Abstract
Alzheimer’s disease (AD) is an age-related progressive neurodegenerative disorder, characterized by the deposition of amyloid-β within the brain parenchyma resulting in a significant decline in cognitive functions. The pathophysiological conditions of the disease are recognized by the perturbation of synaptic function, energy and lipid metabolism. In Addition deposition of amyloid plaques also triggers inflammation upon the induction of microglia. Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors known to play important role in the regulation of glucose ab-sorption, homeostasis of lipid metabolism and are further known to involved in repressing the expression of genes related to inflammation. Therefore, agonists of this receptor represent an attractive therapeutic target for AD. Recently, both clinical and preclinical studies showed that use of Peroxisome proliferator-activated receptor gamma (PPARγ) agonist improves both learning and memory along with other AD related pathology. Thus, PPARγ signifies a significant new therapeutic target in treating AD. In this review, we have shed some light on the recent progress of how, PPARγ agonist selectively modulated different cellular targets in AD and its amazing potential in the treatment of AD.
Keywords: Alzheimer’s disease, Peroxisome proliferator-activated receptors, Transactivation, β-amyloid, Thiazolidinedione, Insulin sensitivity, Rosiglitazone, Blood-brain-barrier
1. INTRODUCTION
Alzheimer’s disease (AD) is the most common form of neurodegenerative disorder that mostly affects the older population in the world. It has been estimated that about 4.7 million individuals were affected with AD in the United States, that will increase to approximately 14 million by 2050 [1]. So far, it is still believed as an irreparable brain disease, which becomes severe with time if not treated and ultimately causes deterioration of memory and reasoning in patients. This is a complex disorder which occurs due to co pathogenic interactions among various constituents like genetic and environmental factors. It is characterized by accumulation of amyloid β (Aβ) plaques in susceptible regions of the brain, extensive neuronal loss and formation of neurofibrillary tangles [2]. These plaques are accompanied by mitochondrial dysfunction which contributes to increase in oxidative stress and inflammatory response and so results in energy failure and synaptic dysfunction [3, 4]. However, several clinical and pre-clinical studies showed that AD could be a degenerative metabolic disease, caused by physiological alterations. Furthermore, AD causes a substantial socio-economic impact on the world and it is estimated that direct and indirect healthcare cost of AD individuals is approximately $203 billion which will increase to approximately $1.2 trillion by the year 2050 [5]. Recently, it was observed that diabetes is the most crucial risk factor for the development of AD and the therapy which controls the diabetes-related metabolic disorders like insulin level, glucose metabolism and hypercholestremia may be helpful in ameliorating the symptoms of AD.
Peroxisome proliferators-activated receptors (PPARs) are lipid sensor nuclear receptors that regulate several cellular processes such as Aβ degradation, anti-inflammatory response and mitochondrial activation in response to various intracellular and extracellular stimuli [6, 7]. Peroxisome proliferator-activated receptor gamma (PPARγ) is the most studied isoform of the PPAR family that holds most promising therapeutic potential in various models of neurodegenerative disorders and generates tremendous interest in developing PPARγ agonist for the treatment of AD [8]. Thiazolidinedione (TZD), is a PPARγ agonist that has been widely used in the treatment of diabetes, whose therapeutic mechanism of action to persuade a decrease the blood glucose level by insulin-sensitizing effect [9]. In this review, we have shed some light on the recent progress of how PPARγ agonist selectively modulated different cellular targets in AD and its amazing potential in the treatment of AD. Our current understanding will be focused to reveal important possible effects of PPARγ agonist in the improvement of cognitive impairment occur due to the oxidative stress, neuroinflammation, deposition of Aβ, energy metabolism and cerebrovascular protection.
2. PEROXISOME PROLIFERATORS ACTIVATED RECEPTORS GAMMA (PPARΓ)
The PPARs belong to the family of nuclear hormone receptors (NHR) that induce signaling and transcription of a unique set of genes in response to various exogenous and endogenous ligands. Generally, they participate in the regulation of glucose and lipid metabolism [10]. More recently it has been reported that PPARγ may play important role in inhibiting inflammation and also regulating insulin sensitivity [11]. Further, few studies also indicated that activation of PPARγ receptor by PPARγ agonist is helpful in the prevention of neurodegeneration and promotes neurogenesis [12].
2.1. Most Common Type of PPARγ Agonist
(I). Natural PPARγ Agonist
Activation of PPARγ and subsequent regulation of gene transcription requires binding of ligand. To date a number of ligands are identified that regulate the PPARγ activity and include both synthetic compounds and natural metabolites. Various natural ligands with different binding affinities to the receptor are produced within the body in response to various metabolic processes. These include dietary lipids and their metabolites. Linoleic acid (9- and 13-HODE) and prostaglandin derivative, 15-deoxi-Δ 12, 14-prostaglandin J2 (15d-PGJ2) is first and perhaps the most powerful endogenous PPARγ ligand. Gamolenic acid, eicosapentaenic acid, and some polyunsaturated fatty acid metabolites are other endogenous ligands [13]. HETE (hydroxyeicosatetraenoic acid) and HODE (hydroxydocosahexaenoic acid), a polyunsaturated fatty acid derived metabolite found in oxidized low-density lipoprotein (oxLDL) in addition to membranes and produce as a result of oxidation of arachidonic acid and linoleic acid, respectively capable of activating PPARγ with effectiveness similar to 15d-PGJ2 [14].
(II). Synthetic PPARγ Agonist
Synthetic PPARγ agonists are widely used as anti-hyperglycemic drugs in treating type 2 diabetes mellitus [15]. The most widely known PPAR-γ agonists include TZDs. TZDs, also known as glitazones, that include pioglitazone (Actos), troglitazone (Rezulin), ciglitazone and rosiglitazone (Avandia). Apart from their anti-diabetic property, emerging evidence indicates that all the TZDs possess anti-inflammatory properties by inhibiting expression of a variety of inflammatory molecules [11]. Pioglitazone (Actos) and rosiglitazone (Avandia) are two FDA approved drugs prescribed widely by physician for treatment of many diseases due to nontoxic nature [16]. Troglitazone which was the first member of this group was approved by FDA, however, due to some reported serious side effect, it was later prohibited by FDA.
Non-steroidal Anti-inflammatory Drug (NSAIDs) like Ibuprofen, indomethacin, and flurbiprofen are also known to have PPARγ agonist activity and have been thoroughly studied in AD due to its anti-inflammatory property. This may make it be ideal for efficacious in significantly delaying incidence and progression of AD [17].
Another class of drug, Telmisartan, blocker of angiotensin receptor II type 1 (AT1), normally used for the hypertension treatment, is recently appreciated PPARγ activation function and executes a range of functions, including anti-apoptotic, anti-inflammatory and ROS scavenging effects [18, 19]. In consistence with this, study on mouse model of AD shows ameliorative effects on the impairment of spatial memory via its anti-inflammatory and antioxidant effect [20-22].
3. REGULATION OF TARGET GENE EXPRESSION BY PPARγ
3.1. Transactivation
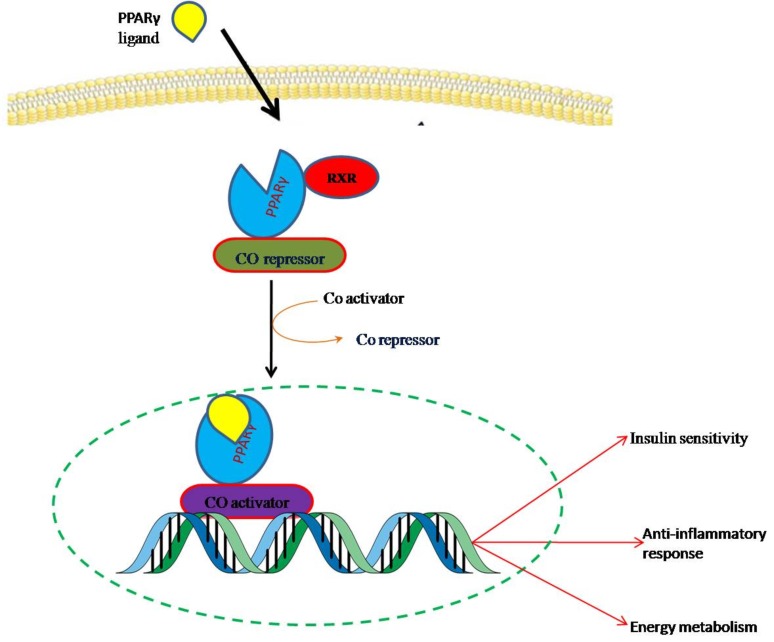
Peroxisome proliferators activated receptors (PPARs) are a kind of ligand-activated transcription factor that provides a direct association between the environment and the genome. Under basal conditions when agonist is absent the transcriptional activity of PPARγ is repressed through its constitutive alliance with nuclear co-repressors like N-CoR/SMRT and histone deacetylases (HDAC). In the presence of ligands, co-repressor complex is exchanged with co-activator complex containing Histone acetyltransferase (HAT) and initiates the transcription of target genes (by forming heterodimers with retinoid-X-receptors (RXRs)) on binding with the specific PPAR-response elements (PPREs) present in the promoter region of target genes. PPARγ has the ability to bind with a variety of compounds derived such as dietary lipids and their metabolites, transcriptionally transactivates specific target genes expression [23]. They act as dominant positive regulators of expression of enzymes involved in lipid metabolism including CD36, prolipoprotein lipase and liver X receptor-α (LXR-α) [24]. PPARγ also regulates genes involved in insulin signaling and the expression of pro-inflammatory cytokines such as tumor necrosis factor-α (Fig. 1) [25].
Fig. (1).
Transactivation of target genes by PPARγ on binding with PPARγ agonist. Upon ligand binding PPARγ and RXR hetrodimer undergoes conformational change that result in exchange of corepressor with coactivator complex and translocates into the nucleus, where it binds to the PPRE to activate the target genes. (The color version of the figure is available in the electronic copy of the article).
3.2. Transrepression
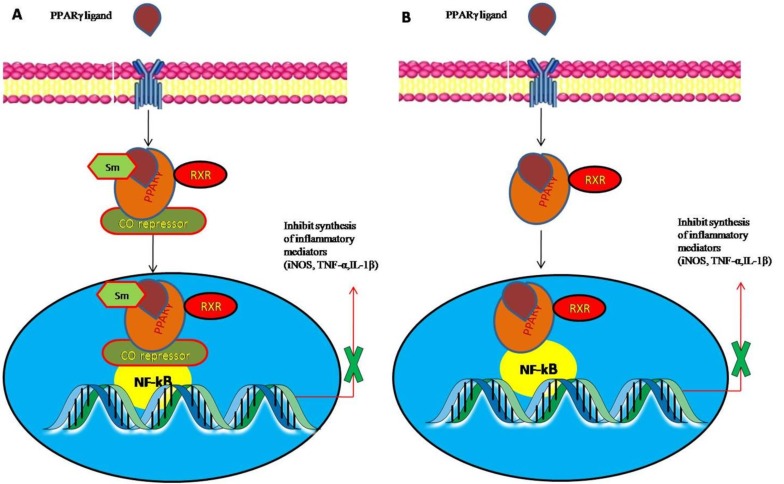
In addition to their potent role in glucose metabolism, recent studies have now focused on the anti-inflammatory action of PPARγ. PPARγ expressions are augmented by specific ligands which further inhibiting expression of pro-inflammatory gene and have been shown to exert a broad spectrum of protective effects in several animal models [26-28]. Even though the exact mechanism remains unknown, it is believed that co-repressor complexes occupied on the promoters region of NF-kB is stabilized by PPARγ agonist by SUMOylation of PPARγ ligand binding domain within PPARγ that targets co-repressor complexes (corepressor interference mechanism) and also by direct binding of PPARγ to NF-kB forming inactive transcriptional complexes (cross-coupling mechanism), thus preventing the expression of various pro-inflammatory genes (Fig. 2) [29-31]. Indeed several studies showed that treatment with 15d-PGJ2 or troglitazone inhibits the overexpression of inflammatory cytokines in phorbol12-myristate13-acetate-stimulated human peripheral monocytes [32]. Thus, PPARγ agonists are an attractive therapeutic target due to their capability to act largely to inhibit expression of various inflammatory genes.
Fig. (2).
Mechanisms of peroxisome proliferator-activated receptor gamma (PPARγ) transrepression- (A) Co repressor interference mechanism- co-repressor complexes occupied on the promoters region of NF-kB is stabilize by PPARγ agonist by SUMOylation of PPARγ ligand binding domain within PPARγ and inhibit proinflammatory inhibitors (B) Cross coupling- direct binding of PPARγ to NF-kB forming inactive transcriptional complexes and inhibit proinflammatory inhibitors. (The color version of the figure is available in the electronic copy of the article).
4. EFFECTS OF PEROXISOME PROLIFERATOR-ACTIVATED RECEPTOR GAMMA (PPARΓ) AGONIST IN AD
4.1. Peroxisome Proliferator-activated Receptor Gamma (PPARγ) Agonist and Inflammation in Alzheimer’s Disease
The ability of PPARγ to inhibit the expression of the inflammatory gene in some peripheral and central nervous system (CNS) disease conditions in response to natural PPARγ agonists provided the idea to keep attention on the inflammatory mechanism in terms of treatment of AD. Accumulative evidence indicates that amyloid deposition in the AD brain coincides with a phenotypic activation of microglial cells that are physically associated with plaque through multi-component cell surface receptor complex [33, 34]. Microglias are the resident brain tissue macrophages. Activation of this cell surface receptor complex initiates intracellular signaling cascades that lead to NF-kB-mediated pro-inflammatory gene transcription and subsequent increase production of wide range of inflammatory mediators including complement proteins, cytokines, chemokines, reactive oxygen species (ROS)/ reactive nitrogen species (RNS) and proteases [35]. This chronic increase in production of inflammatory cytokines and chemokines hypothesizes to speed up the severity of the disease. Consistent with this, a number of clinical and preclinical evidence showed that elevated level of inflammatory cytokines and chemokines including Tumor necrosis factor-α, interleukin-1β, interleukin-6 and monocyte chemotactic protein-1, (MCP-1) was found in stable AD brain. Notably, studies designed for suppressing microglial activation and so inflammation could be a promising approach for AD therapy [28, 35].
As discussed above, treatment of PPARγ agonist appears to regulate the production of inflammatory mediators, metallopeptidases (MMPs) and amyloid protein, each of which is dependent upon NF-kB-dependent transcriptional pathways, with that, prevented the activation of microglia. More recently, APPswe/PS1Δe9 mouse model of AD shows that Pioglitazone reduced activation of microglial and reduction in soluble and insoluble Aβ levels in the specific part of the brain responsible for memory [36-38]. At the same time, treatment with rosiglitazone, prevented the increase of inflammatory cytokines levels in Wistar rats and this is related to improvement in cognitive decline and prevention of microglia activation. 15d-PGJ2 a natural endogenous ligand form by non-enzymatic breakdown product of prostaglandin D2 has been shown to ameliorating effect on various immune response genes in monocytes/macrophages through modulating downstream NF-kB and Janus kinase/signal transducers and activators of transcription (JAK/STAT) inflammatory signaling [39-44].
A number of epidemiological studies have demonstrated that NSAIDs are another kind of PPARγ ligand that modulate the activation of microglia through activation PPARγ activity, which inhibits inflammatory response all of which is beneficial for AD. However, the poor outcome of these conventional NSAIDs was observed in several AD human trials, especially in patients with advanced AD. Compelling the evidence, the beneficial effects of PPARγ ligands lie in modulating microglial activation and subsequent inhibition of inflammation [45, 46].
4.2. Peroxisome Proliferator-activated Receptor Gamma (PPARγ) Agonist and Aβ Clearance
Amyloid β (Aβ) is hallmark of AD and responsible for the progression of the disease. So clearance or removal of plaque is one of the mechanisms through which the disease progression in AD patients is controlled. Interestingly, we will present next several lines of studies that have shown that the treatment with TZDs decreases Aβ accumulation.
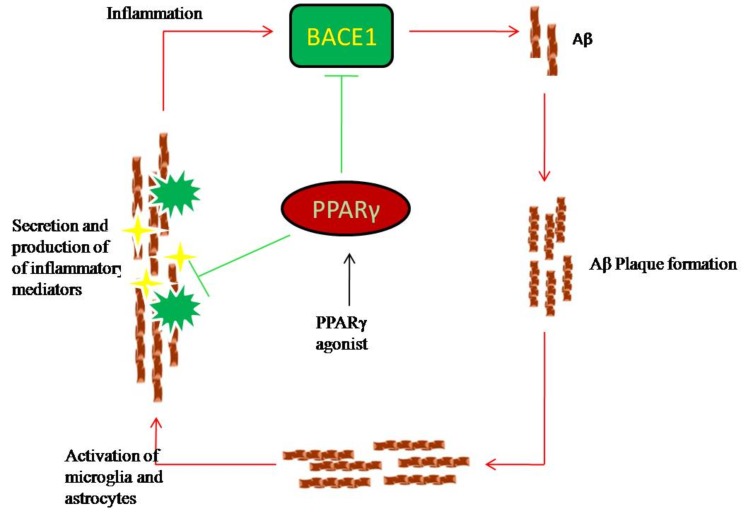
Peroxisome proliferator-activated receptor gamma (PPARγ) agonists elicited a reduction in amyloid pathology due to the ability of PPARγ to affect Aβ homeostasis through ubiquinine mediated degradation of amyloid precursor protein (APP). Earlier Heneka et al showed that pioglitazone treatment reduces the expression of β-amyloid precursor protein cleaving enzyme 1 (BACE1), an enzyme called β-secretase that processes APP protein in APP transgenic mice [47]. BACE1 gene contains PPARγ response element (PPRE) in the promoter region and binding of PPARγ to this response element resulting in suppression of expression of BACE1 and subsequent inhibition of Aβ production, owing to the ability of this receptor to repress the BACE1 [48, 49]. The significance of BACE1 function in Aβ production was confirmed by a number of other studies where they showed, overactivation of BACE1 gene resulted in increase in Aβ deposition in the brain (Fig. 3) [48, 50]. In consistence with this, another study reported that long-term treatment of 9-month-old J20 animals with rosiglitazone for a period of 4 months showed a 50% reduction in levels of Aβ and enhanced Aβ clearance. This could be attributed to an increase in lipidation of Apolipoprotein E (ApoE) by ATP-binding cassette transporter (ABCA1) lipid transporter as lipidated ApoE promotes proteolytic degradation of Aβ [39]. It has also been suggested that NSAIDs act directly on Aβ processing by the γ-secretase complex resulting in selective decrease of Aβ production [51, 52]. These studies suggest that enhancing Aβ clearance by agonist of PPARγ could be the possible novel therapeutic approach for the treatment of AD.
Fig. (3).
Effects of PPARγ agonist on Aβ metabolism. Formation of Amyloid plaques induces the activation of microglia as well as astrocytes which respond with the secretion of inflammatory mediators like pro inflammatory cytokines that are able to increase BACE1 activity thereby stimulating Aβ production. BACE1 gene contain PPARγ response element (PPRE) in the promoter region and binding of PPARγ to this response element resulting in suppression of expression of BACE1 and subsequent inhibition of Aβ production. (The color version of the figure is available in the electronic copy of the article).
4.3. Role of Peroxisome Proliferator-activated Receptor Gamma (PPARγ) Agonist in Lipid Homeostasis
A number of recent research findings as well as some retrospective epidemiological genetic studies and clinical observations indicate a strong relationship between alterations in lipid homeostasis and AD. Elevated level of serum cholesterol level was found in the serum of AD patients that positively correlated with severity of disease and treatment with Cholesterols lowering drugs like Statins, which inhibit cholesterol synthesis, through inhibition of HMG-CoA reductase activity is associated with decreased prevalence of AD [53-56]. Clinical studies found that a slight increase in cholesterol level from borderline (greater than 220 mg/dl) could increase the risk of developing AD [57, 58]. Later this was confirmed by genetic studies that proposed a link between AD and cholesterol regulating genes such as ApoE and ABCA1. ApoE plays a pivotal role in the cholesterol homeostasis and lipid transport. In the brain, ApoE is produced mainly by astrocytes and there are three naturally occurring isoforms of ApoE i.e. (ApoE2, ApoE3, and ApoE4). Few independent studies have confirmed that ApoE4 is associated with greater risk for AD [59]. The exact mechanism through which ApoE confer susceptibility to AD is still unknown but it has been found that ApoE is associated with clearance and deposition of Aβ. Cholesterol may thus be one of the key factors that determines APP processing pathways and Aβ production in AD.
Experimental evidences have shown that as a functional consequence of ApoE4 interaction with APP assists the production of Aβ in ApoE4 transgenic mice [60]. In reference to this lipidation of ApoE facilitate the Aβ degradation and increasing lipidation might be the key for ApoE-based therapy [61]. Lipidation of ApoE is primarily mediated by ABCA1 transporter that, also regulate ApoE function in the CNS [62]. Importantly, the expression of ABCA1 and ApoE is under the control of PPARγ and LXRs and genetic ablation of any of this protein resulted in amyloid pathology [63, 64]. Thus, regulation of ApoE status (lipidation status) is a significant determinant of Aβ clearance and deposition. Sustained PPARγ or LXR activation results in the amelioration of AD-related pathophysiology in AD mouse models [61, 62]. Significantly, it has recently been reported that APP/PS1 mice of 6 and 12 months of age showed significant improvement in the level of ABCA1 and ApoE and as a functional consequence of this reduces the soluble and insoluble levels of Aβ by 50% with treatment of pioglitazone [36]. In consistence with this, another study reported that increased chronic treatment of rosiglitazone increases ApoE transcription, reduces Aβ levels and improves cognitive function in mouse models of AD [65]. Agonists of these receptors also stimulate their alternative pathway which promotes phagocytosis [66]. Thus, agonists might have the potential for modulating lipid homeostasis and neurodegeneration.
4.4. Peroxisome Proliferator-activated Receptor Gamma (PPARγ) Agonist and Cognitive Impairment
AD is a devastating neurodegenerative disease, characterized by the loss of memory and functions necessary to perform complex daily activities. Interestingly evidence suggests a strong connection between abnormal glucose homeostasis, insulin resistance and cognitive impairment [66]. Furthermore, the studies have shown that the dysregulation of insulin signaling pathway exacerbated neurodegeneration and cognitive deficits in AD [67]. Still, insulin and its receptors have a specific pattern of expression in the brain, amid particular abundance in defined areas like the hippocampus and cortex, both of which regions related with memory [68]. Accumulative evidence suggests that with age, decrease in insulin receptors and increased requirement of metabolic energy of brain, put areas like hippocampus at risk for insulin resistance that is quintessential to AD [69]. Conversely, or perhaps reciprocally, few studies reported that dysregulation of insulin signaling arises as a consequence of amyloid deposition in the brain [70, 71]. Evidence from various studies suggests that insulin sensitizer (TZDs) is a key therapeutic target in AD patients with associated insulin dysregulation [72, 73]. Indeed, PPARγ agonist rosiglitazone improved cognition in several clinical and preclinical model through induced expression of proteins critical to presynaptic SNARE complex contain both PPREs and cAMP response element containing extracellular signal-regulated protein kinase mitogen-activated protein kinase [(ERK MAPK) (CREB)] in the promoter region of rosiglitazone targeted genes suggesting that PPAR target genes are also CREB target genes which themselves are highly regulated by ERK MAPK. Denner et al., pointing to a compelling interrelationship between PPARγ and ERK in the hippocampus during memory consolidation [74]. Several lines of studies indicate that common integrator of insulin signaling ERK, which is recruited to PPARγ during consolidation and requisite for hippocampal synaptic plasticity, learning and memory through the maintenance of proper redox, inflammatory and glucose homeostasis within neural networks [75]. Thus, targeting the insulin signaling pathway during AD represents a promising remedial approach based upon practical evidence that insulin resistance and cognitive decline are associated with each other. In other studies, injection of rosiglitazone directly into the brain of Aβ induced AD rat significantly improves cognition and function [41]. In addition, experiments on the transgenic mouse 3xTg-AD showed that pioglitazone treatment improved learning behavior as assessed by Morris Water Maze test [76]. Further studies on the APP/PS1 transgenic mouse model also showed that treatment with rosiglitazone also improves cognition and reduces soluble Aβ [40]. In confirmation to the above study, rosiglitazone treatment also improved cognition in some early AD patients [77, 78]. However, use of these drugs in the treatment of stable AD is under close appraisal due to their poor blood-brain-barrier (BBB) permeability.
Another way of improvement of cognitive impairment and related disorders is the augmentation of the level of acetylcholine (ACh) which is going down in AD and leads to a cholinergic dysfunction [79]. Evidence obtained from a mouse model of dementia having cholinergic dysfunction showed that it was successfully ameliorated by the treatment of pioglitazone and improved learning and memory. This was mediated by an increase in the synthesis of acetylcholine (Ach) regulatory enzymes in the hippocampus and cortex of the mouse model of AD [80, 81].
Taken together, these data suggest a therapeutic role for PPARγ agonism which contributes to combat AD pathology via several pathways including direct modulation of the canonical learning and memory protein cascade in order to ameliorate associated network plasticity and cognitive deficits. The attenuating effects of PPARγ agonists on plaque level and memory retention in animal model of AD have been observed.
4.5. Peroxisome Proliferator-activated Receptor Gamma (PPARγ) Agonist and Cerebrovascular Protection
Peroxisome proliferator-activated receptorγ plays an integral role in maintaining cerebrovascular health. PPAR is highly expressed in the vascular wall and has recently emerged as an important determinant of vascular structure and function. It is of interest that emerging evidence suggests that activation of PPARγ might be a critical regulator of cerebral vascular function as the inaction of PPARγ resulted in vascular hypertrophy and increased oxidative stress [82, 83]. Accordingly, PPARγ agonist can enhance antioxidant systems, inhibit the expression of inflammatory proteins in endothelial cells by virtue of the activation of PPARγ and suppress free radical in the peripheral vasculature of human and animal model of hypertension/diabetes [84-86]. Indeed, TZDs significantly reduced ROS induced cerebrovascular endothelial cell death by inducing pro-survival genes like Bcl-2 and a Wnt target gene [50, 87]. Thus, it might be anticipated that activation of PPARγ with agonists would have beneficial effects on cerebrovascular endothelial function. In addition to this, PPARγ may also induce the oxidative stress response gene hemoxygenase-2 via nuclear factor (erythroid-derived 2)-like 2 pathway. Recently, age-associated increase in the thickness of extracellular matrix (ECM) molecules was observed in humans due to increase in collagen synthesis and decreased in Elastin content. In parallel to these modifications of ECM composition, increased collagen and decrease in elastin contents in AD may be due to an imbalance in Matrix metalloproteinases (MMPs)/ Tissue inhibitors of metalloproteinases (TIMPs). The previous study has revealed that both MMP-2 and MMP-9 are induced by the presence of Aβ that contribute to Aβ -induced neuronal cell death [88, 89]. Furthermore, studies showed that PPARγ agonists (TZDs) can regulate the expression of ECM molecules by reducing MMPs activity which is up-regulated and implicated in the thickening of cerebrovascular basement membranes in AD brain and protects neural cell damage [90].
Adhesion molecules present on the cerebral vascular endothelium facilitate immune cell extravasations across the BBB into the CNS. PPARγ agonists have been shown to selectively modulate the expression of various adhesion molecule and metalloproteinases [91]. Thus blocking the extravasations of immune cells through an endothelial barrier and countering leukocyte-endothelial interactions and BBB compromise that occur in AD [92]. Thus, it might be anticipated that PPARγ ligands offer therapeutic protection against on cerebrovascular endothelial dysfunction in AD. Sato et al., improved cerebral blood flow along with glucose metabolism in AD patients with T2D treated with pioglitazone [73]. Recently, Wang et al., reported that in vivo pioglitazone treatment rescued evoked cerebrovascular dysfunction due to oxidative stress in the rostral ventrolateral medulla by the up-regulation of mitochondrial uncoupling protein 2 (UCP2) positioning the TZD as a valuable tool against chronic hypoperfusion in AD patients devoid of cerebrovascular pathology [93, 94].
4.6. Peroxisome Proliferator-activated Receptor Gamma (PPARγ) Agonist in Energy Metabolism and Synaptic Plasticity
It is well known that energy consumption in the mammalian brain is supplied mainly by the oxidation of glucose. However, several studies documented that impaired glucose metabolism was observed in brain areas mainly responsible for memory and cognition in the majority of AD patients before the symptomatic onset of the disease. One possible reason for impaired glucose metabolism and subsequent decrease in ATP production from mitochondria in the brain is the loss of nerve cells due to the toxic effect of Aβ. However, glucose hypo metabolism developed way before the cognitive loss [95, 96].
Peroxisome proliferator-activated receptor gamma (PPARγ) activation has been thought to play a significant role in improving glucose and energy metabolism in AD due to its immediate effects on mitochondrial biogenesis and function [97]. As mitochondria play decisive roles in both energy metabolism as well as neuronal cell death. Roses and co-workers hypothesize that PPARγ agonist acts on mitochondria through increasing their metabolic efficiency and number, as in diseased state the numbers of mitochondria are greatly reduced with altered morphology and this may be the possible basis of their positive effects on memory and function in AD patients [98]. Supporting this hypothesis, pioglitazone and rosiglitazone, two commonly used TZDs, resulted in a significant increase in mitochondrial DNA copy number as well as mitochondrial biogenesis [99]. Several pieces of evidence show that activation of PPARγ by rosiglitazone exerts these beneficial effects by promoting oxidative metabolism and increases expression of PGC1α which in turn, increases the expression of the variety of mitochondrial genes which positively regulate mitochondrial energy metabolism and mitochondrial biogenesis [100, 101]. In another study, the long-term treatment with pioglitazone restore oxidative damage and attenuate mitochondrial respiratory activity and promotes mitochondrial biogenesis in Aβ animal model of AD [80]. In addition, PPARγ agonist also stimulates the expression of many other key genes that play a vital role in the biogenesis of mitochondria, are independent of PPARγ receptor.
Several lines of studies show that Cdk5 (cyclin-dependent kinase5) is critical in the regulation of tau hyperphosphorylation and synaptic plasticity in the AT8 epitope (found in the AD brain) after activation by Aβ [102]. Interestingly, recent studies showed that the activity of Cdk5 was inhibited by decreasing the level of p35, which is a Cdk5 activator in neurons by activating PPAR𝛾 with pioglitazone. In addition, inhibition of Cdk5 activity by pioglitazone further prohibited long-term potentiation (LTP) defects at CA3-CA1 synapses in transgenic mice, which are a vital form of synaptic plasticity [103]. Recently, Nenov et al., reported putative mechanism for hippocampal cognitive enhancement by rosiglitazone treatment to Tg2576 mice model of AD through amelioration of dysfunctional glutamatergic synaptic transmission, short-term plasticity, and synaptic vesicle regulatory proteins [104]. These assessments are important because recently the use of TZDs showed improvement of synapse plasticity and mitochondrial function untimely which lead to improvement in memory and function. On the other hand, with the involvement of Cdk5 in the regulation of synaptic plasticity, a new attractive promising association opens between their kinase activity and the regulation of tau pathology present in the AD brain.
4.7. Peroxisome Proliferator-activated Receptor Gamma (PPARγ) Agonist Insulin Sensitivity
Recent clinical and epidemiological evidence supports an association between T2D and increased risk of AD [105]. Summary of evidence of the linkage between T2D and AD, pointing toward insulin deficiency, insulin resistance, vascular injuries and humanin (mitochondrial-derived peptide) shared molecular mechanisms that have been strongly implicated as a possible risk factor for AD and memory impairment, a common characteristic feature of diabetes. Humanin appears to act as a signal peptide to inhibit neurotoxicity and cell death caused by Aβ neurotoxicity [106]. Intranasal insulin administration improves working memory in both human and animal studies [107, 108]. However, the exact mechanism that links insulin resistance with AD is obscure, multiple observations have led to the hypothesis that the insulin insensitivity may participate in Aβ accumulation and cognitive deficits. Thus, insulin-based therapies have emerged as potentially successful therapies for AD. PPARγ agonists are the most prescribed drugs by physicians for the treatment of diabetes and known to increase insulin sensitization, modulate glucose metabolism and also proven to have a significant ameliorating effect on cognitive deficits, with decreases in Aβ levels via inhibition of BACE1 receptor for advanced glycation end products (RAGE), and NF-kB in brain as well as insulin sensitivity [27, 48].
5. PPARΓ AGONIST IN HUMAN TRIAL
Few Food and Drug Administration (FDA)-approved PPARγ agonists have been assessed for their effectiveness in AD patients. Watson et al have reported the result of a small clinical study examining 30 subjects with amnestic mild cognitive impairment, treated with rosiglitazone (4 mg daily) for 6 months resulted with an enhanced memory and function as determined by the delayed recall and selective attention [109]. However, authors also reported that APP concentrations in the serum remain unchanged in rosiglitazone treated subjects, while in the control group serum APP levels were reduced. Even though the interpretation to assess Aβ status in the brain using serum levels of APP or Aβ is still controversial, the statistics support the probable curative use of PPARγ agonist for AD. The outcomes of a phase II clinical trial, registering larger scale patients with mild to moderate AD was reported by Risner et al., [110]. A total of 336 patients treated with rosiglitazone and 106 patients receiving placebo for 6 months. Succeeding six months of treatment results were measured by various AD assessments scale and it was suggested that patients receiving rosiglitazone were found to have associated with improved cognitive function compared to those receiving placebo. Notably, patients having ApoE4 allele did not show any response to the treatment compare to those having only E2 and E3 alleles of ApoE. These observations are in accordance with pthe revious finding as reported by Craft and colleagues, which shows the influence of the ApoE4 genotype on insulin action and support the useful effect of PPARγ agonist in AD therapy. However, due to limited permeability of rosiglitazone across the BBB, their outcome in treating AD is not satisfactory. Thus, there is plenty of room for development in future drug discovery [111].
In another clinical trial, an action of pioglitazone was studied in mild AD cases. This study established a small but statistically insignificant improvement in memory. In recent clinical study, Sato et al., proposed that treatment of pioglitazone in patients with mild AD accompanied with T2D shows significant improvement in cognition along with an increase in cerebral blood flow as compared to non-treated groups. The results of this pilot study demonstrated that Pioglitazone may be used for the treatment of AD [73].
6. PRECLINICAL STUDY
Precise animal models are normally used to understand the pathophysiology of disease and the development of new therapeutic strategies. Indeed, we will depict several AD mouse models that showed pathophysiology of AD and after the treatment with PPAR agonists demonstrate significant improvement in disease condition [112]. Lim et al., reported that 6 months treatment of transgenic mouse model of AD with ibuprofen dramatically reduced amyloid deposition and inflammation, may be due to the documented activity of ibuprofen to activate PPARγ [45]. In addition to this, Liu et al., also reported that ibuprofen at a dose of 40 mg/kg orally improve cognition and down regulating the levels of BACE1, inflammation in rat model of diabetic encephalopathy. This led to the hypothesis that targeting PPARγ activity may be beneficial for AD treatment and provided a rationale for studies examining PPARγ action in murine models of AD [27].
In a study carried out by Yan et al., 6 month old Tg2576 animals with plaque pathology were treated orally with pioglitazone and ibuprofen for 4 months at a dose of 20 mg/kg bwt/day [46]. Pioglitazone treatment did not result in a significant decrease in plaque pathology; however, treatment with ibuprofen resulted in dramatic reduction in plaque burden. This result is in accordance with the previous study in which Lim et al., showed about 60% reductions in plaque burden after treatment with ibuprofen in an animal model of AD [45]. Simultaneous treatment of ibuprofen and pioglitazone resulted in a significant reduction in the levels of soluble Aβ peptides. In addition, ibuprofen reduced the number of activated microglia while pioglitazone had no effect. Complimentary to this, NSAIDs improved the amyloid-β-mediated suppression of memory and synaptic plasticity by COX-dependent mechanism, which may play a role in synaptic dysfunction in an experimental model of AD [113]. These findings were argued due to their poor blood-brain barrier permeability of pioglitazone into the brain that compromised its efficacy.
In studies on the AD transgenic mouse J20 and APP/PS1 the treatment with rosiglitazone reduced memory deficits and inflammation [39, 40]. In addition, experiments on the triple transgenic mouse 3xTg-AD, it was found that pioglitazone treatment improved learning by significantly decreasing tau phosphorylation [76, 114]. Consistent with this, pioglitazone treatment causes a significant reduction in microglial activator marker M1 in the surrounding area of amyloid deposits in the brain of 12-month-old APP/PS1 mice, which on activation, is responsible for the production of inflammatory cytokines and oxidative stress. Further using the same mouse model, Mandrekar-Colucci et al., showed that an increase in the expression of M2 markers that generates anti-inflammatory cytokines, promoting phagocytosis and tissue repair [36]. Several studies suggest that the ERK pathway is key for learning and memory. In consistence with these previous studies, treatment of rosiglitazone also improved memory and cognition through PPAR𝛾 mediated pERK pathway in animal model of AD [75]. In addition to this, PPARs𝛾 activation improved the reduction of ACh level, which causes cholinergic dysfunction in AD [79]. This is in accordance with previously reported data that shows pioglitazone improved learning and memory retention in mouse model of the cholinergic deficit in the brain [80, 81]. Furthermore, pioglitazone offers protection against scopolamine-induced dementia by improving in long-term and visuo-spatial memory and therefore, could have a therapeutic potential in AD [115]. Complementary studies on the same transgenic mouse model showed that treatment with pioglitazone improved spatial memory using the same test [103].
The initial studies reviewed by Heneka et al., investigated the activation of PPAR𝛾 by inflammatory drugs on neurological disorders. Interestingly, the activation of PPARγ suppressed inflammatory response through inhibition of NF-kB or AP-1 both in vitro and in vivo that prevented the microglia activation mediated by Aβ [116]. More recently, accumulative studies showed that treatment of TZDs reduces activation of astrocytes and microglia in hippocampus, cortex region of brain of A/T mouse which overexpress Aβ and so inflammation [117]. At the same time, injection of rosiglitazone into the brain of Aβ oligomers treated rats prevented the increase of inflammatory cytokines levels, and this is related to improvement in cognitive decline and prevention of microglia activation [41]. In a latest development, Flesch et al., synthesized a novel compound containing 2-(benzylidene) hexanoic acid scaffold, having a combined effect of γ-secretase-modulation, PPARγ-agonism and inhibition of 5-lipoxygenase that could be multi-target-strategy for AD, concurrently address the causative amyloid pathology and chronic brain inflammation [118]. Recently, T3D-959, an orally active PPARγ agonist remediates neurocognitive deficits in experimental model of AD [119, 120]. List of PPARγ agonists showing ameliorating effect on memory and function is provided in Table 1.
Table 1.
Showing the attenuating effects of PPARγ agonists on plaque level and memory retention in animal model of AD.
| S. No. | PPARγ Agonist | Dose (mg/kg b.wt.) | Treatment | Aβ /Plaque Level | Memory Improvement | Refs. |
|---|---|---|---|---|---|---|
| 1 | Pioglitazone | 20 | 4 months | Decreases | Not assessed | [46] |
| 2 | Pioglitazone | 40 | 7 days | Decreases | Not assessed | [45] |
| 3 | Rosiglitazone | 30 | 7 months | Decreases | Yes | [46] |
| 4 | Pioglitazone | 20 | 6-8 weeks | No change | No | [128] |
| 5 | Rosiglitazone | 3 | 12 weeks | Decreases | Yes | [129] |
| 6 | Rosiglitazone | 5 | 16 weeks | Decreases | Yes | [128] |
| 7 | Rosiglitazone | 30 | 30 days | Not assessed | Yes | [80] |
| 8 | Pioglitazone | 80 | 9 days | Decreases | Yes | [36] |
| 9 | Pioglitazone | 18 | 14 weeks | Decreases | Yes | [76] |
| 10 | Rosiglitazone | 30 | 30 days | No change | Yes | [74] |
| 11 | Pioglitazone | 15 & 30 | 21 days | Not assessed | Yes | [80] |
| 12 | Rosiglitazone | 30 | 30 days | Not assessed | Yes | [75] |
7. THE UNSUCCESSFUL USE OF PPARΓ ACTIVATORS
Even though all evidence that suggests the use of PPARs agonist ameliorates or delayed neurodegenerative changes, however, few studies bring conflicting results [121-123]. Interestingly, a clinical trial that assesses the effects of rosiglitazone on mild-to moderate AD patients exhibited cognitive and functional improvement that did not carry ApoE4 allele while the results were not satisfactory in patients carrying ApoE4 allele [110]. These observations suggest that the improvement of cognition not only depends upon TZDs but also depends on the expression of functional ApoE. In addition, the treatment of rosiglitazone with adjunctive therapy to AChE inhibitors on cognition in participants with mild to moderate AD was unable to find any effect [124]. Recently Hildreth et al., documented the treatment of Pioglitazone in older adults with mild cognitive impairment (MCI) and insulin resistance. In this pilot study, pioglitazone improved insulin resistance, however,showed no sign of improvement in cognitive performance in older adults with MCI and insulin resistance [125].
8. PEROXISOME PROLIFERATOR-ACTIVATED RECEPTOR GAMMA (PPAR𝛾) AGONIST AS FUTURE APPROACH FOR THE TREATMENT OF AD
Alzheimer’s disease causes major impairment of individual health possibly because these patients might not be properly addressed in clinical studies and also limited effective therapeutic approaches. Currently, there are only two types of drug used for the treatment of AD which is limited within two categories such as cholinesterase inhibitors and memantine (N-methyl-D-aspartate receptor antagonist) approved by FDA. Still, unfortunately, the effects and benefits of these drugs are insignificant and provide only symptomatic relief but unable to prevent the occurrence and progression of the disease. Further, combinatorial drug approaches have been tried during the past few decades to improve treatment, but the outcomes have been discouraging because of the major side effects. However, no currently endorsed treatments provide complete relief or reverse the disease progression. Therefore, there is a need to adopt a new, safer approach in the 21st century for the effective treatment of AD. In recent years, fundamental researches focusing on the radical source of AD such as oxidative stress, inflammation, mitochondrial dysfunction, caspase inhibitors paved the way for the development of new treatments. Use of PPARγ agonist is the first step towards the development of new therapeutics, which is safe and without side effects. Due to pleiotropic effects of PPARγ on various signaling pathways; scores of which are obligatory for neuronal homeostasis and plasticity, its activation by PPARγ agonist could potentially have an impact on the amelioration of AD. Markedly, there have already been much significant progressions in the use of various PPARγ agonist based approaches toward diabetes therapeutics [126]. It is the most recent and attractive approach for the treatment of AD that is revolutionizing medicinal research due to its ameliorating effect against various deleterious pathways that affect not only AD but also other disease conditions, which will support its therapeutic use for the treatment of AD in the near future.
CONCLUSION
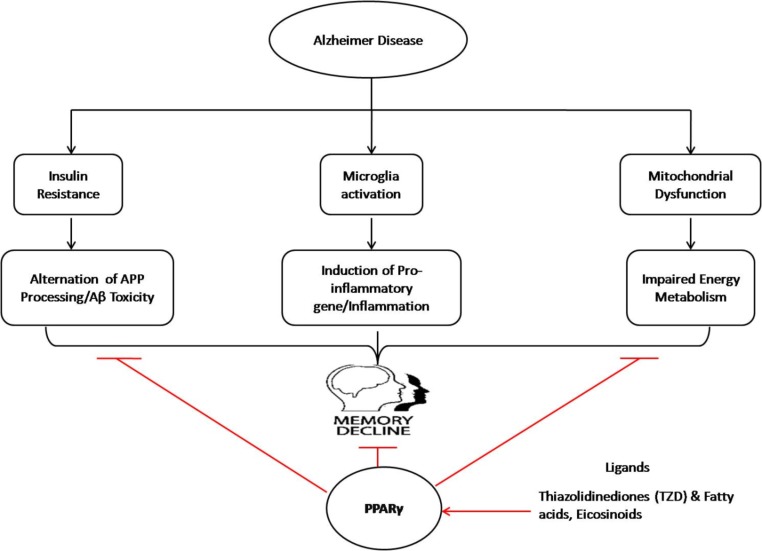
AD is the most commonly occurring disease in the elderly which causes a devastating effect on cognitive function due to the massive neurodegeneration in old aged person. Since, till date, no potential drug has been discovered to cure AD effectively, therefore there is an indispensable need to develop novel therapeutic drugs having simultaneously a broader action in terms of inhibiting a wide array of the targets such as altered cellular and molecular signaling pathways most often afflicted with this disease. So far, the gathered evidence shows that PPAR𝛾 agonist induces neuronal differentiation by regulation and synthesis of various PPARγ-dependent neuroprotetve genes and counter inflammation, oxidative stress and Aβ clearance. Thus, suggesting its pivotal effect on controlling the neuronal abnormality pertaining to AD within brain as further substantiated by the facts that many researchers have demonstrated very encouraging results with some of the derivatives drugs related to PPARγ agonist (Fig. 4) [116, 127-129].
Fig. (4).
Overview of the effect of PPAR𝛾 agonist role in controlling the neuronal degeneration in AD. (The color version of the figure is available in the electronic copy of the article).
ACKNOWLEDGEMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
LIST OF ABBREVIATIONS
- AD
Alzheimer’s disease
- BBB
Blood-brain barrier
- FDA
Food and Drug Administration
- LTP
Long-term potentiation
- NHR
Nuclear hormone receptors
- PPARs
Peroxisome proliferator-activated receptors
- ROS
Reactive oxygen species
- T2D
Type II diabetes
- TNFα
Tumor necrosis factor
- TZD
Thiazolidinedione
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Hebert L.E., Weuve J., Scherr P.A., Evans D.A. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [http://dx.doi.org/ 10.1212/WNL.0b013e31828726f5]. [PMID: 23390181]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Querfurth H.W., LaFerla F.M. Alzheimer’s disease. N. Engl. J. Med. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [http://dx.doi.org/10.1056/NEJMra 0909142]. [PMID: 20107219]. [DOI] [PubMed] [Google Scholar]
- 3.Manczak M., Anekonda T.S., Henson E., Park B.S., Quinn J., Reddy P.H. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum. Mol. Genet. 2006;15(9):1437–1449. doi: 10.1093/hmg/ddl066. [http://dx.doi.org/10.1093/hmg/ ddl066]. [PMID: 16551656]. [DOI] [PubMed] [Google Scholar]
- 4.Reddy P.H., Beal M.F. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol. Med. 2008;14(2):45–53. doi: 10.1016/j.molmed.2007.12.002. [http:// dx.doi.org/10.1016/j.molmed.2007.12.002]. [PMID: 18218341]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fratiglioni L., Qiu C. Prevention of common neurodegenerative disorders in the elderly. Exp. Gerontol. 2009;44(1-2):46–50. doi: 10.1016/j.exger.2008.06.006. [http://dx.doi.org/10.1016/j.exger.2008.06.006]. [PMID: 18620039]. [DOI] [PubMed] [Google Scholar]
- 6.Cameron B., Landreth G.E. Inflammation, microglia, and Alzheimer’s disease. Neurobiol. Dis. 2010;37(3):503–509. doi: 10.1016/j.nbd.2009.10.006. [http:// dx.doi.org/10.1016/j.nbd.2009.10.006]. [PMID: 19833208]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandrekar-Colucci S., Landreth G.E. Nuclear receptors as therapeutic targets for Alzheimer’s disease. Expert Opin. Ther. Targets. 2011;15(9):1085–1097. doi: 10.1517/14728222.2011.594043. [http://dx.doi.org/10.1517/14728222.2011. 594043]. [PMID: 21718217]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zolezzi J.M., Inestrosa N.C. Peroxisome proliferator-activated receptors and Alzheimer’s disease: hitting the blood-brain barrier. Mol. Neurobiol. 2013;48(3):438–451. doi: 10.1007/s12035-013-8435-5. [http://dx.doi.org/10. 1007/s12035-013-8435-5]. [PMID: 23494748]. [DOI] [PubMed] [Google Scholar]
- 9.Wang L., Waltenberger B., Pferschy-Wenzig E.M., Blunder M., Liu X., Malainer C., Blazevic T., Schwaiger S., Rollinger J.M., Heiss E.H., Schuster D., Kopp B., Bauer R., Stuppner H., Dirsch V.M., Atanasov A.G. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): a review. Biochem. Pharmacol. 2014;92(1):73–89. doi: 10.1016/j.bcp.2014.07.018. [http://dx.doi.org/ 10.1016/j.bcp.2014.07.018]. [PMID: 25083916]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bookout A.L., Jeong Y., Downes M., Yu R.T., Evans R.M., Mangelsdorf D.J. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126(4):789–799. doi: 10.1016/j.cell.2006.06.049. [http://dx.doi.org/10.1016/j.cell.2006.06.049]. [PMID: 16923397]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shie F.S., Nivison M., Hsu P.C., Montine T.J. Modulation of microglial innate immunity in Alzheimer’s disease by activation of peroxisome proliferator-activated receptor gamma. Curr. Med. Chem. 2009;16(6):643–651. doi: 10.2174/092986709787458399. [http://dx.doi.org/10.2174/ 092986709787458399]. [PMID: 19199928]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinstein D.L., Galea E., Gavrilyuk V., Brosnan C.F., Whitacre C.C., Dumitrescu-Ozimek L., Landreth G.E., Pershadsingh H.A., Weinberg G., Heneka M.T. Peroxisome proliferator-activated receptor-gamma agonists prevent experimental autoimmune encephalomyelitis. Ann. Neurol. 2002;51(6):694–702. doi: 10.1002/ana.10206. [http://dx.doi.org/ 10.1002/ana.10206]. [PMID: 12112074]. [DOI] [PubMed] [Google Scholar]
- 13.Choi J.M., Bothwell A.L. The nuclear receptor PPARs as important regulators of T-cell functions and autoimmune diseases. Mol. Cells. 2012;33(3):217–222. doi: 10.1007/s10059-012-2297-y. [http://dx.doi.org/10.1007/s10059-012-2297-y]. [PMID: 22382683]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagy L., Tontonoz P., Alvarez J.G., Chen H., Evans R.M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93(2):229–240. doi: 10.1016/s0092-8674(00)81574-3. [http://dx. doi.org/10.1016/S0092-8674(00)81574-3]. [PMID: 9568715]. [DOI] [PubMed] [Google Scholar]
- 15.Azhar S. Peroxisome proliferator-activated receptors, metabolic syndrome and cardiovascular disease. Future Cardiol. 2010;6(5):657–691. doi: 10.2217/fca.10.86. [http://dx.doi.org/10.2217/fca.10.86]. [PMID: 20932114]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheen A.J. Thiazolidinediones and liver toxicity. Diabetes Metab. 2001;27(3):305–313. [PMID: 11431595]. [PubMed] [Google Scholar]
- 17.Kummer M.P., Schwarzenberger R., Sayah-Jeanne S., Dubernet M., Walczak R., Hum D.W., Schwartz S., Axt D., Heneka M.T. Pan-PPAR modulation effectively protects APP/PS1 mice from amyloid deposition and cognitive deficits. Mol. Neurobiol. 2015;51(2):661–671. doi: 10.1007/s12035-014-8743-4. [http://dx.doi.org/10.1007/s12035-014-8743-4]. [PMID: 24838579]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marketou M.E., Kontaraki J.E., Tsakountakis N.A., Zacharis E.A., Kochiadakis G.E., Arfanakis D.A., Parthenakis F., Chlouverakis G., Vardas P.E. Differential effect of telmisartan and amlodipine on monocyte chemoattractant protein-1 and peroxisome proliferator-activated receptor-gamma gene expression in peripheral monocytes in patients with essential hypertension. Am. J. Cardiol. 2011;107(1):59–63. doi: 10.1016/j.amjcard.2010.08.048. [http://dx.doi.org/10.1016/j.amjcard. 2010.08.048]. [PMID: 21146687]. [DOI] [PubMed] [Google Scholar]
- 19.Sukumaran V., Watanabe K., Veeraveedu P.T., Ma M., Gurusamy N., Rajavel V., Suzuki K., Yamaguchi K., Kodama M., Aizawa Y. Telmisartan ameliorates experimental autoimmune myocarditis associated with inhibition of inflammation and oxidative stress. Eur. J. Pharmacol. 2011;652(1-3):126–135. doi: 10.1016/j.ejphar.2010.10.081. [http://dx. doi.org/10.1016/j.ejphar.2010.10.081]. [PMID: 21115000]. [DOI] [PubMed] [Google Scholar]
- 20.Shindo T., Takasaki K., Uchida K., Onimura R., Kubota K., Uchida N., Irie K., Katsurabayashi S., Mishima K., Nishimura R., Fujiwara M., Iwasaki K. Ameliorative effects of telmisartan on the inflammatory response and impaired spatial memory in a rat model of Alzheimer’s disease incorporating additional cerebrovascular disease factors. Biol. Pharm. Bull. 2012;35(12):2141–2147. doi: 10.1248/bpb.b12-00387. [http://dx.doi.org/10.1248/bpb.b12-00387]. [PMID: 23207766]. [DOI] [PubMed] [Google Scholar]
- 21.Singh B., Sharma B., Jaggi A.S., Singh N. Attenuating effect of lisinopril and telmisartan in intracerebroventricular streptozotocin induced experimental dementia of Alzheimer’s disease type: possible involvement of PPAR-γ agonistic property. J. Renin Angiotensin Aldosterone Syst. 2013;14(2):124–136. doi: 10.1177/1470320312459977. [http://dx.doi. org/10.1177/1470320312459977]. [PMID: 23060470]. [DOI] [PubMed] [Google Scholar]
- 22.Tsukuda K., Mogi M., Iwanami J., Min L.J., Sakata A., Jing F., Iwai M., Horiuchi M. Cognitive deficit in amyloid-beta-injected mice was improved by pretreatment with a low dose of telmisartan partly because of peroxisome proliferator-activated receptor-gamma activation. Hypertension. 2009;54(4):782–787. doi: 10.1161/HYPERTENSIONAHA.109.136879. [http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.109.136879]. [PMID: 19635982]. [DOI] [PubMed] [Google Scholar]
- 23.Guo L., Tabrizchi R. Peroxisome proliferator-activated receptor gamma as a drug target in the pathogenesis of insulin resistance. Pharmacol. Ther. 2006;111(1):145–173. doi: 10.1016/j.pharmthera.2005.10.009. [http://dx.doi.org/10. 1016/j.pharmthera.2005.10.009]. [PMID: 16305809]. [DOI] [PubMed] [Google Scholar]
- 24.Fajas L., Fruchart J.C., Auwerx J. Transcriptional control of adipogenesis. Curr. Opin. Cell Biol. 1998;10(2):165–173. doi: 10.1016/s0955-0674(98)80138-5. [http:// dx.doi.org/10.1016/S0955-0674(98)80138-5]. [PMID: 9561840]. [DOI] [PubMed] [Google Scholar]
- 25.Bensinger S.J., Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454(7203):470–477. doi: 10.1038/nature07202. [http://dx.doi.org/10.1038/nature07202]. [PMID: 18650918]. [DOI] [PubMed] [Google Scholar]
- 26.Chu K., Lee S.T., Koo J.S., Jung K.H., Kim E.H., Sinn D.I., Kim J.M., Ko S.Y., Kim S.J., Song E.C., Kim M., Roh J.K. Peroxisome proliferator-activated receptor-gamma-agonist, rosiglitazone, promotes angiogenesis after focal cerebral ischemia. Brain Res. 2006;1093(1):208–218. doi: 10.1016/j.brainres.2006.03.114. [http://dx.doi.org/10.1016/j.brainres. 2006.03.114]. [PMID: 16696956]. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y.W., Zhu X., Zhang L., Lu Q., Zhang F., Guo H., Yin X.X. Cerebroprotective effects of ibuprofen on diabetic encephalopathy in rats. Pharmacol. Biochem. Behav. 2014;117:128–136. doi: 10.1016/j.pbb.2013.11.027. [http://dx.doi.org/10.1016/j.pbb.2013.11.027]. [PMID: 24291733]. [DOI] [PubMed] [Google Scholar]
- 28.Pisanu A., Lecca D., Mulas G., Wardas J., Simbula G., Spiga S., Carta A.R. Dynamic changes in pro- and anti-inflammatory cytokines in microglia after PPAR-γ agonist neuroprotective treatment in the MPTPp mouse model of progressive Parkinson’s disease. Neurobiol. Dis. 2014;71:280–291. doi: 10.1016/j.nbd.2014.08.011. [http://dx.doi.org/10. 1016/j.nbd.2014.08.011]. [PMID: 25134730]. [DOI] [PubMed] [Google Scholar]
- 29.Croasdell A., Duffney P.F., Kim N., Lacy S.H., Sime P.J., Phipps R.P. PPARγ and the Innate Immune System Mediate the Resolution of Inflammation. PPAR Res. 2015;2015:549691. doi: 10.1155/2015/549691. [http://dx.doi.org/10.1155/2015/549691]. [PMID: 26713087]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung S.W., Kang B.Y., Kim S.H., Pak Y.K., Cho D., Trinchieri G., Kim T.S. Oxidized low density lipoprotein inhibits interleukin-12 production in lipopolysaccharide-activated mouse macrophages via direct interactions between peroxisome proliferator-activated receptor-gamma and nuclear factor-kappa B. J. Biol. Chem. 2000;275(42):32681–32687. doi: 10.1074/jbc.M002577200. [http://dx.doi.org/10.1074/ jbc.M002577200]. [PMID: 10934192]. [DOI] [PubMed] [Google Scholar]
- 31.Pascual G., Fong A.L., Ogawa S., Gamliel A., Li A.C., Perissi V., Rose D.W., Willson T.M., Rosenfeld M.G., Glass C.K. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437(7059):759–763. doi: 10.1038/nature03988. [http://dx.doi.org/10.1038/nature03988]. [PMID: 16127449]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang C., Ting A.T., Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–86. doi: 10.1038/34184. [http://dx.doi.org/10.1038/34184]. [PMID: 9422509]. [DOI] [PubMed] [Google Scholar]
- 33.O’Barr S., Cooper N.R. The C5a complement activation peptide increases IL-1beta and IL-6 release from amyloid-beta primed human monocytes: implications for Alzheimer’s disease. J. Neuroimmunol. 2000;109(2):87–94. doi: 10.1016/s0165-5728(00)00291-5. [http://dx.doi.org/10.1016/S0165-5728(00)00291-5]. [PMID: 10996210]. [DOI] [PubMed] [Google Scholar]
- 34.Lue L.F., Rydel R., Brigham E.F., Yang L.B., Hampel H., Murphy G.M., Jr, Brachova L., Yan S.D., Walker D.G., Shen Y., Rogers J. Inflammatory repertoire of Alzheimer’s disease and nondemented elderly microglia in vitro. Glia. 2001;35(1):72–79. doi: 10.1002/glia.1072. [http://dx.doi.org/10.1002/glia.1072]. [PMID: 11424194]. [DOI] [PubMed] [Google Scholar]
- 35.Hoozemans J.J., Rozemuller J.M., van Haastert E.S., Veerhuis R., Eikelenboom P. Cyclooxygenase-1 and -2 in the different stages of Alzheimer’s disease pathology. Curr. Pharm. Des. 2008;14(14):1419–1427. doi: 10.2174/138161208784480171. [http://dx.doi.org/10.2174/138161208784480171]. [PMID: 18537664]. [DOI] [PubMed] [Google Scholar]
- 36.Mandrekar-Colucci S., Karlo J.C., Landreth G.E. Mechanisms underlying the rapid peroxisome proliferator-activated receptor-γ-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer’s disease. J. Neurosci. 2012;32(30):10117–10128. doi: 10.1523/JNEUROSCI.5268-11.2012. [http://dx.doi.org/10.1523/JNEUROSCI.5268-11. 2012]. [PMID: 22836247]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skerrett R., Pellegrino M.P., Casali B.T., Taraboanta L., Landreth G.E. Combined liver X receptor/peroxisome proliferator-activated receptor γ agonist treatment reduces amyloid β levels and improves behavior in amyloid precursor protein/presenilin 1 mice. J. Biol. Chem. 2015;290(35):21591–21602. doi: 10.1074/jbc.M115.652008. [http://dx.doi.org/ 10.1074/jbc.M115.652008]. [PMID: 26163517]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamanaka M., Ishikawa T., Griep A., Axt D., Kummer M.P., Heneka M.T. PPARγ/RXRα-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J. Neurosci. 2012;32(48):17321–17331. doi: 10.1523/JNEUROSCI.1569-12.2012. [http://dx.doi.org/10.1523/JNEUROSCI.1569-12.2012]. [PMID: 23197723]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Escribano L., Simón A.M., Gimeno E., Cuadrado-Tejedor M., López de, Maturana R., García-Osta A., Ricobaraza A., Pérez-Mediavilla A., Del, Río J., Frechilla D. Rosiglitazone rescues memory impairment in Alzheimer’s transgenic mice: mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacol. 2010;35:1593–1604. doi: 10.1038/npp.2010.32. [http://dx.doi.org/10.1038/npp. 2010.32]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Reilly J.A., Lynch M. Rosiglitazone improves spatial memory and decreases insoluble Aβ(1-42) in APP/PS1 mice. J. Neuroimmune Pharmacol. 2012;7(1):140–144. doi: 10.1007/s11481-011-9282-7. [http://dx.doi.org/10.1007/ s11481-011-9282-7]. [PMID: 21617889]. [DOI] [PubMed] [Google Scholar]
- 41.Xu S., Guan Q., Wang C., Wei X., Chen X., Zheng B., An P., Zhang J., Chang L., Zhou W., Mody I., Wang Q. Rosiglitazone prevents the memory deficits induced by amyloid-beta oligomers via inhibition of inflammatory responses. Neurosci. Lett. 2014;578:7–11. doi: 10.1016/j.neulet.2014.06.010. [http://dx.doi.org/10.1016/j.neulet.2014.06.010]. [PMID: 24933538]. [DOI] [PubMed] [Google Scholar]
- 42.Cernuda-Morollón E., Rodríguez-Pascual F., Klatt P., Lamas S., Pérez-Sala D. PPAR agonists amplify iNOS expression while inhibiting NF-kappaB: implications for mesangial cell activation by cytokines. J. Am. Soc. Nephrol. 2002;13(9):2223–2231. doi: 10.1097/01.asn.0000025786.87646.b1. [http://dx.doi.org/10.1097/01.ASN.0000025786.87646.B1]. [PMID: 12191966]. [DOI] [PubMed] [Google Scholar]
- 43.Park E.J., Park S.Y., Joe E.H., Jou I. 15d-PGJ2 and rosiglitazone suppress Janus kinase-STAT inflammatory signaling through induction of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 in glia. J. Biol. Chem. 2003;278(17):14747–14752. doi: 10.1074/jbc.M210819200. [http://dx.doi. org/10.1074/jbc.M210819200]. [PMID: 12584205]. [DOI] [PubMed] [Google Scholar]
- 44.Straus D.S., Pascual G., Li M., Welch J.S., Ricote M., Hsiang C.H., Sengchanthalangsy L.L., Ghosh G., Glass C.K. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc. Natl. Acad. Sci. USA. 2000;97(9):4844–4849. doi: 10.1073/pnas.97.9.4844. [http://dx.doi.org/10.1073/pnas.97.9.4844]. [PMID: 10781090]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim G.P., Yang F., Chu T., Chen P., Beech W., Teter B., Tran T., Ubeda O., Ashe K.H., Frautschy S.A., Cole G.M. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J. Neurosci. 2000;20(15):5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [http://dx.doi.org/10.1523/JNEUROSCI.20-15-05709.2000]. [PMID: 10908610]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan Q., Zhang J., Liu H., Babu-Khan S., Vassar R., Biere A.L., Citron M., Landreth G. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. J. Neurosci. 2003;23(20):7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [http://dx. doi.org/10.1523/JNEUROSCI.23-20-07504.2003]. [PMID: 12930788]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heneka M.T., Sastre M., Dumitrescu-Ozimek L., Hanke A., Dewachter I., Kuiperi C., O’Banion K., Klockgether T., Van Leuven F., Landreth G.E. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain. 2005;128(Pt 6):1442–1453. doi: 10.1093/brain/awh452. [http://dx.doi.org/10.1093/brain/awh452]. [PMID: 15817521]. [DOI] [PubMed] [Google Scholar]
- 48.Liu L.P., Yan T.H., Jiang L.Y., Hu W., Hu M., Wang C., Zhang Q., Long Y., Wang J.Q., Li Y.Q., Hu M., Hong H. Pioglitazone ameliorates memory deficits in streptozotocin-induced diabetic mice by reducing brain β-amyloid through PPARγ activation. Acta Pharmacol. Sin. 2013;34(4):455–463. doi: 10.1038/aps.2013.11. [http://dx.doi. org/10.1038/aps.2013.11]. [PMID: 23524568]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sastre M., Dewachter I., Rossner S., Bogdanovic N., Rosen E., Borghgraef P., Evert B.O., Dumitrescu-Ozimek L., Thal D.R., Landreth G., Walter J., Klockgether T., van Leuven F., Heneka M.T. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proc. Natl. Acad. Sci. USA. 2006;103(2):443–448. doi: 10.1073/pnas.0503839103. [http://dx.doi.org/10. 1073/pnas.0503839103]. [PMID: 16407166]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toledo E.M., Inestrosa N.C. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer’s disease. Mol. Psychiatry. 2010;15(3):272–285. doi: 10.1038/mp.2009.72. 228. [DOI] [PubMed] [Google Scholar]
- 51.Eriksen J.L., Sagi S.A., Smith T.E., Weggen S., Das P., McLendon D.C., Ozols V.V., Jessing K.W., Zavitz K.H., Koo E.H., Golde T.E. NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo. J. Clin. Invest. 2003;112(3):440–449. doi: 10.1172/JCI18162. [http://dx.doi.org/10.1172/JCI18162]. [PMID: 12897211]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prade E., Bittner H.J., Sarkar R., Lopez, Del, Amo J.M., Althoff-Ospelt G., Multhaup G., Hildebrand P.W., Reif B. Structural mechanism of the interaction of alzheimer disease Aβ fibrils with the non-steroidal anti-inflammatory drug (NSAID). Sulindac Sulfide. J. Biol. Chem. 2015;290:28737–28745. doi: 10.1074/jbc.M115.675215. [http://dx.doi.org/ 10.1074/jbc.M115.675215]. [PMID: 26416887]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitmer R.A., Sidney S., Selby J., Johnston S.C., Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [http://dx.doi.org/10.1212/01. WNL.0000149519.47454.F2]. [PMID: 15668425]. [DOI] [PubMed] [Google Scholar]
- 54.Dias H.K., Brown C.L., Polidori M.C., Lip G.Y., Griffiths H.R. LDL-lipids from patients with hypercholesterolaemia and Alzheimer’s disease are inflammatory to microvascular endothelial cells: mitigation by statin intervention. Clin. Sci. (Lond.) 2015;129(12):1195–1206. doi: 10.1042/CS20150351. [http://dx.doi.org/10.1042/CS20150351]. [PMID: 26399707]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hendrie H.C., Hake A., Lane K., Purnell C., Unverzagt F., Smith-Gamble V., Murrell J., Ogunniyi A., Baiyewu O., Callahan C., Saykin A., Taylor S., Hall K., Gao S. Statin use, incident dementia and alzheimer disease in elderly african americans. Ethn. Dis. 2015;25(3):345–354. doi: 10.18865/ed.25.3.345. [http://dx.doi.org/10.18865/ed. 25.3.345]. [PMID: 26673814]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang T., Li R., Cheng O. Statins for treating alzheimer’s disease: Truly ineffective? Eur. Neurol. 2015;73(5-6):360–366. doi: 10.1159/000382128. [http://dx.doi.org/10.1159/000382128]. [PMID: 26021802]. [DOI] [PubMed] [Google Scholar]
- 57.Solomon A., Kivipelto M., Wolozin B., Zhou J., Whitmer R.A. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement. Geriatr. Cogn. Disord. 2009;28(1):75–80. doi: 10.1159/000231980. [http://dx.doi.org/10.1159/000231980]. [PMID: 19648749]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mielke M.M., Zandi P.P., Shao H., Waern M., Östling S., Guo X., Björkelund C., Lissner L., Skoog I., Gustafson D.R. The 32-year relationship between cholesterol and dementia from midlife to late life. Neurology. 2010;75(21):1888–1895. doi: 10.1212/WNL.0b013e3181feb2bf. [http://dx.doi.org/ 10.1212/WNL.0b013e3181feb2bf]. [PMID: 21068429]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strittmatter W. J. Medicine. Old drug, new hope for Alzheimer’s disease. Science. 2012;335(6075):1447–1448. doi: 10.1126/science.1220725. [http://dx.doi.org/ 10.1126/science.1220725]. [PMID: 22442467]. [DOI] [PubMed] [Google Scholar]
- 60.Yin J., Turner G.H., Coons S.W., Maalouf M., Reiman E.M., Shi J. Association of amyloid burden, brain atrophy and memory deficits in aged apolipoprotein ε4 mice. Curr. Alzheimer Res. 2014;11(3):283–290. doi: 10.2174/156720501103140329220007. [http://dx.doi.org/10.2174/156720501103140329220007]. [PMID: 24694076]. [DOI] [PubMed] [Google Scholar]
- 61.Jiang Q., Lee C.Y., Mandrekar S., Wilkinson B., Cramer P., Zelcer N., Mann K., Lamb B., Willson T.M., Collins J.L., Richardson J.C., Smith J.D., Comery T.A., Riddell D., Holtzman D.M., Tontonoz P., Landreth G.E. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58(5):681–693. doi: 10.1016/j.neuron.2008.04.010. [http://dx.doi.org/10.1016/j.neuron.2008.04.010]. [PMID: 18549781]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koldamova R., Fitz N.F., Lefterov I. The role of ATP-binding cassette transporter A1 in Alzheimer’s disease and neurodegeneration. Biochim. Biophys. Acta. 2010;1801(8):824–830. doi: 10.1016/j.bbalip.2010.02.010. [http://dx. doi.org/10.1016/j.bbalip.2010.02.010]. [PMID: 20188211]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonet-Costa V., Herranz-Pérez V., Blanco-Gandía M., Mas-Bargues C., Inglés M., Garcia-Tarraga P., Rodriguez-Arias M., Miñarro J., Borras C., Garcia-Verdugo J.M., Viña J. Clearing amyloid-β through PPARγ/ApoE activation by genistein is a treatment of experimental alzheimer’s disease. J. Alzheimers Dis. 2016;51(3):701–711. doi: 10.3233/JAD-151020. [http://dx.doi.org/10.3233/JAD-151020]. [PMID: 26890773]. [DOI] [PubMed] [Google Scholar]
- 64.Ogata M., Tsujita M., Hossain M.A., Akita N., Gonzalez F.J., Staels B., Suzuki S., Fukutomi T., Kimura G., Yokoyama S. On the mechanism for PPAR agonists to enhance ABCA1 gene expression. Atherosclerosis. 2009;205(2):413–419. doi: 10.1016/j.atherosclerosis.2009.01.008. [http://dx.doi.org/ 10.1016/j.atherosclerosis.2009.01.008]. [PMID: 19201410]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mandrekar-Colucci S., Landreth G.E. Nuclear receptors as therapeutic targets for Alzheimer’s disease. Expert Opin. Ther. Targets. 2011;15(9):1085–1097. doi: 10.1517/14728222.2011.594043. [http://dx.doi.org/10.1517/14728222.2011. 594043]. [PMID: 21718217]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Odegaard J.I., Chawla A. Alternative macrophage activation and metabolism. Annu. Rev. Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [http://dx.doi. org/10.1146/annurev-pathol-011110-130138]. [PMID: 21034223]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Talbot K., Wang H.Y., Kazi H., Han L.Y., Bakshi K.P., Stucky A., Fuino R.L., Kawaguchi K.R., Samoyedny A.J., Wilson R.S., Arvanitakis Z., Schneider J.A., Wolf B.A., Bennett D.A., Trojanowski J.Q., Arnold S.E. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Invest. 2012;122(4):1316–1338. doi: 10.1172/JCI59903. [http://dx.doi.org/10.1172/JCI59903]. [PMID: 22476197]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barbagallo M., Dominguez L.J. Type 2 diabetes mellitus and Alzheimer’s disease. World J. Diabetes. 2014;5(6):889–893. doi: 10.4239/wjd.v5.i6.889. [http://dx.doi.org/10.4239/wjd.v5.i6.889]. [PMID: 25512792]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fehm H.L., Kern W., Peters A. The selfish brain: competition for energy resources. Prog. Brain Res. 2006;153:129–140. doi: 10.1016/S0079-6123(06)53007-9. [DOI] [PubMed] [Google Scholar]
- 70.Abramov A.Y., Duchen M.R. Impaired mitochondrial bioenergetics determines glutamate-induced delayed calcium deregulation in neurons. Biochim. Biophys. Acta. 2010;1800(3):297–304. doi: 10.1016/j.bbagen.2009.08.002. [http:// dx.doi.org/10.1016/j.bbagen.2009.08.002]. [PMID: 19695307]. [DOI] [PubMed] [Google Scholar]
- 71.Agrawal R., Gomez-Pinilla F. ‘Metabolic syndrome’ in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J. Physiol. 2012;590(10):2485–2499. doi: 10.1113/jphysiol.2012.230078. [http://dx.doi.org/10.1113/jphysiol.2012.230078]. [PMID: 22473784]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu J., Wang L.N., Jia J.P. Peroxisome proliferator-activated receptor-gamma agonists for Alzheimer’s disease and amnestic mild cognitive impairment: a systematic review and meta-analysis. Drugs Aging. 2015;32(1):57–65. doi: 10.1007/s40266-014-0228-7. [http://dx.doi.org/10.1007/ s40266-014-0228-7]. [PMID: 25504005]. [DOI] [PubMed] [Google Scholar]
- 73.Sato T., Hanyu H., Hirao K., Kanetaka H., Sakurai H., Iwamoto T. Efficacy of PPAR-γ agonist pioglitazone in mild Alzheimer disease. Neurobiol. Aging. 2011;32(9):1626–1633. doi: 10.1016/j.neurobiolaging.2009.10.009. [http://dx.doi. org/10.1016/j.neurobiolaging.2009.10.009]. [PMID: 19923038]. [DOI] [PubMed] [Google Scholar]
- 74.Denner L.A., Rodriguez-Rivera J., Haidacher S.J., Jahrling J.B., Carmical J.R., Hernandez C.M., Zhao Y., Sadygov R.G., Starkey J.M., Spratt H., Luxon B.A., Wood T.G., Dineley K.T. Cognitive enhancement with rosiglitazone links the hippocampal PPARγ and ERK MAPK signaling pathways. J. Neurosci. 2012;32(47):16725–35a. doi: 10.1523/JNEUROSCI.2153-12.2012. [http://dx.doi.org/10.1523/JNEUROSCI.2153-12.2012]. [PMID: 23175826]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jahrling J.B., Hernandez C.M., Denner L., Dineley K.T. PPARγ recruitment to active ERK during memory consolidation is required for Alzheimer’s disease-related cognitive enhancement. J. Neurosci. 2014;34(11):4054–4063. doi: 10.1523/JNEUROSCI.4024-13.2014. [http://dx.doi.org/10.1523/JNEUROSCI. 4024-13.2014]. [PMID: 24623782]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Searcy J.L., Phelps J.T., Pancani T., Kadish I., Popovic J., Anderson K.L., Beckett T.L., Murphy M.P., Chen K.C., Blalock E.M., Landfield P.W., Porter N.M., Thibault O. Long-term pioglitazone treatment improves learning and attenuates pathological markers in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2012;30(4):943–961. doi: 10.3233/JAD-2012-111661. [http://dx.doi.org/10.3233/JAD-2012-111661]. [PMID: 22495349]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hort J., Laczó J., Vyhnálek M., Bojar M., Bures J., Vlcek K. Spatial navigation deficit in amnestic mild cognitive impairment. Proc. Natl. Acad. Sci. USA. 2007;104(10):4042–4047. doi: 10.1073/pnas.0611314104. [http:// dx.doi.org/10.1073/pnas.0611314104]. [PMID: 17360474]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoefer M., Allison S.C., Schauer G.F., Neuhaus J.M., Hall J., Dang J.N., Weiner M.W., Miller B.L., Rosen H.J. Fear conditioning in frontotemporal lobar degeneration and Alzheimer’s disease. Brain. 2008;131(Pt 6):1646–1657. doi: 10.1093/brain/awn082. [http://dx.doi.org/10. 1093/brain/awn082]. [PMID: 18492729]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barage S.H., Sonawane K.D. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides. 2015;52:1–18. doi: 10.1016/j.npep.2015.06.008. [http://dx.doi.org/10.1016/j.npep. 2015.06.008]. [PMID: 26149638]. [DOI] [PubMed] [Google Scholar]
- 80.Prakash A., Kumar A. Role of nuclear receptor on regulation of BDNF and neuroinflammation in hippocampus of β-amyloid animal model of Alzheimer’s disease. Neurotox. Res. 2014;25(4):335–347. doi: 10.1007/s12640-013-9437-9. [http://dx.doi.org/10.1007/s12640-013-9437-9]. [PMID: 24277156]. [DOI] [PubMed] [Google Scholar]
- 81.Xiang G.Q., Tang S.S., Jiang L.Y., Hong H., Li Q., Wang C., Wang X.Y., Zhang T.T., Yin L. PPARγ agonist pioglitazone improves scopolamine-induced memory impairment in mice. J. Pharm. Pharmacol. 2012;64(4):589–596. doi: 10.1111/j.2042-7158.2011.01432.x. [http://dx.doi.org/10. 1111/j.2042-7158.2011.01432.x]. [PMID: 22420664]. [DOI] [PubMed] [Google Scholar]
- 82.Beyer A.M., Baumbach G.L., Halabi C.M., Modrick M.L., Lynch C.M., Gerhold T.D., Ghoneim S.M., de Lange W.J., Keen H.L., Tsai Y.S., Maeda N., Sigmund C.D., Faraci F.M. Interference with PPARgamma signaling causes cerebral vascular dysfunction, hypertrophy, and remodeling. Hypertension. 2008;51(4):867–871. doi: 10.1161/HYPERTENSIONAHA.107.103648. [http://dx.doi.org/10.1161/HYPERTENSIONAHA. 107.103648]. [PMID: 18285614]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ketsawatsomkron P., Pelham C.J., Groh S., Keen H.L., Faraci F.M., Sigmund C.D. Does peroxisome proliferator-activated receptor-γ (PPAR γ) protect from hypertension directly through effects in the vasculature? J. Biol. Chem. 2010;285(13):9311–9316. doi: 10.1074/jbc.R109.025031. [http://dx.doi.org/10.1074/jbc.R109.025031]. [PMID: 20129921]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Di B.B., Li H.W., Li W.P., Shen X.H., Sun Z.J., Wu X. Pioglitazone inhibits high glucose-induced expression of receptor for advanced glycation end products in coronary artery smooth muscle cells. Mol. Med. Rep. 2015;11(4):2601–2607. doi: 10.3892/mmr.2014.3113. [http://dx.doi.org/ 10.3892/mmr.2014.3113]. [PMID: 25523934]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mukohda M., Stump M., Ketsawatsomkron P., Hu C., Quelle F.W., Sigmund C.D. Endothelial PPAR-γ provides vascular protection from IL-1β-induced oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2016;310(1):H39–H48. doi: 10.1152/ajpheart.00490.2015. [http://dx.doi.org/10.1152/ ajpheart.00490.2015]. [PMID: 26566726]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sakatani Y., Miyoshi T., Oe H., Noda Y., Ohno Y., Nakamura K., Saito Y., Osawa K., Morita H., Kohno K., Ito H. Pioglitazone prevents the endothelial dysfunction induced by ischemia and reperfusion in healthy subjects. J. Cardiovasc. Pharmacol. 2014;64(4):326–331. doi: 10.1097/FJC.0000000000000124. [http://dx.doi.org/10.1097/FJC.0000000000000124]. [PMID: 24887686]. [DOI] [PubMed] [Google Scholar]
- 87.Fuenzalida K., Quintanilla R., Ramos P., Piderit D., Fuentealba R.A., Martinez G., Inestrosa N.C., Bronfman M. Peroxisome proliferator-activated receptor gamma up-regulates the Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial stabilization and protection against oxidative stress and apoptosis. J. Biol. Chem. 2007;282(51):37006–37015. doi: 10.1074/jbc.M700447200. [http://dx.doi.org/10.1074/ jbc.M700447200]. [PMID: 17965419]. [DOI] [PubMed] [Google Scholar]
- 88.Haorah J., Ramirez S.H., Schall K., Smith D., Pandya R., Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J. Neurochem. 2007;101(2):566–576. doi: 10.1111/j.1471-4159.2006.04393.x. [http://dx.doi.org/ 10.1111/j.1471-4159.2006.04393.x]. [PMID: 17250680]. [DOI] [PubMed] [Google Scholar]
- 89.Fujimoto M., Takagi Y., Aoki T., Hayase M., Marumo T., Gomi M., Nishimura M., Kataoka H., Hashimoto N., Nozaki K. Tissue inhibitor of metalloproteinases protect blood-brain barrier disruption in focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2008;28(10):1674–1685. doi: 10.1038/jcbfm.2008.59. [http://dx.doi.org/10.1038/jcbfm.2008.59]. [PMID: 18560439]. [DOI] [PubMed] [Google Scholar]
- 90.Lee S.R., Kim H.Y., Hong J.S., Baek W.K., Park J.W. PPARgamma agonist pioglitazone reduces matrix metalloproteinase-9 activity and neuronal damage after focal cerebral ischemia. Biochem. Biophys. Res. Commun. 2009;380(1):17–21. doi: 10.1016/j.bbrc.2008.12.181. [http://dx.doi.org/10. 1016/j.bbrc.2008.12.181]. [PMID: 19135426]. [DOI] [PubMed] [Google Scholar]
- 91.Huang W., Eum S.Y., András I.E., Hennig B., Toborek M. PPARalpha and PPARgamma attenuate HIV-induced dysregulation of tight junction proteins by modulations of matrix metalloproteinase and proteasome activities. FASEB J. 2009;23(5):1596–1606. doi: 10.1096/fj.08-121624. [http://dx.doi.org/10.1096/fj.08-121624]. [PMID: 19141539]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanz M.J., Albertos F., Otero E., Juez M., Morcillo E.J., Piqueras L. Retinoid X receptor agonists impair arterial mononuclear cell recruitment through peroxisome proliferator-activated receptor-γ activation. J. Immunol. 2012;189(1):411–424. doi: 10.4049/jimmunol.1102942. [http://dx. doi.org/10.4049/jimmunol.1102942]. [PMID: 22661092]. [DOI] [PubMed] [Google Scholar]
- 93.Wang L., Waltenberger B., Pferschy-Wenzig E.M., Blunder M., Liu X., Malainer C., Blazevic T., Schwaiger S., Rollinger J.M., Heiss E.H., Schuster D., Kopp B., Bauer R., Stuppner H., Dirsch V.M., Atanasov A.G. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): a review. Biochem. Pharmacol. 2014;92(1):73–89. doi: 10.1016/j.bcp.2014.07.018. [http://dx.doi.org/10. 1016/j.bcp.2014.07.018]. [PMID: 25083916]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang P., Li B., Cai G., Huang M., Jiang L., Pu J., Li L., Wu Q., Zuo L., Wang Q., Zhou P. Activation of PPAR-γ by pioglitazone attenuates oxidative stress in aging rat cerebral arteries through upregulating UCP2. J. Cardiovasc. Pharmacol. 2014;64(6):497–506. doi: 10.1097/FJC.0000000000000143. [http://dx.doi.org/10.1097/FJC.0000000000000143]. [PMID: 25490415]. [DOI] [PubMed] [Google Scholar]
- 95.Mosconi L. Glucose metabolism in normal aging and Alzheimer’s disease: Methodological and physiological considerations for PET studies. Clin. Transl. Imaging. 2013;1(4):1. doi: 10.1007/s40336-013-0026-y. [http://dx.doi.org/ 10.1007/s40336-013-0026-y]. [PMID: 24409422]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van der Ossenkoppele R., Flier W.M., Verfaillie S.C., Vrenken H., Versteeg A., van Schijndel R.A., Sikkes S.A., Twisk J., Adriaanse S.M., Zwan M.D., Boellaard R., Windhorst A.D., Barkhof F., Scheltens P., Lammertsma A.A., van Berckel B.N. Long-term effects of amyloid, hypometabolism, and atrophy on neuropsychological functions. Neurol. 2014;82:1768–1775. doi: 10.1212/WNL.0000000000000432. [http://dx.doi.org/10.1212/WNL.0000000000000432]. [DOI] [PubMed] [Google Scholar]
- 97.De Nuccio C., Bernardo A., Cruciani C., De Simone R., Visentin S., Minghetti L. Peroxisome proliferator activated receptor-γ agonists protect oligodendrocyte progenitors against tumor necrosis factor-alpha-induced damage: Effects on mitochondrial functions and differentiation. Exp. Neurol. 2015;271:506–514. doi: 10.1016/j.expneurol.2015.07.014. [http://dx. doi.org/10.1016/j.expneurol.2015.07.014]. [PMID: 26210873]. [DOI] [PubMed] [Google Scholar]
- 98.Roses A.D., Saunders A.M., Huang Y., Strum J., Weisgraber K.H., Mahley R.W. Complex disease-associated pharmacogenetics: drug efficacy, drug safety, and confirmation of a pathogenetic hypothesis (Alzheimer’s disease). Pharmacogenomics J. 2007;7(1):10–28. doi: 10.1038/sj.tpj.6500397. [http://dx.doi.org/10.1038/sj.tpj.6500397]. [PMID: 16770341]. [DOI] [PubMed] [Google Scholar]
- 99.Corona J.C., de Souza S.C., Duchen M.R. PPARγ activation rescues mitochondrial function from inhibition of complex I and loss of PINK1. Exp. Neurol. 2014;253:16–27. doi: 10.1016/j.expneurol.2013.12.012. [http://dx.doi.org/ 10.1016/j.expneurol.2013.12.012]. [PMID: 24374061]. [DOI] [PubMed] [Google Scholar]
- 100.Chiang M.C., Cheng Y.C., Lin K.H., Yen C.H. PPARγ regulates the mitochondrial dysfunction in human neural stem cells with tumor necrosis factor alpha. Neuroscience. 2013;229:118–129. doi: 10.1016/j.neuroscience.2012.11.003. [http:// dx.doi.org/10.1016/j.neuroscience.2012.11.003]. [PMID: 23153990]. [DOI] [PubMed] [Google Scholar]
- 101.Chiang M.C., Cheng Y.C., Chen H.M., Liang Y.J., Yen C.H. Rosiglitazone promotes neurite outgrowth and mitochondrial function in N2A cells via PPARgamma pathway. Mitochondrion. 2014;14(1):7–17. doi: 10.1016/j.mito.2013.12.003. [http://dx.doi.org/10.1016/j.mito.2013.12.003]. [PMID: 24370585]. [DOI] [PubMed] [Google Scholar]
- 102.Quintanilla R.A., Orellana D.I., González-Billault C., Maccioni R.B. Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Exp. Cell Res. 2004;295(1):245–257. doi: 10.1016/j.yexcr.2004.01.002. [http://dx.doi.org/10.1016/j.yexcr.2004.01. 002]. [PMID: 15051507]. [DOI] [PubMed] [Google Scholar]
- 103.Chen J., Li S., Sun W., Li J. Anti-diabetes drug pioglitazone ameliorates synaptic defects in AD transgenic mice by inhibiting cyclin-dependent kinase5 activity. PLoS One. 2015;10(4):e0123864. doi: 10.1371/journal.pone.0123864. [http://dx.doi.org/10.1371/journal.pone.0123864]. [PMID: 25875370]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nenov M.N., Laezza F., Haidacher S.J., Zhao Y., Sadygov R.G., Starkey J.M., Spratt H., Luxon B.A., Dineley K.T., Denner L. Cognitive enhancing treatment with a PPARγ agonist normalizes dentate granule cell presynaptic function in Tg2576 APP mice. J. Neurosci. 2014;34(3):1028–1036. doi: 10.1523/JNEUROSCI.3413-13.2014. [http://dx.doi.org/ 10.1523/JNEUROSCI.3413-13.2014]. [PMID: 24431460]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Son S.M., Shin H.J., Byun J., Kook S.Y., Moon M., Chang Y.J., Mook-Jung I. Metformin facilitates amyloid-β generation by β- and γ-secretases via autophagy activation. J. Alzheimers Dis. 2016;51(4):1197–1208. doi: 10.3233/JAD-151200. [http://dx.doi.org/10.3233/JAD-151200]. [PMID: 26967226]. [DOI] [PubMed] [Google Scholar]
- 106.Wang X., Zheng W., Xie J.W., Wang T., Wang S.L., Teng W.P., Wang Z.Y. Insulin deficiency exacerbates cerebral amyloidosis and behavioral deficits in an Alzheimer transgenic mouse model. Mol. Neurodegener. 2010;5:46–58. doi: 10.1186/1750-1326-5-46. [http://dx.doi.org/ 10.1186/1750-1326-5-46]. [PMID: 21044348]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Benedict C., Frey W.H., II, Schiöth H.B., Schultes B., Born J., Hallschmid M. Intranasal insulin as a therapeutic option in the treatment of cognitive impairments. Exp. Gerontol. 2011;46(2-3):112–115. doi: 10.1016/j.exger.2010.08.026. [http://dx.doi.org/10.1016/j.exger.2010.08.026]. [PMID: 20849944]. [DOI] [PubMed] [Google Scholar]
- 108.McNay E.C., Ong C.T., McCrimmon R.J., Cresswell J., Bogan J.S., Sherwin R.S. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol. Learn. Mem. 2010;93(4):546–553. doi: 10.1016/j.nlm.2010.02.002. [http://dx.doi.org/10.1016/ j.nlm.2010.02.002]. [PMID: 20176121]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Watson G.S., Cholerton B.A., Reger M.A., Baker L.D., Plymate S.R., Asthana S., Fishel M.A., Kulstad J.J., Green P.S., Cook D.G., Kahn S.E., Keeling M.L., Craft S. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am. J. Geriatr. Psychiatry. 2005;13(11):950–958. doi: 10.1176/appi.ajgp.13.11.950. [PMID: 16286438]. [DOI] [PubMed] [Google Scholar]
- 110.Risner M.E., Saunders A.M., Altman J.F., Ormandy G.C., Craft S., Foley I.M., Zvartau-Hind M.E., Hosford D.A., Roses A.D. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J. 2006;6(4):246–254. doi: 10.1038/sj.tpj.6500369. [http://dx.doi.org/10.1038/sj.tpj.6500369]. [PMID: 16446752]. [DOI] [PubMed] [Google Scholar]
- 111.Craft S., Asthana S., Schellenberg G., Cherrier M., Baker L.D., Newcomer J., Plymate S., Latendresse S., Petrova A., Raskind M., Peskind E., Lofgreen C., Grimwood K. Insulin metabolism in Alzheimer’s disease differs according to apolipoprotein E genotype and gender. Neuroendocrinology. 1999;70(2):146–152. doi: 10.1159/000054469. [http:// dx.doi.org/10.1159/000054469]. [PMID: 10461029]. [DOI] [PubMed] [Google Scholar]
- 112.Feinstein D.L., Galea E., Gavrilyuk V., Brosnan C.F., Whitacre C.C., Dumitrescu-Ozimek L., Landreth G.E., Pershadsingh H.A., Weinberg G., Heneka M.T. Peroxisome proliferator-activated receptor-gamma agonists prevent experimental autoimmune encephalomyelitis. Ann. Neurol. 2002;51(6):694–702. doi: 10.1002/ana.10206. [http://dx.doi.org/ 10.1002/ana.10206]. [PMID: 12112074]. [DOI] [PubMed] [Google Scholar]
- 113.Doost Mohammadpour J., Hosseinmardi N., Janahmadi M., Fathollahi Y., Motamedi F., Rohampour K. Non-selective NSAIDs improve the amyloid-β-mediated suppression of memory and synaptic plasticity. Pharmacol. Biochem. Behav. 2015;132:33–41. doi: 10.1016/j.pbb.2015.02.012. [http://dx.doi.org/10.1016/j.pbb.2015.02.012]. [PMID: 25697476]. [DOI] [PubMed] [Google Scholar]
- 114.Yu Y., Li X., Blanchard J., Li Y., Iqbal K., Liu F., Gong C.X. Insulin sensitizers improve learning and attenuate tau hyperphosphorylation and neuroinflammation in 3xTg-AD mice. J. Neural Transm. (Vienna) 2015;122(4):593–606. doi: 10.1007/s00702-014-1294-z. [http://dx.doi.org/10. 1007/s00702-014-1294-z]. [PMID: 25113171]. [DOI] [PubMed] [Google Scholar]
- 115.Gupta R., Gupta L.K. Improvement in long term and visuo-spatial memory following chronic pioglitazone in mouse model of Alzheimer’s disease. Pharmacol. Biochem. Behav. 2012;102(2):184–190. doi: 10.1016/j.pbb.2012.03.028. [http://dx.doi.org/10.1016/j.pbb.2012.03.028]. [PMID: 22503969]. [DOI] [PubMed] [Google Scholar]
- 116.Heneka M.T., Fink A., Doblhammer G. Effect of pioglitazone medication on the incidence of dementia. Ann. Neurol. 2015;78(2):284–294. doi: 10.1002/ana.24439. [http://dx.doi.org/10.1002/ana.24439]. [PMID: 25974006]. [DOI] [PubMed] [Google Scholar]
- 117.Papadopoulos P., Rosa-Neto P., Rochford J., Hamel E. Pioglitazone improves reversal learning and exerts mixed cerebrovascular effects in a mouse model of Alzheimer’s disease with combined amyloid-β and cerebrovascular pathology. PLoS One. 2013;8(7):e68612. doi: 10.1371/journal.pone.0068612. [http://dx.doi.org/10.1371/journal.pone.0068612]. [PMID: 23874687]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Flesch D., Ness J., Lamers C., Dehm F., Popella S., Steri R., Ogorek I., Hieke M., Dannhardt G., Werz O., Weggen S., Schubert-Zsilavecz M. SAR-studies of γ-secretase modulators with PPARγ-agonistic and 5-lipoxygenase-inhibitory activity for Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2015;25(4):841–846. doi: 10.1016/j.bmcl.2014.12.073. [http://dx.doi.org/10.1016/j.bmcl.2014.12.073]. [PMID: 25575659]. [DOI] [PubMed] [Google Scholar]
- 119.de la Monte S.M., Tong M., Schiano I., Didsbury J. Improved brain insulin/IGF signaling and reduced neuroinflammation with T3D-959 in an experimental model of sporadic alzheimer’s disease. J. Alzheimers Dis. 2017;55(2):849–864. doi: 10.3233/JAD-160656. [http://dx.doi.org/10. 3233/JAD-160656]. [PMID: 27802237]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tong M., Dominguez C., Didsbury J., de la Monte S.M. Targeting alzheimer’s disease Neuro-Metabolic Dysfunction with a small molecule nuclear receptor agonist (T3D-959) reverses disease pathologies. J. Alzheimers Dis. Parkinsonism. 2016;6(3):238. doi: 10.4172/2161-0460.1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Almad A., Lash A.T., Wei P., Lovett-Racke A.E., McTigue D.M. The PPAR alpha agonist gemfibrozil is an ineffective treatment for spinal cord injured mice. Exp. Neurol. 2011;232(2):309–317. doi: 10.1016/j.expneurol.2011.09.023. [http://dx.doi.org/10.1016/j.expneurol.2011.09.023]. [PMID: 21963672]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Geldmacher D.S., Fritsch T., McClendon M.J., Landreth G. A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Arch. Neurol. 2011;68(1):45–50. doi: 10.1001/archneurol.2010.229. [http://dx.doi.org/10.1001/archneurol.2010.229]. [PMID: 20837824]. [DOI] [PubMed] [Google Scholar]
- 123.Gold M., Alderton C., Zvartau-Hind M., Egginton S., Saunders A.M., Irizarry M., Craft S., Landreth G., Linnamägi U., Sawchak S. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement. Geriatr. Cogn. Disord. 2010;30(2):131–146. doi: 10.1159/000318845. [http://dx.doi.org/10.1159/000318845]. [PMID: 20733306]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Harrington C., Sawchak S., Chiang C., Davies J., Donovan C., Saunders A.M., Irizarry M., Jeter B., Zvartau-Hind M., van Dyck C.H., Gold M. Rosiglitazone does not improve cognition or global function when used as adjunctive therapy to AChE inhibitors in mild-to-moderate Alzheimer’s disease: two phase 3 studies. Curr. Alzheimer Res. 2011;8(5):592–606. doi: 10.2174/156720511796391935. [http://dx.doi.org/10. 2174/156720511796391935]. [PMID: 21592048]. [DOI] [PubMed] [Google Scholar]
- 125.Van Hildreth K.L., Pelt R.E., Moreau K.L., Grigsby J., Hoth K.F., Pelak V., Anderson C.A., Parnes B., Kittelson J., Wolfe P., Nakamura T., Linnebur S.A., Trujillo J.M., Aquilante C.L., Schwartz R.S. Effects of pioglitazone or exercise in older adults with mild cognitive impairment and insulin resistance: a pilot study. Dement. Geriatr. Cogn. Disord. Extra. 2015;5:51–63. doi: 10.1159/000371509. [http:// dx.doi.org/10.1159/000371509]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jiang L.Y., Tang S.S., Wang X.Y., Liu L.P., Long Y., Hu M., Liao M.X., Ding Q.L., Hu W., Li J.C., Hong H. PPARγ agonist pioglitazone reverses memory impairment and biochemical changes in a mouse model of type 2 diabetes mellitus. CNS Neurosci. Ther. 2012;18(8):659–666. doi: 10.1111/j.1755-5949.2012.00341.x. [http://dx.doi.org/10.1111/j.1755-5949.2012.00341.x]. [PMID: 22620268]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cheng H., Shang Y., Jiang L., Shi T.L., Wang L. The peroxisome proliferators activated receptor-gamma agonists as therapeutics for the treatment of Alzheimer’s disease and mild-to-moderate Alzheimer’s disease: a meta-analysis. Int. J. Neurosci. 2016;126(4):299–307. doi: 10.3109/00207454.2015.1015722. [http://dx.doi.org/10.3109/00207454.2015.1015722]. [PMID: 26001206]. [DOI] [PubMed] [Google Scholar]
- 128.Nicolakakis N., Aboulkassim T., Ongali B., Lecrux C., Fernandes P., Rosa-Neto P., Tong X.K., Hamel E. Complete rescue of cerebrovascular function in aged Alzheimer’s disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J. Neurosci. 2008;28(37):9287–9296. doi: 10.1523/JNEUROSCI.3348-08.2008. [http://dx.doi.org/10.1523/JNEUROSCI.3348-08.2008]. [PMID: 18784309]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Inestrosa N.C., Toledo E.M. The role of Wnt signaling in neuronal dysfunction in Alzheimer’s Disease. Mol. Neurodegener. 2008;3:9. doi: 10.1186/1750-1326-3-9. [http://dx.doi.org/10.1186/1750-1326-3-9]. [PMID: 18652670]. [DOI] [PMC free article] [PubMed] [Google Scholar]