Abstract
The research progress of understanding the etiology and pathogenesis of Parkinson's disease (PD) has yet lead to the development of some clinical approaches intended to treat cognitive and behavioral symptoms, such as memory and per-ception disorders. Despite the major advances in different genetic causes and risk factors for PD, which share common pathways to cell dysfunction and death, there is not yet a complete model of PD that can be used to accurately predict the ef-fect of drugs on disease progression. Clinical trials are also important to test any novel neuro-protective agent, and recently there have been great advances in the use of anti-inflammatory drugs and plant flavonoid antioxidants to protect against spe-cific neuronal degeneration and its interference with lipid and cholesterol metabolism. The increasing knowledge of the mo-lecular events underlying the degenerative process of PD has stimulated research to identify natural compounds capable of halting or slowing the progress of neural deterioration. Polyphenols and flavonoids, which play a neuroprotective role in a wide array of in vitro and in vivo models of neurological disorders, emerged from among the multi-target bio-agents found mainly in plants and microorganisms. This review presents a detailed overview of the multimodal activities of neuroprotec-tive bio-agents tested so far, emphasizing their neurorescue/neuroregenerative activity. The brain-penetrating property of bio-agents may make these compounds an important class of natural drugs for the treatment of neurodegenerative diseases. Alt-hough there are numerous studies demonstrating beneficial effects in the laboratory by identifying critical molecular targets, the clinical efficacy of these neuroprotective treatments remains to be proven accurately.
Keywords: Parkinson’s disease, nutraceuticals, neuroprotection, phytobioactivity, degeneration, neuroscience
1. Introduction
Based on limitations concerning the use of synthetic compounds [1-4], natural phytobioactive compounds might play an important role in preventing PD. An ideal neuroprotective property of nutraceuticals, that are natural compounds derived from organic food sources, which have shown verified therapeutic value, should comprise all the mechanisms and strategies involved in the preservation of neuronal integrity and its functions. Therefore, a neuroprotective bio-agent mainly aims to reduce or prevent the pathological mechanisms associated with neurodegeneration, and its efficacy may induce this outcome by either inhibiting primary neurodegenerative events (“neuroprotection”), boosting compensatory responses (“neuroplasticity”) or regenerative mechanisms in the brain (“neurorestoration”) [5].
Neuroprotection or the extraordinary competence of bio-agents in preventing neuronal damage and secondary injuries by limiting oxidative stress, neuroinflammation, mitochondrial dysfunction, excitotoxicity, protein misfolding, disruptive autophagy and apoptosis, is currently leading the therapeutic strategy to slow disease progression [6]. However, various synthetic therapeutic agents, which prevent the death of vulnerable neurons and slow down disease progression, are also used as neuroprotective agents [7]. These agents mainly include antioxidants, anti-inflammatory drugs, glutamate antagonists, cholinesterase inhibitors, dopamine (DA) agonists, antiapoptotics, neuroprotective steroids and some miscellaneous drugs [8]. The main function of a reliable neuroprotective agent is to demonstrate experimentally, first in PD animal models and then in clinical trials, its effects on the preservation of functional nigral dopaminergic neurons, mainly by decreasing apoptotic cell death and activating the glutathione-dependent antioxidant systems which contribute to the reduction of motor dysfunction [9]. Numerous trophic factors, associated receptors and neurotrophins are endogenous plasticity-enhancing and neuroprotective secreted proteins that minimize cell damage and promote compensation in the affected brain cell circuitry, as has been extensively reported in neurons and glia under PD neuropathological conditions [10, 11]. Accordingly, results from treatments that improve neurotrophic factor levels in the brain have been reported to efficiently neutralize adverse degenerative effects such as oxidative damage, mitochondrial dysfunction, microglia activation and apoptosis, while promoting cell regeneration, synaptogenesis and axonal sprouting [12, 13].
Neuroplasticity or the remarkable proven capacity of bio-agents to induce plasticity in the mammalian central nervous system, by reorganizing functionally and structurally their damaged neuronal pathways in response to pathologic insults, provides the basis for both learning and compensatory processes following brain damage or neurodegeneration [14]. In PD, the parkinsonian motor symptoms arise only when more than 30% of nigral dopaminergic neurons or 50% of striatal dopaminergic cells are necrotic or lost, demonstrating the presynaptic compensation response of the nigrostriatal pathway [15]. Preclinical studies from the past decade have demonstrated that monoaminergic systems affected in PD are capable of inducing compensatory changes in an attempt to preserve the proportion of active neurons, and thus to maintain basal aminergic output [9]. The most notable adaptative plastic responses were reported in the dopaminergic systems, particularly in the nigrostriatal pathway, where increased sprouting, branching and thickness of tyrosine hydroxylase (TH)-positive axon terminals were reported in rodent and monkey models intoxicated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [16]. Associated with these compensatory changes, biochemical adjustments involving decreased DA transporter binding, increased activity of L-amino acid decarboxylase (AADC) and up-regulation of DA turnover were also described [17]. However, both noradrenergic neuronal circuitry and the serotonergic projections have been described to participate in the same putative attempt by the dopamine-denervated brain to recruit parallel motor circuits to overcome the functional basal ganglia deficit [18]. Moreover, preclinical studies have also demonstrated that non-monoaminergic systems within the cortical-basal-ganglia-thalamocortical network such as GABAergic [9], enkephalinergic [19] and glutamatergic [20] transmission pathways are involved too, although in an indirect compensatory way. Bezard and colleagues [21] have described clearly three stages of increased compensatory intensity during the presymptomatic period in PD, namely: (i) active dopamine compensation, when dopamine homeostatic compensatory mechanisms can mask the symptoms; (ii) dynamic compensation feedback within the basal ganglia, when striatal dopamine homeostasis breaks down, leading to readjustments in the activity of the basal ganglia output structures; and (iii) emergency compensation activity in structures outside the basal ganglia, when the increased GPi activity does not prevent the emergence of parkinsonian motor symptoms.
Neurorestoration, or the repopulation of dopamine neurons, using cell transplantation or through endogenous neuroprogenitor cells, are types of strategies to promote neuroregeneration and the functional recovery of the affected brain areas, involving the delivery of biological payloads such as gene therapy and stem cells [22]. Therefore, by using neurorestorative and neuroprosthetic technologies, therapeutic treatments aim to change the pathophysiological environment towards the functional recovery of the dopamine phenotype affected. Rasagiline (MAO-B inhibitor) is a good example of a neurorestorative therapeutic agent against PD pathology, as it has been shown to protect in vitro SH-SY5Y neuroblastoma cells from apoptosis [23] and in vivo rodents [24] and primates [25] by restoring dopaminergic neurons previously treated with the neurotoxin MPTP. Moreover, recent studies on neurorestorative therapies for treating PD in 5 dpf zebrafish larvae demonstrate that rasagiline (MAO-B inhibitor) is an effective anti-apoptotic agent for limiting motor symptoms in early disease stages, and also an ideal adjuvant to L-DOPA at more advanced stages [26], as it restored both locomotor function and dopaminergic neurons to control levels [27]. Thus, the prospect of replacing the missing or damaged dopaminergic cells through an effective regenerative therapy that includes the transplanting of developing neural tissue or neural stem cells into the degenerated host brain gains progressively more consistency to overcome the degenerative progress of the disease symptoms.
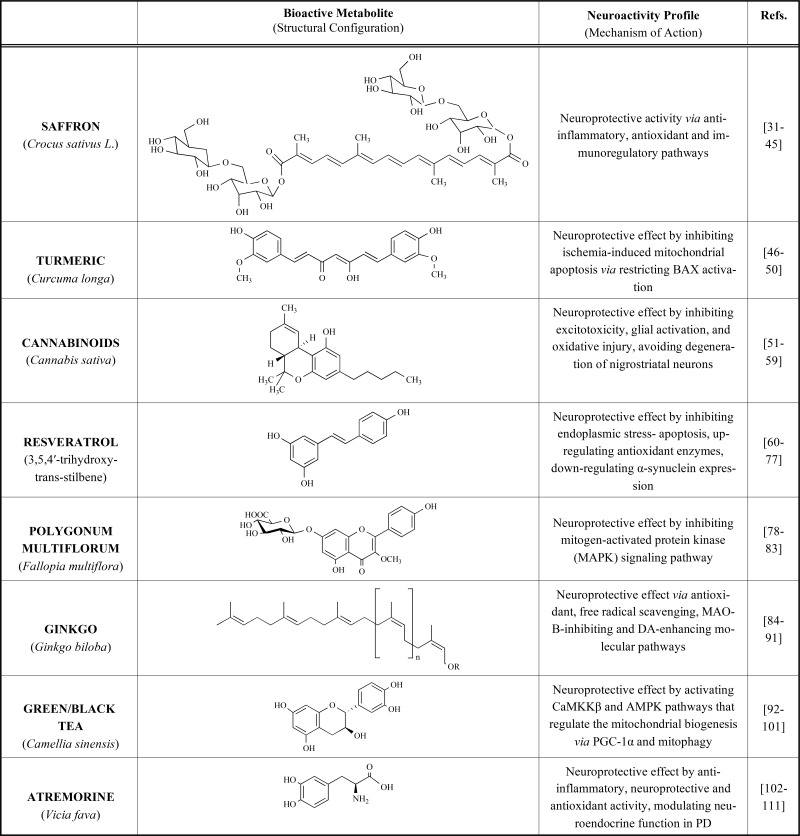
This review will focus on the most studied neuroactive bio-agents (Table 1), based on three approach strategies: neuroprotection, neuroplasticity and neurorestoration, directed against PD pathological symptoms and dysfunctional hallmarks. Natural bio-agents, also called nutraceuticals or functional foods, are currently reliable dietary tools for preventing or slowing down the progression of numerous degenerative processes [28]. Therefore, we will review the most tested natural products against PD, which have been used empirically in current medicine, endorsed by in vitro and in vivo studies validating their biologic properties on scientific bases.
Table 1.
Potential effects of reported plant-derived extracts against PD pathology.
2. Currently used drugs
In the last decades, there have been many remarkable improvements in the treatment approaches for Parkinson's disease. Several new drugs have been developed, tested and used in the daily clinical treatment while updating the knowledge of how to improve the efficiency of older treatments. The result obtained has made a big difference in every day’s life for people with the disease. Currently, there are three main types of medication prescribed for PD patients depending on the symptomatology and the evolution of the disease: levodopa; dopamine agonists and monoamine oxidase-B inhibitors.
2.1. Levodopa and Carbidopa (Sinemet)
Levodopa (also called L-dopa, precursor of dopamine) is the most commonly prescribed medicine for Parkinson’s disease, since it is the best synthetic drug at controlling the main symptoms of the disorder condition, particularly slow movements and rigid body parts. Sinemet is a mix of levodopa and carbidopa (a peripheral DOPA decarboxylase inhibitor used to bypass the excessive peripheral dopamine signaling), potentiating the beneficial effects of levodopa and prevents many common side effects such as nausea, vomiting and irregular heart rhythms.
2.2. Dopamine Agonists
Ropinirole (Requip; stimulate dopamine receptors in the striatum and substantia nigra), Pramipexole (Mirapex; can increase the expression of GDNF and brain-derived neurotrophic factor) and Rotigotine (Neupro; inhibits dopamine uptake and prolactin secretion) are synthetic drugs that act like dopamine in the brain. Since these Dopamine agonists do not have the same risks of long-term problems as levodopa therapy, they are often the first choice of treatment for Parkinson's disease. However, there are some short-term side effects in some patients such as nausea, vomiting, dizziness, light-headedness, confusion and hallucinations.
2.3. Amantadine (Symmetrel)
This synthetic drug raises the concentration of dopamine available to intake by the synaptic neurons by releasing dopamine from the nerve endings of the brain cells, which helps to minimize the Parkinson’s symptoms. Recent studies have also found that Symmetrel reduces the involuntary movements that are triggered by the levodopa therapy, although may cause confusion and memory problems as side effects.
2.4. Trihexyphenidyl (Artane) and Benztropine (Cogentin)
These synthetic drugs demonstrated to restore efficiently the balance between two main neurotransmitters in the brain, dopamine and acetylcholine. The beneficial effects are the reduction of tremors and muscle stiffness in Parkinson's patients, although it may affect memory skills, especially in older people.
2.5. Selegiline (Eldepryl, Zelapar) and Rasagiline (Azilect)
The mechanism of action common among all of these synthetic drugs is the capacity of blocking the neurotransmitters degradation such as Dopamine, enhancing the concentration of available Dopamine in the synaptic areas of the affected brain.
2.6. Tolcapone and Entacapone (Catechol-O-methyltransferase Inhibitors)
These synthetic drugs are prescribed for people in later stages of Parkinson's disease, since it prevents levodopa from being broken down by the enzyme COMT. However, numerous side effects of COMT inhibitors are reported such as nausea or vomiting, diarrhea and abdominal pain.
Unfortunately, no synthetic drugs are yet available that slow efficiently the rate of progression of Parkinson's disease. The initial therapy, for the motor symptoms, should be constitute by direct-acting dopamine agonists while the disease progresses and these agents become insufficient, levodopa can be added. However the efficacy of these synthetic drugs is reduced during the progression of the disease and new modalities presently under investigation are needed to improve the efficiency of clinical treatments.
3. Pluripotent stem cells-derived neurons in PD
In order to explore novel methods for the treatment of PD, a few cell based therapeutics have been developed for the treatment of PD such as embryonic stem cells, induced pluripotent stem cells and somatic stem cells. The human dopaminergic neurons can be generated using a technology called pluripotent induction, where the human fibroblasts can be reprogrammed through a pluripotent stage. In further stages, dopaminergic neurons specific to the patients can be generated, thus avoiding the adverse reaction of immune system as well as the ethical issue of the embryonic stem cells. The major drawbacks associated with this technology is the tumor production, unpredictability in the process of reprogramming, and making prone to the pathogenesis of PD. Although this novel method proved to be effective in PD treatment in animal and clinical studies, it is known to increase the non-motor symptoms. Therefore, more long term clinical trials are required to proof the efficacy of all aspects of PD through cell based therapy including non-motor symptoms.
4. Natural phytobioactive compounds
However, an emerging understanding of nutraceutical science discloses some relevant questions about the level of efficacy of nutraceuticals in comparison with pharmaceutical chemical drugs in the approach of the same pathologic condition. One of these key questions is whether bio-agents (Table 1) are able to activate, at high levels, the endogenous neuroplastic and neuro-regenerative response of the central and peripheral nervous systems, affected in PD, for therapeutic purposes. An even more striking question is whether the improvement in neuroplasticity induced by bio-agents can at the same time enhance dopaminergic function and protect neurons against incipient PD pathology.
Nutraceuticals or nutritional supplements have shown verified therapeutic value by evidence-based studies and have demonstrated no side effects after prolonged intake, opening the pathway toward the use of healthy alternative strategies to battle neurodegenerative diseases. Currently increasing research efforts have been made to better comprehend the role of nutraceuticals in PD [29], demonstrating positive effects via modulating energy metabolism and signaling transduction pathways by inhibiting oxidative stress, inflammation and apoptosis, and regulating mitochondrial homoeostasis and neurotransmission [30]. This review re-examines the most studied nutraceuticals or specific bio-agents against PD and how they can affect each of these biological targets and impact disease processes such as α-synuclein aggregation, ubiquitin proteasome function, lysosomal-autophagy, catecholamine trafficking, DA oxidation, synthesis of toxic DA-quinones, hyperhomocysteinemia, methylation, inflammation and the irreversible oxidation of neuromelanin.
5. SAFFRON (Crocus sativus L.)
In natural medicine there is growing interest in Crocus sativus L. a perennial, stemless herb, belonging to the Iridaceae family, cultivated in most Mediterranean countries and in Iran, India and China. Its active constituents were long studied as antioxidant, anti-inflammatory and neuroprotective bio-agents against degenerative pathologies [31]. Based on traditional natural medicine and recent biomedical research, C. sativus and its bio-active compounds have been indicated to possess anticancer [32], anti-mutagenic [33], antigenotoxic [34] and anti-microbial [35] activities that have proven to be effective treatments for coronary artery diseases [36], gastrointestinal diseases [37], respiratory diseases [38], urinary system disorders [39] and neurodegenerative disorders [40]. Biochemical analysis of the purified C. sativus extract has indicated that carotenoids (crocins, crocetin, picrocrocin, and safranal) and glycosides (quercetin and kaempferol), are its main bioactive constituents [41]. Boskabady and Farkhondeh [31] reported that the immunomodulatory properties of these saffron constituents could be effective against inflammatory and immune system disorders via the modulation of pro-inflammatory cytokines (IL-8, IL-1b, IL-6, TNFα), oxidative stress markers (ROS), immune factors (TGF-β, Leukotriene B4, epidermal growth factor receptor) and an increase in CD8+ lymphocytes, macrophages, neutrophils and epithelial cells. Experimental results using safranal (100 mg/kg) in spinal cord injury (SCI) rat models corroborated these statements by ameliorating neuronal function following SCI, which was associated with its intrinsic anti-inflammatory, anti-apoptotic and edema-attenuating effects [42]. Interestingly, by using crocin (α-crocin; 20 mg/kg) and crocetin (50 mg/kg) against traumatic brain injury in mice, studies have documented their neuroinflammatory protective effects by the amelioration of neurological severity score, brain edema, a decrease in microglial activation, the release of several pro-inflammatory cytokines [43] and a decrease in the lipopolysaccharide (LPS)-induced apoptosis in organotypic hippocampal slice cultures [44]. Broadly speaking, these saffron-derived compounds execute their neuroprotective activity via anti-inflammatory, antioxidant and immunoregulatory pathways, which constitutes a reliable immunomodulation response in neurodegenerative pathologies such as PD [45].
6. TURMERIC (Curcuma longa)
Widely used in Southeast Asia for medical purposes, turmeric is a rhizomatous herbaceous perennial plant of the ginger family (Zingiberaceae). Curcumin (diferuloylmethane) is the main natural turmeric phenol, a flavonoid of the curcuminoids group that displays intrinsic anti-oxidant, anti-inflammatory and anti-cancer properties. In terms of neuroprotection, the capacity of curcumin to cross the blood-brain barrier enables it to exert its anti-oxidant effect on substantia nigra (SN) neurons and to increase striatal dopamine levels and chelates Fe2+ in neurotoxic rat models of PD [46]. In the last decade, several in vitro and in vivo experimental studies in different mouse models of PD have shown that chronic dietary consumption of curcumin exerts a neuroprotective effect against central neurological disease by increasing neuronal viability, tissue perfusion and cerebral blood flow, and reducing ischemic-related apoptosis [47]. The reported results suggested that curcumin intake restored mitochondrial membrane potential and cell viability in 6-OHDA-lesioned mouse embryonic stem cells [48], while increasing striatal dopamine and DOPAC (3,4-Dihydroxyphenylacetic acid) levels in MPTP (1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine)-injected mice [49]. Moreover, in animal models of ischemic stroke, curcumin exerted neuroprotective effects in both in vivo and in vitro experiments, maintaining neuron survival rates through inhibiting ischemia-induced mitochondrial apoptosis via restricting BAX activation [50]. Therefore, the most important neuroprotective function of curcumin against PD is related to its anti-oxidant capability.
7. CANNABINOIDS (CANNABIS SATIVA)
Cannabis is an annual herbaceous flowering plant indigenous to eastern Asia and widely cultivated throughout recorded history, used as a source of industrial fiber, seed oil, food, recreation, religious moods and medicine. It is well known that components of the endocannabinoid system are highly expressed in the basal ganglia circuitry, probably exerting a modulatory function while maintaining an intense interaction with dopaminergic, glutamatergic and GABAergic signaling systems [51]. This endogenous neuroprotective function was revealed by the dramatic increase in endocannabinoid levels upon neuronal injury [52]; these usually being found at low concentrations in the brain. Although over 500 different compounds have been described, the two main plant-derived cannabinoids, Delta9-tetrahydrocannabinol (THC) and cannabidiol (CBD), present neuroprotective effects in animal models of PD, presumably because of their antioxidant properties [53]. Neuroprotective effects of the cannabinoid-based compound (inhibitor of the endocannabinoid inactivation AM404) were investigated in rats with unilateral lesions of nigrostriatal dopaminergic neurons caused by the local application of 6-hydroxydopamine [54]. Results showed that AM404 induced a notable recovery of 6-hydroxydopamine-induced DA depletion and tyrosine hydroxylase deficit in the affected dopaminergic population due to its antioxidant effect, exerted by a phenolic group in its structure. Other cannabinoid agonists have also been suggested as neuroprotective agents in PD, such as WIN-55212-2 [55], due to its capacity to inhibit excitotoxicity, glial activation, and oxidative injury, preventing the degeneration of nigrostriatal neurons. When administered immediately after injury to mouse models of stroke, results showed an enhanced long-term neuronal and oligodendrocyte recovery and regeneration [56]. Moreover, the cannabinoid compounds CE-178253, oleoylethanolamide, nabilone and HU-210, have also demonstrated reliable effects against bradykinesia and levodopa-induced dyskinesia in PD [57]. Pazos and colleagues [58] have also reported that cannabidiol given immediately after hypoxia-ischemia episodes could minimize cerebral injury by preventing hemodynamic impairment and brain edema, and restoring motor and behavioral performance, as results showed after the 72 h analysis. Taken together, reports indicate that cannabinoid bio-agents and their antioxidant properties can exert neuroprotective functions during the different stages of the disease [59], while agonist/antagonist compounds might regulate the behavioral effects of L-Dopa and PD motor symptoms themselves, suggesting that the cannabinoid system plays a crucial role in the therapeutical strategy of PD treatment.
8. RESVERATROL (3,5,4′-trihydroxy-trans-stilbene)
The dietary antioxidant polyphenol found in grapes, cranberries, peanuts and red wine has recently been extensively studied due to its variety of beneficial effects [60]. Although scientific literature has generally focused on its cardioprotective [61], chemopreventive [62], anti-cancer [63] and anti-aging [64] effects, its anti-inflammatory and strong antioxidant properties [65] are other biological effects attributed to resveratrol in the modulation of key molecular mediators directly related to PD-affected pathways. Numerous in vitro studies have demonstrated that resveratrol protects SH-SY5Y cells against rotenone-induced autophagic dysfunction by progressively inducing the degradation of α-synuclein [66]. Zhang and colleagues [67] have corroborated the same protective effect of resveratrol in PC12 cells, significantly reducing the induced damage caused by 6-OHDA via the CXCR4 signaling pathway and by MPPT via the AKT/GSK-3β signaling pathway [68]. Importantly, a recent in vivo study reported that resveratrol has potential therapeutic capacity for rotenone-, 6-OHDA- and MPTP-induced PD in rats by inhibiting endoplasmic reticulum stress-mediated apoptosis [69], up-regulating antioxidant enzymes [70] and down-regulating α-synuclein expression [71], respectively. Nanoencapsulation of the trans-resveratrol form enhances its bioavailability in systematic circulation for prolonged activity in preventing PD by enhancing neuronal survival against oxidative stress, as shown in several studies [72,73]. Moreover, by adding a vitamin E emulsion, studies show a notable increase in availability of resveratrol to the brain, thereby reducing the oxidative stress of PD, improving mouse coordination movements and spatial memory performance [74]. Taken together, experimental studies showed that treatment with resveratrol exerts neuroprotective effects on dopaminergic neurons, probably due to its intrinsic antioxidant properties [75] related to its scavenger activity against hydrogen peroxide. Moreover, resveratrol induces activation and expression of SIRT1, affecting the transcription of heat shock proteins 70 that regulate the homeostasis of cellular proteins, reducing the formation of pathological α-synuclein aggregation [76]. In conclusion, experimental studies found that resveratrol significantly reduced neurotoxic-induced neuronal damage through activating the SIRT1/Akt1 signaling pathway and providing solid evidence for the potential application of resveratrol in treating PD [77].
9. POLYGONUM MULTIFLORUM (FALLOPIA MULTIFLORA)
Polygonum is one of the most popular herbaceous perennial vines in traditional Chinese medicine, known as He shou wu or Fo-ti in China and East Asia [78]. Officially listed in the Chinese Pharmacopoeia, it is used for nourishing the reproductive system, kidney cleansing, liver cleansing and urinary tract cleansing, as well as for reducing triglyceride and cholesterol levels [79]. Recent studies ascribe most of these effects to its main bio-active component, tetrahydroxystilbene glucoside (TSG), directly linked to its antioxidant, anti-inflammatory, free radical-scavenging and cardioprotective activity [80]. In neuroprotection, although the exact molecular pathway has not yet been completely elucidated, TSG has significant neuroprotective effects on the ischemic brain, reported in both in vitro and in vivo experimental studies. In vitro studies indicate that TSG possesses a beneficial potential for PD treatment, as demonstrated by inhibiting microglia activation and the subsequent release of proinflammatory factors in primary rat midbrain neuron-glia cocultures, closely related to the inactivation of the mitogen-activated protein kinase (MAPK) signaling pathway [81]. Moreover, in vitro ischemic models of oxygen–glucose deprivation followed by reperfusion (OGD-R) were used to study the neuroprotective effects of TSG on ischemia/reperfusion brain injury and the related mechanisms. Results obtained demonstrated that OGD-R caused cytotoxicity in primary cultured cortical neurons due to the induction of oxidative stress, which could be rescued by TSG pretreatment. In PD basic studies, results showed that TSG improved both motor and memory functions by attenuating α-synuclein aggregation in the striatum dopaminergic neurons of aged mouse models [82]. Recently, Huang and colleagues [81] demonstrated that TSG promotes neuroprotection from nigral stereotaxic injection of 6-OHDA-induced DA neurotoxicity in rat, by means of the inhibition of microglia-elicited neuroinflammation. Similar results were obtained when administering TSG intraperitoneally before the onset of reperfusion, inducing significant neuroprotection against MCAO-induced neuronal injury due to the induction of oxidative stress in cerebral ischemia mouse models [83]. In summary, TSG has displayed neuroprotection in response to OGD-R-induced oxidative stress, in both in vitro and in vivo experiments, while further research on the main intracellular signaling pathways (MAPK) involved, and their modulation by TSG, may provide additional insights into the molecular basis of the neuroprotective effects that this antioxidative polyphenol exerts.
10. GINKGO (Ginkgo biloba)
Extracts of Ginkgo biloba leaf (EGb) have been studied as antioxidants capable of scavenging various reactive oxygen species, including superoxide, peroxy radical, and hydroxyl radical [84], and of reducing the functional impairments observed after lipid peroxidation [85] in striatum, substantia nigra and hippocampus in Parkinson’s and Alzheimer’s patients. In animal models of hypoxia, EGb exhibits protective effects against excitotoxicity, focal and global cerebral ischemia [86], probably due to its intrinsic inhibitory effects against brain monoamine oxidases, which would prevent the degradation of DA and increase its availability. Previous findings also support MAO-B inhibitors as the main agents involved in decreasing DA re-uptake [87] and delaying disability in Parkinson’s patients [88]. In the neuroprevention of cellular degeneration, the cyclized o-quinones derived from dopamine are efficiently conjugated with glutathione in the presence of human glutathione transferase (GST) catalyzing the detoxification of oxidized metabolites of catecholamine, yielding an effective antioxidant system [89]. Additionally, neurorestoration of striatal DA elements after pre-treatment with EGb has been reported in the MPTP mouse model [90]. Compromised behavioral activity and cellular integrity were also partially restored in PD rat models pre-treated with EGb before lesioning, recovering almost the previous normal levels of TH expression and similar rates of DA, enzymatic and nonenzymatic marker levels related with lipid peroxidation [91]. Taken together, EGb seems to act as a multi-target agent via antioxidant, free radical scavenging, MAO-B-inhibiting and DA-enhancing molecular pathways that rescue the compromised dopaminergic cells, activating the anti-apoptotic, anti-oxidative and anti-inflammatory protective mechanisms in the affected central nervous system.
11. Green/Black Tea (CAMELLIA SINENSIS)
Green or black tea is made from an extract of Camellia sinensis leaves containing phytochemicals, such as polyphenols and caffeine. Polyphenols found in green tea include epigallocatechin-3-gallate (EGCG), epicatechin gallate, epicatechins and flavanols (kaempferol, quercetin, and myricetin) which have antioxidant, anticarcinogenic, anti-inflammatory, and anti-radiation biochemical effects in vitro [92]. EGCG, besides its numerous putative bioactive benefits including anti-oxidative, ROS-scavenging, iron-chelating and anti-apoptotic properties, is frequently featured in PD biological therapy [93]. The two main advantages making it an attractive compound for PD therapy are its complete permeability in crossing the blood-brain barrier and its activation of the adenosine monophosphate-activated protein kinase (AMPK) pathway [94]. This molecular mechanism for EGCG-mediated neuroprotective effects is via the increase of cytosolic Ca2+ levels, thereby influencing the activity of Ca2+-/calmodulin-dependent protein kinase (CaMKKβ), an upstream kinase of AMPK [95]. In in vitro studies, using parkin-null Drosophila as a PD model that bears a resemblance to that of human PD [96], the administration of EGCG significantly reduced the pathological phenotypes of PD flies, such as mitochondrial abnormalities and the progressive degeneration of dopaminergic neurons that induce age-dependent decline in locomotor ability [94]. Consistent with these results, Ng and colleagues [97] also found that LRRK2 mutants of Drosophila ameliorate the parkinsonian phenotypes observed, via pharmacological treatment with EGCG, metformin, AICAR or the co-expression of a constitutively active AMPK mutant. Accordingly, the EGCG-mediated neuroprotective effects requiring AMPK activation may almost completely restore different forms of PD pathological phenotypes in flies. Thus, AMPK activation regulates the mitochondrial biogenesis via PGC-1α and mitophagy, thus maintaining a viable pool of bioenergetically competent mitochondria necessary for dopaminergic neuronal survival [98]. Additionally, in vivo studies have demonstrated that EGCG prevented MPTP-induced loss of dopaminergic neurons in the substantia nigra by inhibiting neuronal nitric oxide synthase [99]. Recently, EGCG has been found to also have immunomodulatory effects in different PD models, regulating peripheral inflammation-related processes, restoring behavior responses and protecting from massive dopaminergic neuronal MPTP-induced degeneration [100]. Hence, it is likely that the EGCG-mediated neuroprotective effects represent a multi-efficient nutraceutical strategy against different forms of PD [101]. Taken together, the exhaustive mechanistic data on the neuroprotective and neurorestorative effects of tea polyphenols suggest that apart from exerting anti-oxidant or anti-chelating properties, they may directly interfere with aggregation of the αS protein and modulate intracellular signaling pathways, in both in vitro and in vivo models.
12. ATREMORINE (Vicia fava)
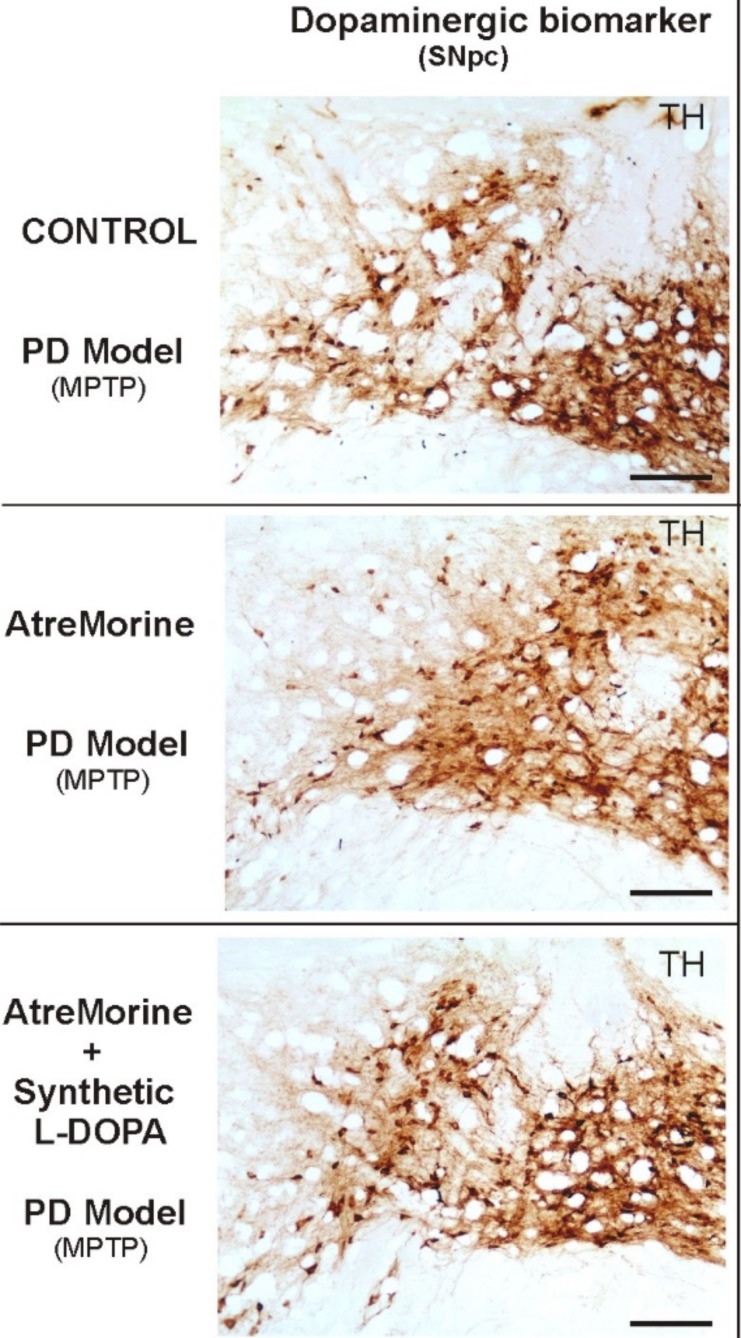
Favalins are a novel class of phytobioactive agents obtained by means of non-denaturing biotechnological procedures from structural components of the Vicia faba L. plant (E-PodoFavalin-15999), for the prevention and treatment of Parkinsonian disorders [102]. Currently, the synthetic dopamine precursor, levodopa (L-DOPA) is still the best available treatment for Parkinson’s disease patients [103] since it increases brain dopamine levels and decreases motor symptoms. However, fluctuating L-DOPA level problems made the testing of some alternative drugs essential [104], in order to prolong its effects by inhibiting DOPA-decarboxylase, the L-DOPA-degrading enzyme (e.g. carbidopa or opicapone), or by inhibiting monoamine-oxidase β, the dopamine-degrading enzyme (e.g. selegiline, rasagiline or safinamide). Therefore, since no conventional therapy can effectively reduce PD progression or even prevent its clinical manifestation during long periods of treatment, natural bioactive strategies should be tested for the purpose of promoting their therapeutic benefits against PD. Thus, Cacabelos and colleagues [105] have recently demonstrated that the favalins of Atremorine are a potent dopaminergic enhancer in preclinical and clinical trials of PD. In vitro studies have reported Atremorine (Fig. 1) as a powerful neuroprotectant in (i) cell cultures of human neuroblastoma SH-SY5Y cells, (ii) hippocampal slices under conditions of oxygen and glucose deprivation, and (iii) striatal slices under conditions of 6-hydroxydopamine (6-OHDA)-induced neurotoxicity [106, 107]. Additionally, in vivo studies conformed to the guidelines established by the European Communities Council (86/609/EEC), have demonstrated that Atremorine (i) protects against MPTP-induced dopaminergic neurodegeneration, (ii) inhibits MPTP-induced microglia activation and neuro-toxicity in substantia nigra, and (iii) improves motor function in MPTP-induced mice [108, 109]. Results showed that approximately the loss of 80% of TH-positive neurons in the basal ganglia of MPTP-induced mice compared to controls, whereas Atremorine treatment diet showed a significant neuroprotective effect in this model after seven weeks of experimentation. Mice models treated with higher atremorine concentration showed 10.5% ([20%]; CI 8.3-12.6%; p < 0.001) of TH-ir loss whereas mice treated with lower atremorine concentration showed 15.0% ([30%]; CI 13.2-17.0%; p < 0.001), when compared with controls, due to a lower reduction in the density of TH-positive neurons in the SNpc [109]. Finally, clinical studies also showed that Atremorine administered for the first time to untreated patients enhances dopaminergic neurotransmission effects and increases plasma dopamine levels by 200- to 500-fold [107]. Moreover, when administered to patients who have been chronically treated with L-DOPA or other antiparkinsonian drugs, Atremorine displayed a dopamine level response of a similar magnitude to that reported in previously untreated patients, responses directly correlated to the pharmacogenetic profile of the patients [110]. According to the results obtained, Atremorine-induced dopamine response is genotype-dependent and is influenced by pleiotropic gene variants, such as APOE, and CYP2D6, CYP2C19, CYP2C9 and CYP3A4/5 pheno-geno-types that control L-DOPA metabolism, as well as other bioactive compounds of E-PodoFavalin-15999. All these data together undoubtedly demonstrate that Atremorine is a safe and effective nutraceutical for PD patients, capable of inducing a significant effect on catecholamine (dopamine and noradrenaline) levels. The collateral effect of Atremorine on prolactin and growth hormone levels observed in patients might be related to the intrinsic hypothalamic regulation circuitry mediated via dopaminergic/noradrenergic neurotransmission, and also to a direct effect on the pituitary gland [111]. In contrast, the effect on cortisol levels might be due to its bioactivity both on the adrenal gland and on the hypothalamus-hypophyseal complex regulated by the adrenocorticotropic hormone. According to these preclinical and clinical results, Atremorine is a valid nutraceutical with anti-inflammatory, neuroprotective and antioxidant effects, showing excellent ability to modulate neuroendocrine function in PD. In the present and future guidelines of anti-parkinsonian therapies, Atremorine should be taken into consideration in the combinations of potent anti-neurodegenerative drugs with this effective bioproduct, which might co-work effectively to revert neuropathological processes and movement disorders such as reducing the progression of PD in patients.
Fig. (1).
Comparative dopaminergic immunoreactivity effects of AtreMorine against PD pathology. Comparative photomicrographs of dopaminergic immunoreactivity (TH) of the substantia nigra pars compacta (SNpc) of MPTP-induced mice with different treatments. Transverse brain sections of mice from groups treated with PBS (control), AtreMorine only [10.5% ([20%]; CI 8.3–12.6%; p<0.001)] and the combination of AtreMorine and synthetic L-DOPA [24.5% ([50%]; CI 21.1–28.0%; p<0.001)], showing the remarkable neuroprotective effect of AtreMorine treatment by reducing the dopaminergic degeneration in the SNpc neurons. Comparative sections show a notable neuroprotection effect of AtreMorine in mice when compared with control mice [31.8% ([60%]; CI 29.0–34.3%; p<0.015)]. Scale bar: 100 µm. (The color version of the figure is available in the electronic copy of the article).
Conclusion
The development and use of neuroprotective phytobioactive agents for therapeutic treatment in PD represents what is probably the most important goal for neuropathological research in this disease. Currently-prescribed drugs can help relieve many symptoms of PD and maintain the quality of life of patients but are inadequate for halting the progression of the disease. However, there is an urgent need for an effective long-term therapy that avoids the adverse effects of the available pharmaceutical drugs on motor and non-motor symptoms. In order to reverse this situation and establish a breakpoint for the disease course, the development of an effective bio-therapeutic agent that exerts DA neuroprotection via its anti-inflammatory action is crucial. Moreover, it is important that the molecular mechanisms through which the bioactive agent potentiates neuroprotection should also regulate compensatory (neuroplastic) and regenerative (neurorestorative) mechanisms in the PD brain. It is well known that MAPK/ERK kinase signaling pathways are vital regulators of neuroinflammation processes in AD and PD models, releasing pro-inflammatory cytokines from activated microglia including NO, IL-1β, and TNF-α [112]. Therefore, currently the intervention of MAPK pathway activation is consequently applied for neurodegenerative disease treatment [113]. The present study reviews the most relevant research data about the phytobioactive agents that inhibit the neuropathological effects of neurotoxic synthetic compounds (6-OHDA, MPTP and others) in PD-induced models, through the active inhibition of the proinflammatory factor production that consequently results from the inactivation of the MAPK pathway. As described previously, these phytobioactive agents (nutraceuticals), by virtue of their origin from naturally available food, have been shown to be not only preventive but also therapeutic for PD while potentially avoiding side effects. Although most of them will require assessment in clinical trials suitably designed to demonstrate their neuroprotective effect in PD patients, some of them, Atremorine in particular, have demonstrated their efficacy in basic and clinical trials by an improvement in cognitive scores, motor function and quality of life. In summary, it is clear that future therapeutic agents will effectively respond to the multi-faceted nature of this disease and will potentiate their beneficial effects in combination with existing drug therapies for PD patients, in order to maximize their neuroprotective effects in PD patients.
Acknowledgements
All authors listed above have contributed equally to the design, performance, analysis and reporting of the present work.
list of Abbreviations
- 6-OHDA
6 Hydroxydopamine
- AADC
Aromatic amino acid decarboxylase
- AICAR
Acadesine
- AKT/GSK-3β
Akt/glycogen synthase kinase-3
- AM404
Endogenous cannabinoid reuptake inhibitor
- AMPK
Adenosine monophosphate-activated protein kinase
- APOE
Apolipoprotein E
- BAX
Apoptosis regulator
- CaMKKβ
Calmodulin-dependent protein kinase β
- CBD
Cannabidiol
- CXCR4
C-X-C-motif Chemokine Receptor-4
- CYP
Cytochrome P450 gene family
- DA
Dopamine
- DOPAC
3,4-Dihydroxyphenylacetic acid
- Dpf
Days post fertilization
- EGb
Ginkgo biloba leaf
- EGCG
Epigallocatechin-3-gallate
- ERK
Extracellular signal-regulated kinases
- GPi
Postero-ventral pallidus
- GST
Human glutathione transferase
- IL
Interleukin
- L-DOPA
l-3,4-dihydroxyphenylalanine (levodopa)
- LRRK2
Leucine-rich repeat kinase 2
- MAO-B
Monoamine oxidase B
- MAPK
Mitogen-activated protein kinase
- MCAO
Intraluminal middle cerebral artery occlusion
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NO
Nitric oxide
- OGD-R
Oxygen-glucose deprivation followed by reperfusion
- PD
Parkinson’s disease
- ROS
Reactive oxygen species
- SCI
Spinal cord injury
- SIRT1/Akt1
Sirtuin 1/Akt enzyme
- SN
Substantia nigra
- TGF-β
Transforming growth factor beta
- TH
Tyrosine hydroxylase
- THC
Delta9-tetrahydrocannabinol
- TNF
Tumor necrosis factor
- TSG
Tetrahydroxystilbene glucoside
- WIN-55212-2
Cannabinoid receptor agonist
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Yuan H., Zhang Z.W., Liang L.W., Shen Q., Wang X.D., Ren S.M., Ma H.J., Jiao S.J., Liu P. Treatment strategies for Parkinson’s disease. Neurosci. Bull. 2010;26(1):66–76. doi: 10.1007/s12264-010-0302-z. [http://dx.doi. org/10.1007/s12264-010-0302-z]. [PMID: 20101274]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cacabelos R. Parkinson’s disease: From pathogenesis to pharmacogenomics. Int. J. Mol. Sci. 2017;18(3):E551. doi: 10.3390/ijms18030551. [http://dx.doi.org/ 10.3390/ijms18030551]. [PMID: 28273839]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cacabelos R. Parkinson’s disease: Old concepts and new challenges. . Sci. Pages Alzheimers Dis. Dement. 2016.
- 4.Cacabelos R., Carrera I., Fernández-Novoa L., Alejo R., Corzo L., Rodríguez S., Alcaraz M., Nebril L., Casas A., Fraile C. Parkinson’s Disease: New solutions to old problems. Euro. Espes J. 2017;11:74–96. [Google Scholar]
- 5.Tang S.W., Helmeste D.M., Leonard B.E. Neurodegeneration, neuroregeneration, and neuroprotection in psychiatric disorders. Mod. Trends Pharmacopsychiatry. 2017;31:107–123. doi: 10.1159/000470811. [http://dx. doi.org/10.1159/000470811]. [PMID: 28738379]. [DOI] [PubMed] [Google Scholar]
- 6.Cummings J. Disease modification and neuroprotection in neurodegenerative disorders. Transl. Neurodegener. 2017;6:25. doi: 10.1186/s40035-017-0096-2. [http:// dx.doi.org/10.1186/s40035-017-0096-2]. [PMID: 29021896]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seidl S.E., Potashkin J.A. The promise of neuroprotective agents in Parkinson’s disease. Front. Neurol. 2011;2:68. doi: 10.3389/fneur.2011.00068. [http://dx. doi.org/10.3389/fneur.2011.00068]. [PMID: 22125548]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bansal R., Singh R. Exploring the potential of natural and synthetic neuroprotective steroids against neurodegenerative disorders: A literature review. Med. Res. Rev. 2018;38(4):1126–1158. doi: 10.1002/med.21458. [http://dx.doi.org/10.1002/med.21458]. [PMID: 28697282]. [DOI] [PubMed] [Google Scholar]
- 9.Francardo V., Schmitz Y., Sulzer D., Cenci M.A. Neuroprotection and neurorestoration as experimental therapeutics for Parkinson’s disease. 2017. [DOI] [PubMed]
- 10.Rangasamy S.B., Soderstrom K., Bakay R.A., Kordower J.H. Neurotrophic factor therapy for Parkinson’s disease. 2010. [DOI] [PubMed] [Google Scholar]
- 11.Ibáñez C.F., Andressoo J.O. Biology of GDNF and its receptors - relevance for disorders of the central nervous system. Neurobiol. Dis., 2017. [DOI] [PubMed]
- 12.Vilar M., Mira H. Regulation of neurogenesis by neurotrophins during adulthood: expected and unexpected roles. Front. Neurosci. 2016;10:26. doi: 10.3389/fnins.2016.00026. [http://dx.doi.org/10.3389/fnins.2016.00026]. [PMID: 26903794]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao C., Deng W., Gage F.H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [http://dx.doi.org/10.1016/j.cell.2008.01.033]. [PMID: 18295581]. [DOI] [PubMed] [Google Scholar]
- 14.Carbon M., Reetz K., Ghilardi M.F., Dhawan V., Eidelberg D. Early Parkinson’s disease: Longitudinal changes in brain activity during sequence learning. Neurobiol. Dis. 2010;37(2):455–460. doi: 10.1016/j.nbd.2009.10.025. [http://dx.doi.org/10.1016/j.nbd.2009.10.025]. [PMID: 19900556]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng H.C., Ulane C.M., Burke R.E. Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 2010;67(6):715–725. doi: 10.1002/ana.21995. [http://dx.doi.org/10.1002/ana.21995]. [PMID: 20517933]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song D.D., Haber S.N. Striatal responses to partial dopaminergic lesion: Evidence for compensatory sprouting. J. Neurosci. 2000;20(13):5102–5114. doi: 10.1523/JNEUROSCI.20-13-05102.2000. [http://dx.doi.org/10.1523/JNEUROSCI.20-13-05102.2000]. [PMID: 10864967]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sossi V., de la Fuente-Fernández R., Holden J.E., Schulzer M., Ruth T.J., Stoessl J. Changes of dopamine turnover in the progression of Parkinson’s disease as measured by positron emission tomography: their relation to disease-compensatory mechanisms. J. Cereb. Blood Flow Metab. 2004;24(8):869–876. doi: 10.1097/01.WCB.0000126563.85360.75. [http://dx. doi.org/10.1097/01.WCB.0000126563.85360.75]. [PMID: 15362717]. [DOI] [PubMed] [Google Scholar]
- 18.Brotchie J., Fitzer-Attas C. Mechanisms compensating for dopamine loss in early Parkinson disease. Neurology. 2009;72(7) Suppl.:S32–S38. doi: 10.1212/WNL.0b013e318198e0e9. [http://dx.doi.org/10.1212/WNL.0b013e318198 e0e9]. [PMID: 19221312]. [DOI] [PubMed] [Google Scholar]
- 19.Maneuf Y.P., Mitchell I.J., Crossman A.R., Brotchie J.M. On the role of enkephalin cotransmission in the GABAergic striatal efferents to the globus pallidus. Exp. Neurol. 1994;125(1):65–71. doi: 10.1006/exnr.1994.1007. [http://dx.doi.org/10.1006/exnr.1994.1007]. [PMID: 8307125]. [DOI] [PubMed] [Google Scholar]
- 20.Vila M., Périer C., Féger J., Yelnik J., Faucheux B., Ruberg M., Raisman-Vozari R., Agid Y., Hirsch E.C. Evolution of changes in neuronal activity in the subthalamic nucleus of rats with unilateral lesion of the substantia nigra assessed by metabolic and electrophysiological measurements. Eur. J. Neurosci. 2000;12(1):337–344. doi: 10.1046/j.1460-9568.2000.00901.x. [http://dx.doi.org/10.1046/j.1460-9568.2000.00901.x]. [PMID: 10651888]. [DOI] [PubMed] [Google Scholar]
- 21.Bezard E., Gross C.E., Brotchie J.M. Presymptomatic compensation in Parkinson’s disease is not dopamine-mediated. Trends Neurosci. 2003;26(4):215–221. doi: 10.1016/S0166-2236(03)00038-9. [http://dx.doi.org/10.1016/S0166-2236 (03)00038-9]. [PMID: 12689773]. [DOI] [PubMed] [Google Scholar]
- 22.Liu C.Y., Lee B., Boulis N., Rezai A.R. Introduction: Neurorestoration: re-animating the CNS. Neurosurg. Focus. 2016;40(5):E1. doi: 10.3171/2016.2.FOCUS1688. [http://dx.doi.org/10.3171/2016.2.FOCUS1688]. [PMID: 27132522]. [DOI] [PubMed] [Google Scholar]
- 23.Maruyama W., Youdim M.B., Naoi M. Antiapoptotic properties of rasagiline, N-propargylamine-1(R)-aminoindan, and its optical (S)-isomer, TV1022. Ann. N. Y. Acad. Sci. 2001;939:320–329. doi: 10.1111/j.1749-6632.2001.tb03641.x. [http://dx.doi.org/10.1111/j.1749-6632.2001.tb03641.x]. [PMID: 11462787]. [DOI] [PubMed] [Google Scholar]
- 24.Sagi Y., Mandel S., Amit T., Youdim M.B. Activation of tyrosine kinase receptor signaling pathway by rasagiline facilitates neurorescue and restoration of nigrostriatal dopamine neurons in post-MPTP-induced parkinsonism. Neurobiol. Dis. 2007;25(1):35–44. doi: 10.1016/j.nbd.2006.07.020. [http://dx.doi.org/10.1016/j.nbd.2006.07.020]. [PMID: 17055733]. [DOI] [PubMed] [Google Scholar]
- 25.Kupsch A., Sautter J., Götz M.E., Breithaupt W., Schwarz J., Youdim M.B., Riederer P., Gerlach M., Oertel W.H. Monoamine oxidase-inhibition and MPTP-induced neurotoxicity in the non-human primate: Comparison of rasagiline (TVP 1012) with selegiline. J. Neural Transm. (Vienna) 2001;108(8-9):985–1009. doi: 10.1007/s007020170018. [http://dx.doi.org/10.1007/s007020170018]. [PMID: 11716151]. [DOI] [PubMed] [Google Scholar]
- 26.Weintraub D., Hauser R.A., Elm J.J., Pagan F., Davis M.D., Choudhry A. Rasagiline for mild cognitive impairment in Parkinson’s disease: A placebo-controlled trial. Mov. Disord. 2016;31(5):709–714. doi: 10.1002/mds.26617. [http://dx.doi.org/10.1002/mds.26617]. [PMID: 27030249]. [DOI] [PubMed] [Google Scholar]
- 27.Cronin A., Grealy M. Neuroprotective and neuro-restorative effects of minocycline and rasagiline in a zebrafish 6-hydroxydopamine model of parkinson’s disease. Neuroscience. 2017;367:34–46. doi: 10.1016/j.neuroscience.2017.10.018. [http://dx.doi.org/10.1016/j.neuroscience.2017. 10.018]. [PMID: 29079063]. [DOI] [PubMed] [Google Scholar]
- 28.Hoyles L., Vulevic J. Diet, immunity and functional foods. Adv. Exp. Med. Biol. 2008;635:79–92. doi: 10.1007/978-0-387-09550-9_7. [http://dx.doi.org/10.1007/978-0-387-09550-9_7]. [PMID: 18841705]. [DOI] [PubMed] [Google Scholar]
- 29.Hang L., Basil A.H., Lim K.L. Nutraceuticals in Parkinson’s Disease. Neuromolecular Med. 2016;18(3):306–321. doi: 10.1007/s12017-016-8398-6. [http://dx.doi.org/10.1007/s12017-016-8398-6]. [PMID: 27147525]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chao J., Leung Y., Wang M., Chang R.C. Nutraceuticals and their preventive or potential therapeutic value in Parkinson’s disease. Nutr. Rev. 2012;70(7):373–386. doi: 10.1111/j.1753-4887.2012.00484.x. [http://dx.doi.org/10.1111/ j.1753-4887.2012.00484.x]. [PMID: 22747840]. [DOI] [PubMed] [Google Scholar]
- 31.Boskabady M.H., Farkhondeh T. Antiinflammatory, antioxidant, and immunomodulatory effects of Crocus sativus L. and its main constituents. Phytother. Res. 2016;30(7):1072–1094. doi: 10.1002/ptr.5622. [http://dx. doi.org/10.1002/ptr.5622]. [PMID: 27098287]. [DOI] [PubMed] [Google Scholar]
- 32.Tseng T.H., Chu C.Y., Huang J.M., Shiow S.J., Wang C.J. Crocetin protects against oxidative damage in rat primary hepatocytes. Cancer Lett. 1995;97(1):61–67. doi: 10.1016/0304-3835(95)03964-x. [http://dx.doi.org/10.1016/ 0304-3835(95)03964-X]. [PMID: 7585479]. [DOI] [PubMed] [Google Scholar]
- 33.Bhandari P.R. Crocus sativus L. (saffron) for cancer chemoprevention: A mini review. J. Tradit. Complement. Med. 2015;5(2):81–87. doi: 10.1016/j.jtcme.2014.10.009. [http://dx.doi.org/10.1016/j.jtcme.2014.10.009]. [PMID: 26151016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Premkumar K., Thirunavukkarasu C., Abraham S.K., Santhiya S.T., Ramesh A. Protective effect of saffron (Crocus sativus L.) aqueous extract against genetic damage induced by anti-tumor agents in mice. Hum. Exp. Toxicol. 2006;25(2):79–84. doi: 10.1191/0960327106ht589oa. [http://dx. doi.org/10.1191/0960327106ht589oa]. [PMID: 16539212]. [DOI] [PubMed] [Google Scholar]
- 35.Yousefi E., Eskandari A., Gharavi M.J., Khademvatan S. In vitro activity and cytotoxicity of Crocus sativus extract against leihmania major (MRHO/IR/75/ER). Infect. Disord. Drug Targets. 2014;14(1):56–60. doi: 10.2174/1871526514666140827101901. [http://dx.doi.org/10.2174/1871526514666140827 101901]. [PMID: 25159304]. [DOI] [PubMed] [Google Scholar]
- 36.Kianbakht S., Mozaffari K. Effects of saffron and its active constituents, crocin and safranal, on prevention of indomethacin induced gastric ulcers in diabetic and nondiabetic rats. Faslnamah-i Giyahan-i Daruyi. 2009;8:30–38. [Google Scholar]
- 37.El-Maraghy S.A., Rizk S.M., Shahin N.N. Gastroprotective effect of crocin in ethanol-induced gastric injury in rats. Chem. Biol. Interact. 2015;229:26–35. doi: 10.1016/j.cbi.2015.01.015. [http://dx.doi.org/10.1016/j.cbi.2015. 01.015]. [PMID: 25637687]. [DOI] [PubMed] [Google Scholar]
- 38.Boskabady M.H., Ghasemzadeh Rahbardar M., Nemati H., Esmaeilzadeh M. Inhibitory effect of Crocus sativus (saffron) on histamine (H1) receptors of guinea pig tracheal chains. Pharmazie. 2010;65(4):300–305. [PMID: 20432629]. [PubMed] [Google Scholar]
- 39.Hazman Ö., Bozkurt M.F. Anti-inflammatory and antioxidative activities of safranal in the reduction of renal dysfunction and damage that occur in diabetic nephropathy. Inflammation. 2015;38(4):1537–1545. doi: 10.1007/s10753-015-0128-y. [http://dx.doi.org/10.1007/s10753-015-0128-y]. [PMID: 25667012]. [DOI] [PubMed] [Google Scholar]
- 40.Rezaee R., Hosseinzadeh H. Safranal: from an aromatic natural product to a rewarding pharmacological agent. Iran. J. Basic Med. Sci. 2013;16(1):12–26. [PMID: 23638289]. [PMC free article] [PubMed] [Google Scholar]
- 41.Abdullaev F., Ortega C.H., Miranda P.R. HPLC quantification of major active components from 11 different saffron (Crocus sativus L.) sources. Food Chem. 2007;100:1126–1131. [http://dx.doi.org/10.1016/j.foodchem.2005.11.020]. [Google Scholar]
- 42.Zhang C., Ma J., Fan L., Zou Y., Dang X., Wang K., Song J. Neuroprotective effects of safranal in a rat model of traumatic injury to the spinal cord by anti-apoptotic, anti-inflammatory and edema-attenuating. Tissue Cell. 2015;47(3):291–300. doi: 10.1016/j.tice.2015.03.007. [http://dx. doi.org/10.1016/j.tice.2015.03.007]. [PMID: 25891268]. [DOI] [PubMed] [Google Scholar]
- 43.Wang K., Zhang L., Rao W., Su N., Hui H., Wang L., Peng C., Tu Y., Zhang S., Fei Z. Neuroprotective effects of crocin against traumatic brain injury in mice: Involvement of notch signaling pathway. Neurosci. Lett. 2015;591:53–58. doi: 10.1016/j.neulet.2015.02.016. [http://dx.doi.org/10.1016/j.neulet.2015.02.016]. [PMID: 25681620]. [DOI] [PubMed] [Google Scholar]
- 44.Nam K.N., Park Y.M., Jung H.J., Lee J.Y., Min B.D., Park S.U., Jung W.S., Cho K.H., Park J.H., Kang I., Hong J.W., Lee E.H. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur. J. Pharmacol. 2010;648(1-3):110–116. doi: 10.1016/j.ejphar.2010.09.003. [http://dx.doi.org/10.1016/j.ejphar.2010.09.003]. [PMID: 20854811]. [DOI] [PubMed] [Google Scholar]
- 45.Hatziagapiou K., Kakouri E., Lambrou G.I., Bethanis K., Tarantilis P.A. Antioxidant Properties of Crocus Sativus L. and its Constituents and Relevance to Neurodegenerative Diseases; Focus on Alzheimer’s And Parkinson’s disease. Curr. Neuropharmacol. 2018 doi: 10.2174/1570159X16666180321095705. [Epub ahead of print]. [http://dx.doi.org/10.2174/1570159 X16666180321095705]. [PMID: 29564976]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mythri R.B., Bharath M.M. Curcumin: a potential neuroprotective agent in Parkinson’s disease. Curr. Pharm. Des. 2012;18(1):91–99. doi: 10.2174/138161212798918995. [http://dx.doi.org/10.2174/138161212798918995]. [PMID: 22211691]. [DOI] [PubMed] [Google Scholar]
- 47.Lee W.H., Loo C.Y., Bebawy M., Luk F., Mason R.S., Rohanizadeh R. Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr. Neuropharmacol. 2013;11(4):338–378. doi: 10.2174/1570159X11311040002. [http://dx.doi.org/10. 2174/1570159X11311040002]. [PMID: 24381528]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J., Du X.X., Jiang H., Xie J.X. Curcumin attenuates 6-hydroxydopamine-induced cytotoxicity by anti-oxidation and nuclear factor-kappa B modulation in MES23.5 cells. Biochem. Pharmacol. 2009;78(2):178–183. doi: 10.1016/j.bcp.2009.03.031. [http://dx.doi.org/10.1016/ j.bcp.2009.03.031]. [PMID: 19464433]. [DOI] [PubMed] [Google Scholar]
- 49.Rajeswari A., Sabesan M. Inhibition of monoamine oxidase-B by the polyphenolic compound, curcumin and its metabolite tetrahydrocurcumin, in a model of Parkinson’s disease induced by MPTP neurodegeneration in mice. Inflammopharmacology. 2008;16(2):96–99. doi: 10.1007/s10787-007-1614-0. [http://dx.doi.org/10.1007/s10787-007-1614-0]. [PMID: 18408903]. [DOI] [PubMed] [Google Scholar]
- 50.Xie C.J., Gu A.P., Cai J., Wu Y., Chen R.C. Curcumin protects neural cells against ischemic injury in N2a cells and mouse brain with ischemic stroke. Brain Behav. 2018;8(2):e00921. doi: 10.1002/brb3.921. [http://dx. doi.org/10.1002/brb3.921]. [PMID: 29484272]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.More S.V., Choi D.K. Promising cannabinoid-based therapies for Parkinson’s disease: Motor symptoms to neuroprotection. Mol. Neurodegener. 2015;10:17. doi: 10.1186/s13024-015-0012-0. [http://dx.doi.org/10.1186/s13024-015-0012-0]. [PMID: 25888232]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berger C., Schmid P.C., Schabitz W.R., Wolf M., Schwab S., Schmid H.H. Massive accumulation of N-acylethanolamines after stroke. Cell signalling in acute cerebral ischemia? J. Neurochem. 2004;88(5):1159–1167. doi: 10.1046/j.1471-4159.2003.02244.x. [http://dx.doi.org/10.1046/j.1471-4159. 2003.02244.x]. [PMID: 15009671]. [DOI] [PubMed] [Google Scholar]
- 53.Stampanoni B.M., Sancesario A., Morace R., Centonze D., Iezzi E. Cannabinoids in parkinson’s disease. Cannabis Cannabinoid Res. 2017;2(1):21–29. doi: 10.1089/can.2017.0002. [http://dx.doi.org/10.1089/can.2017.0002]. [PMID: 28861502]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.García-Arencibia M., González S., de Lago E., Ramos J.A., Mechoulam R., Fernández-Ruiz J. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson’s disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 2007;1134(1):162–170. doi: 10.1016/j.brainres.2006.11.063. [http://dx.doi.org/10.1016/j.brainres.2006.11.063]. [PMID: 17196181]. [DOI] [PubMed] [Google Scholar]
- 55.Fernández-Ruiz J. The endocannabinoid system as a target for the treatment of motor dysfunction. Br. J. Pharmacol. 2009;156(7):1029–1040. doi: 10.1111/j.1476-5381.2008.00088.x. [http://dx.doi.org/10.1111/j.1476-5381.2008.00088.x]. [PMID: 19220290]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hassell K.J., Ezzati M., Alonso-Alconada D., Hausenloy D.J., Robertson N.J. New horizons for newborn brain protection: enhancing endogenous neuroprotection. Arch. Dis. Child. Fetal Neonatal Ed. 2015;100(6):F541–F552. doi: 10.1136/archdischild-2014-306284. [http://dx.doi.org/10.1136/ archdischild-2014-306284]. [PMID: 26063194]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chung E.S., Bok E., Chung Y.C., Baik H.H., Jin B.K. Cannabinoids prevent lipopolysaccharide-induced neurodegeneration in the rat substantia nigra in vivo through inhibition of microglial activation and NADPH oxidase. Brain Res. 2012;1451:110–116. doi: 10.1016/j.brainres.2012.02.058. [http://dx.doi.org/10.1016/j.brainres.2012.02.058]. [PMID: 22436849]. [DOI] [PubMed] [Google Scholar]
- 58.Pazos M.R., Mohammed N., Lafuente H., Santos M., Martínez-Pinilla E., Moreno E., Valdizan E., Romero J., Pazos A., Franco R., Hillard C.J., Alvarez F.J., Martínez-Orgado J. Mechanisms of cannabidiol neuroprotection in hypoxic-ischemic newborn pigs: role of 5HT(1A) and CB2 receptors. Neuropharmacology. 2013;71:282–291. doi: 10.1016/j.neuropharm.2013.03.027. [http://dx.doi.org/10.1016/j.neuropharm. 2013.03.027]. [PMID: 23587650]. [DOI] [PubMed] [Google Scholar]
- 59.Lastres-Becker I., Fernández-Ruiz J. An overview of Parkinson’s disease and the cannabinoid system and possible benefits of cannabinoid-based treatments. Curr. Med. Chem. 2006;13(30):3705–3718. doi: 10.2174/092986706779026156. [http://dx.doi.org/10.2174/092986706779026156]. [PMID: 17168732]. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y., Catana F., Yang Y., Roderick R., van Breemen R.B. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J. Agric. Food Chem. 2002;50(3):431–435. doi: 10.1021/jf010812u. [http://dx.doi.org/10.1021/jf010812u]. [PMID: 11804508]. [DOI] [PubMed] [Google Scholar]
- 61.Renaud S., de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339(8808):1523–1526. doi: 10.1016/0140-6736(92)91277-f. [http://dx.doi.org/10.1016/0140-6736(92)91277-F]. [PMID: 1351198]. [DOI] [PubMed] [Google Scholar]
- 62.Jang M., Cai L., Udeani G.O., Slowing K.V., Thomas C.F., Beecher C.W., Fong H.H., Farnsworth N.R., Kinghorn A.D., Mehta R.G., Moon R.C., Pezzuto J.M. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–220. doi: 10.1126/science.275.5297.218. [http://dx.doi.org/10.1126/science. 275.5297.218]. [PMID: 8985016]. [DOI] [PubMed] [Google Scholar]
- 63.Farooqi A.A., Khalid S., Ahmad A. Regulation of cell signaling pathways and miRNAs by resveratrol in different cancers. Int. J. Mol. Sci. 2018;19(3):E652. doi: 10.3390/ijms19030652. [http://dx.doi.org/10.3390/ijms 19030652]. [PMID: 29495357]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kosmeder J.W., II, Pezzuto J.M., Pezzuto J.M. Biological effects of resveratrol. Antioxid. Redox Signal. 2001;3(6):1041–1064. doi: 10.1089/152308601317203567. [http://dx.doi.org/10.1089/152308601317203567]. [PMID: 11813979]. [DOI] [PubMed] [Google Scholar]
- 65.Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [http://dx.doi.org/10.1038/nrd2060]. [PMID: 16732220]. [DOI] [PubMed] [Google Scholar]
- 66.Potdar S., Parmar M.S., Ray S.D., Cavanaugh J.E. Protective effects of the resveratrol analog piceid in dopaminergic SH-SY5Y cells. Arch. Toxicol. 2018;92(2):669–677. doi: 10.1007/s00204-017-2073-z. [http://dx.doi.org/10. 1007/s00204-017-2073-z]. [PMID: 28980048]. [DOI] [PubMed] [Google Scholar]
- 67.Zhang J., Fan W., Wang H., Bao L., Li G., Li T., Song S., Li H., Hao J., Sun J. Resveratrol protects PC12 cell against 6-OHDA damage via CXCR4 signaling pathway. Evid. Based Complement. Alternat. Med. 2015;2015:730121. doi: 10.1155/2015/730121. [http://dx.doi.org/10.1155/2015/730121]. [PMID: 26681969]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeng W., Zhang W., Lu F., Gao L., Gao G. Resveratrol attenuates MPP+-induced mitochondrial dysfunction and cell apoptosis via AKT/GSK-3β pathway in SN4741 cells. Neurosci. Lett. 2017;637:50–56. doi: 10.1016/j.neulet.2016.11.054. [http://dx.doi.org/10.1016/j.neulet.2016.11.054]. [PMID: 27894919]. [DOI] [PubMed] [Google Scholar]
- 69.Gaballah H.H., Zakaria S.S., Elbatsh M.M., Tahoon N.M. Modulatory effects of resveratrol on endoplasmic reticulum stress-associated apoptosis and oxido-inflammatory markers in a rat model of rotenone-induced Parkinson’s disease. Chem. Biol. Interact. 2016;251:10–16. doi: 10.1016/j.cbi.2016.03.023. [http://dx.doi.org/10.1016/j.cbi.2016.03.023]. [PMID: 27016191]. [DOI] [PubMed] [Google Scholar]
- 70.Khan M.A., Chen H.C., Wan X.X., Tania M., Xu A.H., Chen F.Z., Zhang D.Z. Regulatory effects of resveratrol on antioxidant enzymes: A mechanism of growth inhibition and apoptosis induction in cancer cells. Mol. Cells. 2013;35(3):219–225. doi: 10.1007/s10059-013-2259-z. [http://dx. doi.org/10.1007/s10059-013-2259-z]. [PMID: 23456297]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Z.H., Zhang J.L., Duan Y.L., Zhang Q.S., Li G.F., Zheng D.L. MicroRNA-214 participates in the neuroprotective effect of Resveratrol via inhibiting α-synuclein expression in MPTP-induced Parkinson’s disease mouse. Biomed. Pharmacother. 2015;74:252–256. doi: 10.1016/j.biopha.2015.08.025. [http://dx.doi.org/10.1016/j.biopha.2015.08.025]. [PMID: 26349993]. [DOI] [PubMed] [Google Scholar]
- 72.Singh G., Pai R.S. In-vitro/in-vivo characterization of trans-resveratrol-loaded nanoparticulate drug delivery system for oral administration. J. Pharm. Pharmacol. 2014;66(8):1062–1076. doi: 10.1111/jphp.12232. [PMID: 24779896]. [DOI] [PubMed] [Google Scholar]
- 73.Singh G., Pai R.S. Trans-resveratrol self-nano-emulsifying drug delivery system (SNEDDS) with enhanced bioavailability potential: optimization, pharmacokinetics and in situ single pass intestinal perfusion (SPIP) studies. Drug Deliv. 2015;22(4):522–530. doi: 10.3109/10717544.2014.885616. [http://dx.doi.org/10.3109/10717544.2014.885616]. [PMID: 24512464]. [DOI] [PubMed] [Google Scholar]
- 74.Tellone E., Galtieri A., Russo A., Giardina B., Ficarra S. Resveratrol: A focus on several neurodegenerative diseases. Oxid. Med. Cell. Longev. 2015;2015:392169. doi: 10.1155/2015/392169. [http://dx.doi.org/10. 1155/2015/392169]. [PMID: 26180587]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blanchet J., Longpré F., Bureau G., Morissette M., DiPaolo T., Bronchti G., Martinoli M.G. Resveratrol, a red wine polyphenol, protects dopaminergic neurons in MPTP-treated mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(5):1243–1250. doi: 10.1016/j.pnpbp.2008.03.024. [http://dx.doi.org/10.1016/j.pnpbp.2008.03.024]. [PMID: 18471948]. [DOI] [PubMed] [Google Scholar]
- 76.Donmez G., Arun A., Chung C.Y., McLean P.J., Lindquist S., Guarente L. SIRT1 protects against α-synuclein aggregation by activating molecular chaperones. J. Neurosci. 2012;32(1):124–132. doi: 10.1523/JNEUROSCI.3442-11.2012. [http://dx.doi.org/10.1523/JNEUROSCI.3442-11.2012]. [PMID: 22219275]. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Wang H., Dong X., Liu Z., Zhu S., Liu H., Fan W., Hu Y., Hu T., Yu Y., Li Y., Liu T., Xie C., Gao Q., Li G., Zhang J., Ding Z., Sun J. Anat, Rec, (Hoboken). 2018. Resveratrol suppresses rotenone-induced neurotoxicity through activation of SIRT1/Akt1 signaling pathway. [DOI] [PubMed] [Google Scholar]
- 78.Bounda G.A., Feng Y.U. Review of clinical studies of Polygonum multiflorum Thunb. and its isolated bioactive compounds. Pharmacognosy Res. 2015;7(3):225–236. doi: 10.4103/0974-8490.157957. [http://dx.doi.org/10.4103/ 0974-8490.157957]. [PMID: 26130933]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin L., Ni B., Lin H., Zhang M., Li X., Yin X., Qu C., Ni J. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: a review. J. Ethnopharmacol. 2015;159:158–183. doi: 10.1016/j.jep.2014.11.009. [http://dx.doi.org/10.1016/j.jep. 2014.11.009]. [PMID: 25449462]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang F., Wang Y.Y., Yang J., Lu Y.F., Liu J., Shi J.S. Tetrahydroxystilbene glucoside attenuates neuroinflammation through the inhibition of microglia activation. Oxid. Med. Cell. Longev. 2013;2013:680545. doi: 10.1155/2013/680545. [http://dx.doi.org/10.1155/2013/680545]. [PMID: 24349614]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang C., Lin F., Wang G., Lu D., Wu Q., Liu J., Shi J., Zhang F. Tetrahydroxystilbene glucoside produces neuroprotection against 6-OHDA-induced dopamine neurotoxicity. Oxid. Med. Cell. Longev. 2018;2018:7927568. doi: 10.1155/2018/7927568. [http://dx.doi.org/10.1155/2018/7927568]. [PMID: 29576855]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen C., Sun F.L., Zhang R.Y., Zhang L., Li Y.L., Zhang L., Li L. Tetrahydroxystilbene glucoside ameliorates memory and movement functions, protects synapses and inhibits α-synuclein aggregation in hippocampus and striatum in aged mice. Restor. Neurol. Neurosci. 2015;33(4):531–541. doi: 10.3233/RNN-150514. [http://dx.doi.org/10. 3233/RNN-150514]. [PMID: 26409411]. [DOI] [PubMed] [Google Scholar]
- 83.Wang T., Gu J., Wu P.F., Wang F., Xiong Z., Yang Y.J., Wu W.N., Dong L.D., Chen J.G. Protection by tetrahydroxystilbene glucoside against cerebral ischemia: involvement of JNK, SIRT1, and NF-kappaB pathways and inhibition of intracellular ROS/RNS generation. Free Radic. Biol. Med. 2009;47(3):229–240. doi: 10.1016/j.freeradbiomed.2009.02.027. [http://dx.doi.org/10.1016/j.freeradbiomed.2009.02.027]. [PMID: 19272442]. [DOI] [PubMed] [Google Scholar]
- 84.Nash K.M., Shah Z.A. Current perspectives on the beneficial role of ginkgo biloba in neurological and cerebrovascular disorders. Integr. Med. Insights. 2015;10:1–9. doi: 10.4137/IMI.S25054. [http://dx.doi.org/10.4137/I MI.S25054]. [PMID: 26604665]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoshikawa T., Naito Y., Kondo M. Ginkgo biloba leaf extract: review of biological actions and clinical applications. Antioxid. Redox Signal. 1999;1(4):469–480. doi: 10.1089/ars.1999.1.4-469. [http://dx.doi.org/10.1089/ars. 1999.1.4-469]. [PMID: 11233145]. [DOI] [PubMed] [Google Scholar]
- 86.Yin B., Xu Y., Wei R., Luo B. Ginkgo biloba on focal cerebral ischemia: a systematic review and meta-analysis. Am. J. Chin. Med. 2014;42(4):769–783. doi: 10.1142/S0192415X14500499. [http://dx.doi.org/10.1142/ S0192415X14500499]. [PMID: 25004874]. [DOI] [PubMed] [Google Scholar]
- 87.Riederer P., Jellinger K. Neurochemical insights into monoamine oxidase inhibitors, with special reference to deprenyl (selegiline). Acta Neurol. Scand. Suppl. 1983;95:43–55. doi: 10.1111/j.1600-0404.1983.tb01516.x. [http://dx.doi.org/10.1111/j.1600-0404.1983.tb01516.x]. [PMID: 6145282]. [DOI] [PubMed] [Google Scholar]
- 88.Myllylä V.V., Sotaniemi K.A., Vuorinen J.A., Heinonen E.H. Selegiline as initial treatment in de novo parkinsonian patients. Neurology. 1992;42(2):339–343. doi: 10.1212/wnl.42.2.339. [http://dx.doi.org/10.1212/ WNL.42.2.339]. [PMID: 1736162]. [DOI] [PubMed] [Google Scholar]
- 89.Baez S., Segura-Aguilar J., Widersten M., Johansson A.S., Mannervik B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem. J. 1997;324(Pt 1):25–28. doi: 10.1042/bj3240025. [http://dx.doi.org/10.1042/bj3240025]. [PMID: 9164836]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu W.R., Zhu X.Z. Involvement of monoamine oxidase inhibition in neuroprotective and neurorestorative effects of Ginkgo biloba extract against MPTP-induced nigrostriatal dopaminergic toxicity in C57 mice. Life Sci. 1999;65(2):157–164. doi: 10.1016/s0024-3205(99)00232-5. [http://dx.doi.org/10.1016/S0024-3205(99)00232-5]. [PMID: 10416821]. [DOI] [PubMed] [Google Scholar]
- 91.Ahmad M., Saleem S., Ahmad A.S., Yousuf S., Ansari M.A., Khan M.B., Ishrat T., Chaturvedi R.K., Agrawal A.K., Islam F. Ginkgo biloba affords dose-dependent protection against 6-hydroxydopamine-induced parkinsonism in rats: neurobehavioural, neurochemical and immunohistochemical evidences. J. Neurochem. 2005;93(1):94–104. doi: 10.1111/j.1471-4159.2005.03000.x. [http://dx.doi.org/10.1111/j.1471-4159.2005.03000.x]. [PMID: 15773909]. [DOI] [PubMed] [Google Scholar]
- 92.Sur S., Panda C.K. Molecular aspects of cancer chemopreventive and therapeutic efficacies of tea and tea polyphenols. Nutrition. 2017;43-44:8–15. doi: 10.1016/j.nut.2017.06.006. [http://dx.doi.org/10.1016/j.nut.2017.06.006]. [PMID: 28935149]. [DOI] [PubMed] [Google Scholar]
- 93.Singh N.A., Mandal A.K., Khan Z.A. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr. J. 2016;15(1):60. doi: 10.1186/s12937-016-0179-4. [http://dx.doi.org/10.1186/s12937-016-0179-4]. [PMID: 27268025]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hang L., Basil A.H., Lim K.L. Nutraceuticals in Parkinson’s Disease. Neuromol. Med. 2016;18(3):306–321. doi: 10.1007/s12017-016-8398-6. [http://dx.doi.org/10.1007/s12017-016-8398-6]. [PMID: 27147525]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim J., Shin J., Ha J. Screening methods for AMP-activated protein kinase modulators: a patent review. Expert Opin. Ther. Pat. 2015;25(3):261–277. doi: 10.1517/13543776.2014.995626. [http://dx.doi.org/10.1517/13543776. 2014.995626]. [PMID: 25535089]. [DOI] [PubMed] [Google Scholar]
- 96.Whitworth A.J. Drosophila models of Parkinson’s disease. Adv. Genet. 2011;73:1–50. doi: 10.1016/B978-0-12-380860-8.00001-X. [http://dx.doi.org/10.1016/B978-0-12-380860-8.00001-X]. [PMID: 21310293]. [DOI] [PubMed] [Google Scholar]
- 97.Ng C.H., Guan M.S., Koh C., Ouyang X., Yu F., Tan E.K., O’Neill S.P., Zhang X., Chung J., Lim K.L. AMP kinase activation mitigates dopaminergic dysfunction and mitochondrial abnormalities in Drosophila models of Parkinson’s disease. J. Neurosci. 2012;32(41):14311–14317. doi: 10.1523/JNEUROSCI.0499-12.2012. [http://dx.doi.org/10.1523/JNEUROSCI. 0499-12.2012]. [PMID: 23055502]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ng C.H., Basil A.H., Hang L., Tan R., Goh K.L., O’Neill S., Zhang X., Yu F., Lim K.L. Genetic or pharmacological activation of the Drosophila PGC-1α ortholog spargel rescues the disease phenotypes of genetic models of Parkinson’s disease. Neurobiol. Aging. 2017;55:33–37. doi: 10.1016/j.neurobiolaging.2017.03.017. [http://dx.doi.org/10.1016/j.neurobiolaging. 2017.03.017]. [PMID: 28407521]. [DOI] [PubMed] [Google Scholar]
- 99.Choi J.Y., Park C.S., Kim D.J., Cho M.H., Jin B.K., Pie J.E., Chung W.G. Prevention of nitric oxide-mediated 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease in mice by tea phenolic epigallocatechin 3-gallate. Neurotoxicology. 2002;23(3):367–374. doi: 10.1016/s0161-813x(02)00079-7. [http://dx.doi.org/10.1016/S0161-813X (02)00079-7]. [PMID: 12387363]. [DOI] [PubMed] [Google Scholar]
- 100.Zhou T., Zhu M., Liang Z. (-)-Epigallocatechin-3-gallate modulates peripheral immunity in the MPTP-induced mouse model of Parkinson’s disease. Mol. Med. Rep. 2018;17(4):4883–4888. doi: 10.3892/mmr.2018.8470. [PMID: 29363729]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Caruana M., Vassallo N. Tea Polyphenols in Parkinson’s Disease. Adv. Exp. Med. Biol. 2015;863:117–137. doi: 10.1007/978-3-319-18365-7_6. [http://dx.doi.org/10.1007/978-3-319-18365-7_6]. [PMID: 26092629]. [DOI] [PubMed] [Google Scholar]
- 102.Cacabelos R. Bioactive extract obtained from Vicia faba and its use in the treatment and/or prevention of neurodegenerative diseases. Eur. Patent. 2016.
- 103.Cotzias G.C., Papavasiliou P.S., Gellene R. L-dopa in parkinson’s syndrome. N. Engl. J. Med. 1969;281(5):272. doi: 10.1056/NEJM196907312810518. [http://dx. doi.org/10.1056/NEJM196907312810517]. [PMID: 5791298]. [DOI] [PubMed] [Google Scholar]
- 104.Oertel W.H. Recent advances in treating Parkinson’s disease. F1000 Res. 2017;6:260. doi: 10.12688/f1000research.10100.1. [http://dx.doi.org/10.12688/f1000 research.10100.1]. [PMID: 28357055]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cacabelos R., Lombardi V., Fernandez-Novoa L., Carrera I., Cacabelos P., Corzo L., Carril J.C., Teijido O. Basic and clinical studies with marine Lipo Fishins and vegetal Favalins in neurodegeneration and age-related disorders. 2018.
- 106.Romero A., Parada E., González-Lafuente L., Farré-Alins V., Ramos E., Cacabelos R., Egea J. Neuroprotective effects of E-PodoFavalin-15999 (Atremorine®). CNS Neurosci. Ther. 2017;23(5):450–452. doi: 10.1111/cns.12693. [http://dx.doi.org/10.1111/cns.12693]. [PMID: 28371323]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cacabelos R. Int. J. Mol. Sci. 2017. Parkinson’s Disease: From Pathogenesis to Pharmacogenomics. Angelini S, Ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carrera I., Fernandez-Novoa L., Sampedro C., Cacabelos R., Aliev G. Dopaminergic neuroprotection with Atremorine in Parkinson’s disease. Curr. Med. Chem. 2018 doi: 10.2174/0929867325666180410100559. [Epub ahead of print]. [http://dx.doi.org/10.2174/0929867325666180410100559]. [PMID: 29637853]. [DOI] [PubMed] [Google Scholar]
- 109.Carrera I., Fernandez-Novoa L., Sampedro C., Cacabelos R. Neuroprotective effect of atremorine in an experimental model of parkinson’s disease. Curr. Pharm. Des. 2017;23(18):2673–2684. doi: 10.2174/1381612823666170210143530. [http://dx.doi.org/10.2174/1381612823666170210143530]. [PMID: 28190394]. [DOI] [PubMed] [Google Scholar]
- 110.Cacabelos R., Fernández-Novoa L., Alejo R., Corzo L., Alcaraz M., Nebril L., Cacabelos P., Fraile C., Carrera I., Carril J.C. E-PodoFavalin-15999 (Atremorine®)-induced dopamine response in Parkinson’s Disease: Pharmacogenetics-related effects. J. Genomic Med. Pharmacogenomics. 2016;1:1–26. [Google Scholar]
- 111.Cacabelos R., Fernández-Novoa L., Alejo R., Corzo L., Alcaraz M., Nebril L., Cacabelos P., Fraile C., Carrera I., Carril J.C. E-podofavalin-15999 (Atremorine®)-Induced neurotransmitter and hormonal response in parkinson’s diseasE. J. Exploratory Res. in Pharmacology. 2016;1:1–12. [http://dx.doi.org/10.14218/JERP. 2016.00031]. [Google Scholar]
- 112.Liu H., Deng Y., Gao J., Liu Y., Li W., Shi J., Gong Q. Sodium hydrosulfide attenuates beta-amyloid-induced cognitive deficits and neuroinflammation via modulation of MAPK/NF-κB pathway in rats. Curr. Alzheimer Res. 2015;12(7):673–683. doi: 10.2174/1567205012666150713102326. [http://dx.doi.org/10.2174/1567205012666150713102326]. [PMID: 26165866]. [DOI] [PubMed] [Google Scholar]
- 113.Jeong Y.H., Oh Y.C., Cho W.K., Yim N.H., Ma J.Y. Anti-inflammatory effect of rhapontici radix ethanol extract via inhibition of NF-κB and MAPK and induction of HO-1 in macrophages. Mediators Inflamm. 2016;2016:13. doi: 10.1155/2016/7216912. [http://dx.doi.org/10.1155/2016/7216912]. [DOI] [PMC free article] [PubMed] [Google Scholar]