Abstract
The urothelium of the bladder, renal pelvis, ureter and urethra is maintained through the regulated proliferation and differentiation of urothelial stem and progenitor cells. These cells provide a rich source of a novel urothelial cell therapy approach that could be used to protect, regenerate, repair and restore a damaged urothelium. Urothelial injury caused by physical, chemical and microbial stress is the pathological basis of cystitis (bladder inflammation). The loss of urothelial integrity triggers a series of inflammatory events, resulting in pain and hematuria such as hemorrhage cystitis and interstitial cystitis. Here we investigate a novel cell therapy strategy to treat cystitis by protecting the urothelium from detrimental stresses through intravesically instilling porcine urothelial cells (PUCs) into the bladder. Using a chemical-induced urothelial injury mouse model of cyclophosphamide (CPP)-induced hemorrhagic cystitis, we determined how the intravesical instillation of PUCs could protect the urothelium from toxic attack from CPP metabolites. We show that intravesical PUC instillation protected the bladder from toxic chemical attack in mice receiving CPP with reduced inflammation and edema. Compared with the vehicle control mice, the proliferative response to chemical injury and apoptotic cells within the bladder tissues were reduced by intravesical PUC treatment. Furthermore, the urothelium integrity was maintained in the intravesical PUC-treated group. After xenogeneic PUCs were introduced and adhered to the mouse urothelium, immunological rejection responses were observed with increased neutrophil infiltration in the lamina propria and higher immune-related gene expression. Our findings provide an innovative and promising intravesical PUC cell therapy for cystitis with urothelial injury by protecting the urothelium from noxious agents.
Keywords: intravesical, porcine urothelial cell (PUC), urothelium, cystitis, bladder, cytotherapy, cyclophosphamide (CPP), xenogeneic
Introduction
The urothelium lines not only the inner surface of the urinary bladder, but also the renal pelvis, ureters, and proximal urethra. The urothelium is composed of three distinctive cell layers from the top high-resistance permeability barrier umbrella cells, intermediate cells with limited proliferative potential, to long-term label-retaining basal cells with stem/progenitor cell properties on the bottom1,2. The urothelium is separated by a basement membrane from the lamina propria, a thin layer of fibroblast-like stromal cells, and submucosal, smooth muscle with serous layers lying underneath the lamina propria1. Cystitis, the inflammation of bladder, is the results of urothelial injury due to physical and chemical stress or microbial infection3. The urothelial injury causes hemorrhagic cystitis, resulting in bleeding from the bladder mucosa, which mainly arises from anticancer chemotherapy or radiotherapy for the treatment of pelvic malignancies4.
The currently available therapeutic options have limited efficacy to treat hemorrhagic cystitis. In terms of an anticancer agent, cyclophosphamide (CPP), which is extensively used as an anticancer and immunosuppressive agent with urologic side effects, induces hemorrhagic cystitis, and prophylactic measures are used to dilute the chemicals such as hyperhydration, and bladder irrigation, or to detoxify, such as using 2-mercaptoethane sodium sulphonate (Mesna). For treatment, oral aminocaproic acid, estrogens, sodium pentosane polysulphate, endoscopic laser coagulation, intramural orgotein (free radical scavenger), intravesical regimens of alum and formalin, hyperbaric oxygen, urinary diversion and even cystectomy are used, but their efficacy is variable3,5,6. The isolation and expansion of urothelial cells from bladders have been used in urethral or bladder tissue engineering because their effects in tissue regeneration or wound healing processes in the urinary tract7,8. To find more effective prevention and treatment approaches for cystitis, in our previous work, we have demonstrated that intravesical instillation of normal murine urothelial cells could reduce the urothelial injury in a CPP-induced cystitis mouse model9. And here to apply this cell-based cytotherapy in future clinical application, we explore this novel cell-based approach to treat hemorrhagic cystitis further by intravesical instillation of normal porcine urothelial cells (PUCs) into injured bladders to protect and repair urothelial injury. Normal PUCs isolated from porcine bladders are shown to be capable of self-renewal, proliferation and differentiation into fully mature cells as well as sharing many equivalent cell biological properties to human urothelial cells10–12, suggesting their abilities to repair and restore the injured urothelium.
The direct use of PUCs in clinic is a practice of xenogeneic cell therapy or cellular xenotransplantation13,14. Xenotransplantation has long been proposed as a promising solution for donor shortage in transplantation, but faces enormous immunological barriers from hyperacute, acute vascular rejection to delayed xenograft rejection, sequentially15. However xenogeneic cell therapy or cellular xenotransplantation is more achievable as a clinical treatment because no vascular tissues are involved and only cells are transplanted. The most prominent example is porcine islet transplantation, which has been shown to rescue diabetes after intraportal xenotransplantation in immunosuppressed nonhuman primates16,17. Our intravesical instillation of PUCs to treat cystitis, represents a promising therapeutic approach in clinical xenogeneic cell therapy. In our approach, since the cells are not directly exposed to blood, there is no instant blood-mediated inflammatory reaction triggered, which destructs transplanted cells, but instead, cells are transplanted into the bladder, which acts as a reservoir for folding cells in place for a certain amount of time. The same route of administration has long been applied by intravesical Bacillus Calmette–Guérin (BCG) immunotherapy as an adjuvant therapy after transurethral resection of high grade nonmuscle invasive bladder cancer18.
Although a variety of treatment options are provided for hemorrhagic cystitis, their responses vary in patients. Therefore, it is necessary to find effective treatment modalities and preventive strategies to reduce the morbidity and mortality of hemorrhagic cystitis. Based on the ‘cell drug’ concept, in the present study, we investigate whether innovative intravesical PUC cell therapy could be applied as a well tolerated and effective therapeutic modality to treat hemorrhagic cystitis. In this manuscript, we show that intravesical instillation of PUCs could relieve the injuries inflicted by CPP and protect the integrity of the urothelium as well as the cells from apoptosis as a promising well tolerated and effective cell therapy for cystitis.
Materials and Methods
PUC Isolation and Culture
Porcine urinary bladders for urothelial cell isolation were obtained from a local abattoir. Bladder tissue was dissected into 1–2 cm2 tissue pieces and treated with dispase II dissolved in Hank’s balanced salt solution (HBSS; Gibco, Carlsbad, CA, USA) to strip the urothelium. The stripped urothelium was minced into small pieces and incubated in a cell isolation solution with type VI collagenase (Worthington, Lakewood, NJ, USA) in HBSS (100 U/ml) to disaggregate the cells. Porcine epithelial cells were isolated and grown in Dulbecco’s modified Eagle medium (DMEM)/Ham’s F12 medium supplemented with antibiotics (penicillin 100 U/ml, streptomycin 100 mg/ml, amphotericin B 5 mg/ml) and 10% fetal bovine serum (FBS) following the previously reported mouse urothelial cell culture protocol9.
Western Blotting
PUC cell lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Burlington, MA, USA). PVDF membranes were incubated with primary antibodies: rabbit cytokeratin 5 antibody (LifeSpan BioSciences, Seattle, WA, USA; LS-C352426, 1:500) and mouse cytokeratin 14 antibody (Santa Cruz, Dallas, TX, USA; sc-53253, 1:200) and β-actin (C4) antibody (Santa Cruz; sc-47778, 1:500) respectively and then incubated with second antibodies: goat anti-mouse immunoglobulin (Ig)G-HRP (Santa Cruz), or goat anti-rabbit IgG-HRP (Santa Cruz) respectively. The immunoblotted proteins were detected using an enhanced Immobilon Western chemiluminescent HRP substrate reagent (Millipore) and visualized by the Chemidoc XRS chemiluminescent gel documentation cabinet detection system (Bio-Rad, Hércules, CA, USA).
Immunofluorescent Staining
PUC cells were grown on the chamber slides to 50–70% confluence for staining. The cells were fixed in 4% paraformaldehyde and permeabilized in 0.3% Triton X-100. The slides were incubated with primary antibody against CK5 (1:250) or CK14 (1:100) for overnight in cold room. On the next day, cells were washed with 0.1% Tween-20 in PBS. Subsequently, the cells were incubated with secondary antibodies: anti-rabbit IgG-PE (Santa Cruz; sc-3739,1:100), or goat anti-mouse IgG-Alexa Fluor 488 (Fisher Scientific, Fair Lawn, NJ, USA). In the next step, cells were counterstained with 4′6-diamidino-2-phenylindole (DAPI). Fluorescence images were recorded using a Nikon Eclipse 80i fluorescence microscope with an attached charge-coupled device (CCD) camera.
Mice
Female C3H/HeJ 9-week-old mice were obtained from Lasco (Taipei, Taiwan). To induce chemical injury-induced cystitis, the mice were intraperitoneally injected with 300 mg/kg CPP (Cayman, Ann Arbor, MI, USA) in 100 µl PBS solution. The CPP-induced cystitis mice were randomly divided into two groups: one vehicle-treated control and one PUC-treated group 4 h after CPP injection. In the vehicle-treated group, mice were subjected to intravesical instillation of vehicle and in the PUC-treated group, 106 cells (passage 1 to passage 5) were intravesically instilled into bladders. Intravesical instillation was performed as described before9. The urine remaining in the bladder was removed by mild compression of the lower abdominal regions under isoflurane anesthesia (2.5%). A catheter tube was introduced into the urinary bladder via the urethra, and the vehicle or PUC cells were instilled into the urinary bladder using a syringe and remained for 50 minutes, allowing the PUC cells to adhere. All mice were euthanized 20 h after the treatment and the urinary bladders were quickly removed, weighed, and fixed in 10% neutral-buffered formalin for 24 h. The tissue was cut longitudinally, routinely embedded in paraffin, sectioned and stained with hematoxylin and eosin (H&E) for histopathological examination. Alcian blue staining of urothelium glycosaminoglycan (GAG) layers was also performed to evaluate urothelial integrity. The edema score was determined by examining sections of each bladder to reflect the severity of cystitis. The score was determined as: 0 = no evident sign of edema; 1 = mild edema expanding the lamina propria to less than double the normal size; 2 = moderate edema doubling the size of the lamina propria compared with normal; 3 = moderate edema tripling the size of the lamina propria compared with normal; and 4 = severe edema of the lamina propria and detrusor expanding the lamina propria more than three times the normal size. The animal protocols were approved by the institutional IACUC committee of the China Medical University.
TUNEL Assay
The bladder tissue sections (5 μm). were deparaffinized, rehydrated and detected for DNA fragmentation. DNA fragmentation in apoptotic cells was detected by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL). following the manufacturer’s protocol. (TUNEL BrightGreen Apoptosis Detection Kit, Vazyme Biotec, Nanjing, Jiangsu, China). All images were obtained using a microscope (Nikon Eclipse 80i) with an attached CCD camera.
Carboxyfluorescein Diacetate Succinimidyl Ester Labeling
PUC cells (106 cells/ml) were suspended in PBS containing 0.1% bovine serum albumin. The 5 mM carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) stock (Molecular Probes, Carlsbad, CA, USA) in dimethyl sulfoxide (Fisher Scientific) was diluted to 10 μM, in PBS and added to an equal volume of PUC cells with incubation at 37°C for 30 mins. The labeling reaction was stopped for 1 min by adding an equal volume of 10% FBS medium. The CFDA-SE-labeled cells were washed twice with PBS and resuspend for further use.
Immunohistochemistry
Immunohistochemistry staining was performed on the bladder tissue sections (5 μm) using UltraVision™ Quanto Detection System HRP DAB (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. The following primary antibodies were used in the experiments: anti-Ki67 (Abcam, Cambridge, MA, USA; ab16667, 1:100), and anti-myeloperoxidase (ZETA Corporation, Arcadia, CA, USA; Clone EP151, 1:100). All images were obtained using a microscope (Nikon Eclipse 80i) and a camera (Nikon DS-Qi1Mc).
Quantitative Real Time Polymerase Chain Reaction
For quantitative real time polymerase chain reaction (qRT-PCR) analysis, total RNA was extracted from bladder tissue using TRIzol® (Invitrogen, Carlsbad, CA, USA). The qRT-PCR mRNA analyses were performed using a one-step RT-PCR kit with SYBR Green and a Bio-Rad iCycler (Bio-Rad, Hércules, CA, USA). The relative quantity of gene expression was analyzed by the 2(-ΔΔCt) method with normalization to the endogenous control β-actin and the RNA level in the naïve control was set to 1. The sequences of primers were: COX-2-F 5′-CAGACAACAT AAACTGCGCCTT-3′ and COX-2-R 5′-GA T ACACCTCTCCACCAA TGACC -3′, 71 bp. Inducible nitric oxide synthase (iNOS)-F 5′-CGAAACGCTTCACTTCCAA-3′ and iNOS-R 5′-TGAGCCTATATTGCTGTGGCT-3′, 55 bp. Interleukin (IL)-6-F 5′-GAGGATACCACTCCCAACAGACC-3′ and IL-6-R 5′-AAGTGCATCATCGTTGTTCATACA-3′, 141 bp.
Statistical Analysis
Statistical analysis was performed using PASW Statistics 18. All data are presented as mean ± SD, and two group comparisons were done with a two-tailed Student’s t-test. A value of P<0.05 was taken as statistically significant.
Results
Expanded PUCs Express Urothelial Progenitor/Stem Cell Markers: Cytokeratin 5 (CK5) and Cytokeratin 14 (CK14)
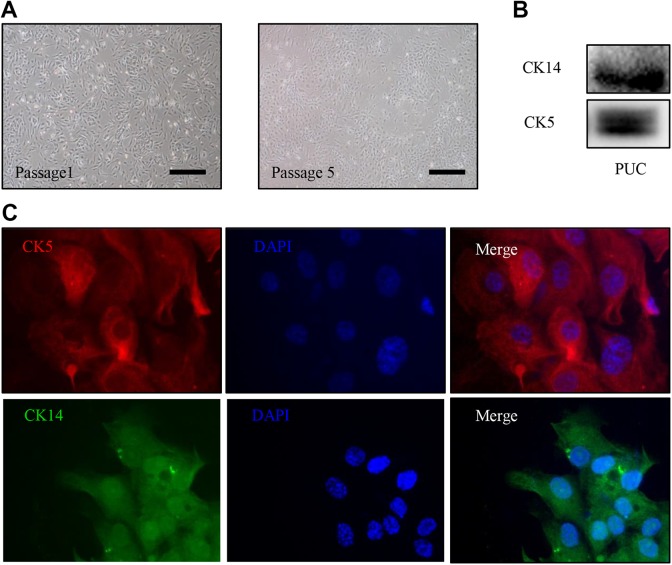
To test our therapeutic hypothesis, we first isolated and expanded urothelial cells for porcine bladder urothelium. The bladder urothelium contains urothelial stem/progenitor cells for repair and regeneration of injured urothelium19, providing a rich source of urothelial cells. Using porcine bladders, we isolated and expanded urothelial cells (Fig. 1A.) The urothelial stem/progenitor cell markers: CK5 and CK14, which are cytokeratin proteins and co-express within the urothelial stem/progenitor cells20 were used to characterize the expanded PUCs. Western blotting assay (Fig. 1B) and immunofluorescent staining (Fig. 1C) of PUCs revealed that both CK5 and CK14 are expressed in PUC cells.
Fig 1.
Expanded PUC cells express urothelial stem/progenitor cell markers. (A) The representative images of PUC cells at passage 1 and 5 are shown. (B) Western blot analysis for CK5 and CK14 expression was performed on passage 3 PUC cells. (C) Immunofluorescence staining of passage 3 PUC cells was carried out with antibodies against CK5 (red) and CK14 (green), nuclei stained with DAPI, 400× magnification. Scale bars represent 50 µm.
DAPI: 4′6-diamidino-2-phenylindole; PUC: porcine urothelial cell.
Intravesical PUC Instillation Attenuates CPP-Induced Cystitis
To demonstrate the therapeutic effect of intravesical instillation of PUCs on hemorrhagic cystitis, we used the CPP-induced cystitis mouse model, which has been widely used as an animal model of urothelial injury and hemorrhagic cystitis2,21,22. We examined whether intravesical instillation of PUCs could protect the urothelium from toxic chemicals. CPP-injected female mice were divided into two groups, the control group received the vehicle control and the treatment group was given 106 PUCs at 4 h after CPP injection and all mice were sacrificed at 24 h post injection of CPP for experiments. The bladder weights and severity of hemorrhage and inflammation were observed. Compared with the vehicle-treated controls, we found that intravesical PUC treatment rescued the injuries caused by CPP injection with reduced bladder hemorrhages, congestion and weight (Fig. 2A and B). The histological analysis of bladder H&E-stained sections also showed that the edema of the lamina propria and edema score was lower as well as less exfoliation was observed (Fig. 2C and D) in the PUC-treated group. These results suggest that intravesical administration of PUCs could protect the urothelium from attacks by noxious chemicals to reduce urothelial injury.
Fig 2.
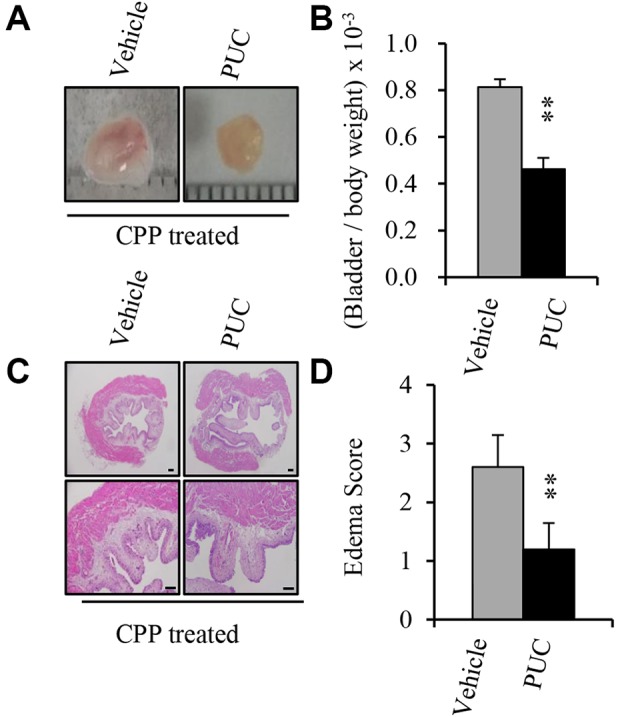
Cyclophosphamide-induced cystitis with changes of the urothelium and Intravesical instillation of PUC attenuates CPP-induced cystitis. The mice were intraperitoneally injected with 300 mg/kg CPP (300 mg/kg) and 106/100 μl, normal PUCs or vehicle control was intravesically instilled into the bladders 4 h after the CPP injection, and the bladder morphology, weight, H&E staining and bladder RNA extractions were performed after 24 h. (A) Representative images of the mouse bladders on day 1 after cyclophosphamide injection from the vehicle control and PUC-treated groups. Hemorrhage is evident. Note the obvious congestion, enlargement and hemorrhaging in the bladder of CPP-treated mice without intravesical PUC instillation. (B) Bladder weight/body weight ratio in the control and PUC-treated groups. (C) Representative histological changes the hematoxylin-eosin-stained bladder sections. Urothelium in the vehicle controls shows some remaining urothelial cells, and denuded areas. (D) Edema index of the bladder sections of vehicle control and PUC-treated mice. The data represent the mean ± SD of three independent experiments. Scale bars represent 50 µm. **P<0.01 versus the vehicle control.
CPP: cyclophosphamide; H&E: hematoxylin and eosin; PUC: porcine urothelial cell.
Intravesical PUC Instillation Represses CPP-Induced Urothelial Injury
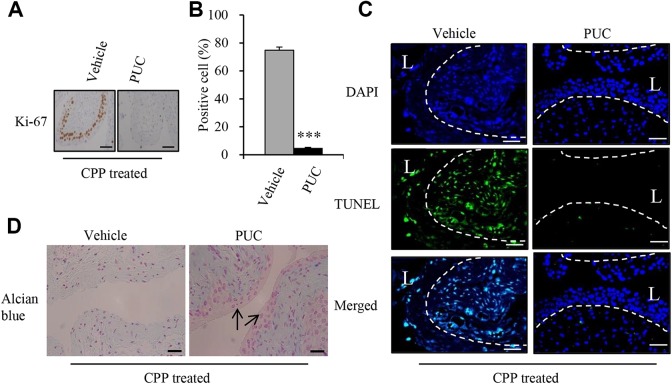
The bladder shifts from near-quiescence to a highly proliferative state of basal cells within the urothelium in response to urothelial injury2. We therefore examined the proliferative response of basal cells to chemical injury within the bladder by the proliferative marker Ki67 Immunohistochemistry (IHC) staining on bladder sections and counted the number of Ki67-positive urothelial cells in the urinary bladder. Ki 67-positive cells were mostly observed in the basal cell layer, but also appeared in intermediate cell layer (Fig. 3A). We found that the proportion of Ki67-postiive cells in urothelium was significantly lower in PUC-treated mice compared with vehicle-treated controls 24 h after CPP injection (Fig. 3A and B), suggesting that injury-induced proliferation in basal urothelial cells was decreased by PUCs. To assess the effect of intravesical instillation of PUCs on cell damage inflicted by CPP, we used a TUNEL assay to detect apoptotic cells caused by CPP. The results showed that CPP-induced apoptotic cells were observed in both urothelial cells in the urothelium and the stromal cells in the lamina propria, but intravesical PUC treatment markedly decreased the apoptotic cells compared with the vehicle controls (Fig. 3C). To study the urothelial integrity affected by CPP treatment, Alcian blue staining was performed and demonstrated a clear absence of the superficial layer of GAGs in vehicle controls, but was preserved in the PUC-treated group (Fig. 3D). These results indicated that intravesical PUC treatment reduced CPP-induced cell proliferation, cell apoptosis and maintained the urothelial integrity.
Fig 3.
Intravesical instillation of PUC reduces urothelial injury. (A) Representative immunostaining images of Ki67 were performed on bladder sections from vehicle control and treated groups to determine injury-induced proliferation during CPP-induced injury. (B) Quantification of urothelial cell proliferation post CPP injection and treatment. Ki67-positive cells are shown as a percent of total cells 24 h after injection of CPP (C). Representative TUNEL staining images of bladder sections. (D) Alcian blue staining of GAG layers. Arrows indicate the GAG layers. Dotted lines demarcate the border between urothelium and lamina propria. Data are presented as mean ± SD and significance was calculated by an unpaired Student’s t-test. ***P < 0.01 versus the vehicle control. Scale bars represent 50 µm.
CPP: cyclophosphamide; DAPI: 4′6-diamidino-2-phenylindole; GAG: glycosaminoglycan; L: bladder lumen; PUC: porcine urothelial cell; TUNEL: terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling.
The Attachment of PUC on the Urothelium and Host Rejection
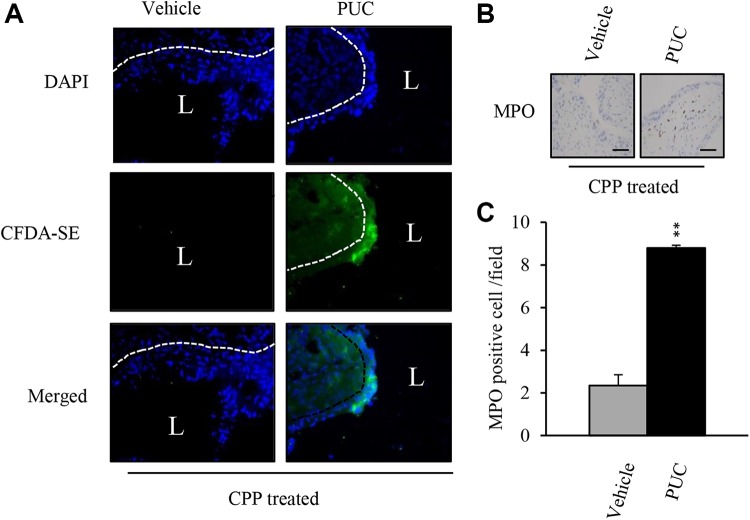
To investigate the cellular events of intravesical PUC treatment, we examined whether PUC could adhere to the urothelium. CFDA-SE labeled PUC cells were intravesically instilled into the bladders of CPP-induced urothelial injury mice. And after 24 h, CFDA-SE labeled PUC cells were observed on the bladder urothelium of intravesical PUC-treated mice, but not the vehicle-treated bladders (Fig. 4A). Since xenogeneic urothelial cells were used, xenograft rejection was expected. Because neutrophils appeared to be the first cells recruited into graft sites due to innate immunity. The neutrophil marker, myeloperoxidase (MPO) IHC was performed on bladder sections. Compared with vehicle-treated mice, More neutrophils (MPO-positive cells) infiltrated the lamina propria of PUC-treated mouse bladders, whereas neutrophils only infiltrated only a few to moderate numbers in the lamina propria of vehicle-treated mouse bladders (Fig. 4B and C).
Fig 4.
PUC cell attachment on the urothelium and neutrophil infiltration. (A) CFDA-SE-labeled PUC cells or vehicle control were intravesically instilled into bladders of CPP-treated mice and bladder tissue cryosections from the two groups of mice were counterstained with DAPI and visualized using fluorescence microscopy for CFDA-SE-labeled PUCs. (B) Representative immunohistological images of infiltrating neutrophils on bladder sections. (C) Quantitation of MPO-positive cells in bladder sections from CPP-treated mice with or without PUC treatment. **P<0.01 versus the vehicle control.
CFDA-SE: carboxyfluorescein diacetate succinimidyl ester; CPP: cyclophosphamide; DAPI: 4′6-diamidino-2-phenylindole; MPO: myeloperoxidase; PUC: porcine urothelial cell.
Intravesical PUC Treatment Induced Immune-Related Gene Expression in CPP-Induced Cystitis Bladders
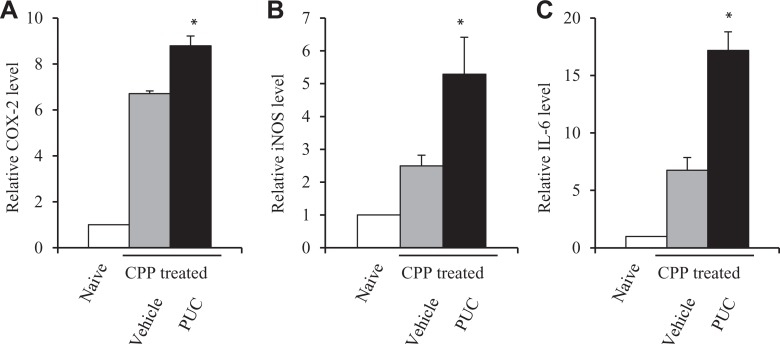
CPP caused bladder inflammation. Furthermore, xenogeneic PUC cells were instilled into the bladder and adhered to the urothelium to protect urothelial injury induced by CPP, but because they were not nonself cells, the host rejection immune responses could be turned on. Therefore, to investigate the inflammation and rejection immune responses, quantitative PCR analysis on related immune gene expression such as COX-223,24, iNOS25 and IL-626 was performed using bladder tissue samples. And the results revealed the increased changes in mRNA expression of immune-related genes: COX-2 (Fig. 5A), iNOS (Fig. 5B) and IL-6 (Fig. 5C) in CPP-treated mice and intravesical PUC treatment further enhanced their expression. The increased expression was associated with bladder inflammation, but although intravesical instillation of PUCs attenuated the inflammation, they induced rejection immune responses and therefore immune-related gene expression was further enhanced.
Fig 5.
Immune-related gene expression in bladder tissues. The expression of immune-related gene mRNA in bladders from vehicle-treated or PUC-treated CPP-injured mice. Relative mRNA expression was measured as the mRNA level in bladder tissue from naïve mice (no any treatment) was set as 1. The expression of COX-2 (A), iNOS (B) and IL-6 mRNA (C) is bladder tissues of vehicle control and PUC-treated mice 24 h after CPP treatment. Data are presented as mean ± SD, and significance was calculated by a paired Student’s t-test. *P<0.05 versus the vehicle control.
CPP: cyclophosphamide; IL: interleukin; iNOS: inducible nitric oxide synthase; PUC: porcine urothelial cell.
Discussion
Urothelial injury is the pathological basis of cystitis. In term of CPP-induced hemorrhagic cystitis, CPP, an alkylating agent for treating both malignant and non-neoplastic diseases, causes mucosal ulceration, transmural edema and epithelial necrosis, resulting gross hematuria and irritative voiding symptoms27. Although many therapeutic options are available, none provides optimal efficacy for most patients. Therefore, it is important to investigate innovative therapeutics with novel mechanisms for treating cystitis. Since the destruction of the urothelial barrier is observed in CPP-induced cystitis, in this article, we adopted a cell-based cytotherapy strategy to target the urothelial injury. We demonstrate that the intravesical instillation of PUCs into bladders reduces the detrimental injury caused by CPP injection, with less injury-induced cell proliferation, cell apoptosis and the maintenance of urothelial integrity. However, because xenogeneic PUCs are transplanted onto the urothelium, the host rejection immunity is triggered, which could eliminate the transplanted cells. The present study confirmed our previously reported therapeutic effect of intravesical urothelial cell instillation on chemical-induced cystitis using PUCs, providing an innovative xenogeneic cell-based therapeutic modality to treat cystitis in future clinical practice.
The pathogeneses of CPP-induced hemorrhagic cystitis is linked to its toxic metabolite (acrolein) concentrated in the bladder, which is a reactive, unsaturated aldehyde that induces the production of reactive oxygen species and nitric oxide to damage the urothelium28,29. In the clinic, Mesna is recommended to be used in patients receiving high-dose CPP as a prophylactic agent to bind acrolein and block its entrance into urothelial cells, causing cell death30. Our results suggest that the intravesical PUC cell therapy could also have effects to protect the urothelium from the toxic chemical attack, not only in decreasing the damage caused by CPP, but also preventing cell death that could be induced by CPP, but in an acrolein-independent action31. There are several intravesical agents used to treat various degree of hemorrhagic cystitis such as alum (aluminum ammonium sulfate or aluminum potassium sulfate), aminocaproic acid, silver nitrate, and hyaluronic acid for persistent or moderate hematuria to stop bleeding32–35. For severe and/or refractory hemorrhagic cystitis, even intravesical instillation of dilute formalin is used to cause coagulation to contain hemorrhage in the mucosa and submucosa36. Our intravesical instillation of PUCs is a xenogeneic cell therapy, which could induce rejection responses by activating blood coagulation through adaptive immune responses and proinflammatory reactions after xenotransplantation. The adhesion of PUCs on the urothelium may trigger coagulation in blood vessels to stop hemorrhage. Therefore, in addition to previously used agents that have either modest effects or severe toxicity, we here provide a safer therapeutic option with normal urothelial cells to treat hemorrhagic cystitis. However, the detailed characterizations of PUCs used in the treatment and whether the therapeutic effect of intravesical PUCs is superior to the current treatments for CPP-induced hemorrhagic cystitis needs further investigating.
Our innovative therapeutic use of intravesical PUC cell therapy for urothelial injury-based cystitis is one type of xenogeneic cell therapy. Xenogeneic cell therapy, or cellular xenotransplantation, has been studied for decades as a regenerative way to treat diseases which could not be cured by molecule-based drugs only, such as diabetes, Parkinson’s disease and Huntington’s disease37,38. However, there are two major challenges to the successful use of xenogeneic cell therapy in treating human diseases: immune-mediated rejection and the risk of xenozoonotic (cross-species) infections. A number of approaches such as the use of immunosuppressive drugs and genetically modified pigs with human transgenes and xenoantigen knockouts, mixed chimerism and induced tolerance have been tested and shown to reduce rejection and extend the survival of xenografts39–41. Mitigation of xenozoonotic risks can be achieved through the use of donor pigs from designated pathogen-free herds42. A recent study reported that it is possible to activate porcine endogenous retroviruses genome-wide by precisely remove porcine endogenous retrovirus genes anywhere in the genome of pigs with CRISPR/Cas9 genome editing technology43, which has made xenotransplantation safer in reducing the risk of xenozoonotic infection and more possible in clinical uses. With these advances in xenotransplantation, our intravesical PUC cell therapy that uses cells instead of vascularized organs, therefore has less immunological barriers and could be potentially applied in clinical settings as a novel, well tolerated and effective therapeutic modality to treat cystitis.
Footnotes
Author Contribution: Chi-Ping Huang and Chi-Cheng Chen contributed equally to this manuscript.
Ethical Approval: The animal study protocol was approved by the Institutional Animal Care and Use Committee of China Medical University, Taiwan.
Statement of Human and Animal Rights: All procedures were performed according to the protocol approved by the Institutional Animal Care and Use Committee, China Medical University.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the MOST grant (106-2314-B-303-017 -), CMU grant (CMU105-S-51), CMUH grant (DMR-CELL-1807), Buddhist Tzu Chi General Hospital grant (TTCRD 104-12) and in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW105-TDU-B-212-133019).
References
- 1. Balsara ZR, Li X. Sleeping beauty: awakening urothelium from its slumber. Am J Physiol. 2017;312(4):F732–F743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA. Hedgehog/wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472(7341):110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee G, Romih R, Zupancic D. Cystitis: from urothelial cell biology to clinical applications. Biomed Res Int. 2014;2014:473536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bon K, Lichtensteiger CA, Wilson SG, Mogil J. Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: species and strain differences. J Urol. 2003;170(3):1008–1012. [DOI] [PubMed] [Google Scholar]
- 5. Sandhu SS, Goldstraw M, Woodhouse CR. The management of haemorrhagic cystitis with sodium pentosan polysulphate. BJU Int. 2004;94(6):845–847. [DOI] [PubMed] [Google Scholar]
- 6. Chong KT, Hampson NB, Corman JM. Early hyperbaric oxygen therapy improves outcome for radiation-induced hemorrhagic cystitis. Urology. 2005;65(4):649–653. [DOI] [PubMed] [Google Scholar]
- 7. Zhang Y, Atala A. Urothelial cell culture. Methods Mol Biol. 2013;1037:27–43. [DOI] [PubMed] [Google Scholar]
- 8. Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367(9518):1241–1246. [DOI] [PubMed] [Google Scholar]
- 9. Huang CP, Chen CC, Shyr CR. The anti-tumor effect of intravesical administration of normal urothelial cells on bladder cancer. Cytotherapy. 2017;19(10):1233–1245. [DOI] [PubMed] [Google Scholar]
- 10. Larsson HM, Gorostidi F, Hubbell JA, Barrandon Y, Frey P. Clonal, self-renewing and differentiating human and porcine urothelial cells, a novel stem cell population. Plos One. 2014;9(2):e90006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turner AM, Subramaniam R, Thomas DF, Southgate J. Generation of a functional, differentiated porcine urothelial tissue in vitro. Eur Urol. 2008;54(6):1423–1432. [DOI] [PubMed] [Google Scholar]
- 12. Pokrywczynska M, Czapiewska M, Jundzill A, Bodnar M, Balcerczyk D, Kloskowski T, Nowacki M, Marszalek A, Drewa T. Optimization of porcine urothelial cell cultures: best practices, recommendations, and threats. Cell Biol Int. 2016;40(7):812–820. [DOI] [PubMed] [Google Scholar]
- 13. Edge AS, Gosse ME, Dinsmore J. Xenogeneic cell therapy: current progress and future developments in porcine cell transplantation. Cell Transplant. 1998;7(6):525–539. [DOI] [PubMed] [Google Scholar]
- 14. Huang CP, Chen CC, Shyr CR. Xenogeneic cell therapy provides a novel potential therapeutic option for cancers by restoring tissue function, repairing cancer wound and reviving anti-tumor immune responses. Cancer Cell Int. 2018;18:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meier RPH, Muller YD, Balaphas A, Morel P, Pascual M, Seebach JD, Buhler LH. Xenotransplantation: back to the future? Transpl Int. 2018;31(5):465–477. [DOI] [PubMed] [Google Scholar]
- 16. Hering BJ, Wijkstrom M, Graham ML, Hardstedt M, Aasheim TC, Jie T, Ansite JD, Nakano M, Cheng J, Li W, Moran K, Christians U, Finnegan C, Mills CD, Sutherland DE, Bansal-Pakala P, Murtaugh MP, Kirchhof N, Schuurman HJ. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12(3):301–303. [DOI] [PubMed] [Google Scholar]
- 17. Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, Bello-Laborn H, Hacquoil B, Strobert E, Gangappa S, Weber CJ, Pearson TC, Rajotte RV, Larsen CP. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12(3):304–306. [DOI] [PubMed] [Google Scholar]
- 18. Sylvester RJ, van der MA, Lamm DL. Intravesical bacillus calmette-guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168(5):1964–1970. [DOI] [PubMed] [Google Scholar]
- 19. Ho PL, Kurtova A, Chan KS. Normal and neoplastic urothelial stem cells: getting to the root of the problem. Nat Rev Urol. 2012;9(10):583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ho PL, Lay EJ, Jian W, Parra D, Chan KS. Stat3 activation in urothelial stem cells leads to direct progression to invasive bladder cancer. Cancer Res. 2012;72(13):3135–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Papafotiou G, Paraskevopoulou V, Vasilaki E, Kanaki Z, Paschalidis N, Klinakis A. Krt14 marks a subpopulation of bladder basal cells with pivotal role in regeneration and tumorigenesis. Nat Commun. 2016;7:11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, Hayward NJ, McNamara CR, Xue F, Moran MM, Strassmaier T, Uykal E, Owsianik G, Vennekens R, De Ridder D, Nilius B, Fanger CM, Voets T. Inhibition of the cation channel trpv4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci U S A. 2010;107(44):19084–19089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang X, Ma N, Szabolcs MJ, Zhong J, Athan E, Sciacca RR, Michler RE, Anderson GD, Wiese JF, Leahy KM, Gregory S, Cannon PJ. Upregulation of cox-2 during cardiac allograft rejection. Circulation. 2000;101(4):430–438. [DOI] [PubMed] [Google Scholar]
- 24. Rangel EB, Moura LA, Franco MF, Pacheco-Silva A. Up-regulation of cyclooxygenase-2 during acute human renal allograft rejection. Clin Transplant. 2005;19(4):543–550. [DOI] [PubMed] [Google Scholar]
- 25. Cannon P, Yang X, Szabolcs MJ, Ravalli S, Sciacca RR, Michler RE. The role of inducible nitric oxide synthase in cardiac allograft rejection. Cardiovasc Res. 1998;38(1):6–15. [DOI] [PubMed] [Google Scholar]
- 26. Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2(10):907–916. [DOI] [PubMed] [Google Scholar]
- 27. Stillwell TJ, Benson RC., Jr Cyclophosphamide-induced hemorrhagic cystitis. A review of 100 patients. Cancer. 1988;61(3):451–457. [DOI] [PubMed] [Google Scholar]
- 28. Korkmaz A, Oter S, Deveci S, Ozgurtas T, Topal T, Sadir S, Bilgic H. Involvement of nitric oxide and hyperbaric oxygen in the pathogenesis of cyclophosphamide induced hemorrhagic cystitis in rats. J Urol. 2003;170(6 Pt 1):2498–2502. [DOI] [PubMed] [Google Scholar]
- 29. Korkmaz A, Topal T, Oter S. Pathophysiological aspects of cyclophosphamide and ifosfamide induced hemorrhagic cystitis; implication of reactive oxygen and nitrogen species as well as parp activation. Cell Biol Toxicol. 2007;23(5):303–312. [DOI] [PubMed] [Google Scholar]
- 30. Vose JM, Reed EC, Pippert GC, Anderson JR, Bierman PJ, Kessinger A, Spinolo J, Armitage JO. Mesna compared with continuous bladder irrigation as uroprotection during high-dose chemotherapy and transplantation: a randomized trial. J Clin Oncol. 1993;11(7):1306–1310. [DOI] [PubMed] [Google Scholar]
- 31. Hughes FM, Jr, Corn AG, Nimmich AR, Pratt-Thomas JD, Purves JT. Cyclophosphamide induces an early wave of acrolein-independent apoptosis in the urothelium. Adv Biosci Biotechnol. 2013;4(88). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Westerman ME, Boorjian SA, Linder BJ. Safety and efficacy of intravesical alum for intractable hemorrhagic cystitis: a contemporary evaluation. Int Braz J Urol. 2016;42(6):1144–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abt D, Bywater M, Engeler DS, Schmid HP. Therapeutic options for intractable hematuria in advanced bladder cancer. Int J Urol. 2013;20(7):651–660. [DOI] [PubMed] [Google Scholar]
- 34. Montgomery BD, Boorjian SA, Ziegelmann MJ, Joyce DD, Linder BJ. Intravesical silver nitrate for refractory hemorrhagic cystitis. Turk J Urol. 2016;42(3):197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shao Y, Lu GL, Shen ZJ. Comparison of intravesical hyaluronic acid instillation and hyperbaric oxygen in the treatment of radiation-induced hemorrhagic cystitis. BJU Int. 2012;109(5):691–694. [DOI] [PubMed] [Google Scholar]
- 36. Donahue LA, Frank IN. Intravesical formalin for hemorrhagic cystitis: analysis of therapy. J Urol. 1989;141(4):809–812. [DOI] [PubMed] [Google Scholar]
- 37. Matsumoto S, Abalovich A, Wechsler C, Wynyard S, Elliott RB. Clinical benefit of islet xenotransplantation for the treatment of type 1 diabetes. EBioMedicine. 2016;12:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fink JS, Schumacher JM, Ellias SL, Palmer EP, Saint-Hilaire M, Shannon K, Penn R, Starr P, VanHorne C, Kott HS, Dempsey PK, Fischman AJ, Raineri R, Manhart C, Dinsmore J, Isacson O. Porcine xenografts in parkinson’s disease and huntington’s disease patients: preliminary results. Cell Transplant. 2000;9(2):273–278. [DOI] [PubMed] [Google Scholar]
- 39. Mohiuddin MM, Singh AK, Corcoran PC, Thomas ML, 3 rd, Clark T, Lewis BG, Hoyt RF, Eckhaus M, Pierson RN, 3 rd, Belli AJ, Wolf E, Klymiuk N, Phelps C, Reimann KA, Ayares D, Horvath KA. Chimeric 2c10r4 anti-cd40 antibody therapy is critical for long-term survival of gtko.Hcd46.Htbm pig-to-primate cardiac xenograft. Nat Commun. 2016;7:11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lan P, Wang L, Diouf B, Eguchi H, Su H, Bronson R, Sachs DH, Sykes M, Yang YG. Induction of human t-cell tolerance to porcine xenoantigens through mixed hematopoietic chimerism. Blood. 2004;103(10):3964–3969. [DOI] [PubMed] [Google Scholar]
- 41. Kalscheuer H, Onoe T, Dahmani A, Li HW, Holzl M, Yamada K, Sykes M. Xenograft tolerance and immune function of human t cells developing in pig thymus xenografts. J Immunol. 2014;192(7):3442–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spizzo T, Denner J, Gazda L, Martin M, Nathu D, Scobie L, Takeuchi Y. First update of the international xenotransplantation association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes--chapter 2a: Source pigs--preventing xenozoonoses. Xenotransplantation. 2016;23(1):25–31. [DOI] [PubMed] [Google Scholar]
- 43. Niu D, Wei HJ, Lin L, George H, Wang T, Lee IH, Zhao HY, Wang Y, Kan Y, Shrock E, Lesha E, Wang G, Luo Y, Qing Y, Jiao D, Zhao H, Zhou X, Wang S, Wei H, Guell M, Church GM, Yang L. Inactivation of porcine endogenous retrovirus in pigs using crispr-cas9. Science. 2017;357(6357):1303–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]