Short abstract
Proanthocyanidin has beneficial features such as free radical scavenging, anti-inflammation, and anti-oxidation. There is no study on whether proanthocyanidin could protect against arsenic-induced respiratory inflammatory damage. The aim of the study is to examine the anti-inflammatory effects of proanthocyanidin and the molecular mechanisms in vivo and in vitro. BEAS-2B cells were treated with As2O3, grape seed proanthocyanidin extract (GSPE), and/or BAY 11–7082. Kunming mice were treated with As2O3 and/or GSPE. p-IκB-α, IκB-α, IKKα/β, NF-κBp65, and NF-κBp50 were assessed by Western blot and qRT-PCR. Lung histology was examined. Arsenic affected the histology of the mouse lungs, but GSPE attenuated those effects. As2O3 increased cell apoptosis, which was reversed by GSPE. In cells and mouse lung tissue, arsenic increased the expression of IL-1β, IL-6, tumor necrosis factor-α, and C-reactive protein, and these effects were attenuated by GSPE. In cells and mouse lung tissue, arsenic enhanced the mRNA and protein levels of IKKα, IKKβ, NF-κBp65, and NF-κBp50, while the IκB-α levels were decreased compared with controls. IKKα, IKKβ, NF-κBp65, and NF-κBp50 mRNA and protein levels in the As2O3+GSPE groups were lower and IκB-α levels were higher than that in the arsenic groups. Arsenic-activated NF-κB signaling induced inflammatory damage through the upregulation of pro-inflammatory cytokines and downregulation of anti-inflammatory cytokines. GSPE plays a beneficial role against arsenic-induced inflammatory damage through, at least in part, the suppression of the arsenic-induced NF-κB signaling pathway.
Impact statement
Arsenic-induced respiratory inflammatory damage is an important occupational hazard in many areas of the world, particularly in underdeveloped and developing countries. Effective treatments are lacking and expensive. Therefore, the aim of the study was to examine the anti-inflammatory effects of proanthocyanidin (PC) and the molecular mechanisms in vivo and in vitro. The present study showed that PC extracted from grape seed could attenuate the lung damage in a mouse model of arsenic poisoning. The effects were observed at the level of lung histology and inflammasome expression. This study suggests that a natural compound is effective in mitigating the toxic effects of arsenic in the lungs, providing an inexpensive and more readily accessible method for treating arsenic exposure in some parts of the world.
Keywords: Arsenic, lung injury, nuclear factor κB, proanthocyanidin
Introduction
Exposure to arsenic can cause significant respiratory damage.1 Workers exposed to arsenic by inhaling dust and particles suffer from faucitis and bronchitis.2 The acute exposure to arsenic also leads to respiratory syndrome.3 Dutta et al.4 assessed the sputum samples from 267 Indian women exposed to arsenic through drinking water and found that the number of inflammatory cells and the levels of tumor necrosis factor (TNF)-α, interleukin (IL)-8, IL-6, IL-12, and C-reactive protein (CRP) were significantly increased. Exposure to arsenic can affect the immune system by altering immune-related gene expression and cytokine secretion by lymphocytes.5 In addition, a number of hormones are dysregulated, including retinoic acid, thyroid hormones, and estrogen receptors.6 Diseases associated with low-grade inflammation (such as type 2 diabetes mellitus and chronic kidney disease) are also associated with chronic exposure to arsenic.7 Many animal models are available for studying the effects of arsenic,8 as well as cell lines.9 Among these, the BEAS-2B cell line has been shown to be an appropriate in vitro model to study the effects of heavy metals on human bronchial epithelium.10
The nuclear factor κB (NF-κB) is a protein complex that is central to transcription control of inflammatory genes, cytokine production, and cell survival. NF-κB is found in all animal cell types and is involved in cellular responses to stress, cytokines, free radicals, heavy metals, radiations, and microbe antigens.11 Incorrect NF-κB regulation is associated with cancer, inflammatory diseases, autoimmune diseases, and incorrect immune development.11 NF-κB plays a crucial role in arsenic-induced damage.12 Arsenic induces the activity of IKK13 and promotes the degradation of IκB.14 The heterodimers of NF-κBp65 and NF-κBp50 are dissociated15 and combine with the DNA binding site of κB.16 The downstream genes related to inflammation are then activated, promoting the expression of inflammatory factors that contribute to inflammatory damage.17
Proanthocyanidin (PC) is a polyphenolic compound that is present at high concentration in the nucleus, pericarp, and seed of grapes.18 PC has beneficial features such as free radical elimination, anti-inflammatory, and anti-oxidation.19–21 A previous study showed that PC prevented endothelial dysfunction by inhibiting NF-κB.19 Limtrakul et al.21 showed that PC inhibited inflammation through the suppression of the AP-1, NF-κB, and MAPK pathways in Raw 264.7 macrophages. PC has been shown to protect against lung inflammation and remodeling in mouse models of lung inflammation through downregulation of inflammatory cytokines.20 Nevertheless, to the best of our knowledge, there is no study on whether PC could protect against arsenic-induced respiratory inflammatory damage and whether the molecular mechanism of the eventual protective effect could be associated with NF-κB signaling.
BAY 11–7082 is a NF-κB inhibitor that has been shown to inhibit the NLRP3 inflammasome and to decrease the pro-inflammatory cytokines IL-1β and IL-18 that participate in chronic inflammatory diseases.22 Lappas et al.23 showed that BAY 11–7082 reduced IL-6, Il-8, and TNF-α secretion by adipocytes. In cancer cells, NF-κB is involved in cell survival, and BAY 11–7082 has been shown to decrease the survival of those cells by inhibiting NF-κB.24 BAY 11–7082 has been shown to decrease inflammatory markers in cell lines treated with arsenic,25 indicating the usefulness of BAY 11–7082 to study the effects of arsenic.
Therefore, the present study examined the anti-inflammatory effects of PC and the molecular mechanisms in vivo and in vitro. The results could provide a reference for the treatment of arsenic poisoning and the development and utilization of PC.
Materials and methods
Cell culture and treatment
Human bronchial epithelial cells (BEAS-2B cells) were obtained from Shanghai FuHeng Biology Co. (Shanghai, China). The BEAS-2B cells were cultured in Dulbecco's modified eagle medium (DMEM; Hyclone, Thermo Fisher Scientific, Waltham, MA, USA), 10% fetal bovine serum (FBS; Hyclone, Thermo Fisher Scientific, Waltham, MA, USA), and penicillin–streptomycin mixture (Hyclone, Thermo Fisher Scientific, Waltham, MA, USA) at 37°C under 5% CO2. The cells were treated with As2O3 (0, 5, 10, or 20 μmol/L in 4 M NaOH solution) (#890314; Beijing Chemical Plant, Beijing, China) and grape seed proanthocyanidin extract (GSPE; 0, 25, and 50 mg/L in deionized water; Tianjin Jianfeng Natural Plants Company, Tianjin, China; >95.0% purity) for 24 or 48 h, according to a previous study.26 BAY 11–7082 was bought from Abcam (Cambridge, United Kingdom; ab141228; >99% purity) and used at 50 mg/L in 0.1% dimethyl sulfoxide (DMSO) (Sigma, St Louis, MO, USA).
MTT assay
BEAS-2B cells in the logarithmic growth phase were inoculated in 96-well culture plates at 5000 cells/well in a total volume of 200 μL/well. All experiments were performed in triplicates (three parallel wells). MTT (Nanjing Jiancheng Inc., Nanjing, China) and DMSO (Sigma, St Louis, MI, USA) were used to detect the viability of the cells using a microplate spectrophotometer to measure the 490-nm OD (reference OD of 620 nm) in each well.27
Flow cytometry
BEAS-2B cells in the logarithmic growth phase were cultured; 1–3 × 106 cells were collected, resuspended in 500 μL of apoptosis positive control solution, and incubated on ice for 30 min. Cold binding buffer (1×; 1.0 mL) was added. The solution was divided equally into three: one was the blank control and the other two were staining tubes. Annexin V-FITC (5 µL; Tianjin Sungene Biotech Co., Ltd, Tianjin, China; AO2001-02P) was added into the two single staining tubes and incubated for 5 min with lucifuge. The samples were analyzed by flow cytometry to determine the apoptosis rates.28
Determination of reactive oxygen species and lipid peroxide
The Coomassie brilliant blue kit (A045-2, Nanjing Jiancheng Inc., Nanjing, China) was used to detect the total protein. The reactive oxygen species (ROS) kit (E004, Nanjing Jiancheng Inc., Nanjing, China) and lipid peroxide (LPO) kit (A106, Nanjing Jiancheng Inc., Nanjing, China) were used to measure the levels of ROS and LPO.29
Determination of inflammatory cytokines
ELISA kits were used to measure the levels of inflammatory cytokines in cell homogenates. IL-1β (L151112469, Cloud-Clone Corp, Wuhan, China), IL-6 (L151124780, Cloud-Clone Corp, Wuhan, China), IL-10 (L151109273, Cloud-Clone Corp, Wuhan, China), CRP (L151112436, Cloud-Clone Corp, Wuhan, China), and TNF-α (151124811, Cloud-Clone Corp, Wuhan, China) were detected. A microplate reader was used to read the plates at 450 nm.30
Western blot
The protein expression levels of the NF-κB signaling pathway (p-IκB-α (9246; Cell Signaling, Danvers, MA, USA), IκB-α (4814; Cell Signaling, Danvers, MA, USA), IKKα/β (ab178870; Abcam, Cambridge, MA, USA), NF-κBp65 (D14E12; Cell Signaling, Danvers, MA, USA), and NF-κBp50 (D4P4D; Cell Signaling, Danvers, MA, USA)) were detected by Western blot, as previously described.31 β-actin (TA-09), HRP-labeled goat anti-rabbit IgG (H + L) (ZB-2301), and HRP-labeled goat anti-mouse IgG (H + L) (ZB-2305) were bought from ZSGB-BIO Inc. (Beijing, China). β-actin was used as a loading control.
Quantitative real-time PCR
According to Zhao and Zhang,32 real-time PCR was used to detect the mRNA expression of IκB-α, IKKα, IKKβ, NF-κBp65, and NF-κBp50 from the cDNA samples. The primers were designed using Primer Premier 5.0 (Supplementary Table 1).
Animals
Forty healthy male Kunming mice were purchased from the Xinjiang Medical University Experimental Animal Center (license XK (Xin) 2011–00040). The mice were housed four/cage. Feed and water were provided ad libitum. A 2 × 2 factorial design protocol was designed using two factors and two levels: As2O3 (0 and 4 mg/kg) and GSPE (0 and 400 mg/kg), resulting in the control group, As2O3 group, GSPE group, and As2O3+GSPE group. There were 10 mice/group, randomly divided into each group with stratification for body weight. The control group was treated with 0.9% normal saline; the As2O3 group was administrated with 4 mg/kg As2O3; the GSPE group received 400 mg/kg GSPE; and the As2O3+GSPE received 4 mg/kg As2O3 and 400 mg/kg GSPE.
All groups were treated by intragastric administration for five weeks. The mice could eat and drink freely during the intervention. Based on the dose of 4.0 mg/kg and the total volume of the stomach is <20 mL/kg, the solution of As2O3 was 0.2 mg/mL, in water adjusted to be slightly alkaline, as previously described.33 For GSPE, 4 g was dissolved in 100 mL of deionized water.
Lung tissue homogenate
Lung tissue homogenate was prepared as previously described.34
ROS and LPO in lung tissue
ROS and LPO were measured in lung tissue homogenate as previously described.29
Inflammatory factors in the lung tissue homogenate
IL-1β (L151214485), IL-6 (L151210437), IL-10 (L151221646), CRP (L151110355), and TNF-α (151102081) ELISA kits were bought from Cloud-Clone Corp. (Wuhan, China), and used as previously described.30
Western blot analysis of lung tissue
Western blot was performed as previously described31 for the detection of p-IκB-α (9246; Cell Signaling, Danvers, MA, USA), IκB-α (4814; Cell Signaling, Danvers, MA, USA), IKKα/β (ab178870; Abcam, Cambridge, MA, USA), NF-κBp65 (D14E12; Cell Signaling, Danvers, MA, USA), and NF-κBp50 (D4P4D; Cell Signaling, Danvers, MA, USA). β-actin was used as a loading control.
Quantitative real-time PCR analysis of lung tissue
Lung tissue (100 mg) was grinded in liquid nitrogen, and added into 1 mL of TRIzol (Invitrogen Inc., Carlsbad, CA, USA). After 5 min, 200 μL chloroform was added and vortexed for 15 min. The solution was centrifuged for 15 min at 4°C and 12,000 ×g. The supernatant was collected for measuring the mRNA expression according to Zhao and Zhang.32
H&E staining of lung tissue
After sacrifice, the lungs were collected and part of the lung tissue was immersed in 10% formaldehyde for 24 h. The fixed lung tissue was embedded in paraffin and sectioned at 5 μm. The pathological changes of lung were observed after hematoxylin-eosin (H&E) staining.
Statistical analysis
The data were analyzed using SPSS 17.0 (Inc., Chicago, IL, USA). The data were expressed as means ± standard deviation. The factorial analysis of variance (4 × 3 × 2 × 2) was used to display the main effects of As2O3, GSPE, BAY 11–7082, and time in vitro. The 2 × 2 factorial analysis was used to compare the main effect of As2O3 and GSPE in vivo. The interaction of As2O3 and GSPE was analyzed by the general linear model. The multiple comparisons among groups were performed using the Bonferroni test. P < 0.05 was regarded as statistically significant.
Results
Cell viability
A previous study showed that as the dosage of As2O3 increased, cell viability gradually decreased (from 100% to 65.3%) after 24 h.35 A similar trend was also seen after 48 h (from 100% to 61.4%). When the same cells were treated with 50 mg/L GSPE, cell viability was 83.4% and 80.4%, respectively. When we applied BAY 11–7082 and GSPE at the same time, cell viability was 94.2% and 91.4%, respectively.
Effects of As2O3 and GSPE on apoptosis by flow cytometry
When cells were treated with As2O3 for 24 h, the apoptotic rate was increased (from 1.7% to 20.6%) and with increasing dose (Table 1, Figure 1(a) to (c)). For 48 h, the apoptotic rate was increased from 1.1% to 30.4%. After the addition of GSPE and BAY 11–7082, the apoptotic rate was decreased (Table 1, Figure 1(e) and (f)).
Table 1.
Effects of As2O3 and GSPE on apoptosis by flow cytometry.
| Time | Dose of GSPE (mg/L) | Dose of As2O3 |
||||
|---|---|---|---|---|---|---|
| 0 µmol/L (%) | 5 µmol/L (%) | 10 µmol/L (%) | 20 µmol/L (%) | |||
| Without BAY 11-7082 | 24 h | 0 | 1.7 | 7.2 | 20.1 | 20.6 |
| 25 | 1.4 | 3.0 | 10.8 | 14.6 | ||
| 50 | 2.3 | 1.8 | 7.2 | 12.3 | ||
| 48 h | 0 | 1.1 | 9.1 | 20.2 | 30.4 | |
| 25 | 1.4 | 5.5 | 11.2 | 16.2 | ||
| 50 | 2.0 | 1.7 | 7.8 | 13.0 | ||
| With BAY 11-7082 | 24 h | 0 | 1.4 | 2.7 | 4.0 | 10.8 |
| 25 | 1.7 | 2.3 | 4.2 | 6.3 | ||
| 50 | 1.3 | 2.7 | 2.6 | 5.1 | ||
| 48 h | 0 | 1.1 | 2.6 | 8.7 | 14.9 | |
| 25 | 0.9 | 3.2 | 6.7 | 11.8 | ||
| 50 | 1.4 | 3.1 | 4.2 | 10.0 | ||
GSPE: grape seed proanthocyanidin extract.
Figure 1.
Effects of As2O3 and GSPE on apoptosis by flow cytometry. Apoptotic cells were quantified by flow cytometry. Q2 shows the apoptotic rate. (a) Blank control group (24 h), (b) 5 μmol/L As2O3 group (24 h), (c) 20 μmol/L As2O3 group (24 h), (d) 50 mg/L GSPE group (48 h), (e) 5 μmol/L As2O3 + 50 mg/L GSPE group (24 h), (f) 5 μmol/L As2O3 + 50mg/L BAY 11–7082 group (24 h). (A color version of this figure is available in the online journal.)
GSPE: grape seed proanthocyanidin extract.
Effects of As2O3, GSPE, and BAY 11–7082 on LPO and ROS in BEAS-2B cells
The concentrations of ROS and LPO decreased with the increasing GSPE concentration. A decreasing trend was shown in ROS and LPO when BAY 11–7082 was applied (Figure 2).
Figure 2.
Effects of As2O3, GSPE, and BAY 11–7082 on LPO and ROS of BEAS-2B cells. The BEAS-2B cells were treated with As2O3 (0, 5, 10, or 20 μmol/L) (n = 3) and/or GSPE (0, 25, or 50 mg/L) (n = 3), all in combination with BAY 11–7082 (50 μmol/L). Values are expressed as means ± SD. aP < 0.05, compared with blank control group; bP < 0.05, compared with arsenic group (5 μmol/L); cP < 0.05, compared with arsenic group (10 μmol/L); dP < 0.05, compared with arsenic group (20 μmol/L).
GSPE: grape seed proanthocyanidin extract; LPO: lipid peroxide.
Effects of As2O3, GSPE and BAY 11–7082 on inflammatory cytokines of BEAS-2B cells
Exposure of BEAS-2B cells to arsenic for 24 and 48 h increased the expression of IL-1β, IL-6, TNF-α, and CRP in a dose-dependent manner while decreased IL-10 expression (Figure 3(a) to (j)). On the other hand, the levels of IL-1β, IL-6, TNF-α, and CRP were attenuated, and IL-10 was enhanced when different concentrations of GSPE and BAY 11–7082 were used (Figure 4(a) to (j)).
Figure 3.
Effects of As2O3, GSPE on inflammatory cytokines of in BEAS-2B cells. The BEAS-2B cells were treated with As2O3 (0, 5, 10, or 20 μmol/L) (n = 3). And GSPE (0, 25, or 50 mg/L) (n = 3) was used to antagonize the effect of arsenic. After 24 h or 48 h, the cells were harvested to measure the expression of inflammatory cytokines. (a, f) IL-1β; (B, G) IL-6; (c, h) IL-10; (d, i) TNF-α; and (e, j) CRP. Values are expressed as means ± SD. aP < 0.05, compared with the blank control group; bP < 0.05, compared with the arsenic group (5 μmol/L); cP < 0.05, compared with the arsenic group (10 μmol/L); dP < 0.05, compared with the arsenic group (20 μmol/L).
CRP: C-reactive protein; TNF-α: tumor necrosis factor-alpha; IL: interleukin; GSPE: grape seed proanthocyanidin extract.
Figure 4.
Effects of As2O3, GSPE, and BAY 11–7082 on inflammatory cytokines of in BEAS-2B cells. The BEAS-2B cells were treated with As2O3 (0, 5, 10, or 20 μmol/L), GSPE (0, 25, or 50 mg/L), and BAY 11–7082 (n = 3) was used to antagonize the effect of arsenic. After 24 h or 48h, the cells were harvested to measure the expression of inflammatory cytokines. (a, f) IL-1β, (b, g) IL-6, (c, h) IL-10, (d, i) TNF-α, and (e, j) CRP. Values are expressed as means ± SD. aP < 0.05, compared with the blank control group; bP < 0.05, compared with the arsenic group (5 μmol/L); cP < 0.05, compared with the arsenic group (10 μmol/L); dP < 0.05, compared with the arsenic group (20 μmol/L).
CRP: C-reactive protein; TNF-α: tumor necrosis factor-alpha; IL: interleukin; GSPE: grape seed proanthocyanidin extract.
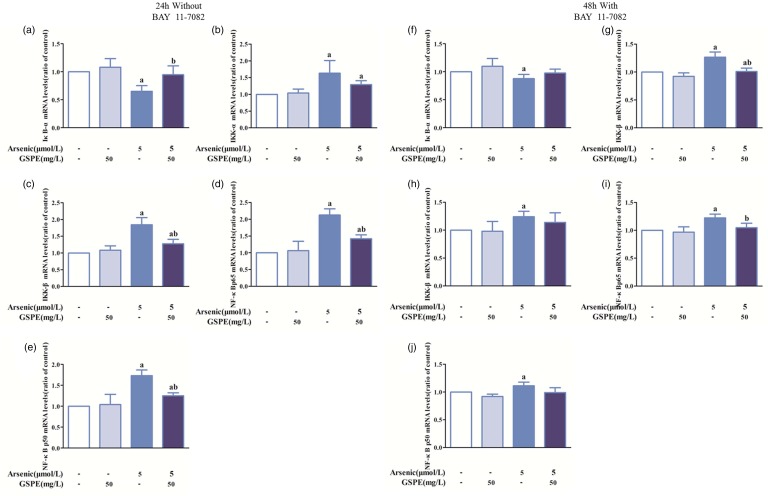
Effects of As2O3, GSPE, and BAY 11–7082 on mRNA expression of IκB-α, IKKα, IKKβ, NF-κBp65, and NF-κBp50 in BEAS-2B cells
Based on the results of cell apoptosis, viability, and inflammatory cytokines, we selected 5 μmol/L of As2O3 for exposure, 50 mg/L of GSPE, and 50 mg/L of BAY 11–7082 for intervention and 24 h for time. Arsenic treatment significantly enhanced the mRNA levels of IKKα, IKKβ, NF-κBp65, and NF-κBp50, while the IκB-α mRNA levels were decreased significantly compared with control. IKKα, IKKβ, NF-κBp65, and NF-κBp50 mRNA levels in the As2O3+GSPE group were lower and IκB-α mRNA levels were higher than that in the arsenic group (Figure 5(a) to (e)). Similar effects were also observed with BAY 11–7082 (Figure 5(f) to (j)).
Figure 5.
Effects of As2O3, GSPE, and BAY 11–7082 on the mRNA expression of IκB-α, IKKα, IKKβ, NF-κBp65, and NF-κBp50 in BEAS-2B cells. The BEAS-2B cells were treated with 5 μmol/L As2O3 (n = 10) and GSPE (50 mg/L) and BAY 11–7082 (50 mg/L) were used to antagonize the effects of arsenic. The relative mRNA levels were determined by RT-PCR using gene-specific primers. Values are expressed as means ± SD. aP < 0.05, compared with the blank control group; bP < 0.05, compared with the arsenic group. (A color version of this figure is available in the online journal.)
GSPE: grape seed proanthocyanidin extract.
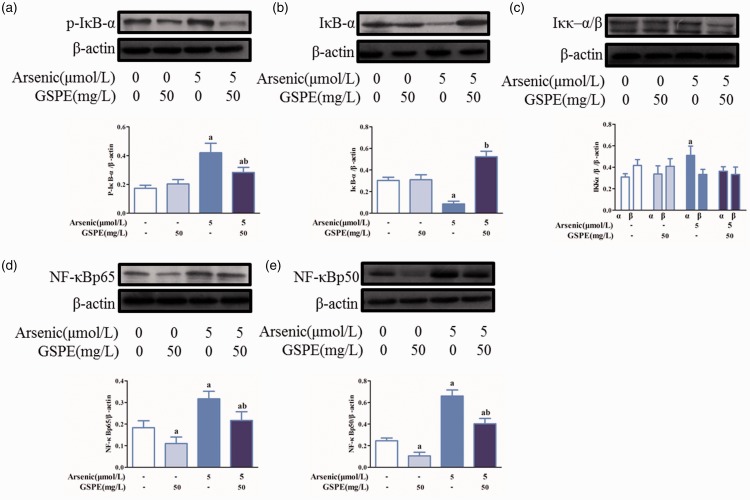
Effects of As2O3, GSPE, and BAY 11–7082 on the protein expression of p-IκB-α, IκB-α, IKKα/β, NF-κBp65, and NF-κBp50 in BEAS-2B cells
Arsenic increased the levels of p-IκB-α, IKKα, NF-κBp65, and NF-κBp50, while IκB-α levels were decreased significantly as compared with control (Figure 6(a) to (e)). Treatment with GSPE weakened the expression of p-IκB-α, IKKα, NF-κBp65, and NF-κBp50, and elevated IκB-α expression compared with the arsenic group (Figure 6(a) to (e)). In comparison with the arsenic group, BAY 11–7082 attenuated the levels of P-IκB-α, IKKα/β, NF-κBp65, and NF-κBp50, and increased IκB-α level (Figure 7(a) to (e)).
Figure 6.
Effects of As2O3, GSPE on the protein expression of p-IκB-α, IκB-α, IKKα/β, NF-κBp65, and NF-κBp50 in BEAS-2B cells. The BEAS-2B cells were treated with 5 μmol/L As2O3 (n = 3). GSPE (50 mg/L) was used to antagonize the effect of arsenic. The expression of the various proteins was detected by Western blot. The levels of p-IκB-α (a), IκB-α (b), IKKα/β (c), NF-κBp65 (d), and NF-κBp50 (e) (without BAY 11–7082), respectively. The Image J software was used to measure the gray value of each blot and β-actin was used as a loading control. Values are expressed as means ± SD. aP < 0.05, compared with the blank control group; bP < 0.05, compared with the arsenic group. (A color version of this figure is available in the online journal.)
GSPE: grape seed proanthocyanidin extract.
Figure 7.
Effects of As2O3, GSPE, and BAY 11–7082 on the protein expression of p-IκB-α, IκB-α, IKKα/β, NF-κBp65, and NF-κBp50 in BEAS-2B cells. The BEAS-2B cells were treated with 5 μmol/L As2O3 (n = 3). GSPE (50 mg/L) was used to antagonize the effect of arsenic. The expression of the various proteins was detected by Western blot. The levels of p-IκB-α (a), IκB-α (b), IKKα/β (c), NF-κBp65 (d), and NF-κBp50 (e) (with BAY 11–7082), respectively. The Image J software was used to measure the gray value of each blot and β-actin was used as a loading control. Values are expressed as means ± SD. aP < 0.05, compared with the blank control group; bP < 0.05, compared with the arsenic group. (A color version of this figure is available in the online journal.)
GSPE: grape seed proanthocyanidin extract.
Effects of arsenic and GSPE on lung histology
Lung histology in mice showed no pathological changes in the lung tissue of the control and GSPE groups (Figure 8(a) and (b)). The alveolar septa of the arsenic group were obviously widened, with capillary congestion and inflammatory cell invasion (Figure 8(c)). In the presence of GSPE, compared with the arsenic group, the alveolar septa were narrowed, capillary congestion was decreased, and the number of inflammatory cell invasion was diminished (Figure 8(d)).
Figure 8.
Representative photomicrographs of H&E stained section of lung after treatment with arsenic and GSPE. (a) Control group, (b) GSPE group, (c) As2O3 group, (d) As2O3+GSPE group (×200). (A color version of this figure is available in the online journal.)
GSPE: grape seed proanthocyanidin extract.
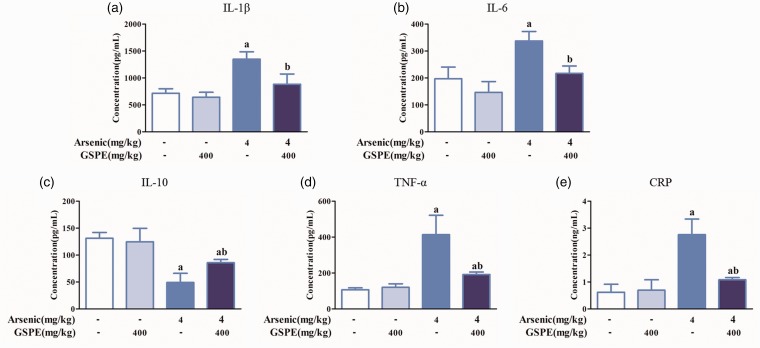
Effects of GSPE on inflammatory cytokines in lung tissue induced by arsenic in mice
Arsenic elevated the levels of IL-1β, IL-6, TNF-α, and CRP, and reduced IL-10 compared with controls. Compared with the arsenic group, IL-1β, IL-6, TNF-α, and CRP levels were decreased in the As2O3+GSPE group, while the IL-10 levels were increased (Figure 9(a) to (e)).
Figure 9.
Effects of arsenic and GSPE on inflammatory cytokines expression in lung tissue induced by arsenic in mice. Mice were exposed to As2O3 (4 mg/kg) (n = 10); 400 mg/kg GSPE was used to antagonize the effects of arsenic. Levels of IL-1β (a), IL-6 (b), IL-10 (c), TNF-α (d), and CRP (e), respectively. Values are expressed as means ± SD. aP < 0.05, compared with the blank control group; bP < 0.05, compared with the arsenic group. (A color version of this figure is available in the online journal.)
GSPE: grape seed proanthocyanidin extract.
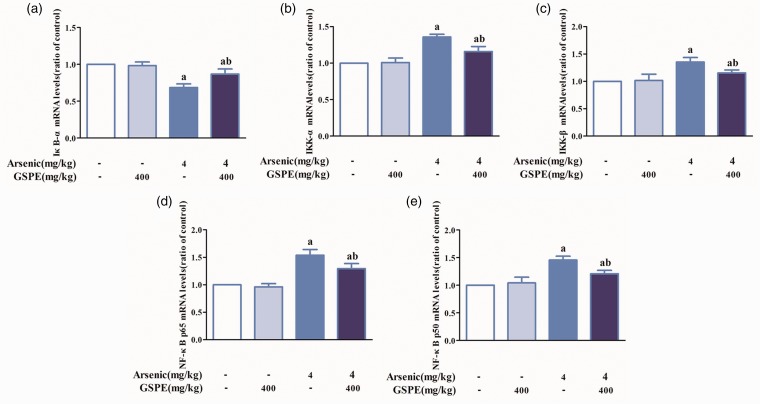
Effects of arsenic and GSPE on the mRNA expression of IκB-α, IKKα, IKKβ, NF-κBp65, and NF-κBp50 in the lung tissues of mice
Arsenic decreased the mRNA levels of IκB-α, while increased the mRNA levels of IKKα, IKKβ, NF-κBp65, and NF-κBp50 compared with controls. Compared with the arsenic group, the mRNA levels of IκB-α in As2O3 + GSPE group were elevated and the mRNA levels of IKKα, IKKβ, NF-κBp65, and NF-κBp50 attenuated (Figure 10(a) to (e)).
Figure 10.
Effects of arsenic and GSPE on mRNA expression of IκB-α, IKKα, IKKβ, NF-κBp65, and NF-κBp50 in lung tissue induced by arsenic in mice. Mice were exposed to As2O3 (4 mg/kg) (n = 10); 400 mg/kg GSPE was used to antagonize the effects of arsenic. The expression of each mRNA in the control, GSPE, AsO3, and As2O3+GSPE groups was detected by RT-PCR. mRNA levels of IκB-α (a), IKKα (b), IKKβ (c), NF-κBp65 (d), and NF-κBp50 (E), respectively. Values are expressed as means ± SD. aP < 0.05, compared with the blank control group; bP < 0.05, compared with the arsenic group. (A color version of this figure is available in the online journal.)
GSPE: grape seed proanthocyanidin extract.
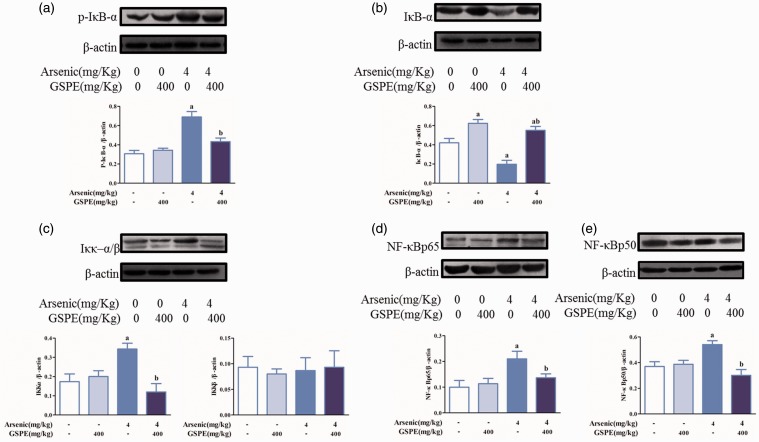
Effects of GSPE on the protein expression of P-IκB-α, IκB-α, IKKα/β, NF-κBp65, and NF-κBp50 in lung tissues induced by arsenic
Higher levels of P-IκB-α, IKKα/β, NF-κBp65, and NF-κBp50, and lower levels of IκB-α in the arsenic group were observed compared with the control group. Lower level of P-IκB-α, IKKα/β, NF-κBp65, and NF-κBp50, and higher level of IκB-α in the As2O3+GSPE group were observed compared with the arsenic group (Figure 11(a) to (e)).
Figure 11.
Effects of GSPE on the protein expression of p-IκB-α, IκB-α, IKKα/β, NF-κBp65, and NF-κBp50 in lung tissues induced by arsenic. Mice were exposed to As2O3 (4 mg/kg) (n = 10); 400 mg/kg GSPE was used to antagonize the effects of arsenic. The expression of each protein in the control, GSPE, AsO3, and As2O3+GSPE groups was detected by Western blot. Protein levels of p-IκB-α (a), IκB-α (b), IKKα/β (c), NF-κBp65 (d), and NF-κBp50 (E), respectively. The Image J software was used to measure the gray value of each blot and β-actin was used as a loading control. Values are expressed as means ± SD. aP < 0.05, compared with the blank control group; bP < 0.05, compared with the arsenic group. (A color version of this figure is available in the online journal.)
GSPE: grape seed proanthocyanidin extract.
Discussion
PC has beneficial features such as free radical scavenging, anti-inflammation, and anti-oxidation. There is no study on whether PC could protect against arsenic-induced respiratory inflammatory damage. Therefore, the aim of the present study was to examine the anti-inflammatory effects of PC and the molecular mechanisms in vivo and in vitro. The results suggest that arsenic induced damage in lung cells and mouse lungs. The NF-κB signaling pathway and inflammatory changes were involved in these effects. GSPE attenuated these changes in vitro and in vivo.
Arsenic is widely distributed in air, food, and water.1,36 Arsenic exposure, through ingestion or contact, can cause toxic damage to multiple organs.37 Arsenic can promote the expression of IL-6, IL-8, and TNF-α, and reduce IL-10 expression, thereby resulting in inflammation and decreased cell viability.4 In the present study, in vitro, arsenic induced cell damage, since decreased cell viability and increased cell apoptosis were observed when BEAS-2B cells were exposed to arsenic. This damage could be alleviated by GSPE or BAY 11–7082 treatment, as previously observed.35 Arsenic activated the NF-κB signaling pathway by increasing the mRNA levels of IKKα, IKKβ, NF-κBp65, and NF-κBp50, and protein levels of P-IκB-α, IKKα/β, NF-κBp65, and NF-κBp50 as well as by decreasing the mRNA and protein expression of IκB-α. These changes led to the altered expression of downstream factors and inflammatory damage of BEAS-2B cells following upregulation of the expression of IL-1β, IL-6, TNF-α, CRP, ROS, and LPO, and downregulation of IL-10. Nevertheless, this arsenic-induced inflammation seemed to be antagonized by GSPE since it decreases the levels of IL-1β, IL-6, TNF-α, CRP, ROS, and LPO, and increases the IL-10 levels, which could result from the suppression of the NF-κB signaling. These effects of arsenic and GSPE were also observed in vivo. A previous study showed that GSPE (400 mg/kg) by intragastric administration, for five weeks, did not alter body weight, lung weight, and viscera coefficient of normal Kunming mice. On the other hand, decreases in body weight and increases in lung weight and viscera coefficient were found after five weeks of intragastric administration of As2O3 (4 mg/kg) in normal mice. GSPE in arsenic-exposed mice for five weeks reduced the lung weight and viscera coefficient.35 Taken together, these results strongly suggest that arsenic activates NF-κB signaling and promotes the expression of downstream inflammatory cytokines, thereby causing inflammatory damage. These effects could be blocked, at least in part, by GSPE.
It has been reported that the activation of NF-κB,38 MAPK,33 Nrf2,39 and other signaling pathways may account for the arsenic-induced inflammatory damage. It is believed that NF-κB signaling pathway plays a prominent role in inflammatory damage, as suggested by the present study. One of the possible mechanisms for these effects was that arsenic promoted the phosphorylation of IκB-α, enhanced the ubiquitination and degradation of IκB-α,40 accelerated the dissociation of the complex of IκB-α, NF-κBp65, and NF-κBp50, released the p50-p65 dimers,41 and then allowed the dimers to become free to translocate to the nucleus from the cytoplasm, to combine with the DNA binding site of κB in order to activate the NF-κB signaling pathway and the expression of inflammatory cytokines.42 It is also likely that arsenic increased the activity and expression of IKK and facilitated the dissociation of the complex, thereby activating the NF-κB signaling pathway.42 In addition, arsenic has been shown to boost the DNA-binding activity of NF-κBp65 and NF-κBp50 that had entered the nucleus.42 It had been reported that arsenic could enhance the expression of INF-γ, TNF-α, IL-6, VEGF, HO-1, ROS, and LPO43,44 and decrease the levels of IL-10 and IL-4.45 A previous study showed that when the BEAS-2B cells were treated with As2O3 for 24 h and 48 h, the levels of ROS and LPO were significantly increased in a dose-dependent manner.35 In contrast, the expression of ROS and LPO were decreased with the increasing GSPE concentration. A decreasing trend in the present study was shown in ROS and LPO when BAY 11–7082 was applied. A previous study showed that the levels of LPO and ROS in the GSPE group were similar with the control group. LPO and ROS were elevated when mice were exposed to As2O3 (4 mg/kg) and attenuated by GSPE.35 In the present study, arsenic caused inflammatory damage with elevated levels of IL-1β, IL-6, and TNF-α, and loss of IL-10 expression. An involved mechanism may be that IL-6 promoted the expression of CRP44 and the elevated TNF-α levels enhanced the content of ROS and LPO.46 Meanwhile, the secreted IL-1β could bind to its receptor on the cell membrane and further act on IKK, aggravating the degree of inflammatory damage.47
BAY 11–7082 is a known inhibitor of the NF-κB signaling pathway and can effectively inhibit the phosphorylation and degradation of IκB-α. In the present study, we investigated whether GSPE, which had been shown to have good effect on anti-inflammatory and scavenging free radicals,21 could diminish the arsenic-induced inflammatory damage by inhibiting NF-κB signaling. We found that GSPE restrained the NF-κB signaling pathway and alleviated the inflammatory damage caused by arsenic, as expected, in that GSPE decreased IKK expression, inhibited IκB-α phosphorylation, and blocked the formation of NF-κBp65 and NF-κBp50, thereby reducing the expression of IL-1β, IL-6, TNF-α, and CRP, and promoting IL-10 expression. Arsenic-induced inflammatory damage (alveolar morphology changes, lung congestion, and inflammatory cells invasion) was prevented by GSPE to a certain extent. The anti-inflammatory effect of GSPE had also been reported in other studies,20,35 and the beneficial effects of GSPE could be derived from the inhibition of the NF-κB signaling pathway.19 GSPE administration could antagonize the effects of arsenic by inhibiting arsenic-induced NF-κB activation, as supported by those previous studies. Supplementary Figure 1 summarizes the effects of arsenic and GSPE, as observed in the present study.
The present study found antagonistic effects of GSPE on NF-κB signaling pathway and inflammatory damage induced by arsenic. Nevertheless, PI3K and Akt have important regulative effects on NF-κB signaling.48 MAPK signaling pathways were also reported to modulate the damage induced by arsenic.49 In addition, arsenic-induced inflammatory damage might also be related to ERK, Stat3, and other signaling pathways.50 Future studies should explore the molecular mechanisms of multiple signaling pathways involved in inflammation and interactions with arsenic and GSPE. Future studies will also have to include a positive control, which was a limitation of the present study. The pathways involved in arsenic-induced damage and rescue by GSPE will also have to be studied using a variety of inhibitors and siRNAs.
Arsenic-activated NF-κB signaling induced inflammatory damage through the upregulation of pro-inflammatory cytokines and downregulation of anti-inflammatory cytokines. GSPE plays a beneficial role against arsenic-induced inflammatory damage through, at least in part, the suppression of the arsenic-induced NF-κB signaling pathway. Therefore, GSPE may represent a potential agent to prevent inflammatory damage induced by arsenic exposure.
Supplemental Material
Supplemental material, Supplemental Figure for Grape seed proanthocyanidin extract alleviates arsenic-induced lung damage through NF-κB signaling by Yunhua Hu, Meng Wei, Qiang Niu, Rulin Ma, Yu Li, Xianhua Wang, Gangling Feng, Shugang Li and Lijuan Pang in Experimental Biology and Medicine
Supplemental Material
Supplemental material, Supplemental Table for Grape seed proanthocyanidin extract alleviates arsenic-induced lung damage through NF-κB signaling by Yunhua Hu, Meng Wei, Qiang Niu, Rulin Ma, Yu Li, Xianhua Wang, Gangling Feng, Shugang Li and Lijuan Pang in Experimental Biology and Medicine
Authors’ contributions
YHH, MW contributed equally to this work. YHH, MW, SGL and LJP carried out the studies, participated in collecting data, and drafted the manuscript. QN, RLM, YL, XHW and GLF performed the statistical analysis and participated in its design. SGL and LJP helped to draft the manuscript. All authors read and approved the final version of the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 81760584, 81560517); the Key Areas of Science and Technology Research Project of Xinjiang Production and Construction Corps (No. 2014BA039 and No. 2015AG014); and the International Cooperative Project of Shihezi University (No. GJHZ201602).
References
- 1.Ramsey K. Arsenic and respiratory disease In: Flora SJS. (ed.) Handbook of arsenic toxicology. London: Academic Press, 2015, pp.45–491 [Google Scholar]
- 2.Saha JC, Dikshit AK, Bandyopadhyay M. A review of arsenic poisoning and its effects on human health. Crit Rev Environ Sci Technol 1999; 29:281–313 [Google Scholar]
- 3.Bolliger CT, van Zijl P, Louw JA. Multiple organ failure with the adult respiratory distress syndrome in homicidal arsenic poisoning. Respiration 1992; 59:57–61 [DOI] [PubMed] [Google Scholar]
- 4.Dutta K, Prasad P, Sinha D. Chronic low level arsenic exposure evokes inflammatory responses and DNA damage. Int J Hyg Environ Health 2015; 218:564–74 [DOI] [PubMed] [Google Scholar]
- 5.Morzadec C, Bouezzedine F, Macoch M, Fardel O, Vernhet L. Inorganic arsenic impairs proliferation and cytokine expression in human primary T lymphocytes. Toxicology 2012; 300:46–56 [DOI] [PubMed] [Google Scholar]
- 6.Barr FD, Krohmer LJ, Hamilton JW, Sheldon LA. Disruption of histone modification and CARM1 recruitment by arsenic represses transcription at glucocorticoid receptor-regulated promoters. PLoS One 2009; 4:e6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orr SE, Bridges CC. Chronic kidney disease and exposure to nephrotoxic metals. Int J Mol Sci 2017; 18:1–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Li C, Sun HJ, Juhasz AL, Luo J, Li HB, Ma LQ. Arsenic relative bioavailability in contaminated soils: comparison of animal models, dosing schemes, and biological end points. Environ Sci Technol 2016; 50:453–61 [DOI] [PubMed] [Google Scholar]
- 9.Tyler CR, Allan AM. The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr Environ Health Rep 2014; 1:132–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, Pratheeshkumar P, Budhraja A, Son YO, Kim D, Shi X. Role of reactive oxygen species in arsenic-induced transformation of human lung bronchial epithelial (BEAS-2B) cells. Biochem Biophys Res Commun 2015; 456:643–8[Mismatch] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol 2007; 8:49–62 [DOI] [PubMed] [Google Scholar]
- 12.Wei M, Liu J, Xu M, Rui D, Xu S, Feng G, Ding Y, Li S, Guo S. Divergent effects of arsenic on NF-kappaB signaling in different cells or tissues: a systematic review and meta-analysis. Int J Environ Res Public Health 2016; 13:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosimo E, McCaig AM, Carter-Brzezinski LJ, Wheadon H, Leach MT, Le Ster K, Berthou C, Durieu E, Oumata N, Galons H, Meijer L, Michie AM. Inhibition of NF-kappaB-mediated signaling by the cyclin-dependent kinase inhibitor CR8 overcomes prosurvival stimuli to induce apoptosis in chronic lymphocytic leukemia cells. Clin Cancer Res 2013; 19:2393–405 [DOI] [PubMed] [Google Scholar]
- 14.Meng F, Liu L, Chin PC, D'Mello SR. Akt is a downstream target of NF-kappa B. J Biol Chem 2002; 277:29674–80 [DOI] [PubMed] [Google Scholar]
- 15.Bouraoui Y, Ben Jemaa A, Rodriguez G, Ben Rais N, Fraile B, Paniagua R, Sellemi S, Royuela M, Oueslati R. Profile of NF-kappaBp(65/NFkappaBp50) among prostate specific antigen sera levels in prostatic pathologies. Pathol Biol (Paris) 2012; 60:301–5 [DOI] [PubMed] [Google Scholar]
- 16.Chenoweth DM, Poposki JA, Marques MA, Dervan PB. Programmable oligomers targeting 5′-GGGG-3′ in the minor groove of DNA and NF-kappaB binding inhibition. Bioorg Med Chem 2007; 15:759–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galao RP, Le Tortorec A, Pickering S, Kueck T, Neil SJ. Innate sensing of HIV-1 assembly by Tetherin induces NFkappaB-dependent proinflammatory responses. Cell Host Microbe 2012; 12:633–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchholz T, Melzig MF. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Med 2015; 81:771–83 [DOI] [PubMed] [Google Scholar]
- 19.Jiang Y, Li Y, Ding Y, Dai X, Ma X, Bao L, Zhang Z, Li Y. Grape seed proanthocyanidin extracts prevent high glucose-induced endothelia dysfunction via PKC and NF-kappaB inhibition. Biosci Biotechnol Biochem 2015; 79:1493–503 [DOI] [PubMed] [Google Scholar]
- 20.Lee T, Kwon HS, Bang BR, Lee YS, Park MY, Moon KA, Kim TB, Lee KY, Moon HB, Cho YS. Grape seed proanthocyanidin extract attenuates allergic inflammation in murine models of asthma. J Clin Immunol 2012; 32:1292–304 [DOI] [PubMed] [Google Scholar]
- 21.Limtrakul P, Yodkeeree S, Pitchakarn P, Punfa W. Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-kappaB pathways in Raw 264.7 macrophages. Nutr Res Pract 2016; 10:251–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, Meng R, Quong AA, Latz E, Scott CP, Alnemri ES. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem 2010; 285:9792–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lappas M, Yee K, Permezel M, Rice GE. Sulfasalazine and BAY 11-7082 interfere with the nuclear factor-kappa B and I kappa B kinase pathway to regulate the release of proinflammatory cytokines from human adipose tissue and skeletal muscle in vitro. Endocrinology 2005; 146:1491–7 [DOI] [PubMed] [Google Scholar]
- 24.Mori N, Yamada Y, Ikeda S, Yamasaki Y, Tsukasaki K, Tanaka Y, Tomonaga M, Yamamoto N, Fujii M. Bay 11-7082 inhibits transcription factor NF-kappaB and induces apoptosis of HTLV-I-infected T-cell lines and primary adult T-cell leukemia cells. Blood 2002; 100:1828–34 [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Kou MC, Weng CY, Hu LW, Wang YJ, Wu MJ. Arsenic modulates heme oxygenase-1, interleukin-6, and vascular endothelial growth factor expression in endothelial cells: roles of ROS, NF-kappaB, and MAPK pathways. Arch Toxicol 2012; 86:879–96 [DOI] [PubMed] [Google Scholar]
- 26.Debaisieux S, Encheva V, Chakravarty P, Snijders AP, Schiavo G. Analysis of signaling endosome composition and dynamics using SILAC in embryonic stem cell-derived neurons. Mol Cell Proteomics 2016; 15:542–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun CG, Zhuang J, Teng WJ, Wang Z, Du SS. PUMA gene transfection can enhance the sensitivity of epirubicin-induced apoptosis of MCF-7 breast cancer cells. Genet Mol Res 2015; 14:5742–9 [DOI] [PubMed] [Google Scholar]
- 28.Diaz D, Prieto A, Reyes E, Barcenilla H, Monserrat J, Alvarez-Mon M. Flow cytometry enumeration of apoptotic cancer cells by apoptotic rate. Methods Mol Biol. 2008; 414:23–33 [DOI] [PubMed] [Google Scholar]
- 29.Muthu M, NCDR, Kalist S, Prabu SM. Defensive role of silibinin against arsenic induced oxidative stress mediated dyslipidemia and neurotoxicity in rats. Int J Pharmacol Toxicol 2016; 4:78 [Google Scholar]
- 30.Ma L, Liang Y, Fang M, Guan Y, Si Y, Jiang F, Wang F. The cytokines (IFN-gamma, IL-2, IL-4, IL-10, IL-17) and Treg cytokine (TGF-beta1) levels in adults with immune thrombocytopenia. Pharmazie 2014; 69:694–7 [PubMed] [Google Scholar]
- 31.Maguire O, Collins C, O'Loughlin K, Miecznikowski J, Minderman H. Quantifying nuclear p65 as a parameter for NF-kappaB activation: Correlation between ImageStream cytometry, microscopy, and Western blot. Cytometry A 2011; 79:461–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao L, Zhang Y. miR-342-3p affects hepatocellular carcinoma cell proliferation via regulating NF-kappaB pathway. Biochem Biophys Res Commun 2015; 457:370–7 [DOI] [PubMed] [Google Scholar]
- 33.Li C, Xu J, Li F, Chaudhary SC, Weng Z, Wen J, Elmets CA, Ahsan H, Athar M. Unfolded protein response signaling and MAP kinase pathways underlie pathogenesis of arsenic-induced cutaneous inflammation. Cancer Prev Res (Phila) 2011; 4:2101–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhat S, Kartha VB, Rai L, Chidangil S. A comparison of protein profiles of cervical tissue homogenate, exfoliated cells from cervix and serum in normal and cervical malignancy conditions. J Chromatogr Sci 2015; 53:167–76 [DOI] [PubMed] [Google Scholar]
- 35.Wei M, Guo F, Rui D, Wang H, Feng G, Li S, Song G. Alleviation of arsenic-induced pulmonary oxidative damage by GSPE as shown during in vivo and in vitro experiments. Biol Trace Elem Res 2017; 183:80–91 [DOI] [PubMed] [Google Scholar]
- 36.Mayer JE, Goldman RH. Arsenic and skin cancer in the USA: the current evidence regarding arsenic-contaminated drinking water. Int J Dermatol 2016; 55:e585–e91 [DOI] [PubMed] [Google Scholar]
- 37.Zhong YX, Sun WC. Research progress on oxidative damage of liver and kidney exposed to arsenic. J Dalian Med Univ 2013; 35:77–80 [Google Scholar]
- 38.Fry RC, Navasumrit P, Valiathan C, Svensson JP, Hogan BJ, Luo M, Bhattacharya S, Kandjanapa K, Soontararuks S, Nookabkaew S, Mahidol C, Ruchirawat M, Samson LD. Activation of inflammation/NF-kappaB signaling in infants born to arsenic-exposed mothers. PLoS Genet 2007; 3:e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miltonprabu S, Muthumani M. Dimethoxycurcumin potentially protects arsenic induced oxidative hepatic injury, inflammation and apoptosis via Nrf2-Keap1 signaling in rats. Biomed Prevent Nutr 2014; 4:561–77 [Google Scholar]
- 40.Shukla S, Shankar E, Fu P, MacLennan GT, Gupta S. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by apigenin through IkappaBalpha and IKK pathway in TRAMP mice. PLoS One 2015; 10:e0138710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhongyi S, Sai Z, Chao L, Jiwei T. Effects of nuclear factor kappa B signaling pathway in human intervertebral disc degeneration. Spine (Phila Pa 1976) 2015; 40:224–32 [DOI] [PubMed] [Google Scholar]
- 42.Ghosh J, Das J, Manna P, Sil PC. Taurine prevents arsenic-induced cardiac oxidative stress and apoptotic damage: role of NF-kappa B, p38 and JNK MAPK pathway. Toxicol Appl Pharmacol 2009; 240:73–87 [DOI] [PubMed] [Google Scholar]
- 43.Tsubaki M, Takeda T, Kino T, Itoh T, Imano M, Tanabe G, Muraoka O, Satou T, Nishida S. Mangiferin suppresses CIA by suppressing the expression of TNF-alpha, IL-6, IL-1beta, and RANKL through inhibiting the activation of NF-kappaB and ERK1/2. Am J Transl Res 2015; 7:1371–81 [PMC free article] [PubMed] [Google Scholar]
- 44.Yin Y, Si X, Gao Y, Gao L, Wang J. The nuclear factor-kappaB correlates with increased expression of interleukin-6 and promotes progression of gastric carcinoma. Oncol Rep 2013; 29:34–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen S, Gao Z, Huang H. Effect of Gancao Xiexin decoction on expression of NF-κB and IL-10 in rats with ulcerative colitis. J Fujian Univ Traditional Chin Med 2014;1–17 [Google Scholar]
- 46.Frances DE, Ingaramo PL, Ronco MT. Diabetes, an inflammatory process: oxidative Stress and TNF-alpha involved in hepatic complication. J Biomed Sci Eng 2013; 6:645–53 [Google Scholar]
- 47.Tarantino N, Tinevez JY, Crowell EF, Boisson B, Henriques R, Mhlanga M, Agou F, Israel A, Laplantine E. TNF and IL-1 exhibit distinct ubiquitin requirements for inducing NEMO-IKK supramolecular structures. J Cell Biol 2014; 204:231–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang CM, Lee IT, Lin CC, Yang YL, Luo SF, Kou YR, Hsiao LD. Cigarette smoke extract induces COX-2 expression via a PKCalpha/c-Src/EGFR, PDGFR/PI3K/Akt/NF-kappaB pathway and p300 in tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2009; 297:L892–902 [DOI] [PubMed] [Google Scholar]
- 49.Liu D, Zhi D, Zhou T, Yu Q, Wan F, Bai Y, Li H. Realgar bioleaching solution is a less toxic arsenic agent in suppressing the Ras/MAPK pathway in Caenorhabditis elegans. Environ Toxicol Pharmacol 2013; 35:292–9 [DOI] [PubMed] [Google Scholar]
- 50.Meng-Dan LI, Liu JY, Wang C. Effects of arsenic on STAT3 m RNA expression in SV-HU-1 cells. J Environ Health 2015; 533:508–14 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Figure for Grape seed proanthocyanidin extract alleviates arsenic-induced lung damage through NF-κB signaling by Yunhua Hu, Meng Wei, Qiang Niu, Rulin Ma, Yu Li, Xianhua Wang, Gangling Feng, Shugang Li and Lijuan Pang in Experimental Biology and Medicine
Supplemental material, Supplemental Table for Grape seed proanthocyanidin extract alleviates arsenic-induced lung damage through NF-κB signaling by Yunhua Hu, Meng Wei, Qiang Niu, Rulin Ma, Yu Li, Xianhua Wang, Gangling Feng, Shugang Li and Lijuan Pang in Experimental Biology and Medicine