Abstract
Individuals with Parkinson’s disease (PD) suffer from motor and mental disturbances due to degeneration of dopaminergic and non-dopaminergic neuronal systems. Although they provide temporary symptom relief, current treatments fail to control motor and non-motor alterations or to arrest disease progression. Aiming to explore safety and possible motor and neuropsychological benefits of a novel strategy to improve the PD condition, a case series study was designed for brain grafting of human neural progenitor cells (NPCs) to a group of eight patients with moderate PD. A NPC line, expressing Oct-4 and Sox-2, was manufactured and characterized. Using stereotactic surgery, NPC suspensions were bilaterally injected into patients’ dorsal putamina. Cyclosporine A was given for 10 days prior to surgery and continued for 1 month thereafter. Neurological, neuropsychological, and brain imaging evaluations were performed pre-operatively, 1, 2, and 4 years post-surgery. Seven of eight patients have completed 4-year follow-up. The procedure proved to be safe, with no immune responses against the transplant, and no adverse effects. One year after cell grafting, all but one of the seven patients completing the study showed various degrees of motor improvement, and five of them showed better response to medication. PET imaging showed a trend toward enhanced midbrain dopaminergic activity. By their 4-year evaluation, improvements somewhat decreased but remained better than at baseline. Neuropsychological changes were minor, if at all. The intervention appears to be safe. At 4 years post-transplantation we report that undifferentiated NPCs can be delivered safely by stereotaxis to both putamina of patients with PD without causing adverse effects. In 6/7 patients in OFF condition improvement in UPDRS III was observed. PET functional scans suggest enhanced putaminal dopaminergic neurotransmission that could correlate with improved motor function, and better response to L-DOPA. Patients’ neuropsychological scores were unaffected by grafting. Trial Registration: Fetal derived stem cells for Parkinson’s disease https://doi.org/10.1186/ISRCTN39104513Reg#ISRCTN39104513
Keywords: Parkinson’s disease, human neural progenitor cells, stem cell transplantation, stereotactic surgery, PET molecular imaging
Introduction
Idiopathic Parkinson’s disease (PD) is a progressive, degenerative neurological disorder of the basal ganglia of unknown origin that affects pigmented dopamine-producing nerve cells in the substantia nigra pars compacta (SNpc) which regulate the excitability of striatal neurons, primarily those in the dorsolateral putamen1,2. When PD depletes pre-synaptic dopamine from nerve terminals in the nigrostriatal tract by ∼80% (that is ∼ 60% loss of dopaminergic neurons of the SNpc)2, post-synaptic dopaminergic receptors in the putamen and head of the caudate nucleus are not adequately stimulated. Consequently, uninhibited striatal neurons fire excessively and alter normal functioning of basal ganglia-thalamo-cortical circuits, leading to motor symptoms such as resting tremor, muscle rigidity, bradykinesia, impaired gait and postural instability, speech, and mask-like facial expression3. Loss of nigral dopaminergic neurons that send axons to the basal ganglia has also been related to non-motor symptoms present in Parkinson’s patients, such as dementia and cognitive impairment, depression, and anxiety4–6. Moreover, non-motor symptoms refractory to available treatments for dopamine deficiency in PD might also be attributed to non-dopaminergic neurotransmitter deficiencies within or outside the basal ganglia that are known to occur in PD such as striatum serotonin, acetylcholine, gamma aminobutyric acid, and noradrenaline, which would imply the requirement of non-dopaminergic drugs besides dopamine to treat PD5,7,8.
The standard pharmacological therapy to replace dopamine with levo-dihydroxyphenylalanine (L-DOPA), although initially effective, gradually produces tolerance, as do other dopamine-linked drugs. Together with the progressive course of the disease these drugs cause motor side effects (motor fluctuations, dyskinesias, and wearing-off phenomena)9,10 that can become as crippling as PD motor symptoms themselves. When PD becomes drug resistant, deep brain stimulation (DBS) in the subthalamic nucleus or globus pallidus11,12 is an alternative neurosurgical treatment available for PD. However, DBS is yet another symptomatic treatment of PD. Not until we know the events that trigger the degenerative processes of PD will it be possible to design a cure for the disease13.
Because current pharmacological treatment (L-DOPA) is unable to block the progression of the disease14, restorative approaches through brain grafting have been designed over the course of the last 30 years to improve PD symptomatology. Autologous adrenal medulla15,16, fetal brain tissue transplantation17–20, and injections of differentiated stem cells producing dopamine21 into the caudate or putamen of PD patients are among the attempted treatments. These approaches aimed to replace degenerated dopaminergic neurons, ameliorate motor function disturbances, and enhance cognitive abilities. However, all of the above treatments resulted in only moderate improvement that could not justify large surgical volume and related clinical risks and complications of these procedures. Moreover, it was shown that robust graft survival and normalized dopaminergic innervation did not result in clinical improvement in a PD patient22.
One of the reasons for this could be attributed to changes in the caudate nuclei of patients with advanced PD23 as well as unattended disturbances of non-dopaminergic neurotransmitter systems within or beyond the basal ganglia which have been linked to PD pathology7,24. An example of such disturbances is a 25% loss of serotonergic receptor 5-HT1A in the median raphe nucleus/midbrain that has been correlated with the severity of resting tremor25. Putaminal serotonin preservation has been related to L-DOPA-induced dyskinesias26. Decline in serotonin in the subthalamic nucleus is related to PD depression27, and acetylcholine down-regulation in the nucleus basalis of Meynert appears to play a significant role in cognition28, although the temporal relationship of damage to specific neurochemical systems is not well established2.
Therefore, we believe it is important to broaden the scope of PD cell therapy by grafting undifferentiated cells that might differentiate into various cell types at various diseased sites of PD brains and improve function in PD patients beyond dopamine. Undifferentiated stem cells are, by definition, more plastic than pre-differentiated ones and are able to tune their proliferation and vector of differentiation in response to micro-environmental cues29,30. These qualities of undifferentiated cells may allow them to address dopaminergic and non-dopaminergic aspects of the disease, as well as to avoid misdosing the patients. In addition, unlike undifferentiated cells of embryonic origin, neural progenitor cells (NPCs) do not produce teratomas in immunocompromised animals in the standard teratoma assay in vivo (in-house data).
PD is a multifactorial disease, where both genetic31,32 and non-genetic environmental factors33 are involved. The genetic architecture of PD is complex and not completely understood, and there are no adequate animal models of PD34. To indirectly address genetic component of PD’s pathogenesis we conducted efficacy testing of NPCs for treatment of cerebellar ataxia in the Spastic Han-Wistar Rat model of cerebellar ataxia35,36. Stereotactic injection of stem cells produced statistically significant improvement in behavioral scores and weight of the animals. Life span of the injected animals doubled. Histological and immunohistochemical examination showed incorporation of the stem cells into hosts’ brains as human mature Calbindin-positive neurons and glial cells. Successful injection of NPCs into a genetic model of cerebellar ataxia allows us to hypothesize that these undifferentiated cells have capabilities to correct pathology caused by gene mutations.
Here we have performed a surgical procedure to treat PD involving bilateral intraputaminal grafting of undifferentiated NPCs in a case series study. Our initial aim has been to explore the safety of the procedure and to identify possible neurological (motor function) and neuropsychological (non-motor symptoms) benefits the procedure might have induced. Patients’ response to grafting was assessed at 1, 2, and 4 years post-surgery using current functional standard neurological and neuropsychological tests, as well as advanced functional positron emission tomography (PET) imaging protocols with the following radiopharmaceuticals to initially assess only the dopaminergic neuronal pathway activity or integrity: [11C]raclopride (RAC) for endogenous dopamine availability; 6-[18F]fluoro-L-DOPA (FDOPA) for dopaminergic nerve terminal metabolic activity, and alpha-[11C] dihydrotetrabenazine (DTBZ) for nigrostriatal terminal integrity (PET examination was not performed at 4 years due to radiation dose safety concerns).
Materials and Methods
Design of the Study
This is a case series study (uncontrolled, longitudinal, and interventional), designed (authors I.M., O.K.) to test the safety and potential benefits of bilateral intraputaminal grafting (I.M., F.J., E.M., C.Z.) of in vitro expanded NPCs (O.K., A.K.) to control PD signs and symptoms. Eight patients with moderate PD were selected for this study. Individually they underwent baseline, 6 months, 1, 2, and 4 years post-surgery neurological (H.C.) and neuropsychological evaluations (F.O., G.G.), and PET imaging (baseline, 1 and 2 year post-operatively) (M.A.A.R., A.A.E.). Magnetic resonance (MR) images (R.C., neuroradiologist) were performed before surgery and 24 h, 6 months, and 1, 2, and 4 years post-surgery. Patients were tested for immune response to stem cells at baseline and 6 months post-surgery. The primary functional outcome of the intervention was patients’ post-surgery neurological performance. Post-surgery neuropsychological performance, MR and PET imaging, and immune response were secondary outcome measures. Patients were monitored for any adverse effects. This was a single-center study (T.V., scientific coordinator). The manuscript was prepared by I.M., O.K., R.E.F.B., and G.G.
Patients were permitted to adjust their L-DOPA medication dosage as to best control their symptoms, and were required to record the dosages in their diaries.
Participants
Inclusion criteria for this study were: (1) Diagnosis of idiopathic PD with tremor, rigidity, or hypokinesia as major symptoms, measured on the Unified Parkinson’s Disease Rating Scale (UPDRS)37, OFF and ON L-DOPA medication. (2) A 2- to 25-year history of PD with significant medical management or difficulty with medical management. (3) A definite response to L-DOPA (which confirms diagnosis) but inadequate relief of symptoms, or severe secondary effects of the drug. (4) Moderate to advanced PD. (5) Good general health. (6) Age between 18 and 75 years. (7) Both genders. (8) A strong desire to participate in the study, although fully aware of its experimental nature (as described to the patients verbally and in their signed informed consents).
Exclusion criteria were: (1) Parkinsonism other than idiopathic PD. (2) Medical unfitness for graft procedure. (3) Active infection(s). (4) Human immunodeficiency virus (HIV) seropositivity. (5) Pregnant or nursing. (6) Self-reported substance abusers. (7) Previous neurosurgical history (e.g. DBS, pallidotomy, etc.). (8) Unsuited for this study in the neurologist’s opinion.
Stopping rules: At the onset, patients were advised that if they acquired a new medical condition in the course of the study that could in any way jeopardize their post-surgery evaluations, they would have to be withdrawn from the study. In addition, patients kept their right to withdraw from the study at any time and for any reason.
Patients were recruited by us from our regular practice. Twenty-three patients were assessed for eligibility through an initial neurological evaluation. Fifteen patients were excluded: seven were found not to be eligible; five declined; and three were not admitted to the study for other reasons. Eight otherwise fit PD patients met the inclusion criteria. Their PD condition was staged according to the Hoehn and Yahr PD scale38. Enrolled patients then underwent baseline neuropsychological, MR imaging and PET evaluations and immunogenicity testing. All pre- and post-surgery neurological and neuropsychological assessments and MR imaging were performed at the Neuroscience Center, Hospital Angeles Pedregal, Mexico City, México; PET studies were conducted at the Unidad Radiofarmacia-Ciclotron, Facultad de Medicina, UNAM, Mexico City, Mexico; immunogenicity testing was performed at Celavie Biosciences, LLC (Oxnard, CA, USA).
Subjects that completed the 4-year follow-up were two females and five males, with ages ranging between 43 and 74 years (mean age 56 years). Their baseline demographics are shown in Table 1. One of the patients was lost to post-surgery follow-up because of a hip fracture 3 months after surgery.
Table 1.
Patient Baseline Demographics.
| P- ID* | Gender | Age, years |
PD evolution, years |
Most affected side |
|---|---|---|---|---|
| 1 | M | 56 | 7 | R |
| 2 | M | 43 | 2 | L |
| 3 | M | 74 | 16 | R |
| 4 | M | 47 | 3 | L |
| 5 | F | 56 | 10 | L |
| 6 | F | 44 | 3 | R |
| 7 | M | 60 | 13 | R |
* Patient ID; M, male; F, female; R, right; L, left.
Intervention: Undifferentiated NPC Brain Grafting for PD
Stereotactic grafting and post-surgery care were performed at the Neuroscience Center, Hospital Angeles Pedregal (Mexico City, Mexico, by F.J., E.M., C.Z., and F.P. (neuro-anesthesiologist). http://www.angeleshealth.com/angeles-pedregal-camino-a-santa-teresa-1055/).
All enrolled patients underwent the same intervention. MR imaging-guided bilateral stereotactic intraputaminal implantation of NPCs into the brains of PD patients was performed using a Leksell Stereotactic System and Stealth Station Surgical Navigation System (Fridley, MN, USA). Ropivacaine was the local anesthetic used for frame placement. For target locations, measurements were made using computed tomography (CT) scan images fused with previous MR images (both in DICOM format, in axial sections 1.0 mm thick). Patients were operated on one a week schedule, four in August and four in October, 2014. With the patient under general anesthesia, the stereotactic frame was fixed to the operating table with a Mayfield head holder. Bilateral parasagittal incisions and corresponding 14 mm burr holes (one for each hemisphere) were made in preparation for cell suspension injections. A gamma sterilized stereotactic needle with external diameter of 2.1 mm, and 180 mm long, compatible with Leksell stereotactic equipment and Medtronic DBS kit attached to a 1 cc Hamilton syringe was used to deploy 1 cc of suspension per needle track. Two different needle tracks through the same burr hole were selected for each side. Target locations were determined by height and length of putaminal nuclei. The lowest Z-coordinates of each track were located in the ventral putamen and spaced 4 mm apart in the Y-direction. Each needle track received 1×106 cells in 1 ml of culture medium. To ensure complete cell suspension delivery, injections were carried out slowly for 2 min with reciprocal withdrawal of the delivery needle to avoid both damage to stem cells and brain tissue, as well as to avert reflux or bubble formation. The first injection was carried out in the posterior putamen followed by the injection to the middle putamen. Post-operative care was given by R.R., an internist specialized in critical care medicine. The day following surgery, MR images were obtained to confirm correct placement of cell suspensions. All patients were discharged 24 h after surgery.
Patients’ immunosuppressant regimen with cyclosporine A at 15 mg/kg/day and indomethacin at 225 mg/day was started 10 days prior to surgery, and continued for 1 month post-operatively. A wide spectrum antibiotic was given pre-operatively and 48 h post-operatively (Cefuroxime, 750 mg IV every 8 h in hospital, and orally at discharge). Antiparkinsonian medications were adjusted to patients’ requirements.
Procedures to Obtain and Release NPCs
Procurement, isolation, expansion, and characterization of NPCs were performed at Celavie Biosciences, LLC (Oxnard, CA, USA). Methods for culturing, propagation, cryopreservation, and manipulation of NPCs are patented (see below, conflict of interest).
Human fetal brain tissue was procured via routine sterile manual aspiration. Maternal blood samples (sera) were tested for: HIV (LabCorp, San Diego, USA) hepatitis A, B and C (LabCorp, San Diego, USA); HTLVI (LabCorp, San Diego, USA); VRDL Serum w/Reflex Titer (LabCorp, San Diego, CA, USA) and cytomegalovirus (LabCorp, San Diego, CA, USA). Potential donors with a history of genital herpes, cancer, asthma, lupus, rheumatoid arthritis, allergies, vasculitis of autoimmune origin, and drug abuse were excluded. Gestation was determined according to Carnegie stages. Fetal tissue was harvested at the sixth week of gestation after elective abortion. Whole fetal forebrain was dissected, minced, and triturated to a single cell suspension and cryopreserved. Cell suspension samples were thawed, expanded, and cultured in flasks incubated at 37°C under hypoxic conditions (5% O2 and 5% CO2) through four doublings, and characterized in 2012.
Cells were cultured in ultra-low attachment culture flasks under feeder-free conditions in serum- and xeno-free Eagle’s essential medium (Hyclone, SH30310, Logan, UT, USA), supplemented with Gem21 (Gemini Bio-Products, 400-660, Sacramento, CA, USA), epidermal growth factor (Peprotech AF-100-15, Rocky Hill, NJ, USA), basic fibroblast growth factor (Peprotech AF-100-18B), transforming growth factor alpha (Peprotech AF-100-16A), insulin-like growth factor I (Peprotech, AF-100-11), leukemia inhibitory factor (Millipore LIF1010, Temecula CA, USA), calcium chloride (Fisher Scientific, 1722, Waltham MA, USA), Glutamax (Invitrogen 35050, Carlsbad, CA, USA), non-essential amino acids (Hyclone, SH30238.01), and an N2 supplement (Invitrogen 17502), all of which were added at proprietary concentrations.
At the second doubling (D2) cell culture was tested for sterility (USP <71>) and at D4 culture was karyotyped (Cell Line Genetics, Madison, WI, USA) and PCR tested for presence of adventitious agents: HTLV-1, HTLV-2, HIV-1 (A, B, D, F, H, N), hepatitis A, B and C, T. p. pallidum, CMV, HSV-1, HSV-2, HPV. Cells were then transferred to a closed bioreactor system (GE WAVE Bioreactor 2/10 System, Uppsala, Sweden), operating under the same physical and chemical conditions. The bioreactor was used to create the master cell bank (MCB), which was harvested, tested, characterized, and rate-control cryopreserved after a total of seven doublings (D7). After the MCB was safety tested and characterized, a portion of the batch was thawed and used to seed the bioreactor to produce the working cell bank (WCB). Cells were cultured in the bioreactor until they reached D13. They were harvested (Centritech LAB-III, CarrCentritech Separation System, Rancho Cucamonga, CA, USA), aliquoted (Fill-It; TAP Biosystems, Wilmington, DE, USA), and cryopreserved.
The WCB was subjected to release testing for safety and characterization assays. Safety testing included sterility (USP <71>), mycoplasma (USP <63>), endotoxin (USP <85>), and karyotyping. Characterization included flow cytometry testing for: Oct-4 >90% (10H11.2, EMD Millipore, Billerica, MA, USA; AF488 conjugated), Sox-2 >90% (Btjce, eBioscience, San Diego CA, USA; AF488 conjugated), MHC-I <10% (A4, eBioscience; APC conjugated), MHC-II <10% (CVS20, Novus Biologicals, Littleton, CO, USA; AF488 conjugated), CD105 <10% (SN6, eBioscience; PE-Cy7 conjugated), and tyrosine hydroxylase <10% (EP1532Y, Abcam, Cambridge, UK; FITC-conjugated goat anti-rabbit IgG; Abcam; polyclonal). Both MCB and WCB were stored in gas phase liquid nitrogen (LN2).
All procedures were performed under aseptic conditions in an ISO 8 clean room, utilizing ISO 5 bio-safety cabinets and laminar airflow hoods, according to validated protocols.
Tumorigenicity of the WCB cells was investigated via a teratoma assay. Briefly, 3 million cells were subcutaneously injected into the flanks of Beige SCID mice. The subject animals have severe immunodeficiency affecting both B and T lymphocytes and are permissive to tumor formation. The mice were monitored for visible tumor formation, changes in vital signs and weight; 12 weeks after stem cell injections, the mice were euthanized and autopsied. The site of the injection, brain, and filtration organs (lungs, liver, spleen, kidneys, and lymph nodes) underwent gross and histological examination for presence of tumors. No tumors were found in any of the animals.
Prior to implantation, a WCB vial was thawed, cells washed with culture medium, counted, resuspended in culture medium at a concentration of 1×106 cells/ml and tested for viability (0.4% trypan blue stain; MP Biomedicals LLC, Santa Ana, CA, USA), gram stain, and endotoxin. Viability of >80%, negative gram stain, and endotoxin <3EU/ml were required for release. A portion of the final formulation was submitted to sterility (USP <71>) and mycoplasma (USP <63>) testing (results were available after implantation). Cell suspension at 2×106 cells in 2 ml of culture medium per vial were shipped overnight in a temperature controlled (2–8°C) container (Pelican BioThermal, Plymouth, MN USA). Temperature inside the container was continuously recorded and checked for deviations prior to implantation. Viability was tested again immediately prior to surgery, and was consistently higher than 90%. After surgery a sample of the remaining cell suspension was submitted to sterility testing (USP <71>).
Immunogenicity Testing
Patients’ whole blood specimens were evaluated by flow cytometry for NPC-specific antibodies (serological assay) and for antibody-dependent cell-mediated cytotoxicity (cytotoxicity assay) 1 month and 6 months after cell implantation as compared with baseline values obtained 1 month prior to grafting.
For serological assays, NPCs in suspension were incubated in patients’ sera, washed, and stained with anti-human IgG Fc FITC antibody (BioLegend, San Diego, CA, USA) according to the vendor’s protocol; immunogenicity was estimated by the amount of NPC-bound labeled antibodies in patients’ sera.
For cytotoxicity assays, a method was used employing the principle of live and dead cell discrimination39. Patients’ peripheral blood mononuclear cells (PBMCs) (effector cells) were isolated and incubated with NPCs (target cells) at 100:1, 50:1, and 25:1 effector to target ratios, using the following staining scheme: live effector cells: no dye; dead effector cells: only 7-amino-actinomycin D (7-AAD) (BioLegend); live target cells: PKH-67 only (Sigma Aldrich, St. Louis, MO, USA), and dead target cells: PKH-67 and -7-aminoactinomycin D (7-AAD)40. Staining was performed according to dye manufacturer’s instructions. This staining arrangement permitted discrimination of cell populations and quantification of NPCs lysed by PBMCs.
Neurological Evaluations
Neurological evaluations were performed on the UPDRS part I (mentation, behavior, and mood), part II (motor activities of daily living), part III (motor performance), and part IV (complications of therapy: response to medication and intervention), as well as the modified Hoehn and Yahr for PD staging38, and Schwab and England activities of daily living scales41. Patients were clinically evaluated in their OFF and ON states at the time of baseline screening, and then at 6 months, 1, 2, and 4 years post-surgery. OFF state was defined as the overnight drug withdrawal condition: patients were asked to discontinue antiparkinsonian medications at least 12 h before evaluation. ON evaluations were performed 1 h after receiving 1.5 times their usual L-DOPA dose. All OFF and ON UPDRS evaluations were videotaped.
Neuropsychological Evaluations
Neuropsychological performance was assessed using the following instruments: our brief neuropsychological (NEUROPSI)42 and computerized test batteries, the Mini-mental Parkinson State Examination (MMPSE)43, and Mexican adaptations of Beck anxiety44 and depression inventories45. Patients reported on their quality of life as related to their daily living activities, physical and mental well-being (health status), cognition, and communication.
Magnetic Resonance Imaging
MR images were obtained before surgery (baseline) and three times after cell implantation at 24 h, 6 months, 1, 2, and 4 years post-surgery with a Philips-Achieva 3.0T(TX) –DS MR System (Best, The Netherlands).
PET Molecular Imaging
In vivo pre- and post-synaptic state of patients’ nigrostriatal dopaminergic system was investigated by PET molecular imaging in their OFF condition (patients were asked to discontinue anti-parkinsonian medications at least 12 h before each study) at baseline (before surgery), and 1 and 2 years post-surgery. Radiopharmaceuticals were synthesized by us at the Unidad Radiofarmacia-Ciclotron, Facultad de Medicina, Universidad Nacional Autonoma de México (Mexico City, Mexico) and were: RAC, a post-synaptic dopamine D2-receptor antagonist46, a measure of dopamine availability; FDOPA, a marker for dopamine storage capacity and aromatic amino acid decarboxylase activity, both of which are affected by the disease47, and by antiparkinsonian medication48; and DTBZ, a high-affinity stereospecific vesicular monoamine transporter 2 (VMAT2) ligand49, which is not very susceptible to dopaminergic pharmacological agents for PD, and therefore regarded as a precise measure of dopaminergic degeneration50, and an objective PD biomarker51,52. Radiochemical purity of all radiopharmaceuticals used here exceeded 98% in every case.
Before scanning, cannulas were placed in antecubital veins for radioligand injection. Sterile solutions containing on average of 370 MBq RAC, 185 MBq FDOPA, and 370 MBq DTBZ were injected intravenously as a bolus. To minimize patients’ exposure to radiation, only two of the three radiopharmaceuticals were used per patient. All patients underwent DTBZ-PET scans and one additional study with either RAC (four patients) or FDOPA (three patients), at least 1 week apart. Patients for either marker were chosen at random. Two healthy male non-PD controls aged 69 and 70 years of age received similar doses of radiopharmaceuticals as PD patients. Here too, only two of the three radiomarkers were used per control individual.
Scans were acquired on a Siemens Biograph 64 PET/CT. Patients were positioned in the scanner with a 3D laser alignment with reference to the orbitomeatal line. Thirty-minute brain emission scans were acquired 20 min after RAC or DTBZ injections; 15 min scans were acquired for FDOPA 75 min post-injection. All patients studied with FDOPA were pre-medicated with 150 mg of carbidopa to prevent peripheral decarboxylation.
Images were reconstructed using an OSEM-2D algorithm and analyzed with the Statistical Parametric Mapping software (SPM v.12). Each individual PET brain image was normalized on an anatomical MRI atlas to be evaluated within a standard space. Following normalization, FSL structural atlases were used to define regions of interest. To facilitate quantitative analysis, specific uptake ratios in the caudate and putamen were calculated by subtracting the background signal of a reference region with nonspecific uptake from striatal activity, and dividing the result by reference region activity, that is [(target uptake – reference uptake)/reference uptake], using cerebellum for RAC, and occipital cortex for DTBZ and FDOPA, as reference regions.
Data Analysis
Standard statistical tests are inappropriate for the present study design (case series). Therefore, data in terms of before versus after intervention are described for each patient.
Results
Safety Issues: Intervention, Tumorigenicity, and Immunogenicity
Intervention: Patients’ hospitalization was uneventful. Neither the craniotomy nor the stereotactic grafting of NPCs to patients’ putamina resulted in any complications during the procedure or within 30 days after surgery; the following 4 years post-surgery went smoothly as well.
Tumorigenicity: Using MRI, no tumors were found in the brains of any of the grafted patients at 4 years post-surgery.
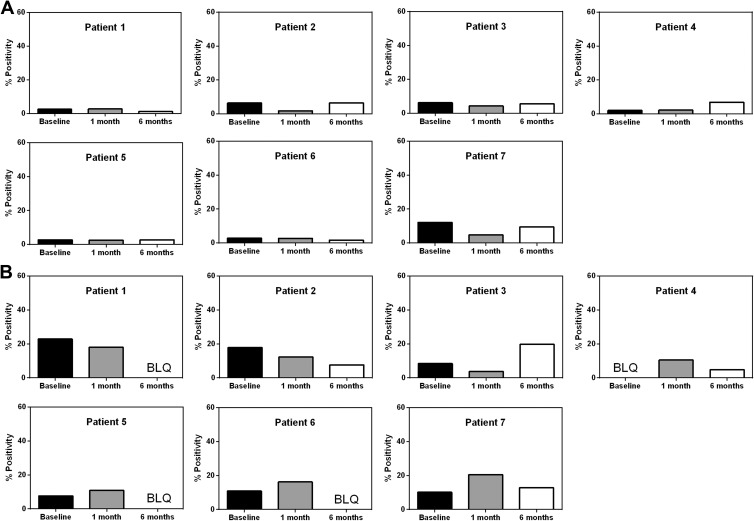
Immunogenicity: In the serological assay, the highest percent NPC-specific antibody reaction at baseline was observed in P7 at 12%. Most of the patients showed similar or lower levels of response at 1 month (while on immunosuppressants) and 6 months (off immunosuppressants) post-implantation, with none exceeding 12% (Fig. 1A). In the cytotoxicity assay the highest of three values for a given time period was used for scoring. The highest baseline observed was for P1 (23%). None of the patients exceeded the 23% baseline at their 1- or 6-month follow-up (Fig. 1B).
Figure 1.
Lack of immune response to NPC grafting. A. In the serological assay, no increase in NPC-specific antibodies was detected at 1 month (on immunosuppressants) or 6 months (off immunosuppressants) after bilateral putaminal stem cell injections, compared with baseline. B. In the cytotoxicity assay, P3 and P7 demonstrated somewhat higher levels of cell lysis at 6 and 1 month post-grafting, respectively, compared with their baselines, but these values were still lower than the highest pre-operative baseline observed (P1). BLQ, below level of quantification.
Neurological Performance
Patients’ neurological performance scores on the UPDRS are given in Table 2. In the first year post-surgery, patients’ UPDRS II, III, and IV scores revealed improved neurological function in their OFF and ON conditions. Motor function examinations showed improvement (UPDRS III), and their motor-related daily living activities improved as well (UPDRS II). Non-motor daily living experiences related to mood and behavior, as rated on the UPDRS I (Table 2), were unequally affected by surgery and showed minor variations or remained unchanged, with two exceptions that showed a poor response (P2 and P5). From patients’ UPDRS II, III, and IV scores at their 2-year post-surgery evaluations, loss of neurological function was evident, although still remaining slightly better than their scores at baseline.
Table 2.
OFF/ON UPDRS (I–IV) Scores.
| Patient/Time | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|
| UPDRS-I OFF/ON | Baseline | 0/0 | 3/3 | 1/1 | 5/3 | 1/1 | 0/0 | 2/2 |
| 1 year | 1/1 | 6/6 | 1/1 | 0/0 | 3/3 | 0/0 | 0/0 | |
| 2 years | 1/1 | 2/2 | 2/2 | 2/2 | 2/2 | 0/0 | 2/2 | |
| 4 years | 1/1 | 3/3 | 2/2 | 1/1 | 2/2 | 1/1 | 1/1 | |
| UPDRS-II OFF/ON | Baseline | 7/2 | 14/12 | 10/4 | 10/6 | 15/10 | 13/6 | 20/14 |
| 1 year | 9/1 | 8/8 | 8/5 | 4/1 | 6/6 | 3/0 | 8/3 | |
| 2 years | 10/10 | 13/10 | 13/10 | 7/0 | 9/7 | 2/2 | 10/9 | |
| 4 years | 17/15 | 11/11 | 13/13 | 8/0 | 9/9 | 2/2 | 12/12 | |
| UPDRS-III OFF/ON | Baseline | 16/2 | 26/13 | 36/25 | 18/4 | 43/24 | 27/2 | 29/11 |
| 1 year | 18/9 | 18/8 | 14/11 | 12/2 | 39/5 | 16/2 | 17/3 | |
| 2 years | 18/5 | 23/6 | 34/28 | 9/3 | 31/9 | 11/6 | 27/14 | |
| 4 years | 25/9 | 22/8 | 34/17 | 11/3 | 31/37 | 15/2 | 23/22 | |
| UPDRS-IV OFF/ON | Baseline | 1/1 | 2/0 | 2/3 | 7/7 | 12/12 | 6/5 | 4/6 |
| 1 year | 7/7 | 4/4 | 1/1 | 2/3 | 4/4 | 1/1 | 3/4 | |
| 2 years | 6/7 | 5/5 | 1/2 | 4/5 | 6/5 | 2/2 | 4/2 | |
| 4 years | 7/7 | 7/7 | 1/1 | 6/6 | 9/9 | 3/3 | 5/5 |
UPDRS-I (non-motor experiences of daily living: mentation, behavior, and mood), score 0–16; UPDRS-II (motor experience performance of daily living), score 0–52; UPDRS-III (motor function examination), score 0–108; UPDRS-IV (motor complications in response to medication and intervention—dyskinesias, fluctuations, and dystonia), score 0–23. Lower scores signify improvement on all UPDRS (I-IV). UPDRS III scores in all but P1 show improvement 4 years after surgery. P2 and P7 demonstrated improvement compared with the both baseline and the second year after surgery. P3, P4, P5, and P6 showed improved response to medication 4 years after surgery (UPDRS IV). P2, P4, P5, P6, and P7 showed improvement in activities of daily living (UPDRS II).
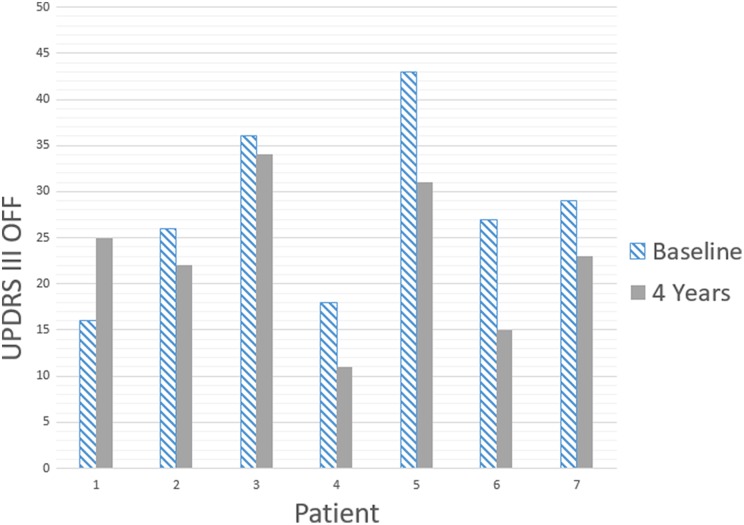
Patients 1, 4, 5, and 6 decreased their L-DOPA equivalent daily dose (LEDD) at year 4 compared with baseline. In patients 3 and 7, 4th year LEDD increased, and in patient 2 remained unchanged compared with baseline (Table 3). In the fourth year all patients except patient 1, improved on UPDRS III OFF as compared with the baseline (Fig. 4) and patients 2, 6, and 7 improved as compared with both baseline and the second year after surgery. Patients 2 and 7, while retaining improvement as compared with the baseline, by the fourth year reversed a certain deterioration observed at the first and second years after surgery.
Table 3.
L-DOPA Medication Regimen in LEDD Values over the Period of 4 Years after Transplantation.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Baseline | 1000mg | 750mg | 562.mg | 1250mg | 1250mg | 750mg | 375mg |
| 1 year | 1000mg | 750mg | 1000mg | 750mg | 375mg | 750mg | 562.5mg |
| 2 years | 937.5mg | 750mg | 562.5mg | 1125mg | 500mg | 750mg | 625mg |
| 3 years | 937.5mg | 750mg | 562.5mg | 1125mg | 375mg | 750mg | 625mg |
| 4 years | 937.5mg | 750mg | 750mg | 750mg | 375mg | 500mg | 750mg |
Figure 4.
UPDRS III in OFF condition at the baseline and four years after surgery. All patients with exception of P1 showed sustained improvement 4 years after surgery.
At their 1-, 2-, and 4-year evaluations, patients’ staging on the Hoehn & Yahr scale (Table 4) and ratings on the Schwab and England scale (Table 4) showed only minor variations, with the exception of P1, whose scores clearly worsened.
Table 4.
PD Staging on the Hoehn and Yahr Scale and Activities of Daily Living Scores on the Schwab and England Scale.
| Patient/Time | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|
| PD Staging | Baseline | 1 | 2 | 3 | 1 | 2 | 2 | 2.5 |
| 1 year | 2.5 | 2 | 1 | 1 | 2 | 2 | 2 | |
| 2 years | 3 | 2 | 2 | 1 | 3 | 2 | 2.5 | |
| 4 years | 3 | 2 | 2 | 1 | 3 | 2 | 2.5 | |
| Activities of daily living, OFF/ON, % |
Baseline | 80/- | 90/- | 80/- | 90/100 | 60/- | 90/- | 80/90 |
| 1 year | 50/90 | 80/100 | 90/90 | 90/100 | 60/90 | 90/100 | 80/100 | |
| 2 years | 50/90 | 80/90 | 90/90 | 90/100 | 70/80 | 100/100 | 80/90 | |
| 4 years | 40/80 | 80/90 | 80/80 | 80/100 | 60/60 | 90/100 | 80/80 |
PD Staging: Hoehn and Yahr scale, lower scores signify better PD condition; Activities of daily living: Schwab and England scale, higher % scores signify enhanced performance of daily living activities. ON scores were not obtained at baseline in 5/7 cases. The table demonstrates absence of significant changes with exception of P1.
Neuropsychological Outcome
Neuropsychological scores are shown in Table 5. At baseline, cognitive performance was normal for all patients, with the exception of P3 whose performance was mildly affected. After surgery their cognitive assessments 1, 2, and 4 years post-surgery were satisfactory for all patients, and showed only minor variations if at all. With regards to their affective assessments, patients were only minimally depressed (patient 2 showed moderate score) throughout the 4-year post-surgery study period but did show mild to severe levels of anxiety which remained unchanged for most patients after surgery, with the exceptions of patients P5 and P6 who appear to have improved, and patient P7 whose anxiety went from mild at baseline to severe 2 years and decreased back to mild 4 years after surgery.
Table 5.
Neuropsychological Scores.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
|---|---|---|---|---|---|---|---|---|---|
| Cognitive assessments* | Baseline | 112 nor | 108 nor | 72 mild | 121 nor | 112 nor | 112 nor | 112 nor | |
| 1 year | 122 nor | 117 nor | 70 mild | 114 nor | 93 nor | 96 mild | 95 nor | ||
| 2 years | 119 nor | 117 nor | 95.5 nor | 124 nor | 90 mild | 124 nor | 80 mod | ||
| 4 years | 119 nor | 116 nor | 64 mod | 88 mod | 120 nor | 86 mod | |||
| Affective assessments | Depression** | Baseline | 1 min | 19 mild | 10 min | 11 min | 15 mild | 5 min | 7 min |
| 1 year | 4 min | 9 min | 9 min | 4 min | 7 min | 8 min | 9 min | ||
| 2 years | 3 min | 19 mild | 5 min | 9 min | 3 min | 0 min | 12 min | ||
| 4 years | 10 min | 28 mod | 6 min | 10 min | 8 min | 7 min | |||
| Anxiety*** | Baseline | 2 min | 30 sev | 13 mild | 8 mild | 33 sev | 31 sev | 12 mild | |
| 1 year | 9 mild | 31 sev | 7 min | 7 min | 26 sev | 4 min | 21 mod | ||
| 2 years | 11 mild | 37 sev | 6 min | 8 mild | 22 mod | 10 mild | 34 sev | ||
| 4 years | 16 mod | 35 sev | 18 mod | 22 mod | 13 mild | 25 mod | |||
* NEUROPSI cognitive performance scale, for ages 31–50 years: 112–102/normal, 101–97/mild, 96–88/moderate, 87–78/severe; for ages 51–65 years: 101–93/normal, 92–88/mild, 87–80/moderate, 79–72/severe; for ages 66–85 years: 91–78, normal; 77–72, mild; 71–59, moderate; 58–46, severe. **BDI, Beck Depression Inventory: 0–13/minimal, 14–19/mild, 20–28/moderate, 29–63/severe. ***BAI, Beck Anxiety Inventory: 0–7/minimal, 8–15/mild, 16–25/moderate, 26–63/severe. P4 had no 4-year follow-up. Neuropsychological performance before and after surgery remained largely unchanged.
Magnetic Resonance Imaging
Representative brain MR images of a grafted patient are shown in Fig. 2. The sites and trajectory paths for cell implantation are shown in Figs. 2A and 2B, respectively. At the end of several needle tracks at 1, 2, and 4-year follow-ups small clusters of hypointensity on T2-weighted images were identified. At the annual MRI monitoring of P4, a robust graft outgrowth was observed in the right putamen (Fig. 2C) with no topographic abnormalities of the brain structures. MRI with gadolinium and PET imaging with 3’-deoxy-3’-[18F]-fluorothymidine (18F-FLT) were performed and showed no evidence of tumor formation. Quantitative volumetric analysis was not performed at this time.
Figure 2.
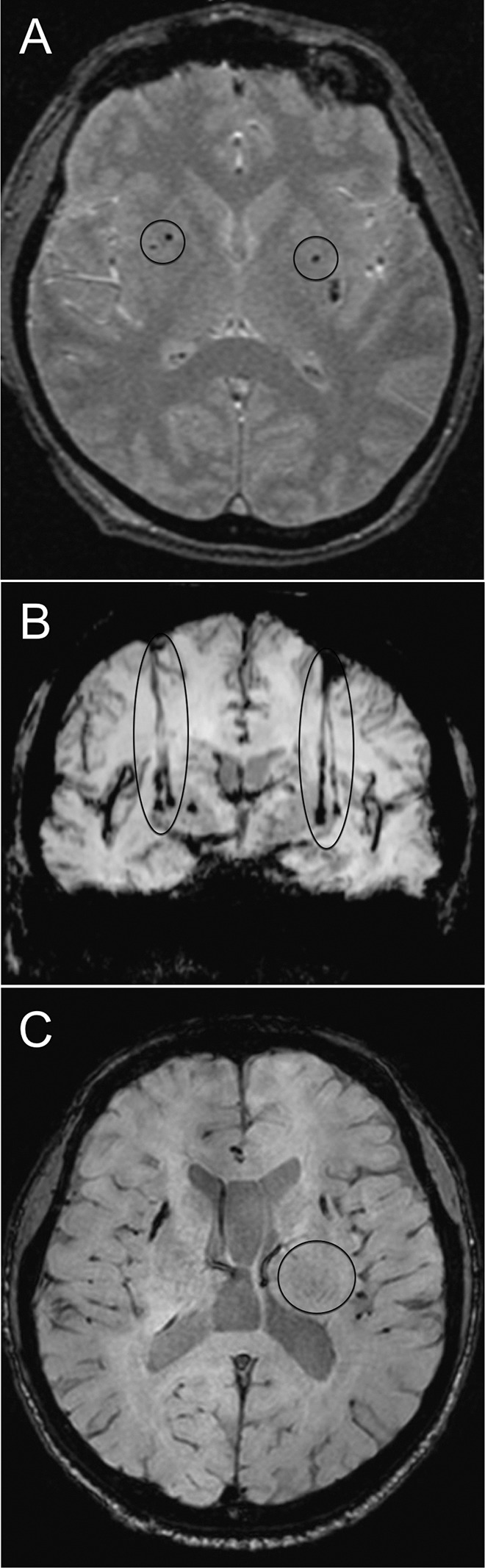
Brain MR images of a grafted patient. A. T2 axial image showing the 2 sites of placement of an NPC suspension in either putamen (circles) at 24 h post-surgery. B. SWAN coronal image showing both implant trajectory paths to either putamen (ellipses) at 1 year postsurgery. C. T2 axial image at 1 year post-surgery showing robust graft outgrowth in the right putamen (circle) in P4 with no topographical abnormalities of the brain structures.
PET Functional Imaging
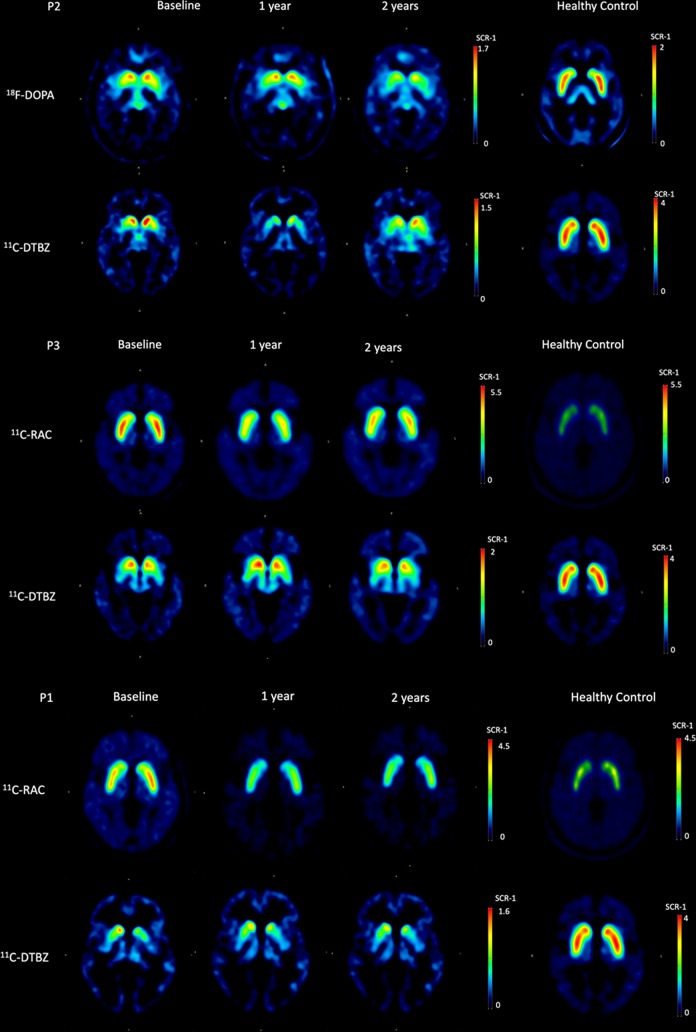
Representative PET images for healthy controls and grafted PD patients are depicted in Fig. 3. All right and left putamina PET imaging scores for RAC, FDOPA, and DTBZ uptake at baseline, and 1 and 2 years post-surgery are shown in Table 6, and the percent average change differences among them are given in Table 7. Compared with baseline, RAC uptake decreased in all four cases tested at patients’ 1-year evaluation; the percent average changes for each patient (P1, P3, P5, P6) were 36%, 45%, 19%, and 21%, respectively. Two years after surgery, RAC average uptake in putamina increased in all cases except one (P6), but remained lower than at baseline by 31%, 18%, and 7% for patients P1, P3, and P5, respectively. The percent average decrease of RAC uptake for P6 was 39%. At their 1-year evaluations, percent average bilateral putaminal FDOPA uptake scores, compared with baseline, showed a gain of 5% and 7% for patients P4 and P7, respectively, but a 12% loss for patient P2. At their 2-year post-surgery evaluations, all patients showed percent average losses of FDOPA uptake, compared with baseline, of 29%, 5%, and 13% for patients P2, P4, and P7, respectively. Percent average bilateral putaminal DTBZ uptake, compared with baseline, 1 year post-surgery showed gains of 15% and 50% for patients P3 and P5, respectively, and losses of 5–27% for patients P2, P4, and P6; at their 2-year evaluations, gains went from 9–17% for patients P2, P3, P5, and P7, and losses from 10–22% for patients P1, P4, and P6.
Figure 3.
PET molecular imaging. Representative (P2 and P3) axial RAC, FDOPA and DTBZ PET images of healthy controls and PD patients in their OFF condition for in vivo pre- and post-synaptic assessment of the state of their nigrostriatal dopaminergic system (putamina and caudate nuclei) at baseline, and 1- and 2 years after surgery. P1 is included as an outlier notable for having a decrease in RAC uptake with lack of improvement in motor performance.
Table 6.
Right and Left Putamina PET Imaging Scores.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
|---|---|---|---|---|---|---|---|---|---|
| RAC | Right | Baseline | 2.8 | – | 3.6 | – | 3.5 | 2.8 | – |
| 1 year | 1.8 | – | 2 | – | 2.8 | 2.3 | – | ||
| 2 years | 1.9 | – | 3 | – | 3.2 | 1.8 | – | ||
| Left | Baseline | 3 | – | 3.7 | – | 3.4 | 3.3 | – | |
| 1 year | 1.9 | – | 2 | – | 2.8 | 2.6 | – | ||
| 2 years | 2.1 | – | 3 | – | 3.2 | 2 | – | ||
| FDOPA | Right | Baseline | – | 0.8 | – | 0.8 | – | – | 0.8 |
| 1 year | – | 0.7 | – | 0.9 | – | – | 0.8 | ||
| 2 years | – | 0.6 | – | 0.8 | – | – | 0.7 | ||
| Left | Baseline | – | 0.9 | – | 1.1 | – | – | 0.7 | |
| 1 year | – | 0.8 | – | 1.1 | – | – | 0.8 | ||
| 2 years | – | 0.6 | – | 1 | – | – | 0.6 | ||
| DTBZ | Right | Baseline | 0.5 | 0.5 | 0.7 | 0.7 | 0.3 | 1.1 | 0.6 |
| 1 year | 0.5 | 0.4 | 0.8 | 0.7 | 0.4 | 0.8 | 0.6 | ||
| 2 years | 0.4 | 0.6 | 0.8 | 0.7 | 0.3 | 0.9 | 0.7 | ||
| Left | Baseline | 0.4 | 0.6 | 0.6 | 1.3 | 0.3 | 0.7 | 0.6 | |
| 1 year | 0.4 | 0.4 | 0.7 | 1.2 | 0.5 | 0.6 | 0.6 | ||
| 2 years | 0.3 | 0.6 | 0.7 | 1.1 | 0.4 | 0.7 | 0.6 | ||
Values correspond to specific uptake ratios [(target uptake – reference uptake)/reference uptake] using cerebellum for RAC, and occipital cortex for FDOPA and DTBZ as reference regions. Regions of interest were defined on FSL structural atlases using PET brain images normalized on an anatomical MRI atlas.RAC, [11C]raclopride (lower values suggest improvement); FDOPA, 6-[18F]fluoro-L-DOPA (higher values suggest improvement); DTBZ, (+)-alpha-[11C]dihidrotetrabenazine (higher values suggest improvement).
Table 7.
Putamina and Caudate Nuclei PET Percent Average Score Differences Compared with Baselines.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| % CHANGES FROM BASELINE | 1 year | PUTAMINA | RAC | +36 | +45 | +19 | +21 | |||
| FDOPA | -12 | +5 | +7 | |||||||
| DTBZ | 0 | -27 | +15 | 5 | +50 | -22 | 0 | |||
| CAUDATE | RAC | +52 | +66 | +29 | +20 | |||||
| FDOPA | -21 | -1 | +20 | |||||||
| DTBZ | -19 | -31 | -17 | -20 | +41 | -42 | -67 | |||
| 2 years | PUTAMINA | RAC | +31 | +18 | +7 | +39 | ||||
| FDOPA | -29 | -5 | -13 | |||||||
| DTBZ | -22 | +9 | +15 | -10 | +17 | -11 | +8 | |||
| CAUDATE | RAC | +72 | +46 | +28 | +55 | |||||
| FDOPA | -34 | -10 | -65 | |||||||
| DTBZ | -131 | -48 | -40 | -12 | -71 | -33 | -51 | |||
The table reflects multidirectional changes in the uptake of the radioligands both from 1 to 2 years and from putamen to caudate in all patients. Of interest, P1 showed the best improvement in the uptake of Raclopride and no improvement in UPDRS III.
+ is improvement.
- is deterioration.
no sign is no change.
Right and left caudate nuclei PET imaging scores for RAC, FDOPA, and DTBZ uptake are shown in Table 8, and the percent average change differences among them are given in Table 7 Comparing caudate nuclei RAC scores for patients P1, P3, P5, and P6 with their corresponding bilateral putaminal scores given in Table 6, it can be seen that at baseline, RAC uptake in the caudate nuclei was less than that seen in the putamina by an approximate factor of 1.6, and that their scores at 1 and 2 years post-surgery reveal, on average, greater decreases in RAC uptake than in the putamina. In the caudate nuclei, percent average improvement compared with baseline was of 52%, 66%, 29%, and 20% in the first year post-surgery, and of 72%, 46%, 28%, and 55% for the second year for patients P1, P3, P5, and P6, respectively. FDOPA levels in the caudate nuclei were similar to those found in the putamina. Compared with baseline, patients P2 and P4 percent average FDOPA uptake went from a loss of 21% and 1% in the first year post-surgery, to a greater loss of 34% and 10% at their second year post-surgery follow-up. P7 showed a percent average increase in FDOPA uptake of 20% in the first year post-surgery that was down by 65% at the patient’s 2-year follow-up. DTBZ uptake values in the caudate nuclei decreased in a range between 17% and 67% the first year post-surgery (excepting P5, who gained 41%), and 12% and 71% the second year post-surgery (excepting P1, who gained 131%).
Table 8.
Right and Left Caudate Nuclei PET Imaging Scores.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
|---|---|---|---|---|---|---|---|---|---|
| RAC | Right | Baseline | 1.6 | – | 1.8 | – | 1.9 | 1.7 | – |
| 1 year | 0.8 | – | 0.5 | – | 1.3 | 1.4 | – | ||
| 2 years | 0.5 | – | 1.1 | – | 1.4 | 0.7 | – | ||
| Left | Baseline | 1.5 | – | 1.6 | – | 1.9 | 1.7 | – | |
| 1 year | 0.7 | – | 0.6 | – | 1.3 | 1.3 | – | ||
| 2 years | 0.3 | – | 0.7 | – | 1.2 | 0.8 | – | ||
| FDOPA | Right | Baseline | – | 1.1 | – | 1 | – | – | 0.5 |
| 1 year | – | 0.8 | – | 0.9 | – | – | 0.5 | ||
| 2 years | – | 0.7 | – | 0.8 | – | – | 0.2 | ||
| Left | Baseline | – | 1.1 | – | 1.2 | – | – | 0.3 | |
| 1 year | – | 0.9 | – | 1.2 | – | – | 0.4 | ||
| 2 years | – | 0.7 | – | 1.1 | – | – | 0.1 | ||
| DTBZ | Right | Baseline | 0.4 | 0.9 | 0.5 | 1.2 | 0.2 | 1.8 | 0.9 |
| 1 year | 0.3 | 0.6 | 0.4 | 0.9 | 0.2 | 1 | 0.3 | ||
| 2 years | 0.9 | 0.5 | 0.2 | 1.1 | 0.1 | 1.2 | 0.4 | ||
| Left | Baseline | 0.3 | 1 | 0.5 | 1.8 | 0.2 | 1.5 | 0.6 | |
| 1 year | 0.3 | 0.7 | 0.4 | 1.4 | 0.3 | 0.9 | 0.2 | ||
| 2 years | 0.8 | 0.5 | 0.3 | 1.5 | 0.1 | 1 | 0.4 | ||
Values correspond to specific uptake ratios[(target uptake – reference uptake)/reference uptake] using cerebellum for RAC, and occipital cortex for FDOPA andDTBZ as reference regions. Regions of interest were defined on FSL structural atlases using PET brain images normalized on an anatomical MRI atlas. RAC, [11C]raclopride (binds to dopamine D2 receptors, a measure of endogenous dopamine depletion, lower values suggest improvement); FDOPA, 6-[18F]fluoro-L-DOPA (a measure of dopamine biosynthesis; higher values suggest improvement); DTBZ, (+)-alpha-[11C]dihidrotetrabenazine (binds to the vesicular monoamine transporter, VMAT2, in pre-synaptic vesicles, a measure of dopaminergic nerve terminal function; higher values suggest improvement).
Discussion
Rationale for Undifferentiated NPC Intraputaminal Grafting
This project was designed to test the safety of stereotactic bilateral grafting to the putamina of PD patients of a highly controlled, standardized, culture-manufactured, undifferentiated NPC cell line. The secondary goals were to explore the potential of this approach to reduce PD motor symptoms, as assessed on the UPDRS, and to evaluate the effects on dopaminergic neurotransmission within the diseased striatal tissue, as inferred from PET functional images of biomarkers for dopaminergic neuronal activity. Although beyond the scope of this study, our intention further on was to assess this cell line’s capability to improve not only dopaminergic neurotransmission but also non-dopaminergic neuronal pathways in the diseased PD brain.
The NPC cell line used here was manufactured by us in large quantities using our patented 13-passage cell culture system. We tested these cells pre-clinically for morphological and behavioral responses35,36. The source of these progenitor stem cells was first trimester human fetal tissue. These cells proliferate easily in culture53,54 and are less likely to be rejected by transplant recipients due to expression of HLA-G55 and reduced immunogenicity56. Furthermore, contrary to embryonic stem cells, the NPCs used here have been shown not to produce tumors in the standard teratoma assay57 (in-house data).
Putamina were chosen for bilateral stereotactic delivery of NPCs because this area has been described as the most affected site in the basal ganglia of PD patients. It shows the greatest dopaminergic cell loss2 and is linked to levodopa-induced dyskinesias9,10. Encouragingly, unilateral58 or bilateral intraputaminal fetal dopaminergic cell grafting20,59,60 or unilateral intraputaminal glial cell line-derived neurotrophic factor delivery61,62 have led to clinical improvement in patients with PD.
A group of eight informed and willing PD patients was formed for the initial trial. They formed a heterogeneous group of 48–75-year-old individuals with a 2–25-year history of the disease, diagnosed with moderate idiopathic PD, responsive to L-DOPA, some with an inadequate relief of symptoms, and others showing mild to severe secondary effects to the drug (dyskinesias).
Safety Issues
Host immune response will always be a matter for concern whenever an allograft is involved63. To accurately evaluate immunogenicity of an allograft would require testing for HLA compatibility. The fact that the NPC cell line used in this trial consistently expressed low levels of MHC-I (<10%) gave us reason to believe that HLA matching was unwarranted for graft survival. Nonetheless, given the fact that the blood–brain barrier was disrupted by cell grafting, thereby altering the central nervous system’s status of immune privilege64, and could potentially result in the host’s immune system to become sensitized to NPCs, all patients were immunosuppressed for 1 month. The safety of the intervention and the lack of tumors and immune response observed after NPC grafting are encouraging reasons to further develop our strategy to treat PD with NPCs.
Efficacy Issues
Although we observed encouraging results, lack of controls, small sample size, and poor understanding of mechanisms of stem cell action do not allow us to draw definite conclusions about efficacy.
The injected cells were suspended in culture media containing supplements and growth factors that could have contributed to the initial improvement observed at 1 year. However, it is highly unlikely that a single injection of trophic factors had an effect that lasted 4 years.
Motor Performance, MRI and Functional PET Imaging
The primary functional outcome tested here was patient motor performance. With one exception, all patients showed motor function improvements in the first year post-surgery which further continued to improve in three cases by their 2-year follow-up (P4, P5, and P6), while the other three still showed improvements, albeit diminished, but better than at baseline. In the fourth year these results remained practically unchanged while patients 2, 6, and 7 improved as compared with both baseline and the second year after surgery.
Small clusters of hypointensity on T2-weighted images were observed at the end of several needle tracks without changes in regional topography of the brain. It is impossible to determine whether these clusters are made up of transplanted cells or the host’s own.
Improved motor function of patients in the RAC/DTBZ group (P1, P3, P5, and P6) seen at both their 1- and 2-year evaluations was correlated with their putaminal PET scans, which suggests enhanced dopaminergic activity. Patients’ motor improvement on the UPDRS-III might be attributed to enhanced activity of their still functional putaminal D2 receptors, in response to the apparently increased availability of endogenous dopamine, as inferred from decreased RAC binding57. Dopamine might have been released from pre-synaptic terminals of newly differentiated dopaminergic neurons derived from the graft, as the moderately enhanced uptake of DTBZ might suggest. Putaminal FDOPA/DTBZ scans for P2, P5, and P7, however, did not correlate well with patients’ motor improvements, especially at their 2-year follow-up. It has to be pointed out that the enhanced dopaminergic activity alone may not result in clinical motor improvement, as evidenced by a finding that robust graft survival and normalized dopaminergic innervation did not result in recovery in a PD patient22.
Graft-induced dyskinesias is a common adverse event described after fetal cell transplantation60,65 and thus was a concern with this trial. At their 12-month follow-ups none of our seven fully evaluated patients have shown graft-induced dyskinesias. In fact, the only one of our patients who showed painful drug-induced dyskinesias at the onset of this trial, namely P5, at her 2-year follow-up, is now mostly free of this symptom. It is possible to speculate that, unlike fetal dopaminergic neurons20 or the embryonic-derived differentiated neurons, our NPCs not only enhance putaminal dopaminergic neurotransmission where it is necessary but might down-regulate it in dyskinetic patients (UPDRS-IV).
Motor dysfunction in PD has been attributed to the loss of dopaminergic innervation in the caudate nuclei. Notably, however, the reduced RAC binding we have observed here in the patients’ caudate nuclei after bilateral putaminal NPC grafting does not appear to be related to enhanced motor performance. P1, for example, showed the greatest RAC uptake decrease in the caudate nuclei (Fig. 3) compared with baseline without motor function improvement (UPDRS III). Furthermore, although it is becoming increasingly clear that this striatal structure may have an even greater role in patients’ neuropsychological condition66, P1’s neuropsychological scores at both his 1- and 2-year post-surgery evaluations also remained basically unchanged. Therefore, it may be reasonable to speculate that reduced post-surgery RAC does not necessarily signify enhanced endogenous dopamine availability (as we suggest occurs in the putamen to explain motor function improvement), but rather an inability of the ligand to bind to down-regulated post-synaptic D2 receptors in the caudate nuclei67. D2 receptor down-regulation has been attributed to chronic dopaminergic therapy or to the need for structural adaptation of the post-synaptic dopaminergic system in response to the progressive loss of nigrostriatal neurons. In fact, the increasing loss of DTBZ uptake in the caudate nuclei of all our grafted patients at their 1- and 2-year post-surgery PET evaluations suggests significant pre-synaptic deterioration, greater in fact than that seen in the putamen (contrary to the PD subjects described by Bohnen et al.52). This state of neurodegeneration in the caudate nuclei of our patients is further supported by the decreased levels of FDOPA uptake (an index of dopaminergic pre-synaptic nerve terminal activity) at all times 1 and 2 years post-surgery for patients P2 and P4; patient P7 showed a short-lived 20% rise in FDOPA uptake 1 year after surgery but then a 65% loss (the greatest loss in the group) at his 2-year follow-up.
For now, we have no way of knowing with certainty if the grafted NPCs did in fact differentiate into dopaminergic neurons or other cell types, or to what extent surgery or grafted cells might have induced changes in the host striatum that could account for the improved motor function. However, we do know that sham surgery does not ameliorate PD-induced behavioral deficits20,59,68,69. Therefore, it would appear that grafted tissue is required for a restorative effect.
The fact that, 4 years after surgery, six out of seven patients improved their UPDRS III scores as compared with their baseline, allows us to suggest that the NPCs can either arrest or reverse the progression of PD. A certain deterioration 2 years after surgery as compared with the first year results can be interpreted as a sign of fading effect. However, reversal of the second-year deterioration as compared with the first year by the fourth year can indicate that the restorative mechanisms of NPCs require longer time to fully materialize their potential.
Neuropsychological Testing
In spite of their motor dysfunctions, patients’ neuropsychological performance before and after grafting was basically normal (cognitive assessments) or only mildly affected (depression and anxiety), with the exception of three cases who showed severe anxiety. This does not seem to be the case for PD groups in other populations70, showing a 40% and 60% incidence of depression and dementia, respectively.
Limitations and Future Prospects
Although we were able to demonstrate the safety of the intervention and obtain an initial impression of the potential of our NPC cell line to affect parkinsonian motor symptoms, a limitation of the present study is the small heterogeneous patient sample size, a drawback we aim to address in future studies.
We are fully aware that an effective PD therapy will require the replacement not only of dopaminergic, but also of non-dopaminergic (serotoninergic, cholinergic, and noradrenergic) innervations that contribute to PD pathology71,72, which is precisely the reason why we have chosen undifferentiated NPCs for brain grafting to treat PD. In the future, we intend to determine if the NPC cell line tested here is able to migrate purposefully to other affected areas of diseased PD brains, besides the striatum, to restore not only motor, but also non-motor functions, and perhaps even support neuroprotection73 and arrest progression of the disease, by responding in these areas to site-specific biochemical and biostructural cues as we have seen occurs in animal models of human neurological diseases grafted with these same cells35,36.
Conclusion
The intervention appears to be safe. At 4 years post-transplantation we can now report that undifferentiated NPCs can be delivered by stereotaxis bilaterally to the putamina of patients with moderate to severe PD, without any complications attributable to the procedure. None of the patients showed unwanted motor disturbances (dyskinesias), tumor formation, or any detectable immune responses to the grafted cells. In six out of seven cases the procedure resulted in improved motor function, and a better response to L-DOPA (particularly in one remarkable case). Given that PD is a progressive disease, the 4-year data suggest that at the very least the NPCs are able to stop or slow down the motor deterioration one would expect to see in this timespan. Patients’ PET functional scans suggest that their improvements might be at least in some cases attributable to enhanced putaminal dopaminergic neurotransmission. Patients’ satisfactory pre-surgery neuropsychological scores were unaffected by grafting.
The encouraging results reported here merit further controlled clinical trials designed to test further safety and efficacy of intraputaminally grafted cell culture-manufactured undifferentiated NPCs to treat multiple deficiencies in PD.
Ethical Considerations
All procedures described herein were performed in compliance with the scientific and ethics committees of the Hospital Angeles Pedregal, the Declaration of Helsinki, and approved by the Comisión Federal para la Protección de Riesgos Sanitarios (COFEPRIS, CMN2012-027). Fetal tissue was obtained in 1998 at Good Samaritan Hospital (Los Angeles, CA) with institutional approval #97-11-01, within their neuro-transplantation program. Human fetal brain tissue donor informed consent forms were obtained in accordance with NIH guidelines for use of fetal tissue (available from: http://stemcells.nih.gov/staticresources/news/newsArchives/fr25au00-136.htm), as well as US federal, and CA state laws. PD patients selected for the study signed letters of informed consent of their own free will, after the protocol had been carefully described to them, emphasizing the use of human fetal tissue, and the requirement for their commitment to lengthy pre- and post-surgery evaluations. Tissue donors and NPC recipients remained unknown to each other.
Acknowledgements
Our gratitude goes out to Jessica Ochoa for her key role in the manufacturing of the cells, to Dr. Carlos Landa Solís for his assistance with cell manipulation prior to grafting, and to Dr. Saúl R. León-Hernández for his help in the course of some issues related to the preparation of the manuscript.
Footnotes
Ethical Approval: This study was approved by the the Comisión Federal para la Protección de Riesgos Sanitarios (COFEPRIS, CMN2012-027) and by the ethics committee of the Hospital Angeles Pedregal (HAP 2330). Fetal tissue was obtained in 1998 at Good Samaritan Hospital (Los Angeles, CA) with institutional approval #97-11-01, within their neuro-transplantation program.
Statement of Human Rights: All of the procedures involving human subjects were conducted in compliance with the Declaration of Helsinki.
Statement of Informed Consent: Human fetal brain tissue donor informed consent forms were obtained in accordance with NIH guidelines for use of fetal tissue (available from: http://stemcells.nih.gov/staticresources/news/newsArchives/fr25au00-136.htm), as well as US federal, and CA state laws. PD patients selected for the study signed letters of informed consent of their own free will, after the protocol had been carefully described to them, emphasizing the use of human fetal tissue, and the requirement for their commitment to lengthy pre- and post-surgery evaluations. Tissue donors and NPC recipients remained unknown to each other.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Oleg Kopyov is a co-owner and CSO of Celavie Biosciences, LLC. Alex Kopyov is Vice-President of Celavie Biosciences, LLC. Celavie Biosciences LLC owns the following patents pertaining to the stem cells used in the trial: (1) Compositions and Methods for Propagation of Neural Progenitor Cells. Issued patents: United States 7632681, China ZL200480039675.1, Japan 4676442. Pending Patents: Canada 2547827, European Union 04812676.7, India 1807/KOLNP/2006. 2) Pluripotent Cells. Issued patents: United States 8367406. Pending Patents: United States 13/744,262.
No fees were charged to patients who received no payment either; tissue donor and center performing the abortion received no payments; researchers were not paid for their work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Authors received material and logistic support from Celavie Biosciences, LLC. Partial research support was also received from an UNAM grant, UNAM-DGAPA-PAPIIT IT201115.
ORCID iD: A. Kopyov  https://orcid.org/0000-0003-0420-138X
https://orcid.org/0000-0003-0420-138X
References
- 1. Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20(4):415–455. [DOI] [PubMed] [Google Scholar]
- 2. Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. [DOI] [PubMed] [Google Scholar]
- 3. Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–376. [DOI] [PubMed] [Google Scholar]
- 4. Ostrosky-Solís F, Quintanar L, Madrazo I, Drucker-Colín R, Franco-Bourland R, Leon-Meza V. Neuropsychological effects of brain autograft of adrenal medullary tissue for the treatment of Parkinson’s disease. Neurology. 1988;38(9):1442–1450. [DOI] [PubMed] [Google Scholar]
- 5. Chaudhuri KR, Healy DG, Schapira AHV, National institute for clinical excellence. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5(3):235–245. [DOI] [PubMed] [Google Scholar]
- 6. Grahn JA, Parkinson JA, Owen AM. The role of the basal ganglia in learning and memory: neuropsychological studies. Behav Brain Res. 2009;199(1):53–60. [DOI] [PubMed] [Google Scholar]
- 7. Hornykiewicz O, Kish SJ. Biochemical pathophysiology of Parkinson’s disease. Adv Neurol. 1987;45:19–34. [PubMed] [Google Scholar]
- 8. Barone P. Neurotransmission in Parkinson’s disease: beyond dopamine. Eur J Neurol. 2010;17(3):364–376. [DOI] [PubMed] [Google Scholar]
- 9. Heumann R, Moratalla R, Herrero MT, Chakrabarty K, Drucker-Colín R, Garcia-Montes JR, Simola N, Morelli M. Dyskinesia in Parkinson’s disease: mechanisms and current non-pharmacological interventions. J Neurochem. 2014;130(4):472–489. [DOI] [PubMed] [Google Scholar]
- 10. Jourdain VA, Schindlbeck KA, Tang CC, Niethammer M, Choi YY, Markowitz D, Nazem A, Nardi D, Carras N, Feigin A, Ma Y, Peng S, Dhawan V, Eidelberg D. Increased putamen hypercapnic vasoreactivity in levodopa-induced dyskinesia. JCI Insight. 2017;2(20):96411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hacker ML, Tonascia J, Turchan M, Currie A, Heusinkveld L, Konrad PE, Davis TL, Neimat JS, Phibbs FT, Hedera P, Wang L, Shi Y, Shade DM, Sternberg AL, Drye LT, Charles D. Deep brain stimulation may reduce the relative risk of clinically important worsening in early stage Parkinson’s disease. Parkinsonism Relat. Disord. 2015;21(10):1177–1183. [DOI] [PubMed] [Google Scholar]
- 12. Odekerken VJJ, van Laar T, Staal MJ, Mosch A, Hoffmann CFE, Nijssen PCG, Beute GN, van Vugt JPP, Lenders MWPM, Contarino MF, Mink MSJ, Bour LJ, van den Munckhof P, Schmand BA, de Haan RJ, Schuurman PR, de Bie RMA. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol. 2013;12(1):37–44. [DOI] [PubMed] [Google Scholar]
- 13. Alexander GE. Biology of Parkinson’s disease: pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin Neurosci. 2004;6(3):259–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kieburtz K. Issues in neuroprotection clinical trials in Parkinson’s disease. Neurology. 2006;66(10 suppl 4):S50–S57. [DOI] [PubMed] [Google Scholar]
- 15. Madrazo I, Drucker-Colín R, Díaz V, Martínez-Mata J, Torres C, Becerril JJ. Open microsurgical autograft of adrenal medulla to the right caudate nucleus in two patients with intractable Parkinson’s disease. N Engl J Med. 1987;316(14):831–834. [DOI] [PubMed] [Google Scholar]
- 16. Goetz CG, Tanner CM, Penn RD, Stebbins GT, Gilley DW, Shannon KM, Klawans HL, Comella CL, Wilson RS, Witt T. Adrenal medullary transplant to the striatum of patients with advanced Parkinson’s disease: 1-year motor and psychomotor data. Neurology. 1990;40(2):273–276. [DOI] [PubMed] [Google Scholar]
- 17. Madrazo I, Franco-Bourland R, Ostrosky-Solis F, Aguilera M, Cuevas C, Zamorano C, Morelos A, Magallon E, Guizar-Sahagun G. Fetal homotransplants (ventral mesencephalon and adrenal tissue) to the striatum of parkinsonian subjects. Arch Neurol. 1990;47(12):1281–1285. [DOI] [PubMed] [Google Scholar]
- 18. Sass KJ, Buchanan CP, Westerveld M, Marek KL, Farhi A, Robbins RJ, Naftolin F, Vollmer TL, Leranth C, Roth RH. General cognitive ability following unilateral and bilateral fetal ventral mesencephalic tissue transplantation for treatment of Parkinson’s disease. Arch Neurol. 1995;52(7):680–686. [DOI] [PubMed] [Google Scholar]
- 19. Freed CR, Breeze RE, Rosenberg NL, Schneck SA, Kriek E, Qi JX, Lone T, Zhang YB, Snyder JA, Wells TH. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson’s disease. N Engl J Med. 1992;327(22):1549–1555. [DOI] [PubMed] [Google Scholar]
- 20. Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344(10):710–719. [DOI] [PubMed] [Google Scholar]
- 21. Armstrong RJ, Rosser AE, Dunnett SB, Barker RA. Neural stem cell technology as a novel treatment for Parkinson’s disease. Methods Mol Med. 2001;62:289–307. [DOI] [PubMed] [Google Scholar]
- 22. Kordower JH, Goetz CG, Chu Y, Halliday GM, Nicholson DA, Musial TF, Marmion DJ, Stoessl AJ, Sossi V, Freeman TB, Olanow CW. Robust graft survival and normalized dopaminergic innervation do not obligate recovery in a Parkinson disease patient. Ann Neurol. 2017;81(1):46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lach B, Grimes D, Benoit B, Minkiewicz-Janda A. Caudate nucleus pathology in Parkinson’s disease: ultrastructural and biochemical findings in biopsy material. Acta Neuropathol (Berl.). 1992;83(4):352–360. [DOI] [PubMed] [Google Scholar]
- 24. Maiti P, Manna J, Dunbar GL. Current understanding of the molecular mechanisms in Parkinson’s disease: targets for potential treatments. Transl. Neurodegener. 2017. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5655877/. [Accessed 2018 Oct 2];6. [DOI] [PMC free article] [PubMed]
- 25. Politis M, Loane C. Serotonergic dysfunction in Parkinson’s disease and its relevance to disability. ScientificWorldJournal. 2011;11:1726–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kish SJ, Tong J, Hornykiewicz O, Rajput A, Chang LJ, Guttman M, Furukawa Y. Preferential loss of serotonin markers in caudate versus putamen in Parkinson’s disease. Brain J Neurol. 2008;131(Pt 1):120–131. [DOI] [PubMed] [Google Scholar]
- 27. Tan SKH, Hartung H, Sharp T, Temel Y. Serotonin-dependent depression in Parkinson’s disease: a role for the subthalamic nucleus? Neuropharmacology. 2011;61(3):387–399. [DOI] [PubMed] [Google Scholar]
- 28. Liu AKL, Chang RCC, Pearce RKB, Gentleman SM. Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol (Berl.). 2015;129(4):527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bjorklund LM, Sánchez-Pernaute R, Chung S, Andersson T, Chen IYC, McNaught KSP, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, Isacson O. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci U S A. 2002;99(4):2344–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martínez-Cerdeño V, Barrilleaux BL, McDonough A, Ariza J, Yuen BTK, Somanath P, Le CT, Steward C, Horton-Sparks K, Knoepfler PS. Behavior of xeno-transplanted undifferentiated human induced pluripotent stem cells is impacted by microenvironment without evidence of tumors. Stem Cells Dev. 2017;26(19):1409–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ibanez L, Dube U, Saef B, Budde J, Black K, Medvedeva A, Del-Aguila JL, Davis AA, Perlmutter JS, Harari O, Benitez BA, Cruchaga C. Parkinson disease polygenic risk score is associated with Parkinson disease status and age at onset but not with alpha-synuclein cerebrospinal fluid levels. BMC Neurol. 2017;17(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lill CM, Klein C. What would Dr. James Parkinson think today? The role of genetics in Parkinson’s disease. Mov Disord Off J Mov Disord Soc. 2017;32(8):1115–1116. [DOI] [PubMed] [Google Scholar]
- 33. Cannon JR, Greenamyre JT. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol Sci Off J Soc Toxicol. 2011;124(2):225–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barker RA, Barrett J, Mason SL, Björklund A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol. 2013;12(1):84–91. [DOI] [PubMed] [Google Scholar]
- 35. Uhlendorf TL, Nuryyev RL, Kopyov AO, Ochoa J, Younesi S, Cohen RW, Kopyov OV. Efficacy of two delivery routes for transplanting human neural progenitor cells (NPCs) into the spastic han-wistar rat, a model of ataxia. Cell Transplant. 2017;26(2):259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nuryyev RL, Uhlendorf TL, Tierney W, Zatikyan S, Kopyov O, Kopyov A, Ochoa J, Trigt WV, Malone CS, Cohen RW. Transplantation of human neural progenitor cells reveals structural and functional improvements in the spastic Han-Wistar rat model of ataxia. Cell Transplant. 2017;26(11):1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N, Holloway RG, Moore CG, Wenning GK, Yahr MD, Seidl L; Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. Movement disorder society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord Off J Mov Disord Soc. 2004;19(9):1020–1028. [DOI] [PubMed] [Google Scholar]
- 38. Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. 1967. Neurology. 2001;57(10 suppl 3):S11–S26. [PubMed] [Google Scholar]
- 39. Srivastava V, Yang Z, Hung IFN, Xu J, Zheng B, Zhang MY. Identification of dominant antibody-dependent cell-mediated cytotoxicity epitopes on the hemagglutinin antigen of pandemic H1N1 influenza virus. J Virol. 2013;87(10):5831–5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tario JD, Muirhead KA, Pan D, Munson ME, Wallace PK. Tracking immune cell proliferation and cytotoxic potential using flow cytometry. Methods Mol Biol Clifton NJ. 2011;699:119–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schwab RS, England AC, Schwab ZJ, England A, Schwab R. Projection technique for evaluating surgery in Parkinson’s disease. Third Symp Park Dis. 1969:152–7. [Google Scholar]
- 42. Ostrosky-Solís F, Ardila A, Rosselli M. NEUROPSI: a brief neuropsychological test battery in Spanish with norms by age and educational level. J Int Neuropsychol Soc JINS. 1999;5(5):413–433. [DOI] [PubMed] [Google Scholar]
- 43. Jacobs DM, Marder K, Côté LJ, Sano M, Stern Y, Mayeux R. Neuropsychological characteristics of preclinical dementia in Parkinson’s disease. Neurology. 1995;45(9):1691–1696. [DOI] [PubMed] [Google Scholar]
- 44. Robles R, Varela R, Jurado S, Páez F. Versión mexicana del inventario de ansiedad de Beck: propiedades psicométricas. Rev Mex Psicol. 2001;18(2):211–218. [Google Scholar]
- 45. Jurado S, Villegas ME, Méndez L. La estandarización del Inventario de Depresión de Beck para los residentes de la ciudad de México. Salud Ment. 1998;21(3):26–31. [Google Scholar]
- 46. Ishibashi K, Ishii K, Oda K, Mizusawa H, Ishiwata K. Competition between 11C-raclopride and endogenous dopamine in Parkinson’s disease. Nucl Med Commun. 2010;31(2):159–166. [DOI] [PubMed] [Google Scholar]
- 47. Snow BJ, Tooyama I, McGeer EG, Yamada T, Calne DB, Takahashi H, Kimura H. Human positron emission tomographic [18F]fluorodopa studies correlate with dopamine cell counts and levels. Ann Neurol. 1993;34(3):324–330. [DOI] [PubMed] [Google Scholar]
- 48. Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow CW, Tanner C, Marek K; Parkinson Study Group. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351(24):2498–2508. [DOI] [PubMed] [Google Scholar]
- 49. Kilbourn M, Lee L, Vander Borght T, Jewett D, Frey K. Binding of alpha-dihydrotetrabenazine to the vesicular monoamine transporter is stereospecific. Eur J Pharmacol. 1995;278(3):249–252. [DOI] [PubMed] [Google Scholar]
- 50. Vander Borght T, Kilbourn M, Desmond T, Kuhl D, Frey K. The vesicular monoamine transporter is not regulated by dopaminergic drug treatments. Eur J Pharmacol. 1995;294(2–3):577–583. [DOI] [PubMed] [Google Scholar]
- 51. Gilman S, Koeppe RA, Adams KM, Junck L, Kluin KJ, Johnson-Greene D, Martorello S, Heumann M, Bandekar R. Decreased striatal monoaminergic terminals in severe chronic alcoholism demonstrated with (+)[11C]dihydrotetrabenazine and positron emission tomography. Ann Neurol. 1998;44(3):326–333. [DOI] [PubMed] [Google Scholar]
- 52. Bohnen NI, Albin RL, Koeppe RA, Wernette KA, Kilbourn MR, Minoshima S, Frey KA. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab Off J Int Soc Cereb. Blood Flow Metab. 2006;26(9):1198–1212. [DOI] [PubMed] [Google Scholar]
- 53. Rutka JT, Giblin JR, Balkissoon R, Wen D, Myatt CA, McCulloch JR, Rosenblum ML. Characterization of fetal human brain cultures. Development of a potential model for selectively purifying human glial cells in culture. Dev Neurosci. 1987;9(3):154–173. [DOI] [PubMed] [Google Scholar]
- 54. Ishii T, Eto K. Fetal stem cell transplantation: past, present, and future. World J Stem Cells. 2014;6(4):404–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J Off Publ Fed Am Soc Exp Biol. 2005;19(7):681–693. [DOI] [PubMed] [Google Scholar]
- 56. Brands K, Colvin E, Williams LJ, Wang R, Lock RB, Tuch BE. Reduced immunogenicity of first-trimester human fetal pancreas. Diabetes. 2008;57(3):627–634. [DOI] [PubMed] [Google Scholar]
- 57. Ben-David U, Kopper O, Benvenisty N. Expanding the boundaries of embryonic stem cells. Cell Stem Cell. 2012;10(6):666–677. [DOI] [PubMed] [Google Scholar]
- 58. Spencer DD, Robbins RJ, Naftolin F, Marek KL, Vollmer T, Leranth C, Roth RH, Price LH, Gjedde A, Bunney BS. Unilateral transplantation of human fetal mesencephalic tissue into the caudate nucleus of patients with Parkinson’s disease. N Engl J Med. 1992;327(22):1541–1548. [DOI] [PubMed] [Google Scholar]
- 59. Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54(3):403–414. [DOI] [PubMed] [Google Scholar]
- 60. Ma Y, Peng S, Dhawan V, Eidelberg D. Dopamine cell transplantation in Parkinson’s disease: challenge and perspective. Br Med Bull. 2011;100:173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Ann Neurol. 2005;57(2):298–302. [DOI] [PubMed] [Google Scholar]
- 62. Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg. 2005;102(2):216–222. [DOI] [PubMed] [Google Scholar]
- 63. Kadereit S, Trounson A. In vitro immunogenicity of undifferentiated pluripotent stem cells (PSC) and derived lineages. Semin Immunopathol. 2011;33(6):551–562. [DOI] [PubMed] [Google Scholar]
- 64. Louveau A, Harris TH, Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 2015;36(10):569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ma Y, Feigin A, Dhawan V, Fukuda M, Shi Q, Greene P, Breeze R, Fahn S, Freed C, Eidelberg D. Dyskinesia after fetal cell transplantation for parkinsonism: a PET study. Ann Neurol. 2002;52(5):628–634. [DOI] [PubMed] [Google Scholar]
- 66. van Beilen M, Portman AT, Kiers HAL, Maguire RP, Kaasinen V, Koning M, Pruim J, Leenders KL. Striatal FDOPA uptake and cognition in advanced non-demented Parkinson’s disease: a clinical and FDOPA-PET study. Parkinsonism Relat. Disord. 2008;14(3):224–228. [DOI] [PubMed] [Google Scholar]
- 67. Antonini A, Schwarz J, Oertel WH, Pogarell O, Leenders KL. Long-term changes of striatal dopamine D2 receptors in patients with Parkinson’s disease: a study with positron emission tomography and [11C]raclopride. Mov Disord Off J Mov. Disord. Soc. 1997;12(1):33–38. [DOI] [PubMed] [Google Scholar]
- 68. Taylor JR, Elsworth JD, Sladek JR, Collier TJ, Roth RH, Redmond DE. Sham surgery does not ameliorate MPTP-induced behavioral deficits in monkeys. Cell Transplant. 1995;4(1):13–26. [DOI] [PubMed] [Google Scholar]
- 69. Nakamura T, Dhawan V, Chaly T, Fukuda M, Ma Y, Breeze R, Greene P, Fahn S, Freed C, Eidelberg D. Blinded positron emission tomography study of dopamine cell implantation for Parkinson’s disease. Ann Neurol. 2001;50(2):181–187. [DOI] [PubMed] [Google Scholar]
- 70. Hughes TA, Ross HF, Musa S, Bhattacherjee S, Nathan RN, Mindham RH, Spokes EG. A 10-year study of the incidence of and factors predicting dementia in Parkinson’s disease. Neurology. 2000;54(8):1596–1602. [DOI] [PubMed] [Google Scholar]
- 71. Lang AE, Obeso JA. Challenges in Parkinson’s disease: restoration of the nigrostriatal dopamine system is not enough. Lancet Neurol. 2004;3(5):309–316. [DOI] [PubMed] [Google Scholar]
- 72. Lang AE, Obeso JA. Time to move beyond nigrostriatal dopamine deficiency in Parkinson’s disease. Ann Neurol. 2004;55(6):761–765. [DOI] [PubMed] [Google Scholar]
- 73. Henchcliffe C, Severt WL. Disease modification in Parkinson’s disease. Drugs Aging. 2011;28(8):605–615. [DOI] [PubMed] [Google Scholar]