The density of malaria parasites is a key determinant of whether an infected individual develops fever. While the pyrogenic threshold for malaria parasite density has been well studied, there are no analogous data on the antigen levels associated with fever during infection.
KEYWORDS: Plasmodium falciparum, pyrogenic threshold
ABSTRACT
The density of malaria parasites is a key determinant of whether an infected individual develops fever. While the pyrogenic threshold for malaria parasite density has been well studied, there are no analogous data on the antigen levels associated with fever during infection. Samples from 797 afebrile and 457 febrile outpatients from two provinces in Angola with known concentrations of histidine-rich protein 2 (HRP2), aldolase, and lactate dehydrogenase (LDH) antigens were analyzed by Bayesian latent class modeling to attribute malarial etiology to the fevers and to estimate the sensitivity and specificity of different antigen thresholds for detection of malaria fevers. Among patients with aldolase or LDH levels detectable with a bead-based assay, the concentrations of these two antigens did not differ between afebrile and febrile patients. In contrast, the concentrations of HRP2 were substantially higher in febrile HRP2-positive patients than in afebrile HRP2-positive patients. When HRP2 concentrations were considered, the malaria-attributable fractions of fever cases were 0.092 in Huambo Province and 0.39 in Uíge Province. Diagnostic tests detecting HRP2 with limits of detection (LODs) in the range of 3,000 to 10,000 pg/µl would provide ideal sensitivity and specificity for determination of malarial etiology among febrile persons.
INTRODUCTION
The presence of malaria parasites (genus Plasmodium) in a human host does not necessarily equate with acute clinical disease. In fact, a large proportion of Plasmodium falciparum-infected individuals are asymptomatic at any given time (1, 2); consequently, many febrile patients in areas in which malaria is endemic have incidental parasitemia. Malaria parasites must be present at sufficiently high densities in order to cause symptoms such as fever, especially among individuals living in high-transmission settings who have been exposed multiple times and developed antimalarial adaptive immunity. The P. falciparum parasite density necessary to provoke fever, i.e., the pyrogenic threshold, is dependent on factors that influence host responses and immunity and can vary substantially according to setting and age (3–6) and between low- and high-transmission seasons (4, 7).
Estimation of the pyrogenic threshold requires comparison of parasite densities in symptomatic (febrile) and asymptomatic (afebrile) persons. Statistical methods can be used to estimate the distribution of parasite densities simultaneously in patients with and without malaria-related febrile disease by comparing the distribution in febrile patients with that in afebrile controls. This in turn allows estimation of sensitivities and specificities of different parasite density thresholds to allow determination of the pyrogenic threshold and to inform the case definition of clinical malaria (8, 9). Despite substantial variation, published pyrogenic thresholds have typically been in the range of 200 to 5,000 parasites/µl (0.05 to 1.25 parasites/1,000 red blood cells) (3–10). Application of pyrogenic thresholds allows estimation of the true attributable fraction of fever cases that are due to malaria, which can be confounded by the presence of malaria parasites in afebrile patients.
Over the past decade, malaria diagnosis has changed drastically with the development and roll out of rapid diagnostic tests (RDTs), which detect the presence of parasite antigens. The majority of malaria laboratory testing performed worldwide is now by RDTs, with over 300 million RDTs per year being used (11). The majority of RDTs detect the Plasmodium falciparum-specific histidine-rich protein 2 (HRP2), although the emergence of HRP2-deleted parasites and the presence of non-falciparum malaria often warrant combination tests that detect essential parasite proteins, such as lactate dehydrogenase (LDH) or aldolase.
Despite the widespread adoption of RDTs, there is no consensus regarding optimal antigen concentration detection limits. RDTs are tested and certified through a systematic product testing process implemented by the Foundation for Innovative New Diagnostics, the World Health Organization, and the U.S. Centers for Disease Control and Prevention. Products are tested against a panel of cultured parasite strains and global isolates at 200 and 2,000 parasites/µl (12). These thresholds were chosen specifically because of previous work showing the pyrogenic threshold to be in this range (10).
Despite being used at fixed parasite densities, reference strains may produce a wide range of antigen levels (12). Different parasite strains are known to express antigens differently, and there are also documented differences in antigen expression in cultured versus wild parasites (10). At a density of 200 parasites/μl, levels of HRP2 (median, 6,800 pg/ml [interquartile range [IQR], 2,000 to 17,000 pg/ml]) and LDH (median, 13,000 pg/ml [IQR, 7,000 to 22,000 pg/ml]) in reference P. falciparum strains are both higher and more variable than aldolase levels (median, 1,300 pg/ml [IQR, 600 to 1,900 pg/ml]). Due to the variations in antigen levels, it follows that pyrogenic thresholds estimated in terms of parasite densities do not translate directly into antigen concentration thresholds. Furthermore, operating characteristics of antigen tests with defined limits of detection (LODs) cannot be inferred directly from sensitivities and specificities estimated from parasite density data. Additionally, it has been suggested that antigen concentrations may be more accurate representations of true P. falciparum infection burdens than microscopy or nucleic acid test results, since sequestered parasites would likely be more consistently overlooked by the latter methods (13, 14).
We are not aware of any studies that have assessed pyrogenic thresholds expressed as antigen concentrations. To address this gap, the concentrations of Plasmodium antigens were compared between febrile and afebrile patients presenting to health facilities in Angola (15). Samples from these patients had been analyzed previously for the concentrations of three malaria antigens, with a subset also having been analyzed by PCR for the presence of parasite nucleic acids (16). These data were used to determine the overall malaria-attributable fraction and to estimate sensitivities and specificities with different diagnostic cutoff values.
MATERIALS AND METHODS
Sample collection.
Previously collected samples of blood on filter paper from 797 afebrile and 457 febrile patients of all ages from 89 randomly selected health facilities in low-transmission Huambo Province (14% malaria test positivity in febrile cases) and high-transmission Uíge Province (53% test positivity) in Angola (15) were analyzed. Patients had been tested with RDTs by survey staff members, but microscopy data were unavailable. Fever was defined as a temperature above 37.5°C measured in the facility or a patient self-report of fever in the past 24 h. Plasmodium falciparum is the primary malaria species in these areas of Angola; of the 466 samples showing evidence of malaria infection, 463 (99.4%) were positive for P. falciparum antigens and/or nucleic acids, with the remaining 3 (0.6%) being positive for Plasmodium ovale.
These samples had been assayed previously for concentrations of P. falciparum-specific HRP2, pan-Plasmodium LDH, and pan-Plasmodium aldolase using a multiplex bead-based immunoassay (16). Briefly, three different bead regions were separately coupled with one of three antibodies specific for the three malaria antigens. After incubation with a blood sample, bound antigen was detected by the fluorescence signal with detection antibodies specific for each antigen. The mean fluorescent intensity value minus the background value for each antigen for each sample was transformed into a concentration by using standard curves for purified recombinant antigens (17). The reportable LODs for the assay were 152 pg/ml for HRP2, 195,205 pg/for LDH, and 325 pg/ml for aldolase.
Statistical analysis.
First, a crude estimate of the malaria-attributable fraction of fevers (λ), taking into account only fever and antigen presence, was estimated as the ratio of excess fevers associated with positivity for the antigen to the total number of fevers,
| (1) |
where is the fraction of the population positive for a given antigen, is the overall prevalence of fever, and and are the prevalences of fever in antigen-positive and antigen-negative patients, respectively. The analysis was performed for antigen positivity for each of the three antigens. Three different definitions of HRP2 positivity were used, namely, any detectable HRP2 above the LOD of the ultrasensitive bead-based assay (152 pg/ml), an HRP2 concentration above 200 pg/ml (the practical LOD of ultrasensitive RDTs [14]), and an HRP2 concentration above 3,000 pg/ml, the estimated LOD of the conventional RDT used in the Angola survey (18).
Next, the continuous values of the three antigen concentrations were characterized and plotted, and pairwise agreement in antigen positivity for the three antigens in patients was assessed using Cohen’s kappa statistic (19). Pairwise correlations between the three antigen concentrations were assessed with Altman-Bland plots, which give clouds of points with no clear pattern when the two variables being compared are measuring the same quantity (20).
A second estimate for the malaria-attributable fraction of fevers was then calculated using a statistical latent class model fit to the continuous antigen levels for each patient (8). Briefly, the antigen levels for febrile patients (x1, x2, … xn) were modelled as a sample from a mixture with two components, (corresponding to negative samples equivalent to control [afebrile] samples) and ϕ (corresponding to positive samples with higher values of x than the controls), so that
| (2) |
where , , and λi is the probability that a fever case in category i has true malarial etiology (which is constrained to increase with i). Cases with negligible antigen levels were assumed by definition not to be true malaria cases, so λ1 = 0, making ϕi, θi, and λi identifiable. The underlying assumption of the model is that, when malaria is not the cause of fever in a patient, the antigen concentration should be the same whether or not the person is sick with another pathogen and whether or not the other pathogen causes fever. A latent class model (9) was used to obtain Bayesian estimates of all of the quantities in equation 2, using a Markov chain Monte Carlo algorithm in the package WinBUGS (21).
The sensitivities and specificities of different candidate HRP2 thresholds were calculated as functions of ϕi, θi, and λi. For the case definition using the ith HRP2 concentration threshold, (corresponding to the lower boundary of the category), the values were computed as and , where k is the total number of HRP2 categories included in the analysis. Correspondingly, the proportion of fever cases attributable to malaria (the latent class attributable fraction, ) was computed as .
This model was separately applied to the HRP2, aldolase, and LDH antigen concentration data from each province. Additionally, the model was fit to HRP2 data stratified according to age (under or over 5 years of age) to investigate differences in malaria attributable fractions according to age.
Ethical review.
Blood samples were collected during a health facility survey (approved by the Office of the Associate Director for Science in the Center for Global Health at the U.S. Centers for Disease Control and Prevention and by the Angolan Ministry of Health). Participants consented to laboratory testing of samples. A separate protocol covering analysis of antigen concentrations in stored samples was approved by the Office of the Associate Director for Science in the Center for Global Health at the U.S. Centers for Disease Control and Prevention (protocol CGH 2018-034).
RESULTS
Fever rates and crude estimates of malaria-attributable fractions.
The percentages of antigen-positive patients with fever ranged from 77.8% HRP2-positive (conventional RDT LOD) in Huambo Province to 91.4% LDH-positive in Huambo Province; the percentages of antigen-negative patients with fever did not exceed 58.0% (Table 1). In the lower-transmission province of Huambo Province, the crude estimates of the malaria-attributable fraction ranged from 0.030 to 0.040. In Uíge Province, which had substantially higher rates of antigen positivity, the crude malaria-attributable fraction (not considering the degree to which antigen concentrations exceeded the LODs) ranged from 0.076 (LDH) to 0.21 (HRP2, with the LOD of an ultrasensitive RDT). These crude attributable fractions were rather similar for the three different LODs for HRP2 and also for the other two antigens, even though the numbers of samples exceeding the LDH and aldolase cutoff values and the different HRP2 cutoff values varied substantially (Table 1).
TABLE 1.
Fever rates and attributable fractions of fever according to antigen positivity among outpatients from Angolan health facilities
| Parameter and province | All samples | HRP2 positive (any level)a | HRP2 positive (ultrasensitive RDT) | HRP2 positive (conventional RDT)b | Aldolase positive (any level)a | LDH positive (any level)a |
|---|---|---|---|---|---|---|
| Huambo Province | ||||||
| No. with fever | 364 | 69 | 67 | 56 | 44 | 32 |
| No. with no fever | 243 | 31 | 25 | 16 | 8 | 3 |
| Proportion with fever (%) | 60.0 | 69.0 | 72.8 | 77.8 | 84.6 | 91.4 |
| Proportion of test-negative cases with fever (%) | 58.2 | 57.7 | 57.6 | 57.7 | 58.0 | |
| Excess proportion with fever (%) | 10.8 | 15.2 | 20.2 | 27.0 | 33.4 | |
| Excess no. of fever cases | 10.8 | 13.9 | 14.5 | 14 | 11.7 | |
| Crude estimate of malaria-attributable fraction of all fever casesc | 0.030 | 0.038 | 0.040 | 0.039 | 0.032 | |
| Latent class estimate (95% CI) of malaria-attributable fraction of all fever casesd | 0.047 (0.004–0.097) | 0.03 (0.003–0.077) | 0.017 (0.001–0.047) | |||
| Uíge Province | ||||||
| No. with fever | 433 | 291 | 282 | 238 | 210 | 112 |
| No. with no fever | 214 | 89 | 80 | 51 | 36 | 16 |
| Proportion with fever (%) | 66.9 | 76.6 | 77.9 | 82.4 | 85.4 | 87.5 |
| Proportion of test-negative cases with fever (%) | 53.2 | 53.0 | 54.5 | 55.6 | 61.8 | |
| Excess proportion with fever (%) | 23.4 | 24.9 | 27.9 | 29.8 | 25.7 | |
| Excess no. of fever cases | 88.9 | 90.2 | 80.6 | 73.2 | 32.8 | |
| Crude estimate of malaria-attributable fraction of all fever casesc | 0.205 | 0.210 | 0.186 | 0.169 | 0.076 | |
| Latent class estimate (95% CI) of malaria-attributable fraction of all fever casesd | 0.329 (0.244–0.405) | 0.271 (0.182–0.361) | 0.117 (0.026–0.200) |
Positive for any antigen level, as assessed with an ultrasensitive, bead-based, laboratory assay.
HRP2 concentration above the threshold of 3,000 pg/ml for a conventional RDT (SD Bioline Pf/Pv).
Assessed as the ratio of excess fever cases to all fever cases.
Assessed using the latent class model fit to quantitative data on protein concentrations (see text).
Characterization of antigen concentrations.
While nonzero HRP2 concentrations were measured in a substantial number of samples, aldolase and LDH were present at measurable levels in much smaller proportions of samples (Table 1). In samples with any detectable HRP2, there was a substantial difference in antigen concentrations between febrile and afebrile patients (see Fig. S1 in the supplemental material). In contrast, among the samples with nonzero concentrations of either aldolase or LDH, there was no clear difference in levels between febrile and afebrile patients (Fig. S1).
The Cohen’s kappa statistics measuring agreement in antigen positivity rates were high and highly statistically significant (P values of <0.0001), indicating that an antigen-positive sample was generally positive for all three antigens (Table S2). Samples with detectable aldolase and LDH levels almost always possessed relatively high HRP2 concentrations. However, the concordance correlation coefficients (19) were much lower, indicating that, among positive samples, the concentrations of the different proteins were not very closely related (Table S2). In all three Altman-Bland pairwise plots, there was a tendency for the differences to decrease with the averages, indicating that the three protein assays were measuring different underlying quantities (Fig. S2).
Estimation of malaria-attributable fractions using continuous antigen levels.
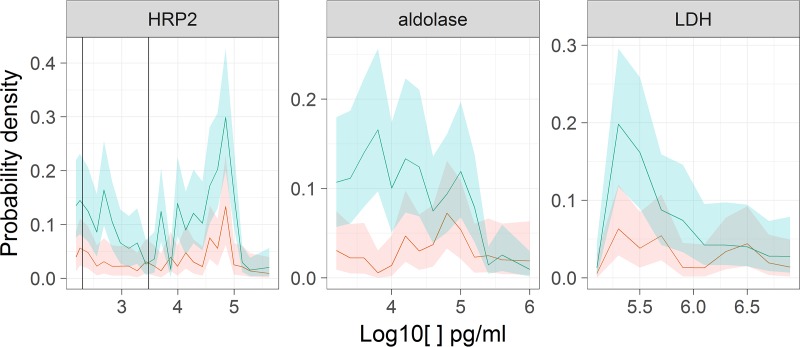
The estimated distribution of HRP2 levels in patients with illnesses of nonmalarial etiology but with detectable antigen levels (θ from equation 1) was generally unimodal in each province, centered between 100 and 300 pg/ml (Fig. 1). The distribution was shifted to the right in Uíge Province, where, in contrast to Huambo Province, there were estimated to be at least some patients with substantial HRP2 concentrations (>10,000 pg/ml) without malaria-induced fever.
FIG 1.
Estimates of θ, the estimated distributions of antigen concentrations in patients without malarial etiology but with detectable antigen levels. Shaded envelopes correspond to 95% CIs (blue, Uíge Province; red, Huambo Province). The vertical lines in the HRP2 panel correspond to the nominal LODs of the different types of RDTs (ultrasensitive RDT, 200 pg/ml; standard RDT, 3,000 pg/ml).
In contrast, the estimated distribution of HRP2 levels in patients with fever of malarial etiology (ϕ from equation 1) was shifted to very high antigen concentrations in both provinces (Fig. 2). It was estimated that virtually all malaria-induced fevers were accompanied by HRP2 concentrations above 10,000 pg/ml. Consequently, the likelihood that a fever case was attributable to malaria increased with the HRP2 concentration, with a sharp rise above 10,000 pg/ml (Fig. 3). The total malaria-attributable fractions estimated using the latent class model were 0.047 (95% confidence interval [CI], 0.004 to 0.097) in Huambo Province and 0.329 (95% CI, 0.244 to 0.405) in Uíge Province, substantially higher than the crude estimates (Table 1). The 95% CIs for the malaria-attributable fractions overlapped for patients under and over 5 years of age in both provinces (Table S1).
FIG 2.
Estimates of ϕ, the distribution of HRP2 antigen levels in febrile patients with true malarial etiology. Shaded envelopes correspond to 95% CIs (blue, Uíge Province; red, Huambo Province). Vertical lines are as in Fig. 1.
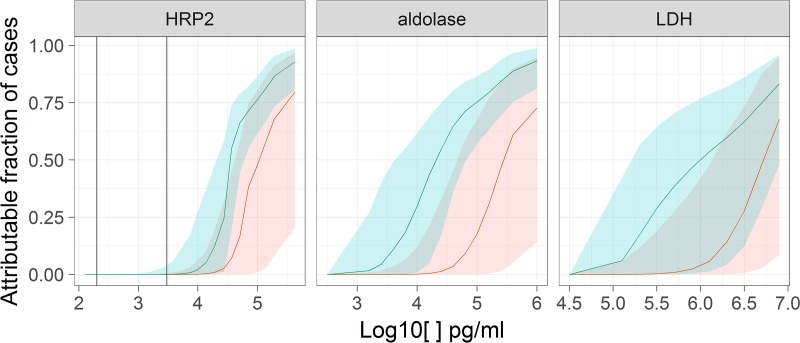
FIG 3.
Estimates of , the probability that cases are malaria attributable, according to antigen concentrations. Shaded envelopes correspond to 95% CIs (blue, Uíge Province; red, Huambo Province). Vertical lines are as in Fig. 1.
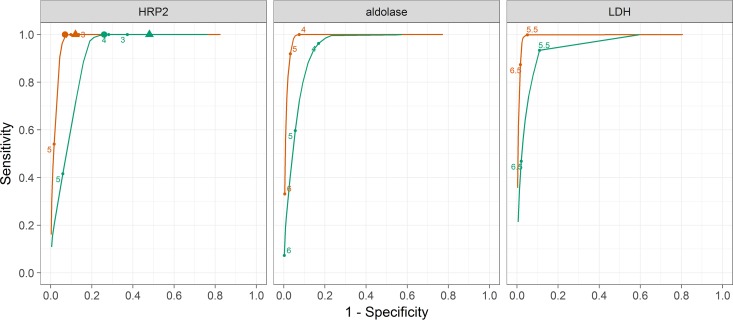
The sensitivities and specificities for different HRP2 cutoff thresholds were similar for the two provinces (Fig. 4 and Table 2). The estimate of the specificity curve was flat for a large range of HRP2 values, spanning several orders of magnitude, in Huambo Province, with virtually no change in specificity between the HRP2 cutoff thresholds for conventional and ultrasensitive tests. In Uíge Province, the specificity for the ultrasensitive test was 25 percentage points lower than that for the conventional test. An HRP2 test with a LOD of 3,000 pg/ml would be expected to provide a sensitivity of 0.99 (95% CI, 0.90 to 1.00) and a specificity of 0.92 (95% CI, 0.88 to 0.95) in Huambo Province and a sensitivity of 0.97 (95% CI, 0.88 to 1.00) and a specificity of 0.72 (95% CI, 0.65 to 0.78) in Uíge Province (Table 2). Increasing the LOD to 10,000 pg/ml would have minimal effects on either the sensitivity or the specificity (Table 2).
FIG 4.
Sensitivities and specificities of different antigen concentration thresholds for diagnosis of malaria fever. Blue indicates Uíge Province, and red indicates Huambo Province. The values are log10 antigen concentrations. Triangles, LOD of the ultrasensitive HRP2 RDT; circles, LOD of the standard HRP2 RDT.
TABLE 2.
Estimated sensitivity and specificity of different HRP2 detection thresholds for diagnosing malaria fever among outpatients from two provinces in Angola
| HRP2 detection threshold (pg/ml) | Estimate (95% CI) |
||||
|---|---|---|---|---|---|
| Parasite density (parasites/µl)a | Huambo Province |
Uíge Province |
|||
| Sensitivity | Specificity | Sensitivity | Specificity | ||
| 100 | 0.35 (0.1–1) | 1.00 (1.00–1.00) | 0.32 (0.14–0.47) | 1.00 (1.00–1.00) | 0.28 (0.20–0.36) |
| 300 | 1.2 (0.5–3) | 1.00 (0.96–1.00) | 0.41 (0.19–0.59) | 0.99 (0.95–1.00) | 0.39 (0.29–0.49) |
| 1,000 | 4.3 (2–8) | 0.99 (0.93–1.00) | 0.91 (0.87–0.94) | 0.98 (0.90–1.00) | 0.66 (0.59–0.73) |
| 3,000 | 14 (8–24) | 0.99 (0.90–1.00) | 0.92 (0.88–0.95) | 0.97 (0.88–1.00) | 0.72 (0.65–0.78) |
| 10,000 | 53 (32–87) | 0.97 (0.83–1.00) | 0.94 (0.90–0.96) | 0.95 (0.84–1.00) | 0.76 (0.70–0.81) |
| 30,000 | 177 (101–311) | 0.91 (0.72–1.00) | 0.95 (0.92–0.97) | 0.86 (0.75–0.97) | 0.81 (0.75–0.86) |
| 100,000 | 660 (322–1,352) | 0.29 (0.16–0.50) | 0.99 (0.98–1.00) | 0.30 (0.23–0.40) | 0.94 (0.90–0.97) |
Estimated by comparison of parasite densities determined by quantitative reverse transcription-PCR and HRP2 concentrations measured with the Luminex assay.
For the aldolase and LDH antigens, the estimated distributions of concentrations among patients whose fever was unrelated to the diagnostic test target (θ from equation 1) were determined predominantly by the test-negative cases (Fig. 1). In contrast, the distributions of concentrations in the malaria-attributable fever cases (ϕ from equation 1) were very similar, for both antigens, to the raw distributions of concentrations among test-positive cases (Fig. 2; also see Fig. S1 in the supplemental material). For both antigens, the probability density was shifted to the right for Huambo Province, compared with Uíge Province.
The probabilities that fever cases were attributable to malaria were much higher in Uíge Province than in Huambo Province at any given concentration of either antigen (Fig. 3). Correspondingly, the estimates of the sensitivities and specificities for different thresholds showed the same patterns for both antigens, with the sensitivities being higher for Huambo Province (Fig. 4). A limitation of this analysis is that it cannot allow for malaria cases with antigen levels below the LOD of the test, which possibly makes the sensitivity appear much higher than it should be. The specificity of any case definition requiring a detectable concentration of either molecule was close to 100%, i.e., any detection was sufficient to indicate malarial disease. Unlike the case of HRP2, for which the latent class model gave higher attributable fraction estimates than the crude estimates based on single cutoff thresholds, the malaria-attributable fractions for Huambo Province estimated using the latent class model for aldolase and LDH levels were lower than the crude estimates, while those for Uíge Province were higher than the crude estimates (Table 1). All of the attributable fraction estimates based on aldolase and LDH data were lower than those based on HRP2 data.
DISCUSSION
The results presented here confirm that the presence of malaria antigen alone does not equate to symptomatic disease, and they suggest that quantitative measures of antigen concentrations can inform characterization of the likelihood that a given fever case is attributable to malaria. This is particularly relevant for the HRP2 antigen, which was detectable in a substantial proportion of samples from afebrile outpatients. Crude comparisons of the presence of HRP2, at levels detectable by the conventional RDT used, in afebrile and febrile patients suggested that 3.8% of fevers in low-transmission Huambo Province and 19% in high-transmission Uíge Province were attributable to malaria. Consideration of the presence of any HRP2 (using the LOD of the ultrasensitive bead-based assay) made little difference to these estimates (3% in Huambo Province and 21% in Uíge Province). However, using a latent class statistical model to analyze continuous data for antigen correlations yielded substantially higher estimates; with the application of that model, 9.2% of fevers in Huambo Province and 39% of fevers in Uíge Province were determined to be attributable to malaria. HRP2-detecting tests with LODs between 3,000 and 10,000 pg/ml would be expected to provide good sensitivity and specificity in identifying clinically relevant HRP2 concentrations. Optimal thresholds did not differ according to age or province, but the sample sizes in the stratified analysis were small.
In contrast to HRP2, aldolase and LDH were generally undetectable in afebrile patients; however, they were also undetectable in many of the patients diagnosed with malaria based on HRP2 levels. The estimates of the overall attributable fractions from the latent class model for aldolase were lower than those for HRP2, and those for LDH were even lower, because only a subset of the samples with high HRP2 levels had measurable concentrations of the other two antigens. This finding suggests that the LODs of the bead-based assay for LDH and aldolase might be too high to provide adequate sensitivity and that RDTs detecting these antigens should aim to have LODs lower than those of the bead-based assay used in this analysis.
Pairwise comparisons of absolute concentrations of the three antigens suggest that each antigen is a different measure of parasite burden. Since all three antigens are produced by blood-stage parasites, the concentrations of these three antigens would all increase in the human host during P. falciparum proliferation. Consequently, the differences can be explained most easily by differences in the degradation kinetics, clearance from the human host, or excretion by the parasite. Because it is a protein completely exogenous to the human body, with no known homology to other human proteins, the unique nature of HRP2 in sequence and structure may lead to delayed clearance from the human host. HRP2 is produced and excreted into the extracellular matrix in large quantities during the ring, trophozoite, and schizont stages (22), which is one of the reasons why it persists in the circulation (23). When HRP2 is used as a diagnostic marker for clinical cases, it may reflect the total parasite load, rather than current peripheral parasite densities. If there is synchronization of the parasite cycle, then HRP2 levels might reflect the total load of parasites (including those that are sequestered) even better than a direct parasitological measure. This consideration has motivated the use of HRP2 levels as a measure of total parasite biomass (13, 14) but is a disadvantage when HRP2 tests are used to estimate the point prevalence of active infections in population surveys, because HRP2 levels can persist for several weeks after parasites have been cleared (23).
Differences in the results for HRP2, aldolase, and LDH antigen concentrations could arise from the differences in the LODs of the bead-based assay. The LODs of the bead-based assay are substantially higher for LDH and aldolase than for HRP2; therefore, the sensitivity, specificity, and population attributable fraction estimates for the three different antigens should not be compared directly. Because detection antibodies for HRP2 would also bind HRP3, this analysis cannot distinguish between HRP2 and HRP3 antigen concentrations. For a wild-type Pfhrp2 and Pfhrp3 population, however, such as in Angola (16), this would have no effect on the interpretation of the assay signals, since every parasite isolate would be considered the same. An important limitation of our study is the limited geographic scope of the original surveys. A variety of factors could influence the pyrogenic antigen threshold estimates presented here, and the estimates may not be generalizable to other settings. Such factors include host immunity and clearance mechanisms and parasite factors such as different levels of antigen expression. Nearly all malaria infections analyzed here were due to P. falciparum, and it is not known how the LDH and aldolase pyrogenic thresholds reported here would translate to other species.
Crucially, the validity of the latent class method for estimation of the thresholds for clinically relevant antigen concentrations is sensitive to the assumption that fever is a proxy for clinical malaria. However, there is evidence that not every clinical manifestation of malaria includes fever (24). Finally, the results presented here are most relevant in the clinical context, where attribution of fever to an etiologic agent is important for appropriate clinical care. From the point of view of elimination or transmission reduction, any malaria infection should be detected and cleared, whether or not it is pyrogenic.
To address some of these limitations, similar studies collecting quantitative data on antigen concentrations for both febrile and afebrile individuals could be performed in other settings and populations. Such data could inform future RDT product testing, especially as it switches from testing products against fixed parasite densities to testing against fixed antigen concentrations. Data on the ideal antigen concentration threshold for attributing fever to malaria can also inform RDT product development, as manufacturers calibrate the LODs of the RDTs. Finally, quantitative antigen detection in samples from controlled human malaria infections could complement determination of pyrogenic antigen thresholds.
Supplementary Material
ACKNOWLEDGMENTS
We thank Camelia Herman, Austin Lu, and Andi Otto for their assistance in sample processing and data collection in the laboratory.
M.M.P. and E.S.H. were supported by the U.S. President’s Malaria Initiative.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01901-18.
REFERENCES
- 1.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. 2013. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther 11:623–639. doi: 10.1586/eri.13.45. [DOI] [PubMed] [Google Scholar]
- 2.Laishram DD, Sutton PL, Nanda N, Sharma VL, Sobti RC, Carlton JM, Joshi H. 2012. The complexities of malaria disease manifestations with a focus on asymptomatic malaria. Malar J 11:29. doi: 10.1186/1475-2875-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mwangi TW, Ross A, Snow RW, Marsh K. 2005. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J Infect Dis 191:1932–1939. doi: 10.1086/430006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bisoffi Z, Sirima SB, Menten J, Pattaro C, Angheben A, Gobbi F, Tinto H, Lodesani C, Neya B, Gobbo M, Van den Ende J. 2010. Accuracy of a rapid diagnostic test on the diagnosis of malaria infection and of malaria-attributable fever during low and high transmission season in Burkina Faso. Malar J 9:192. doi: 10.1186/1475-2875-9-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogier C, Commenges D, Trape J-F. 1996. Evidence for an age-dependent pyrogenic threshold of Plasmodium falciparum parasitemia in highly endemic populations. Am J Trop Med Hyg 54:613–619. [DOI] [PubMed] [Google Scholar]
- 6.Müller I, Genton B, Rare L, Kiniboro B, Kastens W, Zimmerman P, Kazura J, Alpers M, Smith TA. 2009. Three different Plasmodium species show similar patterns of clinical tolerance of malaria infection. Malar J 8:158. doi: 10.1186/1475-2875-8-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dicko A, Mantel C, Kouriba B, Sagara I, Thera MA, Doumbia S, Diallo M, Poudiougou B, Diakite M, Doumbo OK. 2005. Season, fever prevalence and pyrogenic threshold for malaria disease definition in an endemic area of Mali. Trop Med Int Health 10:550–556. doi: 10.1111/j.1365-3156.2005.01418.x. [DOI] [PubMed] [Google Scholar]
- 8.Smith T, Schellenberg JA, Hayes R. 1994. Attributable fraction estimates and case definitions for malaria in endemic areas. Stat Med 13:2345–2358. [DOI] [PubMed] [Google Scholar]
- 9.Vounatsou P, Smith T, Smith A. 2002. Bayesian analysis of two‐component mixture distributions applied to estimating malaria attributable fractions. J R Stat Soc Ser C Appl Stat 47:575–587. doi: 10.1111/1467-9876.00129. [DOI] [Google Scholar]
- 10.Barnwell J. 2009. Implications of parasite density thresholds for product-testing, lot-testing and positive control wells, p 39–41. In Parasitological confirmation of malaria diagnosis: report of a WHO technical consultation, Geneva, 6–8 October 2009. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 11.World Health Organization. 2017. World malaria report 2017. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 12.World Health Organization. 2017. Malaria rapid diagnostic test performance: results of WHO product testing of malaria RDTs: round 7 (2015–2016). World Health Organization, Geneva, Switzerland. [Google Scholar]
- 13.Ochola L, Marsh K, Lowe B, Gal S, Pluschke G, Smith T. 2005. Estimation of the sequestered parasite load in severe malaria patients using both host and parasite markers. Parasitology 131:449–458. doi: 10.1017/S0031182005008085. [DOI] [PubMed] [Google Scholar]
- 14.Dondorp AM, Desakorn V, Pongtavornpinyo W, Sahassananda D, Silamut K, Chotivanich K, Newton PN, Pitisuttithum P, Smithyman AM, White NJ, Day NPJ. 2005. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med 2:e204. doi: 10.1371/journal.pmed.0020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plucinski MM, Ferreira M, Ferreira CMF, Burns J, Gaparayi P, João L, da Costa O, Gill P, Samutondo C, Quivinja J, Mbounga E, Ponce de León G, Halsey ES, Dimbu PR, Fortes F. 2017. Evaluating malaria case management at public health facilities in two provinces in Angola. Malar J 16:186. doi: 10.1186/s12936-017-1843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plucinski MM, Herman C, Jones S, Dimbu R, Fortes F, Ljolje D, Lucchi N, Murphy SC, Smith NT, Cruz KR, Seilie AM, Halsey ES, Udhayakumar V, Aidoo M, Rogier E. 2019. Screening for Pfhrp2/3-deleted Plasmodium falciparum, non-falciparum, and low-density malaria infections by a multiplex antigen assay. J Infect Dis 219:437–. doi: 10.1093/infdis/jiy525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogier E, Plucinski M, Lucchi N, Mace K, Chang M, Lemoine JF, Candrinho B, Colborn J, Dimbu R, Fortes F, Udhayakumar V, Barnwell J. 2017. Bead-based immunoassay allows sub-picogram detection of histidine-rich protein 2 from Plasmodium falciparum and estimates reliability of malaria rapid diagnostic tests. PLoS One 12:e0172139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plucinski M, Dimbu R, Candrinho B, Colborn J, Badiane A, Ndiaye D, Mace K, Chang M, Lemoine JF, Halsey ES, Barnwell JW, Udhayakumar V, Aidoo M, Rogier E. 2017. Malaria surveys using rapid diagnostic tests and validation of results using post hoc quantification of Plasmodium falciparum histidine-rich protein 2. Malar J 16:451. doi: 10.1186/s12936-017-2101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence I, Lin K. 1989. A concordance correlation coefficient to evaluate reproducibility. Biometrics 45:255–268. doi: 10.2307/2532051. [DOI] [PubMed] [Google Scholar]
- 20.Bland JM, Altman DG. 1999. Measuring agreement in method comparison studies. Stat Methods Med Res 8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 21.Spiegelhalter D, Thomas A, Best N, Lunn D. 2003. WinBUGS version 1.4. Institute of Public Health, Cambridge, UK. [Google Scholar]
- 22.Howard RJ, Uni S, Aikawa M, Aley SB, Leech JH, Lew AM, Wellems TE, Rener J, Taylor DW. 1986. Secretion of a malarial histidine-rich protein (Pf HRP II) from Plasmodium falciparum-infected erythrocytes. J Cell Biol 103:1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plucinski MM, Dimbu PR, Fortes F, Abdulla S, Ahmed S, Gutman J, Kachur SP, Badiane A, Ndiaye D, Talundzic E, Lucchi N, Aidoo M, Udhayakumar V, Halsey E, Rogier E. 2018. Post-treatment HRP2 clearance in patients with uncomplicated Plasmodium falciparum malaria. J Infect Dis 217:685–692. doi: 10.1093/infdis/jix622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mutanda AL, Cheruiyot P, Hodges JS, Ayodo G, Odero W, John CC. 2014. Sensitivity of fever for diagnosis of clinical malaria in a Kenyan area of unstable, low malaria transmission. Malar J 13:163. doi: 10.1186/1475-2875-13-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.