Abstract
Background:
Parkinson’s disease (PD) is the second most common neurodegenerative disorder worldwide, the lifetime risk of developing this disease is 1.5%. Motor diagnostic symptoms of PD are caused by degeneration of nigrostria-tal dopamine neurons. There is no cure for PD and current therapy is limited to supportive care that partially alleviates dis-ease signs and symptoms. As diagnostic symptoms of PD result from progressive degeneration of dopamine neurons, drugs restoring these neurons may significantly improve treatment of PD.
Method:
A literature search was performed using the PubMed, Web of Science and Scopus databases to discuss the pro-gress achieved in the development of neuroregenerative agents for PD. Papers published before early 2018 were taken into account.
Results:
Here, we review several groups of potential agents capable of protecting and restoring dopamine neurons in cul-tures or animal models of PD including neurotrophic factors and small molecular weight compounds.
Conclusion:
Despite the promising results of in vitro and in vivo experiments, none of the found agents have yet shown conclusive neurorestorative properties in PD patients. Meanwhile, a few promising biologicals and small molecules have been identified. Their further clinical development can eventually give rise to disease-modifying drugs for PD. Thus, inten-sive research in the field is justified.
Keywords: Neurorestoration, neuroprotection, Parkinson’s disease, neurotrophic factors, GDNF, dopamine neurons, RET agonists, Trk agonists, BDNF, GDNF mimetics, BDNF mimetics
1. INTRODUCTION
Neurons are terminally differentiated cells and many of them live in the organism for the entire life. Neurons need constant trophic support to stay alive and function normally. In the organism, this support is provided by small secretory proteins known as neurotrophic factors. Although many other proteins can affect various aspects of neuronal function, the ability to support neuronal survival is mainly confined to proteins belonging to four different families: (i) neuropoietic cytokines (neurokines); (ii) neurotrophins; (iii) glial cell line-derived neurotrophic factor (GDNF) family ligands (GFLs); (iv) mesencephalic astrocyte-derived neurotrophic factor (MANF), and cerebral dopamine neurotrophic factor (CDNF) families
[1]. Neurotrophic factors activate their respective receptors, thus inducing stimulation of intracellular signaling cascades responsible for the survival, neurite outgrowth and arborization, synapse formation and other processes important for neuronal well-being and function.
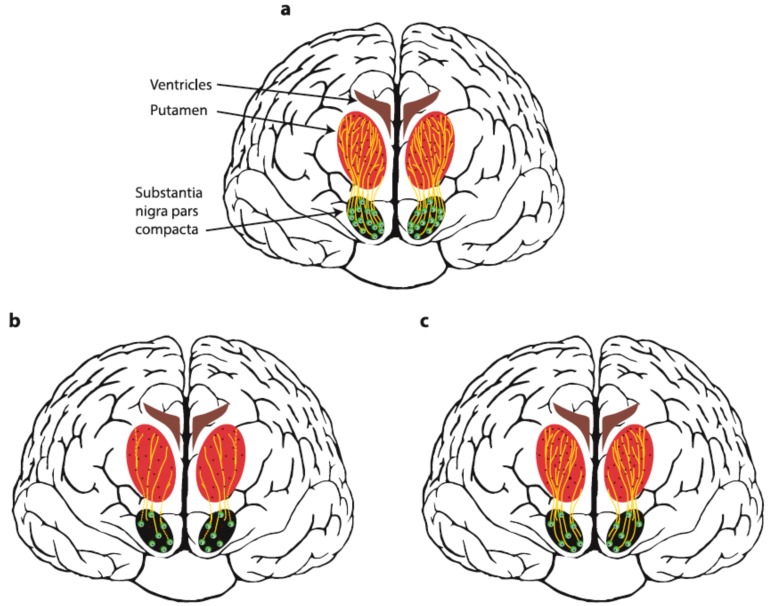
Neurodegenerative disorders are characterized by progressive degeneration and death of specific neuronal populations in the nervous system. There is no cure available for neurodegenerative disorders at the moment. The existing therapies can alleviate some symptoms of these diseases, but none of the marketed drugs is able to rescue or regenerate damaged neurons. In particular, in Parkinson’s disease (PD), diagnostic motor symptoms are caused by degeneration of nigrostriatal dopamine (DA) neurons. The cell bodies of these neurons reside in the brain region known as substantia nigra pars compacta (SNpc) and their projections are found in the brain part known as caudate putamen in humans or striatum in experimental animals (Fig. 1a). Degeneration of dopamine neurons in the brain of PD patients starts in axons. At the moment when a diagnosis of PD is made, approximately 60-80% of DA input in the striatum is lost, although 70% of DA neuronal bodies in SNpc remain alive [2] (Fig. 1b). This fact supports the idea of developing therapeutics that would support the remaining dopamine neurons and stimulate regrowth of dopamine axons into caudate putamen and their arborization (Fig. 1c). Thus, neurotrophic factors or small molecules targeting their receptors attract particular interest as potential neurorestorative therapeutics for Parkinson’s disease. The effects of neurotrophic factors on the dopamine system have been described in detail in Chapter 2 and summarized in Fig. (2). In Chapter 3, we describe small molecules activating receptors of neurotrophic factors and a few other small molecules with their mechanisms of action mostly being unknown, which were shown to restore dopamine neurons in animal models.
Fig. (1).
Schematic representation of nigrostriatal dopamine pathway in healthy people (a) and Parkinsonian patients before (b) and after treatment with a neurorestorative agent (c). Dopamine neurons in healthy patients receive trophic support from neurotrophic factors (shown with dark red dots). In Parkinsonian patients, axons of dopamine neurons in the putamen are either lost or degenerated (dotted line) and the number of dopamine neuron bodies in substantia nigra pars compacta is reduced. Treatment of PD patients with growth factors (or their mimetics) stimulates regrowth and arborization of dopamine axons in the putamen (c), thus restoring the dopamine balance and abolishing motor symptoms. (The color version of the figure is available in the electronic copy of the article).
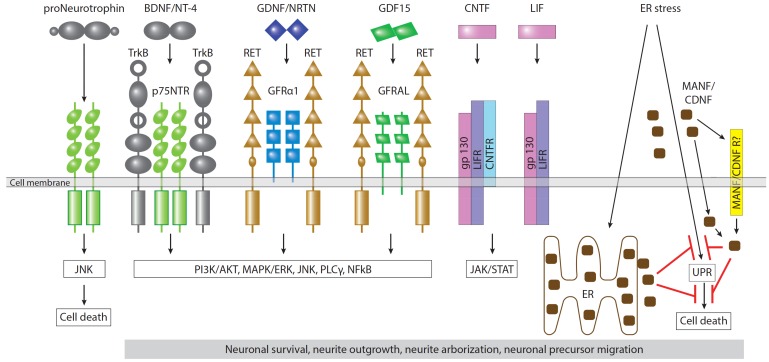
Fig. (2).
Neurotrophic factors and their receptors in dopamine neurons. Dopamine neurons express receptors of neurotrophins (TrkB and p75NTR), GFLs (GFRa and RET), potential distant GFL member GDF15 (GFRAL and RET), neurokines (gp130, LIFR, and CNTFR). Binding of all the afore-mentioned ligands leads to activation of intracellular signaling cascades promoting the survival of neuronal cells, neurite outgrowth and arborization, migration and differentiation of neuronal precursors. MANF and CDNF transmit signals intracellularly via unfolded protein response (UPR) pathways under endoplasmic reticulum (ER) stress. The particular molecular mechanism of action of MANF and CDNF in the cells is poorly understood; however, these proteins can prevent neuronal death induced by ER stress, probably by inhibition of UPR. The ability of MANF and CDNF to protect and restore dopamine neurons when delivered externally implies the existence of a mechanism of their internalization either via yet undiscovered surface receptor (MANF/CDNFR) or via their documented interaction with lipids. Binding of proneurotrophins to p75NTR activates the JNK pathway and leads to cell death. (The color version of the figure is available in the electronic copy of the article).
Development of new drugs requires testing of the candidates in animal models of respective diseases. 1-Methyl- 4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA) models are the most common models of PD used in research [3]. Both of these compounds can selectively kill dopamine neurons in the brain. However, while MPTP readily spreads in the body and crosses the blood brain barrier, 6-OHDA mostly stays near the injection site. Thus, MPTP is injected intraperitoneally and damages dopamine neurons in both hemispheres, while 6-OHDA is often delivered locally to one hemisphere leaving the second part of the brain intact and suitable for comparison. Animals with MPTP-lesioned neurons experience a decrease in general locomotor activity. Animals unilaterally treated with 6-OHDA demonstrate motor imbalance when rotating in a certain direction in response to drugs affecting dopamine release (amphetamine stimulates ipsilateral rotations, while apomorphine stimulates the contralateral ones) or using the paws controlled by the healthy side of the brain more extensively. Although being widely criticized, the models of toxin-induced PD are stably characterized by extensive degeneration of dopamine neurons in contrast to the majority of genetic models [3, 4]. Recently, there have been attempts to use α-synuclein fibrils to induce PD. α-Synuclein is a major component of Lewy bodies, protein aggregates found in the brain of PD patients and believed to disturb the normal function of dopamine neurons. Initial results of such experiments are promising but further tests are needed to elucidate the role played by the α-synuclein fibril model of PD in the discovery of novel therapeutics. Leucine-rich repeat kinase 2 (LRRK2) mutations are often associated with familial and sporadic forms of PD. Thus, attempts were made to develop LRRK2 transgenic models. Although no degeneration of nigrostriatal DA neurons was detected in most of such models, a decrease in the number of DA neurons was observed in aged LRRK2 G2019S mice [5, 6]. Further studies to identify the onset and extent of neurodegeneration in LRRK2 G2019S mice are needed to use these animals for drug testing. Mutations or knocking out of several other PD-related genes, such as PINK1, Parkin, and PJ-1 in particular, were attempted in experimental animals; however, no reliable and reproducible degeneration of dopamine neurons was observed in these models [3]. Some reports indicate that deletion of several other genes, such as VMAT-2, c-REL, or Atg7, results in degeneration of dopamine neurons and related deterioration of motor behavior [3]. Further studies are required to evaluate the applicability of such models for drug testing.
2. NEUROTROPHIC FACTORS AND THEIR NEURO- RESTORATIVE PROPERTIES IN PARKINSON’S DISEASE
2.1. Neuropoietic Cytokines
Neuropoietic cytokines (also known as neurokines) represent a family of neurotrophic factors signaling through cytokine receptors [7]. Neurokines include leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF), interleukin-6 (IL-6), IL-11, IL-27, IL-37, oncostatin M, neuropoietin, cardiotrophin-1 and cardiotrophin-like cytokine [8, 9]. These proteins are well known for their role in immune response and other processes in the organism but are also important for the development, survival and functioning of distinct neuronal populations [7, 8, 10]. All neurokines transmit signals via receptor complex, which includes transmembrane gp130 shared by all members of the family and low affinity receptors being selective for a certain neurokine or a group of neurokines. The first identified low-affinity neurokine receptor was transmembrane Il-6 receptor, which binds into a complex with two molecules of gp130 to form active receptor for IL-6. Low-affinity transmembrane LIF receptor (LIFR), glycosylphosphatidylinositol-anchored CNTF receptor (CNTFR) and oncostatin M receptors were later identified [8, 9]. The signaling complex for LIF consists of LIFR and a single gp130 molecule; the signaling complex for oncostatin M consists of oncostatin M receptor and gp130; CNTF transmits a signal through the complex consisting of LIFR, CNTFR and gp130. Soluble CNTF and IL-6 receptors can also transmit signals from respective neurokines [8, 9]. Binding of ligand to neurokine receptor complex results in activation of the intracellular JAK-STAT and mitogen-activated protein kinase (MAPK) signaling pathways [9, 11].
CNTF and LIF are the most extensively studied neurokines in the context of the nervous system. Both proteins play a role in the regulation of cell fate, neuronal differentiation, self-renewal of neuronal stem cells, neurogenesis and switch between neurogenesis and gliogenesis [7, 9, 11, 12]. The effects of neurokines in the dopamine system have not been very well elucidated. It is known that CNTF seems to mediate dopamine D2 receptor-induced neurogenesis occurring in the subventricular zone [13] and olfactory bulbs and can thus have an effect on two non-motor symptoms of Parkinson’s disease: depression and loss of the sense of smell. Recent studies indicate that in MPTP and α-synuclein animal models of Parkinson’s disease, CNTF endogenously produced by astrocytes mediates the neuroprotective effect of capsaicin on dopamine neurons and alleviates motor symptoms [14]. In addition, continuous intracranial infusion of CNTF to rats with transected nigrostriatal pathway recovered the number of neurons in the substantia nigra pars compacta but not expression of tyrosine hydroxylase (TH), the key enzyme of dopamine synthesis [15].
LIF promotes survival of cultured midbrain embryonic dopamine neurons [16, 17] and differentiation of neuronal progenitor cells into dopamine neurons in vitro, especially in the complex with other cytokines [16, 18, 19]. In mouse striata, LIF prevented proliferation of presynaptic dopamine uptake sites in response to mechanical injury [20]. In the mouse model, LIF treatment increased the number and density of neuronal precursor cells [21, 22] and reduced motor manifestations of PD [22]. Overall, this evidence highlights the potential of neurokines for Parkinson’s disease therapy. However, previous clinical trial with systemically delivered CNTF provided unsatisfactory results. Subcutaneous administration of recombinant human CNTF in phase II–III clinical trials in patients with amyotrophic lateral sclerosis failed to produce statistically significant differences in primary and secondary endpoints and was accompanied by serious adverse effects that resulted in limiting the dose in many patients [23]. At the same time, local intraocular delivery of CNTF-producing capsulated cells was found safe and had signs of efficacy in phase I clinical trial in patients with retinitis pigmentosa [24]. Thus, CNTF has recently been or is being tested in clinical trials for several eye diseases caused by retinal degeneration (the list of clinical trials for CNTF can be found at https://clinicaltrials.gov/).
2.2. Neurotrophins
Described by Stanley Cohen and Rita Levi-Montalcini, the founding member of the neurotrophin family, nerve growth factor (NGF), was the first discovered growth factor and the first neurotrophic factor [25, 26]. This family of neurotrophic factors also includes three other proteins: brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3), and neurotrophin 4 (NT-4) [1, 27]. Neurotrophins transmit signals via tyrosine receptor kinase (Trk) and p75NTR, a non-enzymatic transmembrane protein belonging to the tumor necrosis factor receptor family. Trk receptors are selective: NGF transmits signals via TrkA; BDNF and NT4, via TrkB; and NT3, via TrkC. All Trk receptors bind to mature neurotrophins, while p75NTR transmits signals from all four neurotrophins in both mature and pro-form. Ligand binding to Trk receptors leads to Trk dimerization and activation of the intracellular signaling cascades important for neuronal survival and function. Trk signaling is activated by mature neurotrophins and can be potentiated by binding to p75NTR, which is believed to result in the formation of high-affinity heteromeric receptor complex for neurotrophins [28, 29] or its intracellular domain [30]. Ligand binding promotes dimerization of TrkA, its phosphorylation and subsequent activation of intracellular signaling cascades promoting neuronal survival, neurite outgrowth and functioning. p75NTR can bind both to mature neurotrophins with relatively low affinity and to proneurotrophins with higher affinity. Binding of proneurotrophins to p75NTR results in negative consequences for the cells, such as inhibition of cellular function, growth inhibition and even cell death [31, 32].
Neurotrophin levels in serum and brain are reduced in Parkinsonian patients and in rodent models of PD [33-36]. Dopamine neurons express p75NTR receptor [37], TrkB and TrkC [38], but not TrkA [39]. In agreement with the receptor expression pattern, BDNF, NT-3 and NT-4 are able to support dopamine neurons in cultures [40-42] and to alleviate motor imbalance in animal models of PD. Interestingly, despite the lack of TrkA receptor in dopamine neurons, NGF at high concentrations could stimulate dopamine release from cultured dopamine neurons, presumably via p75NTR [43]. The conflicting evidence regarding the ability of NGF to influence dopamine neurons in vivo was reported: NGF was shown in some studies to be able to protect and/or restore dopamine neurons after injury [44], while no positive effects were seen in other publications [39, 45]. It is possible that the effects of NGF observed in animal models of PD are indirect and mediated by other neuronal populations or glial cells. Besides, it was shown that NGF can interact with the receptor of GDNF [46], the known survival factor for dopamine neurons [47]. Noteworthy, the effect of NGF in the dopamine system was minor if any in all cases. That is why NGF alone has not been considered as a first-line therapeutic agent for PD patients. Nevertheless, NGF has been tested in clinical trials, but it was delivered to PD patients to support survival of adrenal medullary autografts [48, 49]. Although these trials were not designed for rigorous assessment of treatment efficacy, patients reported improvement in the disease status in the absence of severe adverse effects.
Another member of the neurotrophin family, BDNF, is more clearly associated with the nigrostriatal dopamine system. BDNF supports the survival of cultured dopamine neurons, stimulates neurite outgrowth and arborization [42], and protects them against neurotoxic lesion [40, 50]. In animal models of PD, BDNF alleviates motor imbalance and protects nigrostriatal dopamine neurons [45, 51]. Inhibition of BDNF production by MPP+, which is an active MPTP metabolite, in vitro and in an animal model of PD contributes to the neurotoxic effect of this compound; prevention of BDNF downregulation is neuroprotective for dopamine neurons in vivo [52]. Recent studies have indicated that α-synuclein can selectively interact with the kinase domain of BDNF receptor TrkB, impair BDNF-induced TrkB-mediated signaling, and abolish the pro-survival effects of BDNF on dopamine neurons both in vitro and in vivo. This leads to degeneration of dopamine neurons [53]. Interestingly, BDNF/TrkB signaling may underlie the biological effects of several compounds with potential anti-PD activity [54-58], physical exercises [59, 60] and also seems to be important for the neuroprotective effect of deep brain stimulation, which is the most common surgical treatment for PD patients [61]. BDNF is also involved in the development of non-motor symptoms in PD patients: low serum levels of BDNF and BDNF val66/met polymorphism were associated with depression and cognitive impairment [62-66], which is not surprising taking into account the importance of BDNF/TrkB signaling for other neuronal populations. Although BDNF might in general positively influence the nigrostriatal dopamine system and alleviate motor and non-motor symptoms of PD, it has never been tested in clinical trials in PD patients. The effects of BDNF are mostly seen in the neuroprotection paradigm, while its ability to restore lesioned dopamine neurons has not been very well documented. In addition, ablation of the TrkB gene in dopamine neurons of postnatal mice seems to affect neither the number of survived dopamine neurons nor the density of their fibers in the striatum [67]. In line with this, the number of dopamine neurons in SNpc in adult mice (P120) lacking BDNF was not significantly lower compared to that in wild-type controls [68]. Another factor that can limit its clinic utility for PD therapy is the ability of BDNF to induce sprouting of serotonin fibers in the striatum, thus increasing the susceptibility to L-DOPA-induced dyskinesias [69].
The efficacy and potency of another neurotrophin NT-4 in the promotion of survival of cultured dopamine neurons were comparable to or even higher than that of BDNF [41, 70]. This factor also supported the survival of dopamine neurons in animals with axotomy-induced neuronal death [45].
NT-3 promoted survival of cultured dopamine neurons, but its effect was much weaker compared to those of BDNF and NT-4, had bell-shaped dose-response curve and was observed only within a narrow concentration range [41]. Similarly, in animals with the axotomy-induced or 6-OHDA-induced death of dopamine neurons, NT-3 was less potent than other neurotrophins in the protection of dopamine neurons. Interestingly, in contrast to NT-4 and BDNF, NT-3 was able to maintain expression of TH [45]. In 6-OHDA model of PD, NT-3 partially improved motor behavior but failed to influence dopamine metabolism [51]. Poor biological activity with regard to dopamine neurons prevents NT-3 from being considered as an agent that can be used to treat motor symptoms of PD. However, it was studied in a clinical trial as a drug that is able to relieve constipation [71], one of the common non-motor symptoms in PD patients caused by degeneration of enteric neurons.
Despite the potential positive effects in the dopamine system, neurotrophins are not the proteins of choice for developing anti-PD therapeutic agents. They affect multiple neuronal populations, so their use may be associated with adverse effects. It generally seems that neurotrophins can alleviate motor imbalance in animal PD models, stimulate dopamine functions, and possibly protect dopamine neurons against injury; however, their neurorestorative properties in this system require further investigation. The lack of clear degeneration of dopamine neurons in adult and aged mice lacking neurotrophins or their receptors also casts doubt on their possible therapeutic application in PD.
2.3. Glial Cell Line-derived Neurotrophic Factor Family Ligands
GFLs include four proteins: GDNF, neurturin (NRTN), artemin (ARTN) and persephin (PSPN). GDNF, the founding member of this family, was discovered in 1993 as a potent survival factor for cultured dopamine neurons [47].
GFLs transmit a signal via the receptor complex consisting of signal transducing module receptor tyrosine kinase RET shared by all four proteins and the ligand-binding subunit, GPI-anchored GDNF family receptor alpha 1-4 (GFRα1-4) selective for a particular ligand. GDNF preferentially binds to GFRα1; NRTN, to GFRα2; ARTN, to GFRα3; and PSPN, to GFRα4. Some crosstalks have also been described for this system. In particular, all GFLs can signal via GFRα1, although the affinities of ligands to this coreceptor other than GDNF are lower, and GDNF can also interact with GFRα2 [72-75]. It is important to note that soluble GFRα can also transmit signals from GFLs [76]. Binding of ligands to the GFRα/RET receptor complex leads to phosphorylation of tyrosine residues in the kinase domain of RET, which serve as docking sites for adaptor proteins and activate the downstream signaling cascades, such as MAPK, PI3K/Akt, Src and PlCγ, controlling neuronal survival and functioning [77]. Some biological effect of GFLs can also be mediated by other receptors, for instance by NCAM [78] and syndecan-3 (also previously known as N-syndecan) in particular [79]. Recent studies indicate that there is an additional distant member among GFLs, the protein called growth/ differentiation factor-15 (GDF15) transmitting signals via RET and GDNF family receptor alpha-like (GFRAL) [80, 81].
All GFLs and GDF15 were shown to support cultured dopamine neurons [82-86]. Dopamine neurons express RET and high levels of GFRα1 co-receptor, while GFRα2 is mostly found in the adjacent brain regions [76, 84]. Functional GFRα4 is not expressed in the brain [87], and GFRα3 is mostly found in the peripheral nervous system [82]. Thus, it is possible that biological effects of all GFLs in dopamine neurons are mediated via GFRα1/RET.
GDNF and NRTN have been extensively studied in a number of animal models of Parkinson’s disease, since their receptors are expressed in dopamine neurons. In both rodent and nonhuman primate neurotoxin models of PD, GDNF and NRTN alleviated motor symptoms, increased dopamine levels in brain tissues, and protected and restored lesioned dopamine neurons [88-93]. Furthermore, grafting of PSPN overexpressing cells into the striata of 6-OHDA-treated rats had a marked protective effect on dopamine neurons and relieved motor imbalance caused by toxin injection [85, 94].
Based on the encouraging results of preclinical studies, clinical trials were initiated in PD patients using purified GDNF protein and, later, adeno-associated virus (AAV2) vector encoded NRTN. Since neither GDNF nor AAV2-encoded NRTN is able to cross the blood brain barrier, they had to be delivered directly into the brain by means of complicated stereotactic surgery. In the first placebo-controlled phase II clinical trial, GDNF was delivered intraventricularly. In this study, GDNF failed to provide clinical benefits to patients [95]. That is not surprising taking into account the inability of GDNF to cross tissue barriers and spread in tissues, which led to protein incapability to reach the target neurons. Two subsequent small-scale phase I/II clinical trials, in which GDNF was infused into the striatum, showed improvement in motor function of PD patients accompanied by an increase in 18F-dopa uptake in the brain regions adjacent to the delivery catheter [96, 97]. However, the follow-up phase II double-blind, the placebo-controlled clinical trial failed to show statistically significant improvement in motor function of treated patients [98]. Multiple factors could contribute to the lack of efficacy of GDNF in this study. In particular the design of delivery catheter and the rates of GDNF delivery in the study by Lang et al. were different compared to those used in successful phase I/II clinical trials [99]. This issue combined with poor diffusion of GDNF into tissues [100] may lead to the inability of GDNF to reach a sufficient number of dopamine neurons in order to observe clinical effects. Indeed, the follow-up study in rhesus monkeys using the delivery system same as that employed in the unsuccessful phase II clinical trial showed that GDNF mostly concentrated around the catheter tip covering, if extrapolated to the human brain, only 2–9% of the average human putamen volume [101]. Extensive discussion on implications of statistical methods for data analysis indicates that the study might have been underpowered to detect small positive effects of GDNF [102, 103]. The presence of anti-GDNF neutralizing antibodies detected in some patients may indicate that there are problems related to the connection system between GDNF container and the delivery catheter, resulting in protein release into the bloodstream rather than into the brain. The initial results of another phase II double-blind, placebo-controlled clinical trial with GDNF proteins in PD patients conducted by Medgenesis Therapeutic Inc. have recently been released, but actual data are still to be published. Although this study failed to reach its primary endpoint [104], the follow-up phase III clinical trial is being planned by Medgenesis at the moment [105]. In addition, researchers from the National Institute of Neurological Disorders and Stroke (NINDS) have initiated a clinical trial with AAV2-GDNF (ClinicalTrials.gov Identifier: NCT01621581).
AAV2-NRTN (CERE-120) has also been tested in several clinical trials. The results of the first open-label phase I/II clinical trial with intraputamenally delivered CERE-120 showed signs of efficacy in the absence of serious adverse effects [106]. Nevertheless, the phase II clinical trial conducted using a similar setup failed to reach the primary endpoint 12 months after administration of CERE-120, while some improvement was seen in secondary measures and in a subset of patients followed for 18 months [107]. Postmortem analysis of NRTN levels in the brain of two patients from a later trial (both died from treatment-unrelated reasons) revealed that while NRTN-positive immunostaining covered approximately 15% of the putamen volume, very little NRTN staining was found in SNpc [108]. This phenomenon can be explained by extensive degeneration of axons of dopamine neurons in the putamen and/or formation of protein aggregates disrupting retrograde transport of the tested substance to the neuronal bodies. Thus, the CERE-120 delivery paradigm was adjusted and new clinical trials with the drug injected into both the striatum and substantia nigra were conducted [109, 110]. Despite this change and prolonged follow-up period (15- and 24-month evaluation), no statistically significant differences were seen in the primary and most of the secondary endpoints between placebo and AAV2-NRTN treated groups in phase II clinical trial [110]. Noteworthy, consequent analysis revealed significant difference in response of early (≤5 years after diagnosis) and late (≥10 years after diagnosis) stage PD patients to CERE-120: while a trend toward improvement in motor scores of early-stage patients was seen in NRTN-treated group in comparison to placebo control, no difference was observed in late-stage patients [111]. The following fact can explain this difference and lack of CERE-120 efficacy in clinical trials: in patients diagnosed with PD 10 or more years before treatment, a significant portion of dopamine neurons has already died. Since GFLs are unable to resurrect dead neurons but can protect and repair remaining neurons, the lack of efficacy of GFLs in late-stage PD patients is not surprising.
Hence, despite the promising preclinical data, clinical use of GFLs is complicated by multiple factors. Although the first clinical trial by Nutt et al. [95] reported adverse effects of GDNF and some toxicity was also seen in the experiments in monkeys, those were observed only for huge doses of recombinant GDNF (reviewed in [112]). GDNF and AAV2-NRTN administered in reasonable doses were rather save and well-tolerated by patients [96, 97, 106, 110, 113]. However, the necessity for intracranial delivery raises ethical barriers for selection of the target population of patients and makes the treatment reserved for patients with advanced PD who are unlikely to respond to GFLs. High affinity to the extracellular matrix [79] preventing diffusion of GDNF and NRTN in tissues requires careful planning of a delivery system and strategy. The latter problem can be overcome by using GFL variants with reduced affinity to heparin [114] or focusing on PSPN lacking the heparin-binding domain [79]. Other issues hindering the clinical development of GFLs can be associated with differences in biological activity between different preparations of these drugs and their price.
2.4. The MANF/CDNF Family of Neurotrophic Factors
Mesencephalic astrocyte-derived neurotrophic factor (MANF) and cerebral dopamine neurotrophic factor (CDNF) are the most recently discovered neurotrophic factors [115]. They differ from the conventional neurotrophic factors in their structure and function and exert their biological effects as intracellular endoplasmic reticulum (ER)-associated proteins rather than as target-derived factors. Meanwhile, it is known that MANF and CDNF can also be secreted. This fact and the biological activity of exogenously delivered MANF and CDNF indicate that cells should have either a still unidentified cell membrane receptor or use a different uptake mechanism for internalization of these neurotrophic factors, probably via their interactions with lipids [116]. CDNF and MANF seem to be general cytoprotective factors supporting survival of both neuronal and non-neuronal cells [117]. In particular, MANF is essential for survival of pancreatic beta cells: mice lacking MANF develop diabetes soon after birth [118] and are characterized by diabetes unrelated growth retardation [115]. CDNF-/- mice are viable, fertile and have no major defects producing life-treating condition or reducing lifespan [115].
The molecular details of MANF and CDNF signaling are not very well understood. The transmembrane receptor transmitting signals from these neurotrophic factors has either not yet been identified or does not exist. Expression and secretion of MANF is upregulated in response to ER stress, a condition caused by the accumulation of unfolded proteins in the ER [115, 117]. Thus, CDNF and MANF should have a receptor in the ER. ER stress activates unfolded protein response (UPR), an ER homeostasis maintaining mechanism, aiming to reduce a load of misfolded proteins by suppressing translation, activating degradation of unfolded proteins, and producing molecular chaperons to guide proper folding of the proteins. UPR is mediated by ER stress sensors: protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme I (IRE-1), and activating transcription factor 6 (ATF 6). Their activation is triggered by dissociation of glucose-regulated protein 78 (Grp78) from PERK, IRE-1 and ATF-6 caused by competition of unfolded proteins for binding to Grp78. Although short-term UPR is a protective mechanism, chronic ER stress stimulates apoptosis and leads to cell death [119]. MANF regulates ER stress either directly or indirectly via the maintenance of Ca2+ homeostasis. Under normal conditions, MANF seems to be retained in ER by KDEL receptors and GRP78, but it is secreted upon ER stress, which is unusual for ER stress-related proteins. In contrast to MANF, CDNF is expressed constitutively, but similarly to MANF is secreted in response to ER stress [115].
MANF plays an important role in the development and maintenance of non-mammalian dopamine neurons. Knocking it out reduces the number of dopamine neurons in zebrafish [120], diminishes the dopamine levels, the density of dopamine fibers and downregulates expression of the genes playing a role in dopamine synthesis, transport and uptake in fruit flies [121, 122]. It was reported that recombinant MANF supports the survival of cultured mammalian dopamine neurons. Importantly, despite the ubiquitous expression and the general physiological role, MANF is selective towards dopamine neurons [123]. However, naïve dopamine neurons do not seem to respond to MANF. Instead, MANF promotes survival of dopamine neurons under ER stress (Saarma et al., unpublished). Together with the expression pattern of MANF and CDNF, this fact has given grounds for testing these neurotrophic factors in animal models of PD.
When delivered into the striatum as a single bolus, both CDNF and MANF proteins alleviated motor imbalance, protected and restored nigrostriatal dopamine neurons in rat 6-OHDA model of PD, although not all effects reached statistical significance [124, 125]. Intrastriatally delivered CDNF protein also protected and restored dopamine neurons and improved motor performance in MPTP-treated mice [126]. The data on the effects of gene therapy using virally encoded CDNF are contradictory. While delivery of AAV2-encoded CDNF into the striatum alleviated motor imbalance and seemed to protect dopamine neurons [127, 128], no positive effect was seen with CDNF encoded by lentiviral vector in 6-OHDA rat model of PD [129]. Nigral overexpression of lentivirally encoded CDNF in rat 6-OHDA model alleviated motor imbalance and increased the density of dopaminergic innervation in the striatum but had no effect on the number of dopamine neurons in the substantia nigra [129]. However, these results are difficult to interpret because no expression of CDNF and MANF encoded by the lentiviral vector was documented. Positive effects of CDNF protein towards dopamine system were also detected in MPTP model of PD in nonhuman primates, but the exact data have not been published yet. Importantly, CDNF also alleviated PD-related non-motor symptoms in monkeys [130]. Noteworthy, both MANF and CDNF diffuse in the brain better than GDNF and clearly have a different mode of action [115, 131]. Encouraged by these findings, small Finnish company Herantis Pharma has recently initiated the first phase I/II clinical trial of intraputamenally delivered CDNF proteins in PD patients (ClinicalTrials.gov Identifier: NCT03295786). The primary objective of the study is to evaluate the safety and tolerability of treatment; however, efficacy indicators will be also addressed.
2.5. The Effects of other Trophic Factors in Parkinson’s Disease
A few other trophic factors were shown to support the survival of cultured dopamine neurons and exhibit neuroprotective and/or neurorestorative effects in animal models of PD. In particular, such effects were demonstrated for vascular endothelial growth factor (VEGF) [132] and platelet-derived growth factor (PDGF) [133]. The mechanism of their action in dopamine neurons is not very well understood. The primary target for both of these growth factors is vascular system, thus, their effects in animal models of PD can be indirect. Besides, their influence on the vascular system may lead to adverse effects, especially in case of prolonged use in patients. In fact, high doses of VEGF were shown to promote gliogenesis and lead to severe brain edema [134]. Thus, the clinical application of VEGF and PDGF in PD patients is problematic.
Doubtlessly, neurotrophic factors possess an incredible potential for disease modification in patients with neurodegenerative disorders. However, their clinical development is not easy. Being relatively large polypeptides, neurotrophic factors are unable to penetrate through the blood-brain barrier and have to be delivered directly into the brain via invasive surgery. This issue brings certain inconvenience to patients but more importantly reserves the treatment to people with advanced PD only because of ethical considerations. Meanwhile, the target population of patients for neurotrophic factor treatments includes patients with early-stage PD who still have a significant number of dopamine neuron bodies in SNpc that NTF can protect and restore. Another problem is associated with limited diffusion of NTFs in tissues: while different neurotrophic factors show variable distribution volumes in tissues, neither of them outperforms small molecules that spread virtually to every tissue in the body. This fact raises two important issues to be taken into account in the context of the pharmaceutical development of neurotrophic factors for PD. Dopamine neurons degenerating in PD have their cell bodies located in one brain structure (SNpc), while their projections are found in another structure (known as the caudate putamen in humans and the striatum in rodents). NTFs delivered to the striatum most probably have to be retrogradely transported to SNpc to promote survival and this mechanism seems to be greatly impaired in the brains of PD patients [108]. Thus, targeting both the striatum and the substantia nigra might influence the efficacy of NTF treatment [109, 110, 129]. It is important to note that many non-motor symptoms of PD are caused by degeneration of various neuronal populations inside and outside the brain. Many of these neurons are responsive to the same neurotrophic factors as nigrostriatal dopamine neurons. However, limited diffusion of neurotrophic factors confines their effects to delivery sites. In addition, many neurotrophic factors can affect several receptors [30, 41, 77-79], thus activating a plethora of effects, some of which can be even differently directed or affect multiple cell populations [118, 135]. These aspects, together with the history of clinical trials involving GDNF protein in PD patients [95, 111], indicate that clinical development of NTFs is a long-lasting process: although the first clinical trial with GDNF was initiated in 1996, now, more than 20 years later, we still do not have a definite answer regarding efficacy of GFLs in PD treatment.
In this regard, it may be easier to translate small molecules with appropriate pharmacological properties and biological activity into clinical practice. The productive strategies to the development of disease-modifying treatments for PD can include the development of molecules (i) stimulating neurogenesis rendering birth of new dopamine neurons; (ii) mimicking the action of neurotrophic factors, (iii) targeting other proteins that support the survival and regeneration of DA neurons, for instance by inhibiting DA neuron apoptosis, modulating activity of transcription factors regulating survival, and apoptosis, and finally (iv) enhancing the survival and neuroprotection effects of glial cells.
Stimulation of neurogenesis seems to have obvious advantages for the development of disease-modifying treatment for PD; however, this area of research is highly controversial. The published data on neurogenesis in adult human brain are contradictory [136, 137]. Even if neurogenesis in the adult brain does indeed take place, it is a slow process producing a small number of new neurons [136, 138] and thus it is difficult to estimate its therapeutic potential. It is important to note that there are significant interspecies differences, pronounced age-dependent variations and brain region-specific distinctions in the rate of neurogenesis [136, 137]. These issues make translation research in the field of neurogenesis stimulation challenging.
3. SMALL MOLECULES STIMULATING NEURO- REGENERATION AND NEURORESTORATION IN THE NIGROSTRIATAL DOPAMINE SYSTEM
Due to the presence of different and diverse targets in the systems involved in neurogenesis and neuronal regeneration, theoretically there is a vast field for designing low-molecular-weight substances to influence the neuroregeneration and neurorestoration mechanisms for PD cure. Meanwhile, despite many publications concerning the neuroprotective activity in PD models [139-147], just a few types of small molecules were reported to be able to induce neuro- regeneration and neurorestoration in animal models of PD.
3.1. Agonists of Dopaminergic Receptors
It is known that levodopa can increase both proliferation and neuronal differentiation in the subventricular zone (SVZ) in the animal model of PD [148]. Since nigrostriatal DA neurons are mainly concentrated in the SN, the precursors should migrate there from the SVZ to restore the number of neurons lost during PD. Indeed, it has recently been demonstrated in the 6-OHDA-lesioned mouse model of PD that SN newborn dopaminergic neurons were derived from the migration and differentiation of neural progenitor cells [138].
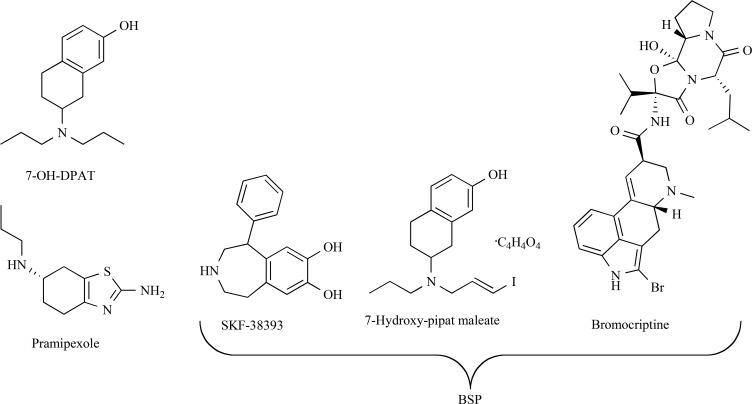
It should be noted that a significant number of DA neurons were already lost before the PD symptoms arise, making levodopa treatment ineffective. It was assumed that DA agonists may be more effective for stimulating regeneration of DA neurons than levodopa. Indeed, D3 receptor agonist 7-OH-DPAT (Fig. 3) infused intraventricularly for 2-8 weeks to rats 4 weeks post 6-OHDA treatment stimulated cell proliferation in the SNpc, protected DA neurons from death and restored retrograde transport of tracer from the striatum, thus indicating axonal regeneration [149]. These changes led to substantial improvement of motor imbalance in the animals. It is noteworthy that 7-OH-DPAT stimulation failed to increase proliferation and neurogenesis in human and murine midbrain neural precursor cells [150].
Fig. (3).
Structures of DA agonists.
The use of a combination of DA receptors agonists, bromocriptine (the agonist of D2, D3 and D4 receptors), SKF 38393 (the agonist of D1 receptors) and 7-hydroxy-pipat maleate (the agonist of D3 receptors), collectively referred to as BSP (Fig. 3) at 0.1 µM concentration was found to increase the number of proliferating precursor cells in cultures by 23% [151]. These results were confirmed by in vivo experiment [151], when BSP was administered to 6-OHDA pretreated rats for 6 days (the dose and the administration route were not specified). Rats receiving the BSP combination exhibited a significant increase in subventricular zone proliferation, both in the control and lesioned groups. Bromodeoxyuridine (BrdU) was used to detect proliferating cells, but no data concerning the neurogenesis of dopamine neurons (or any other neuronal type) were presented.
Oral treatment (1 mg/kg, twice daily for 10 days) with the D2, D3, and D4 dopamine receptor agonist pramipexole (Fig. 3) in 6-OHDA lesioned rats enhanced neuronal proliferation in the lesioned side of the subventricular zone by 31% compared with the control animals receiving phosphate-buffered saline [152]. An even more profound effect (an increase of 46%) was observed on the unlesioned side. The authors emphasized [152] that oral administration of pramipexole was more effective in stimulation of SVZ cell proliferation than intraperitoneal injections of the sufficiently more active D3 receptor agonist, 7-OH-DPAT [150]. Importantly, although the authors of this study observed an increase in the number of TH-positive cells in olfactory bulbs, they failed to detect any newly generated neuroblasts in the nigrostriatal system. Changes in the sense of smell were not addressed [152].
Although DA receptor agonists have been used in PD therapy for many years, there are no data concerning a possible neurogenic effect of these drugs in humans. These concerns are supported by clinical studies that failed to confirm the disease-modifying effect of DA agonists [138]. Lack of efficacy in clinical trials may be attributed to species-specific differences seen in the response to this class of drugs [150].
3.2. Trk Activators
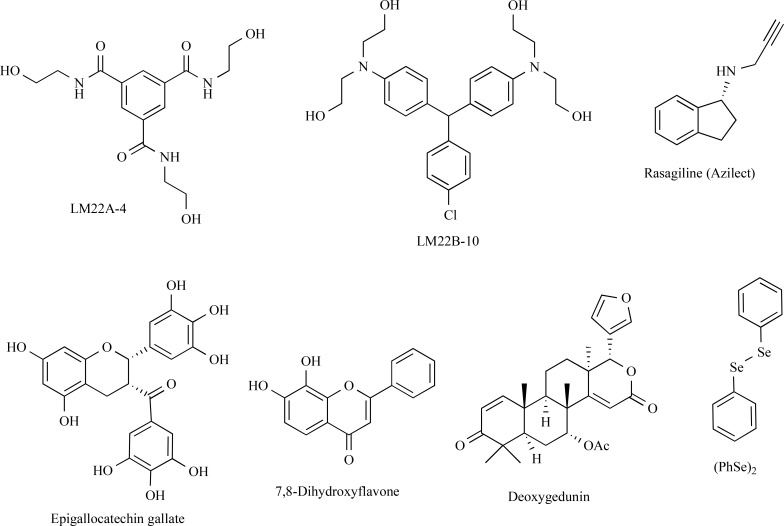
Neurotrophins exert their biological functions partly through Trk (See Chapter 2.2). Thus, activation of TrkA, TrkB or TrkC by small molecules may promote neurorestoration. While TrkA is not expressed in dopamine neurons, TrkB and TrkC can be potential targets to protect DA neurons. Rasagiline (Azilect®) is an irreversible monoamine oxidase-B (MAO-B) inhibitor used to treat PD [153]. Rasagiline (Fig. 4) given chronically in a dose of 0.05 mg/kg/day for 10 days to MPTP-treated mice led to an almost complete recovery of TH-immunopositive cells in the midbrain through activation of cell signaling mediators associated with the TrkA-mediated pathway [154, 155]. Chronic administration of a combination of low-dose (0.01 mg/kg/day) rasagiline and epigallocatechin gallate (10 mg/kg/day) for 14 days was later shown to have a positive synergetic effect on restoration of the nigrostriatal axis in MPTP-treated mice [156]. Moreover, 72-week clinical trials involving more than 1000 subjects with untreated Parkinson’s disease assigned to receive two doses of rasagiline were conducted [157]. The dose of 1 mg/day led to beneficial and possible disease-modifying effect, but the dose of 2 mg/day caused no clear improvements. These mixed results cannot be considered definitive without conducting additional trials [158]. Although the ability of rasagiline to increase the endogenous levels of BDNF and GDNF has been documented, it is still unclear if it exerts any disease-modifying effects in PD.
Fig. (4).
The structures of compounds targeting Trk receptors and/or activating Trk-dependent signaling.
Two TrkB agonists, 7,8-dihydroxyflavone and deoxy- gedunin (Fig. 4), were active in neuroprotective MPTP-based mouse [159, 160] and 6-OHDA rat models of PD [161]. Importantly, in 6-OHDA rat model of PD, post-lesion treatment with 7,8-dihydroxyflavone had an effect on neither motor imbalance nor the density of TH-positive fibers in the striatum, while 7,8-dihydroxyflavone was neuroprotective in the MPTP model with simultaneous administration of the compound and toxin [161, 162]. It was suggested that the effect of the compound in the MPTP model is mediated via downregulation of MPTP-induced α-synuclein overexpression and its anti-oxidant activity, which can explain the discrepancy in the effects seen in these toxin models, thus indicating that the compound might not have any neurorestorative properties [162]. 7,8-Dihydroxyflavone also protected dopamine neurons from MPTP lesion in nonhuman primates, without any severe adverse effects being observed [163]. It is important to note that many laboratories have failed to detect TrkB phosphorylation in response to 7,8-dihydroxyflavone in cultured cells [164, Rantamäki et al., personal communication], indicating that this compound may not be a direct TrkB agonist. Notably, 7,8-dihydroxyflavone was shown to increase the BDNF level in the brain of experimental animals [165]: thus, TrkB activation seen in animal models of different diseases after treatment with this compound can be mediated by BDNF. Meanwhile, chronic treatment of C57BL/6 mice with 7,8-dihydroxyflavone (2.5 and 5 mg/kg, 14 days, i.p.) did not affect the expression of the Htr1a, Htr2a, Bdnf, and TrkB genes in the cortex and hippocampus [166]. In addition, protective effects of 7,8-dihydroxyflavone were also seen in cell lacking TrkB, so its effects in animal models of PD (and other diseases) might be mediated via other molecular mechanisms, such as via anti-oxidant properties [167, 168]. It is noteworthy that many flavonoids might reduce neuroinflammation, thus producing additional neuroprotective effects [169].
Structurally unrelated to 7,8-dihydroxyflavone, selective TrkB agonists, LM22A and TrkB/TrkC agonists LM22B (Fig. 4), are being developed by the group of Prof. F. Longo. LM22A-4 promoted survival of hippocampal neurons expressing TrkB and protected SH-SY5Y cells against MPP+-induced cell death [170]. In vivo LM22A-4 improved motor learning after traumatic brain injury in rats [170]. Another compound, LM22B-10 (Fig. 4), also enhanced survival and neurite outgrowth from cultured hippocampal neurons. LM22B-10 increased TrkB and TrkC phosphorylation, activated pro-survival signaling and increased the density of dendritic spines in murine hippocampus [171]. Although the effects of LMA22 compounds in the dopamine system are yet to be studied, they might have a high potential for development of new drugs for PD treatment, especially if the ability of TrkB agonists to induce neurorestoration in nigrostriatal dopamine neurons is confirmed in further studies.
Intragastric administration of diphenyl diselenide (PhSe)2 (Fig. 4) in a 1 mg/kg/day dose for 30 days to 6-OHDA-treated rats led to the restoration of locomotor function in the cylinder, stepping and bridge tests but did not affect animal behavior in the rotarod test [57]. The Western blotting data allowed the authors to assume that the BDNF/TrkB signaling pathway is involved in the (PhSe)2 effect.
3.3. RET Agonists
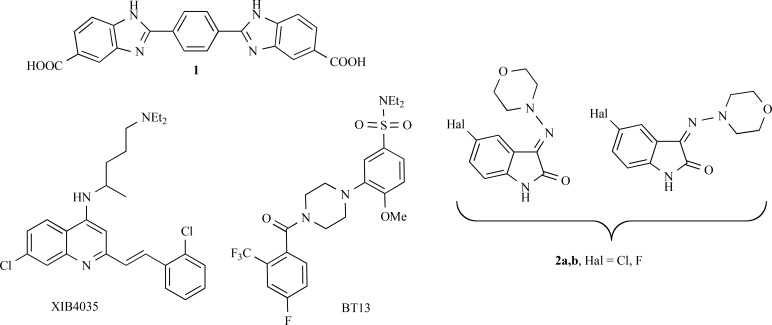
Activation of RET signaling by GDNF is known to be important for the protection and regeneration of dopaminergic neurons in animal models of PD [172, 173]. These data make RET a significant possible target for neuroregenerative therapy of PD [174]. The attempts to develop GDNF mimetics and RET agonists were started over a decade ago. In 2003, a Japanese group published a paper describing a small molecule XIB4035 (Fig. 5), which produced the effects in immortalized cells and cultured neurons similar to those of GDNF [175]. However, further studies revealed that XIB4035 is not able to activate RET in the absence of endogenous ligands. Instead, it increased RET activation only in the presence of GFLs. Although this biological activity was shown to be beneficial in animal model of diabetes-induced neuropathy [176], the applicability of this molecule to PD therapy needs further research. Impairment of retrograde transport and extensive deterioration of dopamine neuron fibers observed in PD patients may limit the availability of endogenous GDNF to dopamine neurons, thus making treatment with XIB4035 or its analogues inefficient.
Fig. (5).
Structures of RET agonists (1, XIB4035, BT13) and LRRK2 inhibitors 2a,b.
BT13 (Fig. 5), a small molecule agonist of RET receptor, was found to attenuate neuropathic pain in the rat neuropathy model and to protect sensory neurons [177]. Meanwhile, no data concerning regenerative activity of small molecule RET agonists in animal models of PD have been published yet.
Another chemical compound (1) activating RET has recently been described (Fig. 5). In contrast to the previously identified agonists, this compound may need a full GDNF receptor complex consisting of GFRα1 co-receptor and RET to exert biological activity [178], thus being more selective than the previously described compounds. However, biological activity of compound 1 is very weak and it has so far been studied only in immortalized cells. This compound needs to be optimized before any in vivo studies in the dopamine system.
3.4. LRRK2 Inhibitors
LRRK2 is a kinase playing multiple but poorly understood roles. Activating mutations in the LRRK2 gene were associated with several familial and sporadic cases of PD. It was proposed that LRRK2 inhibition may prevent neurodegeneration or even lead to neuroregeneration [179, 180].
A set of new selective inhibitors of LRRK2 with indolinone scaffold was designed [180]. Compounds 2a,b (Fig. 5) inhibiting LRRK2 in a low micromolar range were tested in 5 µM concentration for their ability to stimulate neurogenesis of neural stem precursor cells isolated from the ventricular-subventricular zone of adult mice. Indeed, compounds 2a,b increased the size of neurospheres but did not alter their number. The compounds were used as a mixture of stereoisomers. Thus, LRRK2 inhibitors may be useful for stimulating neuroregeneration. However, further research is needed to understand their applicability in PD treatment. The disappointing results of clinical trials with dopamine agonists that were also able to support neurogenesis may indicate potential difficulties in the translation of preclinical solutions to patients.
3.5. 9-Methyl-β-carboline
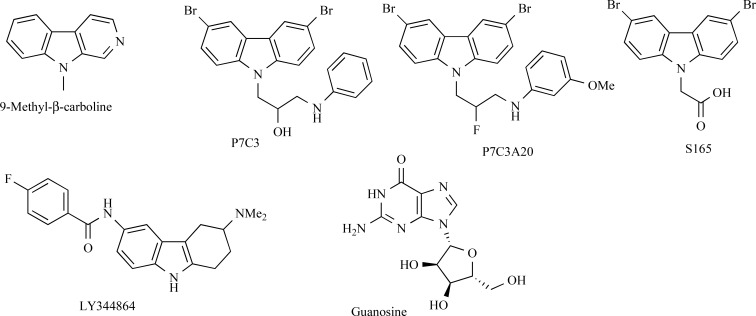
Pyrido [3,4-b]indoles or β-carbolines are widespread in nature and food, with the highest concentration found in charcoal-grilled meat [181]. These compounds are good substrates for the dopamine transporter and thus may affect dopamine neurons. Although many β-carbolines are toxic for these neurons, one of these compounds, 9-methyl-β-carboline (Fig. 6), on the contrary, can promote survival and possibly stimulate neurite outgrowth from cultured dopamine neurons at 25–100 µM concentrations [182, 183]. It was assumed that additional TH-immunoreactive neurons originated exclusively from non-proliferative, preexisting dopa decarboxylase-immunoreactive neurons [183]. 9-Methyl-β-carboline protected cultured neurons against acute lipopolysaccharide and chronic rotenone toxicity as well as reduced the content of α-synuclein protein in the cultures [184]. The compound was also tested in the rat unilateral MPTP model of PD. The following paradigm was used: MPP+ was infused to the left ventricle for 28 days followed by 14-day treatment with 9-methyl-β-carboline. 9-Methyl-β-carboline seemed to restore the dopamine level in the left striatum, but its positive effect on the number of TH-positive neurons in SN claimed by authors is arguable: no statistically significant differences between the vehicle-treated and compound-treated groups were reported. Motor effects of either MPP+ or compound treatment were not observed in this study, although this can be due to very mild lesion of the dopamine system by intraventricular infusion of the administered MPP+ [185]. Although these data may hint at possible neurorestorative properties of 9-methyl-β-carboline, further research is needed to draw any solid conclusions. However, the relatively high concentrations of 9-methyl-β-carboline used in in vitro experiments are unlikely to be reached during systemic administration.
Fig. (6).
Structures of 9-methyl-β-carboline, aminocarbazoles and guanosine.
In addition, the data concerning in vivo toxicity of β-carbolines, including 9-methyl-β-carboline and its possible metabolites, to dopaminergic neurons [186] should also be taken into account before further development of the compound as a neurorestorative agent.
3.6. Aminocarbazoles
Another group of tricyclic nitrogen-containing compounds with possible proneurogenic activity was identified among aminopropyl carbazoles [187]. The parent compound P7C3 (Fig. 6) was discovered by in vivo screening for small molecules capable of stimulating postnatal hippocampal neurogenesis [188]. Moreover, this carbazole, as well as its derivative P7C3A20 (Fig. 6), demonstrated promising activity in the MPTP mouse model (pretreatment with 30 mg/kg MPTP for 5 days) [187]. Thus, 21-day administration of P7C3A20 in a daily dose of 20 mg/kg preserved about 85% of dopaminergic neurons in the SNpc as compared to mice treated with saline, while about 50% of neurons were lost in the control MPTP group. Similar results were obtained using the MPTP model in worms [187]. Among second-generation of P7C3 analogues, acid S165 displayed potency equal to or greater than that of P7C3 in in vivo assays and enhanced bioavailability. Meanwhile, S165 concentration in the murine brain was lower than when the same dose of P7C3 was used [187].
Tetrahydrodiaminocarbazole LY344864 (Fig. 6), which is a 5-HT1F receptor agonist and is known to induce mitochondrial biogenesis [189], was tested for its ability to stimulate neurorestoration in 6-OHDA-lesioned mice [190]. It was found that 14-day administration of LY344864 (2 mg/kg/day, i.p.) significantly increased the number of TH-positive cells in the substantia nigra compared to the vehicle-treated control. It is noteworthy that the number of cells in LY344864-treated 6-OHDA-lesioned mice remained significantly lower than that in the saline-injected control group.
Thus, aminocarbazoles of different structures may be promising agents for stimulating neurorestoration in PD.
3.7. Guanosine
Purine nucleoside guanosine (8 mg/kg, i.p.) was administered for 8 weeks to Sprague-Dawley rats with proteasome inhibitor induced PD [191]. Guanosine (Fig. 6) protected TH-positive cells in the substantia nigra in comparison with proteasome inhibitor-treated mice. The effect of guanosine on cell proliferation may be mediated by basic fibroblast growth factor (FGF-2).
CONCLUSION
Despite the promising developments in neurorestoration of the brain dopamine system, cure for PD is yet to be discovered. Several groups of potential agents capable of protecting and restoring dopamine neurons in cultures or animal models of PD have been described. Nevertheless, none of them have yet shown any conclusive neuroprotective or neurorestorative properties in PD patients. The results of ongoing trials with GDNF and CDNF neurotrophic factors may provide a pathway to the future development of novel therapeutics for PD. Although clinical application of these proteins is complicated, small molecular weight mimetics can replace them in future if druggability of their signaling pathways is confirmed. By now, several small molecular weight compounds with different mechanisms of action have been found to demonstrate neurorestorative properties in in vitro and in vivo experiments. The most advanced are rasagiline and agonists of dopaminergic receptors, but both types of drugs failed to prove their neurorestorative activity when used in PD patients. Meanwhile, promising results obtained with these and other compounds show their potential for the further development of disease-modifying treatment for PD and warrants intensive research in this field.
Acknowledgements
Authors thank Prof. Mart Saarma for critical comments on the manuscript. Dr. Tomi Rantämaki and Prof. Alexander Kulikov are acknowledged for their comments on the biological activity of TrkB agonists. Preparation of this manuscript was financially supported by Sigrid Juselius Foundation.
LIST OF ABBREVIATIoNS
- 6-OHDA
6-hydroxydopamine
- AAV2
Adeno-associated virus
- ARTN
Artemin
- ATF 6
Activating transcription factor 6
- BDNF
Brain-derived neurotrophic factor
- BrdU
Bromodeoxyuridine
- CDNF
Cerebral dopamine neurotrophic factor
- CNTF
Ciliary neurotrophic factor
- CNTFR
CNTF receptor
- DA
Dopamine
- ER
Endoplasmic reticulum
- FGF-2
Fibroblast growth factor
- GDF15
Growth/differentiation factor-15
- GDNF
Glial cell line-derived neurotrophic factor
- GFLs
GDNF family ligands
- GFRAL
GDNF family receptor alpha-like
- GFRα
GDNF family receptor alpha
- Grp78
Glucose-regulated protein 78
- IL
Interleukin
- IRE-1
Inositol-requiring enzyme I
- LIF
Leukemia inhibitory factor
- LIFR
LIF receptor
- LRRK2
Leucine-rich repeat kinase
- MANF
Mesencephalic astrocyte-derived neurotrophic factor
- MAO-B
Monoamine oxidase B
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NGF
Nerve growth factor
- NINDS
National Institute of Neurological Disorders and Stroke
- NRTN
Neurturin
- NT
Neurotrophin
- PD
Parkinson’s disease
- PERK
(PKR)-like endoplasmic reticulum kinase
- PKR
Protein kinase R
- PSPN
Persephin
- SNpc
Substantia nigra pars compacta
- SVZ
Subventricular zone
- TH
Tyrosine hydroxylase
- Trk
Tropomyosin receptor kinase
- UPR
Unfolded protein response
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Nasrolahi A., Mahmoudi J., Akbarzadeh A., Karimipour M., Sadigh-Eteghad S., Salehi R., Farhoudi M. Neurotrophic Factors Hold Promise for the Future of Parkinson’s Disease Treatment: Is There a Light at the End of the Tunnel? Rev. Neurosci. 2018;29(5):475–490. doi: 10.1515/revneuro-2017-0040. [https://doi.org/10.1515/revneuro-2017-0040]. [DOI] [PubMed] [Google Scholar]
- 2.Cheng H.C., Ulane C.M., Burke R.E. Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 2010;67(6):715–725. doi: 10.1002/ana.21995. [http://dx.doi.org/10.1002/ana.21995]. [PMID: 20517933]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blesa J., Przedborski S. Parkinson’s disease: animal models and dopaminergic cell vulnerability. Front. Neuroanat. 2014;8:155. doi: 10.3389/fnana.2014.00155. [http://dx.doi.org/10.3389/fnana.2014.00155]. [PMID: 25565980]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson T.M., Ko H.S., Dawson V.L. Genetic animal models of Parkinson’s disease. Neuron. 2010;66(5):646–661. doi: 10.1016/j.neuron.2010.04.034. [http://dx. doi.org/10.1016/j.neuron.2010.04.034]. [PMID: 20547124]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramonet D., Daher J.P.L., Lin B.M., Stafa K., Kim J., Banerjee R., Westerlund M., Pletnikova O., Glauser L., Yang L., Liu Y., Swing D.A., Beal M.F., Troncoso J.C., McCaffery J.M., Jenkins N.A., Copeland N.G., Galter D., Thomas B., Lee M.K., Dawson T.M., Dawson V.L., Moore D.J. Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS One. 2011;6(4):e18568. doi: 10.1371/journal.pone.0018568. [http://dx.doi.org/10.1371/journal.pone.0018568]. [PMID: 21494637]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C.Y., Weng Y.H., Chien K.Y., Lin K.J., Yeh T.H., Cheng Y.P., Lu C.S., Wang H.L. (G2019S) LRRK2 activates MKK4-JNK pathway and causes degeneration of SN dopaminergic neurons in a transgenic mouse model of PD. Cell Death Differ. 2012;19(10):1623–1633. doi: 10.1038/cdd.2012.42. [http://dx.doi.org/10.1038/cdd.2012.42]. [PMID: 22539006]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stolp H.B. Neuropoietic cytokines in normal brain development and neurodevelopmental disorders. Mol. Cell. Neurosci. 2013;53:63–68. doi: 10.1016/j.mcn.2012.08.009. [http://dx.doi.org/10.1016/j.mcn.2012.08.009]. [PMID: 22926235]. [DOI] [PubMed] [Google Scholar]
- 8.Nathanson N.M. Regulation of neurokine receptor signaling and trafficking. Neurochem. Int. 2012;61(6):874–878. doi: 10.1016/j.neuint.2012.01.018. [http://dx.doi. org/10.1016/j.neuint.2012.01.018]. [PMID: 22306348]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer S., Kerr B.J., Patterson P.H. The neuropoietic cytokine family in development, plasticity, disease and injury. Nat. Rev. Neurosci. 2007;8(3):221–232. doi: 10.1038/nrn2054. [http://dx.doi.org/10.1038/nrn2054]. [PMID: 17311007]. [DOI] [PubMed] [Google Scholar]
- 10.Razavi S., Nazem G., Mardani M., Esfandiari E., Salehi H., Esfahani S.H.Z. Neurotrophic factors and their effects in the treatment of multiple sclerosis. Adv. Biomed. Res. 2015;4:53. doi: 10.4103/2277-9175.151570. [http://dx.doi.org/10.4103/2277-9175.151570]. [PMID: 25802822]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loy B., Apostolova G., Dorn R., McGuire V. a; Arthur, J. S. C.; Dechant, G. P38A and P38B Mitogen-Activated Protein Kinases Determine Cholinergic Transdifferentiation of Sympathetic Neurons. J. Neurosci. 2011;31(34):12059–12067. doi: 10.1523/JNEUROSCI.0448-11.2011. [http://dx.doi.org/ 10.1523/JNEUROSCI.0448-11.2011]. [PMID: 21865449]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer S. Cytokine control of adult neural stem cells. Ann. N. Y. Acad. Sci. 2009;1153(1):48–56. doi: 10.1111/j.1749-6632.2009.03986.x. [http://dx.doi.org/10.1111/j.1749-6632.2009.03986.x]. [PMID: 19236327]. [DOI] [PubMed] [Google Scholar]
- 13.Yang P., Arnold S.A., Habas A., Hetman M., Hagg T. Ciliary neurotrophic factor mediates dopamine D2 receptor-induced CNS neurogenesis in adult mice. J. Neurosci. 2008;28(9):2231–2241. doi: 10.1523/JNEUROSCI.3574-07.2008. [http://dx.doi.org/10.1523/JNEUROSCI.3574-07.2008]. [PMID: 18305256]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam J.H., Park E.S., Won S.Y., Lee Y.A., Kim K.I., Jeong J.Y., Baek J.Y., Cho E.J., Jin M., Chung Y.C., Lee B.D., Kim S.H., Kim E.G., Byun K., Lee B., Woo D.H., Lee C.J., Kim S.R., Bok E., Kim Y.S., Ahn T.B., Ko H.W., Brahmachari S., Pletinkova O., Troconso J.C., Dawson V.L., Dawson T.M., Jin B.K. TRPV1 on astrocytes rescues nigral dopamine neurons in Parkinson’s disease via CNTF. Brain. 2015;138(Pt 12):3610–3622. doi: 10.1093/brain/awv297. [http://dx.doi.org/10.1093/brain/awv297]. [PMID: 26490328]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagg T., Varon S. Ciliary neurotrophic factor prevents degeneration of adult rat substantia nigra dopaminergic neurons in vivo. Proc. Natl. Acad. Sci. USA. 1993;90(13):6315–6319. doi: 10.1073/pnas.90.13.6315. [http://dx. doi.org/10.1073/pnas.90.13.6315]. [PMID: 8101002]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling Z.D., Potter E.D., Lipton J.W., Carvey P.M. Differentiation of mesencephalic progenitor cells into dopaminergic neurons by cytokines. Exp. Neurol. 1998;149(2):411–423. doi: 10.1006/exnr.1998.6715. [http://dx.doi.org/ 10.1006/exnr.1998.6715]. [PMID: 9500954]. [DOI] [PubMed] [Google Scholar]
- 17.Zhao J-W., Dyson S.C., Kriegel C., Tyers P., He X., Fahmy T.M., Metcalfe S.M., Barker R.A. Modelling of a targeted nanotherapeutic ‘stroma’ to deliver the cytokine LIF, or XAV939, a potent inhibitor of Wnt-β-catenin signalling, for use in human fetal dopaminergic grafts in Parkinson’s disease. Dis. Model. Mech. 2014;7(10):1193–1203. doi: 10.1242/dmm.015859. [http://dx.doi.org/10.1242/dmm.015859]. [PMID: 25085990]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim T-S., Misumi S., Jung C-G., Masuda T., Isobe Y., Furuyama F., Nishino H., Hida H. Increase in dopaminergic neurons from mouse embryonic stem cell-derived neural progenitor/stem cells is mediated by hypoxia inducible factor-1alpha. J. Neurosci. Res. 2008;86(11):2353–2362. doi: 10.1002/jnr.21687. [http://dx.doi.org/10.1002/jnr.21687]. [PMID: 18438929]. [DOI] [PubMed] [Google Scholar]
- 19.Storch A., Paul G., Csete M., Boehm B.O., Carvey P.M., Kupsch A., Schwarz J. Long-term proliferation and dopaminergic differentiation of human mesencephalic neural precursor cells. Exp. Neurol. 2001;170(2):317–325. doi: 10.1006/exnr.2001.7706. [http://dx.doi.org/10.1006/exnr. 2001.7706]. [PMID: 11476598]. [DOI] [PubMed] [Google Scholar]
- 20.Howells D.W., Wong J.Y., Churchyard A.J., Donnan G.A. Leukaemia inhibitory factor prevents injury induced proliferation of striatal dopamine uptake sites. Neuroreport. 1995;6(14):1857–1860. doi: 10.1097/00001756-199510020-00009. [http://dx.doi.org/10.1097/00001756-199510020-00009]. [PMID: 8547584]. [DOI] [PubMed] [Google Scholar]
- 21.Liu J., Zang D. Response of neural precursor cells in the brain of Parkinson’s disease mouse model after LIF administration. Neurol. Res. 2009;31(7):681–686. doi: 10.1179/174313209X382368. [http://dx.doi.org/10.1179/ 174313209X382368]. [PMID: 19108756]. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y., Peng M., Zang D., Zhang B. Leukemia inhibitory factor promotes nestin-positive cells, and increases gp130 levels in the Parkinson disease mouse model of 6-hydroxydopamine. Neurosciences (Riyadh) 2013;18(4):363–370. [PMID: 24141460]. [PubMed] [Google Scholar]
- 23.A double-blind placebo-controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis. Neurology. 1996;46(5):1244–1249. doi: 10.1212/wnl.46.5.1244. [http://dx.doi.org/10.1212/WNL.46.5.1244]. [PMID: 8628460]. [DOI] [PubMed] [Google Scholar]
- 24.Sieving P.A., Caruso R.C., Tao W., Coleman H.R., Thompson D.J.S., Fullmer K.R., Bush R.A. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc. Natl. Acad. Sci. USA. 2006;103(10):3896–3901. doi: 10.1073/pnas.0600236103. [http://dx.doi.org/10.1073/ pnas.0600236103]. [PMID: 16505355]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen S., Levi-Montalcini R., Hamburger V. A nerve growth-stimulating factor isolated from sarcom as 37 and 180. Proc. Natl. Acad. Sci. USA. 1954;40(10):1014–1018. doi: 10.1073/pnas.40.10.1014. [http://dx.doi.org/10. 1073/pnas.40.10.1014]. [PMID: 16589582]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levi-Montalcini R., Cohen S. In Vitro and in Vivo Effects of a Nerve Growth-Stimulating Agent Isolated from Snake Venom. Proc. Natl. Acad. Sci. USA. 1956;42(9):695–699. doi: 10.1073/pnas.42.9.695. [http://dx.doi. org/10.1073/pnas.42.9.695]. [PMID: 16589933]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sánchez-Sánchez J., Arévalo J.C. A Review on Ubiquitination of Neurotrophin Receptors: Facts and Perspectives. Int. J. Mol. Sci. 2017;18(3):630. doi: 10.3390/ijms18030630. [http://dx.doi.org/10.3390/ijms18030630]. [PMID: 28335430]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esposito D., Patel P., Stephens R.M., Perez P., Chao M.V., Kaplan D.R., Hempstead B.L. The cytoplasmic and transmembrane domains of the p75 and Trk A receptors regulate high affinity binding to nerve growth factor. J. Biol. Chem. 2001;276(35):32687–32695. doi: 10.1074/jbc.M011674200. [http://dx.doi.org/10.1074/jbc.M011674200]. [PMID: 11435417]. [DOI] [PubMed] [Google Scholar]
- 29.Lad S.P., Peterson D.A., Bradshaw R.A., Neet K.E. Individual and combined effects of TrkA and p75NTR nerve growth factor receptors. A role for the high affinity receptor site. J. Biol. Chem. 2003;278(27):24808–24817. doi: 10.1074/jbc.M212270200. [http://dx.doi.org/10.1074/ jbc.M212270200]. [PMID: 12702729]. [DOI] [PubMed] [Google Scholar]
- 30.Ceni C., Kommaddi R.P., Thomas R., Vereker E., Liu X., McPherson P.S., Ritter B., Barker P.A. The p75NTR intracellular domain generated by neurotrophin-induced receptor cleavage potentiates Trk signaling. J. Cell Sci. 2010;123(Pt 13):2299–2307. doi: 10.1242/jcs.062612. [http://dx.doi.org/10.1242/jcs.062612]. [PMID: 20530577]. [DOI] [PubMed] [Google Scholar]
- 31.Meldolesi J. Neurotrophin receptors in the pathogenesis, diagnosis and therapy of neurodegenerative diseases. Pharmacol. Res. 2017;121:129–137. doi: 10.1016/j.phrs.2017.04.024. [http://dx.doi.org/10.1016/j.phrs.2017.04.024]. [PMID: 28438600]. [DOI] [PubMed] [Google Scholar]
- 32.Nykjaer A., Willnow T.E., Petersen C.M. p75NTR--live or let die. Curr. Opin. Neurobiol. 2005;15(1):49–57. doi: 10.1016/j.conb.2005.01.004. [http://dx.doi.org/ 10.1016/j.conb.2005.01.004]. [PMID: 15721744]. [DOI] [PubMed] [Google Scholar]
- 33.Lorigados Pedre L., Pavón Fuentes N., Alvarez González L., McRae A., Serrano Sánchez T., Blanco Lescano L., Macías González R. Nerve growth factor levels in Parkinson disease and experimental parkinsonian rats. Brain Res. 2002;952(1):122–127. doi: 10.1016/s0006-8993(02)03222-5. [http://dx.doi.org/10.1016/S0006-8993(02)03222-5]. [PMID: 12363411]. [DOI] [PubMed] [Google Scholar]
- 34.Lorigados L., Alvarez P., Pavón N., Serrano T., Blanco L., Macías R. NGF in experimental models of Parkinson disease. Mol. Chem. Neuropathol. 1996;28(1-3):225–228. doi: 10.1007/BF02815226. [http://dx.doi.org/ 10.1007/BF02815226]. [PMID: 8871963]. [DOI] [PubMed] [Google Scholar]
- 35.Mogi M., Togari A., Kondo T., Mizuno Y., Komure O., Kuno S., Ichinose H., Nagatsu T. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci. Lett. 1999;270(1):45–48. doi: 10.1016/s0304-3940(99)00463-2. [http:// dx.doi.org/10.1016/S0304-3940(99)00463-2]. [PMID: 10454142]. [DOI] [PubMed] [Google Scholar]
- 36.Huang Y., Yun W., Zhang M., Luo W., Zhou X. Serum concentration and clinical significance of brain-derived neurotrophic factor in patients with Parkinson’s disease or essential tremor. J. Int. Med. Res. 2018;46(4):1477–1485. doi: 10.1177/0300060517748843. [http://dx.doi.org/10.1177/ 0300060517748843]. [PMID: 29350074]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y.Q., Bian G.L., Bai Y., Cao R., Chen L.W. Identification and kainic acid-induced up-regulation of low-affinity p75 neurotrophin receptor (p75NTR) in the nigral dopamine neurons of adult rats. Neurochem. Int. 2008;53(3-4):56–62. doi: 10.1016/j.neuint.2008.06.007. [http://dx.doi.org/ 10.1016/j.neuint.2008.06.007]. [PMID: 18639597]. [DOI] [PubMed] [Google Scholar]
- 38.Numan S., Seroogy K.B. Expression of trkB and trkC mRNAs by adult midbrain dopamine neurons: a double-label in situ hybridization study. J. Comp. Neurol. 1999;403(3):295–308. doi: 10.1002/(sici)1096-9861(19990118)403:3<295::aid-cne2>3.0.co;2-l. [http://dx.doi. org/10.1002/(SICI)1096-9861(19990118)403:3<295:AID-CNE2> 3.0.CO;2-L]. [PMID: 9886032]. [DOI] [PubMed] [Google Scholar]
- 39.Melchior B., Nerrière-Daguin V., Laplaud D.A., Rémy S., Wiertlewski S., Neveu I., Naveilhan P., Meakin S.O., Brachet P. Ectopic expression of the TrkA receptor in adult dopaminergic mesencephalic neurons promotes retrograde axonal NGF transport and NGF-dependent neuroprotection. Exp. Neurol. 2003;183(2):367–378. doi: 10.1016/s0014-4886(03)00137-7. [http://dx.doi.org/10.1016/S0014-4886(03)00137-7]. [PMID: 14552878]. [DOI] [PubMed] [Google Scholar]
- 40.Hyman C., Hofer M., Barde Y.A., Juhasz M., Yancopoulos G.D., Squinto S.P., Lindsay R.M. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350(6315):230–232. doi: 10.1038/350230a0. [http://dx.doi.org/10.1038/350230a0]. [PMID: 2005978]. [DOI] [PubMed] [Google Scholar]
- 41.Hyman C., Juhasz M., Jackson C., Wright P., Ip N.Y., Lindsay R.M. Overlapping and distinct actions of the neurotrophins BDNF, NT-3, and NT-4/5 on cultured dopaminergic and GABAergic neurons of the ventral mesencephalon. J. Neurosci. 1994;14(1):335–347. doi: 10.1523/JNEUROSCI.14-01-00335.1994. [http://dx.doi.org/10.1523/JNEUROSCI.14-01-00335.1994]. [PMID: 8283241]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Studer L., Spenger C., Seiler R.W., Altar C.A., Lindsay R.M., Hyman C. Comparison of the effects of the neurotrophins on the morphological structure of dopaminergic neurons in cultures of rat substantia nigra. Eur. J. Neurosci. 1995;7(2):223–233. doi: 10.1111/j.1460-9568.1995.tb01058.x. [http://dx. doi.org/10.1111/j.1460-9568.1995.tb01058.x]. [PMID: 7757259]. [DOI] [PubMed] [Google Scholar]
- 43.Blöchl A., Sirrenberg C. Neurotrophins stimulate the release of dopamine from rat mesencephalic neurons via Trk and p75Lntr receptors. J. Biol. Chem. 1996;271(35):21100–21107. doi: 10.1074/jbc.271.35.21100. [http://dx.doi. org/10.1074/jbc.271.35.21100]. [PMID: 8702878]. [DOI] [PubMed] [Google Scholar]
- 44.Chaturvedi R.K., Shukla S., Seth K., Agrawal A.K. Nerve growth factor increases survival of dopaminergic graft, rescue nigral dopaminergic neurons and restores functional deficits in rat model of Parkinson’s disease. Neurosci. Lett. 2006;398(1-2):44–49. doi: 10.1016/j.neulet.2005.12.042. [http://dx.doi.org/10.1016/j.neulet.2005.12.042]. [PMID: 16423459]. [DOI] [PubMed] [Google Scholar]
- 45.Hagg T. Neurotrophins prevent death and differentially affect tyrosine hydroxylase of adult rat nigrostriatal neurons in vivo. Exp. Neurol. 1998;149(1):183–192. doi: 10.1006/exnr.1997.6684. [http://dx.doi.org/10.1006/exnr. 1997.6684]. [PMID: 9454627]. [DOI] [PubMed] [Google Scholar]
- 46.Fusco D., Vargiolu M., Vidone M., Mariani E., Pennisi L.F., Bonora E., Capellari S., Dirnberger D., Baumeister R., Martinelli P., Romeo G. The RET51/FKBP52 complex and its involvement in Parkinson disease. Hum. Mol. Genet. 2010;19(14):2804–2816. doi: 10.1093/hmg/ddq181. [http://dx.doi.org/10.1093/hmg/ddq181]. [PMID: 20442138]. [DOI] [PubMed] [Google Scholar]
- 47.Lin L.F., Doherty D.H., Lile J.D., Bektesh S., Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–1132. doi: 10.1126/science.8493557. [http://dx. doi.org/10.1126/science.8493557]. [PMID: 8493557]. [DOI] [PubMed] [Google Scholar]
- 48.Olson L., Backlund E.O., Ebendal T., Freedman R., Hamberger B., Hansson P., Hoffer B., Lindblom U., Meyerson B., Strömberg I. Intraputaminal infusion of nerve growth factor to support adrenal medullary autografts in Parkinson’s disease. One-year follow-up of first clinical trial. Arch. Neurol. 1991;48(4):373–381. doi: 10.1001/archneur.1991.00530160037011. [http://dx.doi.org/10.1001/archneur.1991.00530160037011]. [PMID: 2012510]. [DOI] [PubMed] [Google Scholar]
- 49.Sydow O., Hansson P., Young D., Meyerson B., Backlund E-O., Ebendal T., Farnebo L.O., Freedman R., Hamberger B., Hoffer B., Seiger A., Strömberq I., Olson L. Long-term beneficial effects of adrenal medullary autografts supported by nerve growth factor in Parkinson’s disease. Eur. J. Neurol. 1995;2(5):445–454. doi: 10.1111/j.1468-1331.1995.tb00154.x. [http://dx.doi.org/10.1111/j.1468-1331.1995.tb00154.x]. [PMID: 24283725]. [DOI] [PubMed] [Google Scholar]
- 50.Spina M.B., Squinto S.P., Miller J., Lindsay R.M., Hyman C. Brain-derived neurotrophic factor protects dopamine neurons against 6-hydroxydopamine and N-methyl-4-phenylpyridinium ion toxicity: involvement of the glutathione system. J. Neurochem. 1992;59(1):99–106. doi: 10.1111/j.1471-4159.1992.tb08880.x. [http://dx.doi.org/10.1111/j.1471-4159.1992. tb08880.x]. [PMID: 1613515]. [DOI] [PubMed] [Google Scholar]
- 51.Altar C.A., Boylan C.B., Fritsche M., Jones B.E., Jackson C., Wiegand S.J., Lindsay R.M., Hyman C. Efficacy of brain-derived neurotrophic factor and neurotrophin-3 on neurochemical and behavioral deficits associated with partial nigrostriatal dopamine lesions. J. Neurochem. 1994;63(3):1021–1032. doi: 10.1046/j.1471-4159.1994.63031021.x. [http://dx.doi.org/ 10.1046/j.1471-4159.1994.63031021.x]. [PMID: 7519657]. [DOI] [PubMed] [Google Scholar]
- 52.Zhang S., Chen S., Liu A., Wan J., Tang L., Zheng N., Xiong Y. Inhibition of BDNF Production by MPP+ through Up-Regulation of MiR-210-3p Contributes to Dopaminergic Neuron Damage in MPTP Model. Neurosci. Lett. 2018;675:133–139. doi: 10.1016/j.neulet.2017.10.014. [https://doi.org/10.1016/j.neulet.2017.10.014]. [DOI] [PubMed] [Google Scholar]
- 53.Kang S.S., Zhang Z., Liu X., Manfredsson F.P., Benskey M.J., Cao X., Xu J., Sun Y.E., Ye K., Trk B. TrkB neurotrophic activities are blocked by α-synuclein, triggering dopaminergic cell death in Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2017;114(40):10773–10778. doi: 10.1073/pnas.1713969114. [http://dx.doi.org/10.1073/pnas.1713969114]. [PMID: 28923922]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goes A.T.R., Jesse C.R., Antunes M.S., Lobo Ladd F.V., Lobo Ladd A.A.B., Luchese C., Paroul N., Boeira S.P. Protective role of chrysin on 6-hydroxydopamine-induced neurodegeneration a mouse model of Parkinson’s disease: Involvement of neuroinflammation and neurotrophins. Chem. Biol. Interact. 2018;279:111–120. doi: 10.1016/j.cbi.2017.10.019. [http://dx.doi.org/10.1016/j.cbi.2017.10.019]. [PMID: 29054324]. [DOI] [PubMed] [Google Scholar]
- 55.Siracusa R., Paterniti I., Cordaro M., Crupi R., Bruschetta G., Campolo M., Cuzzocrea S., Esposito E. Neuroprotective Effects of Temsirolimus in Animal Models of Parkinson’s Disease. Mol. Neurobiol. 2018;55(3):2403–2419. doi: 10.1007/s12035-017-0496-4. [https://doi.org/10.1007/s12035-017-0496-4]. [PMID: 28357809]. [DOI] [PubMed] [Google Scholar]
- 56.Kaur B., Prakash A. Ceftriaxone attenuates glutamate-mediated neuro-inflammation and restores BDNF in MPTP model of Parkinson’s disease in rats. Pathophysiology. 2017;24(2):71–79. doi: 10.1016/j.pathophys.2017.02.001. [http:// dx.doi.org/10.1016/j.pathophys.2017.02.001]. [PMID: 28245954]. [DOI] [PubMed] [Google Scholar]
- 57.Sampaio T.B., Pinton S., da Rocha J.T., Gai B.M., Nogueira C.W. Involvement of BDNF/TrkB signaling in the effect of diphenyl diselenide on motor function in a Parkinson’s disease rat model. Eur. J. Pharmacol. 2017;795(795):28–35. doi: 10.1016/j.ejphar.2016.11.054. [http://dx.doi. org/10.1016/j.ejphar.2016.11.054]. [PMID: 27915043]. [DOI] [PubMed] [Google Scholar]
- 58.Shi X., Chen Y.H., Liu H., Qu H.D. Therapeutic effects of paeonol on methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid-induced Parkinson’s disease in mice. Mol. Med. Rep. 2016;14(3):2397–2404. doi: 10.3892/mmr.2016.5573. [http://dx.doi.org/10.3892/mmr.2016.5573]. [PMID: 27484986]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou W., Barkow J.C., Freed C.R. Running wheel exercise reduces α-synuclein aggregation and improves motor and cognitive function in a transgenic mouse model of Parkinson’s disease. PLoS One. 2017;12(12):e0190160. doi: 10.1371/journal.pone.0190160. [http://dx.doi.org/10.1371/journal. pone.0190160]. [PMID: 29272304]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.da Costa R.O., Gadelha-Filho C.V.J., da Costa A.E.M., Feitosa M.L., de Araújo D.P., de Lucena J.D., de Aquino P.E.A., Lima F.A.V., Neves K.R.T., de Barros Viana G.S. The Treadmill Exercise Protects against Dopaminergic Neuron Loss and Brain Oxidative Stress in Parkinsonian Rats. Oxid. Med. Cell. Longev. 2017;2017:2138169. doi: 10.1155/2017/2138169. [http://dx.doi.org/10.1155/2017/2138169]. [PMID: 28713483]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fischer D.L., Kemp C.J., Cole-Strauss A., Polinski N.K., Paumier K.L., Lipton J.W., Steece-Collier K., Collier T.J., Buhlinger D.J., Sortwell C.E. Subthalamic Nucleus Deep Brain Stimulation Employs trkB Signaling for Neuroprotection and Functional Restoration. J. Neurosci. 2017;37(28):6786–6796. doi: 10.1523/JNEUROSCI.2060-16.2017. [http://dx.doi. org/10.1523/JNEUROSCI.2060-16.2017]. [PMID: 28607168]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y., Liu H., Du X-D., Zhang Y., Yin G., Zhang B-S., Soares J.C., Zhang X.Y. Association of low serum BDNF with depression in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 2017;41(0):73–78. doi: 10.1016/j.parkreldis.2017.05.012. [http://dx.doi.org/10.1016/j. parkreldis.2017.05.012]. [PMID: 28576603]. [DOI] [PubMed] [Google Scholar]
- 63.Cagni F.C., Campêlo C.L. Association of BDNF Val66MET Polymorphism With Parkinson’s Disease and Depression and Anxiety Symptoms. J. Neuropsychiatry Clin. Neurosci. 2017;29(2):142–147. doi: 10.1176/appi.neuropsych.16040062. [https://doi.org/10.1176/appi.neuropsych.16040062]. [DOI] [PubMed] [Google Scholar]
- 64.Caspell-Garcia C., Simuni T., Tosun-Turgut D., Wu I.W., Zhang Y., Nalls M., Singleton A., Shaw L.A., Kang J.H., Trojanowski J.Q., Siderowf A., Coffey C., Lasch S., Aarsland D., Burn D., Chahine L.M., Espay A.J., Foster E.D., Hawkins K.A., Litvan I., Richard I., Weintraub D. Multiple modality biomarker prediction of cognitive impairment in prospectively followed de novo Parkinson disease. PLoS One. 2017;12(5):e0175674. doi: 10.1371/journal.pone.0175674. [http://dx.doi.org/10.1371/journal.pone.0175674]. [PMID: 28520803]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y., Liu H., Zhang B-S., Soares J.C., Zhang X.Y. Low BDNF is associated with cognitive impairments in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 2016;29:66–71. doi: 10.1016/j.parkreldis.2016.05.023. [http://dx.doi.org/10.1016/j.parkreldis.2016.05.023]. [PMID: 27245919]. [DOI] [PubMed] [Google Scholar]
- 66.Khalil H., Alomari M.A., Khabour O.F., Al-Hieshan A., Bajwa J.A. Relationship of circulatory BDNF with cognitive deficits in people with Parkinson’s disease. J. Neurol. Sci. 2016;362:217–220. doi: 10.1016/j.jns.2016.01.032. [http://dx.doi.org/10.1016/j.jns.2016.01.032]. [PMID: 26944151]. [DOI] [PubMed] [Google Scholar]
- 67.Kramer E. R., Aron L., Ramakers G. M. J., Seitz S., Zhuang X., Beyer K., Smidt M. P., Klein R. Absence of Ret Signaling in Mice Causes Progressive and Late Degeneration of the Nigrostriatal System. 2007. [DOI] [PMC free article] [PubMed]
- 68.Baquet Z.C., Bickford P.C., Jones K.R. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J. Neurosci. 2005;25(26):6251–6259. doi: 10.1523/JNEUROSCI.4601-04.2005. [http://dx.doi.org/10.1523/ JNEUROSCI.4601-04.2005]. [PMID: 15987955]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tronci E., Napolitano F., Muñoz A., Fidalgo C., Rossi F., Björklund A., Usiello A., Carta M. BDNF over-expression induces striatal serotonin fiber sprouting and increases the susceptibility to l-DOPA-induced dyskinesia in 6-OHDA-lesioned rats. Exp. Neurol. 2017;297:73–81. doi: 10.1016/j.expneurol.2017.07.017. [http://dx.doi.org/10.1016/j. expneurol.2017.07.017]. [PMID: 28757258]. [DOI] [PubMed] [Google Scholar]
- 70.Hynes M.A., Poulsen K., Armanini M., Berkemeier L., Phillips H., Rosenthal A. Neurotrophin-4/5 is a survival factor for embryonic midbrain dopaminergic neurons in enriched cultures. J. Neurosci. Res. 1994;37(1):144–154. doi: 10.1002/jnr.490370118. [http://dx.doi.org/10.1002/jnr. 490370118]. [PMID: 7908342]. [DOI] [PubMed] [Google Scholar]
- 71.Urbanics R. NT-3. Takeda/Regeneron/Amgen. IDrugs. 2001;4(7):820–824. [PMID: 15995939]. [PubMed] [Google Scholar]
- 72.Sidorova Y.A., Mätlik K., Paveliev M., Lindahl M., Piranen E., Milbrandt J., Arumäe U., Saarma M., Bespalov M.M. Persephin signaling through GFRalpha1: the potential for the treatment of Parkinson’s disease. Mol. Cell. Neurosci. 2010;44(3):223–232. doi: 10.1016/j.mcn.2010.03.009. [http://dx.doi.org/10.1016/j.mcn.2010.03.009]. [PMID: 20350599]. [DOI] [PubMed] [Google Scholar]
- 73.Carmillo P., Dagø L., Day E.S., Worley D.S., Rossomando A., Walus L., Orozco O., Buckley C., Miller S., Tse A., Cate R.L., Rosenblad C., Sah D.W., Grønborg M., Whitty A. Glial cell line-derived neurotrophic factor (GDNF) receptor alpha-1 (GFR alpha 1) is highly selective for GDNF versus artemin. Biochemistry. 2005;44(7):2545–2554. doi: 10.1021/bi049247p. [http://dx.doi.org/10.1021/bi049247p]. [PMID: 15709767]. [DOI] [PubMed] [Google Scholar]
- 74.Baloh R.H., Tansey M.G., Golden J.P., Creedon D.J., Heuckeroth R.O., Keck C.L., Zimonjic D.B., Popescu N.C., Johnson E.M., Jr, Milbrandt J. TrnR2, a novel receptor that mediates neurturin and GDNF signaling through Ret. Neuron. 1997;18(5):793–802. doi: 10.1016/s0896-6273(00)80318-9. [http://dx.doi.org/10.1016/S0896-6273(00)80318-9]. [PMID: 9182803]. [DOI] [PubMed] [Google Scholar]
- 75.Jing S., Yu Y., Fang M., Hu Z., Holst P.L., Boone T., Delaney J., Schultz H., Zhou R., Fox G.M. GFRalpha-2 and GFRalpha-3 are two new receptors for ligands of the GDNF family. J. Biol. Chem. 1997;272(52):33111–33117. doi: 10.1074/jbc.272.52.33111. [http://dx.doi.org/10.1074/ jbc.272.52.33111]. [PMID: 9407096]. [DOI] [PubMed] [Google Scholar]
- 76.Wang L.C., Shih A., Hongo J., Devaux B., Hynes M. Broad specificity of GDNF family receptors GFRalpha1 and GFRalpha2 for GDNF and NTN in neurons and transfected cells. J. Neurosci. Res. 2000;61(1):1–9. doi: 10.1002/1097-4547(20000701)61:1<1::AID-JNR1>3.0.CO;2-J. [http://dx.doi.org/10.1002/1097-4547(20000701) 61:1<1:AID-JNR1>3.0.CO;2-J]. [PMID: 10861794]. [DOI] [PubMed] [Google Scholar]
- 77.Airaksinen M.S., Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 2002;3(5):383–394. doi: 10.1038/nrn812. [http://dx.doi.org/10.1038/nrn812]. [PMID: 11988777]. [DOI] [PubMed] [Google Scholar]
- 78.Paratcha G., Ledda F., Ibáñez C.F. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell. 2003;113(7):867–879. doi: 10.1016/s0092-8674(03)00435-5. [http://dx.doi.org/10. 1016/S0092-8674(03)00435-5]. [PMID: 12837245]. [DOI] [PubMed] [Google Scholar]
- 79.Bespalov M.M., Sidorova Y.A., Tumova S., Ahonen-Bishopp A., Magalhães A.C., Kulesskiy E., Paveliev M., Rivera C., Rauvala H., Saarma M. Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin, and artemin. J. Cell Biol. 2011;192(1):153–169. doi: 10.1083/jcb.201009136. [http://dx.doi.org/10.1083/jcb.201009136]. [PMID: 21200028]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mullican S.E., Lin-Schmidt X., Chin C.N., Chavez J.A., Furman J.L., Armstrong A.A., Beck S.C., South V.J., Dinh T.Q., Cash-Mason T.D., Cavanaugh C.R., Nelson S., Huang C., Hunter M.J., Rangwala S.M. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 2017;23(10):1150–1157. doi: 10.1038/nm.4392. [http://dx.doi.org/10. 1038/nm.4392]. [PMID: 28846097]. [DOI] [PubMed] [Google Scholar]
- 81.Yang L., Chang C.C., Sun Z., Madsen D., Zhu H., Padkjær S.B., Wu X., Huang T., Hultman K., Paulsen S.J., Wang J., Bugge A., Frantzen J.B., Nørgaard P., Jeppesen J.F., Yang Z., Secher A., Chen H., Li X., John L.M., Shan B., He Z., Gao X., Su J., Hansen K.T., Yang W., Jørgensen S.B. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat. Med. 2017;23(10):1158–1166. doi: 10.1038/nm.4394. [http://dx.doi.org/ 10.1038/nm.4394]. [PMID: 28846099]. [DOI] [PubMed] [Google Scholar]
- 82.Baloh R.H., Tansey M.G., Lampe P.A., Fahrner T.J., Enomoto H., Simburger K.S., Leitner M.L., Araki T., Johnson E.M., Jr, Milbrandt J. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3-RET receptor complex. Neuron. 1998;21(6):1291–1302. doi: 10.1016/s0896-6273(00)80649-2. [http://dx.doi.org/10.1016/S0896-6273(00)80649-2]. [PMID: 9883723]. [DOI] [PubMed] [Google Scholar]
- 83.Milbrandt J., de Sauvage F.J., Fahrner T.J., Baloh R.H., Leitner M.L., Tansey M.G., Lampe P.A., Heuckeroth R.O., Kotzbauer P.T., Simburger K.S., Golden J.P., Davies J.A., Vejsada R., Kato A.C., Hynes M., Sherman D., Nishimura M., Wang L.C., Vandlen R., Moffat B., Klein R.D., Poulsen K., Gray C., Garces A., Johnson E.M. Jr Persephin, a novel neurotrophic factor related to GDNF and neurturin. Neuron. 1998;20(2):245–253. doi: 10.1016/s0896-6273(00)80453-5. [http://dx.doi.org/10.1016/S0896-6273(00)80453-5]. [PMID: 9491986]. [DOI] [PubMed] [Google Scholar]
- 84.Horger B.A., Nishimura M.C., Armanini M.P., Wang L-C., Poulsen K.T., Rosenblad C., Kirik D., Moffat B., Simmons L., Johnson E., Jr, Milbrandt J., Rosenthal A., Bjorklund A., Vandlen R.A., Hynes M.A., Phillips H.S. Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J. Neurosci. 1998;18(13):4929–4937. doi: 10.1523/JNEUROSCI.18-13-04929.1998. [http://dx.doi.org/10.1523/ JNEUROSCI.18-13-04929.1998]. [PMID: 9634558]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Akerud P., Holm P.C., Castelo-Branco G., Sousa K., Rodriguez F.J., Arenas E. Persephin-overexpressing neural stem cells regulate the function of nigral dopaminergic neurons and prevent their degeneration in a model of Parkinson’s disease. Mol. Cell. Neurosci. 2002;21(2):205–222. doi: 10.1006/mcne.2002.1171. [http://dx.doi.org/10.1006/mcne.2002. 1171]. [PMID: 12401443]. [DOI] [PubMed] [Google Scholar]
- 86.Strelau J., Sullivan A., Böttner M., Lingor P., Falkenstein E., Suter-Crazzolara C., Galter D., Jaszai J., Krieglstein K., Unsicker K. Growth/differentiation factor-15/macrophage inhibitory cytokine-1 is a novel trophic factor for midbrain dopaminergic neurons in vivo. J. Neurosci. 2000;20(23):8597–8603. doi: 10.1523/JNEUROSCI.20-23-08597.2000. [http://dx. doi.org/10.1523/JNEUROSCI.20-23-08597.2000]. [PMID: 11102463]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lindahl M., Poteryaev D., Yu L., Arumäe U., Timmusk T., Bongarzone I., Aiello A., Pierotti M.A., Airaksinen M.S., Saarma M. Human glial cell line-derived neurotrophic factor receptor alpha 4 is the receptor for persephin and is predominantly expressed in normal and malignant thyroid medullary cells. J. Biol. Chem. 2001;276(12):9344–9351. doi: 10.1074/jbc.M008279200. [http://dx.doi.org/10.1074/jbc. M008279200]. [PMID: 11116144]. [DOI] [PubMed] [Google Scholar]
- 88.Kearns C.M., Cass W.A., Smoot K., Kryscio R., Gash D.M. GDNF protection against 6-OHDA: time dependence and requirement for protein synthesis. J. Neurosci. 1997;17(18):7111–7118. doi: 10.1523/JNEUROSCI.17-18-07111.1997. [http://dx.doi.org/10.1523/JNEUROSCI.17-18-07111.1997]. [PMID: 9278545]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kearns C.M., Gash D.M. GDNF protects nigral dopamine neurons against 6-hydroxydopamine in vivo. Brain Res. 1995;672(1-2):104–111. doi: 10.1016/0006-8993(94)01366-p. [http://dx.doi.org/10.1016/0006-8993(94)01366-P]. [PMID: 7749731]. [DOI] [PubMed] [Google Scholar]
- 90.Gash D.M., Zhang Z., Ovadia A., Cass W.A., Yi A., Simmerman L., Russell D., Martin D., Lapchak P.A., Collins F., Hoffer B.J., Gerhardt G.A. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380(6571):252–255. doi: 10.1038/380252a0. [http://dx. doi.org/10.1038/380252a0]. [PMID: 8637574]. [DOI] [PubMed] [Google Scholar]
- 91.Oiwa Y., Yoshimura R., Nakai K., Itakura T. Dopaminergic neuroprotection and regeneration by neurturin assessed by using behavioral, biochemical and histochemical measurements in a model of progressive Parkinson’s disease. Brain Res. 2002;947(2):271–283. doi: 10.1016/s0006-8993(02)02934-7. [http://dx.doi.org/10.1016/S0006-8993(02)02934-7]. [PMID: 12176170]. [DOI] [PubMed] [Google Scholar]
- 92.Kordower J.H., Herzog C.D., Dass B., Bakay R.A.E., Stansell J., III, Gasmi M., Bartus R.T. Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann. Neurol. 2006;60(6):706–715. doi: 10.1002/ana.21032. [http://dx.doi.org/ 10.1002/ana.21032]. [PMID: 17192932]. [DOI] [PubMed] [Google Scholar]
- 93.Hoffer B.J., Hoffman A., Bowenkamp K., Huettl P., Hudson J., Martin D., Lin L.F.H., Gerhardt G.A. Glial cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic neurons in vivo. Neurosci. Lett. 1994;182(1):107–111. doi: 10.1016/0304-3940(94)90218-6. [http://dx.doi.org/10.1016/0304-3940(94)90218-6]. [PMID: 7891873]. [DOI] [PubMed] [Google Scholar]
- 94.Yin X., Xu H., Jiang Y., Deng W., Wu Z., Xiang H., Sun P., Xie J. The effect of lentivirus-mediated PSPN genetic engineering bone marrow mesenchymal stem cells on Parkinson’s disease rat model. PLoS One. 2014;9(8):e105118. doi: 10.1371/journal.pone.0105118. [http://dx.doi.org/10.1371/ journal.pone.0105118]. [PMID: 25118697]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nutt J.G., Burchiel K.J., Comella C.L., Jankovic J., Lang A.E., Laws E.R., Jr, Lozano A.M., Penn R.D., Simpson R.K., Jr, Stacy M., Wooten G.F. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60(1):69–73. doi: 10.1212/wnl.60.1.69. [http://dx.doi.org/10.1212/WNL.60.1.69]. [PMID: 12525720]. [DOI] [PubMed] [Google Scholar]
- 96.Gill S.S., Patel N.K., Hotton G.R., O’Sullivan K., McCarter R., Bunnage M., Brooks D.J., Svendsen C.N., Heywood P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 2003;9(5):589–595. doi: 10.1038/nm850. [http://dx.doi.org/10. 1038/nm850]. [PMID: 12669033]. [DOI] [PubMed] [Google Scholar]
- 97.Slevin J.T., Gerhardt G.A., Smith C.D., Gash D.M., Kryscio R., Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J. Neurosurg. 2005;102(2):216–222. doi: 10.3171/jns.2005.102.2.0216. [http://dx.doi.org/10.3171/jns.2005.102.2. 0216]. [PMID: 15739547]. [DOI] [PubMed] [Google Scholar]
- 98.Lang A.E., Gill S., Patel N.K., Lozano A., Nutt J.G., Penn R., Brooks D.J., Hotton G., Moro E., Heywood P., Brodsky M.A., Burchiel K., Kelly P., Dalvi A., Scott B., Stacy M., Turner D., Wooten V.G., Elias W.J., Laws E.R., Dhawan V., Stoessl A.J., Matcham J., Coffey R.J., Traub M. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 2006;59(3):459–466. doi: 10.1002/ana.20737. [http://dx.doi.org/10.1002/ana.20737]. [PMID: 16429411]. [DOI] [PubMed] [Google Scholar]
- 99.Penn R.D., Dalvi A., Slevin J., Young B., Gash D., Gerhardt G., Hutchinson M. GDNF in treatment of Parkinson’s disease: response to editorial. Lancet Neurol. 2006;5(3):202–203. doi: 10.1016/S1474-4422(06)70360-X. [http://dx. doi.org/10.1016/S1474-4422(06)70360-X]. [PMID: 16488374]. [DOI] [PubMed] [Google Scholar]
- 100.Piltonen M., Bespalov M.M., Ervasti D., Matilainen T., Sidorova Y.A., Rauvala H., Saarma M., Männistö P.T. Heparin-binding determinants of GDNF reduce its tissue distribution but are beneficial for the protection of nigral dopaminergic neurons. Exp. Neurol. 2009;219(2):499–506. doi: 10.1016/j.expneurol.2009.07.002. [http://dx.doi.org/10.1016/j. expneurol.2009.07.002]. [PMID: 19615368]. [DOI] [PubMed] [Google Scholar]
- 101.Salvatore M.F., Ai Y., Fischer B., Zhang A.M., Grondin R.C., Zhang Z., Gerhardt G.A., Gash D.M. Point source concentration of GDNF may explain failure of phase II clinical trial. Exp. Neurol. 2006;202(2):497–505. doi: 10.1016/j.expneurol.2006.07.015. [http://dx.doi.org/10.1016/j.expneurol. 2006.07.015]. [PMID: 16962582]. [DOI] [PubMed] [Google Scholar]
- 102.Hutchinson M., Gurney S., Newson R. GDNF in Parkinson disease: an object lesson in the tyranny of type II. J. Neurosci. Methods. 2007;163(2):190–192. doi: 10.1016/j.jneumeth.2006.06.015. [http://dx.doi.org/10.1016/j.jneumeth. 2006.06.015]. [PMID: 16876872]. [DOI] [PubMed] [Google Scholar]
- 103.Matcham J., McDermott M.P., Lang A.E. GDNF in Parkinson’s disease: the perils of post-hoc power. J. Neurosci. Methods. 2007;163(2):193–196. doi: 10.1016/j.jneumeth.2007.05.003. [http://dx.doi.org/10.1016/j.jneumeth.2007.05.003]. [PMID: 17540454]. [DOI] [PubMed] [Google Scholar]
- 104.The Science of Parkinson’s https://scienceofparkinsons.com/2016/ 07/07/the-gdnf-trial-bristol-initial-results/
- 105.MedGenesis Therapeutix Announces Successful National Scientific Advice Meetings for GDNF in Parkinson’s Disease http:// medgenesis.com/news.htm#Advice
- 106.Marks W.J., Jr, Ostrem J.L., Verhagen L., Starr P.A., Larson P.S., Bakay R.A., Taylor R., Cahn-Weiner D.A., Stoessl A.J., Olanow C.W., Bartus R.T. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol. 2008;7(5):400–408. doi: 10.1016/S1474-4422(08)70065-6. [http:// dx.doi.org/10.1016/S1474-4422(08)70065-6]. [PMID: 18387850]. [DOI] [PubMed] [Google Scholar]
- 107.Marks W.J., Jr, Bartus R.T., Siffert J., Davis C.S., Lozano A., Boulis N., Vitek J., Stacy M., Turner D., Verhagen L., Bakay R., Watts R., Guthrie B., Jankovic J., Simpson R., Tagliati M., Alterman R., Stern M., Baltuch G., Starr P.A., Larson P.S., Ostrem J.L., Nutt J., Kieburtz K., Kordower J.H., Olanow C.W. Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9(12):1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [http://dx.doi.org/10.1016/S1474-4422(10)70254-4]. [PMID: 20970382]. [DOI] [PubMed] [Google Scholar]
- 108.Bartus R.T., Herzog C.D., Chu Y., Wilson A., Brown L., Siffert J., Johnson E.M., Jr, Olanow C.W., Mufson E.J., Kordower J.H. Bioactivity of AAV2-neurturin gene therapy (CERE-120): differences between Parkinson’s disease and nonhuman primate brains. Mov. Disord. 2011;26(1):27–36. doi: 10.1002/mds.23442. [http://dx.doi.org/10. 1002/mds.23442]. [PMID: 21322017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bartus R.T., Baumann T.L., Siffert J., Herzog C.D., Alterman R., Boulis N., Turner D.A., Stacy M., Lang A.E., Lozano A.M., Olanow C.W. Safety/feasibility of targeting the substantia nigra with AAV2-neurturin in Parkinson patients. Neurology. 2013;80(18):1698–1701. doi: 10.1212/WNL.0b013e3182904faa. [http://dx.doi.org/10.1212/WNL.0b013e3182904faa]. [PMID: 23576625]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Warren Olanow C., Bartus R.T., Baumann T.L., Factor S., Boulis N., Stacy M., Turner D.A., Marks W., Larson P., Starr P.A., Jankovic J., Simpson R., Watts R., Guthrie B., Poston K., Henderson J.M., Stern M., Baltuch G., Goetz C.G., Herzog C., Kordower J.H., Alterman R., Lozano A.M., Lang A.E. Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: A double-blind, randomized, controlled trial. Ann. Neurol. 2015;78(2):248–257. doi: 10.1002/ana.24436. [http://dx.doi.org/10.1002/ana.24436]. [PMID: 26061140]. [DOI] [PubMed] [Google Scholar]
- 111.Bartus R.T., Johnson E.M., Jr 2017.
- 112.Luz M., Mohr E., Fibiger H.C. GDNF-induced cerebellar toxicity: A brief review. Neurotoxicology. 2016;52:46–56. doi: 10.1016/j.neuro.2015.10.011. [http://dx. doi.org/10.1016/j.neuro.2015.10.011]. [PMID: 26535469]. [DOI] [PubMed] [Google Scholar]
- 113.Lang A.E., Langston J.W., Stoessl A.J., Brodsky M., Brooks D.J., Dhawan V., Elias W.J., Lozano A.M., Moro E., Nutt J.G., Stacy M., Turner D., Wooten G.F. GDNF in treatment of Parkinson’s disease: response to editorial. Lancet Neurol. 2006;5(3):200–202. doi: 10.1016/S1474-4422(06)70359-3. [http://dx.doi.org/10.1016/S1474-4422(06)70359-3]. [PMID: 16488373]. [DOI] [PubMed] [Google Scholar]
- 114.Runeberg-Roos P., Piccinini E., Penttinen A-M., Mätlik K., Heikkinen H., Kuure S., Bespalov M.M., Peränen J., Garea-Rodríguez E., Fuchs E., Airavaara M., Kalkkinen N., Penn R., Saarma M. Developing therapeutically more efficient Neurturin variants for treatment of Parkinson’s disease. Neurobiol. Dis. 2016;96:335–345. doi: 10.1016/j.nbd.2016.07.008. [http://dx.doi.org/10.1016/j.nbd.2016.07.008]. [PMID: 27425888]. [DOI] [PubMed] [Google Scholar]
- 115.Lindahl M., Saarma M., Lindholm P. 2017.
- 116.Parkash V., Lindholm P., Peränen J., Kalkkinen N., Oksanen E., Saarma M., Leppänen V.M., Goldman A. The structure of the conserved neurotrophic factors MANF and CDNF explains why they are bifunctional. Protein Eng. Des. Sel. 2009;22(4):233–241. doi: 10.1093/protein/gzn080. [http://dx.doi.org/10.1093/protein/gzn080]. [PMID: 19258449]. [DOI] [PubMed] [Google Scholar]
- 117.Apostolou A., Shen Y., Liang Y., Luo J., Fang S. Armet, a UPR-upregulated protein, inhibits cell proliferation and ER stress-induced cell death. Exp. Cell Res. 2008;314(13):2454–2467. doi: 10.1016/j.yexcr.2008.05.001. [http://dx.doi.org/10.1016/j.yexcr.2008.05.001]. [PMID: 18561914]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lindahl M., Danilova T., Palm E., Lindholm P., Võikar V., Hakonen E., Ustinov J., Andressoo J.O., Harvey B.K., Otonkoski T., Rossi J., Saarma M. MANF is indispensable for the proliferation and survival of pancreatic β cells. Cell Reports. 2014;7(2):366–375. doi: 10.1016/j.celrep.2014.03.023. [http://dx.doi.org/10.1016/j.celrep.2014.03. 023]. [PMID: 24726366]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pitale P.M., Gorbatyuk O., Gorbatyuk M. Neurodegeneration: Keeping ATF4 on a Tight Leash. Front. Cell. Neurosci. 2017;11:410. doi: 10.3389/fncel.2017.00410. [http://dx.doi.org/10.3389/fncel.2017.00410]. [PMID: 29326555]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen Y.C., Sundvik M., Rozov S., Priyadarshini M., Panula P. MANF regulates dopaminergic neuron development in larval zebrafish. Dev. Biol. 2012;370(2):237–249. doi: 10.1016/j.ydbio.2012.07.030. [http://dx.doi.org/10. 1016/j.ydbio.2012.07.030]. [PMID: 22898306]. [DOI] [PubMed] [Google Scholar]
- 121.Palgi M., Lindström R., Peränen J., Piepponen T.P., Saarma M., Heino T.I. Evidence that DmMANF is an invertebrate neurotrophic factor supporting dopaminergic neurons. Proc. Natl. Acad. Sci. USA. 2009;106(7):2429–2434. doi: 10.1073/pnas.0810996106. [http://dx.doi.org/10.1073/ pnas.0810996106]. [PMID: 19164766]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Palgi M., Greco D., Lindström R., Auvinen P., Heino T.I. Gene expression analysis of Drosophilaa Manf mutants reveals perturbations in membrane traffic and major metabolic changes. BMC Genomics. 2012;13(1):134. doi: 10.1186/1471-2164-13-134. [http://dx.doi.org/10.1186/1471-2164-13-134]. [PMID: 22494833]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Petrova P., Raibekas A., Pevsner J., Vigo N., Anafi M., Moore M.K., Peaire A.E., Shridhar V., Smith D.I., Kelly J., Durocher Y., Commissiong J.W. MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J. Mol. Neurosci. 2003;20(2):173–188. doi: 10.1385/jmn:20:2:173. [http://dx.doi.org/10. 1385/JMN:20:2:173]. [PMID: 12794311]. [DOI] [PubMed] [Google Scholar]
- 124.Lindholm P., Voutilainen M.H., Laurén J., Peränen J., Leppänen V.M., Andressoo J.O., Lindahl M., Janhunen S., Kalkkinen N., Timmusk T., Tuominen R.K., Saarma M. Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature. 2007;448(7149):73–77. doi: 10.1038/nature05957. [http://dx.doi.org/ 10.1038/nature05957]. [PMID: 17611540]. [DOI] [PubMed] [Google Scholar]
- 125.Voutilainen M.H., Bäck S., Pörsti E., Toppinen L., Lindgren L., Lindholm P., Peränen J., Saarma M., Tuominen R.K. Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson’s disease. J. Neurosci. 2009;29(30):9651–9659. doi: 10.1523/JNEUROSCI.0833-09.2009. [http://dx.doi.org/10.1523/JNEUROSCI.0833-09.2009]. [PMID: 19641128]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Airavaara M., Harvey B.K., Voutilainen M.H., Shen H., Chou J., Lindholm P., Lindahl M., Tuominen R.K., Saarma M. [DOI] [PMC free article] [PubMed]; Hoffer B., Wang Y. CDNF protects the nigrostriatal dopamine system and promotes recovery after MPTP treatment in mice. Cell Transplant. 2012;21(6):1213–1223. doi: 10.3727/096368911X600948. [http://dx.doi.org/10.3727/ 096368911X600948]. [PMID: 21943517]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bäck S., Peränen J., Galli E., Pulkkila P., Lonka-Nevalaita L., Tamminen T., Voutilainen M.H., Raasmaja A., Saarma M., Männistö P.T., Tuominen R.K. Gene therapy with AAV2-CDNF provides functional benefits in a rat model of Parkinson’s disease. Brain Behav. 2013;3(2):75–88. doi: 10.1002/brb3.117. [http://dx.doi.org/10.1002/brb3. 117]. [PMID: 23532969]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ren X., Zhang T., Gong X., Hu G., Ding W., Wang X. AAV2-mediated striatum delivery of human CDNF prevents the deterioration of midbrain dopamine neurons in a 6-hydroxydopamine induced parkinsonian rat model. Exp. Neurol. 2013;248:148–156. doi: 10.1016/j.expneurol.2013.06.002. [http://dx.doi.org/10.1016/j.expneurol.2013.06.002]. [PMID: 23764500]. [DOI] [PubMed] [Google Scholar]
- 129.Cordero-Llana Ó., Houghton B.C., Rinaldi F., Taylor H., Yáñez-Muñoz R.J., Uney J.B., Wong L-F., Caldwell M.A. Enhanced efficacy of the CDNF/MANF family by combined intranigral overexpression in the 6-OHDA rat model of Parkinson’s disease. Mol. Ther. 2015;23(2):244–254. doi: 10.1038/mt.2014.206. [http://dx.doi.org/10.1038/mt.2014. 206]. [PMID: 25369767]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Subramanian K. Restoration of Motor and Non-Motor Functions by Neurotrophic Factors in Nonhuman Primates with Dopamine Depletion. 2013 http://d-scholarship.pitt.edu/20301/
- 131.Voutilainen M.H., Bäck S., Peränen J., Lindholm P., Raasmaja A., Männistö P.T., Saarma M., Tuominen R.K. Chronic infusion of CDNF prevents 6-OHDA-induced deficits in a rat model of Parkinson’s disease. Exp. Neurol. 2011;228(1):99–108. doi: 10.1016/j.expneurol.2010.12.013. [http://dx. doi.org/10.1016/j.expneurol.2010.12.013]. [PMID: 21185834]. [DOI] [PubMed] [Google Scholar]
- 132.Yasuhara T., Shingo T., Muraoka K., Kameda M., Agari T., Ji W. Y.; Hayase, H.; Hamada, H.; Borlongan, C.V.; Date, I. Neurorescue effects of VEGF on a rat model of Parkinson’s disease. Brain Res. 2005;1053(1-2):10–18. doi: 10.1016/j.brainres.2005.05.027. [http://dx.doi.org/10. 1016/j.brainres.2005.05.027]. [PMID: 16045899]. [DOI] [PubMed] [Google Scholar]
- 133.Padel T., Özen I., Boix J., Barbariga M., Gaceb A., Roth M., Paul G. Platelet-derived growth factor-BB has neurorestorative effects and modulates the pericyte response in a partial 6-hydroxydopamine lesion mouse model of Parkinson’s disease. Neurobiol. Dis. 2016;94:95–105. doi: 10.1016/j.nbd.2016.06.002. [http://dx.doi.org/10.1016/j.nbd. 2016.06.002]. [PMID: 27288154]. [DOI] [PubMed] [Google Scholar]
- 134.Yasuhara T., Shingo T., Muraoka K. wen Ji, Y.; Kameda, M.; Takeuchi, A.; Yano, A.; Nishio, S.; Matsui, T.; Miyoshi, Y.; Hamada, H.; Date, I. The differences between high and low-dose administration of VEGF to dopaminergic neurons of in vitro and in vivo Parkinson’s disease model. Brain Res. 2005;1038(1):1–10. doi: 10.1016/j.brainres.2004.12.055. [http://dx.doi.org/10.1016/j.brainres.2004.12.055]. [PMID: 15748867]. [DOI] [PubMed] [Google Scholar]
- 135.Meng X., Lindahl M., Hyvönen M.E., Parvinen M., de Rooij D.G., Hess M.W., Raatikainen-Ahokas A., Sainio K., Rauvala H., Lakso M., Pichel J.G., Westphal H., Saarma M., Sariola H. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287(5457):1489–1493. doi: 10.1126/science.287.5457.1489. [http://dx.doi.org/ 10.1126/science.287.5457.1489]. [PMID: 10688798]. [DOI] [PubMed] [Google Scholar]
- 136.Bergmann O., Spalding K.L., Frisén J. Adult Neurogenesis in Humans. Cold Spring Harb. Perspect. Biol. 2015;7(7):a018994. doi: 10.1101/cshperspect.a018994. [http://dx.doi.org/10.1101/cshperspect.a018994]. [PMID: 26134318]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sorrells S.F., Paredes M.F., Cebrian-Silla A., Sandoval K., Qi D., Kelley K.W., James D., Mayer S., Chang J., Auguste K.I., Chang E.F., Gutierrez A.J., Kriegstein A.R., Mathern G.W., Oldham M.C., Huang E.J., Garcia-Verdugo J.M., Yang Z., Alvarez-Buylla A. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555(7696):377–381. doi: 10.1038/nature25975. [http://dx.doi.org/10.1038/nature25975]. [PMID: 29513649]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xie M.Q., Chen Z.C., Zhang P., Huang H.J., Wang T.T., Ding Y.Q., Qi S.S., Zhang C., Chen S.X., Zhou P., Shao C.C., Liao M., Sun C.Y. Newborn dopaminergic neurons are associated with the migration and differentiation of SVZ-derived neural progenitors in a 6-hydroxydopamin-injected mouse model. Neuroscience. 2017;352:64–78. doi: 10.1016/j.neuroscience.2017.03.045. [http://dx.doi.org/10.1016/j.neuroscience.2017. 03.045]. [PMID: 28385636]. [DOI] [PubMed] [Google Scholar]
- 139.Krajnak K., Dahl R. Small molecule SUMOylation activators are novel neuroprotective agents. Bioorg. Med. Chem. Lett. 2017;28(3):405–409. doi: 10.1016/j.bmcl.2017.12.028. [http://dx.doi.org/10.1016/j.bmcl.2017.12.028]. [PMID: 29269215]. [DOI] [PubMed] [Google Scholar]
- 140.Kim S.M., Park Y.J., Shin M.S., Kim H.R., Kim M.J., Lee S.H., Yun S.P., Kwon S.H. Acacetin inhibits neuronal cell death induced by 6-hydroxydopamine in cellular Parkinson’s disease model. Bioorg. Med. Chem. Lett. 2017;27(23):5207–5212. doi: 10.1016/j.bmcl.2017.10.048. [http://dx.doi.org/10.1016/j.bmcl.2017.10.048]. [PMID: 29089232]. [DOI] [PubMed] [Google Scholar]
- 141.Salakhutdinov N.F., Volcho K.P., Yarovaya O.I. Monoterpenes as a Renewable Source of Biologically Active Compounds. Pure Appl. Chem. 2017;89(8):1105–1117. [http://dx.doi.org/10.1515/ pac-2017-0109]. [Google Scholar]
- 142.Santos C.M., Santos M. New agents promote neuroprotection in Parkinson’s disease models. CNS Neurol. Disord. Drug Targets. 2012;11(4):410–418. doi: 10.2174/187152712800792820. [http://dx.doi.org/10.2174/187152712800792820]. [PMID: 22483311]. [DOI] [PubMed] [Google Scholar]
- 143.Le Douaron G., Ferrié L., Sepulveda-Diaz J.E., Amar M., Harfouche A., Séon-Méniel B., Raisman-Vozari R., Michel P.P., Figadère B. New 6-Aminoquinoxaline Derivatives with Neuroprotective Effect on Dopaminergic Neurons in Cellular and Animal Parkinson Disease Models. J. Med. Chem. 2016;59(13):6169–6186. doi: 10.1021/acs.jmedchem.6b00297. [http://dx.doi.org/10.1021/acs.jmedchem.6b00297]. [PMID: 27341519]. [DOI] [PubMed] [Google Scholar]
- 144.Zhang Q., Chen S., Yu S., Qin J., Zhang J., Cheng Q., Ke K., Ding F. Neuroprotective effects of pyrroloquinoline quinone against rotenone injury in primary cultured midbrain neurons and in a rat model of Parkinson’s disease. Neuropharmacology. 2016;108:238–251. doi: 10.1016/j.neuropharm.2016.04.025. [http://dx.doi.org/10.1016/j.neuropharm.2016.04.025]. [PMID: 27108097]. [DOI] [PubMed] [Google Scholar]
- 145.Dati L.M., Ulrich H., Real C.C., Feng Z.P., Sun H.S., Britto L.R. Carvacrol promotes neuroprotection in the mouse hemiparkinsonian model. Neuroscience. 2017;356:176–181. doi: 10.1016/j.neuroscience.2017.05.013. [http://dx.doi. org/10.1016/j.neuroscience.2017.05.013]. [PMID: 28526576]. [DOI] [PubMed] [Google Scholar]
- 146.Guo Z., Xu S., Du N., Liu J., Huang Y., Han M. Neuroprotective effects of stemazole in the MPTP-induced acute model of Parkinson’s disease: Involvement of the dopamine system. Neurosci. Lett. 2016;616:152–159. doi: 10.1016/j.neulet.2016.01.048. [http://dx.doi.org/10.1016/j.neulet. 2016.01.048]. [PMID: 26827716]. [DOI] [PubMed] [Google Scholar]
- 147.Singh A.K., Halder-Sinha S., Clement J.P., Kundu T.K. Epigenetic modulation by small molecule compounds for neurodegenerative disorders. Pharmacol. Res. 2018;132(132):135–148. doi: 10.1016/j.phrs.2018.04.014. [http:// dx.doi.org/10.1016/j.phrs.2018.04.014]. [PMID: 29684672]. [DOI] [PubMed] [Google Scholar]
- 148.O’Keeffe G.C., Tyers P., Aarsland D., Dalley J.W., Barker R.A., Caldwell M.A. Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc. Natl. Acad. Sci. USA. 2009;106(21):8754–8759. doi: 10.1073/pnas.0803955106. [http://dx.doi.org/10.1073/pnas.0803955106]. [PMID: 19433789]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Van Kampen J.M., Eckman C.B. Dopamine D3 receptor agonist delivery to a model of Parkinson’s disease restores the nigrostriatal pathway and improves locomotor behavior. J. Neurosci. 2006;26(27):7272–7280. doi: 10.1523/JNEUROSCI.0837-06.2006. [http://dx.doi.org/10.1523/JNEUROSCI.0837-06.2006]. [PMID: 16822985]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Milosevic J., Schwarz S.C., Maisel M., Poppe-Wagner M., Dieterlen M-T., Storch A., Schwarz J. Dopamine D2/D3 receptor stimulation fails to promote dopaminergic neurogenesis of murine and human midbrain-derived neural precursor cells in vitro. Stem Cells Dev. 2007;16(4):625–635. doi: 10.1089/scd.2006.0113. [http://dx.doi.org/10.1089/scd. 2006.0113]. [PMID: 17784836]. [DOI] [PubMed] [Google Scholar]
- 151.O’Keeffe G.C., Barker R.A., Caldwell M.A. Dopaminergic modulation of neurogenesis in the subventricular zone of the adult brain. Cell Cycle. 2009;8(18):2888–2894. doi: 10.4161/cc.8.18.9512. [http://dx.doi.org/10. 4161/cc.8.18.9512]. [PMID: 19713754]. [DOI] [PubMed] [Google Scholar]
- 152.Winner B., Desplats P., Hagl C., Klucken J., Aigner R., Ploetz S., Laemke J., Karl A., Aigner L., Masliah E., Buerger E., Winkler J. Dopamine receptor activation promotes adult neurogenesis in an acute Parkinson model. Exp. Neurol. 2009;219(2):543–552. doi: 10.1016/j.expneurol.2009.07.013. [http://dx.doi.org/10.1016/j.expneurol.2009.07.013]. [PMID: 19619535]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McCormack P.L. Rasagiline: a review of its use in the treatment of idiopathic Parkinson’s disease. CNS Drugs. 2014;28(11):1083–1097. doi: 10.1007/s40263-014-0206-y. [http://dx.doi.org/10.1007/s40263-014-0206-y]. [PMID: 25322951]. [DOI] [PubMed] [Google Scholar]
- 154.Sagi Y., Mandel S., Amit T., Youdim M.B.H. Activation of tyrosine kinase receptor signaling pathway by rasagiline facilitates neurorescue and restoration of nigrostriatal dopamine neurons in post-MPTP-induced parkinsonism. Neurobiol. Dis. 2007;25(1):35–44. doi: 10.1016/j.nbd.2006.07.020. [http://dx.doi.org/10.1016/j.nbd.2006.07.020]. [PMID: 17055733]. [DOI] [PubMed] [Google Scholar]
- 155.Mandel S.A., Sagi Y., Amit T. Rasagiline promotes regeneration of substantia nigra dopaminergic neurons in post-MPTP-induced Parkinsonism via activation of tyrosine kinase receptor signaling pathway. Neurochem. Res. 2007;32(10):1694–1699. doi: 10.1007/s11064-007-9351-8. [http://dx. doi.org/10.1007/s11064-007-9351-8]. [PMID: 17701352]. [DOI] [PubMed] [Google Scholar]
- 156.Reznichenko L., Kalfon L., Amit T., Youdim M.B.H., Mandel S.A. Low dosage of rasagiline and epigallocatechin gallate synergistically restored the nigrostriatal axis in MPTP-induced parkinsonism. Neurodegener. Dis. 2010;7(4):219–231. doi: 10.1159/000265946. [http://dx.doi. org/10.1159/000265946]. [PMID: 20197647]. [DOI] [PubMed] [Google Scholar]
- 157.Olanow C.W., Rascol O., Hauser R., Feigin P.D., Jankovic J., Lang A., Langston W., Melamed E., Poewe W., Stocchi F., Tolosa E. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N. Engl. J. Med. 2009;361(13):1268–1278. doi: 10.1056/NEJMoa0809335. [http://dx.doi.org/10.1056/NEJMoa0809335]. [PMID: 19776408]. [DOI] [PubMed] [Google Scholar]
- 158.Youdim M.B.H. Rasagiline in Parkinson’s disease. N. Engl. J. Med. 2010;362(7):657–658. doi: 10.1056/NEJMc0910491. [http://dx.doi.org/10.1056/ NEJMc0910491]. [PMID: 20164492]. [DOI] [PubMed] [Google Scholar]
- 159.Jang S-W., Liu X., Yepes M., Shepherd K.R., Miller G.W., Liu Y., Wilson W.D., Xiao G., Blanchi B., Sun Y.E., Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA. 2010;107(6):2687–2692. doi: 10.1073/pnas.0913572107. [http://dx.doi.org/10.1073/pnas.0913572107]. [PMID: 20133810]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nie S., Xu Y., Chen G., Ma K., Han C., Guo Z., Zhang Z., Ye K., Cao X. Small molecule TrkB agonist deoxygedunin protects nigrostriatal dopaminergic neurons from 6-OHDA and MPTP induced neurotoxicity in rodents. Neuropharmacology. 2015;99:448–458. doi: 10.1016/j.neuropharm.2015.08.016. [http://dx.doi.org/10.1016/j.neuropharm.2015.08.016]. [PMID: 26282118]. [DOI] [PubMed] [Google Scholar]
- 161.Luo D., Shi Y., Wang J., Lin Q., Sun Y., Ye K., Yan Q., Zhang H. 7,8-dihydroxyflavone protects 6-OHDA and MPTP induced dopaminergic neurons degeneration through activation of TrkB in rodents. Neurosci. Lett. 2016;620:43–49. doi: 10.1016/j.neulet.2016.03.042. [http://dx.doi. org/10.1016/j.neulet.2016.03.042]. [PMID: 27019033]. [DOI] [PubMed] [Google Scholar]
- 162.Li X-H., Dai C-F., Chen L., Zhou W-T., Han H-L., Dong Z-F. 7,8-dihydroxyflavone Ameliorates Motor Deficits Via Suppressing α-synuclein Expression and Oxidative Stress in the MPTP-induced Mouse Model of Parkinson’s Disease. CNS Neurosci. Ther. 2016;22(7):617–624. doi: 10.1111/cns.12555. [http://dx.doi.org/10.1111/cns.12555]. [PMID: 27079181]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.He J., Xiang Z., Zhu X., Ai Z., Shen J., Huang T., Liu L., Ji W., Li T. Neuroprotective Effects of 7, 8-dihydroxyflavone on Midbrain Dopaminergic Neurons in MPP+-treated Monkeys. Sci. Rep. 2016;6:34339. doi: 10.1038/srep34339. [http://dx.doi.org/10.1038/srep34339]. [PMID: 27731318]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Boltaev U., Meyer Y., Tolibzoda F., Jacques T., Gassaway M., Xu Q., Wagner F., Zhang Y.-L., Palmer M., Holson E. Multiplex Quantitative Assays Indicate a Need for Reevaluating Reported Small-Molecule TrkB Agonists. 2017. [DOI] [PubMed]
- 165.Wu C.H., Hung T.H., Chen C.C., Ke C.H., Lee C.Y., Wang P.Y., Chen S.F. Post-injury treatment with 7,8-dihydroxyflavone, a TrkB receptor agonist, protects against experimental traumatic brain injury via PI3K/Akt signaling. PLoS One. 2014;9(11):e113397. doi: 10.1371/journal.pone.0113397. [http://dx.doi.org/10.1371/journal.pone.0113397]. [PMID: 25415296]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sinyakova N.A., Bazhenova E.Y., Bazovkina D.V. Kulikov, A.V. Effect of the TrkB Antagonist-7,8-Dihydroxyflavone on Mice Serotonin System. Zh. Vyssh. Nerv. Deiat. Im. I P Pavlova. 2018;68(1):125–134. [ DOI: 10.7868/S0044467718010100]. [Google Scholar]
- 167.Chen J., Chua K.W., Chua C.C., Yu H., Pei A., Chua B.H.L., Hamdy R.C., Xu X., Liu C.F. Antioxidant activity of 7,8-dihydroxyflavone provides neuroprotection against glutamate-induced toxicity. Neurosci. Lett. 2011;499(3):181–185. doi: 10.1016/j.neulet.2011.05.054. [http://dx. doi.org/10.1016/j.neulet.2011.05.054]. [PMID: 21651962]. [DOI] [PubMed] [Google Scholar]
- 168.Han X., Zhu S., Wang B., Chen L., Li R., Yao W., Qu Z. Antioxidant action of 7,8-dihydroxyflavone protects PC12 cells against 6-hydroxydopamine-induced cytotoxicity. Neurochem. Int. 2014;64(1):18–23. doi: 10.1016/j.neuint.2013.10.018. [http://dx.doi.org/10.1016/j.neuint.2013.10. 018]. [PMID: 24220540]. [DOI] [PubMed] [Google Scholar]
- 169.Spagnuolo C., Moccia S., Russo G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018;153:105–115. doi: 10.1016/j.ejmech.2017.09.001. [http://dx.doi.org/10.1016/j.ejmech.2017.09. 001]. [PMID: 28923363]. [DOI] [PubMed] [Google Scholar]
- 170.Massa S.M., Yang T., Xie Y., Shi J., Bilgen M., Joyce J.N., Nehama D., Rajadas J., Longo F.M. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J. Clin. Invest. 2010;120(5):1774–1785. doi: 10.1172/JCI41356. [http://dx. doi.org/10.1172/JCI41356]. [PMID: 20407211]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yang T. 2016. [Google Scholar]
- 172.Kowsky S., Pöppelmeyer C., Kramer E.R., Falkenburger B.H., Kruse A., Klein R., Schulz J.B. RET signaling does not modulate MPTP toxicity but is required for regeneration of dopaminergic axon terminals. Proc. Natl. Acad. Sci. USA. 2007;104(50):20049–20054. doi: 10.1073/pnas.0706177104. [http://dx.doi.org/10.1073/pnas.0706177104]. [PMID: 18056810]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Drinkut A., Tillack K., Meka D.P., Schulz J.B., Kügler S., Kramer E.R. Ret is essential to mediate GDNF’s neuroprotective and neuroregenerative effect in a Parkinson disease mouse model. Cell Death Dis. 2016;7(9):e2359. doi: 10.1038/cddis.2016.263. [http://dx.doi.org/10.1038/ cddis.2016.263]. [PMID: 27607574]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bespalov M.M., Saarma M. GDNF family receptor complexes are emerging drug targets. Trends Pharmacol. Sci. 2007;28(2):68–74. doi: 10.1016/j.tips.2006.12.005. [http://dx.doi.org/10.1016/j.tips.2006.12.005]. [PMID: 17218019]. [DOI] [PubMed] [Google Scholar]
- 175.Tokugawa K., Yamamoto K., Nishiguchi M., Sekine T., Sakai M., Ueki T., Chaki S., Okuyama S. XIB4035, a novel nonpeptidyl small molecule agonist for GFRalpha-1. Neurochem. Int. 2003;42(1):81–86. doi: 10.1016/s0197-0186(02)00053-0. [http://dx.doi.org/10.1016/S0197-0186(02)00053-0]. [PMID: 12441171]. [DOI] [PubMed] [Google Scholar]
- 176.Hedstrom K.L., Murtie J.C., Albers K., Calcutt N.A., Corfas G. Treating small fiber neuropathy by topical application of a small molecule modulator of ligand-induced GFRα/RET receptor signaling. Proc. Natl. Acad. Sci. USA. 2014;111(6):2325–2330. doi: 10.1073/pnas.1308889111. [http://dx.doi.org/10.1073/pnas.1308889111]. [PMID: 24449858]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sidorova Y.A., Bespalov M.M., Wong A.W., Kambur O., Jokinen V., Lilius T.O., Suleymanova I., Karelson G., Rauhala P.V., Karelson M., Osborne P.B., Keast J.R., Kalso E.A., Saarma M. A novel small molecule gdnf receptor RET agonist, BT13, promotes neurite growth from sensory neurons in vitro and attenuates experimental neuropathy in the rat. Front. Pharmacol. 2017;8(JUN):365. doi: 10.3389/fphar.2017.00365. [PMID: 28680400]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ivanova L., Tammiku-Taul J., Sidorova Y., Saarma M., Karelson M. Small-Molecule Ligands as Potential GDNF Family Receptor Agonists. ACS Omega. 2018;3(1):1022–1030. doi: 10.1021/acsomega.7b01932. [http://dx. doi.org/10.1021/acsomega.7b01932]. [PMID: 30023796]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gilligan P.J. Inhibitors of leucine-rich repeat kinase 2 (LRRK2): progress and promise for the treatment of Parkinson’s disease. Curr. Top. Med. Chem. 2015;15(10):927–938. doi: 10.2174/156802661510150328223655. [http://dx.doi.org/ 10.2174/156802661510150328223655]. [PMID: 25832719]. [DOI] [PubMed] [Google Scholar]
- 180.Salado I.G., Zaldivar-Diez J., Sebastián-Pérez V., Li L., Geiger L., González S., Campillo N.E., Gil C., Morales A.V., Perez D.I., Martinez A. Leucine rich repeat kinase 2 (LRRK2) inhibitors based on indolinone scaffold: Potential pro-neurogenic agents. Eur. J. Med. Chem. 2017;138:328–342. doi: 10.1016/j.ejmech.2017.06.060. [http://dx.doi.org/10.1016/j. ejmech.2017.06.060]. [PMID: 28688273]. [DOI] [PubMed] [Google Scholar]
- 181.Liao G.Z., Wang G.Y., Xu X.L., Zhou G.H. Effect of cooking methods on the formation of heterocyclic aromatic amines in chicken and duck breast. Meat Sci. 2010;85(1):149–154. doi: 10.1016/j.meatsci.2009.12.018. [http:// dx.doi.org/10.1016/j.meatsci.2009.12.018]. [PMID: 20374878]. [DOI] [PubMed] [Google Scholar]
- 182.Hamann J., Wernicke C., Lehmann J., Reichmann H., Rommelspacher H., Gille G. 9-Methyl-β-carboline up-regulates the appearance of differentiated dopaminergic neurones in primary mesencephalic culture. Neurochem. Int. 2008;52(4-5):688–700. doi: 10.1016/j.neuint.2007.08.018. [http:// dx.doi.org/10.1016/j.neuint.2007.08.018]. [PMID: 17913302]. [DOI] [PubMed] [Google Scholar]
- 183.Polanski W., Reichmann H., Gille G. Stimulation, protection and regeneration of dopaminergic neurons by 9-methyl-β-carboline: a new anti-Parkinson drug? Expert Rev. Neurother. 2011;11(6):845–860. doi: 10.1586/ern.11.1. [http://dx.doi.org/10.1586/ern.11.1]. [PMID: 21651332]. [DOI] [PubMed] [Google Scholar]
- 184.Polanski W., Enzensperger C., Reichmann H., Gille G. The exceptional properties of 9-methyl-β-carboline: stimulation, protection and regeneration of dopaminergic neurons coupled with anti-inflammatory effects. J. Neurochem. 2010;113(6):1659–1675. doi: 10.1111/j.1471-4159.2010.06725.x. [PMID: 20374418]. [DOI] [PubMed] [Google Scholar]
- 185.Wernicke C., Hellmann J., Zieba B., Kuter K., Ossowska K., Frenzel M., Dencher N.A., Rommelspacher H. 9-Methyl-beta-carboline has restorative effects in an animal model of Parkinson’s disease. Pharmacol. Rep. 2010;62(1):35–53. doi: 10.1016/s1734-1140(10)70241-3. [http://dx.doi.org/ 10.1016/S1734-1140(10)70241-3]. [PMID: 20360614]. [DOI] [PubMed] [Google Scholar]
- 186.Matsubara K., Gonda T., Sawada H., Uezono T., Kobayashi Y., Kawamura T., Ohtaki K., Kimura K., Akaike A. Endogenously occurring beta-carboline induces parkinsonism in nonprimate animals: a possible causative protoxin in idiopathic Parkinson’s disease. J. Neurochem. 1998;70(2):727–735. doi: 10.1046/j.1471-4159.1998.70020727.x. [http://dx.doi.org/10. 1046/j.1471-4159.1998.70020727.x]. [PMID: 9453568]. [DOI] [PubMed] [Google Scholar]
- 187.De Jesús-Cortés H., Xu P., Drawbridge J., Estill S.J., Huntington P., Tran S., Britt J., Tesla R., Morlock L., Naidoo J., Melito L.M., Wang G., Williams N.S., Ready J.M., McKnight S.L., Pieper A.A. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease. Proc. Natl. Acad. Sci. USA. 2012;109(42):17010–17015. doi: 10.1073/pnas.1213956109. [http://dx.doi. org/10.1073/pnas.1213956109]. [PMID: 23027934]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pieper A.A., Xie S., Capota E., Estill S.J., Zhong J., Long J.M., Becker G.L., Huntington P., Goldman S.E., Shen C.H., Capota M., Britt J.K., Kotti T., Ure K., Brat D.J., Williams N.S., MacMillan K.S., Naidoo J., Melito L., Hsieh J., De Brabander J., Ready J.M., McKnight S.L. Discovery of a proneurogenic, neuroprotective chemical. Cell. 2010;142(1):39–51. doi: 10.1016/j.cell.2010.06.018. [http://dx.doi.org/ 10.1016/j.cell.2010.06.018]. [PMID: 20603013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Garrett S.M., Whitaker R.M., Beeson C.C., Schnellmann R.G. Agonism of the 5-hydroxytryptamine 1F receptor promotes mitochondrial biogenesis and recovery from acute kidney injury. J. Pharmacol. Exp. Ther. 2014;350(2):257–264. doi: 10.1124/jpet.114.214700. [http://dx.doi.org/ 10.1124/jpet.114.214700]. [PMID: 24849926]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Scholpa N.E., Lynn M.K., Corum D., Boger H.A., Schnellmann R.G. 5-HT1F receptor-mediated mitochondrial biogenesis for the treatment of Parkinson’s disease. Br. J. Pharmacol. 2018;175(2):348–358. doi: 10.1111/bph.14076. [http://dx.doi.org/10.1111/bph.14076]. [PMID: 29057453]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Su C., Elfeki N., Ballerini P., D’Alimonte I., Bau C., Ciccarelli R., Caciagli F., Gabriele J., Jiang S. Guanosine improves motor behavior, reduces apoptosis, and stimulates neurogenesis in rats with parkinsonism. J. Neurosci. Res. 2009;87(3):617–625. doi: 10.1002/jnr.21883. [http:// dx.doi.org/10.1002/jnr.21883]. [PMID: 18816792]. [DOI] [PubMed] [Google Scholar]