)1. Among the more notable conclusions of the study, which employed standard density functional theory (DFT) methods, was that nickel norcorrole (NiNc) should exhibit a slightly domed, but otherwise fully symmetric structure. In other words, the calculations did not indicate any difference in bond length between adjacent Cmeso-Cα bonds, as is typically observed for antiaromatic porphyrinoids2–4. Within a few years thereafter, norcorrole was experimentally realized by Bröring and coworkers in the form of an iron(III)-iodido complex5. Subsequently, Kobayashi, Shinokubo, and their coworkers reported a gram-scale synthesis of a nickel meso-diarylnorcorrole, clearing the path for wide-ranging investigations of the norcorrole derivatives6. Interestingly, the X-ray structure reported by these authors (CCDC: YEQKUC) revealed a planar macrocycle geometry with significant bond length alternation6. Furthermore, the experimental 1H NMR spectra and nucleus independent chemical shift (NICS) calculations clearly implicated NiNc as an antiaromatic system6. Together, these findings pose an interesting conundrum: although NiNc is antiaromatic according to the magnetic criterion, DFT geometry optimizations indicate a symmetric structure with little or no bond length alternation. While chemical theory does not rule out such a system, delocalized, antiaromatic systems are virtually unknown among real molecules. Presented herein is a detailed DFT investigation aimed at establishing the true equilibrium geometry of NiNc.
Figure 1.
Free-base (H2Nc, left) and nickel norcorrole (NiNc, right).
Results and Discussion
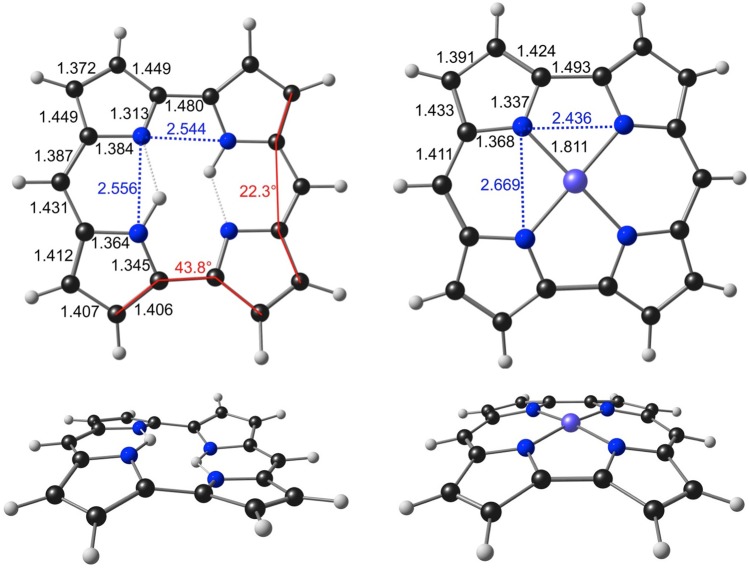
Geometry optimizations with a variety of exchange-correlation functionals reproduced all key geometrical features noted in our earlier study1. Figure 2 depicts highlights of the B3LYP7–9 -D310/STO-TZ2P optimized geometries. The energy minima turned out to be a Ci-symmetric wave conformation for H2Nc11 and a C2v-symmetric dome conformation for NiNc. The planar D2h form of NiNc, just 0.03 eV (0.7 kcal/mol) higher in energy relative to the C2v minimum, was found to correspond to the transition state for the bowl inversion process. The optimized geometry parameters for NiNc are in excellent agreement with the X-ray structures of several Ni meso-diarylnorcorrole derivatives (including CALQIS, CALQOY, CALQUE12; YAFSAC, YAFSEF13; REMGOI14; Table 1). These structures are either planar or slightly domed, consistent with a soft doming potential, and exhibit minimal bond length alternations, in particular minimal differences (<0.02 Å) between adjacent Cmeso-Cα bonds. The X-ray structures of certain other NiNc derivatives (YEQKUC6; CALRAL12; MUJTIW, MUJTOC15, on the other hand, exhibit larger differences (>0.04 Å) between adjacent Cmeso-Cα bonds. To this list may be added a slightly saddled CuNc (YEHTOX)11 and a strongly domed PdNc (YEHTEN)11 structure, which exhibit an intermediate difference (~0.03 Å) between adjacent Cmeso-Cα bonds. Significant bond localization has been observed for several NiNc derivatives with strongly conjugating β-substituents such as cyano15, nitro16 or amino17; these systems are not within the purview of the present study. The overall body of results strongly suggests that although NiNc has a symmetric, delocalized global minimum, bond alternation does correspond to a soft distortion and may manifest itself for certain substitution patterns and crystal environments.
Figure 2.
Selected B3LYP-D3/STO-TZ2P geometry parameters (Å, deg) of H2Nc (left) and NiNc (right).
Table 1.
Selected crystallographic geometry parameters (Å) for relevant norcorrole derivatives.
| Refcode | Compound | d(M-N)meana | d(N-N)||b | d(N-N)⊥c | D(Cα-Cm)mean d | D(Cα-N)mean e | geometry | Metal | Ref |
|---|---|---|---|---|---|---|---|---|---|
| YEHTEN | Palladium meso,meso’-dimesitylnorcorrole | 1.915 | 2.533 | 2.837 | 0.035 | 0.011 | domed | Pd | 11 |
| YEHTOX | Copper meso,meso’-dimesitylnorcorrole | 1.789 | 2.427 | 2.628 | 0.019 | 0.007 | slightly saddled | Cu | 11 |
| CALQIS | Nickel meso-(4-dimethylaminophenyl)-meso’-phenylnorcorrole | 1.783 | 2.419 | 2.618 | 0.007 | 0.004 | slightly domed | Ni | 12 |
| CALQOY | Nickel meso-(4-dimethylaminophenyl)-meso’-phenylnorcorrole | 1.782 | 2.427 | 2.610 | 0.003 | 0.003 | planar | Ni | 12 |
| CALQUE | Nickel meso-(4-cyanophenyl)-meso’-(4-dimethylaminophenyl)norcorrole | 1.771 | 2.423 | 2.583 | 0.000 | 0.000 | planar | Ni | 12 |
| CALRAL | Nickel meso-[3,5-bis(trifluoromethyl)phenyl]-meso’-(4-dimethylaminophenyl)norcorrole | 1.783 | 2.422 | 2.610 | 0.049 | 0.018 | domed | Ni | 12 |
| CALRAL | Nickel meso-[3,5-bis(trifluoromethyl)phenyl]-meso’-(4-dimethylaminophenyl)norcorrole | 1.780 | 2.422 | 2.611 | 0.033 | 0.009 | planar | Ni | 12 |
| CALREP | Nickel meso-(4-dimethylaminophenyl)-meso’-pentafluorophenylnorcorrole, dichloromethane solvate | 1.786 | 2.425 | 2.624 | 0.006 | 0.021 | planar | Ni | 12 |
| YAFSAC | Nickel meso,meso’-diphenylnorcorrole | 1.789 | 2.424 | 2.614 | 0.019 | 0.008 | domed | Ni | 13 |
| YAFSAC | Nickel meso,meso’-diphenylnorcorrole | 1.779 | 2.419 | 2.609 | 0.019 | 0.013 | slightly waved | Ni | 13 |
| YEKQUC | Nickel meso,meso’-dimesitylnorcorrole | 1.779 | 2.406 | 2.622 | 0.044 | 0.015 | planar | Ni | 6 |
| REMGOI | Nickel 3-(4-dimethylaminophenyl)-5,14-dimesitylnorcorrole), dichloromethane solvate | 1.783 | 2.415 | 2.621 | 0.006 | 0.008 | slightly domed | Ni | 14 |
aaverage M-N distance; bN…N distances parallel to direct pyrrole-pyrrole bonds; cN…N distances perpendicular to direct pyrrole-pyrrole bonds; ddifference in length between adjacent Cα-Cmeso bonds, averaged over the whole molecule; edifference in length between adjacent Cα-N bonds, averaged over the whole molecule.
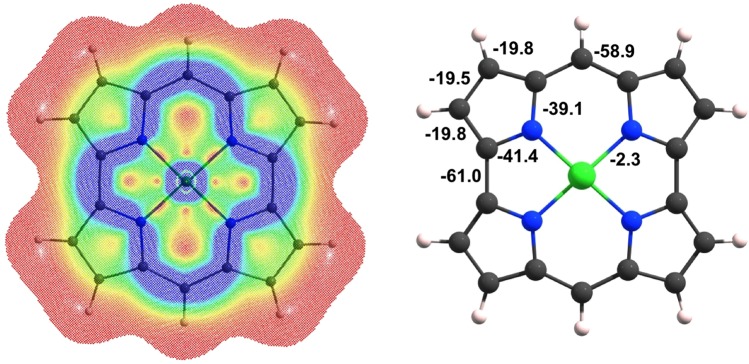
To investigate the question of magnetic antiaromaticity of NiNc, we visualized the magnetically induced current density profile (as previously done for a variety of porphyrin, hydroporphyrin18, carbaporphyrin19, corrole, and isocorrole20 derivatives) and also calculated the bond magnetizabilities21,22 at the B3LYP/def2-TZVP23 level (Fig. 3). Furthermore, we decomposed both the current intensities and the bond magnetizabilities into σ and π components (Table 2). According to the quantum theory of atoms in molecules (QTAIM)24, the total magnetizability of a closed-shell molecule can be decomposed into atomic and bond magnetizabilities, with the latter providing an indirect measure of the total current density flux through the interatomic surface between two neighboring atoms25. Keith and Bader showed that, unlike for aliphatic chains, the atomic and bond magnetizabilities of benzene are significantly anisotropic; the out-of-plane components of the magnetizabilities were found to be about three times larger than the in-plane components26,27. In a series of papers, one of us demonstrated that the out-of-plane bond magnetizability provides a safe index for assessing aromaticity even in complicated cases21,22,28,29, where simple probes such as nucleus independent chemical shift (NICS) and its variants fail30. Table 2 shows that the π-framework of NiNc sustains a strong paratropic electronic current (which is associated with positive bond magnetizabilities) that offsets a much smaller diatropic current along the σ–framework. The strong paratropic current is a clear indication of magnetic antiaromaticity of NiNc.
Figure 3.
Left: Current density profile of NiNc for a magnetic field applied along the C2 axis of symmetry depicted 1 bohr above the ring plane. Red to blue colors represents weak (0.0 au) to strong (0.001 au) current densities. Right: Integrated current intensities (nA/T).
Table 2.
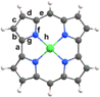
Out-of-plane bond magnetizabilities (, cgs-ppm) and magnetically induced current intensities (MICI, nA/T) for NiNc. See diagram in the leftmost column for definition of bonds a-h.
| a | b | c | d | e | f | g | h | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
Total | +23.4 | +5.1 | +8.6 | +5.4 | +22.2 | +9.3 | +15.5 | +20.7 | |
| σ | −6.8 | −4.8 | −3.1 | −4.7 | −5.7 | −3.9 | −5.2 | −5.7 | ||
| π | +30.2 | +9.9 | +11.7 | +10.1 | +27.9 | +13.2 | +20.7 | +26.4 | ||
| MICI | Total | −61.0 | −19.8 | −19.5 | −19.8 | −58.9 | −39.1 | −41.4 | −2.3 | |
| σ | +6.3 | +3.6 | +11.8 | +4.5 | −0.4 | +2.3 | +4.8 | +0.3 | ||
| π | −67.3 | −23.4 | −31.3 | −24.3 | −58.5 | −42.4 | −46.2 | −2.6 |
As noted above, nothing in chemical theory actually rules out a delocalized structure for an antiaromatic system. Whether bond localization will occur in a given case depends on the relative importance of π and σ distortivities of the system31–33. Typically, for antiaromatic systems, the former wins out2–4. The exceptional nature of NiNc in this regard is perhaps best appreciated in terms of the great stability of the two dipyrrin anion (dipy) fragments that make up the molecule. Such an interpretation is fully in line with a fragment molcular orbital (MO) analysis, which we carried out for the planar D2h complex MgNc. As shown in Fig. 4, all 12 occupied π MOs of MgNc may be regarded as bonding and antibonding combinations of the 6 occupied π MOs of the Mg(dipy)F fragments. This statement is actually not a trivial one, the key implication being that none of the occupied π MOs of MgNc owes its origin to any of the unoccupied π MOs of the Mg(dipy)F fragments. The two dipyrrin halves thus largely maintain their electronic integrity as part of the norcorrole macrocycle. Notably, such an interpretation is consistent with elementary notions of organic functional groups: as vinylogous amidinates, dipyrrin anions are indeed expected to resist structural distortions such as double bond localization.
Figure 4.
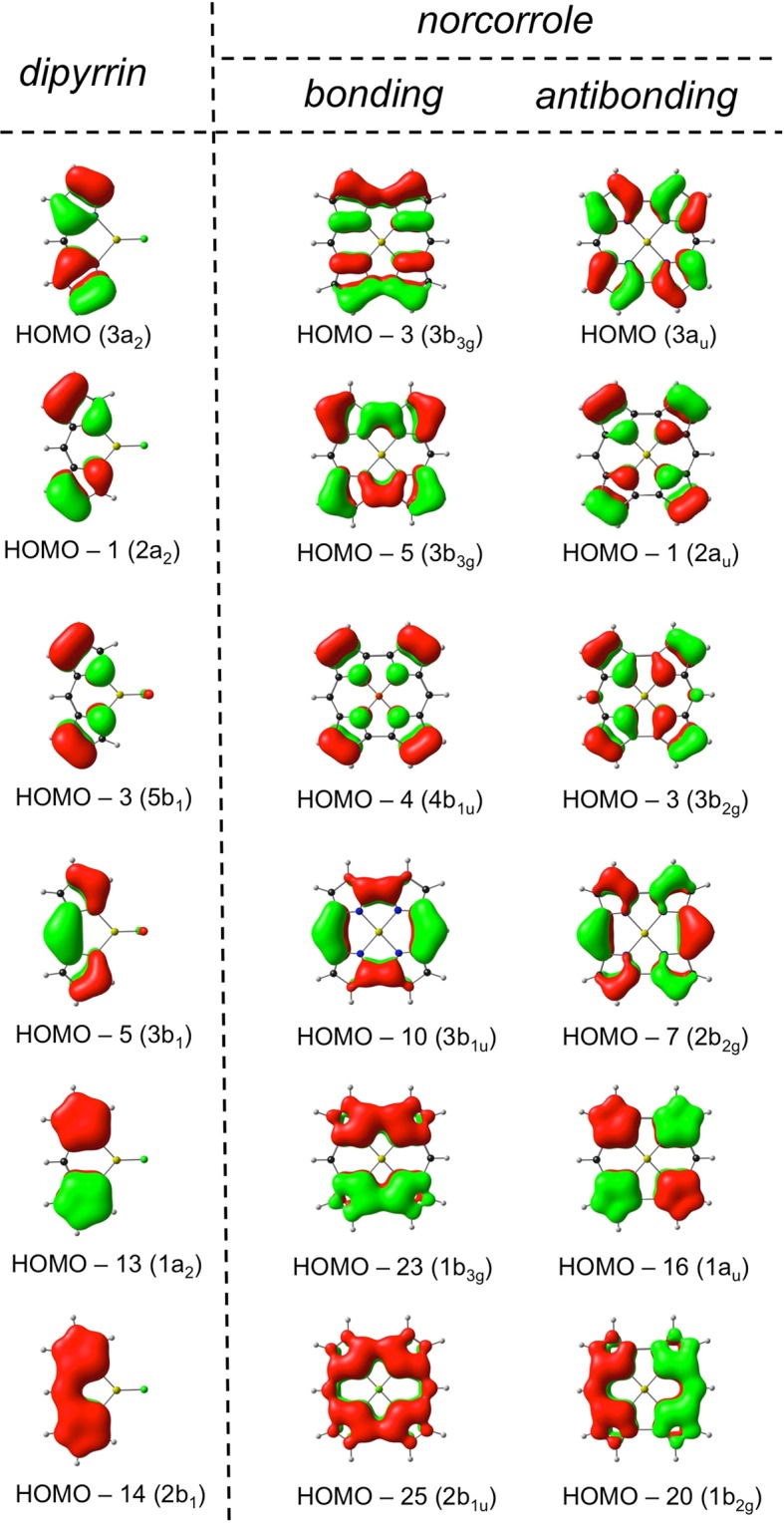
Fragment MO analysis of MgNc in terms of those of Mg(dipy)F.
Conclusion
Our knowledge of antiaromatic porphyrinoid systems has deepened greatly in recent years. Thus, a variety of spectroscopic features have now been recognized as hallmarks of such systems34. As low-bandgap materials, antiaromatic porphyrinoids in general and norcorrole derivatives in particular are potentially of great interest as components of molecular electronic circuits35. Against this exciting backdrop, we have confirmed that simple norcorrole derivatives afford unique examples of symmetric, delocalized, antiaromatic systems. Fragment MO analysis suggests that these seemingly contradictory attributes reflect the great stability of the two dipyrrin halves of the molecule. In other words, the energetic imperative of delocalized bonding within the dipyrrin fragments overrules that of antiaromaticity-related bond length alternation.
Methods
Geometry optimization studies and the fragment MO analysis were carried out with the ADF201736,37 program system using methods described above. All optimized structures (see Supplementary information for coordinates) were confirmed as local minima via frequency analyses. To obtain current density plots and current intensities, geometry optimizations and GIAO NMR calculations were performed at the B3LYP/def2-TZVP level with Gaussian 09 rev. D138. The NMR computations were further analyzed with the AIMAll (version 16.05.18) suite of programs39. Current densities were obtained within the context of quantum theory of atoms in molecules as developed by Keith and Bader40–44.
Electronic supplementary material
Acknowledgements
Financial support from the Research Council of Norway (grant no. 262229 to A.G.) and the National Research Foundation of South Africa (grant no. 113327 to J.C.) is gratefully acknowledged. C.F.-N. acknowledges (1) “Projects of Large Research, Development, and Innovations Infrastructures” for access to the computational resources provided by the CESNET LM2015042 and the CERIT Scientific Cloud LM2015085 and (2) project CEITEC 2020 LQ1601 with financial support from the Ministry of Education, Youth, and Sports of the Czech Republic under the National Sustainability Programme II.
Author Contributions
J.C. carried out the MO and TDDFT analyses, C.F.N. performed the current density calculations and A.G. planned and coordinated the project. All authors contributed to the writing of the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jeanet Conradie, Email: conradj@ufs.ac.nz.
Cina Foroutan-Nejad, Email: canyslopus@yahoo.co.uk.
Abhik Ghosh, Email: abhik.ghosh@uit.no.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-39972-y.
References
- 1.Ghosh, A., Wasbotten, I. H., Davis, W. & Swarts, J. C. Norcorrole and dihydronorcorrole: A predictive quantum chemical study. Eur. J. Inorg. Chem. 4479–4485 (2005).
- 2.Cissell JA, Vaid TP, Yap GP. The Doubly Oxidized, Antiaromatic Tetraphenylporphyrin Complex [Li(TPP)][BF4] Org. Lett. 2006;8:2401–2404. doi: 10.1021/ol060772l. [DOI] [PubMed] [Google Scholar]
- 3.Pawlicki M, Latos-Grażyński L. Aromaticity Switching in Porphyrinoids. Chem. Asian J. 2015;10:1438–1451. doi: 10.1002/asia.201500170. [DOI] [PubMed] [Google Scholar]
- 4.Reddy BK, Basavarajappa A, Ambhore MD, Anand VG. Isophlorinoids: The Antiaromatic Congeners of Porphyrinoids. Chem. Rev. 2017;117:3420–3443. doi: 10.1021/acs.chemrev.6b00544. [DOI] [PubMed] [Google Scholar]
- 5.Bröring M, Köhler S, Kleeberg C. Norcorrole: Observation of the Smallest Porphyrin Variant with a N4 Core. Angew. Chem. Int. Ed. 2008;47:5658–5660. doi: 10.1002/anie.200801196. [DOI] [PubMed] [Google Scholar]
- 6.Ito T, et al. Gram‐Scale Synthesis of Nickel(II) Norcorrole: The Smallest Antiaromatic Porphyrinoid. Angew. Chem. Int. Ed. 2012;51:8542–8545. doi: 10.1002/anie.201204395. [DOI] [PubMed] [Google Scholar]
- 7.Becke AD. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A. 1988;38:3098–3100. doi: 10.1103/PhysRevA.38.3098. [DOI] [PubMed] [Google Scholar]
- 8.Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988;37:785–789. doi: 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- 9.Miehlich B, Savin A, Stoll H, Preuss H. Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989;157:200–206. doi: 10.1016/0009-2614(89)87234-3. [DOI] [Google Scholar]
- 10.Grimme S, Anthony J, Ehrlich S, Krieg HA. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010;132:154104. doi: 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
- 11.Yonezawa T, Shafie SA, Hiroto S, Shinokubo H. Shaping Antiaromatic π-Systems by Metalation: Synthesis of a Bowl-Shaped Antiaromatic Palladium Norcorrole. Angew. Chem. Int. Ed. 2017;56:11822–11825. doi: 10.1002/anie.201706134. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida T, Sakamaki D, Seki S, Shinokubo H. Enhancing the low-energy absorption band and charge mobility of antiaromatic NiII norcorroles by their substituent effects. Chem. Commun. 2017;53:1112. doi: 10.1039/C6CC09444A. [DOI] [PubMed] [Google Scholar]
- 13.Nozawa R, et al. Stacked antiaromatic porphyrins. Nat. Comm. 2016;7:13620. doi: 10.1038/ncomms13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawashima H, Hiroto S, Shinokubo H. Acid-Mediated Migration of Bromide in an Antiaromatic Porphyrinoid: Preparation of Two Regioisomeric Ni(II) Bromonorcorroles. J. Org. Chem. 2017;82:10425–10432. doi: 10.1021/acs.joc.7b01899. [DOI] [PubMed] [Google Scholar]
- 15.Nozawa R, Yamamoto K, Shin J, Hiroto S, Shinokubo H. Regioselective Nucleophilic Functionalization of Antiaromatic Nickel(II) Norcorroles. Angew. Chem. Int. Ed. 2015;54:8454–8457. doi: 10.1002/anie.201502666. [DOI] [PubMed] [Google Scholar]
- 16.Deng Z, Li X, Stępień M, Chmielewski PJ. Nitration of Norcorrolatonickel(II): First Observation of a Diatropic Current in a System Comprising a Norcorrole Ring. Chem. Eur. J. 2016;22:4231–4246. doi: 10.1002/chem.201504584. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida T, Shinokubo H. Direct amination of the antiaromatic NiII norcorrole. Mater. Chem. Front. 2017;1:1853–1857. doi: 10.1039/C7QM00176B. [DOI] [Google Scholar]
- 18.Fliegl H, Sundholm D. Aromatic Pathways of Porphins, Chlorins, and Bacteriochlorins. J. Org. Chem. 2012;77:3408–3414. doi: 10.1021/jo300182b. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh, A., Larsen, S., Conradie, J. & Foroutan-Nejad, C. Local versus global aromaticity in azuliporphyrin and benziporphyrin derivatives. Org. Biomol. Chem. 16, 7964–7970 (2018). [DOI] [PubMed]
- 20.Foroutan-Nejad C, Larsen S, Conradie J, Ghosh A. Isocorroles as Homoaromatic NIR-Absorbing Chromophores: A First Quantum Chemical Study. Sci. Rep. 2018;8:11952. doi: 10.1038/s41598-018-29819-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foroutan-Nejad C. Interatomic Magnetizability: A QTAIM-Based Approach toward Deciphering Magnetic Aromaticity. J. Phys. Chem. A. 2011;115:12555–12560. doi: 10.1021/jp202901f. [DOI] [PubMed] [Google Scholar]
- 22.Foroutan-Nejad C. Al42−: the anion–π interactions and aromaticity in the presence of counter ions. Phys. Chem. Chem. Phys. 2012;14:9738. doi: 10.1039/c2cp40511c. [DOI] [PubMed] [Google Scholar]
- 23.Weigend F, Ahlrichs R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005;7:3297–3305. doi: 10.1039/b508541a. [DOI] [PubMed] [Google Scholar]
- 24.Bader, R. F. W. Atoms in Molecules: A Quantum Theory, Clarendon Press, Oxford, New York (1990).
- 25.Foroutan-Nejad C, Shahbazian S, Marek R. Toward a Consistent Interpretation of the QTAIM: Tortuous Link between Chemical Bonds, Interactions, and Bond/Line Paths. Chem. Eur. J. 2014;20:10140–10152. doi: 10.1002/chem.201402177. [DOI] [PubMed] [Google Scholar]
- 26.Bader RFW, Keith TA. Properties of atoms in molecules: Magnetic susceptibilities. J. Chem. Phys. 1993;99:3683–3693. doi: 10.1063/1.466166. [DOI] [Google Scholar]
- 27.Bader RFW, Keith TA. Use of electron charge and current distributions in the determination of atomic contributions to magnetic properties. Int. J. Quantum Chem. 1996;60:373–379. doi: 10.1002/(SICI)1097-461X(1996)60:1<373::AID-QUA36>3.0.CO;2-C. [DOI] [Google Scholar]
- 28.Badri Z, et al. All-Metal Aromaticity: Revisiting the Ring Current Model among Transition Metal Clusters. J. Chem. Theory Comput. 2013;9:4789–4796. doi: 10.1021/ct4007184. [DOI] [PubMed] [Google Scholar]
- 29.Foroutan-Nejad C. Is NICS a reliable aromaticity index for transition metal clusters? Theor. Chem. Acc. 2015;134:8. doi: 10.1007/s00214-015-1617-7. [DOI] [Google Scholar]
- 30.Chen Z, Wannere CS, Corminboeuf C, Puchta R, Schleyer PVR. Nucleus-Independent Chemical Shifts (NICS) as an Aromaticity Criterion. Chem. Rev. 2005;105:3842–3888. doi: 10.1021/cr030088+. [DOI] [PubMed] [Google Scholar]
- 31.Heilbronner E. Why do some molecules have symmetry different from that expected? J. Chem. Educ. 1989;66:471–478. doi: 10.1021/ed066p471. [DOI] [Google Scholar]
- 32.Shaik S, Shurki A, Danovich D, Hiberty PC. A Different Story of π-Delocalization – The Distortivity of π-Electrons and Its Chemical Manifestations. Chem. Rev. 2001;101:1501–1539. doi: 10.1021/cr990363l. [DOI] [PubMed] [Google Scholar]
- 33.Ceulemans, A., Lijnen, E., Fowler, P. W., Mallion, R. B. & Pisanski, T. Graph theory and the Jahn–Teller theorem. Proc. Roy. Soc. A 1–19 (2011).
- 34.Cho S, et al. Defining Spectroscopic Features of Heteroannulenic Antiaromatic Porphyrinoids. J. Phys. Chem. Lett. 2010;1:895–900. doi: 10.1021/jz100039n. [DOI] [Google Scholar]
- 35.Fujii S, et al. Highly-conducting molecular circuits based on antiaromaticity. Nat. Commun. 2017;8:15984. doi: 10.1038/ncomms15984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.te Velde G, et al. Chemistry with ADF. J. Comput. Chem. 2001;22:931–967. doi: 10.1002/jcc.1056. [DOI] [Google Scholar]
- 37.Guerra CF, Snijders JG, te Velde G, Baerends EJ. Towards an order-N DFT method. Theor. Chem. Acc. 1998;99:391–403. [Google Scholar]
- 38.Frisch, M. J. et al. Gaussian 09, Gaussian, Inc., Wallingford CT (2013).
- 39.Keith, T. A. AIMAll, Gristmill Software: Overland Park KS, USA (2017).
- 40.Keith TA, Bader RFW. Calculation of magnetic response properties using atoms in molecules. Chem. Phys. Lett. 1992;194:1–8. doi: 10.1016/0009-2614(92)85733-Q. [DOI] [Google Scholar]
- 41.Keith TA, Bader RFW. Calculation of magnetic response properties using a continuous set of gauge transformations. Chem. Phys. Lett. 1993;210:223–231. doi: 10.1016/0009-2614(93)89127-4. [DOI] [Google Scholar]
- 42.Keith TA, Bader RFW. Topological analysis of magnetically induced molecular current distributions. J. Chem. Phys. 1993;99:3669–3682. doi: 10.1063/1.466165. [DOI] [Google Scholar]
- 43.Keith TA. Calculation of magnetizabilities using GIAO current density distributions. Chem. Phys. 1996;213:123–132. doi: 10.1016/S0301-0104(96)00272-8. [DOI] [Google Scholar]
- 44.Keith TA, Bader RFW. Properties of atoms in molecules: nuclear magnetic shielding. Can. J. Chem. 1996;74:185–200. doi: 10.1139/v96-022. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.