Abstract
Aniline exposure leads to neuron and spleen toxicity specifically and makes diverse neurological effects and sar-coma that is defined by splenomegaly, hyperplasia, and fibrosis and tumors formation at the end. However, the molecular mechanism(s) of aniline-induced spleen toxicity is not understood well, previous studies have represented that aniline expo-sure results in iron overload and initiation of oxidative/nitrosative disorder stress and oxidative damage to proteins, lipids and DNA subsequently, in the spleen. Elevated expression of cyclins, cyclin-dependent kinases (CDKs) and phosphorylation of pRB protein along with increases in A, B and CDK1 as a cell cycle regulatory proteins cyclins, and reduce in CDK inhibitors (p21 and p27) could be critical in cell cycle regulation, which contributes to tumorigenic response after aniline exposure. Aniline-induced splenic toxicity is corre-lated to oxidative DNA damage and initiation of DNA glycosylases expression (OGG1, NEIL1/2, NTH1, APE1 and PNK) for removal of oxidative DNA lesions in rat. Oxidative stress causes transcriptional up-regulation of fibrogenic/inflammatory factors (cytokines, IL-1, IL-6 and TNF-α) via induction of nuclear factor-kappa B, AP-1 and redox-sensitive transcription factors, in aniline treated-rats. The upstream signalling events as phosphorylation of IκB kinases (IKKα and IKKβ) and mito-gen-activated protein kinases (MAPKs) could potentially be the causes of activation of NF-κB and AP-1. All of these events could initiate a fibrogenic and/or tumorigenic response in the spleen. The spleen toxicity of aniline is studied more and the different mechanisms are suggested. This review summarizes those events following aniline exposure that induce spleen tox-icity and neurotoxicity.
Keywords: Aniline, oxidative stress, neurotoxicity, spleen toxicity, neurology, pharmacology
1. INTRODUCTION
Aniline, a prototypical aromatic amine, is an essential building block of compounds, for instance rubbers, synthetic fibers, indigo pesticides, dyes, diphenylamine (used as antioxidant in the juices industry), and also the precursor of paracetamol/acetaminophen (N-acetyl-para-aminophenol) as one of the most ingested pharmaceutical compounds [1]. Many routes of aniline exposure are encompassing; pharmaceuticals, pesticide residues, cosmetics, textiles, cigarette smoke and colorants used in food [2]. On the other hand, aniline could cause a risk to health through environmental and occupational pollutants exposures. It enters the human body by respiratory tract, skin, and digestion system. Clinical symptoms such as weakness, cyanosis, headache, dizziness, loss of coordination, stupor, and coma occur after aniline exposure [3]. Besides, widely usage of aniline in industrial chemical which induces methemoglobinemia, hemolytic anemia and hemolysis, it could also make a different change to neuron system and destruct the spleen and consequently leads to fibrosis, hyperplasia, splenomegaly, and eventual formation of mesenchymal tumors, or highly malignant soft tissue and sarcomas on chronic and high dose exposure of rats [3, 4]. Spleen is the largest lymphoid tissue, performs important functional roles, such as participation in immune responses, iron metabolism, filtration of blood, phagocytosis, elimination of damaged RBCs, and remove the infectious organisms [5, 6]. Therefore, any damage/injury to the spleen cause reduced ability of spleen. Aniline induced splenotoxic responses is associated with increases in macrophages and fibroblasts, increased red pulp cellularity, and other changes such as iron overload follow by oxidative and nitrosative stress which leads to induction of redox-sensitive transcription factors in the spleen [7]. These events may cause production of pathologic precursors of tumorigenesis [3, 8]. Aniline inducedsplenic toxicity is associated mostly to (1) erythrocyte damage, (2) increases in iron content of spleen (free and total iron), and (3) oxidative stress as evident from increased oxidation (lipid peroxidation, DNA oxidation, protein oxidation) and nitrotyrosine production [9-11]. It has been shown that interaction between aniline and erythrocytes causes the initial step of splenic toxicity, as the damaged erythrocytes induce up-regulation of heme oxygenase-1 (HO-1) and provide an iron source, while aniline and/or its metabolites are released in the spleen [12]. Iron overload is also one of the marked consequences of aniline exposure because of the reactive oxygen species (ROS) generation in spleen [11, 13]. The ROS are considered to play a critical role in the pathogenesis of different diseases, such as rheumatoid arthritis, aging, cardiovascular disease, and cancer [14, 15]. ROS are produced endogenously as a by-product of oxidative metabolism and respiration, and by different environmental agents, exogenously. Neurotoxicity effect of aniline was also discovered and called Excitement-Hypotony Syndrome [16]. Few mechanisms, such as damage to oligodendrocyte which causes encephalopathy, vacuolation of the spinal cord and cerebellum and spongy change could make neurodegenerative effects and neurotoxicity at the end [17]. According to recent studies about splenic toxicity effects and tumorigenic responses of aniline exposure, this review focuses on the recognized molecular mechanisms and consequences by which aniline exerts, such toxic effects (Fig. 1).
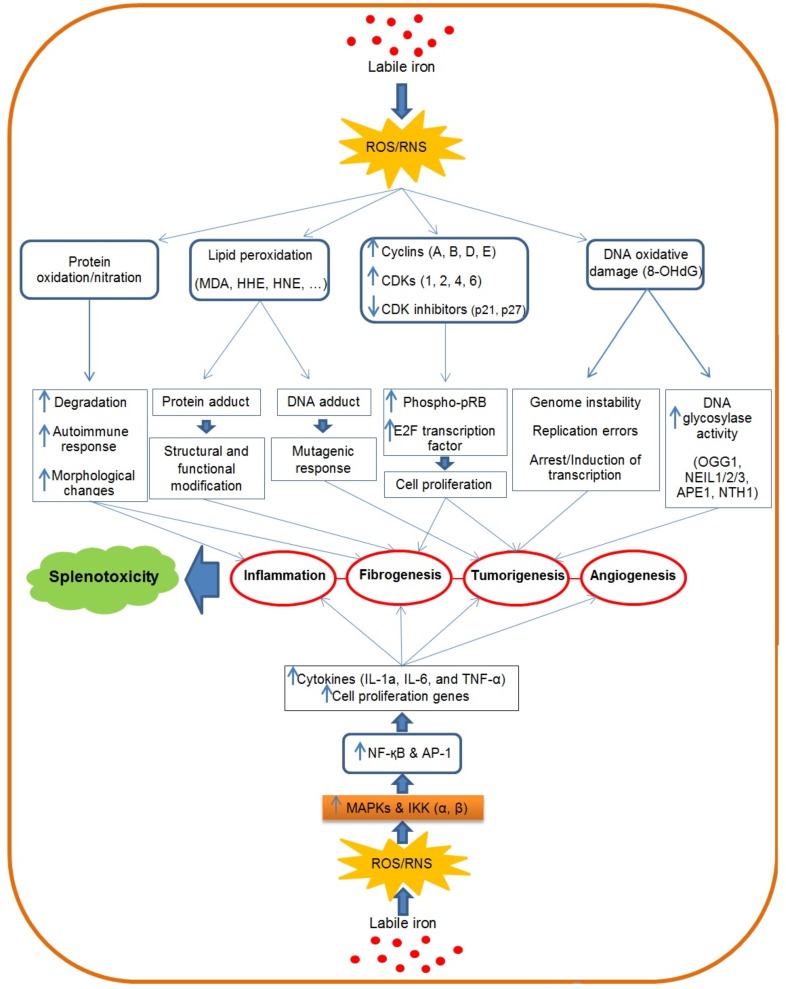
Fig. (1).
Schematic diagram depicting proposed mechanisms responsible for aniline inducedspleen toxicity. Aniline exerts its toxicity through labile iron accumulation and induction of oxidative/nitrosative stress. As depicted, oxidative damage to proteins, lipids and DNA by ROS/RNS can initiate many signaling pathway. ROS/RNS also induce fibrogenic and pro-inflammatory mediators. These phenomena together with other changes result in spleen toxicity. (The color version of the figure is available in the electronic copy of the article).
2. MECHANISMS OF SPLENOTOXICITY
2.1. Oxidative/nitrosative Stress and Outcomes
An association between severity of splenotoxicity and erythrocyte damage was detected in studies of aniline threated rats and information in the literature [18]. The scavenged, removal and breakdown of aniline induceddamaged erythrocyte leads to the release of aniline and/or related metabolites, and particularly result to iron accumulation in the spleen [13, 18, 19]. Iron accumulation results in the generation of different free-radical species, such as ROS in the spleen [7, 20] which react with proteins, nucleic acids, and lipids, and damage them especially the polyunsaturated fatty acid member of cell membrane phospholipids which finally makes cellular dysfunction [7, 21]. It is also probable that splenic phagocytes principally the macrophages, can be active at the time of the removal of damaged erythrocytes, and discharge ROS, which could further oxidase the biomolecules and cause tissue injury [21]. In overall, ROS reaction with proteins could damage different amino acid residues such as tryptophan, histidine, arginine, proline, methionine, cysteine and lysine which leading to formation of carbonyl products as an indicator of proteins oxidative damage [22-24]. Oxidative modification of proteins also makes their more sensitive than native proteins to proteolytic degradation [25]. In this case, aniline inducedprotein oxidation can lead to changing in the normal functions of spleen [7, 21]. These events are followed by morphological changes like elevated red pulp cellularity because of increased fibroblasts and sinusoidal cells, capsular thickening, vascular congestion, and formation of fibrous tissue throughout the parenchyma and in capsule [26, 27]. Thus, oxidative stress, especially lipid peroxidation induced by aniline and/or its metabolites, phenylhydroxylamine and nitrosobenzene, is coupled with iron accumulation in the spleen [26, 28]. Similar results in iron-overloaded rats have shown by Linpisarn 1991 in which lipid peroxidation was correlated with spleen iron concentration [29]. 4-hyroxynonenal (HNE), 4-hydroxy-2-hexenal (HHE) and Malondialdehyde (MDA) as three various cytotoxic degradation products are discovered that produce by lipid peroxidation [30]. These cytotoxic agents are involved in mutagenesis, tumorigenesis, and finally carcionogenesis [31]. The outcome of extend lipid peroxidation leads to more MDA formation in liver, which binds covalently with free amino and sulfhydryl groups of proteins and form covalent adducts (MDA–protein adducts) [32]. Similarly, time-dependent and dose-dependent production of MDA–protein adducts was shown in the spleen after a single but high dose exposure by an enzyme-linked immunosorbent assay (ELISA) [21].
The tight binding of MDA with protein leads to the modification of structural and functional properties of natural proteins, and induce the impairment of cellular integrity and cellular function which contributes to aniline induced splenic toxicity. MDA is also mutagenic because of its binding to DNA and starting the tumor formation [33]. In red pulp of aniline induced splenic toxicity, the MDA–protein adducts are predominantly localized in the sinusoidal macrophages [27]. The same localization of iron with these adducts prepares strong evidence of iron-catalyzed lipid peroxidation which result in MDA–protein adducts formation.
Aniline exposed rats also showed an increased protein tyrosines nitration in the spleen [10]. Compared to physiological conditions, the level of protein tyrosines nitration is increased under oxidative/nitrosative stress. Nitrogen dioxide (•NO2) and peroxynitrite anion (ONOO−), as a reactive nitrogen species (RNS) that are produced by reaction of nitric oxide radical (•NO) with another oxidants like hydrogen peroxide (H2O2), superoxide radicals (O2•−), and/or transition metals, are assumed as the secondary reactive intermediates and cause more toxicity rather than elementary oxidant [34]. The •NO formation was catalyzed by nitric oxide synthase (iNOS) which is inducible [35]. High •NO radical levels could result in increased protein nitration. Therefore, the increased expression of iNOS leads to increase protein tyrosines nitration that could be a probable contributor of nitrosative stress in spleen toxicity following aniline exposure [10]. RNS-induced protein tyrosine nitration has altered the properties, such as inactivation and/or generation of an autoimmune response, perturbed tyrosine phosphorylation and increased degradation [36, 37]. Such events have potential involvement in pathogenesis of most human diseases, such as multiple sclerosis, diabetes, cancer, inflammation, neurodegenerative disorders and coronary artery disease [38-40]. In rats exposed to aniline, the increased protein levels and mRNA of iNOS were contributed to increased protein nitration [41]. Among a variety of proteins, nitration of important structural and functional ones, such as enzymes, chaperones, skeletal proteins, protein transporter, ferric iron transporter and signaling pathway̕ s proteins were observed. The important role of nitrosative stress is shown by different nitrosative modification of proteins in mechanism of aniline induced splenic toxicity.
2.2. Cell Proliferation
High rates of cell proliferation and in cell cycle changing are essential factors in mechanism of chemical-induced cell damage and particularly carcinogenic activities [42-44]. Cell cycle, which consists of G1 (Gap 1), S (synthesis of DNA), G2 (Gap 2) and M (mitosis), is a complex but accurately controlled process which is conducted by cyclins and cyclin-dependent kinases (CDKs), two principal groups of regulatory proteins [45, 46]. Hence, controled cell cycle improvement plays a critical role in accurate proliferation of the cells. In adult tissues, most of the cells maintain in a quiescent state (G0) and they would probably resume proliferation and re-enter the cell cycle just to replace cells lost during normal function or in response to injury, for example in adult spleen [47].
Progression of cell cycle is controlled by changing in formation of cyclin–CDK complexe. In the absence of this control, cell cycle improvement could be stimulated by increased CDK activity and inactivated CDK inhibitors or overexpressed cyclins [46, 48]. For example cyclin E–CDK2, cyclin D–CDK4 and cyclin D–CDK6 complexes regulate the entrance of cell cycle from resting phase, development through the G1 phase and progression from G1 into S phase in response to mitogenic stimulation in mammalian cells [46-48]. It is considered that deregulation of G1 cyclins and altered CDKs in G1 phase is associated with tumorigenesis and oncogenic process, respectively [48]. A checkpoint in G1-S progression which controls normal cellular proliferation is named ‘restriction point’ or ‘R point’ [47, 49]. It determines whether a proper proliferating cell in G1 phase could revert to quiescence or will continue to cycle. The best known inhibitors of the G1-S transition process is a retinoblastoma proteins (pRB) that acts by the regulation of E2 transcription factor (E2F) responsive genes. A variety of cyclin/CDK complexes which are co-overexpressed and modify pRB, formed during different time and phases of cell cycle [50]. Phosphorylated form of pRB which is produced by cyclin E–CDK2 and cyclin D–CDK4/6 complexes, could lead to the release of E2F transcription factors that are necessary for the S-phase entry [51-53].
It is assumed that iron overload along with reactive oxygen generation is one of the reason(s) of mitogenic stimulation and initiation of the cell cycle improvement from G0 and also through the restriction point of G1-S stage that finally leads to proliferative response. In the rats treated with aniline, the spleens appeared dark and singularly enlarged [3] and significant increases in the splenocyte population was observed, suggesting that both deposition of damaged erythrocytes and increased proliferation and/or recruitment of splenocytes are reasons of the splenomegaly.
So, the use of typical cell proliferation indicators, PCNA and Ki67 that express coincides with DNA synthesis [54] shown that aniline exposure induces substantial increases in splenocyte population and cellular proliferation leading to splenomegaly [55]. These phenomena suggesting that splenomegaly is due to the increased proliferation and employment of splenocytes besides the deposition of damaged erythrocytes. These results were confirmed by the overexpressed minichromosome maintenance family of proteins (MCMs 2–7) in proliferating cells which are down-regulated following cell cycle exit [56]. MCMs, as appropriate biomarkers of cell cycle progression, could distinguish progressing cells from quiescent cells, so that perturbation of MCM function might be contributed to tumorigenesis [57]. Taken together, funded biomarkers, besides increases in the spleen weight and splenocyte population, indicate a potential progression of tumorigenic response in the spleen. MTT and flow cytometric analyses data showed that aniline exposure not only induced a proliferative response in vivo, but the splenocytes were also activated and entered cell cycle progression in culture (ex vivo) [55].
The D-type cyclins (D1, D2, and D3) are in core of cell cycle machinery in mammalian cells that regulate the phosphorylation of pRB, and ultimately receive oncogenic and mitogenic signals [58]. In the splenocytes from aniline-treated rats three D-type cyclins (D1, D2, D3) were co-expressed [55]. Even though cyclin D3 is necessary for specific oncogenic pathways in mammalian cells and widely expressed, these three D-type cyclins work to provide the cyclin D–dependent kinase activity that is required to promote G1/S progression, a credible step in the tumorigenesis [59]. The CDKs appear to be the most prominent partners of cyclins and play a key role in cell cycle regulation. Increased expression of CDK6 (556%), CDK4 (343%) and CDK2 (338%) was also observed in the spleens of aniline-treated rats [55]. Overexpression of G1 and G1-S phase cyclins (cyclin D1, D2, D3 and cyclin E) and CDK proteins (CDK2, CDK4 and CDK6) in the spleens from aniline-treated rats would thus be expected to contribute to increased phospho-pRB [55]. Cyclin E–CDK2 complexes become active in late G1-phase, whereas cyclin D–CDK4/6 complexes active in mid to late G1-phase and conformational change of the cyclin D–CDK4/6 permits phosphorylation by cyclin E–CDK2 [49, 53]. These findings suggest that aniline exposure induces dysregulation of G1 phase related cyclins and CDKs, and phosphorylation of pRB which can be associated to increased cell proliferation and potentially lead to a mutagenic and/or carcinogenic response in the spleen.
The G2/M checkpoint also controls normal cellular proliferation. It prevents cells from entering mitosis and a negligent G2/M checkpoint leads to genomic instability and cancer risk [60, 61]. The CDK1 (cdc2) kinase is a key effector of the G2/M checkpoint. Association of CDK1 kinase with cyclin B, together with a series of phosphorylation and dephosphorylation events, activates this kinase that is essential in initiating mitosis [62]. Cyclin B1/CDK1 complexes restrict cell growth prior to cell division and many of the G2/M regulators ultimately target CDK1 and inhibit its activation [46, 63]. In the spleens of aniline-treated rats, enhancement of protein expression in cyclins (A and B), and CDK1, notably p-CDK1 –the required CDK1 form for activation of the cyclin B/CDK1 complexes [46, 60, 62], and greater increases in the mRNA expression of cyclins A and B was observed [64]. Thus, increased expression of cyclin B1 and p-CKD1 at threonine 161, could contribute to uncontrolled cellular proliferation of the spleen. Furthermore, remarkably decreased expression of p21 and p27 as CDK inhibitors [60, 65] and up-regulation of tumor marker proteins Trx-1 and Ref-1 provides evidence to understand the aniline inducedcell proliferation and early indicators of a tumorigenic response in the spleen. Trx-1 and Ref-1 two known markers of cancer, overexpress in a wide variety of human tumors and down-regulate in tumor surgically removed [66]. Thus, increased Trx-1 and Ref-1 expression support the potential of carcinogenic response in aniline exposed rats. Noteworthy, decreased expression of cyclins A, B and CDK1 regulatory miRNAs like Let-7a, miR-15b, miR24, miR-100 and miR-125 which suppress the expression of cyclins A and B, and miRNAs, such as Let-7a, miR24 and miR-125 which regulate activity of CDK1, and increased expression of CDK inhibitory suppress miRNAs such as miR-181a, miR-221 and miR-222 which can target CDK inhibitors further support that splenocytes were promoted to go through an accelerated G2/M progression and tumorigenic response following chronic treatment with aniline [64].
Thus, greater release and presence of iron and increased oxidative stress in the spleen after aniline exposure which may serve as mitogenic stimulator result in uncontrolled cellular proliferation. Taken together, the results from overexpression of proteins as cell proliferation markers and upregulation of cell cycle regulatory proteins, as well as the increases in the splenocyte population and spleen weight, indicate inducement of cellular proliferation and suggest a possible progression to a tumorigenic response in the spleen.
2.3. Base Excision Repair
Iron overload is correlated with carcinogenesis in animal models and human diseases such as asbestosis and genetic hemochromatosis [67]. Normally, iron is store in specific proteins (ferritin, transferrin, heme proteins, and lactoferritin), which minimize or prevent its reaction with reduced derivatives of oxygen [68]. Iron release from these complexes in a free form that is redox active and could increase the production of harmful oxygen species such as hydroxyl radicals, through the Fenton reaction. Hydroxyl radical can induce a multiplicity of oxidative DNA base modifications [69]. Accumulations of DNA damage could have considerable mutagenesis and carcinogenesis consequences for the cells [70]. Continual DNA damage also can result in either induction or arrest of transcription, induction of signal transduction pathways, genomic instability, and replication errors, all of which are known to be associated with carcinogenesis [71].
Increased release of iron is a remarkable feature of aniline toxicity in spleen which could be a probable source for generation of ROS [18, 19, 21]. Formation of RNS and ROS catalyzed by iron and can directly oxidize and damage macromolecules, such as lipids, proteins and DNA, leading to wide-ranging structural and functional changes [72]. DNA oxidative damage following ROS production may be the initiator to inducing diseases associated with iron overload [73]. Indeed, earlier studies of aniline induced oxidative mechanisms including lipid peroxidation, protein oxidation and DNA oxidation is well established in the splenic toxicity of aniline [9]. In fact repeated exposure to aniline led to accumulation and release of free iron in the spleen, which are accompanied by increased 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels, as biomarkers of oxidative stress [11]. The critical role of iron in inducing oxidative stress further supported by administration of single hight-dose of aniline, which does not causes iron overload in the spleen and finally did not lead to oxidative DNA damage [19, 21]. Thus, the intermediate production of ROS and oxidative stress is critical to iron induced DNA oxidative damage. Oxidative DNA damage and increased 8-OHdG formation could induce mutations and could lead to serious consequences, if unrepaired [74].
The organisms possess multiple glycosylases which initiate the base excision repair (BER) pathway through recognize the damaged bases [75]. BER is a main mechanism for the repair of oxidative DNA base lesions induced by ROS and initiated by specialized DNA glycosylases [76]. There are at least five different DNA glycosylases in mammalian cells with overlapping substrate specificities. These include the 8-oxoguanine glycosylase 1 (OGG1), Nei-like DNA glycosylases (NEIL1/2/3), endonuclease III homologue 1 (NTH1), apurinic/apyrimidinic endonuclease (APE1) and polynucleotide kinase (PNK) [77]. BER pathway shows a critical step in the maintenance of genome stability by removal of oxidative DNA lesions from the genome and consequently prevention of mutagenesis and disease. Among DNA base conversions, 8-OHdG is an abundant DNA lesion produced by ROS [78, 79]. One of the DNA glycosylase/ lyase that preferentially removes 8-OHdG and other oxidative guanine adducts from the mitochondrial and nuclear DNA is OGG1 [74]. Disturbance of OGG1 activity and/or accumulation of 8-OHdG was also reported to be a predictive marker of sensitivity to cancer [80]. This is observed, oxidative DNA lesions, mainly 8-OHdG, induced by aniline and repaired by OGG1 in the BER pathway [71]. To examine oxidative DNA damage formation and BER activation in both mitochondrial and nuclear protein extracts, rats exposed subchronically to aniline. Aniline exposure was associated with increased a 2.8-fold formation of 8-OHdG and induction of OGG1 gene expression and 18-OHdG-specific lyase activity [71]. Furthermore, spleens from aniline treated rats showed strong OGG1 immunoreactivity, especially in the pulp areas. Subchronic exposure to aniline could led to persistent generation of ROS in the spleen and higher levels of 8-OHdG (183%) in comparison to repeated-dose study (83% after 7 days) [11]. The higher OGG1 activity was significantly observed in mitochondrial extracts (MEs) (1.2 fold) and nuclear extracts (NEs) (1.3 fold) of spleens from aniline-treated rats than the controls, indicating the induction of OGG1 activity following increased levels of 8-OHdG [71]. The induction of OGG1 in both MEs and NEs was supported also by gene expression data, showing a two-fold increase in splenic OGG1 RNA levels. On the other side, oxidative stress-induced acetylation of OGG1 resulted in significant enhancement of OGG1 activity and increases repair of 8-OHdG [81].
Despite the act on many of the same substrates, NEIL1/2 unlike from OGG1 and NTH1, have high affinity in excising base lesions from unrepaired sequences in bubble DNA or single-stranded DNA [82]. This suggests their involvement in the repair of DNA damage during transcription and/or replication [83]. Hence it is predictable that NEIL1/2 play a unique role in managing the functional integrity of mammalian genomes. NEIL1 and NEIL2 have unique specificity towards oxidized products of 8-oxoG including spiroiminodihydantoin lesions and guanidinohydantoin lesions, which are more mutagenic [84]. Different studies demonstrated activation of NEIL1 in response to ROS [85]. Aniline exposure was associated with variety of oxidized DNA lesions including foramidopyrimidines and oxidation products of cytosine, in addition to 8-OHdG, mentioned earlier [11, 71, 85]. These changes manifested by not only activation of the OGG1-mediated BER pathway also by NEIL1/2-mediated mechanisms of oxidative DNA damage repair. Aniline exposure is associated with induction of NEIL1 and NEIL2 gene (2.0- and 3.8-fold respectively) in the nuclear extracts of spleen and the same rate of fold increase in protein expression and increases in their BER activities in the spleen [85]. Therefore, NEIL2 at both mRNA and protein expression levels had much greater response in compared to NEIL1 that confirmed by relatively stronger immunoreactivity for NEIL2 in the spleens especially in the red pulp areas. This suggests greater production of adducts that are substrates for NEIL2. Thus, the increased BER activity of NEILs could describe an adaptive response for removal of oxidative lesions from transcribed DNA, leading to lesser accumulation of damage in promoter regions of transcribed genes and may be a critical mechanism for the removal of oxidative DNA lesions from aniline exposure.
Third DNA glycosylases of BER pathway is endonuclease III homologue 1 (NTH1). In the spleen of rats, NTH1 and APE1, like NEILs and OGG1, are associated with repair of DNA damage after aniline exposure [86]. Excision of DNA damaged bases by OGG1 and NTH1 glycosylases creating AP sites that are then repair by a common pathway involving an AP-endonuclease (APE). APE, the apurinic/ apyrimidinic endonuclease 1 (APE1), play a central role in BER by removing 3′ blocking groups produced by the β-lyase activity of OGG1 NTH1 [87]. Along whit DNA glycosylase activity, NTH1 also has apurinic/apyrimidinic (AP) lyase activity in the initiation of BER [88]. One of the reasons of aniline inducedsarcomas is an imbalance in DNA damage and repair activities that cause initiation of mutagenic and/or carcinogenic response in spleen [71]. Role of NTH1 and APE1 in the repair of aniline inducedoxidative DNA lesions in the spleen was confirmed by increased NTH1 and APE1-associated BER activity (1.2- and 1.3-fold respectively) in the nuclear extract of spleens, compared to the controls, following aniline exposure [86]. These increases were also observed in transcriptional and translational regulation level of NTH1 and APE1. APE1, in comparison to NTH1, showed greater mRNA and protein expression, higher BER activity, and stronger immunoreactivity because of greater production of AP sites as a substrates for APE1 following aniline exposure [86]. Thus the increased BER activity of APE1 and NTH1 and reported NEILs and OGG1, represent a compatible response against aniline inducedDNA damage and could be a crucial mechanism for the removal of oxidative DNA lesions.
2.4. Inflammation and Fibrosis: Up and Downstream Modulation
Oxidative stress disturbs the cellular redox status, causing oxidative damage to cellular molecules and changing gene expression, likely through post-transcriptional modification of redox-sensitive transcription factors (TFs) [89]. TFs are low-molecular-weight proteins that regulate gene expression through binding with promoter regions of the genes [90]. Two of important redox-sensitive TFs are Nuclear factor-қB (NF-κB) and Activator protein 1 (AP-1), which can regulate transcription of a variety target genes involved in fibrosis, inflammation, and cell proliferation [91]. Aniline inducediron overload and/or oxidative stress was showed to result in activation of both NF-κB and AP-1 in splenocytes, which is correlated with upstream signaling pathways involving MAPKs and IKK and activation of downstream factor such as cytokines [8, 92]. These activation coupled to carcinogenesis by regulation of genes involved in cell proliferation, transformation and angiogenesis [90]. Thus, an early activation of TFs following aniline exposure could lead to inflammatory/ fibrogenic and carcinogenic responses in the spleen (Fig. 2).
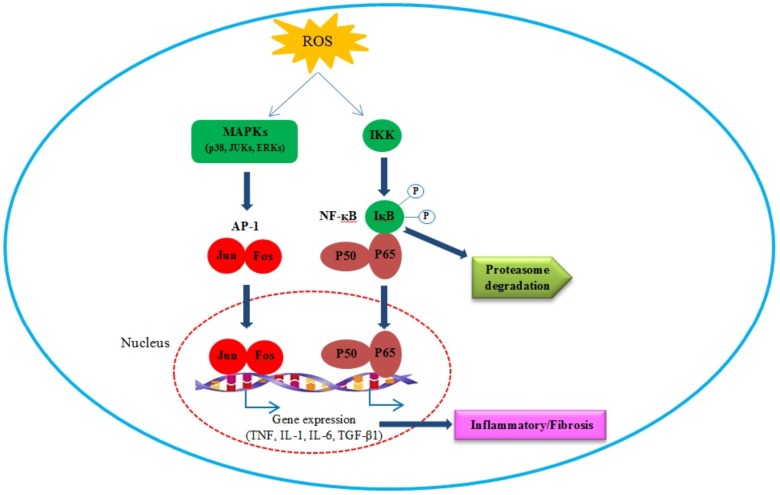
Fig. (2).
Induction of signal transduction cascades and activation of transcription factors AP-1 and NF-B. Two major pathways, the NF-B (right) and the MAPK pathway (left) are activated by ROS after aniline exposure. Excessive reactive oxygen species (ROS) derived from iron accumulation activate MAPKs and IKK. Activated IKK phosphorylates IκB and leads to ubiquitination and proteasome degradation of IκB, releasing NF-κB proteins, such as p50 and p65. Activated MAPKs in other hand, activate AP-1. The free p50 and p65 and Jun/ Fos dimers translocate into nuclei and regulate transcription of a variety of genes including TNF, IL-1, IL-6 that finally induce inflammatory and fibrosis responses. (The color version of the figure is available in the electronic copy of the article).
Increases in lipid peroxidation such as malondialdehyde–protein adducts in the spleen after repeated-dose treatment with aniline were also associated with higher transforming growth factor-beta1 (TGF-β1) mRNA that suggesting a correlation between oxidative stress and the up-regulation of TGF-β1 synthesis [93]. Indeed, aniline induced lipid peroxidation and/or lipid peroxidation-derived reactive aldehydes, caused overexpression of cytokines. Interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) are the major inflammatory cytokines among various cytokines, and known to stimulate extracellular matrix production and fibroblast proliferation [94]. Aniline treatment resulted in overexpression of these three cytokines (IL-1a, IL-6, and TNF-α) at both mRNA and protein levels in the spleen [92]. Up-regulation of these genes in splenocytes under exposure conditions induce fibrogenic response and suggest their contribution in initiating and/or developing splenic fibrosis. Activation of NF-κB along with up-regulation of pro-inflammatory and pro-fibrogenic cytokines and increases in lipid peroxidation [9, 27] suggests an association between oxidative stress and induction of these cytokines, observation that supported by [93].
AP-1 is a redox-sensitive early responsive transcription factor and play a critical role in regulation of a diversity downstream target genes including fibrogenic, inflammatory and cell proliferation genes [95]. AP-1 consists of Jun/ Fos dimers that have different Jun proteins (c-Jun, JunB, and JunD) and Fos proteins (c-Fos, FosB, Fra-1, Fra-2, and FosB2) [96]. Different agents which are known to produce ROS have also been shown to modulate AP-1 activation [97]. Oxidative stress modulate the activation of AP-1 through a diversity of mechanisms, including the phosphorylation of c-Jun or c-Fos by MAPK, or oxidative/reductive modification of the cysteine residues in DNA-binding sites of both c-Jun and c-Fos [98]. Aniline induced oxidative stress led to activation of AP-1, which was evident by the p-c-Jun-based ELISA and Western blot analysis [8]. Among TFs, NF-κB is considered as primary oxidative stress-responsive transcription factor, which is involved in transcriptional regulation of variety genes including those for growth factors and cytokines [99]. NF-κB consists of two subunits, a 50-kDA protein (p50) and a 65-kDA protein (p65). Transcriptional activity of NF-κB depends on inhibitor-κB (IκB) phosphorylation as well as phosphorylation and inducible transactivation activity of p65 [100]. Studies have shown that phosphorylation of multiple serine sites in p65 increase the transcriptional susceptibility of NF-κB in the nucleus [100]. In a normal state, NF-κB is present in the cytoplasm as non-active form and linked to its inhibitor, IκB, through the p65 unit [101]. Following stimulation with one or more stimulatory agents, IκB is phosphorylated (p-IκB), ubiquitinated and finally degraded by the ubiquitin-proteasome [102]. Elimination of IκB results in releasing NF-κB in an activated state and permits translocation of it into the nucleus where it binds to the cognate DNA binding sites and induces transcription [103]. Aniline induced phosphorylation of both p65 and IκB subunits result in NF-κB activation in the spleen. Increased phosphorylation of p65 (Ser 536) and enhanced p-IκB with contemporary decreases in total IκB suggest that aniline inducedoxidative stress has a direct effect on NF-κB transactivation. Thus, activation of AP-1 along with NF-κB as a critical signal transduction mechanism could regulate transcription of a variety of genes in the spleen.
The change in cellular redox status due to oxidative stress can alter many signalling pathways, such as the activation of IκB kinase (IKK) and mitogen-activated protein kinases (MAPKs) [104]. Multiple kinases phosphorylate IκB at particular N-terminal serine residues [105]. Among these kinases, the most studied are IκB kinase, IKKα and IKKβ, which are serine/threonine kinases [106]. Aniline treated rats showed remarkable increases in both p-IKKα and p-IKKβ, which along with increased phosphorylation of p65 and IκB subunits suggest that both p65 and IκB could be the substrates for the IKK complex in the upstream signalling events of NF-κB activation [8]. Aniline exposure also led to increases in p-ERK (p-ERK1, p-ERK2), p-JNK (p-JNK1, p-JNK2) and p-p38 MAPK. MAPKs are essential intermediates in signalling events that have been linked to activation of TFs, cytokines and fibrogenic gene expression [107]. All three MAPKs are implicated in NF-κB activation through phosphorylation of IκBα [108]. The p65 subunit of NF-κB also phosphorylate by MAPKs [109]. AP-1 activation could also regulated by the activation of MAPKs, involving three major pathways of stress-activated protein kinases/c-jun NH2-terminal kinases (JNKs), extracellular signal-related kinases (ERKs) and p38 MAPK at multiple levels [3, 97]. Such findings support by studies where the AP-1 activation and IL-6 mRNA expression inhibited in cells pretreatment by specific inhibitors of ERK and p38 MAPK [110]. Therefore, it is reasonable to expect that capacity of aniline to activate MAPKs contribute to transcriptional activation of cytokine genes.
These observations suggest that activation of upstream pathways such as IKK, MAPKs and TFs (NF-κB, AP-1) is associated with simultaneous increases in gene transcription of TNF-α, and cytokines (IL-1, IL-6) which could initiate inflammation and fibrosis. Up-regulation of fibrogenic cytokines in splenocytes [8] and up-regulation of TGF-β1 reported earlier [93], could be important in initiation and/or developing of a fibrogenic response following aniline exposure in spleen. These early molecular/signalling events finally lead to devastating effects including fibrosis and fibrosarcomas on continued exposure to aniline.
3. Neurotoxicity of Aniline
Several neurological symptoms have been reported following acute aniline exposure such as confusion, headache, nausea, vomiting, tinnitus, vertigo, weakness, lethargy, ataxia, drowsiness or coma in humans [111]. Symptoms from chronic exposure include headaches, decreased body weight, visual disturbances, skin lesions and loss of appetite [29]. Furthermore, neurotoxicity effects of aromatic amines like aniline was discovered and called Excitement-Hypotony Syndrome [16]. However, the in vivo examination of the neurotoxicity effects of aniline has not been fully understood, it has been shown that nitrobenzene (NB), a raw material in the aniline production, induced encephalopathy in rats [112]. NB is produced via the reduction to aniline and hydroxylation to aminophenols. A single dosing or repeated dosing could induce nervous toxicity in rats which shown by intramyelinic vacuolation in the cerebellum and white matter of the brain stem [112]. Morgan et al. reported that NB-related intramyelinic vacuolation was the reason of uncoupling mitochondrial oxidative phosphorylation that occurred in the oligodendrocytes [112]. Although, the occurrence and severity of neurotoxicity was decreased at the same time of the recovery period during the study of repeated dose toxicity [113]. The severity of lesions has been also decreased in recovering animals [114]. In another study, vacuolation of the spinal cord and cerebellum in the white matter, paralysis, ataxia, necrosis of Purkinje’s cells, as well as eosinophilic interstitial pneumonitis and muscular degeneration also observed in rabbits after exposure to anilides and aniline-derivatives [115]. The mechanisms of the neurotoxicity were shown to be related to eosinophil related allergic response or autoimmune toxicity [115]. However, the results showed no inflammatory responses in either skeletal muscles or nervous tissues in this study, and the normal range of white blood cells number was detected in rats after treatment with aniline.
It has been reported that some factors could play role in aniline-induced neurotoxicity such as the age of the animal when they are under treatment. Different ages cause differences in capacity of lipids which induces the separate response between mature myelin and immature one [116]. Immature myelin with lower proportion of galactolipid and higher amounts of phospholipid change to the increased galactolipid within the age, and matures biochemically [116]. In addition, the synthetic activity of lipids remains high (for up to 30 days of age) in the myelin sheath that could be the reason of the age dependent neurotoxicity of aniline in rats [117].
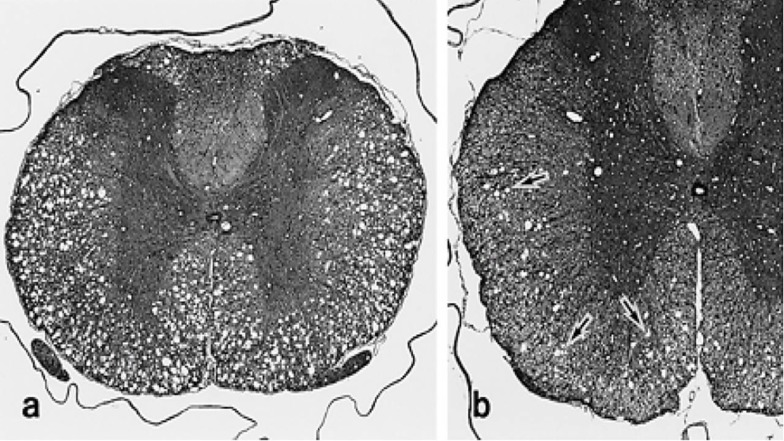
These findings have been shown by a single oral dose (750 or 1,000 mg/kg) administration of aniline which induced neurotoxicity characterized by hindlimb paralysis or paralytic gait, and spongy change especially in the white matter from central nervous system (CNS) and degeneration of fibers in the peripheral nerves in 4-week-old rats aniline [17]. Besides that, no treatment related neurohistopathological change, paralysis or paralytic gait were found in older ages (7- or 10-week-old rats) after receiving 800 mg/kg of aniline. The same changes were found between days 8 and 15 in the clinical observation of the 4-week-old rats which treated once with a bolus dose (1000 mg/kg) of aniline [118]. Histopathologic examination showed spongy change in the white matter of the spinal cord in all rats receiving 1000 mg/kg of aniline and the most sensitivity of the thoracic level of the spinal cord than the other central nervous tissues (Fig. 3).
Fig. (3).
Photomicrographs of the thoracic spinal cord of rats after single injection of aniline at 4 weeks of age and post-treatment day 15. (a) Severe spongy change in the white matter in rat after 1,000 mg/kg injection of aniline. HE. × 50. (b) Vacuoles in the the white matter of rat after 750 mg/kg injection of aniline. HE. × 70 [17].
Oligodendrocyte as a member of nervous system could also be affected in aniline induced neurotoxicity. It is considered as a possible primary target of aniline with transient toxic effect of aniline after a single dosing [118]. The recent in vitro study showed that oligodendrocytes are most sensitive to oxidative stress among the glial cells [119]. Petito et al. also reported that oligodendrocytes are more sensitive to short cerebral global ischemia than neurons in specific brain regions of rats [120]. These are in parallel to the results of the remyelination in the white matter of the CNS tissues and short-term paralysis in the clinical observation [118]. Accordingly, the effect of ROS could not be missed in the aniline neurotoxicity. Production of intramyelinic vacuolation in the cerebellum and white matter of the brain stem after aniline and aniline derrivatives exposure in rat is the result of the inhibition of mitochondrial oxidative phosphorylation in the oligodendrocytes [17, 112]. Regular production and supply of myelin sheath are dependent on the oligodendrocytes. Thus, the impairment of the function of oligodendrocytes could easily lead to the loosening of intraperiod region of the myelin sheath [121]. As a result, the uncoupling mitochondrial oxidative phosphorylation prevents the ATP production, and therefore, the disturbance of the transmembranous energy bound electrolyte transport will be occurred [122].
In overall, although there is some information about aniline related neurotoxicity as described above, the excact mechanism is unclear.
CONCLUSION
Induction of cancers because of exposure to environmental factors, including chemicals agents has been reported by epidemiologists and clinicians more than two centuries ago. Aniline as widely used industrial chemical can be a risk factor to humans either through occupational exposure and/or environmental pollutants. Important routes of exposure have been through pesticide residues, pharmaceuticals, cosmetics, colorants used in food, and cigarette smoke. Exposure to aniline and/or divers substituted anilines are shown to induce splenic toxicity and neuron toxicity in rats. Acute exposure to aniline could induce methemoglobinemia, hemolytic anemia and hemolysis. It is generally assumed that damaged erythrocytes which are scavenged by the spleen, eventually would initiate a chain of toxic events in the spleen. Probably, the most important outcome of these events is the release of iron which catalyzes tissue damaging free radical reactions in the spleen. These damages are characterized by fibrosis, hyperplasia, and alterations in capsular cell morphology, splenomegaly, and the consequent formation of highly mesenchymal tumors or malignant soft tissue, most commonly fibrosarcomas which is pathologic precursors of tumorigenesis. Based on previous studies, splenomegaly and splenotoxicity could be associated with iron overload, oxidative and nitrosative stress, induction of redox sensitive transcription factors and, finally cancer in spleen. Iron overload is also correlated with carcinogenesis both in animal models and human diseases such as genetic hemochromatosis and asbestosis. Aniline induces the significant increases in free iron as well as total iron content. Iron is redox active molecule which could promote the production of harmful oxygen species through the Fenton reaction. Furthermore, aniline exposure significantly results in upregulation of iNOS in the spleen which correlate to increased nitrated protein. The nitrosative modification of varies proteins such as skeletal proteins, enzymes, protein synthesis and signaling pathways suggest the important effect of nitrosative stress in the toxicity of spleen in aniline threated rats. Iron overload is also associated with increases protein oxidation, lipid peroxidation and malondialdehyde–protein adducts that are the critical link between fibrogenesis and tissue injury. Aniline induced oxidative stress correlates to enhanced production/expression of inflammatory and/or fibrogenic cytokines like IL-1a, IL-6, and TNF-a through induction of redox sensitive-transcription factor NF-κB and AP-1 which correlated to fibrosis. Activation of AP-1 and NF-κB is depend on phosphorylation of proteins in the upstream signaling pathway [MAPKs (p38, ERK1/2 and JNK1/2) and IKKα/β], which leads to up-regulation of pro-inflammatory and pro-fibrogenic cytokines.
Iron formation in the spleen of aniline exposure rats, following with enhanced oxidative stress may serve as reason of increased proliferation and/or recruitment of splenocytes associated with splenomegaly. Cell cycle progression has an important role in cell proliferation and aniline could deregulate cell cycle modulator. These modifications include, overexpression of cyclins, enhancement of CDK activity and phosphorylation of pRB that are needed to develop cell cycle entrance from G0, progression through the G1 phase and development from G1 into S phase. Iron overload in spleen, also result in increased expression of the G2/M regulators, specifically overexpression of p-CDK1 and cyclin B1 and knockdown of CDK inhibitors (p21, p27) following aniline exposure that result in uncontrolled cellular proliferation. Aniline induced oxidative stress also leads to DNA base alterations like abasic sites, oxidized bases, and single strand breaks. Increased 8-OHdG levels and G→T transversions have also shown after repeated exposure to aniline. These oxidative DNA damage associated with mutagenic and numerous pathological conditions, including cancer. Repairmen of oxidized DNA damage due to ROS insult is mainly dependent on the activities of BER enzymes, including OGG1, NTH1, NEILs, APE1 and polynucleotide kinase (PNK), which can effectively remove such lesions. Aniline exposure induces the NEIL1/2-associated BER activity and NEIL1/2 gene up-regulation, 8-OHdG-specific lyase (OGG1) activity and NTH1- and APE1-mediated repair activities. The elevated BER activity of mentioned enzymes may represent an adaptive response against ROS-induced DNA damage in aniline exposure.
Neurotoxicity is another toxic effect of aniline whit different outcome such as weakness, confusion, headache, ataxia and drowsiness in humans. Anilides derivatives like aniline have an effect on various part of neuron system, especially oligodendrocytes which are damaged by single oral dosage in 4-week-old rats. Oligodendrocytes considered as a more sensitive member among glial cells to oxidative stress and ischemia could be affected by aniline and results to the loosening of intraperiod region of the myelin sheath and vacuolation in the white matter of CNS.
Our review specifically focuses on known mechanism by which aniline exerts its toxic effects in the spleen and neuron system of rat. This review will help us to better understanding the probable mechanisms of clinical toxicity of aniline in the future. However, the exact molecular mechanisms need to be examined in view of occupational and environmental human exposure to aniline and related chemicals.
ACKNOWLEDGEMENTS
The authors acknowledge all non-financial supports provided by Kermanshah University of Medical Sciences, Iran.
LIST OF ABBREVIATIONS
- 8-OHdG
8-hydroxy-2′-deoxyguanosine
- AP-1
Activator protein 1
- APE1
Apurinic/apyrimidinic endonuclease
- CNS
Central nervous system
- E2F
E2 transcription factor
- HHE
4-hydroxy-2-hexenal
- HNE
4-hyroxynonenal
- IKK
IκB kinase
- IL-1
Interleukin-1
- IL-6
Interleukin-6
- MAPKs
Mitogen-activated protein kinases
- MDA
Malondialdehyde
- NB
Nitrobenzene
- NEIL1/2/3
Nei-like DNA glycosylases
- NF-κB
Nuclear factor-қB
- NTH1
Endonuclease III homologue 1
- OGG1
8-oxoguanine glycosylase 1
- PNK
Polynucleotide kinase
- pRB
Retinoblastoma proteins
- RNS
Reactive Nitrogen Species
- ROS
Reactive Oxygen Species
- TNF-α
Tumor necrosis factor alpha
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Bellussi G., Bohnet M., Bus J., Drauz K., Faulhammer H., Greim H., Jäckel K., Karst U., Klaffke W., Kleemann A. Ullmann’s Encyclopedia of Industrial Chemistry. Germany)(Wiley-VCH Verlag GmbH & Co. KGaA, 2000. [Google Scholar]
- 2.Modick H., Weiss T., Dierkes G., Brüning T., Koch H.M. Ubiquitous presence of paracetamol in human urine: sources and implications. Reproduction. 2014;147(4):R105–R117. doi: 10.1530/REP-13-0527. [http://dx. doi.org/10.1530/REP-13-0527]. [PMID: 24451225]. [DOI] [PubMed] [Google Scholar]
- 3.Khan M.F., Kannan S., Wang J. Activation of transcription factor AP-1 and mitogen-activated protein kinases in aniline-induced splenic toxicity. Toxicol. Appl. Pharmacol. 2006;210(1-2):86–93. doi: 10.1016/j.taap.2005.08.006. [http://dx.doi.org/10.1016/j.taap.2005.08.006]. [PMID: 16169568]. [DOI] [PubMed] [Google Scholar]
- 4.Pauluhn J. Subacute inhalation toxicity of aniline in rats: analysis of time-dependence and concentration-dependence of hematotoxic and splenic effects. Toxicol. Sci. 2004;81(1):198–215. doi: 10.1093/toxsci/kfh187. [http://dx. doi.org/10.1093/toxsci/kfh187]. [PMID: 15187235]. [DOI] [PubMed] [Google Scholar]
- 5.Steiniger B., Barth P. Microanatomy and function of the spleen; Adv. Anal. Embryol. Cell. 1999;151(iii-ix):1–101. doi: 10.1007/978-3-642-57088-9. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee C. Human Physiology; Medical allied Agency. 2000;Vol. 2 [Google Scholar]
- 7.Khairnar U., Upaganlawar A., Upasani C. Ameliorative effect of chronic supplementation of protocatechuic acid alone and in combination with ascorbic acid in aniline hydrochloride induced spleen toxicity in rats. Scientifica (Cairo) 2016;2016:1–9. doi: 10.1155/2016/4306984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J., Wang G., Ansari G.A., Khan M.F. Activation of oxidative stress-responsive signaling pathways in early splenotoxic response of aniline. Toxicol. Appl. Pharmacol. 2008;230(2):227–234. doi: 10.1016/j.taap.2008.02.022. [http://dx.doi.org/10.1016/j.taap.2008.02.022]. [PMID: 18420242]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan M.F., Wu X., Boor P.J., Ansari G.A. Oxidative modification of lipids and proteins in aniline-induced splenic toxicity. Toxicol. Sci. 1999;48(1):134–140. doi: 10.1093/toxsci/48.1.134. [http://dx.doi.org/10.1093/toxsci/ 48.1.134]. [PMID: 10330693]. [DOI] [PubMed] [Google Scholar]
- 10.Khan M.F., Wu X., Kaphalia B.S., Boor P.J., Ansari G.A. Nitrotyrosine formation in splenic toxicity of aniline. Toxicology. 2003;194(1-2):95–102. doi: 10.1016/j.tox.2003.08.008. [http://dx.doi.org/10.1016/j.tox.2003.08.008]. [PMID: 14636699]. [DOI] [PubMed] [Google Scholar]
- 11.Wu X., Kannan S., Ramanujam V.M., Khan M.F. Iron release and oxidative DNA damage in splenic toxicity of aniline. J. Toxicol. Environ. Health A. 2005;68(8):657–666. doi: 10.1080/15287390590921757. [http://dx.doi.org/ 10.1080/15287390590921757]. [PMID: 15901093]. [DOI] [PubMed] [Google Scholar]
- 12.Wang J., Ma H., Boor P.J., Ramanujam V.M., Ansari G.A., Khan M.F. Up-regulation of heme oxygenase-1 in rat spleen after aniline exposure. Free Radic. Biol. Med. 2010;48(4):513–518. doi: 10.1016/j.freeradbiomed.2009.11.027. [http://dx.doi.org/10.1016/j.freeradbiomed.2009.11.027]. [PMID: 19969074]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan M.F., Boor P.J., Kaphalia B.S., Alcock N.W., Ansari G.A. Hematopoietic toxicity of linoleic acid anilide: importance of aniline. Fundam. Appl. Toxicol. 1995;25(2):224–232. doi: 10.1006/faat.1995.1058. [http://dx.doi. org/10.1006/faat.1995.1058]. [PMID: 7665006]. [DOI] [PubMed] [Google Scholar]
- 14.Ames B.N., Shigenaga M.K., Hagen T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA. 1993;90(17):7915–7922. doi: 10.1073/pnas.90.17.7915. [http://dx.doi.org/10.1073/pnas.90.17.7915]. [PMID: 8367443]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Götz M.E., Künig G., Riederer P., Youdim M.B. Oxidative stress: free radical production in neural degeneration. Pharmacol. Ther. 1994;63(1):37–122. doi: 10.1016/0163-7258(94)90055-8. [http://dx.doi.org/10.1016/0163-7258 (94)90055-8]. [PMID: 7972344]. [DOI] [PubMed] [Google Scholar]
- 16.Labont H. Introduction into the study of the antioxydants and the structures of single electrons in biology-1st part-General study. Agressologie. 1960;1:63–79. [Google Scholar]
- 17.Okazaki Y., Yamashita K., Sudo M., Tsuchitani M., Narama I., Yamaguchi R., Tateyama S. Neurotoxicity induced by a single oral dose of aniline in rats. J. Vet. Med. Sci. 2001;63(5):539–546. doi: 10.1292/jvms.63.539. [http://dx.doi.org/10.1292/jvms.63.539]. [PMID: 11411500]. [DOI] [PubMed] [Google Scholar]
- 18.Khan M.F., Kaphalia B.S., Boor P.J., Ansari G.A. Subchronic toxicity of aniline hydrochloride in rats. Arch. Environ. Contam. Toxicol. 1993;24(3):368–374. doi: 10.1007/BF01128736. [http://dx.doi.org/10.1007/BF01128736]. [PMID: 8470936]. [DOI] [PubMed] [Google Scholar]
- 19.Khan M.F., Kaphalia B.S., Ansari G.A. Erythrocyte-aniline interaction leads to their accumulation and iron deposition in rat spleen. J. Toxicol. Environ. Health. 1995;44(4):415–421. doi: 10.1080/15287399509531970. [http://dx.doi. org/10.1080/15287399509531970]. [PMID: 7723074]. [DOI] [PubMed] [Google Scholar]
- 20.Khan R., Upaganlawar A.B., Upasani C. Protective effects of dioscorea alata L. In aniline exposure-induced spleen toxicity in rats: a biochemical study. Toxicol. Int. 2014;21(3):294–299. doi: 10.4103/0971-6580.155371. [http://dx.doi.org/10.4103/0971-6580.155371]. [PMID: 25948969]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan M.F., Boor P.J., Gu Y., Alcock N.W., Ansari G.A. Oxidative stress in the splenotoxicity of aniline. Fundam. Appl. Toxicol. 1997;35(1):22–30. doi: 10.1006/faat.1996.2259. [http://dx.doi.org/10.1006/faat.1996.2259]. [PMID: 9024670]. [DOI] [PubMed] [Google Scholar]
- 22.Stadtman E.R., Oliver C.N. Metal-catalyzed oxidation of proteins. Physiological consequences. J. Biol. Chem. 1991;266(4):2005–2008. [PMID: 1989966]. [PubMed] [Google Scholar]
- 23.Murphy M.E., Kehrer J.P. Oxidation state of tissue thiol groups and content of protein carbonyl groups in chickens with inherited muscular dystrophy. Biochem. J. 1989;260(2):359–364. doi: 10.1042/bj2600359. [http://dx. doi.org/10.1042/bj2600359]. [PMID: 2764876]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stadtman E.R. Metal ion-catalyzed oxidation of proteins: biochemical mechanism and biological consequences. Free Radic. Biol. Med. 1990;9(4):315–325. doi: 10.1016/0891-5849(90)90006-5. [http://dx.doi.org/10.1016/0891-5849(90)90006-5]. [PMID: 2283087]. [DOI] [PubMed] [Google Scholar]
- 25.Stadtman E.R. Protein oxidation and aging. Science. 1992;257(5074):1220–1224. doi: 10.1126/science.1355616. [http://dx.doi.org/10.1126/science.1355616]. [PMID: 1355616]. [DOI] [PubMed] [Google Scholar]
- 26.Khan M.F., Wu X., Alcock N.W., Boor P.J., Ansari G.A. Iron exacerbates aniline-associated splenic toxicity. J. Toxicol. Environ. Health A. 1999;57(3):173–184. doi: 10.1080/009841099157746. [http://dx.doi.org/10.1080/ 009841099157746]. [PMID: 10376884]. [DOI] [PubMed] [Google Scholar]
- 27.Khan M.F., Wu X., Ansari G.A., Boor P.J. Malondialdehyde-protein adducts in the spleens of aniline-treated rats: immunochemical detection and localization. J. Toxicol. Environ. Health A. 2003;66(1):93–102. doi: 10.1080/15287390306464. [http://dx.doi.org/10.1080/15287390306464]. [PMID: 12587293]. [DOI] [PubMed] [Google Scholar]
- 28.Khan M.F., Wu X., Ansari G.A. Contribution of nitrosobenzene to splenic toxicity of aniline. J. Toxicol. Environ. Health A. 2000;60(4):263–273. [http://dx.doi.org/10.1080/00984100050027815]. [PMID: 10914691]. [PubMed] [Google Scholar]
- 29.Linpisarn S., Satoh K., Mikami T., Orimo H., Shinjo S., Yoshino Y. Effects of iron on lipid peroxidation. Int. J. Hematol. 1991;54(3):181–188. [PMID: 1747452]. [PubMed] [Google Scholar]
- 30.Minotti G. Sources and role of iron in lipid peroxidation. Chem. Res. Toxicol. 1993;6(2):134–146. doi: 10.1021/tx00032a001. [http://dx.doi.org/10.1021/ tx00032a001]. [PMID: 8477003]. [DOI] [PubMed] [Google Scholar]
- 31.Cerutti P.A. Prooxidant states and tumor promotion. Science. 1985;227(4685):375–381. doi: 10.1126/science.2981433. [http://dx.doi.org/10.1126/science. 2981433]. [PMID: 2981433]. [DOI] [PubMed] [Google Scholar]
- 32.Mahmoodi H., Hadley M., Chang Y-X., Draper H.H. Increased formation and degradation of malondialdehyde-modified proteins under conditions of peroxidative stress. Lipids. 1995;30(10):963–966. doi: 10.1007/BF02537490. [http://dx.doi.org/10.1007/BF02537490]. [PMID: 8538386]. [DOI] [PubMed] [Google Scholar]
- 33.Stone K., Ksebati M.B., Marnett L.J. Investigation of the adducts formed by reaction of malondialdehyde with adenosine. Chem. Res. Toxicol. 1990;3(1):33–38. doi: 10.1021/tx00013a006. [http://dx.doi.org/10.1021/tx00013a006]. [PMID: 2131822]. [DOI] [PubMed] [Google Scholar]
- 34.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. USA. 2004;101(12):4003–4008. doi: 10.1073/pnas.0307446101. [http://dx.doi. org/10.1073/pnas.0307446101]. [PMID: 15020765]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [http://dx.doi.org/10.1152/physrev.00029.2006]. [PMID: 17237348]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abello N., Kerstjens H.A., Postma D.S., Bischoff R. Protein tyrosine nitration: selectivity, physicochemical and biological consequences, denitration, and proteomics methods for the identification of tyrosine-nitrated proteins. J. Proteome Res. 2009;8(7):3222–3238. doi: 10.1021/pr900039c. [http://dx.doi.org/10.1021/pr900039c]. [PMID: 19415921]. [DOI] [PubMed] [Google Scholar]
- 37.Monteiro H.P., Arai R.J., Travassos L.R. Protein tyrosine phosphorylation and protein tyrosine nitration in redox signaling., Antioxidants &. Redox Signaling. 2008;10:843–890. doi: 10.1089/ars.2007.1853. [http://dx.doi. org/10.1089/ars.2007.1853]. [DOI] [PubMed] [Google Scholar]
- 38.Dalle-Donne I., Scaloni A., Giustarini D., Cavarra E., Tell G., Lungarella G., Colombo R., Rossi R., Milzani A. Proteins as biomarkers of oxidative/nitrosative stress in diseases: the contribution of redox proteomics. Mass Spectrom. Rev. 2005;24(1):55–99. doi: 10.1002/mas.20006. [http://dx.doi.org/10.1002/mas.20006]. [PMID: 15389864]. [DOI] [PubMed] [Google Scholar]
- 39.Dalle-Donne I., Rossi R., Colombo R., Giustarini D., Milzani A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006;52(4):601–623. doi: 10.1373/clinchem.2005.061408. [http://dx.doi.org/10.1373/clinchem.2005. 061408]. [PMID: 16484333]. [DOI] [PubMed] [Google Scholar]
- 40.Miyagi M., Sakaguchi H., Darrow R.M., Yan L., West K.A., Aulak K.S., Stuehr D.J., Hollyfield J.G., Organisciak D.T., Crabb J.W. Evidence that light modulates protein nitration in rat retina. Mol. Cell. Proteomics. 2002;1(4):293–303. doi: 10.1074/mcp.m100034-mcp200. [http://dx.doi. org/10.1074/mcp.M100034-MCP200]. [PMID: 12096111]. [DOI] [PubMed] [Google Scholar]
- 41.Fan X., Wang J., Soman K.V., Ansari G.A., Khan M.F. Aniline-induced nitrosative stress in rat spleen: proteomic identification of nitrated proteins. Toxicol. Appl. Pharmacol. 2011;255(1):103–112. doi: 10.1016/j.taap.2011.06.005. [http://dx.doi.org/10.1016/j.taap.2011.06.005]. [PMID: 21708182]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiu J., Dawes I.W. Redox control of cell proliferation. Trends Cell Biol. 2012;22(11):592–601. doi: 10.1016/j.tcb.2012.08.002. [http://dx.doi.org/10.1016/ j.tcb.2012.08.002]. [PMID: 22951073]. [DOI] [PubMed] [Google Scholar]
- 43.Kakehashi A., Wei M., Fukushima S., Wanibuchi H. Oxidative stress in the carcinogenicity of chemical carcinogens. Cancers (Basel) 2013;5(4):1332–1354. doi: 10.3390/cancers5041332. [http://dx.doi.org/10.3390/cancers 5041332]. [PMID: 24202448]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park D-H., Shin J.W., Park S-K., Seo J-N., Li L., Jang J-J., Lee M-J. Diethylnitrosamine (DEN) induces irreversible hepatocellular carcinogenesis through overexpression of G1/S-phase regulatory proteins in rat. Toxicol. Lett. 2009;191(2-3):321–326. doi: 10.1016/j.toxlet.2009.09.016. [http://dx.doi.org/10.1016/j.toxlet.2009.09.016]. [PMID: 19822196]. [DOI] [PubMed] [Google Scholar]
- 45.Murray A.W. Recycling the cell cycle: cyclins revisited. Cell. 2004;116(2):221–234. doi: 10.1016/s0092-8674(03)01080-8. [http://dx.doi.org/10.1016/S0092-8674(03) 01080-8]. [PMID: 14744433]. [DOI] [PubMed] [Google Scholar]
- 46.Chulu J.L., Liu H.J. Recent patents on cell cycle regulatory proteins. Recent Pat. Biotechnol. 2009;3(1):1–9. doi: 10.2174/187220809787172614. [http://dx.doi.org/ 10.2174/187220809787172614]. [PMID: 19149717]. [DOI] [PubMed] [Google Scholar]
- 47.Pardee A.B. G1 events and regulation of cell proliferation. Science. 1989;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 48.Malumbres M., Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nat. Rev. Cancer. 2001;1(3):222–231. doi: 10.1038/35106065. [http://dx.doi.org/10.1038/35106065]. [PMID: 11902577]. [DOI] [PubMed] [Google Scholar]
- 49.Lundberg A.S., Weinberg R.A. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol. Cell. Biol. 1998;18(2):753–761. doi: 10.1128/mcb.18.2.753. [http://dx.doi.org/10.1128/MCB.18.2.753]. [PMID: 9447971]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lundberg A.S., Weinberg R.A. Control of the cell cycle and apoptosis. Eur. J. Cancer. 1999;35(4):531–539. [http://dx.doi.org/10. 1016/S0959-8049(99)00046-5]. [PMID: 10492624]. [PubMed] [Google Scholar]
- 51.Sherr C.J., Roberts J.M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13(12):1501–1512. doi: 10.1101/gad.13.12.1501. [http://dx.doi.org/10.1101/gad.13.12.1501]. [PMID: 10385618]. [DOI] [PubMed] [Google Scholar]
- 52.Sherr C.J. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 2000;60(14):3689–3695. [PMID: 10919634]. [PubMed] [Google Scholar]
- 53.Harbour J.W., Luo R.X., Dei Santi A., Postigo A.A., Dean D.C. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98(6):859–869. doi: 10.1016/s0092-8674(00)81519-6. [http://dx.doi.org/10.1016/S0092-8674(00)81519-6]. [PMID: 10499802]. [DOI] [PubMed] [Google Scholar]
- 54.Stuart-Harris R., Caldas C., Pinder S.E., Pharoah P. Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast. 2008;17(4):323–334. doi: 10.1016/j.breast.2008.02.002. [http://dx.doi.org/10.1016/j.breast.2008.02.002]. [PMID: 18455396]. [DOI] [PubMed] [Google Scholar]
- 55.Wang J., Wang G., Ma H., Khan M.F. Enhanced expression of cyclins and cyclin-dependent kinases in aniline-induced cell proliferation in rat spleen. Toxicol. Appl. Pharmacol. 2011;250(2):213–220. doi: 10.1016/j.taap.2010.10.026. [http://dx.doi.org/10.1016/j.taap.2010.10.026]. [PMID: 21070798]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korkolopoulou P., Givalos N., Saetta A., Goudopoulou A., Gakiopoulou H., Thymara I., Thomas-Tsagli E., Patsouris E. Minichromosome maintenance proteins 2 and 5 expression in muscle-invasive urothelial cancer: a multivariate survival study including proliferation markers and cell cycle regulators. Hum. Pathol. 2005;36(8):899–907. doi: 10.1016/j.humpath.2005.06.008. [http://dx.doi.org/10.1016/j.humpath.2005. 06.008]. [PMID: 16112007]. [DOI] [PubMed] [Google Scholar]
- 57.Lei M. The MCM complex: its role in DNA replication and implications for cancer therapy. Curr. Cancer Drug Targets. 2005;5(5):365–380. doi: 10.2174/1568009054629654. [http://dx.doi.org/10.2174/1568009054629654]. [PMID: 16101384]. [DOI] [PubMed] [Google Scholar]
- 58.Menon S.G., Goswami P.C. A redox cycle within the cell cycle: ring in the old with the new. Oncogene. 2007;26(8):1101–1109. doi: 10.1038/sj.onc.1209895. [http://dx.doi.org/10.1038/sj.onc.1209895]. [PMID: 16924237]. [DOI] [PubMed] [Google Scholar]
- 59.Sicinska E., Aifantis I., Le Cam L., Swat W., Borowski C., Yu Q., Ferrando A.A., Levin S.D., Geng Y., von Boehmer H., Sicinski P. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell. 2003;4(6):451–461. doi: 10.1016/s1535-6108(03)00301-5. [http:// dx.doi.org/10.1016/S1535-6108(03)00301-5]. [PMID: 14706337]. [DOI] [PubMed] [Google Scholar]
- 60.Stark G.R., Taylor W.R. Control of the G2/M transition. Mol. Biotechnol. 2006;32(3):227–248. doi: 10.1385/MB:32:3:227. [http://dx.doi.org/10.1385/MB: 32:3:227]. [PMID: 16632889]. [DOI] [PubMed] [Google Scholar]
- 61.Löbrich M., Jeggo P.A. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat. Rev. Cancer. 2007;7(11):861–869. doi: 10.1038/nrc2248. [http://dx.doi.org/10.1038/nrc2248]. [PMID: 17943134]. [DOI] [PubMed] [Google Scholar]
- 62.Iliakis G., Wang Y., Guan J., Wang H. DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene. 2003;22(37):5834–5847. doi: 10.1038/sj.onc.1206682. [http://dx.doi.org/10.1038/sj.onc.1206682]. [PMID: 12947390]. [DOI] [PubMed] [Google Scholar]
- 63.Wolgemuth D.J., Manterola M., Vasileva A. Role of cyclins in controlling progression of mammalian spermatogenesis. Int. J. Dev. Biol. 2013;57(2-4):159–168. doi: 10.1387/ijdb.130047av. [http://dx.doi.org/10.1387/ijdb. 130047av]. [PMID: 23784826]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J., Wang G., Khan M.F. Disorder of G2-M checkpoint control in aniline-induced cell proliferation in rat spleen. PLoS One. 2015;10(7):e0131457. doi: 10.1371/journal.pone.0131457. [http://dx.doi.org/10.1371/journal.pone. 0131457]. [PMID: 26192324]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bueno M.J., Malumbres M. MicroRNAs and the cell cycle. Biochimica et Biophysica Acta (BBA)-. Mol. Basis Disease. 2011;1812:592–601. doi: 10.1016/j.bbadis.2011.02.002. [http://dx.doi.org/10.1016/j.bbadis.2011.02.002]. [DOI] [PubMed] [Google Scholar]
- 66.Hillman G.G., Singh-Gupta V. Soy isoflavones sensitize cancer cells to radiotherapy. Free Radic. Biol. Med. 2011;51(2):289–298. doi: 10.1016/j.freeradbiomed.2011.04.039. [http://dx.doi.org/10.1016/j.freeradbiomed.2011.04.039]. [PMID: 21605661]. [DOI] [PubMed] [Google Scholar]
- 67.Toyokuni S. Iron-induced carcinogenesis: the role of redox regulation. Free Radic. Biol. Med. 1996;20(4):553–566. doi: 10.1016/0891-5849(95)02111-6. [http://dx.doi. org/10.1016/0891-5849(95)02111-6]. [PMID: 8904296]. [DOI] [PubMed] [Google Scholar]
- 68.Ferrali M., Signorini C., Sugherini L., Pompella A., Lodovici M., Caciotti B., Ciccoli L., Comporti M. Release of free, redox-active iron in the liver and DNA oxidative damage following phenylhydrazine intoxication. Biochem. Pharmacol. 1997;53(11):1743–1751. doi: 10.1016/s0006-2952(97)82456-2. [http://dx.doi.org/10.1016/S0006-2952(97)82456-2]. [PMID: 9264328]. [DOI] [PubMed] [Google Scholar]
- 69.Hartwig A., Klyszcz-Nasko H., Schlepegrell R., Beyersmann D. Cellular damage by ferric nitrilotriacetate and ferric citrate in V79 cells: interrelationship between lipid peroxidation, DNA strand breaks and sister chromatid exchanges. Carcinogenesis. 1993;14(1):107–112. doi: 10.1093/carcin/14.1.107. [http://dx.doi.org/10.1093/carcin/14.1.107]. [PMID: 8425256]. [DOI] [PubMed] [Google Scholar]
- 70.Feig D.I., Reid T.M., Loeb L.A. Reactive oxygen species in tumorigenesis. Cancer Res. 1994;54(7) Suppl.:1890s–1894s. [PMID: 8137306]. [PubMed] [Google Scholar]
- 71.Ma H., Wang J., Abdel-Rahman S.Z., Boor P.J., Khan M.F. Oxidative DNA damage and its repair in rat spleen following subchronic exposure to aniline. Toxicol. Appl. Pharmacol. 2008;233(2):247–253. doi: 10.1016/j.taap.2008.08.010. [http://dx.doi.org/10.1016/j.taap.2008.08.010]. [PMID: 18793663]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marcon G., Tell G., Perrone L., Garbelli R., Quadrifoglio F., Tagliavini F., Giaccone G. APE1/Ref-1 in Alzheimer’s disease: an immunohistochemical study. Neurosci. Lett. 2009;466(3):124–127. doi: 10.1016/j.neulet.2009.09.039. [http://dx.doi.org/10.1016/j.neulet.2009.09.039]. [PMID: 19782121]. [DOI] [PubMed] [Google Scholar]
- 73.Kang J.O., Jones C., Brothwell B. Toxicity associated with iron overload found in hemochromatosis: possible mechanism in a rat model. Clin. Lab. Sci. 1998;11(6):350–354. [PMID: 10345501]. [PubMed] [Google Scholar]
- 74.Boiteux S., Radicella J.P. The human OGG1 gene: structure, functions, and its implication in the process of carcinogenesis. Arch. Biochem. Biophys. 2000;377(1):1–8. doi: 10.1006/abbi.2000.1773. [http://dx.doi.org/ 10.1006/abbi.2000.1773]. [PMID: 10775435]. [DOI] [PubMed] [Google Scholar]
- 75.Liu M., Bandaru V., Bond J.P., Jaruga P., Zhao X., Christov P.P., Burrows C.J., Rizzo C.J., Dizdaroglu M., Wallace S.S. The mouse ortholog of NEIL3 is a functional DNA glycosylase in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2010;107(11):4925–4930. doi: 10.1073/pnas.0908307107. [http://dx.doi.org/10.1073/pnas.0908307107]. [PMID: 20185759]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Altieri F., Grillo C., Maceroni M., Chichiarelli S. DNA damage and repair: from molecular mechanisms to health implications. Antioxid. Redox Signal. 2008;10:891–938. doi: 10.1089/ars.2007.1830. [http://dx.doi. org/10.1089/ars.2007.1830]. [DOI] [PubMed] [Google Scholar]
- 77.Mori H., Ouchida R., Hijikata A., Kitamura H., Ohara O., Li Y., Gao X., Yasui A., Lloyd R.S., Wang J-Y. Deficiency of the oxidative damage-specific DNA glycosylase NEIL1 leads to reduced germinal center B cell expansion. DNA Repair (Amst.) 2009;8(11):1328–1332. doi: 10.1016/j.dnarep.2009.08.007. [http://dx.doi.org/10.1016/j.dnarep.2009. 08.007]. [PMID: 19782007]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hartwig A., Schlepegrell R. Induction of oxidative DNA damage by ferric iron in mammalian cells. Carcinogenesis. 1995;16(12):3009–3013. doi: 10.1093/carcin/16.12.3009. [http://dx.doi.org/10.1093/carcin/16.12.3009]. [PMID: 8603477]. [DOI] [PubMed] [Google Scholar]
- 79.Cooke M.S., Evans M.D., Dizdaroglu M., Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17(10):1195–1214. doi: 10.1096/fj.02-0752rev. [http://dx.doi.org/10.1096/fj.02-0752rev]. [PMID: 12832285]. [DOI] [PubMed] [Google Scholar]
- 80.Gackowski D., Speina E., Zielinska M., Kowalewski J., Rozalski R., Siomek A., Paciorek T., Tudek B., Olinski R. Products of oxidative DNA damage and repair as possible biomarkers of susceptibility to lung cancer. Cancer Res. 2003;63(16):4899–4902. [PMID: 12941813]. [PubMed] [Google Scholar]
- 81.Bhakat K.K., Mokkapati S.K., Boldogh I., Hazra T.K., Mitra S. Acetylation of human 8-oxoguanine-DNA glycosylase by p300 and its role in 8-oxoguanine repair in vivo. Mol. Cell. Biol. 2006;26(5):1654–1665. doi: 10.1128/MCB.26.5.1654-1665.2006. [http://dx.doi.org/10.1128/MCB.26.5.1654-1665.2006]. [PMID: 16478987]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dou H., Mitra S., Hazra T.K. Repair of oxidized bases in DNA bubble structures by human DNA glycosylases NEIL1 and NEIL2. J. Biol. Chem. 2003;278(50):49679–49684. doi: 10.1074/jbc.M308658200. [http://dx.doi.org/ 10.1074/jbc.M308658200]. [PMID: 14522990]. [DOI] [PubMed] [Google Scholar]
- 83.Dou H., Theriot C.A., Das A., Hegde M.L., Matsumoto Y., Boldogh I., Hazra T.K., Bhakat K.K., Mitra S. Interaction of the human DNA glycosylase NEIL1 with proliferating cell nuclear antigen. The potential for replication-associated repair of oxidized bases in mammalian genomes. J. Biol. Chem. 2008;283(6):3130–3140. doi: 10.1074/jbc.M709186200. [http://dx.doi.org/10.1074/jbc.M709186200]. [PMID: 18032376]. [DOI] [PubMed] [Google Scholar]
- 84.Hailer M.K., Slade P.G., Martin B.D., Rosenquist T.A., Sugden K.D. Recognition of the oxidized lesions spiroiminodihydantoin and guanidinohydantoin in DNA by the mammalian base excision repair glycosylases NEIL1 and NEIL2. DNA Repair (Amst.) 2005;4(1):41–50. doi: 10.1016/j.dnarep.2004.07.006. [http://dx.doi.org/10.1016/j.dnarep.2004.07.006]. [PMID: 15533836]. [DOI] [PubMed] [Google Scholar]
- 85.Ma H., Wang J., Abdel-Rahman S.Z., Hazra T.K., Boor P.J., Khan M.F. Induction of NEIL1 and NEIL2 DNA glycosylases in aniline-induced splenic toxicity. Toxicol. Appl. Pharmacol. 2011;251(1):1–7. doi: 10.1016/j.taap.2010.12.001. [http://dx.doi.org/10.1016/j.taap.2010.12.001]. [PMID: 21145906]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma H., Wang J., Abdel-Rahman S.Z., Boor P.J., Khan M.F. Induction of base excision repair enzymes NTH1 and APE1 in rat spleen following aniline exposure. Toxicol. Appl. Pharmacol. 2013;267(3):276–283. doi: 10.1016/j.taap.2013.01.005. [http://dx.doi.org/10.1016/j.taap.2013.01. 005]. [PMID: 23352893]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hazra T.K., Das A., Das S., Choudhury S., Kow Y.W., Roy R. Oxidative DNA damage repair in mammalian cells: a new perspective. DNA Repair (Amst.) 2007;6(4):470–480. doi: 10.1016/j.dnarep.2006.10.011. [http://dx.doi.org/ 10.1016/j.dnarep.2006.10.011]. [PMID: 17116430]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goto M., Shinmura K., Igarashi H., Kobayashi M., Konno H., Yamada H., Iwaizumi M., Kageyama S., Tsuneyoshi T., Tsugane S., Sugimura H. Altered expression of the human base excision repair gene NTH1 in gastric cancer. Carcinogenesis. 2009;30(8):1345–1352. doi: 10.1093/carcin/bgp108. [http://dx.doi.org/10.1093/carcin/bgp108]. [PMID: 19414504]. [DOI] [PubMed] [Google Scholar]
- 89.Yan J., Hales B.F. Activator protein-1 (AP-1) DNA binding activity is induced by hydroxyurea in organogenesis stage mouse embryos. Toxicol. Sci. 2005;85(2):1013–1023. doi: 10.1093/toxsci/kfi148. [http://dx.doi.org/ 10.1093/toxsci/kfi148]. [PMID: 15772364]. [DOI] [PubMed] [Google Scholar]
- 90.Baldwin A.S., Jr The NF-κ B and I κ B proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [http://dx. doi.org/10.1146/annurev.immunol.14.1.649]. [PMID: 8717528]. [DOI] [PubMed] [Google Scholar]
- 91.Kapahi P., Takahashi T., Natoli G., Adams S.R., Chen Y., Tsien R.Y., Karin M. Inhibition of NF-κ B activation by arsenite through reaction with a critical cysteine in the activation loop of Ikappa B kinase. J. Biol. Chem. 2000;275(46):36062–36066. doi: 10.1074/jbc.M007204200. [http://dx.doi.org/10.1074/jbc.M007204200]. [PMID: 10967126]. [DOI] [PubMed] [Google Scholar]
- 92.Wang J., Kannan S., Li H., Khan M.F. Cytokine gene expression and activation of NF-κ B in aniline-induced splenic toxicity. Toxicol. Appl. Pharmacol. 2005;203(1):36–44. doi: 10.1016/j.taap.2004.07.012. [http://dx.doi. org/10.1016/j.taap.2004.07.012]. [PMID: 15694462]. [DOI] [PubMed] [Google Scholar]
- 93.Firoze Khan M., Wu X., Wang J. Up-regulation of transforming growth factor-β 1 in the spleen of aniline-treated rats. Toxicol. Appl. Pharmacol. 2003;187(1):22–28. doi: 10.1016/s0041-008x(02)00041-8. [http://dx.doi.org/10.1016/ S0041-008X(02)00041-8]. [PMID: 12628581]. [DOI] [PubMed] [Google Scholar]
- 94.Postlethwaite A.E., Seyer J.M. Stimulation of fibroblast chemotaxis by human recombinant tumor necrosis factor alpha (TNF-alpha) and a synthetic TNF-alpha 31-68 peptide. J. Exp. Med. 1990;172(6):1749–1756. doi: 10.1084/jem.172.6.1749. [http://dx.doi.org/10.1084/jem.172.6.1749]. [PMID: 2258704]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pennypacker K. AP-1 transcription factors: short- and long-term modulators of gene expression in the brain. Int. Rev. Neurobiol. 1998;42:169–197. doi: 10.1016/s0074-7742(08)60610-8. [http://dx.doi.org/10.1016/S0074-7742(08)60610-8]. [PMID: 9476173]. [DOI] [PubMed] [Google Scholar]
- 96.Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta. 1991;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [PMID: 1751545]. [DOI] [PubMed] [Google Scholar]
- 97.Hsu T-C., Young M.R., Cmarik J., Colburn N.H. Activator protein 1 (AP-1)- and nuclear factor kappaB (NF-kappaB)-dependent transcriptional events in carcinogenesis. Free Radic. Biol. Med. 2000;28(9):1338–1348. doi: 10.1016/s0891-5849(00)00220-3. [http://dx.doi.org/10.1016/ S0891-5849(00)00220-3]. [PMID: 10924853]. [DOI] [PubMed] [Google Scholar]
- 98.Hirota K., Matsui M., Iwata S., Nishiyama A., Mori K., Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc. Natl. Acad. Sci. USA. 1997;94(8):3633–3638. doi: 10.1073/pnas.94.8.3633. [http://dx.doi.org/10.1073/pnas.94.8.3633]. [PMID: 9108029]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shi M.M., Chong I., Godleski J.J., Paulauskis J.D. Regulation of macrophage inflammatory protein-2 gene expression by oxidative stress in rat alveolar macrophages. Immunology. 1999;97(2):309–315. doi: 10.1046/j.1365-2567.1999.00798.x. [http://dx.doi.org/10.1046/j.1365-2567.1999.00798.x]. [PMID: 10447747]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Utsugi M., Dobashi K., Ishizuka T., Kawata T., Hisada T., Shimizu Y., Ono A., Mori M. Rac1 negatively regulates lipopolysaccharide-induced IL-23 p19 expression in human macrophages and dendritic cells and NF-kappaB p65 trans activation plays a novel role. J. Immunol. 2006;177(7):4550–4557. doi: 10.4049/jimmunol.177.7.4550. [http://dx.doi.org/10.4049/jimmunol.177.7.4550]. [PMID: 16982892]. [DOI] [PubMed] [Google Scholar]
- 101.Chen F., Castranova V., Shi X., Demers L.M. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin. Chem. 1999;45(1):7–17. [PMID: 9895331]. [PubMed] [Google Scholar]
- 102.Chen Z., Hagler J., Palombella V.J., Melandri F., Scherer D., Ballard D., Maniatis T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9(13):1586–1597. doi: 10.1101/gad.9.13.1586. [http://dx.doi.org/10.1101/ gad.9.13.1586]. [PMID: 7628694]. [DOI] [PubMed] [Google Scholar]
- 103.Akira S., Kishimoto T. NF-IL6 and NF-kappa B in cytokine gene regulation. Adv. Immunol. 1997;65:1–46. [http://dx.doi.org/10. 1016/S0065-2776(08)60740-3]. [PMID: 9238507]. [PubMed] [Google Scholar]
- 104.Lee J.Y., Yu B.P., Chung H.Y. Activation mechanisms of endothelial NF-kappaB, IKK, and MAP kinase by tert-butyl hydroperoxide. Free Radic. Res. 2005;39(4):399–409. doi: 10.1080/1071576040002870. [http://dx.doi.org/ 10.1080/1071576040002870]. [PMID: 16028365]. [DOI] [PubMed] [Google Scholar]
- 105.Kamata H., Manabe T., Oka S-i., Kamata K., Hirata H. Hydrogen peroxide activates IkappaB kinases through phosphorylation of serine residues in the activation loops. FEBS Lett. 2002;519(1-3):231–237. doi: 10.1016/s0014-5793(02)02712-6. [http://dx.doi.org/10.1016/S0014-5793(02)02712-6]. [PMID: 12023051]. [DOI] [PubMed] [Google Scholar]
- 106.Karin M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J. Biol. Chem. 1999;274(39):27339–27342. doi: 10.1074/jbc.274.39.27339. [http://dx.doi.org/10.1074/jbc.274.39.27339]. [PMID: 10488062]. [DOI] [PubMed] [Google Scholar]
- 107.Pestka J.J., Zhou H-R., Moon Y., Chung Y.J. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: unraveling a paradox. Toxicol. Lett. 2004;153(1):61–73. doi: 10.1016/j.toxlet.2004.04.023. [http://dx.doi.org/10.1016/j.toxlet.2004.04.023]. [PMID: 15342082]. [DOI] [PubMed] [Google Scholar]
- 108.Castrillo A., de Las Heras B., Hortelano S., Rodríguez B., Villar A., Boscá L. Inhibition of the nuclear factor κ B (NF-κ B) pathway by tetracyclic kaurene diterpenes in macrophages. Specific effects on NF-κ B-inducing kinase activity and on the coordinate activation of ERK and p38 MAPK. J. Biol. Chem. 2001;276(19):15854–15860. doi: 10.1074/jbc.M100010200. [http://dx.doi.org/10.1074/jbc.M100010200]. [PMID: 11278990]. [DOI] [PubMed] [Google Scholar]
- 109.Kefaloyianni E., Gaitanaki C., Beis I. ERK1/2 and p38-MAPK signalling pathways, through MSK1, are involved in NF-kappaB transactivation during oxidative stress in skeletal myoblasts. Cell. Signal. 2006;18(12):2238–2251. doi: 10.1016/j.cellsig.2006.05.004. [http://dx.doi.org/10.1016/ j.cellsig.2006.05.004]. [PMID: 16806820]. [DOI] [PubMed] [Google Scholar]
- 110.Dai J., Huang C., Wu J., Yang C., Frenkel K., Huang X. Iron-induced interleukin-6 gene expression: possible mediation through the extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways. Toxicology. 2004;203(1-3):199–209. doi: 10.1016/j.tox.2004.06.009. [http://dx.doi.org/10.1016/j.tox.2004.06.009]. [PMID: 15363595]. [DOI] [PubMed] [Google Scholar]
- 111.Weiss G. Hazardous chemicals data book. 2nd ed. Park ridge, New Jersey: 1986. [Google Scholar]
- 112.Morgan K.T., Gross E.A., Lyght O., Bond J.A. Morphologic and biochemical studies of a nitrobenzene-induced encephalopathy in rats. Neurotoxicology. 1985;6(1):105–116. [PMID: 3873033]. [PubMed] [Google Scholar]
- 113.Shimo T, Onodera H, Matsushima Y, Todate A, Mitsumori K, Maekawa A, Takahashi M. A 28-day repeated dose toxicity study of nitrobenzene in F344 rats; Eisei Shikenjo hokoku Bulletin of National Institute of Hygienic Sciences. 1994;112:71–81. [PubMed] [Google Scholar]
- 114.Lampert P., O’Brien J., Garrett R. Hexachlorophene encephalopathy. Acta Neuropathol. 1973;23(4):326–333. doi: 10.1007/BF00687462. [http://dx.doi.org/ 10.1007/BF00687462]. [PMID: 4718199]. [DOI] [PubMed] [Google Scholar]
- 115.Rodrigo J., Robles M., Mayo I., Pestaña A., Marquet A., Larraga V., Muñoz E. Neurotoxicity of fatty acid anilides in rabbits. Lancet. 1983;1(8321):414–416. doi: 10.1016/s0140-6736(83)91527-1. [http://dx.doi.org/10.1016/S0140-6736(83)91527-1]. [PMID: 6130403]. [DOI] [PubMed] [Google Scholar]
- 116.Krinke G. Nonneoplastic changes in the brain. . Pathobiology of the aging rat . 1994;2:6–19. [Google Scholar]
- 117.Smith M.E. A regional survey of myelin development: some compositional and metabolic aspects. J. Lipid Res. 1973;14(5):541–551. [PMID: 4354155]. [PubMed] [Google Scholar]
- 118.Okazaki Y., Yamashita K., Sudo M., Tsuchitani M., Narama I., Yamaguchi R., Tateyama S. The progression and recovery of neurotoxicity induced by a single oral dose of aniline in rats. J. Toxicol. Pathol. 2001;14:19–19. doi: 10.1292/jvms.63.539. [http://dx.doi.org/10.1293/tox.14.19]. [DOI] [PubMed] [Google Scholar]
- 119.Smith K.J., Kapoor R., Felts P.A. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9(1):69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [http://dx.doi.org/10.1111/j.1750-3639.1999.tb00212.x]. [PMID: 9989453]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Petito C.K., Olarte J-P., Roberts B., Nowak T.S., Jr, Pulsinelli W.A. Selective glial vulnerability following transient global ischemia in rat brain. J. Neuropathol. Exp. Neurol. 1998;57(3):231–238. doi: 10.1097/00005072-199803000-00004. [http://dx.doi.org/10.1097/00005072-199803000-00004]. [PMID: 9600215]. [DOI] [PubMed] [Google Scholar]
- 121.Graeber M., Blakemore W., Kreutzberg G. Cellular pathology of the central nervous system. Greenfield’s Neuropathology. Arnold; 2002. pp. 123–191. [Google Scholar]
- 122.Verity M. Toxic disorders. Greenfield’s Neuropathol. 1997;1:755–811. [Google Scholar]